Introduction
According to the GLOBOCAN 2018 data interpretation,
breast cancer (BC) is the most commonly diagnosed type of cancer
and the leading cause of cancer-related mortality among women,
followed by colorectal and lung cancer (in terms of incidence), and
vice versa (in terms of mortality) (1). Several breast cancer patients exhibit
immunosuppression, which is enhanced after surgery, radiotherapy
and chemotherapy. Impaired immunity leads to T cell dysfunction,
which allows tumor cells to escape immune surveillance. The
insufficient inhibition of breast tumor growth may be explained by
the heterogeneous expression of tumor antigens within the primary
tumor or its metastases, the modification of the tumor's antigenic
profile during disease progression, and the low levels of
tumor-associated antigens (TAAs), MHC proteins and other
costimulatory proteins required to generate an efficient immune
response (2). However, as recently
exemplified by metastatic non-small-cell lung cancer, tumor types
not traditionally considered as responsive to immunotherapy may
become immunogenic following appropriate immune activation
(3). Thus, immunotherapy is
currently widely recognized as a key element in the treatment of
cancer, including BC (4,5).
Dendritic cells (DCs), which are considered to be
the strongest stimulators of T cell responses, play a crucial role
in the initiation of the primary immune response (5,6). DCs
modulate the activities of immunocompetent cells, and may correct
the disrupted presentation of TAAs and stimulate the production of
antigen-specific cytotoxic T lymphocytes (5–10). It
has also been demonstrated that DC vaccines may display powerful
Th1-polarizing ability that stimulates antitumor activity against
autologous tumor cells in vitro (11). Therapy employing DCs and cytotoxic
DC-induced antigen-specific T lymphocytes efficiently eliminates
residual cancer cells that are the key cause of tumor recurrence
and metastasis. Therefore, the potential effectiveness of DC-based
vaccines has been employed in the treatment of BC (11–13).
The selection of the DC antigen-loading strategy may
affect treatment efficiency. The basic principles of obtaining
antigen-loaded DCs are actively updated with new methods of ex
vivo and in vivo cell modifying. The simplest methods
for antigen loading of DCs are using tumor-associated antigenic
peptides (14) or mRNA from tumor
antigens (15). In addition, DCs
may be transfected ex vivo with DNA constructs encoding
tumor antigens (16,17) or loaded in vivo using DNA
vaccines (18). Although highly
efficient in antigen loading and combating cancer, the DС
transfection technologies and DNA vaccine approaches are
labor-intensive and more costly.
The main advantage of the widely used ex vivo
DC antigen priming approach is that it allows circumventing the
dysfunction of endogenously activated DCs (19) that occurs in numerous patients with
BC, and the transfer of highly active induced cells may improve the
effector mechanisms involved in tumor cell lysis (20). Furthermore, the use of autologous
tumor cell lysates for immune system priming allows the induction
of cellular immune responses against specific tumor tissues and
promotes specific antitumor response, as tumor tissues contain the
most actual set of TAAs that can be unique to each patient
(21). Thus, the approach involving
ex vivo DC activation and T cell priming and the use of
autologous tumor cell lysate for antigen loading is currently
considered as a promising strategy for cancer treatment.
The present study modeled the naturally occurring
activation induced by stimulating type 1 T-helper cell and
cytotoxic T cell cytotoxicity with IL-12 and IL-18 in co-cultures
of DCs and mononuclear cells. IL-18 is a pleiotropic cytokine that
contributes to the regulation of innate and adaptive immunity. In
the presence of IL-12 or IL-15, IL-18 powerfully induces the
secretion of interferon-γ by natural killer cells and type-1 CD4
helper T cells, and modulates the activity of CD8 cytotoxic cells
and neutrophils, depending on their microenvironment (22–24).
Thus, the aim of the present study was to determine
whether the combination of ex vivo priming of DCs with
antigens present in tumor lysates and the in vitro formation
of a pool of antigen-specific cytotoxic cells is promising for
efficient antitumor immune response activation in BC patients.
Materials and methods
Study design and eligibility
criteria
The present phase I/II prospective study was
designed to evaluate the toxicity, antitumor activity and immune
responses to vaccination. The eligibility criteria were as follows:
Age 28–65 years, leukocytes >3,000/mm3, neutrophils
>1,500/mm3, platelets >100,000/mm3, and
negative tests for human immunodeficiency virus, and hepatitis B
and C viruses. The exclusion criteria were as follows: Cerebral
metastases, positive pregnancy test, autoimmune diseases or other
medical conditions, such as decompensated heart failure, severe
anemia and pancytopenia, that constitute contraindications. Prior
chemotherapy and treatment with cytokines was permitted; however,
concomitant immunotherapy was not. The trial was conducted in
accordance with the principles outlined in the Declaration of
Helsinki. Written informed consent was obtained from each patient
prior to inclusion. Patients were enrolled between December 2013
and October 2015 at the Third Oncological Department of the
Novosibirsk City Clinical Hospital No. 1. The patients received DC
therapy in the Clinic of Immunopathology of the Institute of
Clinical and Fundamental Immunology.
Historical controls were recruited from the database
of three oncology departments of the Novosibirsk City Hospital. The
medical records of patients treated between 2012 and 2014 were
reviewed. For each patient from our group, a patient who did not
receive immunotherapy was selected. The patients and control
subjects were matched for age, stage, molecular type and treatment
regimen. Selected cases were analyzed to determine the clinical
outcome at 3 years after surgery.
The study protocol was approved by the local Ethics
Committee of the Institute of Clinical and Fundamental Immunology
(protocol no. 78, September 12, 2013). The study is registered at
www.clinicaltrials.gov (NCT 03113019).
The patent of the Russian Federation was obtained for the approach
described (no. 2645464).
Evaluation of patients and treatment
schedule
Clinical evaluation included a complete medical
history, chest X-ray, tumor staging, histological and
immunohistochemical analysis of tumors, blood chemistry, hematology
and urine analysis. Patients received three vaccinations at 1-week
intervals. Autologous cells were injected into patients with stage
IIa-IIIc BC after completion of chemotherapy. Patients with
underlying progressive disease or those initially diagnosed with
stage IV disease were injected with autologous immune cells during
the interval between hormone therapy cycles.
Preparation of tumor cell lysates
Tumor cell lysates were prepared from tissue samples
after radical surgery or incisional biopsy. A 1–3-cm3
tumor tissue sample was placed into a sterile tube, the adjacent
(macroscopically unaltered) tissues were removed, and the sample
was subjected to mechanical homogenization and four freeze-thaw
cycles (−80°C and room temperature, respectively). Larger particles
were removed by centrifugation at 266 × g and 24°C (room
temperature) for 2 min. The lysate was filter-sterilized (filter
pore diameter=0.45 µm; TPP Techno Plastic Products AG), and the
protein concentration of the lysate was measured using a NanoDrop
2000 (Thermo Fisher Scientific, Inc.). The tumor cell lysate was
divided into aliquots, frozen, and stored at −80°C.
DC preparation and
characterization
Mononuclear cells were isolated from the peripheral
blood of the patients using Ficoll gradient centrifugation. The
isolated peripheral blood mononuclear cells (PBMCs) were washed in
RPMI-1640 medium (Biolot) and centrifuged twice at at 266 × g and
24°C (room temperature) for 10 min. Cells were cultured in
RPMI-1640 medium supplemented with 10% FBS (HyClone; GE Healthcare
Life Sciences), 2 mM L-glutamine (Biolot), 10 mM HEPES (Biolot), 80
µg/ml gentamycin (KRKA), and 100 µg/ml ampicillin (Sintez).
Mononuclear cells (1–1.5×106 cells/ml) in RPMI-1640
complete medium supplemented with 10% FBS were placed into
150-cm2 (690 ml) culture flasks (TPP Techno Plastic
Products AG) with vented caps. The cells were allowed to adhere to
the flasks in a CO2 incubator at 37°C and 100% humidity
for 30 min. Viable non-adherent mononuclear cells (non-adherent
PBMCs) were cultured in complete RPMI-1640 medium with partial
media changes on days 3 and 5. The adherent cells were removed
using a cell scraper and washed with RPMI-1640 medium. The adherent
cell fraction was used to generate mature antigen-activated DCs.
For this purpose, 100 ng/ml granulocyte-macrophage
colony-stimulating factor (GM-CSF; BioVision, Inc.) and 50 ng/ml
IL-4 (BioVision, Inc.) were added to the cells, which were cultured
in a 75-cm2 (270 ml) (TPP Techno Plastic Products AG)
culture flask in complete RPMI-1640 medium supplemented with 10%
FBS for 72 h to generate immature DCs. The tumor tissue lysate (100
µg/ml) was added to immature DCs for 24 h. To obtain mature DCs,
TNF-α (25 ng/ml) (BioVision, Inc.) was added to the fresh medium
within 48 h. The DC preparations were subjected to quality control
tests (viability, cell count, purity) and then to flow cytometry
using a FACSVerse flow cytometer (Becton-Dickinson and Company).
Monoclonal antibodies (mAbs) were as follows: CD11c (cat. no.
371508, cloneS-HCL-3), CD83 (cat. no. 305310, clone HB15e), HLA-DR
(cat. no. 307604, clone L 243), CD86 (cat. no. 305406, clone
IT2.2), CD123 (cat. no. 306012, clone 6H6) (BioLegend, Inc.),
Lineage Cocktail (cat. no. 348801, clone UCHT1, HCD14, 3G8, HIB19,
2H7 and HCD56) (BioLegend, Inc.); CD205 (cat. no. 558069, clone MG
38) and CD209 (cat. no. 558263, clone DCN 46) (Becton-Dickinson and
Company). All antibodies from BioLegend were added as 5 µl per 1
million cells in 100 µl staining volume (antibody:cell suspension
volume ratio, 1:20). The Lineage Cocktail, CD205 and CD209 (Becton,
Dickinson and Company) were added as 20 µl per 1 million cells in
100 µl staining volume (ratio, 1:5). All dilutions mentioned were
as recommended by the manufacturers.
Activation, characterization and
injection of mononuclear cells
Mature DCs and non-adherent PBMCs were mixed at a
ratio of 1:10 and cultured in the presence of 8 ng/ml IL-12 and 80
ng/ml IL-18 (both from BioVision, Inc.) to stimulate Th1
polarization of the activated T cells over 96 h. The resulting cell
suspensions (autologous tumor lysate-loaded mature DCs and
activated PBMCs) were washed and frozen in Freezing Medium (Biolot)
supplemented with 10% FBS and 10% DMSO. The cells were stored at
−150°C and defrosted on the day of injection. The cryotube
containing the cells was placed in a water bath and the cells were
then transferred to a sterile 15-ml test tube containing 4 ml
RPMI-1640 medium and 1 ml FBS. The cells were then thoroughly
resuspended and centrifuged at 266 × g and 24°C (room temperature)
for 10 min. An aliquot of cells was used for analysis. The thawed
cells were cultured for 3 h in RPMI-1640 medium supplemented with
10% FBS, and the non-adherent fraction was prepared for intravenous
administration. The cells were washed with 0.9% sodium chloride
(saline) solution three times, and cell count and viability (≥95%)
were determined. The cells for intravenous administration
(20–30×106) were resuspended in 100 ml 0.9% saline
solution supplemented with 2 ml 10% albumin.
The adherent fraction was removed from the culture
flask surface using a cell scraper, the cells were washed three
times with saline, and cell count and viability (≥95%) were
determined. The cells for subcutaneous administration
(2–3×106) were resuspended in 800–1,000 µl 0.9% saline
solution and injected subcutaneously into three sites within the
intrascapular region. Intravenous administration of the cells was
performed along with 8 mg dexamethasone. All the cell preparations
were subjected to quality control (viability, cell count and
purity) and then to flow cytometry analysis using a FACSVerse flow
cytometer (Becton-Dickinson and Company). The mAbs (20 µl per 100
µl staining volume) used were as follows: 6-color TBNK (cat. no.
644611; Becton-Dickinson and Company) containing CD45, CD3, CD4,
CD8, CD19 and CD16/56.
Evaluation of immunity
To evaluate the relative content of CD3+,
CD4+, CD8+, CD19+,
CD16/56+ and CD4+CD25+
FoxP3+ cells, circulating DCs and the expression of
HLA-DR on monocytes, PBMCs were subjected to flow cytometry before
surgery, before immunotherapy, and at 3 and 6 months after
completion of the immunotherapy.
Cytotoxicity assay
Cytotoxicity was assessed by determining the lactate
dehydrogenase (LDH) activity in the conditioned medium of DC and
non-adherent PBMC co-cultures and the BC-derived cell line MCF-7
(Russian Collection of Cell Cultures, Institute of Cytology), using
a CytoTox 96 Non-Radioactive Cytotoxicity Assay (G1780; Promega
Corporation). PBMCs were isolated using Ficoll gradient
centrifugation (PanEco) from peripheral blood before the
immunotherapy and at 3 and 6 months after completion of the
immunotherapy. Cells were frozen in Freezing Medium (Biolot)
supplemented with 10% FBS and stored at −150°C. Samples acquired
from each patient before and after therapy were tested in the same
experiment. PBMCs (1–105 cells per well) were incubated
in triplicate in 96-well round-bottom tissue culture plates (10:1,
PMBCs:tumor cells) for 16 h. LDH activity in the culture
supernatants was measured using a 30-min coupled enzymatic assay
that measures the conversion of the tetrazolium salt INT into red
formazan. The absorbance of visible light was determined using a
standard 96-well plate reader. The amount of color was proportional
to the number of lysed cells.
Evaluation criteria and statistical
analysis
Adverse events were classified according to the
CTCAE ver. 4.03 (2010) (25). The
World Health Organization criteria (WHO Handbook for Reporting the
Results of Cancer Treatment, 1979, Geneva) (26) were used for patients with stage IV
disease or progression of the underlying disease. A complete
response) was defined as the complete disappearance of all
clinically detectable disease. A partial response was defined as a
≥50% decrease in all measurable lesions, without an increase in the
size of any target lesion or the appearance of new lesions. Stable
disease was defined as the absence of a significant change for 4
weeks or an increase of <25% or a decrease of <50% in tumor
size, and no new lesions. Progressive disease was defined as a ≥25%
increase in the sum of the products of the measurable lesions or
appearance of new lesions. For patients with stages 2a-3c, blood
was collected after 3 and 6 months to evaluate immunological and
clinical parameters.
GraphPad Prism 6 software (GraphPad Software, Inc.)
was used to analyze the data, which are presented as the median and
the interquartile ranges (upper and lower quartile, UQ and LQ,
respectively). Significant differences between the means were
determined using ANOVA for repeated measurements and Tukey's
multiple comparisons test. The Kaplan-Meier method was used to
generate survival curves. The medical records of patients of the
same age and disease stage were analyzed to generate the
Kaplan-Meier curves. P<0.05 was considered to indicate
statistically significant differences.
Results
Patient characteristics
The clinical characteristics of the patients (n=30)
are listed in Table I. A total of
25 patients underwent surgery, 3 patients had stage IV disease, 1
patient experienced disease progression after surgery, and 1
patient progressed during neoadjuvant chemotherapy. In the
remaining 5 patients, tumor samples were obtained using incisional
biopsy of the metastases (skin). The sites of metastasis included
the lungs, skin, lymph nodes and bones. A tumor sample was obtained
during radical surgery (radical mastectomy or radical sector
resection together with axillary lymphadenectomy) in 25 patients.
All patients received chemotherapy (anthracyclines and/or taxanes)
and radiotherapy after surgery.
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
|
Characteristics | Number of patients
(%) |
|---|
| Disease stage |
| T2N0M0
(IIA) | 10 (34.48) |
| T1N1M0
(IIA) | 3 (10.34) |
| T2N1M0
(IIB) | 6 (20.68) |
| T2N2M0
(IIIA) | 2 (6.9) |
| T2N3M0
(IIIC) | 2 (6.9) |
| T1N3M0
(IIIC) | 1 (3.45) |
| TxN3M0
(IIIC) | 1 (3.45) |
| T2N2M0
(IIIA), progression after neoadjuvant therapy | 1 (3.45) |
| T2N2M0
(IIIA), progression after therapy | 1 (3.45) |
| T2NxM1
(IV) | 1 (3.45) |
| T4N3M1
(IV) | 1 (3.45) |
| TxNxM1
(IV) | 1 (3.45) |
| Histological
characteristics of the tumor after radical surgery |
|
Moderately differentiated
invasive ductal carcinoma (GII) | 25 (100.0) |
|
Solid-glandular growth
type | 22 (88.0) |
|
Solid-glandular with areas of
scirrhous growth pattern | 1 (4.0) |
|
Solid-trabecular | 1 (4.0) |
|
Solid-cribriform | 1 (4.0) |
| Molecular subtypes
of the tumor after radical surgery |
|
Isolated expression or
overexpression of HER-2 | 7 (28.0) |
|
Triple-negative | 5 (20.0) |
| Luminal
A | 10 (40.0) |
| Luminal
B (overexpression of HER-2/neu) | 1 (4.0) |
| Luminal
B (Ki-67 >20%). | 2 (8.0) |
Characterization of DCs and activated
mononuclear cells used for injection
DCs were prepared from autologous monocytes cultured
in the presence of GM-CSF and IL-4. The DC preparations were
subjected to quality control tests (viability, cell count and
purity) and then to flow cytometry using a FACSVerse flow
cytometer. Cell viability was 90–95% as assessed by Trypan blue
staining. The purity of the mature DC population was determined by
flow cytometry. To determine the phenotype of mature DCs,
lineage-negative (CD3−, CD14−,
CD16−, CD19−, CD20− and
CD56−) HLA-DR+ cells were isolated from the
population of CD45+ cells, and the levels of
CD123-plasmacytoid and CD11c-myeloid DCs were determined. Their
populations were 2.35% (UQ; LQ: 4.45; 1.25) and 51.75% (UQ; LQ:
64.35; 23.925), respectively. To assess the expression of specific
molecules expressed by DCs in the population of large granular
lymphocytes, the CD11c+HLA-DR+ cell
population was isolated, and the abundance of
CD83+CD86+ and
CD205+CD209+ double-positive DCs was 97.65%
(UQ; LQ: 94.925; 99.3) and 97.5% (UQ; LQ: 99.1; 92.9), respectively
(Table II).
 | Table II.Cellular composition of the vaccine
used to immunize patients who underwent the immunotherapy with
autologous antigen-activated DCs. The data are presented as the
median and interquartile range (n=25). |
Table II.
Cellular composition of the vaccine
used to immunize patients who underwent the immunotherapy with
autologous antigen-activated DCs. The data are presented as the
median and interquartile range (n=25).
| CD45+
leukocytes (100%) | CD45+
leukocytes (100%) |
|---|
|
|
|---|
|
| CD3+
85.8% (UQ; LQ: 88.05; 80.75) | CD3−
14.2% (UQ; LQ: 19.25; 11.95) | Lineage-negative
(CD3−, CD14−, CD16−,
CD19−, CD20− ans CD56−)
HLA-DR |
|---|
|
CD4+ |
CD8+ |
CD16/56+ |
CD19+ | CD123-plasmacytoid
DCs 2.35% (UQ; LQ: 4.45; 1.25) | CD11c-myeloid DCs
51.75% (UQ; LQ: 64.35; 23.925) |
| 59.3% | 21.8% | 36.7% | 23% |
| CD83+
CD86+ 97.65% (UQ; LQ: 94.925; 99.3) |
| (UQ; LQ: 63.83;
51.03) | (UQ; LQ: 31.15;
18.28) | (UQ; LQ: 43.55;
28.83) | (UQ; LQ: 27.25;
15.8) |
| CD205+
CD209+ 97.5% (UQ; LQ: 99.1; 92.9) |
The main subpopulations were measured using the
gating scheme recommended by the manufacturer (6-color TBNK,
Becton, Dickinson and Company). Typical dot plots displaying the
gating scheme used are provided as supplementary material (Fig. S1). The cellular compositions of the
fraction of injected mononuclear cells were characterized
accordingly (Table II).
CD3+ and CD3− T cells were isolated from the
CD45+ T cell population. CD4+ and
CD8+ T cell counts were determined in the
CD3+ T cell population, while
CD16+/56+and CD19+cell counts were
determined in the CD3− T cell population. DCs and
activated non-adherent PBMCs were generated in all patients. The
cultured adherent cells developed elongated, stellate cell
processes, which are characteristic of DCs. Following co-culture
with non-adherent PBMCs, these cells formed characteristic
clusters.
Clinical activity for patients with
stage IV or progressive disease
Among patients with stage IV disease, 2 remained
stable for 6 months. Patient no. 25 developed local progression,
distant foci were undetectable, and chemotherapy was not
administered. Patient no. 23 succumbed to disease progression (two
injections were administered). Three lines of chemotherapy did not
achieve positive response. After cell infusion, the patient noted a
decrease in the pain associated with the affected breast; however,
it was impossible to perform confirmatory tests. The general
condition of patient no. 24 deteriorated (multiple bone metastases,
pathological fracture of the femoral neck). For personal reasons,
the patient refused treatment with the recommended antitumor drugs
(except zoledronic acid). After the introduction of the cells, the
patient noted a decrease in pain in the lumbar spine.
Immune responses
No significant differences were observed in the
numbers of CD3+, CD4+, CD8+ and
CD16/56+ cells before surgery and after immunotherapy.
Statistically significant differences were detected only for
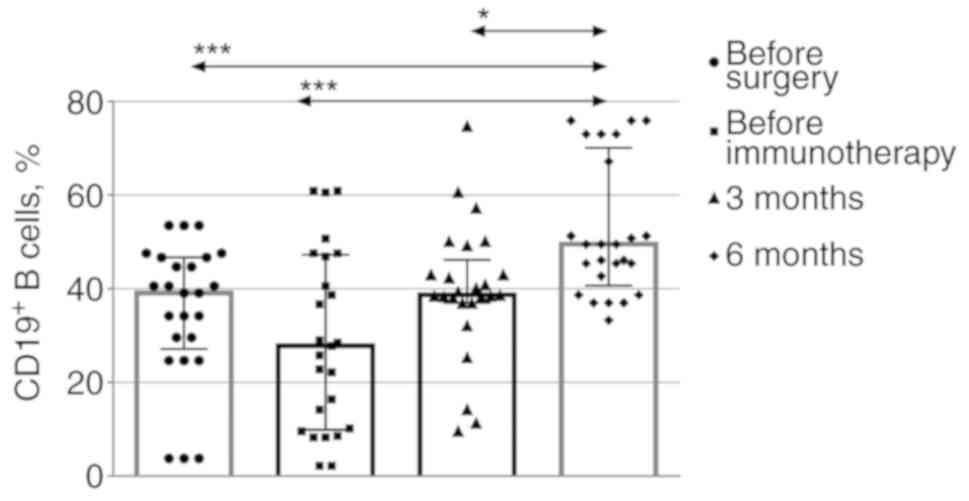
CD19+ B cells (Fig. 1).
There was a consistent reduction in this cell population when the
immunotherapy commenced compared with that prior to surgery, and
the population of these cells was found to be increased at 3 and 6
months after the immunotherapy.
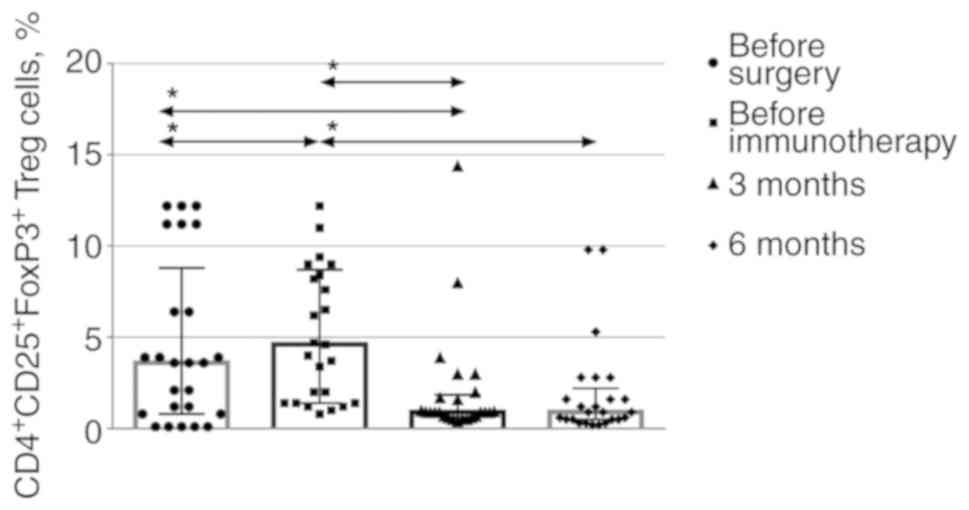
To assess the levels of regulatory T cells among
CD45+ leukocytes, the CD4+CD25+
cell population was isolated, and the number of cells expressing
the FoxP3 marker was determined (Fig.
2). Typical dot plots displaying the gating scheme used for the
FoxP3 expression evaluation are provided as supplementary material
(Fig. S2). There was a consistent
decrease in the level of regulatory T cells 3 months after the
immunotherapy, which was maintained for another 3 months.
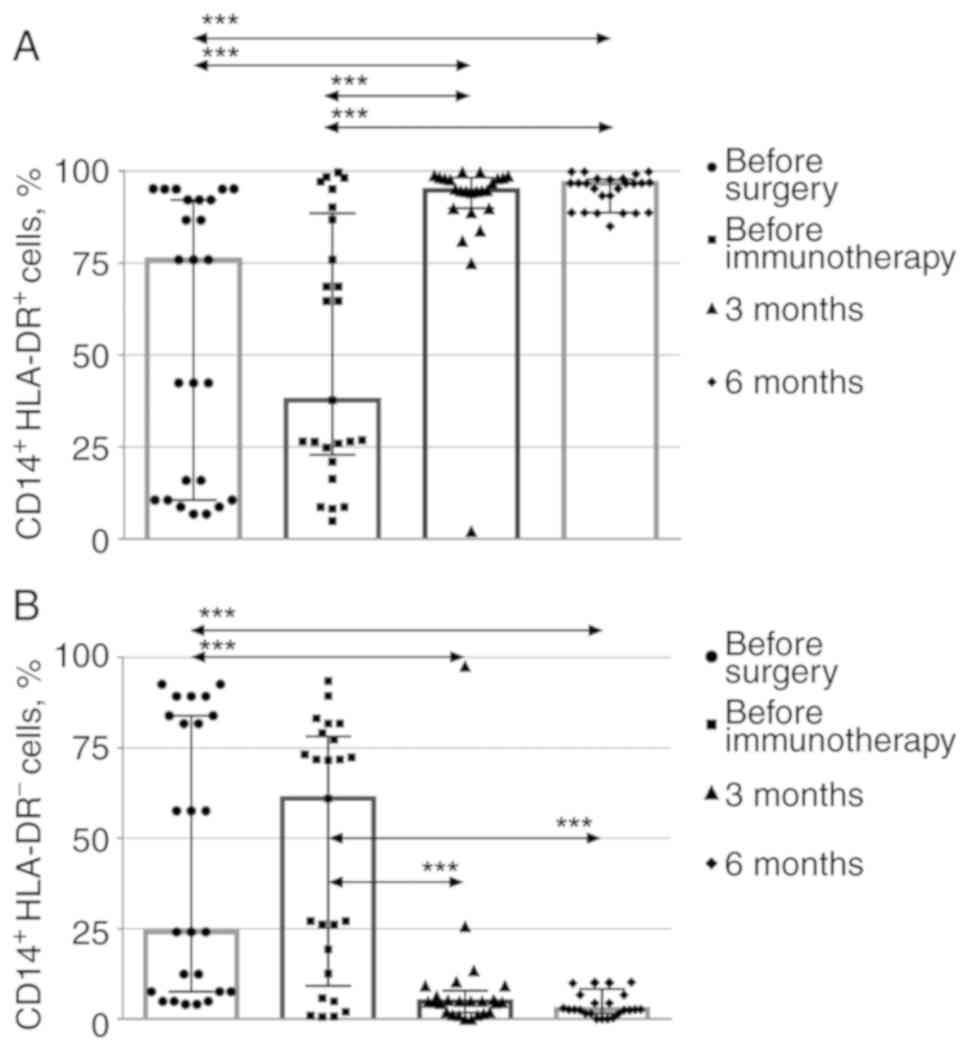
To assess the percentages of activated
HLA-DR-positive and -negative CD14+ monocytes (monocytes
and suppressor myeloid precursor cells, respectively) (27), CD14+ monocytes were
isolated from CD45+ leukocytes, and HLA-DR expression
was assessed (Fig. 3) The relevant
dot plots are provided as supplementary material (Fig. S3). The levels of
CD14+HLA-DR+ monocytes consistently increased
3 months after the immunotherapy, while the levels of myeloid
suppressor cells consistently decreased and remained low 6 months
after the immunotherapy.
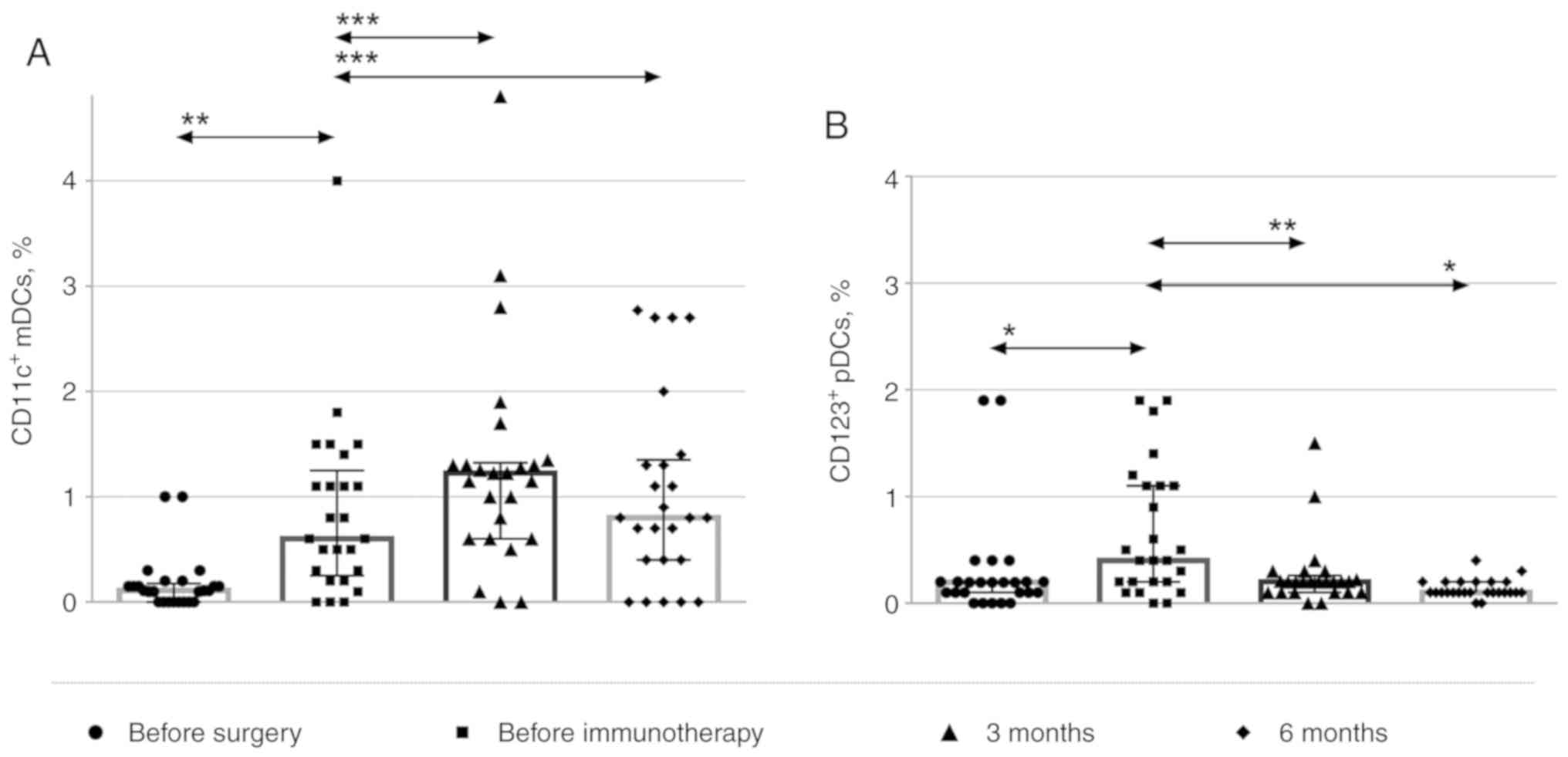
The relative amounts of
CD45+CD123+ cells (plasmacytoid DCs) and
CD45+CD11c+ cells (myeloid DCs) were also
assessed [(typical dot plots displaying the gating scheme used are
provided as supplementary material (Fig. S4)].
Lineage-negative/HLA-DR-positive cells were isolated from the
population of CD45+ leukocytes, and the levels of
CD123+-plasmacytoid and CD11c+-myeloid DCs
were determined (Fig. 4). The data
are presented as the percentage of CD123- and CD11c-positive cells
among the CD45+ cells. During immunotherapy, the numbers
of myeloid DCs increased, while those of plasmacytoid DCs gradually
decreased 3 and 6 months after the immunotherapy.
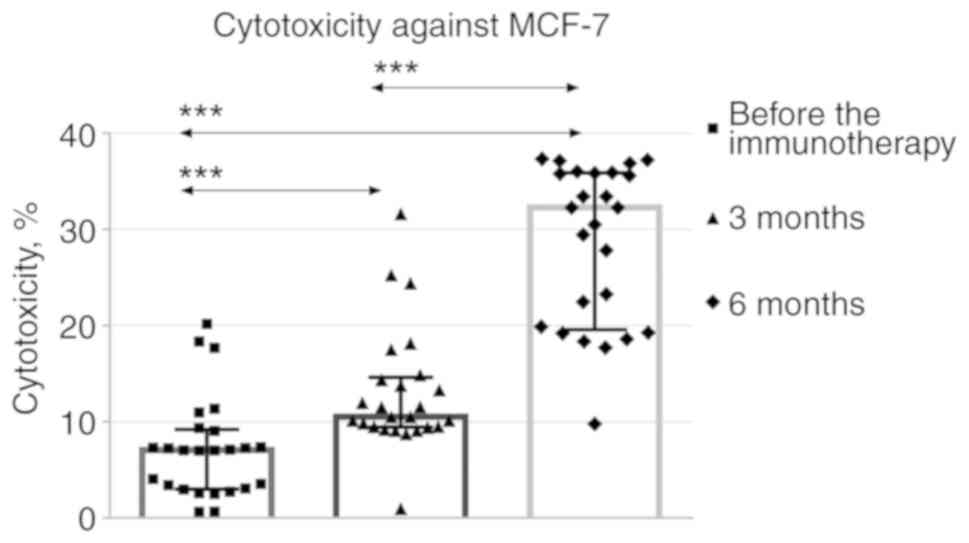
The cytotoxicity of mononuclear cells against the
MCF-7 cell line gradually increased during months 3 and 6 after the
immunotherapy compared with baseline (Fig. 5).
Among the patients who were administered adjuvant
immunotherapy, hyperplasia of the subclavian lymph node, which was
difficult to access, was detected in patient no. 1 who had stage
IIIc BC in 2014. No further progression was observed. Progression
of the underlying illnesses and fatal outcomes of 2 patients was
characterized by metastasis to the brain (patient no. 15), and
recurrence in the axillary lymph nodes and further spread to the
liver (patient no. 17). Patient no. 22 experienced local
recurrence.
Side effects
The treatment was generally well-tolerated. The
patients were monitored by medical personnel for 24 h after
injection to assess toxicity (CTCAE ver. 4.03, 2010). The most
common adverse events were flu-like symptoms, such as fever and
fatigue, that did not require additional treatment or prolonged
hospitalization. All symptoms spontaneously resolved without
treatment after 2–3 h.
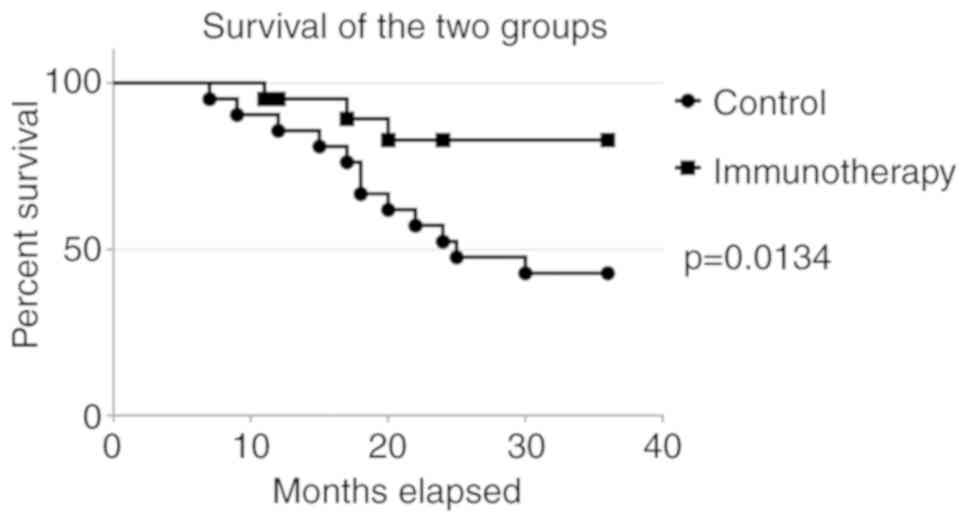
Disease-free period
The 3-year relapse-free periods of the 25 patients
who received adjuvant immunotherapy were compared with those of the
control group who were not immunized (historical controls)
(Fig. 6). The clinical
characteristics of patients in the control group and patients after
immunotherapy are compared in Table
III. The day of surgical treatment was considered as the
starting point.
 | Table III.Clinical characteristics of patients
in the control group (n=28) and after receiving immunotherapy
(n=25). |
Table III.
Clinical characteristics of patients
in the control group (n=28) and after receiving immunotherapy
(n=25).
|
Characteristics | Immunotherapy | Control |
|---|
| Number | 25 | 28 |
| Age, years [mean
(range)] | 48.8 (28–65) | 46.48 (28–62) |
| Disease stage, n
(%) |
|
IIA | 13 (52) | 13 (46.43) |
|
IIB | 6 (24) | 8 (28.57) |
|
IIIA | 2 (8) | 4 (14.29) |
|
IIIC | 4 (24) | 3 (10.71) |
| Molecular subtypes
of the tumor after radical surgery, n (%) |
|
Isolated expression or
overexpression of HER-2 | 7 (28) | 5 (28) |
|
Triple-negative | 5 (20) | 8 (20) |
| Luminal
A | 10 (40) | 12 (40) |
| Luminal
B (overexpression of HER-2/neu) | 1 (4) | 2 (4) |
| Luminal
B (Ki-67 >20%). | 2 (8) | 1 (8) |
| Recurrence rate for
different molecular subtypes, n (%) |
|
Isolated expression or
overexpression of HER-2 | 2 (8) | 3 (10.71) |
|
Triple-negative | 2 (8) | 4 (14.29) |
| Luminal
A | 1 (4) | 5 (17.86) |
| Luminal
B (overexpression of HER-2/neu) | 0 | 2 (7.12) |
| Luminal
B (Ki-67 >20%). | 0 | 0 |
| General
recurrence rate, % | 25 (5/25) | 50 (14/28) |
| Median
time to progression, months | 20.5 | 16.5 |
Discussion
The overall incidence of BC has increased over the
past 30 years, reflecting the increase in absolute and relative
incidence. The absolute increase, which is caused by socioeconomic
factors, reflects the increase in the number of newly diagnosed
patients with BC.
Restoration of antitumor cell-mediated immunity,
particularly that mediated by the T cell component, during
combination cancer therapy is required to destroy cells in the
primary tumor, as well as to eliminate metastatic cells. In our
earlier preclinical studies using DCs to stimulate a cytotoxic
response, the safety and effectiveness of using TAA-loaded
autologous DCs were assessed (28,29). A
number of studies have confirmed the ability of mature
antigen-loaded DCs to successfully present tumor antigens to T
lymphocytes in vitro and in vivo (30,31).
Modification of T cells using both natural adjuvants and genetic
engineering methods may help overcome the mechanisms of tumor
immune escape (30). However, there
remains the question of whether the endogenously activated T cell
response is able to mediate tumor regression, since tumor
progression is often observed, even in the presence of high levels
of circulating blood cells or tumor-infiltrated T cells (33,34).
It is known that cellular immunotherapy is not sufficient to
completely destroy solid tumors, but the approach using T cells
activated ex vivo by antigen-loaded DCs may be efficient for
elimination of minimal residual disease represented by single tumor
cells remaining in the body after eradication of the main tumor
burden via surgery and/or chemotherapy and/or radiotherapy in order
to prevent relapse or metastasis (35).
In the present study, antigen-loaded DCs were
generated in all patients. DCs are adherent cells with an elongated
stellate shape and exhibit the typical mature phenotype. A cell
suspension produced by co-culturing DCs and non-adherent
mononuclear cells in the presence of IL-12 and IL-18 was used for
injection. The viability of the suspension after thawing was
95–98%. Examination of the subpopulations of the resulting
mononuclear cells demonstrated that, compared with the standard
peripheral blood parameters, the percentage of
CD16+/CD56+ cells in the suspension injected
into patients increased to 36.7% from the normal range of
4.2–25.2%, while the percentage of CD8+, CD4+
cells was within the normal range. Cell injection was tolerated
well by all patients, and complications were not reported.
Measurements of the cytotoxic activity of activated
mononuclear cells against the MCF-7 cell line displayed a
consistent increase at 3 and 6 months after the immunotherapy
compared with the baseline. Elevated numbers of CD19+ B
cells and myeloid DCs were detected, while the numbers of
immunosuppressive myeloid precursors and regulatory T cells
simultaneously decreased. Changes in the percentages of
CD8+, CD4+ T cells were undetectable.
The growth of a tumor and its microenvironment leads
to the appearance of immunosuppressive factors as well as the
appearance of cells with suppressive properties, including
regulatory T cells. The absence of tumor load and cell destruction
during chemotherapy may lead to a decrease in the level of
regulatory T cells. In this regard, the present results showing
such a decrease are consistent with those of similar research on
the treatment of kidney cancer (36).
Considering the changes in the numbers of regulatory
T cells and increased cytotoxic activity, it is reasonable to
conclude that a qualitative change in the T cell population
occurred after the therapy. In addition to the changes in the
numbers of regulatory T cells, those of myeloid suppressor cells
and plasmacytoid DCs decreased, while the percentage of myeloid DCs
increased. Thus, the protective response increased in patients
within 6 months after the immunotherapy, while the size of the
suppressor population decreased.
The predicted survival rates and durations of the
relapse-free period of patients with BC are attributed to
characterizing the tumor using the TNM system as well as
determining the molecular subtypes of tumor cells, including
expression levels of estrogen, progesterone and human epidermal
growth factor receptor 2 (HER-2/neu) receptors along with the Ki-67
index. In the present study, patients with operable cancer had all
four tumor molecular subtypes (Table
I). Disease progression during the first year after
immunotherapy was observed in 1 patient with triple-negative BC and
relapse occurred in 2 patients with isolated expression of
HER-2/neu (HER-2/neu 3+) (i.e., patients with an
initially less favorable outcome) (35,37).
Progression in 2 patients occurred towards the end of the 3-year
follow-up period.
The effectiveness of immunotherapy was assessed
according to the regression of tumor foci in patients with tumor
progression, or initially found to have stage IV disease. A total
of 2 patients achieved a positive response, which lasted for 9 and
6 months, respectively. These patients continued treatment
recommended by the case conference. A total of 2 patients received
palliative cellular immunotherapy. Distant changes were
undetectable in 1 patient with disease invading into the soft
tissues of the breast, and only limited local progression was
observed. The tumor in another patient changed its molecular
subtype from luminal A to luminal B during progression (emergence
of HER-2/neu expression), indicating that tumor aggressiveness
increased. Moreover, all tumor samples (biopsies) in this group of
patients were originally luminal A or B subtype.
It should also be taken into consideration that the
therapy was safe and comfortable for the patients. A small sample
of patients in our hospital exhibited fewer relapses after
undergoing a course of immunotherapy and an increase in the median
time to disease progression. Our patients experienced positive
changes in immune responses, such as a decrease in the level of
regulatory T cells. Similar changes, particularly those of
regulatory T cells, in the patients' immune responses after
treatment with DCs and cytokine-induced killer cells was
demonstrated in a study using cellular therapy to treat renal
cancer (36).
The principal features of our research may be
summarized as follows: First, ex vivo DС activation was
used, which enables avoiding impaired maturation and antigen
loading of DСs that usually occur in the presence of a tumor and
its microenvironment. Next, after in vitro antigen-loading
of DCs and further in vitro activation of lymphocytes in
non-adherent PBMC and DC co-culture, antigen-loaded DCs and
DC-activated PBMCs were injected into the patients in order to
eliminate tumor cells via the transferred DC-activated lymphocytes
and to continue the activation of T cells by antigen-loaded DCs
in vivo. There was an observed strong tendency for an
increase in the protective immune response in patients within 6
months after the immunotherapy, while the size of the suppressor
population decreased. Thus, it was inferred that the use of
autologous DCs loaded with antigens in combination with mononuclear
cells with increased cytotoxic activity during Th1 polarization
represents a promising immunotherapeutic approach to the prevention
or treatment of metastatic foci and may be used in the treatment of
patients with stage IV BC.
It should be noted as a limitation of the present
study that no imaging data were provided. The case management of
the patients was performed based on the data provided by the Third
Oncological Department of the Novosibirsk City Clinical Hospital
No. 1, where the patients underwent multispiral computed tomography
examination at different treatment phases. The imaging data were
only provided by the supervising clinics as official textual
conclusions from certified specialists. We could not request
imaging data from the Oncological Department that provided us with
clinical data on the cases, as the results of imaging screenings
are not collected there as images.
We herein demonstrated that the administration of
cell suspensions containing autologous tumor lysate-loaded mature
DCs and activated PBMCs is a feasible approach to inducing an
antitumor response. However, the percentage of patients who
achieved objective long-term tumor regression was very small. The
most common outcome is an extended antigen-specific response in the
absence of a pronounced clinical response (38,39).
The aim of the present approach involved ‘priming’ naïve T cells
using antigen-loaded DCs in the presence of IL-12 and IL-18 to
elicit a T cell antitumor response. It should also be noted that
the approach investigated in the present study helped us i) induce
an antitumor response against breast cancer cells via generation of
activated cytotoxic cells, which is demonstrated in our further
studies (28,29), and ii) generate long-lived memory
cells to prevent late relapses, which was also demonstrated in our
recent research (40).
The clinical effectiveness of our cellular protocol
was demonstrated in the present study and verified by the effects
on the patients' antitumor immunity, which was characterized by
enhanced cell-mediated cytotoxic immune responses and lower
percentages of suppressor cells in the peripheral blood within 6
months after the initiation of immunotherapy. However, we believe
that the maximum effect of cancer immunotherapy can only be
achieved using a combined two-stage strategy. Therefore, it may be
concluded that it is reasonable to conduct anti-suppressor therapy
targeted against suppressor cell populations (41,42) or
their mediators (e.g., targeted immunotherapy using monoclonal
antibodies or immune cells) as the first stage and cellular
immune-stimulating antitumor therapy (cellular immunotherapy) as
the second stage.
Supplementary Material
Supporting Data
Acknowledgements
The authors are grateful to the staff of the
Laboratory of Molecular Immunology, RIFCI for their assistance. We
are also grateful to all the staff of Oncology Department No. 3 of
Clinical Hospital No. 1 for their help.
Funding
The present study was conducted for a government
assignment by state funding.
Availability of data and materials
The authors confirm that the data supporting the
findings of the present study are available in this published
manuscript. Additional data supporting the findings of this study
and basic research materials are available from the corresponding
author on reasonable request.
Authors' contributions
JAS contributed to the design, experimental work,
and optimization of each experimental stage, analysis and
interpretation of data, and drafting of the manuscript. AAK
contributed to the conception, design, and data interpretation. VVK
performed experimental work (flow cytometry) and contributed to
data interpretation. MSK conducted experimental work (optimization
of adherent PBMC isolation protocol) and revision of the
manuscript. DDB performed all the injection procedures and tracking
of clinical indicators and adverse events. NMS supervised the
patients during the immunotherapy. SVSi performed patient
recruiting, oncological treatment and obtained tumor material. SVSe
contributed to the conception, design, revision, and final approval
of the manuscript. All authors have read and approved the final
version of this manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki with written informed consent from all
subjects. The protocol was approved by the local Ethics Committee
of the Institute of Clinical and Fundamental Immunology (protocol
no. 78, September 12, 2013).
Patient consent for publication
The publication of data provided in the study does
not compromise the anonymity or confidentiality of the
participants. Written informed consent was obtained from each
patient prior to inclusion. The informed consent was approved by
the local Ethics committee of the Institute of Clinical and
Fundamental Immunology.
Competing interests
All the authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DCs
|
dendritic cells
|
|
BC
|
breast cancer
|
|
TAAs
|
tumor-associated antigens
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
FBS
|
fetal bovine serum
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eggermont AM, Kroemer G and Zitvogel L:
Immunotherapy and the concept of a clinical cure. Eur J Cancer.
49:2965–2967. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allan CP, Turtle CJ, Mainwaring PN, Pyke C
and Hart DN: The immune response to breast cancer, and the case for
DC immunotherapy. Cytotherapy. 6:154–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan Z and Zhang Q: Molecular mechanisms of
lymphocyte-mediated cytotoxicity. Cell Mol Immunol. 2:259–264.
2005.PubMed/NCBI
|
|
6
|
Reinhard G, Märten A, Kiske SM, Feil F,
Bieber T and Schmidt-Wolf IG: Generation of dendritic cell-based
vaccines for cancer therapy. Br J Cancer. 86:1529–1533. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koski GK, Cohen PA, Roses RE, Xu S and
Czerniecki BJ: Reengineering dendritic cell-based anti-cancer
vaccines. Immunol Rev. 222:256–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palucka K and Banchereau J: Human
dendritic cell subsets in vaccination. Curr Opin Immunol.
25:396–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gardner A and Ruffell B: Dendritic cells
and cancer immunity. Trends Immunol. 37:855–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sabado RL, Balan S and Bhardwaj N:
Dendritic cell-based immunotherapy. Cell Res. 27:74–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomasicchio M, Semple L, Esmail A, Meldau
R, Randall P, Pooran A, Davids M, Cairncross L, Anderson D, Downs
J, et al: An autologous dendritic cell vaccine polarizes a Th-1
response which is tumoricidal to patient-derived breast cancer
cells. Cancer Immunol Immunother. 68:71–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin M, Liang S, Jiang F, Xu J, Zhu W, Qian
W, Hu Y, Zhou Z, Chen J, Niu L, et al: 2003-2013, a valuable study:
Autologous tumor lysate-pulsed dendritic cell immunotherapy with
cytokine-induced killer cells improves survival in stage IV breast
cancer. Immunol Lett. 183:37–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gelao L, Criscitiello C, Esposito A, De
Laurentiis M, Fumagalli L, Locatelli MA, Minchella I, Santangelo M,
De Placido S, Goldhirsch A and Curigliano G: Dendritic cell-based
vaccines: Clinical applications in breast cancer. Immunotherapy.
6:349–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheever MA, Allison JP, Ferris AS, Finn
OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL,
Weiner LM and Matrisian LM: The prioritization of cancer antigens:
A national cancer institute pilot project for the acceleration of
translational research. Clin Cancer Res. 15:5323–5337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benteyn D, Heirman C, Bonehill A,
Thielemans K and Breckpot K: mRNA-based dendritic cell vaccines.
Expert Rev Vaccines. 14:161–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maksyutov AZ, Lopatnikova YA, Kurilin VV,
Shevchenko YA, Khantakova YN, Gavrilova EV, Maksyutov RA, Peregudov
AG, Zaytsev SA, Kozlov VA and Sennikov SV: Efficiency studies of
induced cytotoxic immune response of mononuclear cells by means of
dendritic cells transfected by polyepitopic HER2/ERBB2 constructs.
Meditsinskaya Immunol. 16:417–424. 2014. View Article : Google Scholar
|
|
17
|
Kuznetsova M, Lopatnikova J, Khantakova J,
Maksyutov R, Maksyutov A and Sennikov S: Generation of populations
of antigenspecific cytotoxic T cells using DCs transfected with DNA
construct encoding HER2/neu tumor antigen epitopes. BMC Immunol.
18:312017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garu A, Moku G, Gulla SK and Chaudhuri A:
Genetic immunization with in vivo dendritic cell-targeting
liposomal DNA vaccine carrier induces long-lasting antitumor immune
response. Mol Ther. 24:385–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goyvaerts C and Breckpot K: The journey of
in vivo virus engineered dendritic cells from bench to bedside: A
bumpy road. Front Immunol. 9:20522018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shevchenko YA, Khristin AA, Falaleeva SA,
Kurilin VV, Kuznetsova MS, Sidorov SV and Sennikov SV: Phenotypic
and functional characteristics of dendritic cells and contents of
suppressor cell populations in peripheral blood of breast cancer
patients. Vopr Onkol. 62:519–523. 2016.(In Russian). PubMed/NCBI
|
|
21
|
Thanendrarajan S, Nowak M, Abken H and
Schmidt-Wolf IG: Combining cytokine-induced killer cells with
vaccination in cancer immunotherapy: More than one plus one? Leuk
Res. 35:1136–1142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao D, Li C, Xie X, Zhao P, Wei X, Sun W,
Liu HC, Alexandrou AT, Jones J, Zhao R and Li JJ: Autologous tumor
lysate-pulsed dendritic cell immunotherapy with cytokine-induced
killer cells improves survival in gastric and colorectal cancer
patients. PLoS One. 9:e938862014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Novick D, Kim S, Kaplanski G and Dinarello
CA: Interleukin-18, more than a Th1 cytokine. Semin Immunol.
25:439–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wawrocki S, Druszczynska M,
Kowalewicz-Kulbat M and Rudnicka W: Interleukin-18 (IL-18) as a
target for immune intervention. ActaBiochim Pol. 63:59–63.
2016.
|
|
25
|
http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf
|
|
26
|
WHO Handbook for Reporting the Results of
Cancer Treatment//World Health Organization 1979. Geneva: WHO
Offset Publication. No. 48. https://apps.who.int/iris/handle/10665/37200
|
|
27
|
Huang A, Zhang B, Wang B, Zhang F, Fan KX
and Guo YJ: Increased CD14(+)HLA-DR (−/low)
myeloid-derived suppressor cells correlate with extrathoracic
metastasis and poor response to chemotherapy in non-small cell lung
cancer patients. Cancer Immunol Immunother. 62:1439–1451. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sennikov SV, Shevchenko JA, Kurilin VV,
Khantakova JN, Lopatnikova JA, Gavrilova EV, Maksyutov RA, Bakulina
AY, Sidorov SV, Khristin AA and Maksyutov AZ: Induction of an
antitumor response using dendritic cells transfected with DNA
constructs encoding the HLA-A*02: 01-restricted epitopes of
tumor-associated antigens in culture of mononuclear cells of breast
cancer patients. Immunol Res. 64:171–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shevchenko YA, Khantakova YN, Kurilin VV,
Lopatnikova YA, Sidorov SV, Kozlov VA and Sennikov SV: Stimulation
of the cytotoxic immune response in the culture of mononuclear
cells of patients breast cancer by dendritic cells loaded with
antigens of tumor lysates. Immunology. 134:327–330. 2013.
|
|
30
|
Jeras M, Bergant M and Repnik U: In vitro
preparation and functional assessment of human monocyte-derived
dendritic cells-potential antigen-specific modulators of in vivo
immune responses. Transpl Immunol. 14:231–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Elizalde PV, Cordo Russo RI, Chervo MF and
Schillaci R: ErbB-2 nuclear function in breast cancer growth,
metastasis and resistance to therapy. Endocr Relat Cancer.
23:T243–T257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boudreau J, Bonehill A, Thielemans K and
Wan Y: Engineering dendritic cells to enhance cancer immunotherapy.
Mol Ther. 19:841–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Willimsky G and Blankenstein T: Sporadic
immunogenic tumours avoid destruction by inducing T cell tolerance.
Nature. 437:141–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernhard H, Neudorfer J, Gebhard K, Conrad
H, Hermann C, Nährig J, Fend F, Weber W, Busch DH and Peschel C:
Adoptive transfer of autologous, HER2-specific, cytotoxic T
lymphocytes for the treatment of HER2-overexpressing breast cancer.
Cancer Immunol Immunother. 57:271–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duffy MJ, Harbeck N, Nap M, Molina R,
Nicolini A, Senkus E and Cardoso F: Clinical use of biomarkers in
breast cancer: Updated guidelines from the European Group on Tumor
Markers (EGTM). Eur J Cancer. 75:284–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhan HL, Gao X, Pu XY, Li W, Li ZJ, Zhou
XF and Qiu JG: A randomized controlled trial of postoperative tumor
lysate-pulsed dendritic cells and cytokine-induced killer cells
immunotherapy in patients with localized and locally advanced renal
cell carcinoma. Chin Med J (Engl). 125:3771–3777. 2012.PubMed/NCBI
|
|
37
|
Marmé F: Immunotherapy in Breast Cancer.
Oncol Res Treat. 39:335–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Palucka AK, Ueno H, Connolly J,
Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J and Banchereau J:
Dendritic cells loaded with killed allogeneic melanoma cells can
induce objective clinical responses and MART-1 specific
CD8+ T-cell immunity. J Immunother. 29:545–557. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lowenfeld L, Mick R, Datta J, Xu S,
Fitzpatrick E, Fisher CS, Fox KR, DeMichele A, Zhang PJ, Weinstein
SP, et al: Dendritic cell vaccination enhances immune responses and
induces regression of HER2(pos) DCIS independent of route: Results
of randomized selection design trial. Clin Cancer Res.
23:2961–2971. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuznetsova M, Lopatnikova J, Shevchenko J,
Silkov A, Maksyutov A and Sennikov S: Cytotoxic activity and memory
T cell subset distribution of in vitro-stimulated CD8+ T
cells specific for HER2/neu epitopes. Front Immunol. 10:10172019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Curr Opin Immunol. 27:1–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in tumor immunity. Int J Cancer. 127:759–767. 2010.PubMed/NCBI
|