Introduction
Worldwide, bladder cancer (BC) is the seventh
commonest malignant tumour in men and the eleventh most common
tumour for both sexes. The age-standardised incidence rate (per
100,000 person/years) is 9.0 for men and 2.2 for women worldwide,
and 19.1 for men and 4.0 for women in Europe specifically (1). The majority of bladder tumours are
transitional cell carcinomas (TCCs), with approximately 80% being
noninvasive papillary transitional-cell carcinomas that are
frequently amenable to surgical resection. Unfortunately, 50–70% of
patients develop tumour recurrence and of those that do, 15% have
more advanced disease. These patients with advanced disease
invading the muscle of the bladder, have a significantly reduced
5-year survival rate, due to the increased incidence of metastases
and failure of available treatments (2,3). Of
those patients that present at diagnosis with locally advanced,
bladder muscle invasive TCC, 50% relapse with metastatic diseases
(4). These poor outcomes,
particularly in those with metastatic BC, require a better
understanding of BC disease progression, such that improvements can
be made for BC patients.
Psoriasin (otherwise known as S100A7) is an 11.4-kDa
secreted protein located on chromosome 1q21 and belongs to the S100
protein family (5). In normal human
tissues, expression of Psoriasin is commonly confined to the
epithelia, having been initially identified as highly expressed in
the epidermis of psoriatic skin (6). In terms of cellular functions it is
known to influence calcium binding and signalling, thus affecting
cellular proliferation, differentiation, migration and apoptosis
(7).
It is of no surprise therefore that aberrant
Psoriasin is associated to numerous human diseases including
cancer. Psoriasin overexpression is observed in breast (8), skin (9), head and neck (10), prostate (11) and lung cancers (12). In breast cancer, upregulated
Psoriasin expression is observed particularly in ductal carcinoma
in situ and invasive tumours that are oestrogen and
progesterone receptor negative (13). Higher Psoriasin expression was
associated with poor prognosis and survival. In addition to the
apparent effect on the proliferation, adhesion and invasion of
cancer cells, Psoriasin can also actively engage in tumour
microenvironment and signal transduction. For example, Psoriasin is
able to promote neoangiogenesis by interacting with the receptor
for advanced glycation end products (RAGE), which results in
vascular endothelial growth factor (VEGF) upregulation (14). Psoriasin can also promote
EGF-induced activation of EGFR with a consequent impact on
migration and invasion of a ER-negative breast cancer cell line,
i.e. MDA-MB-231 (15). Psoriasin
was revealed to be highly expressed and secreted by bladder
squamous cancer cells (16,17). Determining the protein level of
Psoriasin in urine has been proposed for early detection and
monitoring the disease progression (18,19).
To date, the expression of Psoriasin in bladder
transitional cell carcinoma and its influence on BC cellular
function remain unknown. The present study aimed to investigate the
role played by Psoriasin in BC, particularly the most common type
of BC, i.e. transitional cell carcinoma.
Materials and methods
Materials and cell lines
The human BC cell lines EJ138 (ECACC
85061108) and RT112 (ECACC cat. no. 85061106; European Collection
of Animal Cell Culture) were routinely cultured in DMEM-F12 medium,
with 10% fetal bovine serum supplementation and antibiotics. The
EJ138 cell line (ECACC 85061108) is the same cell line as T24 shown
by both isoenzyme analysis and human leukocyte antigen (HLA)
profiles. Monoclonal mouse anti-Psoriasin and monoclonal mouse
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies
were obtained from Novus Biologicals (Novus Biologicals LLC) and
Santa Cruz Biotechnology, Inc., respectively. All other reagents
were obtained from Sigma-Aldrich; Merk KGaA. A bladder disease
spectrum (urocystic cancer progression) tissue array (BL804) was
purchased from US Biomax, Inc.
Online data for gene expression
profile in BC
Three different online datasets were included in the
present study. GSE3167 includes normal urothelium (n=9),
superficial transitional cell carcinoma (STCC) without carcinoma
in situ (CIS) (n=15), STCC with CIS (n=13), invasive
carcinoma (n=13), CIS (n=5) and normal mucosa (n=5) (20). Another cohort of BC was GSE32549
which is comprised of BCs of different T stages: Ta T1 and T2
(21). A BC cohort from The Cancer
Genome Atlas (TCGA) was included for a survival analysis using an
online tool (http://ualcan.path.uab.edu) (22).
Vector construction for Psoriasin
overexpression and knockdown
Anti-human Psoriasin hammerhead ribozymes and
Psoriasin overexpression plasmid vectors were developed and used in
our previous study (11). The
Psoriasin overexpression and control plasmids were transfected into
EJ138 cells, while ribozyme transgene Psoriasin knockdown and
control were employed in the RT112 cell line. Stable transfectants
were obtained and verified after 3 weeks of selection using
blasticidin (5 µg/ml). The selected cells were subsequently
maintained in the medium containing 0.5 µg/ml blasticidin and used
for the following experiments.
RNA isolation, reverse
transcription-PCR (RT-PCR) and quantitative PCR (qPCR)
TRIzol Reagent (Sigma-Aldrich; Merck KGaA) was used
for total RNA extraction and cDNA was synthesised using iScript
cDNA Synthesis kit (Bio-Rad Laboratories, Inc.). REDTaq ReadyMix
PCR reaction mix (primer sequences presented in Table I) was utilised for PCR under the
following cycling conditions: 95°C for 5 min, followed by 36 cycles
at 95°C for 30 sec, 55°C for 30 sec, 72°C for 40 sec and a final
extension at 72°C for 10 min.
 | Table I.Primer sequences for PCR and
qPCR. |
Table I.
Primer sequences for PCR and
qPCR.
| Primer | Forward | Reverse |
|---|
| Psoriasin |
5′-GAGGTCCATAATAGGCATGA-3′ |
5′-AGCAAGGACAGAAACTCAGA-3′ |
| Psoriasin
(qPCR) |
5′-TGTGACAAAAAGGGCACAAA-3′ |
5′-ACTGAACCTGACCGTACACCCAGCAAGGACAGAAACTC-3′ |
| GAPDH |
5′-GGCTGCTTTTAACTCTGGTA-3′ |
5′-GACTGTGGTCATGAGTCCTT-3′ |
| GAPDH (qPCR) |
5′-CTGAGTACGTCGTGGAGTC-3′ |
5′-ACTGAACCTGACCGTACAGAGATGATGACCCTTTTG-3′ |
| Psoriasin
ribozyme |
5′-CTGCAGTCACAGGCACTAAGGAAGTTGGGCTGATGAGTCCGTGAGGA-3′ |
5′-ACTAGTGGCTGGTGTTTGACATTTCGTCCTCACGGACT-3′ |
qPCR of BC cell cDNA samples was performed using the
Icycler IQ5 system (Bio-Rad Laboratories, Inc.), the Amplifluor
system (Intergen, Inc.) and qPCR Mastermix (Bio-Rad Laboratories,
Inc.) to identify Psoriasin transcript expression, along with
standards and negative controls. Psoriasin primers were designed
using Beacon design software (Premier Biosoft International), with
additional Z sequence (5′-ACTGAACCTGACCGTACA) on the reverse primer
complementary to the universal Z probe (Intergen, Inc.). Reaction
conditions were as follows: 95°C for 12 min, followed by 90 cycles
at 95°C for 15 sec, 55°C for 40 sec and 72°C for 20 sec.
Immunohistochemical (IHC) staining for
Psoriasin
A bladder disease spectrum tissue array (TMA)
(BL804) was purchased from US Biomax, Inc. The TMA was
de-paraffinised followed by a 20-min incubation with TBS for
rehydration. A further 20-min incubation in 0.6% BSA blocking
solution was followed by the primary antibody (anti-Psoriasin;
dilution 1:100; cat. no. 47C1068; Novus Biologicals, Ltd.) for 1 h
at room temperature. After washing, the sections were incubated in
the secondary biotinylated antibody (dilution 1:25; biotinylated
universal pan-specific antibody, horse anti-mouse/rabbit/goat IgG,
cat. no. BA-1300; Vector Laboratories, Inc.) at room temperature
for 30 min. An avidin-biotin complex (Vector Laboratories, Inc.)
was applied to the TMA and diaminobenzidine chromogen (Vector
laboratories, Inc.) was added in the dark for 5 min.
Counterstaining of the nuclei was performed using Gill's
hematoxylin (H-3401; Vector Laboratories, Inc.). The TMA was
dehydrated with increasing grades of methanol, then cleared in
xylene and mounted.
Cell proliferation assay
Cells were plated into a 96-well plate (2,000
cells/well for EJ138 and 4,000 cells/well for RT112) and
proliferation was determined over a period of up to 5 days. Cells
were stained with 0.5% crystal violet at room temperature for 10
min and the absorbance was read at a wavelength of 540 nm using a
spectrophotometer (Elx800; Bio-Tek Instruments, Inc.).
In vitro invasion assay
Matrigel (50 µg) (BD Matrigel Basement Membrane
Matrix, cat. no. 354234; BD BioSciences) was used to coat Transwell
inserts and air-dried. The coating layer was rehydrated before use.
Cells (20,000) were added to each insert and after 48 h of
incubation at 37°C, any cells remaining in the insert were removed
with a cotton tip swab and the cells that had invaded through the
Matrigel were fixed in 4% formalin at room temperature for 30 min,
stained with 0.5% crystal violet for 10 min at room temperature,
and counted under microscope with a magnification of ×200.
Cell-matrix adhesion assay
Cells (40,000) were placed in each well of
previously prepared 96-well plates coated with Matrigel (5
µg/well). Plates were incubated at 37°C for 45 min. Media was then
discarded and nonadherent cells were removed by BSS buffer wash.
The remaining adherent cells were fixed for 10 min in 4%
formaldehyde, followed by a BSS wash and 0.5% crystal violet
staining at room temperature for 10 min. The number of adherent
cells was then counted under a microscope with a magnification of
×200.
Wound/scratch assay
Cells were seeded at a density of 20,000 cells/well
for EJ138 and 40,000 cells/well for RT112 into a 24-well plate and
cultured until confluency. After creation of a scratch/wound, the
movement of cells to close the wound was recorded using an inverted
microscope equipped with an incubation chamber with a magnification
of ×100 (EVOS FL Auto2; Life Technologies; Thermo Fisher
Scientific, Inc.). The movement of the cells was quantified using
ImageJ (Version 1.48; http://imagej.nih.gov/ij/).
Western blotting
Cells were lysed using a buffer comprising 150 mM
NaCl, 50 mM Tris, 0.02% sodium azide, 0.5% sodium deoxycholate,
1.5% Triton X-100 (v/v), 1 µg/ml aprotinin, 5 mM Na3VO4, 1 µg/ml
leupeptin, 100 µg/ml phenylmethylsulphonylfluoride, 0.1% sodium
dodecyl sulfate and 100 µM dithiothreitol. Protein concentration
was determined using the Bio-Rad DC Protein Assay (500-0116;
Bio-Rad Laboratories, Ltd). Protein (20 µg per lane) was loaded and
separated in 12% sodium dodecyl sulfate-polyacrylamide gel
(SDS-PAGE) followed by tra-nsfer on to polyvinylidene difluoride
membranes. Initial 1-h membrane blocking used 5% fat-free milk in
tris (hydroxymethyl) aminomethane-buffered saline (TBS) (pH 7.5) at
room temperature, followed by incubation with mouse anti-Psoriasin
(1:1,000; cat. no. 47C1068; Novus Biologicals, Ltd.), mouse
anti-MMP2 (1:1,000; cat. no. sc-13595), mouse anti-MMP7 (1:1,000;
cat. no. sc-58388), rabbit anti-MMP9 (1:1,000; cat. no. sc-10737)
or mouse anti-GAPDH (1:1,000; cat. no. sc-47724; all from Santa
Cruz Biotechnology, Inc.) antibodies overnight at 4°C. The
appropriate horseradish peroxidase-conjugated secondary antibodies
(A5278 anti-mouse IgG, A6154 anti-rabbit IgG; Sigma-Aldrich; Merck
KGaA) were applied at room temperature for 1 h. Visualisation of
the membranes after incubation with a chemiluminescence reagent
used the Syngene gel documentation system (G:BOX Chemi XRQ; GeneSys
version 1.5.6.0; Syngene UK).
Statistical analysis
Analysis was performed using SPSS 17.0 software
(SPSS Inc.). The Mann-Whitney U test was employed to analyse
non-normally distributed data, while the two-sample t-test was
utilised for normally distributed data. Pearson correlation test
was used for the correlation analysis. A P-value at <0.05 was
considered to indicate a statistically significant difference.
Results
Expression of Psoriasin in BC tissues,
and correlation with clinicopathological features of BC
The expression of Psoriasin in BC was first
evaluated using two gene array datasets. In the cohort (GSE3167)
comprised of normal urothelium, superficial transitional cell
carcinoma (STCC) without/with carcinoma in situ (CIS),
invasive carcinoma, CIS and normal mucosa, the expression of
Psoriasin was increased in the invasive carcinomas (Fig. 1A). This was further supported by
analyses of Psoriasin in the other cohort of BC (GSE32549), which
revealed an increased expression of Psoriasin in invasive BCs
including both T1 and T2 tumours compared with Ta tumours which
were confined within the innermost layer of the bladder lining
(Fig. 1B). To investigate how
Psoriasin expression may relate to clinical prognosis, Kaplan-Meier
survival analysis was performed for Psoriasin expression in the
TGGA BC cohort using an online tool (http://ualcan.path.uab.edu/cgi-bin/). It was revealed
that a high level of Psoriasin expression was significantly
correlated with poor overall survival in BC patients (P=0.015,
Fig. 1C). With regard to Psoriasin
protein expression levels, IHC analysis of Psoriasin in a human
bladder disease spectrum tissue array demonstrated weak to moderate
staining in normal urocystic tissues, hyperplasia and chronic
inflammation of mucosa (Fig. 1D and
Table II). Psoriasin was weakly
expressed or absent from T1 TCC tumours. Strong staining was
observed in 2 of the 19 T2 TCC tumours. Strong and extensive
staining was observed in 3 out of 5 squamous cell carcinomas.
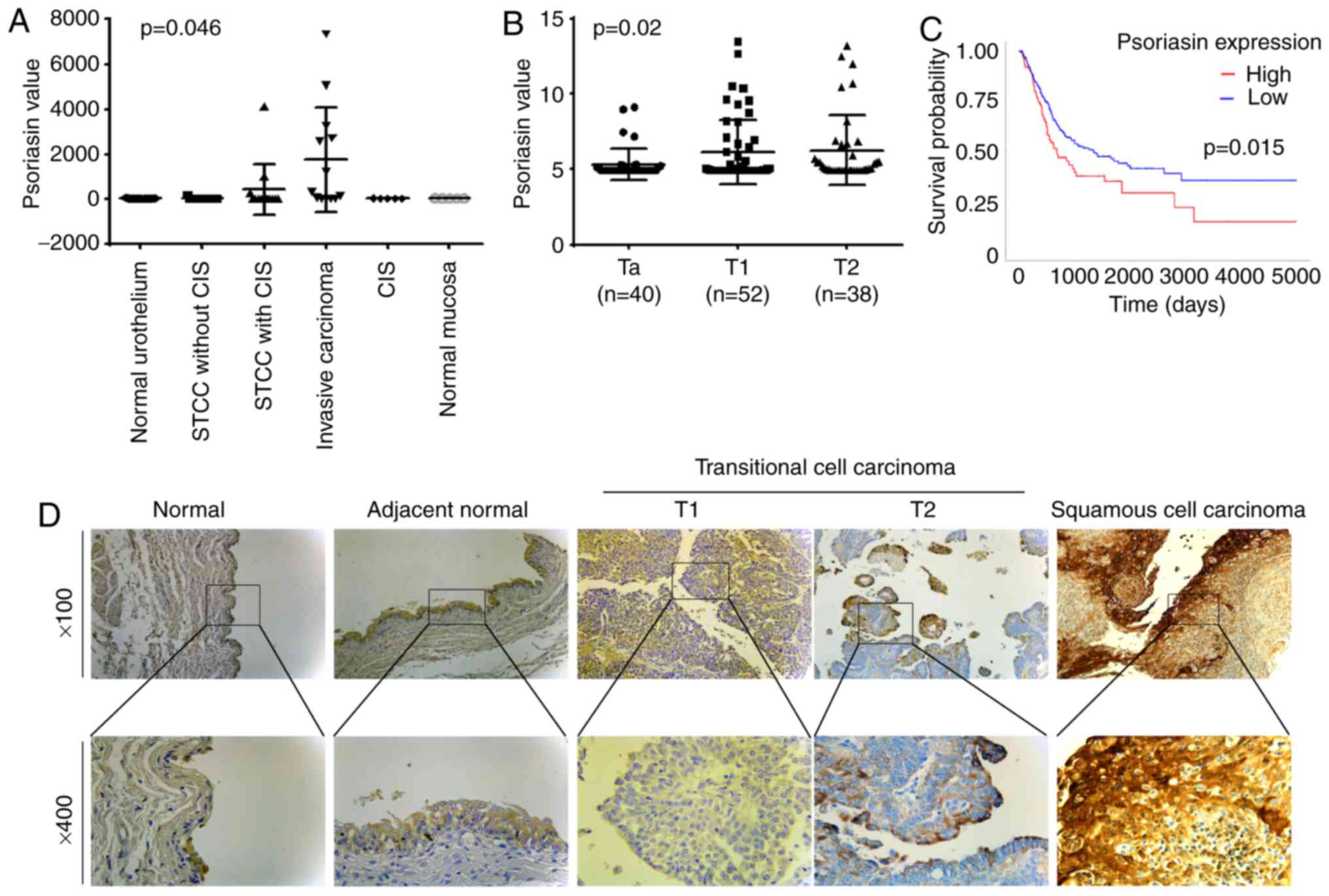 | Figure 1.Expression of Psoriasin in BC. (A)
Psoriasin expression in a cohort of BC (GSE3167) comprised of
normal urothelium (n=9), STCC without CIS (n=15), STCC with CIS
(n=13), invasive carcinoma (n=13), CIS (n=5) and normal mucosa
(n=5) (20). (B) Psoriasin
expression was also analysed in another gene expression array
dataset (GSE32549) which was comprised of non-invasive papillary
carcinoma (Ta, n=40), T1 (n=52) and T2 (n=38) (21). (C) Kaplan-Meier survival analysis
indicated the expression of Psoriasin was associated with a poor
overall survival (TCGA BC). The cutoff value is the 25th
percentile. (D) Differential expression of Psoriasin was detected
in normal urocystic tissues, adjacent normal urocystic tissue,
transitional cell carcinomas and squamous cell carcinoma (BL804, US
Biomax) using IHC. BC, bladder cancer; STCC, superficial
transitional cell carcinoma; CIS, carcinoma in situ; TCGA,
The Cancer Genome Atlas; IHC, immunohistochemical. |
 | Table II.IHC staining of Psoriasin in bladder
tissues. |
Table II.
IHC staining of Psoriasin in bladder
tissues.
|
| N | Negative | Weak | Moderate | Strong |
|---|
| Transitional cell
carcinoma |
| T1 | 10 | 5 | 4 | 1 | 0 |
| T2 | 19 | 8 | 7 | 2 | 2 |
| T3 | 1 | 0 | 1 | 0 | 0 |
| Tx | 1 | 0 | 0 | 1 | 0 |
| Transitional cell
carcinoma | 30 | 13 | 12 | 3 | 2 |
| Squamous cell
carcinoma | 5 | 0 | 1 | 1 | 3 |
| Adenocarcinoma | 2 | 2 | 0 | 0 | 0 |
| Undifferentiated
carcinoma | 1 | 1 | 0 | 0 | 0 |
| Sarcomatoid
carcinoma | 3 | 2 | 1 | 0 | 0 |
| Metastasis | 3 | 0 | 2 | 0 | 1 |
| Normal | 6 | 0 | 6 | 0 | 0 |
| Adjacent
normal | 5 | 1 | 4 | 0 | 0 |
| Inflammation | 8 | 1 | 3 | 3 | 1 |
| Hyperplasia | 12 | 0 | 6 | 5 | 1 |
| Benign | 5 | 2 | 2 | 1 | 0 |
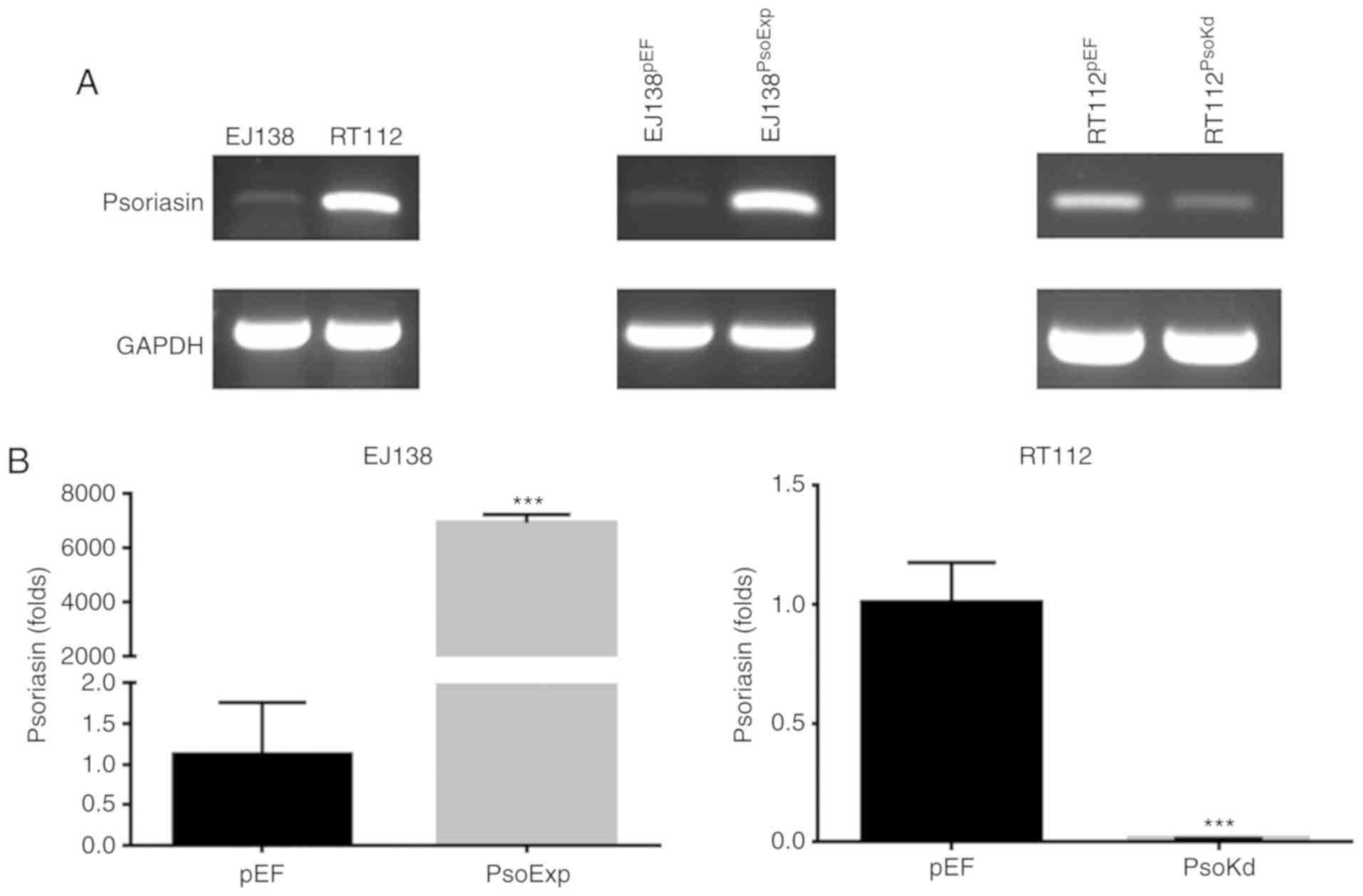
Knockdown and overexpression of
Psoriasin in BC cell lines
Two BC cell lines were examined for mRNA expression
of Psoriasin using RT-PCR (Fig.
2A). The expression of Psoriasin was relatively higher in RT112
cells in comparison with EJ138 cells. This allowed us to examine
the function of Psoriasin in BC cells using these two cell lines to
create a pair of opposite models, i.e. knockdown of Psoriasin in
the RT112 cell line and overexpression of Psoriasin in the EJ138
cell line. Psoriasin knockdown in RT112 and overexpression in EJ138
cell lines was achieved using anti-Psoriasin ribozyme and Psoriasin
expressing plasmid vectors, respectively. Psoriasin expression was
confirmed in the transfected cells using RT-PCR (Fig. 2A) and qPCR (Fig. 2B). Overexpression of Psoriasin was
established in EJ138 (EJ138PsoExp) compared with EJ138
plasmid vector control (EJ138pEF), while decreased
expression of Psoriasin was revealed in the RT112 Psoriasin
knockdown cells (RT112PsoKd) in comparison with and
RT112 plasmid vector control (RT112pEF).
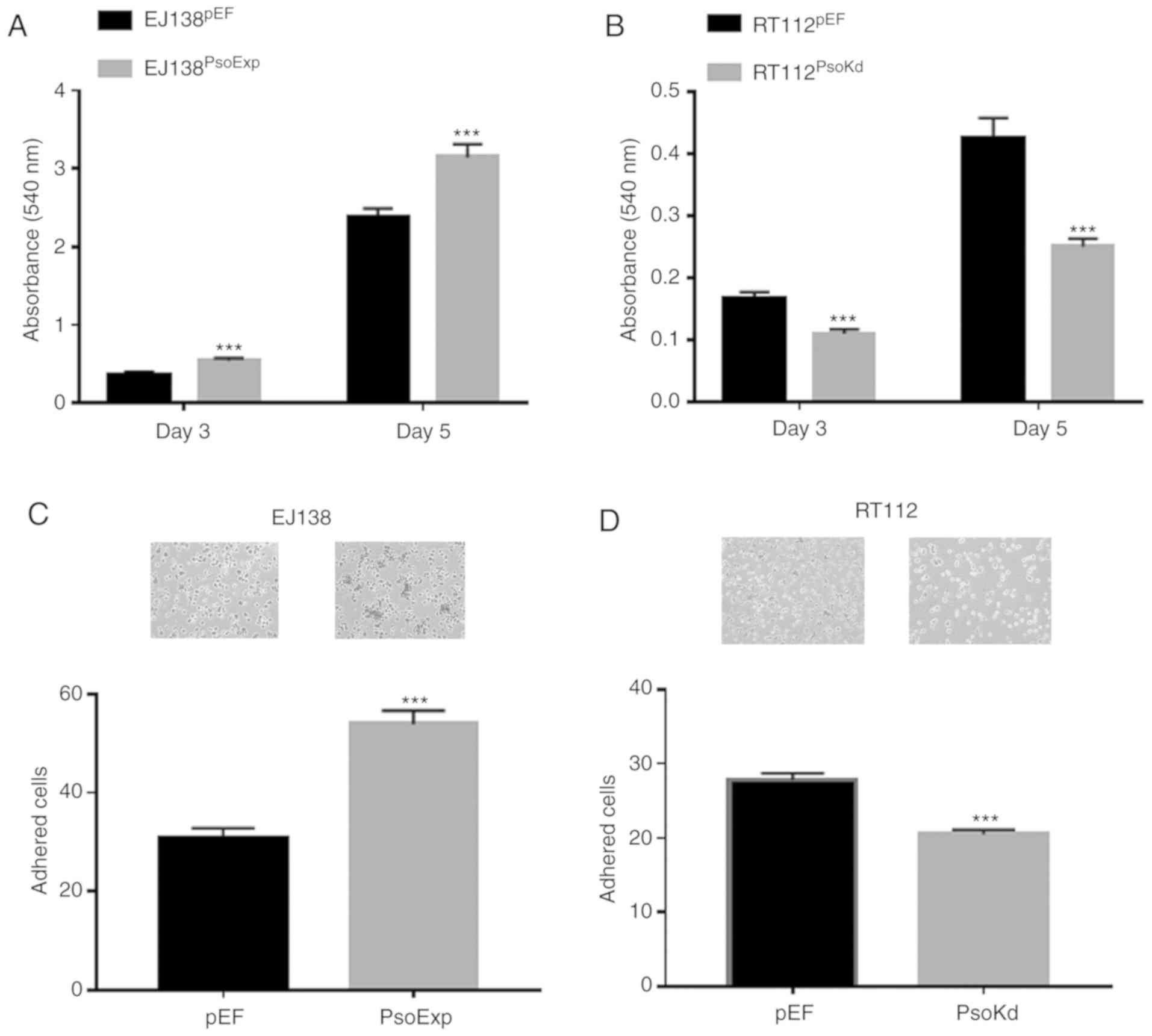
Influence of Psoriasin overexpression
and knockdown on BC cellular proliferation in vitro
Increased proliferation was observed in EJ138 cells
which had Psoriasin overexpressed. EJ138PsoExp cells had
a significantly increased proliferation rate, compared to the
control (P<0.001; Fig. 3A).
Conversely in RT112 cells in which Psoriasin had been knocked down
(RT112PsoKd) there was decreased proliferation, compared
to the control (P<0.001; Fig.
3B).
Influence of Psoriasin overexpression
and knockdown on BC cellular adhesion in vitro
The influence of Psoriasin on the adhesive nature of
BC cells was examined, with EJ138PsoExp cells
significantly increased in their adhesive capacity compared to the
EJ138pEF control cells, (P<0.001; Fig. 3C). Whereas knockdown of Psoriasin
resulted in a significant decrease in adhesiveness of
RT112PsoKd cells, compared with the corresponding
control (P<0.001; Fig. 3D).
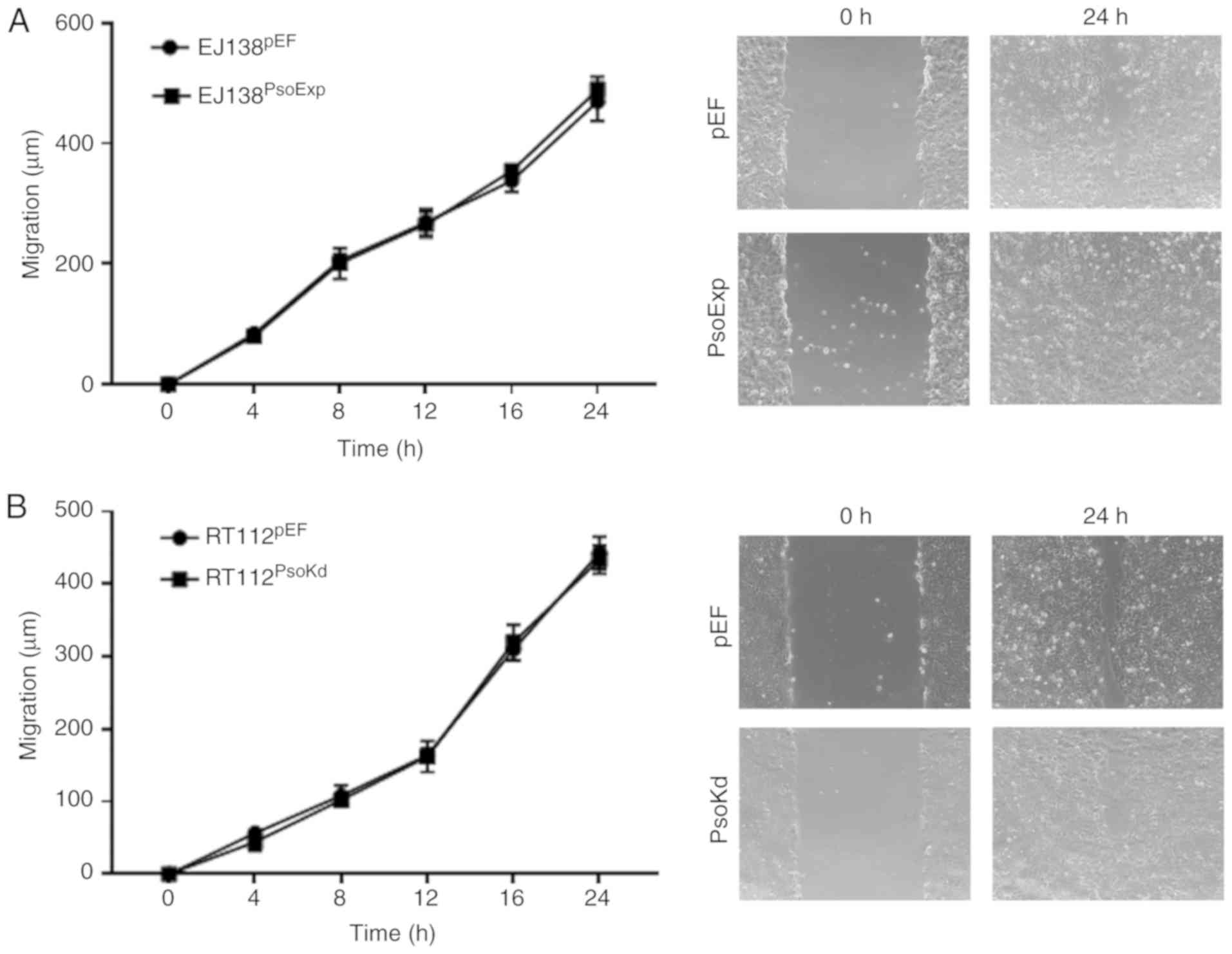
Effects of Psoriasin on in vitro
migration of BC cells
A wound, or scratch assay was utilised to determine
the migratory capacity of BC cells with either Psoriasin
overexpression or knockdown. Notably, the EJ138PsoExp
Psoriasin overexpressing cells did not exhibit any significant
alteration in migration compared to the control (Fig. 4A). A similar result was also
observed in RT112Psokd cells, in which the motility was
similar to that of the RT112pEF cells (Fig. 4B).
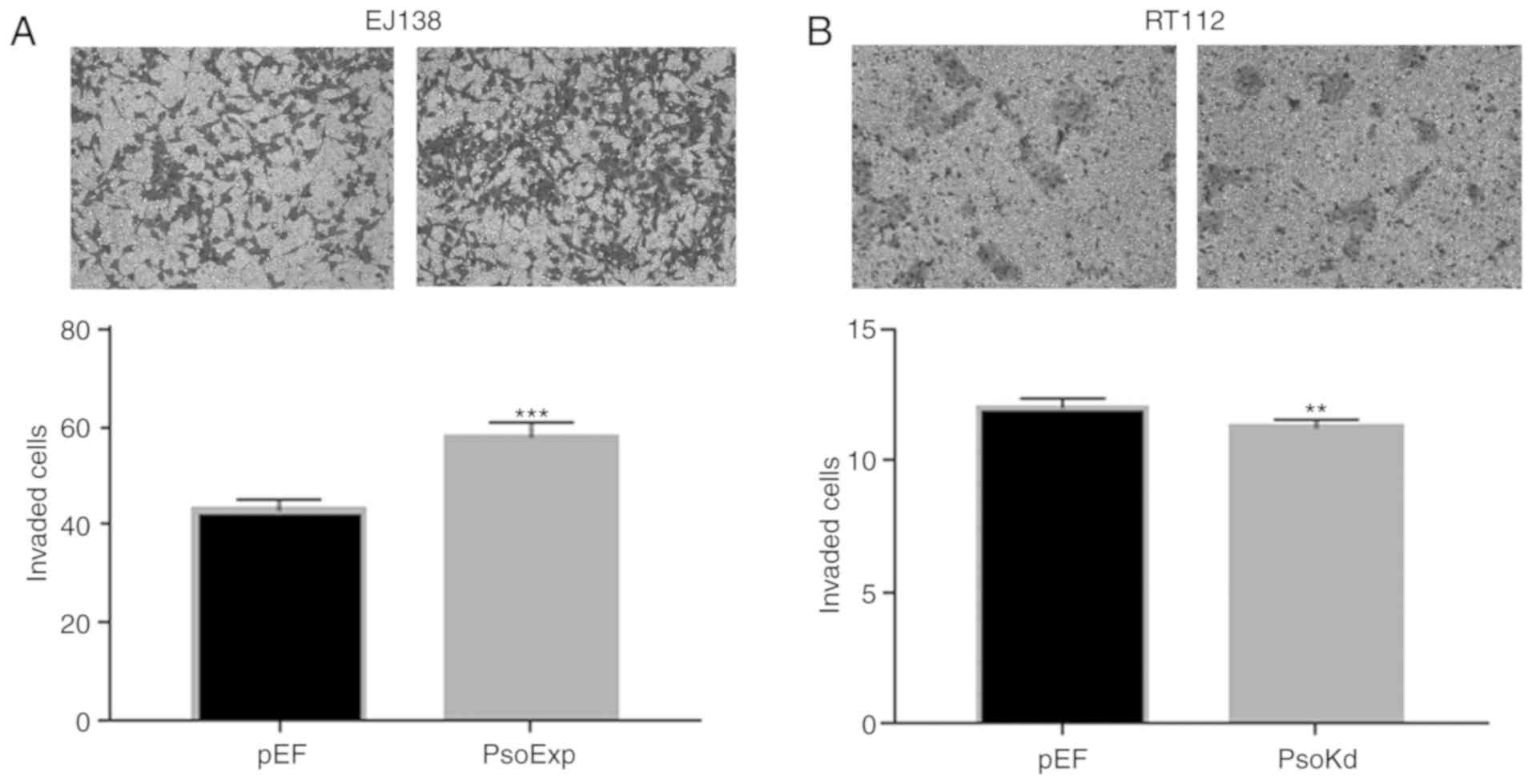
Effects of Psoriasin on invasion of BC
cells
The EJ138PsoExp Psoriasin over-expressing
cells demonstrated a significant increase of invasion, compared to
the control (P<0.001; Fig. 5A).
Conversely, this was also confirmed with RT112Psokd
Psoriasin-knockdown cells that appeared to be significantly less
invasive compared with the RT112pEF cells (Fig. 5B).
Psoriasin regulates the function of BC
cells via MMPs
In our previous study of Psoriasin in prostate
cancer, MMPs were revealed to be involved in Psoriasin-promoted
invasiveness of prostate cancer cells (11). Therefore, the MMP protein present in
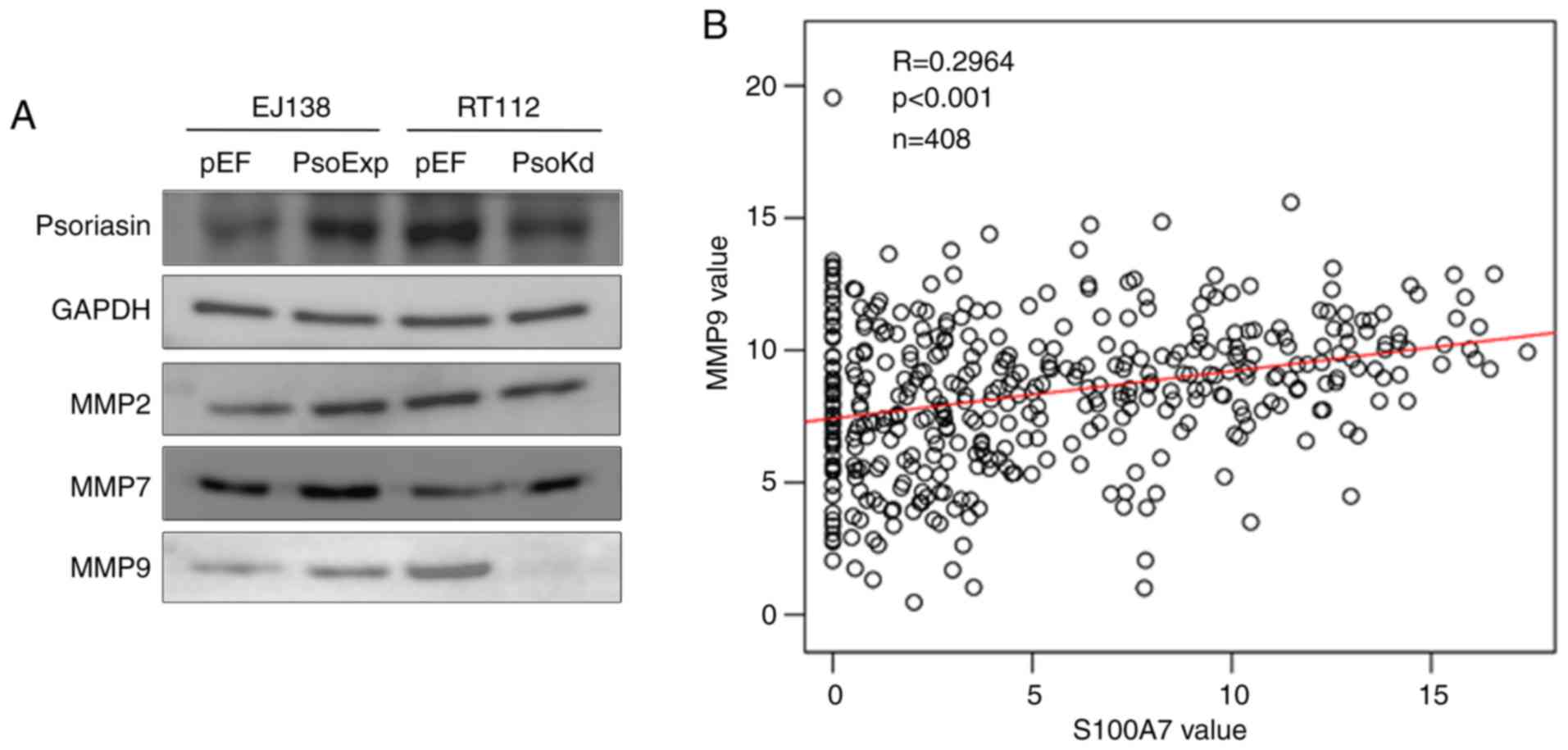
EJ138 and RT112 cells was analysed. Increased expression of MMP9
was observed in EJ138PsoExp cells, and reduced
expression of MMP9 was present in RT112Psokd cells.
There was no evident alterations observed in the expression of MMP2
and MMP7 (Fig. 6A). To further
investigate the relationship between MMP9 and Psoriasin, TCGA
online data was used again (http://www.linkedomics.org/admin.php) and a positive
correlation between the mRNA expression of Psoriasin and MMP9 was
revealed (P<0.05; Fig. 6B).
Discussion
TCC has a five-year survival rate of >95% for
patients with small single foci, well-differentiated papillary
tumours compared to near 0% survival in those patients with locally
advanced disease and gross nodal metastases (23). Despite efforts towards early
detection and treatment, there is still a significant clinical
impact on patients with invasive BC, including the morbidity of
disease recurrence and progression, to the mortality inevitably
associated with metastases.
Psoriasin was first discovered as being highly
expressed in the overproliferative epidermis of psoriatic skin
lesions (24). Subsequent studies
of Psoriasin have implied a role in promoting tumour progression,
particularly in breast cancer and pancreatic cancer, where tumours
with high Psoriasin expression had greater metastasis and poor
prognosis (8,25). Increased Psoriasin protein has also
been detected in the sera of patients with squamous cell carcinomas
of the lung (26). In the present
study, Psoriasin expression was examined in a human bladder disease
spectrum tissue array. Psoriasin was weakly expressed or absent
from the normal bladder tissues, while increased expression was
observed in invasive BCs and squamous cell BCs. Highly expressed
Psoriasin evident in the squamous cell BCs was in line with our
previous observation of Psoriasin in lung cancers (12) and also findings in bladder squamous
cell carcinomas (17). The present
analyses also revealed that Psoriasin expression was upregulated in
invasive BCs. These results indicated that Psoriasin plays an
important role during the progression of BC, particularly with
local invasion. Kaplan-Meier survival analysis revealed that the
elevated expression levels of Psoriasin were associated with the
overall survival of patients with BC.
The present study demonstrated that in vitro,
overexpression of Psoriasin resulted in an increase of
proliferation, invasion and motility of BC cells. Conversely
Psoriasin knockdown exhibited the opposite impact on these cellular
functions. The regulatory role of Psoriasin on the cellular
functions in BC cells is in keeping with findings from studies of
Psoriasin in other malignancies (11,25,27).
It suggests that Psoriasin plays a positive role during the
invasive growth/expansion of BC.
In contrast to the inverse correlation between
Psoriasin expression and adhesion observed in previous studies of
other cancers (11,12,25),
Psoriasin overexpression in EJ138 cells enhanced their adhesion
while an opposite effect on adhesion was evident in the RT112
Psoriasin-knockdown cells. This phenomenon is consistent with some
observations in clinical practice. For example, non-muscle invading
BC cells often exhibit continued growth when they are suspended
without anchorage within the bladder, forming a ‘pedicle’ which
often appears similar to water grasses floating in the bladder. In
contrast, muscle invasive BC often presents with an infiltrating
growth with a ‘moss like’ appearance within the bladder. This
implies the more invasive BCs, with poor prognosis may have a
higher Psoriasin expression, promoting growth and invasion, but
also a more adherent ‘anchored’ appearing moss-like tumour. The
other possibility for the difference in findings regarding cellular
adhesion compared to other studies, is that the present study
examined TCC, whereas other studies focused on adenocarcinomas of
the bladder, within which Psoriasin may have a slightly different
effect. The regulation of Psorisasin on the cellular functions of
BC cells is yet to be fully examined although two BC cell lines
were employed in the present study. In order to understand the
specific adhesion mechanism occurring in these malignant lesions,
further study is yet to be carried out.
It has been well established that MMPs are important
within the tumour microenvironment for promoting cancer cell
invasion and metastatic potential (28). In our previous study of Psoriasin in
prostate cancer and pancreatic cancer, it was revealed that
Psoriasin influenced cancer cell invasion via regulation of certain
MMPs (11,25). Similarly, upregulation of MMPs has
been observed in breast cancer cells overexpressing Psoriasin
(29). In the present study, BC
cells with overexpression of Psoriasin were more invasive and had
increased MMP9 expression, indicating the invasive effects of
Psoriasin in BC could be brought about by MMPs. In the study on
Psoriasin in breast cancer cells, interaction of Psoriasin with the
cytoplasmic domain of the integrin β6 subunit was implicated in the
promotion of cellular invasion, however, is remains to be confirmed
whether this may also be the case for BC cells, and the interaction
of Psoriasin with partner proteins is worthy of further study.
Future investigation using a BC patient-derived xenograft (PDX)
model and organoid model will help to further validate and shed
light on the therapeutic potential. Moreover, better understanding
of the relevant machinery employed by Psoriasin in BC cells will
help to identify the specificity, i.e. in which BC tumours
Psoriasin promotes disease progression. More specific cell line-
derived models, along with PDX and organoid models can then be
employed.
In conclusion, increased expression of Psoriasin in
BC was associated with cellular invasion, and poor survival of
patients with BC. Psoriasin promoted in vitro cell growth,
adhesion, and invasion of BC cell lines. MMP9 may be a key player
in the Psoriasin-promoted invasiveness of BC cells. The prognostic
and therapeutic potential of Psoriasin demonstrated in BC warrants
further investigation.
Acknowledgements
Dr Jia Liu is a recipient of the Chinese Medical
Research Scholarship of Cardiff University. The authors would thank
Dr You Zhou for his help and advices on data analysis of the gene
expression array data.
Funding
No funding was received.
Availability of data and materials
The data generated and/or analysed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY designed the study. JL and LY wrote the
manuscript. JL, ZZ, ZS, CL and XC performed the experiments. JL,
ZZ, WGJ and LY performed the data analyses. FR performed the IHC
analysis. JL, CL, YY and LY performed IHC assessment. JL, ZS, FR,
YY, WGJ and LY made contributions to the revision and proof
reading. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participates
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Droller MJ: Bladder cancer:
State-of-the-art care. CA Cancer J Clin. 48:269–284. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng L, Weaver AL, Leibovich BC, Ramnani
DM, Neumann RM, Scherer BG, Nehra A, Zincke H and Bostwick DG:
Predicting the survival of bladder carcinoma patients treated with
radical cystectomy. Cancer. 88:2326–2332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madsen P, Rasmussen HH, Leffers H, Honoré
B, Dejgaard K, Olsen E, Kiil J, Walbum E, Andersen AH, Basse B, et
al: Molecular cloning, occurrence, and expression of a novel
partially secreted protein ‘psoriasin’ that is highly up-regulated
in psoriatic skin. J Invest Dermatol. 97:701–712. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Algermissen B, Sitzmann J, LeMotte P and
Czarnetzki B: Differential expression of CRABP II, psoriasin and
cytokeratin 1 mRNA in human skin diseases. Arch Dermatol Res.
288:426–430. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salama I, Malone PS, Mihaimeed F and Jones
JL: A review of the S100 proteins in cancer. Eur J Surg Oncol.
34:357–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang WG, Watkins G, Douglas-Jones A and
Mansel RE: Psoriasin is aberrantly expressed in human breast cancer
and is related to clinical outcomes. Int J Oncol. 25:81–85.
2004.PubMed/NCBI
|
|
9
|
Moubayed N, Weichenthal M, Harder J,
Wandel E, Sticherling M and Glaser R: Psoriasin (S100A7) is
significantly up-regulated in human epithelial skin tumours. J
Cancer Res Clin Oncol. 133:253–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tripathi SC, Matta A, Kaur J, Grigull J,
Chauhan SS, Thakar A, Shukla NK, Duggal R, DattaGupta S, Ralhan R
and Siu KW: Nuclear S100A7 is associated with poor prognosis in
head and neck cancer. PLoS One. 5:e119392010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye L, Sun PH, Martin TA, Sanders AJ, Mason
MD and Jiang WG: Psoriasin (S100A7) is a positive regulator of
survival and invasion of prostate cancer cells. Urol Oncol.
31:1576–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu M, Ye L, Ruge F, Zhi X, Zhang L and
Jiang WG: The clinical significance of Psoriasin for non-small cell
lung cancer patients and its biological impact on lung cancer cell
functions. BMC Cancer. 12:5882012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Emberley ED, Alowami S, Snell L, Murphy LC
and Watson PH: S100A7 (psoriasin) expression is associated with
aggressive features and alteration of Jab1 in ductal carcinoma in
situ of the breast. Breast Cancer Res. 6:R308–R315. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shubbar E, Vegfors J, Carlstrom M,
Petersson S and Enerback C: Psoriasin (S100A7) increases the
expression of ROS and VEGF and acts through RAGE to promote
endothelial cell proliferation. Breast Cancer Res Treat. 134:71–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sneh A, Deol YS, Ganju A, Shilo K, Rosol
TJ, Nasser MW and Ganju RK: Differential role of psoriasin (S100A7)
in estrogen receptor α positive and negative breast cancer cells
occur through actin remodeling. Breast Cancer Res Treat.
138:727–739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ostergaard M, Rasmussen HH, Nielsen HV,
Vorum H, Orntoft TF, Wolf H and Celis JE: Proteome profiling of
bladder squamous cell carcinomas: Identification of markers that
define their degree of differentiation. Cancer Res. 57:4111–4117.
1997.PubMed/NCBI
|
|
17
|
Celis JE, Rasmussen HH, Vorum H, Madsen P,
Honoré B, Wolf H and Orntoft TF: Bladder squamous cell carcinomas
express psoriasin and externalize it to the urine. J Urol.
155:2105–2112. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ostergaard M, Wolf H, Orntoft TF and Celis
JE: Psoriasin (S100A7): A putative urinary marker for the follow-up
of patients with bladder squamous cell carcinomas. Electrophoresis.
20:349–354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rasmussen HH, Orntoft TF, Wolf H and Celis
JE: Towards a comprehensive database of proteins from the urine of
patients with bladder cancer. J Urol. 155:2113–2119. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dyrskjot L, Kruhoffer M, Thykjaer T,
Marcussen N, Jensen JL, Møller K and Ørntoft TF: Gene expression in
the urinary bladder: A common carcinoma in situ gene expression
signature exists disregarding histopathological classification.
Cancer Res. 64:4040–4048. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lindgren D, Sjödahl G, Lauss M, Staaf J,
Chebil G, Lövgren K, Gudjonsson S, Liedberg F, Patschan O, Månsson
W, et al: Integrated genomic and gene expression profiling
identifies two major genomic circuits in urothelial carcinoma. PLoS
One. 7:e388632012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oyasu R: World health organization and
international society of urological pathology classification and
two-number grading system of bladder tumors. Cancer. 88:1509–1512.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Glaser R, Harder J, Lange H, Bartels J,
Christophers E and Schroder JM: Antimicrobial psoriasin (S100A7)
protects human skin from Escherichia coli infection. Nat Immunol.
6:57–64. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Bunston C, Hodson N, Resaul J, Sun
PH, Cai S, Chen G, Gu Y, Satherley LK, Bosanquet DC, et al:
Psoriasin promotes invasion, aggregation and survival of pancreatic
cancer cells; association with disease progression. Int J Oncol.
50:1491–1500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Zhao Q, Chen Y, Wang Y, Gao S,
Mao Y, Li M, Peng A, He D and Xiao X: Selective expression of
S100A7 in lung squamous cell carcinomas and large cell carcinomas
but not in adenocarcinomas and small cell carcinomas. Thorax.
63:352–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Winston J and Wolf R: Psoriasin (S100A7)
promotes migration of a squamous carcinoma cell line. J Dermatol
Sci. 67:205–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ellenrieder V, Alber B, Lacher U, Hendler
SF, Menke A, Boeck W, Wagner M, Wilda M, Friess H, Büchler M, et
al: Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int
J Cancer. 85:14–20. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morgan MR, Jazayeri M, Ramsay AG, Thomas
GJ, Boulanger MJ, Hart IR and Marshall JF: Psoriasin (S100A7)
associates with integrin β6 subunit and is required for
αvβ6-dependent carcinoma cell invasion. Oncogene. 30:1422–1435.
2011. View Article : Google Scholar : PubMed/NCBI
|