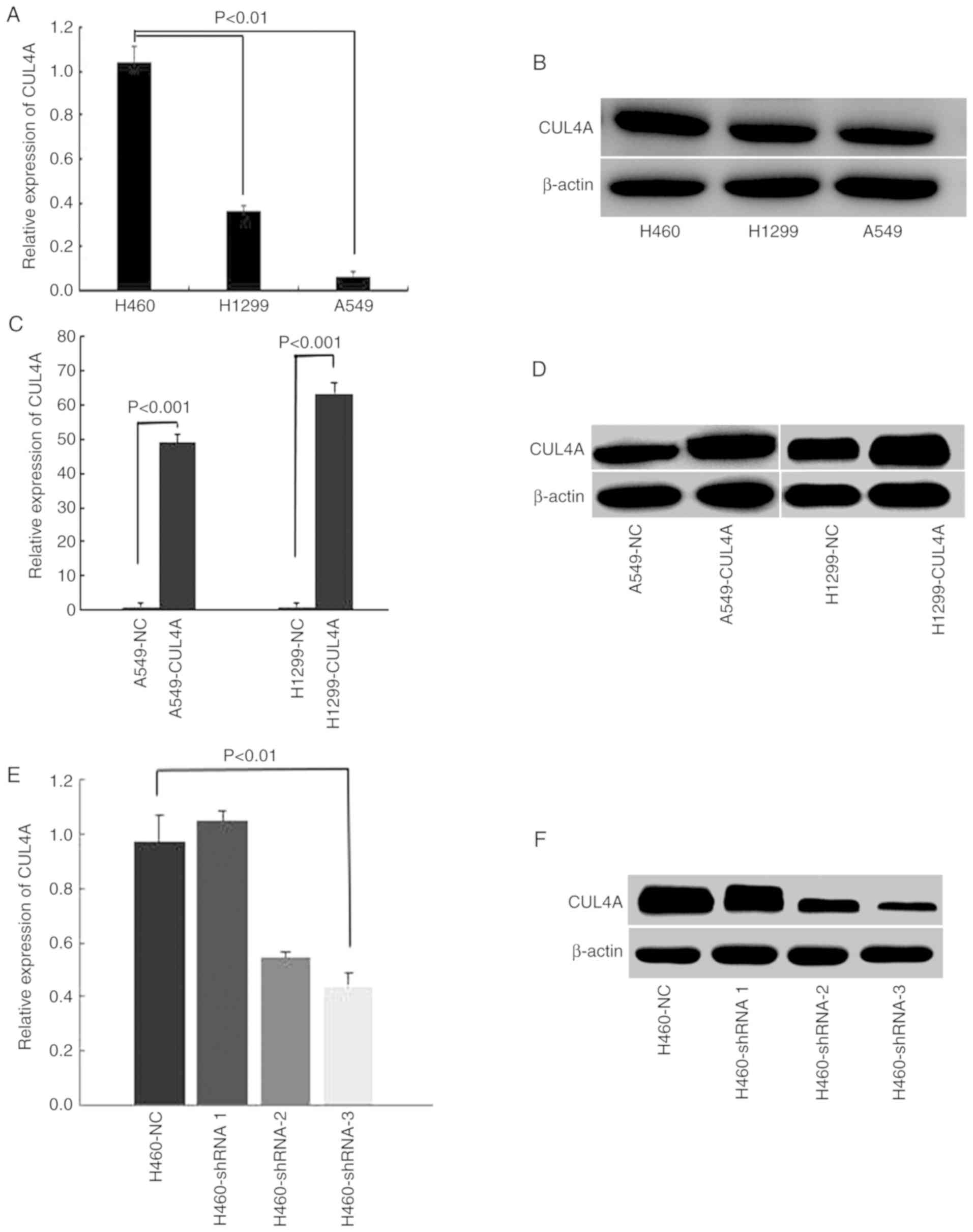
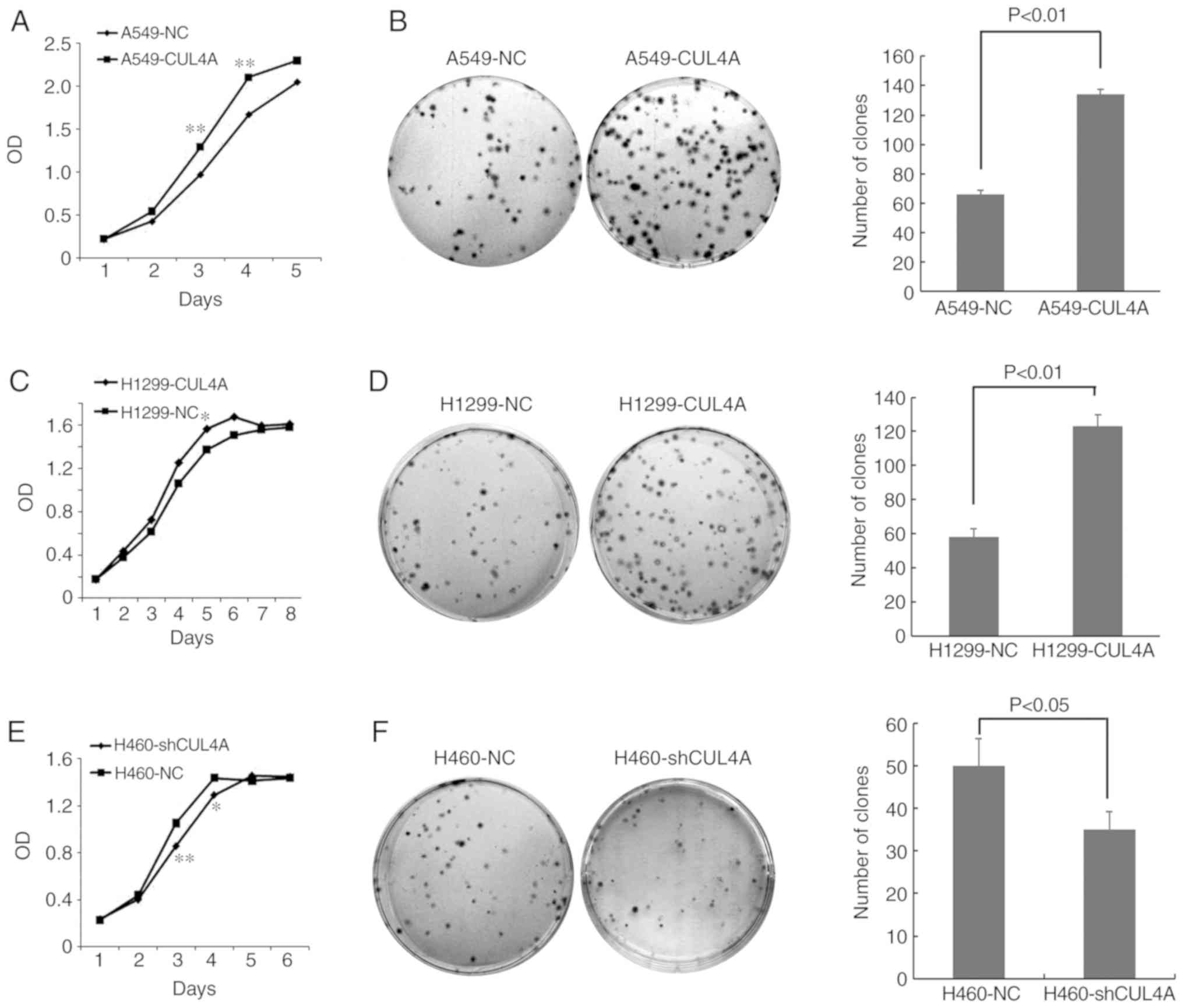
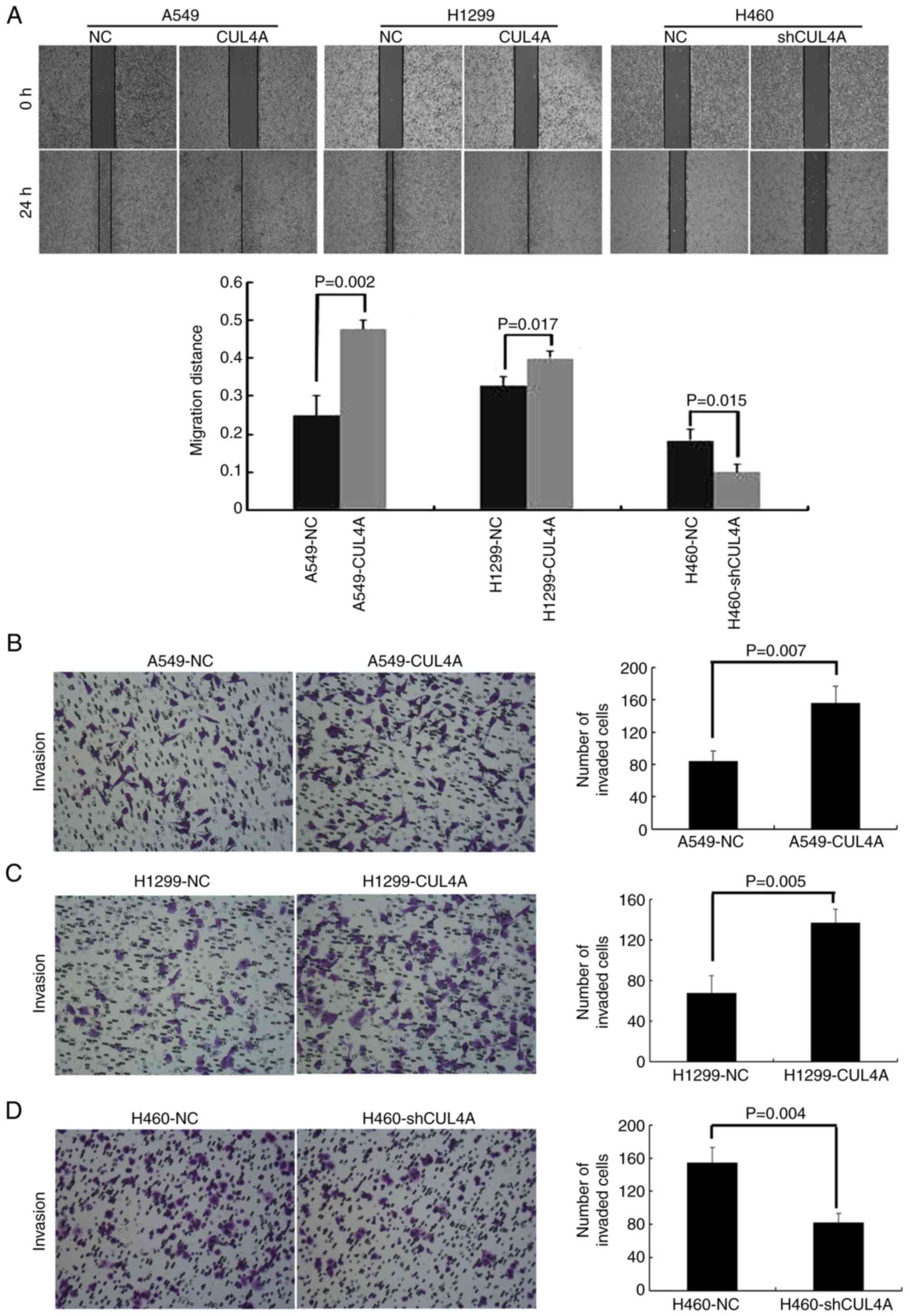
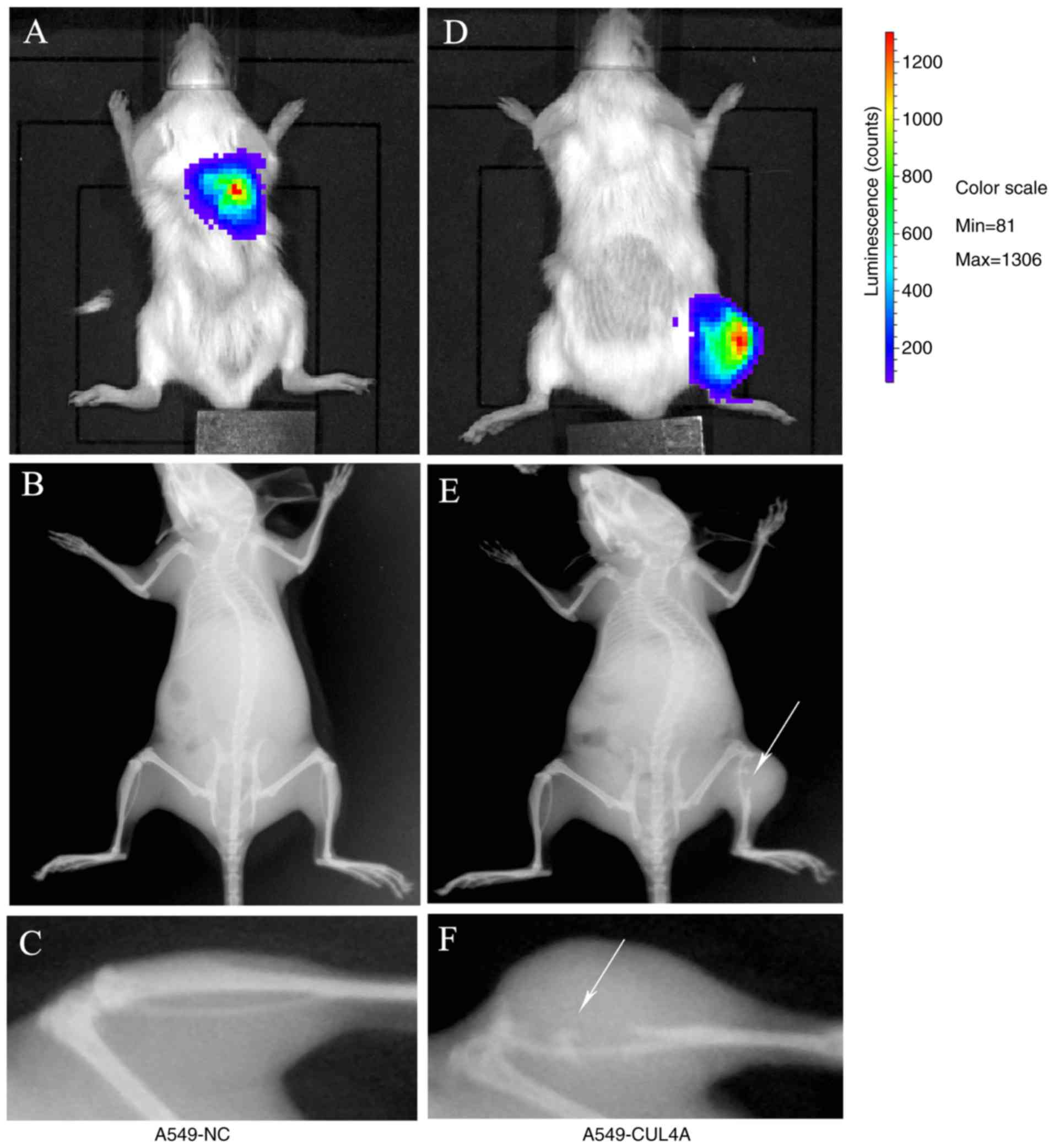
|
1
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI
|
|
2
|
Reck M and Rabe KF: Precision diagnosis
and treatment for advanced non-small-cell lung cancer. N Engl J
Med. 377:849–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000-14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hannah J and Zhou P: Distinct and
overlapping functions of the cullin E3 ligase scaffolding proteins
CUL4A and CUL4B. Gene. 573:33–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma P and Nag A: CUL4A ubiquitin
ligase: A promising drug target for cancer and other human
diseases. Open Biol. 4:1302172014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial- mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Zhang N, Wang Y, Xu M, Liu N, Pang
X, Cao J, Ma N, Pang H, Liu L and Zhang H: Zinc finger E-box
binding homeobox 1 promotes invasion and bone metastasis of small
cell lung cancer in vitro and in vivo. Cancer Sci. 103:1420–1428.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong W, Feng H, Santiago FE and Kipreos
ET: CUL-4 ubiquitin ligase maintains genome stability by
restraining DNA-replication licensing. Nature. 423:885–889. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee J and Zhou P: Pathogenic role of the
CRL4 ubiquitin ligase in human disease. Front Oncol. 2:212012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugasawa K: The CUL4 enigma: Culling DNA
repair factors. Mol Cell. 34:403–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han J, Zhang H, Zhang H, Wang Z, Zhou H
and Zhang Z: A Cul4 E3 ubiquitin ligase regulates histone hand-off
during nucleosome assembly. Cell. 155:817–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu J and Xiong Y: An evolutionarily
conserved function of proliferating cell nuclear antigen for Cdt1
degradation by the Cul4-Ddb1 ubiquitin ligase in response to DNA
damage. J Biol Chem. 281:3753–3756. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li B, Jia N, Kapur R and Chun KT: Cul4A
targets p27 for degradation and regulates proliferation, cell cycle
exit, and differentiation during erythropoiesis. Blood.
107:4291–4299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Wang Y, Ma G, Wang Q and Wei G:
CUL4A is overexpressed in human pituitary adenomas and regulates
pituitary tumor cell proliferation. J Neurooncol. 116:625–632.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melchor L, Saucedo-Cuevas LP, Muñoz-Repeto
I, Rodríguez- Pinilla SM, Honrado E, Campoverde A, Palacios J,
Nathanson KL, García MJ and Benítez J: Comprehensive
characterization of the DNA amplification at 13q34 in human breast
cancer reveals TFDP1 and CUL4A as likely candidate target genes.
Breast Cancer Res. 11:R862009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Zhang P, Liu Z, Wang Q, Wen M,
Wang Y, Yuan H, Mao JH and Wei G: CUL4A overexpression enhances
lung tumor growth and sensitizes lung cancer cells to erlotinib via
transcriptional regulation of EGFR. Mol Cancer. 13:2522014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Birner P, Schoppmann A, Schindl M, Dinhof
C, Jesch B, Berghoff AS and Schoppmann SF: Human homologue for
Caenorhabditis elegans CUL-4 protein overexpression is associated
with malignant potential of epithelial ovarian tumours and poor
outcome in carcinoma. J Clin Pathol. 65:507–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishitani H, Shiomi Y, Iida H, Michishita
M, Takami T and Tsurimoto T: CDK inhibitor p21 is degraded by a
proliferating cell nuclear antigen-coupled Cul4-DDB1Cdt2 pathway
during S phase and after UV irradiation. J Biol Chem.
283:29045–29052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nag A, Bagchi S and Raychaudhuri P: Cul4A
physically associates with MDM2 and participates in the proteolysis
of p53. Cancer Res. 64:8152–8155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Lee S, Zhang J, Peters SB, Hannah
J, Zhang Y, Yin Y, Koff A, Ma L and Zhou P: CUL4A abrogation
augments DNA damage response and protection against skin
carcinogenesis. Mol Cell. 34:451–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hung MS, Chen YC, Lin P, Li YC, Hsu CC,
Lung JH, You L, Xu Z, Mao JH, Jablons DM and Yang CT: Cul4A
modulates invasion and metastasis of lung cancer through regulation
of ANXA10. Cancers (Basel). 11(pii): E6182019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prudkin L, Liu DD, Ozburn NC, Sun M,
Behrens C, Tang X, Brown KC, Bekele BN, Moran C and Wistuba II:
Epithelial-to-mesenchymal transition in the development and
progression of adenocarcinoma and squamous cell carcinoma of the
lung. Mod Pathol. 22:668–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cowin P, Rowlands TM and Hatsell SJ:
Cadherins and catenins in breast cancer. Curr Opin Cell Biol.
17:499–508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mock K, Preca BT, Brummer T, Brabletz S,
Stemmler MP and Brabletz T: The EMT-activator ZEB1 induces bone
metastasis associated genes including BMP-inhibitors. Oncotarget.
6:14399–14412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takeyama Y, Sato M, Horio M, Hase T,
Yoshida K, Yokoyama T, Nakashima H, Hashimoto N, Sekido Y, Gazdar
AF, et al: Knockdown of ZEB1, a master epithelial-to-mesenchymal
transition (EMT) gene, suppresses anchorage-independent cell growth
of lung cancer cells. Cancer Lett. 296:216–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gemmill RM, Roche J, Potiron VA, Nasarre
P, Mitas M, Coldren CD, Helfrich BA, Garrett-Mayer E, Bunn PA and
Drabkin HA: ZEB1-responsive genes in non-small cell lung cancer.
Cancer Lett. 300:66–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang X, Li L, Huang Q, Xu W, Cai X, Zhang
J, Yan W, Song D, Liu T, Zhou W, et al: Wnt signaling through
Snail1 and Zeb1 regulates bone metastasis in lung cancer. Am J
Cancer Res. 5:748–755. 2015.PubMed/NCBI
|
|
32
|
Wang Y, Wen M, Kwon Y, Xu Y, Liu Y, Zhang
P, He X, Wang Q, Huang Y, Jen KY, et al: CUL4A induces
epithelial-mesenchymal transition and promotes cancer metastasis by
regulating ZEB1 expression. Cancer Res. 74:520–531. 2014.
View Article : Google Scholar : PubMed/NCBI
|