Introduction
Osteosarcoma (OS), a common malignant tumor
originating from bone tissue, commonly presents in children and
adolescents. Approximately one-third of OS patients survive less
than 5 years, and the metastasis of osteosarcoma is the main factor
contributing to this poor prognosis. Therefore, the development of
innovative therapies for OS is an urgently needed task in the field
of OS research (1,2). In recent years, basic studies have
shown that long noncoding RNAs (lncRNAs) can play a regulatory role
in tumor progression by extensively regulating proliferation,
invasion and metastasis in tumor cells (3). lncRNAs, as competing endogenous RNAs,
interact with microRNAs (miRNAs) to regulate target genes to play
important roles in the onset and development of cancers (4,5). Along
with miRNAs, lncRNAs have become a ‘hot’ topic in tumor research
due to their antitumor or tumorigenic effects and their potentially
important role in tumor diagnosis, treatment and prognosis
determination.
Although previous studies have shown that abnormal
lncRNA expression occurs in the progression of OS, we still require
more in-depth research in regards to the mechanisms of OS
progression (6–8). In the present study, we discovered and
are reporting for the first time lncRNA PBB12 (named by us), which
is closely related to the proliferation and invasion of OS cells.
Our study not only showed that PBB12 knockdown can effectively
inhibit the proliferation, invasion and metastasis of OS cells by
restoring the function of the tumor-suppressor gene Kruppel-like
factor 4 (KLF4) but also showed that PBB12 is likely to become a
clinical screening marker for patients at high risk for OS
metastasis.
Materials and methods
Cell culture
The OS cell lines MG63 (TCHu124) and SAOS-2
(TCHu114) were obtained from the Cell Bank of the Chinese Academy
of Sciences (CBCAS; Shanghai, China) and maintained in minimal
essential medium (MEM; cat. no. 11095-072; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal calf serum (FBS; cat.
no. 10099-141; Thermo Fisher Scientific, Inc.). 293TN cells (a
lentivirus-producing cell line) were purchased from System
Biosciences (cat. no. LV900A-1) and maintained in Dulbecco's
modified Eagle medium (DMEM; cat. no. 10569-044; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and were passaged via
0.25% trypsin digestion (cat. no 25200056; Thermo Fisher
Scientific, Inc.). All cells were adherently grown in 5%
CO2 at 37°C and in saturated humidity.
Tumor tissues
OS tumors with or without metastasis and the
adjacent normal tissues (within a 2.5-cm radius from the edge of
the tumor) were collected from 24 patients at the Department of
Orthopedics, Huashan Hospital between October 2017 and July 2018
(detailed patient information is presented in Table I). The selected patients were
required to be under 25 years of age; both male and female patients
were diagnosed for the first time, and no patient was treated by
chemotherapy or radiotherapy prior to surgery. This study was
approved by the Ethics Review Committee of Huashan Hospital
Affiliated to Fudan University (2016-037), and written informed
consent was obtained from all participants and/or their guardians.
The tissues were used for total RNA extraction and real-time PCR
for the measurement of PBB12, hsa-miR-204-5p and activating
transcription factor 2 (ATF2) mRNA levels. Total protein extraction
and western blotting were used to assess Kruppel-like factor (KLF4)
and ATF2 expression.
 | Table I.Clinicopathological features of the
24 patients with or without metastatic osteosarcoma. |
Table I.
Clinicopathological features of the
24 patients with or without metastatic osteosarcoma.
| Patient no. | Sex | Age (years) | TNM stage | Site of
metastasis |
|---|
|
1 | M | 12 | T2NxM0 | None |
|
2 | F | 20 | T2N1M0 | None |
|
3 | F | 16 | T1NxM0 | None |
|
4 | M | 12 | T2N1M0 | None |
|
5 | F | 11 | T1N0M0 | None |
|
6 | F | 18 | T1N1M0 | None |
|
7 | M | 15 | T2NxM0 | None |
|
8 | M | 16 | T1N0M0 | None |
|
9 | F | 15 | T2NxM0 | None |
| 10 | M | 13 | T1N1M0 | None |
| 11 | F | 16 | T2NxM0 | None |
| 12 | F | 18 | T2N0M0 | None |
| 13 | F | 14 | T2N2M1 | Liver |
| 14 | M | 16 | T2N1M1 | Lung |
| 15 | F | 12 | T2NxM1 | Bone |
| 16 | M | 15 | T2N2M1 | Bone |
| 17 | M | 17 | T2N1M1 | Kidney |
| 18 | F | 11 | T3N1M1 | Lung |
| 19 | M | 13 | T2N1M1 | Brain |
| 20 | M | 14 | T1NxM1 | Brain |
| 21 | F | 21 | T3N1M1 | Lung |
| 22 | F | 15 | T3N2M1 | Kidney |
| 23 | F | 12 | T2N1M1 | Lymph node |
| 24 | M | 14 | T2N1M1 | Liver |
Recombinant lentivirus
preparation
An siRNA (5′-GCTCGGTGTAAAGGGAGGG-3′) specific for
PBB12 was chosen, and the shRNA oligonucleotide DNA was synthesized
and cloned into the shRNA vector pSIH1-H1 (SI501A-1, System
Biosciences) after double-strand annealing. A nonsense sequence of
siRNA (5′-GGTAGGTGGGCAGCTGAGA-3′) was used as a negative control
(NC). The constructed vectors were named pSIH1-shRNA-PBB12 and
pSIH1-NC. The coding sequence (CDS) of the KLF4 gene (1,542 bp) was
amplified using the primers 5′-GGAATTCGCCACCATGAGGCAGCCACCTGGCG-3′
and 5′-CGGGATCCTTAAAAATGCCTCTTCATGTGTAAGG-3′ using human
complementary DNA (cDNA) as the template. The PCR product was
digested with EcoRI and BamHI and cloned into the
expression vector pcDH-GFP (CD511B-1, System Biosciences) to
construct the recombinant vector pcDH-KLF4. PcDH-PBB12 was
constructed by the same method. The pair of PCR primers were
5′-GGAATTCTAATAGAATGATTTTTATT-3′ (forward) and
5′-CGGGATCCGAGGAAGGAGGGCGCAGGGCA-3′ (reverse), and these primers
produced a 1,121-bp PCR product. All recombinant vectors were
sequenced, and plasmid DNA was prepared using an EndoFree Plasmid
Kit (cat. no. 12362; Qiagen).
A total of 1×106 293TN cells each were
seeded into 10-cm dishes in 10 ml DMEM containing 10% FBS and were
cultured overnight under normal conditions. Two micrograms of each
recombinant vector (pcDH-KLF4, pshRNA-PBB12, pshRNA-NC or
pcDH-PBB12) and 10 µg of the pPACK Packaging Plasmid Mix (LV500A-1;
System Biosciences) were cotransfected using the cationic liposome
transfection reagent Lipofectamine 2000 (cat. no. 11801169;
Invitrogen). The medium was fully replaced with DMEM plus 1% FBS
before transfection. After 48 h, the supernatant was harvested and
cleared by centrifugation at 5,000 × g at 4°C for 10 min and then
passed through a 0.45-µm PVDF membrane (cat. no. IPVH00010,
Millipore). The titer of the virus was determined by a gradient
dilution method. The recombinant lentiviruses were named Lv-KLF4,
Lv-shRNA-PBB12, Lv-NC and Lv-PBB12.
Luciferase assay
Verification of the seed region of
hsa-miR-204-5p in ATF2 3′UTR
Part of the human ATF2 (NM_001880.4) 3′UTR (196 bp)
containing the theoretical seed region was amplified from human
cDNA with the primers 5′-GCTCTAGAAACCTGCAGTACAACAGTT-3′ (forward)
and 5′-GCTCTAGAAATCAGTCTTTTTCCAGAGAC-3′ (reverse). The PCR product
was digested with XbaI and cloned into the pGL3-promoter
vector (E1761, Promega) at the downstream position of the
luciferase gene to construct pGL3-wt-ATF2, which carried the
wild-type seed region of hsa-miR-204-5p; the seed region was
mistranslated from 5′-AAGGGAA-3′ to 5′-GAGAAAG-3′ to construct
pGL3-mt-ATF2 and carried the mutant hsa-miR-204-5p seed region. The
hsa-miR-204-5p-mimic (5′-UUCCCUUUGUCAUCCUAUGCCUTT-3′),
hsa-miR-204-5p-inhibitor (5′-AGGCAUAGGAUGACAAAGGGAATT-3′) and
hsa-miR-204-5p-NC (5′-UGUAUUCUCUCUCACUUCCCUGTT-3′) were obtained
from Shanghai GenePharma (Shanghai, China). 293TN cells were
cotransfected with the miR-204-mimic, inhibitor, or NC and
pGL-wt-ATF2 or pGL3-mt-ATF2 using Lipofectamine 2000 according to
the manufacturer's instructions. Forty-eight hours after
transfection, the cells were harvested, and luciferase assays were
performed using a Dual-Luciferase Reporter Assay System (cat. no.
E1910; Promega).
The experiment to observe the effect of PBB12
depletion on the inhibition of luciferase by the hsa-miR-204-5p
mimic was carried out in 293TN cells by using the same method used
for the seed region validation. We overexpressed PBB12 by
transfecting pcDH-PBB12 in 293TN cells cotransfected with
miR-204-5p mimics and pGL3-wt-ATF2. If overexpression of PBB12PBB12
was able to upregulate intracellular luciferase activity, it would
indicate that PBB12 can significantly attenuate the negative
regulation of miR-204-5p on ATF2.
Validation of the transcription factor
binding site (TFBS) of KLF4 in the promoter of pri-miR-204-5p
We first searched for the location of the precursor
of hsa-miR-204-5p (pri-miR-204) in the human genome and selected a
2.5 kb DNA sequence upstream of the transcription start site as the
promoter region. Then, we predicted the promoter sequence using
Promoter 2.0 software (DTU Health Tech). The KLF4 TFBS in the
pri-miR-204 promoter was predicted by ‘JASPAR’ (9). The promoter of pri-miR-204 (312 bp)
was amplified using human genomic DNA as the template with the
primers 5′-GGGGTACCCTAGCACTCACTCAGTGACT-3′ (forward) and
5′-CCCAAGCTTGGGAATAGTTCTGGGTCCAAACCATGTGAC-3′ (reverse). The PCR
product was digested with KpnI and HindIII and was
cloned into the luciferase reporter vector pGL3-Enhancer (E1771;
Promega) at a position upstream of the luciferase gene to construct
pGL3-TFBS(wt)-miR-204, which carried the wild-type TFBS. Then, the
TFBS in the pGL3-TFBS(wt)-miR-204 vector was mutated from 5-CAC
C-3′ to 5′-CCAC-3′ to construct pGL3-TFBS(mt)-miR-204, which
carried a mutated TFBS. The experiment was carried out in 293TN
cells, and the detection and analysis methods used for transfection
and the measurement of luciferase activity assay were the same as
those used above.
Cell function experiment
MG63 and SAOS-2 OS cells were trypsinized and seeded
into 96-well plates at a density of 1×105 cells per well
72 h after being infected with the recombinant lentiviruses (Lv-NC,
Lv-KLF4, Lv-PBB12+Lv-KLF4 or Lv-shRNA-PBB12+Lv-KLF4). The cells
were cultured under normal conditions, and cell viability was
detected using a Cell Counting Kit-8 assay (CCK-8, CK04; Dojindo
Molecular Technologies, Inc.) at 24, 48, and 72 h. Cell invasion
experiments were performed using the QCM ECMatrix Cell Invasion
Assay, 24-well (8 µm), fluorimetric (ECM554; Chemicon
International) according to the manufacturer's instructions.
Briefly, 500 µl MEM supplemented with 10% FBS was added to the
lower chamber as a chemoattractant. The cells that migrated and
invaded the underside of the membrane were fixed in 4%
paraformaldehyde and stained with crystal violet, and the cells
were counted with the membrane inverted. Simultaneously, tumor
cells that passed through the membrane were also counted by the
fluorescence method according to the instructions.
Gene intervention via the lentiviral
pathway
MG63 OS cells in the logarithmic phase were seeded
into 6-well plates at 1×105 cells/well. One day later,
lentivirus (Lv-NC, Lv-KLF4, Lv-KLF4+Lv-shRNA-PBB12 and
Lv-KLF4+Lv-shRNA-PBB12) was added at a multiplicity of infection
(MOI) of 10. The infection efficiency was evaluated by observing
the fluorescence of green fluorescent protein (GFP) 72 h after
infection. Total RNA and protein were isolated from the cells and
subjected to real-time PCR for the measurement of the levels of
PBB12 and hsa-miR-204-5p and western blotting for the measurement
of the proteins levels of KLF4, ATF2, cyclin D1, E-cadherin and
matrix metalloproteinase-9 (MMP9). In addition, immunofluorescence
was used to detect the expression of MMP9 in each group.
Real-time PCR
Total RNA was isolated and reverse-transcribed into
cDNA using M-MLV reverse transcriptase. Real-time PCR was performed
using the SYBR Premix Ex Taq™ kit and TP800 System (Takara), and
cDNA (200 ng) was used as the template. The levels of PBB12 and
ATF2 were normalized to the level of an endogenous housekeeping
gene, β-actin, using the 2−ΔΔCq method (10). For the determination of the
hsa-miR-204-5p levels, the level of U6 snRNA was used as the
reference. The PCRs were carried out under the following
conditions: 40 cycles of denaturation at 95°C for 10 sec, annealing
at 56°C for 10 sec and extension at 72°C for 10 sec. The specific
primers used for reverse transcription were
5′-TACCTTGCGAAGTGCTTAAAC-3′ for U6 snRNA and
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGAAGGCAT-3′ for
hsa-miR-204-5p. The PCR primers were as follows: PBB12,
5′-AAGGGCGAATGCAGGCA-3′ (forward) and 5′-AAGCAGCCGGCTGGCC-3′
(reverse); ATF2, 5′-AATCTCGACCGCAGTCATT-3′ (forward) and
5′-TTAGCTGCTCTTCTCCGACGACC-3′ (reverse); β-actin,
5′-CCTGTACGCCAACACAGTGC-3′ (forward) and 5′-ATACTCCTGCTTGCTGATCC-3′
(reverse); U6 snRNA, 5′-GTGCTCGCTTCGGCAGCACAT-3′ (forward) and
5′-TACCTTGCGAAGTGCTTAAAC-3′ (reverse); and miR-204-5p,
5′-GCCGGCGCCCGAGCTCTGGCTC-3′ (forward) and
5′-TTCCCTTTGTCATCCTATGCCT-3′ (reverse).
Western blotting
Total protein was extracted from cells or tissues
using the M-PER mammalian protein extraction reagent or the T-PER
tissue protein extraction reagent (78501/78510, Pierce; Thermo
Fisher Scientific, Inc.). Equal amounts of total protein (16 µg)
were loaded onto SDS-PAGE gels (11%) and transferred onto
nitrocellulose membranes. The blots were probed with the primary
antibodies against human KLF4 (cat. no. ab215036, dilution 1:200),
ATF2 (cat. no. ab32019, dilution 1:500), cyclin D1 (cat. no.
ab137867, dilution 1:350) and β-actin (cat. no. ab179467, dilution
1:12,00) (Abcam), followed by probing with the secondary
HRP-conjugated anti-rabbit/mouse antibody (cat. no. ab6721,
1:3,000; cat. no. ab205719, 1:4,000; Abcam). After washing, the
bands were detected by chemiluminescence and imaged with X-ray film
which was used to detect the bands, and the relative optical
densities were analyzed using image processing software TotalLab
v1.10 (TotalLab, Ltd.). β-actin was used as an endogenous reference
for normalization.
Immunofluorescence (IF)
For immunofluorescence, the cells were fixed with 4%
formaldehyde, permeabilized with 0.4% Triton X-100, incubated with
10% donkey serum, and stained with anti-MMP9 (cat. no. ab38898,
dilution 1:400) overnight at 4°C. Hoechst 33342 (cat. no. H21492;
Invitrogen; Thermo Fisher Scientific, Inc.) was used for nuclear
DNA staining.
Statistical analysis
Data are shown as the mean ± SD of three independent
experiments. All statistical data were analyzed using SPSS GradPack
version 20.0 statistical software (IBM Corp.) and GraphPad Prism
7.0 (GraphPad Software, Inc.). Comparisons between groups were
analyzed using a two-tailed Student's t-test or one-way ANOVA with
a post hoc Tukey test. Differences were considered to be
statistically significant at P<0.05.
Results
Differential expression analysis of
KLF4, ATF2, hsa-miR-204-5p and PBB12 during the process of OS
metastasis
KLF4 protein detection data showed that regardless
of whether OS metastasis occurred, KLF4 expression in tumors was
significantly lower than that in adjacent tissues (P<0.01 vs.
autologous adjacent tissues). There was no significant difference
in KLF4 protein expression in OS without metastasis and OS with
metastasis (P>0.05; Fig. 1A).
ATF2 protein showed an opposite trend of KLF4; that is, the ATF2
expression levels were significantly higher in both types of OS
than in adjacent tissues (P<0.01 vs. autologous adjacent
tissues). In contrast to that of KLF4, the expression of ATF2 was
significantly higher in OS with metastasis than in OS without
metastasis (P<0.01 vs. osteosarcoma without metastasis; Fig. 1B). Real-time PCR data indicated that
there were no significant differences in the ATF2 mRNA levels in
all groups (P>0.05; Fig. 1C).
Real-time PCR results also showed that the hsa-miR-204-5p content
in two types of OS and the adjacent tissues was completely
consistent with the changes in the KLF4 protein (Fig. 1D). The level of PBB12 was
significantly higher in OS with metastasis and adjacent tissues
than that in OS without metastasis and adjacent tissues (P<0.01,
vs. non-metastatic osteosarcoma and adjacent tissues), but there
was no significant difference in the PBB12 level in tumors and
autologous adjacent tissues in either type of OS (P>0.05;
Fig. 1E). Real-time PCR data showed
that there was no difference in the PBB12 content of OS and
adjacent tissues regardless of whether metastasis occurred;
however, the content of PBB12 was significantly higher in
metastatic tumors and adjacent tissues than in nonmetastatic tumors
and adjacent tissues, and this result indicated that PBB12 was
highly expressed in patients with metastatic OS. According to the
available data, it is highly probable that the content of PBB12
does not change significantly with time in the progression of OS,
and this hypothesis is completely consistent with the conclusion of
this study. In the case of high PBB12 expression, there was no
correlation between KLF4 and ATF2, and in the case of low PBB12
expression, the two proteins showed a negative correlation; this
correlation is one of the reasons for the existence of the
KLF4/hsa-miR-204-5p/ATF2 regulatory pathway. That is, the
metastasis probability for OS patients with high expression of
PBB12 is significantly increased, and PBB12 has the potential to
become a screening marker for high-risk groups with metastatic
OS.
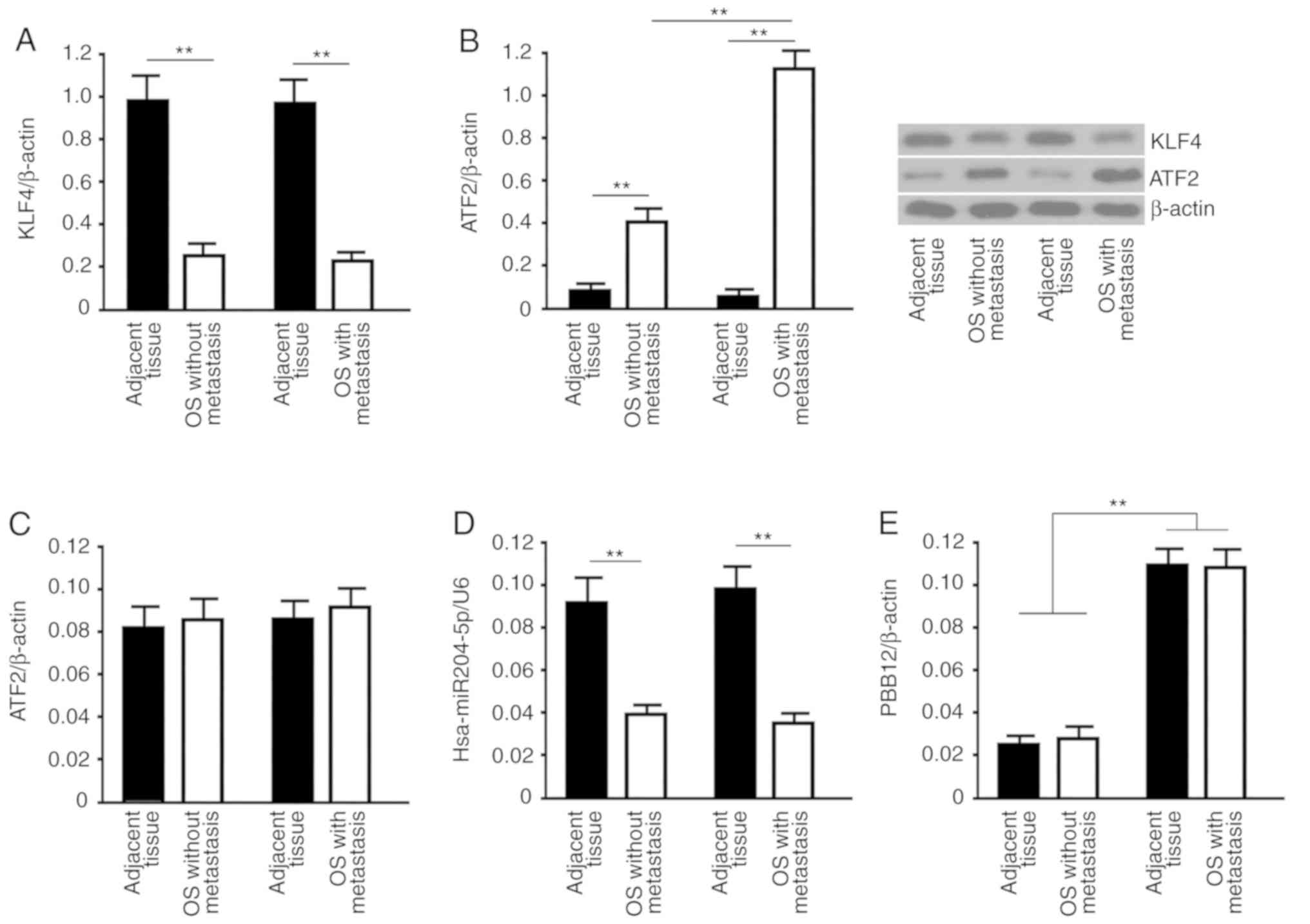 | Figure 1.Analyses of KLF4, ATF2,
hsa-miR-204-5p and PBB12 in OS and adjacent tissues. Analyses of
the levels of (A) KLF4 and (B) ATF2 in OS tissues with and without
metastasis and adjacent tissues were carried out by western blot
analysis. For western blot analysis, β-actin was used as the
internal control protein. The target band sizes of KLF4, ATF2 and
β-actin proteins were 54, 49 and 37 kDa, respectively. Using
real-time PCR, the relative values of (C) ATF2 mRNA, (D)
hsa-miR-204-5p and (E) PBB12 in OS tissues with and without
metastasis and adjacent tissues were analyzed using the
2−ΔΔCq method. β-actin (for the RNA levels of ATF2 mRNA
and PBB12) or U6 (for the hsa-miR-204-5p level) were used as the
internal controls. All data are expressed as the mean ± SD. The
sample size of the group was n=12. **P<0.01, t-test. OS,
osteosarcoma; KLF4, Kruppel-like factor 4; ATF2, activating
transcription factor 2. |
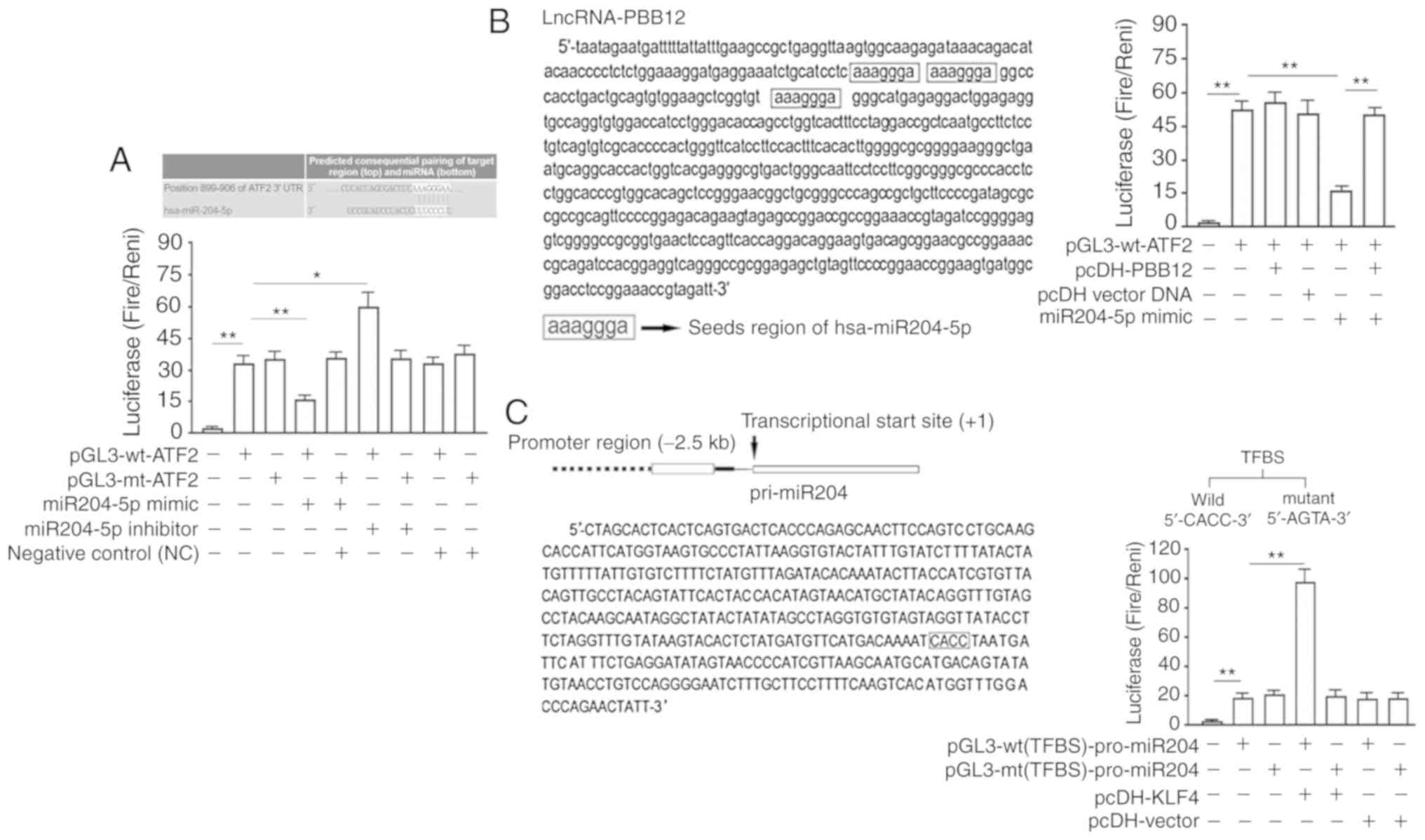
Luciferase experiment
The verification of the miR-204-5p seed region in
the 3′UTR of ATF2 was performed as follows: ‘TargetScan 7.1’
(http://www.targetscan.org/) analysis
showed that the 3′UTR region of the ATF2 gene contained a 7-bp seed
region (5′-AAGGGAA-3′) that could be bound by hsa-miR-204-5p. After
cotransfection of 293T cells for 48 h, a luciferase activity assay
showed that the hsa-miR-204-5p-mimic significantly decreased the
luciferase activity in cells transfected with the wild-type
luciferase reporter vector from 33.51±3.13 to 15.08±1.24 and that
the hsa-miR-204-5p-mimic significantly increased the luciferase
activity in cells transfected with the wild-type luciferase
reporter vector from 33.51±3.13 to 59.32±8.61. The differences
between all cotransfection groups and the group transfected with
pGL3-mt-ATF2 were not significant (Fig.
2A).
PBB12 significantly inhibits the binding of
hsa-miR-204-5p via the seed region to the 3′UTR region of the ATF2
gene. We used the software ‘TargetScan’ to predict the binding
sites of PBB12 and hsa-miR-204-5p. The results showed that there
were at least three hsa-miR-204-5p seed regions in PBB12, and the
positions were distributed over 60 bases. The luciferase activity
assay performed 48 h after the cotransfection of the 293T cells
showed that the luciferase activity was significantly lower in the
miR-204-5p-mimic- and pGL3-wt-ATF2-cotransfected cells than in the
pGL3-wt-ATF2-transfected cells (P<0.01 vs. the
pGL3-wt-ATF2-transfected group). There was no significant change in
the luciferase activity in the pcDH1-PBB12- or pcDH1 vector- and
pGL3-wt-ATF2-cotransfected cells (P<0.01, vs.
pGL3-wt-ATF2-transfected group) compared with that in the
miR-204-5p-mimic- and pGL3-wt-ATF2-cotransfected cells. There was a
significant increase in the luciferase activity in the
miR-204-5p-mimic- and pGL3-wt-ATF2- and pcDH1-PBB12-cotransfected
cells (P<0.01 vs. miR-204-5p-mimic- and
pGL3-wt-ATF2-cotransfected cells) (Fig.
2B).
Analysis of the regulation of the transcription of
hsa-miR-204-5p by KLF4 was performed as follows. To identify the
hsa-miR-204-5p promoter, the hsa-miR-204-5p precursor pri-miR-204
sequence in the human genome was first determined. The 2.5-kb
promoter sequence upstream of the transcription initiation sites
was identified and used to predict the location of the promoter
sequence by using Promoter 2.0 prediction software. The prediction
results showed that the promoter was a 432-bp DNA sequence.
‘JASPAR’ predicted that KLF4 bound to the theoretical TFBS in the
pri-miR-204 promoter. TFBS was validated using a luciferase
reporter gene assay. After 48 h of cotransfection in 293TN cells,
overexpression of KLF4 (pcDH1-KLF4 transfection) significantly
activated the luciferase activity in cells transfected with
pGL3-wt(TFBS)-pro-miR204 (P<0.01, vs.
pGL3-wt(TFBS)-pro-miR204-transfected cells) but did not have a
significant effect on the luciferase activity in cells transfected
with pGL3-mt(TFBS)-pro-miR204 (P>0.05, vs.
pGL3-wt(TFBS)-pro-miR204-transfected cells) (Fig. 2C).
Silencing and overexpression of PBB12
enhance and reverse the inhibition of KLF4 during OS cell
proliferation and invasion
MG63 and SAOS-2 OS cells were reseeded 72 h after
infection. The proliferative activity analysis showed that KLF4
gene overexpression (Lv-KLF4) significantly inhibited the
proliferative activity of MG63 and SAOS-2 cells in the logarithmic
phase (P<0.01, vs. the control group or the NC group, 72 h).
Proliferation was further inhibited in the KLF4 overexpression and
PBB12 silencing (Lv-shRNA-PBB12+LV-KLF4) groups (P<0.05, vs. the
KLF4 overexpression group, 72 h), while the proliferation activity
in the KLF4 overexpression and PBB12 overexpression
(Lv-PBB12+Lv-KLF4) groups was significantly increased (P<0.01,
vs. the KLF4 overexpression group, 72 h) (Fig. 3A). The tumor cell invasion assay
showed that overexpression of the KLF4 gene (Lv-KLF4) significantly
inhibited the invasion of MG63 and SAOS-2 cells (P<0.01, vs. the
control group or the NC group); PBB12 silencing (Lv-shRNA-PBB12)
significantly enhanced the inhibitory effect of KLF4 on tumor cell
invasion (P<0.05, vs the KLF4 overexpression group), while PBB12
overexpression (Lv-PBB12) significantly abrogated the inhibitory
effect of the KLF4 gene on invasion in the overexpression group
(P<0.01, vs. the KLF4 overexpression group; P>0.05, vs. the
control group or the NC group) (Fig.
3B).
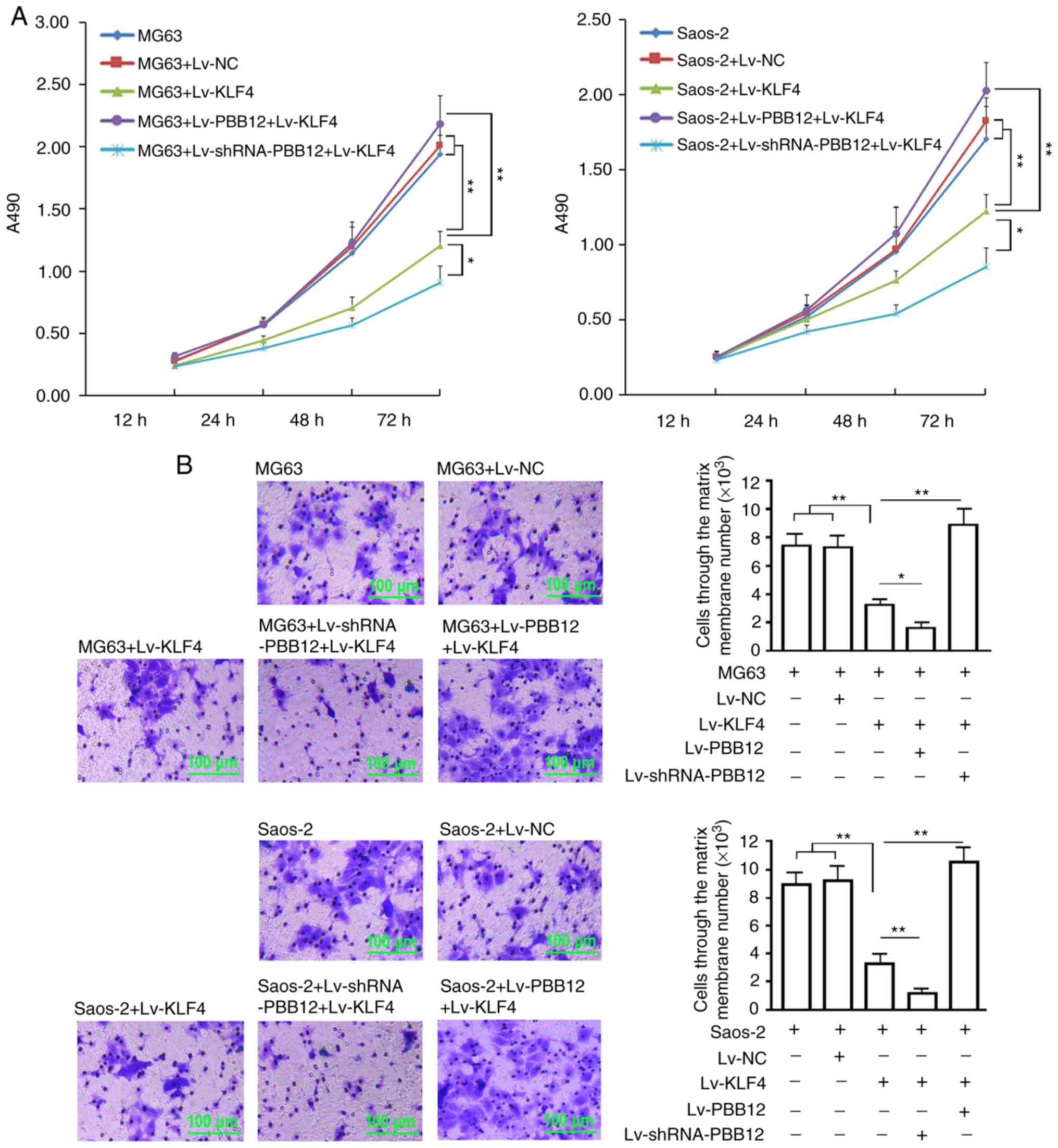 | Figure 3.Cell proliferation and invasion
assay. (A) MG63 and SAOS-2 cells were infected with the indicated
lentiviruses (Lv-NC, Lv-KLF4, Lv-PBB12+Lv-KLF4 or
Lv-shRNA-PBB12+Lv-KLF4), seeded into 96-well plates and subjected
to a cell viability assay at the indicated times. (B) Invasion data
from MG63 and SAOS-2 cells 72 h after being infected with the
indicated viruses (Lv-NC, Lv-KLF4, Lv-PBB12+Lv-KLF4 or
Lv-shRNA-PBB12+Lv-KLF4), as determined by a Transwell assay. The
crystal violet-stained cells are the cells that passed through the
membrane, and the counts reflect the number of stained cells that
passed through the membrane, which were estimated using the
absorbance and the standard curve. The x-coordinate represents the
cell grouping, and the y-coordinate represents the cell number.
**P<0.01, *P<0.05. The tests were carried out with three
biological triplicates, and the data are expressed as the mean ±
SD. KLF4, Kruppel-like factor 4; ATF2, activating transcription
factor 2. |
PBB12 affects downstream functional
proteins in the KLF4/hsa-miR-204-5p/ATF2 pathway during OS
metastasis
The KLF4 protein detection assay showed that
infection with Lv-KLF4 upregulated KLF4 protein expression in the
MG63 cells (P<0.01 vs. the control group or the NC group), and
there were no significant changes in KLF4 expression between the
Lv-KLF4-, Lv-PBB12+Lv-KLF4- and Lv-shRNA-PBB12+Lv-KLF4-infected
groups (P>0.05). The ATF2 protein detection assay showed that
ATF2 protein expression was significantly decreased in the
Lv-KLF4-transfected MG63 cells (P<0.05, vs. the control group or
the NC group), and ATF2 was also significantly decreased in the
Lv-PBB12- or Lv-shRNA-PBB12+Lv-KLF4-transfected MG63 cells
(P<0.01, vs. the control group or the NC group). The expression
of cyclin D1 and MMP9 was consistent with that of ATF2 in all
groups. The expression of the E-cadherin protein in each group was
the complete opposite of that of ATF2 and cyclin D1 in all groups
(Fig. 4A). Immunofluorescence
staining of MMP9 showed that the overexpression of KLF4
significantly inhibited the expression of the MMP9 protein in MG63
cells. The silencing or overexpression of PBB12 significantly
enhanced or reduced the inhibition of KLF4 on the upregulation of
the MMP9 protein (Fig. 4B).
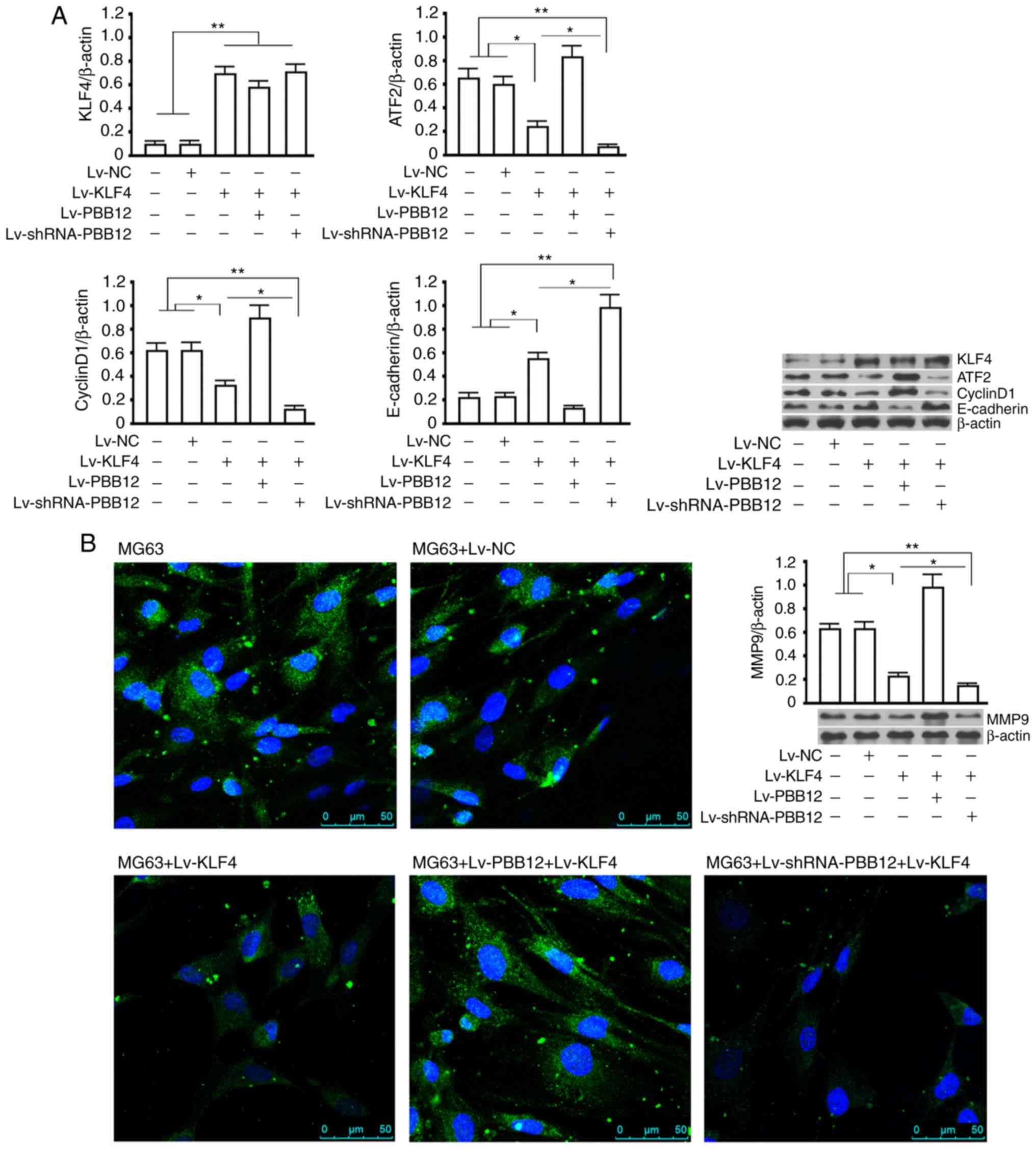 | Figure 4.Western blotting and
immunofluorescence assays for functional proteins downstream of the
KLF4/hsa-miR-204-5p/ATF2 pathway. (A) Western blotting of KLF4 (54
kDa), ATF2 (69 kDa), cyclin D1 (33 kDa), MMP9 (84 kDa) and
E-cadherin (120 kDa) in all groups after 72 h of recombinant virus
infection (Lv-NC, Lv-KLF4, Lv-PBB12+Lv-KLF4 or
Lv-shRNA-PBB12+Lv-KLF4). β-actin was used as the internal control.
(B) Immunofluorescence staining of the MMP9 protein in MG63 cells
from each group after 72 h of recombinant virus infection (Lv-NC,
Lv-KLF4, Lv-PBB12+Lv-KLF4 or Lv-shRNA-PBB12+Lv-KLF4). Blue
represents Hoechst staining, and green represents the FITC-labeled
MMP9 primary antibody. *P<0.05 and **P<0.01. The tests were
carried out with three biological triplicates, and the data are
expressed as the mean ± SD. KLF4, Kruppel-like factor 4; ATF2,
activating transcription factor 2; MMP9, matrix
metalloproteinase-9. |
Discussion
Long non-coding RNA (lncRNA) generally refers to
endogenous RNA sequences with a length greater than 200 bp. In
recent years, many lncRNAs have been discovered and are attracting
research attention. These lncRNAs have no protein-coding function;
however, they can take part in many types of life-sustaining
activities by regulating epigenetic modifications. Compared with
genes that encode proteins, lncRNAs are large in quantity. lncRNAs
can be considered important regulatory molecules in the human
genome and have many biological functions. They are involved in
chromosome silencing, chromatin modification, transcription
regulation and other biological processes (11,12).
lncRNAs interact with microRNAs (miRNAs), and this interaction has
an important influence on tumor progression (13). miRNAs can target and regulate
lncRNAs. miR-21 was found to not only target and inhibit its target
protein gene but also lncRNA-GAS5. Furthermore, lncRNAs can also
affect the tumor regulation function of miRNAs in several ways
(14–16).
The role of miRNAs in molecular regulatory
mechanisms involved in osteosarcoma (OS) has attracted much
research attention (17,18). Studies have shown that the content
of miR-21 in OS is significantly increased. Moreover, the
proliferation, invasion and metastasis of MG63 OS cells were found
to be promoted by the negative regulation of the expression of the
tumor-suppressor gene RECK (19,20).
miR-199a-3p is significantly reduced in OS. Overexpression of
miR-199a-3p can halt the progression of tumor cells in the cell
cycle in the G1 phase, and the growth and migration of tumor cells
are inhibited by the negative regulation of the expression of
STAT3, the target protein of miR-199a (21). Other studies have shown that through
miR-125b and its target protein STAT3, a closed positive feedback
pathway can be generated that jointly regulates the proliferation
and migration of MG63 and SAOS-2 OS cells and their development
into subcutaneous tumors (22).
miR-143-3-p, miR-544, miR-382 and miR-369-3p have all been
indicated to indirectly transcriptionally regulate their downstream
transcription product miR-17-5p through the negative regulation of
the expression of the common target gene MYC. Finally,
apoptosis of SAOS-2 OS cells is affected (23). Let-7 and miR-564 have been shown to
inhibit and promote OS cell invasion, respectively (24,25).
All of these studies show that miRNAs can participate in the
regulation of OS in many ways.
The interaction between miRNAs and lncRNAs in the
cancer field has been studied, and this will lead to important
changes in our understanding of the structural networks and
regulatory networks of tumor cells (26). This has inestimable scientific and
clinical value (27). The lncRNA
TUG1 has been proven to bind to miR-9-5p and miR-335-5p as a
competitive endogenous RNA to inhibit the negative regulation of
the expression of the functional genes POU2F1 and
ROCK1. Ultimately, OS cell proliferation and invasion and
apoptosis inhibition are promoted (28–30).
The lncRNAs PVT1 and H19 have been shown to promote the progression
of OS by causing the functional inactivation of members of the
miR-19 and miR-200 families (31,32).
Studies have shown that the synthesized pyrrolidine polyamide
(Myc-6) can be used to partially downregulate MALAT1 levels and
inhibit the progression of OS. The mechanism of action may be
related to the inhibition of miR-376a function and the upregulation
of the expression of TGF-α, the target protein of miR-376a
(33). These studies have shown
that lncRNAs affect miRNA function, which is generally involved in
the regulation of tumor progression.
hsa-miR-204-5p is located on human chromosome 9.
Previous studies have shown that hsa-miR-204-5p is a
tumor-suppressor gene in many tumor types. Luan and others have
shown that hsa-miR-204-5p can target MMP9 and BCL-2. Therefore, it
functions as a tumor-suppressor gene in malignant melanoma
(34). Research by Sümbül et
al revealed that miR-204-5p is increased in colorectal cancer
to inhibit the increased activity of LC3B-II in autophagy and Bcl2
against apoptosis post-transcriptionally and acts as a tumor
suppressor (35). hsa-miR-204-5 has
also been shown to affect the progression of breast cancer through
the regulation of the expression of its target protein SIX1
(36). However, there have been no
relevant reports on hsa-miR-204-5p and OS progression.
In the present study, low expression of
hsa-miR-204-5p in OS tissues was first identified. This directly
caused high expression of the oncogene ATF2. Interestingly,
the comparative analysis of the hsa-miR-204-5p content in
non-metastatic OS and metastatic OS tissues showed that its
expression was low in both groups of tumors, and there was no
difference between the groups. However, the expression of its
target protein ATF2 in both groups of tumor tissues showed an
opposite trend. To explain this phenomenon, transcription factors
upstream of hsa-miR-204-5p were screened and predicted. Finally, it
was confirmed that the nuclear transcription factor KLF4 can bind
to the hsa-miR-204-5p promoter and positively regulate its
transcription. Surprisingly, regulation by the upstream regulatory
nuclear transcription factor KLF4 of hsa-miR-204-5p appeared to be
unrelated to OS metastasis. However, the expression of KLF4 in the
two groups of tumors and their adjacent tissues was completely
consistent with that of hsa-miR-204-5p. These phenomena indicate
that the regulatory pathway involved in the expression of
KLF4/hsa-miR-204-5p/ATF2 is involved in the progression of OS
metastasis. The comparative analysis of the data obtained from the
study of metastatic and non-metastatic OS showed that there were no
obvious changes in the content of KLF4 and hsa-miR-204-5p in both
groups of tumor tissues. However, the expression of the ATF2
protein was significantly altered. Its content in metastatic tumor
tissue was significantly greater than that in the non-metastatic
tumor tissue. At this point, we believe that there are other
factors involved in the abovementioned pathway. The expression of
the ATF2 protein was further increased, which contributed to tumor
metastasis.
ATF2 belongs to the basic leucine zipper domain bZIP
transcription factor family. Under stress conditions, p38/JNK
kinase can directly phosphorylate the Thr69 and Thr71 sites in the
ATF2 protein; thus, ATF2 transcriptional activity is activated.
Phosphorylated ATF2 can form homodimers or heterodimers and bind to
specific DNA sequences. The expression of target genes is thereby
regulated (37). Studies have shown
that ATF2 has dual functions in carcinogenesis and cancer
suppression during the tumorigenesis of different types of tumors.
In addition to being related to the specific tissue/cell types used
in different studies, the intracellular localization of ATF2 may be
key to its role in tumorigenesis (38). ATF2 can bind to the cyclin D1
promoter. However, its transcription level is upregulated, and
breast cancer progression is promoted (39). Fan and others have shown that ATF2
can promote the proliferation and invasion of tumor cells through
the transcriptional regulation of MMP2 (40). Xu et al also confirmed that
ATF2 can affect the invasive capability of pancreatic tumor cells
through the regulation of the expression of E-cadherin (41). Yet, there have only been a few
studies on the relationship between the ATF2 gene and OS. Only a
few studies have shown that ATF2 is highly expressed in OS
(42). However, there have been no
reports on the specific underlying mechanism. In the present study,
we believe that the transcriptional regulation mechanism of ATF2
during metastasis of OS is deactivated, which explains why its
expression in OS tissues was significantly higher than that in
adjacent tissues, and its protein expression was significantly
higher in metastatic OS than in non-metastatic OS. Furthermore, the
transcription level of the ATF2 gene showed no obvious
change in the two groups of tumor specimens. We believe that the
cause of the abnormal expression of ATF2 may be the abnormal
function of miRNAs, which function as part of a classical
posttranscriptional regulatory mechanism.
In summary, metastasis is the main reason for the
poor prognosis of OS patients. OS treatment must be based on
inhibition of tumor metastasis. Fortunately, after systematic and
in-depth research, lncRNA PBB12 was found to bind to hsa-miR-204-5p
in the form of an RNA sponge; thus, its negative regulation of ATF2
protein expression was greatly reduced. We believe that this
discovery is of great significance for several reasons. First,
PBB12 may serve as a marker for screening high-risk osteosarcoma
patients with tumor metastasis. The research data provide strong
support for this hypothesis. Compared with that from non-metastatic
OS patients, the PBB12 content of tumor tissues from metastatic OS
patients was significantly increased. However, regardless of the
presence of metastasis, the content of PBB12 in tumor tissues was
not different from that in the adjacent tissues. The high
expression of PBB12 demonstrates the characteristics resulting from
individual differences. Second, for the first time, we propose a
possible solution for OS metastasis by targeting KLF4. For patients
with low expression or deletion of PBB12 in bone or other tissues,
KLF4 overexpression can effectively inhibit tumor progression
(proliferation and metastasis) via the KLF4/hsa-miR-204-5p/ATF2
pathway; however, for patients with high expression of PBB12, the
inhibition or knockdown of PBB12 may ensure the normal function of
the KLF4/hsa-miR-204-5p/ATF2 pathway and the inhibition of OS
metastasis.
PBB12 and the members of the
KLF4/hsa-miR-204-5p/ATF2 pathway and their interactions can jointly
regulate the mechanisms involved in OS metastasis. However,
according to our research, the mechanism and pathway involved in
the participation of PBB12 in the progression of OS has been
preliminarily explained. In the present study, PBB12 did not
directly bind to KLF4. PBB12 only blocked the KLF4/miR-204-5p/ATF2
pathway by binding to miR-204-5p. If PBB12 is regarded as a genetic
characteristic of different populations, it is actually a key
factor that is independent of KLF4. Therefore, high expression of
PBB12 can only change the anticancer properties of KLF4 or restore
the anticancer function of KLF4, but it has no correlation with the
expression of KLF4. According to the existing theory, PBB12 has no
correlation with the expression of any member protein or gene in
the KLF4/miR-204-5p/ATF2 pathway. Only when PBB12 is overexpressed
does the KLF4/miR-204-5p/ATF2 pathway fail. When PBB12 is
underexpressed or deleted, the KLF4/miR-204-5p/ATF2 pathway remains
activated. In addition, the expression connection between PBB12 and
miR-204-5p also should be further studied. According to our
existing theory, PBB12 binds to miR-204-5p and inhibits the latter
binding to the 3′UTR of the ATF2 gene, ultimately inhibiting
the expression of the ATF2 protein. However, it is unlikely that
there is a correlation between PBB12 and miR-204-5p. In fact, the
test data of clinical samples also showed no correlation between
PBB12 and miR-204-5p, and the results support the fact that miRNAs
inhibit the translation of target genes into proteins by binding to
the 3′UTR region of the target gene. Whether miRNAs have an effect
on the content of target genes is not fully accepted in the current
mainstream view. In theory, there is no influence (miRNAs are
believed to inhibit translation, not degrade transcripts, and the
classical theory is that the target genes regulated by miRNAs are
posttranscriptionally regulated); however, there are many reports
on the decrease in RNA content. Some scholars believe that miRNAs
have no effect on the contents of target genes. The decrease in the
target gene content results from feedback regulation (43,44).
Although our study initially explained that PBB12 is
involved in the progression of OS, future research still need to be
carried out. Over a long period of time, the large-scale
verification of the differences in the expression of PBB12 in OS
patients and the explanation for these expression differences
require more in-depth and systematic research. In addition, the
addition of in vivo research would have made the present
study more convincing. The main reasons why we did not formally
carry out animal experiments in vivo were as follows: i) The
effect of PBB12 on the subcutaneous tumor-bearing volume can be
observed through subcutaneous tumor-bearing experiments; however,
these experiments are not suitable for observing tumor metastasis;
ii) the expression of PBB12 has certain population characteristics,
but its status in nude mice remains unclear. In addition, the
expression also involves the homology of human and mouse PBB12
sequences. Based on the above, we are preparing and implementing
two in vivo studies. We will first establish a mouse PDX
model of OS and second, aim to establish PBB12 knock-in mice and
then establish a PDX model to avoid the concerns caused by the
homology of human and mouse PBB12 (if the homology between mouse
PBB12 and human PBB12 is not ideal). We believe such animal
experiments will undoubtedly be more meaningful. The significance
of the present study is the revelation that some cancer suppression
regulatory pathways may lose their tumor-suppressive function in
specific patient populations due to particular lncRNAs. Our study
confirmed that PBB12 has the potential to serve as a marker for
screening high-risk OS patients for tumor metastasis. In addition,
for patients with high expression of PBB12, PBB12 can be inhibited
and/or knocked down, which may ensure normal function of the
KLF4/hsa-miR-204-5p/ATF2 cancer suppression pathway. Furthermore,
the anticancer function of the KLF4 gene can be utilized.
This evidence provides strong theoretical support for the
improvement of gene therapy for OS by targeting KLF4.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural
Science Foundation of China (81772267 and 81072070).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM performed the experiments and contributed in
drafting the manuscript. AL and DX performed the analysis and
interpreted the data. TZ conceived and designed the study. All
authors read and approved the final version of the manuscript and
agree to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Ethics Review
Committee of Huashan Hospital Affiliated to Fudan University
(2016–037), and written informed consent was obtained from all
participants and/or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests, and all authors confirm its accuracy.
References
|
1
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: Progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang W, Gao B, Fu P, Xu S, Qian Y and Fu
Q: The miRNAs in the pathgenesis of osteosarcoma. Front Biosci
(Landmark Ed). 18:788–794. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hauptman N and Glavač D: Long non-coding
RNA in cancer. Int J Mol Sci. 14:4655–4669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdelmohsen K and Gorospe M: Noncoding RNA
control of cellular senescence. Wiley Interdiscip Rev RNA.
6:615–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alaei-Mahabadi B and Larsson E: Limited
evidence for evolutionarily conserved targeting of long non-coding
RNAs by microRNAs. Silence. 4:42013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou DK, Yang XW, Li H, Yang Y, Zhu ZJ and
Wu N: Up-regulation of long noncoding RNA CCAL predicts poor
patient prognosis and promotes tumor metastasis in osteosarcoma.
Int J Biol Markers. 32:e108–e112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Xie P and Ruan WH: Overexpression of
lncRNA UCA1 promotes osteosarcoma progression and correlates with
poor prognosis. J Bone Oncol. 5:80–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan H, Liu G, Zhao C, Li X and Yang X:
Transcription factor Oct4 promotes osteosarcoma by regulating
lncRNA AK055347. Oncol Lett. 13:396–402. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fornes O, Castro-Mondragon JA, Khan A, van
der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M,
Baranašić D, et al: JASPAR 2020: Update of the open-access database
of transcription factor binding profiles. Nucleic Acids Res. Nov
8–2019.(Epub ahead of print). View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bergmann JH and Spector DL: Long
non-coding RNAs: Modulators of nuclear structure and function. Curr
Opin Cell Biol. 26:10–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng G and Sui G: Noncoding RNA in
oncogenesis: A new era of identifying key players. Int J Mol Sci.
14:18319–18349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pickard MR and Williams GT: Molecular and
cellular mechanisms of action of tumour suppressor GAS5 lncRNA.
Genes (Basel). 6:484–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pang M, Xing C, Adams N, Rodriguez-Uribe
L, Hughs SE, Hanson SF and Zhang J: Comparative expression of miRNA
genes and miRNA-based AFLP marker analysis in cultivated tetraploid
cottons. J Plant Physiol. 168:824–830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brodersen P, Sakvarelidze-Achard L,
Schaller H, Khafif M, Schott G, Bendahmane A and Voinnet O:
Isoprenoid biosynthesis is required for miRNA function and affects
membrane association of ARGONAUTE 1 in Arabidopsis. Proc Natl Acad
Sci USA. 109:1778–1783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sampson VB, Yoo S, Kumar A, Vetter NS and
Kolb EA: MicroRNAs and potential targets in osteosarcoma: Review.
Front Pediatr. 3:692015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng CJ, Bahal R, Babar IA, Pincus Z,
Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM,
et al: MicroRNA silencing for cancer therapy targeted to the tumour
microenvironment. Nature. 518:107–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ziyan W, Shuhua Y, Xiufang W and Xiaoyun
L: MicroRNA-21 is involved in osteosarcoma cell invasion and
migration. Med Oncol. 28:1469–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren X, Shen Y, Zheng S, Liu J and Jiang X:
miR-21 predicts poor prognosis in patients with osteosarcoma. Br J
Biomed Sci. 73:158–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian R, Xie X, Han J, Luo C, Yong B, Peng
H, Shen J and Peng T: miR-199a-3p negatively regulates the
progression of osteosarcoma through targeting AXL. Am J Cancer Res.
4:738–750. 2014.PubMed/NCBI
|
|
22
|
Wang F, Yu D, Liu Z, Wang R, Xu Y, Cui H
and Zhao T: MiR-125b Functions as a tumor suppressor and enhances
chemosensitivity to cisplatin in osteosarcoma. Technol Cancer Res
Treat. 15:NP105–NP112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thayanithy V, Sarver AL, Kartha RV, Li L,
Angstadt AY, Breen M, Steer CJ, Modiano JF and Subramanian S:
Perturbation of 14q32 miRNAs-cMYC gene network in osteosarcoma.
Bone. 50:171–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu JJ, Pi WS, Cao Y, Peng AF, Cao ZY, Liu
JM, Huang SH, Liu ZL and Zhang W: Let-7a inhibits osteosarcoma cell
growth and lung metastasis by targeting Aurora-B. Cancer Manag Res.
10:6305–6315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang RJ, Shi KR, Zhang J, Zhang J, Gao RR
and Zhu SC: Effects of miR-93 on proliferation and apoptosis of
osteosarcoma cells. Zhonghua Bing Li Xue Za Zhi. 45:866–870.
2016.(In Chinese). PubMed/NCBI
|
|
26
|
Aftab MN, Dinger ME and Perera RJ: The
role of microRNAs and long non-coding RNAs in the pathology,
diagnosis, and management of melanoma. Arch Biochem Biophys.
563:60–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie CH, Cao YM, Huang Y, Shi QW, Guo JH,
Fan ZW, Li JG, Chen BW and Wu BY: Long non-coding RNA TUG1
contributes to tumorigenesis of human osteosarcoma by sponging
miR-9-5p and regulating POU2F1 expression. Tumour Biol.
37:15031–15041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Q, Geng PL, Yin P, Wang XL, Jia JP
and Yao J: Down-regulation of long non-coding RNA TUG1 inhibits
osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J
Cancer Prev. 14:2311–2315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Yang T, Zhang Z, Lu M, Zhao W,
Zeng X and Zhang W: Long non-coding RNA TUG1 promotes migration and
invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells.
Cancer Sci. 108:859–867. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Q, Chen F, Zhao J, Li B, Liang Y, Pan
W, Zhang S, Wang X and Zheng D: Long non-coding RNA PVT1 promotes
osteosarcoma development by acting as a molecular sponge to
regulate miR-195. Oncotarget. 7:82620–82633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan LH, Wang W, Yeung W, Deng Y, Yuan P
and Mak KK: Hedgehog signaling induces osteosarcoma development
through Yap1 and H19 overexpression. Oncogene. 33:4857–4866. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li M, Chen H, Zhao Y, Gao S and Cheng C:
H19 functions as a ceRNA in promoting metastasis through decreasing
miR-200s activity in osteosarcoma. DNA Cell Biol. 35:235–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taniguchi M, Fujiwara K, Nakai Y, Ozaki T,
Koshikawa N, Toshio K, Kataba M, Oguni A, Matsuda H, Yoshida Y, et
al: Inhibition of malignant phenotypes of human osteosarcoma cells
by a gene silencer, a pyrrole-imidazole polyamide, which targets an
E-box motif. FEBS Open Bio. 4:328–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luan W, Qian Y, Ni X, Bu X, Xia Y, Wang J,
Ruan H, Ma S and Xu B: miR-204-5p acts as a tumor suppressor by
targeting matrix metalloproteinases-9 and B-cell lymphoma-2 in
malignant melanoma. Onco Targets Ther. 10:1237–1246. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sümbül AT, Göğebakan B, Ergün S, Yengil E,
Batmacı CY, Tonyalı Ö and Yaldız M: miR-204-5p expression in
colorectal cancer: An autophagy-associated gene. Tumour Biol.
35:12713–12719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng J, Wei M, Shi R, Cai C, Liu X, Li T
and Ma W: MiR-204-5p/Six1 feedback loop promotes
epithelial-mesenchymal transition in breast cancer. Tumour Biol.
37:2729–2735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vlahopoulos SA, Logotheti S, Mikas D,
Giarika A, Gorgoulis V and Zoumpourlis V: The role of ATF-2 in
oncogenesis. Bioessays. 30:314–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsu CC and Hu CD: Critical role of
N-terminal end-localized nuclear export signal in regulation of
activating transcription factor 2 (ATF2) subcellular localization
and transcriptional activity. J Biol Chem. 287:8621–8632. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lewis JS, Vijayanathan V, Thomas TJ,
Pestell RG, Albanese C, Gallo MA and Thomas T: Activation of cyclin
D1 by estradiol and spermine in MCF-7 breast cancer cells: A
mechanism involving the p38 MAP kinase and phosphorylation of
ATF-2. Oncol Res. 15:113–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan Z, Duan X, Cai H, Wang L, Li M, Qu J,
Li W, Wang Y and Wang J: Curcumin inhibits the invasion of lung
cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling
pathway. Oncol Rep. 34:691–698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu Y, Liu Z and Guo K: The effect of JDP2
and ATF2 on the epithelial-mesenchymal transition of human
pancreatic cancer cell lines. Pathol Oncol Res. 18:571–577. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang J, Liao Y, He S, Shi J, Peng L, Xu X,
Xie F, Diao N, Huang J, Xie Q, et al: Autocrine parathyroid
hormone-like hormone promotes intrahepatic cholangiocarcinoma cell
proliferation via increased ERK/JNK-ATF2-cyclinD1 signaling. J
Transl Med. 15:2382017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pu M, Li C, Qi X, Chen J, Wang Y, Gao L,
Miao L and Ren J: MiR-1254 suppresses HO-1 expression through seed
region-dependent silencing and non-seed interaction with TFAP2A
transcript to attenuate NSCLC growth. PLoS Genet. 13:e10068962017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao Y, Qi X, Chen J, Wei W, Yu C, Yan H,
Pu M, Li Y, Miao L, Li C and Ren J: The miR-491-3p/Sp3/ABCB1 axis
attenuates multidrug resistance of hepatocellular carcinoma. Cancer
Lett. 408:102–111. 2017. View Article : Google Scholar : PubMed/NCBI
|