Introduction
Both genetic and epigenetic alterations play
critical roles in the initiation and progression of carcinogenesis.
Compared to genetic defects, epigenetic modifications are more
dynamic and therefore more influenced by the environment (e.g.,
lifestyle, dietary factors) (1,2). DNA
methylation is among the most studied epigenetic mechanisms. It
involves the addition of a methyl (CH3) group to the
cytosine in the CpG dinucleotide, resulting in the formation of
5-methylcytosine. This process is catalyzed by the DNA
methyltransferase (DNMT) family of enzymes: DNMT1, DNMT3a and
DNMT3b. DNMT1 is a maintenance enzyme that guards existing
methylated sites through its preference for hemimethylated DNA
(3). DNMT3a and DNMT3b are de
novo methyltransferases responsible for establishing DNA
methylation patterns during embryogenesis. Any defects in DNMTs
will induce imbalances in DNA modification, resulting in genomic
instability and gene dysregulation (4,5).
However, DNA demethylation involves the
hydroxylation of 5-methylcytosine to 5-hydroxymethylcytosine
(6,7). It is mediated by the ten-eleven
translocation (TET) family of proteins: TET1, TET2 and TET3
(8). TET1 is a maintenance DNA
demethylase enzyme that protects against aberrant demethylation
(9). It acts both as a tumor
suppressor preventing cell proliferation and tumor metastasis and
as an oncogene contributing to aberrant hypomethylation. The
delicate balance between DNA methylation and demethylation is known
to be regulated by a specific class of microRNAs, termed
epi-miRNAs, which target both families of epigenetic enzymes DNMTs
and TETs (10).
MicroRNAs (miRs) are short non-coding RNAs that are
a novel class of cancer-relevant molecules. The miR-29 family,
which consists of miR-29a, miR-29b, and miR-29c, is abnormally
expressed in multiple cancers (10). miR-29b is the most highly expressed
family member. It is classified as an epi-miRNA, regulating the
balance between DNA methylation and demethylation as a regulator
for TET1 and DNMTs (10,11). In breast cancer, miR-29b has been
reported to be both a suppressor and a promoter of proliferation
and metastasis through its regulation of the TET1 gene (12,13).
The BRCA1 gene is a critical DNA
repair-related gene that plays an essential role in the mechanisms
of DNA repair, cell cycle checkpoints, and transcription. Cells
lacking BRCA1 protein are susceptible to mutations and genomic
instability, which can lead to early carcinogenesis. The pathogenic
germline mutations of the BRCA1 gene are highly associated
with familial breast cancers. However, loss-of-function in
BRCA1 resulting from aberrant promoter methylation is
associated with sporadic breast cancer. BRCA1 promoter
methylation has been detected in DNA extracted from white blood
cells (WBCs). Several studies have shown that constitutional
BRCA1 promoter methylation is linked to a high risk of
developing early-onset breast and ovarian cancers (14–19).
The promoter region of the BRCA1 gene contains 30 CpG sites
covering the area from −567 to +44 relative to the transcription
start site (20). This area
includes the binding sites of several transcription factors,
including SP1, E2F and CTCF. The binding of these factors to the
BRCA1 promoter keeps the promoter in a methylation-free
state (21,22). The CTCF and E2F factors are enriched
at the unmethylated BRCA1 promoter, such as in MCF-7, but
not at the methylated promoter in UACC-3199 and HCC-38 cells
(22).
γ synuclein is a member of the synuclein family of
proteins. It is encoded by the gene SNCG, which is also
known as breast cancer-specific gene 1 (BCSG1) (23). This gene is a proto-oncogene that is
highly expressed in stages III and IV of breast ductal carcinomas
but not in normal breast tissues. The expression of SNCG in
the primary breast tumor is associated with metastasis and reduced
disease-free survival (DFS) (24).
Exon 1 of SNCG contains 15 CpG sites covering the region
from −169 to +81 relative to the translation start codon. The
demethylation of these CpG sites is responsible for the aberrant
expression of SNCG in breast carcinomas (25). The inhibition of SNCG
reverses the malignant phenotype of the highly
SNCG-hypomethylated cell line T47D (26).
One of the main differences between genetic and
epigenetic alterations is the reversibility of the latter process.
Accordingly, restoration of the function of defective
tumor-suppressor genes and suppression of constitutively activate
oncogenes is an attractive clinical option for the prevention and
treatment of cancer. Although synthetic demethylating agents, such
as 5-azacytidine and 5-aza-2′-deoxycytidine, are effective DNA
methylation inhibitors, they have unselective demethylation effects
that can lead to the activation of silenced pro-oncogenes. Notably,
no DNA methylation inducers have hitherto been identified.
Curcumin is the active component of the herb
Curcuma longa. It is believed to have chemopreventive and
chemotherapeutic properties. Several studies have shown that this
herb can exert anticancer effects, on breast cancer in particular,
by targeting various signaling pathways (27). Furthermore, it has been reported
that curcumin can function as a DNA methylation inhibitor in breast
cancer cells (28–32). Nevertheless, curcumin's potential as
a DNA methylation inducer remains to be fully explored. In the
present study, we attempted to evaluate the potential of curcumin
molecules to restore normal DNA methylation and gene expression
patterns to the BRCA1 and SNCG genes in breast cancer
cells.
Materials and methods
Cell culture and treatment
The HCC-38, UACC-3199, and T47D breast cancer cell
lines were purchased from the American Type Culture Collection
(ATCC). The cells were tested for mycoplasma. The cells were
cultured in RPMI-1640 media supplemented with 10% FBS, 100 U/ml
penicillin, and 100 µg/ml streptomycin. The supplements were
obtained from Gibco/Life Technologies (Thermo Fisher Scientific,
Inc.). The cells were treated with 5 and 10 µM curcumin
(Sigma-Aldrich; Merck KGaA) when they reached 40–60% confluence and
incubated in a humidified atmosphere at 37°C and 5% CO2
for 6 days. The HCC-38 and UACC-3199 cells were treated with 5 µM
5-aza-2′-deoxycytidine (5′-aza-CDR) (Sigma-Aldrich; Merck KGaA)
when they reached 70% confluence and were incubated at 37°C for 48
h. The cells were then collected for DNA, RNA and protein
extraction.
Cell proliferation
The cells were treated with 5 and 10 µM of curcumin
for 3 days, after which they were re-seeded at a density of 5,000
cells/E-16 plate and re-incubated with the same doses of curcumin
for a further 3 days. The proliferation rate was measured using the
RTCA-DP xCELLigence system (Roche-Germany). The cell index
represents the cell status based on the measured electrical
impendence change divided by a background value.
Methylation-specific PCR
Approximately 2 µg of DNA was treated with sodium
bisulfate and purified using the EpiTect Bisulfite kit (Qiagen,
Inc.) in accordance with the manufacturer's recommendations. The
DNA was then amplified using published PCR primers for BRCA1
and SNCG (33,34) that distinguish between methylated
and unmethylated DNA. PCR products were electrophoresed on 2%
agarose gels and stained with ethidium bromide. Totally methylated
bisulfite-treated DNA was used as a positive control. All PCR
reactions were repeated at least twice.
Bisulfite pyrosequencing
Bisulfite pyrosequencing was used for DNA
methylation quantification. Five different assays (35) were used to assess the methylation
status of 23 CpG sites across the BRCA1 promoter. The PCR
and pyrosequencing reactions were performed using PyroMark products
and reagents (Qiagen, Inc.), as previously described (36). Methylation quantification was
performed using PyroMark Q24 software (Qiagen, Inc.).
Real-time PCR
Superscript III (Invitrogen; Thermo Fisher
Scientific, Inc.) reverse transcriptase and random hexamers were
used for cDNA synthesis. Quantitative real-time PCR was then
performed using primers specific to BRCA1, SNCG, and TET1
transcripts, using GAPDH as an internal control. Primers are listed
in Table I. PCR was performed with
SYBR Green using the CFX96 Real-Time system (Bio-Rad Laboratories,
Inc.). For miR-29b, qPCR was performed using the stem-loop reverse
transcription primer, and the TaqMan microRNA reverse transcription
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). U6 snRNA
was used for normalization. The relative gene expression was
calculated based on the threshold cycle (Ct) value using the
2−ΔΔCq method (37). The
fold change of mRNA expression was performed relative to
DMSO-treated cells.
 | Table I.Real-time and MSP PCR primers. |
Table I.
Real-time and MSP PCR primers.
| Primers | Sequence | Annealing
temperature (°C) |
|---|
| BRCA1 | F
5′-TGTAGGCTCCTTTTGGTTATATCATTC-3′ | 59 |
|
| R
5′-CATGCTGAAACTTCTCAACCAGAA-3′ |
|
| SNCG | F
5′-GGAGGACTTGAGGCCATCTG-3′ | 60 |
|
| R
5′-CTCCTCTGCCACTTCTCTTTTC-3′ |
|
| TET1 | F
5′-CCCGGGCTCCAAAGTTGTG-3′ | 59 |
|
| R
5′-GCAGGAAACAGAGTCATTGGTCCT-3′ |
|
| GAPDH | F
5′-TTCAACGGCACAGTCAAGG-3′ | 60 |
|
| R
5′-CTCAGCACCAGCATCACC-3′ |
|
| M. BRCA1 | F
5′-GGTTAATTTAGAGTTTCGAGAGACG-3′ | 65 |
|
| R
5′-TCAACGAACTCACGCCGCGCAATCG-3′ |
|
| U. BRCA1 | F
5′-GGTTAATTTAGAGTTTTGAGAGATG-3′ | 65 |
|
| R
5′-TCAACAAACTCACACCACACAATCA-3′ |
|
| M. SNCG | F
5′-TCGTATTAATATTTTATCGGCGT-3′ | 60 |
|
| R
5′-CCGCACCCACCACGCCCTCCTTAACGA-3′ |
|
| U. SNCG | F
5′-TTGGTGTTAATAGGAGGTATTGGGGATAGTTGTTGTG-3′ | 59 |
|
| R
5′-CACACCCACCACACCCTCCTTAACAAT-3′ |
|
Western blot analysis
Protein was extracted from the cells using RIPA
lysis buffer (R0278; Sigma-Aldrich; Merck KGaA). Bradford method
was used for protein quantification. Protein (50 µg) was subjected
to 10 and 12% SDS-PAGE and then transferred to PVDF membranes.
After blocking at room temperature with 5% non-fat milk for 1 h,
the membranes were incubated at 4°C overnight with primary
antibodies (dilution 1:1,000), BRCA1 (ab9141), SNCG (ab55424), TET1
(Ab156993), DNMT3a (Ab13888) and DNMT3b (Ab2851) (purchased from
Abcam); DNMT1 (D63A6) and GAPDH (purchased from Cell Signaling
Technology, Inc.). The membranes were visualized using ECL
Detection reagents (Pierce; Thermo Fisher Scientific, Inc.). Images
were visualized using a LAS-4000 Imager (Fujifilm). Band
quantification was carried out using the GelQuant.NET program
(version 1.8.2; Biochem Lab Solutions).
Statistical analysis
For gene expression levels, statistical analysis was
performed using Single Factor ANOVA and Tukey's multiple
comparisons test (GraphPad Prism version 8.3; GraphPad Software,
Inc.) to determine the statistical significance between
multiple-dose curcumin-treated and untreated cells. Student's
t-test was used to determine the statistical significance between
single dose curcumin-treated and untreated cells. All observed
differences were considered significant when associated with
P-values <0.05.
Results
Curcumin suppresses the proliferation
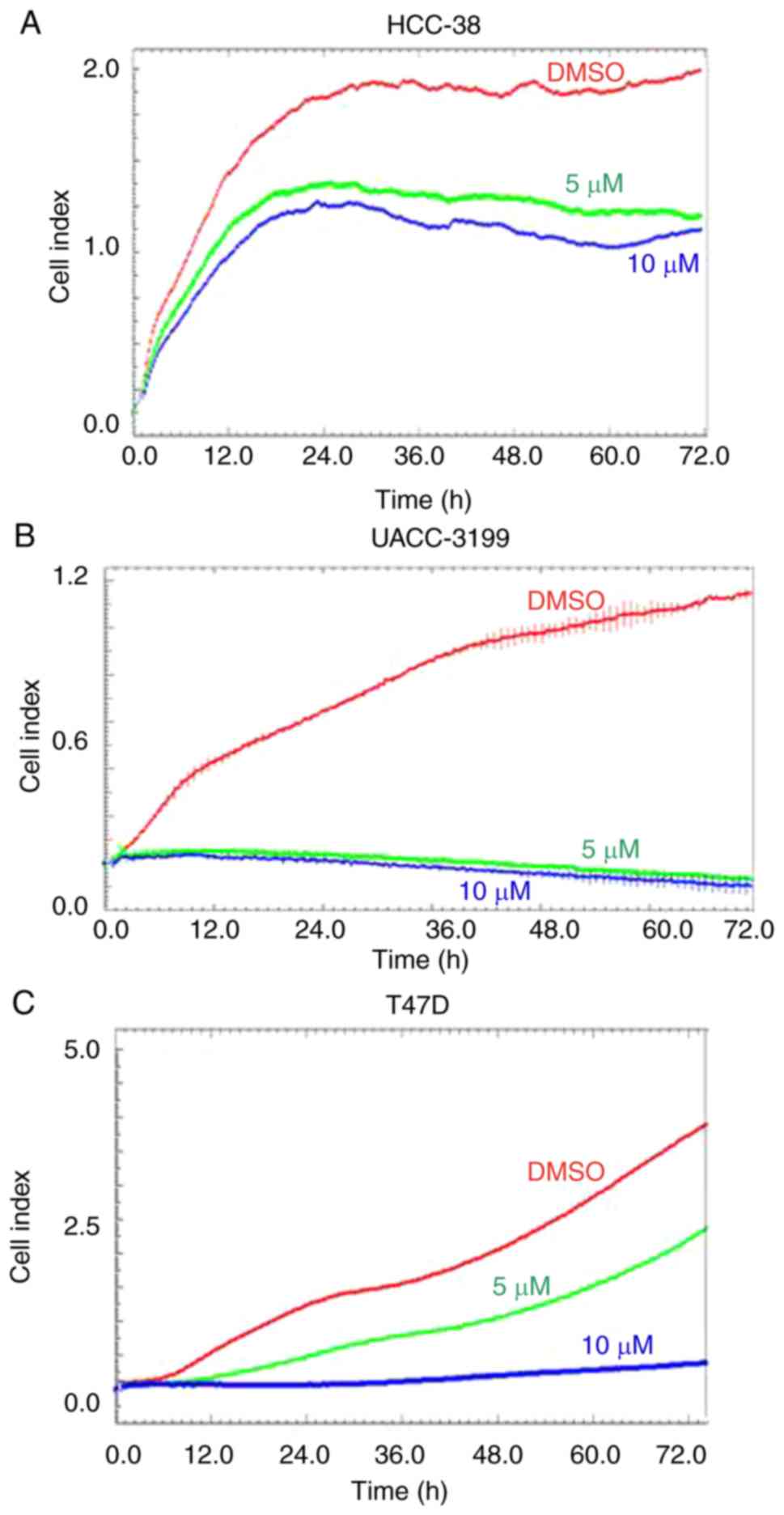
of HCC-38, UACC-3199, and T47D cell lines
In the present study, we used three breast cancer
cell lines, HCC-38 and UACC-3199, which are highly
BRCA1-hypermethylated cell lines (22), and T47D, which is a highly
SNCG-hypomethylated cell line. HCC-38 is a triple-negative
breast cancer (TNBC) cell line, UACC-3199 is estrogen
receptor-negative/progesterone receptor-negative
(ER−/PR−), and T47D is
ER+/PR+. Based on previous studies (29,30,38,39),
we treated the cells with 5 and 10 µM of curcumin. To investigate
the effect of these curcumin concentrations on cell proliferation,
we monitored cell proliferation in the presence of curcumin over a
total period of 6 days. First, we treated the cells with curcumin
for 72 h. Real-time cell proliferation was then monitored over a
further 72 h in the presence of curcumin using the xCELLigence
system E-Plate. Although curcumin reduced the proliferation of
HCC-38 and UACC-3199 cells in a dose-independent manner, the
proliferation of T47D cells was dose-dependently inhibited compared
to the control (Fig. 1). These
results demonstrated that curcumin influenced the proliferation of
all three cell lines studied.
Curcumin increases the mRNA and
protein levels of BRCA1 in HCC-38 and UACC-3199 cells by reducing
promoter methylation
The reactivation of silenced tumor-suppressor genes
is an attractive clinical option for the prevention and treatment
of cancer. Epigenetic silencing of BRCA1 by promoter
hypermethylation results in the low expression of BRCA1 mRNA
and BRCA1 protein in sporadic breast cancer. To ascertain whether
curcumin can re-express BRCA1 in HCC-38 and UACC-3199 cell
lines, the cells were treated with 5 and 10 µM of curcumin for 6
days. Notably, curcumin increased the level of BRCA1 mRNA up
to 2-fold in both cell lines in a dose-dependent manner (Fig. 2A and B). Additionally, a consequent
high increase was observed in the level of BRCA1 protein (Fig. 2C and D). Then, we evaluated whether
curcumin-induced BRCA1 re-expression is associated with the
hypomethylation of its promoter. To this end, we assessed the
status of BRCA1 promoter methylation in curcumin-treated
HCC-38 and UACC-3199 cells using the methylation-specific PCR (MSP)
assay. As shown in Fig. 2F and G,
the intensity of the methylated band was reduced in the
curcumin-treated cells, compared to the control. As further
verification of the hypomethylation of the BRCA1 promoter,
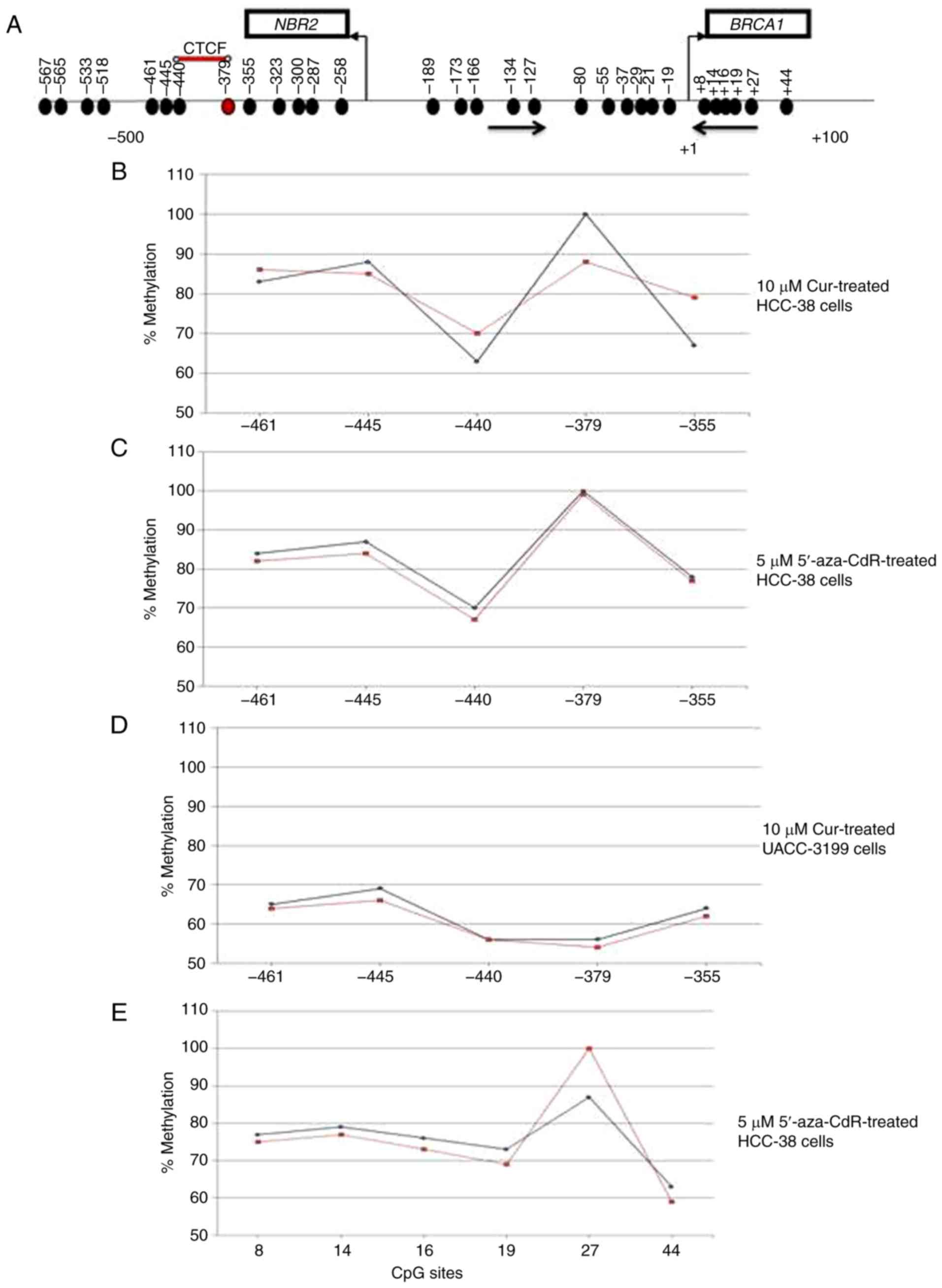
the promoter region spanning six CpG sites (+8, +14, +16, +19, +27
and +44, in which the reverse MSP primer is located) (Fig. 3A) was analyzed using pyrosequencing.
As shown in Fig. 2H and I, the
levels of methylation at +27 and +44 CpG sites were reduced in the
curcumin-treated cells, compared to the control. These results
suggest that the re-expression of BRCA1 in curcumin-treated
cells may be associated with the partial hypomethylation of the
BRCA1 promoter. Next, we investigated whether the
re-expression of the BRCA1 protein is transient or persistent. To
this end, curcumin-treated HCC-38 cells were grown in curcumin-free
media for a further 10 days, with the media changed every 5 days.
Interestingly, BRCA1 protein continued to be highly expressed in
the curcumin-free medium, compared to the control, indicating a
persistent effect of curcumin (Fig.
2E). Next, to compare the demethylating effect of curcumin to
that of the demethylating agent 5′-aza-CdR, we treated the two cell
lines with 5 µM 5′-aza-CdR for 48 h. Notably, 5′-aza-CdR was able
to re-express BRCA1 mRNA and BRCA1 protein only in UACC-3199
(Fig. 2K and M) and not in HCC-38
cells (Fig. 2J). Intriguingly, the
expression of BRCA1 protein was reduced in 5′-aza-CdR-treated
HCC-38 cells (Fig. 2L).
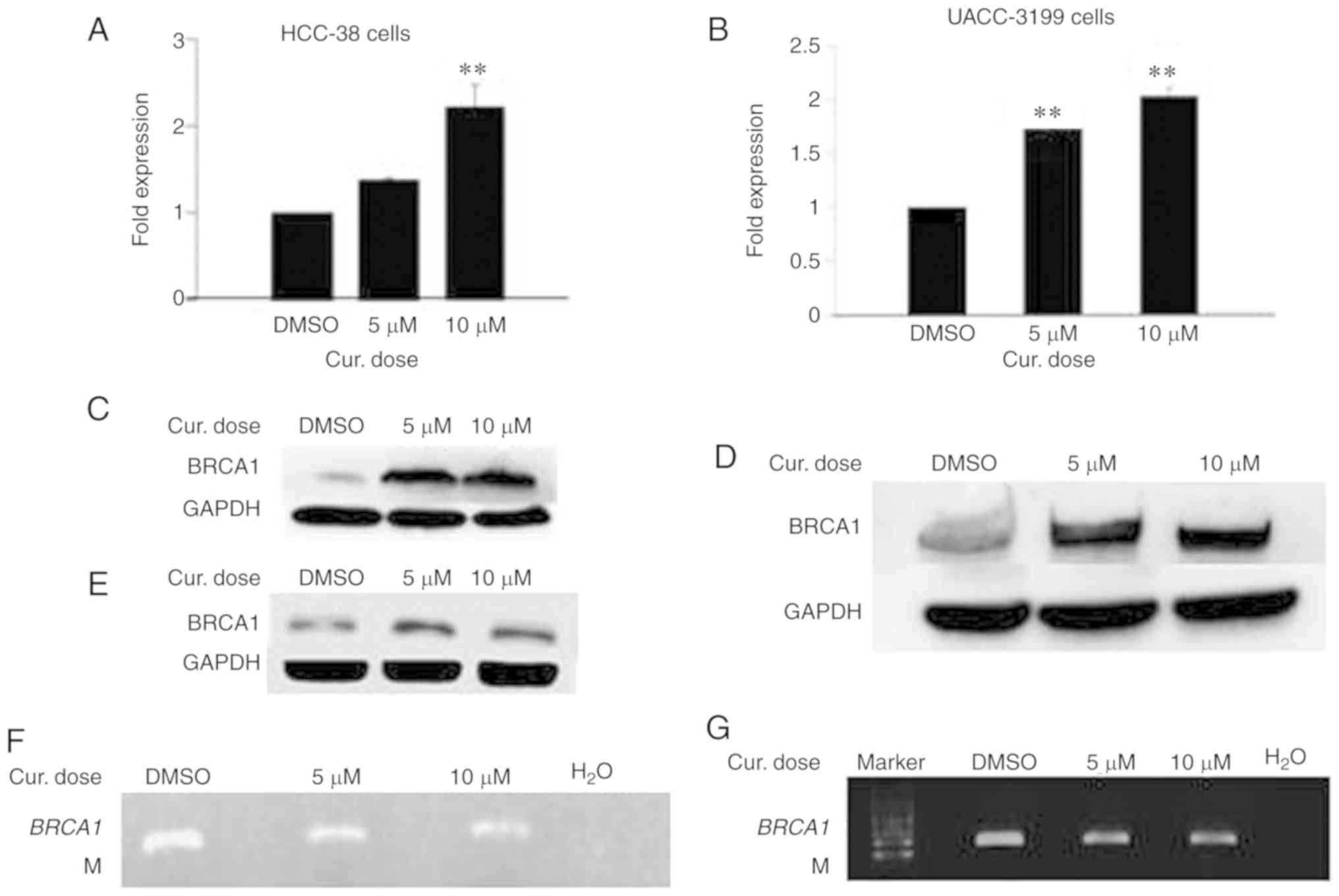 | Figure 2.Curcumin induces the expression of
BRCA1 in HCC-38 and UACC-3199 cells. The cells were treated
with 5 and 10 µM curcumin for 6 days, and the effects of curcumin
on BRCA1 mRNA and protein expression in (A and C) HCC-38 and
(B and D) UACC-3199 cells, respectively, are shown. (E) Effect of
curcumin-free media on the expression of BRCA1 protein in HCC-38
cells. (F and G) MSP analysis of BRCA1 promoter methylation.
M, only the methylated bands are shown. (H and I) Methylation plots
for the BRCA1 promoter as determined by bisulfite
pyrosequencing assay. Black lines represent values for control
DMSO-treated cells, and red lines represent 10 µM curcumin-treated
cells. Numbers represent CpG sites relative to the transcription
start site. (J-M) Cells were treated with 5 µM 5′-aza-CdR for 48 h.
Effect of 5′-aza-CdR on BRCA1 mRNA and protein expression in
(J and L) HCC-38 and (K and M) UACC-3199 cells, respectively.
**P<0.01, vs. the control. Error bars represent the mean ± SD.
BRCA1, BRCA1 DNA repair associated; DMSO, dimethyl
sulfoxide; 5′-aza-CdR, 5-aza-2′-deoxycytidine; Cur, curcumin. |
Curcumin and not 5′-aza-CDR induces
demethylation of the −379 CpG site in the BRCA1 promoter in the
HCC-38 cells
To investigate the difference between the effect of
curcumin and 5′-aza-CdR on the re-expression of BRCA1 in the
HCC-38 cells, we studied the methylation status of 23 CpG sites
located in the BRCA1 promoter region (Fig. 3A) by sodium bisulfite pyrosequencing
in curcumin-treated cells as compared to 5′-aza-CDR. We found that
the CpG site located at −379 was 100% methylated. In comparison to
the control, the methylation level of this CpG site was reduced by
12% in curcumin-treated cells, while in 5′-aza-CdR-treated cells,
the methylation level was only reduced by 1% (Fig. 3B and C). These results suggest that
the methylation of the −379 CpG site is instrumental in controlling
the expression of BRCA1 in the HCC-38 cell line. Notably,
the −379 CpG site was partially methylated (56%) in the UACC-3199
cells, and the methylation level was only reduced by 2% in the
curcumin-treated cells, compared to the control (Fig. 3D). Interestingly, we found also that
the level of methylation at the +27 CpG site was increased by 13%
in 5′-aza-CdR-treated HCC-38 cells, compared to the control
(Fig. 3E).
Curcumin downregulates the expression
of DNMT1 and upregulates TET1 and DNMT3 in HCC-38 cells
DNMTs and TETs are epigenetic enzyme families
responsible for the regulation of DNA methylation and
demethylation, respectively. It has been reported that TET1 acts
both as a tumor suppressor, reducing breast tumor development
through demethylating essential genes (13), and as an oncogene, leading to
hypomethylation and activation of oncogenic pathways (8). However, it has been demonstrated that
curcumin re-activates methylated tumor-suppressor genes by
downregulating the protein level of DNMT1 (30). To investigate which enzyme
participates in the curcumin-induced hypomethylation of the
BRCA1 promoter in HCC-38 cells, the mRNA and protein levels
of TET1, DNMT1, DNMT3a and DNMT3b were analyzed by real-time RT-PCR
and immunoblotting in curcumin-treated cells and compared to those
of the 5′-aza-CdR-treated cells. Notably, while curcumin reduced
the level of DNMT1 protein, it increased TET1 mRNA and TET1 protein
in addition to the protein levels of DNMT3a and DNMT3b, compared to
the control (Fig. 4A-E). However,
in 5′-aza-CdR-treated cells, the TET1 mRNA and TET1 protein levels
were decreased while DNMT1 protein was depleted (Fig. 4F-H). These results suggest that
curcumin-induced hypomethylation of the BRCA1 promoter in
HCC-38 cells may be achieved through the upregulation of TET1.
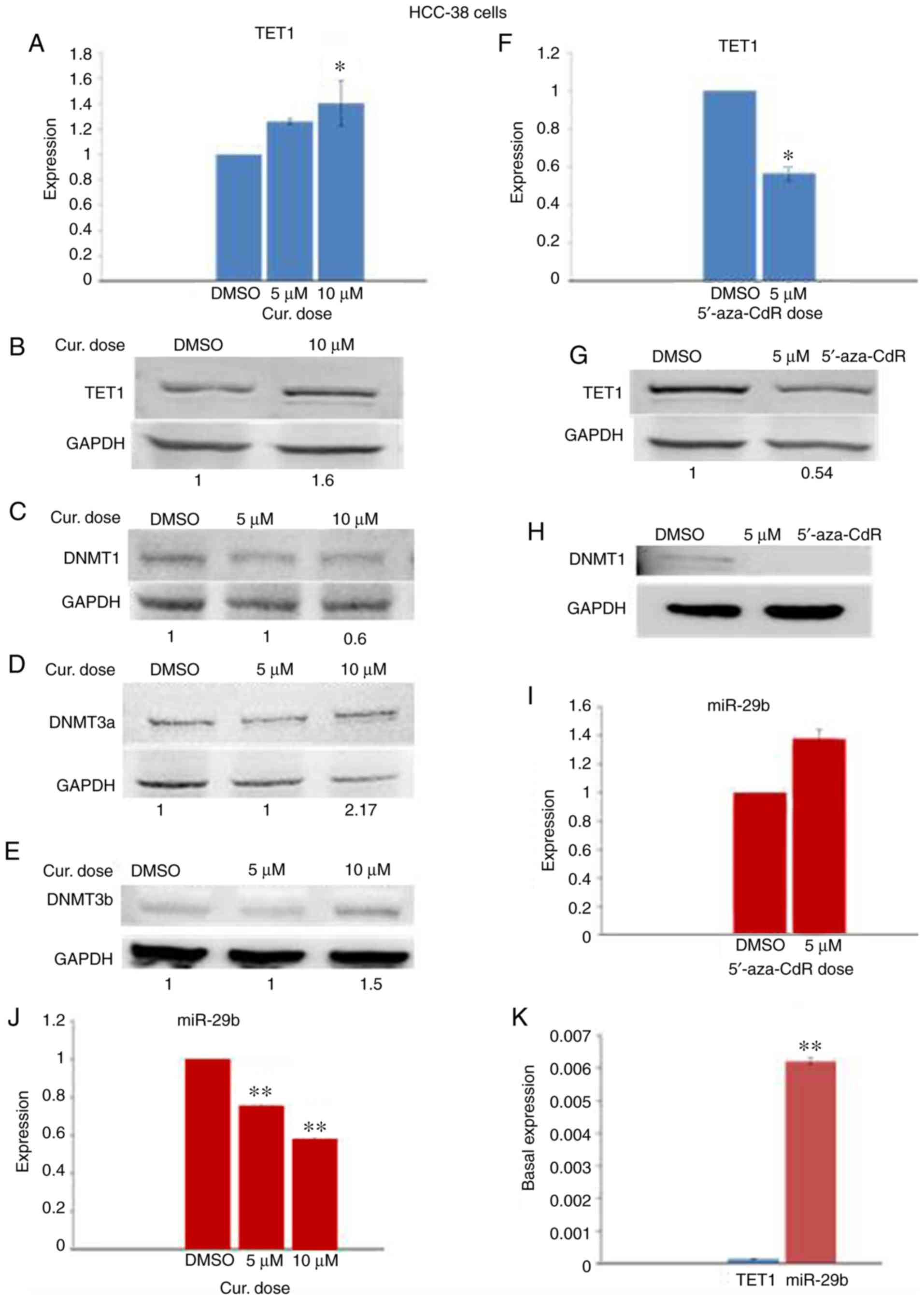 | Figure 4.Effect of curcumin and 5′-aza-CdR on
the expression of TET1, DNMTs, and miR-29b in HCC-38 cells. The
cells were treated with 5 and 10 µM curcumin for 6 days, and with 5
µM 5′-aza-CdR for 48 h. (A) Effect of curcumin on TET1 mRNA
expression. (B-E) Western blots for the expression of TET1, DNMT1,
DNMT3a, and DNMT3b, respectively, in curcumin-treated cells. (F)
Effect of 5′-aza-CdR on TET1 mRNA expression. (G and H) Western
blots for the expression of TET1 and DNMT1, respectively, in
5′-aza-CdR-treated cells. (I and J) Effect of curcumin and
5′-aza-CdR on miR-29b expression, respectively. (K) Basal
expression of TET1 and miR-29b in control HCC-38 cells. *P<0.05
and **P<0.01, vs. the control. Error bars represent the mean ±
SD. TET1, ten-eleven translocation 1; DNMTs, DNA
methyltransferases; DMSO, dimethyl sulfoxide; 5′-aza-CdR,
5-aza-2′-deoxycytidine; Cur, curcumin. |
Curcumin downregulates miR-29b in
HCC-38 cells
It has been reported that TET1 is a target of
miR-29b (13). To investigate
whether the expression of TET1 in HCC-38 cells could be regulated
by miR-29b, we analyzed the expression levels of miR-29b by
real-time RT-PCR in curcumin- and 5′-aza-CdR-treated cells.
Compared to the control, miR-29b was elevated in the
5′-aza-CdR-treated HCC-38 cells (Fig.
4I), and significantly reduced in the curcumin-treated HCC-38
cells (Fig. 4J). These results
revealed a reverse expression pattern of miR-29b and TET1 in cells
that had been treated with curcumin or 5′-aza-CdR, suggesting that
TET1 may be controlled by miR-29b. To support this result, we
evaluated the basal expression levels of TET1 and miR-29b in
control HCC-38 cells. Interestingly, we found that the basal
expression of miR-29b was significantly higher than that of TET1
(Fig. 4K), revealing the possible
involvement of miR-29b in the regulation of TET1 in the HCC-38 cell
line.
Curcumin decreases the mRNA and
protein levels of SNCG in T47D and HCC-38 cells by inducing
promoter methylation
The deactivation of an active oncogene is the other
side of the coin for cancer treatments. Epigenetic activation of
SNCG by promoter hypomethylation results in the high
expression of SNCG mRNA and SNCG protein in breast and
ovarian cancers (40). To ascertain
whether curcumin can deactivate SNCG in the highly
SNCG-hypomethylated cell line T47D, the cells were treated
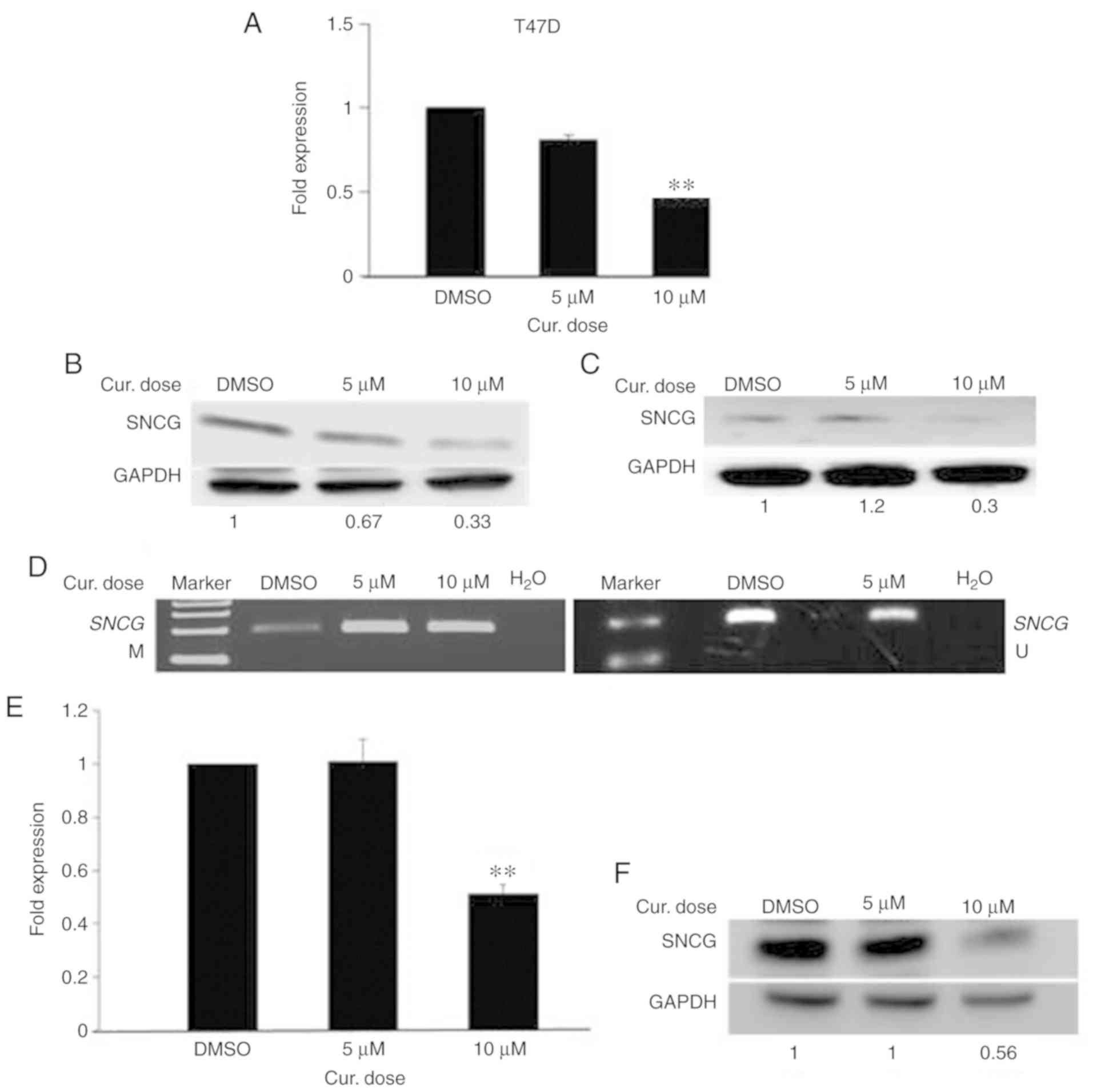
with 5 and 10 µM curcumin for 6 days. As shown in Fig. 5A, curcumin decreased the level of
SNCG mRNA down to 2-fold in a dose-dependent manner.
Additionally, a reduction was observed in the level of SNCG protein
(Fig. 5B). Then, we evaluated
whether the curcumin-induced reduction of SNCG is associated
with the hypermethylation of its promoter. To this end, we assessed
the methylation status of the SNCG promoter in
curcumin-treated T47D cells using the MSP assay. As Fig. 5D illustrates, a high increase was
noted in the intensity of the methylated band with a decrease in
that of the unmethylated band, compared to the control. These
results suggest that the decreased expression of SNCG in
curcumin-treated T47D cells is associated with the hypermethylation
of the SNCG promoter. Next, we assessed whether the
reduction of SNCG protein in curcumin-treated cells is transient or
persistent. To this end, the growth of curcumin-treated T47D cells
in curcumin-free media was continued for a further 10 days, with
the media changed every 5 days. Importantly, as Fig. 5C illustrates, low expression of SNCG
protein continued in the curcumin-free medium, compared to the
control, indicating that curcumin had a sustained effect. Next, to
ascertain whether curcumin can deactivate SNCG in the HCC-38
cell line, we measured the expression of SNCG in the
curcumin-treated HCC-38 cells. In addition to re-expressing BRCA1
in HCC-38, curcumin also decreased SNCG mRNA and SNCG
protein down to 2-fold, with the 10 µM dose (Fig. 5E and F). These results demonstrate
that curcumin has opposing roles in DNA methylation in the same
cell line.
Curcumin downregulates the expression
of DNMT1 and TET1 and upregulates DNMT3 in T47D cells
Next, we sought to investigate which enzyme is
responsible for the curcumin-induced hypermethylation of the
SNCG promoter. To this end, we analyzed the expression
levels of TET1, DNMT1, DNMT3a, and DNMT3b by real-time RT-PCR and
immunoblotting in curcumin-treated T47D cells. In comparison to the
controls, curcumin reduced the expression of TET1 and DNMT1
(Fig. 6A and B) and elevated that
of DNMT3a and DNMT3b (Fig. 6B and
C). These results suggest that both TET1 and DNMT3 may be
involved in the curcumin-induced hypermethylation of the
SNCG promoter in T47D cells.
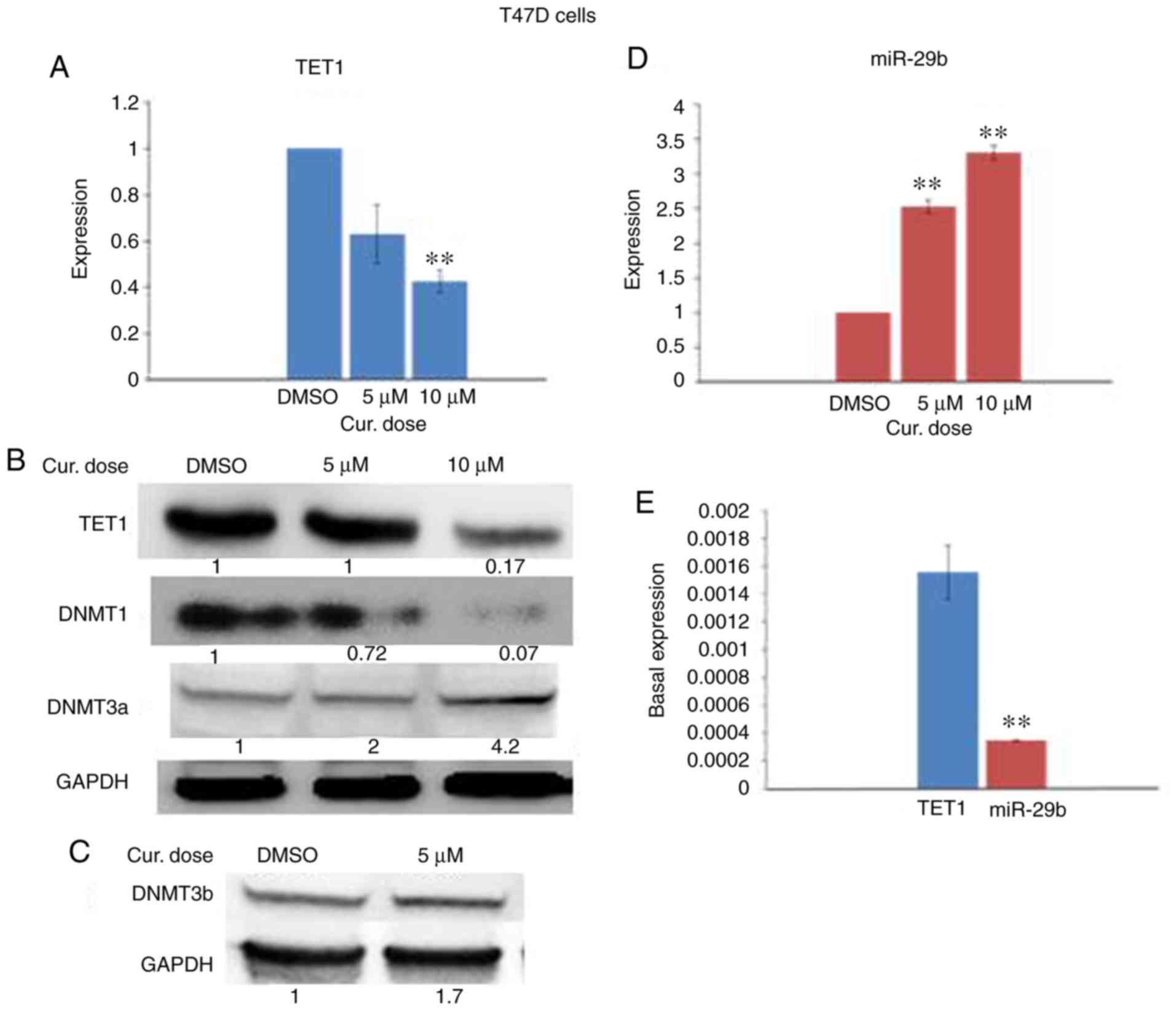 | Figure 6.Effect of curcumin on the expression
of TET1, DNMTs and miR-29b in T47D cells. The cells were treated
with 5 and 10 µM curcumin for 6 days. (A) Effect of curcumin on
TET1 mRNA expression. (B and C) Western blots for TET1, DNMT1,
DNMT3a, and DNMT3b, respectively. (D) Effect of curcumin on miR-29b
expression. (E) Basal expression of TET1 and miR-29b in control
T47D cells. **P<0.01, vs. the control. Error bars represent the
mean ± SD. TET1, ten-eleven translocation 1; DNMTs, DNA
methyltransferases; DMSO, dimethyl sulfoxide; Cur, curcumin. |
Curcumin upregulates miR-29b in T47D
cells
To determine whether miR-29b could regulate the
expression of TET1 in curcumin-treated T47D cells, we analyzed the
expression level of miR-29b by real-time RT-PCR in curcumin-treated
cells. In contrast to the curcumin-treated HCC-38 cells, the
expression of miR-29b was significantly elevated in the T47D cells
(Fig. 6D). However, these results
revealed a reverse expression pattern of miR-29b and TET1 in the
curcumin-treated T47D cells, suggesting that TET1 may be a target
of miR-29b in these cells. To support this result, we evaluated the
basal expression levels of TET1 and miR-29b in control T47D cells.
In contrast to the HCC-38 cells, we found that the basal expression
of miR-29b was significantly lower than that of TET1 (Fig. 6E), also revealing the possible
involvement of miR-29b in the regulation of TET1 in T47D cells.
Discussion
In the present study, it was demonstrated that
curcumin exhibits contradictory functions in regards to DNA
methylation, demethylation and re-expression of the
tumor-suppressor gene BRCA1 DNA repair associated (BRCA1),
as well as the methylation and suppression of the expression of
oncogene γ synuclein (SNCG) in breast cancer cells. We also
found that curcumin is a potent inhibitor of cell proliferation.
This result is consistent with a previous study that demonstrated
that curcumin is a potent growth inhibitor of various breast cancer
cell lines (41). Previous studies
have found that BRCA1 deficiency and the aberrant expression
of SNCG enhance the proliferation of cancer cells (42–44)
and that the restoration of normal expression patterns to these two
genes reduces cell proliferation. In the present study, the
re-expression of BRCA1 in HCC-38 and UACC-3199 cells and the
suppression of SNCG in T47D may have been one mechanism by
which curcumin influenced the inhibition of cell proliferation in
the three cell lines.
Both curcumin and 5′-aza-CdR demethylated and
re-activated BRCA1 in the UACC-3199 cell line. Remarkably,
in HCC-38 cells, only curcumin re-activated BRCA1, while
5′-aza-CdR did not. We may attribute this to curcumin's ability to
reduce the methylation status of the −379 CpG site in the
BRCA1 promoter region. This site flanks the binding site of
the CTCF transcription factor in the BRCA1 promoter. It has
been reported that CTCF binds to the unmethylated BRCA1
promoter simply to function as an insulator, maintaining the
BRCA1 promoter region in a methylation-free state (21,22).
The binding of this transcription factor was found to be affected
by the methylation status of the flanking CpG sites (−440 and −379)
(22). Hence, it is plausible that
the partial demethylation of the −379 CpG site in the
curcumin-treated HCC-38 cells increases the promoter's
accessibility to CTCF leading to the re-expression of BRCA1.
Notably, the −379 CpG site was not affected by 5′-aza-CdR in either
cell line, HCC-38 or UACC-3199. Furthermore, it has been reported
that only E2F1, and not CTCF, was enriched in 5′-aza-CdR-treated
UACC-3199 cells (22). However,
further studies are needed to verify this claim. Remarkably, it has
been reported that zebularine (another demethylating agent) and the
non-nucleoside demethylation drugs EGCG and procaine were also
unable to restore the BRCA1 gene expression in HCC-38 cells
(22). Intriguingly, treating the
HCC-38 cells with 5′-aza-CdR downregulated the expression of BRCA1.
Our findings are consistent with those of a previous study, which
demonstrated that the expression of BRCA1 mRNA was reduced
by 30–40% in 5′-aza-CdR-treated HCC-38 cells, compared to the
control (22).
Previous studies have shown that curcumin
demethylates hypermethylated genes by inhibiting DNA
methyltransferases (DNMTs) (29,30).
In the present study, we found that, while DNMT1 expression was
downregulated in the curcumin-treated HCC-38 cells, DNMT3a and
DNMT3b were upregulated. This result suggests that the
re-expression of BRCA1 in HCC-38 may not result from the modulation
of DNMTs. This suggestion is supported by the fact that 5′-aza-CdR
failed to re-express BRCA1 in HCC-38 despite the depletion of
DNMTs, indicating that another mechanism is involved in the
re-expression of BRCA1. Indeed, we found that TET1 was upregulated
in the curcumin-treated cells, which may indicate its involvement
in the re-expression of BRCA1. This is supported by the fact that
in gastric cancer cells, TET1 binds to the hypermethylated PTEN and
re-activates its transcription through the demethylation of its
promoter (45). Furthermore, it has
been suggested that the decreased expression of TET1 may induce
aberrant DNA methylation (46).
Indeed, we observed an increase in the level of methylation at the
+27 CpG site. This finding may explain the further reduction of the
expression of BRCA1 protein in the 5′-aza-CdR-treated cells, as
TET1 was downregulated in these cells.
The fact that curcumin exerts an effect on both
epigenetic enzyme families DNMTs and TETs suggest that curcumin
plays contradictory roles in the control of DNA methylation status.
Indeed, we found that curcumin could induce methylation to the
hypomethylated SNCG promoter in the breast cancer cell lines
T47D and HCC-38 with a corresponding decrease in its mRNA and
protein expression levels. To the best of our knowledge, our study
is the first to demonstrate the methylation-inducing properties of
curcumin in breast cancer cell lines. However, with regard to
multiple myeloma cells, it has recently been reported that
curcumin-induced promoter methylation to the mTOR gene was
associated with a corresponding downregulation of its expression.
The authors suggested that curcumin-induced hypermethylation of
mTOR may be associated with the upregulation of DNMT3a and DNMT3b
(47). Here, we found that curcumin
downregulated TET1 in T47D and upregulated DNMT3a and DNMT3b in
T47D and HCC-38 cells. This suggests that the curcumin-induced
hypermethylation of SNCG may be associated with the
upregulation of DNMT3. Interestingly, it has been reported that, in
SNCG-positive lung cancer cells H292 endogenous
overexpression of DNMT3b, but not of DNMT3a or DNMT1, suppressed
SNCG expression by inducing DNA methylation of the SNCG CpG
island (48). However, the
possibility that the curcumin-induced hypermethylation of
SNCG may be achieved through the downregulation of TET1
cannot be excluded. This is supported by the finding that the
activation of the oncogenic pathway in breast and ovarian cancers
is TET1 overexpression-dependent and that the deletion of TET1
attenuated the effect of the oncogenic pathway (8). Thus, it is plausible that the
curcumin-induced hypermethylation of SNCG in breast cancer
cells may be achieved through the downregulation of TET1. However,
further studies are needed to clarify the exact mechanisms of this
process.
miR-29b is an epi-miRNA, being a regulator for DNMTs
and TETs by direct inhibition of these enzymes (49). Thus, it has been suggested that
miR-29b may act as a stabilizer of DNA methylation, balancing
between methylation and demethylation (10). Notably, it has been shown that
curcumin re-expressed PTEN in hepatic stellate cells by
downregulating DNMT3b through the upregulation of miR-29b (31). However, miR-29b has also been shown
to affect breast cancer proliferation and metastasis by targeting
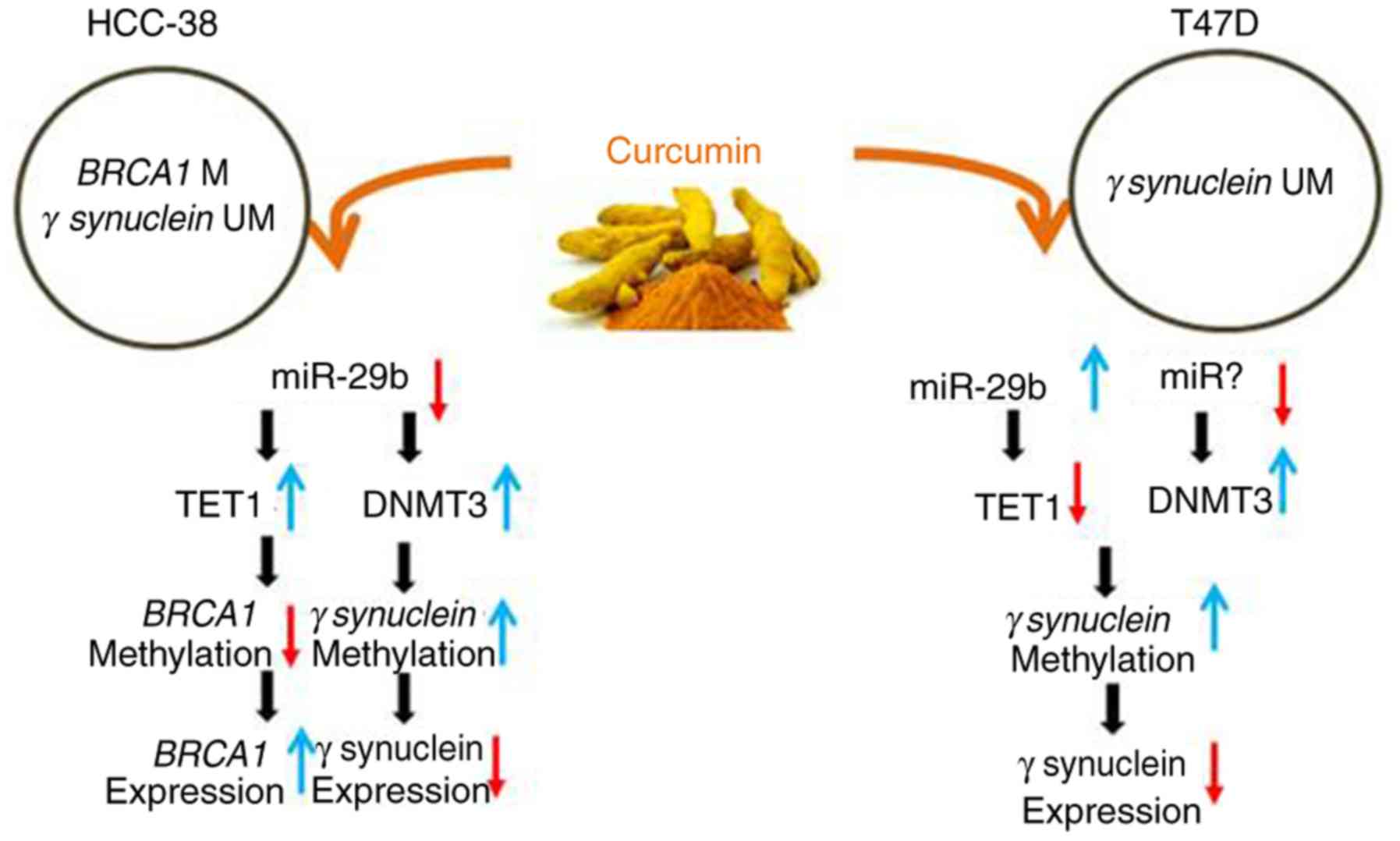
TET1 (12,13). Here, we found that curcumin appears
to re-express BRCA1 and suppress SNCG in HCC-38 cells by
upregulating TET1 and DNMT3, respectively, which may be realized
through the downregulation of miR-29b. However, in T47D cells,
curcumin appears to suppress SNCG by downregulating TET1, which may
be realized through the upregulation of miR-29b and the
upregulation of DNMT3, which may be achieved through another miRNA
(Fig. 7 summarizes the
results).
Overall, our data suggest that curcumin may act as a
stabilizer of DNA methylation balancing between methylation and
demethylation by regulating TET1 and DNMT3, which may be achieved
through the modulation of miR-29b. However, further studies are
needed to confirm the direct role of miR-29b in this epigenetic
regulation.
In conclusion, in the present study, we demonstrated
that curcumin performs a dual function in DNA methylation. As
curcumin is an activator for the hypermethylated BRCA1
promoter, we, therefore, believe that it holds the potential to be
an effective therapeutic option for triple-negative breast cancer
as well as for the prevention of breast and ovarian cancer,
particularly for BRCA1-promoter methylation carriers.
Acknowledgements
We would like to acknowledge Dr B. Karakas and Dr A.
Qattan (Molecular Oncology Department, King Faisal Specialist
Hospital and Research Centre) for revising the article, and for
helping with the statistical analysis, respectively.
Funding
The King Faisal Specialist Hospital and Research
Center supported this research under the RAC #2150 020.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NAY, ZS, BAS and MAS performed the experiments. NAY
and NAM performed the data analysis. NAM conceived and designed the
study and drafted the manuscript with the help from NAY. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SNCG
|
γ synuclein
|
|
BRCA1
|
BRCA1 DNA repair associated
|
|
TETs
|
ten-eleven translocations
|
|
DNMTs
|
DNA methyltransferases
|
|
5′-aza-CdR
|
5-aza-2′-deoxycytidine
|
References
|
1
|
Doll R and Peto R: The causes of cancer:
Quantitative estimates of avoidable risks of cancer in the United
States today. J Natl Cancer Inst. 66:1191–1308. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki K, Suzuki I, Leodolter A, Alonso S,
Horiuchi S, Yamashita K and Perucho M: Global DNA demethylation in
gastrointestinal cancer is age dependent and precedes genomic
damage. Cancer Cell. 9:199–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goll MG and Bestor TH: Eukaryotic cytosine
methyltransferases. Annu Rev Biochem. 74:481–514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito S, Shen L, Dai Q, Wu SC, Collins LB,
Swenberg JA, He C and Zhang Y: Tet proteins can convert
5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine.
Science. 333:1300–1303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maiti A and Drohat AC: Thymine DNA
glycosylase can rapidly excise 5-formylcytosine and
5-carboxylcytosine: Potential implications for active demethylation
of CpG sites. J Biol Chem. 286:35334–35338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tahiliani M, Koh KP, Shen Y, Pastor WA,
Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L and
Rao A: Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in
mammalian DNA by MLL partner TET1. Science. 324:930–935. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scourzic L, Mouly E and Bernard OA: TET
proteins and the control of cytosine demethylation in cancer.
Genome Med. 7:92015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Good CR, Panjarian S, Kelly AD, Madzo J,
Patel B, Jelinek J and Issa JJ: TET1-mediated hypomethylation
activates oncogenic signaling in triple-negative breast cancer.
Cancer Res. 78:4126–4137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu CH, Peng KL, Kang ML, Chen YR, Yang
YC, Tsai CH, Chu CS, Jeng YM, Chen YT, Lin FM, et al: TET1
suppresses cancer invasion by activating the tissue inhibitors of
metalloproteinases. Cell Rep. 2:568–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan B, Guo Q, Fu FJ, Wang Z, Yin Z, Wei YB
and Yang JR: The role of miR-29b in cancer: Regulation, function,
and signaling. Onco Targets Ther. 8:539–548. 2015.PubMed/NCBI
|
|
11
|
Zhang Z, Cao Y, Zhai Y, Ma X, An X, Zhang
S and Li Z: MicroRNA-29b regulates DNA methylation by targeting
Dnmt3a/3b and Tet1/2/3 in porcine early embryo development. Dev
Growth Differ. 60:197–204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chou J, Lin JH, Brenot A, Kim JW, Provot S
and Werb Z: GATA3 suppresses metastasis and modulates the tumour
microenvironment by regulating microRNA-29b expression. Nat Cell
Biol. 15:201–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, An X, Yu H, Zhang S, Tang B, Zhang
X and Li Z: MiR-29b/TET1/ZEB2 signaling axis regulates metastatic
properties and epithelial-mesenchymal transition in breast cancer
cells. Oncotarget. 8:102119–102133. 2017.PubMed/NCBI
|
|
14
|
Wong EM, Southey MC, Fox SB, Brown MA,
Dowty JG, Jenkins MA, Giles GG, Hopper JL and Dobrovic A:
Constitutional methylation of the BRCA1 promoter is specifically
associated with BRCA1 mutation-associated pathology in early-onset
breast cancer. Cancer Prev Res (Phila). 4:23–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupta S, Jaworska-Bieniek K, Narod SA,
Lubinski J, Wojdacz TK and Jakubowska A: Methylation of the BRCA1
promoter in peripheral blood DNA is associated with triple-negative
and medullary breast cancer. Breast Cancer Res Treat. 148:615–622.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iwamoto T, Yamamoto N, Taguchi T, Tamaki Y
and Noguchi S: BRCA1 promoter methylation in peripheral blood cells
is associated with increased risk of breast cancer with BRCA1
promoter methylation. Breast Cancer Res Treat. 129:69–77. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Moghrabi N, Nofel A, Al-Yousef N,
Madkhali S, Bin Amer SM, Alaiya A, Shinwari Z, Al-Tweigeri T,
Karakas B, Tulbah A and Aboussekhra A: The molecular significance
of methylated BRCA1 promoter in white blood cells of cancer-free
females. BMC Cancer. 14:8302014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dobrovic A, Mikeska T, Alsop K, Candiloro
I, George J, Mitchell G and Bowtell D: Constitutional BRCA1
methylation is a major predisposition factor for high-grade serous
ovarian cancer. Cancer Res. 742014.
|
|
19
|
Al-Moghrabi NM: BRCA1 promoter methylation
in peripheral blood cells and predisposition to breast cancer. J
Taibah Univ Med Sci. 12:189–193. 2017.PubMed/NCBI
|
|
20
|
Wei M, Grushko TA, Dignam J, Hagos F,
Nanda R, Sveen L, Xu J, Fackenthal J, Tretiakova M, Das S and
Olopade OI: BRCA1 promoter methylation in sporadic breast cancer is
associated with reduced BRCA1 copy number and chromosome 17
aneusomy. Cancer Res. 65:10692–10699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Butcher DT, Mancini-DiNardo DN, Archer TK
and Rodenhiser DI: DNA binding sites for putative methylation
boundaries in the unmethylated region of the BRCA1 promoter. Int J
Cancer. 111:669–678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu J, Huo D, Chen Y, Nwachukwu C, Collins
C, Rowell J, Slamon DJ and Olopade OI: CpG island methylation
affects accessibility of the proximal BRCA1 promoter to
transcription factors. Breast Cancer Res Treat. 120:593–601. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao
G, Joseph BK, Rosen C and Shi YE: Identification of a breast
cancer-specific gene, BCSG1, by direct differential cDNA
sequencing. Cancer Res. 57:759–764. 1997.PubMed/NCBI
|
|
24
|
Wu K, Quan Z, Weng Z, Li F, Zhang Y, Yao
X, Chen Y, Budman D, Goldberg ID and Shi YE: Expression of neuronal
protein synuclein gamma gene as a novel marker for breast cancer
prognosis. Breast Cancer Res Treat. 101:259–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu A, Gupta A, Li C, Ahlborn TE, Ma Y, Shi
EY and Liu J: Molecular mechanisms for aberrant expression of the
human breast cancer specific gene 1 in breast cancer cells: Control
of transcription by DNA methylation and intronic sequences.
Oncogene. 20:5173–5185. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu A, Zhang F, Gupta A and Liu J: Blockade
of AP1 transactivation abrogates the abnormal expression of breast
cancer-specific gene 1 in breast cancer cells. J Biol Chem.
277:31364–31372. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song X, Zhang M, Dai E and Luo Y:
Molecular targets of curcumin in breast cancer (Review). Mol Med
Rep. 19:23–29. 2019.PubMed/NCBI
|
|
28
|
Jiang A, Wang X, Shan X, Li Y, Wang P,
Jiang P and Feng Q: Curcumin reactivates silenced tumor suppressor
Gene RARβ by reducing DNA methylation. Phytother Res. 29:1237–1245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar U, Sharma U and Rathi G: Reversal of
hypermethylation and reactivation of glutathione S-transferase pi 1
gene by curcumin in breast cancer cell line. Tumour Biol.
39:10104283176922582017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du L, Xie Z, Wu LC, Chiu M, Lin J, Chan
KK, Liu S and Liu Z: Reactivation of RASSF1A in breast cancer cells
by curcumin. Nutr Cancer. 64:1228–1235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng J, Wu C, Lin Z, Guo Y, Shi L, Dong
P, Lu Z, Gao S, Liao Y, Chen B and Yu F: Curcumin up-regulates
phosphatase and tensin homologue deleted on chromosome 10 through
microRNA-mediated control of DNA methylation-a novel mechanism
suppressing liver fibrosis. FEBS J. 281:88–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Link A, Balaguer F, Shen Y, Lozano JJ,
Leung HC, Boland CR and Goel A: Curcumin modulates DNA methylation
in colorectal cancer cells. PLoS One. 8:e577092013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Birgisdottir V, Stefansson OA,
Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG and Eyfjord JE:
Epigenetic silencing and deletion of the BRCA1 gene in sporadic
breast cancer. Breast Cancer Res. 8:R382006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu H, Liu W, Wu Y, Zhou Y, Xue R, Luo C,
Wang L, Zhao W, Jiang JD and Liu J: Loss of epigenetic control of
synuclein-gamma gene as a molecular indicator of metastasis in a
wide range of human cancers. Cancer Res. 65:7635–7643. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Al-Moghrabi N, Al-Showimi M, Al-Yousef N,
Al-Shahrani B, Karakas B, Alghofaili L, Almubarak H, Madkhali S and
Al Humaidan H: Methylation of BRCA1 and MGMT genes in white blood
cells are transmitted from mothers to daughters. Clin Epigenetics.
10:992018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tost J and Gut IG: DNA methylation
analysis by pyrosequencing. Nat Protoc. 2:2265–2275. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hendrayani SF, Al-Khalaf HH and
Aboussekhra A: Curcumin triggers p16-dependent senescence in active
breast cancer-associated fibroblasts and suppresses their paracrine
procarcinogenic effects. Neoplasia. 15:631–640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vaughan RA, Garcia-Smith R, Dorsey J,
Griffith JK, Bisoffi M and Trujillo KA: Tumor necrosis factor alpha
induces Warburg-like metabolism and is reversed by
anti-inflammatory curcumin in breast epithelial cells. Int J
Cancer. 133:2504–2510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gupta A, Godwin AK, Vanderveer L, Lu A and
Liu J: Hypomethylation of the synuclein gamma gene CpG island
promotes its aberrant expression in breast carcinoma and ovarian
carcinoma. Cancer Res. 63:664–673. 2003.PubMed/NCBI
|
|
41
|
Hu S, Xu Y, Meng L, Huang L and Sun H:
Curcumin inhibits proliferation and promotes apoptosis of breast
cancer cells. Exp Ther Med. 16:1266–1272. 2018.PubMed/NCBI
|
|
42
|
Feilotter HE, Michel C, Uy P, Bathurst L
and Davey S: BRCA1 haploinsufficiency leads to altered expression
of genes involved in cellular proliferation and development. PLoS
One. 9:e1000682014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma Z, Niu J, Sun E, Rong X, Zhang X and Ju
Y: Gamma-synuclein binds to AKT and promotes cancer cell survival
and proliferation. Tumour Biol. 37:14999–15005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
He JS, Xie N, Yang JB, Guan H, Chen WC,
Zou C, Ouyang YW, Mao YS, Luo XY, Pan Y and Fu L: BCSG1 siRNA
delivered by lentiviral vector suppressed proliferation and
migration of MDA-MB-231 cells. Int J Mol Med. 41:1659–1664.
2018.PubMed/NCBI
|
|
45
|
Pei YF, Tao R, Li JF, Su LP, Yu BQ, Wu XY,
Yan M, Gu QL, Zhu ZG and Liu BY: TET1 inhibits gastric cancer
growth and metastasis by PTEN demethylation and re-expression.
Oncotarget. 7:31322–31335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kai M, Niinuma T, Kitajima H, Yamamoto E,
Harada T, Aoki H, Maruyama R, Toyota M, Sasaki Y, Sugai T, et al:
TET1 Depletion induces aberrant CpG methylation in colorectal
cancer cells. PLoS One. 11:e01682812016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen J, Ying Y, Zhu H, Zhu T, Qu C, Jiang
J and Fang B: Curcumin-induced promoter hypermethylation of the
mammalian target of rapamycin gene in multiple myeloma cells. Oncol
Lett. 17:1108–1114. 2019.PubMed/NCBI
|
|
48
|
Liu H, Zhou Y, Boggs SE, Belinsky SA and
Liu J: Cigarette smoke induces demethylation of prometastatic
oncogene synuclein-gamma in lung cancer cells by downregulation of
DNMT3B. Oncogene. 26:5900–5910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Morita S, Horii T, Kimura M, Ochiya T,
Tajima S and Hatada I: miR-29 represses the activities of DNA
methyltransferases and DNA demethylases. Int J Mol Sci.
14:14647–14658. 2013. View Article : Google Scholar : PubMed/NCBI
|