Introduction
Cholangiocarcinoma (CCA) is a primary liver cancer
arising from bile duct epithelial cells and characteristically
manifests as an abundant stromal reaction (1). CCA is associated with a poor patient
prognosis and a low survival rate due to its high capability to
invade and metastasize (2).
Increased α-smooth muscle actin (ASMA)-positive fibroblasts have
been indentified in the stroma of CCA tissues and higher levels
have been correlated with shorter patient survival times (1). These fibroblasts were found to have
upregulated expression of several secreted proteins, and periostin
(PN) is a crucial one exclusively overexpressed in stromal
fibroblasts and significantly correlated with poor patient
prognosis (3). PN (also known as
osteoblast-specific factor OSF-2) is a protein that in humans is
encoded by the POSTN gene and has been reported to enhance
several tumorigenic properties in various types of cancers
including ovarian (4), breast
(5), colon (6), head and neck (7), and pancreatic cancer (8). Enhancement was shown to occur through
interactions with membrane receptor integrin (ITG)αvβ3 or αvβ5 in
ovarian cancer (4), ITGα6β4 in
pancreatic cancer (9), and ITGα5β1
in CCA (10). Mino et al
revealed that the malignant potential of PN is expressed through
the induction of epithelial-to-mesenchymal transition (EMT) in CCA
via ITGαv (11). Although PN acts
through ITGα5β1 in CCA cell invasion (10), understanding of the role of ITGα5β1
in the EMT phenotype of CCA cells is still limited.
EMT has been strongly implicated in several types of
cancer in regards to a key impact on cell invasion and metastasis
(12,13). Characteristics of cells undergoing
EMT include an increase in mesenchymal markers such as vimentin
(VIM), N-cadherin (CDH-2), ASMA, and fibronectin (FN-1) and a
reduction in epithelial markers, in particular, E-cadherin (CDH-1)
and cytokeratin (CK) (14,15). Three transcription factors, zinc
finger protein SNAI1 (SNAIL-1), SLUG (SNAIL-2) and TWIST have been
demonstrated to function in the regulation of EMT in cancers
(14). Zinc finger protein SNAI1
(SNAIL-1) has been identified as a key molecule in transforming
growth factor (TGF)-β1-activated EMT in pancreatic cancer (16,17).
TWIST was shown to induce breast cancer cells to undergo EMT
(18). However, the role of TWIST-2
in EMT that follows PN activation of ITGα5β1 has not been well
elucidated. In the present study, recombinant PN activated EMT
which led to CCA migration and TWIST-2 activation, and
additionally, the potential clinical use of TWIST-2 as a marker for
poor prognosis in human CCA was revealed. These findings also
highlight the potential impact of targeting the PN/ITGα5β1/TWIST-2
pathway driving CCA migration to attenuate the progression of
disease.
Materials and methods
CCA cell line culture
Human CCA cell lines KKU-100, KKU-139 and KKU-213
were cultured in DMEM medium (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin, 100 µg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.), and an anti-fungal agent. Cells were cultured in
a 5% CO2 incubator at 37°C, and passaged with 0.25%
trypsin-EDTA. Cells with more than 90% viability were used
throughout this study.
Migration assay (wound healing
assay)
KKU-100, KKU-139, and KKU-213 tumor cell lines
(50,000 cells/well) were cultured in 6-well plates until they
reached approximately 90% confluence. A reference midline was drawn
under the plate. Cells were scraped off along the line using a
sterile 200-µl pipette tip and the detached cells were washed away
with serum-free medium. The remaining cells were then treated with
medium containing either 100 ng/ml recombinant PN or medium without
PN. The scraped area indicated by the reference line was recorded
at the beginning of treatment and again at 24 h. The efficiency of
migration into the scraped area was taken as a measure of wound
healing and was calculated by the following formula: % wound
healing=[(wound space at 0 h-wound space at 24 h)/wound space at 0
h] ×100.
Transwell migration assay
A total of 5×104 KKU-213 cells in DMEM
containing 1% FBS with or without 100 ng/ml PN was plated in the
upper chamber of a 24-well Corning Transwell plate (Corning #3428
Transwell) and 600 µl of 1% FBS DMEM was added to the lower
chamber. After culture in a humidified incubator at 37°C for 12 h,
the upper chamber was fixed in 70% ethanol for 30 min and stained
with 0.5% crystal violet for 15 min. After drying, migrating cells
were counted under an inverted microscope (original magnification,
×400).
Measurement of EMT gene expression in
PN-treated CCA cell lines by real-time PCR
Total RNA was extracted using a Perfect Pure RNA
Cultured Cell Kit (5 PRIME; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The cDNA was
synthesized from 1 µg of total RNA using SuperScript™ III
First-Strand Synthesis System for RT-PCR (M-MLV; Invitrogen; Thermo
Fisher Scientific, Inc.) according to the instructions. The
sequences of all genes in this study were retrieved from PubMed
(www.ncbi.nln.nih.gov) and the primers
were designed using Primer 3 software (Version 0.4.0) (Table I). The PCR cycles consisted of: 95°C
for 10 min, followed by 50 cycles of 95°C for 15 sec and 60°C
(except 58°C for TWIST-2) for 15 sec. Crossing point data were
automatically obtained by the fit-point of Lightcycler®
480 software version 1.5 (https://lifescience.roche.com/en_th/products/lightcycler14301-480-software-version-15.html).
ACTB (β-actin gene) was used as an internal control. The
expression level of each gene in the PN-treated CCA cells was
compared to that of the control cells without PN treatment
(2−ΔΔCp) as follows (19):
2−ΔΔCp=2−(CpPN-treated
sample-CpACTB)-(Cpuntreated control-CpACTB) (To note
Cp is the crossing point as calculated by Lightcycler
480 software). The expression of EMT genes in TGF-β-treated cells
was used as the positive control for EMT.
 | Table I.Primer sequences used in the
study. |
Table I.
Primer sequences used in the
study.
| Gene | Accession no. | Primer (5′-3′) | Size (bp) |
|---|
| MMP-1 | NM_002421 | F:
TTCGGGGAGAAGTGATGTTC | 156 |
|
|
| R:
ACCGGACTTCATCTCTGTCG |
|
| MMP-7 | NM_002423 | F:
TGTATGGGGAACTGCTGACA | 131 |
|
|
| R:
GAGCATCTCCTCCGAGACCT |
|
| MMP-9 | NM_004994 | F:
GCACGACGTCTTCCAGTACC | 105 |
|
|
| R:
TAGCCCACTTGGTCCACCT |
|
| MMP-10 | NM_002425 | F:
TGGCCCTCTCTTCCATCATA | 95 |
|
|
| R:
CTGATGGCCCAGAACTCATT |
|
| MMP-13 | NM_002427 | F:
GCAGCTGTTCACTTTGAGGA | 136 |
|
|
| R:
CACCAATTCCTGGGAAGTCT |
|
| MMP-14 | NM_004995 | F:
GTGGTCTCGGACCATGTCTC | 154 |
|
|
| R:
GGGAGGCAGGTAGCCATATT |
|
| SNAIL-1 | NM_005985 | F:
TCTGAGGCCAAGGATCTCCAGGC | 243 |
|
|
| R:
CAGGTTGGAGCGGTCAGCGAA |
|
| SLUG | NM_003068 | F:
AATATGTGAGCCTGGGCGCCCT | 163 |
|
|
| R:
GCTCTGTTGCAGTGAGGGCAAGAA |
|
| TWIST-2 | NM_057179 | F:
GCAAGAAGTCGAGCGAAGAT | 221 |
|
|
| R:
CAGCTTGAGCGTCTGGATCT |
|
| CK-19 | NM_002276 | F:
AGCTAGAGGTGAAGATCCGCGAC | 155 |
|
|
| R:
GGCATTGTCGATCTGCAGGACAA |
|
| CDH-1 | NM_004360 | F:
GCCTGGGACTCCACCTACA | 147 |
|
|
| R:
TCTGAGGCCAGGAGAGGAG |
|
| ASMA | NM_001613 | F:
AGGAAGCAGCTCTATGCTAACAAT | 379 |
|
|
| R:
AACACATAGGTAACGAGTCAGAGC |
|
| FN-1 | NM_054034 | F:
GGAAGCCGAGGTTTTAACTGCGAG | 186 |
|
|
| R:
ATGGCAGCGGTTTGCGATGGT |
|
| VIM | NM_003380 | F:
CAGGTGGGACCAGCTAACCAA | 152 |
|
|
| R:
TGCCAGACGCATTGTCA |
|
| ACTB | NM_001101 | F:
CACACTGTGCCCATCTACGA | 162 |
|
|
| R:
CTCCTTAATGTCACGCACGA |
|
Western blot analysis of EMT
markers
Cells with or without PN treatment were collected
after refrigerated centrifugation of the cell suspensions at 400 ×
g for 5 min. The cell pellets were lysed in 1X sample buffer
containing 50 mM Tris-HCl pH 6.8, 2% (w/v) SDS, 10% (v/v) glycerol,
5% (v/v) β-mercaptoethanol and 0.05% (w/v) bromophenol blue. Cell
lysates were boiled for 10 min and centrifuged at 8,000 × g for 1
min to remove the undissolved proteins and cell debris. Cell
extracts were then separated using 10% SDS-PAGE and transferred
onto PVDF membranes (Amersham). The membranes were blocked in 5%
skim milk containing TBST for 1 h at room temperature (RT). The
immunodetection steps for TWIST-2, ASMA, VIM, CK-19, MMP-9, MMP-13,
and ITGα5 were performed using mouse anti-TWIST-2 (dilution 1:250;
cat. no. ab57997; Abcam), mouse anti-ASMA (dilution 1:400; cat. no.
A5228; Sigma-Aldrich; Merck KGaA), mouse anti-VIM (dilution 1:200;
cat. no. sc-6260; Santa Cruz Biotechnology, Inc.), mouse anti-CDH-1
(dilution 1:50; cat. no. sc-71008; Santa Cruz Biotechnology), mouse
anti-CK-19 (dilution 1:200; cat. no. sc-6278; Santa Cruz
Biotechnology), mouse anti-MMP-9 (dilution 1:100; cat. no.
sc-21733; Santa Cruz Biotechnology, Inc.), anti-MMP13 (dilution
1:1,000; cat. no. sc-30073; Santa Cruz Biotechnology, Inc.), and
mouse anti-ITGα5 (dilution 1:500, sc-376199; Santa Cruz
Biotechnology). The incubation periods of primary antibodies for
TWIST-2, ASMA, VIM, CK-19, and ITGα5 were overnight at 4°C, and
those of MMP-9 and MMP-13 were 2 and 1 h, respectively, at RT. The
secondary antibodies were HRP-conjugated goat anti-mouse IgG
antibody (dilution 1:2,000; cat. no. 62-6620; Zymed, Thermo Fisher
Scientific, Inc.) for mouse primary antibody, and HRP-conjugated
goat anti-rabbit IgG antibody (dilution 1:3,000; Ab6717; Abcam) for
rabbit primary antibody. The immunoreactive signals were visualized
by ECL (Thermo Fisher Scientific, Inc.) under Gel Document Syngene
(Syngene). The β-actin level was used as an internal control with
mouse anti-β-actin (dilution 1:10,000; sc-47778; Santa Cruz). The
bands were quantified by ImageJ software (Version 1.52a; National
Institutes of Health, Bethesda, MD, USA). The obtained staining
intensities of the proteins of interest were then normalized
against the intensity of the staining of β-actin protein
(ACTB).
Zymography
The conditioned-media of CCA cells that had been
treated with PN, or that had been left untreated, were centrifuged
at 400 × g for 5 min to remove cell debris, and supernatants were
then collected. The supernatants were concentrated with Vivaspin 20
(28932358; GE Healthcare; Merck KGaA) (MWCO 3,000) by
centrifugation at 3,750 × g at 4°C for 2 h. The protein
concentrations were measured using the Coomassie Plus Assay Kit
(Thermo Fisher Scientific, Inc.). The samples were loaded using 12%
SDS-PAGE containing 1 mg/ml gelatin (Ajax Finechem) as a substrate.
The electrophoresis was performed at 200 V for 5 min in pre-cooled
SDS-PAGE running buffer, followed by prolonged electrophoresis in a
4°C refrigerator for 1 h 20 min. Afterward, the zymogram gels were
twice washed for 30 min with 2.5% Triton X-100 to renature the
enzymes. Zymographic activities were processed with developing
buffer at 37°C for 18 h. The results were visualized as clear bands
after staining with 0.006% Coomassie blue for 2 h at RT The bands
were quantified by ImageJ software. The cut-off at 1.5 for
upregulated gene expression was used as it typically causes enough
upregulated protein production to affect cell phenotype (3).
Immunocytochemistry for localization
of CDH-1
KKU-213 and KKU-139 CCA cells (5,000 cells/well)
were grown in 96-well plates to 40% confluence and treated with 100
ng/ml PN for 48 h. Cells were washed with 1X PBS pH 7.4 and fixed
with 4% paraformaldehyde at RT for 10 min. For cell
permeabilization, 0.5% Triton X-100 in 1X PBS was added and
incubated at RT for 5 min. The non-specific binding was blocked
with 10% (w/v) FBS in 1X PBS with 0.1% triton X-100, and incubated
at RT for 1 h. Cells were washed with 1X PBS 3 times and incubated
with mouse anti-human CDH-1 antibody (dilution 1:50; cat. no.
sc-71008; Santa Cruz Biotechnology, Inc.) overnight at 4°C. Then,
the cells were washed with a blocking solution comprised of 10%
(w/v) FBS in 1X PBS with 0.1% Triton X-100, and then incubated with
goat anti-mouse IgG-Cy3 (dilution 1:2,000; cat. no. 115-166-071;
Jackson ImmunoResearch). The nuclei were stained with Hoechst at
1:1,000 (Invitrogen Thermo Fisher Scientific, Inc.). After washing,
the cells were fixed with 4% paraformaldehyde at RT for 10 min and
mounted in 50% glycerol. The positive staining cells were
visualized under a fluorescence inverted microscope (Olympus; ×400
original magnification). The patterns and localizations
(cytoplasmic or nuclear) were recorded.
Immunofluorescence staining of
ITGα5β1
Cells were plated onto 12-mm glass coverslips in
DMEM containing 10% FBS in a 24-well plate at 2×104
cells/well and cultured overnight. Cells were fixed with 4% (w/v)
paraformaldehyde for 15 min at RT. The cells were blocked with 1X
PBS containing 0.5% BSA for 30 min at RT. The cells were stained
with primary antibody, mouse anti-human ITGα5 (dilution 1:100, C-9,
Santa Cruz Biotechnology, Inc.), for 2 h at RT in 1X PBS containing
0.5% BSA, and incubated with Cy3-conjugated anti-mouse IgG
secondary antibody (dilution 1:2,000; cat. no. 115-166-071; Jackson
ImmunoResearch Laboratory Inc.). Based on the fact that the ITGα5
subunit can bind only to the ITGb1 subunit (20), changes in ITGα5 were taken as
equivalent to changes in ITGα5β1. The nuclei were stained with
Hoechst 33342 (1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.)
for 1 h at RT. The stained samples were mounted in 50% glycerol and
immunofluorescence images were obtained on an inverted microscope
and a confocal microscope (LSM800, Carl Zeiss, Jena, Germany; ×63
original magnification).
Transient knockdown of ITGα5 in CCA
cells
Silencing the expression of ITGα5β1 was performed by
transfection with siITGα5 (Santa Cruz Biotechnology, Inc.)
according to the manufacturer's instructions. Briefly,
1.5×105 cells/well were seeded onto 6-well plates and
cultured with complete medium at 37°C in a 5% CO2
incubator for 24 h. A siITGα5 transfection solution was
prepared by separately mixing 100 µl of OptiMEM® I
(Invitrogen; Thermo Fisher Scientific, Inc.) with either 10 µl of
10 pmole siITGα5 or 5 µl of Lipofectamine™ 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). The solutions were then mixed and
incubated for 20 min at RT. In addition, 200 µl of
OptiMEM® I mixed with 5 µl of Lipofectamine™ 2000 was
simultaneously used as a mock solution. Cells were washed three
times with antibiotic-serum-free medium. The siITGα5
transfection solution (cat. no. STCSC-29372, Santa Cruz
Biotechnology, Inc.) was slowly dropped into each well and
incubated at 37°C in a 5% CO2 incubator for 6 h. Then
the complete medium was replaced. The efficiency of ITGα5 silencing
was confirmed by real-time PCR and immunofluorescence staining.
Immunohistochemistry of ITGα5β1 and
TWIST-2 in CCA tissues
Paraffin-embedded tissue samples from 50 CCA cases
were collected from the Cholangiocarcinoma Research Institute,
Faculty of Medicine, Khon Kaen University (Khon Kaen, Thailand)
under the approval of the Human Research Ethics Committee, Khon
Kaen University (HE490143). The median age of the patients was 58
years with a range of 37–75 years. The percentage of females was
38% (19/50) and males was 62% (31/50). The samples were recruited
during 2006–2011. The tissues were baked at 60–65°C for 4 h,
deparaffinized, soaked in 10 mM citrate buffer pH 6, and boiled at
95°C for 40 min. Endogenous peroxidase was blocked with 3%
H2O2 in methanol for 30 min. The tissues were
incubated with 2% BSA in 0.05 M Tris-HCl pH 7.6 to block
non-specific binding, then mouse anti-human TWIST-2 (dilution
1:200; ab57997; Abcam) was applied and the tissues were incubated
at 4°C overnight. The tissues were then incubated with
EnVision+ System horseradish peroxidase (Dako) for 30
min. The signal was developed using 0.05% 3,3′-diaminobenzidine
(DAB) and counterstained with hematoxylin. The scoring was assessed
using the percentage of positive staining cells (P) and the
intensity of the staining signal (I). For P, samples containing
0–25, 26–50, 51–75, and 76–100% positive cells were classified as
grades 0, 1, 2, and 3, respectively. For I, unstained, slightly
stained, intermediately stained, and strongly stained cells were
classified as 0, 1, 2, and 3, respectively. The multiplied score (P
× I) as a total score of 0–9 was determined for each stained slide
by 2 investigators double-blinded to the clinical data. A total
score of more than 4 was classified as high TWIST-2 level whereas
scores less than or equal to 4 were in the low-level group. This
cut-off value was derived from the median of the scoring of all
cases. In this research study, the normal adjacent area which was
approximately 5–10 mm distant from the border of the cancerous area
in the same slide of the CCA tissue section was used to represent
normal bile duct.
For ITGα5β1, the level in CCA tissues was
detected using the same protocols as for TWIST-2 except that the
antigen retrieval was performed using samples microwave-heated with
10 mM sodium citrate buffer with 0.05% Tween 20, pH 6.0 for 10 min,
and the mouse anti-human ITGα5 antibody (dilution 1:100,
sc-376199; Santa Cruz Biotechnology, Inc.) was used as the primary
antibody.
Statistical analysis
All quantified data are expressed as mean ± standard
deviation (SD), except for survival time for which median values
were used. Statistics for the in vitro studies were compared
with controls using the Student's t-test. One-way analysis of
variance (ANOVA) with all pairwise multiple comparison by Tukey
test was utilized to analyze the significant results of more than 2
groups. The statistical analysis of two variables and the
interaction between the groups was performed by two-way ANOVA. The
correlation of TWIST-2 level with clinicopathological data was
verified by Cox regression multivariate analysis, and the
Kaplan-Meier Log Rank test was used for survival analysis. A
P-value of <0.05 was considered to be statistically
significant.
Results
PN-induced alterations in the EMT
phenotype and EMT-related markers in CCA cells
The results revealed with statistical significance
that KKU-213 CCA cells treated with 100 ng/ml PN had a greater
ability to migrate than the untreated control cells, which had a
migration ability similar to CCA cells treated with 10 ng/ml TGF-β
(Fig. S1). Upregulated genes in
the PN-treated cells were defined as those with relative gene
expression 1.2-fold over those in the untreated cells.
Downregulated genes had a relative gene expression equal to or less
than 0.8-fold. The results showed that PN treatment induced the
expression of both SNAIL-1 and TWIST-2, with
statistical significance only for TWIST-2 (Fig. 1A). Notably, SNAIL-1 was
activated by both PN and TGF-β, whereas SLUG was not
changed. Upregulation of ASMA and VIM mesenchymal
markers was observed in the PN-treated CCA cells (Fig. 1B). Downregulation of the
CK-19 epithelial marker was significant, whereas no
significant change was observed in the CDH-1 mRNA level
(Fig. 1C). The Western blot results
confirmed that the PN-induced EMT of CCA cells was accompanied by
significant downregulation of CK-19, and a significant
increase in the production of TWIST-2 and VIM (Fig. 1D).
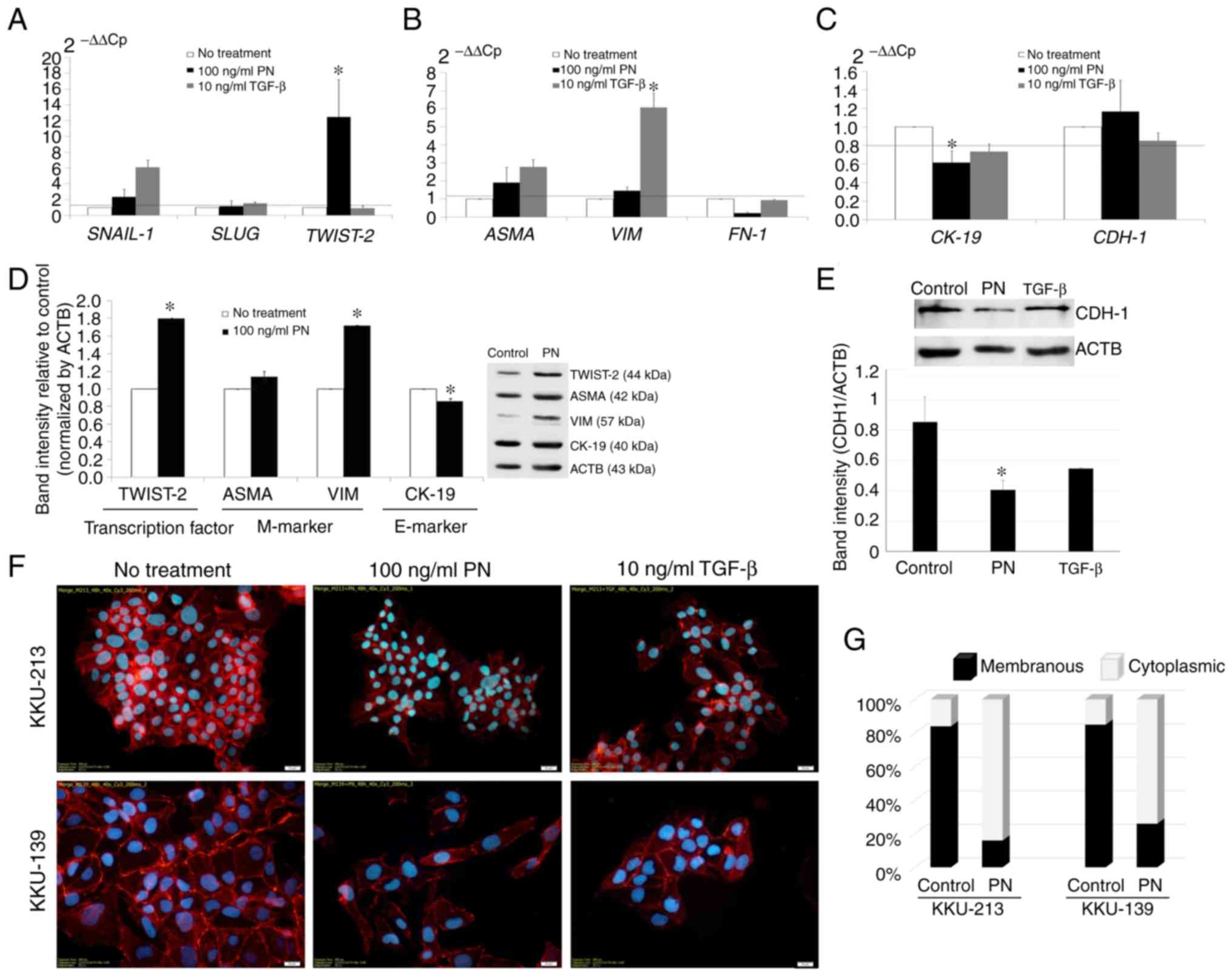 | Figure 1.PN-induced alterations in EMT-related
genes. (A-C) Real-time PCR analysis of mRNA in KKU-213 CCA cells
was conducted after treatment with 100 ng/ml PN. Gene expression in
excess of 1.2 times that of the negative control, (i.e. above the
dashed line), was scored as upregulation. (A) EMT-regulated
transcription factors. (B) Mesenchymal markers. (C) Epithelial
markers. Cells cultured in serum-free media without PN were used as
the negative control. Results are represented as mean ± SD of
triplicate reactions in one to three independent experiments.
*P<0.05 compared to the untreated control which was assumed to
be one. (D) Western blot analysis of TWIST-2, ASMA, VIM, and CK-19.
Band intensity of EMT markers quantified by ImageJ software was
determined relative to the control without PN treatment, and
normalized by ACTB. The bars represent the mean ± SD of two
measurements. (E) The alteration of CDH-1 after PN treatment
compared to ACTB. (F) Immunocytochemical localization of CDH-1 in
CCA cell lines induced by PN. TGF-β was used as an EMT stimulant.
Original magnification, ×400 (scale bar, 20 mm). (G) Bar graphs
representing the distribution of CDH-1 staining in CCA cells with
and without PN treatment. PN, periostin; EMT,
epithelial-to-mesenchymal transition; CCA, cholangiocarcinoma;
TWIST-2, Twist-related protein 2; ASMA, α-smooth muscle actin; VIM,
vimentin; MMP, matrix metalloproteinase; CK, cytokeratin;
ACTB, β-actin; CDH-1, E-cadherin; FN-1, fibronectin; TGF-β,
transforming growth factor-β; SNAIL-1, zinc finger protein
SNAI1; SLUG, human embryonic protein SNAI2. |
Interestingly, although the difference in the
expression level of CDH-1 mRNA was not significant, the
protein level revealed by western blot analysis was significantly
reduced in the PN-treated cancer cells (Fig. 1E). This was supported by the finding
in TGF-β-treated cells. Moreover, CDH-1, expressed predominantly on
the cell membranes of both KKU-213 and KKU-139 CCA cells, was
translocated after PN treatment to the cytoplasm (Fig. 1F). Thus, the membrane localization
of CDH-1 in the untreated cells was found on approximately 84 and
85% of the KKU-213 and KKU-139 cells (Fig. 1G). In cells treated with PN, the
membranous protein staining pattern of CDH-1 was reduced to 16 and
26% of KKU-213 and KKU-139 cells respectively, and the percentages
of cells displaying a cytoplasmic staining pattern was increased to
approximately 84 and 74%.
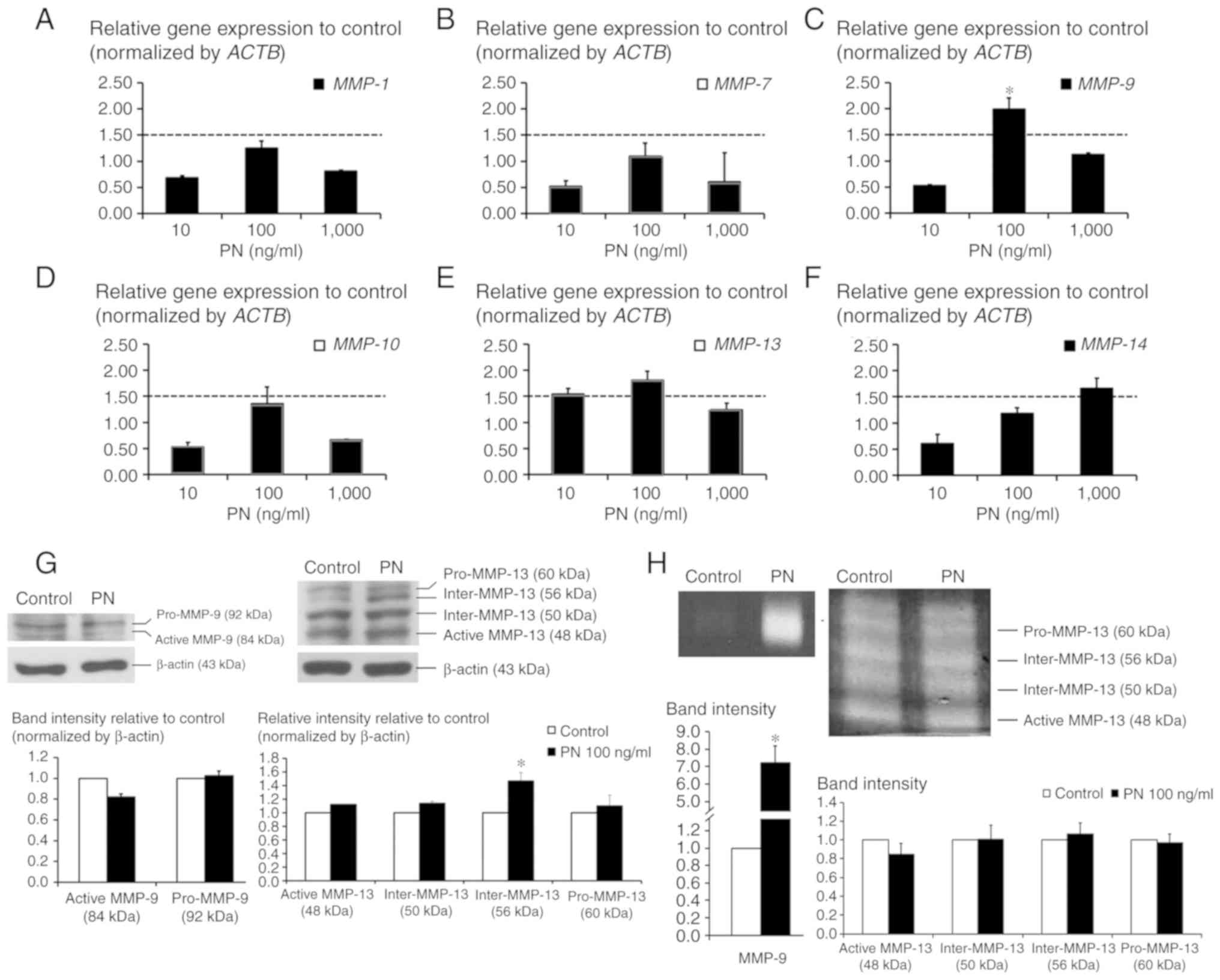
Effect of PN on the induction of MMPs
in CCA cells
No alterations of MMP-1, MMP-7, MMP-10, or
MMP-14 were detected in PN-treated CCA cells, whereas 100
ng/ml PN induced levels of MMP-9 and MMP-13 to
1.99±0.21- and 1.81±0.17-fold that of the untreated control cells
(Fig. 2A-F). However, the western
blot analysis results showed that neither pro-MMP-9 (92 kDa) nor
active MMP-9 (84 kDa) were induced by PN whereas the intermediate
form of MMP-13 (Inter-MMP-13) at 56 kDa showed a significant
increase (Fig. 2G). The zymography
results showed that after exposure to PN, CCA cells secreted MMP-9
into the conditioned-medium with a marked increase in activity
compared to the activity observed in the control condition
(Fig. 2H). Densitometric analysis
showed that MMP-9 activity was significantly increased in the
PN-treated CCA cells to 7-fold that of the control cells. No
significant change in MMP-13 in the PN-treated CCA cells was
observed.
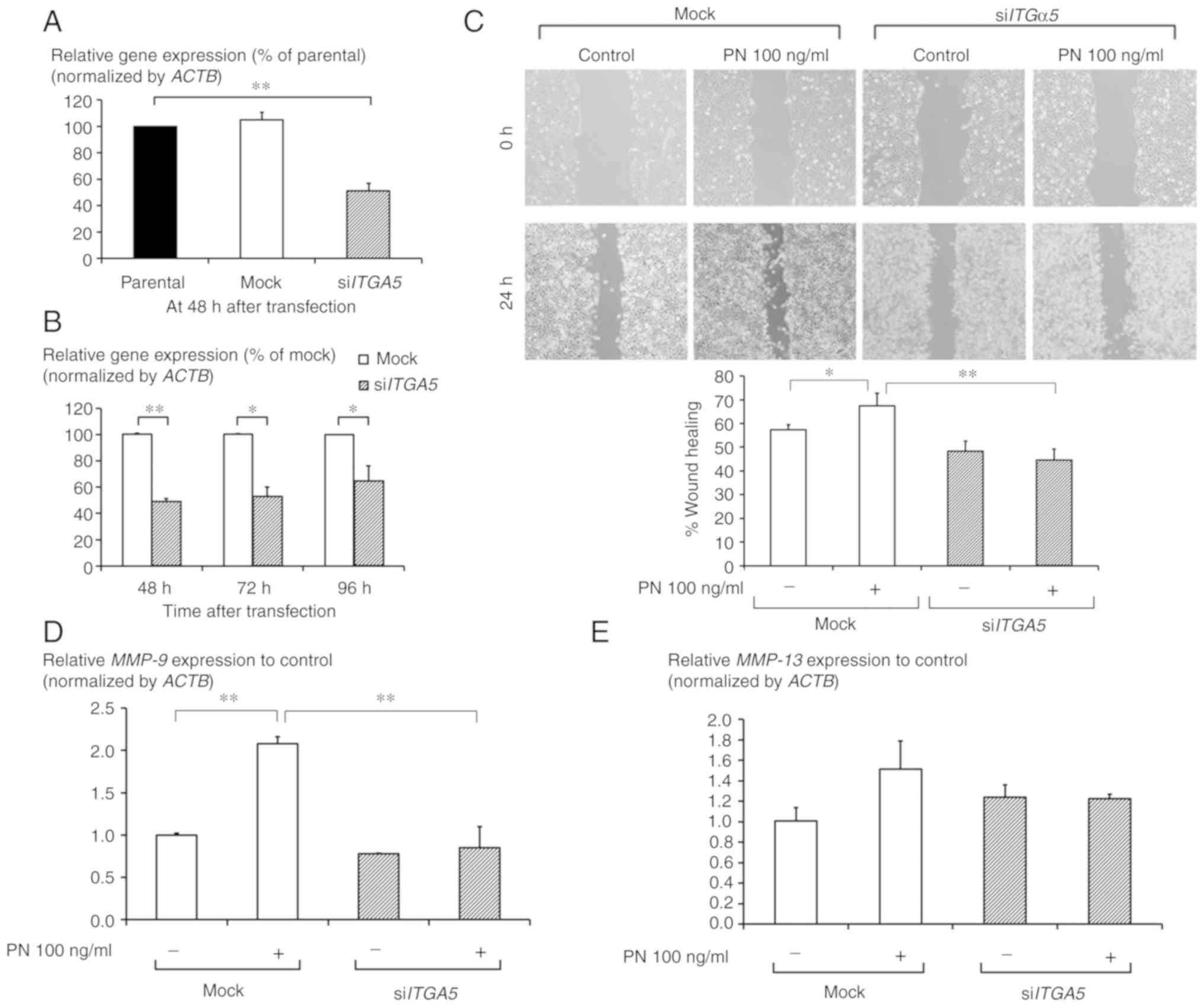
ITGα5β1-mediated CCA cell migration
and MMP expression
The siITG5-transfection significantly
downregulated intrinsic ITG5 mRNA expression with an
efficiency of approximately 35–50% (Fig. 3A). The efficiency of knockdown was
significant in cells up to 96 h post-siRNA treatment (Fig. 3B). The wound-closure assay showed
that PN significantly induced CCA cell migration after 24 h of
treatment, while in the siITGα5-transfected cells, the
migration was slower than that in the mock-transfected cells
(Fig. 3C). In cells in which
ITGα5β1 was diminished, the induction of expression of both
MMP-9 and MMP-13 by PN treatment was less than that
in the mock-transfected cells. This difference was significant for
MMP-9 (Fig. 3D and E).
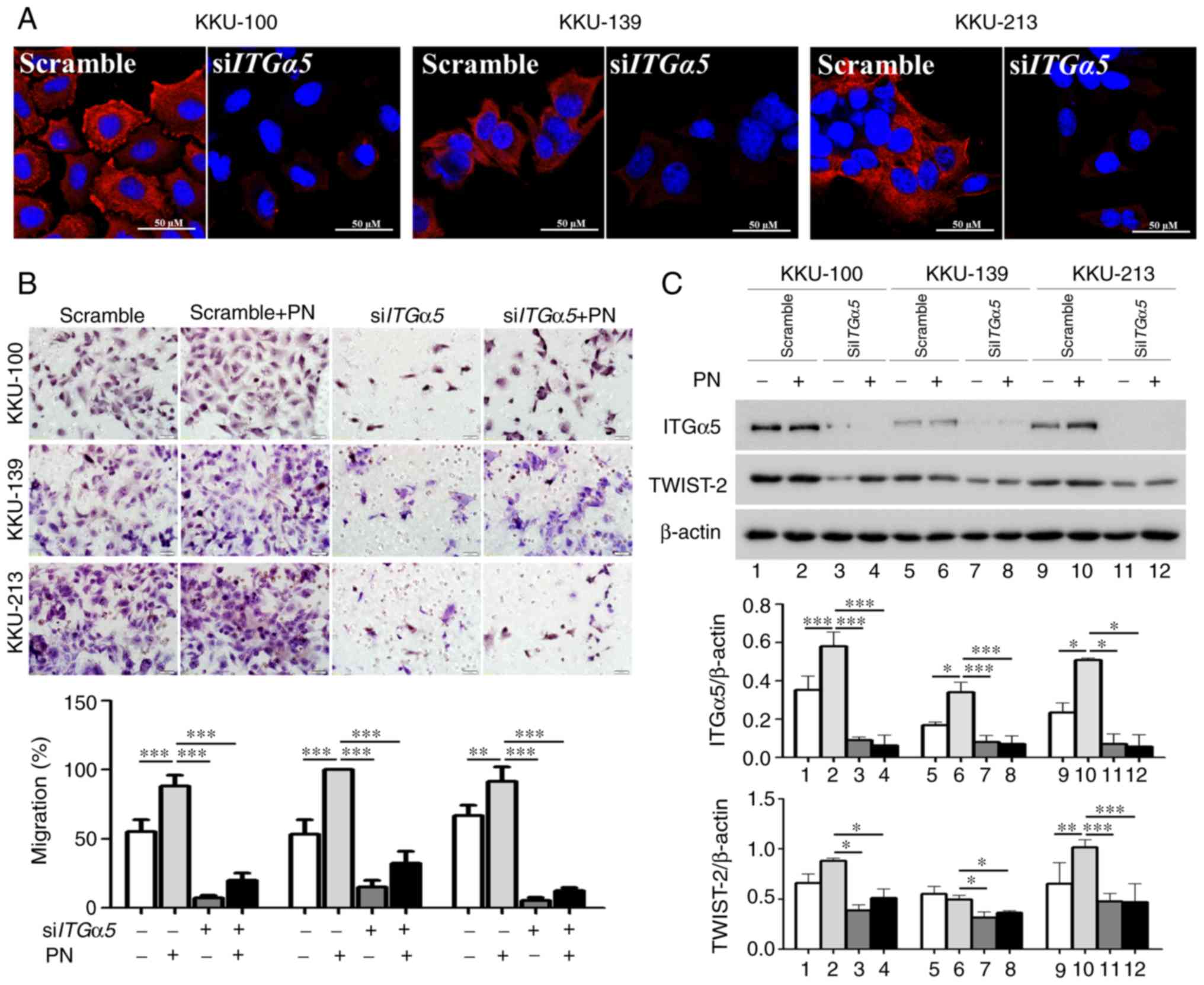
ITGα5β1/TWIST2-mediated CCA cell
migration
The results showed that siITGα5 treatment
successfully inhibited the production of membrane ITGα5β1 in CCA
cell lines (Fig. 4A). Moreover, the
siITGα5-transfected cells had a significantly lower response
rate in the PN-induced cell migration assay compared to the
scrambled-siRNA-treated cells (Fig.
4B). These results were observed in all three CCA cell lines.
TWIST-2 levels in these ITGα5-transient-knockdown CCA cells
were not induced by PN and this was in accord with the decreased
level of cell migration induced by PN (Fig. 4C).
Expression of ITGα5β1 and TWIST-2 in
CCA tissues and the clinicopathological relevance
The demographic data of the enrolled patients are
shown in Table II. Most of the
cases (60%) were male and more than 90% of the enrolled cases were
diagnosed with late-stage disease. Various positive stain-intensity
scores of TWIST-2 were found in CCA tissues (Fig. 5A-C) whereas normal bile duct showed
no TWIST-2 expression (Fig. 5D).
Most of the CCA cases, (33/50, 66%), showed high TWIST-2 levels.
Multivariate Cox regression analysis revealed that elevated TWIST-2
level and a categorization as a poorly differentiated cancer type
were independent parameters associated with poor prognosis. Other
parameters showed no significant correlations. Interestingly, CCA
patients with high TWIST-2 had shorter mean survival times (211±14
days) compared to those with low TWIST-2 (637±503 days). The
overall survival analysis by Kaplan-Meier test showed, with
statistical significance, that patients exhibiting high TWIST-2
levels had shorter survival times than those with low TWIST-2 using
both 3-year and 5-year survival as the cut-off values (Fig. 5E). The level of ITGα5β1 in CCA
clinical samples was also explored, and the results showed elevated
expression in 70% (7/10) of the CCA cases. The levels ranged from
low to high expression (Fig.
5F).
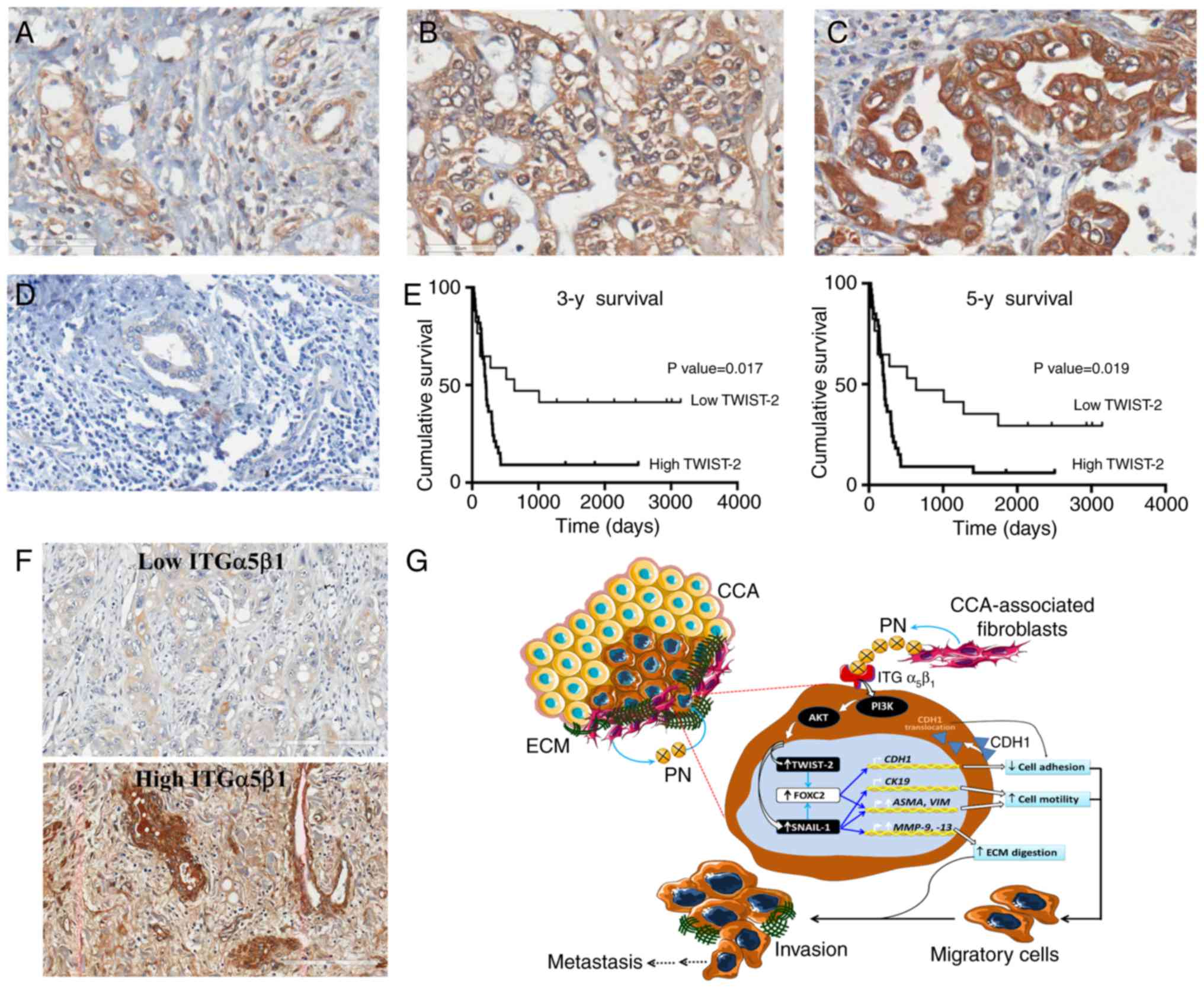 | Figure 5.Immunohistochemical staining of
TWIST-2 and ITGα5β1 in CCA tissues. (A-D) Representative images
showing the stain intensity range. A, grade 1; B, grade 2; C, grade
3; D, no staining in a normal bile duct (original magnification
×400; scale bar, 50 µm). (E) Kaplan-Meier analysis of the 3-year
and 5-year survival of patients with high and low TWIST-2 levels.
(F) Representative images of low and high expression of ITGα5 in
CCA (original magnification ×200; scale bar, 200 µm). (G) Proposed
mechanism of PN-induced EMT in CCA. The PN-mediated PI3K-dependent
pathway leads to the increased level of TWIST-2 and SNAIL-1,
facilitating: i) The reduction in membranous CDH-1 and CK-19
causing loss of cell-to-cell adhesio; ii) decreased levels of ASMA
and VIM causing cell motility, and iii) increased levels of MMPs
causing ECM degradation. TWIST-2, Twist-related protein 2; ITG,
integrin; CCA, cholangiocarcinoma; PN, periostin; EMT,
epithelial-to-mesenchymal transition; SNAIL-1. zinc finger protein
SNAI1; CDH-1, E-cadherin; CK-19, cytokeratin 19; ASMA, α-smooth
muscle actin; VIM, vimentin; MMPs, matrix metalloproteinases; ECM,
extracellular matrix. |
 | Table II.TWIST-2 covariates to the survival of
CCA patients in the multivariate Cox regression analysis. |
Table II.
TWIST-2 covariates to the survival of
CCA patients in the multivariate Cox regression analysis.
|
| 3-year
survival |
| 5-year
survival |
|
|---|
|
|
|
|
|
|
|---|
| Variable
(n=50) | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| TWIST-2 level |
|
|
0.022a |
|
|
0.023a |
| Low
(n=17) | 2.687 | 1.153–6.259 |
| 2.485 | 1.135–5.439 |
|
| High
(n=33) |
|
|
|
|
|
|
| Age, in years |
|
| 0.338 |
|
| 0.281 |
| ≤57
(n=24) | 1.405 | 0.701–2.816 |
| 1.458 | 0.734–2.893 |
|
| >57
(n=26) |
|
|
|
|
|
|
| Sex |
|
| 0.219 |
|
| 0.414 |
| Male
(n=32) | 0.643 | 0.319–1.299 |
| 0.759 | 0.392–1.470 |
|
| Female
(n=18) |
|
|
|
|
|
|
| Histological
type |
|
|
|
|
|
|
| WD
(n=21) | 1.000 |
|
| 1.000 |
|
|
| MD
(n=8) | 1.336 | 0.563–3.170 |
0.511 | 1.186 | 0.510–2.759 | 0.692 |
| PD
(n=6) | 4.670 | 1.648–13.24 | 0.004a | 4.466 | 1.617–12.33 |
0.004a |
| Pap
(n=15) | 2.070 | 0.681–6.292 | 0.199 | 2.158 | 0.772–6.032 | 0.143 |
| Vascular
invasion |
|
| 0.878 |
|
|
|
|
Presence (n=8) | 1.071 | 0.444–2.582 |
| 0.900 | 0.380–2.129 | 0.900 |
| Absence
(n=42) |
|
| |
|
|
|
| Stage |
|
| 0.450 |
|
|
|
| I–II
(n=4) | 1.1763 | 0.404–7.668 |
| 1.247 | 0.369–4.215 | 0.723 |
| III–IV
(n=46) |
|
|
|
|
|
|
| Tumor size
(cm) |
|
| 0.713 |
|
|
|
| ≤5
(n=27) | 1.147 | 0.553–2.378 |
| 1.185 | 0.581–2.417 | 0.641 |
| >5
(n=23) |
|
|
|
|
|
|
Discussion
Epithelial-to-mesenchymal transition (EMT) is a
transient phenomenon but is an important step in cancer cell
activation by many intracellular and extracellular stimuli,
including soluble substances from both cancer cells and cancer
stromal fibroblasts in the cholangiocarcinoma (CCA)
microenvironment (21–23). The aberration of secretomes in
ASMA-positive stromal fibroblasts led to the discovery of periostin
(PN) as a major protein having a tumorigenic impact in intrahepatic
CCA (3), and a variety of EMT
markers have been proposed as indicators for the poor patient
prognosis (24). The present study
confirms the effect of PN on the EMT phenotype in CCA cells and is
consistent with recently published research indicating that this
protein acts in an autocrine manner (11).
The high level of ITGα5β1 in CCA cell lines and its
role as a receptor for PN have been reported in our previous
publication (10). In the present
study, it was revealed that PN treatment of CCA cell lines induced
an EMT pattern of gene expression that included upregulation of
ASMA and VIM, an increase in MMP-9 activity, and downregulation of
CK-19 through the ITGα5β1 and TWIST-2 signaling pathway.
Additionally, the level of TWIST-2 was correlated with patient
survival time, with statistical significance. These findings
highlight the potential impact of inhibiting the
PN/ITGα5β1/TWIST-2-pathway that drives CCA migration, thereby
attenuating the progression of this disease.
The expression pattern of EMT markers in PN-induced
CCA cell lines revealed alterations and translocation of membranous
CDH-1 to the cytoplasm of cells. This hallmark of EMT gene
expression has also been found in TNF-α and HGF-α-treated CCA cells
(21,23). Although CDH1 mRNA in
periostin-treated cells was not significantly changed compared to
the control untreated cells, the protein level was reduced. The
level of protein, the final product, is considered to be more
relevant than the mRNA level. Moreover, there is an evident showing
that PN regulated EMT and EMT transcriptional factor expression by
repressing microRNA-38 expression in lung cancer (25). In the same way, CDH-1 was shown in
breast cancer to be downregulated by miR-9 resulting in increased
cell motility and invasiveness (26). These findings highlight the
potential of PN to regulate gene expression at the
post-transcriptional or translational level which supports the
finding of unaltered mRNA but an altered protein level of CDH-1 in
the study herein. Cells need membranous CDH-1 to provide
intercellular molecular adhesion and attachment to extracellular
matrices (27) and a decrease in
membranous CDH-1 in PN-treated CCA cells facilitates subsequent
cell detachment and migration. The level of CK-19 was also reduced
by PN, as previously reported in cells treated with TGF-β and TNF-α
(22,23). Evidently, the PN-induced EMT
phenomenon in CCA cells displays the characteristic decrease in
CK-19 and cytoplasmic translocation of CDH-1, which are important
steps allowing cells to detach and become ready to move. The
reduction in the CK level is an important characteristic of EMT in
CCA (24) and is associated with
neural invasion, intrahepatic metastasis, and shortened overall
survival time of patients (28).
Hence, the lower CK-19 level in PN-treated cells than that noted in
the untreated controls represents the induction of EMT in
PN-treated CCA cells. To accomplish cell motility, cytoskeletal
proteins must increase their expression. In CCA cells activated by
PN, the expression levels of ASMA and VIM were found
to be increased which is consistent with increased motility through
ASMA-mediated cell-generated mechanical tension, cytoskeleton
remodeling (29) and VIM-regulated
cell adhesion and migration (30).
VIM appears to be a consistent characteristic of EMT in CCA as it
was observed both using TGF-β1 as the positive control EMT inducer
(22), and using PN as presented in
this present study. Interestingly, PN-treated CCA cells that have
undergone EMT in highly confluent cultures were found to exhibit
epithelial cell morphology (cobblestone shape) while spindle-shaped
cells were observed in areas with low confluence, which is
consistent with previous findings (31). Serum MMP-9 has been reported as
being increased in CCA patients (32) and high expression of tissue MMP-9
has been correlated with poor patient prognosis (33). Herein, PN-stimulated CCA cells had
increased levels of MMP-9 and this alteration may help CCA cells to
invade through the degraded extracellular matrix consequently
leading to distant metastases. PN-induced MMP expression was
observed to display a dose-dependent trend in the range of 10–100
ng/ml, for which 1,000 ng/ml was an excessive level, and a feedback
inhibition effect is expected (3).
We previously showed that ITGα5β1 is the major receptor for PN on
the CCA cell membrane (10). The
involvement of ITGα5β1 in the regulation of the PN-activated EMT
pathway was confirmed here by the finding that cells silenced for
ITGα5β1 had both less migration ability induced by PN treatment and
reduced MMP production.
SNAIL-1 has been postulated to control the EMT
phenotype in CCA cells after stimulation with TGF-β1 and TNF-α
(22,23). In a similar way to these previous
findings, the present study showed that PN-treated CCA cells
exhibited increments of SNAIL-1 and TWIST-2 mRNA.
Increased SNAIL-1 and TWIST-2 proteins were also found and the
increase in TWIST-2 was statistically significant. This may imply
an involvement of TWIST-2 in PN-induced EMT in CCA cells,
particularly as cells with suppressed ITGα5β1 had a
decreased capability to activate TWIST-2 and this corresponded to a
decreased ability of the cells to migrate. TWIST-2 has been shown
to control the membrane translocation of CDH-1 through FOXC-2
activation (34) which mediates
several EMT phenotypes including those of detached and motile cells
(35). This function of TWIST-2 was
supported by a finding in cervical cancer cell lines demonstrating
that lower intrinsic expression of cytoplasmic CDH-1 was exhibited
in cells with high TWIST-2 levels (36). In addition, SNAIL-1 was found to
play an important role in CCA activation by PN, probably through
the downregulation of CK-19 and upregulation of ASMA, VIM, and MMPs
(22,23). Herein, it was evident that TWIST-2
may have had a strong impact after activation via the PN/ITGα5β1
pathway in EMT-mediated CCA progression. However, TWIST-2
overexpression should be carried out in ITGα5-knockdown
cells to rescue the cell response to PN to further validate TWIST-2
in regards to the effects of PN through ITGα5β1 on CCA cells.
Increased expression of TWIST-2 has been
associated with metastases in the progression of breast and
cervical cancers, and is also correlated with poor cell
differentiation and a short patient survival rate (36,37).
This is similar to the findings of the present study; those
patients with high levels of TWIST-2 had significantly shorter
survival times compared to those with low TWIST-2. Increases in the
SNAIL-1 level in CCA tissues were previously reported to
significantly correlated with lymph node metastasis and a poor
survival rate in CCA patients (22). This is in contrast to the findings
with extrahepatic CCA, in which SNAIL, SLUG, TWIST, ZEB1, ZEB2 were
not valuable to predict poor outcomes of the patients, whereas VIM,
FN, S100A4, CDH1, and CDH2 were associated with short survival
times (38).
In conclusion, PN enrichment found in the CCA
microenvironment, mainly produced by cancer-associated fibroblasts,
activates CCA cells by binding to ITGα5β1 to trigger an
AKT-dependent signaling pathway (10) that activates TWIST-2 (Fig. 5G). Although the limitation of FOXC2
was not identified, this study identified alterations in
EMT-related genes underlying changes in the phenotype for EMT and
driving CCA progression after the binding of PN to ITGα5β1. The
change in TWIST-2 levels can be suggested as a marker of poor
prognosis that predicts the aggressiveness of CCA. Moreover,
clinically targeting this ITGα5β1-mediated pathway and the
activated signaling molecules and/or associated transcription
factors may help attenuate PN-induced CCA progression.
Supplementary Material
Supporting Data
Acknowledgements
We thank Dr Jan Davies of Mahidol University for
assisting with the English editing of the manuscript.
Funding
This research project was co-supported by MAG Window
II, TRF (MRG-WII525S084) and the Faculty of Medicine of Siriraj
Hospital. JS was supported by the Development and Promotion of
Science and Technology Talents Project (DPST). ST and TK are
research assistants supported by the Research Division, Faculty of
Medicine, Siriraj Hospital, Mahidol University.
Availability of data and material
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS, SS, KU, ST and CT designed the experiments; JS,
SS, ST, TK and KU performed the main research work. AP and SW
prepared the samples in preparation for the experiments and
facilitated the research work. PT and CT discussed and analyzed the
data and results. CT reviewed the paper for research grant
application, prepared figures, wrote, and improved the scientific
quality of the manuscript, and finally submitted the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Fifty cases of CCA tissues were obtained from
patients who had undergone hepatectomy using the protocol approved
by the Human Research Ethics Committee, Khon Kaen University
(HE490143). Each patient was informed and written informed consent
was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
Authors' information
JS and SS were enrolled in the Graduate Program in
Immunology, Department of Immunology, Faculty of Medicine, Siriraj
Hospital, Mahidol University. ST and TK are research assistants
under the Research Unit, Faculty of Medicine, Siriraj Hospital,
Mahidol University.
Glossary
Abbreviations
Abbreviations:
|
CCA
|
cholangiocarcinoma
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
PN
|
periostin
|
|
ITG
|
integrin
|
|
ASMA
|
α-smooth muscle actin
|
|
VIM
|
vimentin
|
|
MMP
|
matrix metalloproteinase
|
|
CDH-1
|
E-cadherin
|
|
CDH-2
|
N-cadherin
|
|
FN-1
|
fibronectin
|
|
CK
|
cytokeratin
|
|
TGF-β
|
transforming growth factor-β
|
|
TNF-α
|
tumor necrosis factor-α
|
|
HNPCC
|
head and neck squamous cell cancer
|
|
FBS
|
fetal bovine serum
|
|
HGF-α
|
hepatocyte growth factor α
|
References
|
1
|
Chuaysri C, Thuwajit P, Paupairoj A,
Chau-In S, Suthiphongchai T and Thuwajit C: Alpha-smooth muscle
actin-positive fibroblasts promote biliary cell proliferation and
correlate with poor survival in cholangiocarcinoma. Oncol Rep.
21:957–969. 2009.PubMed/NCBI
|
|
2
|
Nakagohri T, Kinoshita T, Konishi M,
Takahashi S and Gotohda N: Surgical outcome and prognostic factors
in intrahepatic cholangiocarcinoma. World J Surg. 32:2675–2680.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Utispan K, Thuwajit P, Abiko Y, Charngkaew
K, Paupairoj A, Chau-in S and Thuwajit C: Gene expression profiling
of cholangiocarcinoma-derived fibroblast reveals alterations
related to tumor progression and indicates periostin as a poor
prognostic marker. Mol Cancer. 9:132010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gillan L, Matei D, Fishman DA, Gerbin CS,
Karlan BY and Chang DD: Periostin secreted by epithelial ovarian
carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5)
integrins and promotes cell motility. Cancer Res. 62:5358–5364.
2002.PubMed/NCBI
|
|
5
|
Puglisi F, Puppin C, Pegolo E, Andreetta
C, Pascoletti G, D'Aurizio F, Pandolfi M, Fasola G, Piga A, Damante
G and Di Loreto C: Expression of periostin in human breast cancer.
J Clin Pathol. 61:494–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu
M, Shao R, Anderson RM, Rich JN and Wang XF: Periostin potently
promotes metastatic growth of colon cancer by augmenting cell
survival via the Akt/PKB pathway. Cancer Cell. 5:329–339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudo Y, Ogawa I, Kitajima S, Kitagawa M,
Kawai H, Gaffney PM, Miyauchi M and Takata T: Periostin promotes
invasion and anchorage-independent growth in the metastatic process
of head and neck cancer. Cancer Res. 66:6928–6935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baril P, Gangeswaran R, Mahon PC, Caulee
K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T and
Lemoine NR: Periostin promotes invasiveness and resistance of
pancreatic cancer cells to hypoxia-induced cell death: Role of the
beta4 integrin and the PI3k pathway. Oncogene. 26:2082–2094. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ben QW, Jin XL, Liu J, Cai X, Yuan F and
Yuan YZ: Periostin, a matrix specific protein, is associated with
proliferation and invasion of pancreatic cancer. Oncol Rep.
25:709–716. 2011.PubMed/NCBI
|
|
10
|
Utispan K, Sonongbua J, Thuwajit P,
Chau-In S, Pairojkul C, Wongkham S and Thuwajit C: Periostin
activates integrin α5β1 through a PI3K/AKT-dependent pathway in
invasion of cholangiocarcinoma. Int J Oncol. 41:1110–1118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mino M, Kanno K, Okimoto K, Sugiyama A,
Kishikawa N, Kobayashi T, Ono J, Izuhara K, Kobayashi T, Ohigashi
T, et al: Periostin promotes malignant potential by induction of
epithelial-mesenchymal transition in intrahepatic
cholangiocarcinoma. Hepatol Commun. 1:1099–1109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cervantes-Arias A, Pang LY and Argyle DJ:
Epithelial-mesenchymal transition as a fundamental mechanism
underlying the cancer phenotype. Vet Comp Oncol. 11:169–184. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adhes Migr. 9:317–324. 2015. View Article : Google Scholar
|
|
14
|
Son H and Moon A: Epithelial-mesenchymal
transition and cell invasion. Toxicol Res. 26:245–252. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song L, Wang P, Tian Y, Chang D, Li K, Fan
Y, Shen J, Du H, Mi R, Bian X and Tang X: Lung metastasis of
pancreatic carcinoma is regulated by TGFβ signaling. Tumour Biol.
36:2271–2276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yue B, Ren QX, Su T, Wang LN and Zhang L:
ERK5 silencing inhibits invasion of human osteosarcoma cell via
modulating the Slug/MMP-9 pathway. Eur Rev Med Pharmacol Sci.
18:2640–2647. 2014.PubMed/NCBI
|
|
18
|
Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu
L, Tang X, Du YE, Hu P and Liu M: Twist induces
epithelial-mesenchymal transition and cell motility in breast
cancer via ITGB1-FAK/ILK signaling axis and its associated
downstream network. Int J Biochem Cell Biol. 71:62–71. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li ZH, Zhou Y, Ding YX, Guo QL and Zhao L:
Roles of integrin in tumor development and the target inhibitors.
Chin J Nat Med. 17:241–251. 2019.PubMed/NCBI
|
|
21
|
Menakongka A and Suthiphongchai T:
Involvement of PI3K and ERK1/2 pathways in hepatocyte growth
factor-induced cholangiocarcinoma cell invasion. World J
Gastroenterol. 16:713–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sato Y, Harada K, Itatsu K, Ikeda H,
Kakuda Y, Shimomura S, Shan Ren X, Yoneda N, Sasaki M and Nakanuma
Y: Epithelial-mesenchymal transition induced by transforming growth
factor-{beta}1/Snail activation aggravates invasive growth of
cholangiocarcinoma. Am J Pathol. 177:141–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Techasen A, Namwat N, Loilome W,
Bungkanjana P, Khuntikeo N, Puapairoj A, Jearanaikoon P, Saya H and
Yongvanit P: Tumor necrosis factor-α (TNF-α) stimulates the
epithelial-mesenchymal transition regulator Snail in
cholangiocarcinoma. Med Oncol. 29:3083–3091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vaquero J, Guedj N, Clapéron A, Nguyen
Ho-Bouldoires TH, Paradis V and Fouassier L: Epithelial-mesenchymal
transition in cholangiocarcinoma: From clinical evidence to
regulatory networks. J Hepatol. 66:424–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu WW, Chen PC, Chen JM, Wu YM, Liu PY, Lu
CH, Lin YF, Tang CH and Chao CC: Periostin promotes
epithelial-mesenchymal transition via the MAPK/miR-381 axis in lung
cancer. Oncotarget. 8:62248–62260. 2017.PubMed/NCBI
|
|
26
|
Ma L, Young J, Prabhala H, Pan E, Mestdagh
P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S,
et al: miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin
and cancer metastasis. Nat Cell Biol. 12:247–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ryu HS, Chung JH, Lee K, Shin E, Jing J,
Choe G, Kim H, Xu X, Lee HE, Kim DG, et al: Overexpression of
epithelial-mesenchymal transition-related markers according to cell
dedifferentiation: Clinical implications as an independent
predictor of poor prognosis in cholangiocarcinoma. Hum Pathol.
43:2360–2370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Zohar R and McCulloch CA: Multiple
roles of alpha-smooth muscle actin in mechanotransduction. Exp Cell
Res. 312:205–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ivaska J, Pallari HM, Nevo J and Eriksson
JE: Novel functions of vimentin in cell adhesion, migration, and
signaling. Exp Cell Res. 313:2050–2062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leggett SE, Sim JY, Rubins JE, Neronha ZJ,
Williams EK and Wong IY: Morphological single cell profiling of the
epithelial-mesenchymal transition. Integr Biol. 8:1133–1144. 2016.
View Article : Google Scholar
|
|
32
|
İnce AT, Yıldız K, Gangarapu V, Kayar Y,
Baysal B, Karatepe O, Kemik AS and Şentürk H: Serum and biliary
MMP-9 and TIMP-1 concentrations in the diagnosis of
cholangiocarcinoma. Int J Clin Exp Med. 8:2734–2740.
2015.PubMed/NCBI
|
|
33
|
Sun Q, Zhao C, Xia L, He Z, Lu Z, Liu C,
Jia M, Wang J and Niu J: High expression of matrix
metalloproteinase-9 indicates poor prognosis in human hilar
cholangiocarcinoma. Int J Clin Exp Pathol. 7:6157–6164.
2014.PubMed/NCBI
|
|
34
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watanabe A, Suzuki H, Yokobori T, Altan B,
Kubo N, Araki K, Wada S, Mochida Y, Sasaki S, Kashiwabara K, et al:
Forkhead box protein C2 contributes to invasion and metastasis of
extrahepatic cholangiocarcinoma, resulting in a poor prognosis.
Cancer Sci. 104:1427–1432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Wang W, Wang W, Yang R, Wang T, Su
T, Weng D, Tao T, Li W, Ma D and Wang S: Correlation of TWIST2
up-regulation and epithelial-mesenchymal transition during
tumorigenesis and progression of cervical carcinoma. Gynecol Oncol.
124:112–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mao Y, Zhang N, Xu J, Ding Z, Zong R and
Liu Z: Significance of heterogeneous Twist2 expression in human
breast cancers. PLoS One. 7:e481782012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nitta T, Mitsuhashi T, Hatanaka Y,
Miyamoto M, Oba K, Tsuchikawa T, Suzuki Y, Hatanaka KC, Hirano S
and Matsuno Y: Prognostic significance of epithelial-mesenchymal
transition-related markers in extrahepatic cholangiocarcinoma:
Comprehensive immunohistochemical study using a tissue microarray.
Br J Cancer. 111:1363–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|