Introduction
Pancreatic cancer (PC) is one of the deadliest human
cancer types, ranking as the seventh leading cause of
cancer-related deaths worldwide (1,2). The
5-year survival rate of PC patients remains less than 8%, despite
the fact that surgical and adjuvant treatment approaches have
improved greatly during recent years (3,4).
Identification of the key mechanisms involved in the development
and progression of PC is still urgently needed.
Mitochondria are highly dynamic organelles that
constantly change their morphology by fission and fusion to adapt
to the cellular environment (5,6).
Several recent studies have revealed close links between unbalanced
mitochondrial dynamics and cancers (7–9). In
addition, deregulated expression of mitochondrial fission and
fusion proteins such as dynamin-related protein 1 (DRP1), mitofusin
1 (MFN1) and mitofusin 2 (MFN2) have also been observed in human
cancer of the liver (10,11), lung (12), thyroid (13), and breast (14). MiD49 (mitochondrial dynamics protein
of 49 kDa) is a newly identified mitochondrial anchored protein,
which is involved in the regulation of mitochondrial fission
(15–17). In the present study, we conducted
the first study to elucidate the expression pattern and biological
functions of MiD49 in PC.
Materials and methods
PC cell lines and tissue samples
Six human PC cell lines (BxPC-3, SW1990, PANC-1,
PaCa-2, AsPC-1 and HPAC) and one normal pancreatic cell line
hTERT-HPNE were obtained from The American Type Culture Collection
(ATCC), and cultured in Dulbecco's modified Eagle's medium (DMEM)
(Hyclone Laboratories, Inc.) supplemented with 10% fetal bovine
serum (FBS) (Hyclone Laboratories, Inc.). All cells were cultured
in a humidified atmosphere at 37°C with 5% CO2.
In addition, tumor and adjacent normal pancreatic
tissues were obtained from 20 PC patients (13 males and 7 females)
between March 2016 and July 2017 from the Department of
Hepato-Biliary Surgery, The First Affiliated Hospital of Xi'an
Jiaotong University (Xi'an, China). The median age was 73 years
(ranging from 63 to 85). Written informed consent was obtained from
the patients for their tissues to be used for biomedical research.
The specimens were stored at −80°C prior to quantitative real-time
PCR (qPCR) and western blot analysis. All experimental protocols
were approved by the Ethics Committee of The First Affiliated
Hospital of Xi'an Jiaotong University and carried out in accordance
with the Declaration of Helsinki.
Oncomine data analysis
The Oncomine database (https://www.oncomine.org) (18), which compiles a large amount of
previously published microarray data, was employed to compare the
mRNA expression levels of MiD49 in tumor and normal tissue of
pancreatic cancer.
Knockdown or forced expression of
target genes in PC cells
PC cells were transfected with the MiD49 expression
vector or siRNAs by using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions. In
addition, synthetic miR-424 precursor (pre-miR-424; Ambion; Thermo
Fisher Scientific, Inc.) was used to study the function of miR-424.
The MiD49 RNA interference sequences were
5′-ACACCTAAGTTCAGCACTATAGCAC-3′ (siMiD49#1) and
5′-GCCATGCCTTGAAGATGTGAATAAA-3′ (siMiD49#2). A scramble control
siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) was also used. A pSilencer™
3.1-H1 puro vector (Ambion; Thermo Fisher Scientific, Inc.) was
used for generation of shRNA expression vectors targeting MiD49.
For MiD49 overexpression, the coding sequence of MiD49 was
amplified from cDNA derived from BxPC-3 cells and subsequently
cloned into a pcDNA™ 3.1 (+) vector (Invitrogen; Thermo Fisher
Scientific, Inc., V790-20).
Quantitative real-time PCR (qPCR)
analysis
Total RNA from PC tissues or cell lines was
extracted by using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Then, a PrimeScript RT Reagent Kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for the reverse transcription of mRNA
into cDNA. Finally, qPCR was carried out using a SYBR Premix Ex
Taq™ (Takara, Japan) on a Corbett 6200. The primer sequences used
in the study are listed in Table
I.
 | Table I.Primers used in qPCR analysis. |
Table I.
Primers used in qPCR analysis.
| Gene | Forward primer | Reverse primer |
|---|
| MiD49 |
CAGAAACGGGGGAAGCGG |
CACCAGGAGACGCACATGG |
| miR-424 |
GGCTAGTCAGCAGCAATTCATGT |
GTGCAGGGTCCGAGGT-3 |
|
E-cadherin |
ATTTTTCCCTCGACACCCGAT |
TCCCAGGCGTAGACCAAGA |
|
N-cadherin |
TCAGGCGTCTGTAGAGGCTT |
ATGCACATCCTTCGATAAGACTG |
|
Vimentin |
CCTGAACCTGAGGGAAACTAA |
GCAGAAAGGCACTTGAAAGC |
| ZO-1 |
CACGCAGTTACGAGCAAG |
TGAAGGTATCAGCGGAGG |
| U6 |
GCTTCGGCAGCACATATACTAAAAT |
CGCTTCACGAATTTGCGTGTCAT |
| GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
Western blot analysis
RIPA buffer was used for the extraction of whole
protein from both PC cell lines and tissue samples. A total of 20
µg extracted proteins were loaded per lane and separated by 10%
SDS-polyacrylamide gels and transferred onto the polyvinylidene
fluoride (PVDF) membrane. After blocked with 5% BSA (SABC,
Shanghai, China) for 1 h at room temperature, the membranes were
immunostained with the primary antibody overnight at 4°C and the
secondary antibody for 1 h at room temperature. Equal amount of
protein sample loading was monitored using an anti-β-actin
antibody. Blots were detected by an enhanced chemiluminescence
system (Thermo Fisher Scientific, Inc.) and images were captured
with a Molecular Imager VersaDoc™ MP 5000 system (Bio-Rad
Laboratories). The primary antibodies used and their dilutions are
listed in Table II.
 | Table II.Primary antibodies used in the
western blot and IHC analyses. |
Table II.
Primary antibodies used in the
western blot and IHC analyses.
| Antibody | Company (cat.
no.) | Working
concentration dilutions |
|---|
| MiD49 | Proteintech
(16413-1-AP) | WB: 1/8 IHC:
1/150 |
| Caspase-9 | Proteintech
(66169-1-Ig) | WB: 1/800 |
| Caspase-3 | Proteintech
(25546-1-AP) | WB: 1/800 |
| Cytochrome
c | Proteintech
(10993-1-AP) | WB: 1/1,000 |
| COX IV | Abgent
(#AP9153a) | WB: 1/1,000 |
| E-cadherin | Proteintech
(20874-1-AP) | WB: 1/1,000 |
| N-cadherin | Cell Signaling
(13116) | WB: 1/1,000 |
| Vimentin | Proteintech
(10366-1-AP) | WB: 1/1,000 |
| ZO-1 | Proteintech
(21773-1-AP) | WB: 1/1,000 |
| Ki-67 | MAB (MAB-0129) | IHC:1/200 |
| β-actin | Beijing TDY
(TDY051F) | WB: 1/3,000 |
Immunohistochemical analysis
An IHC detection kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for immunohistochemical (IHC) analysis.
Sections (4 µm) from paraffin-embedded tissues were deparaffinized
and rehydrated and then treated with boiling sodium citrate buffer
(pH 6.0) under pressure to unmask epitopes. Samples were blocked
with 5% goat serum and then incubated with primary antibodies,
including MiD49 and Ki-67 overnight at 4°C. The color was developed
using DAB and counterstained with hematoxylin. Images were captured
using a a light microscope (Olympus Corp.) at ×400 magnification.
Semi-quantitative IHC staining scores were evaluated on a scale of
1 to 12 according to both the intensity and percentage of positive
staining. Briefly, the staining intensities were graded on a scale
of 0–3 as follows: 0 for no staining, 1 for weak staining, 2 for
moderate staining, and 3 for strong staining. The percentages of
the stained area were graded on a scale of 0–4 as follows: 0 is 0%,
1 (0–25%), 2 (25–50%), 3 (51–75%), and 4 (75–100%). The intensity
grade multiplied by the percentage grade was calculated to equal
the final IHC staining score.
MTS cell proliferation assay
Cell proliferation ability was determined by the MTS
assay (Promega, G3581) according to the manufacturer's protocol.
Briefly, 2,000 PC cells were seeded onto 96-well plates and cell
viability was determined by the absorbance at 490 nm.
Colony formation assay
Two hundred PC cells were seeded into 6-well plates
and cultured for 14 days. After that, the colonies were fixed with
4% paraformaldehyde and stained with crystal violet for 20 min at
28°C. Images were captured using a microscope with a light
microscope (Olympus Corp.) at ×400 magnification. Finally, the
number of colonies in each well was counted.
EdU (5-ethynyl-2′-deoxyuridine)
incorporation assay
For EdU staining, 50 mM EdU in culture media was
added to PC cells and incubated for 2 h at 37°C. Cells were then
fixed with 4% paraformaldehyde for 20 min and treated with 0.5%
Triton X-100 for 30 min. Afterwards, cells were incubated with DAPI
at 28°C for 20 min, followed by observation under an Olympus
confocal microscope (Olympus Corp.).
Flow cytometric analysis for cell
apoptosis and cell cycle distribution
An Annexin V and PI apoptosis kit (F-6026, US
Everbright Inc.) was used for cell apoptosis analysis according to
the manufacturer's instructions. Briefly, 5 µl Annexin 5-FITC and 2
µl propidium iodide (PI) solutions were added to PC cells and
incubated on ice for 15 min. Data were analyzed with a flow
cytometer (Beckman Coulter).
A cell cycle kit from Everbright Inc. (C6031) was
used for analysis of cell cycle distribution according to the
manufacturer's instructions. Briefly, PC cells were fixed overnight
in 80% cold ethanol at −20°C, followed by staining with 0.5 ml PI
for 20 min at 4°C. Finally, data were analyzed with a flow
cytometer (Beckman Coulter).
Scratch wound healing and Matrigel
invasion assays
Cell migration ability was assessed by the scratch
wound healing assay. Briefly, PC cells (2×106) were
seeded into a 6-well plate. A single vertical scratch was made in
the middle of the wells with a plastic pipette tip when cells had
grown to 90% confluence. Finally, the PC cells that had migrated
into the wounded area were photographed under a light microscope
(Olympus Corp.) at ×400 magnification.
For the Transwell Matrigel invasion assay,
1×105 PC cells were seeded into the top of a Transwell
chamber containing a Matrigel-coated membrane and cultured for 48
h. Penetrated PC cells were stained with crystal violet for 20 min
at 28°C and counted under a light microscope (Olympus Corp.) at
×400 magnification.
In vivo tumor growth and metastasis
assays
A total of 24 mice (12 for tumor growth and 12 for
tumor metastasis) with body weight ranging from 17 to 21 g were
used in our study. The nude mice were purchased from the Kunming
Institute of Zoology, Chinese Academy of Sciences (Kunming, Yunnan,
China) and group-housed (3 mice per cage) in plastic shoebox cages
with autoclaved bedding and filtered air with access to sterilized
food and water with a 12-h light/dark cycle at 28°C. Animal health
and behavior were monitored daily. For in vivo tumor growth
assay, 1×107 PC cells were subcutaneously injected into
the right flanks of 6-week-old male BALB/c nude mice (one tumor per
mice, six mice per group). Tumor volume (V) was calculated
according to the formula: V=0.52 × L × W2 (L, length; W,
width) every week for 5 weeks. At the end of the experiment, the
mice were euthanized with CO2 rodent euthanasia chamber
with a flow rate of CO2 not more than 30% of the chamber
volume/min, according to the University of Minnesota guidelines.
The death was verified by the absence of respiratory movement and
heart beat. For in vivo metastasis assay, 1×106
PC cells were injected intravenously into the mice. The mice were
euthanized on day 28 after injection and their lungs were removed.
The metastatic nodes in the lung were counted. Images were captured
and photographed under a light microscope (Olympus Corp.) at ×400
magnification. All animal experiments were performed in accordance
with the relevant guidelines and regulations of the Animal Ethics
Committee of the First Affiliated Hospital of Xian Jiaotong
University.
Mitochondrial morphology analysis
MitoTracker Green staining was used for
mitochondrial morphology analysis. Briefly, PC cells with different
MiD49 levels were seeded into a confocal dish and cultured
overnight. Then, cells were fixed in 4% paraformaldehyde for 20 min
and incubated with MitoTracker green probe (Molecular Probes,
M7514) for 1.5 h. An Olympus confocal microscope (Olympus) was used
for the photography of immunofluorescence images. Mitochondrial
length was measured with an ImageJ software (National Institutes of
Health).
Detection of reactive oxygen species
(ROS) levels
Cellular ROS levels were detected by the fluorescent
probe DCFH-DA (Beyotime Institute of Biotechnology) following to
the manufacturer's instructions. Briefly, a final concentration of
10 mM of DCFH-DA in serum-free medium was added to PC cells in each
group. Cell suspensions in each group were incubated with DCFH-DA
at 37°C for 30 min. Finally, the fluorescence was assessed by flow
cytometry.
Statistical analysis
Experiments were performed independently at least
three times and the results are expressed as mean ± SEM. Paired or
unpaired student's t-test was used for comparisons between two
groups. One-way analysis of variance (ANOVA) followed by Dunnett's
t-test was used for comparisons between multiple groups.
Correlations between measured variables were tested by Spearman's
rank correlation analysis. The SPSS 17.0 software program (SPSS,
Inc.) was used for statistical analysis and a P-value <0.05 was
considered statistically significant.
Results
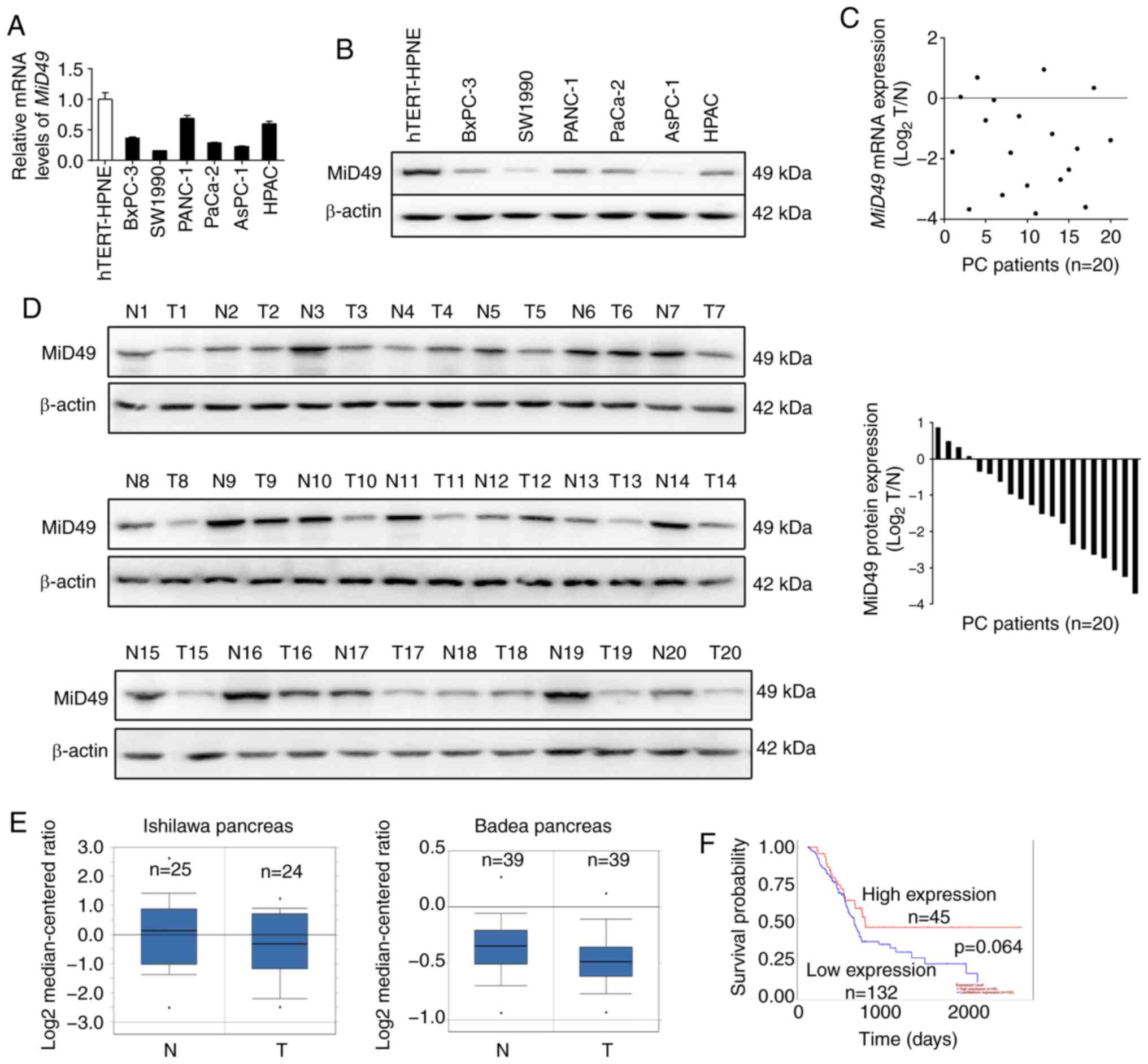
MiD49 is downregulated in pancreatic
cancer cell lines and tissues
We firstly determined the expression of MiD49 in six
pancreatic cancer (PC) cell lines and one immortalized normal
pancreatic cell line hTERT-HPNE by quantitative real-time PCR
(qPCR) and western blot analyses. As shown in Fig. 1A and B, MiD49 expression was
significantly lower in all of the six PC cell lines when compared
with that noted in the nonmalignant hTERT-HPNE cells, especially in
the SW1990 and AsPC-1 cell lines with high metastatic potential. To
determine whether MiD49 is also downregulated in tumor tissues of
human pancreatic cancer, MiD49 expression was determined in
20-paired pancreatic tumor and adjacent non-tumor tissues by qPCR
and western blot analyses. In concordance with the results from the
PC cell lines, MiD49 expression was significantly decreased in the
pancreatic tumor tissues than that found in the adjacent non-tumor
tissues (Fig. 1C and D). In
addition, two datasets (19,20)
from the Oncomine platform consistently indicated that the level of
MiD49 was significantly lower in tumor tissues of PC than in their
normal counterparts (Fig. 1E).
Moreover, prognostic significance analysis using the online web
portal UALCAN (21) (http://ualcan.path.uab.edu) indicated that PC patients
with a low expression level of MiD49 had clearly poorer overall
survival than those with high MiD49 (Fig. 1F), although the difference was not
significant (P=0.064). These findings indicate that MiD49 is
significantly downregulated in PC, indicating a potential
tumor-suppressive role in PC.
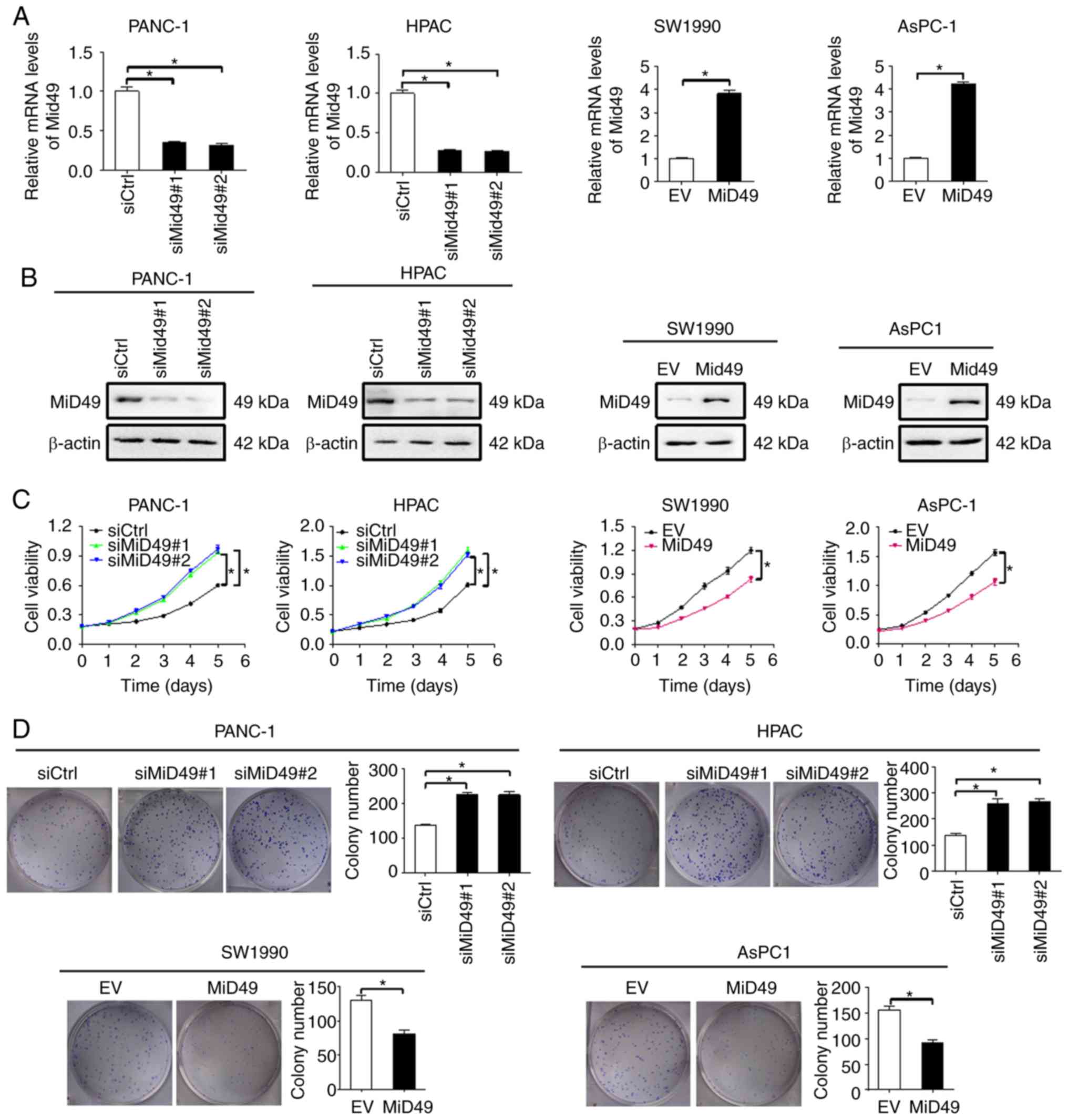
MiD49 suppresses PC growth by inducing
G1-S phase cell cycle arrest and cell apoptosis
To investigate the functional roles of MiD49 in PC
cells, gain- and loss-of-function studies were applied in human PC
cell lines. Efficient knockdown of MiD49 in PANC-1 and HPAC cells
and overexpression of MiD49 in SW1990 and AsPC-1 cells was
confirmed and is shown in Fig. 2A and
B. MTS cell viability and colony formation assays indicated
that downregulation of MiD49 significantly promoted PC cell growth
in the PANC-1 and HPAC cells, whereas forced expression of MiD49
suppressed PC cell growth in the SW1990 and AsPC-1 cell lines
(Fig. 2C and D).
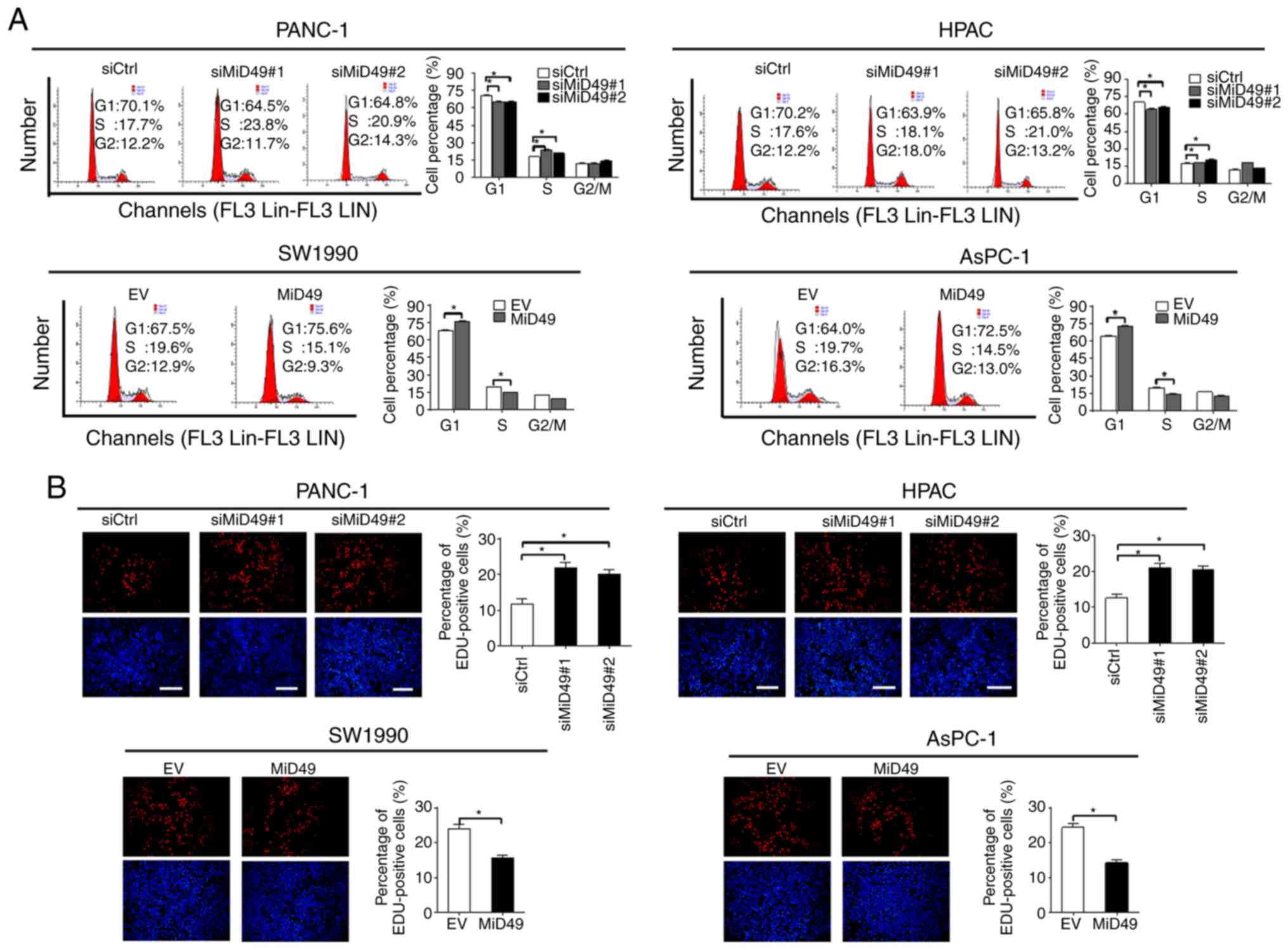
Increased cell growth could be caused by decreased
apoptosis or accelerated cell cycle progression, or both. To
determine the underlying mechanism by which MiD49 suppresses PC
cell growth, the effects of MiD49 on apoptosis and cell cycle
distribution were analyzed. Knockdown of MiD49 significantly
decreased the number of cells in the G1 phase but increased the
number of cells in the S phase in PANC-1 and HPAC cells.
Conversely, forced expression of MiD49 arrested the cell cycle at
the G1-S transition in SW1990 and AsPC-1 cells (Fig. 3A). Consistently, significantly more
proliferating cells were detected by the EdU (5-ethynyl-2′-
deoxyuridine) incorporation assay in PANC-1 and HPAC cells when
MiD49 was knocked down, whereas fewer proliferating cells were
observed in the SW1990 and AsPC-1 cells when MiD49 was
overexpressed (Fig. 3B). Cell
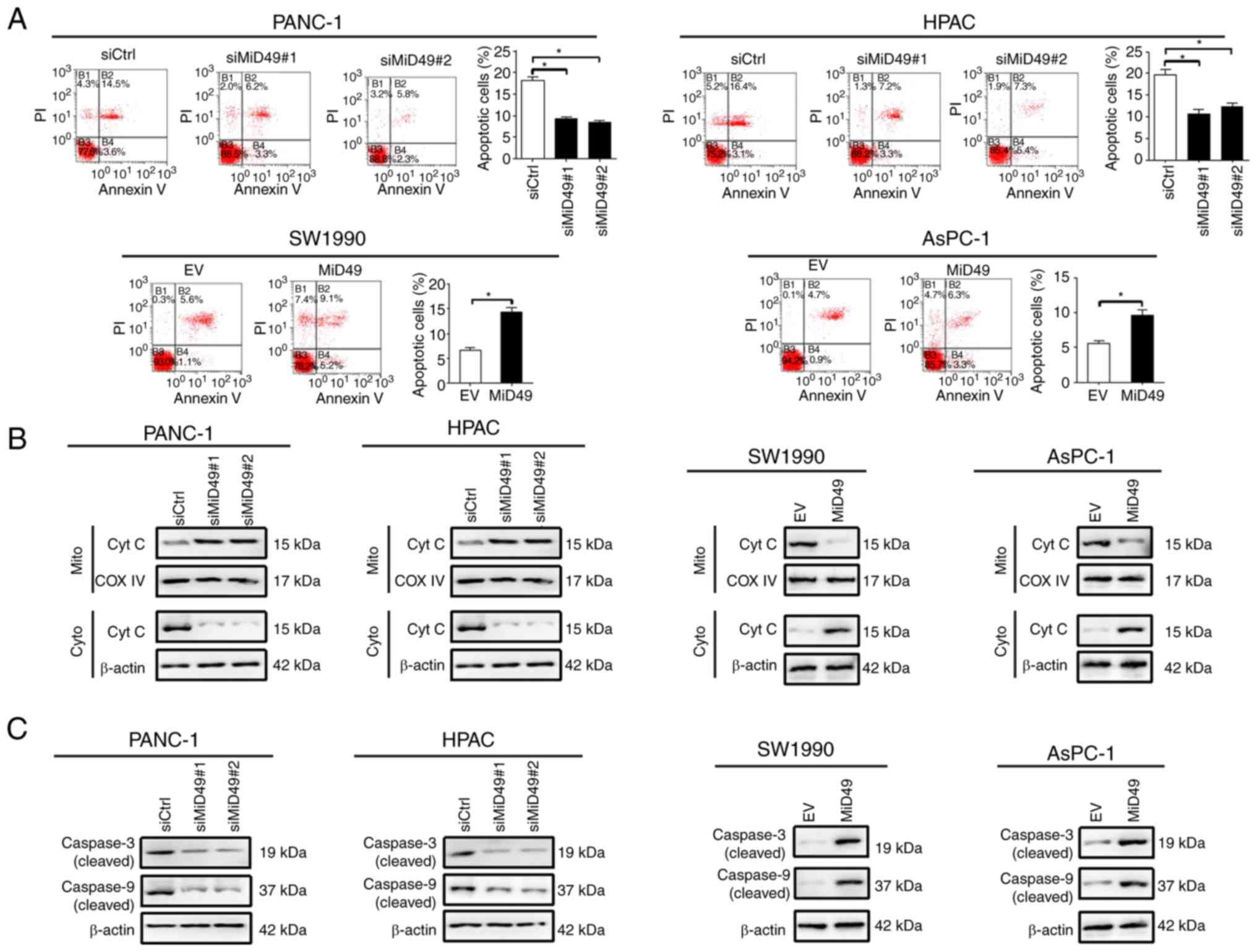
apoptosis analysis by flow cytometry revealed that knockdown of
MiD49 significantly suppressed PC cell apoptosis, whereas forced
expression of MiD49 exhibited an opposite effect (Fig. 4A). Consistently, knockdown of MiD49
suppressed the release of cytochrome c and the cleavage of
caspase 3 and caspase 9, whereas forced expression of MiD49 had the
opposite effect (Fig. 4B and C).
Collectively, these results indicated that MiD49 suppresses the
growth of PC cells by inducing G1-S cell cycle arrest and cell
apoptosis.
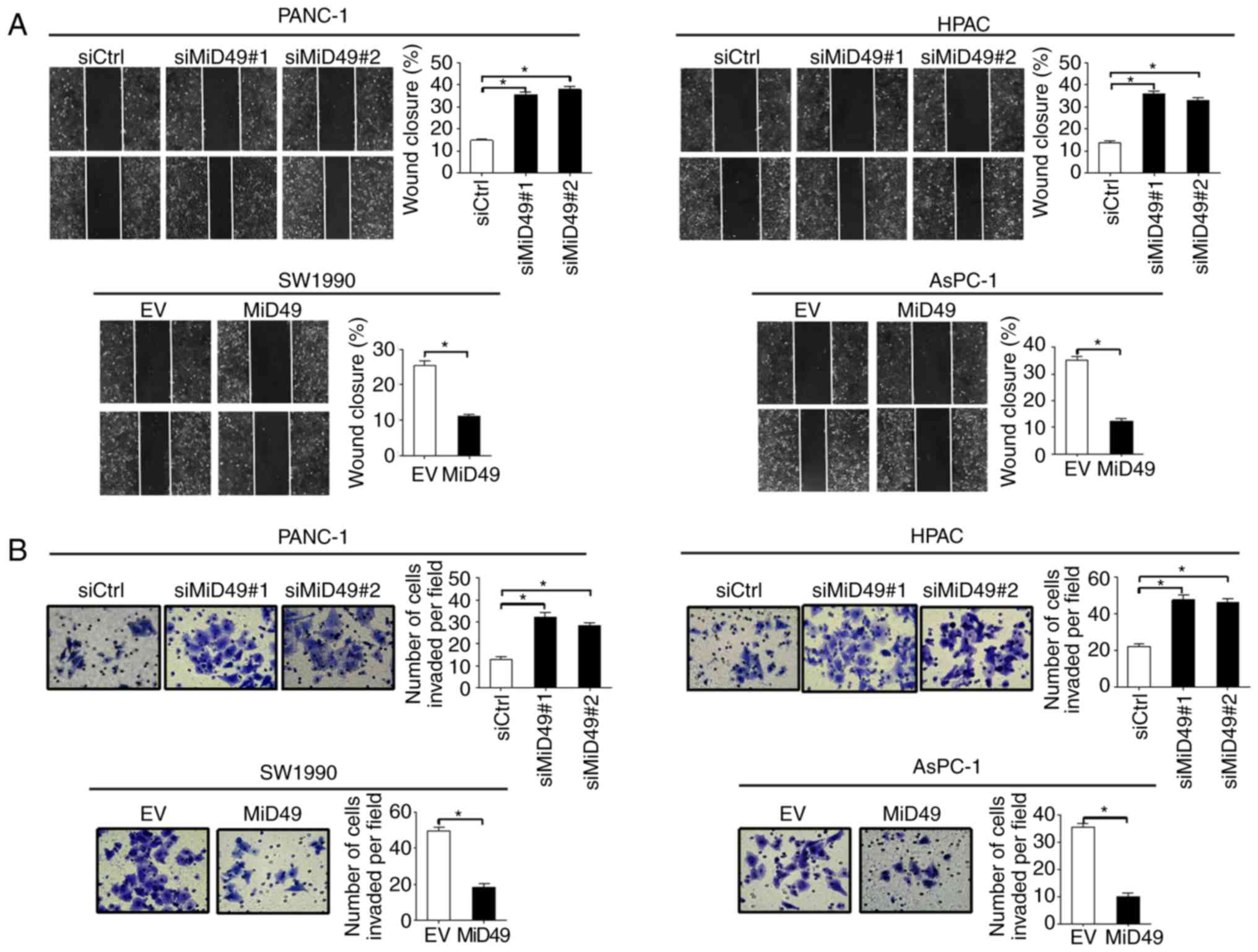
MiD49 suppresses the migration and
invasion of PC cells through inhibition of epithelial-mesenchymal
transition (EMT)
To further evaluate the role of MiD49 in the
metastasis of PC cells, scratch wound healing and Transwell
invasion assays were performed. Downregulation of MiD49
significantly increased the wound closure of PANC-1 and HPAC cells,
whereas overexpression of MiD49 significantly decreased the
migration abilities of the SW1990 and AsPC-1 cells (Fig. 5A). Similarly, the invasion potential
was also significantly increased when MiD49 was knocked down, while
the invasion potential was significantly decreased upon MiD49 was
overexpressed (Fig. 5B).
Epithelial-mesenchymal transition (EMT) is one of
the major mechanisms underlying tumor metastasis (22). To determine the underlying mechanism
by which MiD49 suppresses PC cell metastasis, the expression levels
of key EMT markers were examined in PC cells with different MiD49
level. As shown in Fig. 6A and B,
knockdown of MiD49 significantly increased the expression levels of
mesenchymal markers, N-cadherin and vimentin, and significantly
reduced the expression levels of epithelial markers E-cadherin and
ZO-1, indicating an induction of EMT. In contrast, forced
expression of MiD49 inhibited the EMT of PC cells.
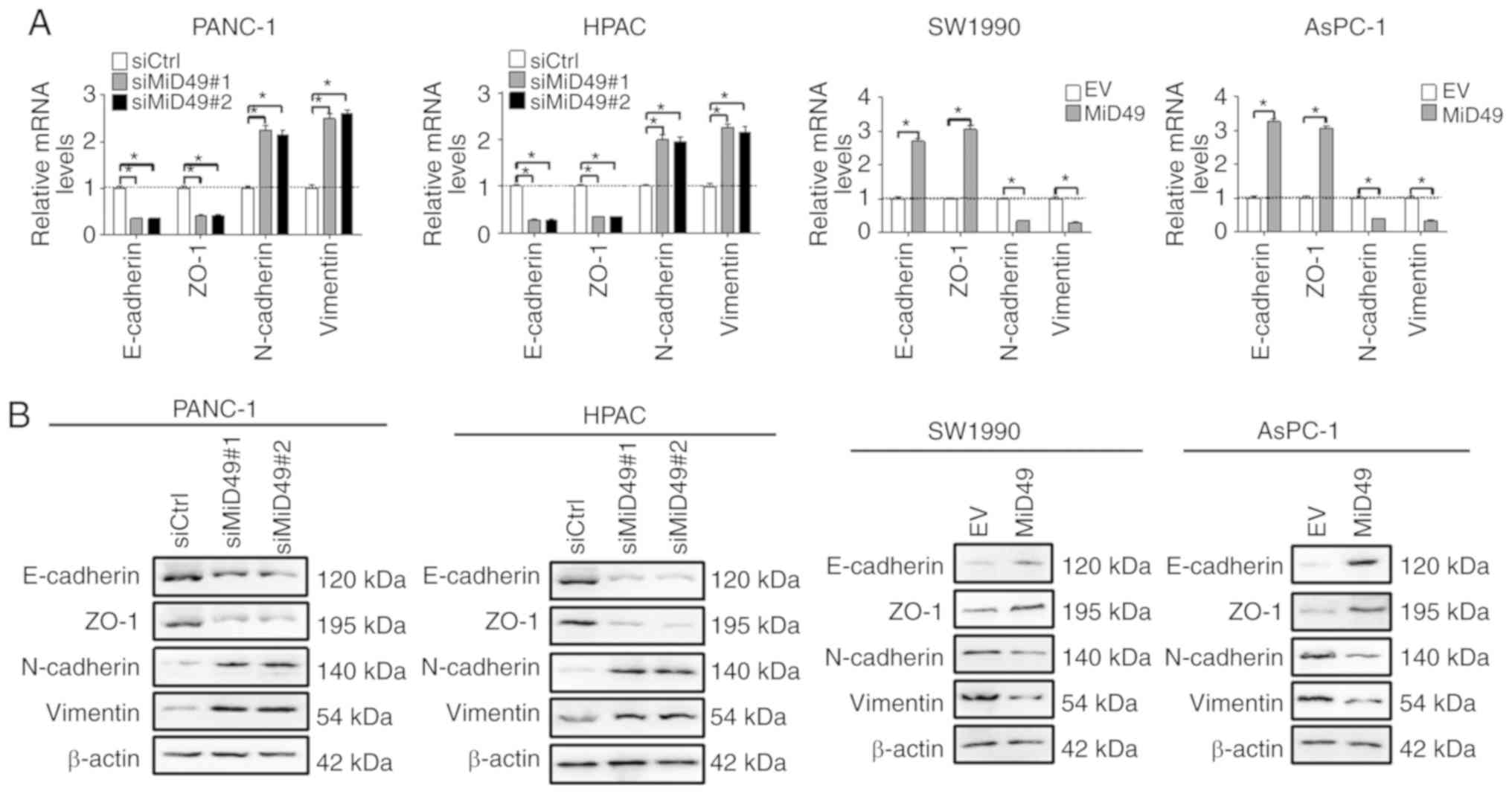 | Figure 6.MiD49 suppresses the migration and
invasion of pancreatic cancer (PC) cells through inhibition of
epithelial-mesenchymal transition. (A) The expressions of EMT
markers E-cadherin, ZO-1, N-cadherin, and vimentin were determined
by qPCR analysis in PC cells with different levels of MiD49 as
indicated. (B) The expression of EMT markers E-cadherin, ZO-1,
N-cadherin, and vimentin were determined by western blot analysis
in PC cells with different levels of MiD49 as indicated. MiD49,
mitochondrial dynamics protein of 49 kDa; siMiD49, siRNA against
MiD49; siCtrl, control siRNA; MiD49, expression vector encoding
MiD49; EV, empty vector. *P<0.05. |
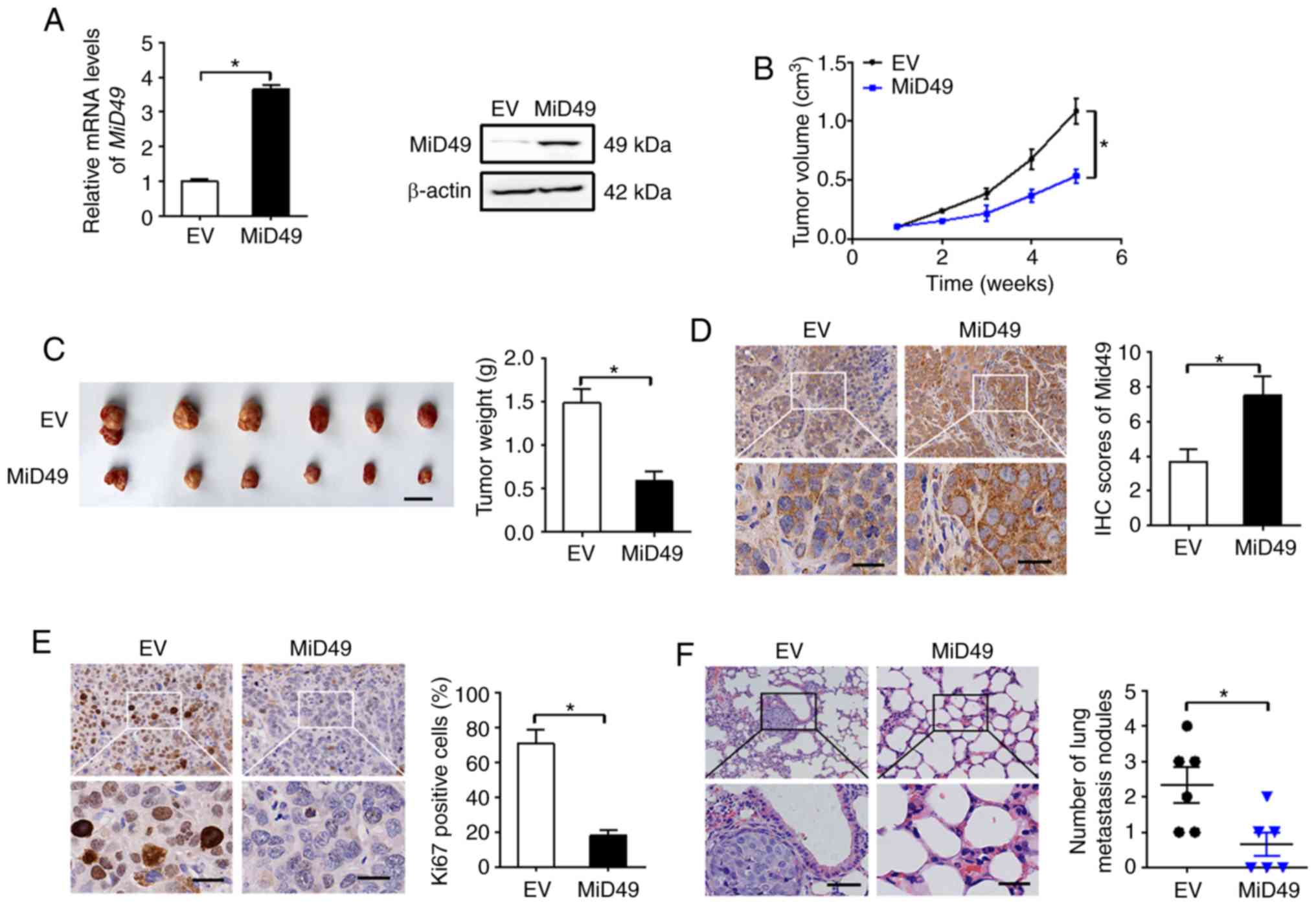
MiD49 suppresses the growth and
metastasis of PC cells in vivo
To study the role of MiD49 in PC growth in
vivo, SW1990 cells overexpressing MiD49 (Fig. 7A) were injected into the right
flanks of nude mice. As shown in Fig.
7B, tumors developed from SW1990 cells with stable MiD49
overexpression grew much slower than those developed from control
cells. Consistently, markedly reduced tumor volume (length, width
and volume of the largest tumor was 2.82 cm, 0.95 cm and 1.32
cm3, respectively) and wet weight were also observed in
the MiD49 overexpression group (Fig.
7C). Immunohistochemical (IHC) analysis showed significantly
higher expression of MiD49 in tumors developed from
MiD49-overexpressing SW1990 cells than in the controls (Fig. 7D), implying that the tumor growth
inhibitory effects were exerted by forced expression of MiD49.
Similar to the in vitro experiments, significantly fewer
proliferating cells were observed in the MiD49-overexpressing
xenografts, as indicated by Ki-67 staining assay (Fig. 7E). Moreover, forced expression of
MiD49 in the SW1990 cells significantly suppressed lung metastasis
compared to the control cells.
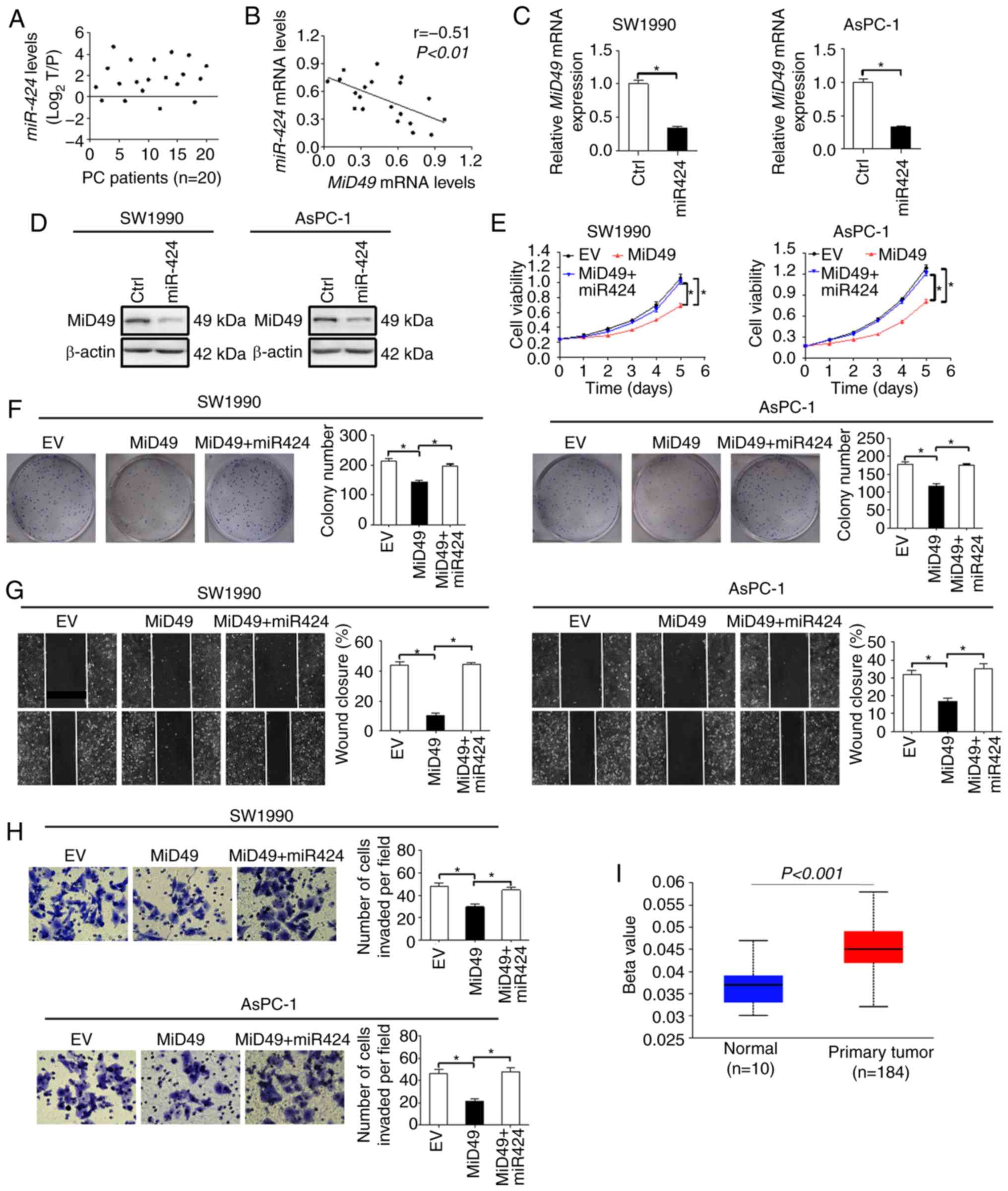
Downregulation of MiD49 is mainly
mediated by elevated miR-424 level in PC cells
MicroRNAs are important regulators of gene
expression at the post-transcriptional level. We thus explored the
possibility that downregulation of MiD49 in PC cells was mediated
by miRNA. Using the publicly available database miRDIP (microRNA
Data Integration Portal) (23), we
identified miR-424, an aberrantly overexpressed miRNA in PC
(24), that could potentially
target MiD49 (data not shown). We firstly evaluated the level of
miR-424 in 20-paired tumor tissues and adjacent non-tumor tissues
of PC. As shown in Fig. 8A, the
level of miR-424 was significant higher in tumor tissues of PC
compared with that observed in the adjacent non-tumor tissues. In
addition, a significant inverse correlation (r=−0.51, P<0.01)
between the expression of miR-424 and MiD49 was observed in the PC
tumor tissues (Fig. 8B). To further
test whether miR-424 is involved in the downregulation of MiD49 in
PC cells, synthetic precursor of miR-424 was transfected into
SW1990 and AsPC-1 cells. As shown in Fig. 8C and D, introduction of the
synthetic precursor of miR-424 significantly decreased the
expression of MiD49 at both the mRNA and protein levels in SW1990
and AsPC-1 cells. Moreover, miR-424 significantly reversed the
tumor growth and metastasis-suppressive effects of MiD49 in SW1990
and AsPC-1 cells (Fig. 8E-H).
Considering that genetic changes could also contribute to the
downregulation of MiD49 in PC cells, we further applied promoter
methylation profile analysis using the online web portal UALCAN
(21). The promoter methylation of
MiD49 is significantly higher in primary PC tumor tissues than that
in normal pancreatic tissues (Fig.
8I), suggesting that promoter hypermethylation may also
contribute to the downregulation of the expression of MiD49 in PC
cells.
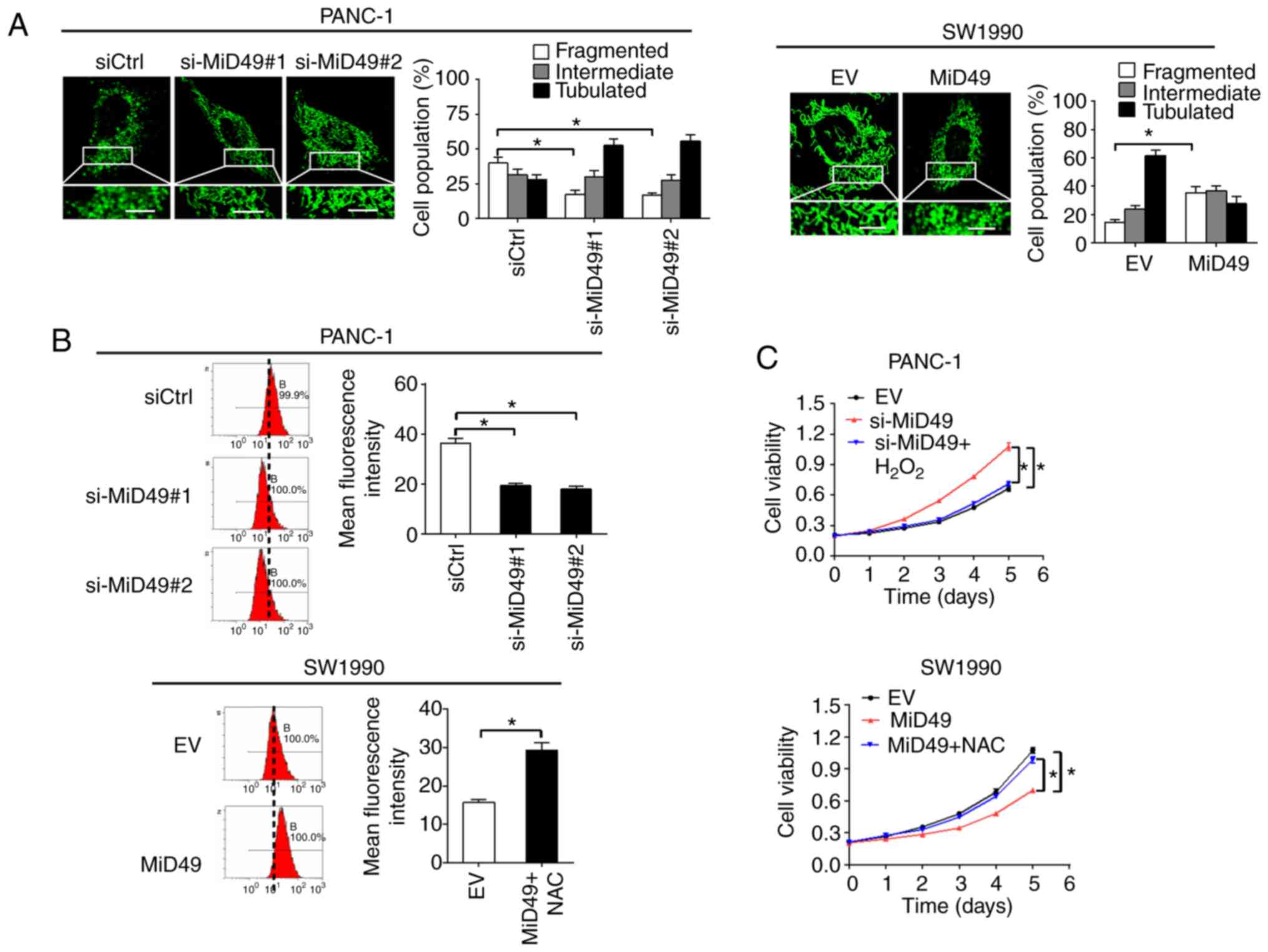
MiD49 suppresses PC growth and
metastasis through decreased ROS production
To explore the mechanistic basis of the
tumor-suppressive effects of MiD49 in PC, the changes in
mitochondria morphological were firstly investigated in PC cells
when MiD49 was overexpressed or knocked down. MitoTracker Green
staining results indicated that knockdown of MiD49 resulted in an
extensive elongation of mitochondria in the PANC-1 cells, while
overexpression of MiD49 induced a marked fragmentation of
mitochondria in the SW1990 cells (Fig.
9A). Mitochondria are a major source of reactive oxygen species
(ROS) production, which contributes to tumor development and
progression through activation of multiple oncogenic signaling
pathways (25,26). To explore the potential role of ROS
in MiD49-mediated suppressed tumor growth and metastasis in PC, the
production of ROS was evaluated in PC cells with different MiD49
expression levels. Knockdown of MiD49 significantly decreased the
production of ROS, whereas overexpression of MiD49 significantly
increased ROS levels in the PC cells (Fig. 9B). Moreover, treatment with
H2O2 significantly inhibited the growth and
metastasis of PANC-1 cells induced by MiD49 knockdown, whereas
N-acetyl-L-cysteine (NAC) (a ROS scavenger) treatment reversed the
growth and metastasis of SW1990 cells suppressed by MiD49
overexpression (Fig. 9C-F). These
results collectively indicate that MiD49 suppresses the growth and
metastasis of PC cells through decreased ROS production.
Discussion
In the present study, we demonstrated that
mitochondrial dynamics protein of 49 kDa (MiD49) is commonly
downregulated in pancreatic cancer (PC) cell lines and tissues
mainly due to elevated miR-424 expression. Consistently, abnormal
expression of other mitochondrial dynamic proteins, including
dynamin-related protein 1 (DRP1), mitofusin 1 (MFN1) and mitofusin
2 (MFN2), have also been observed in several types of human cancers
(11,12,14).
For example, Zhan et al reported that the mitochondrial
fission protein DRP1 was significantly upregulated and the
mitochondrial fusion protein MFN1 was downregulated in liver
cancer, both of which contributed to poor prognosis in liver cancer
patients (10). These observations
support the notion that dysfunction of mitochondrial dynamics plays
a crucial role in tumor progression. However, considering that
tumors are heterogeneous tissues composed of a mixture of cancer
and non-tumor cells, we cannot rule out the possibility that mid49
is off in tumor cells but on in infiltrating non-tumor cells, which
still needs further confirmation by immunohistochemical staining of
mid49 in clinical PC tissues.
Given the common downregulation of MiD49 in PC
cells, we hypothesized that MiD49 functions as a tumor suppressor
in pancreatic carcinogenesis. However, the biological function of
MiD49 in tumor progression remains largely unexplored. Our present
study showed that MiD49 overexpression suppressed PC growth and
metastasis both in vitro and in vivo, whereas
knockdown of MiD49 exhibited the opposite effects. In agreement
with our results, previous studies in several other cancer types
have also demonstrated that dysregulation of mitochondrial dynamics
proteins are involved in the promotion of tumor growth and
metastasis (10,14,27).
Additionally, we found that MiD49 suppressed PC growth and
metastasis through induction of G1-S phase cell cycle arrest and
inhibition of epithelial-mesenchymal transition.
MicroRNAs are crucial post-transcriptional
regulators of gene expression. We thus explored the possibility
that downregulation of MiD49 in PC cells was mediated by miRNA.
Using the publicly available database miRDIP, we proposed that
miR-424, an aberrantly overexpressed miRNA in PC (24), could potentially target MiD49.
However, the target of miR-424 and its biological functions in PC
cells are still unclear. Our present study demonstrated that MiD49
is a novel target of miR-424 in PC cells and downregulation of
MiD49 is mainly due to the overexpression of miR-424, suggesting
that miR-424 plays a crucial oncogenic role in PC. In addition to
miR-424, other mechanisms such as promoter hypermethylation may
also contribute to the downregulation of MiD49 in PC cells, which
still needs further investigation.
MiD49 functions as a receptor on the outer membrane
of mitochondria that recruits Drp1 to facilitate mitochondrial
fission (17). Expectedly, our
results indicated a marked fragmentation of mitochondria when MiD49
was overexpressed in PC cells. Mounting evidence indicates that
mitochondria are a main source of reactive oxygen species (ROS),
which contributes to tumor development and progression through
activation of multiple oncogenic signaling pathways (25). We revealed that overexpression of
MiD49 significantly increased ROS production in PC cells.
Additionally, we found that increased ROS production is involved in
the suppression of PC growth and metastasis by MiD49. However, a
previous study in liver cancer has reported a promoting effect of
mitochondrial fragmentation-induced ROS production on tumor growth
and metastasis (11). This
contradictory finding may be explained by the fact that moderate
levels of ROS function as signals to promote tumor growth and
metastasis, while excessive ROS induces tumor cell death.
In summary, our data indicate for the first time
that MiD49 is frequently downregulated in PC cells, which
contributes to the growth and metastasis of PC, suggesting a
crucial tumor-suppressive role of MiD49 in pancreatic cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Basic Research Plan in Shaanxi Province of China
(program no. 2017JM8071).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LB and EL designed the experiments. LB and JL and LL
collected, analyzed the data and prepared the figures. LB and JL
wrote the manuscript. All authors reviewed the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patients for their tissues to be used for biomedical research. All
experimental protocols were approved by the Ethics Committee of The
First Affiliated Hospital of Xi'an Jiaotong University and carried
out in accordance with the Declaration of Helsinki. All procedures
involving animals study were approved by the Animal Care Committee
of Xi'an Jiao Tong University in accordance with institutional
requirements and Chinese government guidelines for animal
experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ansari D, Tingstedt B, Andersson B,
Holmquist F, Sturesson C, Williamsson C, Sasor A, Borg D, Bauden M
and Andersson R: Pancreatic cancer: Yesterday, today and tomorrow.
Future Oncol. 12:1929–1946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta R, Amanam I and Chung V: Current and
future therapies for advanced pancreatic cancer. J Surg Oncol.
116:25–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loc WS, Smith JP, Matters G, Kester M and
Adair JH: Novel strategies for managing pancreatic cancer. World J
Gastroenterol. 20:14717–14725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee H and Yoon Y: Mitochondrial fission
and fusion. Biochem Soc Trans. 44:1725–1735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Bliek AM, Shen Q and Kawajiri S:
Mechanisms of mitochondrial fission and fusion. Cold Spring Harb
Perspect Biol. 5:a0110722013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trotta AP and Chipuk JE: Mitochondrial
dynamics as regulators of cancer biology. Cell Mol Life Sci.
74:1999–2017. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maycotte P, Marín-Hernández A,
Goyri-Aguirre M, Anaya- Ruiz M, Reyes-Leyva J and Cortés-Hernández
P: Mitochondrial dynamics and cancer. Tumour Biol.
39:10104283176983912017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prieto J and Torres J: Mitochondrial
dynamics: In cell reprogramming as it is in cancer. Stem Cells Int.
2017:80737212017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhan L, Cao H, Wang G, Lyu Y, Sun X, An J,
Wu Z, Huang Q, Liu B and Xing J: Drp1-mediated mitochondrial
fission promotes cell proliferation through crosstalk of p53 and
NF-κB pathways in hepatocellular carcinoma. Oncotarget.
7:65001–65011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Q, Zhan L, Cao H, Li J, Lyu Y, Guo
X, Zhang J, Ji L, Ren T, An J, et al: Increased mitochondrial
fission promotes autophagy and hepatocellular carcinoma cell
survival through the ROS-modulated coordinated regulation of the
NF-KB and TP53 pathways. Autophagy. 12:999–1014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rehman J, Zhang HJ, Toth PT, Zhang Y,
Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C and Archer SL:
Inhibition of mitochondrial fission prevents cell cycle progression
in lung cancer. FASEB J. 26:2175–2186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferreira-da-Silva A, Valacca C, Rios E,
Pópulo H, Soares P, Sobrinho-Simões M, Scorrano L, Máximo V and
Campello S: Mitochondrial dynamics protein Drp1 is overexpressed in
oncocytic thyroid tumors and regulates cancer cell migration. PLoS
One. 10:e01223082015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao J, Zhang J, Yu M, Xie Y, Huang Y,
Wolff DW, Abel PW and Tu Y: Mitochondrial dynamics regulates
migration and invasion of breast cancer cells. Oncogene.
32:4814–4824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Atkins K, Dasgupta A, Chen KH, Mewburn J
and Archer SL: The role of Drp1 adaptor proteins MiD49 and MiD51 in
mitochondrial fission: Implications for human disease. Clin Sci
(Lond). 130:1861–1874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palmer CS, Osellame LD, Laine D,
Koutsopoulos OS, Frazier AE and Ryan MT: MiD49 and MiD51, new
components of the mitochondrial fission machinery. EMBO Rep.
12:565–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loson OC, Meng S, Ngo H, Liu R, Kaiser JT
and Chan DC: Crystal structure and functional analysis of MiD49, a
receptor for the mitochondrial fission protein Drp1. Protein Sci.
24:386–394. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Badea L, Herlea V, Dima SO, Dumitrascu T
and Popescu I: Combined gene expression analysis of whole-tissue
and microdissected pancreatic ductal adenocarcinoma identifies
genes specifically overexpressed in tumor epithelia.
Hepatogastroenterology. 55:2016–2027. 2008.PubMed/NCBI
|
|
20
|
Ishikawa M, Yoshida K, Yamashita Y, Ota J,
Takada S, Kisanuki H, Koinuma K, Choi YL, Kaneda R, Iwao T, et al:
Experimental trial for diagnosis of pancreatic ductal carcinoma
based on gene expression profiles of pancreatic ductal cells.
Cancer Sci. 96:387–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L and Li W: Epithelial-mesenchymal
transition in human cancer: Comprehensive reprogramming of
metabolism, epigenetics, and differentiation. Pharmacol Ther.
150:33–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tokar T, Pastrello C, Rossos AEM, Abovsky
M, Hauschild AC, Tsay M, Lu R and Jurisica I: mirDIP
4.1-integrative database of human microRNA target predictions.
Nucleic Acids Res. 46:D360–D370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohuchida K, Mizumoto K, Kayashima T,
Fujita H, Moriyama T, Ohtsuka T, Ueda J, Nagai E, Hashizume M and
Tanaka M: MicroRNA expression as a predictive marker for
gemcitabine response after surgical resection of pancreatic cancer.
Ann Surg Oncol. 18:2381–2387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabharwal SS and Schumacker PT:
Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles'
heel? Nat Rev Cancer. 14:709–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rigoulet M, Yoboue ED and Devin A:
Mitochondrial ROS generation and its regulation: Mechanisms
involved in H(2)O(2) signaling. Antioxid Redox Signal. 14:459–468.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun X, Cao H, Zhan L, Yin C, Wang G, Liang
P, Li J, Wang Z, Liu B, Huang Q and Xing J: Mitochondrial fission
promotes cell migration by Ca2+/CaMKII/ERK/FAK pathway
in hepatocellular carcinoma. Liver Int. 38:1263–1272. 2018.
View Article : Google Scholar : PubMed/NCBI
|