Introduction
Lung cancer remains the leading cause of
cancer-associated mortality for males and females worldwide.
According to statistics, >2 million new cases were diagnosed in
2018 and ~1.7 million patients succumbed to lung cancer (1). According to the pathological features,
lung cancer may be categorized into two major types: Non-small cell
lung cancer (NSCLC) and SCLC (2,3).
Compared with SCLC, NSCLC is a common lung cancer subtype,
accounting for >80% of diagnosed cases (4). NSCLC is insensitive to chemotherapy
and the prognosis of patients with NSCLC is unsatisfactory with a
five-year overall survival rate of <50% (5,6).
Further investigation of the molecular mechanisms of NSCLC is
imperative to provide valuable targets for the development of novel
therapeutic approaches for patients with NSCLC (7).
Non-coding RNAs are single-stranded RNAs with no
protein-coding function (8). Long
non-coding RNAs (lncRNAs) are non-coding RNAs of >200
nucleotides in length (9).
Previously considered as ‘junk’ RNAs, recent studies revealed that
dysregulation of lncRNAs is relevant to human diseases, including
tumorigenesis (10,11). Mechanistically, lncRNAs may interact
with micro (mi)RNAs, mRNAs or proteins to regulate gene expression
and protein localization (12,13).
The competing endogenous (ce)RNA hypothesis suggests that the
expression of lncRNAs controls the mRNAs levels via sponging miRNAs
(14). Several lncRNAs have been
reported to be oncogenes or tumor suppressors in NSCLC (15–17).
For instance, Yang et al (18) indicated that lncRNA insulin-like
growth factor binding protein 4-1 was significantly upregulated in
lung cancer and promoted tumor cell metabolism to facilitate cancer
cell proliferation. lncRNA-HIT interacted with E2F transcription
factor 1 to regulate target gene expression and promoted cell
proliferation of NSCLC cells (19).
lncRNA TPTE pseudogene 1 (TPTEP1) was identified as one of most
significantly downregulated lncRNAs in NSCLC via a bioinformatics
analysis of The Cancer Genome Atlas (TCGA) dataset (20). However, the roles of TPTEP1 in NSCLC
have remained elusive.
Src kinase signaling inhibitor 1 (SRCIN1), also
known as p140CAP, is an adapter protein that binds to Src and
inactivates Src kinase through C-terminal Src kinase (21). Non-receptor protein tyrosine kinase
Src is a well-characterized oncogene and its activity is associated
with the progression of cancer (22,23).
Src is known to mediate several oncogenic signaling pathways in
cancer cells, including the PI3K and STAT3 pathways (24,25).
Via inactivation of Src, SRCIN1 functions as a tumor suppressor in
multiple cancer types (26,27). However, it has remained elusive how
SCRIN1 expression is regulated in NSCLC.
The present study aimed to investigate the
clinicopathological significance and prognosis of TPTEP1 as well as
its functional role in NSCLC. A bioinformatics analysis, reverse
transcription-quantitative (RT-q)PCR, western blot analysis and
dual-luciferase reporter assays were performed to explore the
molecular mechanisms of TPTEP1 in NSCLC cells. The results
demonstrated a tumor suppressor role of TPTEP1 in NSCLC.
Materials and methods
Patients and samples
Human NSCLC tumors and matched normal tissues were
collected from 56 patients (41 males and 15 females; age range,
35–76 years) with NSCLC who underwent surgery at Shangqiu First
People's Hospital and the First Affiliated Hospital of Henan
University between June 2015 and July 2016. The information of sex,
age and smoking history was obtained from patients. Written
informed consent was obtained from all participants prior to the
study. The patients did not receive any chemotherapy or
radiotherapy prior to surgery. The NSCLC samples were staged
according to surgical and pathological results, which were based on
the guidelines described by the 7th edition of the American Joint
Committee on Cancer/Union for International Cancer Control
(28). All experiments were
approved by the Ethics Committee of Shangqiu First People's
Hospital and the First Affiliated Hospital of Henan University.
Tissues were stored in liquid nitrogen at the time of surgery and
stored in a −80°C refrigerator.
Cell lines and culture
Human NSCLC cell lines (A549 and NCI-H1299) and the
human lung epithelial cell line BEAS-2B were purchased from the
American Type Culture Collection. These cells were maintained in
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator
with 5% CO2.
RNA extraction and RT-qPCR
Total RNA was extracted from BEAS-2B, A549,
NCI-H1299 cells and tissue samples with the RNeasy Mini Kit
(Qiagen) following the manufacturer's protocol. The RNA
concentration was measured with a NanoDrop 2000 (Thermo Fisher
Scientific, Inc.). First-strand complementary (c) DNA was
synthesized with a SuperScript III First-Strand kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Realtime qPCR was performed using TB Green Premix Ex Taq
(Takara Bio, Inc.) with the following protocol: Initial
pre-denaturation at 98°C for 30 sec, followed by 35 cycles of
denaturation at 98°C for 5 sec and elongation/annealing at 60°C for
30 sec. GAPDH and U6 were used as internal controls for mRNA and
miRNA, respectively. The relative expression of genes were
calculated with the 2−ΔΔCq method (29). The primer sequences were listed as
follows: Stem-loop,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCTGA-3′;
miR-328-5p-forward, 5′-GCCGAGGGGGGGGCAGGAGG-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′; TPTEP1 forward, 5′-CTGGGAGAAGTGCCCTTGC-3′
and reverse, 5′-CACCTCATCAGTCATTTGCTCA-3′; SRCIN1 forward,
5′-GAGGCTCGCAACGTCTTCTAC-3′ and reverse,
5′-GCGATGCGTACACCATCTCTC-3′; GAPDH forward,
5′-TCAACAGCAACTCCCACTCTTCCA-3′ and reverse,
5′-ACCCTGTTGCTGTAGCCGTATTCA-3′.
Overexpression of TPTEP1 and silencing
of SRCIN1
Full-length TPTEP1 was amplified by PCR (TPTEP1
forward, 5′-GTGAATTCCTCGAGACTAGTTCTGCCTCTCCCGGTACCTGCT-3′ and
reverse, 5′-GGATCCGCGGCCGCTCTAGCACTAGTTTTTGATGGAATTTTTAGTTT-3′)
from A549 cDNA and ligated into pcDNA3.1 plasmid. pcDNA3.1 or
pcDNA3.1-TPTEP1 was transfected into A549 or NCI-H1299 cells with
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. SRCIN1 siRNA and control
siRNA were purchased from GenePharma Co., Ltd. SRCIN1 siRNA
(5′-GCCCGCUGAGCGCCUCCAGAC-3′) or control siRNA
(5′-UUCUCCGAACGUGUCACGU-3′) was transfected into A549 or NCI-H1299
cells with Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
72 h, the cells were collected and the RNA or proteins were
extracted for the subsequent experiments.
Knockdown and overexpression of
miR-328-5p
miR-negative control (NC) mimics
(5′-UUCUCCGAACGUGUCACGU-3′), miR-328-5p mimics
(5′-AGGGGGGGCAGGAGGGGCUCAGGG-3′), miR-NC inhibitor
(5′-UUCUCCGAACGUGUCACGU-3′) and miR-328-5p inhibitor
(5′-CCCUGAGCCCCUCCUGCCCCCCCU-3′) were synthesized and provided by
GenePharma Co., Ltd. miR-NC mimics, miR-328-5p mimics, miR-NC
inhibitor or miR-328-5p inhibitor was transfected into A549 or
NCI-H1299 cells with Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. At 72 h
after transfection, the cells were collected and subjected to
RT-qPCR or western blot analysis.
Dual-luciferase reporter assay
Full-length TPTEP1 and the full length of
3′untranslated region (3′UTR) of SRCIN1 were amplified from A549
cDNA and ligated into pGL3-basic plasmid. Two site mutations were
introduced into pGL3-TPTEP1 or pGL3-SRCIN1 3′UTR plasmid with a
Quick Site-directed mutation kit (Agilent Technologies, Inc.). For
the dual-luciferase reporter assay, cells were transfected with a
combination of pGL3-TPTEP1 or pGL3-SRCIN1 3′UTR or pGL3-TPTEP1
mutated (Mut) or pGL3-SRCIN1 3′UTR-Mut with miR-NC mimics or
miR-328-5p mimics by using Lipofectamine 3000. At 48 h after
transfection, the relative luciferase activity was measured with a
Dual-Luciferase Reporter Assay System (Promega Corp.).
Protein extraction and western blot
analysis
Primary antibodies of SRCIN1 (product number 3757;
dilution 1:1,000), P-SRC (TYR416) (product number 6943; dilution
1:1,000), SRC (product number 2108; dilution 1:1,000) p-STAT3
(product number 9145; dilution 1:1,000) and STAT3 (product number
9139; dilution 1:1,000) were purchased from Cell Signaling
Technology, Inc. GAPDH (product code ab8245; dilution 1:5,000)
antibody was obtained from Abcam. HRP-conjugated secondary
antibodies of mouse (cat. no. SA00001-1; dilution 1:5,000) and
rabbit (cat. no. SA00001-2; dilution 1:5,000) were purchased from
ProteinTech Group. Radioimmunoprecipitation assay lysis buffer
(RIPA; Thermo Fisher Scientific, Inc.) was used to prepare protein
lysates from cells following the manufacturer's protocol.
Subsequently, the protein concentration was determined with a BCA
Protein Assay Kit (Thermo Fisher Scientific, Inc.). Lysates
containing 25 µg protein were loaded in each well of 8% SDS gels
and separated by SDS-PAGE. Proteins were transferred to
polyvinylidene difluoride membranes (EMD Millipore) and blocked
with non-fat milk for 30 min at room temperature. The membrane was
then washed and incubated with a primary antibody for 1 h at room
temperature. Subsequently, the membranes were washed and incubated
with secondary antibodies for 1 h at room temperature. Finally, the
blots were developed with ECL Western Blotting Substrate (Thermo
Fisher Scientific, Inc.). The relative expression of protein was
quantified with ImageJ software (v. 1.52r; National Institutes of
Health).
Bioinformatics analysis
The expression of TPTEP1 in multiple cancer types
and normal tissues of different organs was analyzed using the Gene
Expression Profiling Interactive Analysis database (GEPIA;
http://gepia.cancer-pku.cn/) based on
TCGA datasets. To study the association between TPTEP1 expression
and prognosis of patients with NSCLC, the gene expression data and
patient survival information from the dataset GSE30219 (30) comprising 307 patients with lung
cancer were downloaded. The samples were divided into two groups
based on the expression of TPTEP1 (above or below the median
expression level of TPTEP1 (relative expression of TPTEP1=4.20554)
(n=141 for each group) and were analyzed using Kaplan-Meier
analysis with log-rank testing. The miRNAs containing putative
binding sites for TPTEP1 were predicted with miRDB software
(http://mirdb.org/). The potential target genes of
miR-328-5p were predicted with TargetScan software (http://www.targetscan.org/vert_72/).
Cell proliferation assay
The proliferation ability of cells was determined
with a CCK-8 kit (Dojindo Molecular Technologies, Inc.) following
the manufacturer's protocol. On the first day, 5,000 cells were
seeded in each well of 96-well plates. On the second day, cells
were transfected with plasmids and/or miRNA inhibitor. At 0, 24, 48
and 72 h after transfection, 10 µl CCK-8 solution was added to each
well, followed by incubation for 2 h. The medium containing CCK-8
was transferred to another 96-well plate and the absorbance at 450
nM was detected with a microplate reader (Bio-Rad Laboratories,
Inc.) to determine the number of cells.
Cell apoptosis assay
The cell apoptosis rate was detected with a Dead
Cell Apoptosis Kit with Annexin V Alexa Fluor™ 488 and propidium
iodide (PI) (Thermo Fisher Scientific, Inc.). In brief, at 48 h
after transfection, cells were harvested and resuspended in 1X
Binding Buffer provided with the kit. Subsequently, 5 µl PI and 1
µl Annexin V was added to the cells, followed by incubation at room
temperature for 15 min. The cells were immediately subjected to
flow cytometric analysis on a MACSQuant Analyzer X (Miltenyi
Biotec, Inc.). The data were analyzed using FlowJo 10 software
(FlowJo LLC).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.0 (GraphPad Software, Inc.). Student's t-test (paired test
for specimens and unpaired test for cell-based assays) was applied
to analyze differences between two groups. For comparison of three
groups, one-way analysis of variance followed by
Student-Neuman-Keuls analysis was performed. The association
between TPTEP1 expression and characteristics of patients was
analyzed by Chi-square test. All statistical analyses were
two-tailed and P<0.05 was considered to indicate a statistically
significant difference.
Results
lncRNA TPTEP1 is downregulated in
NSCLC and associated with good prognosis
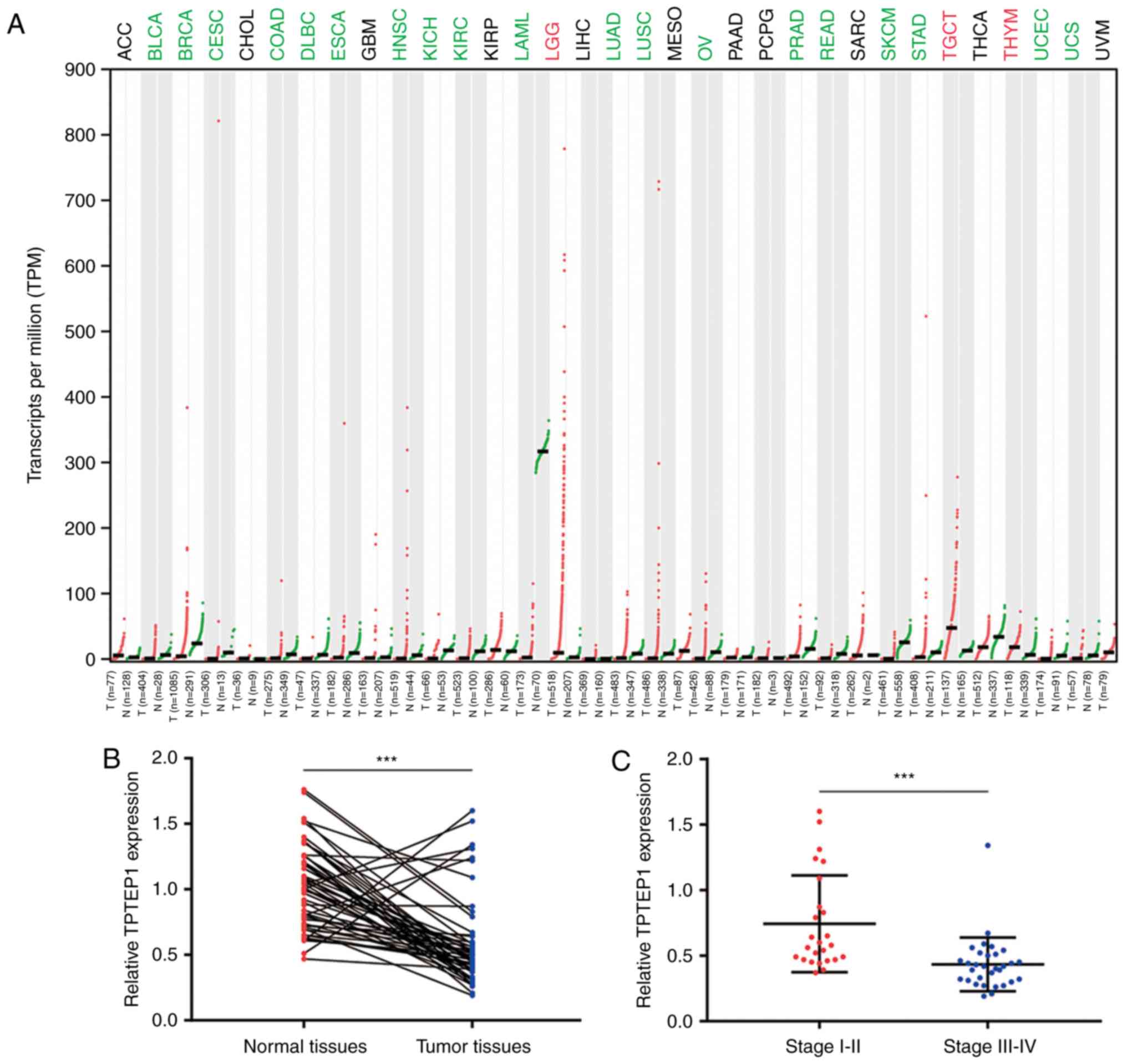
To investigate the expression pattern of TPTEP1 in
NSCLC, the GEPIA database was used to explore its expression in
cancer and normal tissues of various origins from TCGA datasets.
TPTEP1 was downregulated in a majority of cancer types, including
bladder urothelial carcinoma and breast invasive carcinoma.
Notably, TPTEP1 was significantly downregulated in lung
adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC)
(Fig. 1A). For confirmation,
RT-qPCR was performed to detect TPTEP1 expression in 56 pairs of
tumors and adjacent normal tissues from patients with NSCLC. The
results revealed that TPTEP1 was downregulated in most tumors
(Fig. 1B). It was also indicated
that TPTEP1 expression was lower in high-grade (stage III–IV)
tumors compared with that in low-grade tumors (stage I–II; Fig. 1C). Low expression of TPTEP1 was
associated with high-grade tumors (stage III–IV; P=0.006), but no
significant association was determined between TPTEP1 expression
and sex, age and smoking history (Table
I). The prognostic value of TPTEP1 in patients with NSCLC was
then analyzed. For the dataset GSE30219, the Kaplan-Meier plot
indicated that high expression of TPTEP1 was associated with a
favorable overall survival for patients with NSCLC (log-rank
P=0.001; Fig. 1D). In addition, in
the tumor tissues collected from patients with NSCLC, high
expression of TPTEP1 was further confirmed to be associated with a
relatively good prognosis (log-rank P=0.036; Fig. 1E). Next, TPTEP1 expression was
analyzed in a panel of NSCLC cell lines and lung epithelial cells.
TPTEP1 was ~2-fold decreased in NSCLC cells (A549 and NCI-H1299)
compared with that in lung epithelial cells (BEAS-2B) (Fig. 1F).
 | Table I.Association between
clinicopathological characteristics and TPTEP1 expression in
patients with NSCLC. |
Table I.
Association between
clinicopathological characteristics and TPTEP1 expression in
patients with NSCLC.
|
|
| TPTEP1
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Total cases | High
expression | Low expression | P-value |
|---|
| Sex |
|
|
| 0.227 |
|
Male | 41 | 23 | 18 |
|
|
Female | 15 | 5 | 10 |
|
| Age |
|
|
| 0.391 |
|
<60 | 18 | 7 | 11 |
|
|
≥60 | 38 | 21 | 17 |
|
| Smoking
history |
|
|
| 0.503 |
| No | 11 | 7 | 4 |
|
|
Yes | 45 | 21 | 24 |
|
| TNM stage |
|
|
| 0.006 |
|
I–II | 23 | 17 | 6 |
|
|
III–IV | 33 | 11 | 22 |
|
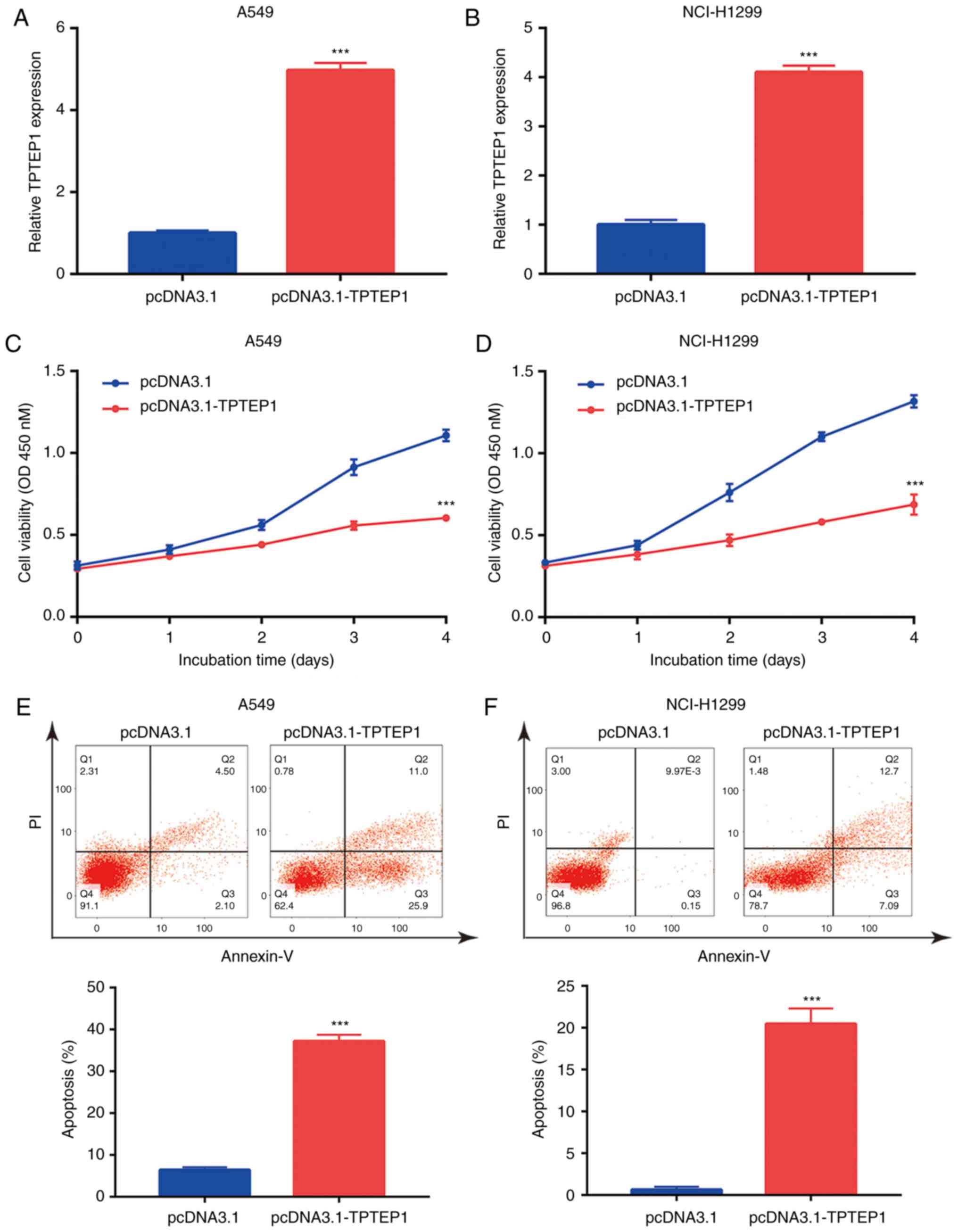
Overexpression of TPTEP1 inhibits
NSCLC cell proliferation and induces apoptosis
To study the role of TPTEP1 in NSCLC,
pcDNA3.1-TPTEP1 with full-length TPTEP1 was constructed and
transfected into NSCLC cells. Transfection of TPTEP1 led to a
>4-fold increase of TPTEP1 in A549 and NCI-H1299 cells (Fig. 2A and B). In the cell proliferation
assay, overexpression of TPTEP1 led to a significant decrease in
the proliferation rate of A549 and NCI-H1299 cells (Fig. 2C and D). The strong cell
growth-inhibitory effect of TPTEP1 suggested that cell apoptosis
may be involved in this process. Flow cytometry was used to
determine the ratio of apoptotic cells following overexpression of
TPTEP1. The results indicated that overexpression of TPTEP1
significantly increased the apoptotic ratio of A549 cells (Fig. 2E). Similarly, a significant increase
of cell apoptosis was observed in NCI-H1299 cells transfected with
TPTEP1 (Fig. 2F). The results
collectively indicated that TPTEP1 was pivotal for resistance to
apoptosis of NSCLC cells.
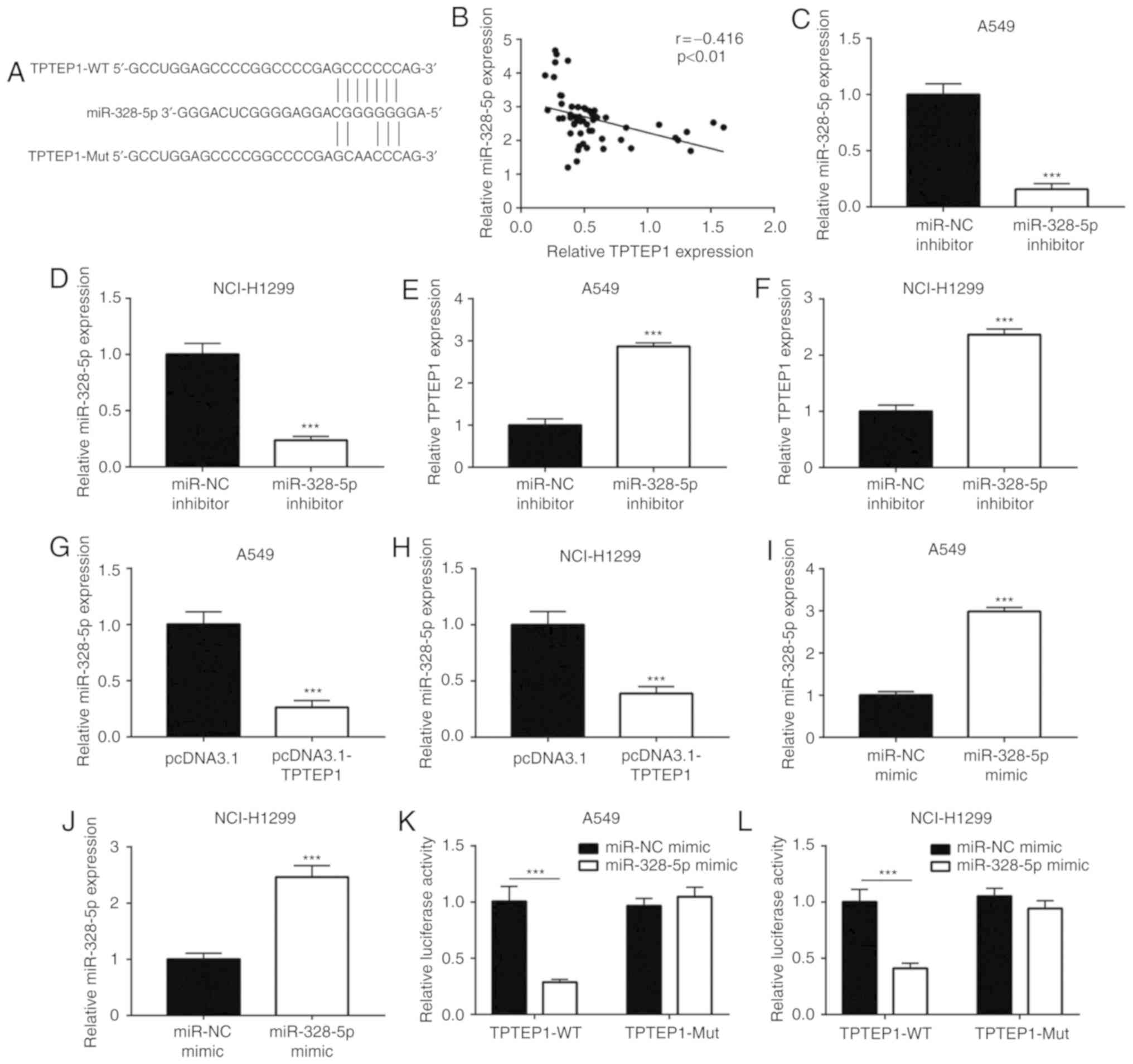
TPTEP1 sponges miR-328-5p in NSCLC
cells
lncRNAs may function as ceRNAs to sponge certain
miRNAs and thereby regulate cancer progression (31). By using miRDB, 38 miRNAs containing
a putative binding site for TPTEP1 were predicted. Among them,
miR-328-5p was previously reported as an upregulated miRNA in NSCLC
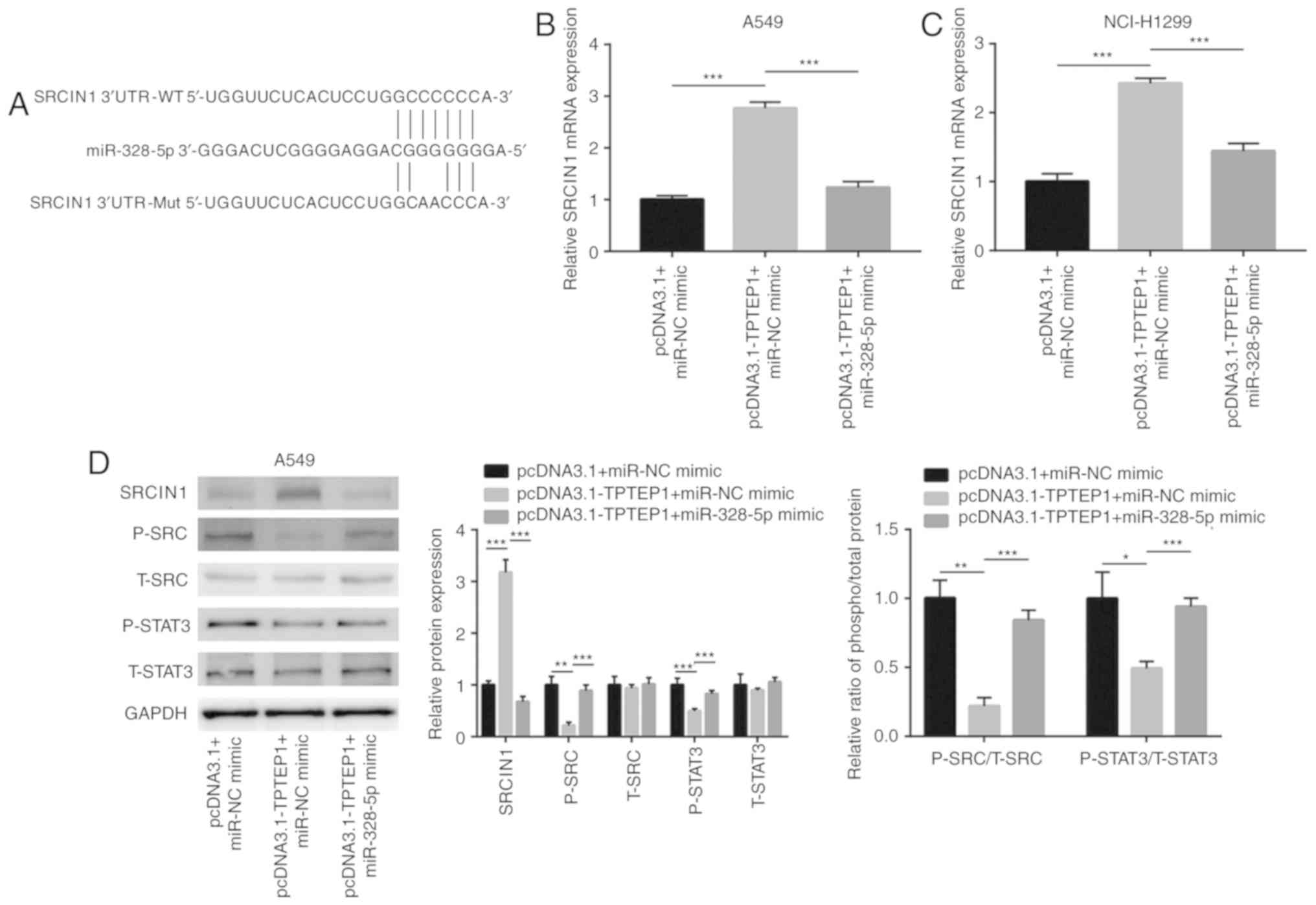
(32). As indicated in Fig. 3A, a putative binding site for
miR-328-5p on TPTEP1 was predicted. In the 56 NSCLC tumors, a
significant negative correlation between miR-328-5p levels and
TPTEP1 expression was observed (r=−0.416, P<0.01; Fig. 3B). To explore the regulatory
association between miR-328-5p and TPTEP1, miR-328-5p was knocked
down in A549 and NCI-H1299 cells by transfection of miR-328-5p
inhibitor (Fig. 3C and D).
Downregulation of miR-328-5p led to an increase of TPTEP1
expression in A549 cells (Fig. 3E).
A similar effect was also observed in NCI-H1299 cells (Fig. 3F). Conversely, overexpression of
TPTEP1 decreased miR-328-5p levels in A549 and NCI-H1299 cells
(Fig. 3G and H). Transfection of
miR-328-5p mimics was confirmed to increase miR-328-5p levels in
A549 and NCI-H1299 cells (Fig. 3I and
J). In the dual-luciferase reporter assay, overexpression of
miR-328-5p was indicated to decrease the relative luciferase
activity of pGL3-TPTEP1-wild-type (WT) plasmid but did not
influence the relative luciferase activity of pGL3-TPTEP1-Mut in
A549 cells (Fig. 3K). Similarly,
miR-328-5p mimics also suppressed the relative luciferase activity
of pGL3-TPTEP1-WT in NCI-H1299 cells (Fig. 3L). These results indicated that
TPTEP1 directly interacts with miR-328-5p in NSCLC cells.
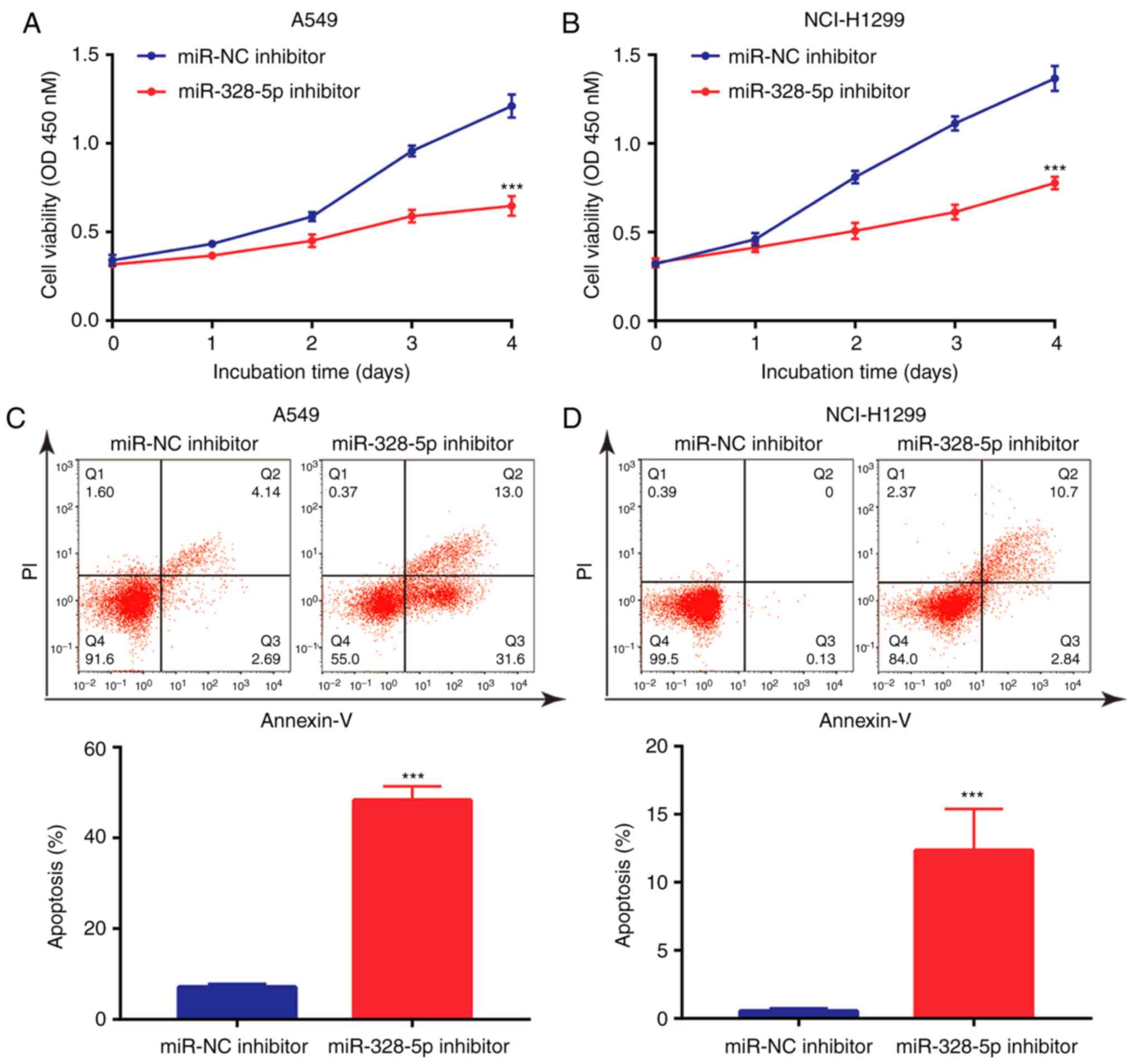
Downregulation of miR-328-5p inhibits
NSCLC cell proliferation and induces cell apoptosis
Although it has been previously indicated that
miR-328-5p was overexpressed in NSCLC and may serve as a diagnostic
biomarker (32), the function of
miR-328-5p in NSCLC has remained elusive. The present results
indicated that in A549 and NCI-H1299 cells, downregulation of
miR-328-5p inhibited cell proliferation (Fig. 4A and B). In addition, miR-328-5p
inhibitor induced apoptosis in A549 cells (Fig. 4C), which was also observed in
NCI-H1299 cells (Fig. 4D).
Collectively, similar to the effect of overexpression of TPTEP1,
downregulation of miR-328-5p inhibited cell proliferation and
induced apoptosis in NSCLC cells.
TPTEP1 causes upregulation of SRCIN1
via sponging miR-328-5p
Using TargetScan, several target genes of miR-328-5p
were predicted, and among them, SRCIN1 is a well-characterized
tumor suppressor (33) which was
indicated to share a putative binding site for miR-328-5p (Fig. 5A). Overexpression of TPTEP1
increased SRCIN1 mRNA levels, and transfection of miR-328-5p mimics
attenuated the upregulation of SRCIN1 mRNA in A549 and NCI-H1299
cells (Fig. 5B and C). Previous
studies reported that SRCIN1 directly inactivated Src signaling and
indirectly inactivated STAT3 signaling in cancer cells (34,35).
In the present study, western blot analysis revealed that TPTEP1
overexpression increased SRCIN1 protein expression and decreased
p-Src and p-STAT3 protein levels in A549 cells (Fig. 5D). The decrease of the p-SRC/t-SRC
and p-STAT3/t-STAT3 ratios indicated inactivation of SRC and STAT3
signaling in response to TPTEP1 overexpression in A549 cells.
Similar to the effect in A549 cells, TPTEP1 overexpression
increased SRCIN1 protein expression and decreased p-SRC and p-STAT3
protein levels in NCI-H1299 cells (Fig.
5E). SRC and STAT3 signaling became inactivated in NCI-H1299
after TPTEP1 overexpression. In the dual-luciferase reporter assay,
miR-328-5p mimics suppressed the relative luciferase activity of
the plasmid driven by the SRCIN1 3′UTR in A549 and NCI-H1299 cells
(Fig. 5F and G), revealing that
miR-328-5p directly binds to the 3′UTR of SRCIN1 mRNA.
Hyperactivation of Src and STAT3 signaling are pivotal for cancer
cell survival (36,37). The present results indicated that
TPTEP1 sponged miR-328-5p to upregulate SRCIN1 and inactivated Src
and STAT3 signaling to promote NSCLC cell apoptosis.
TPTEP1 inhibits cell proliferation and
induces apoptosis via upregulation of SRCIN1
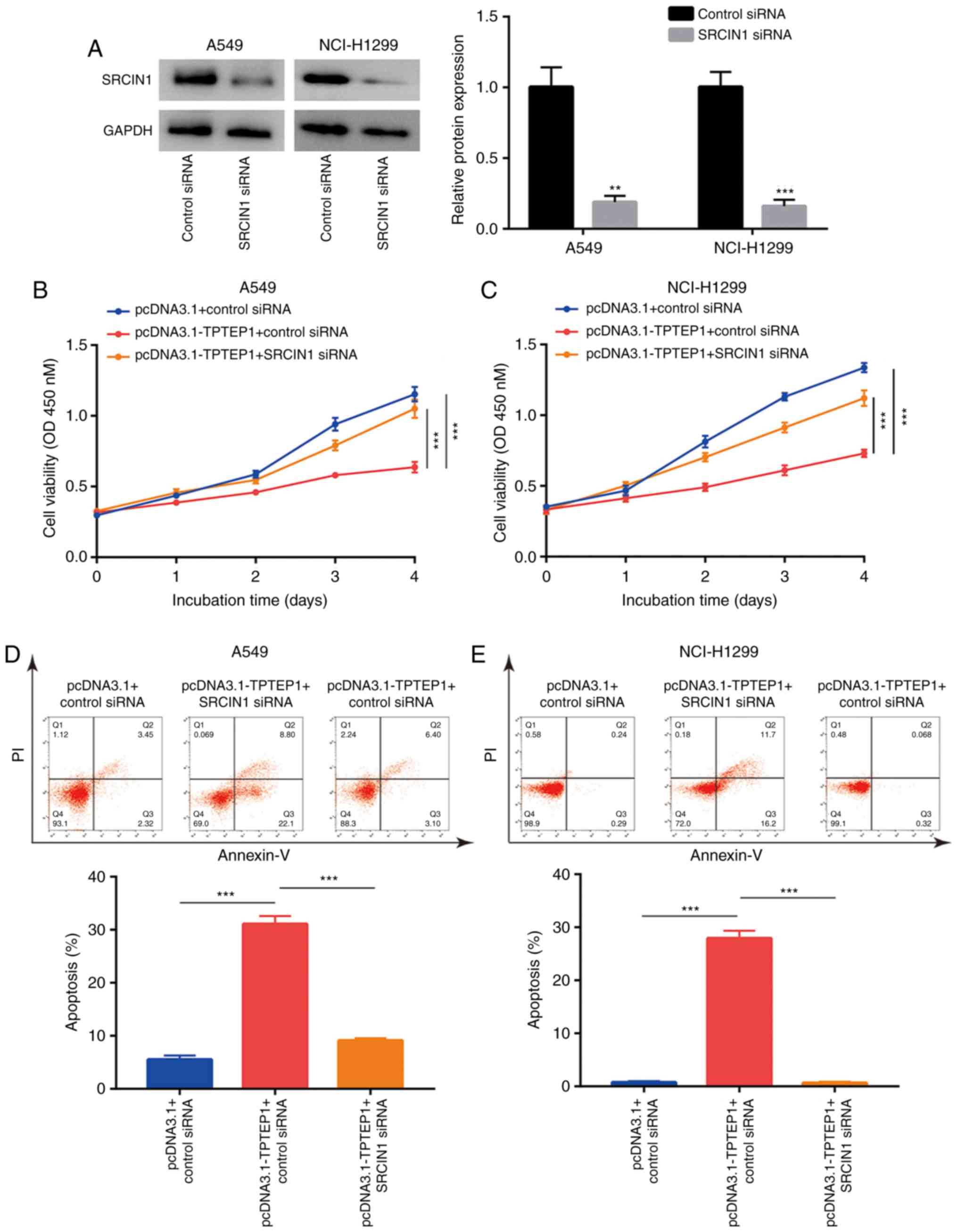
To investigate whether SRCIN1 is involved in
TPTEP1-meditated cell growth arrest and cell apoptosis, SRCIN1
siRNA was transfected into A549 and NCI-H1299 cells to knock down
SRCIN1 expression. Western blot analysis confirmed that
transfection of SRCIN1 siRNA decreased SRCIN1 protein expression in
A549 and NCI-H1299 cells (Fig. 6A).
The cell proliferation assay indicated that silencing of SRCIN1
abrogated the inhibitory effect of TPTEP1 overexpression on the
proliferation of A549 and NCI-H1299 cells (Fig. 6B and C). Furthermore, flow
cytometric analysis revealed that silencing of SRCIN1 inhibited the
effect of TPTEP1 overexpression to induce apoptosis in A549 cells
(Fig. 6D), which was also observed
in NCI-H1299 cells (Fig. 6E).
TPTEP1 expression is positively
correlated with SRCIN1 mRNA levels in NSCLC tumors
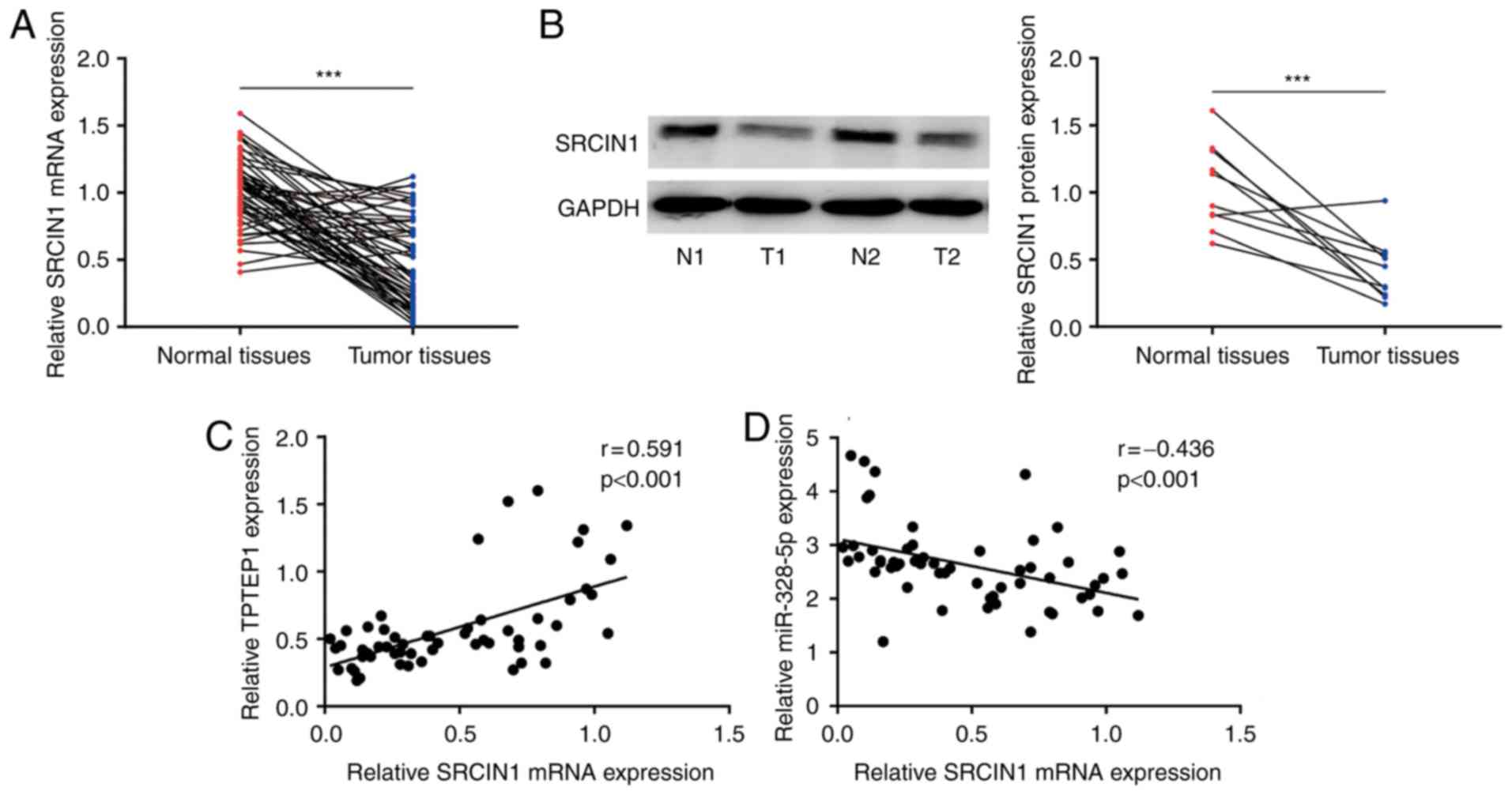
To examine the association between SRCIN1 and TPTEP1
in clinical samples, RT-qPCR was performed to detect SRCIN1 mRNA
levels in 56 pairs of NSCLC tumors and adjacent normal tissues.
Downregulation of SRCIN1 was observed in NSCLC tumors (P<0.001;
Fig. 7A), which was consistent with
the results of a previous study (33). In addition, SRCIN1 protein
expression was detected in 10 pairs of NSCLC tumors and normal
tissues by western blot analysis. Consistently, SRCIN1 protein
expression was significantly downregulated in tumors (P<0.001;
Fig. 7B). Pearson correlation
analysis revealed a strong positive correlation between TPTEP1 and
SRCIN1 mRNA expression (r=0.591, P<0.001; Fig. 7C) and a negative correlation between
SRCIN1 mRNA and miR-328-5p levels (r=−0.436, P<0.001; Fig. 7D) in tumors.
Discussion
Due to the development of RNA sequencing technology,
numerous aberrantly expressed lncRNAs have been identified in NSCLC
(38). Several lncRNAs were
experimentally identified to function as tumor suppressors or
oncogenes during NSCLC (38–40).
DiGeorge syndrome critical region gene 5, an upregulated lncRNA in
lung adenocarcinoma identified through integrated analysis of TCGA
data (20), was indicated to be
pivotal for proliferation and metastasis of lung cancer cells via
sponging miR-1180 and miR-873-5p (41,42).
The present study focused on TPTEP1, another differentially
expressed lncRNA in lung adenocarcinoma, and indicated for the
first time to the best of our knowledge, that TPTEP1 regulated cell
proliferation and apoptosis in NSCLC.
Downregulation of TPTEP1 was first reported in
kidney, liver, lung and stomach cancers as a result of DNA
methylation (43). In the present
study, the expression pattern of TPTEP1 was analyzed in several
cancer types and normal tissues according to TCGA datasets,
indicating that TPTEP1 was downregulated in a majority of cancers,
including kidney renal clear cell carcinoma, LUAD, LUSC and stomach
adenocarcinoma. The present RT-qPCR data further confirmed the
downregulation of TPTEP1 in NSCLC tumors. In addition, based on an
analysis of previously published data (30), it was indicated that the high
expression of TPTEP1 was able to predict a prolonged overall
survival of patients with lung cancer. The result was verified in
the samples collected from 56 patients with NSCLC. Certain lncRNAs
are crucial for cancer cell proliferation and survival (44,45).
In hepatocellular carcinoma, overexpression of TPTEP1 suppressed
cell proliferation and metastasis but did not affect cell apoptosis
(46). The present results
indicated that overexpression of TPTEP1 significantly inhibited
cell proliferation and induced apoptosis in NSCLC cells. These
results indicated that TPTEP1 may have a pivotal role by inducing
cell death to inhibit overgrowth of NSCLC cells.
The role of miR-328-5p in cancers is controversial.
miR-328-5p levels were high in the serum of patients with NSCLC and
may serve as an excellent biomarker for early prediction of NSCLC
(32). In breast cancer, loss of
miR-328-5p expression was observed in tumors and miR-328-5p exerted
an anti-proliferative effect on breast cancer cells by targeting
RAGE (47). In the present study,
miR-328-5p was determined to be overexpressed in NSCLC tumors. This
is consistent with the RT-qPCR data from a previous study of NSCLC
tumors (48). Similar to the effect
in NSCLC cells transfected with TPTEP1, knockdown of miR-328-5p
inhibited cell proliferation and induced cell apoptosis in NSCLC
cells. The dual-luciferase assay confirmed the direct binding of
TPTEP1 and miR-328-5p in NSCLC cells. Aberrant expression of
miR-328-5p has been reported in several cancer types, and was
negatively regulated by circRNA (circRNA-5692) and lncRNA
(LINC00210) (47,49,50).
Our data implied that TPTEP1 downregulation may contribute to the
increase of miR-328-5p in NSCLC, indicating TPTEP1 as a novel
regulator of miR-328-5p.
As a non-receptor kinase, Src drives NSCLC cell
proliferation, drug resistance and cell survival by activating
pathways including STAT3 signaling (51,52).
SRCIN1 suppresses Src activity and according to a previous study,
its expression was relatively low in tumors (53). Upregulation of miR-873 and miR-346
were indicated to be responsible for the downregulation of SRCIN1
in various tumor types (33,54).
In the present study, a bioinformatics analysis indicated that
SRCIN1 is a potential target gene of miR-328-5p. In addition,
downregulation of miR-328-5p increased SRCIN1 expression and
decreased the phosphorylation levels of Src and STAT3, indicating
inactivation of Src and STAT3 signaling. Furthermore, SRCIN1 was
confirmed as a target gene of miR-328-5p in the dual-luciferase
reporter assay. miR-328-5p targeted mRNA of several genes such as
RAGE, PLCE1 and PTEN to exert its role during cancer progression
(47,55,56).
To the best of our knowledge, the present study was the first to
indicate that miR-328-5p promotes NSCLC cell proliferation and
sustains cell survival at least partially via targeting of SRCIN1
to activate the Src and STAT3 pathways.
In conclusion, the present results revealed that
TPTEP1 was downregulated in NSCLC and predicted a good prognosis
for patients with NSCLC. Mechanistically, TPTEP1 inactivated Src
and STAT3 signaling via sponging miR-328-5p to upregulate SRCIN1.
These results may provide novel insights into the molecular basis
of NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was funded by Hebei Keynote
Research and Development Plan Self-Financing Project
(182777234).
Availability of materials and methods
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
FC, ZW, YF, HZ and MY carried out the experimental
studies and analyzed the data. XW, HZ and SZ designed the study,
and performed the literature research. XW and SZ also carried out
the experimental studies and prepared the manuscript preparation.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shangqiu First People's Hospital and the First
Affiliated Hospital of Henan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin X, Chen Y, Chen H, Fei S, Chen D, Cai
X, Liu L, Lin B, Su H, Zhao L, et al: Evaluation of tumor-derived
exosomal miRNA as potential diagnostic biomarkers for Early-stage
non-small cell lung cancer using next-generation sequencing. Clin
Cancer Res. 23:5311–5319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schrodl K, Oelmez H, Edelmann M, Huber RM
and Bergner A: Altered Ca2+-homeostasis of cisplatin-treated and
low level resistant non-small-cell and small-cell lung cancer
cells. Cell Oncol. 31:301–315. 2009.PubMed/NCBI
|
|
4
|
Koh ES, Sun A, Tran TH, Tsang R, Pintilie
M, Hodgson DC, Wells W, Heaton R and Gospodarowicz MK: Clinical
dose-volume histogram analysis in predicting radiation pneumonitis
in Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys. 66:223–228.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing J, Stewart DJ, Gu J, Lu C, Spitz MR
and Wu X: Expression of methylation-related genes is associated
with overall survival in patients with non-small cell lung cancer.
Br J Cancer. 98:1716–1722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wakelee H, Kelly K and Edelman MJ: 50
Years of progress in the systemic therapy of non-small cell lung
cancer. Am Soc Clin Oncol Educ Book. 177–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vitiello M, Tuccoli A and Poliseno L: Long
non-coding RNAs in cancer: Implications for personalized therapy.
Cell Oncol (Dordr). 38:17–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harries LW: Long non-coding RNAs and human
disease. Biochem Soc Trans. 40:902–906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li DY, Chen WJ, Luo L, Wang YK, Shang J,
Zhang Y, Chen G and Li SK: Prospective lncRNA-miRNA-mRNA regulatory
network of long non-coding RNA LINC00968 in non-small cell lung
cancer A549 cells: A miRNA microarray and bioinformatics
investigation. Int J Mol Med. 40:1895–1906. 2017.PubMed/NCBI
|
|
11
|
Yang X, Gao L, Guo X, Shi X, Wu H, Song F
and Wang B: A network based method for analysis of lncRNA-disease
associations and prediction of lncRNAs implicated in diseases. PLoS
One. 9:e877972014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong G, Lou W, Yao M, Du C, Wei H and Fu
P: Identification of novel mRNA-miRNA-lncRNA competing endogenous
RNA network associated with prognosis of breast cancer.
Epigenomics. 11:1501–1518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu J, Fu H, Wu Y and Zheng X: Function of
lncRNAs and approaches to lncRNA-protein interactions. Sci China
Life Sci. 56:876–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng Q, Ren M, Li Y and Song X:
LncRNA-RMRP acts as an oncogene in lung cancer. PLoS One.
11:e01648452016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H, Yan L, Sun K, Sun X, Zhang X, Cai
K and Song T: lncRNA BCAR4 increases viability, invasion, and
migration of non-small cell lung cancer cells by targeting
glioma-associated oncogene 2 (GLI2). Oncol Res. 27:359–369. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng ZH, Wu DM, Fan SH, Zhang ZF, Chen GQ
and Lu J: Upregulation of miR-675-5p induced by lncRNA H19 was
associated with tumor progression and development by targeting
tumor suppressor p53 in non-small cell lung cancer. J Cell Biochem.
120:18724–18735. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang B, Zhang L, Cao Y, Chen S, Cao J, Wu
D, Chen J, Xiong H, Pan Z, Qiu F, et al: Overexpression of lncRNA
IGFBP4-1 reprograms energy metabolism to promote lung cancer
progression. Mol Cancer. 16:1542017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L, Fang F, Lu S, Li X, Yang Y and Wang
Z: lncRNA-HIT promotes cell proliferation of non-small cell lung
cancer by association with E2F1. Cancer Gene Ther. 24:221–226.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sui J, Li YH, Zhang YQ, Li CY, Shen X, Yao
WZ, Peng H, Hong WW, Yin LH, Pu YP and Liang GY: Integrated
analysis of long non-coding RNA-associated ceRNA network reveals
potential lncRNA biomarkers in human lung adenocarcinoma. Int J
Oncol. 49:2023–2036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Stefano P, Damiano L, Cabodi S, Aramu
S, Tordella L, Praduroux A, Piva R, Cavallo F, Forni G, Silengo L,
et al: p140Cap protein suppresses tumour cell properties,
regulating Csk and Src kinase activity. EMBO J. 26:2843–2855. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Summy JM and Gallick GE: Src family
kinases in tumor progression and metastasis. Cancer Metastasis Rev.
22:337–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park SI, Shah AN, Zhang J and Gallick GE:
Regulation of angiogenesis and vascular permeability by Src family
kinases: Opportunities for therapeutic treatment of solid tumors.
Expert Opin Ther Targets. 11:1207–1217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu Y, Wang H and Mills GB: Targeting
PI3K-AKT pathway for cancer therapy. Rev Clin Exp Hematol.
7:205–228. 2003.PubMed/NCBI
|
|
25
|
Thakur R, Trivedi R, Rastogi N, Singh M
and Mishra DP: Inhibition of STAT3, FAK and Src mediated signaling
reduces cancer stem cell load, tumorigenic potential and metastasis
in breast cancer. Sci Rep. 5:101942015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kennedy S, Clynes M, Doolan P, Mehta JP,
Rani S, Crown J and O'Driscoll L: SNIP/p140Cap mRNA expression is
an unfavourable prognostic factor in breast cancer and is not
expressed in normal breast tissue. Br J Cancer. 98:1641–1645. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang P, Wang H, Li X, Liu Y, Zhao C and
Zhu D: SRCIN1 suppressed osteosarcoma cell proliferation and
invasion. PLoS One. 11:e01555182016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rousseaux S, Debernardi A, Jacquiau B,
Vitte AL, Vesin A, Nagy-Mignotte H, Moro-Sibilot D, Brichon PY,
Lantuejoul S, Hainaut P, et al: Ectopic activation of germline and
placental genes identifies aggressive metastasis-prone lung
cancers. Sci Transl Med. 5:186ra1662013. View Article : Google Scholar
|
|
31
|
Castro-Oropeza R, Melendez-Zajgla J,
Maldonado V and Vazquez-Santillan K: The emerging role of lncRNAs
in the regulation of cancer stem cells. Cell Oncol (Dordr).
41:585–603. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ulivi P, Foschi G, Mengozzi M, Scarpi E,
Silvestrini R, Amadori D and Zoli W: Peripheral blood miR-328
expression as a potential biomarker for the early diagnosis of
NSCLC. Int J Mol Sci. 14:10332–10342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao Y, Xue Q, Wang D, Du M, Zhang Y and
Gao S: miR-873 induces lung adenocarcinoma cell proliferation and
migration by targeting SRCIN1. Am J Transl Res. 7:2519–2526.
2015.PubMed/NCBI
|
|
34
|
Portillo JC, Muniz-Feliciano L, Lopez
Corcino Y, Lee SJ, Van Grol J, Parsons SJ, Schiemman WP and
Subauste CS: Toxoplasma gondii induces FAK-Src-STAT3 signaling
during infection of host cells that prevents parasite targeting by
autophagy. PLoS Pathog. 13:e10066712017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao M, Hou D, Liang H, Gong F, Wang Y, Yan
X, Jiang X, Wang C, Zhang J, Zen K, et al: miR-150 promotes the
proliferation and migration of lung cancer cells by targeting SRC
kinase signalling inhibitor 1. Eur J Cancer. 50:1013–1024. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang N, Liang H, Zhou Y, Wang C, Zhang S,
Pan Y, Wang Y, Yan X, Zhang J, Zhang CY, et al: miR-203 suppresses
the proliferation and migration and promotes the apoptosis of lung
cancer cells by targeting SRC. PLoS One. 9:e1055702014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang M, Meng B and Liu Y, Yu J, Chen Q and
Liu Y: MiR-124 Inhibits Growth and enhances radiation-induced
apoptosis in non-small cell lung cancer by inhibiting STAT3. Cell
Physiol Biochem. 44:2017–2028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Lin J, Liu T, Chen T, Pan S, Huang
W and Li S: Analysis of lncRNA expression profiles in non-small
cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer.
85:110–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang T, Li H, Chen T, Ren H, Shi P and
Chen M: LncRNA MALAT1 depressed Chemo-sensitivity of NSCLC cells
through directly functioning on miR-197-3p/p120 Catenin Axis. Mol
Cells. 42:270–283. 2019.PubMed/NCBI
|
|
40
|
Zhang B, Wang H, Wang Q, Xu J, Jiang P and
Li W: Knockout of lncRNA UCA1 inhibits drug resistance to gefitinib
via targeting STAT3 signaling in NSCLC. Minerva Med. 110:273–275.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen EG, Zhang JS, Xu S, Zhu XJ and Hu HH:
Long non-coding RNA DGCR5 is involved in the regulation of
proliferation, migration and invasion of lung cancer by targeting
miR-1180. Am J Cancer Res. 7:1463–1475. 2017.PubMed/NCBI
|
|
42
|
Luo J, Zhu H, Jiang H, Cui Y, Wang M, Ni X
and Ma C: The effects of aberrant expression of LncRNA
DGCR5/miR-873-5p/TUSC3 in lung cancer cell progression. Cancer Med.
May 23–2018.(Epub ahead of print). View Article : Google Scholar
|
|
43
|
Liang Q, Ding J, Xu R, Xu Z and Zheng S:
The novel human endogenous retrovirus-related gene, psiTPTE22-HERV,
is silenced by DNA methylation in cancers. Int J Cancer.
127:1833–1843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie JJ, Guo QY, Jin JY and Jin D:
SP1-mediated overexpression of lncRNA LINC01234 as a ceRNA
facilitates non-small-cell lung cancer progression via regulating
OTUB1. J Cell Physiol. 234:22845–22856. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan R, Jiang Y, Lai B, Lin Y and Wen J:
The positive feedback loop FOXO3/CASC11/miR-498 promotes the
tumorigenesis of non-small cell lung cancer. Biochem Biophys Res
Commun. 519:518–524. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ding H, Liu J, Zou R, Cheng P and Su Y:
Long non-coding RNA TPTEP1 inhibits hepatocellular carcinoma
progression by suppressing STAT3 phosphorylation. J Exp Clin Cancer
Res. 38:1892019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Luo T, Yan Y, He Q, Ma X and Wang W:
miR-328-5p inhibits MDA-MB-231 breast cancer cell proliferation by
targeting RAGE. Oncol Rep. 39:2906–2914. 2018.PubMed/NCBI
|
|
48
|
Wang C, Wang S, Ma F and Zhang W:
miRNA-328 overexpression confers cisplatin resistance in nonsmall
cell lung cancer via targeting of PTEN. Mol Med Rep. 18:4563–4570.
2018.PubMed/NCBI
|
|
49
|
Liu Z, Yu Y, Huang Z, Kong Y, Hu X, Xiao
W, Quan J and Fan X: CircRNA-5692 inhibits the progression of
hepatocellular carcinoma by sponging miR-328-5p to enhance DAB2IP
expression. Cell Death Dis. 10:9002019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang S, Li P, Zhao L and Xu L: LINC00210
as a miR-328-5p sponge promotes nasopharyngeal carcinoma
tumorigenesis by activating NOTCH3 pathway. Biosci Rep. 38(pii):
BSR201811682018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Johnson FM and Gallick GE: SRC family
nonreceptor tyrosine kinases as molecular targets for cancer
therapy. Anticancer Agents Med Chem. 7:651–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Byers LA, Sen B, Saigal B, Diao L, Wang J,
Nanjundan M, Cascone T, Mills GB, Heymach JV and Johnson FM:
Reciprocal regulation of c-Src and STAT3 in non-small cell lung
cancer. Clin Cancer Res. 15:6852–6861. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen R, Liao JY, Huang J, Chen WL, Ma XJ
and Luo XD: Downregulation of SRC Kinase signaling inhibitor 1
(SRCIN1) expression by MicroRNA-32 promotes proliferation and
Epithelial-mesenchymal transition in human liver cancer cells.
Oncol Res. 26:573–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang F, Luo LJ, Zhang L, Wang DD, Yang SJ,
Ding L, Li J, Chen D, Ma R, Wu JZ and Tang JH: MiR-346 promotes the
biological function of breast cancer cells by targeting SRCIN1 and
reduces chemosensitivity to docetaxel. Gene. 600:21–28. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Han N, Zhao W, Zhang Z and Zheng P:
MiR-328 suppresses the survival of esophageal cancer cells by
targeting PLCE1. Biochem Biophys Res Commun. 470:175–180. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liang F, Cui ZJ, Liu JD, Liu KP, Li L and
Chen YL: Downregulated miR-328 suppressed cell invasion and growth
in hepatocellular carcinoma via targeting PTEN. Eur Rev Med
Pharmacol Sci. 22:6324–6332. 2018.PubMed/NCBI
|