Introduction
Breast cancer remains the most common cancer type in
women worldwide and accounts for more than 25% of all cancer types
and 15% of all cancer-related deaths in females (1). Breast cancer is classified into
estrogen receptor-positive (ER+) breast cancer,
epidermal growth factor receptor-2-positive (Her2+)
breast cancer and triple-negative breast cancer
(ER−/progesterone receptor-negative
(PR−)/Her2−; TNBC) according to the
expression of ER, PR and Her2 (2).
Hormonal therapy and Her2-targeted monoclonal antibodies have
significantly improved the clinical outcome of ER+ and
Her2+ breast cancer (3,4).
However, the relatively low response rate and the development of
resistance during treatment limits their therapeutic efficacy in
numerous ER+ or Her2+ breast cancer patients
(5,6). The treatment of TNBC patients depends
solely on chemotherapy, which reduces the overall survival rate
(7). Therefore, it is urgent to
investigate the molecular mechanisms that promote breast cancer
progression and develop new treatment strategies for patients with
breast cancer.
The successful application of deep sequencing
technologies has disproved the notion that large proportion of
non-translated DNA is ‘junk’ (8).
Accumulating evidence demonstrates that non-coding RNAs (ncRNAs)
are involved in normal cellular processes and that their
deregulation is closely associated with disease progression
(9). Based on length, ncRNAs can be
divided into short ncRNA (miRNA, siRNA and piRNA) and long ncRNA
(lncRNA) (10). Aberrant expression
of several lncRNAs has been reported in human diseases, including
breast cancer (11,12). However, the number of lncRNAs that
have been experimentally identified as oncogenes or tumor
suppressors in breast cancer is considerably low (13–15).
lncRNAs can regulate gene expression by sponging miRNAs, binding to
promoters or directly interacting with proteins (16–18).
Small nucleolar RNA host gene 1 (SNHG1) is a recently discovered
lncRNA with oncogenic potential in various cancer types (19,20).
An increase in the levels of SNHG1 was found to promote cancer cell
proliferation and migration by sponging several miRNAs (21,22). A
recent study revealed that the upregulation of SNHG1 promoted
breast cancer cell proliferation and invasion (23). The molecular mechanisms of the
contribution of SNHG1 to breast cancer development require further
investigation.
LIM domain only 4 (LMO4) is a family member of the
LIM-only subclass of LIM proteins. Its expression is tightly
regulated in mammary gland and aberrant expression of LMO4 leads to
differentiation blockade of mammary epithelial cells (24). Overexpression of LMO4 is frequently
observed in several cancer types including breast cancer (25,26).
It was previously reported that the transcriptional regulation of
LMO4 expression is mediated by p53 in breast cancer (27).
In the present study, the results demonstrated that
SNHG1 levels were elevated in breast cancer tumor tissues and cell
lines. Knockdown of SNHG1 inhibited cell proliferation and cell
migration of breast cancer cells and induced cell cycle arrest at
the G2/M phase. Additional analysis demonstrated that SNHG1 could
sponge miR-573 to increase LMO4 expression in the breast cancer
cell lines tested. Overexpression of LMO4 was able to reverse SNHG1
knockdown-induced cell proliferation and cell cycle alteration in
breast cancer cells as demonstrated by in vitro assays. In
addition, SNHG1 knockdown inhibited MDA-MB-231 tumor growth in
vivo, which was reversed by LMO4 overexpression. Moreover,
SNHG1 expression exhibited a positive correlation with LMO4
mRNA expression in breast cancer tumor tissues. The present
findings revealed an oncogenic role of SNHG1 in breast cancer and
suggested that it may promote cell proliferation and cell cycle
progression via the miR-573/LMO4 axis.
Materials and methods
Bioinformatic analysis
Bioinformatic analysis of SNHG1 expression was
performed in 1,063 breast cancer cases and 102 normal breast cases
using the Human Cancer Metastasis Database (HCMDB, http://hcmdb.i-sanger.com/). The Cancer Genome Atlas
Breast Invasive Carcinoma (TCGA-BRCA) dataset was selected. The
prediction of the potential binding site between miR-573 and SNHG1
and LMO4 was carried out by miRDB (http://www.mirdb.org/) and miRanda software
(http://www.microrna.org). The PROGgeneV2
(http://genomics.jefferson.edu/proggene/index.php)
was used to study the association between LMO4 expression and the
overall survival of patients with breast cancer based on the
GSE42568 dataset (28).
Human tissue samples
Human breast cancer tumor tissues and matched normal
breast tissues were collected from 50 patients with breast cancer
at The Second Xiangya Hospital of Central South University from
June 2014 to July 2017. All tissues were obtained following surgery
of primary breast cancer tumors and were immediately frozen in
liquid nitrogen for subsequent experiments. Prior to project
initiation, written informed consent was provided by all patients
enrolled in the present study and the experimental procedures were
conducted under the supervision of the Ethics Committee of the
Second Xiangya Hospital of the Central South University. The
protocol of the experiments was approved by the Ethics Committee of
the Second Xiangya Hospital of the Central South University
(approval no. 2014S057).
Cell culture
293 cells, the human breast epithelial cell line
MCF10A, the human ER+ breast cancer cell lines MCF7, and
T47D, and the human triple-negative breast cancer (TNBC) cell lines
(ER−/PR−/Her2−) MDA-MB-231 and
MDA-MB-468 were purchased from the American Type Culture Collection
(ATCC). The cell lines were used within 6 months following receipt.
MCF10A cells were cultured in Mammary Epithelial Cell Growth Medium
(MEGM; Lonza) supplemented with 100 ng/ml cholera toxin
(Sigma-Aldrich; Merck KGaA). 293, MCF7 and T47D cells were cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (HyClone; GE Healthcare). MDA-MB-231 and
MDA-MB-468 cells were maintained in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (HyClone; GE Healthcare). All
cell lines were cultured in a humidified incubator with 5%
CO2.
Plasmid construction and cell
transfection
The full length of the LMO4 open reading frame was
amplified from the cDNA of 293 cells and ligated into a pcDNA3.1
plasmid. Plasmid transfection was performed using Lipofectamine
3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer protocol. SNHG1 siRNA and control siRNA were purchased
from GenePharma. The sequence for SNHG1 siRNA was
CAGCAGTTGAGGGTTTGCTGTGTAT. The transfection of SNHG1 siRNA or
control siRNA sequences was achieved using LipoRNAiMax reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) in serum-free medium
and sustained for 5 min until addition into the culture medium.
miR-NC mimic, miR-573 mimic, miR-NC inhibitor and miR-573 inhibitor
were purchased from Applied Biological Materials (ABM). The
transfection of the mimic or inhibitor sequences was carried out
using Lipofectamine 3000.
Establishment of stable cell
lines
For stable knockdown of SNHG1, lentiviral particles
were prepared by co-transfection of pLko.1-SNHG1 shRNA, pMD2G and
pCMV-dR8.91 into 293 cells using Lipofectamine 2000. Following 72 h
of culture, the medium containing lentiviral particles was obtained
and filtered through a 0.45-µm filter (Millipore). The medium
containing lentiviral particles was added to the MDA-MB-231 cells
in 6-well plates. Following 48 h of culture, the culture medium was
replaced with fresh medium containing 5 mg/ml puromycin (Solarbio)
for 24 h to select the cells successfully infected with the
lentivirus. For establishment of SNHG1-knockdown and
LMO4-overexpression models, MDA-MB-231 cells and shSNHG1 MDA-MB-231
cells were transfected with the pcDNA3.1-LMO4 plasmid using
Lipofectamine 3000. Following 24 h of cell culture, the cells were
screened with 4 mg/ml G418 (Sigma-Aldrich) for the following 72 h
of growth.
RNA extraction and real-time
RT-PCR
Total RNA from tissues and cells was prepared using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
separation of nuclear and cytoplasmic RNA was accomplished using a
PARIS kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Gene expression was detected
following reverse transcription of RNA into cDNA using PrimeScript™
RT Reagent Kit (Takara Bio, Inc.). miR-573 expression was performed
using a stem-loop specific primer method. Real-time RT-PCR was
conducted using the SYBR Premix Ex Taq (Takara Bio, Inc.). The
expression levels of genes or miRNAs was analyzed using the
2−ΔΔCq method (29).
GAPDH and U6 were used as internal controls for gene and miRNA
expression analysis, respectively. The primer sequences are listed
in Table I.
 | Table I.List of primer sequences used for
real-time RT-PCR. |
Table I.
List of primer sequences used for
real-time RT-PCR.
| miRNA/gene
name | Sequences |
|---|
| miR-573-RT |
CTCAACTGGTGTCGTGGAGTCGG |
|
|
CAATTCAGTTGAGCACAGGGC |
|
miR-573-forward |
GCCGAGCTGAAGTGATGTGT |
|
miR-573-reverse |
CTCAACTGGTGTCGTGGA |
| U6-RT |
AACGCTTCACGAATTTGCGT |
|
U6-forward |
CTCGCTTCGGCAGCACA |
|
U6-reverse |
TGGTGTCGTGGAGTCG |
|
SNHG1-forward |
AGGCTGAAGTTACAGGTC |
|
SNHG1-reverse |
TTGGCTCCCAGTGTCTTA |
|
GAPDH-forward |
AAGGTGAAGGTCGGAGTCA |
|
GAPDH-reverse |
GGAAGATGGTGATGGGATTT |
Protein extraction and western blot
analysis
Protein lysates were prepared using RIPA lysis
buffer (Sigma-Aldrich; Merck KGaA). The antibodies for LMO4 (cat.
no. ab131030; dilution 1:2,000) and GAPDH (cat. co. AMAB91153;
dilution 1:10,000) detection were purchased from Abcam and
Sigma-Aldrich/Merck KGaA, respectively. Cyclin D1 (cat. no. 2978;
dilution 1:2,000) and cyclin E1 (cat. no. 4129; dilution 1:2,000)
antibodies were obtained from Cell Signaling Technology. Secondary
antibodies for mouse (cat. no. SA00001-1; dilution 1:10,000) and
rabbit (cat. no. SA00001-2; dilution 1:10,000) were obtained from
Proteintech. Briefly, 25 µg proteins per lane were separated on an
8% SDS gel and transferred to a PVDF membrane. Following transfer,
the membrane was blocked in 5% non-fat milk and incubated in the
presence of the primary antibodies overnight at 4°C. On the next
day, the membrane was incubated with secondary antibodies for an
additional 1 h at room temperature. The protein bands were
developed using ECL substrate (Thermo Fisher Scientific, Inc.). The
intensity of the bands was quantified using ImageJ (V. 1.6.0;
National Institutes of Health).
Dual luciferase reporter assay
The 3′ untranslated region (3′UTR) of the pGL3
construct containing wild-type LMO4 3′UTR (LMO4 3′UTR-WT) and the
pGL3 construct containing wild-type SNHG1 (SNHG1 3′UTR-WT) were
prepared by PCR of the cDNA derived from 293 cells. Two site
mutations were introduced into the putative seed regions of LMO4
3′UTR-WT and SNHG1 3′UTR-WT in order to produce mutant constructs.
The dual luciferase assay was performed using cells that were
co-transfected with either wild-type or mutant-type luciferase
reporter plasmids, pRL-TK plasmid, miR-NC mimic or miR-573 mimic
sequences. The transfections were performed using Lipofectamine
3000. The relative luciferase activity was measured at 24 h
following transfection using a Dual-Luciferase reporter assay
system (Promega Corp.) according to the manufacturer's
protocol.
Cell proliferation assay
The cell proliferative ability was measured using a
Cell Counting Kit-8 (CCK-8; Dojindo Laboratories). On the first
day, the cells were seeded in each well of a 96-well plate. On the
next day, the cells were transfected with the indicated siRNA,
miRNA mimic or miRNA inhibitor sequences. The cell viability was
subsequently examined at 24-h time points between days 1 to 4, by
addition of 10 µl CCK-8 solution into the culture medium. The
solution was incubated for 2 h and the medium containing CCK-8 was
aspirated from the wells and added to another 96-well plate. The
absorbance at 450 nm of each well was measured to estimate the cell
number.
Cell cycle analysis
Transfected cells were harvested and fixed with
ice-cold 70% ethanol at 4°C overnight. The cells were stained with
propidium iodide (PI; Sigma-Aldrich; Merck KGaA) for 30 min and
analyzed using flow cytometry. The percentage of the cells present
in each cell cycle phase was counted.
Cell migration assay
The wound healing assay was applied to detect cell
migratory activity. The cells were grown in 6-well plates at 90%
confluence and subsequently transfected with siRNA for 24 h. The
cell layer was scratched with a 10-µl pipette tip at the central
area and subsequently washed with PBS. Serum-free DMEM was added.
The images of the wound area were captured at the 0 and 24 h time
points following the initial scratch. The closure areas of all
wells were quantified using Image-Pro Plus (V. 6.0; Media
Cybernetics).
Tumorigenesis in nude mice
Female, 5-week-old nude mice (BALB/c-null) were
purchased from the Shanghai Laboratory Animal Center (Chinese
Academy of Sciences, China) and bred under SPF conditions. The
present study was approved by the Ethics Committee of the Second
Xiangya Hospital of the Central South University. The mice were
randomly divided into the three following groups (n=3): MDA-MB-231,
shSNHG1-MDA-MB-231 and shSNHG1+LMO4 OE MDA-MB-231. The cells from
each group were subcutaneously injected into the mammary armpit of
the mouse fat pad. The tumor size was measured every five days with
a caliper. The tumor volume was estimated according to the
following formula: Volume = 0.5×LengthxWidth2. The mice
were sacrificed by decapitation at 35 days following cell injection
and the tumors were dissected.
Immunohistochemistry
A total of 10 formalin-fixed, paraffin-embedded
biopsy breast tumors were available. Immunohistochemistry was
performed as described previously (30). The samples were stained with the
LMO4 antibody (dilution, 1:100) used in the western blotting
experiments. Diaminobenzidine (DAB; Boster Inc.) was used for color
development. The specimens were observed via a Olympus BX51 light
microscope (Olympus Corp.) at ×100 magnification.
Statistical analysis
The data presented in the present study were
calculated using GraphPad Prism 7 software (GraphPad Software,
Inc.) and are represented as mean ± SD. The comparison between the
two groups was achieved using the paired Student's t-test. The
differences among the three groups were analyzed using one-way
ANOVA followed by the Student-Newman-Keul test. The comparison for
more than 3 groups was performed using one-way ANOVA followed by
the Tukey's test. The values were considered significantly
different at P<0.05.
Results
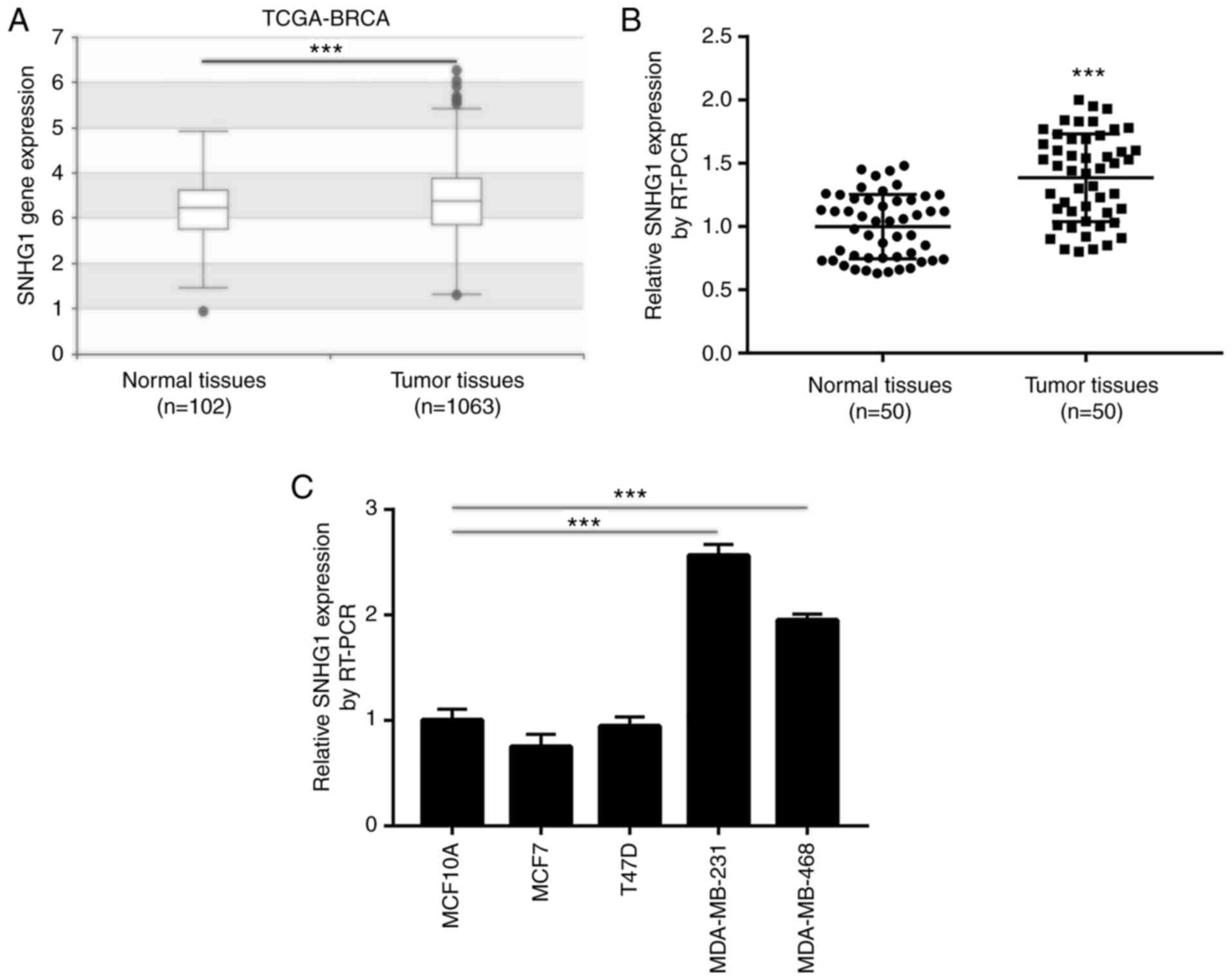
SNHG1 is overexpressed in breast
cancer tumor tissues and cell lines, notably in
ER−/PR− breast cancer
The expression levels of SNHG1 in breast cancer have
not been previously studied. To explore the expression profile of
SNHG1 in breast cancer, its expression levels were assessed in 102
normal breast and 1,063 primary breast cancer tissues derived from
the TCGA-BRCA dataset. A slight yet significant elevation in SNHG1
levels was observed in breast cancer tissues compared with that
noted in normal tissues (Fig. 1A).
Subsequently, RT-PCR was applied to detect SNHG1 expression in 50
tissue pairs derived from breast cancer and normal breast tissues.
The results demonstrated that SNHG1 expression was significantly
higher in breast cancer tissues compared with that noted in normal
breast tissues (Fig. 1B). In
addition, the increase in the levels of SNHG1 was associated with
advanced pathological stage, as well as ER− and
PR− status (Table II).
However, SNHG1 expression levels were not associated with factors,
such as age, lymph node metastasis and Her2 status (Table II). Furthermore, RT-PCR indicated
that SNHG1 levels were increased in TNBC MDA-MB-231 and MDA-MB-468
cell lines compared with those noted in the normal epithelial
breast cell line MCF10A (Fig. 1C).
SNHG1 expression levels were not increased significantly in the
ER+ breast cancer cell lines MCF7 and T47D compared with
those noted in the MCF10A cell line (Fig. 1C). These results indicated that high
expression levels of SNHG1 were associated with breast cancer
incidence, notably with ER−/PR− breast
cancer. Therefore, MDA-MB-231 and MDA-MB-468 cells were used for
the following experiments.
 | Table II.Association between
clinicopathological variables and SNHG1 expression in 50 breast
cancer patients. |
Table II.
Association between
clinicopathological variables and SNHG1 expression in 50 breast
cancer patients.
|
|
| SNHG1
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | No. of cases | High | Low | P-value |
|---|
| Age (years) |
|
|
| 0.567 |
|
>50 | 29 | 13 | 16 |
|
|
≤50 | 21 | 12 | 9 |
|
| Lymph node
metastasis |
|
|
| 0.087 |
| No | 23 | 8 | 15 |
|
|
Yes | 27 | 17 | 10 |
|
| Pathological
stage |
|
|
| 0.001 |
|
I–II | 18 | 3 | 15 |
|
|
III–IV | 32 | 22 | 10 |
|
| ER status |
|
|
| 0.001 |
|
Negative | 20 | 16 | 4 |
|
|
Positive | 30 | 9 | 21 |
|
| PR status |
|
|
| 0.045 |
|
Negative | 22 | 15 | 7 |
|
|
Positive | 28 | 10 | 18 |
|
| Her2 status |
|
|
| 0.538 |
|
Negative | 35 | 19 | 16 |
|
|
Positive | 15 | 6 | 9 |
|
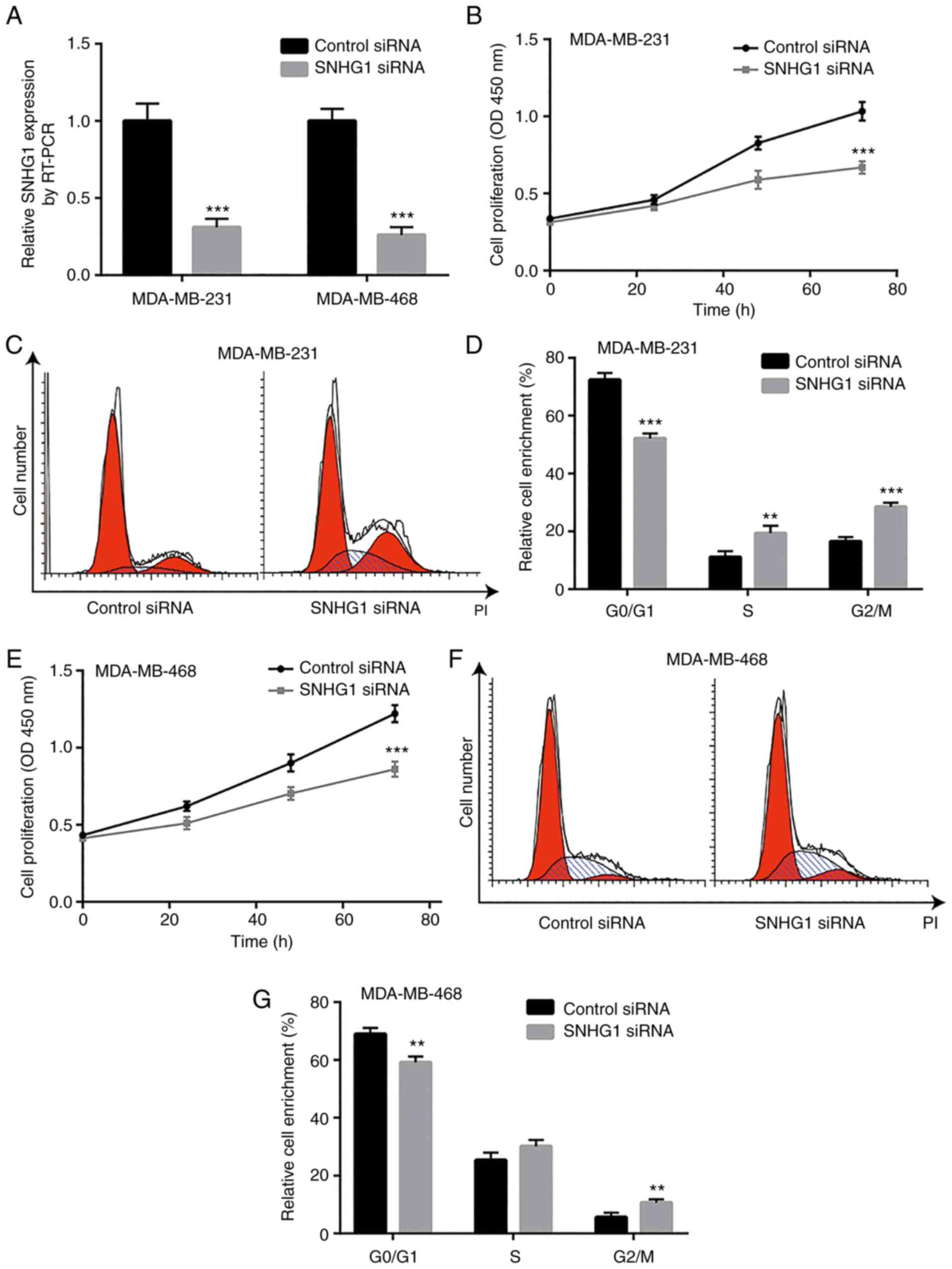
Knockdown of SNHG1 disrupts cell cycle
progression and induces cell cycle arrest in breast cancer
cells
The increase in the levels of SNHG1 in breast cancer
cells suggest a potential oncogenic role of this lncRNA. To
evaluate the function of SNHG1 in breast cancer cells,
siRNA-mediated knockdown of SNHG1 was performed to assess cell
proliferation of MDA-MB-231 and MDA-MB-468 cells. Transfection of
the cells with SNHG1 siRNA significantly decreased SNHG1 expression
in both MDA-MB-231 and MDA-MB-468 cells compared with control siRNA
transfection (Fig. 2A). In
MDA-MB-231 cells, knockdown of SNHG1 significantly inhibited cell
growth as determined by CCK-8 cell viability assay (Fig. 2B). Although significant activation
of cell apoptosis was not observed following SNHG1 downregulation
(data not shown), flow cytometric analysis of cell cycle
distribution demonstrated that SNHG1 knockdown resulted in cell
cycle redistribution with an accumulation of cells in the S and
G2/M phases (Fig. 2C and D). In the
MDA-MB-468 cell line, knockdown of SNHG1 also induced cell growth
arrest (Fig. 2E). Specifically, the
number of MDA-MB-468 cells that accumulated in the G2/M phase was
significantly increased (Fig. 2F and
G). These data suggest that SNHG1 may promote breast cancer
cell proliferation via the G2/M cell cycle checkpoint.
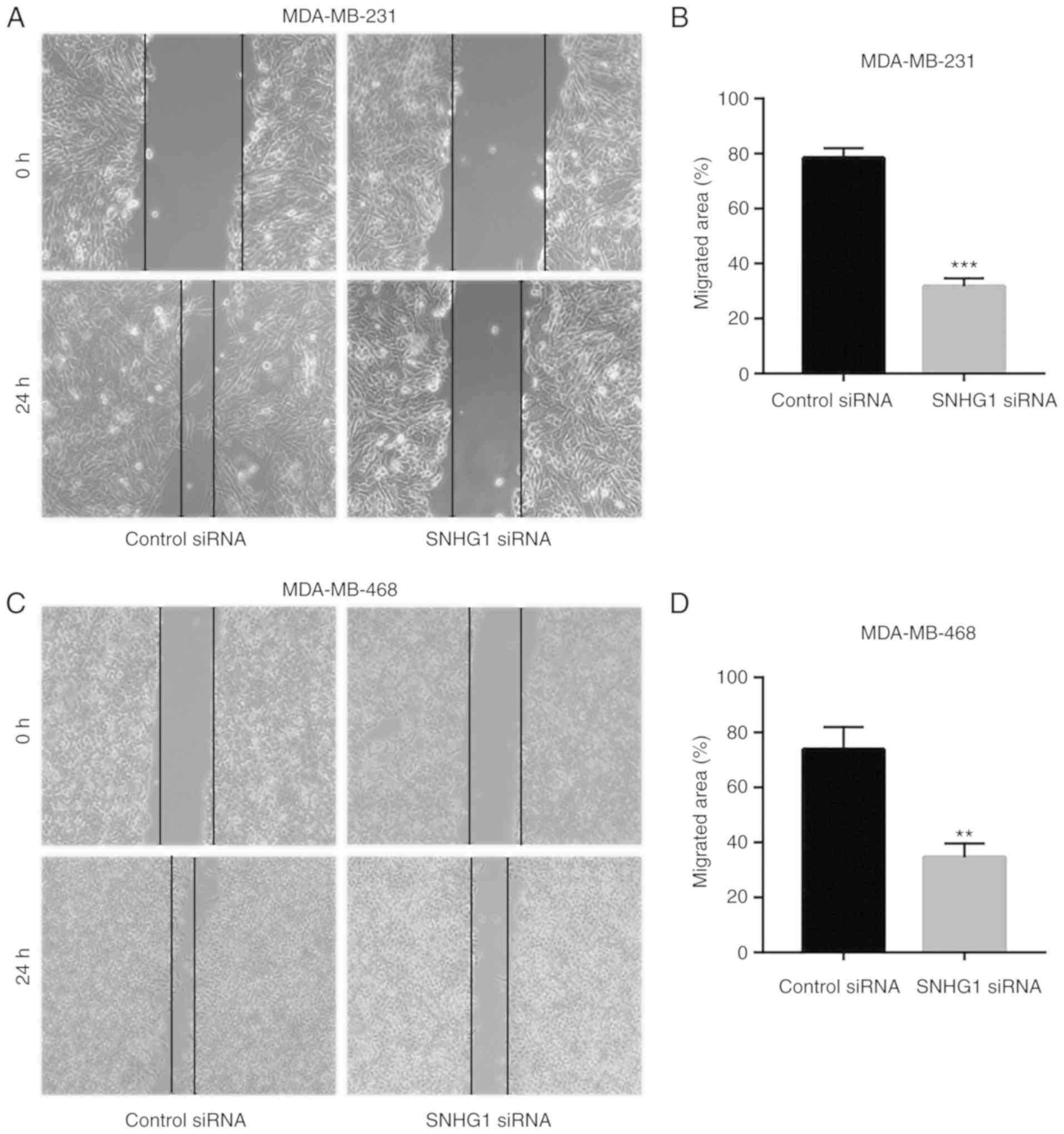
SNHG1 knockdown inhibits cell
migration of breast cancer cells
Tumor metastasis is considered a major mortality
cause in breast cancer patients (31). To investigate whether SNHG1
regulates breast cancer cell migration, wound healing assays were
performed to detect cell migration following SNHG1 knockdown.
Knockdown of SNHG1 significantly decreased the wound closure area
of the MDA-MB-231 cells (Fig. 3A and
B), suggesting that SNHG1 regulates the cell migratory activity
of breast cancer cells. Similarly, SNHG1 knockdown reduced the cell
migratory activity of the MDA-MB-468 cells (Fig. 3C and D).
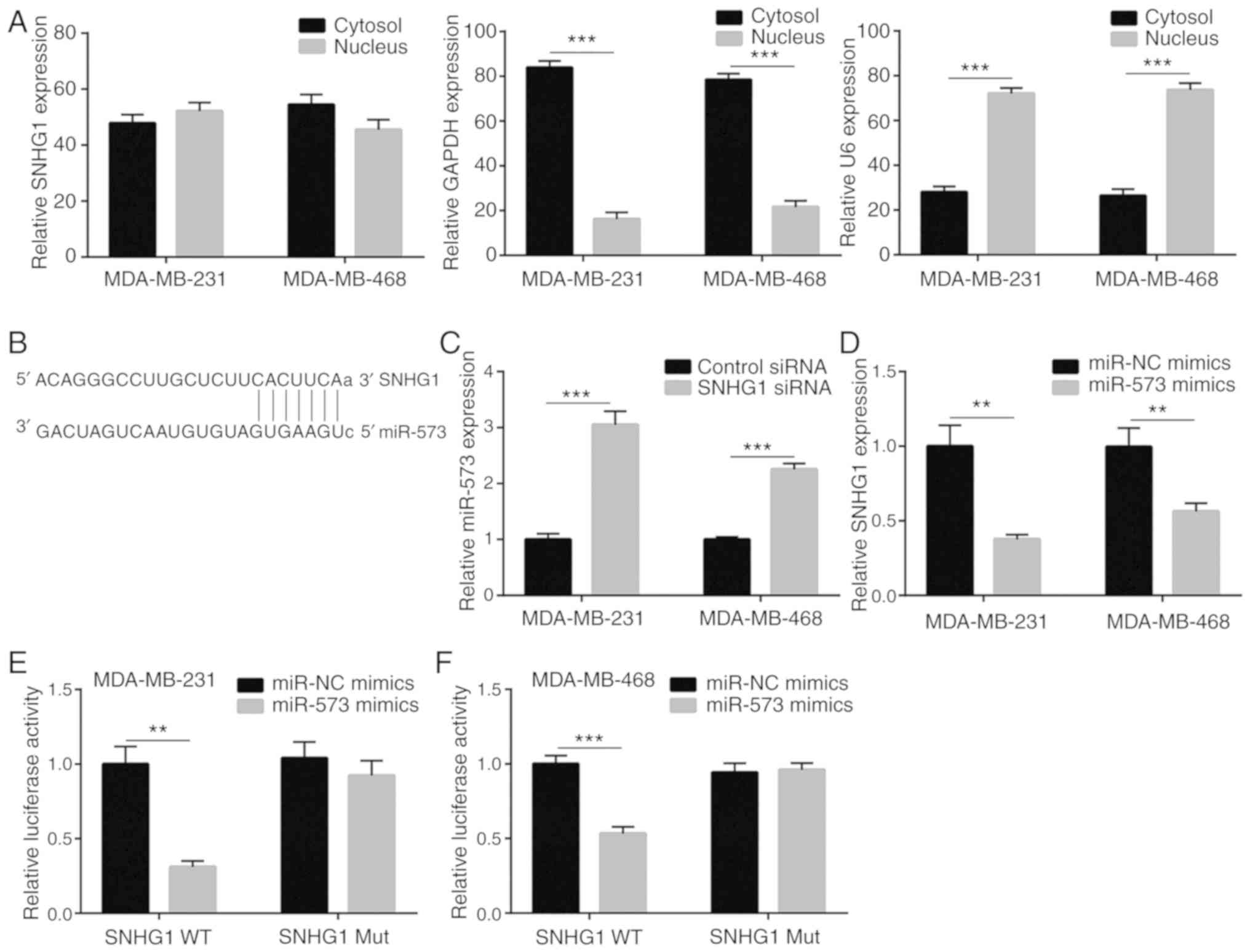
SNHG1 is located in nuclear and
cytoplasmic regions and sponges miR-573 in breast cancer cells
The molecular mechanism of SNHG1 was examined in
breast cancer by detecting its nuclear and cytoplasmic
localization. Following isolation of the nuclear and cytoplasmic
RNA of MDA-MB-231 and MDA-MB-468 cells, RT-PCR analysis
demonstrated that nearly half of SNHG1 was present in the cytoplasm
(Fig. 4A), suggesting that it may
act as an miRNA and ceRNA, as previously reported (32). Bioinformatic analysis using the
miRDB software suggested a potential binding site between SNHG1 and
miR-573 (Fig. 4B). Based on the
prediction, it was speculated that SNHG1 could negatively regulate
miR-573 in breast cancer cells. Knockdown of SNHG1 significantly
increased miR-573 levels in both MDA-MB-231 and MDA-MB-468 cell
lines (Fig. 4C). Moreover,
overexpression of miR-573 by transfection of miR-573 mimic
significantly decreased SNHG1 expression in both cell lines tested
(Fig. 4D). To further validate the
direct binding of SNHG1 with miR-573, luciferase plasmids
containing wild-type WT and mutant Mut SNHG1 (with two site
mutations in the complementary sequence) were constructed. Using
dual luciferase reporter assays, co-transfection of miR-573 mimic
and WT SNHG1 exhibited significantly decreased luciferase activity
in the MDA-MB-231 cells (Fig. 4E).
Similar results were also observed in the MDA-MB-468 cells
(Fig. 4F). Therefore, SNHG1
functions as a negative regulator of miR-573 in breast cancer
cells.
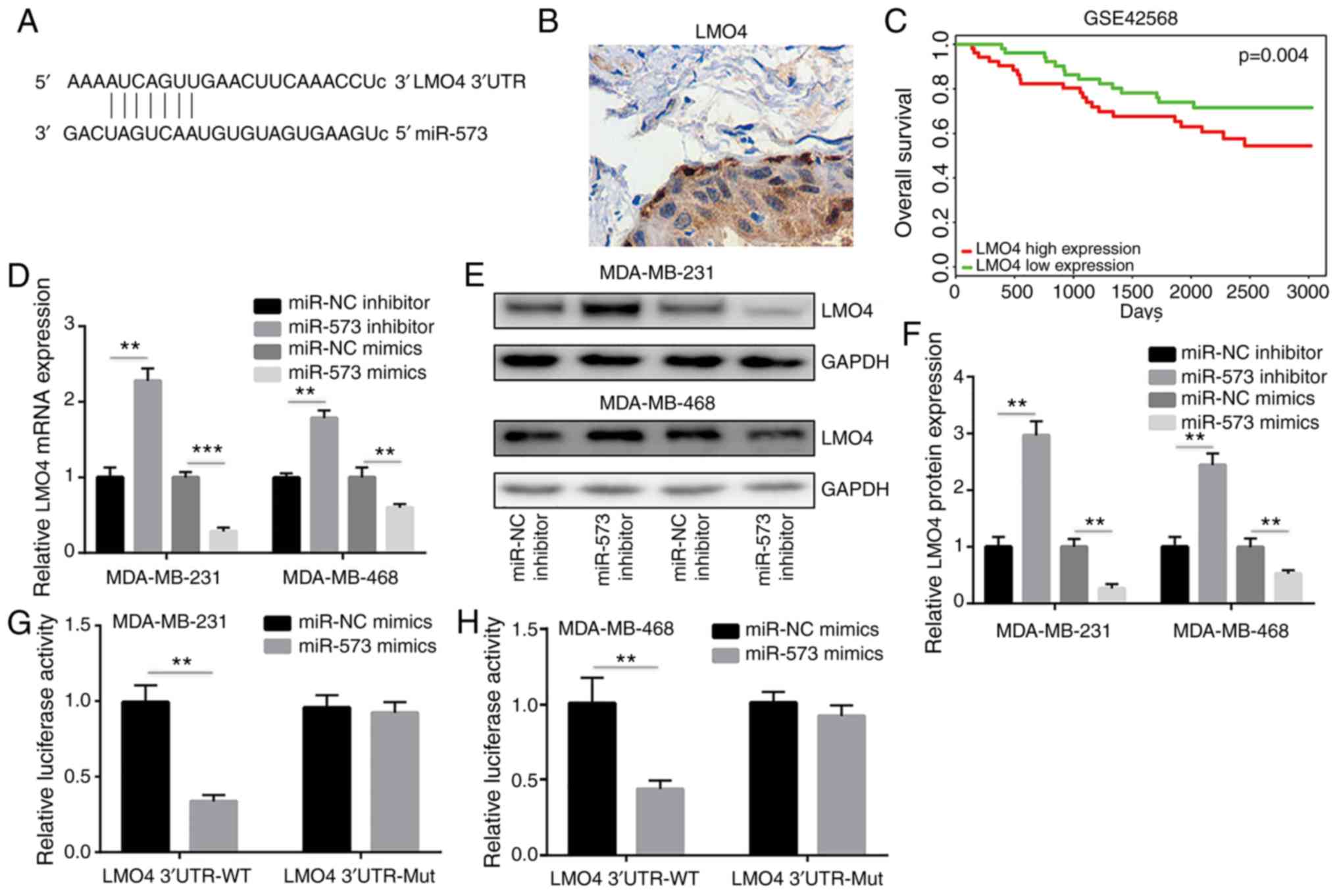
miR-573 binds directly to LMO4 to
repress its expression
miRNAs regulate gene expression by binding to the
3′UTR of target gene mRNA sequences leading to their degradation or
the inhibition of their translation (33). The miRanda software was used to
demonstrate that the seed region of miR-573 matched the 3′UTR of
LMO4 mRNA (Fig. 5A).
LMO4 is an oncogene and is overexpressed in breast cancer
(34). Immunohistochemical analysis
was used to detect LMO4 expression in 10 breast cancer tumors.
Positive expression of LMO4 was observed in the majority of breast
cancer tumors (8/10) (Fig. 5B). The
prognostic value of LMO4 was analyzed in breast cancer. Based on
the GSE42568 (n=104) dataset, high expression levels of LMO4 were
associated with reduced overall survival time of the patients with
breast cancer (Fig. 5C), suggesting
that LMO4 may promote breast cancer progression. In MDA-MB-231 and
MDA-MB-468 cells, the transfection of the miR-573 inhibitor
significantly increased LMO4 mRNA expression, while the
miR-573 mimic decreased LMO4 mRNA levels (Fig. 5D). Western blot analysis further
revealed that miR-573 inhibition significantly increased LMO4
protein expression, whereas transfection of the cells with miR-573
mimic significantly reduced LMO4 protein levels (Fig. 5E and F). The direct binding of
miR-573 to the LMO4 3′UTR sequence was confirmed in a dual
luciferase reporter assay. The overexpression of miR-573 decreased
the relative luciferase activity in MDA-MB-231 cells transfected
with the LMO4 3′UTR WT sequence, while the luciferase activity of
the cells transfected with LMO4 3′UTR Mut sequences was not altered
compared with that of the miR-573 mimic (Fig. 5G). Similarly, miR-573 overexpression
reduced luciferase activity of MDA-MB-468 cells transfected with
the LMO4 3′UTR WT sequence (Fig.
5H). These results indicated that miR-573 directly repressed
LMO4 expression in breast cancer cells.
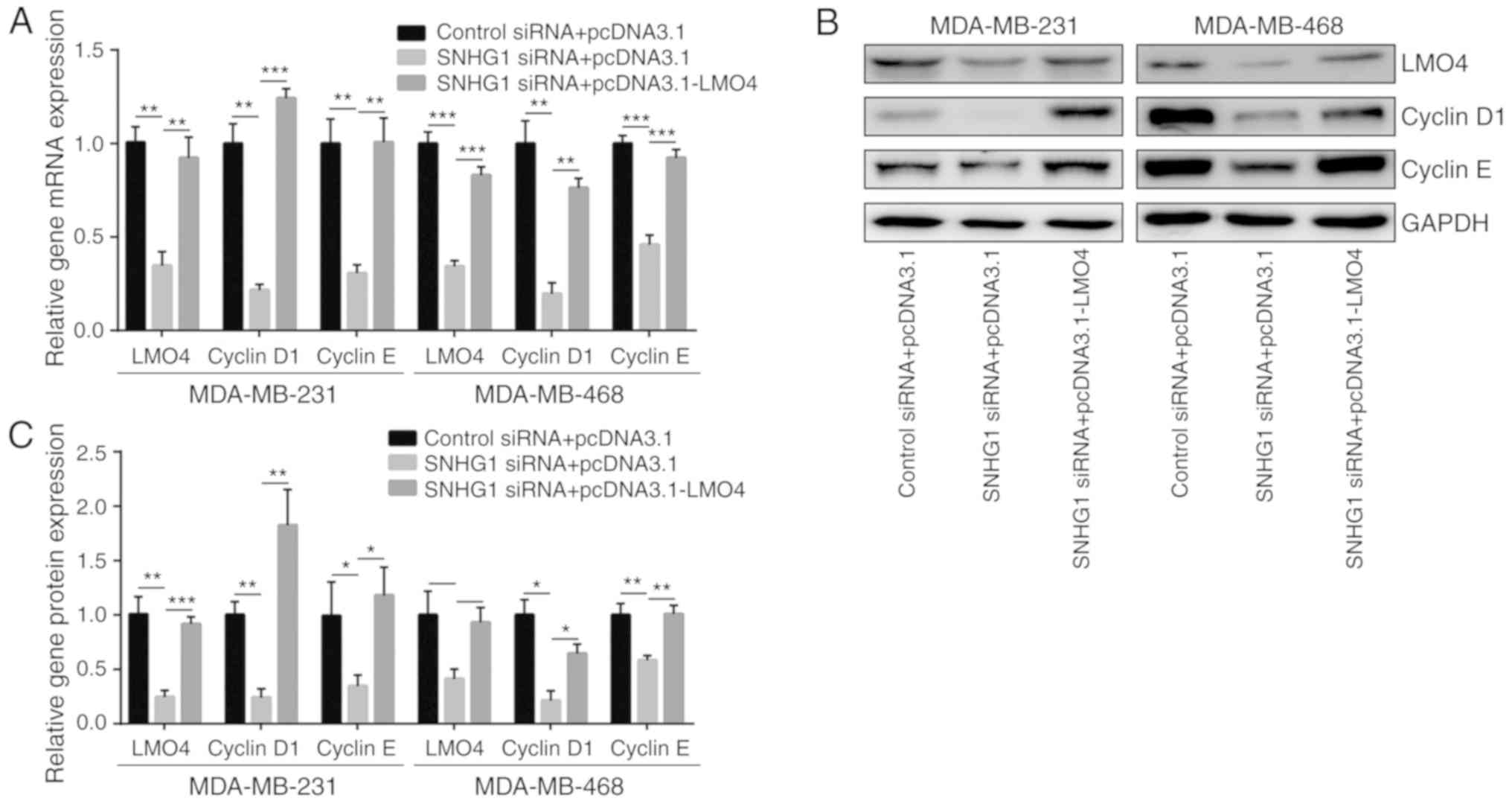
SNHG1 knockdown downregulates LMO4
expression and the expression of key cell cycle regulator proteins
in breast cancer cells
The aforementioned results demonstrated that SNHG1
could sponge miR-573, which in turn suppressed LMO4 expression,
suggesting that SNHG1 may control LMO4 expression in breast cancer
cells. As expected, SNHG1 knockdown led to a decrease in
LMO4 mRNA levels in MDA-MB-231 and MDA-MB-468 cells
(Fig. 6A). LMO4 is a transcription
factor that activates cyclin D1 and E1 transcription and mediates
cell cycle progression (35).
Knockdown of SNHG1 reduced cyclin D1 and cyclin E1 mRNA levels
(Fig. 6A). Moreover, the decrease
in the levels of cyclin D1 and cyclin E1 was reversed by
transfection of recombinant LMO4 (Fig.
6A). Western blot analysis further indicated that SNHG1
knockdown reduced LMO4, cyclin D1 and cyclin E1 protein levels.
These changes were reversed by pcDNA3.1-LMO4 transfection in both
cell lines (Fig. 6B and C). The
RT-PCR and western blot results revealed the effects of the
SNHG1/miR-573/LMO4 axis in breast cancer cell lines.
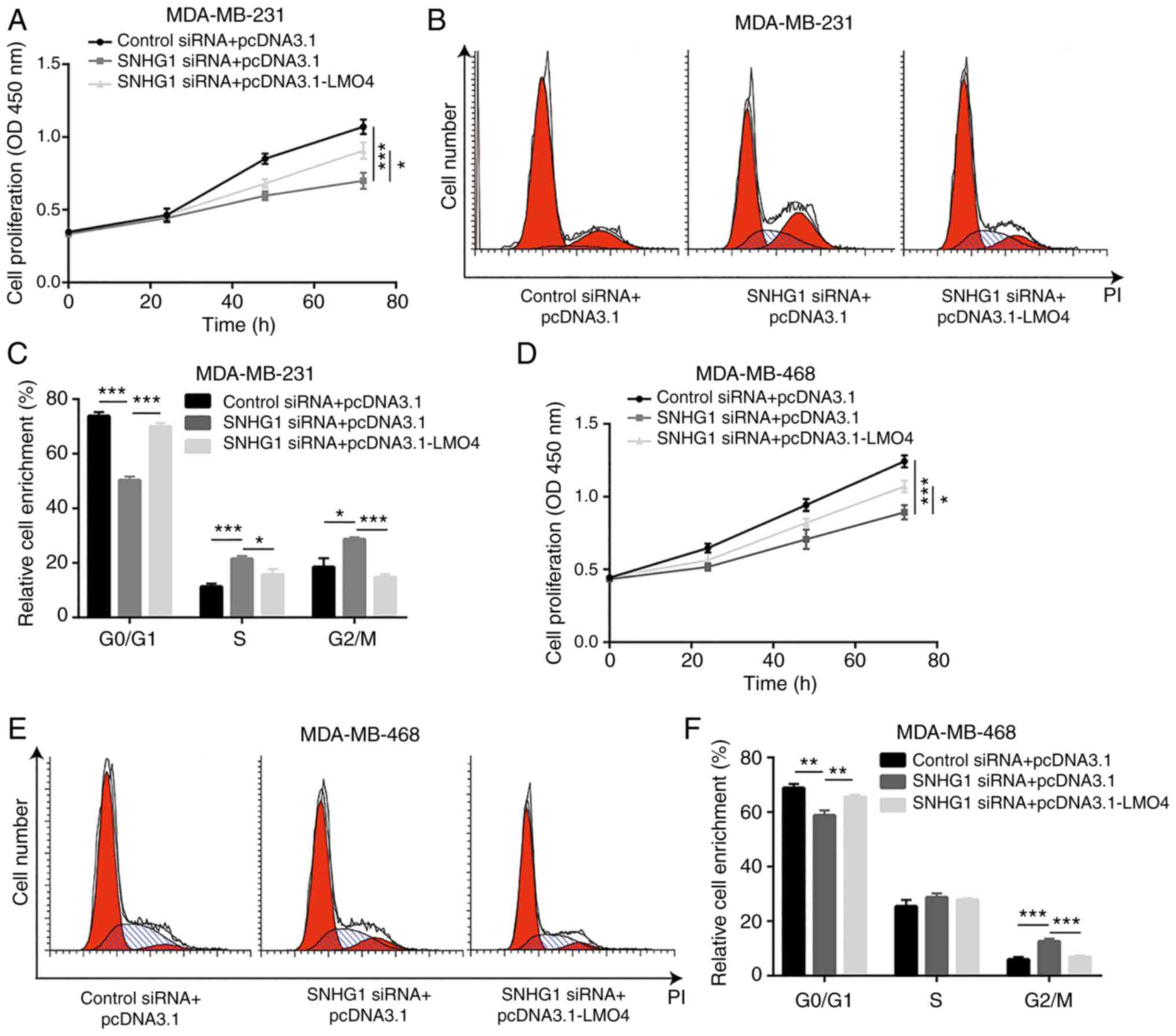
LMO4 is required for SNHG1-mediated
regulation of cell proliferation in breast cancer cells
The regulation of breast cancer cell proliferation
by SNHG1 was assessed by cell proliferation of MDA-MB-231 cells
transfected with SNHG1 siRNA or SNHG1 siRNA in the presence of
pcDNA3.1-LMO4. The increase in the levels of LMO4 partially
reversed SNHG1 knockdown-mediated cell growth arrest in MDA-MB-231
cells (Fig. 7A). In addition,
overexpression of LMO4 reversed the accumulation of cells at the
G2/M phase following SNHG1 knockdown (Fig. 7B and C). Similarly, LMO4
overexpression reversed cell proliferation inhibition and cell
cycle redistribution induction by SNHG1 knockdown in MDA-MB-468
cells (Fig. 7D-F). However,
overexpression of SNHG1 did not reverse inhibition of cell
migration induced by SNHG1 knockdown (data not shown), suggesting
that SNHG1 could control cell migration by other mechanisms. These
data indicated that LMO4 plays a major role in the SNHG1-mediated
cell growth and cell cycle regulation of breast cancer cells.
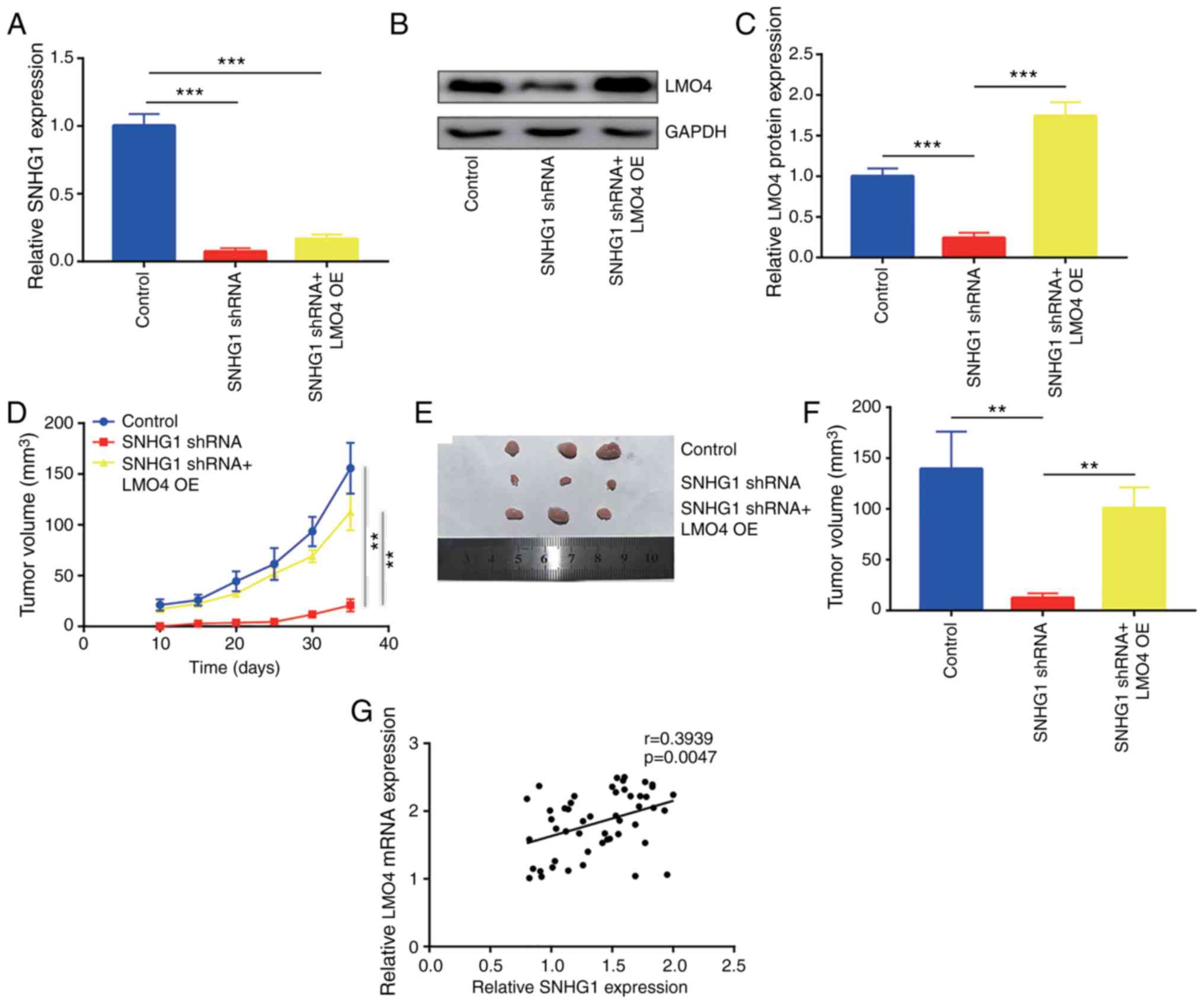
SNHG1 knockdown inhibits tumor growth
in vivo
In addition to the in vitro results, the
regulation of breast cancer cell progression by SNHG1 was examined
in vivo. MDA-MB-231 cells were infected with a lentiviral
vector carrying the SNHG1 or control shRNA sequences to construct
stable SNHG1-knockdown and control MDA-MB-231 cell lines. SNHG1
shRNA MDA-MB-231 cells were transfected with pcDNA3.1-LMO4 plasmid
and screened with G418 to build LMO4-overexpressing and
SNHG1-knockdown MDA-MB-231 cell lines. RT-qPCR indicated that SNHG1
expression was significantly downregulated in the SNHG1 shRNA
MDA-MB-231 and SNHG1 shRNA+LMO4 OE MDA-MB-231 cells (Fig. 8A). Western blot analysis confirmed
that LMO4 protein expression was decreased in SNHG1 shRNA
MDA-MB-231 cells, whereas it was slightly elevated in SNHG1
shRNA+LMO4 OE MDA-MB-231 cells (Fig. 8B
and C). Tumor growth was very slow in mice injected with SNHG1
shRNA MDA-MB-231 cells compared with mice in the control and SNHG1
shRNA+LMO4 OE groups (Fig. 8D).
Following 35 days of in vivo tumor cell growth, the mice
were sacrificed and the tumors were dissected. SNHG1 knockdown
significantly reduced the tumor size, whereas overexpression of
LMO4 reversed the inhibitory effects on breast cancer cells
(Fig. 8E and F). Moreover, the
expression levels of SNHG1 and LMO4 were examined in 50 breast
cancer tumor tissues. The results indicated a positive correlation
between SNHG1 expression and LMO4 mRNA expression in tumor
tissues (Fig. 8G).
Discussion
High-throughput deep sequencing and microarray
analysis have revealed several differentially expressed long
non-coding RNAs (lncRNAs) in breast cancer (36,37).
Subsequent experimental analysis identified several lncRNAs which
act as oncogenes or tumor suppressors in this type of cancer
(38–40). Moreover, the overexpression or
downregulation of lncRNAs has been used as a breast cancer
biomarker, aiming to improve detection of this disease at an early
stage or predict the sensitivity of the patients to chemotherapy
(41–43). The present study demonstrated that
lncRNA small nucleolar RNA host gene 1 (SNHG1) was overexpressed in
breast cancer and that it promoted breast cancer cell growth, cell
cycle and migration, suggesting its oncogenic role. These findings
are consistent with a recent study (23). The data further revealed a novel
molecular mechanism of SNHG1 in breast cancer progression.
Previous studies have shown that high expression
levels of SNHG1 predict poor prognosis in hepatocellular carcinoma,
glioma, non-small cell lung cancer (NSCLC), colon cancer and
prostate cancer (20,44–46).
Functional assays indicated that SNHG1 promoted cell proliferation
and metastasis of cancer cells, such as glioma and colorectal
cancer (20,45). To date, the expression and role of
SNHG1 in breast cancer remains elusive. Using the TCGA-BRCA
dataset, the present study demonstrated that SNHG1 was
significantly elevated in breast cancer tumor tissues compared with
the corresponding expression in normal tissues. Subsequently, the
overexpression of SNHG1 was confirmed in the tumor counterpart by
the detection of SNHG1 expression in 50 pairs of breast cancer and
adjacent normal tissues using RT-PCR. Notably, high expression of
SNHG1 was associated with ER−/PR− status and
advanced clinical stage. Furthermore, loss of function assays
indicated that SNHG1 promoted cell proliferation and cell migration
of breast cancer cells. Flow cytometric analysis indicated that
SNHG1 knockdown led to an accumulation of cells at the G2/M phase
without a concomitant increase in the cell apoptotic rate. These
data collectively demonstrated that SNHG1 is overexpressed in
breast cancer cells and that it promoted cell proliferation,
progression and migration.
The molecular mechanism of SNHG1 is
well-characterized in colorectal cancer and NSCLC. Sun et al
found that approximately 60% of SNHG1 is expressed in the nuclei of
colorectal cancer and NSCLC cells and that it regulated gene
expression of cis and trans elements in order to
control AKT signaling activity and MYC levels (46,47).
In addition to the regulation of transcription, the cytoplasmic
form of SNHG1 functions as a ceRNA in order to determine gene
expression. In NSCLC cells, SNHG1 was found to directly bind to
miR-145-5p in order to increase MTDH levels (45). In colorectal cancer cells, SNHG1
sponged miR-145 to promote cell proliferation and cell metastasis
(22). In the present study, SNHG1
was expressed in both the nuclear and cytoplasmic regions of breast
cancer cells. miRDB software was used to predict the potential
binding of miRNAs and SNHG1. Among the candidate miRNAs, miR-573 is
a well-known tumor suppressor involved in several cancer types
(48,49). miR-573 has been reported to target
several oncogenes (VEGFA, HIF1A, FAK and ANGPT2) in
breast cancer (48,49). Using RT-PCR, a mutual inhibition
between miR-573 and SNHG1 was noted in breast cancer cells. Their
direct binding was verified using the dual luciferase reporter
assay. Furthermore, bioinformatic analysis suggested that the
zinc-finger protein LMO4 is one of the potential target
genes of miR-573. LIM domain only 4 (LMO4) expression is frequently
elevated in breast cancer (34).
Additional experiments in breast cancer cells from various subtypes
demonstrated that LMO4 induced cyclin D1 and cyclin E1 expression
to promote cell cycle progression and facilitate cell proliferation
(35). The expression of LMO4 was
regulated by miR-409-3p in colorectal cancer (50). However, its regulation by miRNAs in
breast cancer has not yet been investigated. RT-PCR and western
blot analyses demonstrated that LMO4 is negatively regulated by
miR-573. The dual luciferase reporter assay verified that
LMO4 is a target gene of miR-573 in breast cancer cells.
Moreover, SNHG1 knockdown decreased LMO4 expression and the
expression of its target genes cyclin D1 and cyclin E1. In breast
cancer tumor tissues, the expression of LMO4 mRNA was found
to be positively associated with SNHG1 levels. The decrease in the
expression levels of LMO4 contributed to G2/M arrest and cell
proliferation inhibition in breast cancer cells (35), suggesting that LMO4 may play a major
role in SNHG1-induced inhibition of breast cancer progression.
These effects were similar to those noted following SNHG1
knockdown. Indeed, the in vitro and in vivo assays
presented in the present study revealed that LMO4 overexpression
could reverse cell cycle redistribution, cell proliferation
inhibition and tumor growth inhibition in breast cancer
SNHG1-knockdown mouse xenografts. Zheng et al reported that
SNHG1 controlled ZEB1 expression to regulate cell proliferation and
migration of breast cancer cells (23). The present findings demonstrated
that SNHG1 could modulate cell cycle progression to control cell
proliferation in breast cancer cells. The study provided novel
insights into the potential mechanism of SNHG1 action in breast
cancer progression.
In conclusion, the present study demonstrated that
SNHG1 acts as an oncogene in breast cancer. This lncRNA sponged
miR-573, leading to elevated levels of LMO4 that in turn promoted
cell proliferation and migration. These results support a promising
role of SNHG1 in the treatment of patients with breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Author's contributions
XX, MZ, PZ and FH participated in the design and
performance of the experiments. XX and JY contributed to the data
analysis. YF and LL contributed to the collection of samples and
clinical data analyses. LZ and LY supervised the performance of the
experiments and data analysis and wrote the manuscript. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were approved by the Ethic Committee
of Second Xiangya Hospital of Central South University (Changsha,
Hunan, China). Written informed consent for the publication of any
associated data and accompanying images was obtained from all
patients prior to surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Criscitiello C, Fumagalli D, Saini KS and
Loi S: Tamoxifen in early-stage estrogen receptor-positive breast
cancer: Overview of clinical use and molecular biomarkers for
patient selection. Onco Targets Ther. 4:1–11. 2010.PubMed/NCBI
|
|
4
|
Amiri-Kordestani L, Blumenthal GM, Xu QC,
Zhang L, Tang SW, Ha L, Weinberg WC, Chi B, Candau-Chacon R, Hughes
P, et al: FDA approval: Ado-trastuzumab emtansine for the treatment
of patients with HER2-positive metastatic breast cancer. Clin
Cancer Res. 20:4436–4441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esteva FJ, Yu D, Hung MC and Hortobagyi
GN: Molecular predictors of response to trastuzumab and lapatinib
in breast cancer. Nat Rev Clin Oncol. 7:98–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y, Liu Y, Zhang C, Chu J, Wu Y, Li Y,
Liu J, Li Q, Li S, Shi Q, et al: Tamoxifen-resistant breast cancer
cells are resistant to DNA-damaging chemotherapy because of
upregulated BARD1 and BRCA1. Nat Commun. 9:15952018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neophytou C, Boutsikos P and Papageorgis
P: Molecular mechanisms and emerging therapeutic targets of
triple-negative breast cancer metastasis. Front Oncol. 8:312018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richard JL and Eichhorn PJ: Deciphering
the roles of lncRNAs in breast development and disease. Oncotarget.
9:20179–20212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Costa FF: Non-coding RNAs, epigenetics and
complexity. Gene. 410:9–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding X, Zhu L, Ji T, Zhang X, Wang F, Gan
S, Zhao M and Yang H: Long intergenic non-coding RNAs (LincRNAs)
identified by RNA-seq in breast cancer. PLoS One. 9:e1032702014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Ye C, Xiong H, Shen Y, Lu Y, Zhou
J and Wang L: Dysregulation of long non-coding RNA in breast
cancer: An overview of mechanism and clinical implication.
Oncotarget. 8:5508–5522. 2017.PubMed/NCBI
|
|
13
|
Wang M, Wang M, Wang Z, Yu X, Song Y, Wang
C and Xu Y, Wei F, Zhao Y and Xu Y: Long non-coding
RNA-CTD-2108O9.1 represses breast cancer metastasis by influencing
leukemia inhibitory factor receptor. Cancer Sci. 109:1764–1774.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng R, Lin S, Guan L, Yuan H, Liu K, Liu
C, Ye W, Liao Y, Jia J and Zhang R: Long non-coding RNA XIST
inhibited breast cancer cell growth, migration, and invasion via
miR-155/CDX1 axis. Biochem Biophys Res Commun. 498:1002–1008. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao W, Geng D, Li S, Chen Z and Sun M:
LncRNA HOTAIR influences cell growth, migration, invasion, and
apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer
Med. 7:842–855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tuo YL, Li XM and Luo J: Long noncoding
RNA UCA1 modulates breast cancer cell growth and apoptosis through
decreasing tumor suppressive miR-143. Eur Rev Med Pharmacol Sci.
19:3403–3411. 2015.PubMed/NCBI
|
|
17
|
Mao C, Wang X, Liu Y, Wang M, Yan B, Jiang
Y, Shi Y, Shen Y, Liu X, Lai W, et al: A G3BP1-interacting lncRNA
promotes ferroptosis and apoptosis in cancer via nuclear
sequestration of p53. Cancer Res. 78:3484–3496. 2018.PubMed/NCBI
|
|
18
|
Wang H, Li W, Guo R, Sun J, Cui J, Wang G,
Hoffman AR and Hu JF: An intragenic long noncoding RNA interacts
epigenetically with the RUNX1 promoter and enhancer chromatin DNA
in hematopoietic malignancies. Int J Cancer. 135:2783–2794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin SX, Jiang H, Xiang GZ, Zhang WR, Weng
YH, Qiu FD, Wu J and Wang HG: Up-regulation of long non-coding RNA
SNHG1 contributes to proliferation and metastasis in laryngeal
squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 22:1333–1341.
2018.PubMed/NCBI
|
|
20
|
Wang Q, Li Q, Zhou P, Deng D, Xue L, Shao
N, Peng Y and Zhi F: Upregulation of the long non-coding RNA SNHG1
predicts poor prognosis, promotes cell proliferation and invasion,
and reduces apoptosis in glioma. Biomed Pharmacother. 91:906–911.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan H, Zhao L, Song R, Liu Y and Wang L:
The long noncoding RNA SNHG1 promotes nucleus pulposus cell
proliferation through regulating miR-326 and CCND1. Am J Physiol
Cell Physiol. 315:C21–C27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian T, Qiu R and Qiu X: SNHG1 promotes
cell proliferation by acting as a sponge of miR-145 in colorectal
cancer. Oncotarget. 9:2128–2139. 2017.PubMed/NCBI
|
|
23
|
Zheng S, Li M, Miao K and Xu H: SNHG1
contributes to proliferation and invasion by regulating miR-382 in
breast cancer. Cancer Manag Res. 11:5589–5598. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Visvader JE, Venter D, Hahm K, Santamaria
M, Sum EY, O'Reilly L, White D, Williams R, Armes J and Lindeman
GJ: The LIM domain gene LMO4 inhibits differentiation of mammary
epithelial cells in vitro and is overexpressed in breast cancer.
Proc Natl Acad Sci USA. 98:14452–14457. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wittlin S, Sum EY, Jonas NK, Lindeman GJ
and Visvader JE: Two promoters within the human LMO4 gene
contribute to its overexpression in breast cancer cells. Genomics.
82:280–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taniwaki M, Daigo Y, Ishikawa N, Takano A,
Tsunoda T, Yasui W, Inai K, Kohno N and Nakamura Y: Gene expression
profiles of small-cell lung cancers: Molecular signatures of lung
cancer. Int J Oncol. 29:567–575. 2006.PubMed/NCBI
|
|
27
|
Zhou X, Sang M, Liu W, Gao W, Xing E, Lü
W, Xu Y, Fan X, Jing S and Shan B: LMO4 inhibits p53-mediated
proliferative inhibition of breast cancer cells through interacting
p53. Life Sci. 91:358–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clarke C, Madden SF, Doolan P, Aherne ST,
Joyce H, O'Driscoll L, Gallagher WM, Hennessy BT, Moriarty M, Crown
J, et al: Correlating transcriptional networks to breast cancer
survival: A large-scale coexpression analysis. Carcinogenesis.
34:2300–2308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu X, Jin H, Liu Y, Liu L, Wu Q, Guo Y, Yu
L, Liu Z, Zhang T, Zhang X, et al: The expression patterns and
correlations of claudin-6, methy-CpG binding protein 2, DNA
methyltransferase 1, histone deacetylase 1, acetyl-histone H3 and
acetyl-histone H4 and their clinicopathological significance in
breast invasive ductal carcinomas. Diagn Pathol. 7:332012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koleva-Kolarova RG, Greuter MJ, Feenstra
TL, Vermeulen KM, de Vries EF, Parkin D, Buskens E and de Bock GH:
Molecular imaging with positron emission tomography and computed
tomography (PET/CT) for selecting first-line targeted treatment in
metastatic breast cancer: A cost-effectiveness study. Oncotarget.
9:19836–19846. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lan X and Liu X: LncRNA SNHG1 functions as
a ceRNA to antagonize the effect of miR-145a-5p on the
down-regulation of NUAK1 in nasopharyngeal carcinoma cell. J Cell
Mol Med. 23:2351–2361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sum EY, Segara D, Duscio B, Bath ML, Field
AS, Sutherland RL, Lindeman GJ and Visvader JE: Overexpression of
LMO4 induces mammary hyperplasia, promotes cell invasion, and is a
predictor of poor outcome in breast cancer. Proc Natl Acad Sci USA.
102:7659–7664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Montañez-Wiscovich ME, Shelton MD,
Seachrist DD, Lozada KL, Johnson E, Miedler JD, Abdul-Karim FW,
Visvader JE and Keri RA: Aberrant expression of LMO4 induces
centrosome amplification and mitotic spindle abnormalities in
breast cancer cells. J Pathol. 222:271–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Shen X, Xie B, Ma Z, Chen X and
Cao F: Transcriptional profiling of differentially expressed long
non-coding RNAs in breast cancer. Genom Data. 6:214–216. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen C, Li Z, Yang Y, Xiang T, Song W and
Liu S: Microarray expression profiling of dysregulated long
non-coding RNAs in triple-negative breast cancer. Cancer Biol Ther.
16:856–865. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Q, Gao H, Zhou S and Liao Y: LncRNA
PlncRNA-1 overexpression inhibits the growth of breast cancer by
upregulating TGF-β1 and downregulating PHGDH. Breast Cancer.
25:619–625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pan Y, Pan Y, Cheng Y, Yang F, Yao Z and
Wang O: Knockdown of lncRNA MAPT-AS1 inhibites proliferation and
migration and sensitizes cancer cells to paclitaxel by regulating
MAPT expression in ER-negative breast cancers. Cell Biosci.
8:72018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Sharma S and Watabe K: Roles of
lncRNA in breast cancer. Front Biosci (Schol Ed). 7:94–108. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Wang W, Xia P, Wan L, Zhang L, Yu L,
Wang L, Chen X, Xiao Y and Xu C: Identification of a five-lncRNA
signature for predicting the risk of tumor recurrence in patients
with breast cancer. Int J Cancer. 143:2150–2160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang S, Wang J, Ghoshal T, Wilkins D, Mo
YY, Chen Y and Zhou Y: lncRNA gene signatures for prediction of
breast cancer intrinsic subtypes and prognosis. Genes (Basel).
9:2018. View Article : Google Scholar
|
|
43
|
Zhu QN, Wang G, Guo Y, Peng Y, Zhang R,
Deng JL, Li ZX and Zhu YS: LncRNA H19 is a major mediator of
doxorubicin chemoresistance in breast cancer cells through a
cullin4A-MDR1 pathway. Oncotarget. 8:91990–92003. 2017.PubMed/NCBI
|
|
44
|
Zhang M, Wang W, Li T, Yu X, Zhu Y, Ding
F, Li D and Yang T: Long noncoding RNA SNHG1 predicts a poor
prognosis and promotes hepatocellular carcinoma tumorigenesis.
Biomed Pharmacother. 80:73–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu Q, Shan S, Li Y, Zhu D, Jin W and Ren
T: Long noncoding RNA SNHG1 promotes non-small cell lung cancer
progression by up-regulating MTDH via sponging miR-145-5p. FASEB J.
32:3957–3967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun X, Wang Z and Yuan W: Down-regulated
long non-coding RNA SNHG1 inhibits tumor genesis of colorectal
carcinoma. Cancer Biomark. 20:67–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun Y, Wei G, Luo H, Wu W, Skogerbø G, Luo
J and Chen R: The long noncoding RNA SNHG1 promotes tumor growth
through regulating transcription of both local and distal genes.
Oncogene. 36:6774–6783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang L, Song G, Tan W, Qi M, Zhang L, Chan
J, Yu J, Han J and Han B: MiR-573 inhibits prostate cancer
metastasis by regulating epithelial-mesenchymal transition.
Oncotarget. 6:35978–35990. 2015.PubMed/NCBI
|
|
49
|
Danza K, De Summa S, Pinto R, Pilato B,
Palumbo O, Merla G, Simone G and Tommasi S: MiR-578 and miR-573 as
potential players in BRCA-related breast cancer angiogenesis.
Oncotarget. 6:471–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bai R, Weng C, Dong H, Li S, Chen G and Xu
Z: MicroRNA-409-3p suppresses colorectal cancer invasion and
metastasis partly by targeting GAB1 expression. Int J Cancer.
137:2310–2322. 2015. View Article : Google Scholar : PubMed/NCBI
|