Introduction
Globally, head and neck cancers account for 5–10% of
all malignancies and >500,000 new cases are diagnosed each year
(1,2). Among them, ~95% of head and neck
cancers are classified as squamous cell carcinoma. The poor
prognosis associated with this type of cancer is related to local
recurrence and distant metastasis. It has previously been reported
that 50–60% of patients exhibit recurrence and regional lymph node
metastasis following treatment, and 20% of patients exhibit distant
metastasis (3). Even if the
surgical margin appears negative, as determined by histopathology,
recurrence still occurs in 20% of patients (2,4,5).
In the past, the diagnosis of recurrence and
metastasis was mainly based on imaging, serum tumor marker levels
and histopathology. However, these methods are often limited by
tumor size and location, low compliance rate, and the inability to
achieve real-time monitoring. Previous studies have revealed that
circulating tumor cells (CTCs) are reliable indicators that may be
used for the early prediction of tumor recurrence and metastasis,
thereby facilitating clinical intervention, and improving patient
survival and quality of life (6–10).
Liquid biopsies, specifically for CTC detection, can
make up for the deficiencies of tissue biopsy. For example, tissue
biopsy specimens must be solid lesions, which are unable to respond
to the current state of the disease; however, CTC specimens are
obtained from peripheral blood and can reflect the current state of
the disease. The advantages of CTC detection are its safety,
non-invasiveness and reliability (11). Numerous studies have reported the
usefulness of CTCs in the evaluation of recurrence, metastasis and
prognosis of breast cancer (12,13),
prostate cancer (14), colorectal
cancer (15), esophageal cancer
(16), etc.; therefore, CTCs may be
used as an independent predictor of tumor prognosis (12,17–23).
In addition, CTCs were defined as a tumor marker by the American
Society of Clinical Oncology in 2007 (24), and in 2010, the American Joint
Committee on Cancer designated CTCs as a novel M-segment (remote
metastasis) standard, which appeared between M0 and M1 as cM0 (i+)
(25). In 2017, CTCs were included
in the TNM staging system in accordance with the breast cancer
guidelines of the National Comprehensive Cancer Network (26).
Tumor recurrence and metastasis are the leading
causes of death in patients with head and neck squamous cell
carcinoma (HNSCC). To date, only a few studies have focused on the
detection of CTCs in this type of cancer (1,2,27–32).
Furthermore, these studies have several limitations, including few
cases analyzed (1,2,27), low
technical detection rate (2,28–31)
and difficulty in obtaining specimens (32). To investigate the prognostic effect
of CTCs on locally advanced HNSCC (LA-HNSCC), as well as the
association between CTCs and clinical tumor features, the CTCs
detection rate of patients with LA-HNSCC was studied and changes in
CTC detection before and after treatment were analyzed.
Materials and methods
Study population and sample
collection
Between October 2015 and September 2018, 264
patients that were admitted to the Chinese PLA General Hospital
(Beijing, China), and were histopathologically diagnosed with
LA-HNSCC via an endoscopic biopsy, were recruited to the present
study. Notably, 86 patients were excluded; therefore, 178 patients
were assessed. All patients had an Eastern Cooperative Oncology
Group performance status score (33) of 0–1. A complete review of their
medical history, as well as a thorough physical examination, was
conducted for each patient prior to treatment. CTC counts were
determined within 3 days prior to chemoradiotherapy and 1 month
after radiotherapy (at first follow-up). All patients were followed
prospectively, and all patients read and signed informed consent
forms.
Inclusion and exclusion criteria
Initially, 264 patients were recruited and 86
patients were excluded, including patients that had undergone
relevant treatment before CTC detection and patients with some
types of cancer of which there were few cases (including 5 patients
that had undergone chemoradiotherapy, 27 postoperative patients, 12
patients with non-squamous cell carcinoma,6 patients with unknown
primary sites, 13 patients with laryngeal squamous cell carcinoma,
12 patients with nasal sinus squamous cell carcinoma and 11
patients with oropharyngeal squamous cell carcinoma). Finally, 178
patients were included in the present analysis. Inclusion criteria
were as follows: i) Squamous cell carcinoma; ii) expected survival
of >3 months; and iii) no treatment prior to CTC detection.
Exclusion criteria were as follows: i) Non-squamous cell carcinoma;
ii) cachexia or serious medical disease; and iii) treatments were
performed prior to CTC detection.
Treatment protocols
All patients received induction chemotherapy with
concurrent chemoradiotherapy. The induction chemotherapy regimen
consisted of cisplatin + docetaxel + 5-fluorouracil/cisplatin +
docetaxel. The concurrent chemoradiotherapy regimen consisted of
cisplatin (nidaplatin) + nimotuzumab (cetuximab)/docetaxel +
nimotuzumab. Patients with increased CTCs and patients whose CTCs
changed from negative to positive post-treatment, and who did not
exhibit disease progression or succumb to the disease were treated
with thymopentin (TP5). Subsequently, CTCs were reanalyzed.
Detection of CTCs
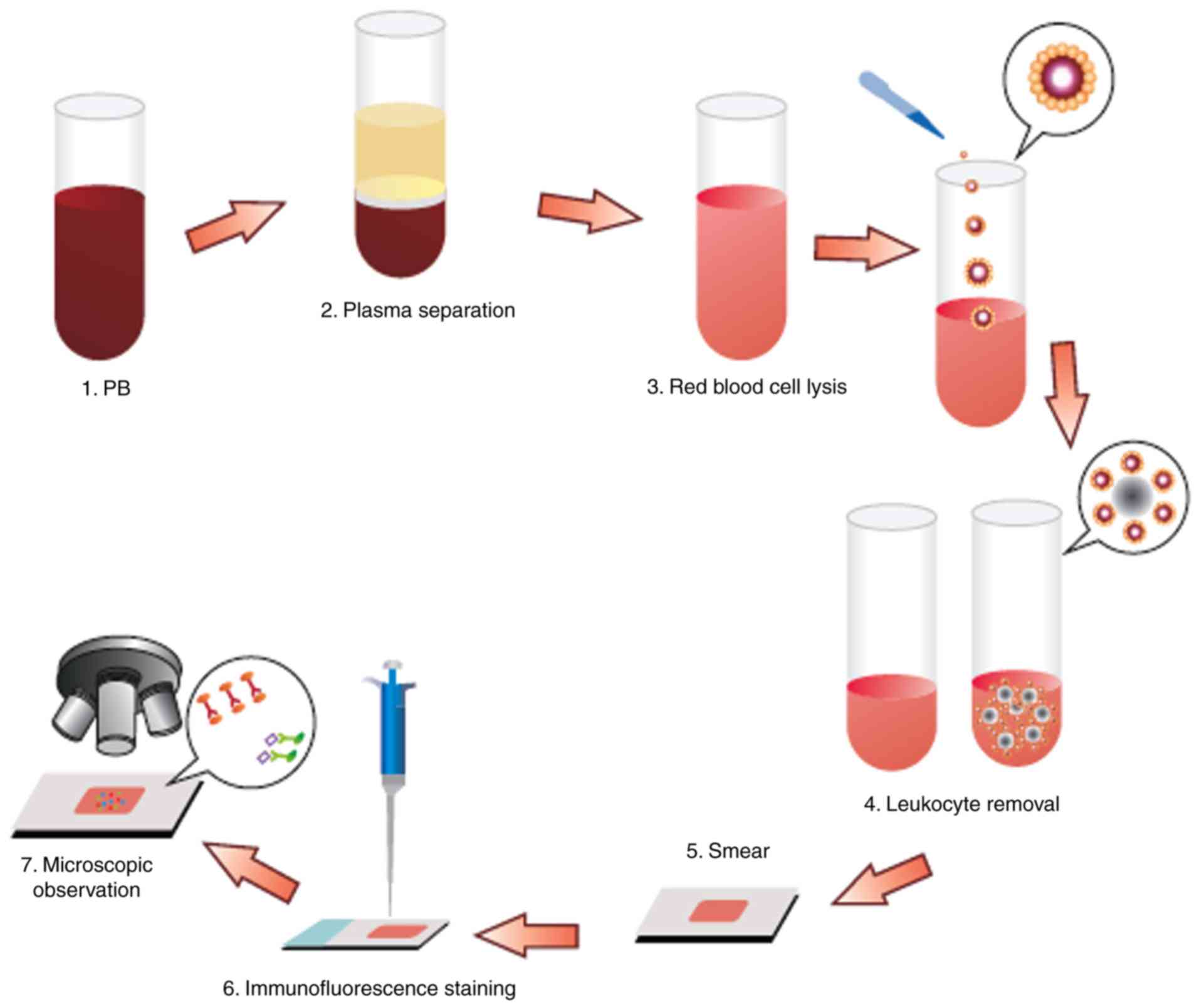
The CTCs were enriched and identified as described
previously (34). Briefly, a 3.2-ml
peripheral blood sample was drawn into an acid citrate dextrose
anticoagulant tube (BD Biosciences) and centrifuged (650 × g; 5
min; room temperature) to separate the cells from the plasma. The
red blood cells were lysed with CS2 buffer (Cyttel), followed by
resuspension of cell particulates in CS1 buffer (Cyttel) and
incubation with an anti-CD45 antibody conjugated to magnetic beads
(Cyttel) for 20 min at 15–30°C. The immunomagnetic beads were
collected using a magnetic stand (Promega Corporation) and the
resulting CTC sample was applied to a glass microscope slide for
observation.
The enriched cells (30–100 CTCs/µl) were then fixed
in CF1 buffer (Cyttel) for 8 min at 15–30°C. The slides were
immersed in saline-sodium citrate buffer for 10 min at 37°C and
dehydrated in a gradient series of ethanol baths (75, 85 and 100%)
for 2 min each. The slides were then incubated with hybridization
solution containing chromosome 8 centromere probe (200–1,000 bp;
Abbott Laboratories) at 76°C for 5 min and 37°C for 1.5 h, and
placed in a hybridizer (Dako; Agilent Technologies, Inc.) for 1.5 h
at 37°C. The CTCs were then immunostained for 1 h at room
temperature with an anti-CD45 antibody conjugated to Alexa
Fluor® 594 (Invitrogen; Thermo Fisher Scientific, Inc.).
After staining the nuclei with 4,6-diamidino-2-phenylindole (DAPI;
Invitrogen; Thermo Fisher Scientific, Inc.), the slides were
mounted for image analysis under a fluorescence microscope. For
image analysis, samples underwent double-probe staining with
fluorescence in situ hybridization (FISH)-probe A and
FISH-probe B (Abbott Laboratories) at 76°C for 5 min and 37°C for
1.5 h. This protocol was conducted by Cyttel Biosciences, Inc. All
assessments were performed by investigators who were blinded to the
clinical characteristics of the patients (Fig. 1).
Comparison of the enrichment and
identification methods adopted in this study with other
methods
The negative immunomagnetic bead enrichment method
used in the present study can enrich all CTCs, whereas other
enrichment methods, such as positive immunomagnetic bead enrichment
and two-dimensional electrophoresis, are unable to capture CTCs
that no longer possess epithelial cell adhesion molecules after
undergoing epithelial-mesenchymal transition. In addition,
centrifugation may result in loss of CTCs that have migrated to the
plasma, red cell and granulocyte layers, and filtration is not
advisable to detect tumor cells <8 µm.
The FISH identification methods adopted in this
study are non-radioactive, safe, fast and sensitive, and can be
used for analysis of metaphase chromosomes and interphase cells. In
addition, the probes used can be detected simultaneously on the
same specimen and stored for a long time. Conversely, other
identification methods are radioactive, the probes used must be
relabeled for each test and the labeled probe is unstable. In
addition, when observing the results, more cell divisions are
required for statistical analysis.
Follow-up
Follow-up was conducted through outpatient
interviews. In some cases, telephone interviews were conducted.
Survival was defined as the interval from the time of diagnosis to
the time of death or last follow-up. The last follow-up was
conducted in November 2018.
Classification of changes in CTC
counts
Changes in the CTC counts were classified into three
categories: Increasing, stable and decreasing. A positive CTC count
(above the threshold) at first follow-up compared with a negative
CTC count at baseline was considered an increase in CTCs. A
negative CTC count (below the threshold) at first follow-up
compared with a positive CTC count at baseline was considered a
decrease in CTCs. A negative CTC count at baseline and at first
follow-up was considered stable. A positive CTC count at baseline
and at first follow-up with an increase in CTC count >2/ml was
considered increasing, whereas a decrease in CTC count >2/ml was
considered decreasing, and a change in CTC count <2/ml was
considered stable (35).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 22; IBM Corp.). Youden's index and the receiver
operating characteristic (ROC) curve were used to determine the
best diagnostic cutoff value. The associations between CTC
detection rate, CTC ploidy number at different cutoff values (CTCs
≥1, CTCs ≥2, CTCs ≥3, CTCs ≥4 and CTCs ≥5), CTC count (tumor load)
and clinical characteristics were evaluated. Progression-free
survival (PFS) and overall survival (OS) was assessed in the groups
stratified according to selected CTC cutoff values for all patients
with different types of cancer, and the association between changes
in CTC count and treatment response and prognosis was evaluated.
χ2 test or Fisher's exact test was used to analyze
associations. The log-rank test and Cox proportional hazards model
were used to identify prognostic factors independently associated
with survival. Survival rates were assessed using the Kaplan-Meier
method. PFS and OS were defined as the time from the collection of
blood to the time of confirmed disease progression or last
follow-up, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
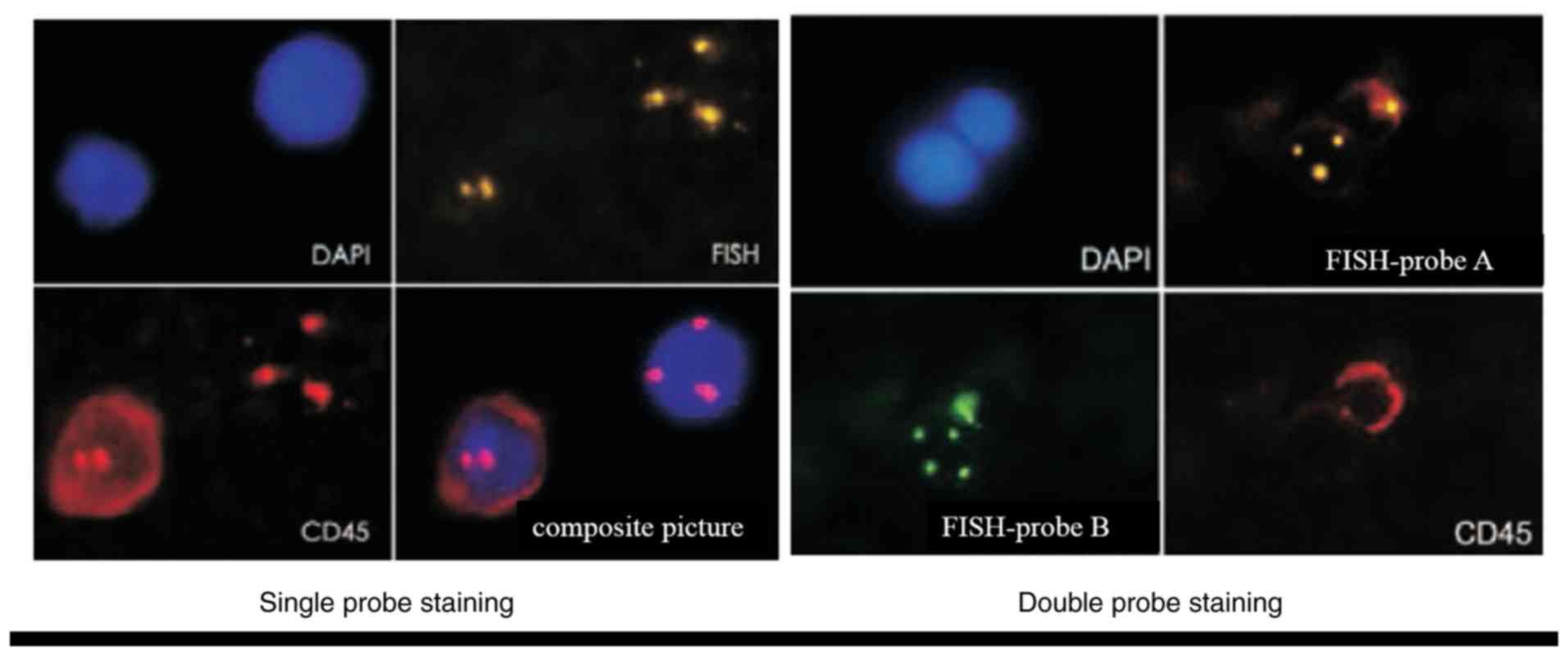
Identification of CTCs
The clinical characteristics of the patients,
including cancer type, differentiation and clinical stage are shown
in Table I. Abnormally
proliferating and CD45-negative CTCs with ≥3 nuclear chromosome
enumeration probe signals were identified. The nuclei of CTCs were
stained with DAPI (Fig. 2).
 | Table I.Clinical characteristics of the
patients recruited to the present study. |
Table I.
Clinical characteristics of the
patients recruited to the present study.
|
|
|
| Clinical staging
type |
|
|---|
|
|
|
|
|
|
|---|
| Cancer type | Ratio of male to
female | Most common degree
of differentiation | III | IV | Total number of
cases |
|---|
| Nasopharyngeal
squamous cell carcinoma | 2.65:1 | Low | 75 | 60 | 135 |
| Hypopharyngeal
squamous cell carcinoma | 42:1 | Medium and
high | 12 | 31 | 43 |
| Total | 3.68:1 | Medium and low | 87 | 91 | 178 |
CTC detection rate in patients with
LA-HNSCC
Before treatment, the CTC detection rate was 73.8%.
The minimum, maximum and median CTC counts were 1, 22 and 2/3.2 ml,
respectively. The overall distribution was skewed.
Association between CTCs or the CTC
detection rate and clinicopathological variables
No significant associations were observed between
CTC count or CTC detection rate and clinical characteristics,
including sex, age, clinical stage, cancer type and degree of
differentiation (data not shown). However, an association was
observed between polyploid CTC number and metastasis (P<0.05;
Table II).
 | Table II.Clinical association between the
number of polyploid CTCs before treatment and locally advanced head
and neck squamous cell carcinoma. |
Table II.
Clinical association between the
number of polyploid CTCs before treatment and locally advanced head
and neck squamous cell carcinoma.
|
| Trisomic CTCs | Tetrasomic
CTCs | Multibody CTCs |
|---|
|
|
|
|
|
|---|
|
| No | Yes |
| No | Yes |
| No | Yes |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variable | n | % | n | % | P-value | n | % | n | % | P-value | n | % | n | % | P-value |
|---|
| M stage |
|
|
|
| 0.777 |
|
|
|
| 0.705 |
|
|
|
| 0.026a |
| M0 | 75 | 44.9 | 92 | 55.1 |
| 115 | 68.9 | 52 | 31.1 |
| 158 | 94.6 | 9 | 5.4 |
|
| M1 | 4 | 50.0 | 4 | 50.0 |
| 5 | 62.5 | 3 | 37.5 |
| 6 | 75.0 | 2 | 25.0 |
|
Association between CTCs and the
survival rate of patients with LA-HNSCC
Five patients were lost during follow-up; therefore,
a total of 173 patients were followed-up, with a follow-up rate of
97.2%. The median follow-up time was 16 months (range, 2–37
months); over the follow-up period, 30 cases exhibited recurrence
or metastasis and 12 patients died. According to Youden's index and
the receiver operating characteristic (ROC) curve, the best
diagnostic cutoff value was determined (Figs. S1 and S2; Tables
SI and SII). All patients had
a cutoff value of 2 before treatment and 3 after treatment. There
was no significant difference in the PFS and OS of all patients
with CTCs above the cutoff value compared to those with CTCs below
the cutoff value (P>0.05; Figs.
S3 and S4). For patients with
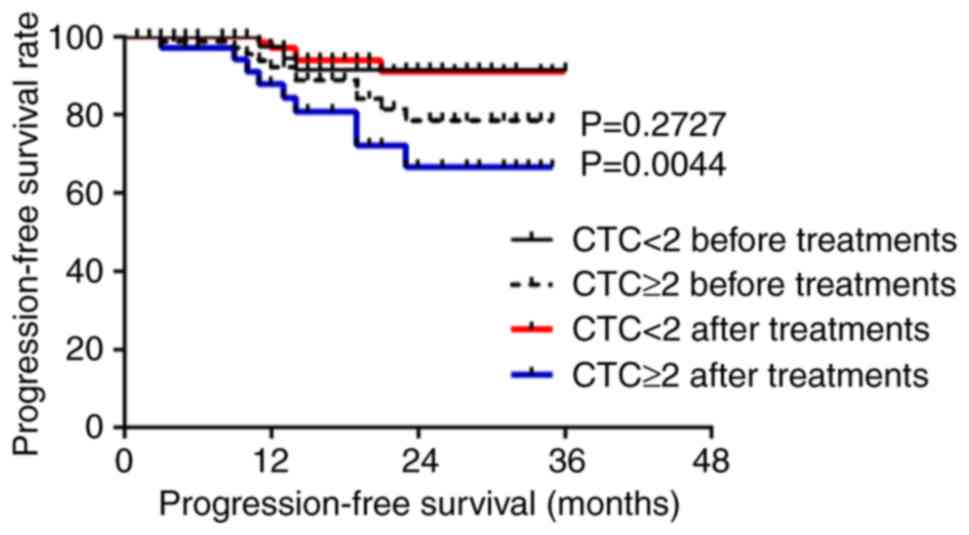
nasopharyngeal squamous cell carcinoma, the PFS was significantly
lower for those with ≥2 CTCs than those with <2 CTCs after
treatment (P<0.05 for a threshold of 2 CTCs/3.2 ml blood;
Fig. 3), but the OS before and
after treatment were not statistically significant (P>0.05;
Fig. S5). For patients with
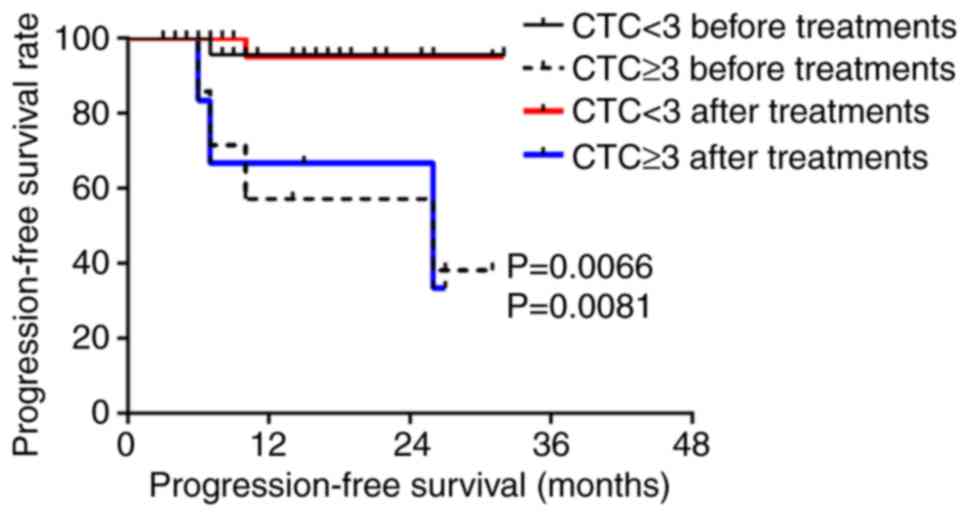
hypopharyngeal squamous cell carcinoma, the PFS and OS of patients
with ≥3 CTCs were significantly lower than those with <3 CTCs
before and after treatment (P<0.05 for a threshold of 3 CTCs/3.2
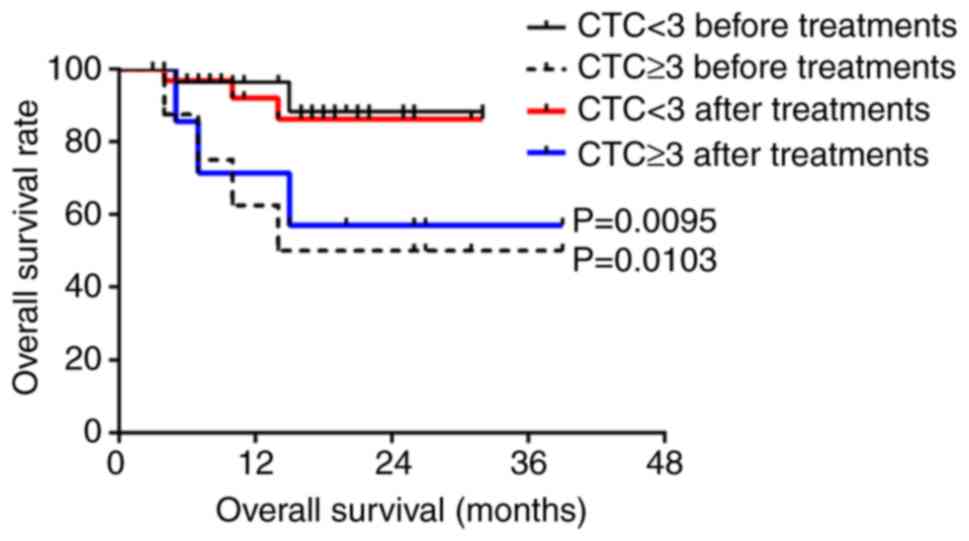
ml blood; Figs. 4 and 5).
Association between CTC changes and
the survival rate of patients with LA-HNSCC before and after
treatment
Blood samples were obtained from 178 patients
following treatment. The CTCs of 140 out of 178 patients decreased
or remained unchanged after treatment, whereas the CTCs of 38 out
of 178 patients increased. In addition, the CTCs of 90 patients
were positive before and after treatment, 46 patients were positive
before treatment but negative after treatment, and 42 patients were
negative before and after treatment. The results revealed that
patients with negative CTCs after treatment lived longer than those
with positive CTCs after treatment (P<0.05; Figs. S6 and S7). The PFS of these patients was
1.21-fold higher than that of patients with positive CTCs (93.31
vs. 77.40%). The 3-year OS was also significantly reduced by 1.17
times (95.75 vs. 81.70%). In addition, PFS and OS were shorter in
patients whose CTCs increased after treatment compared with in
patients whose CTCs decreased or remained unchanged (P<0.0001
and P=0.0301; Figs. S8 and
S9).
Association between CTCs and survival
in patients with nasopharyngeal squamous cell carcinoma and
hypopharyngeal squamous cell carcinoma
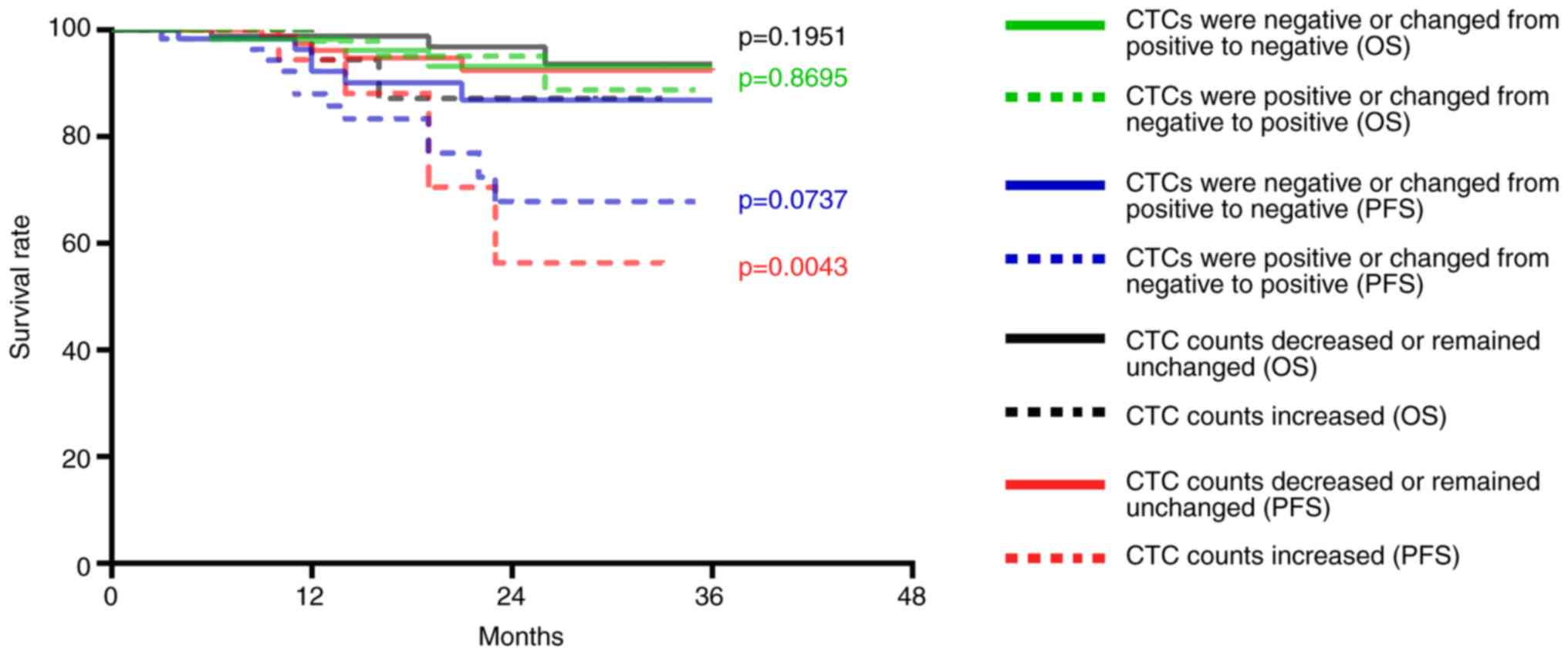
The CTC counts of 114 patients with nasopharyngeal
squamous cell carcinoma were decreased or remained unchanged after
treatment; of these 114 patients, disease progression occurred in
eight patients and three patients died. The CTC counts of 21
patients with nasopharyngeal squamous cell carcinoma were
increased; of these 21 patients, disease progression occurred in
seven patients and two patients died. Compared with patients with
decreased or unchanged CTC counts following treatment, the PFS of
patients with increased CTC counts was significantly decreased
(P=0.0043; Fig. 6), whereas no
significant change was observed for OS (P=0.1951; Fig. 6). In addition, the CTCs of 67
patients were positive post-treatment; of these 67 patients disease
progression occurred in nine patients and three patients died. The
CTCs of 68 patients were negative post-treatment; of these 68
patients, six exhibited disease progression and two patients died.
No differences were observed in the PFS (P=0.0737) and OS
(P=0.8695; Fig. 6).
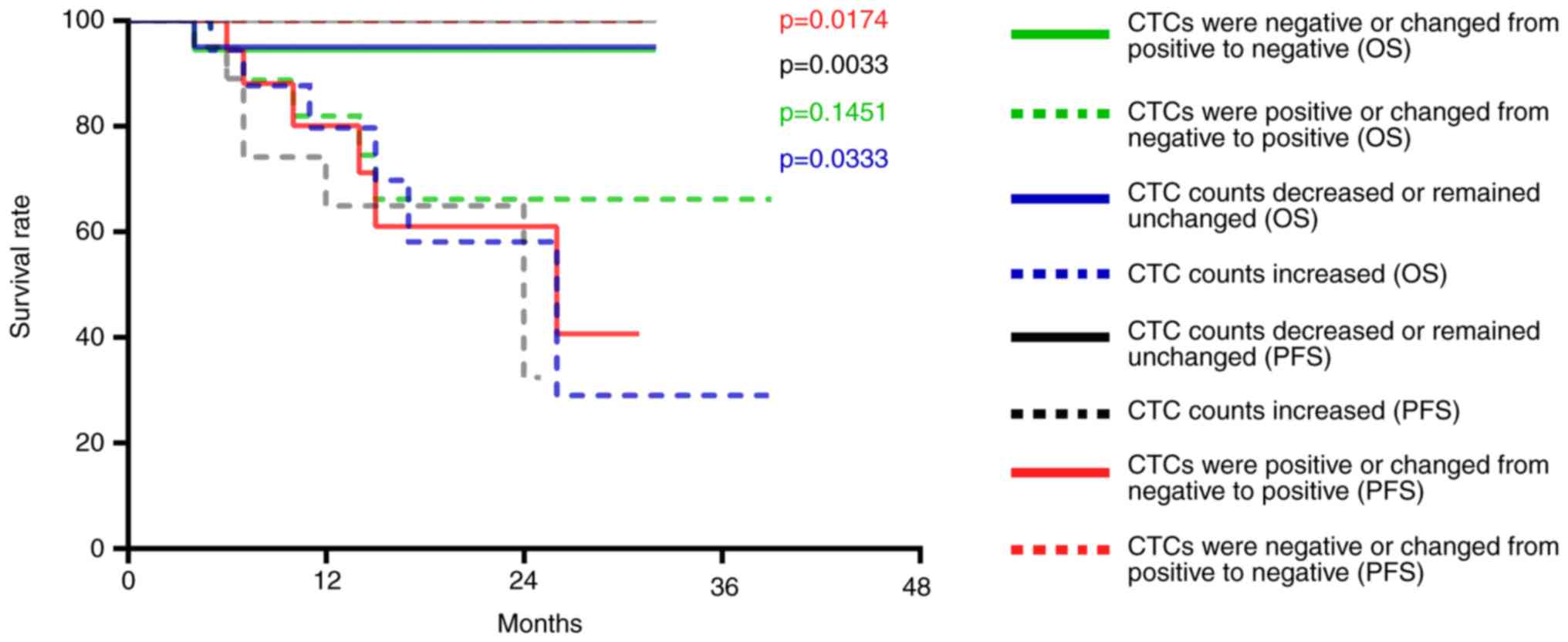
The CTC counts of 26 patients with hypopharyngeal
squamous cell carcinoma were decreased or remained unchanged after
treatment; no disease progression was observed in this group and
one patient died. The CTC counts of 17 patients with hypopharyngeal
squamous cell carcinoma were increased; of these 17 patients, six
exhibited disease progression and five patients died. Compared with
patients with decreased or unchanged CTC counts post-treatment, the
PFS (P=0.0033) and OS (P=0.0333) of patients with increased CTCs
was significantly decreased (Fig.
7). In addition, the CTCs of 23 patients were positive after
treatment; of these 23 patients, six exhibited disease progression
and five patients died. The CTCs of 20 patients were negative after
treatment; no disease progression was observed in this group and
one patient died. Compared with patients with negative CTCs or
whose CTCs changed from positive to negative post-treatment, PFS
was significantly decreased (P=0.0174) in patients whose CTCs were
positive after treatment, whereas OS was not (P=0.1451) (Fig. 7).
Multivariate analysis of predictors of
overall survival for patients with nasopharyngeal and
hypopharyngeal squamous cell carcinoma
Univariate analysis revealed that clinical stage,
baseline CTC counts, and CTC count at first follow-up were clinical
factors affecting OS. Multivariate analysis revealed all of these
factors to be independent prognostic markers of OS in patients with
hypopharyngeal squamous cell carcinoma (Table III), but not for patients with
nasopharyngeal squamous cell carcinoma (data not shown).
 | Table III.Multivariate Cox regression analysis
for overall survival prediction of patients with hypopharyngeal
squamous cell carcinoma. |
Table III.
Multivariate Cox regression analysis
for overall survival prediction of patients with hypopharyngeal
squamous cell carcinoma.
| Variable | Univariate
P-value | Multivariate
P-value | Hazard ratio | 95% CI |
|---|
| Sex |
|
|
|
|
| M | 0.770 |
|
|
|
| F |
|
|
|
|
| Age, years |
|
|
|
|
|
≥60 | 0.240 |
|
|
|
|
<60 |
|
|
|
|
| PS, n |
|
|
|
|
| 0 or
1 | 0.372 |
|
|
|
| ≥2 |
|
|
|
|
| Stage at
diagnosis |
|
|
|
|
|
Limited | 0.027a | 0.031a | 1.021 | 1.004 |
|
Extensive |
|
|
|
|
| CTC count at
baseline |
|
|
|
|
| ≥3 | 0.010a | 0.014a | 0.127 | 0.016 |
|
<3 |
|
|
|
|
| CTC count at first
follow-up |
|
|
|
|
| ≥3 | 0.009a | 0.010a | 6.992 | 3.781 |
|
<3 |
|
|
|
|
TP5 treatment
TP5 is a synthetic pentapeptide that corresponds to
position 32–36 of thymopoietin, and exhibits similar biological
activity to thymopoietin, which is responsible for phenotypic
differentiation of T cells and regulation of the immune system. TP5
has been clinically used for the treatment of patients with
immunodeficiency diseases, including rheumatoid arthritis, cancer,
hepatitis B virus infection, and acquired immunodeficiency syndrome
(36).
Patients with increased CTCs or patients whose CTCs
changed from negative to positive post-treatment, and did not
exhibit disease progression or succumb to the disease were treated
with TP5. Subsequently, CTCs were measured again and were decreased
(data not shown). These findings indicated that increased CTCs or
positive CTCs post-treatment, which did not result in disease
progression, were caused by low immunity.
Discussion
In the present study, the number of polyploid CTCs
was associated with distant metastasis (P=0.026). Furthermore,
patients with undetectable CTCs, and decreasing CTCs or negative
CTCs after treatment tended to have a good prognosis (P<0.05).
For nasopharyngeal squamous cell carcinoma, the PFS of patients
with increased CTCs and CTCs ≥2/3.2 ml after treatment was
significantly lower (P<0.05). For hypopharyngeal squamous cell
carcinoma, CTCs with a cutoff value of 3 may be used to evaluate
PFS and OS before and after treatment. The present findings
suggested that CTCs may be used to monitor disease progression and
the response to chemoradiotherapy for patients with LA-HNSCC.
Notably, the results indicated that CTCs are a better predictor of
the prognosis of hypopharyngeal squamous cell carcinoma than
nasopharyngeal squamous cell carcinoma.
During tumor metastasis, tumor cells interact with
their surrounding microenvironment and undergo epithelial to
mesenchymal transition (EMT). EMT causes epithelial cells to lose
their epithelial cell phenotype and to acquire a mesenchymal cell
phenotype, leading to various morphological and functional changes,
thereby promoting the migration and invasion of tumor cells. Tumor
cells can leave the primary site, and enter vascular or lymphatic
systems as CTCs to induce metastasis (37). CTCs can also return to the bone
marrow reserve pool in a resting state; under certain conditions,
CTCs can again enter the vascular or lymphatic systems, and travel
to other organs to form distant metastases (38,39).
CTCs are tumor cells that can be used to screen and
classify high-risk tumors (40).
Studies have shown that CTC counts are variable in different
subtypes and stages of breast cancer, and that CTCs are more
frequently observed in advanced stages compared with in early
stages (41,42). In addition, CTC counts in patients
with non-small cell lung cancer are significantly increased from
stages I–II to III–IV (43).
Conversely, this study demonstrated that there was no association
between CTC count or CTC detection rate and clinical stage in
LA-HNSCC.
CTC counts are lower in patients with LA-HNSCC than
other types of cancer. Notably, there are few comparative studies
on the clinical relevance of CTCs in LA-HNSCC, and these studies
have reported different conclusions (1,2,27–32)
(Table IV). It may be hypothesized
that the relevance of CTCs is associated with cancer type, number
of cases and the technology used.
 | Table IV.Association between the detection
rate of CTCs and clinical risk parameters and outcome of patients
with head and neck squamous cell carcinoma in previous studies. |
Table IV.
Association between the detection
rate of CTCs and clinical risk parameters and outcome of patients
with head and neck squamous cell carcinoma in previous studies.
| Author, year | CTC-positive
patients/total number of patients | Sampling
site/volume | Sampling time | Tumor stage
(UICC) | Detection
method | Type of tumor
markers | Associations | (Refs.) |
|---|
| Buglione et
al, 2012 | 11/73 | PB/≥7.5 ml | Before TM | I–IV | CellSearch | EpCAM, CD45, CK,
DAPI | Tumor stage, tumor
burden, progression | (27) |
| Wollenberg et
al, 2004 | 54/176 | BM/- | Before TM | I–IV | IHC-APAAP | CK19 | Progression | (32) |
| Nichols et
al, 2012 | 6/15 | PB/10 ml | Before TM | III–IV | CellSearch | EpCAM, CD45, CK,
DAPI | Prognosis,
treatment outcome and efficacy of adjuvant treatments | (28) |
| Tinhofer et
al, 2012 | 42/144 | PB/7.5 ml | Before TM | III–IV | PCR | EGFR | DFS, OS | (30) |
| Bozec et al,
2013 | 8/49 | PB/7.5 ml | Before TM | III–IV | CellSearch | EpCAM, CD45, CK,
DAPI | No association
detected | (31) |
| Jatana et
al, 2010 | 34/48 | PB/10–18 ml | Before TM | I–IV | ICC | CK, CD45, DAPI | PFS | (1) |
| Hristozova et
al, 2012 | 18/42 | PB/7.5 ml | Before TM | I–IV | Flow cytometry | EpCAM, CK | N stage | (29) |
CellSearch is the most common method used to isolate
CTCs from the blood samples of patients with epithelial carcinoma,
using epithelial cell adhesion molecule (EpCAM) as the target for
cell capture. However, this technique cannot capture CTCs that no
longer express EpCAM after they undergo EMT, resulting in a reduced
CTC detection rate (44). Buglione
et al (27) studied 73
patients with oropharyngeal, hypopharyngeal, nasopharyngeal,
laryngeal and nasal sinus cancer using the CD45− +
CellSearch approach. The results revealed that the CTC detection
rate was 15.1%, and more CTCs were detected at stage IV than at
stages I–III. The decrease or complete disappearance of CTCs during
treatment meant that the progression of the disease was halted. The
present results revealed that decreased or unchanged CTC counts
after treatment was associated with a good prognosis, which was
consistent with the aforementioned study. However, CTC detection
rate was not associated with clinical stage, which may due to the
fact that only stage III and IV cases were studied; therefore, the
difference in detection rate was not apparent.
Jatana et al (1), studied 48 patients with oral,
oropharyngeal, laryngeal and hypopharyngeal carcinoma at stages
I–IV using the CD45− + DAPI approach with a detection
rate of 70.8%. The results revealed that the CTC detection rate was
not associated with clinical stage, tumor site and lymphatic
metastasis, which was consistent with this study. Wollenberg et
al (32) studied 176 patients
with stage I–IV HNSCC using the immunohistochemistry-alkaline
phosphatase-anti-alkaline phosphatase + cytokeratin 19 (CK19)
method. The results revealed that individual CK19-expressing tumor
cells were detected in the bone marrow of 30.7% of patients, and
there was an association between occult tumor cells in the bone
marrow and recurrence. Univariate and multivariate analyses
indicated that metastases in locoregional lymph nodes and
disseminated tumor cells in the bone marrow were all important
predictors of prognosis. Notably, none of the aforementioned
studies investigated the association between CTC counts and
prognosis of different HNSCC subtypes. In the present study,
polyploid CTCs were associated with distant metastases, indicating
that highly proliferating tumor cells may be more likely to
metastasize.
In this study, CTC detection rate was increased,
compared with in other studies (1,27–32),
by increasing the number of cases and improving the detection
technology used. The best diagnostic threshold value for the
different types of cancer was determined according to the ROC
curve. An increase in CTCs post-treatment was associated with a
poor prognosis, which often indicates drug resistance and the use
of ineffective treatments, whereas a decrease or no change in the
CTCs was associated with a better prognosis, which often indicates
the use of effective treatments. The 3-year PFS of patients with
positive CTCs post-treatment was significantly lower than that of
patients with negative CTCs post-treatment. The PFS of patients
with negative CTCs was 1.21-fold higher than that of patients with
positive CTCs (93.31% vs. 77.40%). The 3-year OS was also
significantly reduced by 1.17 times (95.75% vs. 81.70%).
Notably, there were still patients with increased
CTCs or patients whose CTCs changed from negative to positive
post-treatment that did not exhibit disease progression or succumb
to the disease. After TP5 was administered to these patients, CTCs
were measured again and were decreased. This finding may be
associated with a decline in immunity; after using TP5 to improve
immunity, the CTC counts may decrease (45).
For patients with nasopharyngeal carcinoma, CTC
counts <2/3.2 ml post-treatment were associated with a
significantly higher PFS than in patients with CTC counts ≥2/3.2 ml
(P=0.0044). For patients with hypopharyngeal carcinoma, CTC counts
≥3/3.2 ml before and after treatment were associated with a
significantly reduced PFS and OS compared with patients with CTC
counts <3/3.2 ml (P<0.05). CTCs were related to PFS and OS
before and after treatment for hypopharyngeal squamous cell
carcinoma; however, CTCs were only associated with PFS after
treatment for nasopharyngeal squamous cell carcinoma.
The PFS of patients with nasopharyngeal squamous
cell carcinoma and decreased or unchanged CTCs post-treatment was
1.64 times higher than that of patients with increased CTCs (93.62%
vs. 87.18%); however, no difference was observed with regards to
OS. In addition, PFS and OS were significantly lower in patients
with nasopharyngeal squamous cell carcinoma whose CTCs remained
positive after treatment than those whose CTCs remained negative.
The PFS of patients with hypopharyngeal squamous cell carcinoma and
decreased or unchanged CTCs post-treatment was 3.08 times higher
than that of patients with increased CTCs (100% vs. 32.47%), and
the OS was 3.27 times higher (95% vs. 29.07%); these findings were
significant. When CTCs changed from positive to negative or
remained negative, the PFS of patients with hypopharyngeal squamous
cell carcinoma was 2.46 times higher than that of patients whose
CTCs changed from negative to positive or remained positive after
treatment (100% vs. 40.7%); however, the OS was not significantly
decreased. These findings indicated that CTCs may be a better
predictor of the prognosis of hypopharyngeal squamous cell
carcinoma than nasopharyngeal squamous cell carcinoma.
Notably, the present study had many novel aspects
compared with previous studies; in particular: i) This study used
negative enrichment of immunomagnetic beads, meaning all CTCs could
be enriched, combined with fluorescence in situ
hybridization, which is a non-radioactive, safe, fast and sensitive
technique, the probe for which can be stored for a long time, to
detect CTCs. The detection rate was 73.8%. ii) This study
investigated the clinical significance of CTCs in patients with
nasopharyngeal squamous cell carcinoma and hypopharyngeal squamous
cell carcinoma with different prognoses. iii) Youden's index and
the ROC curve were used to select the optimal CTC baseline value.
iv) The associations between increased/decreased CTCs,
positive/negative CTCs and prognosis before and after treatment
were analyzed. v) It was demonstrated that after using TP5 to
improve immunity, the CTC counts were decreased; thus, the present
study analyzed the relationship between immunity and CTCs. vi)
Polyploid CTCs were revealed to be associated with distant
metastases, indicating that highly proliferating tumor cells are
more likely to metastasize. This study provided an explanation as
to why CTCs are associated with metastasis.
Conversely, in previous studies: i) The detection
rate was 6.0–89.0% (46,47), and in the majority of studies the
detection rate was <40%. ii) Numerous cancer species were
studied with no separate subtype analysis (1,2,27–32).
iii) Youden's index and the ROC curve were not used to select the
optimal CTC baseline value (1,2,27–32).
iv) Only the relationship between increased/decreased CTCs and
prognosis was analyzed (28). v)
The relationship between immunity and CTC was not assessed
(1,27–32).
vi) The fact that CTCs are related to metastasis was mentioned, but
the underlying mechanism was not discussed (34).
In conclusion, this study revealed that the number
of polyploid CTCs was associated with distant metastasis (P=0.026).
In addition, patients with undetectable CTCs, and decreasing or
negative CTCs post-treatment tended to have a good prognosis
(P<0.05). For nasopharyngeal squamous cell carcinoma, the PFS of
patients with increased CTCs and CTCs ≥2/3.2 ml after treatment was
significantly lower (P<0.05). For hypopharyngeal squamous cell
carcinoma, it was suggested that CTCs with a cutoff value of 3 may
be used to evaluate PFS and OS before and after treatment. These
findings indicated that CTCs may be used to monitor disease
progression and the response to chemoradiotherapy for patients with
LA-HNSCC. Furthermore, CTCs are a better predictor of the prognosis
of hypopharyngeal squamous cell carcinoma than that of
nasopharyngeal squamous cell carcinoma.
In future research, lymphocyte subsets will be
examined and the changes in immune function will be evaluated by
changes in CD4, CD8, B cells and T cells prior to chemoradiotherapy
and 1 month after radiotherapy. In conclusion, a large prospective
multi-institutional validation study is required to confirm these
results.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KL, SY and XZ contributed to the conception of this
study and performed the preliminary documentation. All authors
participated in the design of the study and implemented the
research. KL, NC, JW and LM examined the archives and identified
the cases included in the study, examined the slides and collected
the pathological information. KL and JW enrolled patients in the
study, performed clinical diagnosis and collected clinical data.
All authors participated in the statistical analysis and
contributed to the interpretation of the results, as well as the
writing of the study. All authors reviewed the data and approved
the final manuscript.
Ethics approval and consent to
participate
This research abides by international and national
regulations in accordance with the Declaration of Helsinki. This
study was approved by the Ethics Committee of the Chinese PLA
General Hospital. All patients provided written informed consent
before being included in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jatana KR, Balasubramanian P, Lang JC,
Yang L, Jatana CA, White E, Agrawal A, Ozer E, Schuller DE, Teknos
TN and Chalmers JJ: Significance of circulating tumor cells in
patients with squamous cell carcinoma of the head and neck: Initial
results. Arch Otolaryngol Head Neck Surg. 136:1274–1279. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fanelli MF, Oliveira TB, Braun AC, Corassa
M, Abdallah EA, Nicolau UR, da Silva Alves V, Garcia D, Calsavara
VF, Kowalski LP and Chinen LTD: Evaluation of incidence,
significance, and prognostic role of circulating tumor microemboli
and transforming growth factor-β receptor I in head and neck
cancer. Head Neck. 39:2283–2292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Denaro N, Merlano MC and Russi EG:
Follow-up in head and neck cancer: Do more does it mean do better?
A systematic review and our proposal based on our experience. Clin
Exp Otorhinolaryngol. 9:287–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Economopoulou P, Kotsantis I, Kyrodimos E,
Lianidou ES and Psyrri A: Liquid biopsy: An emerging prognostic and
predictive tool in head and neck squamous cell carcinoma (HNSCC).
Focus on circulating tumor cells (CTCs). Oral Oncol. 74:83–89.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tognela A, Spring KJ, Becker T, Caixeiro
NJ, Bray VJ, Yip PY, Chua W, Lim SH and de Souza P: Predictive and
prognostic value of circulating tumor cell detection in lung
cancer: A clinician's perspective. Crit Rev Oncol Hematol.
93:90–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mateo J, Gerlinger M, Rodrigues DN and de
Bono JS: The promise of circulating tumor cell analysis in cancer
management. Genome Biol. 15:4482014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li F, Liu J, Song D, Zhang Q, Ding N and
He X: Circulating tumor cells in the blood of poorly differentiated
nasal squamous cell carcinoma patients: Correlation with treatment
response. Acta Otolaryngol. 136:1164–1167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu X, Shen C, Wang H, Chen F, Li G and Wen
Z: Joint quantitative measurement of hTERT mRNA in both peripheral
blood and circulating tumor cells of patients with nasopharyngeal
carcinoma and its clinical significance. BMC Cancer. 17:4792017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu SH, Tsai WS, Chang YH, Chou TY, Pang
ST, Lin PH, Tsai CM and Chang YC: Identifying cancer origin using
circulating tumor cells. Cancer Biol Ther. 17:430–438. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang HD and Yu ZK: Enrichment and
detection of circulating tumor cells and its application in head
and neck squamous cell carcinoma. Zhonghua Er Bi Yan Hou Tou Jing
Wai Ke Za Zhi. 52:147–151. 2017.(In Chinese). PubMed/NCBI
|
|
11
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6:224ra242014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramirez JM, Fehm T, Orsini M, Cayrefourcq
L, Maudelonde T, Pantel K and Alix Panabières C: Prognostic
relevance of viable circulating tumor cells detected by EPISPOT in
metastatic breast cancer patients. Clin Chem. 60:214–221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reeh M, Effenberger KE, Koenig AM,
Riethdorf S, Eichstädt D, Vettorazzi E, Uzunoglu FG, Vashist YK,
Izbicki JR, Pantel K and Bockhorn M: Circulating tumor cells as a
biomarker for preoperative prognostic staging in patients with
esophageal cancer. Ann Surg. 261:1124–1130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Racila E, Euhus D, Weiss AJ, Rao C,
McConnell J, Terstappen LW and Uhr JW: Detection and
characterization of carcinoma cells in the blood. Proc Natl Acad
Sci USA. 95:4589–4594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiltermann TJN, Pore MM, Van Den Berg A,
Timens W, Boezen HM, Liesker JJ, Schouwink JH, Wijnands WJ, Kerner
GS, Kruyt FA, et al: Circulating tumor cells in small-cell lung
cancer: A predictive and prognostic factor. Ann Oncol.
23:2937–2942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krebs MG, Sloane R, Priest L, Lancashire
L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G,
et al: Evaluation and prognostic significance of cir-culating tumor
cells in patients with non-small-cell lung cancer. J Clin Oncol.
29:1556–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsusaka S, Chin K, Oqura M, Suenaga M,
Shinozaki E, Mishima Y, Terui Y, Mizunuma N and Hatake K:
Circulating tumor cells as a surrogate marker for determining
response to chemotherapy in patients with advanced gastric cancer.
Cancer Sci. 101:1067–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rao C, Bui T, Connelly M, Doyle G, Karydis
I, Middleton MR, Clack G, Malone M, Coumans FA and Terstappen LW:
Circulating melanoma cells and survival in metastatic melanoma. Int
J Oncol. 38:755–760. 2011.PubMed/NCBI
|
|
22
|
Gazzaniga P, Gradilone A, de Berardinis E,
Busetto GM, Raimondi C, Gandini O, Nicolazzo C, Petracca A,
Vincenzi B, Farcomeni A, et al: Prognostic value of circulating
tumor cells in nonmuscle invasive bladder cancer: A cellsearch
analysis. Ann Oncol. 23:2352–2356. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poveda A, Kaye SB, McCormack R, Wang S,
Parekh T, Ricci D, Lebedinsky CA, Tercero JC, Zintl P and Monk BJ:
Circulating tumor cells predict progression free survival and
overall survival in patients with relapsed/recurrent advanced
ovarian cancer. Gynecol Oncol. 122:567–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harris L, Firtsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr; American
Society of Clinical Oncology, : American Society of Clinical
Oncology 2007 update of recommendations for the use of tumor
markers in breast cancer. J Clin Oncol. 25:5287–5312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Riethdorf S, Wu G, Wang T, Yang
K, Peng G, Liu J and Pantel K: Meta-analysis of the prognostic
value of circulating tumor cells in breast cancer. Clin Cancer Res.
18:5701–5710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buglione M, Grisanti S, Almici C, Mangoni
M, Polli C, Consoli F, Verardi R, Costa L, Paiar F, Pasinetti N, et
al: Circulating tumour cells in locally advanced head and neck
cancer: Preliminary report about their possible role in predicting
response to non-surgical treatment and survival. Eur J Cancer.
48:3019–3026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nichols AC, Lowes LE, Szeto CCT, Basmaji
J, Dhaliwal S, Chapeskie C, Todorovic B, Read N, Venkatesan V,
Hammond A, et al: Detection of circulating tumor cells in advanced
head and neck cancer using the CellSearch system. Head Neck.
34:1440–1444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hristozova T, Konschak R, Budach V and
Tinhofer I: A simple multicolor flow cytometry protocol for
detection and molecular characterization of circulating tumor cells
in epithelial cancers. Cytometry A. 81:489–495. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tinhofer I, Hristozova T, Stromberger C,
Keilhoiz U and Budach V: Monitoring of circulating tumor cells and
their expression of EGFR/phospho-EGFR during combined radiotherapy
regimens in locally advanced squamous cell carcinoma of the head
and neck. Int J Radiat Oncol Biol Phys. 83:e685–e690. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bozec A, Ilie M, Dassonville O, Long E,
Poissonnet G, Santini J, Chamorey E, Ettaiche M, Chauvière D,
Peyrade F, et al: Significance of circulating tumor cell detection
using the cellsearch system in patients with locally advanced head
and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol.
270:2745–2749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wollenberga B, Walza A, Kolbow K, Pauli C,
Chaubal S and Andratschke M: Clinical relevance of circulating
tumour cells in the bone marrow of patients with SCCHN. Onkologie.
27:358–362. 2004.PubMed/NCBI
|
|
33
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Z, Xiao Y, Zhao J, Chen M, Xu Y,
Zhong W, Xing J and Wang M: Relationship between circulating tumour
cell count and prognosis following chemotherapy in patients with
advanced non-small-cell lung cancer. Respirology. 21:519–525. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka M, Takeuchi H, Osaki Y, Hiraiwa K,
Nakamura R, Oyama T, Takahashi T, Wada N, Kawakubo H, Saikawa Y, et
al: Prognostic significance of circulating tumor cells in patients
with advanced esophageal cancer. Esophagus. 12:352–359. 2015.
View Article : Google Scholar
|
|
36
|
Cao Q, Gao X, Lin Y, Yue C, Wang Y, Quan
F, Zhang Z, Liu X, Lu Y, Zhan Y, et al: Thymopentin ameliorates
dextran sulfate sodium-induced colitis by triggering the production
of IL-22 in both innate and adaptive lymphocytes. Theranostics.
9:7490–7505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu X, Li X, Fu Q, Cao Q, Chen X, Wang M,
Yu J, Long J, Yao J, Liu H, et al: AKR1B1 promotes basal-like
breast cancer progression by a positive feedback loop that
activates the EMT program. J Exp Med. 214:1065–1079. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang C, Wang L, Guan Y, Sun Y, Liu X, Zhu
D and Guo Q: Progress of circulating tumor cells in cancer
management. Technol Cancer Res Treat. 15:509–516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Wei L, Li J, Zheng J, Zhang S and
Zhou J: Epithelial-mesenchymal transition phenotype of circulating
tumor cells is associated with distant metastasis in patients with
NSCLC. Mol Med Rep. 19:601–608. 2019.PubMed/NCBI
|
|
40
|
Castro J, Sanchez L, Nuñez MT, Lu M,
Castro T, Sharifi HR and Ericsson C: Screening circulating tumor
cells as a noninvasive cancer test in 3388 individuals from
high-risk groups (ICELLATE2). Dis Markers. 2018:46531092018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bidard FC, Peeters DJ, Fehm T, Nolé F,
Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz
JA, Stebbing J, et al: Clinical validity of circulating tumour
cells in patients with metastatic breast cancer: A pooled analysis
of individual patient data. Lancet Oncol. 15:406–414. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li L, Liu Y, Zhang S, Wang T, Bian L, Wu
S, Song S, Liu B and Jiang Z: Detection of circulating tumor cells
and its clinical value for different stages and various subtypes of
breast cancer. Zhonghua Yi Xue Za Zhi. 94:2812–2815. 2014.(In
Chinese). PubMed/NCBI
|
|
43
|
Bu XM, Xu FF, Ma J and Jiang B: The
expression of circulating tumor cells in peripheral blood of
patients with non-small cell lungcancer and its detection. J Biol
Regul Homeost Agents. 32:843–849. 2018.PubMed/NCBI
|
|
44
|
Gorges TM, Tinhofer I, Drosch M, Röse L,
Zollner TM, Krahn T and von Ahsen O: Circulating tumour cells
escape from EpCAM-based detection due to epithelial-to-mesenchymal
transition. BMC Cancer. 12:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yin SL, Liu FK, Cai DM, et al: Effect of
perioperative administration of thymopentin on cellular immunity
and cytokine level following cardiopulmonary bypass. Xinfei
Xueguanbing Zazhi. 30:116–118, 121. 2011.(In Chinese).
|
|
46
|
Tinhofer I and Staudte S: Circulating
tumor cells as biomarkers in head and neck cancer: Recent advances
and future outlook. Expert Rev Mol Diagn. 18:897–906. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McMullen KP, Chalmers JJ, Lang JC, Kumar P
and Jatana KR: Circulating tumor cells in head and neck cancer: A
review. World J Otorhinolaryngol Head Neck Surg. 2:109–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|