Introduction
Worldwide, endometrial adenocarcinoma is one of the
most common types of gynecological malignancies and the incidence
rate is increasing (1). In the USA
in 2019, it was estimated that endometrial adenocarcinoma was the
fourth most common newly diagnosed type of cancer in women, with an
estimated 61,880 new cases from 2015–2019 and 12,160 deaths between
2016 and 2019 and the incidence rate continues to increase
(2). The clinical factors
(surgical-pathological staging, depth of myometrial invasion and
lymph node metastases) and biological factors (steroid receptors,
growth factors, oncogenes and suppressor genes) were found to be
associated with reduced survival and a less favorable disease
prognosis (3,4). Due to tumor recurrence and metastasis,
as well as sensitivity to hormone therapy, radiotherapy and
chemotherapy in certain patients, the five-year survival rate of
endometrial cancer with recurrence or distant metastases is 30.8%
(5), with vaginal-only recurrence
it is 61% (6), which is
considerably lower than the median overall five-year survival rate
87% or 85% (7,8).
BMI-1 is an oncogene and a member of the
Polycomb-group family of proteins (9,10).
Several studies have shown that the expression levels of BMI-1 are
upregulated in a variety of different types of cancer (11–15).
Upregulated expression of BMI-1 promotes tumor cell proliferation,
invasion and metastasis (16–18).
In several types of cancer, BMI-1 has been reported to be poor
prognostic factor (19–21). However, the expression profile of
BMI-1 in endometrial cancer remains controversial. Engelsen et
al (22) demonstrated that low
levels of BMI-1 expression were correlated with a more aggressive
phenotype of endometrial adenocarcinoma. However, Honig et
al (15) showed that BMI-1
expression was higher in endometrial adenocarcinoma compared with
benign endometrium samples. Our previous study showed that miR-200c
inhibits epithelial-mesenchymal transition (EMT) by targeting BMI-1
via a phospho-AKT signaling pathway in endometrial cancer cells
(23). However, the prognostic
predictive ability of BMI-1 and its association with EMT in
endometrial adenocarcinoma were not assessed. Therefore, the aim of
the present study was to investigate the expression profile of
BMI-1 and its association with clinicopathologic parameters as well
as the prognostic value of BMI-1 expression in endometrial
adenocarcinoma. BMI-1 expression was also knocked down in
endometrial cancer cells to determine its role in the regulation of
tumor invasion, metastasis and EMT in vitro.
Materials and methods
Patients and specimens
A total of 60 cases (age range 38–79 years, median
age 59 years) of patients with endometrial adenocarcinoma, who had
undergone total hysterectomy or pelvic and para-aortic
lymphadenectomy simultaneously, were recruited for the present
study. Cancer tissue samples were collected during the operation. A
total of 40 normal endometrial specimens (age range, 38–77 years;
median age, 58 years), from patients with abnormal uterine bleeding
who had undergone an endometrium biopsy where the pathology results
found proliferation or secretion, were collected. All tissue
specimen were collected at Yantai Affiliated Hospital of Binzhou
Medical University between January 2007 and December 2008. The
tumors of all patients with endometrial adenocarcinoma were staged
according to the 2009 International Federation of Gynecology and
Obstetrics (FIGO) staging system (24). All of the specimens were fixed in
10% buffered formalin solution at room temperature for 48 h,
embedded in paraffin and consecutive 4-µm-thick sections were cut.
None of the patients received preoperative radiotherapy,
chemotherapy, hormone therapy or treatment with other medications,
and had no previous history of other types of cancer. Regular
follow-ups began at the day of surgery and ended after 120 months,
or upon death. The present study was approved by the Ethics
Committee of the Yantai Affiliated Hospital of Binzhou Medical
University (Approval no. 2018-016) and the study adhered to the
principles of the Declaration of Helsinki (25). Oral informed consent was obtained
from each patient prior to collection of tissues.
Immunohistochemistry (IHC)
Tissue sections were stained as described previously
(15). Briefly, following
deparaffinization in dimethylbenzene and rehydration in a series of
decreasing concentrations of alcohol (100% for 5 min, 95% for 2
min, 80% for 2 min and 75% for 2 min) the sections were heated in
an antigen retrieval buffer pH 6.0 (EDTA, 1:300 dilution; cat. no.
C1034; Beijing Solarbio Science & Technology Co., Ltd.) at
120°C for 5 min. After endogenous peroxidases were quenched with 3%
hydrogen peroxide for 30 min, the sections were incubated at 4°C
for 24 h with a primary antibody against BMI-1 (1:200; cat. no.
ab126738; rabbit anti-human monoclonal antibody; Abcam). The
samples were subsequently incubated with biotinylated secondary
goat-anti-rabbit antibodies (1:5,000; cat. no. A0208; Beyotime
Institute of Biotechnology) and horseradish peroxidase labelled
avidin, and the staining was developed using DAB (OriGene
Technologies, Inc.).
BMI-1 staining was analyzed by two investigators who
were blinded to the clinical and prognostic data. The proportion of
stained cells were scored as follows: 0, <5% of cells stained;
1, 5–25% of cells stained; 2, 26–50% of cells stained; 3, 51–75% of
cells stained; or 4 >75% of cells stained. The staining
intensity was scored as follows: 0, no staining; 1, weak staining;
2, medium staining; or 3, strong staining. The overall staining
score was calculated as follows: Staining intensity × proportion of
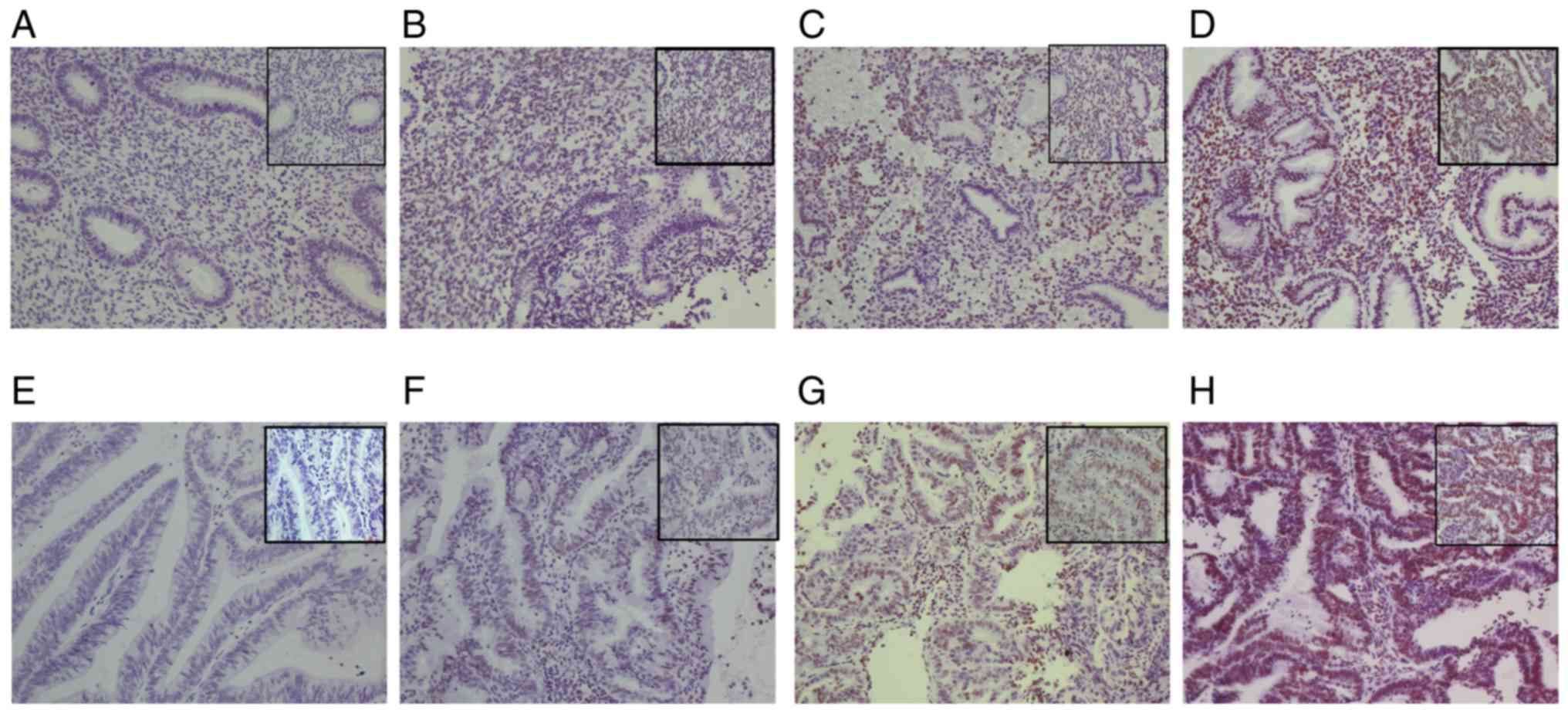
stained cells. Representative examples of the tissues with
different staining intensities are presented as follows: -, 0–2
points; +, 3–4 points; ++, 5–8 points; and +++, 9–12 points
(Fig. 1).
Cell culture
The human endometrial cancer cell lines, Ishikawa
cells were obtained from The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences; cat. no. EB081). JEC cells were
purchased from Shanghai Fusheng Industrial Co., Ltd.; cat. no.
FS-0129). Both cell lines were validated using short tandem repeat
DNA profiling. Both cell lines were cultured in RPMI 1640 medium
(HyClone; GE Healthcare Life Sciences) with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 100 µg/ml penicillin, and 100 mg/ml
streptomycin and cultured at 37°C in 5% CO2 and 95%
atmospheric air.
BMI-1 gene small interfering RNA
(siRNA) transfection
siRNA targeting the BMI-1 gene and scrambled siRNA
control were purchased from Shanghai GenePharma Co., Ltd. The
si-BMI-1 sequences were as follows: Sense,
5′-CCAGAUUGAUGUCAUGUAUTT-3′ and antisense,
5′-AUACAUGACAUCAAUC-UGGTT-3′; and scramble siRNA sense
5′-UUCUCCGAACGUGCACGUTT-3′, and antisense,
5′-ACGUGACAGGUUCGGAGAATT-3′. Cells were plated into 6-well plates
overnight, to reach 50–60% confluency, and transfected with the
BMI-1 siRNA/NC using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. After 6 h of incubation with the
transfection reagent and DNA, the cells were incubated in fresh
supplemented medium for a further 24 or 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.), treated with DNase I
(Takara Bio, Inc.) to eliminate contaminating genomic DNA, and then
reverse transcribed using a PrimeScript™ RT reagent kit (Perfect
Real Time kit; Dalian Meilun Biology Technology Co., Ltd.). RT-qPCR
was performed in a reaction volume of 20 µl containing SYBR green
PCR mix, according to the manufacturer's protocol. Each sample was
run in triplicate. GAPDH was used as the internal control, to which
all samples were normalized. Results were calculated using the
2−ΔΔCq method (26). The
primer sequences for BMI-1 and GAPDH used were: BMI-1 forward,
TCATGGTCATCCTTCTGCTGATGCTG and reverse, GCATGAGCATCACAGTCATTGCTGCT;
and GAPDH forward, CATATGCAAGGTCATCCATGACAACTTTG and reverse,
AAGCTTGTCCACCACCCTGTTGCTGTAG.
MTT cell viability assay
A total of 2×103 cells were plated per
well in 200 ml medium in a 96-well plate, with six wells per a
group, and the cells were transfected with si-BMI-1 or negative
control-siRNA as described above. After 24, 48, 72 or 96 h, 20 µl 1
mg/ml MTT solution (Sigma-Aldrich; Merck KGaA) was added to each
well, and cells were incubated for a further 4 h at 37°C with 5%
CO2 and 95% air. Subsequently, the medium was removed,
and the precipitated formazan was dissolved in 200 µl DMSO. After
agitation for 15 min, the absorbance of the medium was measured at
495 nm using a microplate reader (Omega Bio-Tek, Inc.).
Transwell assays
Briefly, 24-well transwell chambers, with 8 µm pores
(Costar; Corning, Inc.) were used to assess the migratory and
invasive properties. For the transwell migration assays, frozen
Matrigel® (Corning, Inc.) was dissolved at room
temperature and was diluted to a working solution of 1:8 Matrigel:
Serum-free medium. The upper chambers were coated with 100 µl
Matrigel solution per well, and incubated for 24 h to allow the
Matrigel to polymerize. For the invasion assays, the chambers were
not coated prior to use. A total of 24 h after transfection, the
cells were seeded in the upper chamber of the transwell inserts in
200 µl serum-free medium (1×105 cells/ml). After 24 h of
incubation, cells which had not migrated or invaded were removed.
The cells on the underside of the chambers were fixed using
methanol and 3.7%formaldehyde solution at room temperature, each
for 5 min. Subsequently, the cells were stained with 0.1% crystal
violet at room temperature for 30 min, imaged using an Olympus IX51
inverted microscope (Olympus Corporation) with a UIS2 optical
system and phase contrast objectives, and the number of cells in
five randomly chosen fields of view (magnification, ×200) were
counted. A total of three independent experiments were performed
for statistical analysis.
Western blotting
After transfection for 48 h, proteins were
extracted, and western blotting was performed. Proteins were
extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology) with 1% PMSF (Thermo Fisher Scientific Inc.) and 1%
NaF (Beyotime Institute of Biotechnology). Protein concentrations
were determined using a bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology). A total of 40 mg of protein
was loaded per lane on 10% SDS-gel, resolved by SDS-PAGE and
subsequently transferred to a PVDF membrane. Membranes were blocked
with 5% fat-free dry milk at room temperature for 2 h, followed by
incubation with primary rabbit anti-BMI-1 monoclonal antibodies
(1:1,000 dilution; cat. no. ab126738; Abcam), and rabbit monoclonal
antibodies against E-cadherin, N-cadherin, vimentin, Keratin and
slug (1:1,000 dilution; cat. nos. 3195, 4061, 5741s, 4546p and
58613, respectively; Cell Signaling Technology, Inc.) overnight at
4°C. After washing with TBS-Tween three times 5 min each, the
membranes were incubated with secondary goat anti-rabbit IgG
antibody conjugated to horseradish peroxidase (1:5,000; cat. nos.
ab6721; Abcam) for 2 h at room temperature. GAPDH was used as the
loading control with a rabbit anti-GAPDH antibody (1:1,000; cat.
no. cst2118; Cell Signaling Technology, Inc.) overnight at 4°C.
Densitometry analysis was performed using ImageJ software version
1.46 (National Institutes of Health). A total of three independent
experiments were performed for statistical analysis.
Statistical analysis
Experiments were performed at least three times for
statistical analysis. SPSS version 23.0 (IBM Corp.) and GraphPad
version 6.0 (GraphPad Software, Inc.) were used for statistical
analysis. Results are presented as the mean ± standard error of the
mean. Comparison among groups was performed using a one-way ANOVA
with a post hoc Tukey's test for multiple comparisons. Unpaired
nominal-scale data was analyzed using a χ2 test.
Pearson's correlation coefficient tests were used to analyze the
correlation between BMI-1 expression and the clinicopathological
parameters. A log-rank test was used for Kaplan-Meier survival
analysis. The significance of the clinicopathological
characteristics on survival were analyzed using a Cox proportional
hazards model in univariate and multivariate analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of BMI-1 in endometrial
adenocarcinoma tissues
IHC was performed to detect BMI-1 expression levels
in endometrial adenocarcinoma and normal endometrial tissues. BMI-1
expression levels were significantly increased in endometrial
adenocarcinoma tissues compared with the normal endometrial tissue
(χ2=25.0, P<0.001). A total of 10% (4/40) of normal
endometrial tissues exhibited high expression of BMI-1
(representative example in Fig. 1C and
D), and the remaining tissues (90%, 36/40) had no or low
expression levels of BMI-1 (representative example in Fig. 1A and B). However, 60% (36/60) of the
cancer tissues exhibited high BMI-1 expression levels
(representative example in Fig. 1G and
H) and the remainder (40%, 24/60) exhibited low expression
(representative example in Fig. 1E and
F). The intensity of staining observed in endometrial
adenocarcinoma tissue was higher compared with the normal
endometrial tissue (Table II).
Interestingly, BMI-1 expression was detected in both the nucleus
and cytoplasm in normal and cancer cells (Fig. 1C, D, G and H). These results suggest
that BMI-1 expression is increased in endometrial adenocarcinoma
tissues compared with normal endometrial tissues (Table I).
 | Table II.Patient characteristics and
percentage of positive cells and staining intensity of BMI-1
expression. |
Table II.
Patient characteristics and
percentage of positive cells and staining intensity of BMI-1
expression.
|
|
| Proportion of
stained cells, n (%) | Staining intensity,
n |
|---|
|
|
|
|
|
|---|
|
Characteristics | n | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 |
|---|
| Age |
|
|
|
|
|
|
|
|
|
|
|
≤60 | 21 | 1 (2.0) | 2 (18.5) | 4 (34.6) | 7 (68.2) | 7 (89.6) | 1 | 2 | 4 | 14 |
|
>60 | 39 | 1 (3.6) | 3 (21.4) | 5 (40.2) | 10 (60.6) | 20 (92.5) | 1 | 4 | 11 | 23 |
| FIGO stage |
|
|
|
|
|
|
|
|
|
|
| I | 26 | 1 (2.8) | 5 (15.6) | 5 (32.5) | 7 (71.5) | 8 (84.2) | 1 | 8 | 10 | 7 |
| II | 20 | 1 (3.2) | 3 (16.5) | 6 (38.2) | 5 (78.6) | 5 (89.7) | 1 | 4 | 5 | 10 |
|
III | 14 | 0 | 1 (19.3) | 0 | 1 (64.0) | 12 (91.4) | 0 | 0 | 6 | 8 |
| Histologic
type |
|
|
|
|
|
|
|
|
|
|
|
Endometrioid-adenocarcinoma | 29 | 1 (3.0) | 3 (15.2) | 4 (33.2) | 10 (79.4) | 11 (86.7) | 1 | 6 | 12 | 10 |
|
Serous-adenocarcinoma | 31 | 1 (3.5) | 3 (17.5) | 7 (38.0) | 8 (79.2) | 12 (89.6) | 1 | 4 | 10 | 16 |
| Grade |
|
|
|
|
|
|
|
|
|
|
| G1 | 28 | 1 (3.2) | 3 (14.8) | 6 (36.0) | 8 (78.2) | 10 (88.5) | 1 | 6 | 12 | 9 |
| G2 | 18 | 1 (3.5) | 2 (16.6) | 5 (36.5) | 5 (78.6) | 5 (88.5) | 1 | 4 | 5 | 8 |
| G3 | 14 | 1 (3.6) | 1 (20.8) | 2 (37.6) | 4 (62.5) | 6 (91.8) | 1 | 1 | 3 | 9 |
| Myometrial
invasion |
|
|
|
|
|
|
|
|
|
|
|
<50% | 45 | 2 (2.8) | 4 (20.6) | 8 (40.1) | 15 (64.8) | 16 (90.7) | 2 | 12 | 8 | 23 |
|
≥50% | 15 | 0 | 1 (22.3) | 1 (35.7) | 1 (64.0) | 12 (91.4) | 0 | 0 | 5 | 10 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
|
|
| No | 47 | 2 (2.8) | 5 (19.9) | 9 (37.4) | 13 (63.2) | 18 (91.1) | 2 | 11 | 10 | 24 |
|
Yes | 13 | 0 | 0 | 1 (39.7) | 2 (65.6) | 10 (91.2) | 0 | 0 | 3 | 10 |
 | Table I.BMI-1 expression between normal
endometrial and endometrial adenocarcinoma tissues. |
Table I.
BMI-1 expression between normal
endometrial and endometrial adenocarcinoma tissues.
| Group | n (% of total) |
|---|
| Normal
endometrium | 40 |
| Low
BMI-1 expression | 36 (90%) |
| High
BMI-1 expression | 4
(10%) |
| Endometrial
adenocarcinoma | 60 |
| Low
BMI-1 expression | 24 (40.0%) |
| High
BMI-1 expression | 36 (60.0%) |
Association between BMI-1 expression
and clinicopathological characteristics of endometrial
adenocarcinoma
The possible correlations between BMI-1 expression
and clinicopathological characteristics were assessed. As shown in
Tables II and III, BMI-1 expression levels were
correlated with the FIGO stage, myometrial invasion and lymph node
metastasis (P<0.05). However, there was no correlation between
BMI-1 expression levels and age or grade.
 | Table III.Correlation between BMI-1 expression
and clinicopathological characteristics in patients with
endometrial adenocarcinoma. |
Table III.
Correlation between BMI-1 expression
and clinicopathological characteristics in patients with
endometrial adenocarcinoma.
| Clinicopathological
characteristics | n | Low BMI-1
expression (%) | High BMI-1
expression (%) | Correlation
coefficient | P-value |
|---|
| Age |
|
|
| 0.100 | 0.448 |
|
≤60 | 21 | 7 (33.33) | 14 (66.67) |
|
|
|
>60 | 39 | 17 (43.59) | 22 (56.41) |
|
|
| FIGO stage |
|
|
| 0.290 | 0.025a |
|
I–II | 46 | 22 (47.83) | 24 (52.17) |
|
|
|
III | 14 | 2 (14.29) | 12 (85.71) |
|
|
| Grade |
|
|
| 0.048 | 0.714 |
|
G1/G2 | 46 | 19 (41.30) | 27 (58.70) |
|
|
| G3 | 14 | 5 (35.71) | 9 (64.29) |
|
|
| Myometrial
invasion |
|
|
| 0.314 | 0.014a |
|
<50% | 45 | 22 (48.89) | 23 (51.11) |
|
|
|
≥50% | 15 | 2 (13.3) | 13 (86.67) |
|
|
| Lymph node
metastasis |
|
|
| 0.347 | 0.007b |
| No | 47 | 23 (48.94) | 24 (51.06) |
|
|
|
Yes | 13 | 1
(7.69) | 12 (92.31) |
|
|
High BMI-1 expression is associated
with a less favorable prognosis
As shown in Table
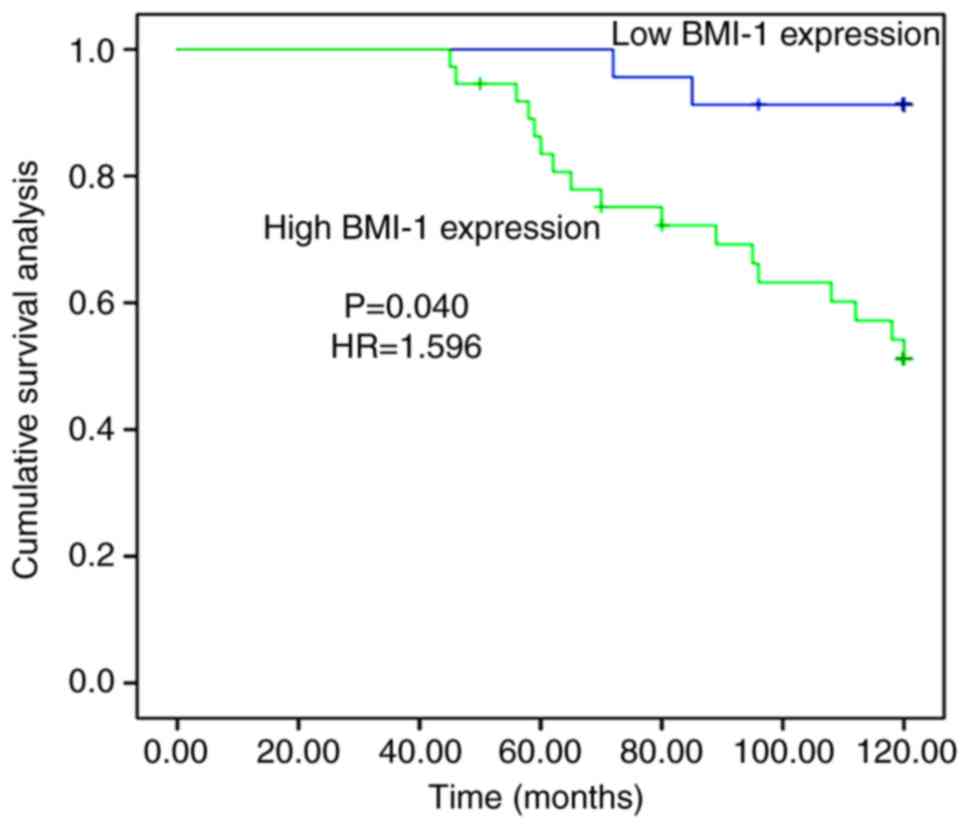
IV, in the 120-month follow-up study, Kaplan-Meier analyses
showed that 36 patients with high expression levels of BMI-1 had a
survival rate of 52.8% and 24 patients with low expression levels
of BMI-1 had a survival rate of 91.7%. There was a significant
difference in survival rate in patients with endometrial
adenocarcinoma with high and low expression levels of BMI-1
(P=0.040, HR=1.596). Kaplan-Meier analyses revealed that the
overall survival time of patients with high expression levels of
BMI-1 was significantly shorter compared with patients with low
expression levels (Fig. 2).
 | Table IV.Kaplan-Meier analyses of the
association between BMI-1 expression and survival time. |
Table IV.
Kaplan-Meier analyses of the
association between BMI-1 expression and survival time.
| BMI-1
expression | Survival rate
(%) | 95% confidence
interval | χ2 | P-value |
|---|
| Low | 91.7 |
115.365–121.051 | 12.036 | <0.001 |
| High | 52.8 |
87.564–107.007 |
|
|
Cox regression proportional hazard analyses were
used to determine the risk factors associated with death. As shown
in Table V, univariate Cox
regression analyses revealed that the risk of death with high BMI-1
expression levels was significantly increased compared with low
BMI-1 expression levels in patients with endometrial adenocarcinoma
(P=0.040, HR=1.596). Additionally, late-stage (III, P=0.006,
HR=1.67), myometrial invasion (P=0.006, HR=1.509) and lymph node
metastasis (P=0.004, HR=1.703) were also significantly associated
with less favorable prognosis. However, a high grade was not
associated with increased risk of death (P=0.234). After adjustment
for confounding factors, BMI-1 expression levels were still shown
to predict a less favorable prognosis using multivariate Cox
regression analysis (P=0.037, HR=1.698; Table VI). Furthermore, late-stage
(P=0.017, HR=1.645), myometrial invasion (P=0.010, HR=1.305) and
lymph node metastasis (P=0.016, HR=1.352; Table VI) were still shown to predict a
less favorable prognosis.
 | Table V.Univariate Cox-regression analysis of
clinicopathological characteristics in patients with endometrial
adenocarcinoma. |
Table V.
Univariate Cox-regression analysis of
clinicopathological characteristics in patients with endometrial
adenocarcinoma.
| Clinicopathological
characteristics | 95% Confidence
interval | P-value | Hazard ratio |
|---|
| Age | 0.173–1.482 | 0.214 | −0.680 |
|
≤60 |
|
|
|
|
>60 |
|
|
|
| FIGO stage | 1.598–7.66 | 0.006b | 1.670 |
|
I–II |
|
|
|
|
III |
|
|
|
| Grade |
0.597–8.244 | 0.234 | 0.797 |
|
G1/G2 |
|
|
|
| G3 |
|
|
|
| Myometrial
invasion | 1.547–3.228 | 0.006b | 1.509 |
|
<50% |
|
|
|
|
≥50% |
|
|
|
| Lymph node
metastasis | 1.750–7.236 | 0.004b | 1.703 |
| No |
|
|
|
|
Yes |
|
|
|
| BMI-1
expression | 1.076–2.617 | 0.040a | 1.596 |
|
Low |
|
|
|
|
High |
|
|
|
 | Table VI.Multivariate Cox-regression analysis
of clinicopathological characteristics in patients with endometrial
adenocarcinoma. |
Table VI.
Multivariate Cox-regression analysis
of clinicopathological characteristics in patients with endometrial
adenocarcinoma.
| Clinicopathological
characteristics | 95% Confidence
interval | P-value | Hazard ratio |
|---|
| FIGO stage | 1.515–6.217 | 0.008 | 1.601 |
|
I–II |
|
|
|
|
III |
|
|
|
| Myometrial
invasion | 1.365–9.967 | 0.010 | 1.305 |
|
<50% |
|
|
|
|
≥50% |
|
|
|
| Lymph node
metastasis | 1.290–5.579 | 0.016 | 1.352 |
| No |
|
|
|
|
Yes |
|
|
|
| BMI-1
expression | 1.102–4.329 | 0.037 | 1.645 |
|
Low |
|
|
|
|
High |
|
|
|
Knockdown of BMI-1 expression in-vitro
reduces endometrial adenocarcinoma cell growth and
proliferation
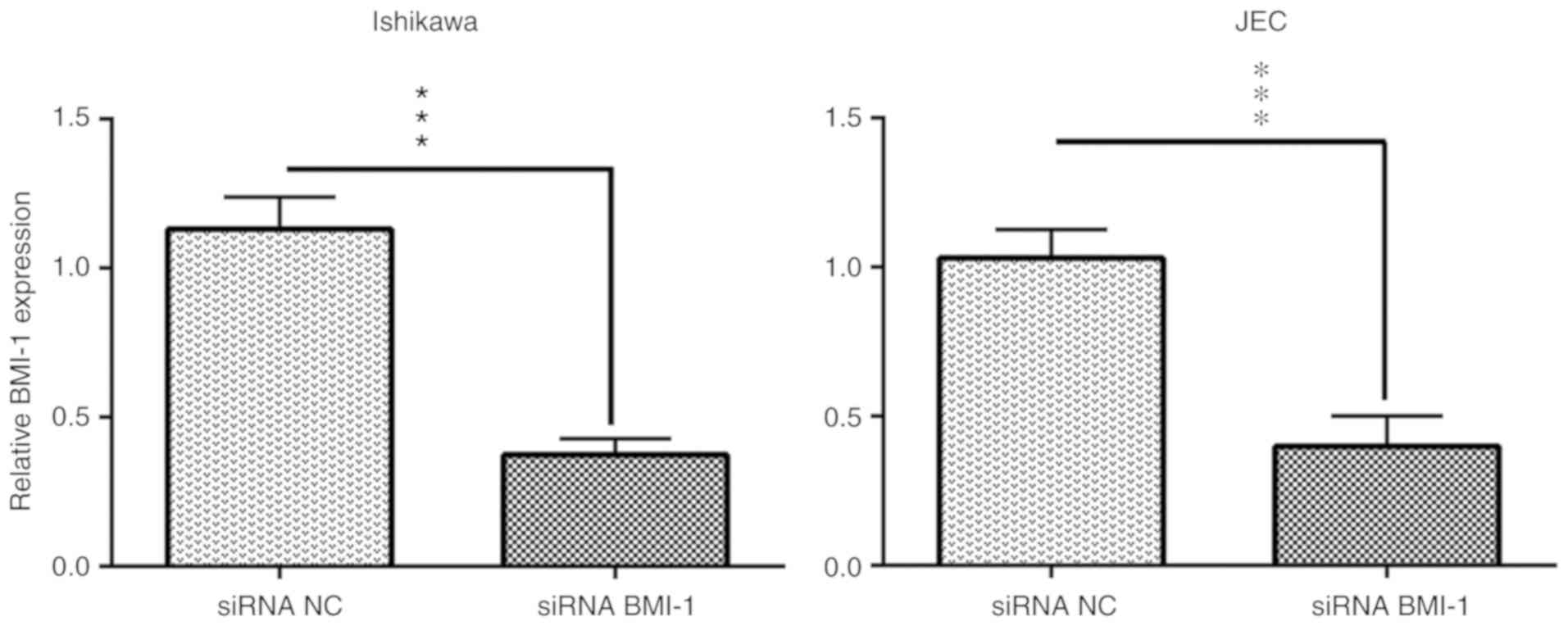
To determine the potential functional roles of BMI-1
in the Ishikawa and JEC endometrial cancer cell lines, si-BMI-1 was
transfected into cells to knockdown the expression levels of BMI-1,
and RT-qPCR was used to assess the transfection efficiency. The
results showed that the quantitative expression of BMI-1 mRNA
decreased 37.45±2.7% in Ishikawa cells and 39.95±5.0% in JEC cells
following si-BMI-1 transfection (Fig.
3).
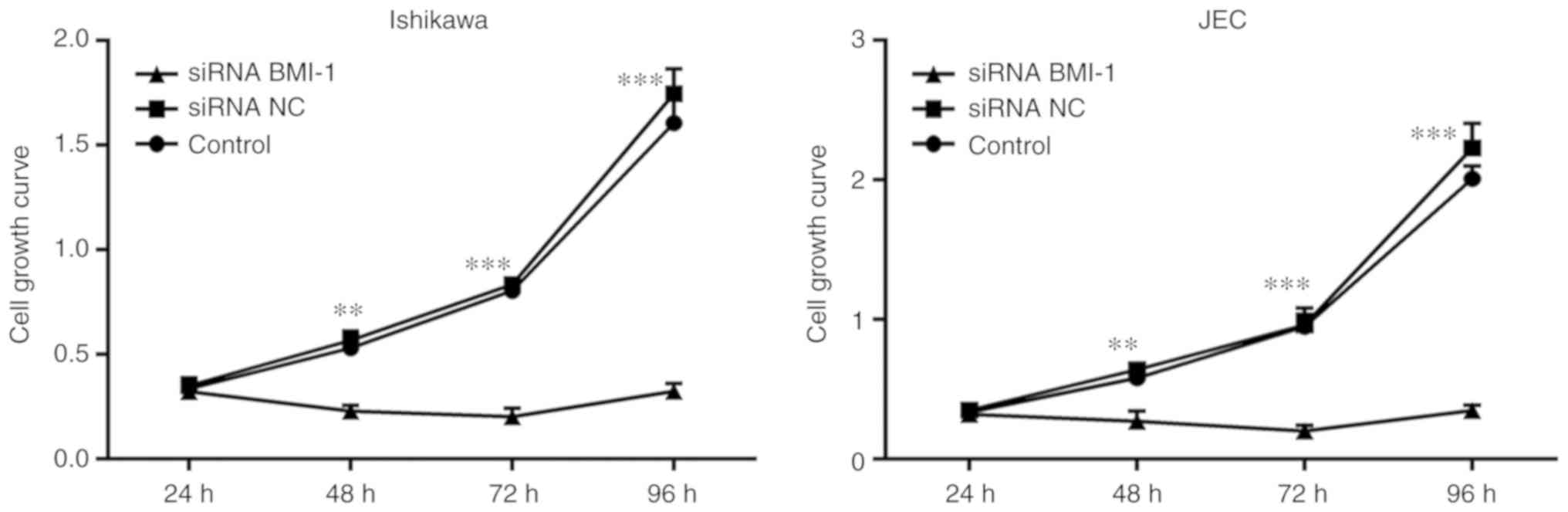
The MTT assay results showed that cell growth and
proliferation were decreased in the si-BMI-1 transfected Ishikawa
and JEC cells compared with the respective controls (Fig. 4).
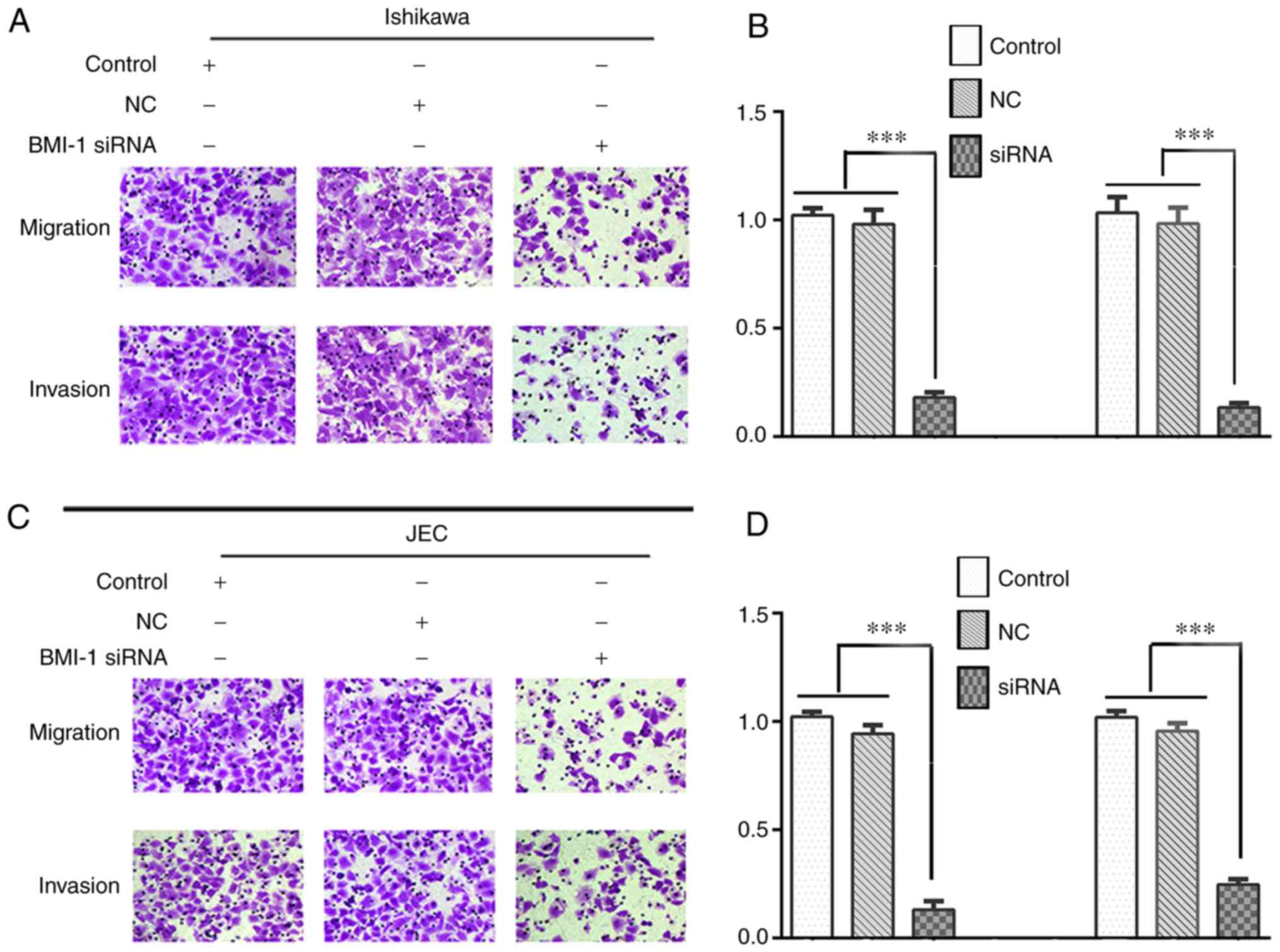
Knockdown of BMI-1 expression
decreases migration and invasion of Ishikawa and JEC cells
After the cells were transfected with si-BMI-1, the
effects of BMI-1 on cell migration and invasion were assessed using
transwell assays. The results showed that knockdown of BMI-1
expression significantly reduced the migratory and invasive
capacities of Ishikawa (Fig. 5A and
B) and JEC cells (Fig. 5C and
D; P<0.001).
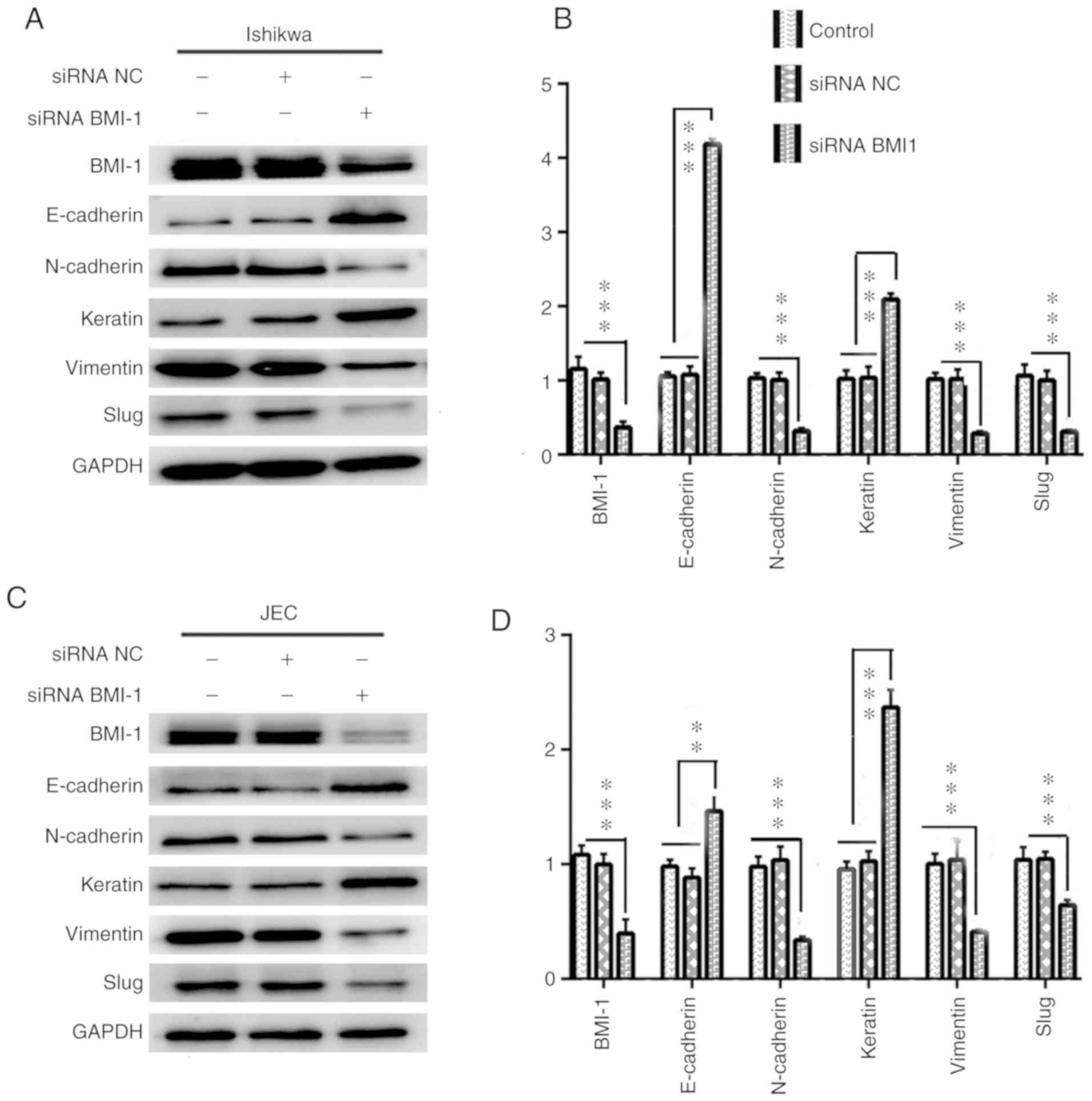
Knockdown of BMI-1 expression inhibits
EMT in Ishikawa and JEC cells
To further investigate the functional roles of BMI-1
expression on the migratory and invasive capacities of Ishikawa and
JEC cells, the expression of EMT-associated proteins was assessed
in cells transfected with si-BMI-1. Western blotting showed that
the expression levels of the mesenchymal markers N-cadherin,
vimentin and the downstream EMT transcription factor, Slug, were
decreased in the si-BMI-1 transfected cells, whereas the expression
levels of the epithelial markers E-cadherin and keratin were
increased relative to the respective control cells (Fig. 6).
Discussion
Endometrial adenocarcinoma is one of the most common
gynecological malignancies in women and is prone to invade adjacent
regions and to metastasize to lymph nodes (27–29).
To develop effective treatments for treatment of endometrial
adenocarcinoma, it is important to identify the factors underlying
tumorigenesis, invasion and metastasis. One of the clinical
features of endometrial adenocarcinoma is uterine bleeding
(30,31). Normal endometrium exhibits
proliferative, secretory and atrophic properties (32,33).
Therefore, patients with abnormal uterine bleeding who had
undergone an endometrium biopsy (34,35)
where the results of pathological analysis showed proliferation or
secretion were used as control group in the present study, and it
was shown that BMI-1 expression was significantly higher in
endometrial adenocarcinoma tissues compared with the control
tissues. BMI-1 was demonstrated to serve an important functional
role in the progression of endometrial adenocarcinoma. In
endometrial adenocarcinoma tissues, 60% of tissues exhibited high
levels of expression of BMI-1 in the present study, consistent with
that of a previous study which found that BMI-1 expression was
significantly upregulated in endometrial cancer (15). In the present study, high BMI-1
expression levels were correlated with myometrial invasion and
lymph node metastasis. Of the samples classed as FIGO stage III,
deep myometrial invasion and lymph nodes metastasis, >85%
exhibited high expression levels of BMI-1. These results showed
that higher BMI-1 expression levels were associated with more
aggressive behavior. A previous study also found that low BMI-1
expression levels were associated with histological grade 3 and
deep myometrial infiltration (22).
The difference in results between the present and previous studies
may reflect differences in BMI-1 status of the samples used in the
different studies, with tissue samples obtained from varying
populations. In the present study, high BMI-1 expression levels
were not correlated with tumor differentiation, suggesting that
high BMI-1 expression levels were associated with tumor progression
but not differentiation.
Upregulated expression of BMI-1 was a poor
prognostic factor in patients with endometrial adenocarcinoma,
consistent with previous studies (16,18–21,36–38).
In the present study, it was found that patients with high BMI-1
expression levels had a worse prognosis compared with patients with
low BMI-1 expression levels. Late-stage, myometrial invasion and
lymph node metastasis were also unfavorable prognostic factors.
However, high expression levels of BMI-1 was an independent
prognostic indicator, alongside late-stage, myometrial invasion and
lymph node metastasis.
Invasion and metastasis of cancer is commonly
associated with a poor prognosis in patients (27,39,40).
Knockdown of BMI-1 expression levels reduced the proliferative,
migratory and invasive capacities of Ishikawa and JEC cells. EMT is
characterized by a loss of the epithelial markers E-cadherin and
keratin, and increased the expression levels of the mesenchymal
markers vimentin and N-cadherin (41–46).
There was also activation of the EMT related pathways, leading to
an increase in migratory and invasive behavior (43). The BMI-1 gene has been reported to
stimulate EMT by reducing the expression levels of the epithelial
marker E-cadherin, and increasing expression of the mesenchymal
marker, N-cadherin (16,17,47–52).
In the present study, western blotting showed that knockdown of
BMI-1 expression was associated with increased E-cadherin and
keratin expression, whilst simultaneously reducing N-cadherin,
vimentin and Slug expression levels. These results are consistent
with the results of previous study (17,23).
One limitation of the present study is that the expression and
function of BMI-1 in normal endometrial cells were not determined
and compared with endometrial adenocarcinoma cells.
In conclusion, the present study showed that BMI-1
expression was increased in endometrial adenocarcinoma tissues
compared with the control tissues, and that knockdown of BMI-1
expression may inhibit EMT in endometrial adenocarcinoma cells.
Additionally, BMI-1 expression was correlated with tumor invasion
and metastasis, contributing to lymph node metastases and deep
myometrial invasion in endometrial adenocarcinoma. These results
suggest that BMI-1 may serve as a potential target for treatment of
endometrial adenocarcinoma. However the mechanisms underlying the
development of endometrial adenocarcinoma development are complex
and require further study.
Acknowledgements
We appreciate the valuable work carried out by Dr Yu
Yunliang (Binzhou Medical University) for her interpretation of the
immunohistochemistry staining.
Funding
The present study was supported by grants from the
Key Research Items of Shandong Province (grant no. 2018GSF118190)
and Science and Technology Planning Project of Yantai (grant no.
2018SFGY100).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL, LL and GL designed the study. JY, LC, ZB and YL
performed the experiments and analyzed the data. FL wrote the
manuscript. LL and GL revised the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Yantai Affiliated Hospital of Binzhou Medical University
(approval no. 2018-016) and the study adhered to the principles of
the Declaration of Helsinki. Informed consent was obtained from
each patient prior to tissue collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sheikh MA, Althouse AD, Freese KE, Soisson
S, Edwards RP, Welburn S, Sukumvanich P, Comerci J, Kelley J,
LaPorte RE and Linkov F: USA endometrial cancer projections to
2030: Should we be concerned? Future Oncol. 10:2561–2568. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kulhan M, Kulhan G, Nayki U, Nayki C, Ulug
P, Sipahi M and Yildirim Y: Assessment of clinicopathological
features, evaluation of treatment, and prognosis of clear cell and
serous papillary endometrial carcinoma. Ginekol Pol. 87:570–574.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dessai SB, Adrash D, Geetha M, Arvind S,
Bipin J, Nayanar S, Sachin K, Biji MS and Balasubramanian S:
Pattern of care in operable endometrial cancer treated at a
rural-based tertiary care cancer center. Indian J Cancer.
53:416–419. 2016.PubMed/NCBI
|
|
5
|
Ouldamer L, Bendifallah S, Body G, Touboul
C, Graesslin O, Raimond E, Collinet P, Coutant C, Bricou A, Lavoué
V, et al: Incidence, patterns and prognosis of first distant
recurrence after surgically treated early stage endometrial cancer:
Results from the multicentre FRANCOGYN study group. Eur J Surg
Oncol. 45:672–678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francis SR, Ager BJ, Do OA, Huang YJ,
Soisson AP, Dodson MK, Werner TL, Sause WT, Grant JD and Gaffney
DK: Recurrent early stage endometrial cancer: Patterns of
recurrence and results of salvage therapy. Gynecol Oncol.
154:38–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bogani G, Dowdy SC, Cliby WA, Ghezzi F,
Rossetti D, Frigerio L and Mariani A: Management of endometrial
cancer: Issues and controversies. Eur J Gynaecol Oncol. 37:6–12.
2016.PubMed/NCBI
|
|
8
|
Evans T, Sany O, Pearmain P, Ganesan R,
Blann A and Sundar S: Differential trends in the rising incidence
of endometrial cancer by type: data from a UK population-based
registry from 1994 to 2006. Br J Cancer. 104:1505–1510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rajasekhar VK and Begemann M: Concise
review: Roles of polycomb group proteins in development and
disease: A stem cell perspective. Stem Cells. 25:2498–2510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang L, Li J and Song L: Bmi-1, stem
cells and cancer. Acta Biochim Biophys Sin (Shanghai). 41:527–534.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beà S, Tort F, Pinyol M, Puig X, Hernández
L, Hernández S, Fernandez PL, van Lohuizen M, Colomer D and Campo
E: BMI-1 gene amplification and overexpression in hematological
malignancies occur mainly in mantle cell lymphomas. Cancer Res.
61:2409–2412. 2001.PubMed/NCBI
|
|
12
|
Kim JH, Yoon SY, Jeong SH, Kim SY, Moon
SK, Joo JH, Lee Y, Choe IS and Kim JW: Overexpression of Bmi-1
oncoprotein correlates with axillary lymph node metastases in
invasive ductal breast cancer. Breast. 13:383–388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Pan K, Zhang HK, Weng DS, Zhou J,
Li JJ, Huang W, Song HF, Chen MS and Xia JC: Increased
polycomb-group oncogene Bmi-1 expression correlates with poor
prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol.
134:535–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Wang CX, Zhu CB, Zhang J, Kan SF,
Du LT, Li W, Wang LL and Wang S: Overexpression of Bmi-1 in uterine
cervical cancer: Correlation with clinicopathology and prognosis.
Int J Gynecol Cancer. 20:1597–1603. 2010.PubMed/NCBI
|
|
15
|
Honig A, Weidler C, Häusler S,
Krockenberger M, Buchholz S, Köster F, Segerer SE, Dietl J and
Engel JB: Overexpression of polycomb protein BMI-1 in human
specimens of breast, ovarian, endometrial and cervical cancer.
Anticancer Res. 30:1559–1564. 2010.PubMed/NCBI
|
|
16
|
Guo BH, Feng Y, Zhang R, Xu LH, Li MZ,
Kung HF, Song LB and Zeng MS: Bmi-1 promotes invasion and
metastasis, and its elevated expression is correlated with an
advanced stage of breast cancer. Mol Cancer. 10:102011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan W, Yuan Y, Zhang T and Wu S: Role of
Bmi-1 in regulation of ionizing irradiation-induced
epithelial-mesenchymal transition and migration of breast cancer
cells. PLoS One. 10:e01187992015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang MC, Jiao M, Wu T, Jing L, Cui J, Guo
H, Tian T, Ruan ZP, Wei YC, Jiang LL, et al: Polycomb complex
protein BMI-1 promotes invasion and metastasis of pancreatic cancer
stem cells by activating PI3K/AKT signaling, an ex vivo, in vitro,
and in vivo study. Oncotarget. 7:9586–9599. 2016.PubMed/NCBI
|
|
19
|
Silva J, García V, García JM, Peña C,
Domínguez G, Díaz R, Lorenzo Y, Hurtado A, Sánchez A and Bonilla F:
Circulating Bmi-1 mRNA as a possible prognostic factor for advanced
breast cancer patients. Breast Cancer Res. 9:R552007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abe S, Yamashita SI, Miyahara SO, Wakahara
J, Yamamoto L, Mori R, Imamura N, Yoshida Y, Waseda R, Hiratsuka M,
et al: Prognostic significance of BMI-1 But Not MEL-18 expression
in pulmonary squamous cell carcinoma. Anticancer Res. 37:1923–1929.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Tian T, Sun W, Liu C and Fang X:
Bmi-1 overexpression as an efficient prognostic marker in patients
with nonsmall cell lung cancer. Medicine (Baltimore). 96:e73462017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Engelsen IB, Mannelqvist M, Stefansson IM,
Carter SL, Beroukhim R, Øyan AM, Otte AP, Kalland KH, Akslen LA and
Salvesen HB: Low BMI-1 expression is associated with an activated
BMI-1-driven signature, vascular invasion, and hormone receptor
loss in endometrial carcinoma. Br J Cancer. 98:1662–1669. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li F, Liang A, Lv Y, Liu G, Jiang A and
Liu P: MicroRNA-200c inhibits epithelial-mesenchymal transition by
targeting the BMI-1 gene through the Phospho-AKT pathway in
endometrial carcinoma cells in vitro. Med Sci Monit. 23:5139–5149.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
FIGO Committee on Gynecologic Oncology, .
FIGO staging for carcinoma of the vulva, cervix, and corpus uteri.
Int J Gynaecol Obstet. 125:97–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hellmann F, Verdi M, Schlemper BR Jr and
Caponi S: 50th anniversary of the Declaration of Helsinki: The
double standard was introduced. Arch Med Res. 45:600–601. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Makker A and Goel MM: Tumor progression,
metastasis, and modulators of epithelial-mesenchymal transition in
endometrioid endometrial carcinoma: An update. Endocr Relat Cancer.
23:R85–R111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mahdi H, Jernigan A, Nutter B, Michener C
and Rose PG: Lymph node metastasis and pattern of recurrence in
clinically early stage endometrial cancer with positive
lymphovascular space invasion. J Gynecol Oncol. 26:208–213. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Devis L, Moiola CP, Masia N,
Martinez-Garcia E, Santacana M, Stirbat TV, Brochard-Wyart F,
García Á, Alameda F, Cabrera S, et al: Activated leukocyte cell
adhesion molecule (ALCAM) is a marker of recurrence and promotes
cell migration, invasion, and metastasis in early-stage
endometrioid endometrial cancer. J Pathol. 241:475–487. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giannella L, Cerami LB, Setti T, Bergamini
E and Boselli F: Prediction of endometrial hyperplasia and cancer
among premenopausal Women with abnormal uterine bleeding. Biomed
Res Int. 2019:85981522019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khafaga A and Goldstein SR: Abnormal
uterine bleeding. Obstet Gynecol Clin North Am. 46:595–605. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goldstein SR and Lumsden MA: Abnormal
uterine bleeding in perimenopause. Climacteric. 20:414–420. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Munro MG, Critchley HOD and Fraser IS;
FIGO Menstrual Disorders Committee, : The two FIGO systems for
normal and abnormal uterine bleeding symptoms and classification of
causes of abnormal uterine bleeding in the reproductive years: 2018
revisions. Int J Gynaecol Obstet. 143:393–408. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stovall TG, Photopulos GJ, Poston WM, Ling
FW and Sandlers LG: Pipelle endometrial sampling in patients with
known endometrial carcinoma. Obstet Gynecol. 77:954–959.
1991.PubMed/NCBI
|
|
35
|
Guido RS, Kanbour-Shakir A, Rulin MC and
Christopherson WA: Pipelle endometrial sampling. Sensitivity in the
detection of endometrial cancer. J Reprod Med. 40:553–555.
1995.PubMed/NCBI
|
|
36
|
Peng HX, Liu XD, Luo ZY, Zhang XH, Luo XQ,
Chen X, Jiang H and Xu L: Upregulation of the proto-oncogene Bmi-1
predicts a poor prognosis in pediatric acute lymphoblastic
leukemia. BMC Cancer. 17:762017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song W, Tao K, Li H, Jin C, Song Z, Li J,
Shi H, Li X, Dang Z and Dou K: Bmi-1 is related to proliferation,
survival and poor prognosis in pancreatic cancer. Cancer Sci.
101:1754–1760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Zhe H, Ding Z, Gao P, Zhang N and
Li G: Cancer stem cell marker Bmi-1 expression is associated with
basal-like phenotype and poor survival in breast cancer. World J
Surg. 36:1189–1194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lei X, Li YF, Chen GD, Ou DP, Qiu XX, Zuo
CH and Yang LY: Ack1 overexpression promotes metastasis and
indicates poor prognosis of hepatocellular carcinoma. Oncotarget.
6:40622–40641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang M, Xie X and Ding Y: SALL4 is a
marker of poor prognosis in serous ovarian carcinoma promoting
invasion and metastasis. Oncol Rep. 35:1796–1806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kalluri R and Weinberg RA: The basics of
epithelial--mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu
LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al: The polycomb group
protein Bmi-1 represses the tumor suppressor PTEN and induces
epithelial-mesenchymal transition in human nasopharyngeal
epithelial cells. J Clin Invest. 119:3626–3636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li H, Song F, Chen X, Li Y, Fan J and Wu
X: Bmi-1 regulates epithelial-to-mesenchymal transition to promote
migration and invasion of breast cancer cells. Int J Clin Exp
Pathol. 7:3057–3064. 2014.PubMed/NCBI
|
|
49
|
Yi C, Li BB and Zhou CX: Bmi-1 expression
predicts prognosis in salivary adenoid cystic carcinoma and
correlates with epithelial-mesenchymal transition-related factors.
Ann Diagn Pathol. 22:38–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Y, Chu Z, Li Q, Peng B, Xu S, Lian CG
and Geng S: Downregulation of Bmi-1 suppresses
epithelial-mesenchymal transition in melanoma. Oncol Rep.
37:139–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zheng Z, Bao F, Chen X, Huang H and Zhang
X: MicroRNA-330-3p expression indicates good prognosis and
suppresses cell proliferation by targeting Bmi-1 in osteosarcoma.
Cell Physiol Biochem. 46:442–450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Joensuu K, Hagstrom J, Leidenius M,
Haglund C, Andersson LC, Sariola H and Heikkilä P: Bmi-1, c-myc and
Snail expression in primary breast cancers and their
metastases-elevated Bmi-1 expression in late breast cancer
relapses. Virchows Arch. 459:31–39. 2011. View Article : Google Scholar : PubMed/NCBI
|