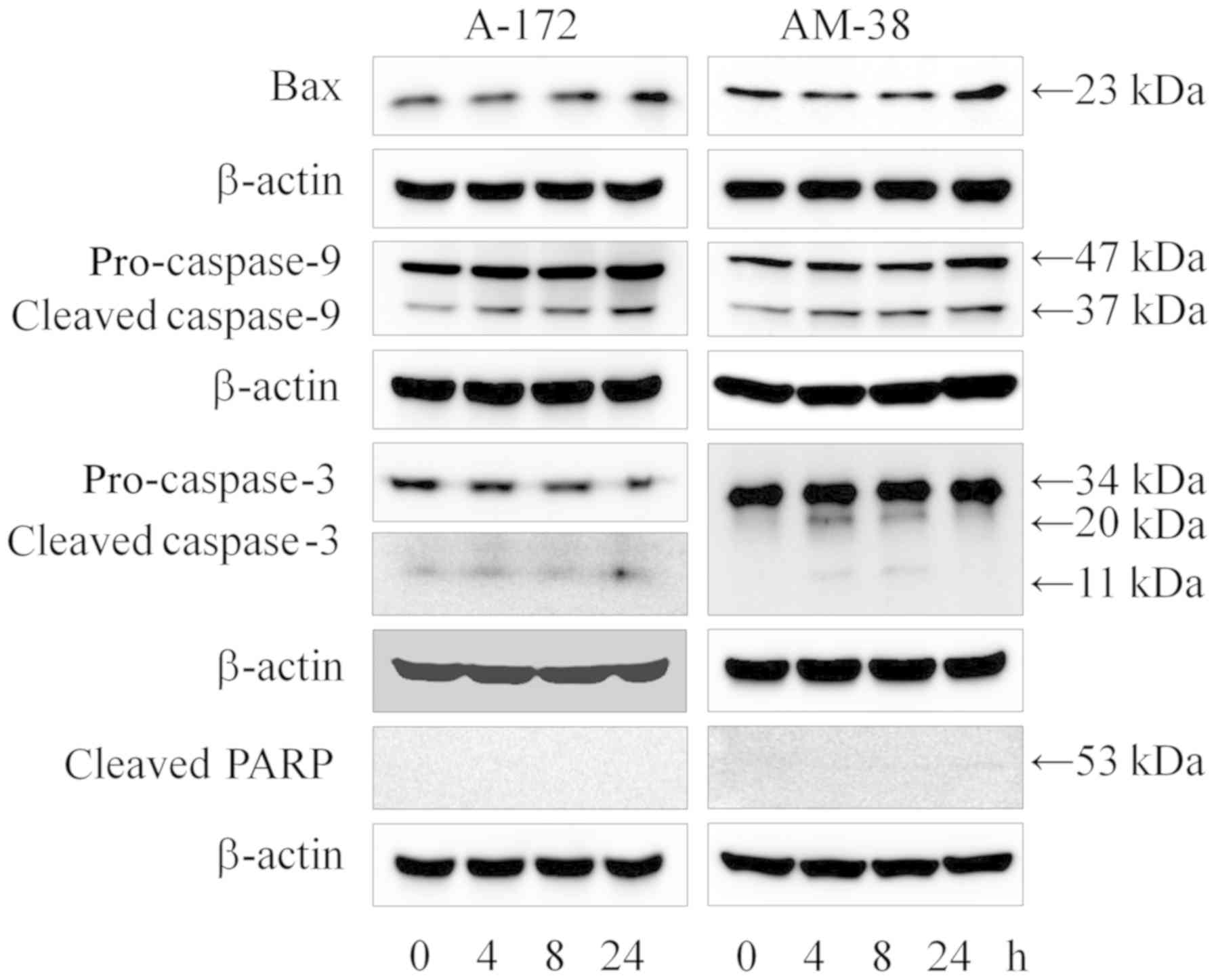
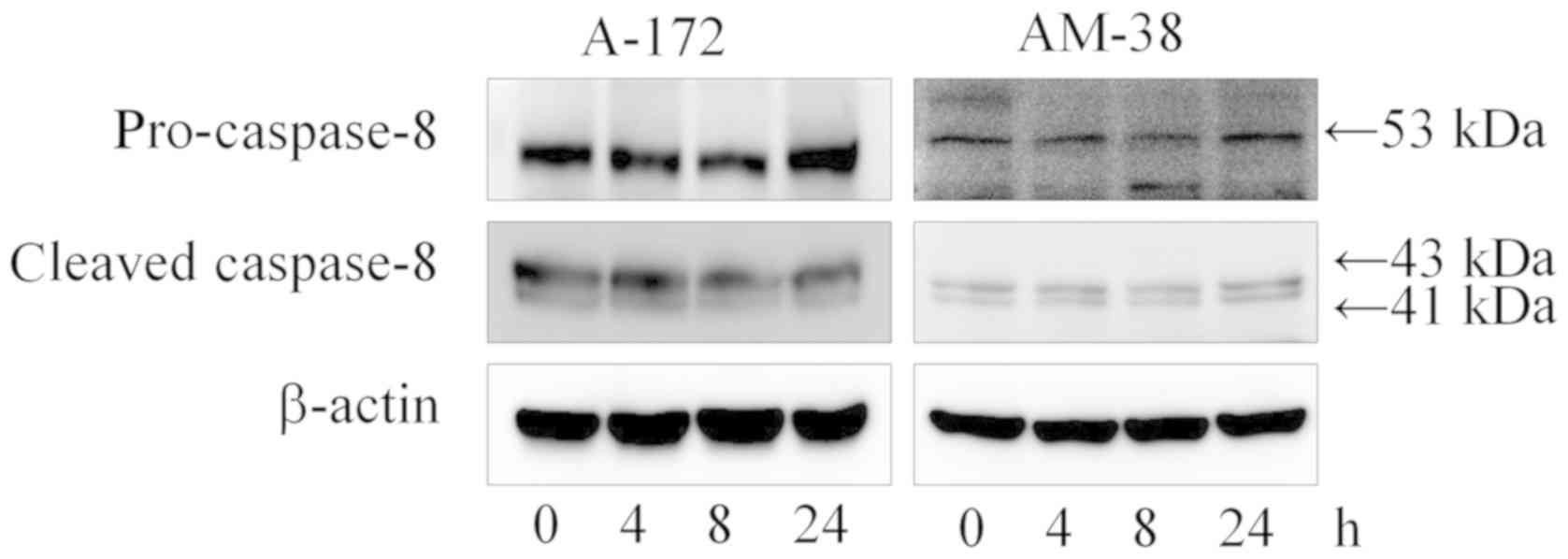
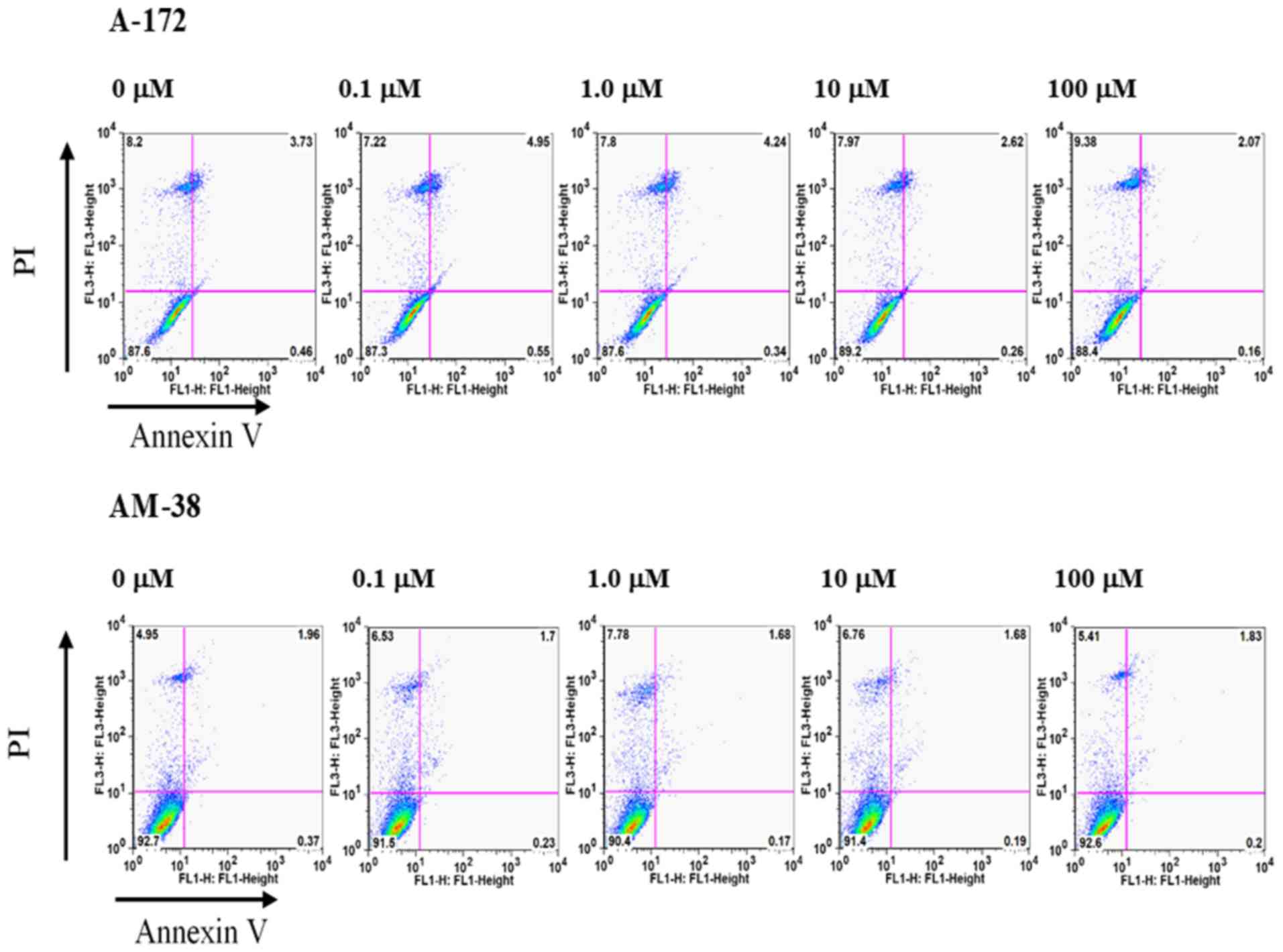
|
1
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singhal S, Mehta J, Desikan R, Ayers D,
Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar
M, et al: Antitumor activity of thalidomide in refractory multiple
myeloma. N Engl J Med. 341:1565–1571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palumbo A, Bringhen S, Caravita T, Merla
E, Capparella V, Callea V, Cangialosi C, Grasso M, Rossini F, Galli
M, et al: Oral melphalan and prednisone chemotherapy plus
thalidomide compared with melphalan and prednisone alone in elderly
patients with multiple myeloma: Randomised controlled trial.
Lancet. 367:825–831. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Facon T, Mary JY, Hulin C, Benboubker L,
Attal M, Pegourie B, Renaud M, Harousseau JL, Guillerm G, Chaleteix
C, et al: Melphalan and prednisone plus thalidomide versus
melphalan and prednisone alone or reduced-intensity autologous stem
cell transplantation in elderly patients with multiple myeloma (IFM
99-06): A randomised trial. Lancet. 370:1209–1218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waage A, Gimsing P, Fayers P, Abildgaard
N, Ahlberg L, Björkstrand B, Carlson K, Dahl IM, Forsberg K,
Gulbrandsen N, et al: Melphalan and prednisone plus thalidomide or
placebo in elderly patients with multiple myeloma. Blood.
116:1405–1412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wijermans P, Schaafsma M, Termorshuizen F,
Ammerlaan R, Wittebol S, Sinnige H, Zweegman S, van Marwijk Kooy M,
van der Griend R, Lokhorst H, et al: Phase III study of the value
of thalidomide added to melphalan plus prednisone in elderly
patients with newly diagnosed multiple myeloma: The HOVON 49 study.
J Clin Oncol. 28:3160–3166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benboubker L, Dimopoulos MA, Dispenzieri
A, Catalano J, Belch AR, Cavo M, Pinto A, Weisel K, Ludwig H,
Bahlis N, et al: Lenalidomide and dexamethasone in
transplant-ineligible patients with myeloma. N Engl J Med.
371:906–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fenaux P, Giagounidis A, Selleslag D,
Beyne-Rauzy O, Mufti G, Mittelman M, Muus P, Te Boekhorst P, Sanz
G, Del Cañizo C, et al: A randomized phase 3 study of lenalidomide
versus placebo in RBC transfusion-dependent patients with
Low-/Intermediate-1-risk myelodysplastic syndromes with del5q.
Blood. 118:3765–3776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gay F, Hayman SR, Lacy MQ, Buadi F, Gertz
MA, Kumar S, Dispenzieri A, Mikhael JR, Bergsagel PL, Dingli D, et
al: Lenalidomide plus dexamethasone versus thalidomide plus
dexamethasone in newly diagnosed multiple myeloma: A comparative
analysis of 411 patients. Blood. 115:1343–1350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stadtmauer EA, Weber DM, Niesvizky R,
Belch A, Prince MH, San Miguel JF, Facon T, Olesnyckyj M, Yu Z,
Zeldis JB, et al: Lenalidomide in combination with dexamethasone at
first relapse in comparison with its use as later salvage therapy
in relapsed or refractory multiple myeloma. Eur J Haematol.
82:426–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zonder JA, Crowley J, Hussein MA, Bolejack
V, Moore DF Sr, Whittenberger BF, Abidi MH, Durie BG and Barlogie
B: Lenalidomide and high-dose dexamethasone compared with
dexamethasone as initial therapy for multiple myeloma: A randomized
Southwest Oncology Group trial (S0232). Blood. 116:5838–5841. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knop S, Gerecke C, Liebisch P, Topp MS,
Platzbecker U, Sezer O, Vollmuth C, Falk K, Glasmacher A, Maeder U,
et al: Lenalidomide, adriamycin, and dexamethasone (RAD) in
patients with relapsed and refractory multiple myeloma: A report
from the German Myeloma Study Group DSMM (Deutsche Studiengruppe
Multiples Myelom). Blood. 113:4137–4143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schey SA, Morgan GJ, Ramasamy K, Hazel B,
Ladon D, Corderoy S, Jenner M, Phekoo K, Boyd K and Davies FE: The
addition of cyclophosphamide to lenalidomide and dexamethasone in
multiply relapsed/refractory myeloma patients; a phase I/II study.
Br J Haematol. 150:326–333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palumbo A, Hajek R, Delforge M, Kropff M,
Petrucci MT, Catalano J, Gisslinger H, Wiktor-Jędrzejczak W,
Zodelava M, Weisel K, et al: Continuous lenalidomide treatment for
newly diagnosed multiple myeloma. N Engl J Med. 366:1759–1769.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hideshima T, Chauhan D, Shima Y, Raje N,
Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P, et
al: Thalidomide and its analogs overcome drug resistance of human
multiple myeloma cells to conventional therapy. Blood.
96:2943–2950. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lopez-Girona A, Mendy D, Ito T, Miller K,
Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M, et
al: Cereblon is a direct protein target for immunomodulatory and
antiproliferative activities of lenalidomide and pomalidomide.
Leukemia. 26:2326–2335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fecteau JF, Corral LG, Ghia EM, Gaidarova
S, Futalan D, Bharati IS, Cathers B, Schwaederlé M, Cui B,
Lopez-Girona A, et al: Lenalidomide inhibits the proliferation of
CLL cells via a cereblon/p21(WAF1/Cip1)-dependent mechanism
independent of functional p53. Blood. 124:1637–1644. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fine HA, Figg WD, Jaeckle K, Wen PY,
Kyritsis AP, Loeffler JS, Levin VA, Black PM, Kaplan R, Pluda JM
and Yung WK: Phase II trial of the antiangiogenic agent thalidomide
in patients with recurrent high-grade gliomas. J Clin Oncol.
18:708–715. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giglio P, Dhamne M, Hess KR, Gilbert MR,
Groves MD, Levin VA, Kang SL, Ictech SE, Liu V, Colman H, et al:
Phase 2 trial of irinotecan and thalidomide in adults with
recurrent anaplastic glioma. Cancer. 118:3599–3606. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Penas-Prado M, Hess KR, Fisch MJ, Lagrone
LW, Groves MD, Levin VA, De Groot JF, Puduvalli VK, Colman H,
Volas-Redd G, et al: MD Anderson Community Clinical Oncology
Program; Brain Tumor Trials Collaborative: Randomized phase II
adjuvant factorial study of dose-dense temozolomide alone and in
combination with isotretinoin, celecoxib, and/or thalidomide for
glioblastoma. Neuro Oncol. 17:266–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fine HA, Kim L, Albert PS, Duic JP, Ma H,
Zhang W, Tohnya T, Figg WD and Royce C: A phase I trial of
lenalidomide in patients with recurrent primary central nervous
system tumors. Clin Cancer Res. 13:7101–7106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Warren KE, Goldman S, Pollack IF,
Fangusaro J, Schaiquevich P, Stewart CF, Wallace D, Blaney SM,
Packer R, Macdonald T, et al: Phase I trial of lenalidomide in
pediatric patients with recurrent, refractory, or progressive
primary CNS tumors: Pediatric Brain Tumor Consortium study
PBTC-018. J Clin Oncol. 29:324–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ochiai Y, Sano E, Okamoto Y, Yoshimura S,
Makita K, Yamamuro S, Ohta T, Ogino A, Tadakuma H, Ueda T, et al:
Efficacy of ribavirin against malignant glioma cell lines:
Follow-up study. Oncol Rep. 39:537–544. 2018.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuramitsu S, Ohno M, Ohka F, Shiina S,
Yamamichi A, Kato A, Tanahashi K, Motomura K, Kondo G, Kurimoto M,
et al: Lenalidomide enhances the function of chimeric antigen
receptor T cells against the epidermal growth factor receptor
variant III by enhancing immune synapses. Cancer Gene Ther.
22:487–495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kühnöl CD, Staege MS and Kramm CM:
Lenalidomide in an in vitro dendritic cell model for malignant
gliomas. Anticancer Agents Med Chem. 16:1468–1473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen N, Zhou S and Palmisano M: Clinical
pharmacokinetics and pharmacodynamics of Lenalidomide. Clin
Pharmacokinet. 56:139–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Amato RJ, Loughnan MS, Flynn E and
Folkman J: Thalidomide is an inhibitor of angiogenesis. Proc Natl
Acad Sci USA. 91:4082–4085. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rao KV: Lenalidomide in the treatment of
multiple myeloma. Am J Health Syst Pharm. 64:1799–1807. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Escoubet-Lozach L, Lin IL, Jensen-Pergakes
K, Brady HA, Gandhi AK, Schafer PH, Muller GW, Worland PJ, Chan KW
and Verhelle D: Pomalidomide and lenalidomide induce p21 WAF-1
expression in both lymphoma and multiple myeloma through a
LSD1-mediated epigenetic mechanism. Cancer Res. 69:7347–7356. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holstein SA, Hillengass J and McCarthy PL:
Next-generation drugs targeting the cereblon ubiquitin ligase. J
Clin Oncol. 36:2101–2104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tao J, Yang J and Xu G: The interacting
domains in cereblon differentially modulate the immunomodulatory
drug-mediated ubiquitination and degradation of its binding
partners. Biochem Biophys Res Commun. 507:443–449. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng J, Sha Y, Roof L, Foreman O,
Lazarchick J, Venkta JK, Kozlowski C, Gasparetto C, Chao N, Ebens
A, et al: Pan-PIM kinase inhibitors enhance Lenalidomide's
anti-myeloma activity via cereblon-IKZF1/3 cascade. Cancer Lett.
440-441:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krönke J, Udeshi ND, Narla A, Grauman P,
Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, et al:
Lenalidomide causes selective degradation of IKZF1 and IKZF3 in
multiple myeloma cells. Science. 343:301–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu G, Middleton RE, Sun H, Naniong M, Ott
CJ, Mitsiades CS, Wong KK, Bradner JE and Kaelin WG Jr: The myeloma
drug lenalidomide promotes the cereblon-dependent destruction of
Ikaros proteins. Science. 343:305–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mori T, Ito T, Liu S, Ando H, Sakamoto S,
Yamaguchi Y, Tokunaga E, Shibata N, Handa H and Hakoshima T:
Structural basis of thalidomide enantiomer binding to cereblon. Sci
Rep. 8:1294. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao R, Han D, Sun X, Xie Y, Wu Q, Fu C,
Yao Y, Li H, Li Z and Xu K: Scriptaid inhibits cell survival, cell
cycle, and promotes apoptosis in multiple myeloma via epigenetic
regulation of p21. Exp Hematol. 60:63–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qian Z, Zhang L, Cai Z, Sun L, Wang H, Yi
Q and Wang M: Lenalidomide synergizes with dexamethasone to induce
growth arrest and apoptosis of mantle cell lymphoma cells in vitro
and in vivo. Leuk Res. 35:380–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pitter KL, Tamagno I, Alikhanyan K,
Hosni-Ahmed A, Pattwell SS, Donnola S, Dai C, Ozawa T, Chang M,
Chan TA, et al: Corticosteroids compromise survival in
glioblastoma. Brain. 139:1458–1471. 2016. View Article : Google Scholar : PubMed/NCBI
|