Introduction
The indications for the use of oral therapies in the
field of digestive oncology are increasing. Whether oral
chemotherapy or targeted therapies, their use is favored by
patients. Adherence to treatment is directly linked with the
patient's understanding, the ability to remember the information
provided by the physician, treatment length and psychological
distress (1). A good coordination
between oncologists, general practitioners, pharmacists and nurses
is essential, information about drug monitoring and management of
the side effects has to be widely distributed. Drug and food
interactions have to be known by the oncologist. We herein propose
an updated review of the literature about oral therapies in
digestive oncology and a short reminder of how to use them and to
manage their side effects.
Oral chemotherapy
Capecitabine
Capecitabine is an antimetabolite, an oral prodrug
of 5-fluorouracil (5-FU), which is metabolized to
5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluouridine and 5
fluorouracil by cytidine deaminase and thymidine phosphorylase.
Capecitabine is a uracil-based nucleic acid analog that inhibits
thymidylate synthase, resulting in inhibition of DNA synthesis and
RNA damage. It has been shown that 5-FU concentrations after
capecitabine intake are higher in colorectal tumors than levels in
healthy tissue, due to cytidine deaminase and thymidine
phosphorylase concentrations in these tumors (2).
A systematic screening for dihydropyrimidine
dehydrogenase (DPD) deficiency is recommended before any
administration of 5-FU-based chemotherapy (3).
Capecitabine is administered orally, twice a day, in
a divided dose 12 h apart, 30 min after a meal. Tablets come in 2
doses; 150 and 500 mg.
In a neoadjuvant setting, capecitabine is indicated
in association with radiotherapy. The standard neoadjuvant CAP50
regimen is recommended in rectal T3-4M0 cancer (4), with a dose of concomitant capecitabine
800 mg/m2 twice daily, 5 days per week.
In adjuvant situations, capecitabine is applied in
monotherapy with a 1250 mg/m2 dose twice daily, 14
days/21 after complete resection of a cholangiocarcinoma, as shown
in the recent BILCAP study (5).
Capecitabine monotherapy can be discussed in adjuvant therapy for
stage II colorectal cancer (CRC), or in stage III after 70 years of
age (6). In association with
oxaliplatin, capecitabine is administered at the dose of 1,000
mg/m2 twice daily, 14 days/21 in adjuvant for stage
II/III CRC, for 3 or 6 months, as shown in the IDEA study (7). In association with gemcitabine after
surgery for pancreatic adenocarcinoma, capecitabine is administered
at the dose of 1,660 mg/m2 daily, 21 days/28 (ESPAC-4
study) (8).
In metastatic situations, capecitabine can replace
intravenous 5-FU in monotherapy, doublet, or triplet with targeted
therapy (9). Capecitabine is
preferentially used in maintenance therapy in association with
bevacizumab in CRC (10). In
pancreatic neuroendocrine tumors (NETs), administration of
capecitabine at a dosage of 750 mg/m2 twice daily from
day 1 to 14 and temozolomide at 200 mg/m2 once daily
from day 10 to 14 every 28 days was found to be associated with
durable response rate (11).
Oncologists and pharmacists have to be aware of drug
interactions, and the most common is in regards to warfarin, which
is prohibited during capecitabine treatment (12). It has also been shown that proton
pump inhibitors (PPIs) negatively affect capecitabine efficacy, by
modifying gastric pH leading to altered absorption (13). The use of PPIs must be withdrawn
when possible.
Toxicity associated with capecitabine is described
in nearly 80% of patients (14),
and up to 40% grade III. Nausea and vomiting from 3 to 35% is
observed depending on the studies (5,6,9,15,16),
mucositis is observed in 2 to 22% of cases, diarrhea up to 30%,
hand and foot syndrome, 17% and hematologic toxicity less than 10%.
Cardiac toxicity is described at a range of 3 to 6%, and its
incidence increases with the presence of underlying cardiopathy.
The incidence of toxicity and grade depends on the dose,
combination therapy and the DPD status.
Systematic education for hand and foot syndrome
management, mucositis management and treatment for digestive side
effects must be provided at initial capecitabine prescription.
TAS 102
TAS-102 is an orally administered combination of a
thymidine-based nucleic acid analogue, trifluridine and a thymidine
phosphorylase inhibitor, tipiracil. Tipiracil improves the
bioavailability of trifluridine by inhibiting its catabolism by
thymidine phosphorylase.
The only indication validated to this day was for
refractory metastatic CRC after oxaliplatin and 5 FU-based
chemotherapy and irinotecan and 5 FU-based chemotherapy (targeted
therapies included). TAS-102 was administered at a dose of 35
mg/m2 twice a day, 5 days a week, with 2 days of rest
for 2 weeks followed by a 14-day rest period. Overall survival was
7.1 months (vs. 5.3 months in the placebo group; P<0.001)
(17).
Toxicity was found to be mainly hematologic, with
38% of ≥grade III neutropenia, with 4% febrile neutropenia. Mild
nausea (grade I–II) and vomiting have been described in 48 and 28%
of cases, respectively (17).
Temozolomide
Temozolomide is an oral alkylating agent indicated
for the treatment of neuroendocrine pancreatic carcinoma, in
association with capecitabine. Temozolomide is an alkylating agent
prodrug, delivering a methyl group to purine bases of DNA. DNA
alkylation induces cell cycle arrest at G2/M phase of mitosis
eventually leading to apoptosis (18).
The indication for temozolomide in the treatment of
pancreatic NETs was studied by Strosberg et al (11). The regimen consisted of oral
capecitabine at 750 mg/m2 twice a day for 14 days (days
1–14) and oral temozolomide at 200 mg/m2 once a day at
bedtime for 5 days (days 10–14) every 28 days. The dose was adapted
in the case of renal insufficiency. Median progression-free
survival was 18 months.
The first prospective randomized study was presented
this year (ASCO) (19) comparing
temozolomide alone vs. temozolomide and capecitabine in patients
with advanced pancreatic neuroendocrine carcinoma, in progression
after targeted therapy or somatostatin analogues. Progression-free
survival was 22.7 months in the temozolomide+capecitabine group vs.
14.4 months in the temozolomide group (P=0.023). Overall survival
was not reached in the bi-chemotherapy group, vs. 38 months in the
temozolomide group (P=0.012). Predictive value of MGMT status for
temozolomide response was studied in pancreatic NETs. Combination
therapy induced more side effects (44%), with 13% grade III or more
neutropenia. Digestive side effects were present in 8% of
cases.
Targeted therapies
Sorafenib
Sorafenib is an oral multikinase inhibitor of the
vascular endothelial growth factor receptor (VEGFR), the
platelet-derived growth factor receptor (PDGFR) and Raf kinase.
Sorafenib inhibits tumor cell proliferation and angiogenesis
(20).
Sorafenib is indicated in the first-line treatment
of advanced HCC (21) at the dose
of 400 mg twice daily. It is recommended to take sorafenib as far
away as possible from meal-times or with a low-fat diet for better
absorption. No adaptation is necessary in case of renal
dysfunction. In case of cirrhosis, sorafenib is indicated in the
case of Child A score. In a study by Llovet et al (21) enrolling 602 patients with advanced
HCC without previous systemic treatment, the median overall
survival of the patients was 10.7 months in the sorafenib group vs.
7.9 months in the placebo group (P<0.001). Toxicity described in
the sorafenib group included diarrhea (55%), weight loss (30.9%),
anorexia (28.6%), hand and foot syndrome (21.2%), vomiting (14.8%)
and alopecia (14.1%). A treatment discontinuation was necessary in
31.6% of the patients due to these side effects.
Lenvatinib
Lenvatinib is a multikinase inhibitor, targeting
VEGF receptors 1–3, fibroblast growth factor (FGF) receptors 1–4,
PDGF receptor α, RET and KIT. Lenvatinib is indicated in τηε
first-line treatment for unresectable or metastatic hepatocellular
carcinoma (22). The recommended
dose is 12 mg/day for body weight ≥60 kg or 8 mg/day for <60 kg.
Kudo et al (22) enrolled
1,492 unresectable hepatocellular carcinoma patients randomly
assigned to lenvatinib or sorafenib as first-line treatment. The
median survival time for lenvatinib was 13.6 months (95% CI,
12.1–14.9), not inferior to sorafenib [(12.3 months, 10.4–13.9), HR
0.93, 95% CI, 0.79–1.06]. The most common any-grade adverse events
for lenvatinib were fatigue (30%, grade ≥3: 4%), hypertension (42%,
grade ≥3: 23%), diarrhea (39%; grade ≥3: 4%), anorexia (34%, grade
≥3: 5%), decreased weight (31%, grade ≥3: 8%). A similar tolerance
profile was observed in an elderly population (23).
Regorafenib
Regorafenib is an oral multikinase inhibitor which
targets angiogenic, stromal and oncogenic tyrosine kinase
receptors. Inhibition of angiogenesis is accomplished by inhibition
of VEGF, fibroblast growth factor (FGF) and PDGF receptors.
Inhibition of metastatic invasion was found to be due to the
inhibition of VEGFR2 and VEGFR3. Oncogenic inhibition was found to
be induced by inhibition of the MAP kinase pathway. Regorafenib was
administered at a dose of 160 mg daily, in the morning (4×40 mg)
associated with a low-fat breakfast, during 3 weeks over a 4-week
cycle (24).
Regorafenib is indicated for patients with
metastatic CRC previously treated with fluoropyrimidine-based
chemotherapy, anti-VEGF or anti-EGFR therapy. The CORRECT study
(25) included 760 metastatic CRC
PS 0 or 1 patients after failure of fluoropyrimidine, oxaliplatin,
irinotecan, bevacizumab and anti-EGFR therapy of RAS wild-type
tumors. They received either placebo or regorafenib at 160 mg per
day. The median overall survival was 6.4 months in the regorafenib
group vs. 5 months in the placebo group (HR=0.77, 95% CI,
0.64–0.94).
A multicentric randomized phase II study was carried
out and enrolled 123 metastatic CRC patients treated with
regorafenib; randomized between a dose-escalation strategy
(starting dose 80 mg/day taken orally with weekly escalation, per
40 mg increment, to 160 mg/day regorafenib) vs. a standard dose
strategy (160 mg/day) (26). A
comparable anti-tumoral activity was observed, with lower incidence
of adverse events in the ‘dose escalation strategy’ group. This
strategy is due to be implemented in clinical practice.
Regorafenib is also indicated in advanced HCC after
sorafenib treatment. The RESORCE study (27) randomized 573 HCC (Child A) patients
between best supportive care or regorafenib 160 mg daily 3 weeks/4.
Patients had to have tolerated sorafenib at least 400 mg/day for at
least 20 of the last 28 days of treatment. Regorafenib improved
overall survival: 10.6 vs. 7.8 months (HR=0.63, 95% CI
0.50–0.79).
Regorafenib is also indicated in metastatic or
unresectable gastrointestinal (GI) stromal tumors after intolerance
or failure of imatinib and sunitinib (28), with a dose of 160 mg per day 3
weeks/4. The median progression-free survival vs. placebo was 4.8
vs. 0.9 months (P<0.001).
In the CORRECT study (25) the most common adverse events of
grade III or higher were hand-foot syndrome (18%), asthenia (10%),
diarrhea (36.7%), hypertension (36.7%), rash or desquamation
(29.6%). In the RESORCE study (27), at least grade III or higher adverse
events consisted of hypertension (15%), hand-foot syndrome (13%),
asthenia (9%) and diarrhea (3%).
Biological monitoring during regorafenib treatment
includes phosphoremia, blood cell count (cytopenia) and hepatic
biology. Regorafenib is forbidden in case of recent (<6 months)
arterial thrombosis.
Cabozantinib
Cabozantinib is a tyrosine kinase inhibitor which
targets VEGF1, VEGF2, VEGF3, hepatocyte growth factor receptor
(MET) and AXL receptor tyrosine kinase (AXL), which are implicated
in the progression of HCC and the development of resistance to
sorafenib. Inhibition of VEGFR and c-MET decreases resistance of
VEGFR inhibitor via c-Met axis (29).
Cabozantinib is indicated for the treatment of
non-resectable HCC after progression with sorafenib. A randomized
double-blind phase III trial recruited 707 HCC patients after
progression with sorafenib. The patients received either
cabozantinib 60 mg once daily or placebo (30). The median overall survival was
longer with cabozantinib, 10.2 months vs. 8 months (HR for death
0.76, 95% CI, 0.63–0.92, P=0.005). Principal grade III or higher
adverse events included palmar-plantar-erythrodysesthesia (17%),
arterial hypertension (16%), increased alanine aminotransferase
(ALT) (12%), fatigue (10%) and diarrhea (10%).
Biological monitoring for this agent includes
testing for blood cell count, hepatic biology, TSH, glycemia and
electrolytes.
Sunitinib
Sunitinib is a multi-targeted tyrosine kinase
inhibitor indicated for the treatment of advanced
well-differentiated pancreatic neuroendocrine carcinoma. Sunitinib
has been identified as an inhibitor of VEGFR1, VEGFR2, VEGFR3, KIT
proto-oncogene, receptor tyrosine kinase (KIT), proto-oncogene
tyrosine-protein kinase receptor Ret (RET) and PDGFRA. The
recommended dose is 37.5 mg daily continuously (31).
A phase III randomized trial of sunitinib treatment
vs. a placebo included 171 patients who had progressive pancreatic
well-differentiated neuroendocrine carcinoma (32). The median progression-free survival
was 11.4 months in the sunitinib group vs. 5.5 months for the
placebo group (HR for progression or death 0.42, IC 95% 0.26–0.66,
P<0.001). The HR for death was 0.41 (IC 95% 0.19 to 0.89,
P=0.02).
Sunitinib is also indicated for the treatment of
metastatic or unresectable GI stromal tumors, after imatinib
resistance or intolerance (32,33).
The recommended dose is 50 mg per day for 4 weeks/6, but a dose of
37.5 mg per day continuously showed an acceptable toxicity profile
and similar efficiency in a non-randomized phase II study (33).
Grade III or greater adverse events included
neutropenia (12%), hypertension (10%),
palmar-plantar-erythrodysesthesia (6%), diarrhea (5%), fatigue
(5%), abdominal pain (5%), stomatitis (4%) and thrombocytopenia
(4%) (32,33).
A transthoracic ultrasonography is recommended
before treatment initiation and every 3 months, due to a possible
decreased left ventricular ejection fraction (LVEF). Urine analysis
(protein) is recommended every 6 weeks. Co-medication with P450
cytochrome treatments must be monitored (34).
Everolimus
Everolimus is a selective mammalian target of
rapamycin (mTOR) inhibitor. Everolimus inhibits tumor cell growth
as well as that of endothelial cells and fibroblasts. Everolimus is
indicated for the treatment of advanced and progressive
well-differentiated digestive neuroendocrine tumors (35–37).
The dose recommended is 10 mg per day continuously.
In the RADIANT-3 study (37), 410 patients with advanced low-grade
or intermediate-grade pancreatic progressive neuroendocrine tumors
were randomized between an everolimus 10 mg/day group and a placebo
group. The median progression-free survival was 11 months in the
everolimus group vs. 4.6 months in the placebo group
(P<0.001).
In the RADIANT-4 study (36), 302 patients with advanced
progressive well-differentiated neuroendocrine tumors of pulmonary
or gastrointestinal origin were enrolled, and randomized between an
everolimus 10 mg per day group and a placebo group. The median
progression-free survival was 11 months in the everolimus group vs.
3.9 months in the placebo group (P<0.00001) and no significance
in overall survival.
Grade 3 or 4 events with everolimus included anemia
(6%), hyperglycemia (5%), stomatitis (9%), diarrhea (7%),
infections (7%) and fatigue (3%). A non-infectious pneumopathy was
observed in 12% of cases; therefore, a systematic clinical
investigation for dyspnea, auscultation and pulmonary evaluation on
TDM is recommended (36,37). A systematic follow-up of metabolic
disorders induced by everolimus should be performed at day 15, and
then once a month (38).
Additionally there is a high risk of toxicity linked
with the association of inhibitors of p450 cytochrome, which could
lead to a dose reduction and should justify a close watch (39).
Imatinib mesylate
Imatinib is a tyrosine kinase inhibitor targeting
BCR-ABL tyrosine kinase and stem cell factor receptor, encoded by
the proto-oncogene c-Kit, and PDGFR. Competitive inhibition at the
ATP-binding site of BCR-ABL tyrosine kinase leads to inhibition of
tyrosine phosphorylation of proteins involved in BCR-ABL signal
transduction. Imatinib also inhibits the receptor PDGFR and KIT
(40). The recommended dose is 400
to 800 mg once daily during a meal.
Imatinib is indicated as an adjuvant therapy for
high risk stromal tumors or in cases of metastatic stromal
tumors.
In an adjuvant situation, a phase III randomized
trial (41) included patients who
underwent surgery for a gastrointestinal stromal tumor of at least
3 cm, and who were randomized between a placebo (N=354) and
imatinib 400 mg per day (N=359) daily group, for 1 year after tumor
resection. At 1 year, imatinib significantly improved the
recurrence-free survival of the imatinib group compared with the
placebo group [HR=0.35 (0.22–0.53), P<0.0001]. In the imatinib
group, grade III or IV adverse events occurred in 30.9% of the
cases. Grade III edema was noted in 2%, grade III or IV neutropenia
in 3% and grade III or IV fatigue in 2% of the cases.
A secondary analysis concerning the function of
tumor genotype was performed (42),
confirming that KIT exon 11 mutation was associated with the
significantly improved prognosis in the imatinib treatment,
contrary to exon 9 mutation or wild-type genotype. Imatinib did not
benefit patients with a PDGFRA mutation type D 842V of exon 18.
Joensuu et al (43) assessed adjuvant therapy with
imatinib for 1 to 3 years in 400 patients with operable GI stromal
tumors with a high risk of recurrence (size, mitosis, tumoral
breach). Recurrence-free survival was 66% in the 3-year treatment
group vs. 48% in the 1-year treatment group (P<0.0001). After 5
years, the overall survival was 93 vs. 87% (P=0.032), in favor of
the 3-year treatment. A 3-year treatment was also recommended in
adjuvant therapy for high-recurrence risk stromal tumors.
Imatinib is recommended for cases of metastatic GI
stromal tumors (44,45). In cases with KIT exon 9
mutation, a higher dose of 800 mg per day is recommended, as well
as in the event of progression at the dose of 400 mg per day
(46).
Table I provides a
summary of drug indications, dose, principal side effects and
monitoring.
 | Table I.Oral therapies in digestive oncology:
indications, dose, side effects and surveillance. |
Table I.
Oral therapies in digestive oncology:
indications, dose, side effects and surveillance.
| Name | Indications | Dose and treatment
intake modalities | Side effects | % ≥grade 3 | Surveillance |
|---|
| Capecitabine | CRC, esophageal,
gastric, biliary tract cancer, NETs | Monotherapy 1,250
mg/m2 twice a day; 14 days/21 | Nausea, vomiting,
diarrhea, mucitis, hand foot syndrome, cardiac toxicity | 40 | Clinical monitoring
Biological monitoring before each cycle |
| TAS 102 | CRC ≥3rd line | 35 mg/m2
J1-J5 and J8-J12 J1=J28 | Neutropenia | 38 | Biological
monitoring before each cycle |
| Temozolomide | In association with
capecitabine; pancreatic NETs | Capecitabine 750
mg/m2 2×/day (days 1–14), Temozolomide 200 mg/m2/day
(days 10–14) J1=J28 | Neutropenia | 13 | Biological
monitoring before each cycle; Renal function adaptation |
| Sorafenib | Advanced HCC | 400 mg twice
daily | Hand foot
syndrome | 8 | Clinical monitoring
and |
|
|
|
| Diarrhea | 8 | biological surv/4
weeks |
|
|
|
| Fatigue | 3 |
|
|
|
|
| Weight loss | 2 |
|
| Lenvatinib | Advanced HCC | 12 mg/day for ≥60
kg or | Fatigue | 4 | Clinical monitoring
and |
|
|
| 8 mg/day for <60
kg | Hypertension | 23 | biological surv/4
weeks |
|
|
|
| Diarrhea | 4 |
|
|
|
|
| Anorexia | 5 |
|
|
|
|
| Decreased
weight | 8 |
|
| Regorafenib | Metastatic CCR
after | 160 mg daily 3
weeks/4 | Hand foot
syndrome | 17 | Clinical,
biological monitoring |
|
| 5-FU,
oxaliplatin, |
| Fatigue | 10 | (phosphoremia, BCC,
hepatic) |
|
| irinotecan,
targeted therapy |
| Diarrhoea |
36.7 | every 15 days. |
|
| Advanced HCC after
sorafenib |
| Hypertension |
36.7 |
|
|
|
|
| Rash |
29.6 |
|
| Cabozantinib | Advanced HCC after
sorafenib | 60 mg daily | Hand-foot
syndrome | 17 | Clinical
monitoring, biology |
|
|
|
| Hypertension | 16 | monitoring
(phosphoremia, |
|
|
|
| Increased ALT | 12 | BCC, glycemia,
hepatic) |
|
|
|
| Fatigue | 10 | every 15 days |
|
|
|
| Diarrhea | 10 |
|
| Sunitinib | Advanced
pancreatic | 37.5 mg daily | Neutropenia | 12 | Clinical
monitoring, biological |
|
| well-differentiated
NETs |
| Hypertension | 10 | monitoring BCC,
ionogram, |
|
|
|
| Hand foot
syndrome | 6 | (renal
function) |
|
|
|
| Diarrhea | 5 | Proteinury/6
weeks |
|
|
|
| Fatigue | 5 | Cardiac
ultrasonography/3 |
|
|
|
| Abdominal pain | 5 | months |
|
|
|
|
Thrombocytopenia | 4 |
|
|
|
|
| Stomatitis | 4 |
|
| Capecitabine | CRC, esophageal,
gastric, | Monotherapy | Nausea, vomiting,
diarrhea, mucitis, | 40 | Clinical
monitoring |
| Everolimus | Advanced
progressive | 10 mg daily | Stomatitis | 9 | Clinical
monitoring, biological |
|
| well-differentiated
NETs |
| Anemia | 6 | monitoring (BCC,
glycemia, |
|
|
|
| Hyperglycemia | 5 | cholesterol,
triglycerides) at |
|
|
|
| Diarrhea | 7 | 15 days and once a
month, |
|
|
|
| Infections | 7 | HBA1C/3 months |
|
|
|
| Fatigue | 3 |
|
|
|
|
| Non-infectious
pneumopathy | 8 to 12 |
|
| Imatinib | Adjuvant in
high-risk GI | 400 mg daily, 800
mg in | Fatigue | 2 | Clinical
monitoring, biological |
|
| stromal tumors or
first-line | case of KIT
exon 9 mutation | Neutropenia | 3 | monitoring (BCC
once a |
|
| metastatic GI
stromal tumors | or failure at the
dose of 400 mg | Edema
Gastrointestinal | 2 | week/1 month, twice
a month during 3 months and once every 3 months; ionogram, renal
and hepatic function once a month/3 months and/3 months) |
Practical aspects
Initiating oral therapy
Similar to every treatment in oncology, the
prescription must follow a multidisciplinary decision. Before
initiating oral therapy, medical history of the patient must be
known by the clinician, as well as co-medications. Therapeutic
education for the treatment's modalities and side effects is
essential, completed by the physician or specially trained oncology
nurses (47). Patients therefore
tend to handle side effects and critical situations better.
Pharmaceutical counseling is suggested (48), to alert the patient to the
importance of compliance with therapy, as well as for interactions
with concomitant medication and food. Treatment trough levels
should be assessed in the case of a suspicion of pharmaceutical
interactions, primary treatment failure, before dose optimization
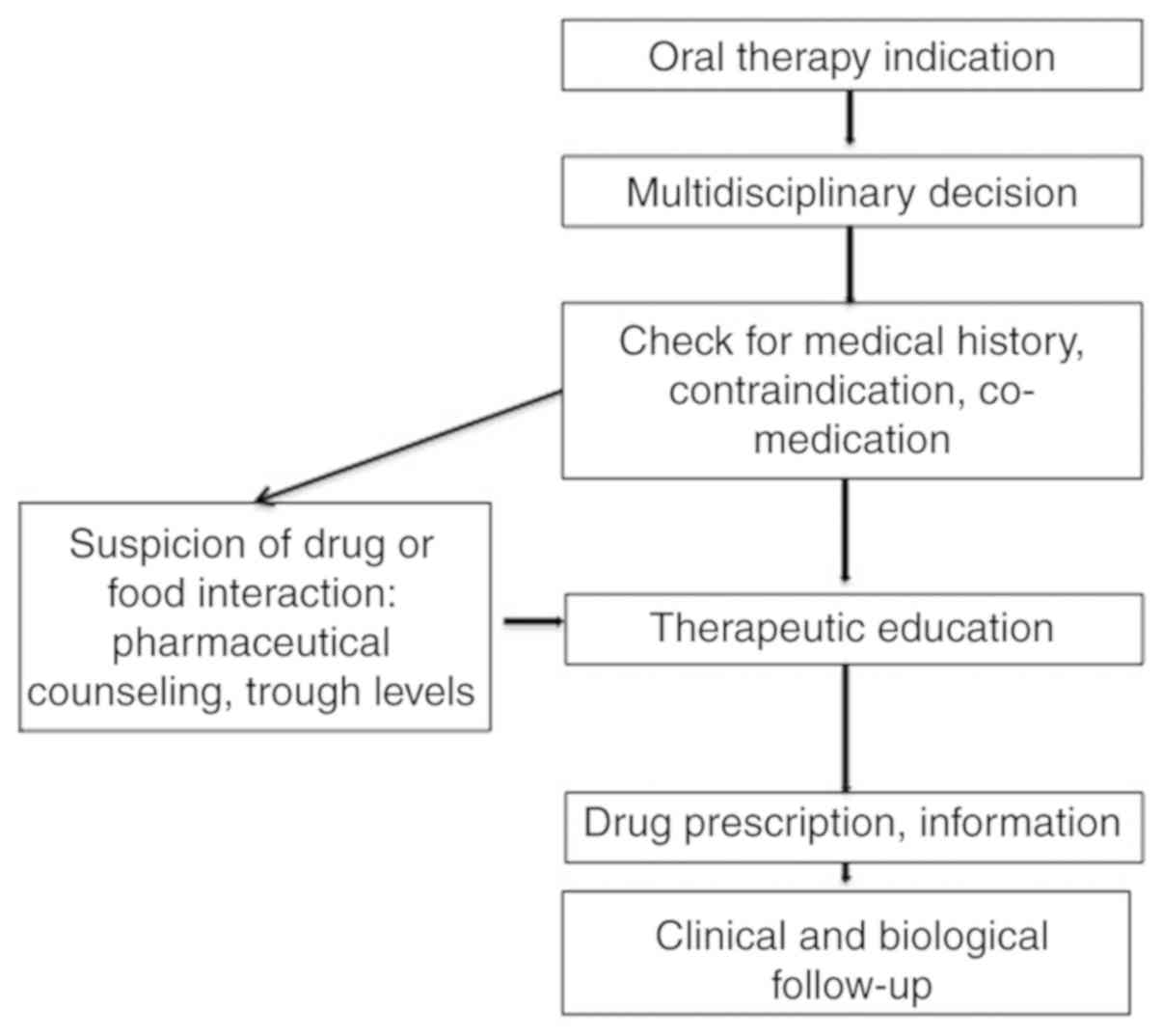
(Fig. 1).
Fig. 1 illustrates
the proposition of a decisional pathway for initiating oral therapy
in digestive oncology. A multidisciplinary decision must lead to
the initial prescription, with an assessment of the patient's
medical history co-medication or contraindications. Pharmaceutical
counseling can be useful in these cases. Therapeutic education and
clinical close follow-up must be organized.
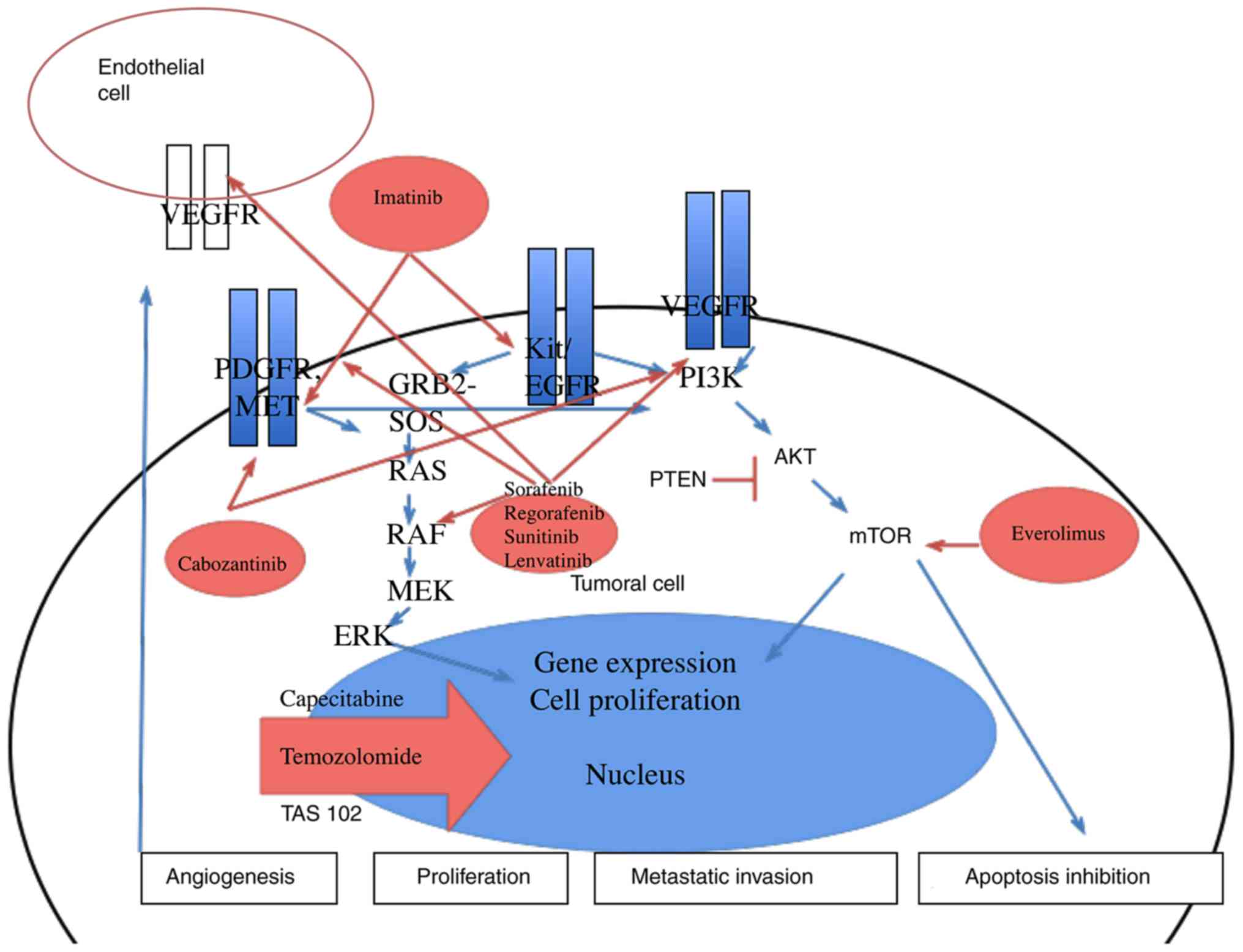
Fig. 2 summarizes
the oral therapies used in digestive oncology, the simplified
pathway of tumoral proliferation and the mechanisms of action of
the various oral therapies. As cytotoxic therapies are mainly
mitosis inhibitors, targeted therapies such as multikinase
inhibitors act on pathways related to cell proliferation,
angiogenesis, and metastatic invasion.
Selection of personalized therapy
The therapeutic choice is sometimes difficult, as in
some clinical situations there are no head-to-head clinical trials.
As an example, in the treatment of refractory metastatic CRC,
regorafenib or TAS 102 are both approved (17,25).
Moreover, patient preferences, medical history and tolerance to
previous medication must be taken into account.
A retrospective study of 550 Japanese patients
(49) observed no difference in
overall survival between patients receiving regorafenib or TAS 102.
In a subgroup analysis, a significant interaction with age was
observed; regorafenib treatment showed favorable survival in
patients aged <65 years (HR 1.29, 95% CI 0.98–1.69); and TAS 102
showed favorable survival in patients aged >65 years (HR 0.78;
95% CI 0.59–1.03). Tolerance of side effects and adherence to
treatment must be taken into account in interpretation of such
retrospective results.
An observational study enrolled 469 patients treated
with TAS 102 and 311 treated with regorafenib for metastatic CRC,
aiming to describe real-world adherence to treatment (50). Patients treated with TAS 102 had
higher compliance to treatment, better persistence and lower risk
of discontinuation than patients treated with regorafenib (HR 0.76,
P=0.006).
A dose optimization (ReDOS study) (26) with regorafenib could be a way to
strengthen treatment continuation and lower incidence of adverse
events.
Main adverse events management
Nausea and vomiting
Up to 80% of patients receiving chemotherapy
experience nausea or vomiting. Preventive treatment is important
and is currently used in i.v. chemotherapy; depending on the
emetogenical potential of agents (high emetic risk, >90%;
moderate risk, 30–90%; low risk, 10–30%; minimal risk, <10%).
The ESMO guidelines recently classified oral therapies by their
emetic potential, based upon a full course of therapy and not a
single dose (51). For example,
imatinib and temozolomide were classified as moderately emetogenic.
Capecitabine, everolimus, regorafenib and sunitinib were classified
as having low emetic risk, and sorafenib as having minimal risk.
For low or minimal risk there is no recommendation for an
anti-emetic prophylaxis (51).
Metoclopramide or a setron (granisetron, odansetron) can be
prescribed in the case of delayed nausea. In the case of refractory
nausea or vomiting, dexamethasone and olanzapine (10 mg orally for
3 days) is available (51).
Diarrhea
Treatment-related diarrhea is described as a
frequent side effect with capecitabine and tyrosine kinase
inhibitors. Its severity is assessed by the number of bowel
movements, a need of hospitalization and the effects on activities
of self-care. In mild-to-moderate diarrhea, a simple treatment with
oral hydration, dietary modifications (fibers decreased) and
loperamide (4 mg to start and then 2 mg after each loose stool) is
recommended. In the case of severe diarrhea hospitalization can be
necessary for an i.v. hydroelectrolytic supplementation, stool
bacteriological examination, discussing CT scan or endoscopic
explorations. Somatostatin and budesonide have shown to be
effective for refractory i.v. chemotherapy-induced diarrhea
(52).
Hand and foot syndrome
Severe hand and foot syndrome, often occurring in
patients treated with multikinase inhibitors or capecitabine can be
painful and interfere with normal daily activities. Supportive
measures must be initiated as soon as the symptoms appear. To
relieve inflammation, the application of cold packs, the use of
moisturizing creams, topical preparations containing
vasoconstrictor (eg. phenylephrine), astringents, anesthetics or
dermocorticoids can be used (53).
In hyperkeratosis, keratolytic agents such as urea-based cream are
useful. Hydrocolloid or alginate dressing may be used to protect
pressure points and aid healing. Treatment discontinuation should
be discussed in case of toxicity≥grade 2.
Mucositis
Oral mucositis can be painful and affect nutritional
intake, oral treatment intake and quality of life. Oral
decontamination by brushing with a soft toothbrush, flossing and
the use of sodium bicarbonate rinses are the first step in
treatment (54). Pain control with
the use of viscous lidocaine, which can be mixed with equal volumes
of soothing covering agents such as kaopectate may provide
short-term relief. Treatment of an associated candidosis infection
with fluconazole can be helpful. Nutritional support and treatment
discontinuation should be discussed in the case of severe
mucositis.
Conclusions
Various oral therapies can be prescribed in
digestive oncology. Classic chemotherapies such as capecitabine,
TAS 102 and temozolomide are often well known by physicians, from
their prescription modalities to the monitoring of the side
effects. However, targeted therapies and the risk of drug or food
interactions and their specific side effects are challenging. In
neuroendocrine tumors as well as in stromal tumors, drug exposure
spreads over an extended period of time. Therefore, dealing with
side effects and drug interactions is a common occurrence and
digestive oncologists should be able to know how to handle them. A
multidisciplinary association with the pharmacist and a trained
nurse should be developed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
CPK was involved in the conception and design of the
research, drafting and revision of the review. SD, LJP and JL were
involved in acquisition and analysis of the work, and drafting of
the manuscript. SC, CB and RC carried out the interpretation of the
data for the review and revision of the manuscript. All authors
contributed to the literature review and gathering of the data and
information. All authors read and approved the final manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This article does not contain any studies with human
participants or animals performed by any of the authors.
Patient consent for publication
Not applicable.
Competing interests
CPK, SD, LJP, JL, SC and CB declare no competing
interest. RC acts as consultant or speaker for Bayer, Amgen,
Novartis, Servier, Ipsen, Keocyt, AAA, Roche, and Merck.
References
|
1
|
Bassan F, Peter F, Houbre B, Brennstuhl
MJ, Costantini M, Speyer E and Tarquinio C: Adherence to oral
antineoplastic agents by cancer patients: Definition and literature
review. Eur J Cancer Care (Engl). 23:22–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schüller J, Cassidy J, Dumont E, Roos B,
Durston S, Banken L, Utoh M, Mori K, Weidekamm E and Reigner B:
Preferential activation of capecitabine in tumor following oral
administration to colorectal cancer patients. Cancer Chemother
Pharmacol. 45:291–297. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loriot MA, Ciccolini J, Thomas F,
Barin-Le-Guellec C, Royer B, Milano G, Picard N, Becquemont L,
Verstuyft C, Narjoz C, et al: Dihydropyrimidine déhydrogenase (DPD)
deficiency screening and securing of fluoropyrimidine-based
chemotherapies: Update and recommendations of the French
GPCO-Unicancer and RNPGx networks. Bull Cancer. 105:397–407.
2018.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gérard JP, Azria D, Gourgou-Bourgade S,
Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E,
de La Roche G, Bouché O, et al: Comparison of two neoadjuvant
chemoradiotherapy regimens for locally advanced rectal cancer:
Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin
Oncol. 28:1638–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Primrose JN, Fox RP, Palmer DH, Malik HZ,
Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, et
al: Capecitabine compared with observation in resected biliary
tract cancer (BILCAP): A randomised, controlled, multicentre, phase
3 study. Lancet Oncol. 20:663–673. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Twelves C, Wong A, Nowacki MP, Abt M,
Burris H III, Carrato A, Cassidy J, Cervantes A, Fagerberg J,
Georgoulias V, et al: Capecitabine as adjuvant treatment for stage
III colon cancer. N Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grothey A, Sobrero A, Meyerhardt JA,
Yoshino T, Paul J, Taieb J, Souglakos I, Kerr R, Labianca R,
Shields AF, et al: Prospective pooled analysis of six phase III
trials investigating duration of adjuvant (adjuv) oxaliplatin-based
therapy (3 vs. 6 months) for patients (pts) with stage III colon
cancer (CC): Updated results of IDEA (International Duration
Evaluation of Adjuvant Chemotherapy). Ann Oncol. 28 (Suppl
5):v605–v649. 2017. View Article : Google Scholar
|
|
8
|
Neoptolemos JP, Palmer DH, Ghaneh P,
Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA,
Cunningham D, Wadsley J, et al: Comparison of adjuvant gemcitabine
and capecitabine with gemcitabine monotherapy in patients with
resected pancreatic cancer (ESPAC-4): A multicentre, open-label,
randomised, phase 3 trial. Lancet. 389:1011–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR;
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom, : Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simkens LH, van Tinteren H, May A, ten
Tije AJ, Creemers GJ, Loosveld OJ, de Jongh FE, Erdkamp FL, Erjavec
Z, van der Torren AM, et al: Maintenance treatment with
capecitabine and bevacizumab in metastatic colorectal cancer
(CAIRO3): A phase 3 randomised controlled trial of the Dutch
colorectal cancer group. Lancet. 385:1843–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strosberg JR, Fine RL, Choi J, Nasir A,
Coppola D, Chen DT, Helm J and Kvols L: First-line chemotherapy
with capecitabine and temozolomide in patients with metastatic
pancreatic endocrine carcinomas. Cancer. 117:268–275. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikenishi M, Ueda M, Kuroda A, Tsukazaki H,
Nakao M, Takeuchi M, Konishi Y, Matsuda T, Figoni W, Ohtori T, et
al: A study on drug interaction between warfarin and capecitabine
with special reference to the co-administered term or the
discontinuation term of capecitabine. Gan To Kagaku Ryoho.
42:833–839. 2015.PubMed/NCBI
|
|
13
|
Chu MP, Hecht JR, Slamon D, Wainberg ZA,
Bang YJ, Hoff PM, Sobrero A, Qin S, Afenjar K, Houe V, et al:
Association of proton pump inhibitors and capecitabine efficacy in
advanced gastroesophageal cancer: Secondary analysis of the
TRIO-013/LOGiC randomized clinical trial. JAMA Oncol. 3:767–773.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baan J, Bos MMEM, Gonesh-Kisoensingh SU,
Meynaar IA, Alsma J, Meijer E and GVulto A: Capecitabine-induced
toxicity: An outcome study into drug safety. J Integr Oncol.
3:1132014. View Article : Google Scholar
|
|
15
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hofheinz RD, Wenz F, Post S, Matzdorff A,
Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E, et
al: Chemoradiotherapy with capecitabine versus fluorouracil for
locally advanced rectal cancer: A randomised, multicentre,
non-inferiority, phase 3 trial. Lancet Oncol. 13:579–588. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
New drug extends lives of brain cancer
patients. FDA Consum. 39:32005.
|
|
19
|
Kunz PL, Catalano PJ, Nimeiri H, Fisher
GA, Longacre TA, Suarez CJ, Yao JC, Kulke MH, Hendifar AE, Shanks
JC, et al: A randomized study of temozolomide or temozolomide and
capecitabine in patients with advanced pancreatic neuroendocrine
tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J
Clin Oncol. 36 (15 Suppl):S40042018. View Article : Google Scholar
|
|
20
|
Marx J: Cancer. Encouraging results for
second-generation antiangiogenesis drugs. Science. 308:1248–1249.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tada T, Kumada T, Hiraoka A, Michitaka K,
Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama
K, et al: Safety and efficacy of lenvatinib in elderly patients
with unresectable hepatocellular carcinoma: A multicenter analysis
with propensity score matching. Hepatol Res. 50:75–83. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ettrich TJ and Seufferlein T: Regorafenib.
Recent Results Cancer Res. 211:45–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bekaii-Saab TS, Ou FS, Ahn DH, Boland PM,
Ciombor KK, Heying EN, Dockter TJ, Jacobs NL, Pasche BC, Cleary JM,
et al: Regorafenib dose-optimisation in patients with refractory
metastatic colorectal cancer (ReDOS): A randomised, multicentre,
open-label, phase 2 study. Lancet Oncol. 20:1070–1082. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Demetri GD, Reichardt P, Kang YK, Blay JY,
Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M,
Joensuu H, et al: Efficacy and safety of regorafenib for advanced
gastrointestinal stromal tumours after failure of imatinib and
sunitinib (GRID): An international, multicentre, randomised,
placebo-controlled, phase 3 trial. Lancet. 381:295–302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grüllich C: Cabozantinib: A MET, RET, and
VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res.
201:207–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blumenthal GM, Cortazar P, Zhang JJ, Tang
S, Sridhara R, Murgo A, Justice R and Pazdur R: FDA approval
summary: Sunitinib for the treatment of progressive
well-differentiated locally advanced or metastatic pancreatic
neuroendocrine tumors. Oncologist. 17:1108–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raymond E, Dahan L, Raoul JL, Bang YJ,
Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A,
et al: Sunitinib malate for the treatment of pancreatic
neuroendocrine tumors. N Engl J Med. 364:501–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
George S, Blay JY, Casali PG, Le Cesne A,
Stephenson P, Deprimo SE, Harmon CS, Law CN, Morgan JA, Ray-Coquard
I, et al: Clinical evaluation of continuous daily dosing of
sunitinib malate in patients with advanced gastrointestinal stromal
tumour after imatinib failure. Eur J Cancer. 45:1959–1968. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi BS: Risks associated with sunitinib
use and monitoring to improve patient outcomes. Korean J Intern
Med. 29:23–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pavel ME, Hainsworth JD, Baudin E, Peeters
M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM,
et al: Everolimus plus octreotide long-acting repeatable for the
treatment of advanced neuroendocrine tumours associated with
carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled,
phase 3 study. Lancet. 378:2005–2012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao JC, Fazio N, Singh S, Buzzoni R,
Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, et al:
Everolimus for the treatment of advanced, non-functional
neuroendocrine tumours of the lung or gastrointestinal tract
(RADIANT-4): A randomised, placebo-controlled, phase 3 study.
Lancet. 387:968–977. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lombard-Bohas C, Cariou B, Vergès B,
Coriat R, N'guyen T, François E, Hammel P, Niccoli P and Hentic O:
Management of metabolic disorders induced by everolimus in patients
with differentiated neuroendocrine tumors: Expert proposals. Bull
Cancer. 101:175–183. 2014.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Klümpen HJ, Beijnen JH, Gurney H and
Schellens JH: Inhibitors of mTOR. Oncologist. 15:1262–1269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mesa RA: Imatinib and tyrosine kinase
inhibition, in the management of BCR-ABL negative
myeloproliferative disorders. Biologics. 1:129–138. 2007.PubMed/NCBI
|
|
41
|
Dematteo RP, Ballman KV, Antonescu CR,
Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von
Mehren M, Brennan MF, et al: Adjuvant imatinib mesylate after
resection of localised, primary gastrointestinal stromal tumour: A
randomised, double-blind, placebo-controlled trial. Lancet.
373:1097–1104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Corless CL, Ballman KV, Antonescu CR,
Kolesnikova V, Maki RG, Pisters PW, Blackstein ME, Blanke CD,
Demetri GD, Heinrich MC, et al: Pathologic and molecular features
correlate with long-term outcome after adjuvant therapy of resected
primary GI stromal tumor: The ACOSOG Z9001 trial. J Clin Oncol.
32:1563–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Joensuu H, Eriksson M, Sundby Hall K,
Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster
J, Al-Batran SE, et al: One vs three years of adjuvant imatinib for
operable gastrointestinal stromal tumor: A randomized trial. JAMA.
307:1265–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
ESMO/European Sarcoma Network Working
Group, : Gastrointestinal stromal tumours: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25
(Suppl 3):iii21–iii26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Blanke CD, Rankin C, Demetri GD, Ryan CW,
von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki
RG, et al: Phase III randomized, intergroup trial assessing
imatinib mesylate at two dose levels in patients with unresectable
or metastatic gastrointestinal stromal tumors expressing the kit
receptor tyrosine kinase: S0033. J Clin Oncol. 26:626–632. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zalcberg JR, Verweij J, Casali PG, Le
Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M
and Judson IR; EORTC Soft Tissue and Bone Sarcoma Group and the
Italian Sarcoma Group and Australasian Gastrointestinal Trials
Group, : Outcome of patients with advanced gastro-intestinal
stromal tumours crossing over to a daily imatinib dose of 800 mg
after progression on 400 mg. Eur J Cancer. 41:1751–1757. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Riese C, Weiß B, Borges U Jr, Beylich A,
Dengler R, Hermes-Moll K, Welslau M and Baumann W: Effectiveness of
a standardized patient education program on therapy-related side
effects and unplanned therapy interruptions in oral cancer therapy:
A cluster-randomized controlled trial. Support Care Cancer.
25:3475–3483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Favier-Archinard C, Leguelinel-Blache G,
Cousin C, Gillet C, Bourquard P, Dubois F, Passemard N, Tora S, Rey
A, Heraut B, et al: Élaboration d'un guide standardisé
d'informations d'optimisation de l'adhésion médicamenteuse à
dispenser lors d'une consultation pharmaceutique au patient atteint
de myélome multiple: Validation finale. Bull Cancer. 105:1157–1172.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Moriwaki T, Fukuoka S, Taniguchi H,
Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama
C, Denda T, et al: Propensity score analysis of regorafenib versus
trifluridine/tipiracil in patients with metastatic colorectal
cancer refractory to standard chemotherapy (REGOTAS): A Japanese
society for cancer of the colon and rectum multicenter
observational study. Oncologist. 23:7–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Patel AK, Barghout V, Yenikomshian MA,
Germain G, Jacques P, Laliberté F and Duh MS: Real-world adherence
in patients with metastatic colorectal cancer treated with
trifluridine plus tipiracil or regorafenib. Oncologist. 25:e75–e84.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roila F, Molassiotis A, Herrstedt J, Aapro
M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer
P, et al: 2016 MASCC and ESMO guideline update for the prevention
of chemotherapy- and radiotherapy-induced nausea and vomiting and
of nausea and vomiting in advanced cancer patients. Ann Oncol. 27
(Suppl 5):v119–v33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gebbia V, Carreca I, Testa A, Valenza R,
Curto G, Cannata G, Borsellino N, Latteri MA, Cipolla C, Florena M,
et al: Subcutaneous octreotide versus oral loperamide in the
treatment of diarrhea following chemotherapy. Anticancer Drugs.
4:443–445. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bryce J and Boers-Doets CB: Non-rash
dermatologic adverse events related to targeted therapies. Semin
Oncol Nurs. 30:155–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lalla RV, Sonis ST and Peterson DE:
Management of oral mucositis in patients who have cancer. Dent Clin
North Am. 5261–77. (viii)2008. View Article : Google Scholar : PubMed/NCBI
|