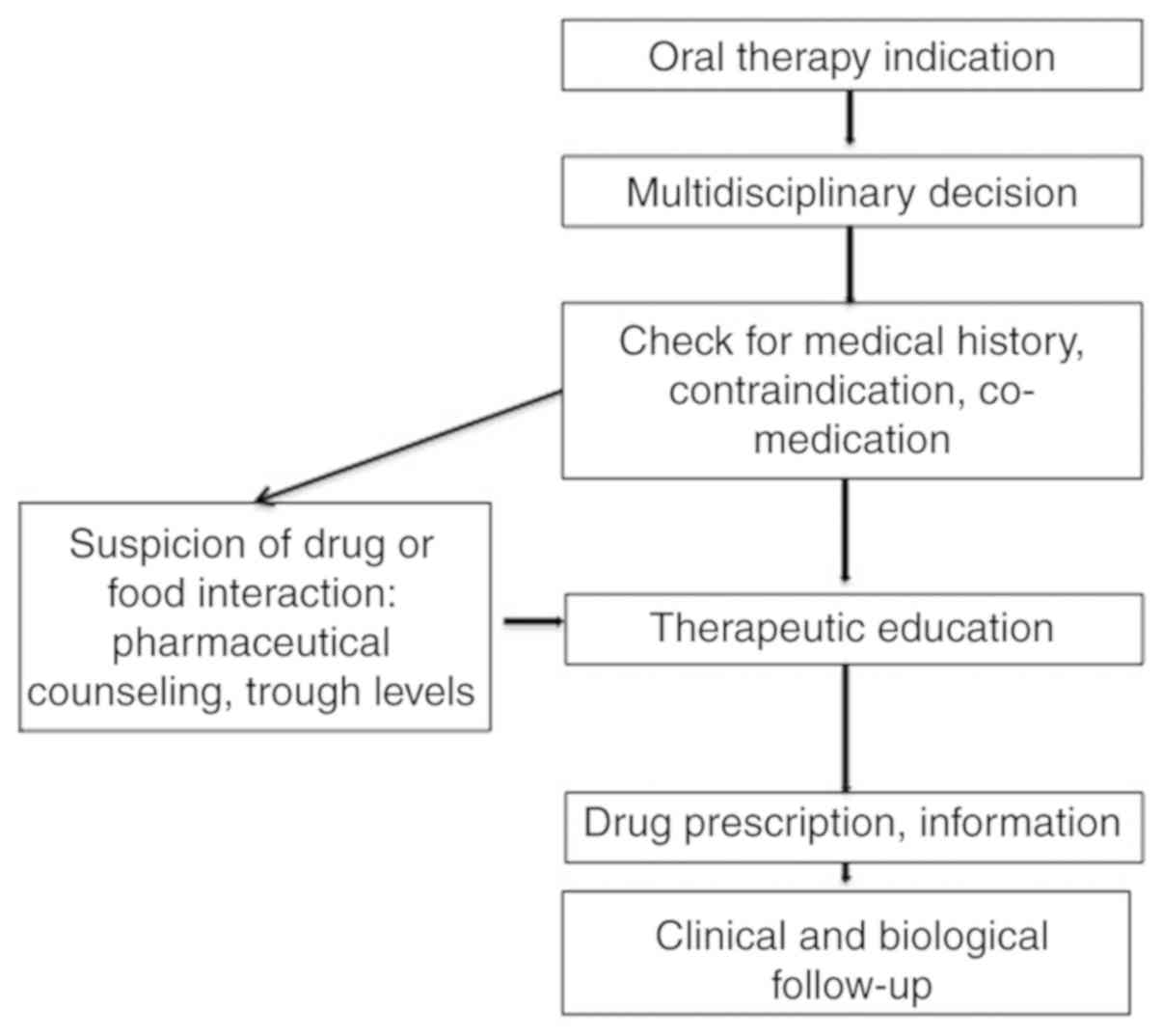
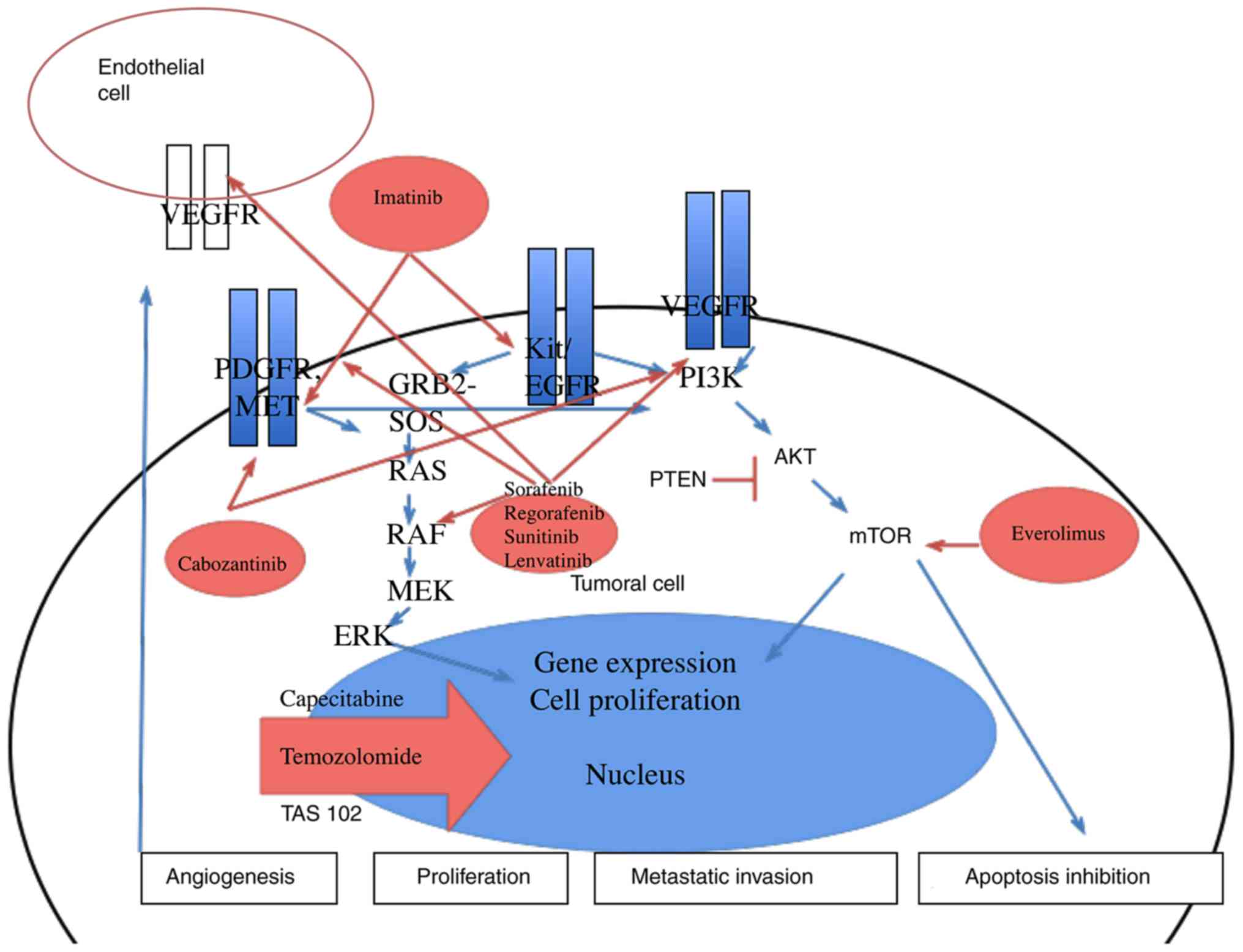
|
1
|
Bassan F, Peter F, Houbre B, Brennstuhl
MJ, Costantini M, Speyer E and Tarquinio C: Adherence to oral
antineoplastic agents by cancer patients: Definition and literature
review. Eur J Cancer Care (Engl). 23:22–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schüller J, Cassidy J, Dumont E, Roos B,
Durston S, Banken L, Utoh M, Mori K, Weidekamm E and Reigner B:
Preferential activation of capecitabine in tumor following oral
administration to colorectal cancer patients. Cancer Chemother
Pharmacol. 45:291–297. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loriot MA, Ciccolini J, Thomas F,
Barin-Le-Guellec C, Royer B, Milano G, Picard N, Becquemont L,
Verstuyft C, Narjoz C, et al: Dihydropyrimidine déhydrogenase (DPD)
deficiency screening and securing of fluoropyrimidine-based
chemotherapies: Update and recommendations of the French
GPCO-Unicancer and RNPGx networks. Bull Cancer. 105:397–407.
2018.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gérard JP, Azria D, Gourgou-Bourgade S,
Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E,
de La Roche G, Bouché O, et al: Comparison of two neoadjuvant
chemoradiotherapy regimens for locally advanced rectal cancer:
Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin
Oncol. 28:1638–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Primrose JN, Fox RP, Palmer DH, Malik HZ,
Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, et
al: Capecitabine compared with observation in resected biliary
tract cancer (BILCAP): A randomised, controlled, multicentre, phase
3 study. Lancet Oncol. 20:663–673. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Twelves C, Wong A, Nowacki MP, Abt M,
Burris H III, Carrato A, Cassidy J, Cervantes A, Fagerberg J,
Georgoulias V, et al: Capecitabine as adjuvant treatment for stage
III colon cancer. N Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grothey A, Sobrero A, Meyerhardt JA,
Yoshino T, Paul J, Taieb J, Souglakos I, Kerr R, Labianca R,
Shields AF, et al: Prospective pooled analysis of six phase III
trials investigating duration of adjuvant (adjuv) oxaliplatin-based
therapy (3 vs. 6 months) for patients (pts) with stage III colon
cancer (CC): Updated results of IDEA (International Duration
Evaluation of Adjuvant Chemotherapy). Ann Oncol. 28 (Suppl
5):v605–v649. 2017. View Article : Google Scholar
|
|
8
|
Neoptolemos JP, Palmer DH, Ghaneh P,
Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA,
Cunningham D, Wadsley J, et al: Comparison of adjuvant gemcitabine
and capecitabine with gemcitabine monotherapy in patients with
resected pancreatic cancer (ESPAC-4): A multicentre, open-label,
randomised, phase 3 trial. Lancet. 389:1011–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR;
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom, : Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simkens LH, van Tinteren H, May A, ten
Tije AJ, Creemers GJ, Loosveld OJ, de Jongh FE, Erdkamp FL, Erjavec
Z, van der Torren AM, et al: Maintenance treatment with
capecitabine and bevacizumab in metastatic colorectal cancer
(CAIRO3): A phase 3 randomised controlled trial of the Dutch
colorectal cancer group. Lancet. 385:1843–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strosberg JR, Fine RL, Choi J, Nasir A,
Coppola D, Chen DT, Helm J and Kvols L: First-line chemotherapy
with capecitabine and temozolomide in patients with metastatic
pancreatic endocrine carcinomas. Cancer. 117:268–275. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikenishi M, Ueda M, Kuroda A, Tsukazaki H,
Nakao M, Takeuchi M, Konishi Y, Matsuda T, Figoni W, Ohtori T, et
al: A study on drug interaction between warfarin and capecitabine
with special reference to the co-administered term or the
discontinuation term of capecitabine. Gan To Kagaku Ryoho.
42:833–839. 2015.PubMed/NCBI
|
|
13
|
Chu MP, Hecht JR, Slamon D, Wainberg ZA,
Bang YJ, Hoff PM, Sobrero A, Qin S, Afenjar K, Houe V, et al:
Association of proton pump inhibitors and capecitabine efficacy in
advanced gastroesophageal cancer: Secondary analysis of the
TRIO-013/LOGiC randomized clinical trial. JAMA Oncol. 3:767–773.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baan J, Bos MMEM, Gonesh-Kisoensingh SU,
Meynaar IA, Alsma J, Meijer E and GVulto A: Capecitabine-induced
toxicity: An outcome study into drug safety. J Integr Oncol.
3:1132014. View Article : Google Scholar
|
|
15
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hofheinz RD, Wenz F, Post S, Matzdorff A,
Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E, et
al: Chemoradiotherapy with capecitabine versus fluorouracil for
locally advanced rectal cancer: A randomised, multicentre,
non-inferiority, phase 3 trial. Lancet Oncol. 13:579–588. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
New drug extends lives of brain cancer
patients. FDA Consum. 39:32005.
|
|
19
|
Kunz PL, Catalano PJ, Nimeiri H, Fisher
GA, Longacre TA, Suarez CJ, Yao JC, Kulke MH, Hendifar AE, Shanks
JC, et al: A randomized study of temozolomide or temozolomide and
capecitabine in patients with advanced pancreatic neuroendocrine
tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J
Clin Oncol. 36 (15 Suppl):S40042018. View Article : Google Scholar
|
|
20
|
Marx J: Cancer. Encouraging results for
second-generation antiangiogenesis drugs. Science. 308:1248–1249.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tada T, Kumada T, Hiraoka A, Michitaka K,
Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama
K, et al: Safety and efficacy of lenvatinib in elderly patients
with unresectable hepatocellular carcinoma: A multicenter analysis
with propensity score matching. Hepatol Res. 50:75–83. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ettrich TJ and Seufferlein T: Regorafenib.
Recent Results Cancer Res. 211:45–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bekaii-Saab TS, Ou FS, Ahn DH, Boland PM,
Ciombor KK, Heying EN, Dockter TJ, Jacobs NL, Pasche BC, Cleary JM,
et al: Regorafenib dose-optimisation in patients with refractory
metastatic colorectal cancer (ReDOS): A randomised, multicentre,
open-label, phase 2 study. Lancet Oncol. 20:1070–1082. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Demetri GD, Reichardt P, Kang YK, Blay JY,
Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M,
Joensuu H, et al: Efficacy and safety of regorafenib for advanced
gastrointestinal stromal tumours after failure of imatinib and
sunitinib (GRID): An international, multicentre, randomised,
placebo-controlled, phase 3 trial. Lancet. 381:295–302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grüllich C: Cabozantinib: A MET, RET, and
VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res.
201:207–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blumenthal GM, Cortazar P, Zhang JJ, Tang
S, Sridhara R, Murgo A, Justice R and Pazdur R: FDA approval
summary: Sunitinib for the treatment of progressive
well-differentiated locally advanced or metastatic pancreatic
neuroendocrine tumors. Oncologist. 17:1108–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raymond E, Dahan L, Raoul JL, Bang YJ,
Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A,
et al: Sunitinib malate for the treatment of pancreatic
neuroendocrine tumors. N Engl J Med. 364:501–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
George S, Blay JY, Casali PG, Le Cesne A,
Stephenson P, Deprimo SE, Harmon CS, Law CN, Morgan JA, Ray-Coquard
I, et al: Clinical evaluation of continuous daily dosing of
sunitinib malate in patients with advanced gastrointestinal stromal
tumour after imatinib failure. Eur J Cancer. 45:1959–1968. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi BS: Risks associated with sunitinib
use and monitoring to improve patient outcomes. Korean J Intern
Med. 29:23–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pavel ME, Hainsworth JD, Baudin E, Peeters
M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM,
et al: Everolimus plus octreotide long-acting repeatable for the
treatment of advanced neuroendocrine tumours associated with
carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled,
phase 3 study. Lancet. 378:2005–2012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao JC, Fazio N, Singh S, Buzzoni R,
Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, et al:
Everolimus for the treatment of advanced, non-functional
neuroendocrine tumours of the lung or gastrointestinal tract
(RADIANT-4): A randomised, placebo-controlled, phase 3 study.
Lancet. 387:968–977. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lombard-Bohas C, Cariou B, Vergès B,
Coriat R, N'guyen T, François E, Hammel P, Niccoli P and Hentic O:
Management of metabolic disorders induced by everolimus in patients
with differentiated neuroendocrine tumors: Expert proposals. Bull
Cancer. 101:175–183. 2014.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Klümpen HJ, Beijnen JH, Gurney H and
Schellens JH: Inhibitors of mTOR. Oncologist. 15:1262–1269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mesa RA: Imatinib and tyrosine kinase
inhibition, in the management of BCR-ABL negative
myeloproliferative disorders. Biologics. 1:129–138. 2007.PubMed/NCBI
|
|
41
|
Dematteo RP, Ballman KV, Antonescu CR,
Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von
Mehren M, Brennan MF, et al: Adjuvant imatinib mesylate after
resection of localised, primary gastrointestinal stromal tumour: A
randomised, double-blind, placebo-controlled trial. Lancet.
373:1097–1104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Corless CL, Ballman KV, Antonescu CR,
Kolesnikova V, Maki RG, Pisters PW, Blackstein ME, Blanke CD,
Demetri GD, Heinrich MC, et al: Pathologic and molecular features
correlate with long-term outcome after adjuvant therapy of resected
primary GI stromal tumor: The ACOSOG Z9001 trial. J Clin Oncol.
32:1563–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Joensuu H, Eriksson M, Sundby Hall K,
Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster
J, Al-Batran SE, et al: One vs three years of adjuvant imatinib for
operable gastrointestinal stromal tumor: A randomized trial. JAMA.
307:1265–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
ESMO/European Sarcoma Network Working
Group, : Gastrointestinal stromal tumours: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25
(Suppl 3):iii21–iii26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Blanke CD, Rankin C, Demetri GD, Ryan CW,
von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki
RG, et al: Phase III randomized, intergroup trial assessing
imatinib mesylate at two dose levels in patients with unresectable
or metastatic gastrointestinal stromal tumors expressing the kit
receptor tyrosine kinase: S0033. J Clin Oncol. 26:626–632. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zalcberg JR, Verweij J, Casali PG, Le
Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M
and Judson IR; EORTC Soft Tissue and Bone Sarcoma Group and the
Italian Sarcoma Group and Australasian Gastrointestinal Trials
Group, : Outcome of patients with advanced gastro-intestinal
stromal tumours crossing over to a daily imatinib dose of 800 mg
after progression on 400 mg. Eur J Cancer. 41:1751–1757. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Riese C, Weiß B, Borges U Jr, Beylich A,
Dengler R, Hermes-Moll K, Welslau M and Baumann W: Effectiveness of
a standardized patient education program on therapy-related side
effects and unplanned therapy interruptions in oral cancer therapy:
A cluster-randomized controlled trial. Support Care Cancer.
25:3475–3483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Favier-Archinard C, Leguelinel-Blache G,
Cousin C, Gillet C, Bourquard P, Dubois F, Passemard N, Tora S, Rey
A, Heraut B, et al: Élaboration d'un guide standardisé
d'informations d'optimisation de l'adhésion médicamenteuse à
dispenser lors d'une consultation pharmaceutique au patient atteint
de myélome multiple: Validation finale. Bull Cancer. 105:1157–1172.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Moriwaki T, Fukuoka S, Taniguchi H,
Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama
C, Denda T, et al: Propensity score analysis of regorafenib versus
trifluridine/tipiracil in patients with metastatic colorectal
cancer refractory to standard chemotherapy (REGOTAS): A Japanese
society for cancer of the colon and rectum multicenter
observational study. Oncologist. 23:7–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Patel AK, Barghout V, Yenikomshian MA,
Germain G, Jacques P, Laliberté F and Duh MS: Real-world adherence
in patients with metastatic colorectal cancer treated with
trifluridine plus tipiracil or regorafenib. Oncologist. 25:e75–e84.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roila F, Molassiotis A, Herrstedt J, Aapro
M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer
P, et al: 2016 MASCC and ESMO guideline update for the prevention
of chemotherapy- and radiotherapy-induced nausea and vomiting and
of nausea and vomiting in advanced cancer patients. Ann Oncol. 27
(Suppl 5):v119–v33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gebbia V, Carreca I, Testa A, Valenza R,
Curto G, Cannata G, Borsellino N, Latteri MA, Cipolla C, Florena M,
et al: Subcutaneous octreotide versus oral loperamide in the
treatment of diarrhea following chemotherapy. Anticancer Drugs.
4:443–445. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bryce J and Boers-Doets CB: Non-rash
dermatologic adverse events related to targeted therapies. Semin
Oncol Nurs. 30:155–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lalla RV, Sonis ST and Peterson DE:
Management of oral mucositis in patients who have cancer. Dent Clin
North Am. 5261–77. (viii)2008. View Article : Google Scholar : PubMed/NCBI
|