Introduction
Oral cancer is a prevalent malignant tumor. Owing to
the frequent mechanical stimulation, the incidence of tongue
squamous cell carcinoma ranks first among oral cancer cases, and
its incidence continues to increase (1,2). The
incidence of tongue cancer is obviously higher among older
patients, as the majority of the patients are aged ~60 years
(3). Oral cancer is characterized
as highly malignant, with high rates of local recurrence and
cervical lymph node metastasis (4,5).
Currently, surgery combined with postoperative radiotherapy and
chemotherapy is the preferred treatment for tongue cancer (6). Due to the short-term recurrence and
poor therapeutic efficacy, oral cancer has a dismal prognosis and
severely affects the life quality of the affected patients
(7). The 5 year survival rate of
oral cancer is reported to be ~50% (8). The symptoms of early-stage oral cancer
are atypical and they are often mistaken for a bite or a mild
stabbing pain. Consequently, several patients with oral cancer
already have intermediate- or advanced-stage disease at initial
diagnosis and, thus, have missed the optimal window for treatment.
Tongue cancer must be predicted and diagnosed as early as possible;
thus, it is crucial to elucidate the molecular mechanisms
underlying its etiology and pathogenesis.
Bioinformatics analyses of gene expression profiles
have been extensively performed in recent years. As an assemblage
of RNAs, the transcriptome is mainly transcribed from specific
tissues or cells at a certain phase or functional state. Analyzing
transcriptome data enables assessing overall gene function and
structure, thereby elucidating the potential molecular mechanisms
underlying pathological conditions, and has previously been applied
extensively in cancer research (9).
Non-coding RNAs (ncRNAs) are a type of RNA transcribed from DNA
that lacks protein-encoding ability. Critical functions of ncRNAs
have been highlighted in almost all aspects of cancer progression
(10,11).
Transcriptome analyses have been well documented in
cancer research; however, few transcriptome analyses have been
reported for tongue cancer, and traditional in vivo
experiments or single-gene studies are currently preferred. To the
best of our knowledge, this is the first study to date to
investigate differentially expressed mRNAs and ncRNAs in tongue
cancer and paracancerous tissues via transcriptome analysis, in the
hope that the findings may help identify potential targets for the
diagnosis and clinical treatment of early-stage tongue cancer.
Materials and methods
Subjects
The present study was approved by the Medical Ethics
Committee of Nantong Municipal Tumor Hospital (approval no.
2018037). Tongue cancer tissues and matched paracancerous tissues
(located ~3 cm from the cancerous tissues) were surgically
extracted from 4 patients with tongue cancer (cases 1–4, Table I). A total of 3 men and 1 woman were
enrolled, with a mean age of 67 years. Pathologically, 3 of the
patients had well-differentiated squamous cell carcinoma (n=2 with
T3N2bM0, stage IVb and n=1 with T2N2cM0, stage IVb), and 1 patient
had moderately differentiated squamous cell carcinoma (T2N0M0,
stage II). Based on the tongue cancer subtypes, 3 patients had
ulcerative infiltrating tongue cancer, and 1 had exophytic tongue
cancer. Samples used for reverse transcription-quantitative PCR
(RT-qPCR) were extracted from 20 patients, whose data are shown in
supplementary Table SI. The mean
age of these patients was 61.6 years (range, 51–73 years).
 | Table I.Basic patient information. |
Table I.
Basic patient information.
| Case no. | Age, years | Sex | Stage |
Differentiation | Type |
|---|
| 1 | 66 | Male | T3N2bM0, IVb |
Well-differentiated | Ulcerative
infiltrating |
| 2 | 73 | Male | T2N0M0, II |
Well-differentiated | Exogenous |
| 3 | 57 | Male | T3N2bM0, IVb | Moderately
differentiated | Ulcerative
infiltrating |
| 4 | 73 | Female | T2N2cM0, IVb |
Well-differentiated | Ulcerative
infiltrating |
Hematoxylin and eosin (H&E)
staining
Tongue cancer and paracancerous tissues were used
for morphological observation with H&E staining as previously
described (12). All the following
steps are performed at room temperature. In brief, the tissues were
fixed in paraformaldehyde for 4 h, dehydrated in graded
concentrations of ethanol (70 to 100%, 1 min each), permeabilized
in xylene for 5 min, and embedded in paraffin. After embedding, the
tissues were cut into 4 µm sections, washed in descending
concentrations of ethanol to remove the xylene (100 to 75%, 1 min
each, with a final wash in water), stained with hematoxylin for 5
min followed by washing in water, and then stained with eosin for 2
min. The sections were observed and images were captured at a
magnification of ×100 using the Olympus IX71 inverted microscope
(Olympus Corporation).
cDNA library construction and
sequencing
Total RNA was extracted from the tongue cancer and
paracancerous tissues using TRIzol reagent (Thermo Fisher
Scientific, Inc.). The RNA concentration and purity were determined
using an ultraviolet spectrophotometer (RAY-757CRT; Raylabel
Instrument Co., Ltd.). The cDNA library was constructed and
sequenced as previously described (13). In brief, as much rRNA as possible
was removed to obtain the purified RNA. Subsequently, mRNA and
ncRNA were separated by poly (A) splicing. The RNA was randomly
sliced into short fragments that were used as templates. The
first-strand cDNA was synthesized alongside 6-bp random primers.
The second-strand cDNA was synthesized using a commercial kit
(Takara Biotechnology Co., Ltd.) following the manufacturer's
instructions. After purification, end-repair, A-tailing and
addition of adaptor sequences, the cDNA was fragmentated using
uracil glycosylase.
cDNA fragments were subjected to PCR amplification,
and the complementary cDNA library was constructed. ncRNAs and
mRNAs were sequenced using the high-throughput and high-sensitivity
HiSeq 2500 sequencing platform (Illumina, Inc.). Sequenced data
were analyzed and processed to dynamically remove the sequence
fragments at the 3′-end and low-quality fragments using Trim Galore
−0.6.5 software (Babraham Institute). Finally, Fast-QC version
0.11.8 (Babraham Institute) was used to evaluate the quality of the
preprocessed data.
Comparison with reference
sequences
RNA-Seq data were compared with the reference
database (GRCh, version 38) using Hisat2 software version 2.1.0
(Johns Hopkins University). Data were mapped with a 56-kb index by
genetic fate mapping. Reads were quickly and accurately located on
the genome to obtain the genomic structure of the sequencing
data.
Analysis of differentially expressed
genes
Gene expression was quantified via reads per
kilobase per million mapped reads (RPKM), which was calculated
using Htseq software version 0.9.0 (GitHuB, Fabio Zanini,
University of New South Wales, Sydney) as follows: RPKM = total
exon reads/[mapped reads × exon length (kb)]. Fold changes (FCs) of
the differentially expressed genes in the tongue cancer and
paracancerous tissues were calculated. Genes with log2
FC >1 or <-1 and a false discovery rate ≤0.05 were
selected.
Gene Ontology (GO) analysis
GO analysis revealed significant enrichment of the
terms associated with biological processes, molecular functions and
cellular components. GO terms were assigned based on differentially
expressed mRNAs and host genes with significantly different
circRNAs. The P-value of each GO term was calculated using Fisher's
exact test, and P<0.05 was considered to indicate statistically
significant differences.
Kyoto Encyclopedia of Genes and
Genomes (KEGG) analysis
Pathway annotation of differentially expressed genes
was performed using the KEGG database. The P-value of each pathway
involved was analyzed using Fisher's exact test based on
hypergeometric distribution. P<0.05 was considered to indicate
statistically significant differences.
RT-qPCR
Tongue cancer and paracancerous tissues from each
patient were considered as a group of samples to verify the changes
of cancer-related genes by RT-qPCR. The total sample size was 20,
and basic patient information is provided in Table SI.
According to the results of gene expression and
signaling pathway analysis, 10 genes that were significantly
different and were associated with cancer were selected for
RT-qPCR. Their primers are listed in Table II (Sangon Biotech, Co., Ltd.). The
reverse transcription kit used was PrimeScript™ RT reagent Kit with
gDNA Eraser (Takara Biotechnology Co., Ltd.), first at 42°C for 2
min with gDNA eraser and buffer 1, and then at 37°C for 15 min and
85°C for 5 sec with enzyme mix, RT primer and buffer 2. qPCR
(Takara Biotechnology Co., Ltd.) was performed at 95°C for 10 min,
then at 95°C for 15 sec, 60°C for 30 sec and 72°C for 40 sec for 40
cycles, with a final step at 72°C for 5 min. The reactions were set
up in 96-well format Microseal PCR plates (Bio-Rad Laboratories,
Inc.) in triplicates.
 | Table II.Primers used in reverse
transcription-quantitative PCR. |
Table II.
Primers used in reverse
transcription-quantitative PCR.
| Gene | Primers
(5′-3′) |
|---|
| COL4A6 | F:
CTAACTATCTAAGGGGCTGTGC |
|
| R:
ATTGGCTGATGGTGAGATTTGTATC |
| SPP1 | F:
AGAAGTTTCGCAGACCTGAC |
|
| R:
TTTCAGCACTCTGGTCATCC |
| FN1 | F:
TACCATCAGAGAACAAACACTAATG |
|
| R:
AAGAACTCTAAGCTGGGTCTGC |
| MMP13 | F:
TCTGGACAGACTGGCTGTTG |
|
| R:
TTGAAGGGATGTGATGGTCA |
| CXCL13 | F:
CTCTGCTTCTCATGCTGCTG |
|
| R:
CAGCTTGAGGGTCCACACAC |
| MMP9 | F:
TTCAGGAGACGCCCATTTC |
|
| R:
GTCGTCGGTGTCGTAGTTGG |
| COL10A1 | F:
CATGCCTGATGGCTTCATAAA |
|
| R:
AAGCAGACACGGGCATACCT |
| COL27a1 | F:
GGAACGGACAGGTCTTTGAA |
|
| R:
GGGTCCGGAAGGTGAATAGT |
| COL4A1 | F:
GAACGGGCCCATGGACAGGACTTG |
|
| R:
AGGTGGACGGCGTAGGCTTCTTG |
| CXCL10 | F:
GTACGCTGTACCTGCATCAGCATTAG |
|
| R:
CTGGATTCAGACATCTCTTCTCACCC |
Signaling pathway network
The signaling pathway networks were depicted using
Cytoscape 3.4.0 software (Institute for Systems Biology). Each
pathway network was depicted based on the pathway terms, and those
with P<0.05 were analyzed by KEGG.
Statistical analysis
The data are presented as mean ± standard error of
the mean. RNA-sequencing data were obtained from 4 independent
experiments, while RT-qPCR data were obtained from 20. These data
were mainly compared by log2FC to explain the
upregulation of gene expression in cancer tissues.
Results
Tissue characteristics and
morphology
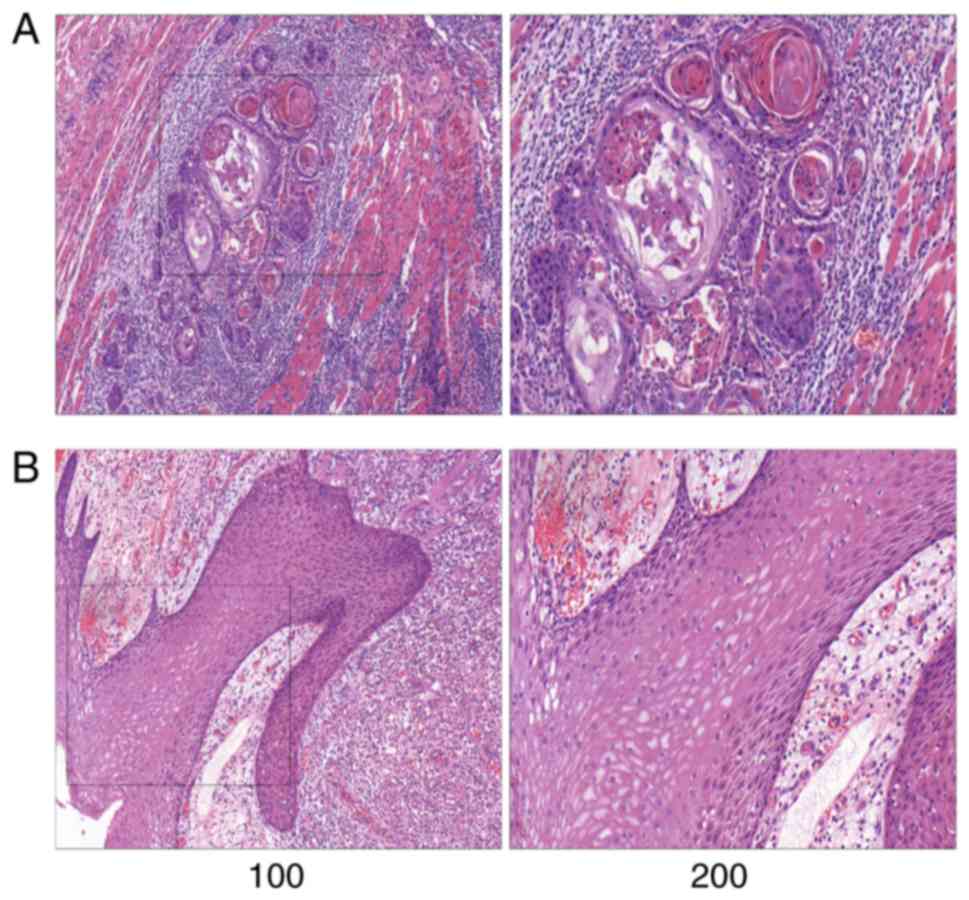
Representative pathological images are shown in
Fig. 1. The normal tongue mucosa is
composed of stratified squamous epithelium, while the tongue cancer
tissues were mostly highly differentiated, accompanied by large
amounts of extracellular keratinization and intercellular
bridges.
Mass analysis of the RNA-Seq data
Transcriptome sequencing and data filtering were
performed on 4 matched pairs of tongue cancer and paracancerous
tissues. After removing adaptor sequences, the acquired data were
compared with the reference genome. Large data sequences and high
unique mapping rates of the samples indicated that the quantity and
quality of the sequenced data were in accordance with the
requirements. The raw data have been uploaded to the NCBI online
database, and the accession number is GSE143950.
Genome composition of the sequenced
samples
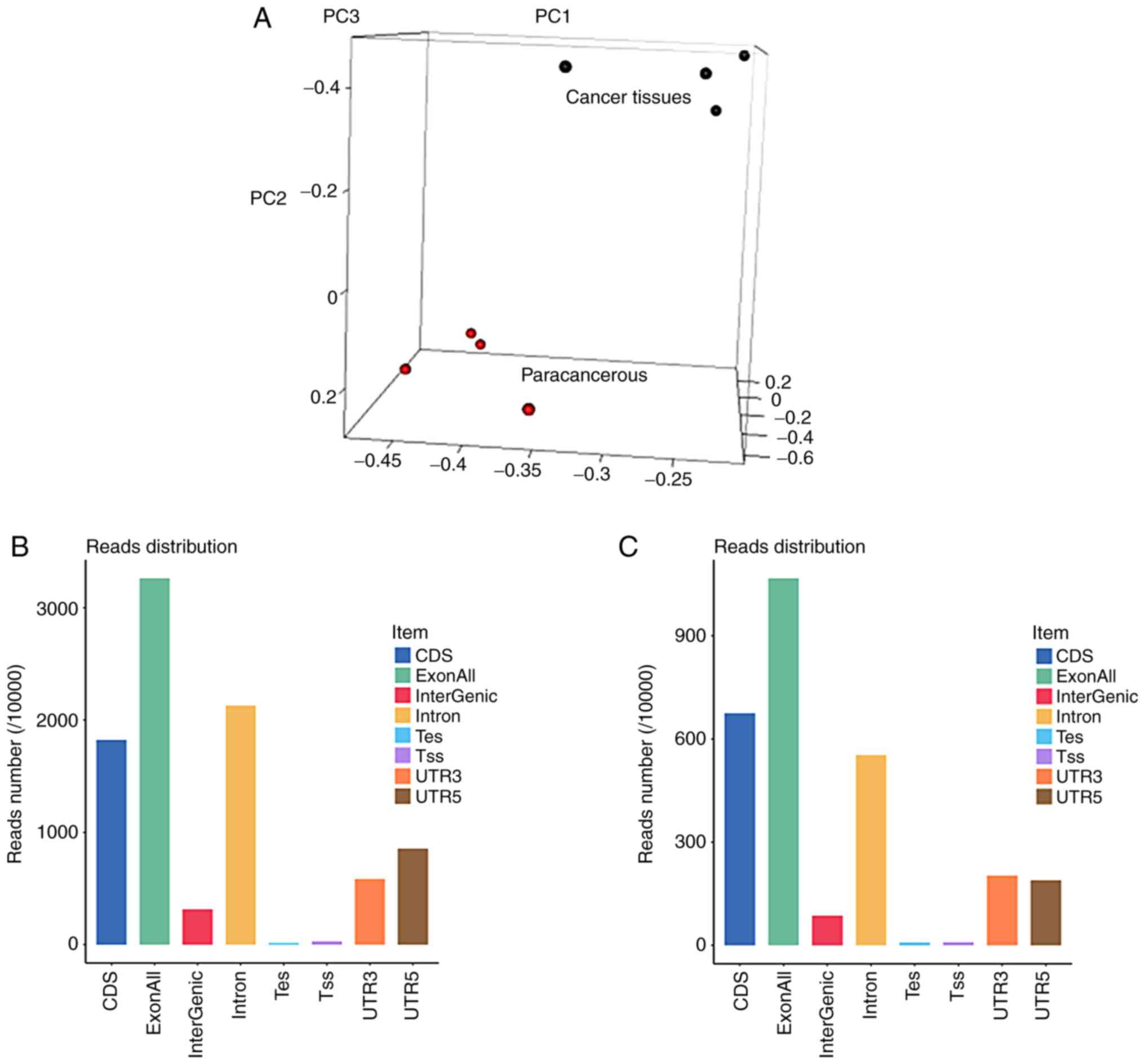
Principal component analysis (Fig. 2A) revealed a good clustering effect
for both the cancerous and adjacent tissues. The genetic
composition of the samples in the exons, introns and intergenic
regions is shown in Fig. 2B.
Abundant RNA transcripts were detected in both exons and introns,
suggesting the presence of numerous ncRNAs. The gene composition
and distribution on the chromosomes is shown in Fig. 2C. Compared with the paracancerous
tissues, Chr.14 was upregulated, while Chr.MT was downregulated in
tongue cancer tissues. The gene expression in each patient's
cancerous and paracancerous tissues is shown in Fig. 2D.
Analyses of differentially expressed
genes
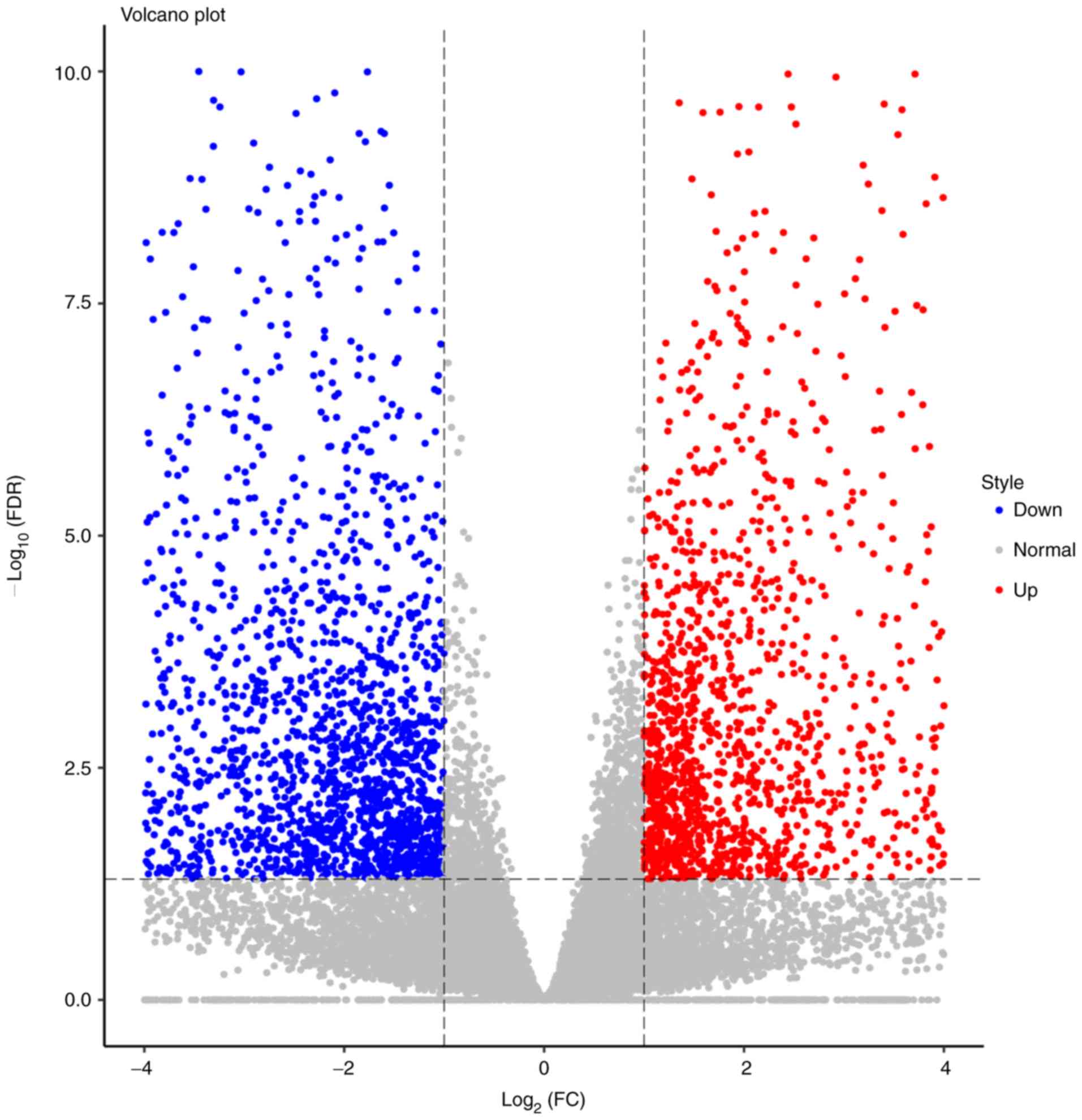
Gene expressions were quantified via RPKM.
Expression abundances were calculated and depicted as volcano
plots. In total, 24,582 genes were examined, of which 1,700 were
upregulated and 2,249 were downregulated (Fig. 3). The top 20 differentially
expressed mRNAs and ncRNAs are listed in Table III. The top 4 genes were MMP13
(log2FC=11.35), KRTAP13-2 (log2FC=−10.52),
KRT36 (log2FC=−10.07) and S100A7A
(log2FC=10.00). The heatmap revealed that the trend in
gene expression change was consistent in all 4 patients, and the
differences between the cancerous and paracancerous tissues were
statistically significant.
 | Table III.Top 10 differentially expressed genes
among mRNAs and non-coding (nc)RNAs. |
Table III.
Top 10 differentially expressed genes
among mRNAs and non-coding (nc)RNAs.
| A, mRNAs |
|---|
|
|---|
| Gene ID |
log2FC |
Up/downregulation |
|---|
| KRTAP13-2 | −10.52 | Down |
| KRT36 | −10.07 | Down |
| KRTAP13-1 | −9.95 | Down |
| MYOC | −9.26 | Down |
| KRT84 | −8.96 | Down |
| CA9 | 9.07 | Up |
| IL24 | 9.27 | Up |
| MMP10 | 9.85 | Up |
| S100A7A | 10.00 | Up |
| MMP13 | 11.35 | Up |
|
| B,
ncRNAs |
|
| Gene ID |
log2FC |
Up/downregulation |
|
| RMST | −8.62 | Down |
| LOC105375180 | −7.20 | Down |
| DIO2-AS1 | −6.83 | Down |
| LOC105372641 | −6.82 | Down |
| TATDN2P3 | −6.78 | Down |
| LINC00520 | 6.83 | Up |
| LOC101928272 | 7.26 | Up |
| AFAP1-AS1 | 7.33 | Up |
| RFTN1P1 | 7.47 | Up |
| LINC01322 | 8.86 | Up |
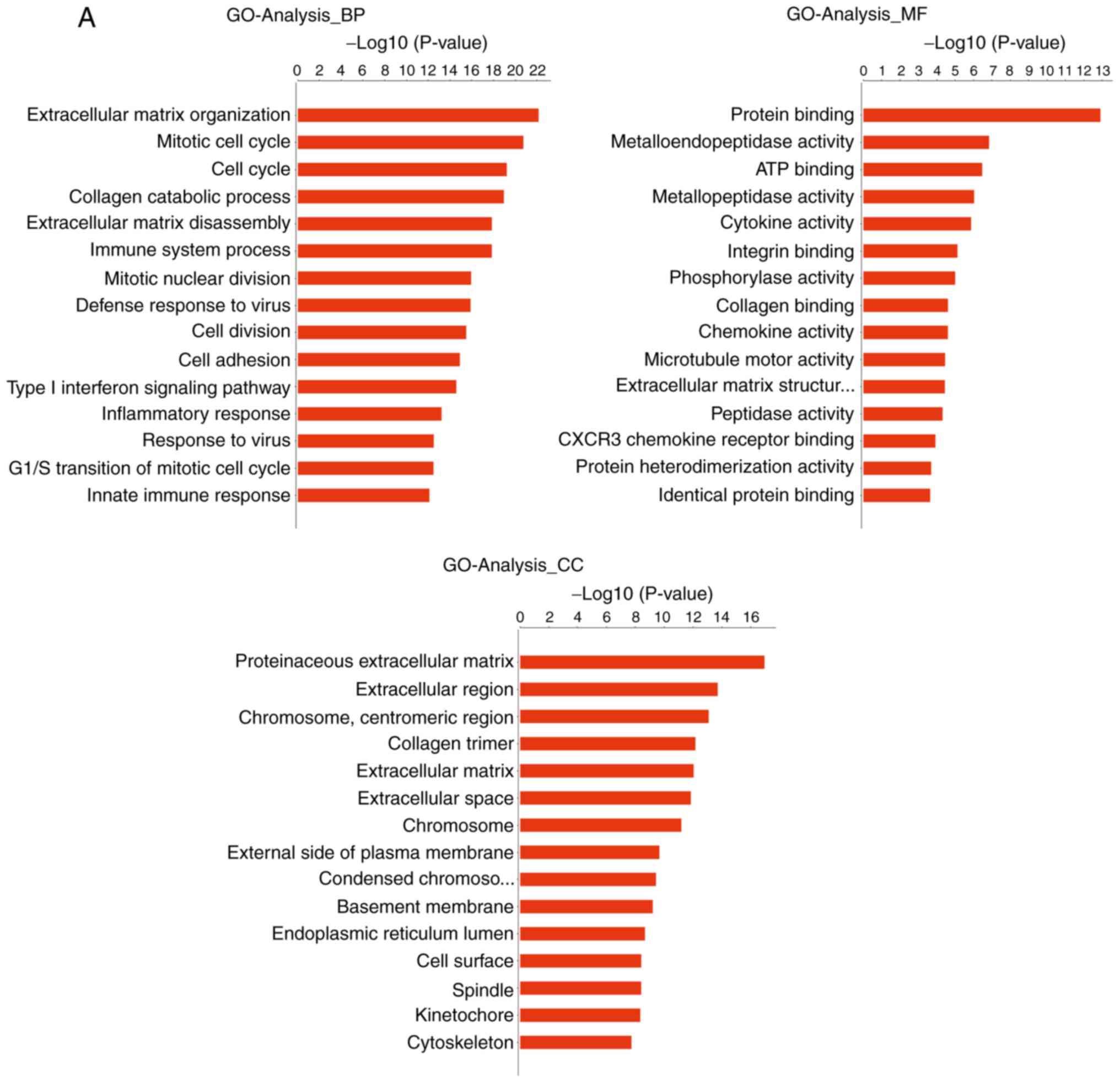
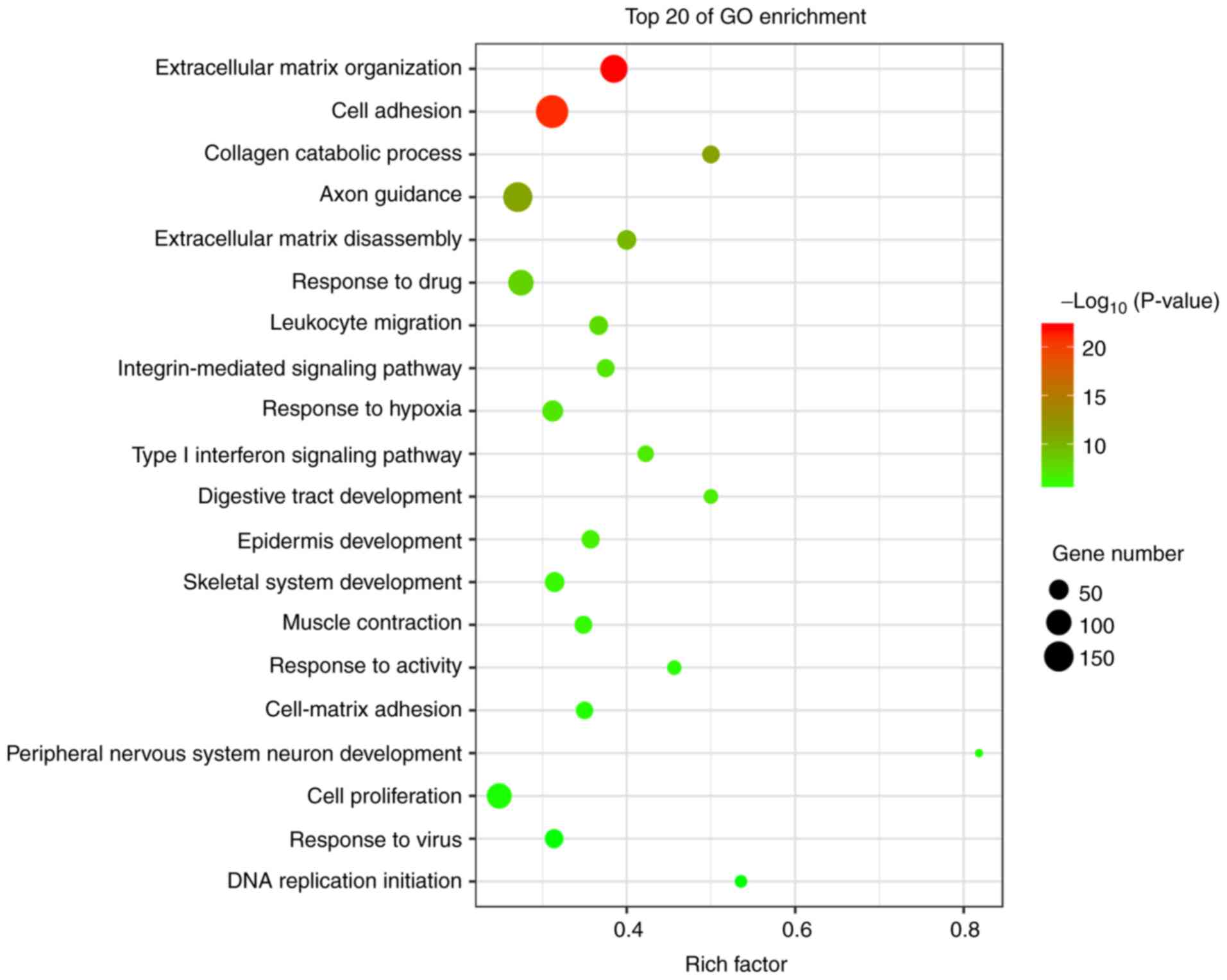
GO analysis
The genes that were differentially expressed in
tongue cancer and paracancerous tissues were analyzed. GO analysis
uncovered significantly enriched terms associated with three items:
i) Biological processes: Extracellular matrix (ECM) organization,
cell adhesion, collagen catabolic processes, ECM disassembly and
the integrin-mediated signaling pathway; ii) molecular functions:
Calcium ion binding, integrin binding, growth factor activity and
ECM structural constituents; and iii) cellular components:
Proteinaceous ECM, extracellular region and ECM. The most
significantly enriched terms were analyzed as the ECM organization
and cell adhesion (Figs. 4 and
5).
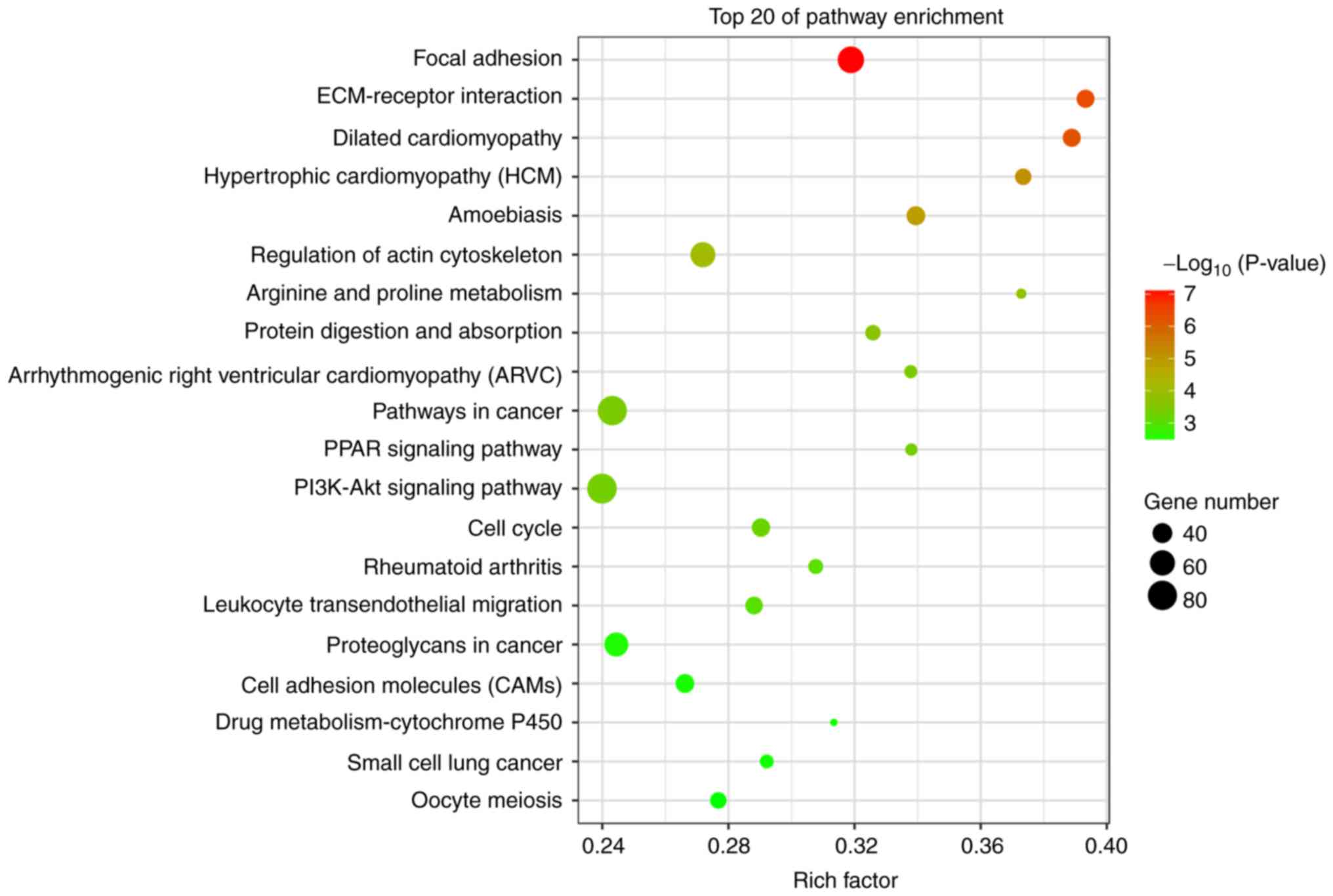
KEGG analysis
Of the pathways identified by KEGG pathway
annotation and analysis, 61 were significantly downregulated and 43
were upregulated (Fig. 6). The most
differentially activated pathways were focal adhesion, ECM-receptor
interaction, pathways in cancer, small-cell lung cancer,
phosphoinositide 3-kinase (PI3K)-Akt signaling and cell adhesion
molecules (CAMs).
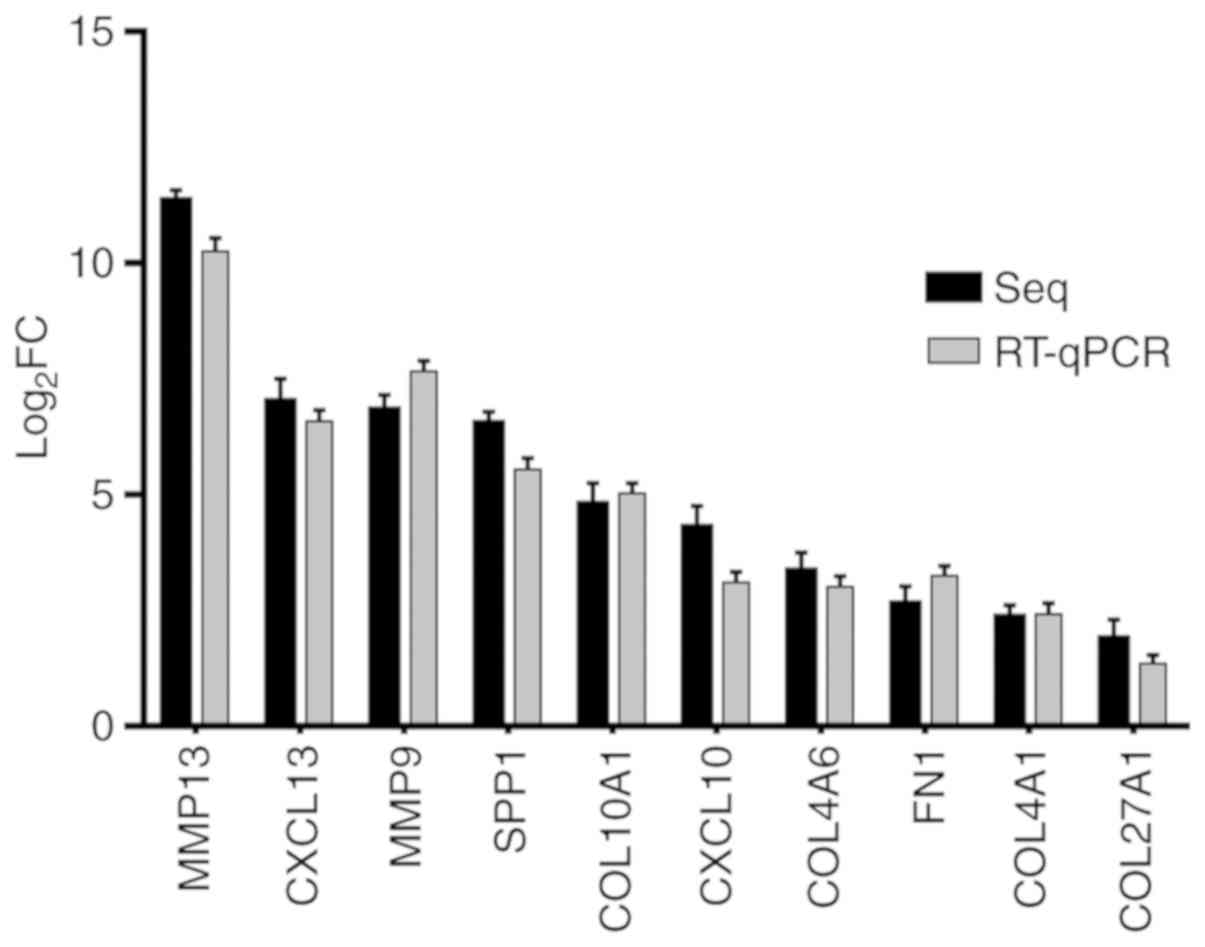
RT-qPCR
The differences of fold changes were compared
between RNA-seq and RT-qPCR. The results are shown as Fig. 7. These 10 genes were all upregulated
in cancer tissues, and there was no significant biological
difference between RT-qPCR and RNA-seq, indicating that the RNA-seq
results were consistent with those of RT-qPCR.
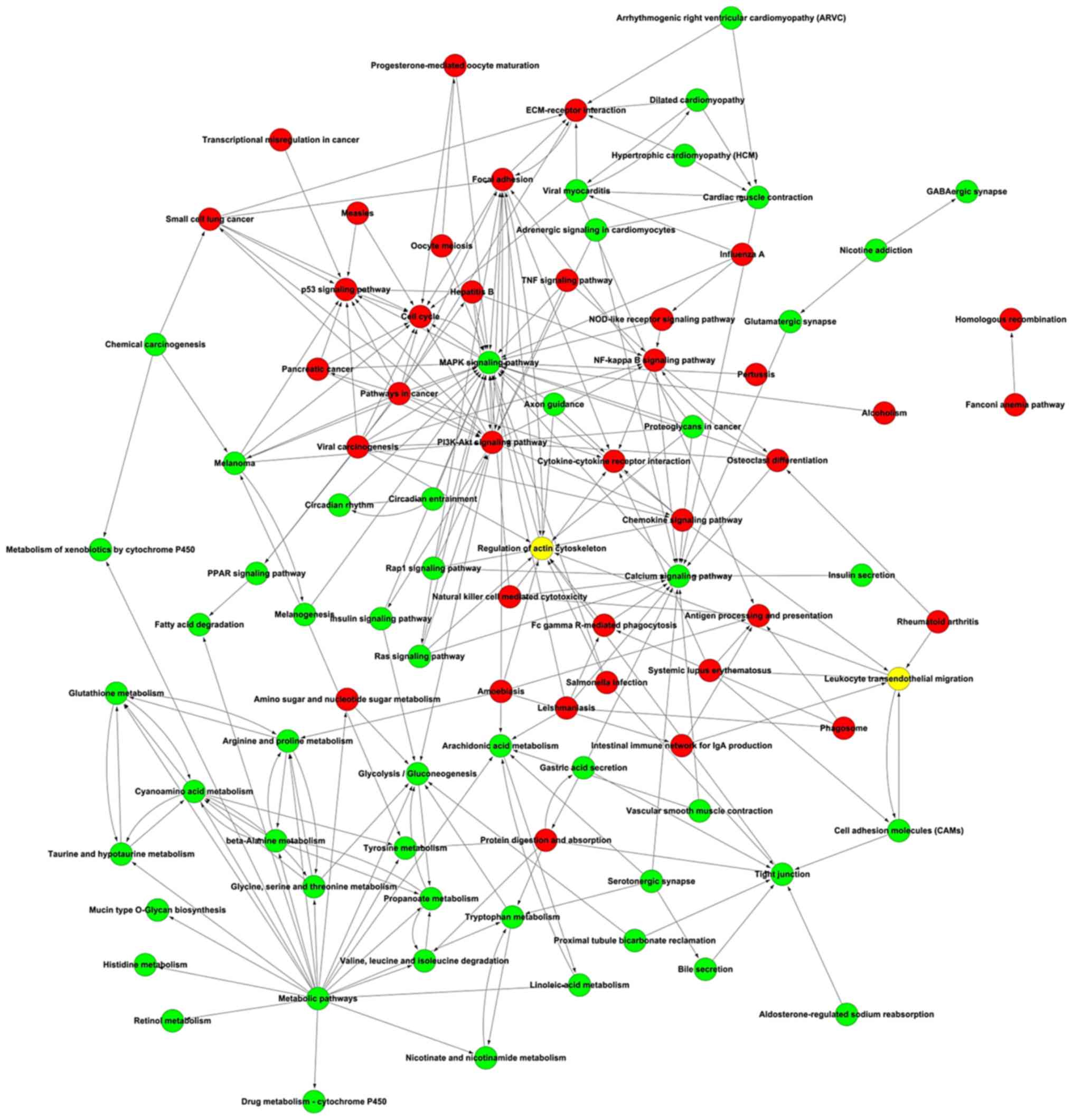
Signaling pathway network
Potential interactions among the differentially
expressed pathways were depicted in the pathway network, including
mitogen-activated protein kinase signaling, PI3K-Akt signaling,
cancer, calcium signaling and focal adhesion pathways (Fig. 8).
Discussion
Tongue cancer is a prevalent malignant disease that
is difficult to diagnose in its early stages and is characterized
by high rates of metastasis and postoperative recurrence (4). Hence, the molecular mechanisms
implicated in tongue cancer must be elucidated to improve the
clinical outcomes of affected patients. In the complicated
pathological network of tumorigenesis, abnormal upregulation or
downregulation of a single gene cannot adequately illustrate
complex transcriptome changes during tumor development.
Consequently, GO and KEGG pathway annotations were used in the
present study to analyze the transcriptome data of tongue
cancer.
GO analysis uncovered significantly enriched terms
associated with ECM organization (upregulated), cell adhesion
(downregulated) and collagen catabolic processes (upregulated).
KEGG analysis demonstrated that these differentially expressed
genes were mainly enriched in the focal adhesion pathway
(upregulated), ECM-receptor interaction pathway (upregulated),
PI3K-Akt pathway (upregulated) and CAMs (downregulated). RT-qPCR
was used to verify the sequencing results.
The occurrence, progression and metastasis of tongue
cancer are closely linked to the ECM (14). Proliferation and fibrosis of
connective tissues have been reported to accompany tumorigenesis
and progression of solid tumors (15,16).
Upregulated type I collagen, fibronectin (FN1) and other ECM
proteins in breast cancer (17,18)
and upregulated collagens, non-collagen glycoproteins and
proteoglycans in hepatocellular carcinoma (19) suggest that the expression and
component changes in the ECM are crucial indicators of tumor
progression. Our RNA-Seq results revealed decreased elastin
assembly and increased collagen degradation in tongue cancer
tissues. Current evidence has demonstrated that fibrous tissue
hyperplasia is a protective response of the body, which may be used
as a prognostic indicator (20).
Abnormal deposition and increased ECM rigidity are apparent in
fibrotic and malignant cancer tissues (21). Neovascularization is a hallmark of
cancer. The collagen (COL) gene family encodes collagen in the ECM,
which can participate in inducing angiogenesis in cancer (22–24).
Sequencing and qPCR results demonstrated that COL4A1 and COL4A6
were significantly upregulated in tongue cancer, suggesting that
these genes may play important roles in the occurrence and
development of tongue cancer. Matrix metalloproteinases (MMPs) are
important enzymes in the ECM, which can degrade the basement
membrane and ECM and promote tumor cell invasion and metastasis
(25–27). MMP-9 and MMP-13 were found to be
significantly upregulated in tongue cancer, further confirmed this
function.
The mammalian ECM consists of ~300 proteins
(28). The ECM acts as a structural
support and infiltration mediator for tissues and organs, and as a
cellular signal mediator that regulates cell phenotypes by
transmitting signals via membrane surface receptors. A relevant
study on hepatocellular carcinoma demonstrated that prostaglandin
E2 (PGE2) activates prostaglandin EP3 receptor (PTGER3) in the
mesenchymal cells surrounding tumor cells, thereby promoting the
activation and release of vascular endothelial growth factor, MMP-2
and MMP-9; in this manner, PGE2 ultimately promotes angiogenesis
and tumor cell growth (29). In
addition, PTGER3 was found to regulate prostate cancer cell growth
by targeting androgen receptors (30). KEGG analysis revealed that several
ECM-receptor interactions were significantly upregulated and
enriched. As depicted in the KEGG network, the ECM-receptor
interaction pathway was directly linked to pathways in cancer,
namely the small-cell lung cancer pathway, the PI3K-Akt pathway and
CAMs in tongue cancer tissues. The interaction between the ECM and
cell membrane receptors is considered to play a key role in tongue
cancer development, suggesting that blocking such an interaction
may suppress tongue cancer development and metastasis. Chemokines
are cytokines that mobilize cells via chemotaxis. It was previously
demonstrated that CXCL10, CXCL13 and other chemokines are closely
associated with the occurrence and development of cancer (31,32).
The sequencing and PCR results in the present study confirmed that
the expression levels of CXCL10, CXCL13 and other chemokines in
tongue cancer tissues were significantly increased, suggesting
their importance in tongue cancer.
The PI3K-Akt pathway is widely distributed in
various cells and is known to regulate cellular behavior, protein
synthesis and angiogenesis (33).
Under pathological conditions, dysregulation of the PI3K-Akt
pathway may trigger cancer occurrence and progression (34). Three mechanisms are considered to be
responsible for the biological functions of the PI3K-Akt pathway in
cancer. First, the PI3K-Akt pathway suppresses apoptosis and
stimulates cell proliferation. p-Akt can suppress the mitochondrial
apoptotic pathway by downregulating caspase-9 (35). p-Akt can also inhibit cell apoptosis
and promote proliferation by activating glycogen synthase kinase
3β, FOX proteins, the MDM2 protooncogene and the transcription
factor NF-κB (36). Second, the
PI3K-Akt pathway regulates cell cycle progression. By transmitting
the mitotic signal to p70s6k, the PI3K-Akt pathway upregulates the
expression of cell cycle-related proteins and CDK4. Subsequently,
the upregulated CDK4 inhibits downregulation of p21Cip1/WAF1 and
p27Kip1, thus triggering the progression from the G1 to
the S phase (37). Finally, the
PI3K-Akt pathway stimulates tumor angiogenesis and tumor cell
migration by upregulating the hypoxia-inducible factors, nitric
oxide and cyclooxygenase 2. After activating the PI3K-Akt pathway,
downregulated E-cadherin, which is regulated by phosphorylated
glycogen synthase, inactivates intercellular adhesion molecules and
enhances the metastatic ability of tumor cells (38).
CAMs are functional molecules that mediate the
contact and binding between cells, or between cells and the ECM.
CAMs exert their biological effects via receptor-ligand binding,
and participate in physiological and pathological processes,
including cell proliferation, differentiation, movement, immune and
inflammatory responses, coagulation, and cancer cell metastasis.
Generally, CAMs comprise the integrin family, the immunoglobulin
superfamily, the selectin family, the cadherin family, and other
adhesion molecules. Epithelial-to-mesenchymal transition (EMT)
usually occurs during tumor progression and embryonic development,
leading to transformation of cells from an epithelial phenotype
with strong adhesions to a mesenchymal phenotype with invasive
ability and increased motility (39). EMT causes weakening of intercellular
connections in cadherin-based isotypes and attenuates the ability
of cells to anchor to the cytoskeleton via cadherin-catenin.
Subsequently, activated CAMs with weak adhesion or heterotypic CAMs
induce detachment of in situ tumor cells and entry into the
blood or lymphatic circulation. CAMs regulate cell-cell adhesion
and interactions between cells and the surrounding
microenvironment. Moreover, CAMs and their relevant pathways play
important roles during different stages of tumor progression
(40).
Focal adhesions (FAs) are the main mediators of the
connection between cells and the ECM. FAs induce enhanced tumor
cell motility and EMT through integrin-based signaling transition
and mechanical structural support. Upregulation of genes in the FA
pathway is closely associated with tongue cancer progression.
During malignant progression from atypical dysplasia of the oral
mucosal epithelium into oral squamous cell carcinoma, focal
adhesion kinase (FAK) is gradually upregulated. Thus, FAK may be
used as a diagnostic marker for precancerous oral epithelial
lesions (41). In FAK−/−
mice, the rate of papilloma formation decreased by 50%; once benign
tumors had formed, loss of FAK inhibited malignant progression
(42). Blocking the FAK pathway was
shown to markedly decrease cell adhesion ability and cell invasion
and motility in head and neck squamous cell carcinoma (43). FAK is upregulated in most tumors,
and its level is associated with the malignant behavior of the
tumors. FAK levels are markedly higher in malignant metastatic
tumor tissues compared with those in normal tissues or invasive
tumor tissues (44). A relevant
clinical trial reported that FAK expression levels are inversely
correlated with the survival of patients with tumors (45). Therefore, FAK may be a potential
diagnostic marker for early-stage tongue cancer, and FAK receptors
may represent promising therapeutic targets. In the present study,
FN1 was found to be highly expressed in tongue cancer tissues. In
addition, secreted phosphoprotein 1 (SPP1; also referred to as
osteopontin), is a secreted phosphorylated glycoprotein involved in
cell adhesion, proliferation, migration, inflammation, immune
response and signal transduction, and can induce new angiogenesis
(46,47). The present study demonstrated that
SPP1 was significantly upregulated in tongue cancer, suggesting
that FN1 and SPP1 play important roles in the development of tongue
cancer.
Collectively, the activity of the FAK, ECM-receptor,
PI3K-Akt and CAM pathways varies greatly during tongue cancer
occurrence and progression. The activated PI3K-Akt pathway
stimulates cell proliferation, alters the cell cycle, inhibits
apoptosis and induces angiogenesis. Phosphorylated glycogen
synthases further downregulate E-cadherin and, thus, block the CAM
pathway. Impaired CAMs result in compositional changes in the
signaling molecules in the ECM, which, in turn, cause alterations
in the relevant pathways. In addition, a unique microenvironment
affected by component and rigidity alterations of the ECM further
affect tumor cell metastasis. In summary, the progression, invasion
and metastasis of malignant tumors is a dynamic process. The
interaction between the PI3K-Akt pathway and CAMs was shown to
trigger changes in the ECM, thus further aggravating an unfavorable
microenvironment in tongue cancer.
In the present study, potential molecular mechanisms
and tumor-related pathways during tongue cancer progression were
analyzed through RNA-Seq. However, certain limitations should be
addressed. First, the small sample size may have led to individual
bias. Since transcriptome sequencing is a sensitive detection
method, our sequencing data only provided referential information.
Second, differentially expressed pathways were abundant, and some
were significant during tongue cancer progression. The critical
pathways and underlying mechanisms of tongue cancer require further
investigation.
In conclusion, transcriptomes from four tongue
cancer tissues and four paired paracancerous tissues were analyzed,
and 1,700 upregulated and 2,249 downregulated genes were
identified. These differentially expressed genes were mainly
enriched in the FA, ECM-receptor interaction, PI3K-Akt and CAM
pathways. These pathways synergistically promoted tongue cancer
occurrence and progression, and may be potential biological markers
and therapeutic targets for early-stage tongue cancer. However, due
to the small sample size and sensitivity of RNA-Seq, further
molecular biology research is required to elucidate the roles of
differentially expressed pathways in tongue cancer progression, in
order to provide a wider theoretical and experimental basis for the
clinical diagnosis, treatment and prognosis of patients with tongue
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Nantong Science
and Technology Projects (grant nos. MS22018011, GJZ17103,
MS12018059 and MS32017004).
Availability of materials and data
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MMT and LH designed the study and drafted the
manuscript. MMT, HW, WCD, XJX and BJ conducted the experiments, and
contributed to data collection and analysis. YZW, HYQ and LH
contributed to and reviewed the data analysis. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Nantong Municipal Tumor Hospital (no. 2018037).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Wade J, Smith H, Hankins M and Llewellyn
C: Conducting oral examinations for cancer in general practice:
What are the barriers? Fam Pract. 27:77–84. 2010. View Article : Google Scholar
|
|
2
|
Nemeth Z, Somogyi A, Takacsi-Nagy Z,
Barabas J, Nemeth G and Szabo G: Possibilities of preventing
osteoradionecrosis during complex therapy of tumors of the oral
cavity. Pathol Oncol Res. 6:53–58. 2000. View Article : Google Scholar
|
|
3
|
Mann J, Julie D, Mahase SS, D'Angelo D,
Potters L, Wernicke AG and Parashar B: Elective neck dissection,
but not adjuvant radiation therapy, improves survival in stage I
and II oral tongue cancer with depth of invasion >4 mm. Cureus.
11:e62882019.
|
|
4
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
5
|
Hao SP and Tsang NM: The role of
supraomohyoid neck dissection in patients of oral cavity carcinoma.
Oral Οncol. 38:309–312. 2002. View Article : Google Scholar
|
|
6
|
St John MA, Abemayor E and Wong DT: Recent
new approaches to the treatment of head and neck cancer.
Anti-cancer Drugs. 17:365–375. 2006. View Article : Google Scholar
|
|
7
|
Calabrese L, Bruschini R, Giugliano G,
Ostuni A, Maffini F, Massaro MA, Santoro L, Navach V, Preda L,
Alterio D, et al: Compartmental tongue surgery: Long term oncologic
results in the treatment of tongue cancer. Oral Oncol. 47:174–179.
2011. View Article : Google Scholar
|
|
8
|
Uma RS, Naresh KN, D'Cruz AK, Mulherkar R
and Borges AM: Metastasis of squamous cell carcinoma of the oral
tongue is associated with down-regulation of epidermal fatty acid
binding protein (E-FABP). Oral Oncol. 43:27–32. 2007. View Article : Google Scholar
|
|
9
|
Sun QL, Zhao CP, Wang TY, Hao XB, Wang XY,
Zhang X and Li YC: Expression profile analysis of long non-coding
RNA associated with vincristine resistance in colon cancer cells by
next-generation sequencing. Gene. 572:79–86. 2015. View Article : Google Scholar
|
|
10
|
Iorio MV and Croce CM: Causes and
consequences of microRNA dysregulation. Cancer J. 18:215–222. 2012.
View Article : Google Scholar :
|
|
11
|
Wang F, Ren X and Zhang X: Role of
microRNA-150 in solid tumors. Oncol Lett. 10:11–16. 2015.
View Article : Google Scholar :
|
|
12
|
Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu
H, Zhai B, Tan YX, Shan L, Liu Q, et al: Profound impact of gut
homeostasis on chemically-induced pro-tumorigenic inflammation and
hepatocarcinogenesis in rats. J Hepatol. 57:803–812. 2012.
View Article : Google Scholar
|
|
13
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar :
|
|
14
|
Rucklidge GJ, Dean V, Robins SP, Mella O
and Bjerkvig R: Immunolocalization of extracellular matrix proteins
during brain tumor invasion in BD IX rats. Cancer Res.
49:5419–5423. 1989.
|
|
15
|
Ohlund D, Elyada E and Tuveson D:
Fibroblast heterogeneity in the cancer wound. J Exp Med.
211:1503–1523. 2014. View Article : Google Scholar :
|
|
16
|
Schafer M and Werner S: Cancer as an
overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell
Biol. 9:628–638. 2008. View
Article : Google Scholar
|
|
17
|
Bergamaschi A, Tagliabue E, Sorlie T,
Naume B, Triulzi T, Orlandi R, Russnes HG, Nesland JM, Tammi R,
Auvinen P, et al: Extracellular matrix signature identifies breast
cancer subgroups with different clinical outcome. J Pathol.
214:357–367. 2008. View Article : Google Scholar
|
|
18
|
Emery LA, Tripathi A, King C, Kavanah M,
Mendez J, Stone MD, de las Morenas A, Sebastiani P and Rosenberg
CL: Early dysregulation of cell adhesion and extracellular matrix
pathways in breast cancer progression. Am J Pathol. 175:1292–1302.
2009. View Article : Google Scholar :
|
|
19
|
Lai KK, Shang S, Lohia N, Booth GC, Masse
DJ, Fausto N, Campbell JS and Beretta L: Extracellular matrix
dynamics in hepatocarcinogenesis: A comparative proteomics study of
PDGFC transgenic and pten null mouse models. PLoS Genet.
7:e10021472011. View Article : Google Scholar :
|
|
20
|
Rasmussen BB, Pedersen BV, Thorpe SM and
Rose C: Elastosis in relation to prognosis in primary breast
carcinoma. Cancer Res. 45:1428–1430. 1985.
|
|
21
|
Frantz C, Stewart KM and Weaver VM: The
extracellular matrix at a glance. J Cell Sci. 123:4195–4200. 2010.
View Article : Google Scholar :
|
|
22
|
Liu Y, Carson-Walter EB, Cooper A, Winans
BN, Johnson MD and Walter KA: Vascular gene expression patterns are
conserved in primary and metastatic brain tumors. J NeuroOncol.
99:13–24. 2010. View Article : Google Scholar :
|
|
23
|
Ameur N, Lacroix L, Roucan S, Roux V,
Broutin S, Talbot M, Dupuy C, Caillou B, Schlumberger M and Bidart
JM: Aggressive inherited and sporadic medullary thyroid carcinomas
display similar oncogenic pathways. Endocr Relat Cancer.
16:1261–1272. 2009. View Article : Google Scholar
|
|
24
|
Bianchini G, Qi Y, Alvarez RH, Iwamoto T,
Coutant C, Ibrahim NK, Valero V, Cristofanilli M, Green MC,
Radvanyi L, et al: Molecular anatomy of breast cancer stroma and
its prognostic value in estrogen receptor-positive and -negative
cancers. J Clin Oncol. 28:4316–4323. 2010. View Article : Google Scholar
|
|
25
|
Lahmann C, Young AR, Wittern KP and
Bergemann J: Induction of mRNA for matrix metalloproteinase 1 and
tissue inhibitor of metalloproteinases 1 in human skin in vivo by
solar simulated radiation. Photochem Photobiol. 73:657–663. 2001.
View Article : Google Scholar
|
|
26
|
Gomes JR, Omar NF, dos Santos Neves J,
Narvaes EA and Novaes PD: Immunolocalization and activity of the
MMP-9 and MMP-2 in odontogenic region of the rat incisor tooth
after post shortening procedure. J Mol Histol. 42:153–159. 2011.
View Article : Google Scholar
|
|
27
|
Brummer O, Bohmer G, Hollwitz B, Flemming
P, Petry KU and Kuhnle H: MMP-1 and MMP-2 in the cervix uteri in
different steps of malignant transformation-an immunohistochemical
study. Gynecol Oncol. 84:222–227. 2002. View Article : Google Scholar
|
|
28
|
Hynes RO and Naba A: Overview of the
matrisome-an inventory of extracellular matrix constituents and
functions. Cold Spring Harb Perspect Biol. 4:a0049032012.
View Article : Google Scholar :
|
|
29
|
Fang T, Hou J, He M, Wang L, Zheng M, Wang
X and Xia J: Actinidia chinensis planch root extract (acRoots)
inhibits hepatocellular carcinoma progression by inhibiting EP3
expression. Cell Biol Toxicol. 32:499–511. 2016. View Article : Google Scholar
|
|
30
|
Kashiwagi E, Shiota M, Yokomizo A, Itsumi
M, Inokuchi J, Uchiumi T and Naito S: Prostaglandin receptor EP3
mediates growth inhibitory effect of aspirin through androgen
receptor and contributes to castration resistance in prostate
cancer cells. Endocr Relat Cancer. 20:431–441. 2013. View Article : Google Scholar
|
|
31
|
Kawada K, Hosogi H, Sonoshita M, Sakashita
H, Manabe T, Shimahara Y, Sakai Y, Takabayashi A, Oshima M and
Taketo MM: Chemokine receptor CXCR3 promotes colon cancer
metastasis to lymph nodes. Oncogene. 26:4679–4688. 2007. View Article : Google Scholar
|
|
32
|
Zipin-Roitman A, Meshel T, Sagi-Assif O,
Shalmon B, Avivi C, Pfeffer RM, Witz IP and Ben-Baruch A: CXCL10
promotes invasion-related properties in human colorectal carcinoma
cells. Cancer Res. 67:3396–3405. 2007. View Article : Google Scholar
|
|
33
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar
|
|
34
|
Juric D, Krop I, Ramanathan RK, Wilson TR,
Ware JA, Sanabria Bohorquez SM, Savage HM, Sampath D, Salphati L,
Lin RS, et al: Phase I dose-escalation study of taselisib, an oral
PI3K inhibitor, in patients with advanced solid tumors. Cancer
Discov. 7:704–715. 2017. View Article : Google Scholar :
|
|
35
|
Shultz JC, Goehe RW, Wijesinghe DS,
Murudkar C, Hawkins AJ, Shay JW, Minna JD and Chalfant CE:
Alternative splicing of caspase 9 is modulated by the
phosphoinositide 3-kinase/Akt pathway via phosphorylation of
SRp30a. Cancer Res. 70:9185–9196. 2010. View Article : Google Scholar :
|
|
36
|
Jin G, Kim MJ, Jeon HS, Choi JE, Kim DS,
Lee EB, Cha SI, Yoon GS, Kim CH, Jung TH and Park JY: PTEN
mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations
in non-small cell lung cancers. Lung Cancer. 69:279–283. 2010.
View Article : Google Scholar
|
|
37
|
Yoon MK, Mitrea DM, Ou L and Kriwacki RW:
Cell cycle regulation by the intrinsically disordered proteins p21
and p27. Biochem Soc Trans. 40:981–988. 2012. View Article : Google Scholar :
|
|
38
|
Cheng JC and Leung PC: Type I collagen
down-regulates E-cadherin expression by increasing PI3KCA in cancer
cells. Cancer Lett. 304:107–116. 2011. View Article : Google Scholar
|
|
39
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar
|
|
40
|
Cavallaro U and Christofori G: Cell
adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev
Cancer. 4:118–132. 2004. View
Article : Google Scholar
|
|
41
|
Min A, Zhu C, Wang J, Peng S, Shuai C, Gao
S, Tang Z and Su T: Focal adhesion kinase knockdown in
carcinoma-associated fibroblasts inhibits oral squamous cell
carcinoma metastasis via downregulating MCP-1/CCL2 expression. J
Biochem Mol Toxicol. 29:70–76. 2015. View Article : Google Scholar
|
|
42
|
McLean GW, Komiyama NH, Serrels B, Asano
H, Reynolds L, Conti F, Hodivala-Dilke K, Metzger D, Chambon P,
Grant SG and Frame MC: Specific deletion of focal adhesion kinase
suppresses tumor formation and blocks malignant progression. Genes
Dev. 18:2998–3003. 2004. View Article : Google Scholar :
|
|
43
|
Canel M, Secades P, Garzon-Arango M,
Allonca E, Suarez C, Serrels A, Frame M, Brunton V and Chiara MD:
Involvement of focal adhesion kinase in cellular invasion of head
and neck squamous cell carcinomas via regulation of MMP-2
expression. Br J Cancer. 98:1274–1284. 2008. View Article : Google Scholar :
|
|
44
|
Gabriel B, zur Hausen A, Stickeler E,
Dietz C, Gitsch G, Fischer DC, Bouda J, Tempfer C and Hasenburg A:
Weak expression of focal adhesion kinase (pp125FAK) in patients
with cervical cancer is associated with poor disease outcome. Clin
Cancer Res. 12:2476–2483. 2006. View Article : Google Scholar
|
|
45
|
Yuan Z, Zheng Q, Fan J, Ai KX, Chen J and
Huang XY: Expression and prognostic significance of focal adhesion
kinase in hepatocellular carcinoma. J Cancer Res Clin Oncol.
136:1489–1496. 2010. View Article : Google Scholar
|
|
46
|
Ramaiah SK and Rittling S:
Pathophysiological role of osteopontin in hepatic inflammation,
toxicity, and cancer. Toxicol Sci. 103:4–13. 2008. View Article : Google Scholar
|
|
47
|
Wai PY and Kuo PC: Osteopontin: Regulation
in tumor metastasis. Cancer Metastasis Rev. 27:103–118. 2008.
View Article : Google Scholar
|