Introduction
Breast cancer is the common type of cancer in
females, and its morbidity rates have been increasing since the
1970s (1). Moreover, the health
burden of breast cancer is increasing in China, with >1.6
million individuals being diagnosed and 1.2 million mortalities
each year. Breast cancer is the most common type of cancer in
Chinese female patients; cases in China account for 12.2% of all
newly diagnosed cancers and 9.6% of all mortalities from breast
cancer worldwide (2). Furthermore,
in America, ~231,840 new cases of invasive breast cancer and 40,290
breast cancer mortalities were estimated to occur among women in
2015 (3). In addition, from
2012–2016, the breast cancer incidence rate increased by ~0.3% per
year (4). Therefore, it is
important to investigate the etiological factors and the related
factors that may accelerate the progression of breast cancer.
It has been revealed that the immune system plays an
important role in carcinogenesis and cancer progression.
Chemokines, which are immune substances, can act as cancer
promoters in tumor development (5),
but also have an antitumor immune response function (6). Therefore, it is important to
investigate dual-directional regulation substances, such as
chemokines. Thymus-expressed chemokine (TECK), also known as CCL25,
is an important chemokine. Its receptor is CCR9.
TECK is mainly expressed in the thymus and small
intestine (7), however it can also
be secreted from the spleen and pancreatic stellate cells (8). Li et al (9) revealed that TECK could be secreted
from endometrial stromal cells and may mediate regulatory T-cell
differentiation. Moreover, TECK plays a role in the development of
T cells. TECK is a chemotactic factor that acts on thymocytes,
macrophages and dendritic cells, and results in the recruitment of
thymic precursors, migration of thymocytes within the thymus and
recruitment of thymic dendritic cells (10).
Previous studies revealed that TECK is associated
with survival rate (11) and
drug-resistance in breast cancer (12). But as a chemokine expressed by the
thymus, the role of TECK on carcinogenesis and progression of
breast cancer is not fully understood. Furthermore, whether breast
cancer cells can secrete TECK requires further investigation.
The aims of the present study were to investigate
the role and underlying mechanism of TECK on cell migration,
invasion, epithelial-mesenchymal transition, proliferation and
apoptosis in breast cancer. Furthermore, the effect of TECK on
carcinogenesis and progression in breast cancer was examined, which
may facilitate the development of novel therapeutic strategies for
breast cancer treatment.
Materials and methods
Cell culture and reagents
The two human breast cancer cell lines MCF-7 and
MDA-MB-231 were obtained from the American Type Culture Collection.
MCF-7 and MDA-MB-231 cells were cultured in RPMI-1640 medium
(Hyclone; GE Healthcare Life Sciences) with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) in 5% CO2 at 37°C. Both cell
lines were stimulated with TECK (PeproTech, Inc.) at 0, 25, 50, 100
ng/ml to assess the effect of TECK on the expression of
epithelial-mensenchymal transition (EMT) biomarkers, and cell
migration and invasion. LY294002 (Cell Signaling Technology, Inc.)
was used to inhibit the phosphorylation of Akt.
Immunohistochemistry
This study was approved by the Ethics Committees of
Shandong Provincial Hospital (NSFC: approval no. 2019-201). Thymus
tissues from three healthy volunteers (37, 55 and 61 years old)
were collected from Shandong University Qilu Hospital from June to
July 2019, and written informed consents were obtained prior to
sample collection. Tissues were embedded with paraffin and cut into
5 µm-thick sections. Tissue sections were dewaxed in xylene and
rehydrated in graded alcohol concentrations. Sodium citrate buffer
was used for antigen retrieval. The endogenous peroxidase activity
of tissues was blocked, and tissues were then incubated with the
primary antibody anti-TECK (1:1,000; product code ab200343)
overnight at 4°C: and the secondary antibody anti-rabbit (1:1,000;
product code ab97080; both from Abcam). DAB was used to reveal the
area targeted by the primary antibodies, and nuclei were
counterstained with hematoxylin.
Interference and enhance of gene
expression
MCF-7 and MDA-MB-231 cells were seeded
(1×105) in a 6-well plate (NEST Biotechnology) and
cultured overnight. Cells were transfected with TECK
overexpression, silencing and negative control vectors, which
contained green fluorescent protein (GFP) gene. Viral vectors
Ubi-MCS-3FLAG-CBh-gcGFP-IRES-puromycin for overexpression and
hU6-MCS-CBH-gcGFP-IRES-puromycin for silencing were used. The
sequences for TECK silencing were as follows: Small interfering RNA
(si)TECK1, 3′-GCUCCUGGAUGCUCGAAAUTT-5′; siTECK2,
3′-CCAAGUUUAGCAAUCCCAUTT-5′; and siTECK3,
3′-CCUCCUGAUAUCAGCUAAUTT-5′. The sequence for CCR9 silencing was:
3′-GCUUGAAGCUGUCGUCUAUTT-5′. Culture medium was changed after 12 h,
and then puromycin was added into the culture medium in order to
select cells transfected with vectors. The transfection efficiency
was assessed by fluorescence microscope, reverse
transcription-quantitative PCR (RT-qPCR) and western blotting.
Western blotting
Cells were lysed by RIPA buffer (Beyotime Institute
of Biotechnology) with protease inhibitor cocktail (20X),
ultrasonication was performed and then cells were centrifuged at
12,000 × g at 4°C for 30 min. The supernatant was collected and the
concentration of protein was determined using the BCA Protein Assay
kit (Thermo Fisher Scientific), and then, loading buffer (5X) was
added. The sample was separated with 10% SDS-PAGE gel (40 µg
protein per lane) by electrophoresis. Then, proteins were
electrotransferred onto a PVDF membrane (Invitrogen; Thermo Fisher
Scientific, Inc.). The membrane was blocked with 5% BSA (Beijing
Solarbio Science & Technology Co., Ltd.), and incubated at 4°C
overnight with the primary antibodies: Akt (1:1,000; product no.
4691), and phosphorylated (p)-Akt (1:1,000; product no. 4060; Cell
Signaling Technology, Inc.), TECK (1:250; product code ab400343;
Abcam), E-cadherin (1:1,000; product no. 3195), vimentin (1:1,000;
product no. 5741), Snail (1:250; product no. 3879), β-actin
(1:1,000; product no. 4970), cleaved caspase-3 (1:500; product no.
9664) and Bax (1:500; product no. 5203; all from Cell Signaling
Technology, Inc.). Membranes were washed with TBST three times (10
min per time), incubated with horseradish peroxidase-conjugated
Rabbit secondary antibodies (1:10,000; cat. no. ZB-2301; Zhongshan
Golden Bridge Biotechnology; OriGene Technologies), and then washed
thrice with TBST. Protein bands were visualized with an
electrochemiluminescence reagent (Advansta, Inc.) and the software
ImageJ (version 1.48; National Institutes of Health) was used for
densitometry.
RT-qPCR
Total RNA was isolated using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. cDNA was reverse transcribed from the
mRNA using Revert Aid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.) following the manufacturer's protocols.
mRNAs of GAPDH and TECK, and the EMT biomarkers E-cadherin,
vimentin and Slug were detected by RT-qPCR using Maxima SYBR Green
qPCR Master mix (Thermo Fisher Scientific, Inc.). Data were
obtained and analyzed using the Mx4000 Multiplex qPCR System
(Stratagene; Agilent Technologies, Inc.) equipped with version 3.0
software. RT-qPCR reaction conditions were as follows: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles at 95°C for
5 sec and 60°C for 34 sec. The 2−ΔΔCq method was
utilized for the quantification of gene expression, with GAPDH as
an endogenous control (13). The
primers used were as follows: E-cadherin forward,
5′-CTGATGCTGATGCCCCCAATA-3′ and reverse,
5′-CAGTTTCTGCATCTTGCCAGG-3′; Snail forward,
5′-AGGCAGCTATTTCAGCCTCC-3′ and reverse, 5′-CACATCGGTCAGACCAGAGC-3′;
TECK forward, 5′-TTTGAAGACTGCTGCCTGG-3′ and reverse,
5′-GTCTTCTTCCTAACAAGCC-3′; and GAPDH forward,
5′-GCCGCATCTTCTTTTGCGTCGC-3′ and reverse,
5′-TCCCGTTCTCAGCCTTGACGG-3′.
Wound-healing assay
Human breast cells were cultured in 6-well dishes
with RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences) with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.). When the cells
grew to >90% confluency, a scratch was performed with a 200 µl
pipette tip to make a linear wound in the central area. Then cells
were washed with PBS, and migrated cells were counted under a
phase-contrast microscope (magnification ×100) after 24 and 48
h.
Matrigel invasion assay
Matrigel (BD Biosciences) was thawed at 4°C
overnight and diluted in RPMI-1640 cell culture media. Then, 60 µl
diluted Matrigel was added into the upper chamber of a 24-well
Transwell plate (Corning, Inc.) and incubated at 37°C for 30 min.
Cells were seeded (5×104) on the Matrigel in the upper
chamber with DMEM, which was supplemented with 1% FBS, and the
lower chamber was coated with RPMI-1640 medium with 10% FBS.
Non-invaded cells were removed with a cotton swab. The invaded
cells, which adhered to the lower surface of the filter, were fixed
with methanol at room temperature for 30 min and stained with 0.1%
crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 20 min. The number of invaded cells
in five random fields (magnification ×100) on the underside of the
filter was counted under a light microscope (14).
Cell Counting Kit-8 (CCK-8) assay
Cells (5,000) with 100 µl medium were seeded in
96-well dishes and cultured for 48 h under different stimulations.
Medium in each well was replaced with 100 µl serum-free medium
containing 10 µl CCK-8 reagent and cultured for 2 h. The absorbance
was measured at 450 nm using a microplate reader.
Annexin V/PI assay
Cells were seeded (0.4 million in 6-well dishes.
After 12 h, cells were treated with the blank, 100 ng/ml TECK,
LY294002 +100 ng/ml TECK and LY294002 for 24 h. Cells were then
treated with 750 µM H2O2 for 12 h in order to
induce apoptosis. Cells were collected and stained according to the
manufacturer's protocol. The ratio of apoptotic cells was detected
with flow cytometry (BD Biosciences) and the data were analyzed
using FlowJo software (version 7.6; BD Biosciences).
Hoechest 33342 staining
Cells were treated as aforementioned in the ‘Annexin
V/PI assay’ section. Hoechest solution 33342 was purchased from
Beyotime Institute of Biotechnology. Staining solution was added
into wells to cover cells for 5 min. The staining solution was then
removed and cells were washed thrice with PBS before observation
under a fluorescence microscope. The apoptotic cells in five random
fields were counted with at a magnification of ×100.
Cell cycle detection
Cells were seeded (0.1 million) in 6-well plates.
The treatment was the same as aforementioned in the CCK-8 assay. PI
was used for DNA staining. The fluorescence signals were detected
by flow cytometry and data were processed using ModFit (Windows
Version 3.2; Verity Software House).
Nude mice xenograft model
Forty-eight female nude mice (4 weeks old) were
purchased from Model Animal Research Center of Nanjing University
and housed four animals per cage under standard conditions
(24°C±2°C, 50±10% relative humidity, 12 h light/dark cycles) and
with unlimited access to standard rodent maintenance feed (KEAO
XIELI FEED Co., Ltd.) and water. Hygienic conditions were
maintained by weekly cage changes. Animal health and behavior were
monitored every day and body weights were assessed weekly over the
course of the study. The animals were divided into 6 groups (MCF-7:
TECK-OE, NC and siTECK; MDA-MB-231: TECK-OE, NC and siTECK) with 8
animals in each group. Newly digested cells (2×106) were
injected to the oxter region of the animal. The tumors were
measured every other day and the tumor volume was estimated
according to the following formula: Volume=length ×
width2 ×0.52. All animals were sacrificed by overdose
(>120 mg/kg body weight) intraperitoneal injection of
pentobarbital after 40 days. The death was verified by loss of
spontaneous breathing. All procedures were approved by The Use
Committee for Animal Care of Shandong University.
Statistical analysis
Data from experiments are presented as the mean ±
SD. The comparisons among different groups (>2 groups) were
analyzed by one-way analysis of variance (one-way ANOVA) with a
Tukey's post hoc test, while Student's t-test was used for
comparisons between 2 groups. SPSS 22.0 (IBM Corp.) was used for
statistical analysis.
Results
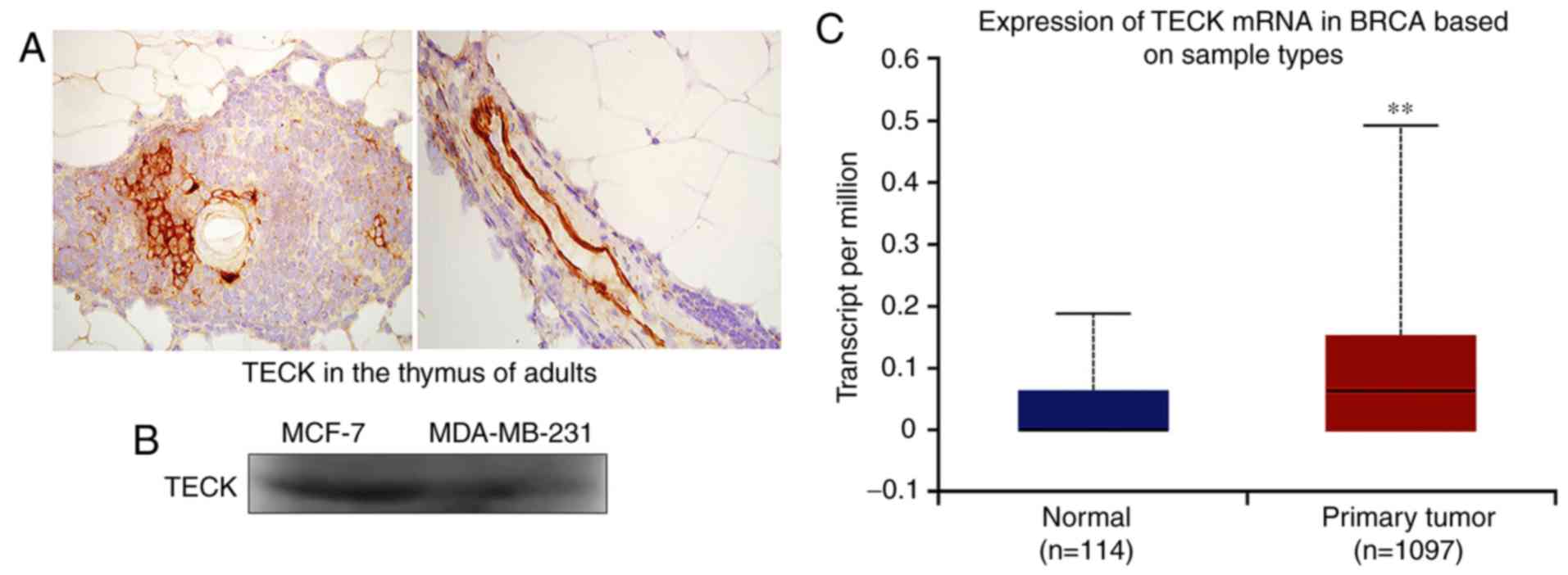
TECK is secreted by breast cancer
cells in an autocrine manner, and is highly expressed in adult
atrophic thymus tissue
Western blotting was used to detect the protein
expression of TECK in breast cancer cells, and IHC was used to
detect TECK expression in thymus tissue. It was revealed that adult
atrophic thymus tissue had high TECK expression (Fig. 1A). Furthermore, it was demonstrated
that breast cancer cells could synthesize and secrete TECK
(Fig. 1B). Based on analysis of The
Cancer Genome Atlas (TCGA) database, the present results indicated
that primary breast cancer tissues have a high TECK expression
compared with healthy breast tissues (Fig. 1C; P=0.0067).
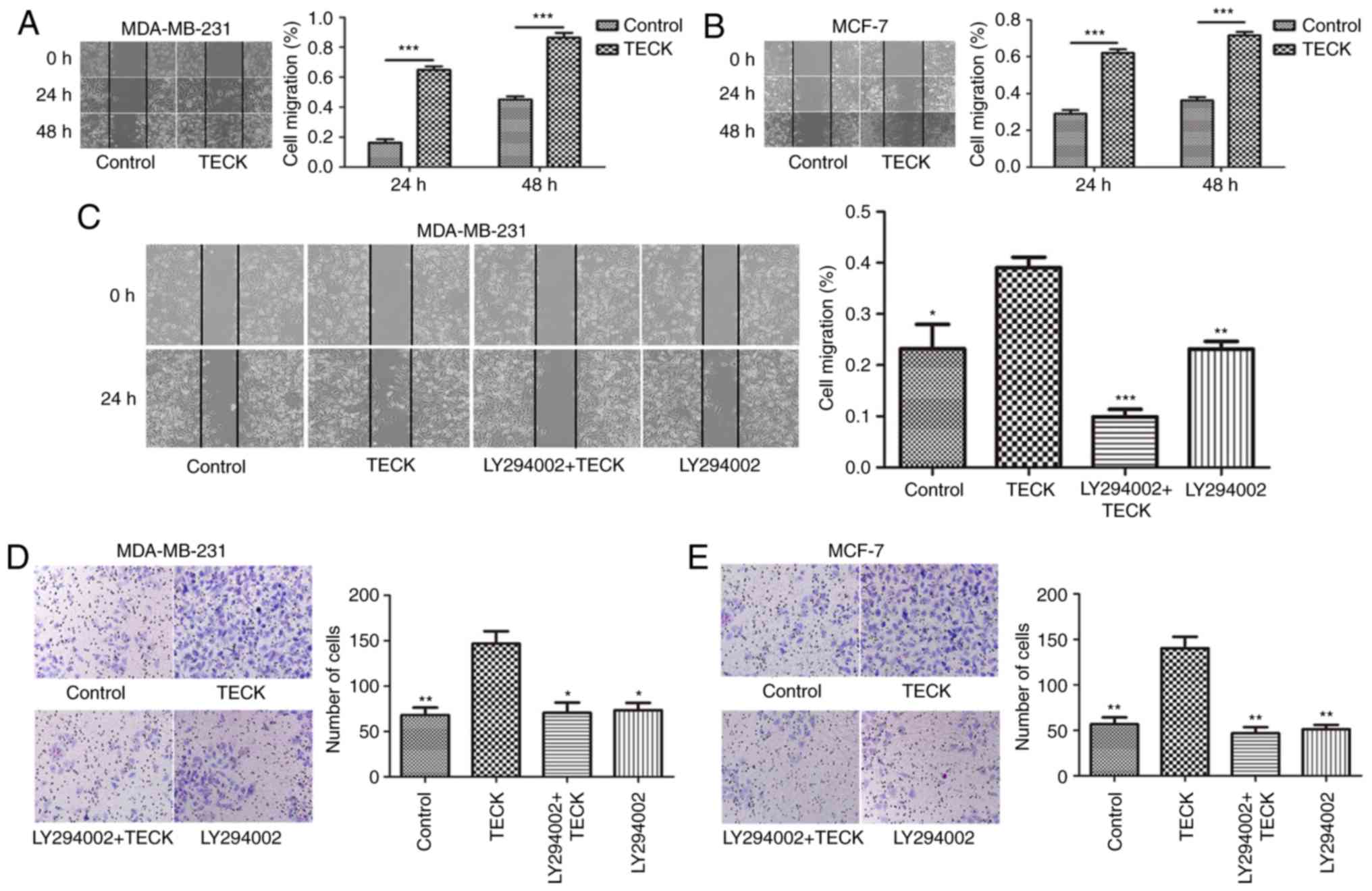
TECK promotes cell migration and
invasion via the Akt signaling pathway
The present results indicated that TECK
significantly promoted cell migration in breast cancer (Fig. 2A and B). Furthermore, the percentage
of migrated cells in the TECK treatment group was increased
compared with the control group, and the effect could be suppressed
by blocking the phosphorylation of Akt using LY294002 (TECK group
vs. LY294002+TECK group) (Fig. 2C).
Thus, TECK may promote cell migration via the Akt signaling pathway
in breast cancer. The present results also indicated that TECK
treatment promoted cell invasion compared to the control group, and
similarly, this effect could also be suppressed by blocking the Akt
signaling pathway (TECK group vs. LY294002+TECK group) (Fig. 2D and E).
TECK promotes the EMT process in a
dose-dependent manner
To investigate whether TECK could affect the process
of EMT in breast cancer, MCF-7 and MBA-MD-231 cells were treated
with TECK (0, 25, 50 and 100 ng/ml) for 48 h. Then, the expression
levels of the EMT biomarkers E-cadherin, vimentin and Snail were
detected by western blotting. It was revealed that there was a
dose-dependent relationship between TECK treatment and the EMT
process, as, EMT was enhanced as the dose of TECK increased.
Moreover, this effect was demonstrated by the decreased protein
expression of E-cadherin, and the increased expression levels of
vimentin and Snail (Fig. 3A and
B).
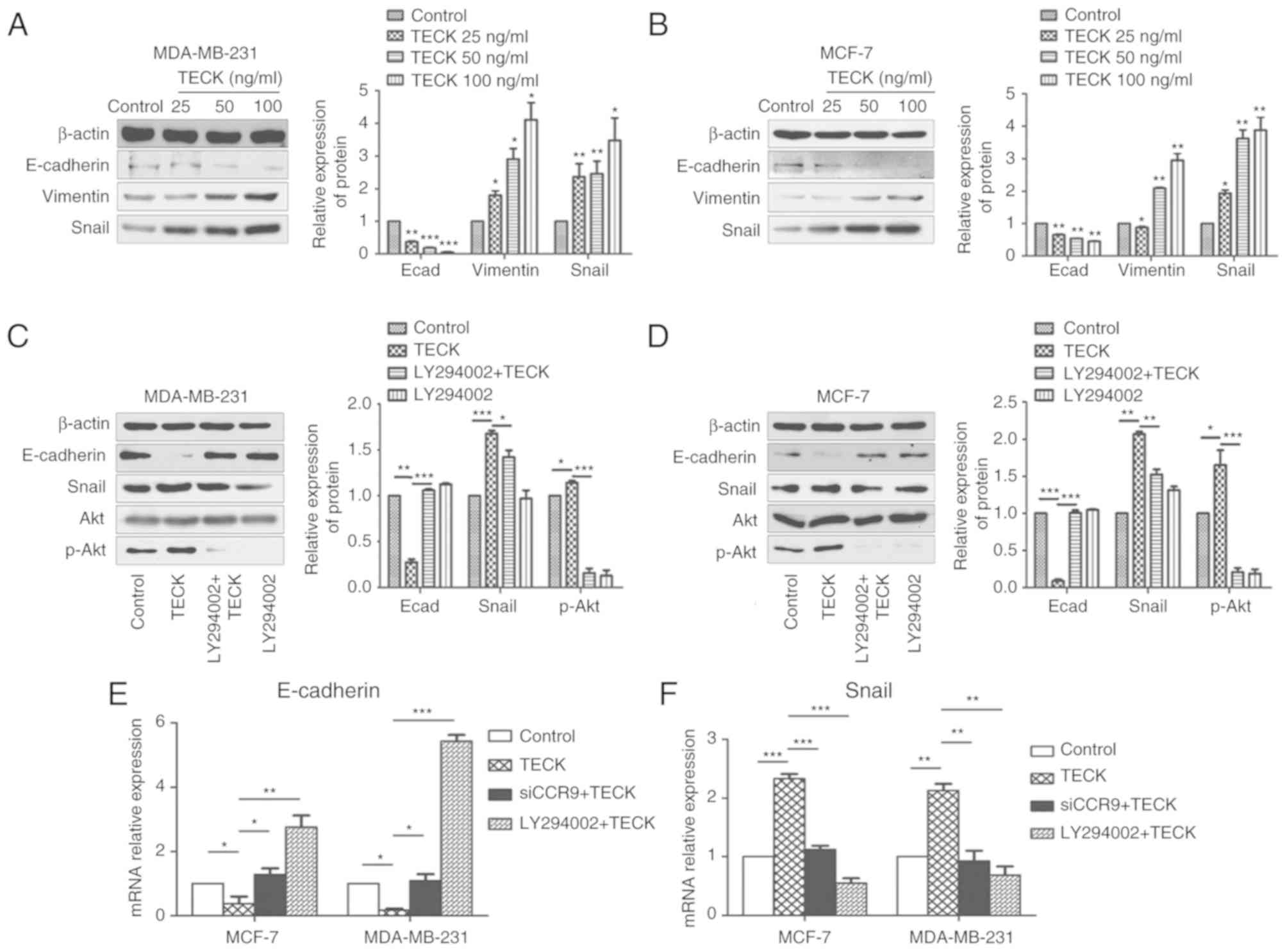 | Figure 3.TECK promotes EMT mediated by the Akt
signaling pathway in a dose-dependent manner in breast cancer
cells. (A) MDA-MB-231 and (B) MCF-7 were treated with TECK 0, 25,
50 and 100 ng/ml, and then the expression levels of E-cadherin,
vimentin and Snail were detected by western blotting. ImageJ was
used for densitometry. *P<0.05, **P<0.01, ***P<0.001
compared with the control group. (C) MDA-MB-231 and (D) MCF-7 cells
were treated with the Akt inhibitor LY294002 before adding TECK.
The expression levels of E-cadherin, Snail, Akt and p-Akt were
detected by western blotting after 24-h 100 ng/ml TECK stimulation.
(E and F) Cells were treated with the Akt inhibitor LY294002 or
CCR9 siRNA before adding TECK. The expression levels of E-cadherin
and Snail were detected by RT-qPCR after 24 h 100 ng/ml TECK
stimulation. *P<0.05, **P<0.01, ***P<0.001. TECK,
thymus-expressed chemokine; EMT, epithelial-mesenchymal
transition. |
TECK promotes the EMT process via the
Akt pathway
In order to further examine the possible signal
molecule underlying the effect of TECK on EMT, the Akt
phosphorylation inhibitor LY294002 was used to block signal
transduction. Moreover, the biomarkers of EMT Akt and p-Akt were
detected by western blotting. It was revealed that 100 ng/ml TECK
treatment could significantly increase the level of EMT compared to
the control group, and the promotion could be suppressed by Akt
phosphorylation inhibitor LY294002. Therefore, the present results
indicated that TECK promoted the EMT process via the Akt signaling
pathway (Fig. 3C and D).
Furthermore, similar results were obtained from the RT-qPCR
results, which detected the mRNA expression levels of EMT markers.
As revealed in Fig. 3E and F, EMT
was promoted by TECK treatment (TECK group vs. control group),
which could be suppressed by CCR9 interference (siCCR9+TECK group
vs. TECK group) or Akt phosphorylation inhibition (LY294002+TECK
group vs. TECK group).
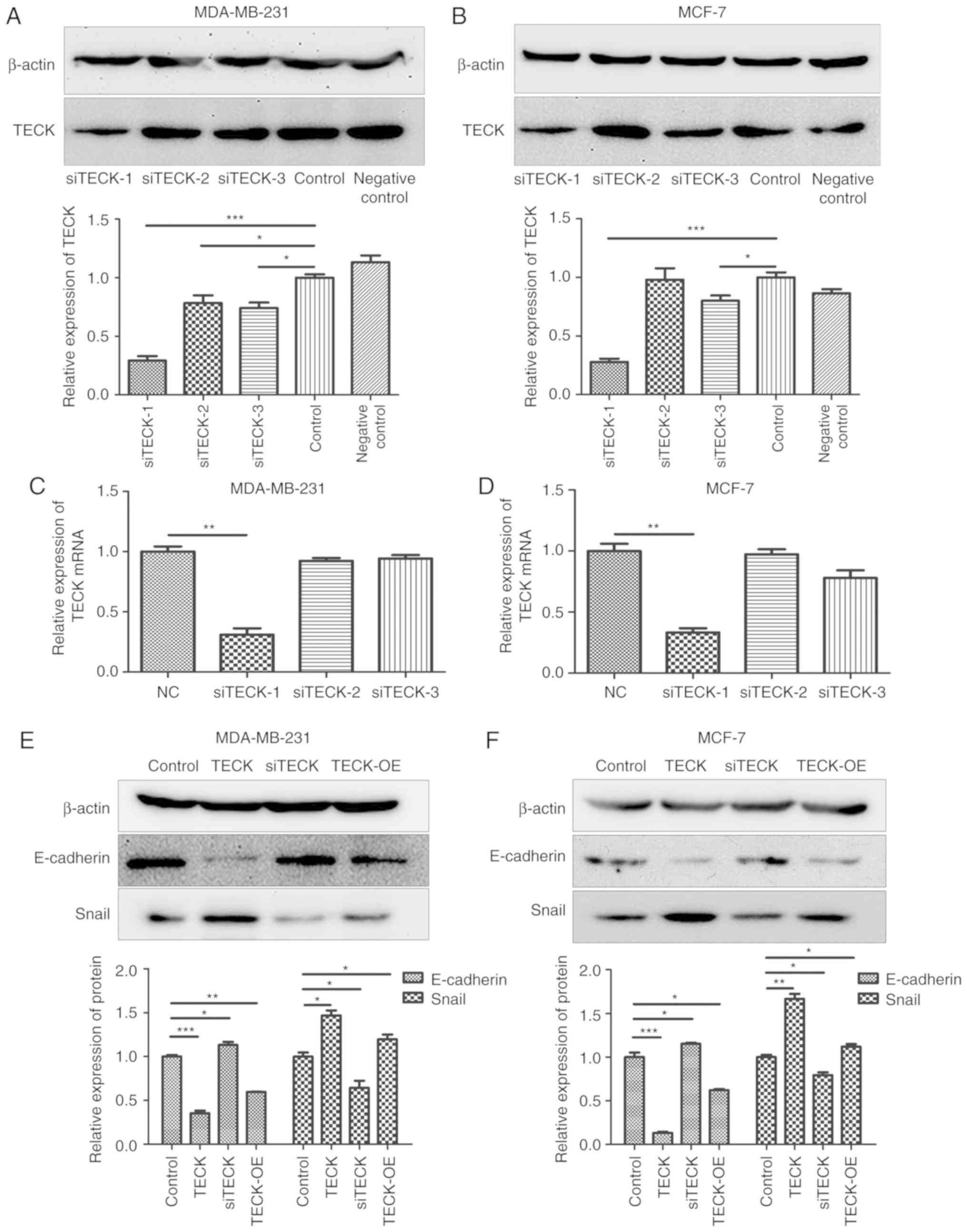
Overexpressing or inhibiting TECK
expression can promote or inhibit EMT
The transfection efficiency of three different
siRNAs was detected using western blotting, and it was revealed
that siTECK-1 was the most effective at silencing TECK expression
(Fig. 4A and B). Furthermore, the
effect of siTECK was detected at the mRNA expression level
(Fig. 4C and D) and similar effects
were observed, thus siTECK-1 was used in follow-up experiments. The
present results indicated that TECK expression, exogenous TECK or
overexpressing TECK, contributed to increased EMT, marked with
E-cadherin decrease and Snail increase compared to the control,
while silenced TECK expression revealed a decrease of the EMT level
(Fig. 4E and F).
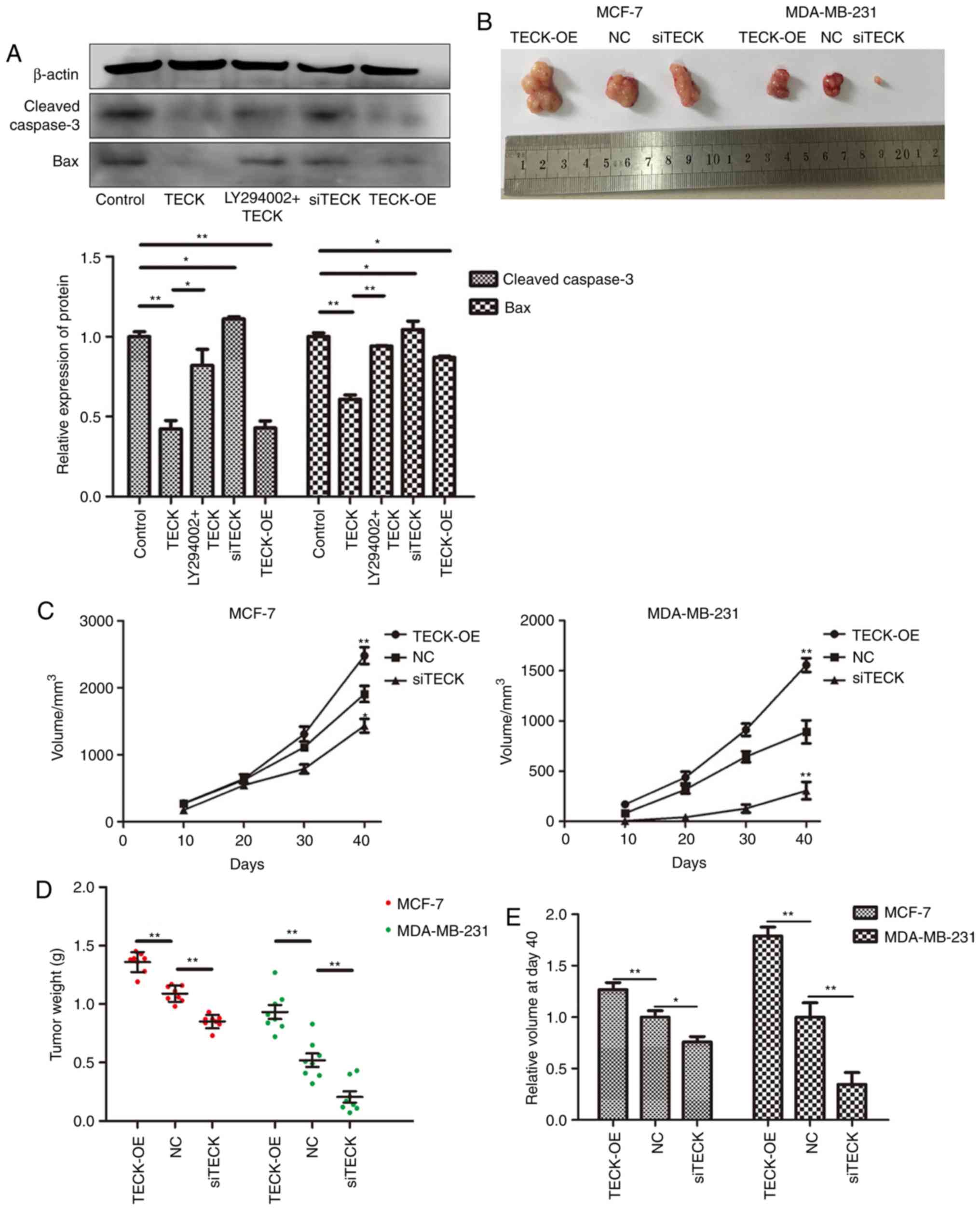
TECK promotes cell growth by
decreasing apoptosis instead of enhancing proliferation
During cell culture, it was revealed that TECK
treatment contributed to increased cell proliferation. Moreover,
the CCK-8 results (Fig. 5A and B)
indicated that cell proliferation was increased compared to the
control. Therefore, the present study examined the effect of TECK
on cell proliferation and apoptosis in breast cancer. Cell cycle
detection (Fig. 5C and D) results
revealed that there was no difference in the cell cycle in the
absence or presence of TECK. However, Hoechst 33342 staining
(Fig. 5E) and AnnexinV/PI assay
(Fig. 5F) results indicated that
TECK could decrease the ratio of apoptotic cells induced by
H2O2, and this change was disrupted by
LY294002. Moreover, apoptosis-related protein markers were detected
by western blotting, and similar results were obtained (Fig. 6A).
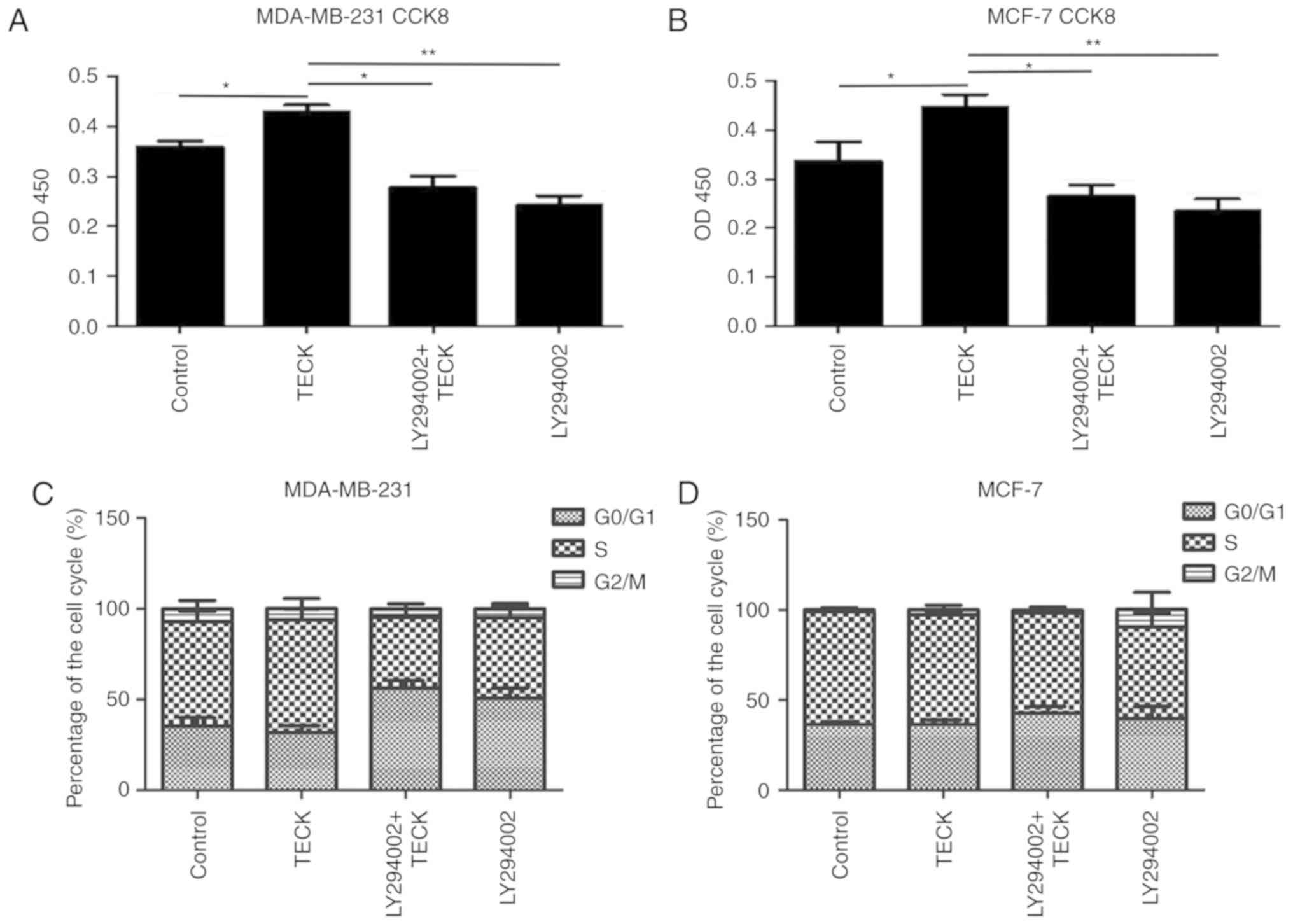 | Figure 5.TECK stimulation increases the number
of cells by inhibiting apoptosis, instead of influencing the cell
cycle. (A) MDA-MB-231 and (B) MCF-7 cells were treated with/without
LY294002 before 48 h TECK stimulation. The OD450 was measured to
determine the number of cells. *P<0.05, **P<0.01. (C and D)
Cells were treated with/without LY294002 before 48 h TECK
stimulation, and stained with PI. Fluorescence was measured by flow
cytometry and data were processed with ModFit. (E) MDA-MB-231 and
MCF-7 cells were treated with blank, 100 ng/ml TECK, LY294002+100
ng/ml TECK and LY294002 for 24 h, and then treated with 750 µM
H2O2 to induce apoptosis. After 12 h, the
cells were stained with Hoechst33342. *P<0.05, **P<0.01,
***P<0.001. (F) Cells were treated with/without LY294002 before
48 h TECK stimulation. Then, cells were stained using an Annexin
V/PI kit and detected with flow cytometry. *P<0.05. TECK,
thymus-expressed chemokine. |
TECK expression affects the growth of
breast cancer in vivo
For the tumor-bearing model experiment, there was no
mice found dead over the course of the study and it was revealed
that after 40 days the TECK overexpression xenograft volume was the
largest, while the silenced TECK xenografts were the smallest
(Fig. 6B and C). Furthermore, the
same trend was identified with regards to the tumor weight
(Fig. 6D; P<0.05). From the
result of the relative tumor volume (Fig. 6E), it was observed that TECK was
more effective in the MDA-MB-231 cell line than the MCF-7 cell
line.
Discussion
EMT is an important biological process in
tumorigenesis and cancer progression, and during this process
epithelial cells gain mesenchymal phenotypes (15), which is characterized by the
reduction of an epithelial marker and increased mesenchymal
markers. In the EMT process, epithelial cells lose cell polarity
and cell-cell adhesion, and also acquire migratory and invasive
properties similar to mesenchymal stem cells (16). EMT can contribute to many cellular
activities to enhance survival and improve the metastasis capacity
of cancer cells. Moreover, previous studies have reported that EMT
is associated with breast cancer stem cell development and
multi-drug resistance (17), which
are the primary reasons for failure of cancer treatment. Therefore,
EMT is a target for clinical oncology therapy (18). The present results indicated that
TECK could promote the EMT process in a dose-dependent manner. The
relationship between the capacity of the migration and invasion and
EMT was also investigated, and it was revealed that TECK affected
cell migration and invasion. Moreover, TECK could promote the EMT
level in breast cancer and increase cell migration and
invasion.
TECK has been widely studied in relation to the
immune system and immune cells (19–22),
but its effect in cancer cells requires further investigation.
Heinrich et al (4) reported
that pancreatic stellate cells can secrete TECK to activate
pancreatic cancer cells. Furthermore, it has been revealed that
serum TECK expression is elevated in patients with non-small cell
lung cancer (23). However, it is
not fully understood whether TECK can be secreted by cancer cells,
and the role of TECK in cancer development remains unknown.
To the best of our knowledge, the present study is
the first to demonstrate that breast cancer cells could secrete
TECK in an autocrine manner, and that this secretion can promote
breast cancer cell development. Thus, breast cancer cells may be
activated by autocrine release of TECK.
As an endogenous immune substance, TECK can be
secreted from thymus tissues, and a small amount from the small
intestine and endometrial stromal cells. The results of IHC also
demonstrated that an adult thymus secretes large amounts of TECK.
The limitation of the present study is that a negative control was
not set in the IHC experiments although we all know that thymus
tissues can secrete TECK. TECK is important for immune cell
development, but can also promote carcinogenesis and progression in
cancers (24). However, breast
cancer cells can secrete TECK themselves, and TECK expression may
be associated with survival rate (11). Therefore, it is important to
investigate the effect of TECK in breast cancer.
The present results indicated that TECK could play a
role in breast cancer progression, the EMT process, and cell
migration, invasion and apoptosis, which were mediated by the Akt
signaling pathway. Thymus tissues are replaced by adipose tissues
in adults, but thymus tissues can still secrete abundant levels of
TECK. Furthermore, TECK can be transported to the cancer site via
the blood flow, which acts as a stimulation factor to breast cancer
cells.
Metastasis and drug-resistance are the main reasons
for the failure of treatment for breast cancer. A previous study
revealed that TECK was associated with breast cancer metastasis and
drug-resistance (12), and it could
also regulate immune cell functions around the cancer cells. Common
metastatic sites include the lymph nodes, lung, bone and brain,
however small intestine and thymus metastases are often identified
in patients with breast cancer, with a reported rate of 16 and 11%,
respectively (25), but the
mechanism of this is not fully understood.
Studies have shown that chemokines cause the
transfer of cancer cells to specific organs in the same way as
causing movement and displacement of leukocytes given their
chemotaxis properties (26,27). TECK, which is also a chemokine, may
have a potential role in the process of breast cancer cell
metastasis to specific organs, which is thought to be similar to
the mechanism of leukocytes chemotaxis. Thus, it is speculated that
TECK may be a key substance in the process of breast cancer cell
metastasis to the thymus and small intestine, where a high
expression level of TECK was identified, which may be related to
its strong chemotaxis ability.
During carcinogenesis and the progression of breast
cancer, the immune organ, thymus and its secretory protein TECK may
act as helpers, despite the primary function of the immune system
to attack cancer cells.
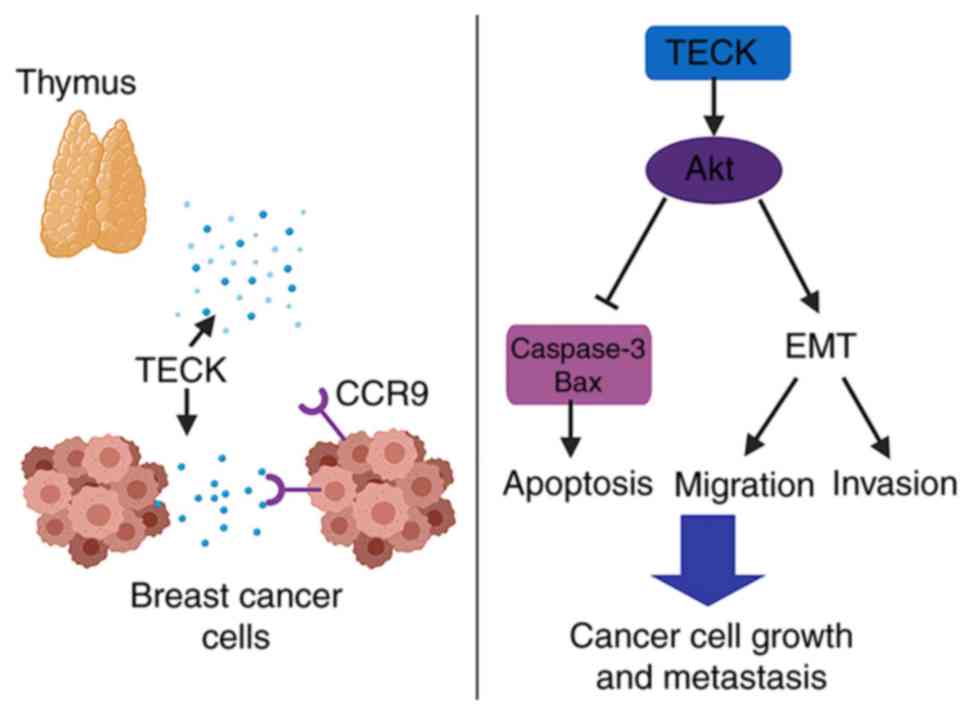
The present results revealed that TECK could
regulate several cellular functions in breast cancer cells via the
Akt signaling pathway (Fig. 7). The
present results may facilitate the development of novel therapeutic
strategies to treat breast cancer. It may be possible to develop
new drugs to block TECK secretion or function, or inhibit the
phosphorylation of Akt, which cannot only reduce the level of EMT,
but also inhibit cell migration and invasion in breast cancer.
Currently, there are few effective therapies to
treat triple-negative breast cancer (TNBC), and the present results
indicated that TECK was most effective in the TNBC cell line,
particularly in the apoptosis assay and xenograft model. Therefore,
the present results could provide a basis for effective treatments
for patients with TNBC. Moreover, inhibition of TECK may be a key
target to prevent breast cancer specific organ-oriented metastasis
to the small intestine and thymus.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC, YS and LQ performed the experiments. ZZo and HT
made substantial contributions to the concept and design of the
present study. SZ and ZZa conducted data analysis and
interpretation of the results. LC and SZ wrote the manuscript. All
authors read, revised and approved the manuscript and agreed to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Ethics committees of
Shandong Provincial Hospital (NSFC: approval no. 2019-201).
Non-retrospective ethical approval for the animal experiments
conducted in the study were obtained by the Use Committee for
Animal Care of Shandong University. In addition, it is confirmed
that the tumor burden did not exceed the recommended dimensions and
that animals were anesthetized and sacrificed using acceptable
methods/techniques. Informed consent was obtained before using
thymus tissue paraffin sections.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Fedewa SA, Goding Sauer A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marcuzzi E, Angioni R, Molon B and Calì B:
Chemokines and chemokine receptors: Orchestrating tumor
metastasization. Int J Mol Sci. 20:E962018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franciszkiewicz K, Boissonnas A, Boutet M,
Combadière C and Mami-Chouaib F: Role of chemokines and chemokine
receptors in shaping the effector phase of the antitumor immune
response. Cancer Res. 72:6325–6332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Svensson M and Agace WW: Role of
CCL25/CCR9 in immune homeostasis and disease. Expert Rev Clin
Immunol. 2:759–773. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heinrich EL, Arrington AK, Ko ME, Luu C,
Lee W, Lu J and Kim J: Paracrine activation of chemokine receptor
CCR9 enhances the invasiveness of pancreatic cancer cells. Cancer
Microenviron. 6:241–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li MQ, Wang Y, Chang KK, Meng YH, Liu LB,
Mei J, Wang Y, Wang XQ, Jin LP and Li DJ: CD4+Foxp3+ regulatory T
cell differentiation mediated by endometrial stromal cell-derived
TECK promotes the growth and invasion of endometriotic lesions.
Cell Death Dis. 5:e14362014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vicari AP, Figueroa DJ, Hedrick JA, Foster
JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon
KB and Zlotnik A: TECK: A novel CC chemokine specifically expressed
by thymic dendritic cells and potentially involved in T cell
development. Immunity. 7:291–301. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas JK, Mir H, Kapur N, Bae S and Singh
S: CC chemokines are differentially expressed in breast cancer and
are associated with disparity in overall survival. Sci Rep.
9:40142019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Liu Z, Xu Z, Wu X, Zhang D, Zhang
Z and Wei J: The role of chemokine receptor 9/chemokine ligand 25
signaling: From immune cells to cancer cells. Oncol Lett.
16:2071–2077. 2018.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Liu R, Zhu W, Chu H, Yu H, Wei P,
Wu X, Zhu H, Gao H, Liang J, et al: UDP-glucose accelerates SNAI1
mRNA decay and impairs lung cancer metastasis. Nature. 571:127–131.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greenburg G and Hay ED: Epithelia
suspended in collagen gels can lose polarity and express
characteristics of migrating mesenchymal cells. J Cell Biol.
95:333–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomaskovic-Crook E, Thompson EW and Thiery
JP: Epithelial to mesenchymal transition and breast cancer. Breast
Cancer Res. 11:2132009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mallini P, Lennard T, Kirby J and Meeson
A: Epithelial-to-mesenchymal transition: What is the impact on
breast cancer stem cells and drug resistance. Cancer Treat Rev.
40:341–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Santamaria PG, Moreno-Bueno G, Portillo F
and Cano A: EMT: Present and future in clinical oncology. Mol
Oncol. 11:718–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou C, Wu J, Borillo J, Torres L, McMahon
J and Lou YH: Potential roles of a special CD8 alpha alpha + cell
population and CC chemokine thymus-expressed chemokine in ovulation
related inflammation. J Immunol. 182:596–603. 2019. View Article : Google Scholar
|
|
20
|
Meurens F, Whale J, Brownlie R, Dybvig T,
Thompson DR and Gerdts V: Expression of mucosal chemokines
TECK/CCL25 and MEC/CCL28 during fetal development of the ovine
mucosal immune system. Immunology. 120:544–555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zabel BA, Agace WW, Campbell JJ, Heath HM,
Parent D, Roberts AI, Ebert EC, Kassam N, Qin S, Zovko M, et al:
Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is
selectively expressed on intestinal homing T lymphocytes, mucosal
lymphocytes, and thymocytes and is required for thymus-expressed
chemokine-mediated chemotaxis. J Exp Med. 190:1241–1256. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kunkel EJ, Campbell JJ, Haraldsen G, Pan
J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB,
Genovese MC, et al: Lymphocyte CC chemokine receptor 9 and
epithelial thymus-expressed chemokine (TECK) expression distinguish
the small intestinal immune compartment: Epithelial expression of
tissue-specific chemokines as an organizing principle in regional
immunity. J Exp Med. 192:761–768. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gupta P, Sharma PK, Mir H, Singh R, Singh
N, Kloecker GH, Lillard JW Jr and Singh S: CCR9/TECK expression in
non-small cell lung cancer correlates with aggressive disease and
mediates key steps of metastasis. Oncotarget. 30:10170–10179.
2014.
|
|
24
|
Tu Z, Xiao R, Xiong J, Tembo KM, Deng X,
Xiong M, Liu P, Wang M and Zhang Q: CCR9 in cancer: Oncogenic role
and therapeutic targeting. J Hematol Oncol. 16:102016. View Article : Google Scholar
|
|
25
|
Cifuentes N and Pickren JW: Metastases
from carcinoma of mammary gland: An autopsy study. J Surg Oncol.
11:193–205. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rezaeeyan H, Shirzad R, McKee TD and Saki
N: Role of chemokines in metastatic niche: New insights along with
a diagnostic and prognostic approach. APMIS. 126:359–370. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ben-Baruch A: Organ selectivity in
metastasis: Regulation by chemokines and their receptors. Clin Exp
Metas. 25:345–356. 2008. View Article : Google Scholar
|