Introduction
Worldwide each year, approximately 500,000 women are
diagnosed with cervical cancer, and more than 300,000 deaths are
recorded from this malignancy (1).
In most cases, persistent infection with high-risk human
papillomavirus (HPV) is reported to be the cause of the cervical
carcinoma (2). Approximately 90% of
new cases and deaths from cervical cancer occur in low- and
middle-income countries, where mortality rates are 18 times higher
than developed countries (1,3).
Traditional treatments for cervical cancer involve radical
hysterectomy, chemotherapy, radiotherapy, or a combination of both.
Currently, nearly 70% of cancer patients will undergo radiotherapy,
which is considered to be an effective treatment modality for
locally advanced cervical cancer (4).
During conventional radiotherapy, most of the X-ray
energy or γ-irradiation is deposited in normal tissue before
reaching tumors, therefore, it may not be possible to deliver
sufficient radiation target doses using single-field irradiation
(5). As an advancement of current
radiotherapy approaches, 12C6+ radiotherapy
provides a promising therapeutic option for various malignancies
resistant to conventional photon radiotherapy. The unique factors
that improve the killing effects on tumor cells when compared to
X-ray or γ-irradiation include: i) superior physical dose
distribution as a result of the spread-out Bragg peak effect
(6), enabling radiation to
intensively target cancer cells while sparing surrounding tissues
(7,8); ii) increased relative biological
effectiveness (9); iii) lower
oxygen enhancement ratios and almost constant radio-sensitivity
within the cell cycle (10,11), thereby inducing more cell cycle- and
oxygenation-independent, irreparable DNA damage, and killing more
radio-resistant tumor cells than photon beams (12). A large body of evidence has revealed
that 12C6+ radiation exhibits strong
cell-killing effects, approximately 2–3 times greater than photon
techniques (13,14). Ever since the first clinical
experience in 1977, approximately 20,000 patients worldwide have
received 12C6+ radiotherapy, with patient
numbers growing steadily (15,16).
In spite of heavy-ion therapy alone exhibiting favorable
therapeutic effects on a variety of tumors, the approach does not
achieve a satisfactory overall 5-year survival rate for patients
with cervical cancer (17). Thus,
there is a growing interest in combined modalities for cervical
cancer, especially for molecularly targeted therapies.
The Wnt/β-catenin signaling pathway is crucial
during cell development, and is implicated in numerous biological
events, including embryogenesis, cell proliferation (18), invasion (19), apoptosis, and cellular
differentiation (20,21). During normal adult stage, the Wnt
signaling pathway is inactive or silent. However, once the body is
stimulated by inflammation, neurological disease and cancer, Wnt
signaling becomes dysregulated (22,23).
Accumulating evidence suggests that the canonical Wnt signaling
pathway is indispensable for tumorigenesis and tumor propagation
(24). Activation of Wnt signaling
contributes to tumor proliferation and metastasis, while Wnt
signaling suppression blocks cancer stemness, and induces cell
senescence (25). Previous studies
have revealed that activation of the Wnt/β-catenin signaling
pathway is implicated in a number of tumor types, such as
gastrointestinal, breast, melanoma, glioblastoma (26), colorectal, lung and liver (27,28).
Moreover, it has been reported that the aberrant activation of
canonical Wnt signaling is involved in the multistep pathogenesis
and progression of cervical cancer (29,30).
Previously, we demonstrated that Wnt signaling pathway inhibition
enhances radiosensitivity in human cervical cancer HeLa cells
(31). Thus, the Wnt/β-catenin
signaling pathway is involved in the tumorigenesis of numerous
cancers, indicating its potential value as a target for cancer
treatment.
HLY78 is a new small-molecule activator of the
Wnt/β-catenin signaling pathway, that acts in a Wnt
ligand-dependent manner. Mounting evidence suggests that HLY78
directly binds to the DAX domain of Axin, enhancing the interaction
of Axin-LRP6, which promotes LRP6 phosphorylation and Wnt signal
transduction (32–34). A previous study has revealed that
zebrafish embryo supplementation with HLY78 significantly reduced
developmental toxicity induced by carbon ion radiation alone
(34). Although the protective
effects of HLY78 on cancer cell damage have been reported, its
effects on ionizing radiation (IR)-induced damage have not been
well defined.
In the present study, a HeLa cell model was used to
explore Wnt signaling pathway functions in cervical cancer
progression. To provide a biological basis for the combined use of
the Wnt agonist with 12C6+ beam radiotherapy,
the effects of HLY78 on IR-induced damage in HeLa cells was
evaluated. These findings may provide new insights into potential
therapeutic applications relevant to the Wnt signaling pathway.
Materials and methods
Cell culture and treatments
Human cervical carcinoma cells (HeLa) were gifted by
the First Hospital of Lanzhou University, and cultured in
Dulbecco's modified Eagle's medium (DMEM), supplemented with 10%
fetal bovine serum (FBS; both from HyClone™; GE Healthcare Life
Sciences). All cells were maintained in a humidified 5%
CO2 atmosphere at 37°C.
Chemical and irradiation exposure
HeLa cells in exponential growth were cultured in
35-mm culture dishes, and incubated overnight to facilitate
adherence. The Wnt activator, HLY78 (purity ≥98%) was dissolved in
dimethyl sulfoxide (DMSO; both from Sigma-Aldrich; Merck KGaA).
Cells were pre-incubated with DMSO and HLY78 for 2 h before
12C6+ irradiation, and maintained in the
medium until the samples were collected.
Then, HeLa cells were exposed to
12C6+ beam at an energy of 80 MeV/u and
linear energy transfer (LET) of 50 keV/µm, with a dose rate of 4
Gy/min. Heavy-ion beam irradiation was performed at the Heavy Ion
Research Facility in Lanzhou (HIRFL; Institute of Modern Physics,
Chinese Academy of Sciences, Lanzhou, China). All experiments were
separately completed three times.
Cell Counting Kit-8 (CCK-8) cell
viability assay
Cells from various treatment groups (control, HLY78,
IR, IR+HLY78) were transferred to 96-well plates at a density of
3,000 cells/well, and cultured for 6, 12, 24, 48, 72 and 96 h in a
humidified 5% CO2 incubator. At the end of incubation,
20 µl CCK-8 (Dojindo Molecular Technologies, Inc.) reagent was
added to each well 2 h before assaying. Cell viability was
determined by colorimetry, with optical density at 450 nm measured
on a microplate reader (Tecan Infinite 200 M).
Clonogenic survival assay
Immediately after irradiation, cells from various
treatment groups (control, HLY78, IR, IR+HLY78) were detached and
plated in triplicate onto 60-mm culture dishes at 1,000 cells/well.
All cells were cultured for 10–14 days at 37°C in a humidified 5%
CO2 incubator. After this period, surviving colonies
were fixed in ice-cold 4% paraformaldehyde for 15 min, and then
stained with 1% crystal violet for 20 min. The number of colonies
containing at least 50 cells were scored as surviving units.
Plating efficiency (PE) was calculated as the number of colonies
formed, divided by the cell inoculation number in the control. The
surviving fraction (SF) of the irradiated group was defined as: SF
= colonies counted/(cells seeded × PE). The parameters of the
survival curve were determined using a linear-quadratic
equation.
Cell apoptosis assay
Following incubation for 24 h at 37°C, various
treatment group of cells (control, HLY78, IR, IR+HLY78) were
detached and washed twice in chilled PBS, diluted to 100 µl in 1X
binding buffer, then mixed with 5 µl FITC Annexin V and 5 µl
propidium iodide (PI) (Annexin V-FITC Apoptosis Detection Kit I; BD
Biosciences). Samples were incubated in the dark for 15 min at
25°C, after which flow cytometry (Amnis FlowSight; Luminex
Corporation) was performed to quantify cell apoptosis. A minimum of
10,000 cells were counted in each sample. IDEAS Application v6.0
(Merck Millipore) software was used to identify apoptotic
cells.
Cell cycle assay
After incubation for 24 h at 37°C, various treatment
group of cells (control, HLY78, IR, IR+HLY78) were detached and
fixed overnight in ice-cold 70% ethanol at −20°C. Then, the cells
were centrifuged at 1,000 rpm for 4 min, and the supernatant was
removed. To the pellet, 2–5 ml PBS was added to rehydrate the cells
for 15 min. Finally, the cells were gently resuspended in 75 µl DNA
staining solution (MultiSciences Biotech Co., Ltd.) for 30 min, and
maintained in the dark at room temperature (RT) until analysis.
Data were processed using FlowJo-V10 software (FlowJo, LLC) with at
least 10,000 cells examined per sample.
Immunofluorescence
Phosphorylated histone H2AX (γ-H2AX) and cytosolic
and nuclear β-catenin expression in HeLa cells was detected using
immunofluorescence. After irradiation for 4 h, cells were exposed
to various treatments (control, HLY78, IR, IR+HLY78) and fixed
overnight at 4°C in 4% paraformaldehyde. The following day, the
cells were permeabilized with 0.5% Triton X-100 for 5 min, and
rinsed three times in PBS, followed by blocking in 10% bovine serum
albumin (BSA) in TBS with 0.1% Tween-20 (TBST) for 1 h at RT.
Subsequently, the cells were incubated with rabbit anti-γ-H2AX
(1:200; ab11174) (Abcam), or anti-β-catenin (1:300; GTX26302)
(GeneTex, Inc.) overnight at 4°C, and then the cells were incubated
with a 1:1,000 dilution of FITC-labelled secondary antibody, goat
anti-rabbit IgG (H&L) (cat. no. RS3211; ImmunoWay Biotechnology
Company) for 1 h in the dark. Cells were counterstained with DAPI
(Vector Laboratories, Inc.) for nuclear staining. A Zeiss LSM-700
confocal microscope (Carl Zeiss AG) captured cell images
(magnification, ×400).
Western blotting
Following 12C6+ irradiation
for 24 h, cells (treated and untreated) were lysed in RIPA buffer
(Beijing Solarbio Science & Technology Co, Ltd.) supplemented
with protease inhibitors for 30 min on ice. Protein concentrations
were quantified using the BCATM protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Equivalent protein
lysates (30 µg) were fractionated on 15% SDS-PAGE and transferred
to PVDF membranes (EMD Millipore). To prevent non-specific binding,
the membranes were first blocked in TBST containing 5% BSA for 1 h
at RT, and then probed with primary rabbit antibodies for anti-p53
(1:1,000; cat. no. GTX50438), anti-Bax (Bcl-2-Associated X protein)
(1:500; cat. no. GTX32465), anti-Bcl-2 (B-cell lymphoma-2) (1:500;
cat. no. GTX100440), anti-Wnt5a (1:500; cat. no. GTX111187),
anti-Wnt3a (1:1000; cat. no. GTX128101) and anti-β-catenin (1:4000;
cat. no. GTX26302) (all from GeneTex, Inc.) at 4°C overnight. After
washing three times in TBST for 15 min, the corresponding
HRP-conjugated secondary antibody, goat anti-rabbit IgG (H+L)
(1:10,000; cat. no. BS13278; Bioworld Technology, Inc.) was added
to membranes and incubated for 1 h at RT. Subsequently, the protein
expression was visualized by ECL reagent (WBKLS0500; EMD
Millipore). Densitometric analysis was performed using the
AlphaView imaging software (version 3.4.0; ProteinSimple). GAPDH
(1:5,000; cat. no. GTX100118; GeneTex, Inc.) was used as a loading
control to normalize data.
Statistical analyses
All assays were repeated at least three times and
values were presented as the mean ± standard error of the mean
(SEM). Statistical analyses were performed using IBM SPSS
Statistics 20 software (IBM, Corp.). One-way analysis of variance
(ANOVA), with Tukey's post hoc tests were applied to detect
statistically significant differences between groups. Differences
with a P-value <0.05 were considered statistically significant.
Figures were plotted using Origin 8.0 (OriginLab).
Results
Determination of optimal HLY78
concentrations in HeLa cells
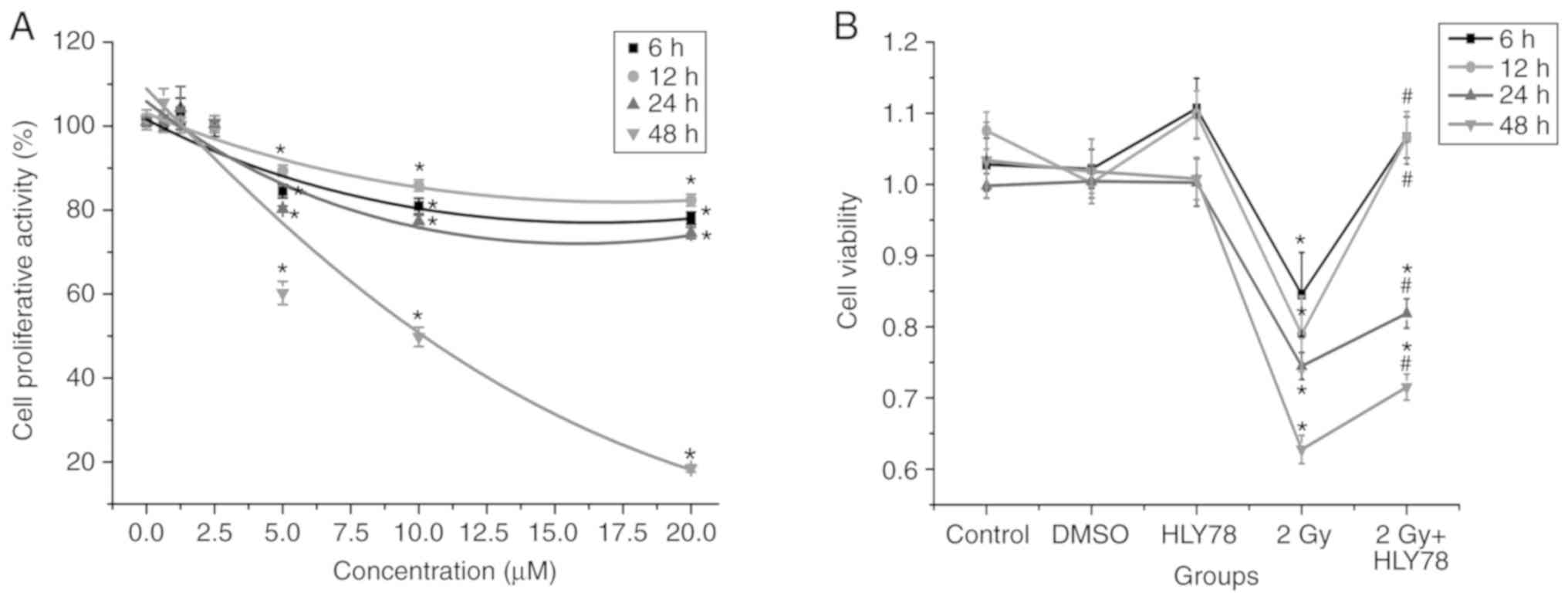
Firstly, HLY78 cytotoxicity was evaluated in HeLa
cells over the dose range, 0.625–20 µM. A CCK-8 assay indicated
that cell viability was significantly decreased between 2.5–20 µM,
and that the decrease was dose-dependent. There were no obvious
toxic effects at doses <2.5 µM in comparison with the control
cells (Fig. 1A), therefore, 2.5 µM
HLY78 was used for all subsequent experiments in cells.
Combinatorial effects of
12C6+ radiation and HLY78 on HeLa cell
viability
To investigate the effects of HLY78 on HeLa cells
exposed to high-LET 12C6+ beams, cell
viability was assessed after irradiation at 2 Gy
12C6+ beams. The present data revealed a
significant difference in cell viability among groups (P<0.05).
12C6+ radiation induced a significant
decrease in cell viability, whereas the addition of HLY78 markedly
attenuated the cellular inhibitory effects induced by irradiation
alone (P<0.05). The results (Fig.
1B) revealed that treatment with both DMSO and HLY78 alone had
no effects on cell proliferation within 48 h, when compared with
the controls (P>0.05). Cell viability was reduced by ~40.62%
(P<0.05) at 48 h after 12C6+ irradiation
when compared with the controls. For cells administered HLY78 plus
irradiation, viability was significantly increased from 62.77±1.98
to 71.53±1.83% (P<0.05) when compared with irradiation-only
cells. These data indicated that HLY78 protects HeLa cells from
cellular toxicity after 12C6+ radiation.
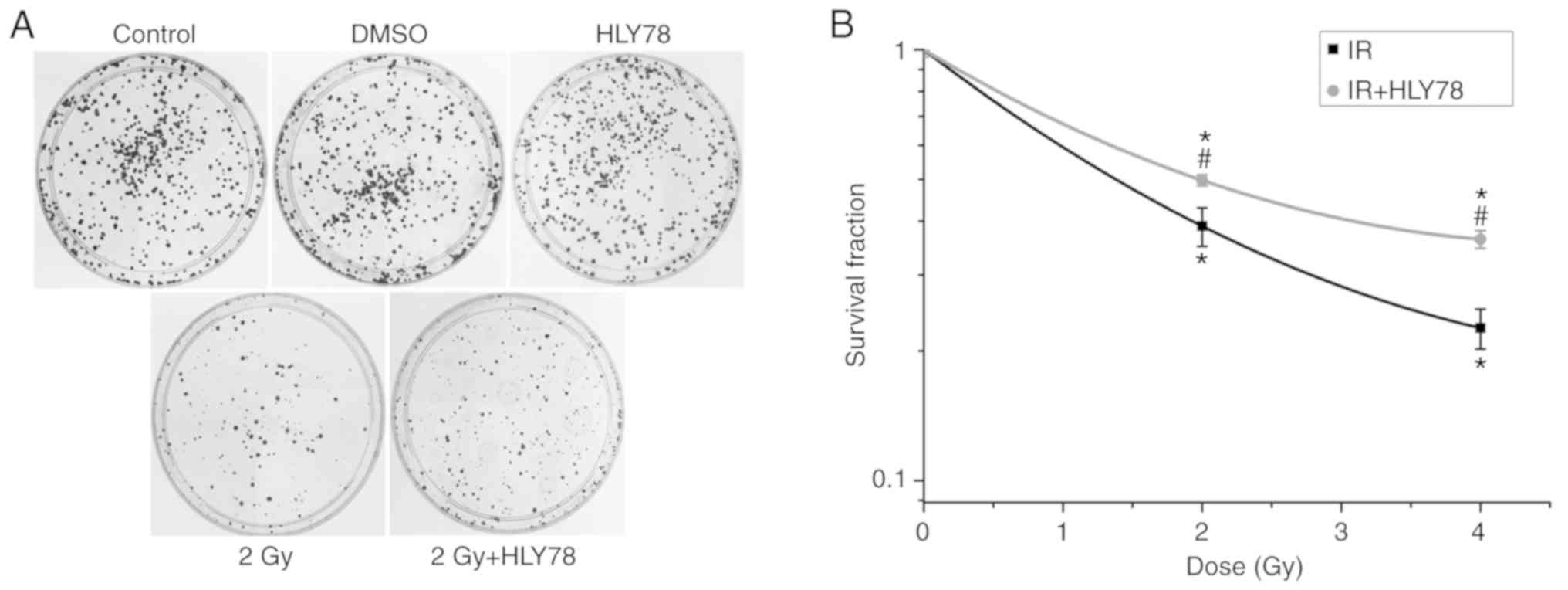
HeLa cytotoxicity at 2 Gy
12C6+ irradiation alone, and in combination
with HLY78 was evaluated by clonogenic survival assay. The results
(Fig. 2) revealed that treatment
with HLY78 alone exerted no notable effects on colony forming
efficiency. Moreover, there was a significant decrease in colonies
formed in the radiation group at 2 and 4 Gy (~2.57- and 4.42-fold,
respectively), in comparison with the control group (P<0.05).
However, when 12C6+ beam-exposed HeLa cells
were pre-treated with HLY78, colony formation efficiency was
increased when compared with the irradiation alone cells (from
38.96±4.00 to 49.78±1.45% for 2Gy+HLY78; and 22.60±4.39 to
36.32±1.72% for 4Gy+HLY78 respectively) (Fig. 2B), indicating that HLY78 decreased
radiation-induced radio-sensitivity.
Combinatorial effects of carbon ion
radiation and HLY78 on Wnt-related protein expression in HeLa
cells
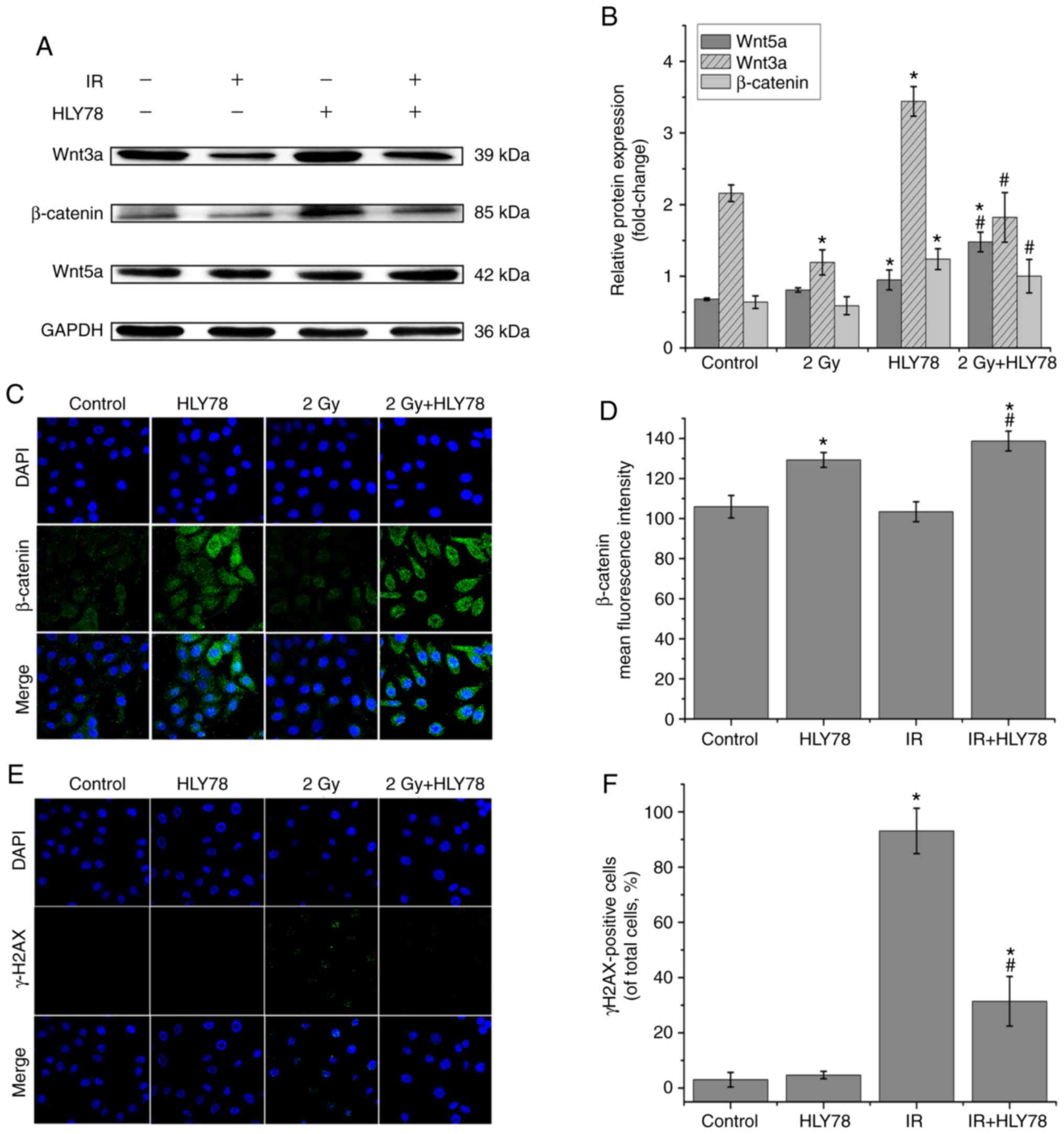
Western blotting evaluated the net effects of
combined HLY78 and 12C6+ radiation on
Wnt-related protein expression in HeLa cells. The present data
indicated that HLY78 alone induced a significant increase in
β-catenin (1.94-fold, P<0.05), Wnt3a (1.59-fold, P<0.05) and
Wnt5a (1.39-fold, P<0.05) expression, relative to the control
group. Moreover, when compared with HeLa cells treated with
12C6+ beam alone, β-catenin, Wnt3a and Wnt5a
expression levels were increased by ~1.70-, 1.53- and 1.83-fold,
respectively in HeLa cells that received combined treatment of
HLY78 after radiation (Fig. 3A and
B). Moreover, immunofluorescence staining was used to examine
the expression and location of β-catenin in HeLa cells treated with
the HLY78-irradiation combination. These data revealed that HLY78
significantly reduced the inhibitory effects of the
12C6+ beam on nuclear β-catenin protein
expression in HeLa cells (Fig. 3C and
D). Thus, HLY78 may protect HeLa cells from IR-induced damage
by promoting Wnt-related protein expression. Collectively, these
data indicated that the Wnt signaling pathway was responsible for
the radio-sensitivity of HeLa cells.
Combinatorial effects of carbon ion
radiation and HLY78 on cell cycle progression in HeLa cells
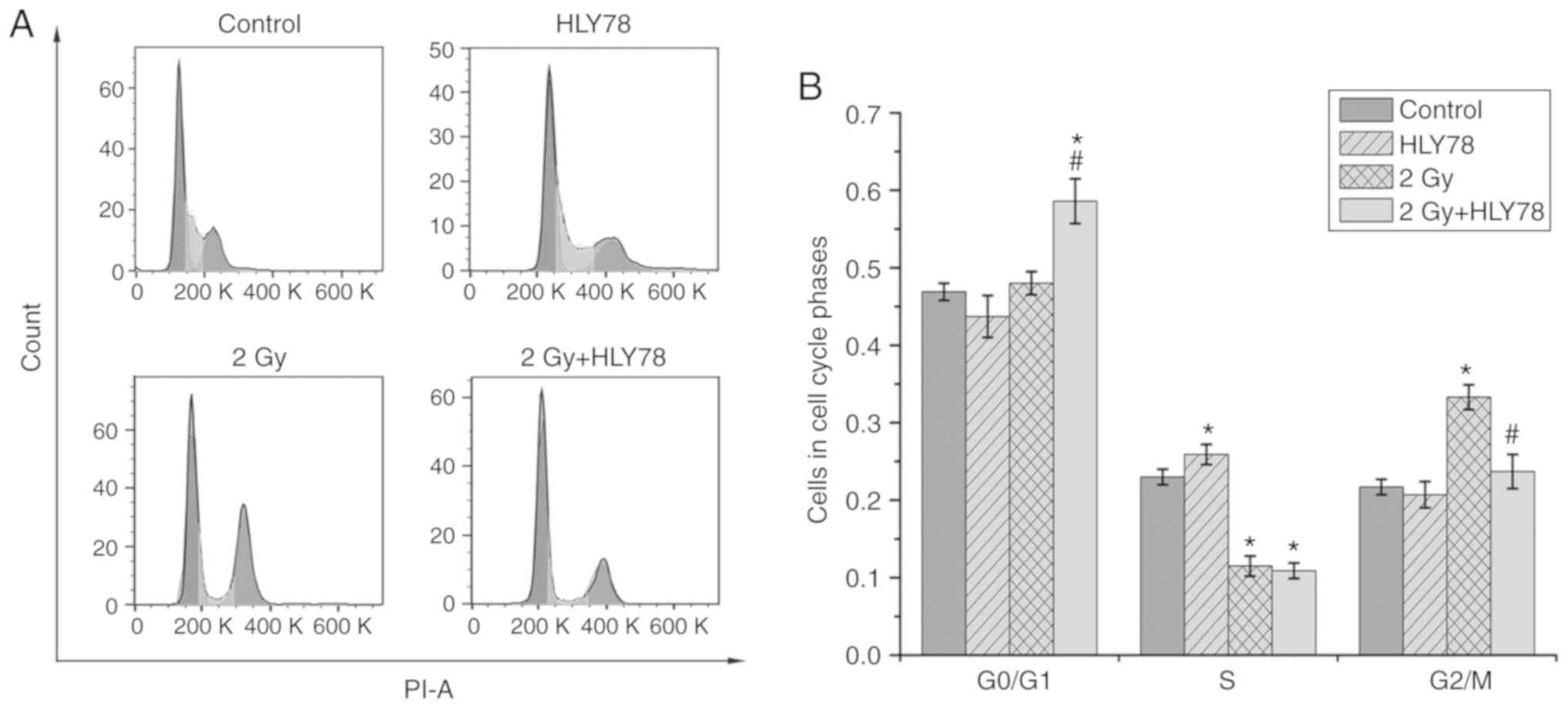
Increasing evidence suggests that alterations in
cell cycle distribution post-irradiation, are closely associated
with sensitivity in tumor cell lines (35). Hence, the effects of
12C6+ radiation alone and in combination with
HLY78 on cell cycle progression were examined by flow cytometry
after irradiation for 24 h. The results (Fig. 4) revealed that treatment with HLY78
alone without radiation, did not induce an appreciable G2/M arrest.
However, the proportion of cells in the G2/M phase increased upon
12C6+ radiation alone, when compared with the
control (33.30±1.60 vs. 21.70±1.00%, P<0.05). In addition, a
significant decrease was observed in the proportion of cells in the
G2/M phase (23.70±2.20 vs. 33.30±1.60%, P<0.05) after combined
treatment with radiation and HLY78, when compared with cells
exposed to 12C6+ radiation alone.
Combinatorial effects of carbon ion
radiation and HLY78 on DNA damage in HeLa cells
The expression of γ-H2AX expression, which is an
important biomarker of DNA double-strand breaks (DSBs), was
assessed after irradiation treatment. Immunofluorescence was used
to examine the effects of HLY78 on the accumulation of DNA damage
in irradiated HeLa cells. The results (Fig. 3E and F) revealed that the addition
of HLY78 significantly decreased γ-H2AX expression, when compared
with cells that received 12C6+ radiation
alone. These data indicated that HLY78 potentially blocked the
accumulation of unrepaired lethal DNA lesions to protect HeLa cells
from radiation-induced damage.
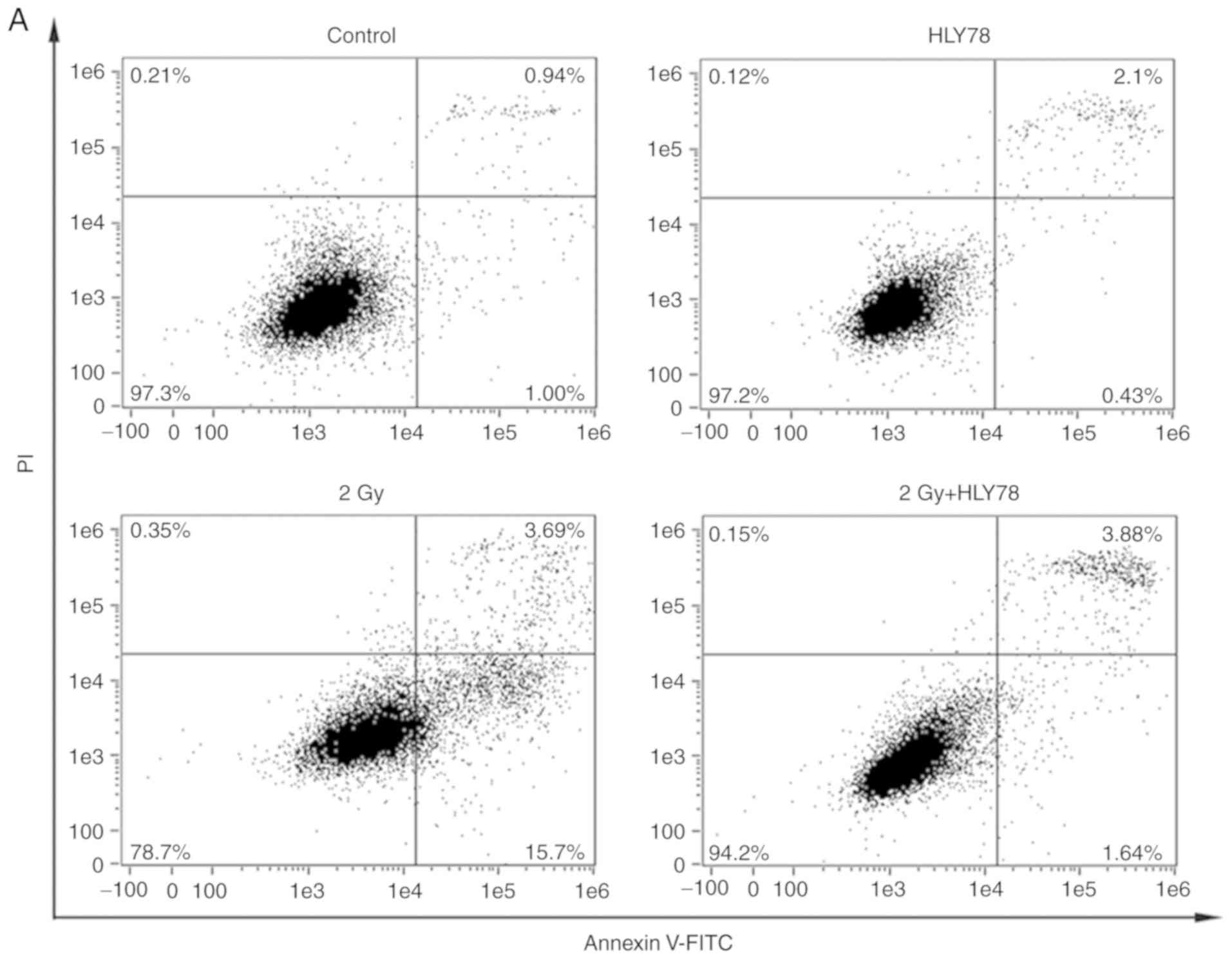
Combinatorial effects of carbon ion
radiation and HLY78 on HeLa apoptosis
Apoptotic HeLa cells were assessed using flow
cytometry, after irradiation with 2 Gy 12C6+
beam for 24 h. The results (Fig. 5A and
B) revealed that treatment with HLY78 in the absence of
12C6+ beam irradiation, exerted no
significant changes in HeLa apoptosis. However, a significant
increase was observed in apoptosis after
12C6+ irradiation, when compared with the
control (17.95±1.25 vs. 1.97±0.42%, P<0.05, respectively). When
compared to the irradiation group alone, the combined treatment,
HLY78 and radiation group generated a significant decrease in
apoptosis, and thus an advantage for HeLa cell survival (17.95±1.25
vs. 5.63±0.47%, P<0.05).
Since HLY78 administration had significant responses
in 12C6+ radiation-induced apoptosis, the
potential functional mechanisms were investigated. Induction of
apoptosis by 12C6+ radiation, with or without
HLY78, was analyzed by western blotting. As revealed in Fig. 5C and D, in cells treated with HLY78
immediately after 12C6+ irradiation, p53 and
Bax expression were significantly decreased (by ~1.40- and
1.21-fold, respectively, P<0.05), whereas Bcl-2 expression was
significantly increased (by ~1.44-fold, P<0.05), when compared
to cells receiving the 12C6+ beam alone.
These data indicated that the canonical Wnt signaling pathway
effectively regulated irradiation-induced apoptosis in HeLa
cells.
Discussion
Although 12C6+ radiotherapy
has become a promising therapeutic option for locally advanced
cervical cancer, due to its unique physical and biological effects,
the 5-year survival rate for patients is unsatisfactory. Therefore,
there is an urgent need to identify effective therapeutic targets
to improve efficacy approaches for cervical cancer. Previously,
aberrant activation of the Wnt/β-catenin signaling pathway was
implicated in the initial steps and progression of a variety of
cancers, including cervical cancer (36). HLY78, as a small-molecule activator
of the Wnt/β-catenin pathway protects cancer cells against damage;
HLY78 was revealed to exert cytoprotective effects against
resveratrol-induced cellular toxicity in breast cancer cell lines
(37). However, its effects on
12C6+ radiation-induced damage in HeLa cells
are unknown.
HLY78 was used to improve our understanding of the
Wnt/β-catenin signaling pathway in regulating irradiation-induced
toxicity in HeLa cells. A previous study indicated that the
upregulation of the Wnt pathway by HLY78, significantly attenuated
decreases in gastric cancer cell viability, induced by the
overexpression of oxysterol-binding protein-related protein 8
(ORP8) (38). Moreover, other
studies have indicated that Wnt/β-catenin pathway activation
promotes cell growth and proliferation in cervical cancer (39). Considering that HLY78 may have
protective effects on irradiated HeLa cells, HLY78-mediated
cytotoxicity in these cells was firstly evaluated using a CCK-8
assay. The present data data indicated that increasing drug
concentrations (2.5, 5, 10 and 20 µM) caused cell proliferation to
decrease in a dose-dependent manner. Similarly, there were no
significant differences in cell proliferation rates as incubation
duration increased (6, 12, 24, and 48 h). Therefore, HLY78 at 2.5
µM was used in the present study.
Continuous activation of Wnt/β-catenin signaling is
closely related to the uncontrolled, self-renewal of tumor cells
(24,40). The present study also demonstrated
that cell viability decreased after 12C6+
irradiation at a dose of 2 Gy, when compared with control cells.
Notably, this decrease in cell viability was effectively alleviated
by HLY78, indicating that HLY78 exerted protective effects against
radiation-induced damage. Similar data were observed for the
colony-formation abilities of HeLa cells treated with HLY78 and
irradiation; colony forming efficiencies were increased in
comparison with radiation alone. These observations agreed with
previous research which revealed that the aberrant activation of
the Wnt/β-catenin pathway could prevent radiation-induced damage to
salivary glands (41), and rescue
intestinal stem cells against radiation (42). Similarly, another study identified
HLY78 as having a protective role against
12C6+ radiation-induced developmental
toxicity in zebrafish (33).
Collectively, these results indicated that the Wnt signaling
pathway is implicated in 12C6+
radiation-induced cytotoxicity.
Next, the possible mechanisms behind HLY78-mediated
protective effects were explored. Increasing evidence has revealed
that HLY78 acts upstream of the β-catenin degradation complex, and
functions by stabilizing cytosolic and nuclear β-catenin in the
presence of the Wnt3a ligand (43).
β-Catenin is known as the central effector of the canonical Wnt
signaling pathway, thus when the Wnt/β-catenin pathway is
activated, β-catenin translocates to the nucleus from the cytoplasm
in coordination with the TCF/LEF complex and co-activators, to
regulate the expression of downstream target genes (39,44).
Research has revealed that high levels of β-catenin are expressed
during tumor progression in cervical cancer tissues (45), and the protein is a poor prognostic
factor of cervical carcinoma (27).
Furthermore, HLY78 protected zebrafish embryos from IR-induced
damage, by activating the Wnt/β-catenin pathway (34). In the present study, the expression
levels of Wnt-related proteins (β-catenin, Wnt3a, and Wnt5a) were
evaluated using western blotting, revealing that the expression of
these three proteins was significantly increased when HLY78 was
administered immediately after 12C6+
radiation. In our previous study, nuclear β-catenin concentrations,
after combined treatments with XAV939 (Wnt signaling pathway
inhibitor) and 12C6+ radiation, were
significantly lower than irradiation alone treatments (31). Immunofluorescence staining revealed
that β-catenin expression in the nucleus was notably increased when
HLY78 was administrated after 12C6+
radiation, in comparison to radiation alone cells. These data when
combined with western blotting results, indicated that HLY78
altered the nuclear localization of β-catenin, leading to increased
canonical Wnt signaling, and the promotion of cell survival.
Collectively, these results revealed that HLY78 promoted the
progression of cervical cancer via activation of the Wnt/β-catenin
signaling pathway. Notably, the non-canonical Wnt pathway appears
to be activated, however, the mechanism remains poorly
understood.
Evidence has revealed that
12C6+ particles produce high levels of
clustered DNA damage, including DSBs, which are implicated in cell
lethality after irradiation (46,47).
Since γ-H2AX has been demonstrated to form nuclear foci upon DSB,
and previous studies have revealed that after irradiation with
12C6+, a number of γ-H2AX foci increased at 4
h (48,49), in the present study, γ-H2AX
expression was assessed 4 h post-irradiation, and further detected
by immunofluorescence. The results indicated that after irradiation
with 12C6+, HeLa cells exhibited an increased
number of γ-H2AX foci. However, Wnt signaling activation by HLY78
alleviated this effect. This result could support our theory that
the Wnt/β-catenin pathway activator HLY78 could reduce the DNA
damage to protect HeLa cells from radiation-induced damage.
Research has revealed that prolonged cell cycle arrest occurs after
exposure to high-LET ionizing radiation (47). In addition, it is reported that
aberrant activation of Wnt signaling by HLY78 could significantly
alleviate the overexpression of ORP8-induced mitochondrial
apoptotic events in gastric cancer cells (38). In the present study, HeLa cells
irradiated with 12C6+ exhibited increased
cell apoptosis, and G2/M phase arrest at 24 h, in comparison with
control cells. The addition of HLY78 significantly intensified the
effects induced by 12C6+ radiation alone. p53
activation and the mitochondrial apoptotic pathway contribute to
IR-induced cellular damage (34).
Equally, Wnt signaling may prevent apoptosis by antagonizing p53
and mitochondrial apoptosis pathways. The present findings revealed
that the addition of HLY78 after 12C6+
radiation, suppressed the increase of p53 and Bax expression, and
the decrease of Bcl-2 expression induced by radiation alone. This
was in agreement with our previous study, which indicated that
HLY78 had a protective role against 12C6+
radiation-induced cell apoptosis (34). Thus, HLY78, as a specific Wnt
synergistic activator, exhibited protective roles against
IR-induced cytotoxicity. Overall, the Wnt/β signaling pathway may
be a promising molecular target for the treatment of cervical
cancer.
In conclusion, the association between the canonical
Wnt signaling pathway and cervical cancer was investigated. It was
observed that HLY78 promoted the growth and proliferation of
12C6+ radiated HeLa cells through the
Wnt/β-catenin pathway. These key observations revealed that the
Wnt/β-catenin signaling pathway played particularly important roles
in 12C6+ radiation-induced cytotoxicity, and
may provide fundamental clinical applications relevant to the Wnt
pathway in cancer therapeutics.
Acknowledgements
We express our thanks to the accelerator crew at the
HIRFL, Institute of Modern Physics, Chinese Academy of Sciences
(Lanzhou, China) and to all the institutions which provided
financial support.
Funding
Financial support was provided by the National Key
R&D Program of China (2018YFE0205100), the National Natural
Science Foundation of China (nos. 11675234, 11875061 and 31560254),
the Science and Technology Plan Project of Chengguan, Lanzhou
(2019RCCX0071), the Lanzhou Talent Innovation and Entrepreneurship
Project (2019-RC-76) and the Key Program of the National Natural
Science Foundation of China (U1632270).
Availability of data and materials
The datasets analysed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
Experiments were conceived and designed by JS and
HZ. Experiments were performed by JZ, LG and MG. Data were analyzed
by FW, YX, CD, CS and JY. The study was written by JZ. All authors
carefully reviewed, read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sawaya GF, Smith-McCune K and Kuppermann
M: Cervical cancer screening: More choices in 2019. JAMA.
321:2018–2019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Denny L, de Sanjose S, Mutebi M, Anderson
BO, Kim J, Jeronimo J, Herrero R, Yeates K, Ginsburg O and
Sankaranarayanan R: Interventions to close the divide for women
with breast and cervical cancer between low-income and
middle-income countries and high-income countries. Lancet.
389:861–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohamad O, Sishc BJ, Saha J, Pompos A,
Rahimi A, Story MD, Davis AJ and Kim DWN: Carbon ion radiotherapy:
A review of clinical experiences and preclinical research, with an
emphasis on DNA Damage/Repair. Cancers (Basel). 9:piiE662017.
View Article : Google Scholar
|
|
5
|
Hagiwara Y, Oike T, Niimi A, Yamauchi M,
Sato H, Limsirichaikul S, Held KD, Nakano T and Shibata A:
Clustered DNA double-strand break formation and the repair pathway
following heavy-ion irradiation. J Radiat Res. 60:69–79. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamada N, Imaoka T, Masunaga S, Ogata T,
Okayasu R, Takahashi A, Kato TA, Kobayashi Y, Ohnishi T, Ono K, et
al: Recent advances in the biology of heavy-ion cancer therapy. J
Radiat Res. 51:365–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loeffler JS and Durante M: Charged
particle therapy-optimization, challenges and future directions.
Nat Rev Clin Oncol. 10:411–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Onishi M, Okonogi N, Oike T, Yoshimoto Y,
Sato H, Suzuki Y, Kamada T and Nakano T: High linear energy
transfer carbon-ion irradiation increases the release of the immune
mediator high mobility group box 1 from human cancer cells. J
Radiat Res. 59:541–546. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsumoto Y, Furusawa Y, Uzawa A, Hirayama
R, Koike S, Ando K, Tsuboi K and Sakurai H: Antimetastatic effects
of carbon-ion beams on malignant melanomas. Radiat Res.
190:412–423. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamada T, Tsujii H, Blakely EA, Debus J,
De Neve W, Durante M, Jäkel O, Mayer R, Orecchia R, Pötter R, et
al: Carbon ion radiotherapy in Japan: An assessment of 20 years of
clinical experience. Lancet Oncol. 16:e93–e100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Hu X, Liu B, Liu Z, Liu C, Cai J,
Gao F, Cui J, Li B and Yang Y: Effects of
12C6+ heavy ion radiation on dendritic cells
function. Med Sci Monit. 24:1457–1463. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sai S, Vares G, Kim EH, Karasawa K, Wang
B, Nenoi M, Horimoto Y and Hayashi M: Carbon ion beam combined with
cisplatin effectively disrupts triple negative breast cancer
stem-like cells in vitro. Mol Cancer. 14:1662015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oike T, Niimi A, Okonogi N, Murata K,
Matsumura A, Noda SE, Kobayashi D, Iwanaga M, Tsuchida K, Kanai T,
et al: Visualization of complex DNA double-strand breaks in a tumor
treated with carbon ion radiotherapy. Sci Rep. 6:222752016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ando K and Kase Y: Biological
characteristics of carbon-ion therapy. Int J Radiat Biol.
85:715–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lazar AA, Schulte R, Faddegon B, Blakely
EA and Roach M III: Clinical trials involving carbon-ion radiation
therapy and the path forward. Cancer. 124:4467–4476. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fossati P, Matsufuji N, Kamada T and
Karger CP: Radiobiological issues in prospective carbon ion therapy
trials. Med Phys. 45:e1096–e1110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wakatsuki M, Kato S, Ohno T, Karasawa K,
Kiyohara H, Tamaki T, Ando K, Tsujii H, Nakano T, Kamada T, et al:
Clinical outcomes of carbon ion radiotherapy for locally advanced
adenocarcinoma of the uterine cervix in phase 1/2 clinical trial
(protocol 9704). Cancer. 120:1663–1669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Danieau G, Morice S, Redini F, Verrecchia
F and Royer BB: New insights about the Wnt/β-catenin signaling
pathway in primary bone tumors and their microenvironment: A
promising target to develop therapeutic strategies? Int J Mol Sci.
20(pii): E37512019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hua F, Liu S, Zhu L, Ma N, Jiang S and
Yang J: Highly expressed long non-coding RNA NNT-AS1 promotes cell
proliferation and invasion through Wnt/β-catenin signaling pathway
in cervical cancer. Biomed Pharmacother. 92:1128–1134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loh KM, van Amerongen R and Nusse R:
Generating cellular diversity and spatial form: Wnt signaling and
the evolution of multicellular animals. Dev Cell. 38:643–655. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Tian T, Kalland KH, Ke X and Qu Y:
Targeting Wnt/beta-catenin signaling for cancer immunotherapy.
Trends Pharmacol Sci. 39:648–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oren O and Smith BD: Eliminating cancer
stem cells by targeting embryonic signaling pathways. Stem Cell
Rev. 13:17–23. 2017. View Article : Google Scholar
|
|
23
|
vallée A, Lecarpentier Y and Vallée JN:
Curcumin: A therapeutic strategy in cancers by inhibiting the
canonical WNT/β-catenin pathway. J Exp Clin Cancer Res. 38:3232019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bahrami A, Hasanzadeh M, ShahidSales S,
Yousefi Z, Kadkhodayan S, Farazestanian M, Joudi Mashhad M, Gharib
M, Mahdi Hassanian S and Avan A: Clinical significance and
prognosis value of wnt signaling pathway in cervical cancer. J Cell
Biochem. 118:3028–3033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ramachandran I, Ganapathy V, Gillies E,
Fonseca I, Sureban SM, Houchen CW, Reis A and Queimado L: Wnt
inhibitory factor 1 suppresses cancer stemness and induces cellular
senescence. Cell Death Dis. 5:e12462014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghosh N, Hossain U, Mandal A and Sil PC:
The Wnt signaling pathway: A potential therapeutic target against
cancer. Ann N Y Acad Sci. 1443:54–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma S, Deng X, Yang Y, Zhang Q, Zhou T and
Liu Z: The lncRNA LINC00675 regulates cell proliferation,
migration, and invasion by affecting Wnt/β-catenin signaling in
cervical cancer. Biomed Pharmacother. 108:1686–1693. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang G, Shen T, Yi X, Zhang Z, Tang C,
Wang L, Zhou Y and Zhou W: Crosstalk between long non-coding RNAs
and Wnt/beta-catenin signalling in cancer. J Cell Mol Med.
22:2062–2070. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lan K, Zhao Y, Fan Y, Ma B, Yang S, Liu Q,
Linghu H and Wang H: Sulfiredoxin may promote cervical cancer
metastasis via Wnt/β-catenin signaling pathway. Int J Mol Sci.
18(pii): E9172017.PubMed/NCBI
|
|
30
|
Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM,
Leung TH, Wong OG, Cheung AN and Ngan HY: AMPK activators suppress
cervical cancer cell growth through inhibition of DVL3 mediated
Wnt/βand -catenin signaling activity. PLoS One. 8:e535972013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Si J, Gan M, Yan J, Chen Y, Wang
F, Xie T, Wang Y and Zhang H: Inhibition of Wnt signalling pathway
by XAV939 enhances radiosensitivity in human cervical cancer HeLa
cells. Artif Cells Nanomed Biotechnol. 48:479–487. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen D, Zhang H, Jing C, He X, Yang B, Cai
J, Zhou Y, Song X, Li L and Hao X: Efficient synthesis of new
phenanthridine Wnt/β-catenin signaling pathway agonists. Eur J Med
Chem. 157:1491–1499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen DZ, Yang BJ, He XL, Fan SR, Cai JY,
Jing CX, Zhang H, Zhang Y, Li L and Hao XJ: Design, synthesis and
structure-activity relationship optimization of phenanthridine
derivatives as new Wnt/β-catenin signalling pathway agonists.
Bioorg Chem. 84:285–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Si J, Zhou R, Zhao B, Xie Y, Gan L, Zhang
J, Wang Y, Zhou X, Ren X and Zhang H: Effects of ionizing radiation
and HLY78 on the zebrafish embryonic developmental toxicity.
Toxicology. 411:143–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mao A, Zhao Q, Zhou X, Sun C, Si J, Zhou
R, Gan L and Zhang H: MicroRNA-449a enhances radiosensitivity by
downregulation of c-Myc in prostate cancer cells. Sci Rep.
6:273462016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ayala-Calvillo E, Mojica-Vazquez LH,
Garcia-Carranca A and Gonzalez-Maya L: Wnt/β-catenin pathway
activation and silencing of the APC gene in HPVpositive human
cervical cancerderived cells. Mol Med Rep. 17:200–208.
2018.PubMed/NCBI
|
|
37
|
Venkatadri R, Iyer AKV, Kaushik V and Azad
N: A novel resveratrol-salinomycin combination sensitizes
ER-positive breast cancer cells to apoptosis. Pharmacol Rep.
69:788–797. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo X, Zhang L, Fan Y, Zhang D, Qin L,
Dong S and Li G: Oxysterol-binding protein-related protein 8
inhibits gastric cancer growth through induction of er stress,
inhibition of Wnt signaling, and activation of apoptosis. Oncol
Res. 25:799–808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J and Gao Y: CCAT-1 promotes
proliferation and inhibits apoptosis of cervical cancer cells via
the Wnt signaling pathway. Oncotarget. 8:68059–68070. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Liu B, Zhao Q, Hou T and Huang X:
Nuclear localizaiton of beta-catenin is associated with poor
survival and chemo-/radioresistance in human cervical squamous cell
cancer. Int J Clin Exp Pathol. 7:3908–3917. 2014.PubMed/NCBI
|
|
41
|
Hai B, Yang Z, Shangguan L, Zhao Y, Boyer
A and Liu F: Concurrent transient activation of Wnt/β-catenin
pathway prevents radiation damage to salivary glands. Int J Radiat
Oncol Biol Phys. 83:e109–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saha S, Aranda E, Hayakawa Y, Bhanja P,
Atay S, Brodin NP, Li J, Asfaha S, Liu L, Tailor Y, et al:
Macrophage-derived extracellular vesicle-packaged WNTs rescue
intestinal stem cells and enhance survival after radiation injury.
Nat Commun. 7:130962016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang S, Yin J, Chen D, Nie F, Song X, Fei
C, Miao H, Jing C, Ma W, Wang L, et al: Small-molecule modulation
of Wnt signaling via modulating the Axin-LRP5/6 interaction. Nat
Chem Biol. 9:579–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiao CH, Yu HZ, Guo CY, Wu ZM, Cao HY, Li
WB and Yuan JF: Long non-coding RNA TUG1 promotes the proliferation
of colorectal cancer cells through regulating Wnt/β-catenin
pathway. Oncol Lett. 16:5317–5324. 2018.PubMed/NCBI
|
|
45
|
Pereira-Suárez AL, Meraz MA, Lizano M,
Estrada-Chávez C, Hernández F, Olivera P, Pérez E, Padilla P, Yaniv
M, Thierry F and García-Carrancá A: Frequent alterations of the
β-catenin protein in cancer of the uterine cervix. Tumour Biol.
23:45–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sunada S, Hirakawa H, Fujimori A, Uesaka M
and Okayasu R: Oxygen enhancement ratio in radiation-induced
initial dsbs by an optimized flow cytometry-based Γ-H2AX analysis
in A549 human cancer cells. Radiat Res. 188:591–594. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takahashi A, Yamakawa N, Kirita T, Omori
K, Ishioka N, Furusawa Y, Mori E, Ohnishi K and Ohnishi T: DNA
damage recognition proteins localize along heavy ion induced tracks
in the cell nucleus. J Radiat Res. 49:645–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yan J, Xie Y, Zhang Q, Gan L, Wang F, Li
H, Si J and Zhang H: Dynamic recognition and repair of DNA complex
damage. J Cell Physiol. 234:13014–13020. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu X, Li P, Hirayama R, Niu Y, Liu X,
Chen W, Jin X, Zhang P, Ye F, Zhao T, et al: Genistein sensitizes
glioblastoma cells to carbon ions via inhibiting DNA-PKcs
phosphorylation and subsequently repressing NHEJ and delaying HR
repair pathways. Radiother Oncol. 129:84–94. 2018. View Article : Google Scholar : PubMed/NCBI
|