Introduction
Acute myeloid leukemia (AML) is a heterogeneous
malignant hematopoietic stem cell disease, which is commonly
characterized by an abnormal accumulation of myeloblasts (1). Chemotherapy remains the most common
treatment for AML (2). The current
standard clinical approach is known as ‘3+7 induction’, and
consists of a combination of daunorubicin (DNR), at a dosage of
60–90 mg/m2 for 3 days, and cytarabine (Ara-C), at a
dosage of 100–200 mg/m2 for 7 days (3,4).
Although most patients with AML exhibit a response to induction
chemotherapy, relapse represents the main cause of treatment
failure (5). Patients with
secondary AML have a 5-year survival rate of 5–10% (6,7).
Moreover, it has previously been demonstrated that high-dose
chemotherapy is not safe for treating elderly patients (>60
years old) due to poor drug tolerance and high toxicity (8,9). The
overall prognosis of AML remains unsatisfactory (10,11);
therefore, the development of safe and effective therapy for AML is
necessary to improve the prognosis of patients with this
malignancy.
Due to advances in disease and drug research,
numerous novel treatments have been developed to treat and manage
AML (12,13); however, few novel drugs have been
approved for the treatment of AML in the past 20 years (14). The effectiveness of some complexes,
including CPX-351 (15–17), liposome-encapsulated DNR and Ara-C,
prompted the identification of a more potent and effective hybrid.
NL-101 (also known as EDO-S101) is an alkylate, and is a novel
chemotherapeutic agent structurally consisting of the DNA
damage-inducing agent bendamustine hydrochloride and the histone
deacetylase inhibitor vorinostat (SAHA) (18). NL-101 was designed to allow the
simultaneous function of the two drugs and has been reported to
have an increased efficacy compared with the single agents
(19). It has previously been shown
that NL-101 upregulated the protein expression levels of
cleaved-poly (ADP-ribose) polymerase (PARP) and cleaved-caspase-3
in Kasumi and NB4 cells. In addition, NL-101 could increase the
survival rate in mice compared with bendamustine and SAHA in
vivo (20). However, the
application of NL-101 with other drugs needs to be further
investigated.
Anthracyclines are one of the most effective
anticancer drugs developed (21).
DNR is an anthracycline that has been broadly used for the
treatment of cancer (22). The
mechanism underlying DNR-mediated cytotoxicity has been reported to
include the induction of DNA damage, causing DNA double-strand
breaks (DSBs) and apoptosis (23,24).
The hybrid NL-101 in combination with DNR may represent a novel
strategy to improve the therapeutic effects of NL-101, thus
facilitating the development of more effective combinatorial
therapies to treat AML.
The present study aimed to investigate the potential
of the novel hybrid NL-101 to treat AML and to examine its
synergistic effect in combination with DNR. In addition, the
mechanisms underlying the effect of NL-101 in combination with DNR
were examined in vitro, and cell-mediated xenograft AML
models were used to investigate the efficacy and safety of this
combinatorial regimen in vivo.
Materials and methods
Patients
Clinical data were collected from the medical
records of patients with AML at Key Laboratory of Hematopoietic
Malignancies, Diagnosis and Treatment, The First Affiliated
Hospital, Zhejiang University College of Medicine, (Hangzhou,
China). From March 2017 to July 2018, 10 patients were included in
this study with demographic and clinical information (Table SI), including diagnosis, age,
French-American-British classification system type (25), cytogenetics and molecular mutation.
Patients with secondary AML or acute promyelocytic leukemia were
excluded. This study was approved by the Research Ethics Committee
of the First Affiliated Hospital, College of Medicine, Zhejiang
University (Hangzhou, China). Informed consent was obtained from
each patient according to institutional guidelines.
Antibodies and reagents
Antibodies against caspase-3 (cat. no. 9662),
caspase-7 (cat. no. 9492), PARP (cat. no. 9532), BAD (cat. no.
9239), BIM (cat. no. 2933), cyclin B1 (cat. no. 4135), cell
division cycle protein 2 (CDC2; cat. no. 9116), γ-H2AX (Ser139;
cat. no. 9718), phosphorylated (p)-ATR (Thr1981; cat. no. 30632),
p-ATM (Ser1981; cat. no. 5883), p-CHK1 (Ser345; cat. no. 2348),
p-CHK2 (Thr68; cat. no. 2197), GAPDH (cat. no. 5174) and β-actin
(cat. no. 4970) were purchased from Cell Signaling Technology, Inc.
ATR (cat. no. 19787–1-AP), ATM (cat. no. 27156-1-AP), CHK1 (cat.
no. 10362-1-AP) and CHK2 (cat. no. 13954-1-AP) antibodies were
purchased from ProteinTech Group, Inc. Anti-human
(h)CD45-fluorescein isothiocyanate (FITC) (cat. no. ab134199) was
obtained from Abcam. DNR was purchased from Selleck Chemicals.
NL-101 was provided from Minsheng Institute of Pharmaceutical
Research. DNR was diluted to 1-100 µM with PBS, whereas NL-101 was
diluted to 100-1,000 µM with dimethyl sulfoxide (DMSO). Aliquots of
DNR and NL-101 were stored at −20°C. Female
NOD-Prkdcscid IL2rgtm1/Bcgen (B-NSG) mice
(age, 6–8 weeks; weight, 18–22 g; Biocytogen) were used to generate
a xenograft model of AML.
Cell lines and primary cells
AML cell lines MV4-11, Molm13 and THP-1 were
provided by Professor Ravi Bhatia (City of Hope National Medical
Center, Duarte, CA, USA). HL-60 and Kasumi-1 cell lines were
purchased from American Type Culture Collection. NOMO-1, OCI-AML-2
and OCI-AML-3 cell lines were gifted by Professor Jianjun Chen
(City of Hope, Gehr Family Center for Leukemia Research, Duarte,
CA, USA). The MV4-11-luciferase (luc) cell line was gifted by
Professor Rongzhen Xu (The Second Affiliated Hospital of Zhejiang
University, Hangzhou, China). MV4-11 and Molm13 cells were cultured
in Iscove's Modified Dulbecco's Medium (Gibco; Thermo Fisher
Scientific, Inc.) and the other cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at
37°C in a humidified incubator containing 5% CO2.
Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque
(Sigma-Aldrich; Merck KGaA) density gradient centrifugation after
obtaining written informed consent from patients with AML. All
procedures were conducted in accordance with the ethical standards
of the Research Ethics Committee of the First Affiliated Hospital,
Zhejiang University College of Medicine.
Cell viability assay
This assay is also known as the cell proliferation
assay (26,27). AML cells (2×105) or
primary AML cells (1×106; PBMCs isolated from patients
with AML) were plated into 96-well plates. After treatment with
NL-101 (150, 300, 450, 600 and 750 nM in MV4-11; 250, 500, 750,
1,000 and 1,250 nM in HL-60; 100, 200, 400 and 800 nM in primary
AML cells), DNR (4, 8, 12, 16 and 20 nM in MV4-11; 8, 16, 24, 32
and 40 nM in HL-60; 4, 8, 16 and 32 nM in primary AML cells), the
combination or DMSO at 37°C for 48 h, 20 µl MTS solution (Promega
Corporation) was added. Subsequently, the plates were read at an
absorbance of 490 nm after 3–4 h incubation at 37°C. Experiments
were repeated three times.
Flow cytometric analysis
For assessment of apoptosis, cells
(2×105/ml) were treated with corresponding drugs for 48
h and then collected. Cells were resuspended in 1X binding buffer,
then co-stained with 5 µl Annexin V-FITC and 10 µl propidium iodide
(PI), obtained from an apoptosis detection kit (Multi Sciences),
for 15 min at room temperature in the dark. To analyze cell cycle
distribution, cells were treated with drugs for 24 h. After fixing
with 75% ethanol at 4°C overnight, the cells were resuspended in
buffer with 50 µg/ml PI and 100 µg/ml RNase A, obtained from a cell
cycle detection kit (Multi Sciences), for 30 min at room
temperature. To assess hCD45+ cells, the bone marrows of
AML mice were incubated with 1:25 anti-hCD45 as aforementioned. The
engrafted MV4-11 cells were analyzed using anti-hCD45 FITC-labeled
antibody (Abcam). The apoptotic cells, DNA content and
hCD45+ cells were analyzed using NovoExpress software
(version 1.2.1; ACEA Biosciences, Inc.) and FACScan flow cytometer
(BD Biosciences).
Western blot analysis
Cells (2×105/ml) were treated with
NL-101, DNR, NL-101 + DNR or DMSO for 24 or 48 h at 37°C, and were
then collected. After lysing in 1X RIPA buffer (Cell Signaling
Technology, Inc.) on ice for 30 min, cells were centrifuged at
12,000 × g for 15 min at 4°C. The supernatant was then collected
and protein concentration was determined using bicinchoninic acid
reagent. Protein samples (50 µg) were separated by 10–12% SDS-PAGE
(Thermo Fisher Scientific, Inc.) and transferred to PVDF membranes
(EMD Millipore). Subsequently, the membranes were blocked in 5%
non-fat milk for 1–1.5 h at room temperature and incubated with
primary antibodies (1:1,000) at 4°C for ≥4 h. The membranes were
then washed with TBS-Tween (0.1%) three times and were incubated
with 1:5,000 anti-rabbit or anti-mouse secondary antibodies
(1:5,000; cat. nos. 7074 and 7076; Cell Signaling Technology, Inc.)
at room temperature for 1–1.5 h. The protein bands were visualized
with SuperSignal™ West Femto Maximum Sensitivity Substrate (cat.
no. 34096; Thermo Fisher Scientific, Inc.), and images were
captured using a chemiluminescence imager (ChemiDoc XRS+; Bio-Rad
Laboratories, Inc.) and were analyzed using Image Lab software
(version 5.2.1; Bio-Rad Laboratories, Inc.).
AML mice models
All mice were raised under standard conditions (room
temperature, 22–24°C; 12-h light/dark cycle; relative humidity,
45–50%), with free access to food and water. For the MV4-11-luc
model, 1×106 MV4-11-luc cells were implanted
intravenously into the tail vein of mice. Mice were randomly sorted
into four groups (n=5/group), and were observed daily and weighed
every 3 days. The leukemic burden was estimated by non-invasive
luciferin imaging (28) every 7
days. Mice were treated with 12 mg/kg NL-101, 1.5 mg/kg DNR, the
combination or the same volume of PBS (vehicle). NL-101 was given
intravenously on days 1 and 2, and DNR was given intravenously on
days 1, 3, and 5 after AML symptoms were observed (after 7 days).
Mice were observed daily and hind limb paralysis was used as an
endpoint (28). When lower
extremities appeared paralyzed, the mice were euthanized in a
non-pre-inflated chamber. The chamber displacement rate of
CO2 was set at 20% per min. Euthanasia was confirmed by
cervical dislocation. All mice were euthanized before the study
finished. Subsequently, the bone marrows obtained from the lower
extremities of AML mice were washed three times and resuspended in
250 µl PBS. Anti-hCD45 antibody (10 µl; equivalent to 1:25) was
then added to the samples for 30 min at 4°C in the dark. The
hCD45+ cells were detected by flow cytometric analysis,
as aforementioned. Animal experiments were approved by the Animal
Experimental Ethical Inspection of the First Affiliated Hospital,
College of Medicine, Zhejiang University.
Statistical analysis
The combination index (CI) was calculated by
CalcuSyn software (version 2.1; Biosoft). GraphPad Prism software
(version 6.0; GraphPad Software, Inc.) was used to calculate
IC50. The experiments were repeated three times. The
cell viability assay was analyzed by one-way ANOVA followed by LSD
multiple comparisons test, whereas the other biological and in
vivo assays were analyzed by one-way ANOVA followed by Tukey's
test using SPSS version 19.0 (IBM Corp.).
Results
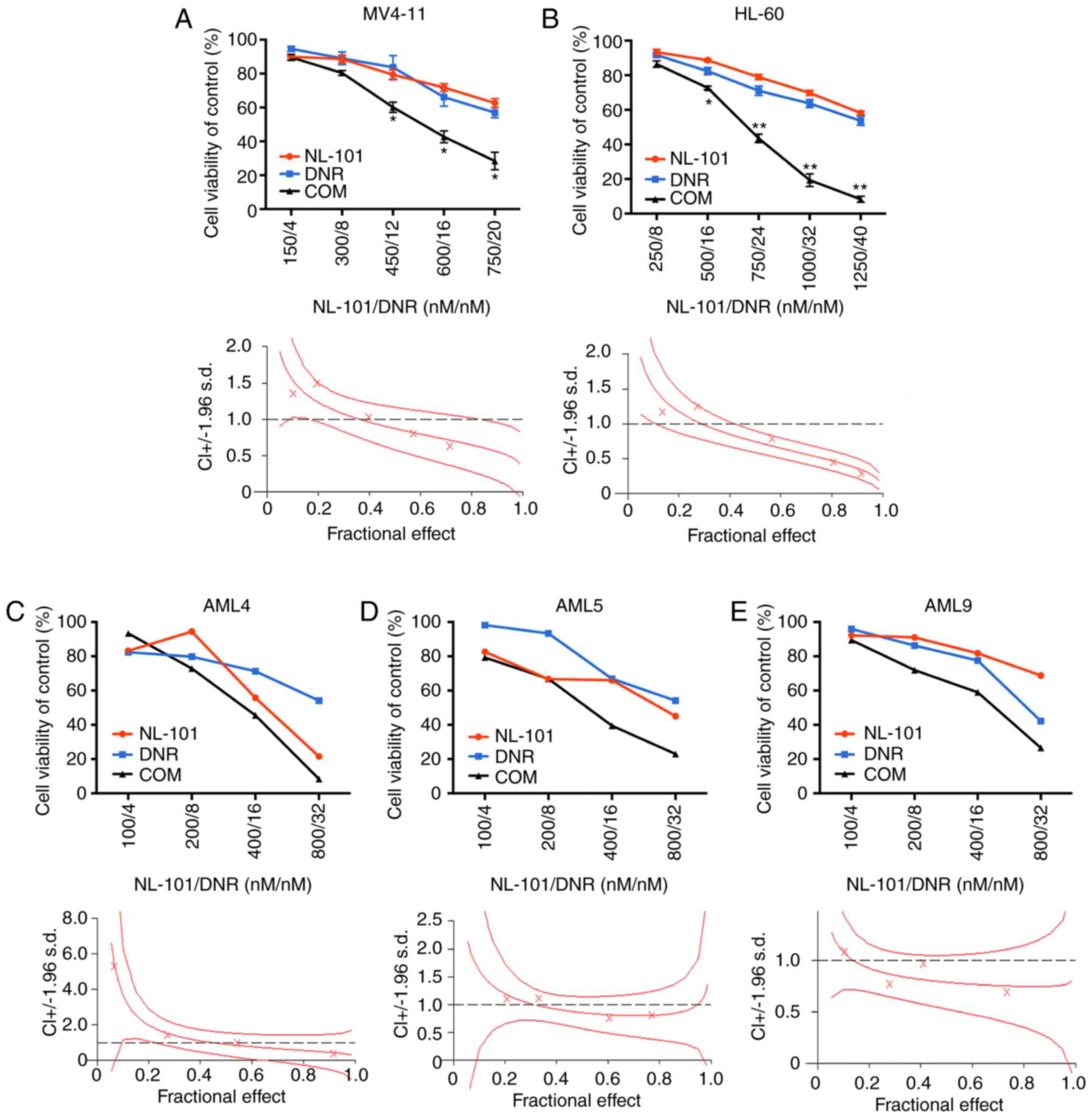
NL-101 in combination with DNR
synergistically inhibits proliferation of AML cell lines and
primary AML cells
The present study examined the inhibitory effects of
the single agent NL-101 on the proliferation of AML cells. AML
cells were plated into 96-well plates and exposed to increasing
concentrations of NL-101 (200, 400, 600, 800, 1,000, 1,500 and
2,000 nM) and equal volumes of DMSO for 48 h. The results revealed
that NL-101 inhibited the growth of AML cell lines and primary AML
cells; the IC50 values are shown in Table I. Subsequently the synergistic
effects of NL-101 and DNR were assessed on two cell lines: MV4-11
and HL-60 cells. A previous study indicated that the concentration
at which the inhibition rates of the two single agents were similar
was the most suitable as the combined concentration to calculate
the combination index (28). Thus
we confirmed the concentration of NL-101 and DNR in the combination
experiments. In the present study, NL-101 and DNR synergistically
inhibited the growth of AML cell lines (Fig. 1A and B) and primary AML cells
(Fig. 1C-E). The synergistic effect
curves were generated using CalcuSyn. CIs at the 50, 75 and 90%
effective dose are presented in Tables
II and III. The present study
demonstrated that NL-101 in combination with DNR had a synergistic
inhibitory effect on the proliferation of AML cell lines and
primary AML cells. The demographic information of the included
patients is presented in Table
SI.
 | Table I.IC50 values of NL-101 in
AML cell lines and primary AML cells. |
Table I.
IC50 values of NL-101 in
AML cell lines and primary AML cells.
| Cell type | IC50
value (nM) |
|---|
| AML cell lines |
|
Molm13 | 666.2±24.0 |
|
MV4-11 | 805.7±58.8 |
|
Kasumi-1 | 973.1±61.4 |
|
OCI-AML-3 | 941.4±105.9 |
|
OCI-AML-2 | 962.5±49.5 |
|
HL-60 | 1,124.0±68.4 |
|
NOMO-1 | 1,338.0±53.9 |
|
THP1 | 1,466.0±62.4 |
| Primary AML
cells |
|
AML1 | 281.1 |
|
AML2 | 312.5 |
|
AML3 | 324.4 |
|
AML4 | 473.2 |
|
AML5 | 528.2 |
|
AML6 | 632.0 |
|
AML7 | 661.0 |
|
AML8 | 741.4 |
|
AML9 | 1,017.0 |
|
AML10 | 1,034.0 |
 | Table II.Synergistic effect of NL-101 in
combination with DNR on MV4-11 and HL-60 cells. |
Table II.
Synergistic effect of NL-101 in
combination with DNR on MV4-11 and HL-60 cells.
|
| CI values |
|---|
|
|
|
|---|
| Cell line |
ED50 |
ED75 |
ED90 |
|---|
| MV4-11 | 0.87436 | 0.68794 | 0.55617 |
| HL-60 | 0.74289 | 0.51788 | 0.36108 |
 | Table III.Synergistic effect of NL-101 in
combination with DNR on primary AML cells. |
Table III.
Synergistic effect of NL-101 in
combination with DNR on primary AML cells.
|
| CI values |
|---|
|
|
|
|---|
|
|
ED50 |
ED75 |
ED90 |
|---|
| AML4 | 0.91606 | 0.61831 | 0.46426 |
| AML5 | 0.85689 | 0.80696 | 0.89024 |
| AML9 | 0.78682 | 0.74980 | 0.74815 |
Combination treatment with NL-101 and
DNR induces cell apoptosis and G2/M cell cycle
arrest
To explore the mechanism underlying the synergistic
effect of NL-101 and DNR, cell apoptosis and cell cycle arrest was
assessed after exposure to drugs. Cells were treated with NL-101,
DNR or the combination for 48 h. Compared with untreated cells and
single agent-treated cells, combination treatment with NL-101 and
DNR resulted in a significant increase in apoptosis (early + late)
of MV4-11 and HL-60 cells (P<0.05; Fig. 2A and B). Subsequently, the key
signaling proteins in the apoptotic pathway were analyzed by
western blot analysis. As shown in Fig.
2C, the combination treatment markedly increased the expression
of cleaved-PARP, cleaved-caspase-3 and cleaved-caspase-7 after 48
h. In addition, the expression levels of BAD and BIM were increased
by 48-h treatment with NL-101 in combination with DNR (Fig. 2D). After treatment for 24 h, cell
cycle distribution was analyzed. There was no significant evidence
to suggest that the combination of NL-101 and DNR synergistically
impeded cell cycle progression of MV4-11 cells (Fig. S1A). However, significant
G2/M accumulation was observed following treatment of
HL-60 cells with the combination (P<0.05; Fig. S1B). Western blot analysis revealed
an upregulation of the G2/M regulatory molecules, cyclin
B1 and CDC2, in the combination treatment group compared with the
single agent groups in HL-60 cells (Fig. S1C). These findings indicated that
NL-101 in combination with DNR may have the ability to induce
apoptosis and G2/M cell cycle arrest of HL-60 cells.
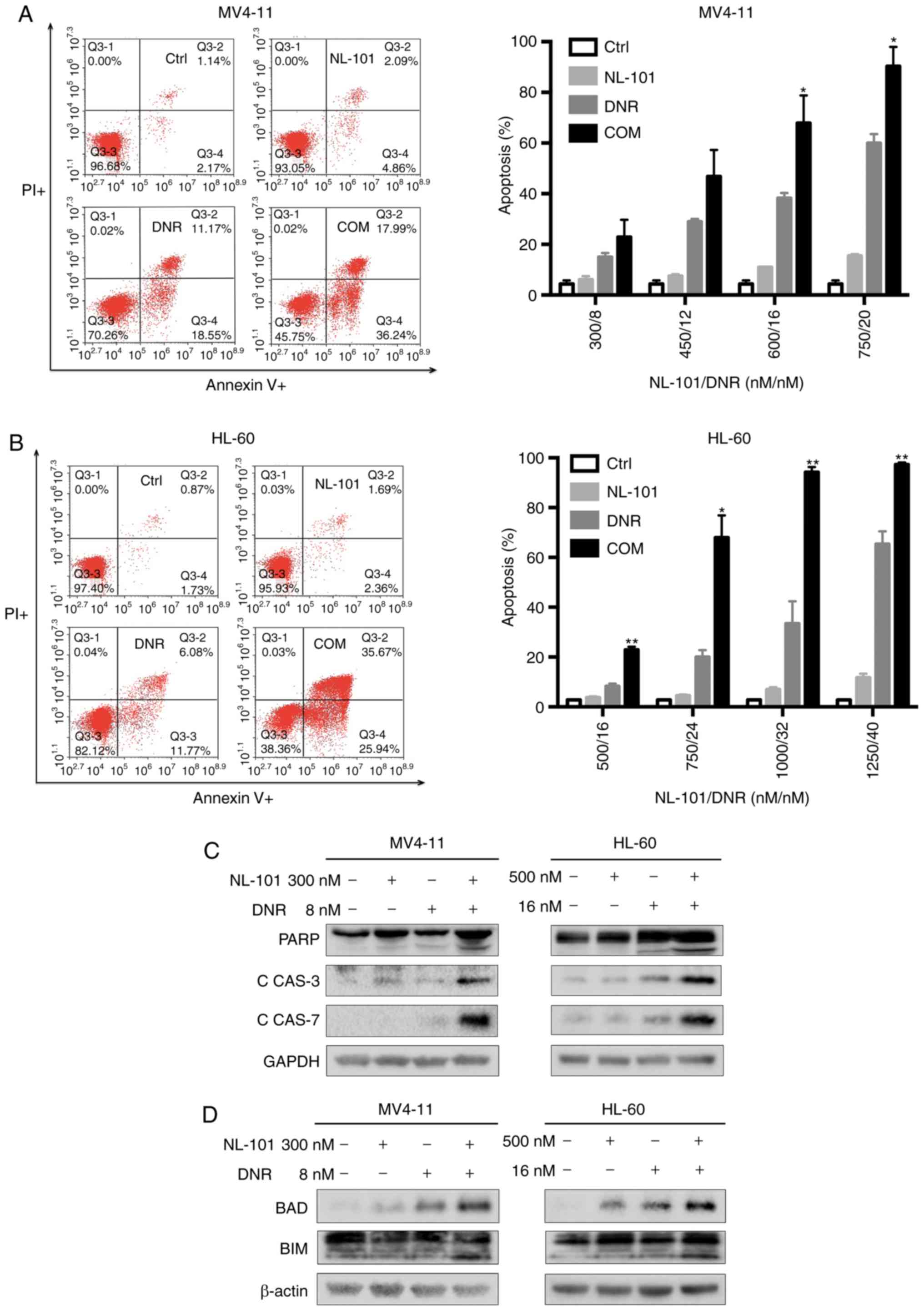 | Figure 2.Apoptosis is induced by NL-101, DNR
and a combination of these agents in acute myeloid leukemia cells.
(A) MV4-11 and (B) HL-60 cells were treated with NL-101, DNR and
the combination for 48 h. Cells co-stained with Annexin V and PI
were measured by flow cytometry. Expression of (C) cleaved-PARP,
cleaved-CAS3, cleaved-CAS7, (D) BAD and BIM were detected in MV4-11
and HL-60 cells by western blotting after cells were exposed to
drugs for 48 h. Data are presented as the mean apoptosis rates ±
SD. P-values were determined using one-way ANOVA. *P<0.05 and
**P<0.001 vs. NL-101 and DNR groups. C, cleaved; CAS, caspase;
COM, combination; Ctrl, control; DNR, daunorubicin; PARP, poly
(ADP-ribose) polymerase; PI, propidium iodide. |
NL-101 in combination with DNR
increases the DNA damage response
By further investigating the cause of apoptosis,
western blot analysis revealed that the protein expression levels
of γ-H2AX were increased in cells treated with the drug combination
compared with the single agents. In addition, the expression levels
of DNA damage checkpoint proteins, p-ATM, p-CHK1 and p-CHK2, were
upregulated after treatment (Fig.
3). P-ATR was upregulated in HL-60 cells, whereas no change was
observed in MV4-11 cells. ATR, ATM, CHK1 and CHK2 exhibited no
change in MV4-11 and HL-60 cells. Briefly, increased DNA damage may
contribute to apoptosis of cells induced by the combination
treatment.
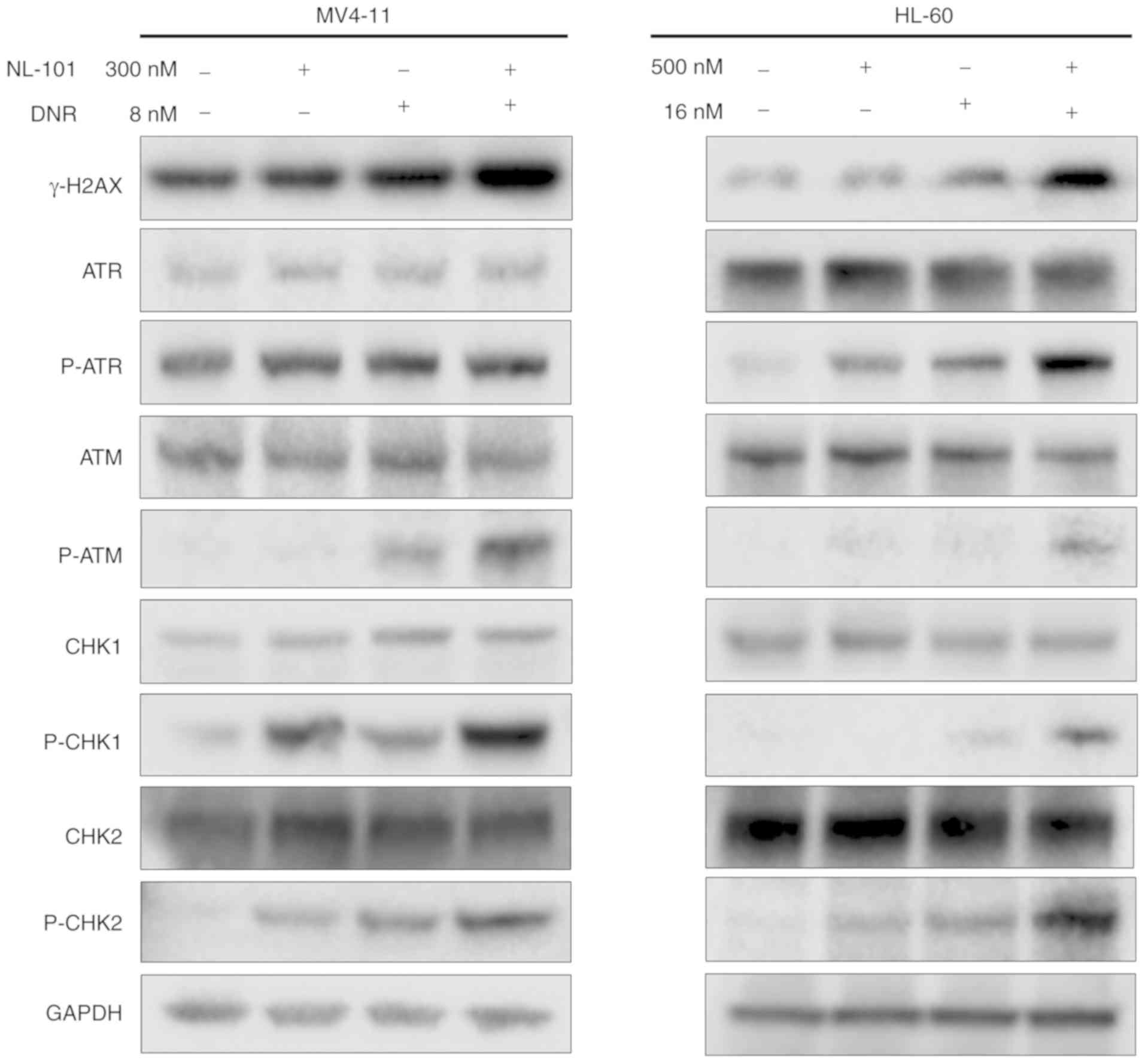 | Figure 3.NL-101 in combination with DNR
promotes DNA damage-related signaling. MV4-11 cells were treated
with 300 nM NL-101, 8 nM DNR and the combination; HL-60 cells were
treated with 500 nM NL-101, 16 nM DNR and the combination for 48 h.
Western blot analysis was conducted for γ-H2AX, ATR, p-ATR, ATM,
p-ATM, CHK1, p-CHK1, CHK2 and p-CHK2 protein levels. DNR,
daunorubicin; p, phosphorylated. |
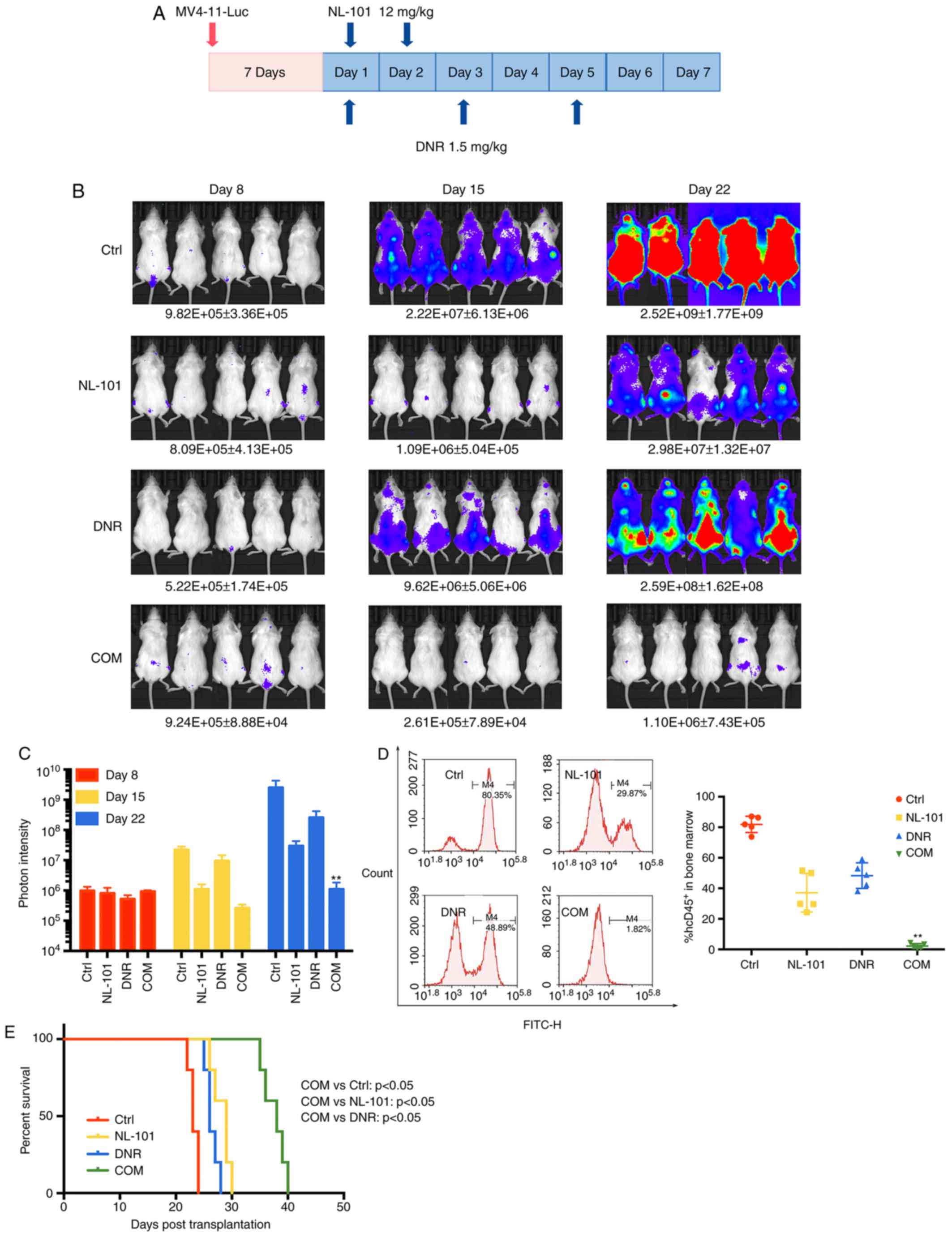
NL-101 in combination with DNR delays
AML progression and prolongs survival in vivo
This study also investigated the efficacy and safety
of NL-101 in combination with DNR on female B-NSG MV4-11-luc
xenograft mouse models. Mice were randomly sorted into four
treatment groups. Briefly, 1×106 MV4-11-Luc cells were
injected into mice; 7 days after cell injection, engraftment was
measured by photon intensity and leukemia burden was assessed
weekly. The treatment regimen is presented in Fig. 4A. On day 22 after transplantation,
the fluorescence intensity of the combination group was distinctly
reduced compared with the single drug treatment group
(P<0.001; Fig. 4B and C).
Blast cell number in the bone marrow was quantified and the number
of hCD45+ cells was significantly decreased in the
combination treatment group (P<0.001; Fig. 4D). In addition, combination
treatment significantly prolonged survival time (P<0.05;
Fig. 4E). These data indicated that
NL-101 in combination with DNR enhanced the therapeutic effect on
AML, compared with untreated and single agents, in vivo.
Discussion
In the present study, the efficacy of NL-101 was
investigated in AML cells, and the synergistic anti-AML effects of
NL-101 in combination with DNR were revealed. Notably, the
mechanism underlying NL-101 function was investigated and it was
suggested that this synergistic effect was due to an increase in
DNA damage-induced apoptosis. In addition, NL-101 + DNR treatment
delayed AML progression and prolonged survival time in
vivo.
The SAHA-bendamustine hybrid NL-101 has dual
molecular mechanisms and has been reported to exhibit improved
pharmaceutical effects in treating AML compared with single agents
(20). In the present study,
treatment with NL-101 alone inhibited proliferation of AML cell
lines and primary AML cells in a dose-dependent manner. In order to
identify a novel regimen that could potentially improve the
prognosis of patients with AML, NL-101 treatment was combined with
DNR, one of the most effective chemotherapeutic drugs used to treat
patients with AML. Subsequently, the synergistic effects of this
combinatorial therapy were identified in various types of AML
cells, including MV4-11 and HL-60 cell lines, and primary AML
cells; the synergistic effect was reflected by CI <1. Notably,
an increase in cell apoptosis may be responsible for this effect.
Cleaved-PARP has been reported to serve a role in apoptosis and in
numerous types of programmed cell death (29,30).
In addition, caspases (31) and the
Bcl-2 family proteins (32,33) have been demonstrated to serve a role
in apoptosis. In the present study, a significant upregulation in
the protein expression levels of cleaved-PARP, cleaved-caspase-3,
cleaved-caspase-7, BAD and BIM was detected in AML cells following
treatment. Furthermore, G2/M cell cycle arrest was
observed, and an upregulation in the G2/M regulatory
molecules, cyclin B1 and CDC2, was detected in HL-60 cells. In some
AML cells, it has been suggested that cell cycle arrest contributes
to cell death; however, this process is not absolute in AML and
requires further validation. Therefore, the present findings
suggested that NL-101 in combination with DNR had the ability to
synergistically inhibit AML cell proliferation by principally
enhancing apoptosis.
Subsequently, the mechanisms underlying enhancement
of apoptosis following combination therapy were investigated; it
was revealed that γ-H2AX, p-ATM, p-CHK1 and p-CHK2 were upregulated
in MV4-11 and HL-60 cells, whereas no changes in the expression
levels of ATR, ATM, CHK1 and CHK2 were detected, following
combination treatment. Since the bendamustine subunit in NL-101
(34,35) and DNR (36,37)
are known to induce the DNA damage stress response, it was
hypothesized that the synergistic effects of NL-101 and DNR may be
caused by an enhancement of the DNA damage stress response. The
protein expression of γ-H2AX is a sensitive DSB response marker
(38,39), and ATR, in combination with ATM, is
required for the precise repair of DSBs (40,41).
ATR and ATM can phosphorylate and activate CHK1 and CHK2, which
have important roles in the DNA damage checkpoint (42). However, in the present study, p-ATR
was significantly upregulated in HL-60 cells but not in MV4-11
cells. This discrepancy may be responsible for the inconsistency in
cell cycle distribution in MV4-11 and HL-60 cells. Collectively, it
was suggested that, by enhancing DNA damage, NL-101 + DNR
synergistically increased cell apoptosis and led to AML cell
death.
In order to investigate the in vivo
biocompatibility of this combinatorial therapy, tumor progression
was investigated in a B-NSG MV4-11-luc xenograft mouse model by
detecting fluorescence intensity. In addition, the
hCD45+ cells in the bone marrow were quantified
following lower extremity paralysis and the survival time of mice
was then recorded. These in vivo experiments indicated that
NL-101 in combination with DNR could significantly delay AML
progression and prolong survival time, which supported the
aforementioned in vitro results. The present findings
further confirmed the efficacy and safety of this combinatorial
therapy and may facilitate the future development of novel
treatments.
The present study had certain limitations. Although
the synergistic effects of the combination of NL-101 and DNR were
examined and the underlying mechanisms were investigated, the most
effective relative concentration between the two agents was not
identified. With regards to in vivo experiments, the
measurements of complete blood count and bone marrow smear could
provide more information; therefore, a more comprehensive study is
required. As DNR and Ara-C/idarubicin and Ara-C (DA/IA) regimen is
the standard therapy for AML, it might be better to compare the
combination regimen with the DA/IA regimen in vivo.
Moreover, the present study is a preliminary investigation that may
facilitate the development of novel strategies based on the
combination of NL-101 with other chemotherapeutics to improve the
prognosis of patients with AML. However, it is unclear whether a
combination of two chemotherapeutics may lead to an improved
prognosis. Therefore, future studies are required to test
additional combinations and to confirm the effectiveness of NL-101
+ DNR in treating AML.
In conclusion, the present results suggested that
the combinatorial treatment of NL-101 with DNR was effective in
treating AML, and the key mechanism underlying this combination
therapy was also identified. The combination of the
SAHA-bendamustine hybrid NL-101 with DNR may represent a promising
therapeutic strategy to treat patients with AML, thus improving
their prognosis.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Ravi
Bhatia (City of Hope National Medical Center, Duarte, CA, USA) and
Professor Jianjun Chen (City of Hope, Gehr Family Center for
Leukemia Research, Duarte, CA, USA) for providing cell lines;
Professor Rongzhen Xu (The Second Affiliated Hospital of Zhejiang
University, Hangzhou, China) for supplying the MV4-11-luciferase
cells, and Hangzhou Minsheng Institute of Pharmaceutical Research
for providing NL-101.
Funding
This study was supported by Zhejiang Provincial Key
Innovation Team (grant no. 2011R50015) and Zhejiang Provincial
Natural Science Foundation of China (grant no. LY16H080002). The
funders were not involved in experimental design, data analysis,
manuscript writing and submission.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JJ designed the experiments. JRJ, XL and WJG
performed the experiments, and JRJ was a major contributor in
writing the manuscript. FLL, JSH and XH analyzed the data. JJP and
SJH collected the patient samples and information. WLY and QL
contributed to the writing of the manuscript and performed the
primary cells experiment. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by Animal
Experimental Ethical Inspection of the First Affiliated Hospital,
College of Medicine, Zhejiang University. All procedures performed
involving human patients were approved by Research Ethics Committee
of the First Affiliated Hospital, College of Medicine, Zhejiang
University. Informed consent was obtained from all patients
according to institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
SAHA
|
vorinostat
|
|
DNR
|
daunorubicin
|
|
Ara-C
|
cytarabine
|
|
CI
|
combination index
|
|
DSBs
|
DNA double-strand breaks
|
|
DMSO
|
dimethyl sulfoxide
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
B-NSG
|
NOD-Prkdcscid
IL2rgtm1/Bcgen
|
References
|
1
|
Lam SS, Ho ES, He BL, Wong WW, Cher CY, Ng
NK, Man CH, Gill H, Cheung AM, Ip HW, et al: Homoharringtonine
(omacetaxine mepesuccinate) as an adjunct for FLT3-ITD acute
myeloid leukemia. Sci Transl Med. 8:359ra1292016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrara F and Schiffer CA: Acute myeloid
leukaemia in adults. Lancet. 381:484–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quintás-Cardama A, Santos FP and
Garcia-Manero G: Histone deacetylase inhibitors for the treatment
of myelodysplastic syndrome and acute myeloid leukemia. Leukemia.
25:226–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Papaemmanuil E, Gerstung M, Bullinger L,
Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F,
Bolli N, et al: Genomic classification and prognosis in acute
myeloid leukemia. N Engl J Med. 374:2209–2221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenblat TL, McDevitt MR, Mulford DA,
Pandit-Taskar N, Divgi CR, Panageas KS, Heaney ML, Chanel S,
Morgenstern A, Sgouros G, et al: Sequential cytarabine and
alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195)
for acute myeloid leukemia. Clin Cancer Res. 16:5303–5311. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stein EM, DiNardo CD, Pollyea DA, Fathi
AT, Roboz GJ, Altman JK, Stone RM, DeAngelo DJ, Levine RL, Flinn
IW, et al: Enasidenib in mutant IDH2 relapsed or refractory acute
myeloid leukemia. Blood. 130:722–731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walter RB and Estey EH: Management of
older or unfit patients with acute myeloid leukemia. Leukemia.
29:770–775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Löwenberg B and Rowe JM: Introduction to
the review series on advances in acute myeloid leukemia (AML).
Blood. 127:12016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Döhner H, Estey EH, Amadori S, Appelbaum
FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson
RA, et al: Diagnosis and management of acute myeloid leukemia in
adults: Recommendations from an international expert panel, on
behalf of the European LeukemiaNet. Blood. 115:453–474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei AH and Tiong IS: Midostaurin,
enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring
new hope to AML. Blood. 130:2469–2474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burnett A, Wetzler M and Löwenberg B:
Therapeutic advances in acute myeloid leukemia. J Clin Oncol.
29:487–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feldman EJ, Lancet JE, Kolitz JE, Ritchie
EK, Roboz GJ, List AF, Allen SL, Asatiani E, Mayer LD, Swenson C
and Louie AC: First-in-man study of CPX-351: A liposomal carrier
containing cytarabine and daunorubicin in a fixed 5:1 molar ratio
for the treatment of relapsed and refractory acute myeloid
leukemia. J Clin Oncol. 29:979–985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lancet JE, Cortes JE, Hogge DE, Tallman
MS, Kovacsovics TJ, Damon LE, Komrokji R, Solomon SR, Kolitz JE,
Cooper M, et al: Phase 2 trial of CPX-351, a fixed 5:1 molar ratio
of cytarabine/daunorubicin, vs. cytarabine/daunorubicin in older
adults with untreated AML. Blood. 123:3239–3246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stein EM and Tallman MS: Emerging
therapeutic drugs for AML. Blood. 127:71–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Besse L, Kraus M, Besse A, Bader J, Silzle
T, Mehrling T and Driessen C: The first-in-class alkylating HDAC
inhibitor EDO-S101 is highly synergistic with proteasome inhibition
against multiple myeloma through activation of multiple pathways.
Blood Cancer J. 7:e5892017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mehrling T and Chen Y: The alkylating-HDAC
inhibition fusion principle: Taking chemotherapy to the next level
with the first in class molecule EDO-S101. Anticancer Agents Med
Chem. 16:20–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu J, Qiu S, Ge Q, Wang Y, Wei H, Guo D,
Chen S, Liu S, Li S, Xing H, et al: A novel SAHA-bendamustine
hybrid induces apoptosis of leukemia cells. Oncotarget.
6:20121–20131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishida Y, Mizutani N, Inoue M, Omori Y,
Tamiya-Koizumi K, Takagi A, Kojima T, Suzuki M, Nozawa Y, Minami Y,
et al: Phosphorylated Sp1 is the regulator of DNA-PKcs and DNA
ligase IV transcription of daunorubicin-resistant leukemia cell
lines. Biochim Biophys Acta. 1839:265–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burden DA and Osheroff N: Mechanism of
action of eukaryotic topoisomerase II and drugs targeted to the
enzyme. Biochim Biophys Acta. 1400:139–154. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy T and Yee KWL: Cytarabine and
daunorubicin for the treatment of acute myeloid leukemia. Expert
Opin Pharmacother. 18:1765–1780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drexler HG: Classification of acute
myeloid leukemias-a comparison of FAB and immunophenotyping.
Leukemia. 1:697–705. 1987.PubMed/NCBI
|
|
26
|
Zhao F, Sun X, Lu W, Xu L, Shi J, Yang S,
Zhou M, Su F, Lin F and Cao F: Synthesis of novel, DNA binding
heterocyclic dehydroabietylamine derivatives as potential
antiproliferative and apoptosis-inducing agents. Drug Deliv.
27:216–227. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zoi I, Karamouzis MV, Xingi E, Sarantis P,
Thomaidou D, Lembessis P, Theocharis S and Papavassiliou AG:
Combining RANK/RANKL and ERBB-2 targeting as a novel strategy in
ERBB-2-positive breast carcinomas. Breast Cancer Res. 21:1322019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Li C, Jin J, Wang J, Huang J, Ma Z,
Huang X, He X, Zhou Y, Xu Y, et al: High PARP-1 expression predicts
poor survival in acute myeloid leukemia and PARP-1 inhibitor and
SAHA-bendamustine hybrid inhibitor combination treatment
synergistically enhances anti-tumor effects. EBioMedicine.
38:47–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tewari M, Quan LT, O'Rourke K, Desnoyers
S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS and Dixit VM:
Yama/CPP32 beta, a mammalian homolog of CED-3, is a
CrmA-inhibitable protease that cleaves the death substrate
poly(ADP-ribose) polymerase. Cell. 81:801–809. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaufmann SH, Desnoyers S, Ottaviano Y,
Davidson NE and Poirier GG: Specific proteolytic cleavage of
poly(ADP-ribose) polymerase: An early marker of
chemotherapy-induced apoptosis. Cancer Res. 53:3976–3985.
1993.PubMed/NCBI
|
|
31
|
Testa U and Riccioni R: Deregulation of
apoptosis in acute myeloid leukemia. Haematologica. 92:81–94. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benito A, Silva M, Grillot D, Nuñez G and
Fernández-Luna JL: Apoptosis induced by erythroid differentiation
of human leukemia cell lines is inhibited by Bcl-XL. Blood.
87:3837–3843. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nuñez G and Clarke MF: The Bcl-2 family of
proteins: Regulators of cell death and survival. Trends Cell Biol.
4:399–403. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheson BD and Rummel MJ: Bendamustine:
Rebirth of an old drug. J Clin Oncol. 27:1492–1501. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garnock-Jones KP: Bendamustine: A review
of its use in the management of indolent non-Hodgkin's lymphoma and
mantle cell lymphoma. Drugs. 70:1703–1718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pang B, de Jong J, Qiao X, Wessels LF and
Neefjes J: Chemical profiling of the genome with anti-cancer drugs
defines target specificities. Nat Chem Biol. 11:472–480. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pang B, Qiao X, Janssen L, Velds A,
Groothuis T, Kerkhoven R, Nieuwland M, Ovaa H, Rottenberg S, van
Tellingen O, et al: Drug-induced histone eviction from open
chromatin contributes to the chemotherapeutic effects of
doxorubicin. Nat Commun. 4:19082013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pilch DR, Sedelnikova OA, Redon C, Celeste
A, Nussenzweig A and Bonner WM: Characteristics of gamma-H2AX foci
at DNA double-strand breaks sites. Biochem Cell Biol. 81:123–129.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zou L and Elledge SJ: Sensing DNA damage
through ATRIP recognition of RPA-ssDNA complexes. Science.
300:1542–1548. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reilly NM, Novara L, Di Nicolantonio F and
Bardelli A: Exploiting DNA repair defects in colorectal cancer. Mol
Oncol. 13:681–700. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y and Poon RY: The multiple
checkpoint functions of CHK1 and CHK2 in maintenance of genome
stability. Front Biosci. 13:5016–5029. 2008.PubMed/NCBI
|