Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide, with ~1.8 million new cases
and 1.2 million mortality cases annually (1). NSCLC accounts for ~80% of lung cancer
cases (2). Advances in the
diagnosis and treatment of lung cancer, including percutaneous lung
biopsy, tumor marker detection, surgical, medicine and radiological
intervention, have been achieved in the last decade (3). However, the 5-year survival rate of
patients with lung cancer is only 15% due to metastasis (4). The development of novel molecular
therapies for preventing patient mortality due to metastasis is
therefore urgently required (5),
which would subsequently improve the current strategies and
outcomes.
Propofol is one of the most frequently used
intravenous anesthetics during tumor resection due to its rapid
onset and short duration of action (6). Propofol was reported to inhibit cancer
cell proliferation and migration and cancer invasiveness in various
types of cancer, including ovarian (7), breast (8), colon (9) and liver (10) cancers. Considering the widespread
use of propofol in clinical practice, exploring the connection
between propofol and NSCLC and determining the underlying
mechanisms might be essential.
MicroRNAs (miRNAs or miRs) are highly conserved,
single-stranded small non-coding RNAs (~22 nucleotides in length),
which inhibit targets post-transcriptionally by binding to their
3′-UTRs (11). miRNAs are involved
in numerous biological processes, including cell proliferation,
migration, invasion and apoptosis (12). Numerous links between propofol and
miRNAs have been reported in NSCLC. For example, propofol has been
demonstrated to suppress A549 cell proliferation, migration and
invasion by decreasing miR-372 expression (13). In addition, it has been demonstrated
that propofol inhibits A549 cell proliferation and
epithelial-mesenchymal transition (EMT) by increasing miR-1284
expression (14), and that it can
inhibit H1299 and H1792 cell viability and induce their apoptosis
by increasing miR-486 expression (15).
The present study investigated whether the effects
of propofol on the viability and apoptosis of A549 and H1299 cells
could be mediated by the regulation of another miRNA in particular
and its target.
Materials and methods
Tissue samples
A total of 31 NSCLC tissues and 31 adjacent
non-tumor tissues (5 cm from tumor tissue) were collected from 31
patients with NSCLC (16 men and 15 women; age range, 40–57 years;
mean age: 47.21±8.33 years) who underwent tumor resection at the
People's Hospital of Xinjiang Uygur Autonomous Region between
February 2015 and January 2017. Patients who had received
radiotherapy or chemotherapy prior to the surgery were excluded
from the study. The tumors were staged according to the
8th edition of American Joint Committee on Cancer
Tumor-Node-Metastasis (TNM) system (16). This study was approved by the Ethics
Committee of People's Hospital of Xinjiang Uygur Autonomous Region.
Patients provided written informed consent.
Bioinformatics analysis
The interaction between miR-21-5p and MAPK10 was
predicted by TargetScan/miRanda. Starbase V2.0 project (http://starbase.sysu.edu.cn/) was used for the
analysis of miR-21-5p and MAPK10 expression levels in NSCLC tumor
and normal tissues, and the relation between miR-21-5p and MAPK10
in NSCLC tumor tissues from The Cancer Genome Atlas (TCGA) datasets
(TCGA-LUAD and TCGA-LUSC) (17).
Survival analysis
GEPIA software version 1.0 developed by Zhang
Laboratory at the Peking University was used for the survival
analysis based on TCGA data (960 patients) (18). Long-rank test was used to perform
the analysis. For group cutoff, MAPK10 median expression threshold
was chosen to split the cohort between high expression and low
expression. P<0.05 was used for significance threshold.
Cell lines
The human NSCLC cell lines A549 and H1299 and the
human lung epithelial cell line BEAS-2B were purchased from the
American Type Culture Collection. Cells were cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (HyClone; GE
Healthcare Life Sciences) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) and placed at 37°C in
a humidified incubator containing 5% CO2.
Cell transfection
miR-21-5p mimic and negative control (NC) were
synthesized by Shanghai GenePharma Co., Ltd. pcDNA3-MAPK10 and
pcDNA3 were purchased from Addgene (cat. no. 13758). A549
(1×103/well) and H1299 (1×103/well) cells
were seeded in 96-well plates, cultured for 24 h and transfected
with 50 nM miR-21-5p mimic, 50 nM miR-NC mimic, 2 µg pcDNA3 or 2 µg
pcDNA3-MAPK10 using Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. After 48 h, A549 and H1299 cells were collected for
subsequent experiments.
Cell treatment
A549 and H1299 cells were treated with 8 µg/ml
propofol (Sigma-Aldrich; Merck KGaA) for 48 h as previously
described (13). Following propofol
treatment for 48 h, and/or transfection with miR-21-5p mimic and/or
pcDNA3-MAPK10 for 48 h, the A549 and H1299 cells were harvested for
use in subsequent experiments.
CCK-8 assay
Cell viability was determined using a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). Briefly, A549
and H1299 cells (5×103 cells/well) were seeded into
96-well plates. Following propofol treatment and/or transfection
with miR-21-5p mimic and/or pcDNA3-MAPK10 for 48 h, 10 µl CCK-8
solution was added to each well. Plates were then incubated for 1
at 37°C in a humidified incubator containing 5% CO2.
Absorbance was read at 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc.).
Cell apoptosis assay
Cell apoptosis was determined using an
Annexin-V/Dead Cell Apoptosis kit (cat. no. V13242; Invitrogen;
Thermo Fisher Scientific, Inc.). Briefly, A549 (1×106
cells/ml) and H1299 cells (1×106 cells/ml) were diluted
in 100 µl binding buffer. Subsequently, Annexin V (5 µl) and
propidium iodide (1 µl) were added to the cell suspensions. A549
and H1299 cells were then incubated in the dark at room temperature
for 15 min. Subsequently, the cell apoptosis rate was determined by
flow cytometric analysis using a FACSCalibur flow cytometer (BD
Biosciences). Results were analyzed using FlowJo software (version
10.2; FlowJo LLC).
RNA isolation and reverse
transcription-quantitative (RT-q) PCR
Total RNA was extracted from NSCLC and adjacent
tissues, and from the BEAS-2B, A549 and H1299 cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.). miRNA was extracted from
NSCLC and adjacent tissues, and from the BEAS-2B, A549 and H1299
cells using a miRNA Extraction kit (cat. no. 217004; Qiagen GmbH).
RNA integrity was determined using a Nano Drop ND-1000
spectrophotometer. RT was carried out using a miRNA cDNA Synthesis
kit (Qiagen GmbH) and TransScript First-Strand cDNA Synthesis
SuperMix (Beijing TransGen Biotech co., ltd.). qPCR was carried out
using SYBR-Green Premix Ex Taq II (Qiagen GmbH) with the ABI Prism
7500 Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). qPCR reactions were performed as follows:
Initial denaturation at 95°C (10 min), then 95°C (5 sec), 63°C (30
sec) and 72°C (30 sec) for 40 cycles, followed by an extension step
at 72°C (5 min). The sequences of the primers were as followed:
MAPK10, forward 5′-CTTCCCAGATTCCCTCTTCC-3′, reverse
3′-GCTGGGTCATACCAGACGTT-5′; GAPDH, forward
5′-GAGTCAACGGATTTGGTCGT-3′, reverse 3′-TTGATTTTGGAGGGATCTCG-5′;
miR-193-3p, forward 5′-ACACTCCAGCTGGGAACTGGCCTACAAAGT-3′, reverse
3′-TGGTGTCGTGGAGTCG-5′; miR-221-3p, forward
5′-CUUUGGGUCGUCUGUUACAUCGA-3′, reverse
3′-AUUGCUUCUCUUCAUGCAAGUCA-5′; miR-203a-3p, forward
5′-GAUCACCAGGAUUUGUAAAGU-3′, reverse
3′-GCAAAUUAAAUUUGGGCUUGUUCU-5′; miR-22-3p, forward
5′-UGUCAAGAAGUUGACCGUCGAA-3′ and reverse,
3′-AUGGUUGUUUCAAAUGCAUUGGG-5′; miR-125-5p, forward
5′-AGUGUUCAAUCCCAGAGUCCCU-3′, reverse
3′-CAGGCUUCAUGAUGUCUCCAUAUGAA-5′; miR-21-5p, forward
5′-AGUUGUAGUCAGACUAUUCGAU-3′, reverse 3′-CUUUACGAUGAAUUCAUUUCC-5′;
U6, forward 5′-CGCTTCGGCAGCACATATACTAA-3′ and reverse
3′-TATGGAACGCTTCACGAATTTGC-5′. GAPDH was used as the endogenous
control for MAPK10. U6 was used as the endogenous control for
miR-21-5p. The expression levels of genes were calculated using the
2−ΔΔCq method (19).
Western blotting
Proteins were extracted from NSCLC and adjacent
tissues, and from the BEAS-2B, A549 and H1299 cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) that contains
protease inhibitors (Roche Diagnostics) on ice for 30 min.
Following the quantification of protein concentration using BCA
(Beyotime Institute of Biotechnology), proteins (20 µg/lane) were
separated by 8% SDS-PAGE and transferred onto PVDF membranes.
Membranes were blocked with 5% non-fat milk for 2 h at room
temperature and were incubated with primary antibodies against BAX
(cat. no. 2772; 1:1,000), Bcl-2 (cat. no. 4223; 1:1,000), MAPK10
(cat. no. 2305; 1:1,000) and GAPDH (cat. no. 5174; 1:1,000) at 4°C
overnight. Membranes were then incubated with goat-anti rabbit
secondary antibody (cat. no. 7074; 1:2,000; Cell Signaling
Technology, Inc.) at room temperature for 2 h. All antibodies were
purchased from Cell Signaling Technology, Inc. Bands were detected
using enhanced chemiluminescence substrate (EMD Millipore). The
band intensities were analyzed using Image Lab™ (version 4.0;
Bio-Rad Laboratories, Inc.).
Dual-luciferase reporter assay
The binding between miR-21-5p and the 3′-UTR of
MAPK10 was predicted by TargetScan version 7.1 (http://www.targetscan.org/vert_71/) and validated
by dual-luciferase reporter assay. The 3′-UTR of MAPK10 mRNA was
amplified from the cDNA, which was obtained from 293T cells, and
inserted into the pGL3-basic vector (Promega Corporation) between
the KpnI and Xhol restriction enzyme sites. Two site
mutations were introduced into the pGL3-MAPK10 3′-UTR-wild-type
(WT) sequence to obtain the pGL3-MAPK10 3′-UTR-Mutant (MT) sequence
by using a Quick Site-directed mutation kit (cat. no. 200518;
Agilent Technologies, Inc.). 293T cells (1×105/well)
were plated in 24-well plates, co-transfected with pGL3-MAPK10
3′-UTR-WT (0.4 mg) or pGL3-MAPK10 3′UTR-MT (0.4 mg) together with
miR-NC mimic (20 nM) or miR-21-5p mimic (20 nM) using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. After 48 h, the relative luciferase
activity in each group was determined using the dual luciferase
reporter system kit (Promega Corporation). Renilla
luciferase activity (Promega Corporation) acted as the reference
control.
Statistical analysis
Each experiment was repeated at least three times in
the present study. Statistical analysis was carried out using
GraphPad Prism 7.0 software (GraphPad Software, Inc.). Differences
between two groups were analyzed by Student's t-test, and
differences among three or more groups were analyzed by one-way
analysis of variance followed by Newman-Keuls test. The association
between MAPK10 expression and the clinicopathological
characteristics of patients was analyzed using χ2
test. Pearson's correlation test was performed to analyze the
correlation between the miR-21-5p and MAPK10 mRNA levels. P<0.05
was considered to indicate a statistically significant
difference.
Results
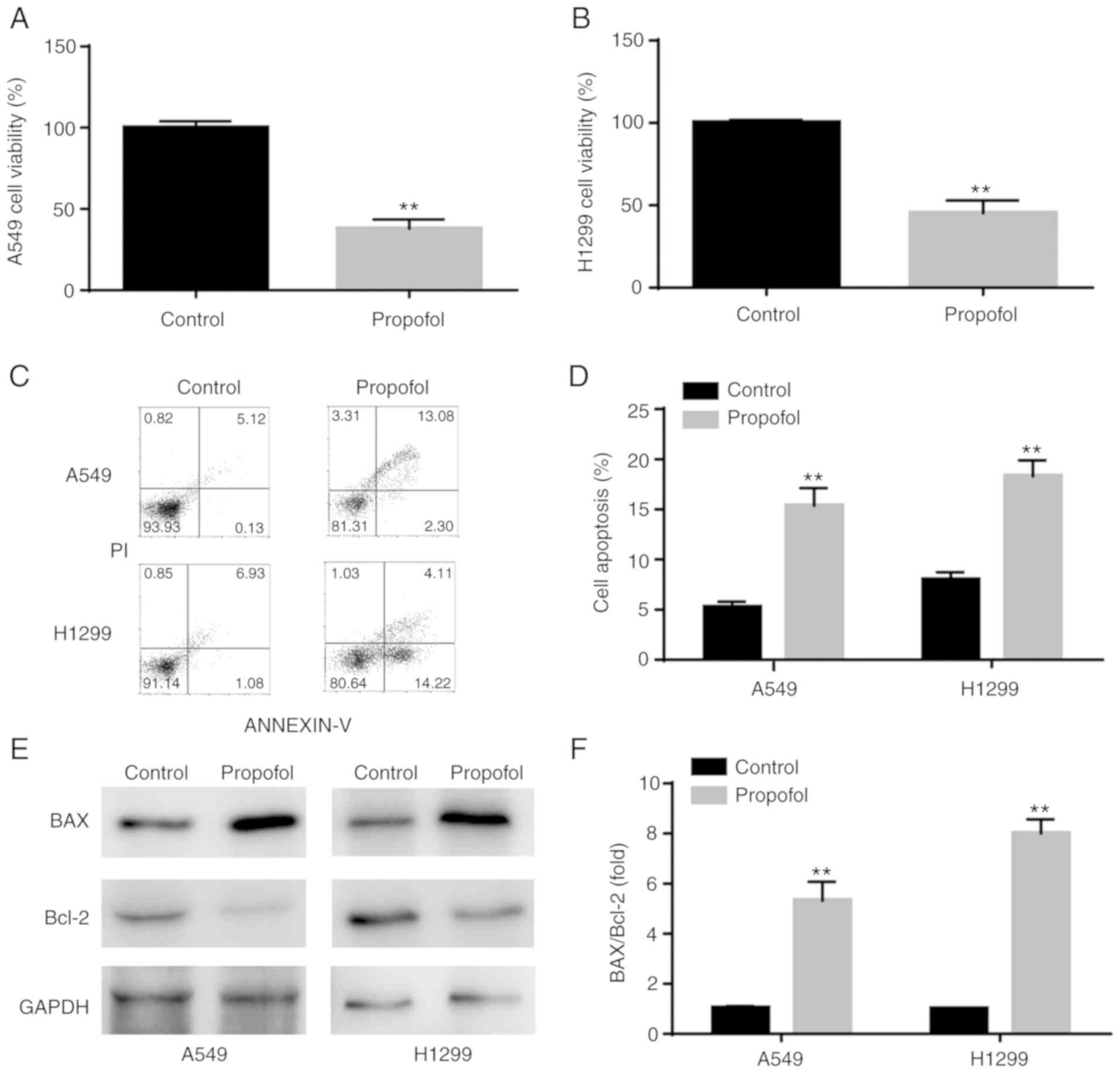
Propofol decreases viability and
induces apoptosis in NSCLC cell lines
The results from CCK-8 assay demonstrated that
propofol significantly decreased A549 and H1299 cell viability
compared with control group (Fig. 1A
and B). Furthermore, results from flow cytometry indicated that
propofol significantly increased NSCLC cell apoptosis compared with
control group (Fig. 1C and D). In
addition, western blotting analysis revealed that BAX/Bcl-2 ratio
was significantly increased in NSCLC cells following propofol
treatment, which was consistent with induction of cell apoptosis
(Fig. 1E and F).
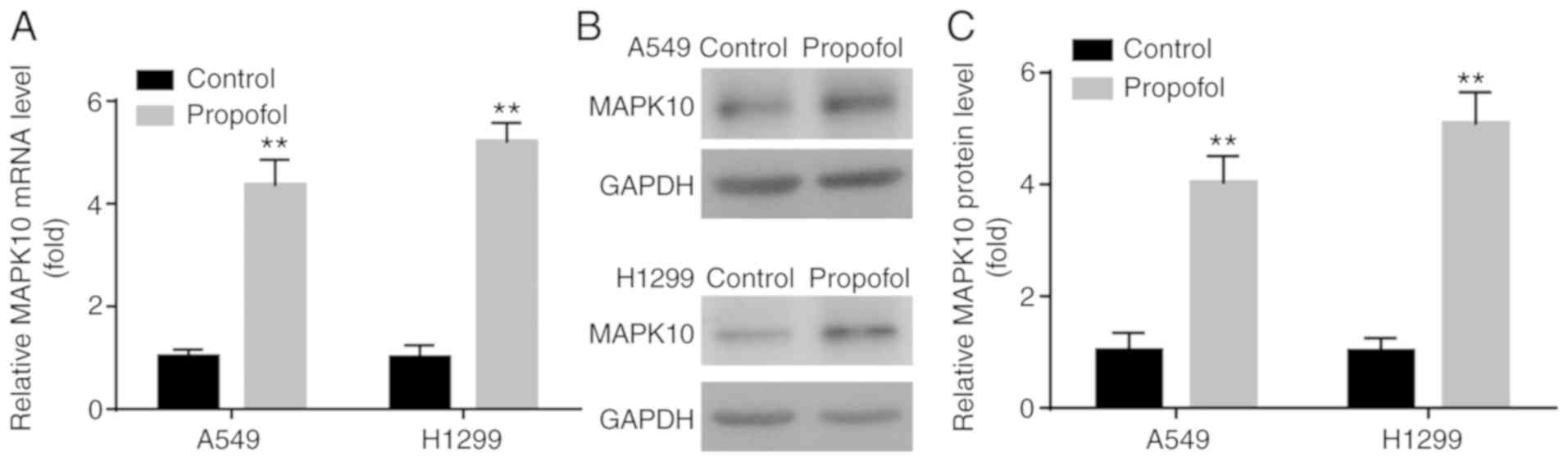
Propofol increases MAPK10 expression
in NSCLC cell lines
The levels of apoptotic-related molecules were
examined following propofol treatment. As an important
pro-apoptotic gene (20), a
decrease in MAPK10 expression has been reported in breast, gastric,
hepatocellular carcinoma and nasopharyngeal carcinomas (21,22).
However, to the best of our knowledge, the effects of propofol on
MAPK10 expression in NSCLC remain unknown, which was investigated
in the present study. In the present study, propofol significantly
increased MAPK10 mRNA and protein expression in A549 and H1299
cells compared with control group (Fig.
2A-C).
MAPK10 expression is decreased in
NSCLC cell lines and tumor tissues
To the best of our knowledge, expression profile of
MAPK10 in NSCLC remains unknown. In the present study, results from
RT-qPCR and western blotting demonstrated that MAPK10 mRNA
(Fig. 3A) and protein expression
(Fig. 3B and C) was significantly
lower in the human NSCLC cell lines A549 and H1299 compared with
the human lung epithelial cell line BEAS-2B.
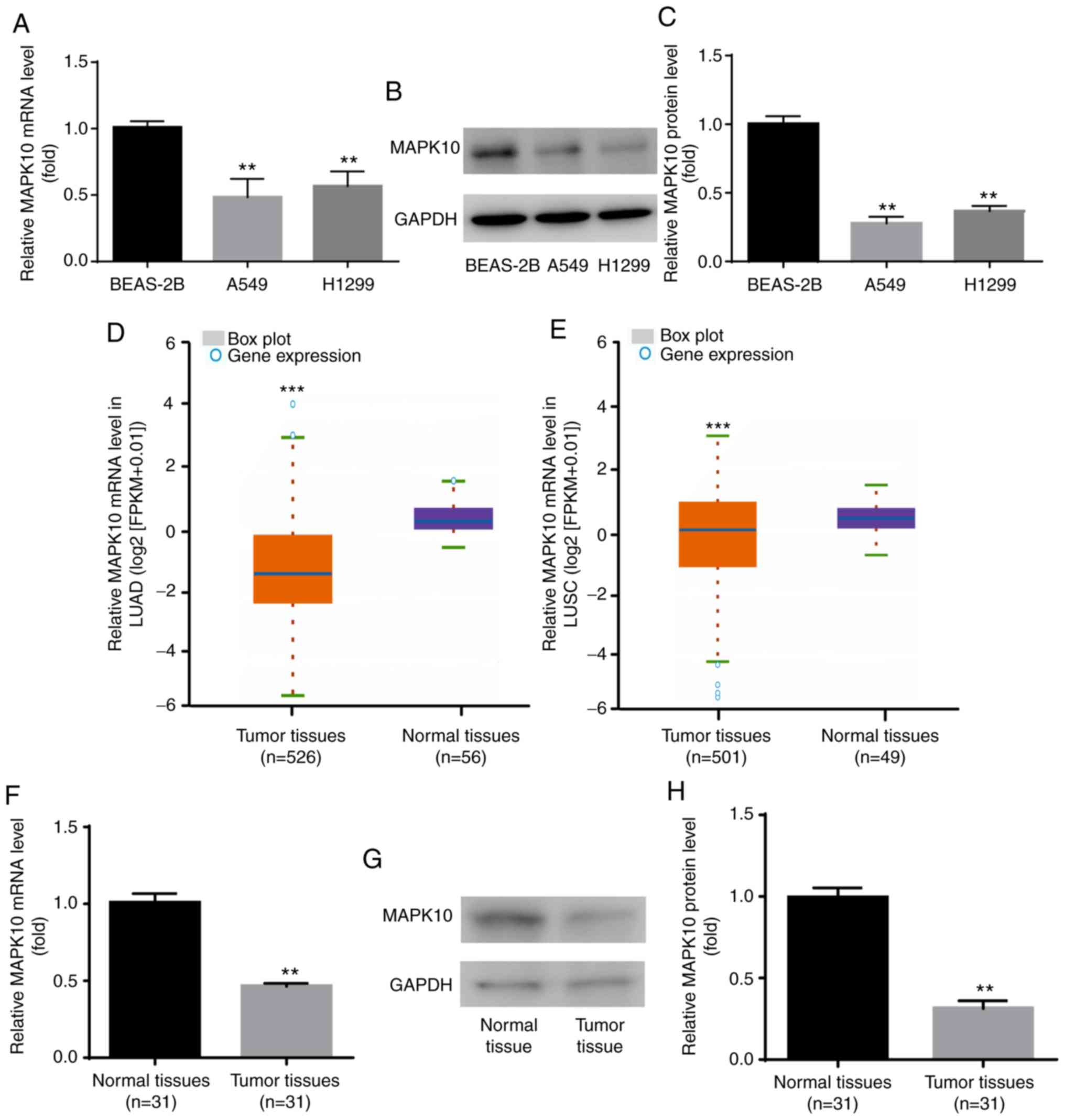 | Figure 3.MAPK10 expression was decreased in
A549 and H1299 cells and NSCLC tumor tissues. Compared with BEAS-2B
cells, MAPK10 (A) mRNA and (B and C) protein expression was
significantly decreased in A549 and H1299. (D-H) Compared with
normal samples, MAPK10 expression was significantly decreased in
NSCLC tissues, according to data from Starbase V3.0 project,
reverse transcription quantitative PCR and western blotting.
**P<0.01, A549, H1299 vs. BEAS-2B and tumor tissues vs. normal
tissues; ***P<0.001, tumor tissues vs. normal tissues. MAPK10,
mitogen-activated protein kinase 10; LUAD, lung adenocarcinoma;
LUSC, lung squamous cell carcinoma. |
The expression profile of MAPK10 in NSCLC was
subsequently identified via Starbase V2.0 project. The database
showed that compared with the 56 normal samples, MAPK10 expression
was significantly lower in the 526 tumor samples with lung
adenocarcinoma (TCGA-LUAD; Fig.
3D). Compared with the 49 normal samples, MAPK10 expression was
significantly lower in the 501 tumor samples with lung squamous
cell carcinoma (TCGA-LUSC; Fig.
3E). In addition, MAPK10 mRNA and protein levels were
significantly lower in tumor tissues from patients with NSCLC
compared with adjacent normal tissues (Fig. 3F-H). These findings suggested that
MAPK10 may serve a tumor-suppressive role in NSCLC.
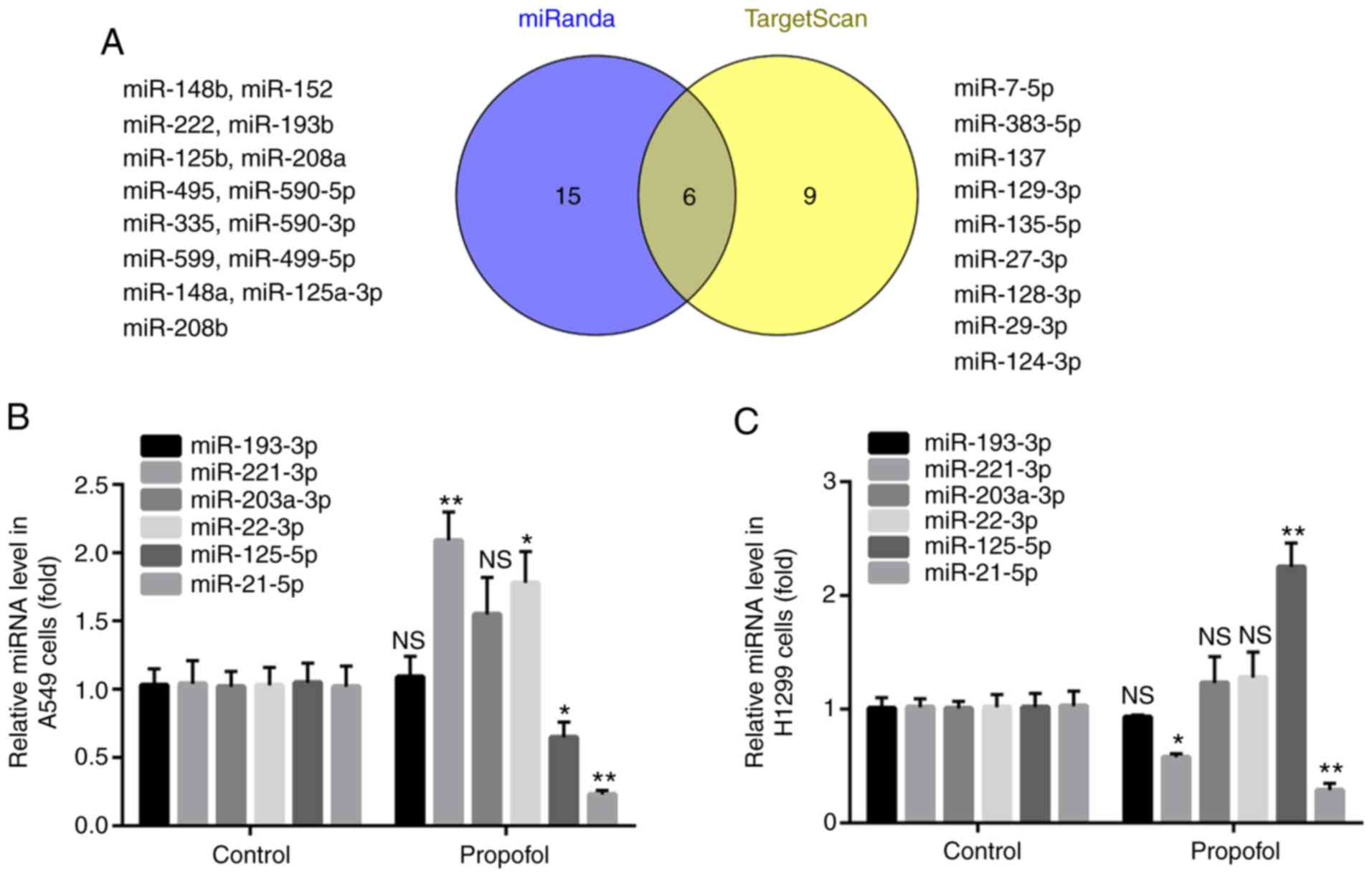
Propofol decreases miR-21-5p
expression in NSCLC cell lines
The present study aimed to determine the oncogenes
that target MAPK10. By using the online softwares miRanda and
TargetScan, it was found that the 3′UTR of MAPK10 included the
complementary sites for 21 miRNAs in miRanda and 15 miRNAs in
TargetScan, and that six miRNAs overlapped, including miR-193-3p,
miR-203a-3p, miR-22-3p, miR-125-5p, miR-221-3p and miR-21-5p
(Fig. 4A).
Compared with the control group, propofol had no
effect on miR-193-3p or miR-203a-3p expression level in A549 and
H1299 cells; however, propofol treatment significantly increased
miR-221-3p expression level in A549 cells and significantly
decreased miR-221-3p expression level in H1299 cells. In addition,
propofol significantly increased miR-22-3p expression level in A549
cells; however, it had no effect on miR-22-3p expression level in
H1299 cells. Furthermore, propofol treatment significantly
decreased and increased miR-125-5p level in A549 and H1299 cells,
respectively. However, treatment with propofol downregulated
miR-21-5p expression level in both A549 and H1299 cells (Fig. 4B and C). miR-21-5p was therefore
selected in the present study and its role in NSCLC was further
investigated.
miR-21-5p targets and inhibits MAPK10
expression in NSCLC cell lines
The binding site between MAPK10 and miR-21-5p is
illustrated in Fig. 5A. Before
exploring the interaction between MAPK10 and miR-21-5p by
dual-luciferase reporter assay, the present study aimed to verify
the successful transfection of miR-21-5p mimic in A549 and H1299
cells. The results demonstrated a significantly higher miR-21-5p
expression level in the miR-21-5p mimic group compared with the
miR-NC group, confirming the successful transfection (Fig. 5B). Furthermore, results from
dual-luciferase reported assay demonstrated that miR-21-5p mimic
inhibited the relative luciferase activity in cells transfected
with WT-MAPK10 but not with MT-MAPK10 in A549 and H1299 cells
(Fig. 5C and D).
In addition, compared with the miR-NC mimic group,
miR-21-5p mimic significantly inhibited the mRNA and protein levels
of MAPK10 in A549 and H1299 cells (Fig.
5E-G).
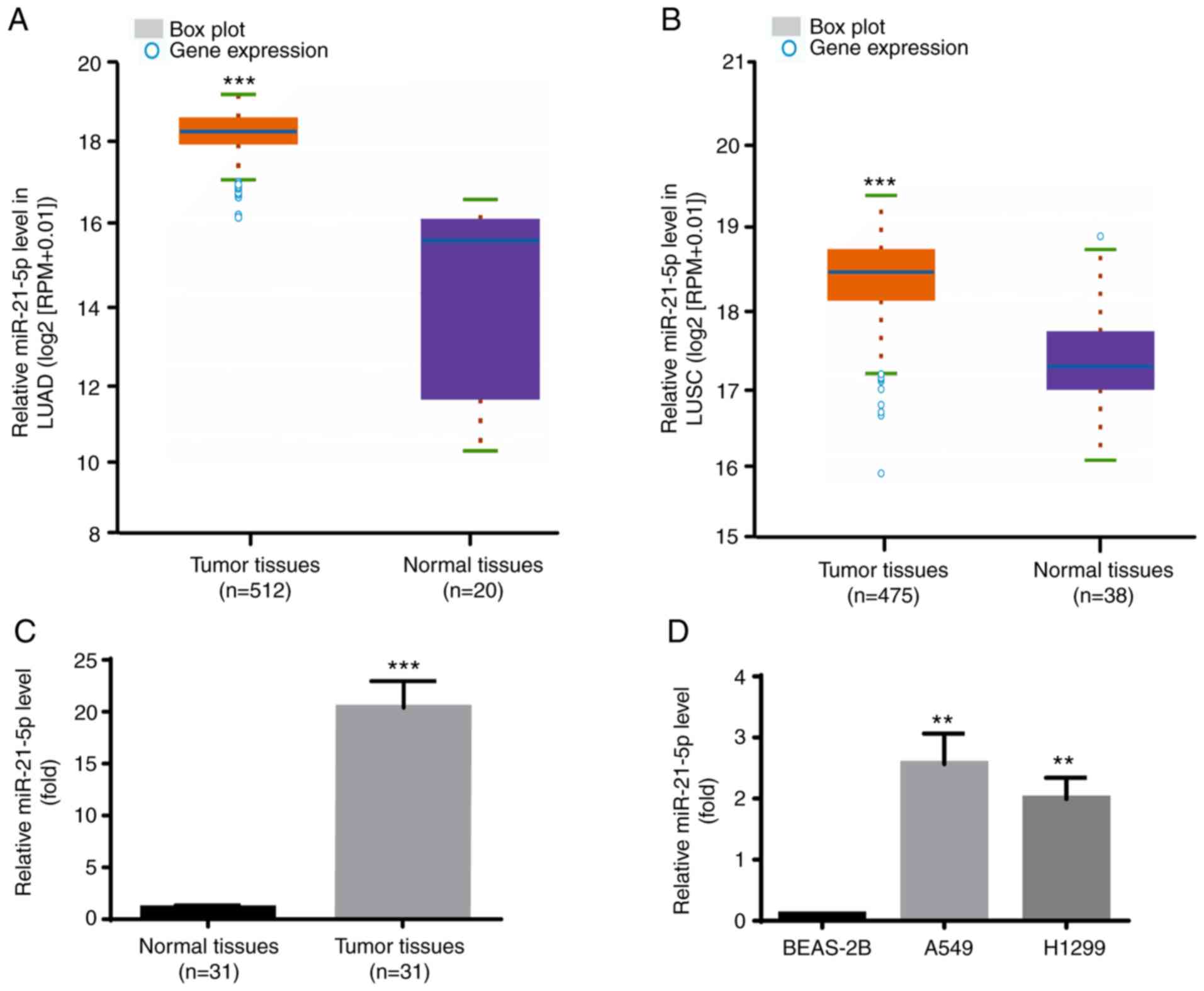
miR-21-5p is increased in NSCLC cell
lines and tumor tissues
The expression profile of miR-21-5p in NSCLC was
identified via Starbase V3.0 project. As presented in Fig. 6A, miR-21-5p expression level was
significantly increased in the 521 tumor tissues compared with the
20 normal samples in LUAD. Furthermore, as presented in Fig. 6B, miR-21-5p expression level was
also significantly increased in the 475 tumor tissues compared with
the 38 normal samples in LUSC.
Consistently, miR-21-5p expression level was higher
in tumor tissues compared with adjacent normal tissues from the
patients with NSCLC included in the present study (Fig. 6C). Furthermore, results from RT-qPCR
demonstrated that miR-21-5p expression level was significantly
increased in the human NSCLC cell lines A549 and H1299 compared
with the human lung epithelial cell line BEAS-2B (Fig. 6D). Taken together, these results
further indicated that miR-21-5p may act as an oncogene in
NSCLC.
miR-21-5p negatively regulates MAPK10
expression in NSCLC tumor tissues
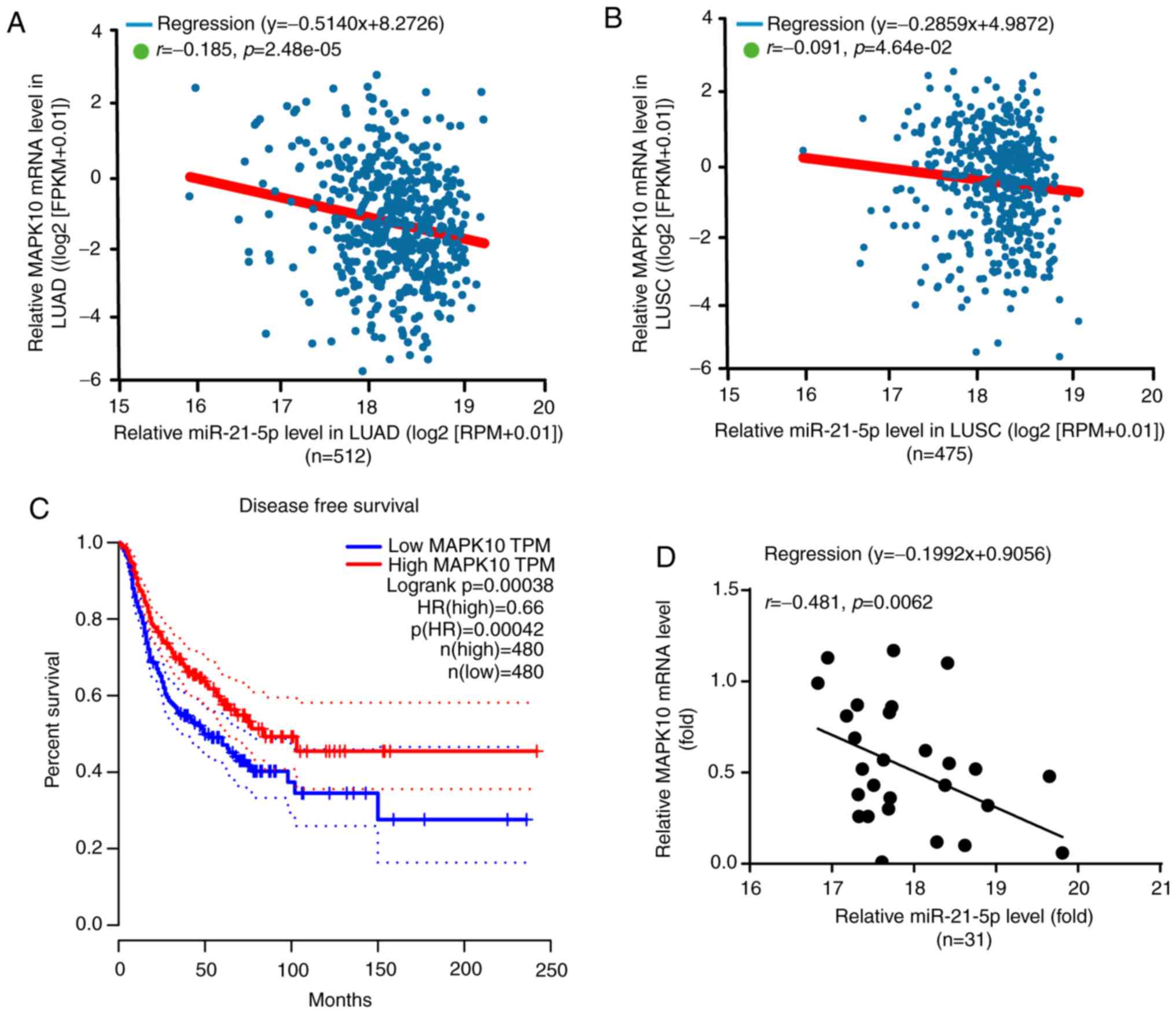
According to the results from Starbase V3.0 project,
there was a negative correlation between miR-21-5p and MAPK10
expression in LUAD (n=512) and LUSC (n=475) (Fig. 7A and B). Furthermore, miR-21-5p is
known to be associated with the DFS of patients with NSCLC
(23). Subsequently, results from
GEPIA software analysis demonstrated that a higher MAPK10
expression (n=480) was significantly associated with the prolonged
DFS of patients with NSCLC in LUAD and LUSC, which was not the case
for patients with lower MAPK10 expression (n=480; Fig. 7C).
Furthermore, miR-21-5p expression was negatively
correlated with MAPK10 expression in the tumor tissues of patients
with NSCLC included in the present study (Fig. 7D). In addition, the characteristics
of patients with NSCLC are presented in Table I, and MAPK10 expression was
demonstrated to be significantly associated with lymph node
metastasis and Tumor-Node-Metastasis stage; however, MAPK10
expression was not associated with sex, age, histological type,
EGFR mutation or KRAS mutation.
 | Table I.Association between MAPK10 expression
and the characteristics of patients with NSCLC (n=31). |
Table I.
Association between MAPK10 expression
and the characteristics of patients with NSCLC (n=31).
|
| MAPK10
expression |
|
|---|
|
|
|
|
|---|
|
Characteristics | High | Low | P-value |
|---|
| Sex |
|
| 0.724 |
|
Male | 9 | 7 |
|
|
Female | 7 | 8 |
|
| Age, years |
|
| 0.999 |
|
>55 | 10 | 9 |
|
|
≤55 | 6 | 6 |
|
| Histological
type |
|
| 0.285 |
|
Squamous cell carcinoma | 9 | 5 |
|
|
Adenocarcinoma | 7 | 10 |
|
| Lymph node
metastasis |
|
| 0.012 |
|
Yes | 12 | 4 |
|
| No | 4 | 11 |
|
| TNM stage |
|
| 0.029 |
|
I–II | 3 | 9 |
|
|
III–IV | 13 | 6 |
|
| EGFR mutation |
|
| 0.722 |
|
Yes | 8 | 6 |
|
| No | 8 | 9 |
|
| KRAS mutation |
|
| 0.458 |
|
Yes | 4 | 6 |
|
| No | 12 | 9 |
|
Subsequently, the present study investigated whether
the effects of propofol on NSCLC cell viability and apoptosis were
associated with miR-21-5p and MAPK10 expression. A549 and H1299
cells were therefore randomly divided into four different groups,
including the control group, propofol group, propofol + miR-21-5p
mimic group and the propofol + miR-21-5p mimic + pcDNA3-MAPK10
group.
Propofol decreases NSCLC cell
viability via the miR-21-5p/MAPK10 axis
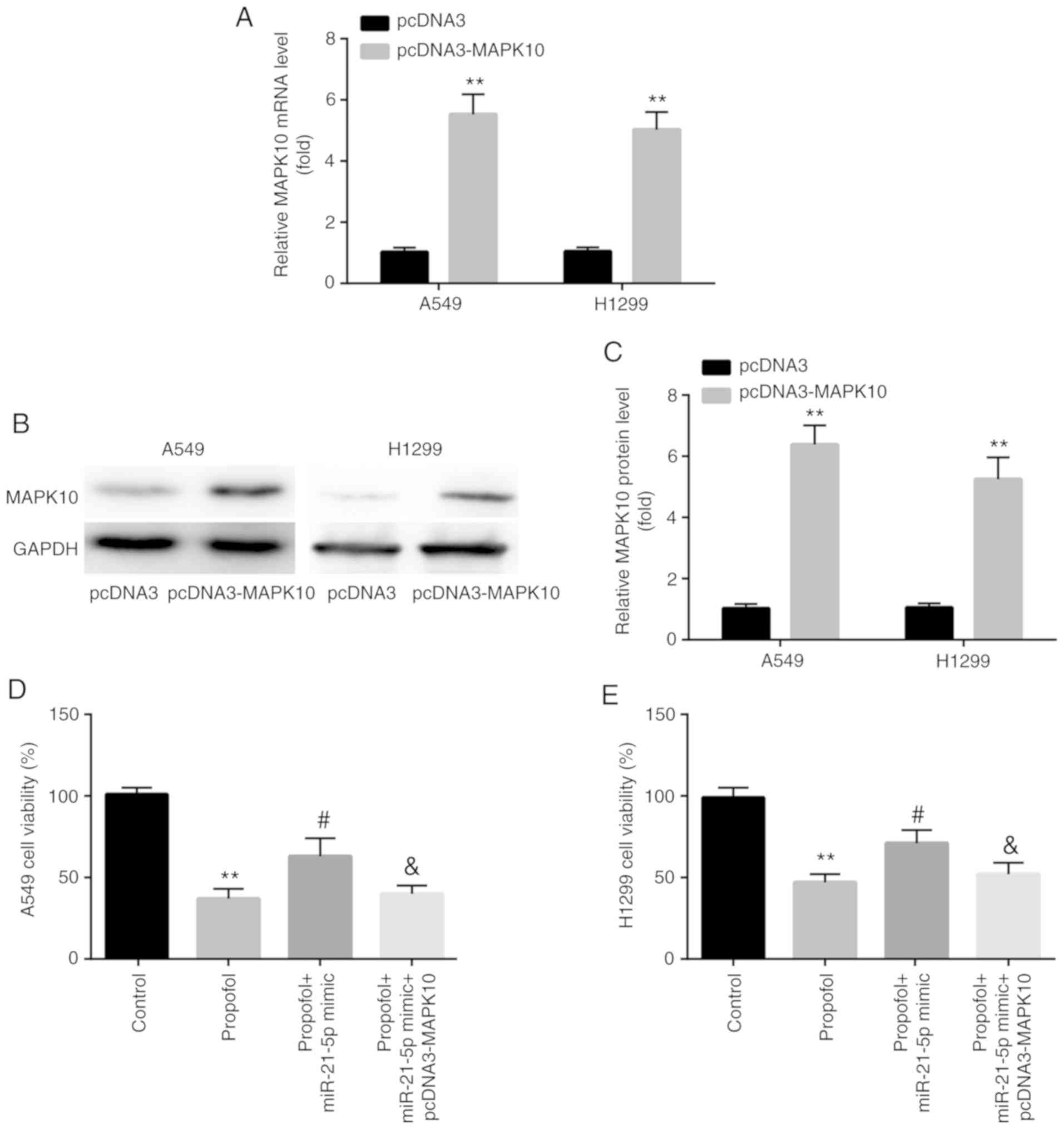
In A549 and H1299 cells, increased expression of
MAPK10 mRNA and protein was confirmed following transfection with
pcDNA3-MAPK10 (Fig. 8A-C).
Furthermore, results from CCK-8 assay demonstrated that
transfection with miR-21-5p mimic attenuated the propofol-induced
decrease in cell viability, which was reversed by pcDNA3-MAPK10
(Fig. 8D and E).
Propofol induces NSCLC apoptosis via
the miR-21-5p/MAPK10 axis
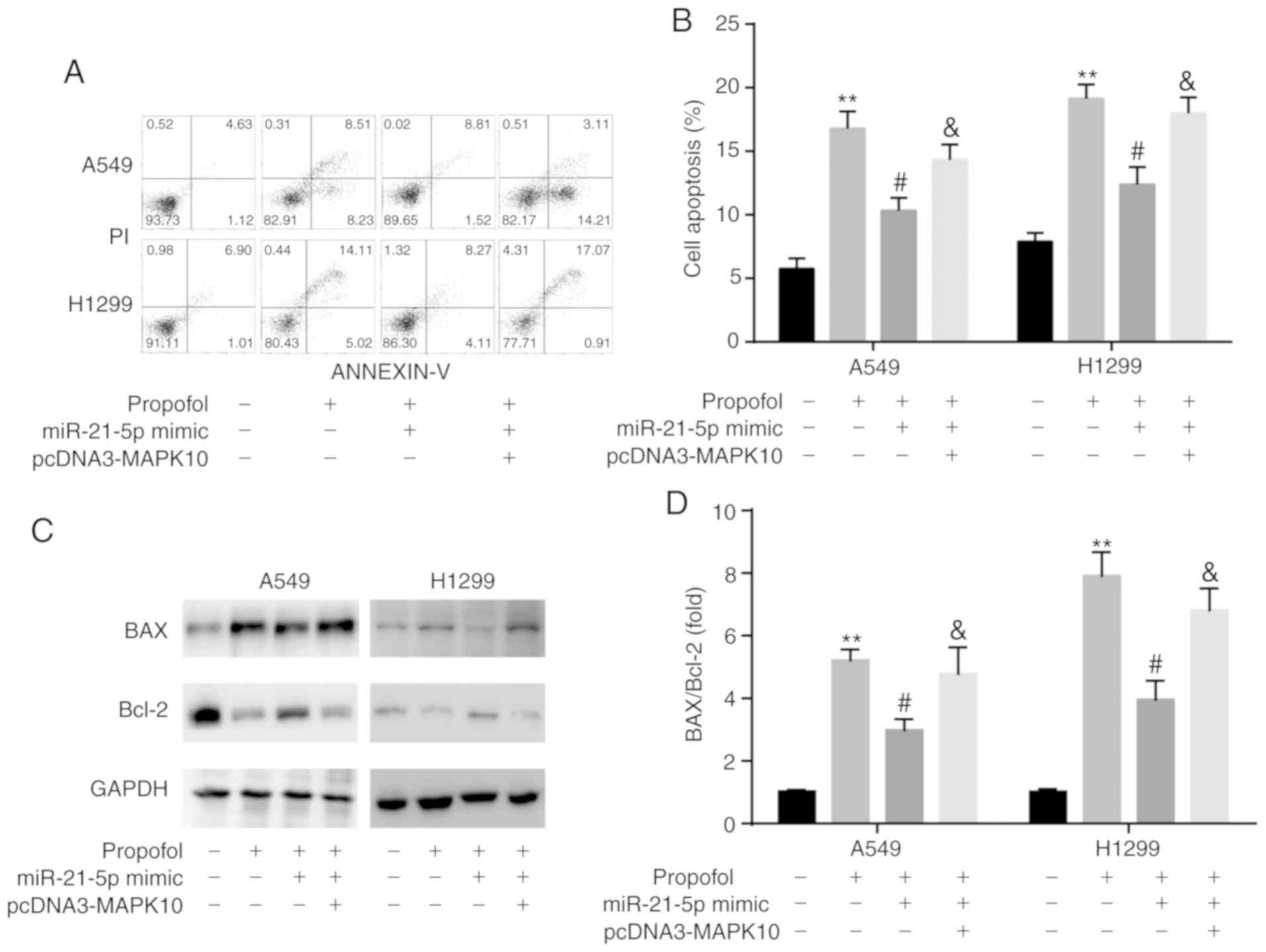
The results from flow cytometric assay demonstrated
that transfection with miR-21-5p mimic attenuated the
propofol-induced increase in A549 and H1299 cell apoptosis, which
was reversed by pcDNA3-MAPK10 (Fig. 9A
and B). In addition, results from western blotting revealed
that the ratio BAX/Bcl-2 was consistent with an increase in cell
apoptosis (Fig. 9C and D).
Discussion
In the present study, propofol inhibited NSCLC cell
proliferation and promoted NSCLC cell apoptosis by targeting the
miR-21-5p/MAPK10 axis. A recent study reported the association
between miR-21-5p and MAPK10 in breast cancer (24), and further confirmed the findings
from the present study.
Propofol is a frequently used intravenous anesthetic
agent (25). The clinical roles of
propofol in cancer are as follows: i) Propofol exhibits less
adverse effect on postoperative cognitive function from patients
with lung cancer than sevoflurane (26); ii) propofol is applied to solve
secondary cerebral lesions in patients with lung cancer (27); and iii) propofol-based total
intravenous anesthesia is associated with prolonged recurrence-free
survival and overall survival of patients who underwent tumor
resection (28). Propofol has been
demonstrated to decrease the LPS-induced apoptosis of the lung
epithelial cell line BEAS-2B (29).
In addition, it was reported that propofol serves crucial roles in
lung cancer. For example, propofol inhibits LPS-induced metastasis
by decreasing hypoxia-inducible factor-1α expression in NSCLC
(30). In addition, propofol
induces cell apoptosis by increasing the expression level of p53
upregulated modulator of apoptosis in NSCLC (31). It has also been demonstrated that
propofol decreases the expression of the inflammatory factor matrix
metalloproteinase 9 and the cognitive impairment in patients
undergoing lung cancer resection (32). Consistently, the present study
demonstrated that propofol inhibited NSCLC cell proliferation and
promoted NSCLC cell apoptosis.
miRNAs are novel biomarkers for NSCLC (33). Recent studies described a novel
therapeutic strategy based on miRNAs for patients with NSCLC
(34). For example, miR-1298
suppresses tumor progression in NSCLC (35). In addition, the inhibition of
miR-425 suppresses cell proliferation, migration and apoptosis in
NSCLC (36), and miR-421 predicts
the poor survival of patients with NSCLC (37). Propofol also inhibits the
progression of NSCLC by regulating the expression level of some
miRNAs, including miR-372 (13),
miR-1284 (14) and miR-486
(15). As an important oncogene in
NSCLC, a higher miR-21-5p level is associated with recurrence
(38), tumor stage (39) and poor overall survival (40) of patients with NSCLC. The link
between Fpropofol and miR-21 has been reported in several diseases,
but not in NSCLC. For example, propofol suppresses breast cancer
cell proliferation and EMT by inhibiting miR-21 expression
(41). In addition, propofol has
been reported to inhibit pancreatic cancer cell proliferation and
invasion by decreasing miR-21 expression level (42). The decrease in miR-21 expression is
associated with propofol-induced neurotoxicity in human stem
cell-derived neurons (43). The
present study demonstrated that propofol inhibited miR-21-5p
expression in NSCLC cell lines.
miRNAs function by targeting mRNAs (11). MAPK10 is an important pro-apoptotic
gene (20). MAPK10 has been
demonstrated to function as a novel epigenetic marker for
chromophobe kidney cancer (44).
Furthermore, MAPK10, which is targeted by miR-27a-3p, also serves a
promotional role in nasopharyngeal carcinoma (22). In addition, MAPK10, which is
targeted by miR-29b, is involved in ovarian carcinoma (45). However, to the best of our
knowledge, there is no study on the link between NSCLC and MAPK10,
propofol and MAPK10 or miR-21-5p and MAPK10 in NSCLC. In addition,
the association between propofol and MAPK10 has not been reported
in other diseases. The present study first verified that propofol
increased MAPK10 expression, which was decreased in NSCLC cell
lines, and that miR-21-5p targeted 3′-UTR of MAPK10.
Resistance to cell apoptosis contributes to the
progression of NSCLC (46). For
example, Acetyl-11-Keto-β-Boswellic acid has some anti-cancer
effects by stimulating NSCLC apoptosis (47), KCP10043F reduces NSCLC proliferation
by inducing caspase-mediated apoptosis (48) and ASB16-AS1 promotes the progression
of NSCLC by decreasing cell apoptosis (49). In addition, propofol was reported to
induce cell apoptosis by regulating the miR-21-5p/MAPK10 axis in
NSCLC cell lines. In conclusion, the present study demonstrated
that miR-21-5p and MAPK10 were associated with propofol-induced
inhibition of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All the data generated or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contribution
XW and GX performed the experiments. XW, XL and GX
analyzed the data. XL designed the experiments and prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of People's Hospital of Xinjiang Uygur Autonomous Region.
All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meza R, Meernik C, Jeon J and Cote ML:
Lung cancer incidence trends by gender, race and histology in the
United States, 1973–2010. PLoS One. 10:e01213232015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forde PM and Ettinger DS: Targeted therapy
for non-small-cell lung cancer: Past, present and future. Expert
Rev Anticancer Ther. 13:745–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pei K, Zhu JJ, Wang CE, Xie QL and Guo JY:
MicroRNA-185-5p modulates chemosensitivity of human non-small cell
lung cancer to cisplatin via targeting ABCC1. Eur Rev Med Pharmacol
Sci. 20:4697–4704. 2016.PubMed/NCBI
|
|
5
|
Johnson KN, Gooden G, Gonzalez P,
Sepulveda M, Gorgol L, Petricoin EF, Pierobon M, Byron S, Glen J,
Ahluwalia M, et al: BM-15 targeting MEK is a novel and effective
treatment strategy of lung CNS metastasis. Neuro Oncol. 16 (Suppl
5):v352014. View Article : Google Scholar
|
|
6
|
Gholipour Baradari A, Firouzian A, Zamani
Kiasari A, Aarabi M, Emadi SA, Davanlou A, Motamed N and Yousefi
Abdolmaleki E: Effect of etomidate versus combination of
propofol-ketamine and thiopental-ketamine on hemodynamic response
to laryngoscopy and intubation: A randomized double blind clinical
trial. Anesth Pain Med. 6:e300712016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang X, Teng Y, Yang H and Ma J: Propofol
inhibits invasion and growth of ovarian cancer cells via regulating
miR-9/NF-kB signal. Braz J Med Biol Res. 49:e57172016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ecimovic P, Murray D, Doran P and Buggy
DJ: Propofol and bupivacaine in breast cancer cell function in
vitro-role of the NET1 gene. Anticancer Res. 34:1321–1331.
2014.PubMed/NCBI
|
|
9
|
Xu YJ, Li SY, Cheng Q, Chen WK, Wang SL,
Ren Y and Miao CH: Effects of anaesthesia on proliferation,
invasion and apoptosis of LoVo colon cancer cells in vitro.
Anaesthesia. 71:147–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Zhang D, Wu GQ, Feng ZY and Zhu
SM: Propofol inhibits the adhesion of hepatocellular carcinoma
cells by upregulating microRNA-199a and downregulating MMP-9
expression. Hepatobiliary Pancreat Dis Int. 12:305–309. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun H and Gao D: Propofol suppresses
growth, migration and invasion of A549 cells by down-regulation of
miR-372. BMC Cancer. 18:12522018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu WZ and Liu N: Propofol inhibits lung
cancer A549 cells growth and epithelial-mesenchymal transition
process by upregulation of microRNA-1284. Oncol Res. 27:1–8. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang N, Liang Y, Yang P, Yang T and Jiang
L: Propofol inhibits lung cancer cell viability and induces cell
apoptosis by upregulating microRNA-486 expression. Braz J Med Biol
Res. 50:e57942017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cserni G, Chmielik E, Cserni B and Tot T:
The new TNM-based staging of breast cancer. Virchows Arch.
472:697–703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cancer Genome Atlas Research Network, .
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang DD, Kuan CY, Whitmarsh AJ, Rincón M,
Zheng TS, Davis RJ, Rakic P and Flavell RA: Absence of
excitotoxicity-induced apoptosis in the hippocampus of mice lacking
the Jnk3 gene. Nature. 389:865–870. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ying J, Li H, Cui Y, Wong AH, Langford C
and Tao Q: Epigenetic disruption of two proapoptotic genes
MAPK10/JNK3 and PTPN13/FAP-1 in multiple lymphomas and carcinomas
through hypermethylation of a common bidirectional promoter.
Leukemia. 20:1173–1175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L and Luo Z: Dysregulated miR-27a-3p
promotes nasopharyngeal carcinoma cell proliferation and migration
by targeting Mapk10. Oncol Rep. 37:2679–2687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ulivi P, Petracci E, Marisi G, Baglivo S,
Chiari R, Billi M, Canale M, Pasini L, Racanicchi S, Vagheggini A,
et al: Prognostic role of circulating miRNAs in early-stage
non-small cell lung cancer. J Clin Med. 8:E1312019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie Y, Liu Y, Fan X, Zhang L, Li Q, Li S,
Wang H and Xiao Y: MicroRNA-21 promotes progression of breast
cancer via inhibition of mitogen-activated protein kinase10
(MAPK10). Biosci Rep. Aug 2–2019.(Epub ahead of print).
|
|
25
|
Vasileiou I, Xanthos T, Koudouna E, Perrea
D, Klonaris C, Katsargyris A and Papadimitriou L: Propofol: A
review of its non-anaesthetic effects. Eur J Pharmacol. 605:1–8.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun H, Zhang G, Ai B, Zhang H, Kong X, Lee
WT, Zheng H, Yan T and Sun L: A systematic review: Comparative
analysis of the effects of propofol and sevoflurane on
postoperative cognitive function in elderly patients with lung
cancer. BMC Cancer. 19:12482019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gheorghita E, Pruna VM, Neagoe L, Bucur C,
Cristescu C and Gorgan MR: Perioperative management of patients
with lung carcinoma and cerebral metastases. Maedica (Buchar).
5:28–33. 2010.PubMed/NCBI
|
|
28
|
Yap A, Lopez-Olivo MA, Dubowitz J, Hiller
J and Riedel B; Global Onco-Anesthesia Research Collaboration
Group, : Anesthetic technique and cancer outcomes: A meta-analysis
of total intravenous versus volatile anesthesia. Can J Anaesth.
66:546–561. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lv X, Zhou X, Yan J, Jiang J and Jiang H:
Propofol inhibits LPS-induced apoptosis in lung epithelial cell
line, BEAS-2B. Biomed Pharmacother. 87:180–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang N, Liang Y, Yang P and Ji F: Propofol
suppresses LPS-induced nuclear accumulation of HIF-1α and tumor
aggressiveness in non-small cell lung cancer. Oncol Rep.
37:2611–2619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xing SG, Zhang KJ, Qu JH, Ren YD and Luan
Q: Propofol induces apoptosis of non-small cell lung cancer cells
via ERK1/2-dependent upregulation of PUMA. Eur Rev Med Pharmacol
Sci. 22:4341–4349. 2018.PubMed/NCBI
|
|
32
|
Wang G, Liu J, Gao J and Zheng X:
Comparison of the effects of sevoflurane and propofol anesthesia on
pulmonary function, MMP-9 and postoperative cognition in patients
receiving lung cancer resection. Oncol Lett. 17:3399–3405.
2019.PubMed/NCBI
|
|
33
|
Wojczakowski W, Kobylarek D, Lindner J,
Limphaibool N and Kaczmarek M: MicroRNAs-novel biomarkers for
malignant pleural effusions. Contemp Oncol (Pozn). 23:133–140.
2019.PubMed/NCBI
|
|
34
|
Li G, Fang J, Wang Y, Wang H and Sun CC:
MiRNA-based therapeutic strategy in lung cancer. Curr Pharm Des.
23:6011–6018. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du Z, Wu J, Wang J, Liang Y, Zhang S,
Shang Z and Zuo W: MicroRNA-1298 is downregulated in non-small cell
lung cancer and suppresses tumor progression in tumor cells. Diagn
Pathol. 14:1322019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang L, Ge W and Geng J: MiR-425
regulates cell proliferation, migration and apoptosis by targeting
AMPH-1 in non-small-cell lung cancer. Pathol Res Pract.
215:1527052019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Duan FG, Wang MF, Cao YB, Dan Li, Li RZ,
Fan XX, Khan I, Lai HL, Zhang YZ, Hsiao WW, et al: MicroRNA-421
confers paclitaxel resistance by binding to the KEAP1 3′UTR and
predicts poor survival in non-small cell lung cancer. Cell Death
Dis. 10:8212019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang M, Shen H, Qiu C, Ni Y, Wang L, Dong
W, Liao Y and Du J: High expression of miR-21 and miR-155 predicts
recurrence and unfavourable survival in non-small cell lung cancer.
Eur J Cancer. 49:604–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abdollahi A, Rahmati S, Ghaderi B, Sigari
N, Nikkhoo B, Sharifi K and Abdi M: A combined panel of circulating
microRNA as a diagnostic tool for detection of the non-small cell
lung cancer. QJM. 112:779–785. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song F, Xuan Z, Yang X, Ye X, Pan Z and
Fang Q: Identification of key microRNAs and hub genes in
non-small-cell lung cancer using integrative bioinformatics and
functional analyses. J Cell Biochem. 121:2690–2703. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Du Q, Zhang X, Zhang X, Wei M, Xu H and
Wang S: Propofol inhibits proliferation and epithelial-mesenchymal
transition of MCF-7 cells by suppressing miR-21 expression. Artif
Cells Nanomed Biotechnol. 47:1265–1271. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Z, Zhang J, Hong G, Quan J, Zhang L
and Yu M: Propofol inhibits growth and invasion of pancreatic
cancer cells through regulation of the miR-21/slug signaling
pathway. Am J Transl Res. 8:4120–4133. 2016.PubMed/NCBI
|
|
43
|
Twaroski DM, Yan Y, Olson JM, Bosnjak ZJ
and Bai X: Down-regulation of microRNA-21 is involved in the
propofol-induced neurotoxicity observed in human stem cell-derived
neurons. Anesthesiology. 121:786–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yoo KH, Park YK, Kim HS, Jung WW and Chang
SG: Identification of MAPK10 as a novel epigenetic marker for
chromophobe kidney cancer. Pathol Int. 61:52–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dai F, Zhang Y and Chen Y: Involvement of
miR-29b signaling in the sensitivity to chemotherapy in patients
with ovarian carcinoma. Hum Pathol. 45:1285–1293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu G, Pei F, Yang F, Li L, Amin AD, Liu
S, Buchan JR and Cho WC: Role of autophagy and apoptosis in
non-small-cell lung cancer. Int J Mol Sci. 18:E3672017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lv M, Shao S, Zhang Q, Zhuang X and Qiao
T: Acetyl-11-Keto- β-Boswellic acid exerts the anti-cancer effects
via cell cycle arrest, apoptosis induction and autophagy
suppression in non-small cell lung cancer cells. Onco Targets Ther.
13:733–744. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee JH, Lee HH, Ryu KD, Kim M, Ko D, Chung
KS, Hassan AHE, Lee SH, Lee JY and Lee KT: KCP10043F represses the
proliferation of human non-small cell lung cancer cells by
caspase-mediated apoptosis via STAT3 inactivation. J Clin Med.
9:E7042020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tan LJ, Liu JT, Yang M, Ju T and Zhang YS:
LncRNA ASB16-AS1 promotes proliferation and inhibits apoptosis of
non-small cell lung cancer cells by activating the Wnt/β catenin
signaling pathway. Eur Rev Med Pharmacol Sci. 24:1870–1876.
2020.PubMed/NCBI
|