Introduction
Laryngeal carcinoma (LCC) is a common malignant
tumor of the head and neck, which accounts for 5.7–7.6% of all
malignant tumors (1). Approximately
40% of patients with LCC have late-stage (III–IV) disease at the
time of diagnosis (1,2). Due to the basic laryngeal and
hypopharyngeal dysfunction, the presenting symptoms, such as
hoarseness, difficulty swallowing, or difficulty breathing, lead to
a significant decline in the quality of life of the patients
(3,4). Therefore, in addition to achieving
optimal local control, it is crucial to maintain vocalization and
swallowing function. Radiotherapy, one of the main treatment
modalities for LCC, may fully preserve the vocal function of the
patients and is considered to be a valuable alternative to total
laryngectomy for advanced tumors (5,6). A
comprehensive treatment strategy has been developed for LCC that
includes surgical resection combined with radiotherapy or
chemotherapy (7,8). However, despite this comprehensive
treatment regimen, tumors often exhibit low sensitivity to
radiotherapy and the response rates are generally poor,
particularly in patients with advanced and/or recurrent tumors
(8). Therefore, improved strategies
for the prevention and treatment of LCC, including the exploration
of radiotherapy and therapeutic targets, are urgently needed, which
represents an important and pressing issue in the field of
biomedical research.
The ubiquitin-proteasome system (UPS) is a regulator
of protein homeostasis and cellular signaling. Defective UPS may
lead to abnormal protein expression, interaction and cellular
localization (9). Among the three
known components of the UPS, the E3 ubiquitin ligases are primarily
responsible for determining substrate specificity and ubiquitin
chain topology (10). Recently,
targeting E3 ligases has attracted interest as a strategy for
cancer treatment (9,10). A previous study demonstrated that
inhibition of the E3 ubiquitin ligase CHIP promotes
radiosensitivity in human lung cancer cells via the CHIP-HSP70-p21
ubiquitination/degradation axis (11). Increased expression of the E3 ligase
cIAP2 resulted in altered MRE11 ubiquitination models and mediated
radiosensitization in response to histone deacetylase inhibition
(12). In addition, an increasing
number of studies have indicated E3 ubiquitin ligases as novel
effectors linking the p38/mitogen-activated protein kinase (MAPK)
and phosphoinositide 3-kinase signaling pathways to the cell cycle
(13,14). These findings prompt further
investigation of E3 ligases as potential regulators of
radiosensitivity in cancer.
Ubiquitin protein ligase E3 component n-recognin 5
(UBR5), also referred to as EDD, is a nuclear phosphoprotein
involved in the regulation of DNA damage responses, β-catenin
activity, metabolic processes and apoptosis (9). UBR5 was also recently identified as a
key regulator of the UPS and ciliogenesis, which may have important
implications in elucidating cancer pathophysiology (10,15).
As a downstream factor of BMI1, UBR5 is enriched to ultraviolet
(UV) radiation-induced damage along with the FACT component SPT16
(16). UBR5 was identified as a
mediator of the activating phosphorylation of checkpoint kinase 2
in response to DNA damage following exposure to ionizing radiation
(IR), revealing its potential importance in cancer (17). Moreover, UBR5 is a key component of
ataxia-telangiectasia mutated (ATM) activation in response to IR.
Upon stimulation by IR, UBR5 catalyzes the ubiquitination of ATM,
which decreases the interaction of the ATM interacting protein with
ATM and promotes Mre11/Rad50/NBS1-mediated signaling, thus
impairing checkpoint activation and increasing radiosensitivity
(18). In addition, increased UBR5
expression has been shown to promote metastasis in triple-negative
breast cancer (19), while high
UBR5 expression in ovarian cancer is associated with poor prognosis
(20). Therefore, UBR5 may play an
important role in regulating sensitivity to radiotherapy in these
types of cancer. However, the function of UBR5 in LCC and its
potential role in radiosensitivity remain unclear. A previous study
indicated that p38/MAPK phosphorylation is associated with
radiosensitivity in liver cancer (21). The aim of the present study was to
analyze the expression and function of UBR5 in LCC cell lines, and
to further investigate whether UBR5 regulates radiosensitivity in
LCC via p38/MAPK signaling, with the hope of uncovering the
mechanism underlying the development of radiosensitivity and
identifying a novel target for the clinical treatment of LCC.
Materials and methods
Patients and variables
A total of 171 LCC patients from the First People's
Hospital of Foshan were consecutively recruited between August 2011
and May 2018. LCC and adjacent tissue samples were collected during
surgery or biopsy. The clinicopathological characteristics of LCC
patients, including age, sex, TNM stage and absence of radiotherapy
history, were reviewed to identify their association with UBR5
expression. Biopsies from LCC patients were obtained prior to
radiotherapy. The present study was approved by the Ethics
Committee of the First People's Hospital of Foshan. Written
informed consent was obtained from all patients who participated in
this study, according to the committee regulations.
Raw biological microarray data and
functional enrichment analysis
Raw DNA microarray data of patients with LCC were
obtained from Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo) (22). Corresponding probes were converted
into symbols according to the annotation information in the
platform. Three chip datasets GSE51985 (10 LCC and 10 normal
samples) were downloaded from GEO (Illumina GPL10558 platform).
Back-ground correction of probe data, normalization and
summarization were executed by robust multi-array average analysis
algorithm17 in the affy package of R. The follow-up duration was
estimated using the Kaplan-Meier method with 95% confidence
interval (95%CI) and log-rank test in separate curves. In addition,
related long non-coding RNAs (lncRNAs), targeted miRNA and
protein-protein interaction (PPI) networks were predicted using R
software. The biological process from Gene Ontology and Kyoto
Encyclopedia of Genes and Genomes pathway analysis of PPI genes was
performed and visualized using ClueGO, version 2.5.3 (23) and CluePedia, version 1.5.3 (24).
Cell lines and culture
The human LCC cell lines M2E and M4E were purchased
from Central South University (http://gdyjzx.csu.edu.cn/info/1034/1204_2.htm). The
HuLa-PC cell line was a kind gift from Dr Deng (Tongren Hospital of
WuHan University). All cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100
µg/ml penicillin. The cells were cultured in a 37°C humidified
incubator with an atmosphere of 5% CO2 and were
harvested in the logarithmic growth phase. Both M2E and M4E cell
lines were authenticated by STR profiling.
Immunohistochemistry (IHC)
staining
The tissue samples were fixed in formalin at room
temperature for 24 h, embedded in paraffin, cut into 10-µm sections
and deparaffinized. IHC staining was performed using a Dako
Envision System (Dako; Agilent Technologies, Inc.) following the
manufacturer's protocol at room temperature. The sections were
blocked using serum-free protein blocking buffer (Dako; Agilent
Technologies, Inc.) for 30 min at room temperature, after which
time they were incubated with anti-UBR5 antibody (1:200, cat. no.
ab70311, Abcam). Images were captured using a Nikon light
microscope (magnification, ×200), and staining intensity was
analyzed using Nikon software (NIS-Elements AR 3.2; Nikon
Corporation). The IHC score was calculated according to the amount
and level of immunoreactivity as follows: The percentage of stained
cells was scored on a scale from 0 to 4 [0 (<1%), 1 (1-24%), 2
(25-49%), 3 (50-74%) and 4 (75-100%)], and the staining intensity
was scored from 0 to 3 [0 (no staining), 1 (pale yellow), 2
(yellow), and 3 (reddish-brown)]. The final IHC score was
determined by multiplying the intensity score and the positivity
score to achieve a final score ranging between 0 and 12. Expression
scores higher than the median score were categorized as
positive.
RNA interference and UBR5
overexpression vector construction
Small interfering RNAs (si-RNAs) targeting UBR5 were
synthesized; the sequences were as follows: si-UBR5-1 sense
5′-GCAGUGUUCCUGCCUUCU-3′ and antisense 5′-AGAAGGCAGGAACACUGC-3′;
si-UBR5-2 sense 5′-GCGACUCUCCAUGGUUUCU-3′ and antisense
5′-AGAAACCAUGGAGAGUCGC-3′; scrambled control sense
5′-UUCUCCGAACGUGUCACGU-3′; scrambled control antisense
5′-ACGUGACACGUUCGGAGAA-3′. In order to construct a plasmid for
overexpressing UBR5, the coding sequence of UBR5 was inserted into
the p-Enter vector (Vigene Bio.) according to the manufacturer's
instructions. The empty p-Enter vector was used as control. UBR5
si-RNAs or scrambled si-RNA were transfected into cells at a final
concentration of 20 nM/ml. UBR5 overexpression plasmid or the empty
vector control were transfected in LCC cells at a final
concentration of 1 µg/µl with Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Successful transfection was confirmed by reverse
transcription-quantitative PCR (RT-qPCR) analysis and western
blotting. All subsequent experiments were performed 48 h after the
transfection.
RT-qPCR analysis
Total RNA was extracted from cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. RNAs were then reverse-transcribed
into cDNA using the PrimeScript™ RT Master Mix (Takara Bio, Inc.)
at 42°C for 20 min and 90°C for 5 min. qPCR reactions were
performed using SYBR® Premix Ex Taq™ II (Takara Bio,
Inc.) following standard protocols. Briefly, an initial
denaturation at 95°C for 3 min was followed by 40 cycles of
denaturation (at 95°C for 20 sec), annealing (at 55°C for 45 sec),
and elongation (at 72°C for 30 sec), with a final extension at 72°C
for 5 min. The PCR primers used for UBR5 were as follows: Forward
5′-GACGCGAGAACTCTTGGAAC-3′ and reverse 5′-TTCAAATGGATTTGGGGGTA-3′.
The PCR primers used for β-actin were as follows: Forward
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse
5′-CTCCTTAATGTCACGCACGAT-3′. The relative UBR5 expression quantity
was calculated using the 2−ΔΔCq method (25). Each sample was replicated three
times.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Takara Bio, Inc.) according to the manufacturer's
instructions, and the protein concentration was quantified using a
bicinchoninic acid protein assay kit. A total of 15 µg protein per
lane was separated by 10% SDS/PAGE and then transferred onto a
polyvinylidene fluoride membrane. The membrane was incubated with
blocking buffer (1X TBS, 0.1% Tween-20, 5% non-fat dry milk) for 2
h at room temperature and then with the appropriate primary
antibody overnight at 4°C. The following primary antibodies were
used: Anti-UBR5 (1:1,000, cat. no. ab70311, Abcam), anti-Bax
(1:1,000, cat. no. ab32503, Abcam), anti-Bcl-2 (1:1,000, cat. no.
ab185002, Abcam), anti-p38 (1:1,000, cat. no. ab170099, Abcam),
anti-phosphorylated (p)-p38 (1:1,000, cat. no. ab178867, Abcam),
and anti-β-actin (1:1,000, cat. no. ab115777, Abcam) antibody. The
membrane was then incubated with secondary antibody for 2 h at room
temperature. Protein bands were visualized using ECL plus western
blotting detection reagents (BD Biosciences) and detected with an
enhanced chemiluminescence kit.
Cell proliferation assays
LCC cells were seeded in a 96-well plate
(5×103 cells/well) and cultured in a 5% CO2
incubator at 37°C for 24 h. Next, 10 µl CCK8 solution (KeyGen Bio.)
was added to each well, and the cells were incubated for 4 h. The
OD value of the medium was detected using a spectrophotometer at
450 nm. In some experiments, 5 µM SB203580 (Selleck Chemicals), a
p38/MAPK inhibitor, was added to UBR5-overexpressing cells
overnight before analyses.
Cell migration and invasion
assays
A Transwell 24-well Boyden chamber with a 8-µm
polycarbonate membrane (Corning, Inc.) was used to examine the
migration capacity. For invasion assay, the Transwell insert was
pretreated with Matrigel (BD Biocoat) at 37°C for 8 h. LCC cells
(1×105 cells) were seeded in serum-free medium in the
upper chamber of the insert; medium with 20% FBS was included in
the lower chamber. After 24 h, cells on the bottom surface of the
filter were fixed with 4% polyoxymethylene at room temperature for
15 min and then 1% crystal violet dye was used to stain the cells
at room temperature for 10 min. Cells were visualized using a Leica
AF6000 LX fluorescence microscope (Meyer Instruments, Inc.) and
counted in 5 randomly selected fields of vision at a magnification,
×100.
Cell cycle analysis
Flow cytometry was used to examine the cell cycle
distribution of LCC cells using a cell cycle assay kit (KeyGen
Bio.) according to the manufacturer's instructions. The cells were
washed twice with PBS and assayed on the machine. The percentage of
cells in each phase of the cell cycle was then calculated.
Radiosensitivity assays
To investigate the impact of UBR5 on
radiosensitivity, M2E and M4E cells transfected with si-RNA,
overexpression plasmid or controls were seeded in 96-well plates at
a density of 5,000 cells/well. The cells were then exposed to 0, 2,
5 or 8 Gy, and CCK8 assay was performed 48 h later, as described
above. Cell numbers of treated and control groups were converted by
the CCK8 standard curve method. The cell viability rate was
calculated as the cell number of the treated group/5,000 ×100%.
For survival fraction assays, cells seeded in a 6-cm
culture plate were exposed to 0, 2, 4, 6 or 8 Gy, after which time
the cells were routinely cultured for 2 weeks. Cells were fixed in
methanol at room temperature for 15 min, stained with 1% crystal
violet-ethanol solution at room temperature for 10 min, and the
number of clones was calculated in 5 randomly selected fields of
vision at a magnification of ×100, using a Leica DM2500 system
microscope (Meyer Instruments, Inc.). The survival rate was
calculated as the number of clones in the treated group/the number
of clones in the control group. All experiments were replicated
three times.
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. Statistical analysis was performed using R
software (version 3.5.1; http://cran.r-project.org/bin/windows/base/old/3.5.1/),
R studio software (version 1.2.1335; http://rstudio.com/products/rstudio/download/) and
GraphPad Prism 5 software (version 7.0; GraphPad Software, Inc.).
Comparisons between two groups were analyzed using a two-tailed
unpaired Student's t-test. One-way ANOVA with Bonferroni correction
was used to compare multiple groups. The χ2 test was
used for cell cycle distribution analysis. The median mRNA and
protein levels were used as cut-off values in the two cohorts of
LCC cases. The Kaplan-Meier method with log-rank hazard ratio (95%
CI) was used to construct survival plots. P<0.05 was considered
to indicate statistically significant differences in all tests.
Results
UBR5 expression pattern and prognostic
value in human LCC tissues
To investigate the potential role of UBR5 in LCC
development, the expression pattern of UBR5 was first examined in
171 LCC tissue samples and adjacent non-tumor tissues. IHC
demonstrated that UBR5 was significantly overexpressed in LCC
tissues compared with its levels in adjacent non-tumor tissues
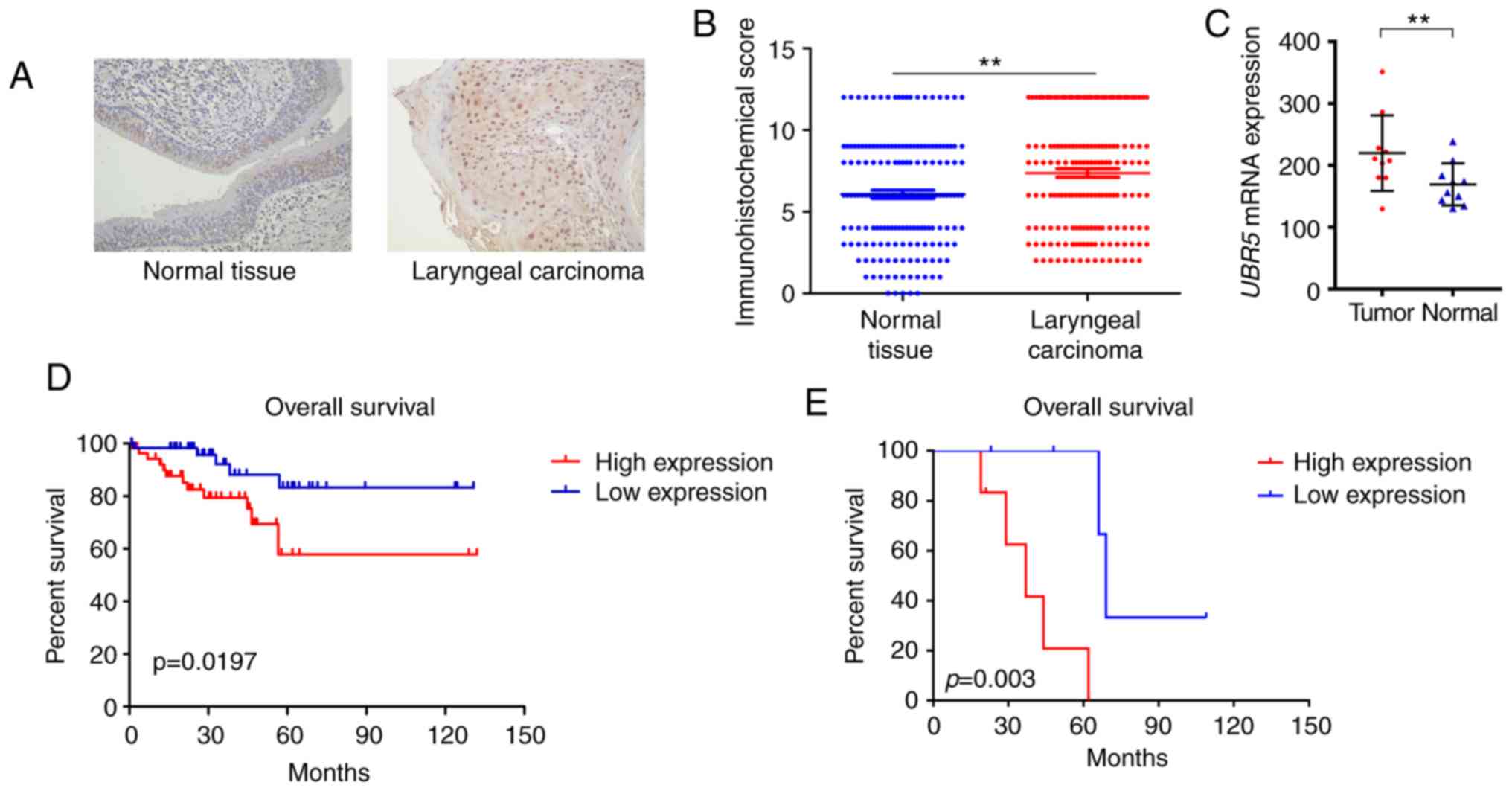
(P<0.05; Fig. 1A and B).
Patients positive for UBR5 expression were likely to be male with
T3-4, N2-3 or M1 disease, while patients with T1-2, N0-1 or M0
stage tended to be UBR5-negative (Table
I).
 | Table I.Association of clinicopathological
characteristics with UBR5 expression status in patients with LCC
(n=171). |
Table I.
Association of clinicopathological
characteristics with UBR5 expression status in patients with LCC
(n=171).
|
|
| UBR5 expression, n
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Entire cohort, n
(%) | IHC negative
(n=85) | IHC positive
(n=86) | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.874 | 0.350 |
|
<70 | 128 (74.8) | 60 (70.6) | 68 (79.1) |
|
|
|
≥70 | 43 (25.2) | 25 (29.4) | 18 (20.9) |
|
|
| Sex |
|
|
| 0.491 | 0.712 |
|
Male | 131 (76.7) | 62 (72.9) | 69 (80.2) |
|
|
|
Female | 40 (23.3) | 23 (27.1) | 17 (19.8) |
|
|
| T
stagea |
|
|
| 7.754 |
<0.001 |
|
T1-T2 | 101 (59.1) | 64 (75.3) | 37 (43.0) |
|
|
|
T3-T4 | 70 (40.9) | 21 (24.7) | 49 (57.0) |
|
|
| N
stagea |
|
|
| 9.173 |
<0.001 |
|
N0-N1 | 151 (88.3) | 80 (94.1) | 71 (82.6) |
|
|
|
N2-N3 | 20 (11.7) | 5 (5.9) | 15 (17.4) |
|
|
| M
stagea |
|
|
| 4.229 | 0.037 |
| M0 | 152 (88.9) | 78 (91.8) | 74 (86.0) |
|
|
| M1 | 19 (11.1) | 7 (8.2) | 12 (14.0) |
|
|
| Radiotherapy |
|
|
| 0.917 | 0.203 |
|
Yes | 43 (25.0) | 18 (21.2) | 25 (29.1) |
|
|
| No | 128 (75.0) | 67 (78.8) | 61 (70.9) |
|
|
In addition, transcriptional expression data from
the GEO database indicated that UBR5 expression was markedly
increased in tumor samples (213.23±74.1) compared with that in
non-tumor tissues (172.4±47.23; Fig.
1C). The median survival time of the high UBR5 expression group
was 43 months (interquartile range, 12–57 months) compared with 67
months (interquartile range, 23–79 months) in the low UBR5
expression group. The median survival time of patients in our
cohort has not been reached. Survival curves demonstrated that
increased UBR5 mRNA and protein expression was significantly
correlated with poor overall survival in LCC patients (P<0.05;
Fig. 1D and E).
Expression of UBR5 in LCC cell
lines
Using RT-qPCR and western blot analyses, UBR5
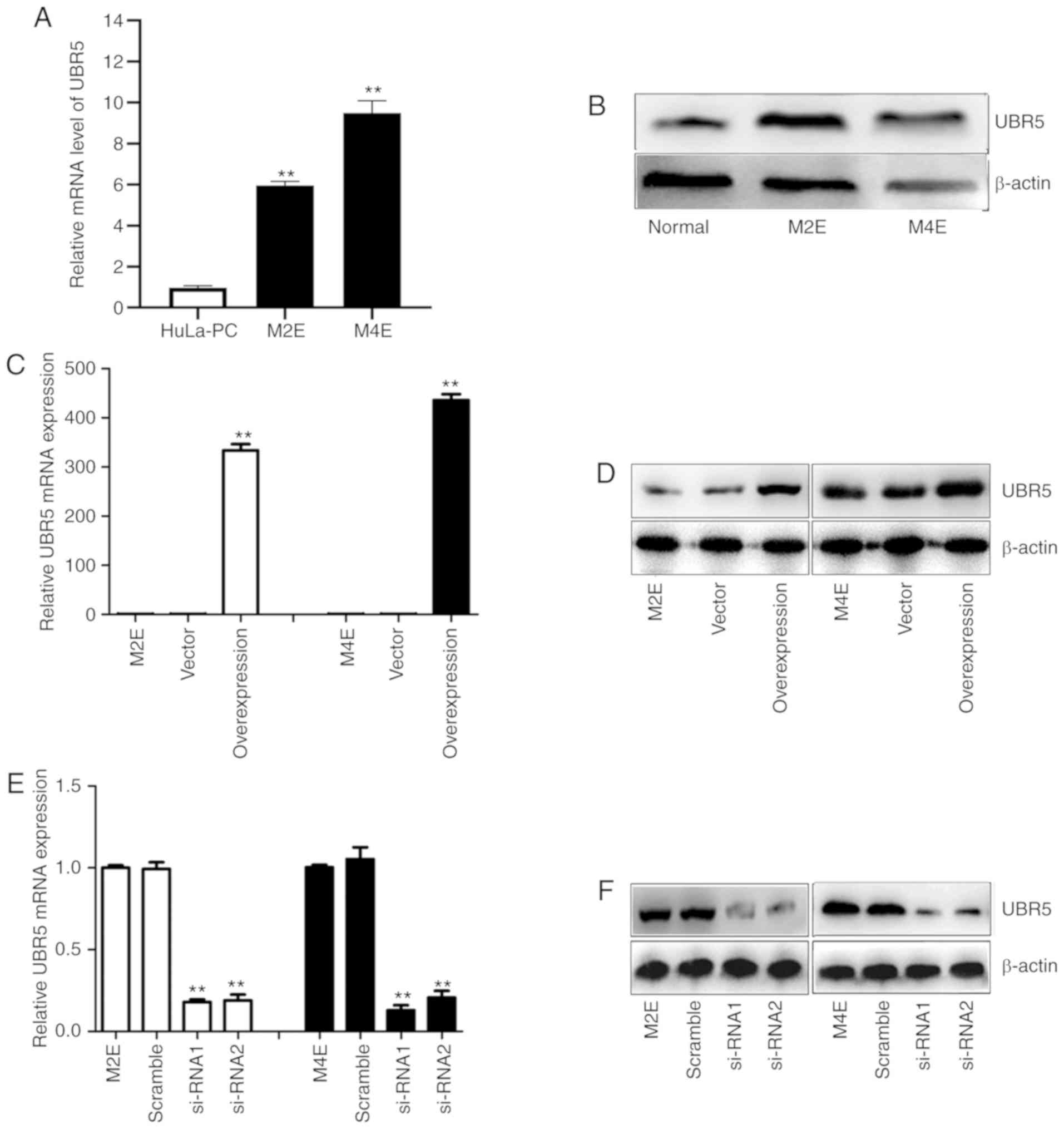
expression was examined in two LCC cell lines. As illustrated in
Fig. 2A and B, the UBR5 mRNA and
protein expression levels were significantly higher in M2E and M4E
cells compared with HuLa-PC cells.
To more closely examine the function of UBR5 in LCC,
a UBR5 overexpression construct was generated and si-RNA was used
to target UBR5 in LCC cells. Transfection of M2E and M4E cells with
the overexpression plasmid resulted in increased UBR5 mRNA
expression by 300-400-fold compared with its levels in cells
transfected with vector (Fig. 2C).
Western blotting revealed a similar trend (Fig. 2D). After transfection of M2E and M4E
cells with UBR5 si-RNAs, UBR5 mRNA expression decreased
significantly (0.27±0.07 in M2E cells and 0.31±0.09 in M4E cells)
compared with its levels in the negative control groups (P<0.05;
Fig. 2E). Western blotting
similarly confirmed that UBR5 expression was downregulated by
transfection of UBR5 si-RNA in both M2E and M4E cells (Fig. 2F).
UBR5 regulates the proliferation of
M2E and M4E cells
CCK-8 assay was next performed to evaluate the
effect of UBR5 on the proliferation capacities of M2E and M4E LCC
cells. UBR5 silencing significantly decreased cell proliferation
(Fig. 3A and B), whereas UBR5
overexpression significantly increased the proliferation of both
cell lines compared with the control groups (Fig. 3C and D). We further analyzed the
impact of URB5 expression on the cell cycle, as shown in Fig. 3E-J. The percentage of cells in the S
phase was relatively similar in the untreated, vector and UBR5
overexpression groups (45, 42 and 45%, respectively). However,
compared to the control group (Fig.
3H), the percentage of S phase cells significantly decreased in
both UBR5 si-RNA-1- and UBR5 si-RNA-2-transfected cells (38.2 and
39.4%, respectively; P<0.05; Fig. 3I
and J).
Effect of UBR5 on the invasion and
migration of LCC cells
To explore the role of UBR5 in the invasion and
migration of LCC cells, invasion and migration assays were
performed. As shown in Fig. 4A and
B, the number of invading cells slightly decreased in M4E cells
transfected with si-RNA-1 (382±14) compared with controls (413±15),
but the difference was not statistically significant (P>0.05).
Similarly, no statistically significant differences were observed
in terms of migration or invasion among the four groups of M2E
cells. We also observed no statistically significant difference in
the number of migrating cells in the UBR5-overexpressing M2E and
M4E cells compared with the control or vector groups (Fig. 4E and F). The invasion assay revealed
similar trends in M4E and M2E cells (Fig. 4C, D, G and H), wit up- or
downregulation of UBR5 exerting no effect on cell invasion.
UBR5 regulates radiosensitivity in M2E
and M4E cells
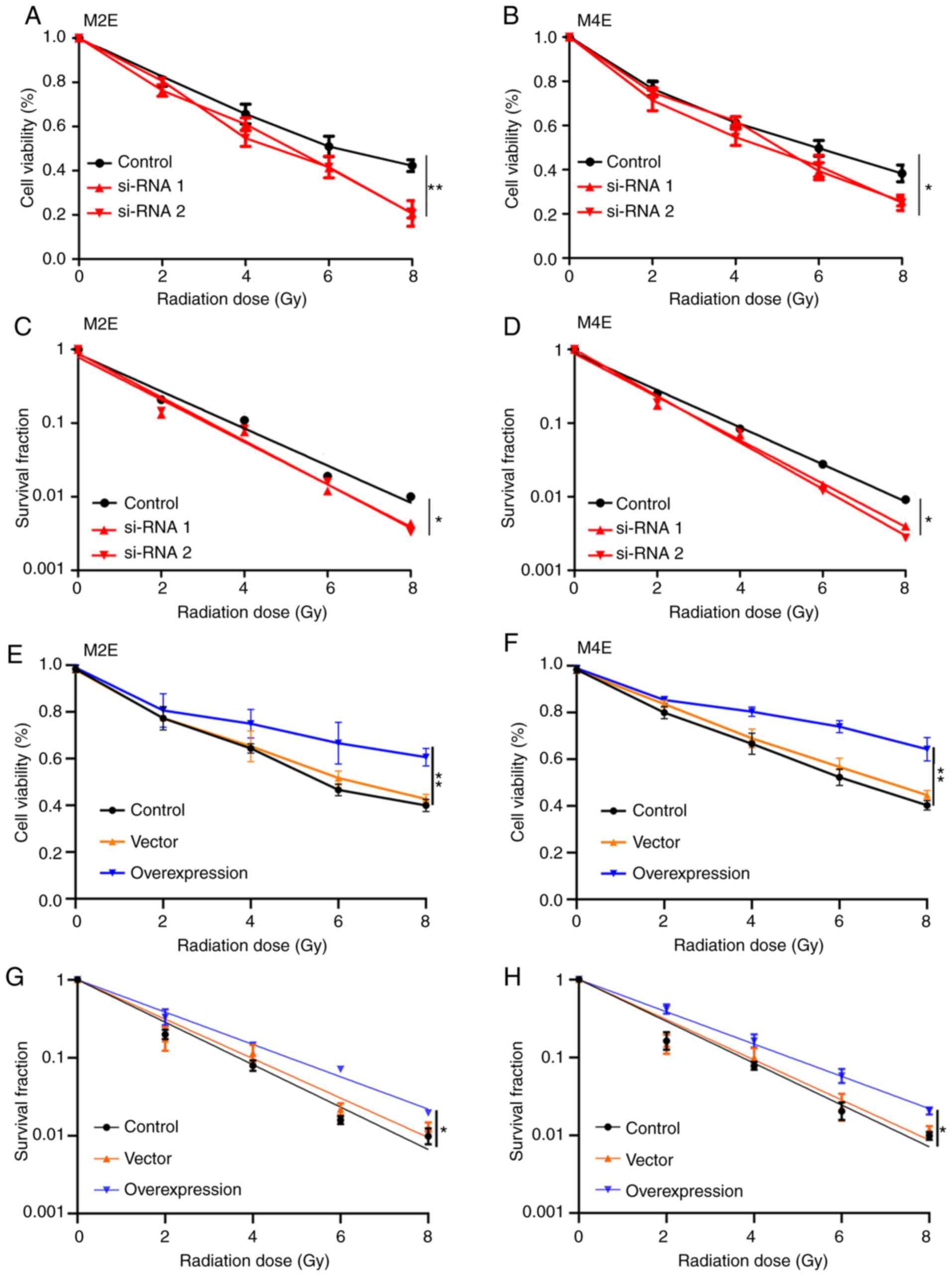
The role of UBR5 in the radiosensitivity of LCC
cells was next investigated. As shown in Fig. 5, downregulation of UBR5 expression
in M2E and M4E cells significantly suppressed the viability and
survival fraction of both cell lines after exposure to radiation
(Fig. 5A-D). Conversely,
overexpression of UBR5 increased cell viability and the survival
fraction of M2E and M4E cells with increasing radiation dose
compared with the control or vector groups (Fig. 5E-H).
UBR5 silencing increases the Bax/Bcl2
ratio and activates the p38/MAPK signaling pathway following
radiotherapy
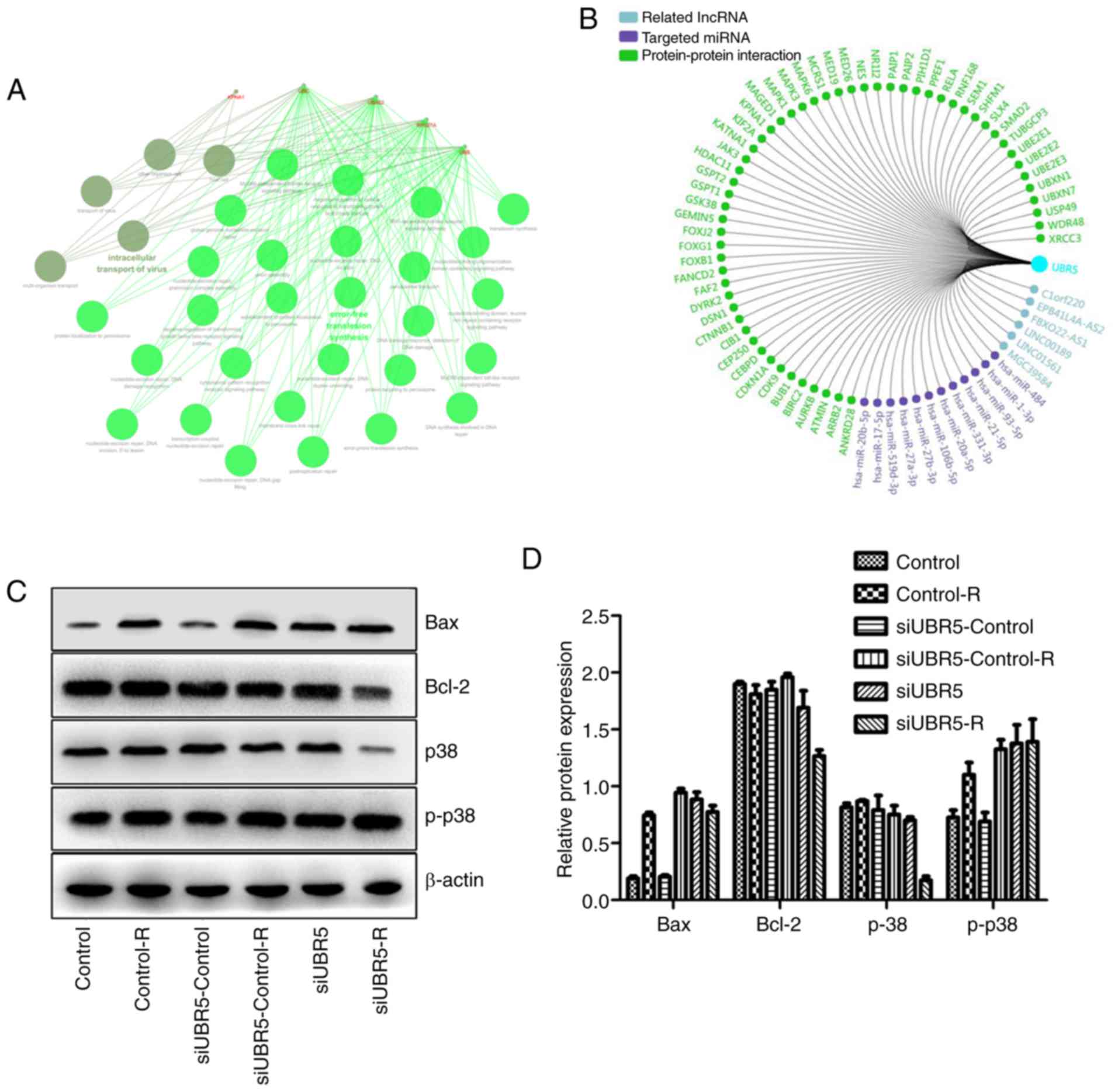
Using bioinformatics, related lncRNA, targeted miRNA
and PPI networks were obtained and a functional enrichment analysis
of significant co-regulated nodes of UBR5 was performed, as shown
in Fig. 6A and B. Alterations in
the expression of p38/MAPK were measured in different groups.
Western blot analysis revealed that radiation increased Bax
expression in the control-R group compared with the control group,
whereas it exerted no effect on the expression of p38. However,
when M2E cells transfected with UBR5 si-RNA were exposed to
radiation, Bcl-2 expression decreased significantly compared with
its levels in cells transfected with si-RNA control and then
exposed to radiation. Bax expression was the same in the two groups
mentioned above. In addition, the p-p38 protein level was higher in
the UBR5 si-RNA combined with radiation group compared with that in
the radiation alone group, while total p38 protein was
significantly decreased in the UBR5 si-RNA and radiation
combination group (P<0.05; Fig. 6C
and D).
As shown in Fig.
6E-H, significantly increased proliferation was observed in the
UBR5 overexpression group compared with the normal control.
Following radiation exposure, the cell viability and survival
fraction significantly increased in cells transfected with the UBR5
overexpression plasmid compared with the vector group. Moreover,
UBR5-overexpressing cells were treated with SB203580, an inhibitor
of the p38/MAPK signaling pathway, and cell proliferation,
viability and survival fraction were rescued to the original
levels. However, SB203580 treatment exerted no effect on the
percentage of LCC cells in the S phase (Figs. 6F and S1). Taken together, these
findings suggest that UBR5 regulates cell proliferation and
radiosensitivity via p38/MAPK in LCC cells.
Discussion
The treatment strategy for LCC currently involves a
comprehensive treatment based on surgery. However, with the
development of molecular biology and technologies in medical
research, increasing attention has been focused on developing
approaches that improve the quality of life of patients with LCC
after surgery, such as preservation of vocal and swallowing
functions. Currently, the preservation of laryngeal function while
maximally eradicating tumors is a major challenge in LCC (26,27).
Radiotherapy can preserve the integrity of the laryngeal stent to
the maximum extent, thus maintaining the full function of the
larynx. However, the major drawback of radiotherapy for LCC is that
individual sensitivity varies greatly (26). The present study demonstrated that
survival fraction was decreased when IR was administered in
conjunction with downregulation of UBR5 expression, suggesting that
downregulation of UBR5 enhances radiosensitivity in LCC cells.
These findings also suggest the possibility that increased UBR5
expression may represent a mechanism of radioresistance in LCC,
which, to the best of our knowledge, has not been reported to
date.
Recent studies indicated that the development of
resistance to radiotherapy involves various signaling pathways that
regulate DNA repair, cell survival, proliferation and apoptosis
(8). Previous reports revealed that
IR regulates the expression of Bcl-2 family genes, and its
expression depends on the downregulation of Bcl-2 and the
upregulation of Bax expression in wild-type p53 cancer (28,29).
Nix et al reported that Bcl-2 expression in LCC predicted
radioresistance with an accuracy of 71%, suggesting a potential
mechanism by which LCC cells avoid the destructive effects of
radiotherapy (30). In acute
leukemia, regulating the response of cells to apoptotic stimuli
affects radiosensitivity (31).
IR-induced DNA double-strand breaks are among the most cytotoxic
types of DNA damage. In LCC cells, DNA damage checkpoint 1 and
p53-binding protein 1 limit tumor cell radiosensitivity via
upstream mediators of the ATM pathway (32).
UBR5 is an important nuclear phosphoprotein involved
in the regulation of DNA damage response, β-catenin activity,
metabolism, transcription and apoptosis (33). The UBR5 gene is localized to
chromosome 8q22, a region that is disrupted in a number of cancers.
The UBR5 gene encodes a progestin-induced protein that belongs to
the homologous to E6-AP carboxyl terminus (HECT) family (34,35).
HECT family proteins function as E3 ubiquitin-protein ligases,
targeting specific proteins for ubiquitin-mediated proteolysis
(36). Recently, targeting E3
ligases as a strategy for radiotherapy treatment in several
neoplasms has attracted significant interest (9,10). For
example, increased E3 ligase cIAP2 expression resulted in altered
MRE11 ubiquitination models and mediated radiosensitization in
response to histone deacetylase inhibition (12). The E3 ligase UBR5 is a key regulator
of the UPS in cells and in cancer development, and a potential gene
that regulates IR sensitivity. In addition, UBR5-knockout cells are
hypersensitive to UV radiation, which supports the role of UBR5 in
IR-induced lesions in cancers (16). These results indicate that UBR5 may
be a new potential independent prognostic marker of outcome or
radiosensitivity in cancer. To the best of our knowledge, the
present study was the first to demonstrate that UBR5 plays a key
role in regulating the malignant behavior and radiosensitivity of
LCC cells through the p38/MAPK pathway, thereby highlighting
possible approaches to the development of new therapeutic
strategies and targets for the treatment of this disease. However,
there was a major limitation to the present study: Of all the
included LCC patients from a retrospective cohort, only 43 received
radiotherapy, and biopsy was performed on these patients prior to
radiotherapy. Further studies are required to explore the
association between UBR5 expression and response to radiotherapy in
clinical biopsies following radiotherapy.
In conclusion, the present study demonstrated that
UBR5 is highly expressed in LCC tissues and that downregulation of
UBR5 in LCC cells may reduce their proliferation and increase their
radiosensitivity. The mechanism underlying reduced proliferation
and increased radiosensitivity may be associated with the
activation of p38-MAPK signaling and downregulation of the
apoptosis-related proteins Bax and Bcl-2. These findings indicate
that UBR5 may be a novel treatment target in patients with
radiation-resistant LCC and should be further investigated in
future clinical studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Science and Technology Planning Project of Guangdong Province of
China (nos. 2014A020212004 and 2016A02021500), the Natural Science
Foundation of Guangdong Province of China (no. 2016A030313245), the
Project of Administration of Traditional Chinese Medicine of
Guangdong Province of China (no. 20161238), the Medical Scientific
Research Foundation of Guangdong Province of China (no. A2015212),
and the Capital Health Research and Development of Special (no.
2018-4-2082).
Availability of materials and data
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ and WC conceived and designed the study. KW and
JT performed the experiments. KW and XL wrote the paper. YW and RZ
reviewed and edited the manuscript. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First People's Hospital of Foshan. Written
informed consent was obtained from all patients who participated in
this study according to the committee regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Society of Clinical Oncology, .
Pfister DG, Laurie SA, Weinstein GS, Mendenhall WM, Adelstein DJ,
Ang KK, Clayman GL, Fisher SG, Forastiere AA, et al: American
Society of Clinical Oncology clinical practice guideline for the
use of larynx-preservation strategies in the treatment of laryngeal
cancer. J Clin Oncol. 24:3693–3704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tobias JS, Monson K, Gupta N, Macdougall
H, Glaholm J, Hutchison I, Kadalayil L and Hackshaw A; UK Head and
Neck Cancer Trialists' Group, : Chemoradiotherapy for locally
advanced head and neck cancer: 10-year follow-up of the UK Head and
Neck (UKHAN1) trial. Lancet Oncol. 11:66–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Langendijk JA, Doornaert P, Verdonck-de
Leeuw IM, Leemans CR, Aaronson NK and Slotman BJ: Impact of late
treatment-related toxicity on quality of life among patients with
head and neck cancer treated with radiotherapy. J Clin Oncol.
26:3770–3776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duke RL, Campbell BH, Indresano AT, Eaton
DJ, Marbella AM, Myers KB and Layde PM: Dental status and quality
of life in long-term head and neck cancer survivors. Laryngoscope.
115:678–683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Zhang Y, Li Z, Liu S, Li H and Xu
Z: Benefit of salvage total pharyngolaryngoesophagectomy for
recurrent locally advanced head and neck cancer after radiotherapy.
Radiat Oncol. 12:1642017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pujo K, Philouze P, Scalabre A, Céruse P,
Poupart M and Buiret G: Salvage surgery for recurrence of laryngeal
and hypopharyngeal squamous cell carcinoma: A retrospective study
from 2005 to 2013. Eur Ann Otorhinolaryngol Head Neck Dis.
135:111–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Popovtzer A, Burnstein H, Stemmer S, Limon
D, Hili O, Bachar G, Sopov V, Feinmesser R, Groshar D and Shvero J:
Phase II organ-preservation trial: Concurrent cisplatin and
radiotherapy for advanced laryngeal cancer after response to
docetaxel, cisplatin, and 5-fluorouracil-based induction
chemotherapy. Head Neck. 39:227–233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shearer RF, Iconomou M, Watts CK and
Saunders DN: Functional roles of the E3 Ubiquitin ligase UBR5 in
cancer. Mol Cancer Res. 13:1523–1532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shearer RF, Frikstad KM, McKenna J, McCloy
RA, Deng N, Burgess A, Stokke T, Patzke S and Saunders DN: The E3
ubiquitin ligase UBR5 regulates centriolar satellite stability and
primary cilia. Mol Biol Cell. 29:1542–1554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Biswas K, Sarkar S, Du K, Brautigan DL,
Abbas T and Larner JM: The E3 Ligase CHIP Mediates p21 degradation
to maintain radioresistance. Mol Cancer Res. 15:651–659. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nicholson J, Jevons SJ, Groselj B,
Ellermann S, Konietzny R, Kerr M, Kessler BM and Kiltie AE: E3
Ligase cIAP2 mediates downregulation of MRE11 and
radiosensitization in response to HDAC inhibition in bladder
cancer. Cancer Res. 77:3027–3039. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dogan T, Gnad F, Chan J, Phu L, Young A,
Chen MJ, Doll S, Stokes MP, Belvin M, Friedman LS, et al: Role of
the E3 ubiquitin ligase RNF157 as a novel downstream effector
linking PI3K and MAPK signaling pathways to the cell cycle. J Biol
Chem. 292:14311–14324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rom O, Kaisari S, Aizenbud D and Reznick
A: Involvement of E3 Ubiquitin ligases in Cigarette Smoke
associated muscle catabolism. Free Radic Biol Med. 75 (Suppl
1):S52014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie Z, Liang H, Wang J, Xu X, Zhu Y, Guo
A, Shen X, Cao F and Chang W: Significance of the E3 ubiquitin
protein UBR5 as an oncogene and a prognostic biomarker in
colorectal cancer. Oncotarget. 8:108079–108092. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanchez A, De Vivo A, Uprety N, Kim J,
Stevens SM Jr and Kee Y: BMI1-UBR5 axis regulates transcriptional
repression at damaged chromatin. Proc Natl Acad Sci USA.
113:11243–11248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henderson MJ, Munoz MA, Saunders DN,
Clancy JL, Russell AJ, Williams B, Pappin D, Khanna KK, Jackson SP,
Sutherland RL and Watts CK: EDD mediates DNA damage-induced
activation of CHK2. J Biol Chem. 281:39990–40000. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang T, Cronshaw J, Kanu N, Snijders AP
and Behrens A: UBR5-mediated ubiquitination of ATMIN is required
for ionizing radiation-induced ATM signaling and function. Proc
Natl Acad Sci USA. 111:12091–12096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alpsoy A, Yasa S and Gündüz U: Gunduz,
Etoposide resistance in MCF-7 breast cancer cell line is marked by
multiple mechanisms. Biomed Pharmacother. 68:351–355. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Brien PM, Davies MJ, Scurry JP, Smith
AN, Barton CA, Henderson MJ, Saunders DN, Gloss BS, Patterson KI,
Clancy JL, et al: The E3 ubiquitin ligase EDD is an adverse
prognostic factor for serous epithelial ovarian cancer and
modulates cisplatin resistance in vitro. Br J Cancer. 98:1085–1093.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chun SY, Kim S, Nam KS and Lee KS:
Anti-metastatic potential of a proton beam is regulated by p38
MAPK/c-Fos signaling pathway in TPA-treated HepG2 human
hepatocellular carcinoma. Biomed Pharmacother. 99:904–912. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edgar R, Domrachev M and Lash AE: Gene
Expression Omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A Cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bindea G, Galon J and Mlecnik B: CluePedia
Cytoscape plugin: Pathway insights using integrated experimental
and in silico data. Bioinformatics. 29:661–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Graboyes EM, Zhan KY, Garrett-Mayer E,
Lentsch EJ, Sharma AK and Day TA: Effect of postoperative
radiotherapy on survival for surgically managed pT3N0 and pT4aN0
laryngeal cancer: Analysis of the National Cancer Data Base.
Cancer. 23:2248–2257. 2017. View Article : Google Scholar
|
|
27
|
Dyckhoff G, Plinkert PK and Ramroth H: A
change in the study evaluation paradigm reveals that larynx
preservation compromises survival in T4 laryngeal cancer patients.
BMC Cancer. 17:6092017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tong QS, Zheng LD, Chen FM, Zeng FQ, Wang
L, Dong JH and Lu GC: Selection of optimal antisense accessible
sites of survivin and its application in treatment of gastric
cancer. World J Gastroenterol. 11:634–640. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bokarewa M, Lindblad S, Bokarew D and
Tarkowski A: Balance between survivin, a key member of the
apoptosis inhibitor family, and its specific antibodies determines
erosivity in rheumatoid arthritis. Arthritis Res Ther. 7:R349–R358.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nix P, Cawkwell L, Patmore H, Greenman J
and Stafford N: Bcl-2 expression predicts radiotherapy failure in
laryngeal cancer. Br J Cancer. 92:2185–2189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wrzesień-Kuś A, Smolewski P, Sobczak-Pluta
A, Wierzbowska A and Robak T: The inhibitor of apoptosis protein
family and its antagonists in acute leukemias. Apoptosis.
9:705–715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gou Q, Xie Y, Liu L, Xie K, Wu Y, Wang Q,
Wang Z and Li P: Downregulation of MDC1 and 53BP1 by short hairpin
RNA enhances radiosensitivity in laryngeal carcinoma cells. Oncol
Rep. 34:251–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen Q, Qiu Z, Wu W, Zheng J and Jia Z:
Characterization of interaction and ubiquitination of
phosphoenolpyruvate carboxykinase by E3 ligase UBR5. Biol Open.
7(pii): bio0373662018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matta-Camacho E, Kozlov G, Menade M and
Gehring K: Structure of the HECT C-lobe of the UBR5 E3 ubiquitin
ligase. Acta Crystallogr Sect F Struct Biol Cryst Commun.
68:1158–1163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Safdar K, Gu A, Xu X, Au V, Taylor J,
Flibotte S, Moerman DG and Maine EM: UBR-5, a Conserved HECT-Type
E3 Ubiquitin Ligase, negatively regulates Notch-type signaling in
Caenorhabditis Elegans. G3 (Bethesda). 6:2125–2134. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Flack JE, Mieszczanek J, Novcic N and
Bienz M: Wnt-Dependent inactivation of the Groucho/TLE Co-repressor
by the HECT E3 Ubiquitin Ligase Hyd/UBR5. Mol Cell. 67:181–193.e5.
2017. View Article : Google Scholar : PubMed/NCBI
|