Introduction
Ovarian cancer (OC) continues to be one of the most
lethal malignant tumors among women worldwide. In the United
States, OC accounts for only 2.5% of all malignancies among females
but for 5% of cancer deaths, making it the fifth leading cause of
cancer-related deaths in females (1,2). In
China, the mortality rate of OC is ranked tenth among females
throughout the country (3). The low
survival rates in OC are largely driven by a late-stage diagnosis
and a high rate of tumor recurrence. Although most OC patients have
no evidence of disease after first treatment, approximately 19% of
early OC patients and 60 to 85% of advanced OC patients experience
a relapse, and recurrent OC after treatment is almost incurable
(4–6). Current treatment for OC is not
confined merely to surgery and classical chemotherapy. Targeted
therapy, such as polyADP ribose polymerase (PARP) inhibitors, as
well as immunotherapy, are promising treatments (7–10).
The Notch signaling pathway is an important system
that regulates cell proliferation, differentiation, and apoptosis.
Integrated genomic analyses revealed that Notch is the main
signaling pathway involved in the pathophysiology of OC (11,12).
The expression of notch reporter 3 (NOTCH3) was found to be
elevated in OC and closely correlated with the clinical stage,
pathological grade, lymph node metastasis, drug-resistant
recurrence, and survival rate of OC patients (13–15).
NOTCH3 promotes the malignant progression of OC by enhancing the
proliferation of tumor cells, maintaining their stemness, and
resisting apoptosis (16,17). A better understanding of the
tumor-specific regulation of NOTCH3 is crucial and may contribute
to targeted therapy for OC. However, limited information regarding
the molecules that regulate the expression of NOTCH3 is
available.
Approximately 70% of the human genome can be
transcribed, while less than 2% of the genome encodes proteins,
generating thousands of non-coding transcripts (ncRNAs) (18,19).
Among them, microRNAs (miRNAs) and long noncoding RNAs (lncRNAs)
are key regulators of gene expression at the post-transcriptional
level. miRNAs are single-stranded non-coding small RNAs with a
length of 17–22 nt. They are destabilizers and repressors of
translation of mRNAs and regulate diverse biological functions,
including proliferation, migration, apoptosis, angiogenesis, and
metabolic progression in various cancers, including OC (20–24).
lncRNAs are ncRNAs that are longer than 200 nt. They are implicated
in multiple cancers and play critical roles in tumor initiation and
progression (25–28). Recent studies have revealed that
lncRNAs can act as competing endogenous RNA (ceRNA) or miRNA
‘sponges’ to modulate crucial genes in cancer (29–32),
providing a feasible way to understand the regulatory mechanisms
underlying cancer pathogenesis and identify novel diagnostic
biomarkers or potential therapeutic candidates.
Given the significant oncogenic role of NOTCH3 in
OC, we aimed to determine the functional significance of
NOTCH3-mediated regulatory ncRNAs in OC. In this study, we found
that microRNA-1299 (miR-1299) was a novel negative regulator of
NOTCH3 expression in OC with clinical significance. Downregulation
of miR-1299 promoted the malignant progression of OC by tumor cell
proliferation. The lncRNA taurine upregulated gene 1 (TUG1) was
found to function as a ceRNA, regulating NOTCH3 expression by
sponging miR-1299 in OC. TUG1 was also identified as a potential
downstream target of NOTCH3 and formed a miR-1299/NOTCH3/TUG1
feedback loop. Our results elucidate the regulatory mechanism
underlying NOTCH3 expression in OC and provide potential
therapeutic targets for the anticancer therapy of OC.
Materials and methods
Cell lines/cell culture
The human OC cell lines A2780, CAOV3, and SKOV3 were
purchased from the National Infrastructure of Cell Line Resources
in China (Beijing, China). Cells were cultured in DMEM or RPMI-1640
medium with 10% FBS (Thermo Fisher Scientific, Inc.) and incubated
at 37°C with 5% CO2. To block Notch signaling in OC
cells, the γ-secretase inhibitor
N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl
ester (DAPT, #HY-13027; MedChem Express) dissolved in DMSO was
added in the medium.
Clinical samples
Thirty-five epithelial serous OC and 16
pathologically confirmed normal ovary tissues were collected from
patients (medium age 54 years; range 30–72 years and medium age 51
years; range 35–64 years, respectively) undergoing surgery at the
Cancer Hospital, Chinese Academy of Medical Sciences and Peking
Union Medical College (Beijing, China) from July 2018 to June 2019.
All OC cases were diagnosed by pathological evaluation and had
complete clinical information. All tissue samples were immediately
frozen in liquid nitrogen after resection from patients and stored
at −80°C for RNA extraction. This study was conducted according to
the ethical guidelines of the 1975 Declaration of Helsinki and
approved by the Ethics Committee of Peking Union Medical College
Cancer Hospital (Beijing, China) (grant no: NCC2017G-115). Written
informed consent was obtained from all of the subjects.
The OC samples were divided into high and low
expression of miR-1299 and NOTCH3 groups by the cut-off value,
which was defined as the cohort median.
Oligonucleotide transfection, plasmid
construction, and lentiviral infection
miR-1299 mimics, inhibitors, and scramble miRNA
controls were purchased from GenePharma (Shanghai, China). The
lentivirus-containing short hairpin RNA (shRNA) targeting TUG1 or
NOTCH3, and the plasmid expressing the active intracellular domain
of NOTCH3 (NICD3) were purchased from GeneChem (Shanghai, China).
The wild-type and mutated 3′UTR sequence of NOTCH3 was cloned into
the pmirGLO vector (Promega) to construct recombinant plasmids
named pmirGLO-NOTCH3-WT and pmirGLO-NOTCH3-MUT. Sequences of lncRNA
TUG1 and XIST containing the predictive binding sites of miR-1299
and containing point mutations at the site region were cloned into
the pmirGLO vector and were named pmirGLO-TUG1-WT, pmirGLO-XIST-WT,
and pmirGLO-TUG1-MUT. OC cells were transfected with
oligonucleotides using Lipofectamine® RNAiMAX reagent
(Thermo Fisher Scientific, Inc.) and plasmids using TurboFect™
transfection reagent (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions.
RNA extraction and RT-qPCR
Total RNA was extracted from OC tissues and cultured
cell lines using TRIzol reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. mRNA and lncRNA
levels were quantified with RT-qPCR using the SYBR Premix Ex Taq
reverse transcription PCR kit (Takara, China), and β-actin was used
as an internal control. miRNA was quantified with RT-qPCR using the
hairpin-it™ microRNA and U6 snRNA normalization RT-PCR quantitation
kit (GenePharma). Each assay was carried out in triplicates in a
Light Cycler 480 Instrument (Roche), and the relative expression of
mRNA and miRNA was calculated using the 2−ΔΔCq method
(33). The primers for RT-qPCR are
shown in Table SI.
Cell cycle, proliferation, and colony
formation assays
For cell cycle analysis, cells were collected and
adjusted to 1×106/ml 48 h after transfection. Ethanol
(70%) was used to fix the cells, and RNase A (100 µg/ml) was used
to remove RNAs. Finally, cells were stained with propidium iodide
(PI) (50 µg/ml) at room temperature for 30 min and analyzed using
LSRII flow cytometry (BD Biosciences). Cell viability was detected
using the Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) according to
the manufacturer's instructions. Twenty-four hours after
transfection, the cells were seeded in 96-well plates at 3,000
cells/well. The proliferative ability of cells was determined at 0,
24, 48, 72 and 96 h by measuring the absorbance values at a
wavelength of 450 nm. For colony formation assay, OC cells (800
cells per well) were seeded into 6-well plates 24 h after
transfection. After 10 days of incubation, the cells were fixed in
methanol and stained with 0.1% crystal violet. The colonies were
counted using a GBOX F3 gel documentation system (Syngene,
Cambridge, UK).
Immunofluorescence (IF) assay
A total of 10,000 cells were seeded on glass
coverslips in 24-well plates and grown overnight. For the EdU
incorporation assay, 10 µM EdU was used to treat the cells, and an
EdU cell proliferation kit (Sangon) was used for measurement.
Cellular nuclei were stained using 5 µg/ml Hoechst 33342 at 26°C
for 30 min. Images were observed under a DMI 4000 confocal laser
scanning microscope at ×200 magnification (Leica, Frankfurt,
Germany).
Luciferase reporter assay
Cells were seeded in 96-well plates at
1.5×104 cells/well. When the cells reached 60%
confluence, vectors containing the wild-type or mutant 3′UTR of
NOTCH3 and wild-type or mutant binding site sequence of TUG1 were
co-transfected with miR-1299 mimics and scramble miRNA using
Turbofect (Thermo Fisher Scientific, Inc.). Forty-eight hours after
transfection, luciferase activity was measured using the
Dual-Luciferase Reporter assay system (Promega) and expressed as
the ratio of firefly and Renilla luciferase activities.
Western blotting
Proteins were extracted from cells or tissues using
RIPA lysis buffer (Solarbio) with protease inhibitors and
phosphatase inhibitors. A total of 30 µg proteins were loaded per
lane, separated on 10% SDS-PAGE gels, and blotted on polyvinylidene
difluoride membrane. After being blocked with 5% skim milk for 2 h
at room temperature, the membranes were incubated with primary
antibodies (dilution 1:1,000) overnight at 4°C. The primary
antibodies used in this study were NOTCH3 antibody (product code
ab23426; Abcam) and GAPDH (cat. no. 2118; Cell Signaling
Technology, Inc.). Anti-mouse IgG, peroxidase-linked antibody was
used as secondary antibody (dilution 1:2,500; cat. no. 5174; Cell
Signaling Technology, Inc.) and was incubated at room temperature
for 1 h. The blots were detected using a chemiluminescence kit
(cat. no. 34577; Thermo Fisher Scientific, Inc.) and imaged using
MiniChemi 610 system (Sage Creation Science, Co., Ltd.).
In vivo animal experiments
Eight four-week-old female BALB/c nude mice (body
weight range, 17.1–18.2 g) were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. and randomly divided into
two groups. The mice were housed with filtered air, 12 h light/dark
cycle, constant temperature (25°C) and had free access to
sterilized food and water. After 24-h transfection,
2×106 cells containing miR-1299 or NC agomir were
infected into the right armpit of the mice. miR-1299 agomir or NC
agomir (RiboBio) was directly injected into the implanted tumor at
the dose of 2 nmol/30 µl phosphate-buffered saline (PBS) per mouse
every 6 days for 6 times. Tumor growth was monitored by measuring
the tumor volume (V) every 6 days with a Vernier caliper and
calculated as: V=length × width2/2. All the mice were
anesthetized with sodium pentobarbital (500 mg/kg,
intraperitoneally) and sacrificed by cervical vertebra dislocation
on day 42 or when the tumor volume reached the threshold of 1,500
mm3, and tumors were weighed and snap-frozen for protein
and RNA extraction.
Animal experiments were conducted with the approval
of the Animal Ethics Committee of Cancer Hospital, Chinese Academy
of Medical Sciences and Peking Union Medical College, Beijing
(ACC2019A060) and in accordance with the Guide for the Care and Use
of Laboratory Animals by the US National Institutes of Health.
Statistical analysis
SPSS software version 25.0 (IBM Corp.) and GraphPad
Prism 8 (GraphPad Software, Inc.) were used for statistical
analysis. Each experiment was performed at least in triplicate, and
numerical data are expressed as means ± SD. The difference in
clinicopathological features between two groups was determined by
independent-sample Student's t-test or Mann-Whitney U test for
continuous variables and the Chi-square test or Fisher exact test
for categorical variables. One-way ANOVA analysis with Tukey post
hoc test was used for comparisons among multiple groups. Pearson's
correlation analysis was used to examine the relationship between
two gene expression levels. A value of P<0.05 was indicative of
statistical significance.
Results
miR-1299 is a negative regulator of
NOTCH3 in OC with clinical significance
To investigate the potential miRNAs that regulate
NOTCH3, we firstly performed a bioinformatics analysis using the
miRWalk database (34), which
integrated predicted gene-miRNA target information from 13
databases and compared the results with miRNAs reported to be
significantly downregulated in previously published miRNA profiles
(NCBI/GEO/GSE47841) (35) (Table SII). The two screening methods
overlapped on only 11 miRNAs among which miR-1299 had the highest
score in miRWalk. Therefore, we focused our subsequent analysis on
miR-1299 as a putative regulator of NOTCH3 in OC. We measured the
level of mature miR-1299 and NOTCH3 in 35 fresh OC tissues and 16
normal ovarian tissues, as well as in four OC cell lines. miR-1299
expression was significantly downregulated in the OC tissues
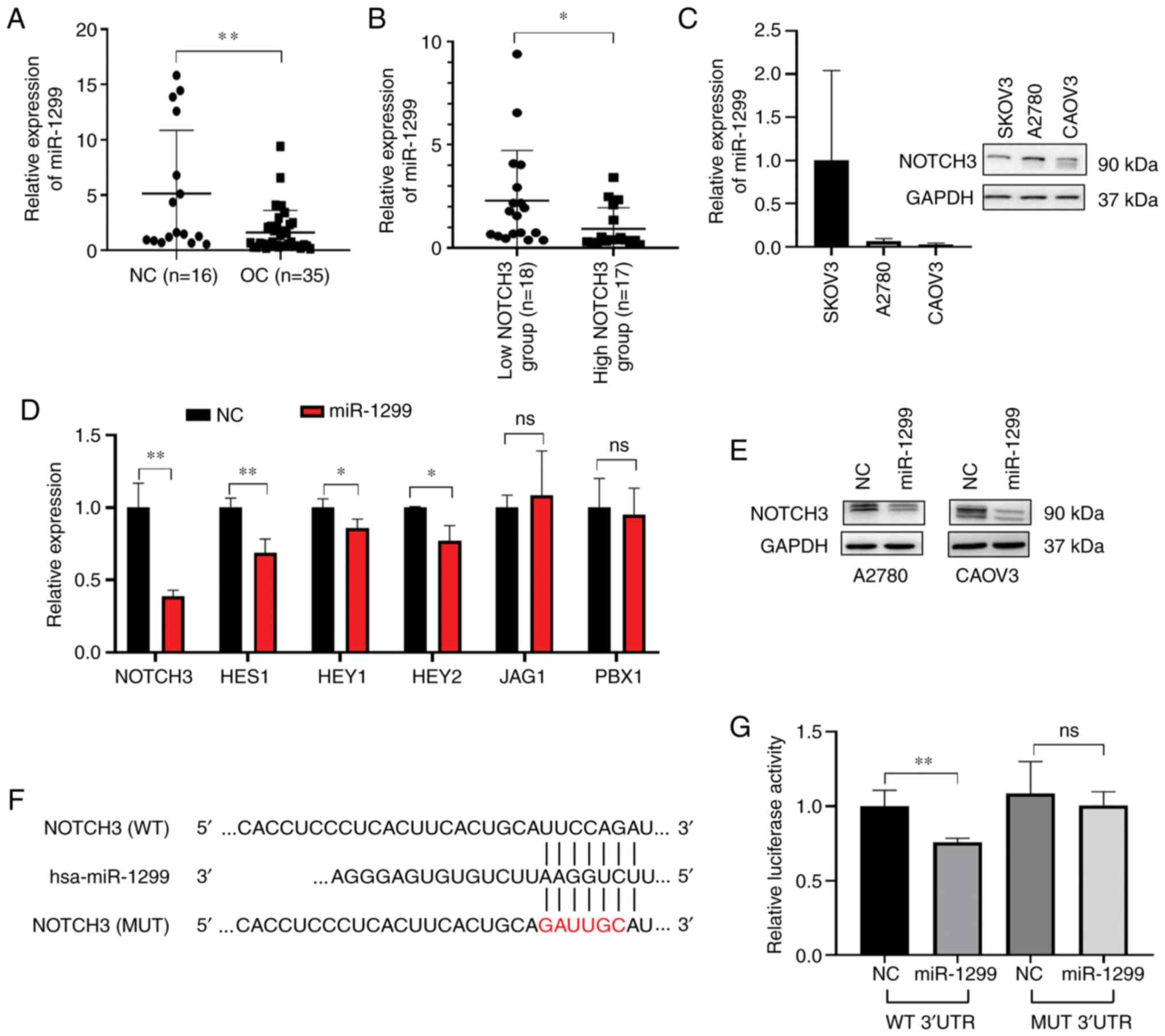
compared with that in the normal tissue (P<0.05, Fig. 1A). There was an inverse correlation
between the level of miR-1299 and NOTCH3 both in OC tissues
(Fig. 1B) and in OC cell lines
(Fig. 1C).
To confirm the regulation of miR-1299 on NOTCH3
expression, we first transfected miR-1299 mimics and NC scramble
into miR-1299 low-expressing cell A2780 and CAOV3. RT-qPCR results
showed that NOTCH3 and its pathway genes were largely reduced in
the A2780 (Fig. 1D) and CAOV3 cells
(Fig. S1) when transfected with
miR-1299 mimics, and western blotting results confirmed the
decreased protein level of NOTCH3 (Fig.
1E). In turn, when we knocked down NOTCH3 or overexpressed
NICD3 in OC cells, we observed a subsequent increase or decrease in
the miR-1299 level (Fig. S2).
Furthermore, we cloned wild-type 3′UTR sequence of NOTCH3 and 3′UTR
containing point mutations in the putative binding sites into a
luciferase reporter plasmid (pmirGLO-NOTCH3-WT and
pmirGLO-NOTCH3-MUT) (Fig. 1F). Both
plasmids were co-transfected with miR-1299 mimics or NC mimics
separately in A2780 cells. The results showed that overexpression
of miR-1299 suppressed luciferase activity significantly in cells
transfected with pmirGLO-NOTCH3-WT plasmid but not in those
transfected with pmirGLO-NOTCH3-mutant vectors (Fig. 1G). These observations suggested that
miR-1299 bound directly to the predicted binding sites in the
NOTCH3 3′UTR region and negatively regulated NOTCH3 expression.
The association of miR-1299 level and
clinicopathological features was also analyzed in OC patients
(Table I). Low expression of
miR-1299 was significantly correlated with low tumor
differentiation. Decreased miR-1299 level was associated with
younger age, advanced tumor stage, and necessity to receive
neoadjuvant chemotherapy before surgery; however, the association
was not statistically significant because of the limited number of
cases. Thus, we considered miR-1299 as a regulator of NOTCH3 and
hypothesized that decreased expression of miR-1299 promoted OC
progression and development.
 | Table I.Association between miR-1299 levels
and clinicopathological characteristics of the OC patients. |
Table I.
Association between miR-1299 levels
and clinicopathological characteristics of the OC patients.
|
| miR-1299
expression |
|
|---|
|
|
|
|
|---|
|
Characteristics | High expression
(n=17) | Low expression
(n=18) | P-value |
|---|
| Mean age
(years) | 55.2±2.8 | 52.9±1.8 | 0.121 |
| Tumor stage |
|
I–II | 3 | 0 | 0.057 |
|
III | 13 | 13 |
|
| IV | 1 | 5 |
|
| Tumor
differentiation |
|
| 0.045 |
|
High/median | 4 | 0 |
|
|
Low | 13 | 18 |
|
| Nodal
metastasis |
|
| 0.305 |
| No | 12 | 9 |
|
|
Yes | 5 | 9 |
|
| Neoadjuvant
chemotherapy |
|
| 0.060 |
| No | 15 | 10 |
|
|
Yes | 2 | 8 |
|
| Serum CA125 level
at initial diagnosis | 534.1 (108.0,
1,362.0) | 1017.3 (197.4,
1,676.0) | 0.211 |
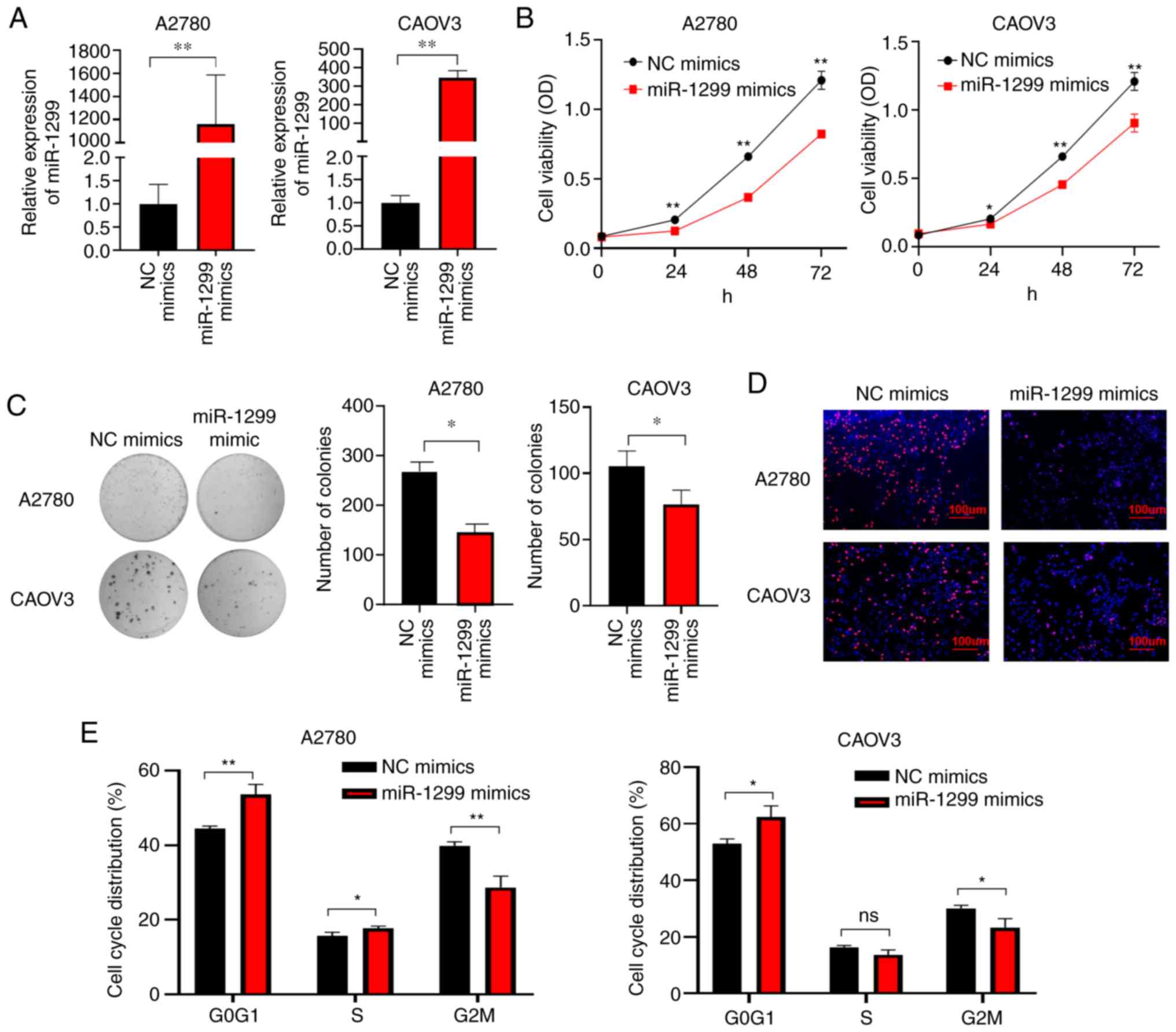
miR-1299 inhibits OC cell
proliferation, colony formation, and cell cycle in vitro
Subsequently, we investigated the role of miR-1299
in OC by transfecting miR-1299 mimics in OC cell lines A2780 and
CAOV3 (Fig. 2A), both of which had
an endogenous low miR-1299 expression. Overexpression of miR-1299
in OC cells significantly reduced cell proliferation, as observed
with a CCK-8 assay compared to cells transfected with scrambled
miRNA (NC mimic) (Fig. 2B). Colony
formation assay showed that treatment with miR-1299 mimics
decreased the number of colonies formed by OC cell lines (Fig. 2C). EdU incorporation assay revealed
that miR-1299 inhibited DNA synthesis in cell proliferation
(Fig. 2D). Additionally, cell cycle
analysis showed that transfection of miR-1299 mimics significantly
blocked the cells in the G0/G1 phase, accompanied by a smaller
population in S and G2 phases (Fig.
2E).
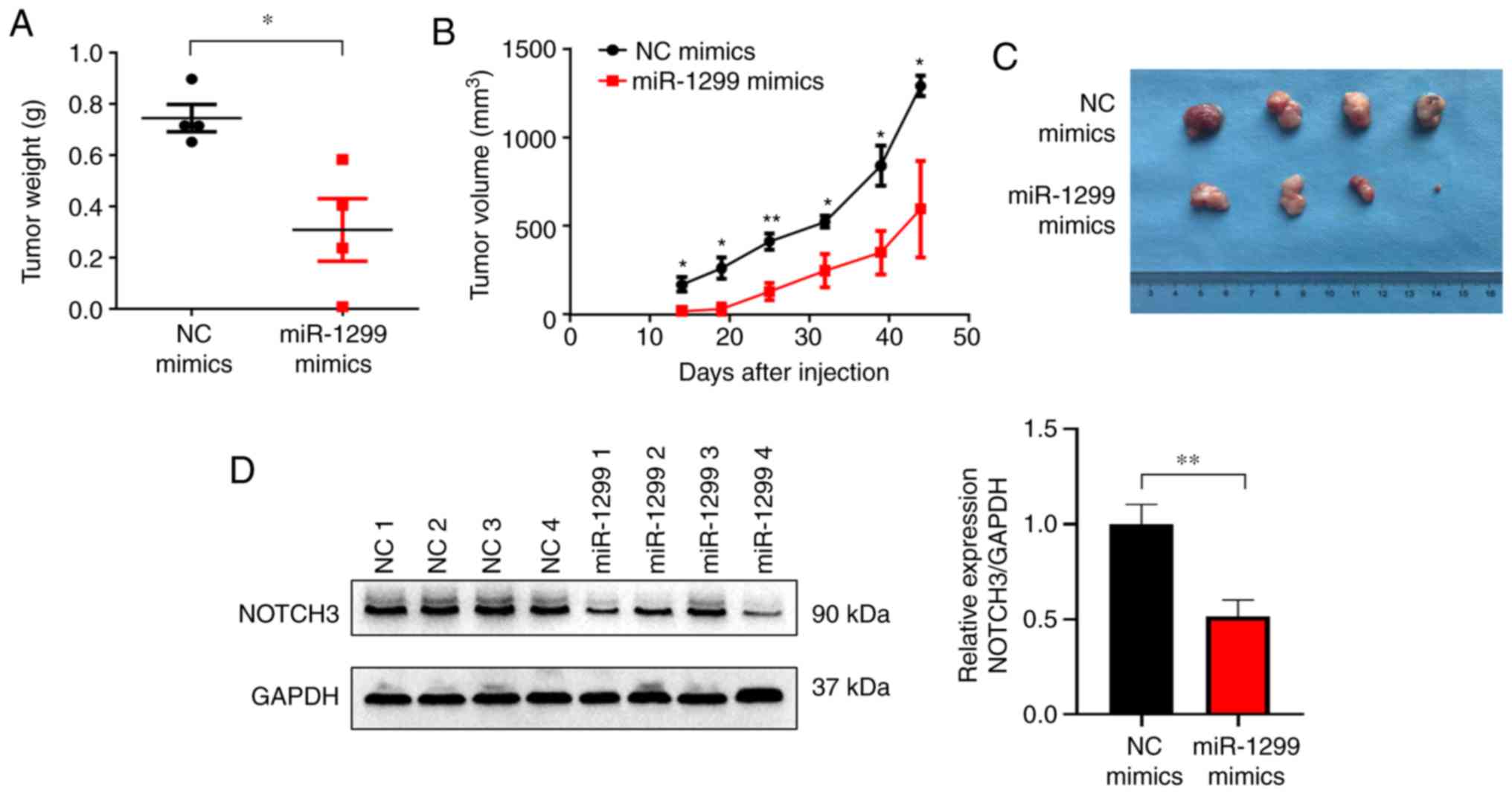
miR-1299 suppresses tumor growth in
vivo
We further examined the impact of miR-1299 on OC in
a xenograft nude mouse model. A2780 cells transfected with miR-1299
or NC mimics were implanted subcutaneously in BALB/c nude mice.
Starting on day 8 post-implantation, miR-1299 or NC mimics were
injected intratumorally every 6 days for 6 times. In vivo
tumor growth was evaluated by measuring tumor volume and final
weight. We observed that treatment with miR-1299 mimics
significantly inhibited tumor growth in vivo. The tumor
volume and weight were significantly lower in the miR-1299 mimic
group than in the NC group (Fig.
3A-C), substantiating the tumor suppressor function of miR-1299
in OC tumorigenesis. Moreover, decreased NOTCH3 protein level in
the xenograft tissues with overexpression of miR-1299 was further
confirmed (Fig. 3D).
lncRNA TUG1 functions as a sponge of
miR-1299 and promotes cell proliferation in OC
To investigate the potential regulators of NOTCH3 by
ceRNA mechanism, we first performed a comprehensive bioinformatics
analysis using predicted miR-1299-targeted lncRNAs from DIANA
databases (36), and OC-relevant
lncRNAs from Starbase database (37) and lncRNA disease database (38) (Fig.
4A). The three-screening data overlapped on only two lncRNAs,
TUG1 and XIST (X-inactive specific transcript). Therefore, we
constructed luciferase reporter plasmids containing the two highest
scoring putative miR-1299 binding sites in TUG1 (pmirGLO-TUG1-WT1
and pmirGLO-TUG1-WT2) and XIST (pmirGLO-XIST-WT1 and
pmirGLO-XIST-WT2). After co-transfecting the plasmids with miR-1299
mimics or NC mimics in A2780, we found that overexpression of
miR-1299 suppressed luciferase activity significantly in two
wild-type plasmids of TUG1 (Fig. 4B and
C) but not in those of XIST (Fig.
S3). We also constructed plasmids carrying point mutations in
either binding site of TUG1 (pmirGLO-TUG1-MUT1 and
pmirGLO-TUG1-MUT2), and no significant changes in luciferase
activity was observed in cells transfected with the two mutant
plasmids (Fig. 4B and C). RT-qPCR
further revealed that miR-1299 could be upregulated in OC cells
after knockdown of TUG1 by lentiviral shRNA particles (Fig. 4D and E). Taken together, these
results indicated that the lncRNA TUG1 was a direct sponge of
miR-1299 in OC.
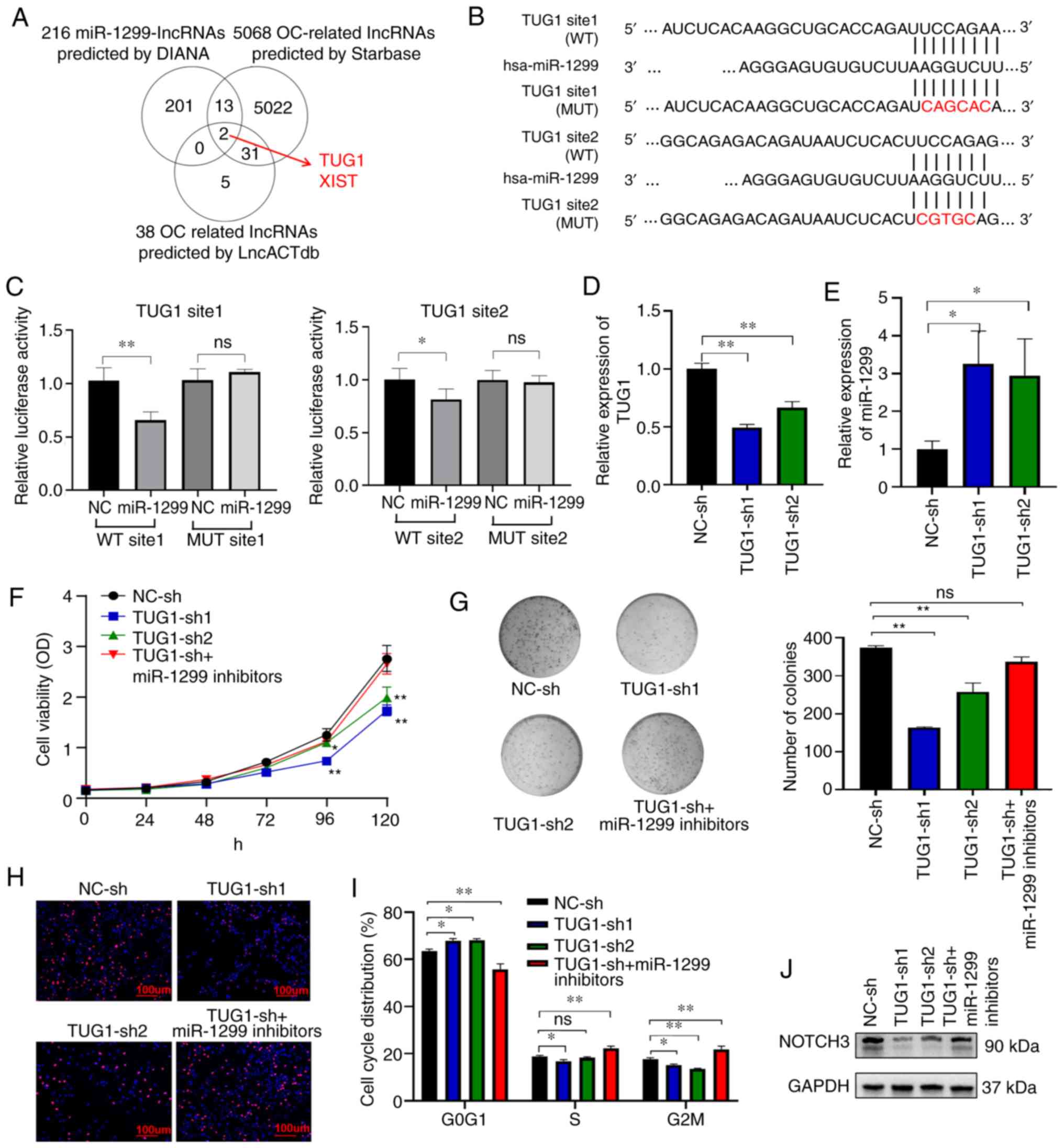 | Figure 4.lncRNA TUG1 functions as a sponge of
miR-1299 and promotes cell proliferation in OC. (A) Screening
methods for regulatory lncRNAs of miR-1299 in OC. (B) The two
highest scoring putative miR-1299 binding sites in the TUG1
sequence (WT) and the point mutations in either binding site (MUT).
(C) Relative luciferase activity of reporter plasmids carrying
wild-type (WT) or mutant (MUT) TUG1 binding sites in A2780 cells
co-transfected with NC or miR-1299 mimics. (D) Confirmation of TUG1
knockdown in A2780 cells transfected with TUG1 shRNA by RT-qPCR
analysis. (E) RT-qPCR analysis of miR-1299 level in A2780 cells
transfected with TUG1 shRNA or scrambled shRNA. Results of the (F)
cell proliferation, (G) colony formation, (H) EdU incorporation
assays, and (I) cell cycle analysis of A2780 cells transfected with
NC-sh, TUG1-sh1, TUG1-sh2, and TUG1-sh+miR-1299 inhibitors. (J)
Representative blot image of NOTCH3 protein level in A2780 cells
transfected with NC-sh, TUG1-sh1, TUG1-sh2, and TUG1-sh+miR-1299
inhibitors by western blotting. Means ± SD are shown. Statistical
analysis was conducted using Student's t-test and one-way ANOVA
with Tukey post hoc test. *P<0.05 and **P<0.01; ns, not
significant; OC, ovarian cancer; NOTCH3, notch receptor 3; TUG1,
lncRNA taurine upregulated gene 1. |
We next evaluated the potential tumorigenicity of
TUG1 in OC. As expected, downregulation of TUG1 by shRNA resulted
in the inhibition of OC cells in terms of cell proliferation,
colony formation, EdU incorporation, and cell cycle, which could be
partially rescued by inhibition of miR-1299 (Fig. 4F-I). Moreover, knockdown of TUG1
reduced NOTCH3 expression in OC cells, and miR-1299 inhibitors
partially rescued the NOTCH3 level (Fig. 4J). This suggested that TUG1, acting
as a ceRNA, affects NOTCH3 expression and promotes cell
proliferation by sponging miR-1299.
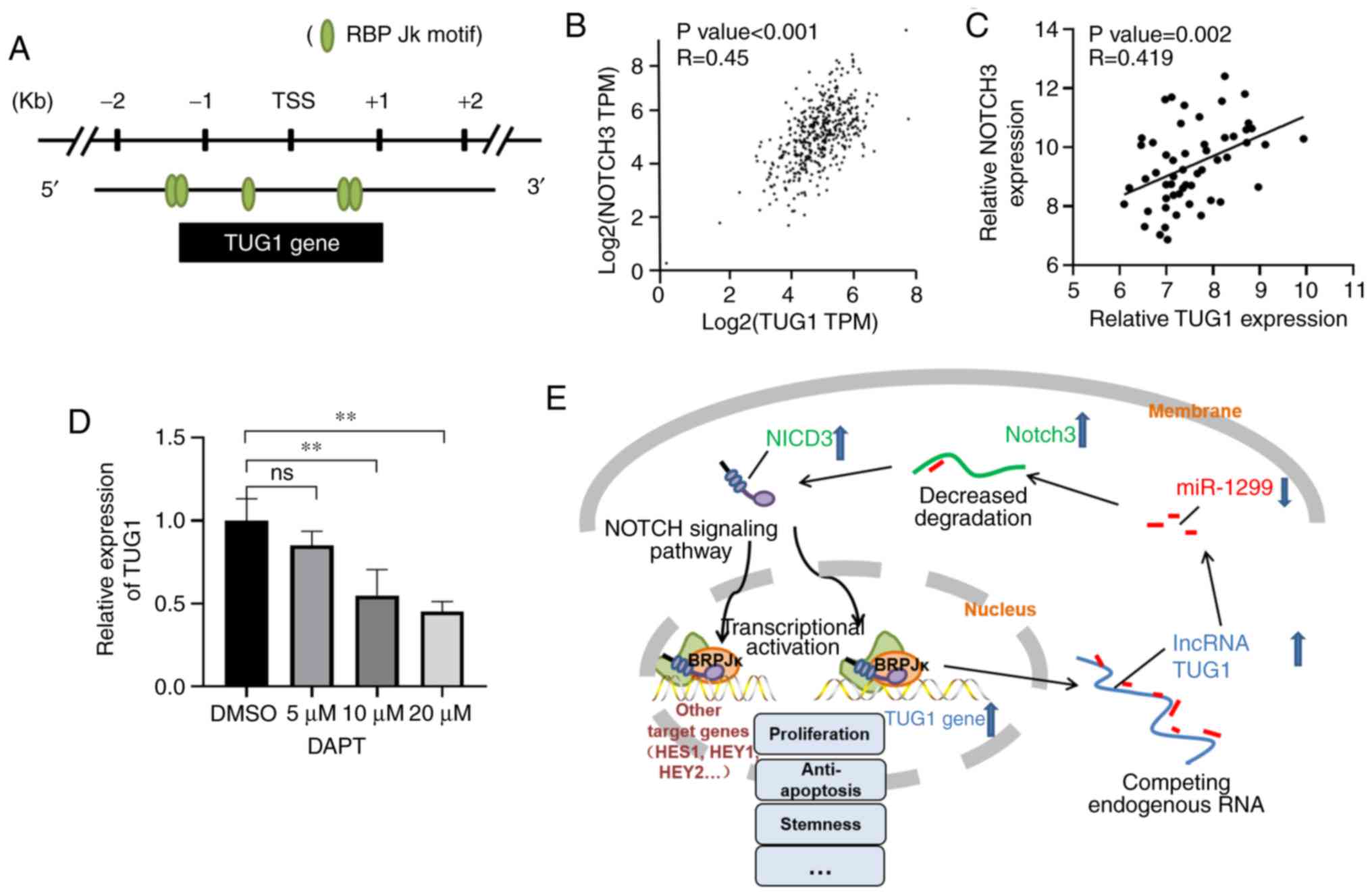
TUG1 is a potential target of NOTCH3
and forms a miR-1299/NOTCH3/TUG1 feedback loop
In the NOTCH signaling pathway, after the cleavage
of γ-secretase, the intracellular domain of NOTCH3 protein (NICD3)
is translocated to the nucleus and binds the transcription factor
complex RBP Jκ (39). When we
analyzed the transcriptional start site (TSS, −2 kb to +1 kb) of
TUG1 in the JASPAR CORE database (40), we found 5 RBP Jκ binding motifs
around its TSS (Fig. 5A),
indicating that TUG1 may be directly regulated by NOTCH3.
Additionally, there was a significant positive correlation between
the expression of TUG1 and NOTCH3 both in our tissues and in the
TCGA database (41) (Fig. 5B and C). When we treated A2780 cells
with DAPT, an inhibitor of γ-secretase, that can block the NOTCH
signaling pathway without interference in NOTCH3 mRNA activity, we
found a subsequent decrease in TUG1 expression in a concentration
gradient manner (Fig. 5D).
Therefore, we considered TUG1 as a potential downstream target of
NOTCH3, and that there may exist a miR-1299/NOTCH3/TUG1 feedback
loop in the development of OC (Fig.
5E).
Discussion
Recently, miR-1299 was found to be downregulated in
several types of cancer and to act as a tumor suppressor. Zhu et
al (42) showed that in
hepatocellular cancer, miR-1299 overexpression inhibited cell
proliferation and arrested the cell cycle in the G0/G1 phase, while
miR-1299 knockdown promoted cell proliferation and accelerated G1/S
transition. In breast cancer, miR-1299 was found to inhibit CDK6
expression and to bind to its 3′UTR region, suppressing cell
proliferation and migration ability (43). A similar tumor suppressive role of
miR-1299 was found in esophageal squamous cell carcinoma (44), prostate cancer (45), triple-negative breast cancer
(46), and cholangiocarcinoma
(47). In this study, we
demonstrated that miR-1299 was significantly decreased in OC
tissues and was associated with clinical features, and
overexpression of miR-1299 inhibited tumor growth both in
vitro and in vivo, suggesting that miR-1299 also acted
as a tumor suppressor in ovarian cancer (OC). In addition, we first
demonstrated that miR-1299 was a negative regulator of NOTCH3, a
vital oncogene in OC. Transcriptome analysis showed that
suppression of NOTCH signaling in ovarian and breast cancer cells
led to downregulation of genes in pathways involved in cell-cycle
regulation (48). Both NOTCH3 siRNA
and pathway inhibitors caused cell cycle arrest by reducing cyclin
D1 and cyclin D3 levels while elevating p21 and p27 levels. In this
study, we observed a similar cellular phenotype and protein change
(data not shown) after transfection of miR-1299. However, there is
also a limitation to our study as we used miR-1299 mimics to
transfect two cell lines, and did not use inhibitors in the
functional experiments considering the low expression of miR-1299
in OC.
The lncRNA taurine upregulated gene 1 (TUG1), a
7.1-kb lncRNA, was first discovered in a genomic screen for genes
upregulated by taurine treatment in mouse retinal cells (49). Recently, TUG1 was characterized as a
new oncogene and found to be upregulated in multiple human cancers
(50–56). TUG1 was upregulated in OC tissues
and cells and was positively correlated with advanced disease and
poor prognosis. Knockdown of TUG1 significantly inhibited cell
proliferation and epithelial-mesenchymal transition (EMT) and
induced cell apoptosis in OC (56–58).
The biological functions of lncRNAs largely rely on their distinct
subcellular localization. Cytoplasmic lncRNAs can regulate mRNA
stability or translation by acting as sponges for miRNAs (59). TUG1 localizes both in the nucleus
and the cytoplasm, indicating that it could have a multi-oncogenic
role. Using bioinformatics analyses and luciferase reporter assays,
our study provided the first evidence that TUG1 directly bound to
and inhibited miR-1299 expression. Knockdown of TUG1 was found to
inhibit cell proliferation, colony formation, and cell cycle
progression, which could be partially rescued by inhibition of
miR-1299. Our observations were consistent with previous studies
and confirmed the involvement of TUG1 in the ceRNA mechanism.
Notably, there have been several other miRNAs negatively regulated
by TUG1 in cancers (60–62), among which quite a few miRNAs have
been reported to have a tumor suppressive role in OC (63–65).
TUG1 could sponge more than one miRNA in OC and affect the
development of OC through different signaling pathways.
Interestingly, we found that TUG1 possessed five RBP
Jk motifs around its promoter region, which can be bound by the
NOTCH intracellular domain and trigger transcriptional activation
by classical NOTCH signaling pathway. Additionally, treatment with
DAPT, an inhibitor of γ-secretase, in OC cells decreased the TUG1
level in a concentration gradient-dependent manner. Therefore, we
hypothesized that there could be a miR-1299/NOTCH3/TUG1 feedback
loop in OC development. Downregulation of miR-1299 in OC
upregulated NOTCH3 level, and overexpression of NOTCH3 activated
the expression of various oncogenes by the NOTCH signaling pathway,
including the downstream molecule TUG1. In addition, overexpression
of TUG1 was a sponge for miR-1299, triggering a positive feedback
in tumorigenesis. A recent study in glioma showed that TUG1 was a
NOTCH-regulated lncRNA (66), which
confirmed our hypothesis. However, we have provided preliminary
evidence on this feedback loop hypothesis. The direct regulation of
TUG1 by NOTCH3 at the molecular level requires further experimental
evidence such as RIP or RNA pulldown results. Therefore, further
confirmation of the miR-1299/NOTCH3/TUG1 feedback loop, as well as
its role in other important issues, including stemness and
drug-resistance in OC, will be the main aim of our future work.
To date, the possible regulators and mechanisms
underlying NOTCH3-mediated regulatory behaviors in OC have not been
completely elucidated. Our study showed that miR-1299 is a novel
negative regulator of NOTCH3 and is downregulated in OC.
Overexpression of miR-1299 was found to play a tumor suppressor
role both in vitro and in vivo by partially
inhibiting cell proliferation. lncRNA TUG1 acted as a sponge for
miR-1299 and promoted cell proliferation by upregulating NOTCH3.
TUG1 was also a potential target of NOTCH3, forming a
miR-1299/NOTCH3/TUG1 feedback loop in OC cells. Our findings
improve the understanding of OC pathogenesis and facilitate the
development of miRNA- and lncRNA-targeted diagnostics and
therapeutics against this disease.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgement
Not applicable.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant no. 81772272) and the
Graduate Innovation Fund of Peking Union Medical College (grant no.
2018-1002-01-27).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YP, DZ and WC designed the research and analyzed the
data. YP, KL and XL performed the cell function experiments. YP and
XD performed the animal experiments. YW, WW and NL collected the OC
and normal ovary tissues and reviewed the clinical features. YP
prepared the images and drafted the original manuscript. WC and DZ
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the Cancer Hospital, Chinese Academy of Medical
Sciences and Peking Union Medical College, Beijing [grant no:
NCC2017G-115], and informed consent was obtained from all
participating patients. Animal experiments were conducted with the
approval of the Animal Ethics Committee of Cancer Hospital, Chinese
Academy of Medical Sciences and Peking Union Medical College,
Beijing (ACC2019A060) and in accordance with the Guide for the Care
and Use of Laboratory Animals by the US National Institutes of
Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OC
|
ovarian cancer
|
|
NOTCH3
|
notch receptor 3
|
|
lncRNA
|
long noncoding RNA
|
|
miRNA
|
microRNA
|
|
ceRNA
|
competing endogenous RNA
|
|
NICD3
|
NOTCH3 intracellular domain
|
|
TSS
|
transcriptional start site
|
|
EMT
|
epithelial-mesenchymal transition
|
|
TUG1
|
taurine upregulated gene 1
|
References
|
1
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou
XN, Chen R, Gu XY, Wei WW and He J: Report of cancer epidemiology
in China, 2015. Zhonghua Zhong Liu Za Zhi. 41:19–28. 2019.(In
Chinese). PubMed/NCBI
|
|
4
|
Marchetti C, Palaia I, De Felice F,
Musella A, Donfracesco C, Vertechy L, Romito A, Piacenti I, Musio
D, Muzii L, et al: Tyrosine-kinases inhibitors in recurrent
platinum-resistant ovarian cancer patients. Cancer Treat Rev.
42:41–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
du Bois A, Reuss A, Pujade-Lauraine E,
Harter P, Ray-Coquard I and Pfisterer J: Role of surgical outcome
as prognostic factor in advanced epithelial ovarian cancer: A
combined exploratory analysis of 3 prospectively randomized phase 3
multicenter trials: By the Arbeitsgemeinschaft Gynaekologische
Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe
d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire
(GINECO). Cancer. 115:1234–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zang RY, Harter P, Chi DS, Sehouli J,
Jiang R, Tropé CG, Ayhan A, Cormio G, Xing Y, Wollschlaeger KM, et
al: Predictors of survival in patients with recurrent ovarian
cancer undergoing secondary cytoreductive surgery based on the
pooled analysis of an international collaborative cohort. Br J
Cancer. 105:890–896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ottevanger PB: Ovarian cancer stem cells
more questions than answers. Semin Cancer Biol. 44:67–71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mirza MR, Monk BJ, Herrstedt J, Oza AM,
Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I,
et al: Niraparib maintenance therapy in platinum-sensitive,
recurrent ovarian cancer. N Engl J Med. 375:2154–2164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee EK and Konstantinopoulos PA: Combined
PARP and immune checkpoint inhibition in ovarian cancer. Trends
Cancer. 5:524–528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitsuhashi Y, Horiuchi A, Miyamoto T,
Kashima H, Suzuki A and Shiozawa T: Prognostic significance of
Notch signalling molecules and their involvement in the
invasiveness of endometrial carcinoma cells. Histopathology.
60:826–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park JT, Li M, Nakayama K, Mao TL,
Davidson B, Zhang Z, Kurman RJ, Eberhart CG, Shih IeM and Wang TL:
Notch3 gene amplification in ovarian cancer. Cancer Res.
66:6312–6318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Yun R, Yu X, Hu H, Huang G, Tan B
and Chen T: Overexpression of Notch3 and pS6 is associated with
poor prognosis in human ovarian epithelial cancer. Mediators
Inflamm. 2016:59534982016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung SG, Kwon YD, Song JA, Back MJ, Lee
SY, Lee C, Hwang YY and An HJ: Prognostic significance of Notch 3
gene expression in ovarian serous carcinoma. Cancer Sci.
101:1977–1983. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park JT, Chen X, Tropè CG, Davidson B,
Shih IeM and Wang TL: Notch3 overexpression is related to the
recurrence of ovarian cancer and confers resistance to carboplatin.
Am J Pathol. 177:1087–1094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu W, Liu T, Ivan C, Sun Y, Huang J,
Mangala LS, Miyake T, Dalton HJ, Pradeep S, Rupaimoole R, et al:
Notch3 pathway alterations in ovarian cancer. Cancer Res.
74:3282–3293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta N, Xu Z, El-Sehemy A, Steed H and Fu
Y: Notch3 induces epithelial-mesenchymal transition and attenuates
carboplatin-induced apoptosis in ovarian cancer cells. Gynecol
Oncol. 130:200–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Derrien T, Guigó R and Johnson R: The long
non-coding RNAs: A new (P)layer in the ‘Dark Matter’. Front Genet.
2:1072012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Ishak Gabra MB, Hanse EA, Lowman
XH, Tran TQ, Li H, Milman N, Liu J, Reid MA, Locasale JW, et al:
MiR-135 suppresses glycolysis and promotes pancreatic cancer cell
adaptation to metabolic stress by targeting phosphofructokinase-1.
Nat Commun. 10:8092019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang W, Zhou W, Xiang L, Wu X, Zhang P,
Wang J, Liu G, Zhang W, Peng Y, Huang X, et al: The
p300/YY1/miR-500a-5p/HDAC2 signalling axis regulates cell
proliferation in human colorectal cancer. Nat Commun. 10:6632019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang T, Wan X, Alvarez AA, James CD, Song
X, Yang Y, Sastry N, Nakano I, Sulman EP, Hu B and Cheng SY: MIR93
(microRNA-93) regulates tumorigenicity and therapy response of
glioblastoma by targeting autophagy. Autophagy. 15:1100–1111. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Zhang L, Wang B, Wei R, Wang Y,
Wan J, Zhang C, Zhao L, Zhu X, Zhang Y, et al: MiR-337-3p
suppresses proliferation of epithelial ovarian cancer by targeting
PIK3CA and PIK3CB. Cancer Lett. 469:54–67. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tung CH, Kuo LW, Huang MF, Wu YY, Tsai YT,
Wu JE, Hsu KF, Chen YL and Hong TM: MicroRNA-150-5p promotes cell
motility by inhibiting c-Myb-mediated Slug suppression and is a
prognostic biomarker for recurrent ovarian cancer. Oncogene.
39:862–876. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin C and Yang L: Long noncoding RNA in
cancer: Wiring signaling circuitry. Trends Cell Biol. 28:287–301.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klingenberg M, Matsuda A, Diederichs S and
Patel T: Non-coding RNA in hepatocellular carcinoma: Mechanisms,
biomarkers and therapeutic targets. J Hepatol. 67:603–618. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang CJ, Zhu CC, Xu J, Wang M, Zhao WY,
Liu Q, Zhao G and Zhang ZZ: The lncRNA UCA1 promotes proliferation,
migration, immune escape and inhibits apoptosis in gastric cancer
by sponging anti-tumor miRNAs. Mol Cancer. 18:1152019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H,
Kong J, Ding K, Shen HM, Wu H, et al: Long non-coding RNA linc00673
regulated non-small cell lung cancer proliferation, migration,
invasion and epithelial mesenchymal transition by sponging
miR-150-5p. Mol Cancer. 16:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du Z, Sun T, Hacisuleyman E, Fei T, Wang
X, Brown M, Rinn JL, Lee MG, Chen Y, Kantoff PW and Liu XS:
Integrative analyses reveal a long noncoding RNA-mediated sponge
regulatory network in prostate cancer. Nat Commun. 7:109822016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL,
Lv JW, Huang XD, Liu RQ, Chen F, He XJ, et al: Long noncoding RNA
FAM225A promotes nasopharyngeal carcinoma tumorigenesis and
metastasis by acting as ceRNA to sponge miR-590-3p/miR-1275 and
upregulate ITGB3. Cancer Res. 79:4612–4626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sticht C, De La Torre C, Parveen A and
Gretz N: miRWalk: An online resource for prediction of microRNA
binding sites. PLoS One. 13:e02062392018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vilming Elgaaen B, Olstad OK, Haug KB,
Brusletto B, Sandvik L, Staff AC, Gautvik KM and Davidson B: Global
miRNA expression analysis of serous and clear cell ovarian
carcinomas identifies differentially expressed miRNAs including
miR-200c-3p as a prognostic marker. BMC Cancer. 14:802014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paraskevopoulou MD, Georgakilas G,
Kostoulas N, Reczko M, Maragkakis M, Dalamagas TM and Hatzigeorgiou
AG: DIANA-LncBase: Experimentally verified and computationally
predicted microRNA targets on long non-coding RNAs. Nucleic Acids
Res. 41((Database Issue)): D239–D245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42((Database Issue)): D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen G, Wang Z, Wang D, Qiu C, Liu M, Chen
X, Zhang Q, Yan G and Cui Q: LncRNADisease: A database for
long-non-coding RNA-associated diseases. Nucleic Acids Res.
41((Database Issue)): D983–D986. 2013.PubMed/NCBI
|
|
39
|
Groeneweg JW, Foster R, Growdon WB,
Verheijen RH and Rueda BR: Notch signaling in serous ovarian
cancer. J Ovarian Res. 7:952014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khan A, Fornes O, Stigliani A, Gheorghe M,
Castro-Mondragon JA, van der Lee R, Bessy A, Chèneby J, Kulkarni
SR, Tan G, et al: JASPAR 2018: Update of the open-access database
of transcription factor binding profiles and its web framework.
Nucleic Acids Res. 46:D12842018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu H, Wang G, Zhou X, Song X, Gao H, Ma
C, Chang H, Li H, Liu FF, Lu J and Ma J: miR-1299 suppresses cell
proliferation of hepatocellular carcinoma (HCC) by targeting CDK6.
Biomed Pharmacother. 83:792–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu LH, Tian QQ, Liu J, Zhou Y and Yong H:
Upregulation of hsa_circ_0136666 contributes to breast cancer
progression by sponging miR-1299 and targeting CDK6. J Cell
Biochem. 120:12684–12693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Meng L, Liu S, Ding P, Chang S and Sang M:
Circular RNA ciRS-7 inhibits autophagy of ESCC cells by functioning
as miR-1299 sponge to target EGFR signaling. J Cell Biochem.
121:1039–1049. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Morgan SL, Wyant GA and Dinulescu DM:
‘Take it up a NOTCH’: Novel strategies for cancer therapy. Cell
Cycle. 12:191–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sang M, Meng L, Liu S, Ding P, Chang S, Ju
Y, Liu F, Gu L, Lian Y and Geng C: Circular RNA ciRS-7 maintains
metastatic phenotypes as a ceRNA of miR-1299 to target MMPs. Mol
Cancer Res. 16:1665–1675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shah MM, Zerlin M, Li BY, Herzog TJ,
Kitajewski JK and Wright JD: The role of Notch and gamma-secretase
inhibition in an ovarian cancer model. Anticancer Res. 33:801–808.
2013.PubMed/NCBI
|
|
48
|
Chen X, Thiaville MM, Chen L, Stoeck A,
Xuan J, Gao M, Shih IeM and Wang TL: Defining NOTCH3 target genes
in ovarian cancer. Cancer Res. 72:2294–2303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Young TL, Matsuda T and Cepko CL: The
noncoding RNA taurine upregulated gene 1 is required for
differentiation of the murine retina. Curr Biol. 15:501–512. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guo S, Zhang L, Zhang Y, Wu Z, He D, Li X
and Wang Z: Long non-coding RNA TUG1 enhances chemosensitivity in
non-small cell lung cancer by impairing microRNA-221-dependent PTEN
inhibition. Aging (Albany NY). 11:7553–7569. 2019.PubMed/NCBI
|
|
51
|
Yu G, Zhou H, Yao W, Meng L and Lang B:
lncRNA TUG1 Promotes cisplatin resistance by regulating CCND2 via
epigenetically silencing miR-194-5p in bladder cancer. Mol Ther
Nucleic Acids. 16:257–271. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Z, Wang X, Cao S, Han X, Wang Z,
Zhao X, Liu X, Li G, Pan and Lei D: The long noncoding RNA TUG1
promotes laryngeal cancer proliferation and migration. Cell Physiol
Biochem. 49:2511–2520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barbagallo C, Brex D, Caponnetto A,
Cirnigliaro M, Scalia M, Magnano A, Caltabiano R, Barbagallo D,
Biondi A, Cappellani A, et al: LncRNA UCA1, upregulated in CRC
biopsies and downregulated in serum exosomes, controls mRNA
expression by RNA-RNA interactions. Mol Ther Nucleic Acids.
12:229–241. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
He C, Liu Z, Jin L, Zhang F, Peng X, Xiao
Y, Wang X, Lyu Q and Cai X: lncRNA TUG1-mediated miR-142-3p
downregulation contributes to metastasis and the
epithelial-to-mesenchymal transition of hepatocellular carcinoma by
targeting ZEB1. Cell Physiol Biochem. 48:1928–1941. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun J, Hu J, Wang G, Yang Z, Zhao C, Zhang
X and Wang J: LncRNA TUG1 promoted KIAA1199 expression via miR-600
to accelerate cell metastasis and epithelial-mesenchymal transition
in colorectal cancer. J Exp Clin Cancer Res. 37:1062018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xu Y, Ge Z, Zhang E, Zuo Q, Huang S, Yang
N, Wu D, Zhang Y, Chen Y, Xu H, et al: The lncRNA TUG1 modulates
proliferation in trophoblast cells via epigenetic suppression of
RND3. Cell Death Dis. 8:e31042017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kuang D, Zhang X, Hua S, Dong W and Li Z:
Long non-coding RNA TUG1 regulates ovarian cancer proliferation and
metastasis via affecting epithelial-mesenchymal transition. Exp Mol
Pathol. 101:267–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li TH, Zhang JJ, Liu SX and Chen Y: Long
non-coding RNA taurine-upregulated gene 1 predicts unfavorable
prognosis, promotes cells proliferation, and inhibits cells
apoptosis in epithelial ovarian cancer. Medicine (Baltimore).
97:e05752018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen LL: Linking long noncoding RNA
localization and function. Trends Biochem Sci. 41:761–772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ren K, Li Z, Li Y, Zhang W and Han X: Long
noncoding RNA taurine-upregulated gene 1 promotes cell
proliferation and invasion in gastric cancer via negatively
modulating miRNA-145-5p. Oncol Res. 25:789–798. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang Y, Yang T, Zhang Z, Lu M, Zhao W,
Zeng X and Zhang W: Long non-coding RNA TUG1 promotes migration and
invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells.
Cancer Sci. 108:859–867. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhao L, Sun H, Kong H, Chen Z, Chen B and
Zhou M: The lncRNA-TUG1/EZH2 axis promotes pancreatic cancer cell
proliferation, migration and EMT phenotype formation through
sponging miR-382. Cell Physiol Biochem. 42:2145–2158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sheng Q, Zhang Y, Wang Z, Ding J, Song Y
and Zhao W: Cisplatin-mediated down-regulation of miR-145
contributes to up-regulation of PD-L1 via the c-Myc transcription
factor in cisplatin-resistant ovarian carcinoma cells. Clin Exp
Immunol. 200:45–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tan H, He Q, Gong G, Wang Y, Li J, Wang J,
Zhu D and Wu X: miR-382 inhibits migration and invasion by
targeting ROR1 through regulating EMT in ovarian cancer. Int J
Oncol. 48:181–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhu X, Li Y, Xie C, Yin X, Liu Y, Cao Y,
Fang Y, Lin X, Xu Y, Xu W, et al: miR-145 sensitizes ovarian cancer
cells to paclitaxel by targeting Sp1 and Cdk6. Int J Cancer.
135:1286–1296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Katsushima K, Natsume A, Ohka F, Shinjo K,
Hatanaka A, Ichimura N, Sato S, Takahashi S, Kimura H, Totoki Y, et
al: Targeting the Notch-regulated non-coding RNA TUG1 for glioma
treatment. Nat Commun. 7:136162016. View Article : Google Scholar : PubMed/NCBI
|