Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors worldwide, and its incidence has significantly
increased during the past 20 years (1,2). While
surgical treatments have significantly increased the 5-year
survival rate of patients with most types of primary tumors,
late-stage CRC patients still have a 5-year overall survival rate
of <10% (3). Radiotherapy and
chemotherapy can be used for patients with different stages of CRC,
but are not recommended for patients with functional organs and for
extended treatment of >6 months (4). Recently, molecular-targeting agents
have emerged as promising treatments for prolonging the overall
survival of CRC patients, (5). This
has created an urgent need to identify new targets that can be used
for the early diagnosis and personalized treatment of CRC.
Testis-specific protein Y-encoded-like 5
(TSPYL5), located on chromosome 8q22.1, is a member of the
TSPY-L gene family (6), and
is also a member of the nucleosome assembly protein (NAP)
superfamily (7). TSPYL5 has been
shown to interact with ubiquitin-specific protease to reduce the
tumor-suppressor activity of p53 (8). Accumulating evidence suggests a
critical role for TSPYL5 in tumor progression. For example, TSPYL5
was shown to modulate the growth of A549 cells and their
sensitization to the detrimental effects of toxic agents via
regulation of p21(WAF1/Cip1) and the PTEN/AKT pathway (9). Restoration of TSPYL5 by a DNA
methyltransferase inhibitor was demonstrated to suppress the growth
of gastric cancer cells (10).
Furthermore, TSPYL5 functions as a tumor suppressor in
ovarian cancer (11) and an
oncogene in breast cancer (12).
Endoplasmic reticulum (ER) is made up of membranous
tubules and vesicles. An accumulation of unfolded and misfolded
proteins usually leads to ER stress (ERS) (13,14).
ERS is mediated by pancreatic endoplasmic reticulum kinase (PERK),
inositol-requiring enzyme 1 (IRE1), and activating transcription
factor 6 (ATF6) for the purpose of maintaining protein homeostasis
(15). As a major
signal-transducing event, ERS can induce apoptosis to enhance the
cytotoxicity of various chemotherapeutic drugs (16,17).
It is now well-established that targeting the ERS response is an
effective strategy for suppressing the growth of human
hepatocellular carcinoma (18),
breast cancer (19), and ovarian
cancer cells (20). Although some
investigators have focused on the role of ERS in CRC, few studies
have examined the mechanism by which TSPYL5 affects ERS and CRC
progression.
In the present study, we investigated the expression
patterns and clinical significance of TSPYL5 in CRC patients via a
GEPIA database analysis and an analysis of clinical samples.
Furthermore, we explored the biological function of TSPYL5 and its
effects on ERS-associated factors for the purpose of identifying
molecular pathways involved in the malignant behaviors of CRC
cells.
Materials and methods
GEPIA database analysis
The levels of TSPYL5 expressed in CRC tumors
and normal tissues were identified using the online Gene Expression
Profiling Interactive Analysis (GEPIA) database (http://gepia.cancer-pku.cn/index.html),
which is an interactive website that includes information for 9,736
tumor samples and 8,587 normal tissue samples obtained from TCGA
and GTEx projects. The GEPIA database was also used to generate
survival curves based on the levels of TSPYL5 gene
expression in CRC tissues, as determined by the log-rank test.
Clinical tissues
Thirty pairs of CRC and para-carcinoma tissue
samples were collected from CRC patients who underwent surgical
resection of their tumors at the Renmin Hospital of Wuhan
University from February 2017 to December 2018 (age range, 45–86
years; Females, 41%). None of the patients had received any
radiotherapy or chemotherapy prior to surgery, and each patient
provided a written informed consent. All the tissue samples were
immediately frozen in liquid nitrogen and stored at −80°C until
use. The protocol for this study was approved by the Ethics
Committee of The Renmin Hospital of Wuhan University (Wuhan, Hubei,
China). All procedures involving human subjects were performed in
accordance with the 1964 Helsinki declaration.
Quantitative real time PCR
Total RNA was isolated from frozen tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
reversed transcribed into cDNA with an iScript cDNA Synthesis Kit
(Bio-Rad Laboratories, Inc.). Quantitative real-time PCR was
performed using iTaq Universal SYBR Green Supermix (Bio-Rad
Laboratories, Inc.) on an ABI 7500 Fast Real-Time PCR System
(Applied Biosystems). The PCR reaction conditions consisted of 95°C
for 3 min, followed by 40 cycles of 95°C for 30 sec and 60°C for 30
sec. The primer sequences used were as follows: TSPYL5
forward, GGTTGTTTTTTGTGTAGTTGTAGT and reverse,
CATCACAAACATACAACTATACCA. TSPYL5 expression was normalized
to that of GAPDH, and analyzed using the 2−ΔΔCq method
(21).
Cell culture and transfection
Human CRC cell lines HCT116 and HT29 were obtained
from the American Type Culture Collection (ATCC) and cultured in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.). Both cell lines were maintained in a humidified
atmosphere containing 5% CO2 at 37°C.
For TSPYL5 overexpression, the cDNA for TSPYL5
without its 3′-UTR was inserted into a pcDNA3.1 vector using
Lipofectamine 2000 transfection reagent (both from Invitrogen;
Thermo Fisher Scientific, Inc.) to generate the recombinant vector,
pcDNA3.1-TSPYL5. Subsequently, HCT116 and HT29 cells were seeded
into 6-well plates and transfected with pcDNA3.1-TSPYL5 or empty
pcDNA3.1, to produce TSPYL5 and negative control (NC) groups,
respectively. Non-transfected cells served as a blank group.
EdU proliferation assay
A Cell Light™ EDU Apollo®488 In Vitro
Imaging Kit (Guangzhou RiboBio) was used to detect the
proliferation rates of the transfected CRC cells, according to
instructions provided by the manufacturer. Briefly, HCT116 and HT29
cells were fixed with 4% paraformaldehyde for 15 min, washed three
times with PBS, and then stained with 200 µl of 1X Apollo solution
for 30 min. After another wash with PBS, the cells with
EdU-positive signals were detected by flow cytometry (BD
Biosciences).
Flow cytometry
Cell apoptosis was detected with an Annexin
V-fluorescein Isothiocyanate Propidium Iodide (FITC/PI) apoptosis
detection kit (cat. no. 70-AP101-100; Hangzhou MultiSciences
Biotech Co., Ltd.). Briefly, HCT116 and HT29 cells were harvested,
washed twice in PBS, and then stained with Annexin V-FIFC/PI for 15
min; after which, they were analyzed by flow cytometry (BD
Biosciences). The total apoptotic rate, including early apoptosis
and late apoptosis, was calculated and averaged for three
experiments.
Hoechst 33342 staining
HCT116 and HT29 cells in their logarithmic growth
phase were plated into 6-well plates and incubated for 48 h. The
cells were then washed twice with PBS, incubated in the dark for 10
min with Hoechst 33342 nucleic acid stain (Sigma-Aldrich; Merck
KGaA), and subsequently examined for their nuclear morphology under
a fluorescence microscope (Olympus Corp.).
Transwell assay
Cell migration and invasion abilities were examined
using Transwell assays. Briefly, ~2×104 HCT116 or HT29
cells suspended in 1 ml of serum-free medium were added into the
upper chamber (without Matrigel for migration or with Matrigel for
invasion) of a Transwell plate (Costar, cat. no. 3422; Corning Life
Sciences). The lower chamber was filled with 500 µl of culture
medium containing 10% FBS. After a 48-h incubation, the migrated
cells in the lower chamber were fixed with formaldehyde for 5 min,
and then stained with 0.5% crystal violet solution (Sigma-Aldrich;
Merck KGaA). The stained cells were observed under a phase-contrast
microscope (Olympus Corp.) at a magnification of ×200, and various
visual fields were randomly selected for cell counting.
Transmission electron microscopy
ERS was examined by transmission electron microscopy
as previously described (22). In
brief, transfected HCT116 and HT29 cells were harvested, fixed in
glutaraldehyde, and then dehydrated in serial dilutions of acetone
(30, 80 and 90%). Next, ultrathin sections were produced by
embedding cells in Ultracut (Leica, Germany) and cutting into 60-nm
sections. After staining with uranyl acetate, the ultrathin
sections were examined with a JEM-1230 transmission electron
microscope (JEOL Ltd.).
Western blot analysis
Total cellular protein was obtained by lysing CRC
cells in RIPA buffer (Pierce; Thermo Fisher Scientific, Inc.) that
contained a protease inhibitor cocktail and phosphatase inhibitors
(Sigma-Aldrich; Merck KGaA). Protein concentrations were detected
using a BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.).
Samples containing ~30 µg of protein were separated by 10%
SDS-PAGE, and the protein bands were transferred onto PVDF
membranes (Invitrogen; Thermo Fisher Scientific, Inc.). The
membranes were then blocked with 5% skim milk and subsequently
incubated overnight with primary antibodies against TSPYL5 (cat.
no. ab203657, dilution: 1:800; Abcam), caspase-1 (cat. no. ab62698,
dilution: 1:800; Abcam), caspase-3 (cat. no. ab49822, dilution:
1:500; Abcam), Bax (cat. no. M00183-2, dilution: 1:1,000, Boster),
ATF4 (cat. no. A00371, dilution: 1:500; Boster), CHOP (cat. no.
A00311, dilution: 1:500; Boster), and GAPDH (cat. no. ab9485,
dilution: 1:800; Abcam) at 4°C. On the following day, the membranes
were incubated with an HRP conjugated secondary antibody (cat. no.
ab97080, dilution: 1:30,000; Abcam). The immunostained proteins
were detected using an enhanced chemiluminescence kit (Thermo
Fisher Scientific, Inc.). Quantification of western blot bands was
performed using Image-Pro Plus (Version 6; Media Cybernetics,
Inc.).
Statistical analysis
All statistical analyses were performed using IBM
SPSS Statistics for Windows, version 21.0 (IBM Corp.). Quantitative
results are expressed as the mean ± SD of data obtained from at
least three experiments. Differences between two groups were
analyzed using Student's t-test and differences among groups were
assessed by one-way analysis of variance (ANOVA). A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of TSPYL5 was downregulated
in CRC tissues
To determine differences in TSPYL5 expression
in CRC tumor tissues vs. normal tissues, the TSPYL5 mRNA
levels in COAD (colon adenocarcinoma) and READ (rectum
adenocarcinoma) were analyzed using the GEPIA database. As shown in
Fig. 1A, TSPYL5 expression
was significantly lower in the COAD and READ tissues when compared
to levels in the respective adjacent normal tissues (P<0.05). To
verify the expression results obtained from the GEPIA database, we
determined the levels of TSPYL5 expression in 30 pairs of
CRC tumor and para-carcinoma tissues by quantitative real-time PCR.
As shown in Fig. 1B, TSPYL5
expression was significantly downregulated in the tumor tissues
when compared with that noted in the para-carcinoma tissues
(P<0.05). Western blot analyses of 8 pairs of representative
tissues showed a result similar to that obtained by quantitative
real-time PCR (Fig. 1C and D,
P<0.01). These results suggest the role of TSPYL5 as a possible
tumor suppressor in CRC.
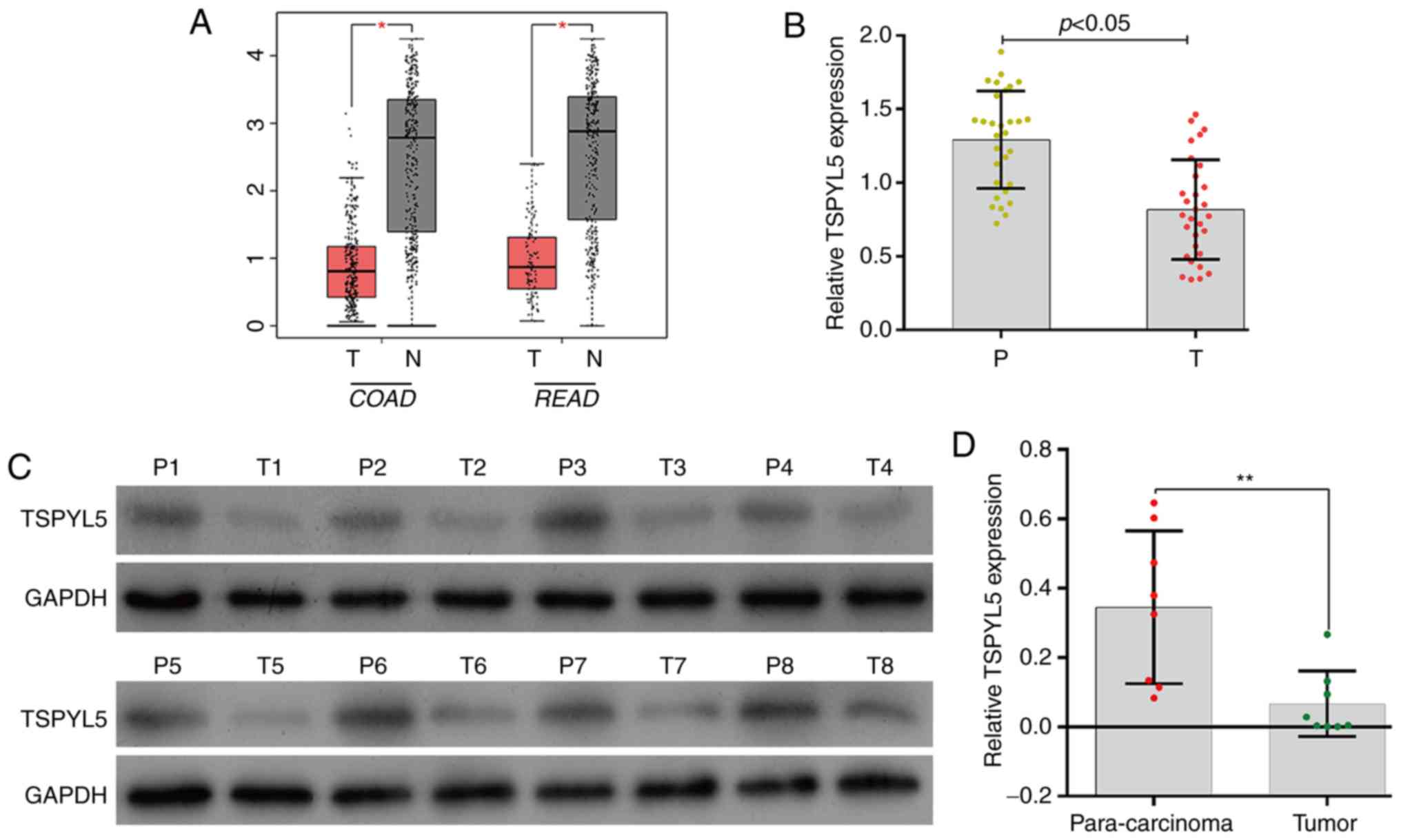 | Figure 1.Expression of TSPYL5 was downregulated
in CRC tissues. (A) TSPYL5 expression levels in COAD (colon
adenocarcinoma) T, tumor [n=275], N, normal [n=349] and READ
(rectum adenocarcinoma) T [n=92], N [n=318] were investigated via
the GEPIA database. (B) The levels of TSPYL5 mRNA expression
in 30 paired tumor tissues and para-carcinoma tissues were
investigated by quantitative real-time PCR analysis. (C) The levels
of TSPYL5 protein expression in 8 paired tumor tissues (T1-T8) and
para-carcinoma tissues (P1-P8) were investigated by western
blotting. (D) Quantitative analysis of TSPYL5 protein levels in 8
paired tumor tissues and para-carcinoma tissues. *P<0.05,
**P<0.01. P, para-carcinoma tissues; T, CRC tumor tissues. CRC,
colorectal cancer; TSPYL5, testis-specific protein Y-encoded-like
5; GEPIA, Gene Expression Profiling Interactive Analysis. T, Tumor
tissues; N, non-tumor tissues; n, number of samples used. |
Overexpression of TSPYL5 suppresses
the proliferation and promotes the apoptosis of CRC cells
To further confirm that TSPYL5 acts as a tumor
suppressor in CRC in vitro, two CRC cell lines, HCT116 and
HT29, were transfected with pcDNA3.1-TSPYL5, and then used in a
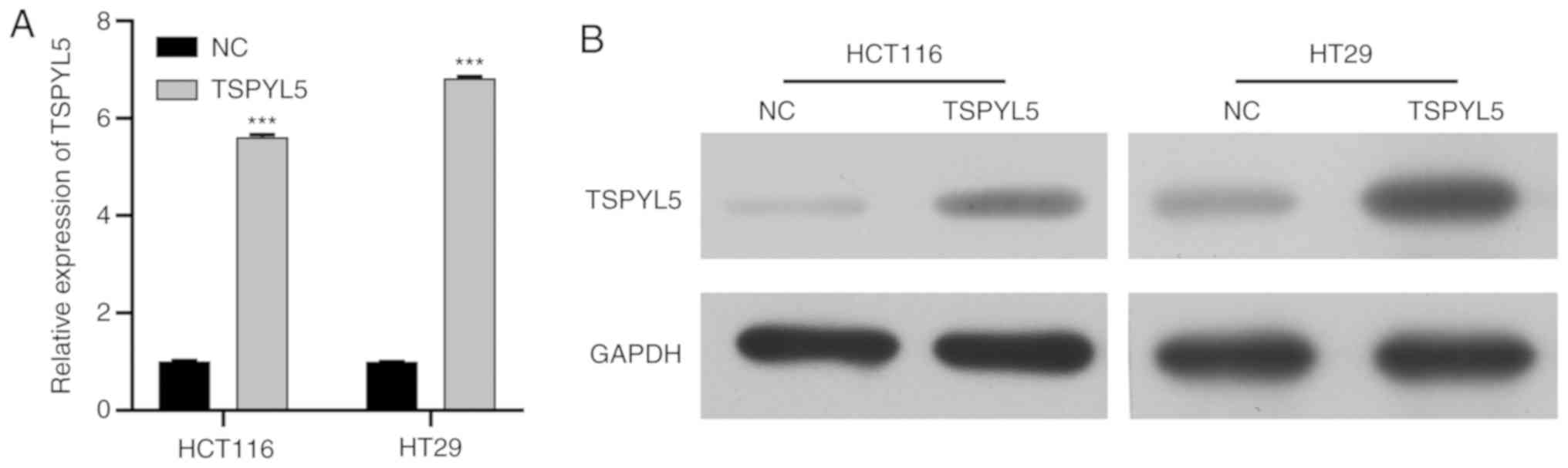
series of functional experiments. Firstly, RT-qPCR and western blot
analysis were performed to validated the overexpression of TSPYL5
in HCT116 and HT29 cell lines. Results showed that expression of
TSPYL5 was enhanced after cell transfection (Fig. 2A and B).
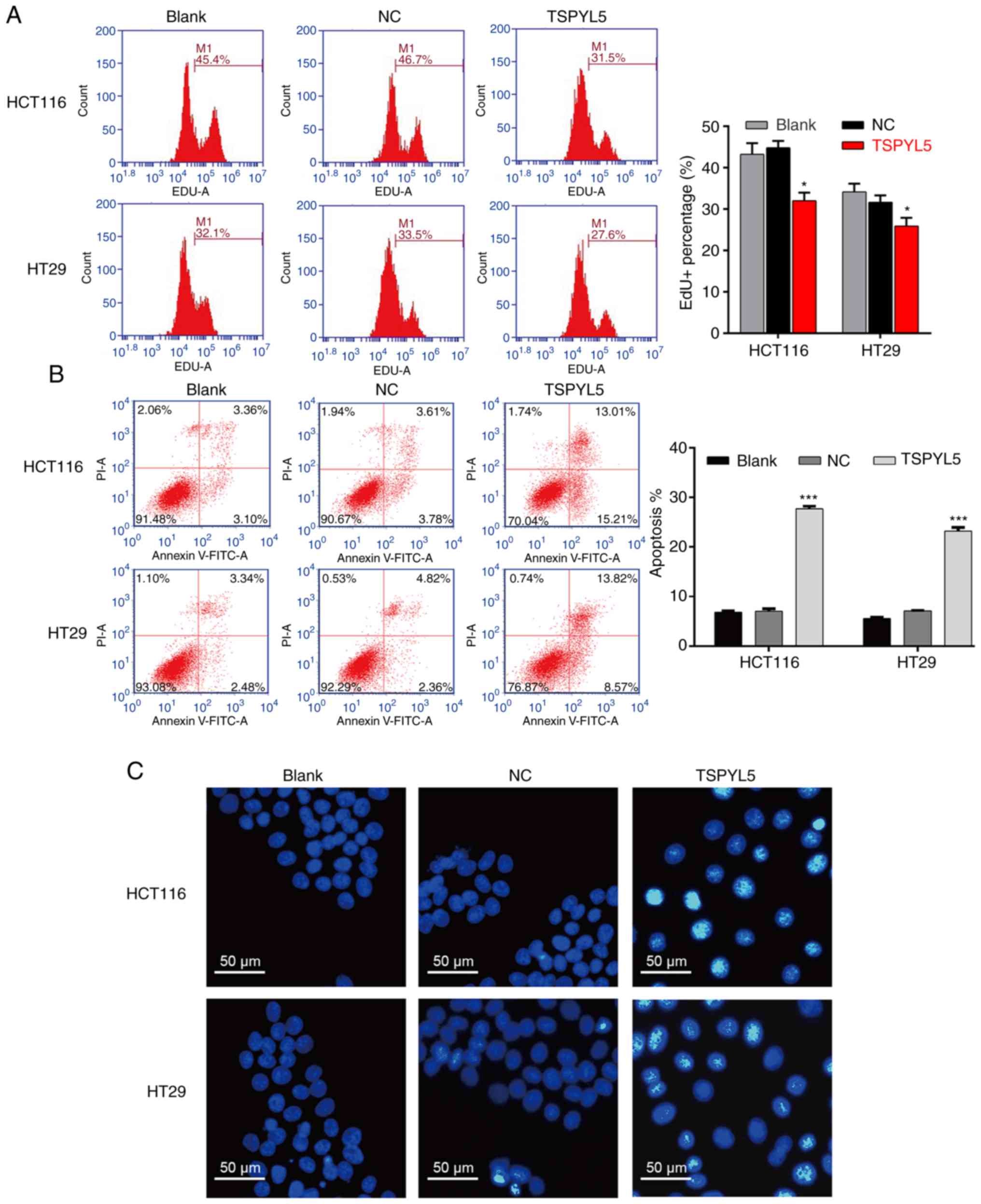
As shown in Fig. 3A,
flow cytometry of EdU-positive cells revealed that TSPYL5
overexpression decreased the percentage of EDU-positive HCT116
cells from 46.7 to 31.5% and the percentage of EDU-positive HT29
cells from 33.5 to 27.6%. In addition, flow cytometry with Annexin
V/PI double staining was performed to evaluate cell apoptosis. As
illustrated in Fig. 3B, the
apoptotic rate of the TSPYL5-overexpressing HCT116 cells was
significantly increased (27.69±0.52%) when compared to the
apoptotic rates of cells in the blank (6.81±0.30%) and NC
(7.05±0.49%) groups (P<0.001). Similarly, overexpression of
TSPYL5 promoted the apoptosis of HT29 cells (Fig. 3B, P<0.001). Next, HCT116 and HT29
cells stained with a DNA-specific dye (Hoechst 33342) were examined
for morphologic changes. The results showed that
TSPYL5-overexpressing HCT116 and HT29 cells exhibited bright
fluorescence and characteristic features of apoptosis, including
chromatin condensation when compared with cells in the NC and blank
groups (Fig. 3C).
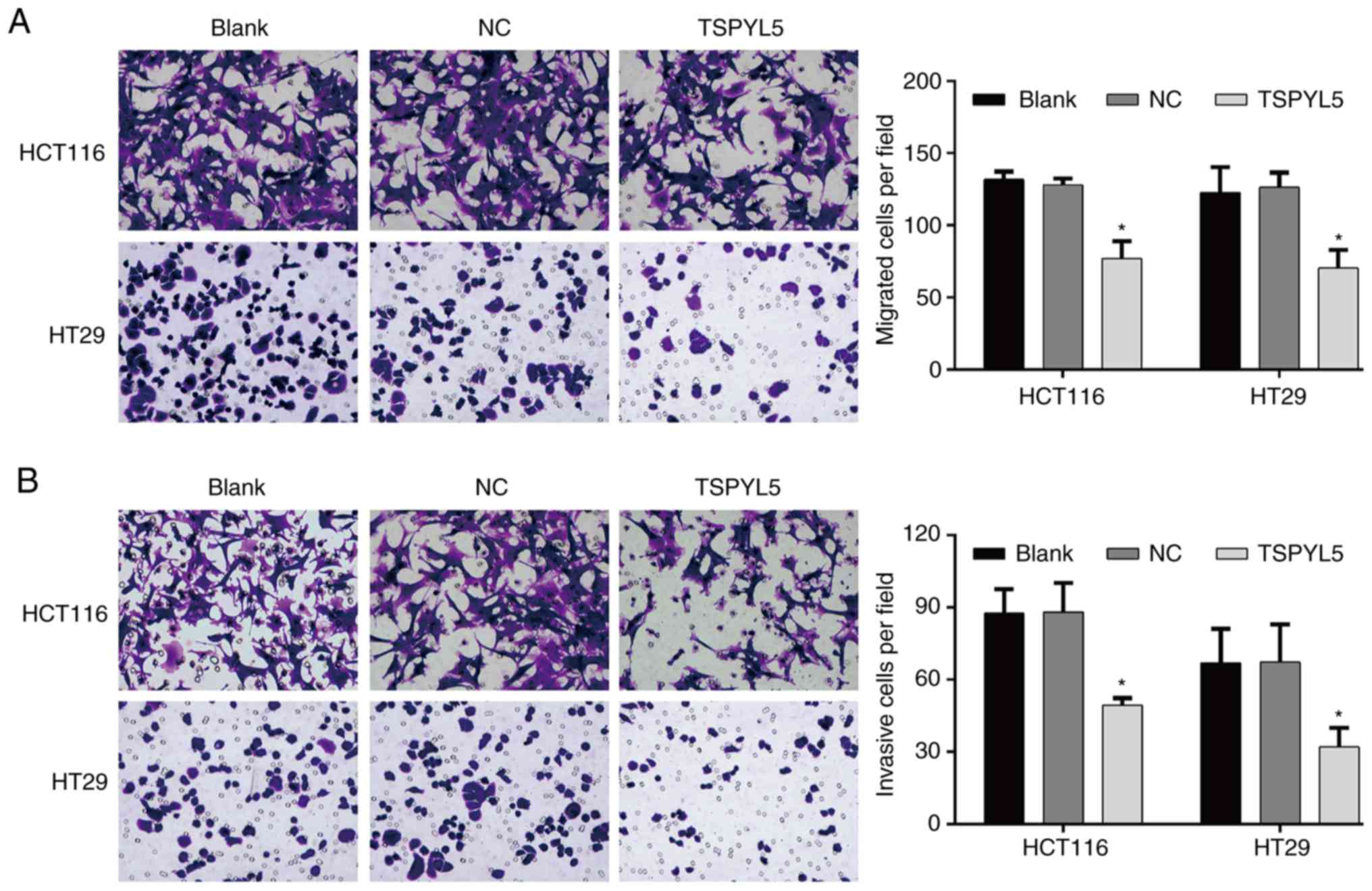
Overexpression of TSPYL5 suppresses the migration
and invasion abilities of CRC cells. Transwell assays were
performed to investigate the effect of TSPYL5 on the migration and
invasion abilities of CRC cells. As shown in Fig. 4A, overexpression of TSPYL5
significantly reduced the numbers of migrated HCT116 cells when
compared to the numbers of migrated control cells
(TSPYL5-overexpressing HCT116 cells vs. NC cells: 77.00±12.12 vs.
128.00±4.36; P<0.05), and similar results were obtained for the
TSPYL5-overexpressing HT29 cells (TSPYL5-overexpressing HT29 cells
vs. NC cells: 70.33±12.66 vs. 126.33±10.26, P<0.05). Moreover,
after transfection with pcDNA3.1-TSPYL5, the numbers of invading
HCT166 and HT29 (Fig. 4B) cells
were also decreased when compared to the numbers of invading NC
cells (TSPYL5-overexpressing HCT116 cells vs. NC cells: 49.33±3.06
vs. 88.00±12.12, P<0.05 and TSPYL5-overexpressing HT29 cells vs.
NC cells: 32.00±7.94 vs. 67.33±15.57, P<0.05).
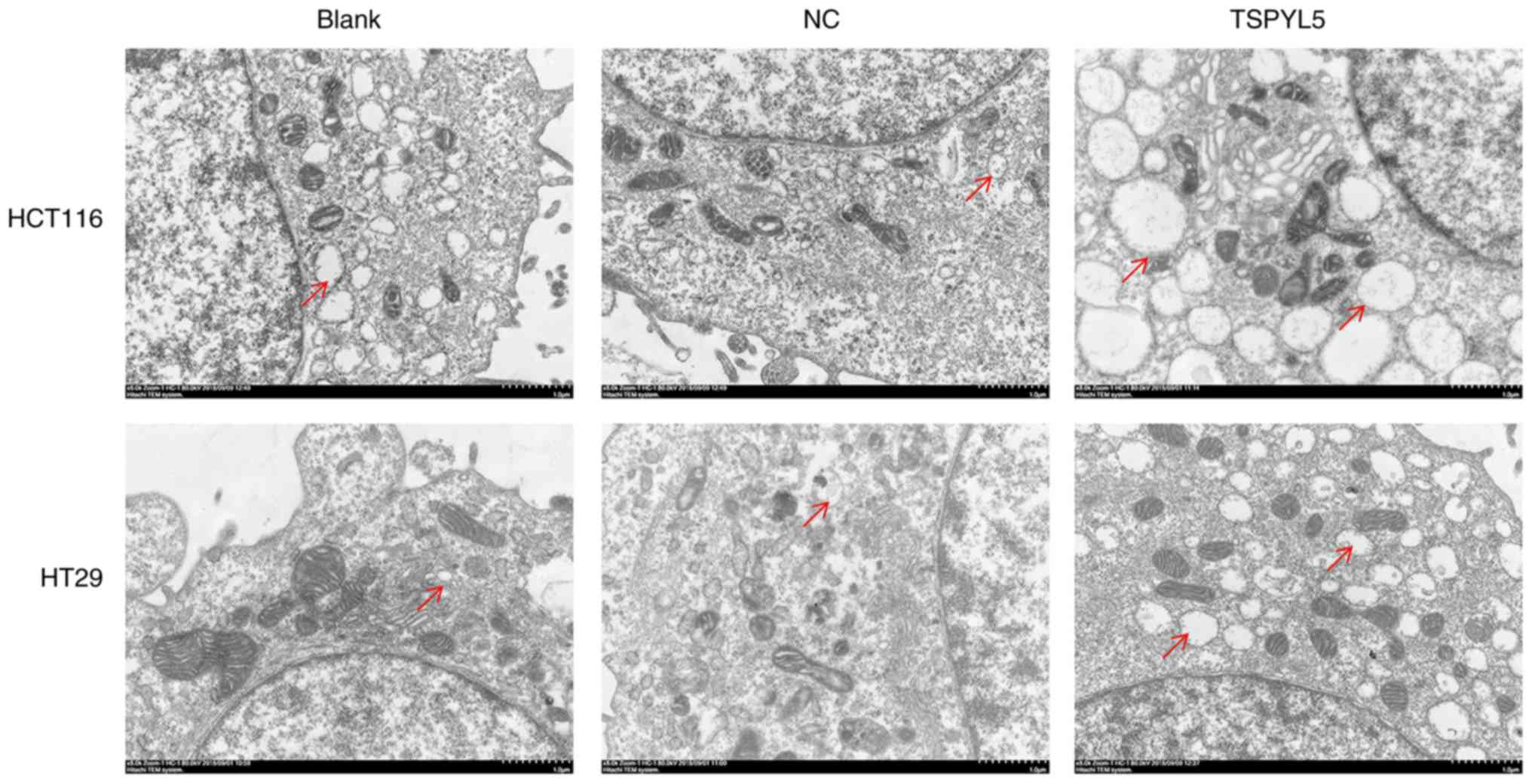
Effects of TSPYL5 overexpression on
ERS and associated proteins
As the effect of TSPYL5 on ERS in CRC cells remains
unknown, we next investigated whether TSPYL5 overexpression could
induce ERS. First, ERS was detected using a transmission electron
microscope. As shown in Fig. 5,
larger amounts of swollen ER were observed in the cytoplasm of
HCT116 and HT29 cells that overexpressed TSPYL5, and that change
was not observed in the control or NC cells. Next, western blot
analyses were performed to examine the direct effects of TSPYL5 on
ERS and apoptosis. As shown in Fig.
6, the levels of caspase-1, caspase-3, bcl-2-like protein 4
(Bax), activating transcription factor 4 (ATF4) and
CCAAT-enhancer-binding protein homologous protein (CHOP) proteins
were upregulated after TSPYL5 overexpression. These observed
changes indicated that TSPYL5 overexpression-induced apoptosis may
be correlated with activated ERS in CRC cells.
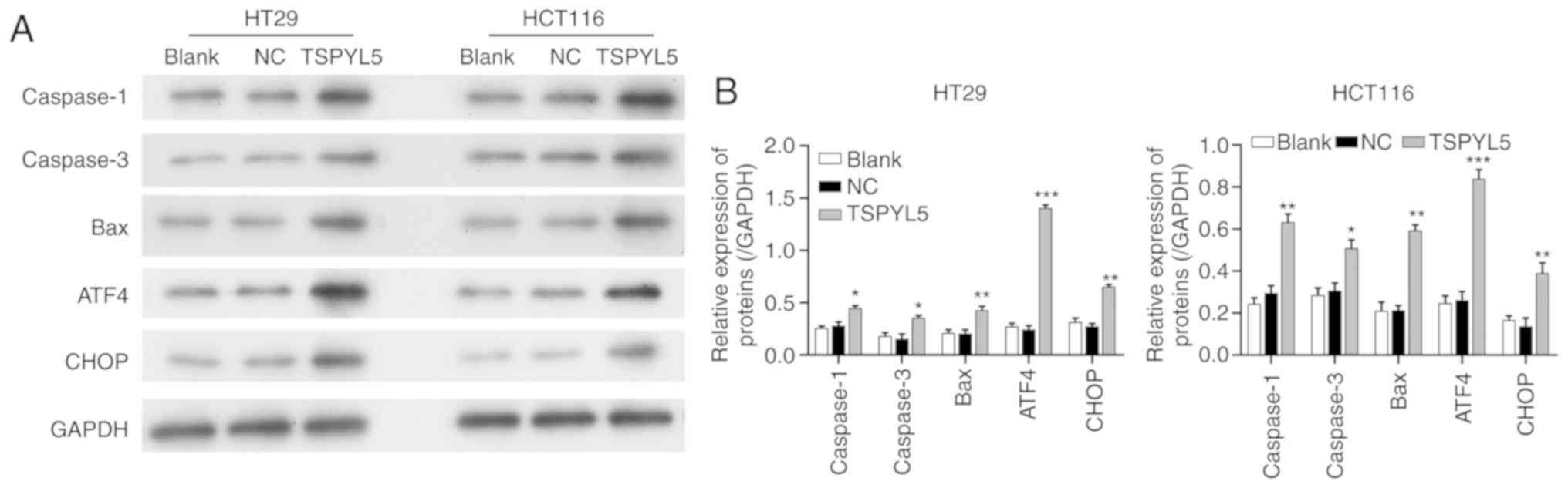 | Figure 6.Effects of TSPYL5 overexpression on
ERS and its associated proteins. HCT116 and HT29 cells were
transfected with pcDNA3.1-TSPYL5 or empty pcDNA3.1, and
subsequently divided into TSPYL5 and negative control (NC) groups,
respectively. (A) Western blot analysis of the protein levels of
caspase-1, caspase-3, bcl-2-like protein 4 (Bax), activating
transcription factor 4 (ATF4) and CCAAT-enhancer-binding protein
homologous protein (CHOP) in the HT29 and HCT116 cells in the
TSPYL5 overexpression and NC groups. (B) Quantification of the
levels of caspase-1, caspase-3, Bax, ATF4, and CHOP protein
expression as determined by western blot analysis. *P<0.05,
**P<0.01 and ***P<0.001 vs. the NC group. CRC, colorectal
cancer; TSPYL5, testis-specific protein Y-encoded-like 5. |
Discussion
Several studies have reported that TSPYL5
acts as a tumor-suppressor gene in gastric (10) and ovarian cancer (11), yet its clinical significance and
biological role in CRC remain unclear. In the present study, we
first found that TSPYL5 expression was significantly lower in CRC
tissues when compared with that noted in adjacent normal tissues.
The methylation levels of TSPYL5 were found to be
significantly increased in HCC tissues when compared with those
levels in adjacent non-tumor tissues, and that may be an
independent unfavorable factor affecting disease-free survival
(6). Aberrant methylation of the
TSPYL5 promoter has been reported to be associated with
high-risk oral leukoplakia (23).
In addition, TSPYL5 gene expression has been correlated with
the survival of patients with all grades of endometrial cancer
(24).
Next, we further demonstrated that overexpression of
TSPYL5 significantly suppressed cell proliferation, migration and
invasion, and induced apoptosis in two human CRC cell lines. Our
findings were consistent with those reported by Shao et al
(11), who observed that ovarian
cancer inhibition ability could be elevated by miR-629
inhibitor-mediated upregulation of TSPYL5. Lakshmanan et al
(25) showed that MUC16 regulates
TSPYL5 via the JAK2/STAT3/GR signaling axis in regards to lung
cancer cell growth and metastasis. Kumar et al (26) revealed that TSPYL5 overexpression in
prostate cancer cells increased the sensitivity of those cells to
chemotherapy drugs. Strikingly, a recent study by Huang and Luo
(27) demonstrated that
overexpression of TSPYL5 promoted apoptosis in HT29 cells and
reduced cell proliferation, migration and invasion, which further
validates our results (27).
Recently, interest has developed in determining how
to utilize the endoplasmic reticulum stress (ERS) response as a
method for treating cancer. In the present study, larger amounts of
swollen ER were observed in the cytoplasm of HCT116 and HT29 cells
that exhibited overexpression of TSPYL5, indicating an increase in
ERS. We also found that TSPYL5 overexpression triggered ERS that
significantly increased the levels of ATF4, CHOP, caspase-1,
caspase-3, and Bax proteins in CRC cells. These findings suggest
that ER stress can trigger apoptosis via ER stress-specific
cell-death signals, including CHOP and caspase, as previously
described (28). ERS-induced
apoptosis in CRC was previously described in another report
(29,30). Shikonin was found to induce
apoptotic cell death by activating ERS, accompanied by increases in
Bax and CHOP protein levels (30).
Moreover, interleukin-1 receptor associated kinase 2
(IRAK2), as a potential tumor suppressor to counterbalance
oncogenic Smad ubiquitylation regulatory factor 1 (Smurf1) in
response to ERS, also induced cell death (31). To the best of our knowledge, the
effects of TSPYL5 on ERS activation have not been previously
investigated. Our present findings suggest that TSPYL5
overexpression suppresses cell proliferation, migration, and
invasion via activation of ERS. While knockdown experiments of
TSPYL5 must also be performed in further in vitro and in
vivo investigations.
Overall, the data presented here demonstrated that
TSPYL5 exerted anticancer effects on HCT116 and HT29 cells by
activating ERS-induced apoptosis, as evidenced by an accumulation
of caspase-1, caspase-3, and Bax proteins, induction of ERS
markers, and induction of ATF4 and CHOP. These findings indicate
that targeting the ERS response using TSPYL5 may be a promising
strategy for treating CRC. This approach should be investigated in
future clinical trials.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data will be provided based on requirement from the
corresponding author upon reasonable request.
Authors' contributions
CH and RZ conceived and designed the study. CH and
CH performed the experiments. CH and PR wrote the manuscript. CH
and PR collected the data. PR and RZ reviewed, revised it
critically for important intellectual content and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The protocol for this study was approved by the
Ethics Committee of The Renmin Hospital of Wuhan University (Wuhan,
Hubei, China). All procedures involving human subjects were
performed in accordance with the 1964 Helsinki declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declared no competing interest.
References
|
1
|
Smeby J, Sveen A, Merok MA, Danielsen SA,
Eilertsen IA, Guren MG, Dienstmann R, Nesbakken A and Lothe RA:
CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in
primary colorectal cancer. Ann Oncol. 29:1227–1234. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Somers E: International Agency for
Research on Cancer. Encyclopedia of Toxicology. 133:1067–1069.
2014.
|
|
3
|
Tauriello DV, Calon A, Lonardo E and
Batlle E: Determinants of metastatic competency in colorectal
cancer. Mol Oncol. 11:97–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang JP: Chinese standard for the
diagnosis and treatment of colorectal cancer (2010). Zhonghua Wei
Chang Wai Ke Za Zhi. 14:1–4. 2011.(In Chinese). PubMed/NCBI
|
|
5
|
Sepulveda AR, Hamilton SR, Allegra CJ,
Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE, Lieu C,
Lindor NM, Minsky BD, et al: Molecular biomarkers for the
evaluation of colorectal cancer: Guideline From the American
Society for Clinical Pathology, College of American Pathologists,
Association for Molecular Pathology, and American Society of
Clinical Oncology. J Clin Oncol. 35:1453–1486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu X, Hu B, Huang Y, Deng Y, Wang X and
Zheng F: Hypermethylation of ACP1, BMP4, and TSPYL5 in
hepatocellular carcinoma and their potential clinical significance.
Dig Dis Sci. 61:149–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogel T, Dittrich O, Mehraein Y, Dechend
F, Schnieders F and Schmidtke J: Murine and human TSPYL genes:
Novel members of the TSPY-SET-NAP1L1 family. Cytogenet Cell Genet.
81:265–270. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Epping MT, Meijer LA, Krijgsman O, Bos JL,
Pandolfi PP and Bernards R: TSPYL5 suppresses p53 levels and
function by physical interaction with USP7. Nat Cell Biol.
13:102–108. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim EJ, Lee SY, Kim TR, Choi SI, Cho EW,
Kim KC and Kim IG: TSPYL5 is involved in cell growth and the
resistance to radiation in A549 cells via the regulation of
p21(WAF1/Cip1) and PTEN/AKT pathway. Biochem Biophys Res Commun.
392:448–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung Y, Park J, Bang YJ and Kim TY: Gene
silencing of TSPYL5 mediated by aberrant promoter methylation in
gastric cancers. Lab Invest. 88:153–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shao L, Shen Z, Qian H, Zhou S and Chen Y:
Knockdown of miR-629 Inhibits ovarian cancer malignant behaviors by
targeting testis-specific Y-like protein 5. DNA Cell Biol.
36:1108–1116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lyu JH, Park DW, Huang B, Kang SH, Lee SJ,
Lee C, Bae YS, Lee JG and Baek SH: RGS2 suppresses breast cancer
cell growth via a MCPIP1-dependent pathway. J Cell Biochem.
116:260–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ansari SS, Sharma AK, Soni H, Ali DM, Tews
B, König R, Eibl H and Berger MR: Induction of ER and mitochondrial
stress by the alkylphosphocholine erufosine in oral squamous cell
carcinoma cells. Cell Death Dis. 9:2962018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khaket TP, Singh MP, Khan I, Bhardwaj M
and Kang SC: Targeting of cathepsin C induces autophagic
dysregulation that directs ER stress mediated cellular cytotoxicity
in colorectal cancer cells. Cell Signal. 46:92–102. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang F and Luo J: Endoplasmic reticulum
stress and ethanol neurotoxicity. Biomolecules. 5:2538–2553. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mishra RR, Belder N, Ansari SA, Kayhan M,
Bal H, Raza U, Ersan PG, Tokat ÜM, Eyüpoğlu E, Saatci Ö, et al:
Reactivation of cAMP pathway by PDE4D inhibition
represents a novel druggable axis for overcoming tamoxifen
resistance in ER-positive breast cancer. Clin Cancer Res.
24:1987–2001. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bakhtou H, Olfatbakhsh A, Deezagi A and
Ahangari G: The expression of dopamine receptors gene and their
potential role in targeting breast cancer cells with selective
agonist and antagonist drugs. Could it be the novel insight to
therapy? Curr Drug Discov Technol. 16:184–197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Zhang L, Gao M, Han M, Liu K, Zhang
Z, Gong Z, Xing L, Shi X, Lu K and Gao H: Endoplasmic reticulum
stress triggers Xanthoangelol-induced protective autophagy via
activation of JNK/c-Jun Axis in hepatocellular carcinoma. J Exp
Clin Cancer Res. 38:82019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Z, Yang Y, Di S, Feng X, Liu D, Jiang
S, Hu W, Qin Z, Li Y, Lv J, et al: Pterostilbene exerts anticancer
activity on non-small-cell lung cancer via activating endoplasmic
reticulum stress. Sci Rep. 7:80912017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Gong W, Yang ZY, Zhou XS, Gong C,
Zhang TR, Wei X, Ma D, Ye F and Gao QL: Quercetin induces
protective autophagy and apoptosis through ER stress via the
p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis. 22:544–557. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ba-Omar TA, Al-Jardani S and Victor R:
Effects of pesticide temephos on the gills of Aphanius
dispar (Pisces: Cyprinodontidae). Tissue Cell. 43:29–38. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abe M, Yamashita S, Mori Y, Abe T, Saijo
H, Hoshi K, Ushijima T and Takato T: High-risk oral leukoplakia is
associated with aberrant promoter methylation of multiple genes.
BMC Cancer. 16:3502016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Witek Ł, Janikowski T, Bodzek P, Olejek A
and Mazurek U: Expression of tumor suppressor genes related to the
cell cycle in endometrial cancer patients. Adv Med Sci. 61:317–324.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lakshmanan I, Salfity S, Seshacharyulu P,
Rachagani S, Thomas A, Das S, Majhi PD, Nimmakayala RK, Vengoji R,
Lele SM, et al: MUC16 regulates TSPYL5 for lung cancer cell growth
and chemoresistance by suppressing p53. Clin Cancer Res.
23:3906–3917. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar SR, Bryan JN, Esebua M,
Amos-Landgraf J and May TJ: Testis specific Y-like 5: Gene
expression, methylation and implications for drug sensitivity in
prostate carcinoma. BMC Cancer. 17:1582017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang C and Luo H: miR-19-5p enhances
tumorigenesis in human colorectal cancer cells by targeting TSPYL5.
DNA Cell Biol. 37:23–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim IY, Shim MJ, Lee DM, Lee AR, Kim MA,
Yoon MJ, Kwon MR, Lee HI, Seo MJ, Choi YW and Choi KS: Loperamide
overcomes the resistance of colon cancer cells to bortezomib by
inducing CHOP-mediated paraptosis-like cell death. Biochem
Pharmacol. 162:41–54. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han X, Kang KA, Piao MJ, Zhen AX, Hyun YJ,
Kim HM, Ryu YS and Hyun JW: Shikonin exerts cytotoxic effects in
human colon cancers by inducing apoptotic cell death via the
endoplasmic reticulum and mitochondria-mediated pathways. Biomol
Ther (Seoul). 27:41–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Chen Y, Huang Q, Liu W, Ji X, Hu F,
Zhu Y, Zhang L and Dong G: IRAK2 counterbalances oncogenic Smurf1
in colon cancer cells by dictating ER stress. Cell Signal.
48:69–80. 2018. View Article : Google Scholar : PubMed/NCBI
|