Introduction
Lung, prostate and colorectal cancers are among the
most frequent causes of mortality associated with cancer in men,
accounting for 42% of all cases (1). In particular, prostate cancer (PCa)
alone account for ~20% of new diagnoses in the United States
(1). Although factors associated
with dietary, lifestyle and androgens have been recognized as
contributors to increasing the risk of PCa (2), the molecular mechanisms of PCa
tumorigenesis have not been fully elucidated.
The E2F family of transcription factors have been
previously demonstrated to be important in the regulation of gene
transcription involved in cell proliferation, differentiation and
apoptosis in mamm alian cells (3,4). The
expression of a variety of E2F isoforms can be found in most cell
types and have been characterized (3). It has been demonstrated that E2F
serves a role in regulating the transcription profile associated
with G1-S phase progression (5,6). In
addition, E2F7 has been previously revealed to suppress the
transcription of a subset of E2F target genes, suggesting that E2F7
may function as a novel member of the mammalian E2F transcription
factor family involved in the negative regulation of cellular
proliferation (7). Recent studies
implied that E2F7 functions as an oncogene in a multitude of
malignant tumors, including cervical cancer (8) and liver cancer (9). E2F7 has been found to be overexpressed
in primary blasts isolated from patients with acute myeloid
leukemia (AML), where it promoted cell cycle progression and
inhibited the monocytic differentiation of AML cells (10). A previous study suggested that
miR-26a and E2F7 may form a double-negative feedback loop,
resulting in the downregulation of miR-26a and upregulation of E2F7
in estrogen receptor-positive breast cancer, where Both miR-26a
knockdown and E2F7 overexpression conferred resistance to tamoxifen
in MCF-7 cells (11). In addition,
E2F7 has also been demonstrated to be overexpressed in cutaneous
squamous cell carcinoma compared with that in normal epidermis,
where suppression of E2F7 expression in a squamous cell carcinoma
cell line resulted in increased UV-induced and doxorubicin-induced
apoptosis (12). It has been
reported that inhibition of E2F7 nuclear export reinstated squamous
cell carcinoma sensitivity to anthracyclines. Inhibition of E2F7
has also been similarly demonstrated to suppress Ishikawa and
HEC-1B cell growth, suggesting that E2F7 is important for the
tumorigenesis of endometrial carcinoma (13). In gallbladder cancer cells, E2F7
knockdown resulted in reduced tumor cell growth, whilst the
upregulation of E2F7 resulted in the opposite effect (14). Additionally, E2F7 has been
demonstrated to negatively regulate cyclin-dependent kinase (CDK)
inhibitor p21 (p21CIP1/WAF1) transcription in HeLa cells (12), which is critical in promoting cell
cycle arrest downstream of a variety of stimuli, including p53,
transforming grwoth factor-β and double homeobox 4 (15).
MicroRNAs (miRNAs) are a family of small non-coding
RNA molecules 18–25 nucleotides in length that serve important
functions across a variety of biological processes. Downregulation
of miRNA expression in tumors compared with normal tissues has been
frequently observed (16). miRNAs
can bind to the 3′-untranslated region (3′-UTR) of target messenger
RNAs (mRNAs) by complementary base pairing, which in turn interfere
with the translation process or induce mRNA degradation (17,18).
It has been found that miR-30c may serve a tumor suppressor role in
a number of tumor types by inhibiting physiological processes
including cell migration, invasion and epithelial-to-mesenchymal
transition (EMT) (19).
Downregulation of miR-30c has been revealed to promote EMT in human
renal cell carcinoma (20), whilst
miR-30c can suppress PCa survival by negatively targeting the
serine/arginine-rich splicing factor 1 (ASF/SF2) (21). In addition, it has been found that
overexpression of miR-30c inhibited breast cancer cell growth
(22).
In the present study, the role of miR-30c and E2F7
on PCa cell physiology was investigated by utilizing the transient
transfection of the PCa cell lines DU145 and PC3. Dual-luciferase
reporter assays were performed to verify if E2F7 is one of the
potential targets of miR-30c. E2F7 was found to be overexpressed in
PCa cells and tissues compared with that in their non-cancerous
counterparts. In addition, inhibition of E2F7 and introduction of
miR-30c into PCa cells led to significantly reduced cell
proliferation. Dual-luciferase reporter assays demonstrated E2F7 to
be one of the targets for miR-30c. Transfection with E2F7 siRNA or
miR-30c mimics resulted in the upregulation of p21. These
observations suggest that the dysregulation of E2F7 in PCa may
contribute to PCa tumorigenesis, which may serve as a viable
therapeutic target for PCa.
Materials and methods
Ethics
The procedures performed in this study were approved
(approval no. YB M-05-01 V.2) by the Ethics Committee of the
Shanghai Outdo Biotech Company, the member of National Human
Genetic Resources Sharing Service Platform (Shanghai, China). The
present study was performed in accordance with the ethical
standards of The Institutional and National Research Committee
(23) and with The Declaration of
Helsinki. Written informed consent was acquired from all
patients.
Tissue arrays
In this study, core tissue arrays (cat. no.
HProA150CS01), including 50 paired samples of matched PCa samples
and adjacent normal prostate samples and 50 additional PCa samples,
were purchased from Shanghai Outdo Biotech Co., Ltd.
Cell lines and culture
The human PCa cell lines DU145, PC3 and the normal
prostate epithelial cell line RWPE1 were kind gifts from Professor
Xin Gao (Urology department, The Third Affiliated Hospital of Sun
Yat-sen University, Guangzhou, China). PCa cells were cultured in
RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences) containing
10% FBS (Hyclone; GE Healthcare Life Sciences) in a humidified
sterile atmosphere containing 5% CO2 at 37°C.
Cell transfection
The human miR-30c mimics (50 nM; cat. no.
miR10000244-1-5; 5′-UGUAAACAUCCUACACUCUCAGC-3′), the miR-30
inhibitor (100 nM; cat. no. miR20000244-1-5;
5′-GCUGAGAGUGUAGGAUGUUUACA-3′) and the negative control (cat. no.
miR01101-1-5; 5′-UUUGUACUACACAAAAGUACUG-3′) were synthesized by
Guangzhou RiboBio Co., Ltd. For E2F7 knockdown, siRNAs were used
(50 nM; cat. no. sc-44590; sense, 5′-GUAAACCAGCCUUCAAGUGdTdT-3′ and
anti-sense, 5′-dTdTCAUUUGGUCGGAAGUUCAC-3′; Santa Cruz
Biotechnology, Inc.). Cells were transfected using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to manufacturer's
protocols. Cells were harvested 48 h after transfection for Cell
Counting Kit-8 (CCK-8), cell cycle, apoptosis and western blotting
analyses.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the PCa and normal
prostate cell lines using the TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and examined on a
NanoDrop machine according to the manufacturer's protocols
(Eppendorf). Electrophoresis and spectrophotometric methods were
used to examine the concentrations and purity of the RNA samples.
cDNA was prepared using ImProm-II™ Reverse Transcription System
according to manufacturer's protocols (cat. no. A3800; Promega
Corporation). The temperature protocol of reverse transcription
reactions was as follows: i) mRNA, 85°C for 5 min, 30°C for 10 min,
42°C for 60 min and 85°C for 10 min; and ii) 85°C for 5 min, 42°C
for 60 min and 85°C 10 min for miRNA. Platinum™ SYBR™ Green qPCR
SuperMix-UDG (cat. no. 11733046, Invitrogen; Themo Fisher
Scientific, Inc.) was used for qPCR and each reaction was performed
in triplicate. The thermocycling conditions used for RT-qPCR were
as follows: Activation at 50°C for 2 min, Initial denaturation at
95°C for 2 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 32 sec. The 2−∆∆Cq method (24) was utilized to calculate the relative
expression levels of mRNA and miRNA (primers used can be seen in
Table SI), using GAPDH and U6 as
reference genes for mRNA and miRNA, respectively.
Western blotting
Western blotting was performed as previously
described (25,26). DU145 and PC3 cells were lysed in
RIPA Buffer (Beyotime Institute of Biotechnology) containing
protease inhibitors on ice followed by centrifugation at 1,000 × g
for 15 min at 4°C. The protein concentration was quantified using
bicinchoninic acid Protein Assay Kit (Beyotime Institute of
Biotechnology), following which the protein samples (30 µg) were
separated using 12% SDS polyacrylamide gels and transferred onto
PVDF membranes (EMD Millipore). Membranes were blocked with 5%
non-fat milk powder at room temperature for 1 h and incubated
overnight at 4°C with primary antibodies. Anti-E2F7 primary
antibody (1:200; cat. no. ab56022) was obtained from Abcam,
Anti-p21 primary antibody (1:200; cat. no. 10355-1-AP) was obtained
from Proteintech Group, Inc., whilst the anti-GAPDH primary
antibody (1:600; cat. no. 10494-1-AP) was purchased from
ProteinTech Group, Inc. Membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (1:2,000; cat. no.
SA00001-2; ProteinTech Group, Inc.) for 1 h at room temperature
after three 10 min washes in TBS supplemented with 0.1% Tween-20.
Finally, immunodetection and visualization of the membranes were
performed using the ECL kit (Pierce; Thermo Fisher Scientific,
Inc.) which were subsequently scanned and using a Bio-Rad ChemiDoc
XRS+ imaging system (Bio-Rad Laboratories, Inc.) and analyzed using
the Image J software (version 1.8.0; National Institutes of
Health). The protein expression was normalized to an endogenous
reference (GAPDH) and relative to the control.
miRNA target prediction
To predict potential miRNA target genes, the
microRNA.org website (2010 version; http://www.microrna.org/microrna/home.do) and
TargetScan Human 5.1 (http://www.targetscan.org/vert_72/) were used by
entering ‘E2F7’ into the search box to see if there are binding
sites for miR-30c. Free binding energy was calculated and binding
sites were analyzed using the BiBiServ website (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid).
Construction of luciferase reporter
plasmids, transfection and dual-luciferase assay
In the present study, the plasmid containing the
3′-UTR of E2F7 was constructed using the psiCHECK-2 dual luciferase
vector (cat. no. C8011; Promega Corporation). The fragments
containing the predicted wild-type (wt) and mutant (mut) sites of
E2F7 3′UTR were first directly synthesized by Guangzhou RiboBio
Co., Ltd., followed by subcloning into the psiCHECK-2 vector at the
XhoI and NotI restriction sites to generate the
E2F7-3′-UTR-wild-type and E2F7-3′-UTR-mutant vectors. The following
primer pairs were used to generate the E2F7-3′UTR mut plasmid:
Forward,
5′-TTTATCAACACTAAACTTTTAAAACCCGTGAGTTTTTTTTTTCTTTTTTACAGTCTTC-3′
and reverse,
5′-GAAGACTGTAAAAAAGAAAAAAAAAACTCACGGGTTTTAAAAGTTTAGTGTTGATAAA-3′.
The following primer pairs were used to generate the E2F7-3′UTR wt
plasmid: Forward, 5-′CCGCTCGAGCCTGCCGCTTTGCCAGGTGGG-3′ and reverse,
5′-ATAAGAATGCGGCCGCTTCTTCTTAAATGAATTATTTTTTATTG-3′.
Du145 and PC3 cells (1×105/well) were seeded into a
24-well plates and co-transfected with E2F7-3′-UTR-wild or
E2F7-3′-UTR-mutant vectors (50 ng/well) and miR-30c mimic (50
nM/well). Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for transfection. NC mimics (50 nM/well;
5′-UUUGUACUACACAAAAGUACUG-3′) and NC inhibitor (100 nM/well;
5′-CAGUACUUUUGUGUAGUACAAA-3′) were used as control. Cells were
collected 48 h later and luciferase activities were measured using
the Dual-Luciferase® Reporter Assay kit according to
manufacturer's protocols (Promega Corporation) on a
GloMax® 96 Microplate Luminometer (Promega Corporation).
All results were normalized to those of Renilla luciferase
activities to verify the luciferase activities.
Cell viability assay
Cell Counting kit-8 (CCK-8; Nanjing KeyGen Biotech
Co., Ltd.) was used according to the manufacturer's protocols to
examine cell viability. Du145 and PC3 cells were seeded onto
96-well plates (1×104 cells/well), following which cell
viability was measured every 24 h for 5 days. The number of the
viable cells was evaluated by assessment of absorbance values at
450 nm using a Multiskan MK3 microplate reader (Thermo Fisher
Scientific, Inc.).
Cell cycle analysis
PC3 and DU145 were cultured using the RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific) supplemented with 10%
FBS in 6-well plates for 48 h before being collected. Transfection
was performed when cells reached 80–90% confluency. A total of
1×104 cells cells were then collected 48 h after
transfection, washed twice using PBS and fixed in 70% ethanol at
4°C overnight, followed by incubation with 5 µl propidium iodide
(50 µg/ml; cat. no. KGA511; Nanjing KeyGen Biotech Co., Ltd) for
each sample at room temperature for 1 h. Finally, the cells were
analyzed using flow cytometry (BD FACScalibur™; BD Biosciences)
using the ModFitLT™ software (version 3.0; Verity Software House,
Inc.).
Apoptosis assay
Transfection was performed when DU145 and PC3 cells
reached 80–90% confluency for 48 h, followed by cell collection for
apoptosis analysis. The degree of cell apoptosis was measured using
the Annexin V-FITC/PI apoptosis detection kit (cat. no. KGA106;
Nanjing KeyGen Biotech Co., Ltd.) according to the manufacturer's
protocols. A total of 1×104 cells were used per staining
reaction. Cell apoptosis rates were assessed using flow cytometry
(BD FACScalibur™; BD Biosciences) using the ModFitLT™ software
(version 3.0; Verity Software House, Inc.).
5-Ethynyl-2′-deoxyuridine (EdU)
assay
DU145 and PC3 cells (5×105 cells/cell)
were seeded into six-well plates at 37°C 24 h before transfection
was performed and were incubated overnight to reach 30–50%
confluence. Transfection was then performed for 48 h. EdU solution
(Guangzhou RiboBio Co., Ltd.) was subsequently diluted using
RPMI-1640 medium at a ratio of 1:1,000 to prepare a 50 µM EdU
solution. When 80–90% confluency was reached, each well was treated
with 100 µl 50 µM EdU solution for 2 h at room temperature,
following which 4% paraformaldehyde was added to each well and the
cells were incubated for 15–30 min at room temperature. The cells
were then incubated with 2 mg/ml glycine for 2 min, then 100 µl
detergent (0.5% Triton X-100 in PBS) was used to permeabilize cells
in each well at room temperature for 5 min. A total of 100 µl 1X
Apollo®567 staining solution (cat. no. C10316-1;
Guangzhou RiboBio Co., Ltd.) was added to each well, where the
cells were then protected from light and incubated for 30 min at
room temperature. Finally, 100 µl 1X Hoechst 33342 reaction
solution (Guangzhou RiboBio Co., Ltd.) was added to each well in
the dark for 10–30 min followed by observation and analysis using a
fluorescent inverted microscope (Magnification, ×100; DMI6000B;
Leica Microsystems GmbH. Images were analyzed using the Image J
software (version 1.44p; National Institutes of Health).
Immunohistochemical (IHC)
staining
All sections were first deparaffinized by washing in
xylene I for 15 min, xylene II for 15 min and xylene III for 15
min, followed by rehydration using a descending ethanol gradient.
Antigen retrieval was then performed, where the tissue microarrays
were incubated in EDTA antigen repair buffer solution (pH 8.0; cat.
no. G1206; Wuhan Servicebio Technology Co., Ltd.) at 95°C for 10
min. Tissue sections were permeabilized by incubation with Triton
X-100 solution at room temperature for 10 min. The slides were then
washed with PBS (pH 7.4) for three times at 5 min each, following
which they were treated with 3% endogenous peroxidase blocking
buffer (cat. no. P0100A; Beyotime Institute of Biotechnology) at
room temperature in the dark for 25 min block endogenous peroxidase
activities. After another round of washing using PBS for three
times at 5 min each, the sections were blocked with 3% BSA (Wuhan
Servicebio Technology Co., Ltd.) at room temperature for 30 min.
IHC staining of tissue microarrays was performed by incubation with
the anti-E2F7 (1:200; cat. no. ab56022; Abcam) and anti-p21 (1:200;
cat. no. 10355-1-AP; Proteintech Group, Inc.) primary antibodies
overnight at 4°C. The tissue microarrays were then incubated with
HRP-conjugated secondary antibodies (1:500; cat. no. G23303; Wuhan
Servicebio Technology Co., Ltd.) at room temperature for 1 h after
washing three times with PBS. Finally, the microarrays were
immersed in 3,3′-diaminobenzidine for 5–10 min at room temperature
and counterstained with 10% Mayer's hematoxylin at room temperature
for 3 min. Images of the microarrays were obtained using a digital
scanner (Pannoramic MIDI; 3DHISTECH, Ltd.). An independent
pathologist (XJP) blinded to the clinical data scored the samples.
The stained slides were graded according to the estimated
proportion of cells whose nuclei stained positive: i) 0, 0% of
cells; ii) 1, 1 −25%; iii) 2, 26–50%; iv) 3, 51–75%; and iv) 4,
76–100%. In addition, the staining intensity was also graded as
follows: i) 0, none; ii) 1, weak; iii) 2, intermediate; and iv) 3,
strong. Total score=‘staining intensity score’ × ‘staining positive
rate score’ (27,28).
Statistical analysis
Data were presented as the mean ± SD from three
experimental repeats. The SPSS statistical software (version 17.0;
SPSS, Inc.) was used to perform statistical analyses. Wilcoxon
signed-rank test was used to compare the immunohistochemistry
scores of unpaired and paired cancer and matched adjacent
non-cancerous tissues, separately. Comparison between multiple
groups was performed using one-way ANOVA followed by Tukey's post
hoc test. P<0.05 were considered to indicate a statistically
significant difference.
Results
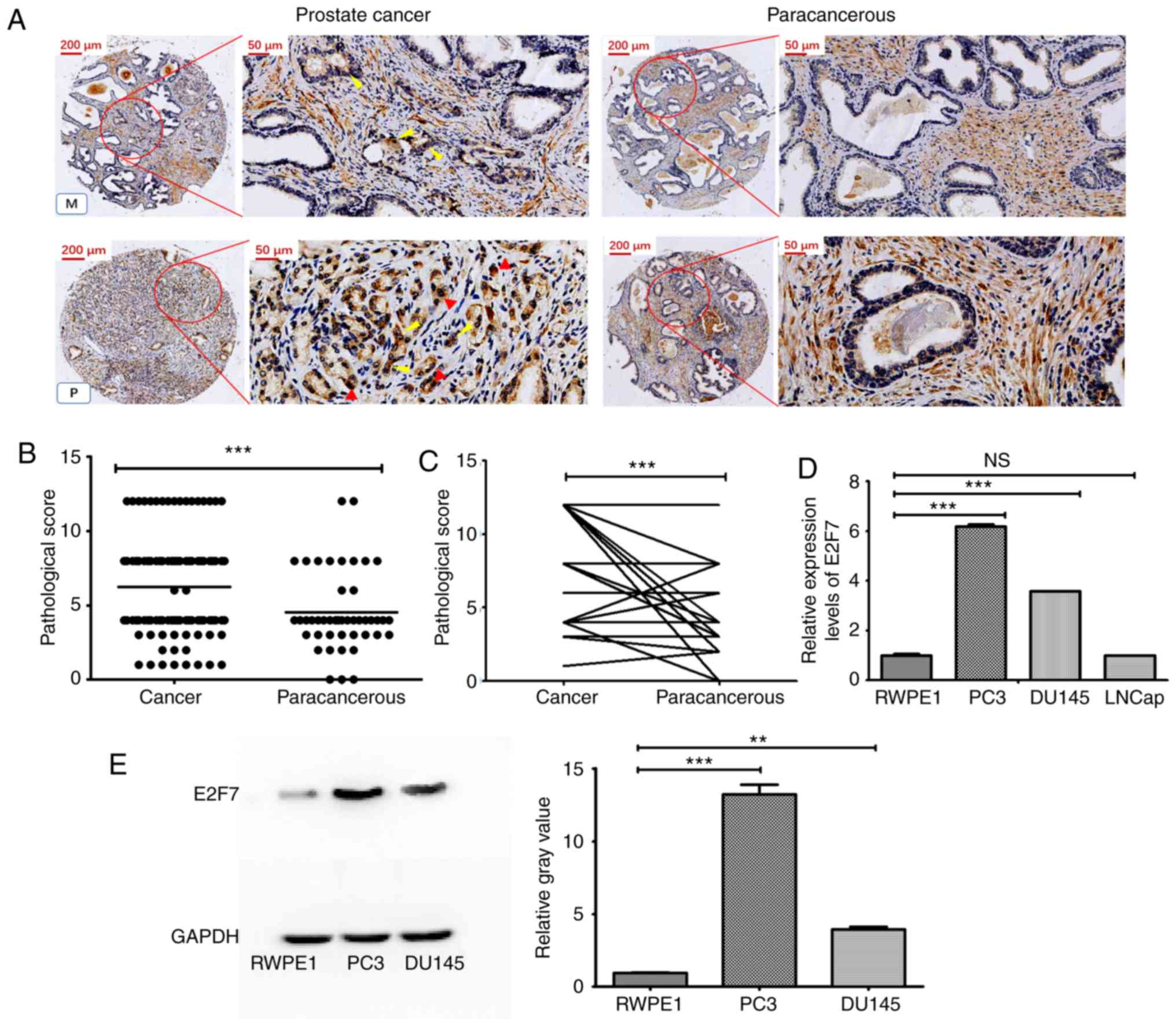
Upregulation of E2F7 expression in PCa
tissues and cell lines
To assess the expression levels of E2F7 in PCa, E2F7
expression of 93 PCa tissues and 45 adjacent non-cancerous prostate
tissues were examined using IHC. It was found that E2F7 was mainly
expressed in the nuclei of the poorly differentiated PCa tissues,
whilst it was mainly expressed in the cytoplasm of highly or
moderately differentiated PCa tissues (Figs. 1A and S1). The staining scores of E2F7 of PCa
tissues were found to be markedly higher compared with those of
adjacent normal tissues (Fig. 1B).
The further comparison revealed that the staining scores in PCa
tissues was significantly higher compared with their corresponding
paired matched adjacent non-cancerous tissues (Fig. 1C). E2F7 expression was subsequently
evaluated in three different PCa cell lines. The mRNA expression
levels of E2F7 in PCa cell lines DU145 and PC3 were found to be
significantly higher compared with those in the normal prostate
cell line RWPE1 (Fig. 1D). Western
blotting also showed that the levels of E2F7 expression in PC3 and
DU145 cells were significantly higher compared with those of the
RWPE1 cells (Fig. 1E).
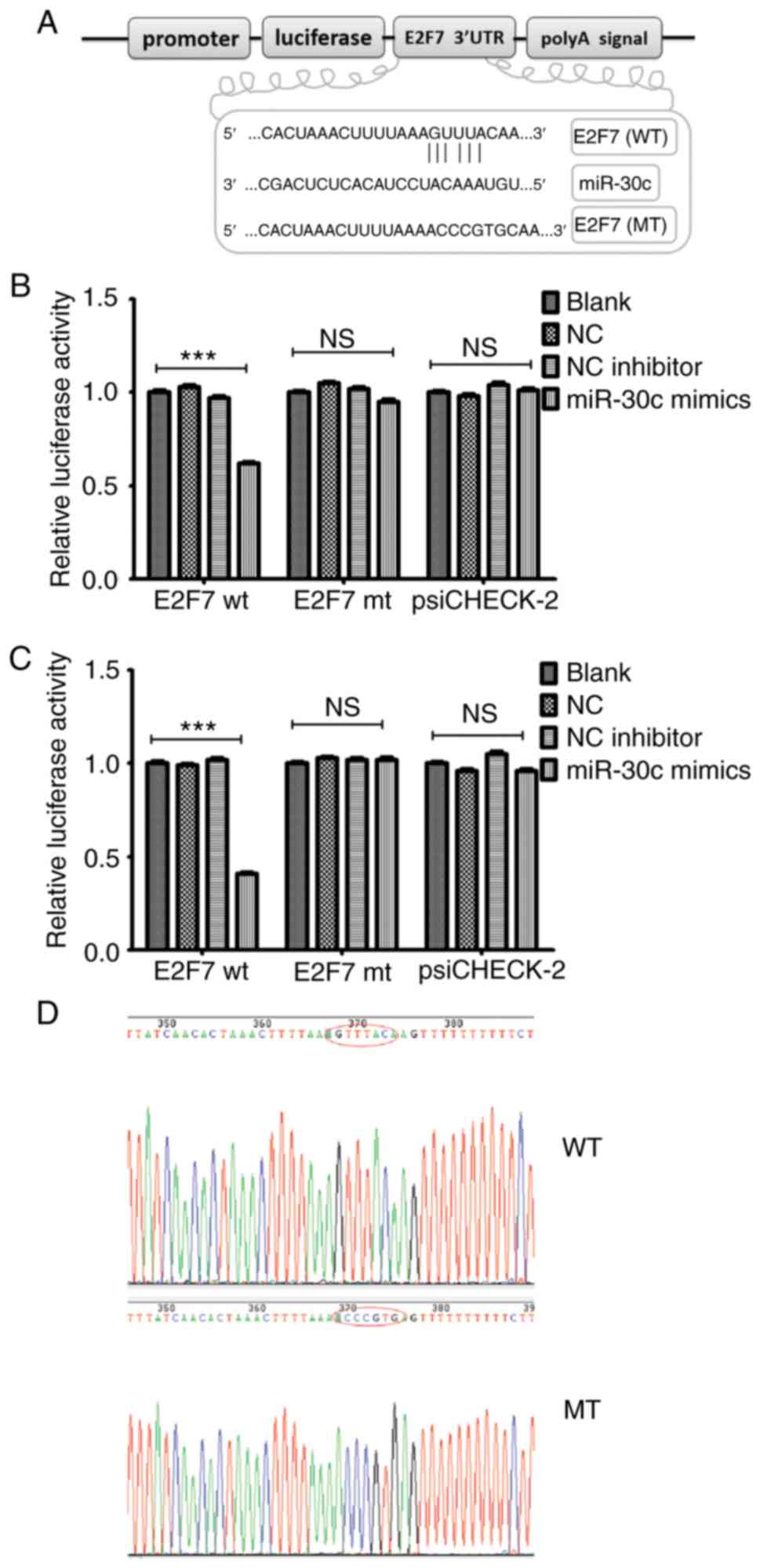
E2F7 is a functional target of
miR-30c
The aforementioned observations suggested that E2F7
may function as an oncogene in PCa. Therefore, potential downstream
targets of miR-30c were searched using online tools TargetScan
Human 5.1 (Conserved) (http://www.targetscan.org/vert_72/) and microRNA.org (http://www.microrna.org/microrna/home.do). E2F7 was
found to be one of the potential targets of miR-30c in PCa, as it
predicted to have putative miR-30c binding sites within its 3′-UTR
(Fig. 2A). Luciferase reporter
assays were subsequently performed to verify whether E2F7 is a
direct target of miR-30c using DU145 and PC3 cell lines. DU145 and
PC3 cell lines were co-transfected with a psiCHECK-2 vector
encoding the 3′-UTR of either the wild-type or mut E2F7 and miR-30c
mimics or miR-30c inhibitors. Wild-type and mut E2F7-3′-UTR
containing the putative binding sites for miR-30c were cloned into
the psiCHECK-2 vector downstream of the luciferase gene (Fig. S2). Co-transfection with the miR-30c
mimic significantly reduced the luciferase activities of the E2F7
3′-UTR reporter vector, but not those of the E2F7-3′-UTR-mut vector
(Fig. 2B and C). Sequencing data of
the wild-type and mut E2F7-3′-UTR showed that miR-30c may directly
regulate the expression levels of E2F7, where miR-30c may bind to
the 3′-UTR of E2F7 mRNA and downregulate its expression (Fig. 2D).
E2F7 knockdown and upregulation of
miR-30c reduces cell viability by increasing apoptosis whilst
suppressing S phase progression in PCa cells
To study the potential role of E2F7 and miR-30c in
PCa cells, E2F7 siRNA, miR-30c mimics and miR-30c inhibitors were
transiently transfected into DU145 and PC3 cell lines (Fig. S3). Cell counting Kit-8 (CCK-8) was
used to calculate cell viability. Transfection with E2F7 siRNA and
miR-30c mimics lead to significantly reduced PCa cell viability
compared those transfected with negative controls 48 h after
transfection and onwards (Fig. 3A and
B). By contrast, transfection with the miR-30c inhibitors
resulted in significantly increased cell viability compared with
cells transfected with negative controls within the same timeframe
(Fig. 3A and B). Further study
showed that compared with cells transfected with the negative
control, transfection with either the miR-30c mimics or E2F7 siRNA
lead to significantly increased rates of apoptosis (Fig. 3C), significantly reduced the
proportion of cells in the S phase and increased the number of
cells at the G1 phase of the cell cycle (Fig. 4). Transfection with the miR-30c
inhibitor resulted in significant reductions in apoptosis and
significant increases in the percentage of cells in the S phase in
both DU145 and PC3 cells, but did not significantly alter the
percentage of cell PC3 cells in the G1 phase (Figs. 3 and 4).
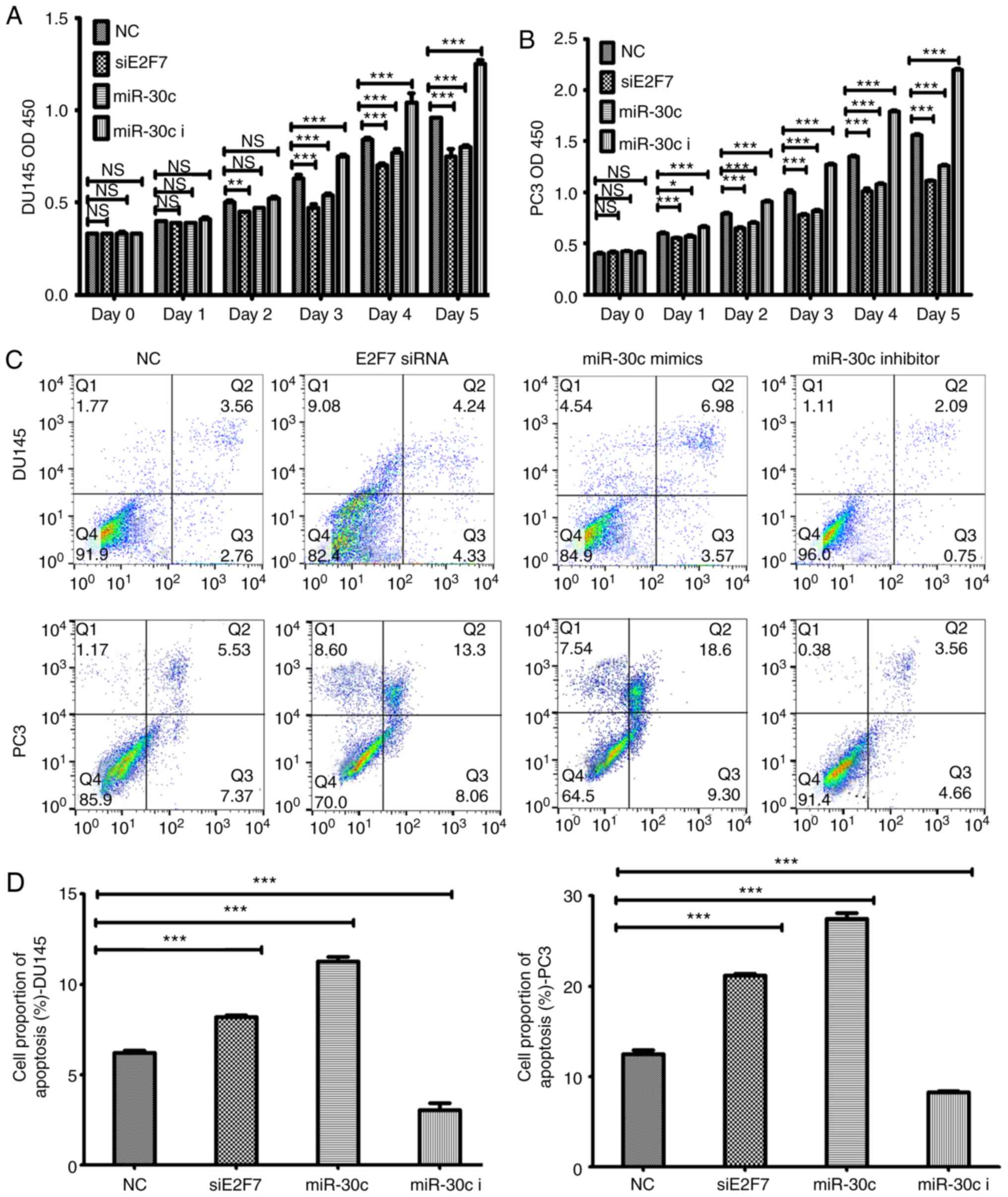 | Figure 3.Effects of E2F7 knockdown, miR-30c
overexpression and miR-30c inhibition on prostate cancer cell
viability and apoptosis. CCK-8 assay results of (A) DU145 and (B)
PC3 cells, where cell viability was measured every 24 h over 5 days
following their respective transfections. (C) Representative flow
cytometry dot plots showing that transfection with the miR-30c
mimics or E2F7 siRNA increased the apoptotic rates of DU145 and PC3
cells compared with the those transfected with NC, whilst
transfection with the miR-30c inhibitor resulted in the opposite
effect. (D) Quantified data using cell counts from the Q2 and Q3
quadrants (C). The values represent the mean ± SD from three
experimental repeats. *P<0.05, **P<0.01 and ***P<0.0001.
miR, microRNA; siRNA, small interfering RNA; miR-30c, miR-30c
mimics; miR-30c I, miR-30c inhibitor; siE2F7, E2F7 siRNA; OD,
optical density; NS, not significant. |
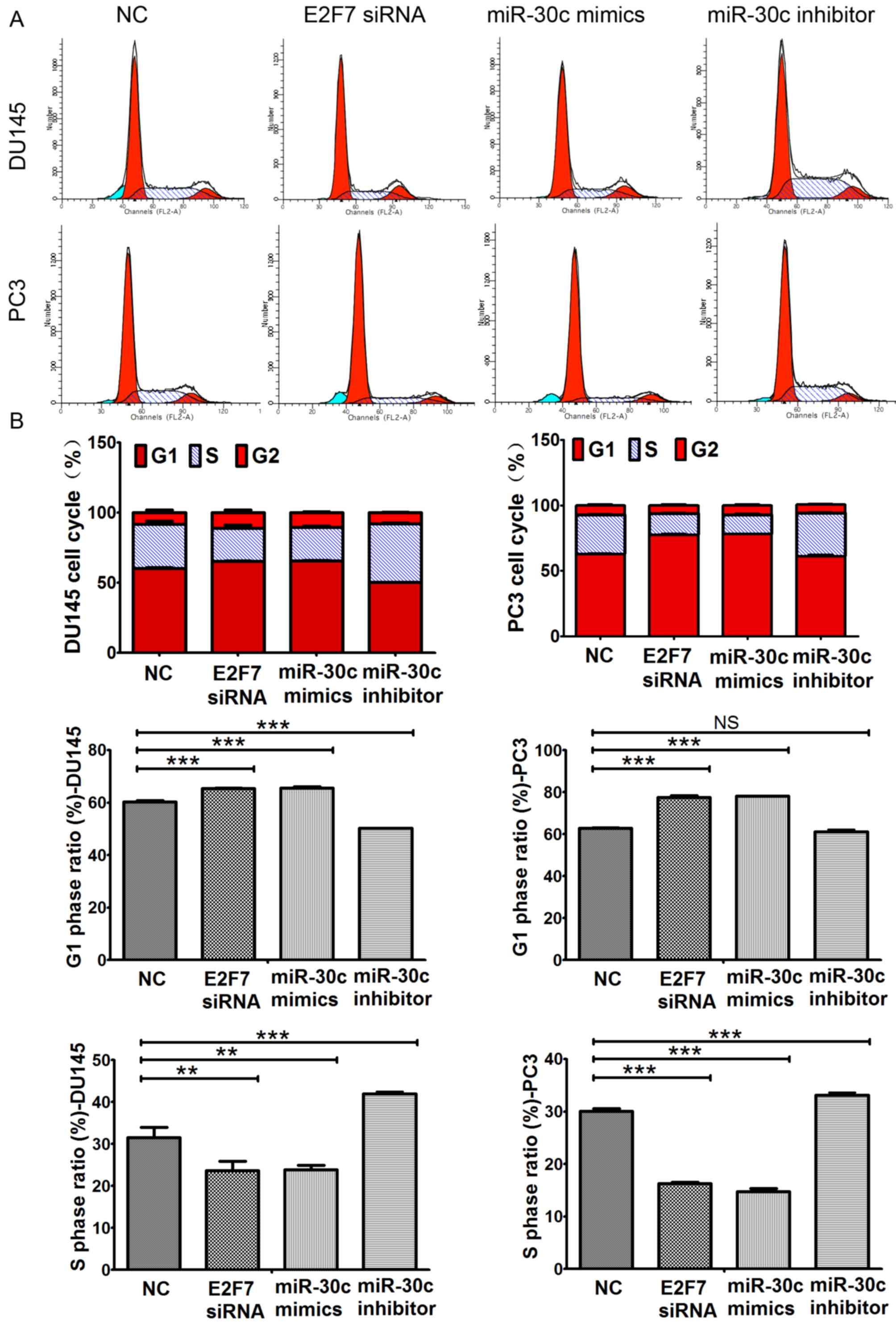 | Figure 4.Effects of E2F7 knockdown, miR-30c
overexpression and miR-30c knockdown on prostate cancer cell cycle
progression. (A) Representative histograms showing the proportion
of Du145 and PC3 cells in the G1, S and G2
phases of the cell cycle following transfection with either NC,
miR-30c mimics, E2F7 siRNA or miR-30c inhibitors. Dark red
indicates cells in G1 phase; Light red indicates cells
in G2 phase; Teal indicates apoptotic cells; Blue
indicates cells in S phase. (B) Transfection with the miR-30c mimic
and E2F7-siRNA increased the percentage of DU145 and PC3 cells in
the G1 phase of the cell cycle whilst transfection with
the miR-30c inhibitor resulted in the opposite effect. No
significant difference was observed in the G1 phase
ratio between miR-30c inhibitor transfection group and the NC group
in PC3 cells. Transfection with miR-30c mimics and E2F7-siRNA
reduced the percentage of DU145 and PC3 cells in the S phase of the
cell cycle whilst transfection with the miR-30c inhibitor resulted
in the opposite effect. The values represent the mean ± SD from
three experimental repeats. **P<0.01 and ***P<0.0001, NS, not
significant. miR, microRNA; miR-30c, miR-30c mimics; miR-30c I,
miR-30c inhibitor; NC, negative control. |
E2F7 mediates the function of
miR-30c
To study the relationship between miR-30c and E2F7,
PCa cell lines were co-transfected with the miR-30 inhibitor and
E2F7 siRNA. Co-transfection with the miR-30c inhibitor and E2F7
siRNA significantly increased cell viability compared with E2F7
siRNA transfection alone in both DU145 and PC3 cells from day 2
onwards (Fig. 5A and B).
Transfection with E2F7 siRNA did not significantly change the
expression levels of miR-30c in either PCa cell lines (Fig. 5C and D). By contrast, transfection
with the miR-30c mimics lead to a significant downregulation in
E2F7 expression, whilst transfection with the miR-30c inhibitor
resulted in higher expression levels of E2F7. The effects mediated
by the miR-30c inhibitor was significantly reversed by
co-transfection with the E2F7 siRNA in both DU145 and PC3 cells
(Fig. 5E and F).
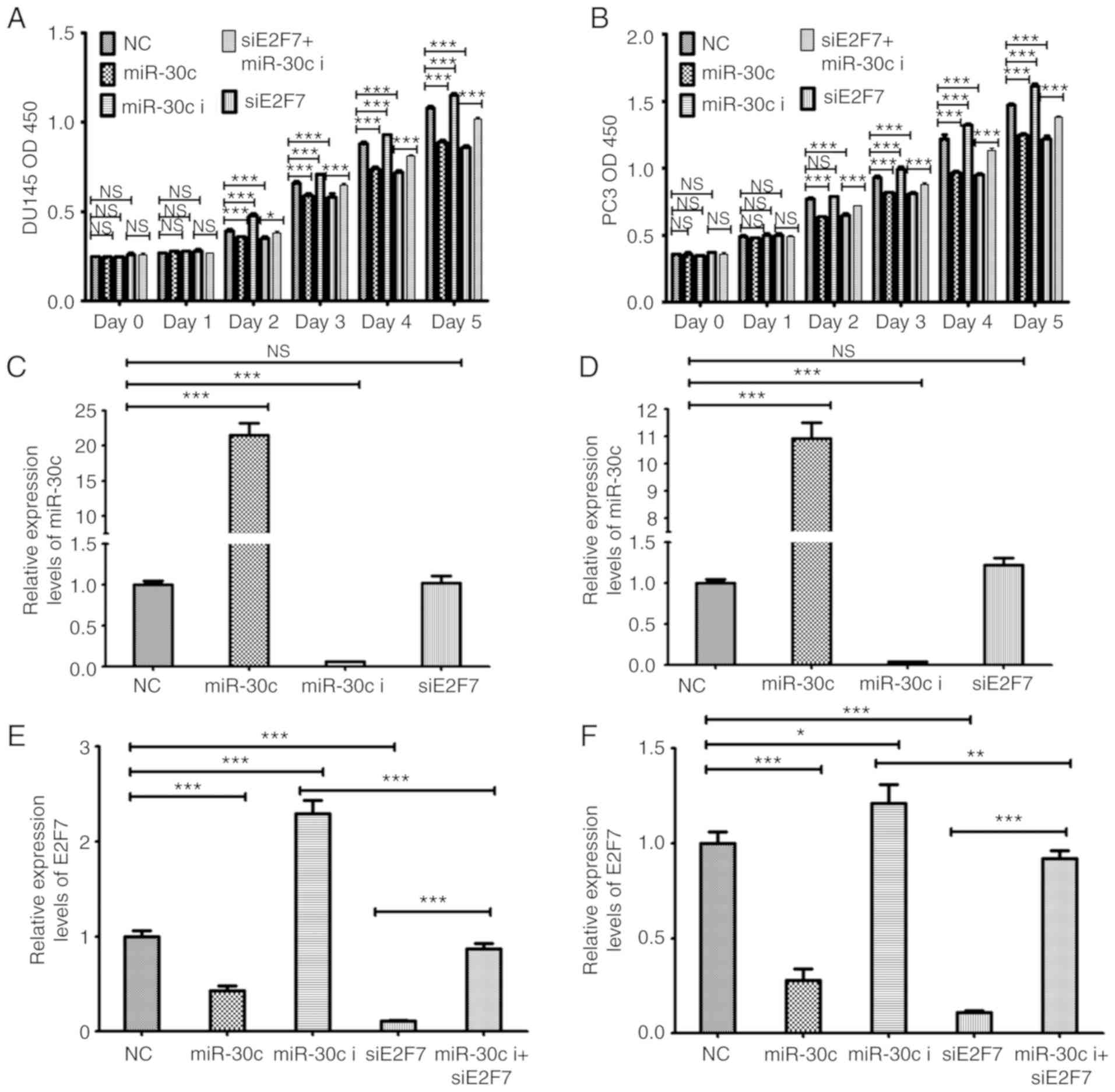 | Figure 5.Investigation into the functional
relationship between of E2F7 and miR-30c. Cell Counting kit-8 assay
of (A) DU145 and (B) PC3 cells following transfection with miR-30c
mimic, miR-30c inhibitor, siE2F7 or NC, or co-transfected with
miR-30c inhibitor and siE2F7. miR-30c expression levels as measured
in (C) DU145 and (D) PC3 cells following transfection with miR-30c
mimic, miR-30c inhibitor, siE2F7 or NC or co-transfected with
miR-30c inhibitor and siE2F7. The E2F7 expression levels in (E)
DU145 and (F) PC3 cells following transfection with miR-30c mimic,
miR-30c inhibitor, siE2F7 or NC, or co-transfected with miR-30c
inhibitor and siE2F7. The values represent the mean ± SD from three
experimental repeats. *P<0.05, **P<0.01 and ***P<0.0001.
OD, optical density; miR, microRNA; siRNA, small interfering RNA;
miR-30c, miR-30c mimics; miR-30c i, miR-30c inhibitor; siE2F7, E2F7
siRNA; NC, negative control; NS, not significant. |
In addition, EdU assay was subsequently performed,
which found that transfection with the miR-30c inhibitor
significantly increased EdU uptake in DU145 and PC3 cells (Fig. 6A and B), which was reversed by
co-transfection with E2F7 siRNA (Fig.
6A and B). Additionally, western blotting experiments also
showed that overexpression and inhibition of miR-30c expression led
to significant downregulation and upregulation of E2F7 expression
in both PCa cell lines (Fig. 6C-F),
respectively. These findings suggested that miR-30c overexpression
inhibited PCa cell proliferation by potentially inhibiting E2F7
expression.
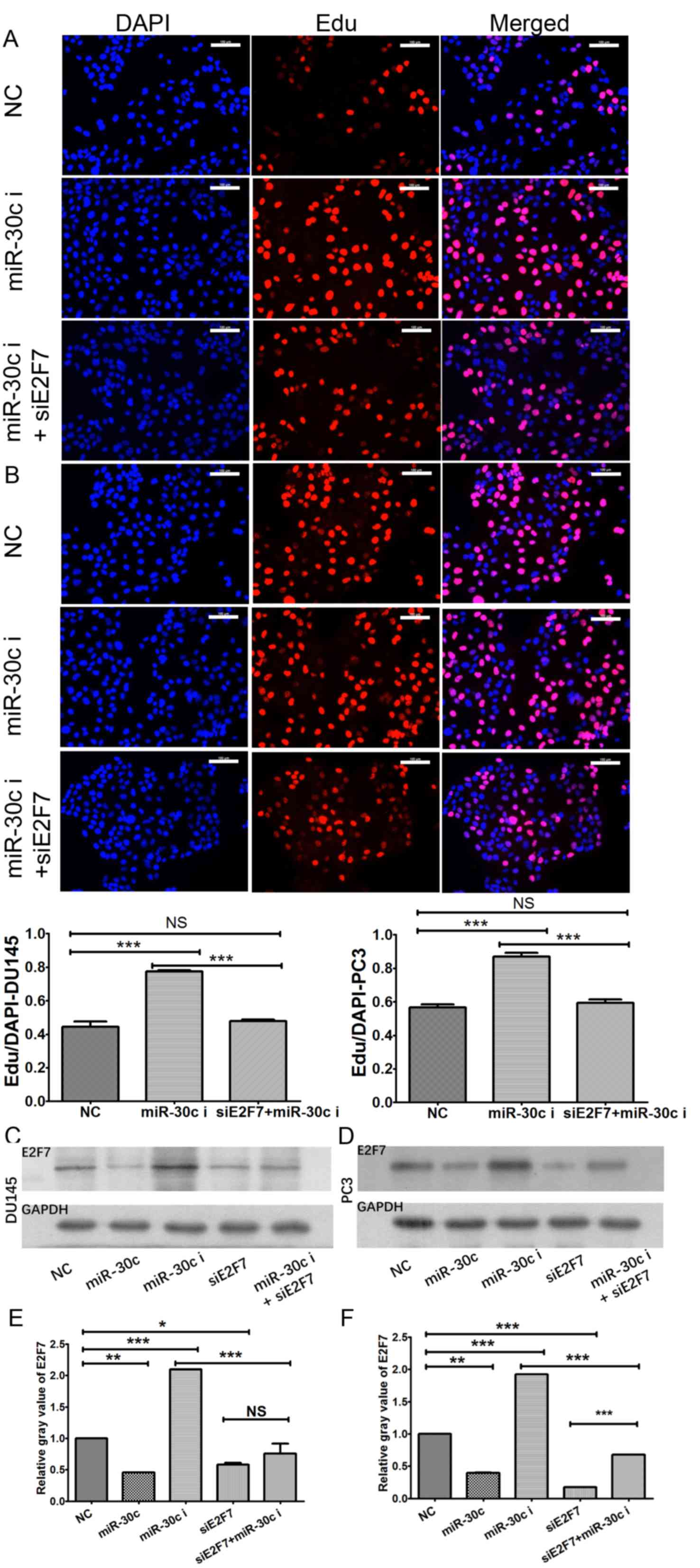 | Figure 6.Effect of E2F7 on miR-30c function.
According to the results from EdU assay, miR-30c knockdown
significantly promoted EdU uptake in (A) DU145 and (B) PC3 cell
lines, which was reversed by co-transfection with E2F7 siRNA.
Western blotting experiments showed that upregulation and
downregulation of miR-30c resulted in the downregulation and
upregulation of E2F7 expression in (C) DU145 and (D) PC3 cell
lines, respectively. The values represent the mean ± SD from three
experimental repeats of (E) DU145 and (F) PC3 cell lines.
*P<0.05, **P<0.01 and ***P<0.0001. Scale bar, 100 µm. EdU,
5-Ethynyl-2′-deoxyuridine; miR, microRNA; miR-30c, miR-30c mimics;
miR-30c i, miR-30c inhibitor; siE2F7, E2F7 siRNA. |
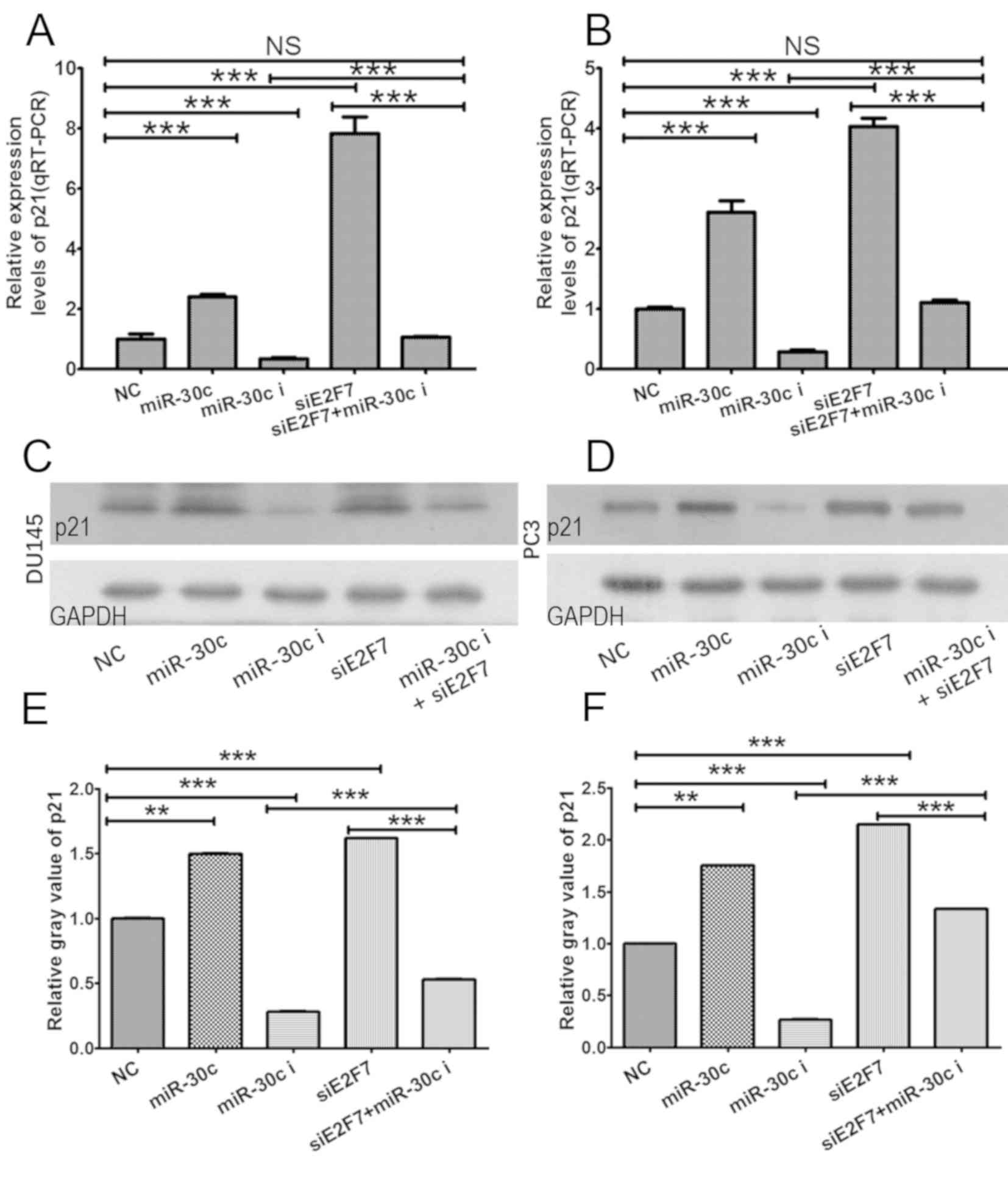
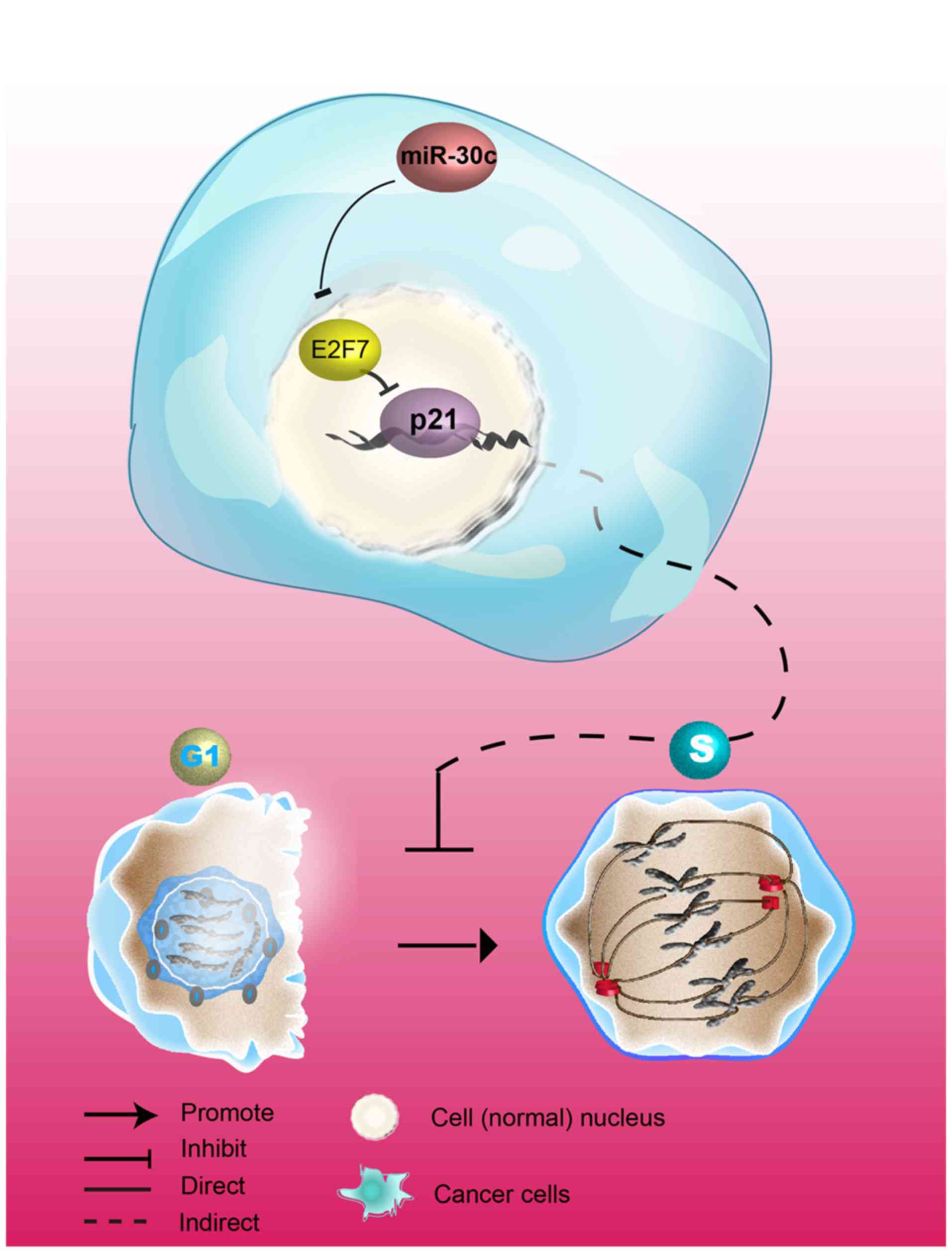
miR-30c/E2F7/p21 may serve as a
pathway to promote the development of PCa
E2F7 has been previously shown to negatively
regulate the expression of the universal cell cycle inhibitor p21
(9,10). To investigate the mechanism by which
miR-30c/E2F7 promotes the development of PCa, p21 expression in PCa
cell lines following transfection with E2F7 siRNA, miR-30c mimics,
miR-30c inhibitor or NC was then investigated further. In both cell
lines, compared with cells transfected with NC, p21 expression was
revealed to be significantly increased on both mRNA (Fig. 7A and B) and protein (Fig. 7C and D) levels following
transfection with E2F7 siRNA or miR-30c mimics, whilst transfection
with the miR-30c inhibitor resulted in significant downregulation
of p21. Co-transfection of both cell lines with the miR-30c
inhibitor and E2F7 siRNA led to significantly increased
upregulation in p21 expression compared with cells transfected with
the miR-30c inhibitor alone (Fig.
7C-F). The association between E2F7 and p21 expression was
analyzed further by immunohistochemistry, which showed that the
levels of E2F7 expression associated negatively with p21
expression, especially in identical locations within the same
sample of the tissue array (Fig.
S4). These observations suggest that p21 expression may be
negatively regulated by E2F7. In addition, it was found that E2F7
knockdown led to a higher magnitude of upregulation in p21
expression compared with that induced by the introduction of
miR-30c mimics in both cell lines tested (Fig. 7). Therefore, these findings suggest
that the miR-30c/E2F7/p21 pathway promotes the development of PCa
(Fig. 8).
Discussion
The E2F family of transcription factors serve
important roles in the regulation of cell cycle progression
(29). E2F7 and E2F8 are considered
to be atypical members of the E2F family due to reported
suppressive roles on the transcription of genes associated with DNA
replication, resulting in S phase cell cycle arrest and inhibition
of tumorigenesis (30,31). Interestingly, E2F7/8 double-knockout
mice have been previously found to exhibit vascular defects and
extensive apoptosis (32). In
addition, it has been found that E2F7, which is reported to be
involved in the DNA damage (33,34).
response and control of E2F target genes associated with cell cycle
progression as well as the proliferation and apoptosis of
keratinocytes (35), has been
revealed to promote tumorigenesis in some malignant tumors
(11,36).
The role of E2F7/8 in the development of tumors
remain unclear. Ma et al (9)
reported that high levels of E2F7 expression was correlated with
shorter median overall survival and progress-free survival in
hepatocellular carcinoma patients. Despite their classification as
transcriptional repressors, Weijts et al (37) demonstrated that E2F7/8 is essential
for the opportune development of blood vessels. Similarly, the high
expression of E2F7 was found to be correlated with higher risks of
relapse and poor prognosis in patients with breast cancer that were
treated with tamoxifen (38).
In the present study, it was found that the staining
scores of E2F7 in PCa tissues was higher compared with those of
adjacent normal tissues. Transfections of PCa cells with E2F7 siRNA
resulted in significantly reduced cell viability, increased
proportion of cells in the G1 phase and higher apoptotic
rates. Strategies combining cell cycle inhibitors in
castration-resistant prostate cancer (CRPC) have been considered to
have beneficial effects with CDK4/6 and Wee1 inhibitors (39). S phase inhibitors, including M-6620
and prexasertib, G1 phase inhibitors including AZD-5363
(39), palbociclib (39), and ipatasertib (40), G2 phase inhibitors such
as adavosertib (39) and M phase
inhibitors such as alisertib (41)
are all undergoing clinical trials and may prove promising in
targeted therapies for CRPC in the future. Linking cell cycle to
the inhibition of prostate cancer pathophysiology, Kang et
al (42) reported that TJ001
promoted G1/S cell cycle arrest by upregulating
p21Cip1/WAF1 expression whilst downregulating cyclin E and cyclin
D1 expression. The mechanism underlying the E2F7-mediated
regulation of tumorigenesis could be through the inhibition of gene
expression associated with the maintenance of genomic stability
(43).
The present study showed E2F7 to be one of the
targets of miR-30c, which was examined using Dual-luciferase
reporter assay. Previous studies have demonstrated that miR-30c
involvement is critical for the development of a variety of human
cancers. It has also been found that miR-30c functioned as a tumor
suppressor (44), where it
inhibited cancer metastasis (36)
by directly targeting genes associated with metastasis (37,38).
Huang et al (21) reported
that miR-30c reduced PCa survival by targeting the ASF/SF2 splicing
factor oncoprotein whilst Ling et al (46) found that the B-cell lymphoma 9
protein, a coactivator for Wnt/β-catenin transcription, was
targeted by miR-30c, which was associated with PCa progression. In
the present study, it was demonstrated that transfection with the
miR-30c mimics led to increased apoptotic rates compared with the
corresponding negative control, consistent with a previous
conclusion (45). In addition,
previous data suggested that downregulation of the tumor suppressor
miR-30c was a frequent pathological event in PCa (46), where it was revealed that miR-30c
appears to be a tumor suppressor gene in DU145 cells (21). In the present study, luciferase
reporter assays were performed to verify if E2F7 is a direct target
of miR-30c using DU145 and PC3 cell lines. In addition, the
androgen-dependent VCaP cell line, was used to examine the miR-30c
effect on E2F7 and p21 expression by western blotting method, and
the results were consistent with that of the DU145 and PC3 cell
lines (data not shown).
In terms of cell cycle progression, it would be
ideal to perform these types of experiments in a synchronized
manner. In the present study, it was confirmed that the inhibition
of proliferation mediated by miR-30c in PCa cell lines was by
targeting E2F7 expression; specifically, co-transfection of the
miR-30 inhibitor with E2F7 siRNA resulted in lower cell viability
compared with E2F7 siRNA transfection alone. E2F7 siRNA
transfection did not significantly change the expression levels of
miR-30c in PCa cell lines. However, transfection with the miR-30c
mimic led to the significant reductions in E2F7 expression, whilst
transfection with the miR-30c inhibitor resulted in increased E2F7
expression which was reversed by co-transfection with E2F7 siRNA in
both DU145 and PC3 cells.
E2F7 has been previously demonstrated to negatively
regulate p21 (9), a universal
cell-cycle inhibitor (10) which
can be controlled by p53 and p53-independent pathways (17). In the present study, it was found
that p21 expression was significantly increased by transfection
with either E2F7 siRNA or miR-30c mimics but significantly reduced
by miR-30c inhibitor transfection compared with the negative
control in PCa cells. In addition, E2F7 knockdown appeared to be
more effective in upregulating p21 expression compared with miR-30c
mimics in PCa cells, suggesting that p21 expression might be
negatively regulated by E2F7 in PCa cells.
In conclusion, the present study demonstrated that
E2F7 may serve a proliferative role in PCa cells by inhibiting p21.
In addition, E2F7 was found to be negatively regulated by miR-30c,
suggesting that miR-30c/E2F7/p21 may be a viable therapeutic target
pathway for interventions against PCa.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Xin Gao,
Department of Urology, The Third Affiliated Hospital, Sun Yat-Sen
University (Guangzhou, China) for his generous donation of prostate
cell lines DU145, PC3 and the normal prostate cell line RWPE1. The
abstract of this manuscript was presented online (abstract no.
e16568) at the 2019 American Society of Clinical Oncology meeting
(Chicago, USA) in Journal of Clinical Oncology 37 (Suppl 15):
2019.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81572849, 81772631
and 81301763) and Sanming Project of Medicine in Shenzhen (grant
no. SZSM201612023)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW initiated the project, designed and performed the
experiments, interpreted the data and wrote the manuscript. XJP
performed the IHC experiment and data analysis, PX performed cell
cycle data analysis, ZBT, ZWZ and ZYJ performed the reverse
transcription-quantitative PCR and apoptosis data analysis. GPZ and
ZD interpreted the data and edited figures. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The procedures used in the present study were
approved (approval no. YB M-05-01 V.2) by the Ethics Committee of
the Shanghai Outdo Biotech Company, a member of the National Human
Genetic Resources Sharing Service Platform (Shanghai, China) and
were performed in accordance with the ethical standards of The
Institutional and National Research Committee and with The
Declaration of Helsinki. Written informed consent was acquired from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PCa
|
Prostate cancer
|
|
E2F7
|
E2F transcription factor 7
|
|
IHC
|
immunohistochemistry
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
CCK-8
|
Cell Counting Kit-8
|
|
siRNA
|
small interfering RNA
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
NC
|
negative control
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 60:277–300. 2018.
|
|
2
|
Nelson WG, De Marzo AM and Isaacs WB:
Prostate cancer. N Engl J Med. 349:366–681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nevins JR: The Rb/E2F pathway and cancer.
Human Mol Genet. 10:699–703. 2001. View Article : Google Scholar
|
|
4
|
Dyson N: The regulation of E2F by
pRB-family proteins. Genes Dev. 12:2245–2262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thwaites MJ, Cecchini MJ and Dick FA:
Analyzing RB and E2F during the G1-S transition. Methods
Mol Biol. 1170:449–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Subtil-Rodríguez A, Vázquez-Chávez E,
Ceballos-Chávez M, Rodríguez-Paredes M, Martín-Subero JI, Esteller
M and Reyes JC: The chromatin remodeller CHD8 is required for
E2F-dependent transcription activation of S-phase genes. Nucleic
Acids Res. 42:2185–2196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De AB, Maiti B, Jakoi L, Timmers C, Buerki
R and Leone G: Identification and characterization of E2F7, a novel
mammalian E2F family member capable of blocking cellular
proliferation. J Biol Chem. 278:42041–42049. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zong S, Liu X, Zhou N and Yue Y: E2F7,
EREG, miR-451a and miR-106b-5p are associated with the cervical
cancer development. Arch Gynecol Obstet. 299:1089–1098. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma YS, Lv ZW, Yu F, Chang ZY, Cong XL,
Zhong XM, Lu GX, Zhu J and Fu D: MicroRNA-302a/d inhibits the
self-renewal capability and cell cycle entry of liver cancer stem
cells by targeting the E2F7/AKT axis. J Exp Clin Cancer Res.
37:2522018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salvatori B, Iosue I, Mangiavacchi A,
Loddo G, Padula F, Chiaretti S, Peragine N, Bozzoni I, Fazi F and
Fatica A: The microRNA-26a target E2F7 sustains cell proliferation
and inhibits monocytic differentiation of acute myeloid leukemia
cells. Cell Death & Disease. 3:e4132012. View Article : Google Scholar
|
|
11
|
Jian L, Xiang L, Meng W, Xiao G, Yang G,
Wang H, Li Y, Sun X, Qin S, Du N, et al: A miR-26a/E2F7 feedback
loop contributes to tamoxifen resistance in ER-positive breast
cancer. Int J Oncol. 53:1601–1612. 2018.PubMed/NCBI
|
|
12
|
Endo-Munoz L, Dahler A, Teakle N, Rickwood
D, Hazar-Rethinam M, Abdul-Jabbar I, Sommerville S, Dickinson I,
Kaur P, Paquet-Fifield S and Saunders N: E2F7 can regulate
proliferation, differentiation, and apoptotic responses in human
keratinocytes: Implications for cutaneous squamous cell carcinoma
formation. Cancer Res. 69:1800–1808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quan L, Qiu XM, Li QH, Wang XY, Li L, Xu
M, Dong M and Xiao YB: MicroRNA-424 may function as a tumor
suppressor in endometrial carcinoma cells by targeting E2F7.
Oncology Rep. 33:2354–2360. 2015. View Article : Google Scholar
|
|
14
|
Ye YY, Mei JW, Xiang SS, Li HF, Ma Q, Song
XL, Wang Z, Zhang YC, Liu YC, Jin YP, et al: MicroRNA-30a-5p
inhibits gallbladder cancer cell proliferation, migration and
metastasis by targeting E2F7. Cell Death Dis. 9:4102018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karimian A, Ahmadi Y and Yousefi B:
Multiple functions of p21 in cell cycle, apoptosis and
transcriptional regulation after DNA damage. DNA Repair (Amst.).
42:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jun L, Gad G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Behmansmant I, Rehwinkel J, Doerks T,
Stark A, Bork P and Izaurralde E: mRNA degradation by miRNAs and
GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping
complexes. Genes Dev. 20:1885–1898. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lytle JR, Yario TA and Steitz JA: Target
mRNAs are repressed as efficiently by microRNA-binding sites in the
5′ UTR as in the 3′ UTR. Proc Natl Acad Sci USA. 104:9667–9672.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao JM, Li GZ, Han M, Xu HL and Huang KM:
MiR-30c-5p suppresses migration, invasion and epithelial to
mesenchymal transition of gastric cancer via targeting MTA1. Biomed
Pharmacother. 93:554–560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Yao X, Zhang J, Dong B, Chen Q,
Xue W, Liu D and Huang Y: Hypoxia-induced downregulation of miR-30c
promotes epithelial-mesenchymal transition in human renal cell
carcinoma. Cancer Sci. 104:1609–1617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang YQ, Ling XH, Yuan RQ, Chen ZY, Yang
SB, Huang HX, Zhong WD and Qiu SP: miR-30c suppresses prostate
cancer survival by targeting the ASF/SF2 splicing factor
oncoprotein. Mol Med Rep. 16:2431–2438. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanic M, Yanowsky K, Rodriguez-Antona C,
Andrés R, Márquez-Rodas I, Osorio A, Benitez J and Martinez-Delgado
B: Deregulated miRNAs in hereditary breast cancer revealed a role
for miR-30c in regulating KRAS oncogene. PLoS One. 7:e388472012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
GJ L: ETHICS. The Pelople's publishing
house. 1989.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurien BT and Scofield RH: Western
blotting. Methods. 38:283–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Madamanchi NR and Runge MS: Western
blotting. Methods Mol Med. 51:245–256. 2001.PubMed/NCBI
|
|
27
|
Miyake M, Goodison S, Lawton A,
Gomes-Giacoia E and Rosser CJ: Angiogenin promotes tumoral growth
and angiogenesis by regulating matrix metallopeptidase-2 expression
via the ERK1/2 pathway. Oncogene. 34:890–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giacoia EG, Miyake M, Lawton A, Goodison S
and Rosser CJ: PAI-1 leads to G1-phase cell-cycle
progression through cyclin D3/cdk4/6 upregulation. Mol Cancer Res.
12:322–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
La Thangue NB: DRTF1/E2F: An expanding
family of heterodimeric transcription factors implicated in
cell-cycle control. Trends Biochem Sci. 19:108–114. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao LJ, Subramanian T, Vijayalingam S and
Chinnadurai G: CtBP2 proteome: Role of CtBP in E2F7-mediated
repression and cell proliferation. Genes Cancer. 5:31–40.
2014.PubMed/NCBI
|
|
31
|
Weijts BGMW, Westendorp B, Hien BT,
Martínez-López LM, Zijp M, Thurlings I, Thomas RE, Schulte-Merker
S, Bakker WJ and de Bruin A: Atypical E2Fs inhibit tumor
angiogenesis. Oncogene. 37:271–276. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Ran C, Li E, Gordon F, Comstock G,
Siddiqui H, Cleghorn W, Chen HZ, Kornacker K, Liu CG, et al:
Synergistic function of E2F7 and E2F8 is essential for cell
survival and embryonic development. Dev Cell. 14:62–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zalmas LP, Zhao X, Graham AL, Fisher R,
Reilly C, Coutts AS and La Thangue NB: DNA-damage response control
of E2F7 and E2F8. EMBO Rep. 9:252–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zalmas LP, Coutts A, Helleday T and La
Thangue NB: E2F-7 couples DNA damage-dependent transcription with
the DNA repair process. Cell Cycle. 12:3037–3051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carvajal LA, Hamard PJ, Tonnessen C and
Manfredi JJ: E2F7, a novel target, is up-regulated by p53 and
mediates DNA damage-dependent transcriptional repression. Genes
Dev. 26:1533–1545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin WW, Wang B, Ding M, Huo Y, Hu H, Cai
R, Zhou T, Gao Z, Wang Z and Chen D: Elevated E2F7 expression
predicts poor prognosis in human patients with gliomas. J Clin
Neurosci. 33:187–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weijts BG, Bakker WJ, Cornelissen PW,
Liang KH, Schaftenaar FH, Westendorp B, de Wolf CA, Paciejewska M,
Scheele CL, Kent L, et al: E2F7 and E2F8 promote angiogenesis
through transcriptional activation of VEGFA in cooperation with
HIF1. EMBO J. 31:3871–3884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chu J, Zhu Y, Liu Y, Sun L, Lv X, Wu Y, Hu
P, Su F, Gong C, Song E, et al: E2F7 overexpression leads to
tamoxifen resistance in breast cancer cells by competing with E2F1
at miR-15a/16 promoter. Oncotarget. 6:31944–31957. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Batra A and Winquist E: Emerging cell
cycle inhibitors for treating metastatic castration-resistant
prostate cancer. Expert Opin Emerg Drugs. 23:271–282. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu R, Poland B, Wada R, Liu Q, Musib L,
Maslyar D, Cho E, Yu W, Ma H, Jin JY and Budha N:
Exposure-response-based product profile-driven clinical utility
index for ipatasertib dose selection in prostate cancer. CPT
Pharmacometrics Syst Pharmacol. 8:240–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Beltran H, Danila D, Montgomery B,
Szmulewitz R, Vaishampayan U, Armstrong A, Stein M, Hoimes C,
Pinski J, Scher H, et al: A phase 2 study of the aurora kinase A
inhibitor alisertib for patients with neuroendocrine prostate
cancer (NEPC). Ann Oncol. 27 (Suppl 6):V15652016. View Article : Google Scholar
|
|
42
|
Kang SY, Kim HI, Hong SH, Ku JM, Lee K,
Kim MS, Choi YJ, Cheon C, Ko Y, Huang CW, et al: Abstract 300:
Taeumjowi-tang (TJ001) induces G2/M cell cycle arrest
but not apoptosis in p53-mutant prostate cancer via up-regulation
of p21 WAF/CIP1. Cancer Res. 77 (Suppl 13):3002017.
|
|
43
|
Mitxelena J, Apraiz A, Vallejo-Rodríguez
J, García-Santisteban I, Fullaondo A, Alvarez-Fernández M,
Malumbres M and Zubiaga AM: An E2F7-dependent transcriptional
program modulates DNA damage repair and genomic stability. Nucleic
Acids Res. 46:4546–4559. 2019. View Article : Google Scholar
|
|
44
|
Bockhorn J, Yee K, Chang YF, Prat A, Huo
D, Nwachukwu C, Dalton R, Huang S, Swanson KE, Perou CM, et al:
MicroRNA-30c targets cytoskeleton genes involved in breast cancer
cell invasion. Breast Cancer Res Treat. 137:373–382. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu X, Li M, Peng Y, Hu X, Xu J, Zhu S, Yu
Z and Han S: miR-30c regulates proliferation, apoptosis and
differentiation via the Shh signaling pathway in P19 cells. Exp Mol
Med. 48:e2482016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ling XH, Han ZD, Xia D, He HC, Jiang FN,
Lin ZY, Fu X, Deng YH, Dai QS, Cai C, et al: MicroRNA-30c serves as
an independent biochemical recurrence predictor and potential tumor
suppressor for prostate cancer. Mol Biol Rep. 41:2779–2788. 2014.
View Article : Google Scholar : PubMed/NCBI
|