Introduction
RNase 7, an antimicrobial peptide (AMP) with
ribonuclease activity, belongs to the RNase A superfamily and was
first identified in squamous epithelium obtained from healthy skin
(1). It is constitutively expressed
in many types of epithelial tissues, such as those of the
genitourinary, respiratory, and gastrointestinal systems, including
the oral epithelium (1–4). The expression of this peptide is
induced by stimulation with several types of microorganisms and
pro-inflammatory cytokines (1,5,6). Other
types of epithelial AMPs, such as human beta-defensins (hBD),
psoriasin, cathelicidin (LL-37), and calprotectin, have been
involved in various epithelial diseases, including inflammatory
diseases and cancer (7–13). In cancer, the AMPs were found to be
involved in both tumor suppression and progression (8,9,11). For
example, hBD-1 and −2 may function as tumor suppressors, whereas
hBD-3 is often associated with tumor progression (8). Although RNase 7 has been detected in
the oral epithelium (4), its role
in malignancy remains unknown. A recent immunohistochemical study
indicated that the levels of RNase 7 gradually reduced with the
progression of cancer in the skin, suggesting that the peptide may
act as a tumor suppressor (14).
Nonetheless, biological evidence of the involvement of RNase 7 in
malignant tumors has not been presented thus far. Therefore, the
aim of the present study was to evaluate the expression patterns of
RNase 7 in oral squamous cell carcinoma (OSCC) in vitro and
in vivo, and examine whether alterations in the expression
levels of this gene affected the malignant potentialities of the
cancer cells.
Materials and methods
Tissue samples
Tissue sections of patients at the Department of
Oral Medicine and Pathology, at the Health Sciences University of
Hokkaido (Hokkaido, Japan) were collected. The study was designed
to include 20 specimens from OSCC patients (well-differentiated
OSCC, 7; moderately-differentiated OSCC, 7; poorly-differentiated
OSCC, 6); adjacent normal oral epithelium was used as healthy
control. The differentiation stages of all the samples were
determined by an experienced pathologist.
The protocol for the study was approved by the
ethics review board of the Health Sciences University of
Hokkaido.
Immunohistochemistry
Formalin-fixed paraffin-embedded sections obtained
from the tissue samples were deparaffinized in xylene, rehydrated
in ethanol series, and rinsed with demineralized water. Antigen
binding sites were exposed using the heat-induced antigen retrieval
method in a pressure cooker containing citrate buffer (pH 6.0).
Endogenous peroxidase activity was blocked with 3% hydrogen
peroxide for 10 min. Primary antibody [anti-RNase 7 antibody
(CL0224); dilution, 1:50; catalog number, ab 154143; Abcam] was
added and incubated for 1 h at 37°C. After washing with PBS, 100 µl
of the secondary antibody (HRP-labeled polymer anti-mouse antibody;
Dako EnVision + System, Dako North America, Inc.) was added to the
sections, which were then incubated for 30 min at 37°C. The
sections were visualized with DAB (3,3′-diaminobenzidine; code
K3468; Dako North America, Inc.) for 3 min at room temperature,
followed by immersion in distilled water to avoid overstaining.
Subsequently, the sections were counterstained with hematoxylin,
dehydrated, and mounted in malinol. Normal rabbit serum was used
instead of the primary antibody for the negative controls. The
sections were observed under a light microscope (Olympus BX 50,
Olympus Corporation) and the expression levels of RNase 7 were
evaluated based on the intensity of staining (brown color). The
findings were interpreted using the following scores: Negative, no
reactivity; weakly positive, mild brown-colored staining; positive,
immunoreactivity showing brown-colored staining of moderate
intensity; and strongly positive, strong brown-colored
staining.
Cell culture
Four different types of OSCC cell lines (BSC-OF,
SAS, OSC-19, and HSC-2) and an immortalized human keratinocytes
cell line (HaCaT) were used in this study. BSC-OF was isolated from
excised tissues diagnosed as basaloid-squamous cell carcinoma of
the floor of the mouth, and cultured in our laboratory (15). OSCC cell lines SAS, HSC-2 and OSC19
were obtained from the Human Science Research Resource Bank (Osaka,
Japan). HaCaT was obtained from Cell Line Services (CLS, Germany).
The cells were grown in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich Biotechnology) supplemented with 10% fetal bovine
serum (FBS; Invitrogen), 2% 100 IU/ml penicillin and 100 IU/ml
streptomycin (Sigma-Aldrich) at 37°C in a humidified atmosphere and
5% CO2.
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was extracted from the cell lines using
the acid guanidine thiocyanate/phenol-chloroform method with
TRIzol® (Invitrogen) according to the manufacturer's
instructions. The RNA was reverse transcribed using oligo (dT)
12–18 primers (Invitrogen), Superscript II reverse transcriptase
(SuperScript Reverse Transcriptase; Invitrogen), DTT, and dNTPs.
Nucleotide contents were measured on a nanodrop ND-1000 spectral
photometer (Nanodrop Technologies). Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
performed using the KAPA SYBR Fast qPCR Kit (Nippon Genetics); cDNA
was mixed with the primer and RT-qPCR was performed on a Light
Cycler® Nano System (Software version 1.1, Roche
Diagnostics). The following RNase 7 primers were used in the study:
Forward-GGAGTCACAGCACGAAGACCA, and reverse CATGGCTGAGTTGCATGCTTGA.
The PCR conditions included denaturation at 95°C for 10 min, 45
cycles of denaturation at 95°C for 10 sec, and annealing at 60°C
for 30 sec. The relative mRNA expression levels were calculated
using the ΔΔCq method (16). The
experiment was performed in triplicate and values were normalized
to GAPDH (forward: GAGTCAACGGATTTGGTCGT and reverse:
GACAAGCTTCCCGTTCTCAG primers) as a reference gene. Data were
expressed as the ratio of the target mRNA to the GAPDH mRNA.
Immunocytochemical examination of
RNase 7 in the cultured cells
Approximately 50,000 cells/well were seeded on
sterilized chamber slides and left to grow overnight. After 24 h,
the cells were fixed in 95% alcohol for 5 min at room temperature
and immunostaining was carried out using the primary antibody
(CL0224; dilution, 1:50; catalog number ab 154143; Abcam). The
subsequent steps were performed as described for the
immunohistochemistry protocol under the same conditions.
Enzyme-linked immunosorbent assay
The OSCC and HaCaT cells were seeded at a density of
5×106 cells/well for three days. The supernatant was
collected and centrifuged at 4,760 × g for 5 min at 4°C.
Subsequently, the supernatant was separated from the cell debris
and stored at −80°C. To collect the cell extract, the cells in the
wells were scraped and mixed with 1 ml of cell lysate. After 30
min, the lysate was centrifuged at 11,290 × g for 10 min at 4°C;
the cell extract was collected and stored at −80°C. The
concentration of RNase 7 was determined by enzyme-linked
immunosorbent assay (ELISA; Hycult Biotech) according to the
manufacturer's instructions.
Knockdown of RNase 7 in OSCC
cells
The OSCC and HaCaT cells were seeded in a 6-well
plate at a density of 1×106 cells/well and incubated in
2 ml of DMEM supplemented with 10% FBS without antibiotics, at 37°C
with 5% CO2. The next day, the cells were transfected
with 100 pmol of RNase 7 siRNA and 100 pmol of the negative control
siRNA (control) in test and control wells, respectively, along with
the transfecting reagent containing Lipofectamine 2000 (Life
Technologies) and an optimum medium. After 24 h of transfection,
total RNA was extracted using the acid guanidine
thiocyanate/phenol-chloroform method. The RNA was reverse
transcribed to cDNA and RNase 7 mRNA expression was determined in
both the negative control and the siRNA-OSCC cells via RT-qPCR.
Cell proliferation
The CyQUANT® cell proliferation assay
(Invitrogen) was used for the cell proliferation assay. The
standard curve was prepared according to the manufacturer's
instruction. In brief, the OSCC and HaCaT cells were seeded in
96-well plates (Corning Incorporated; surface area, 0.3
cm2/well; density of 1×104 cells/well) and
incubated in 100 µl of DMEM supplemented with 10% FBS without
antibiotics. After 24 h, the cells were transfected with 5 pmol
siRNA along with the transfecting media. After 24 and 48 h of
incubation, the wells were stored at −80°C for two days to lyse the
cells. The cells were thawed at room temperature; 200 µl of CyQUANT
GR dye/cell-lysis buffer (Invitrogen) was added to the well and
incubated for 5 min at room temperature in the dark. The
fluorescence of the nucleic acid was measured using a fluorescence
microplate reader with filters appropriate for 480 nm excitation
and 520 nm emission. The fluorescence values were converted into
DNA concentrations and measured using a standard curve.
Invasion assay
Cell invasion was evaluated using a 24-multiwell
Falcon TC Companion plate with Falcon cell culture inserts
containing an 8.0-µm pore size PET membrane with a thin layer of
Matrigel basement membrane matrix (Corning Biocoat Matrigel
Invasion Chamber). The experiment was carried out according to the
manufacturer's instructions. In brief, 500 µl of a suspension of
OSCC cells (2.5×104 cells) and HaCaT were grown in the
serum media within the insert. After 24 h, the cells were
transfected with 20 pmol of siRNA along with the transfecting
reagent. The transfected cells were allowed to invade through the
Matrigel matrix for 24 h. The non-invading cells were removed and
invading cells were stained with Diff-Quick stain
(Hemacolor®). Images of the invaded cells were taken
from three fields at high power. The numbers of invaded cells were
counted using the ImageJ software, version 1.5 ob (National
Institute of Health).
RT-qPCR for expression of matrix
metalloproteinase 9, involucrin, and K14
The mRNA expression levels of matrix
metalloproteinase (MMP) 9, involucrin (INV), and K14 were
determined by RT-qPCR in both the control and knockdown OSCC cells.
The primers used were: MMP-9 (forward: GGCAATGCTGATGGGAAACC,
reverse: CTTGTCCCGGTCGTAGTTGG); INV (forward:
ATGCCAGAAGGTGCCTGTCGA, reverse: TGTGTTTTCTCCTGCTTAAGC); and K14
(forward: GCCTCTCAGGGCATTCATCTC, reverse:
GAGTGTGGAAGCCGACATCA).
Statistical analysis
Statistical analysis was performed using the IBM
SPSS Statistical tool for iOS (Version 25; SPSS Inc.). The results
were compared using the Mann-Whitney U test and Student's t-test
for two groups and one way of variance (ANOVA) followed by
Dunnett's post hoc test for multiple groups. P<0.05 was
considered statistically significant.
Results
Immunohistochemical localization of
RNase 7 was observed in the OSCC tissue sections
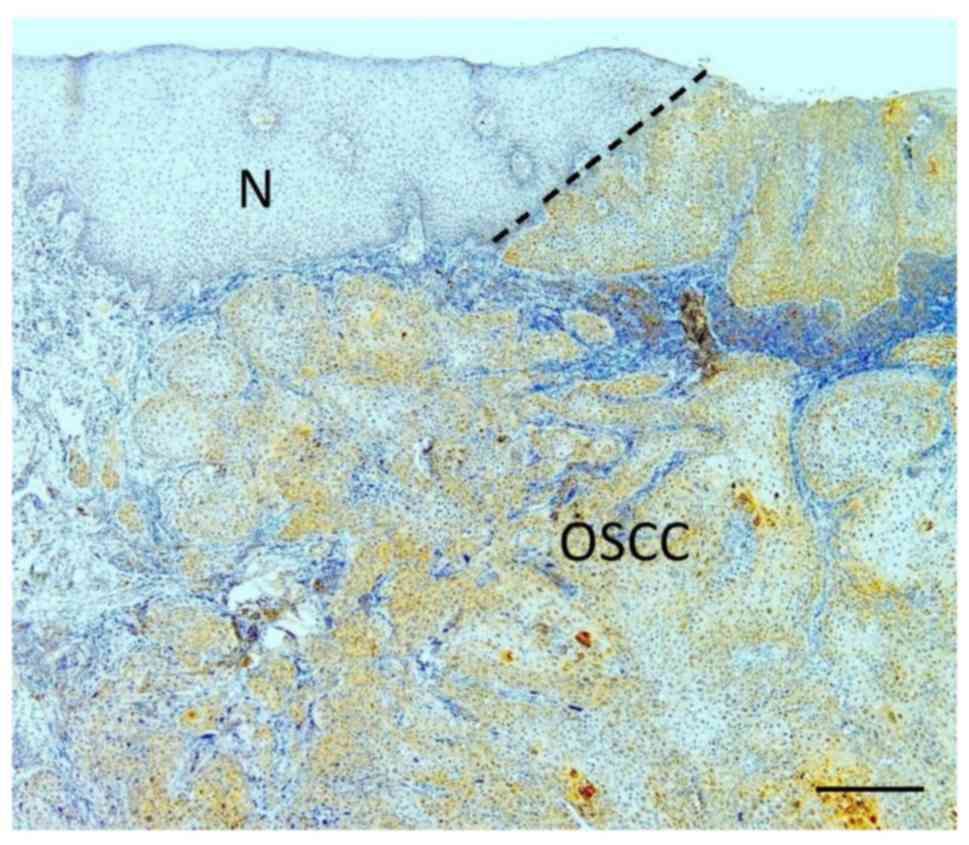
Weak immunoreaction for RNase 7 was localized mainly
in the surface layers of the normal oral epithelium adjacent to the
tumor (Fig. 1). In
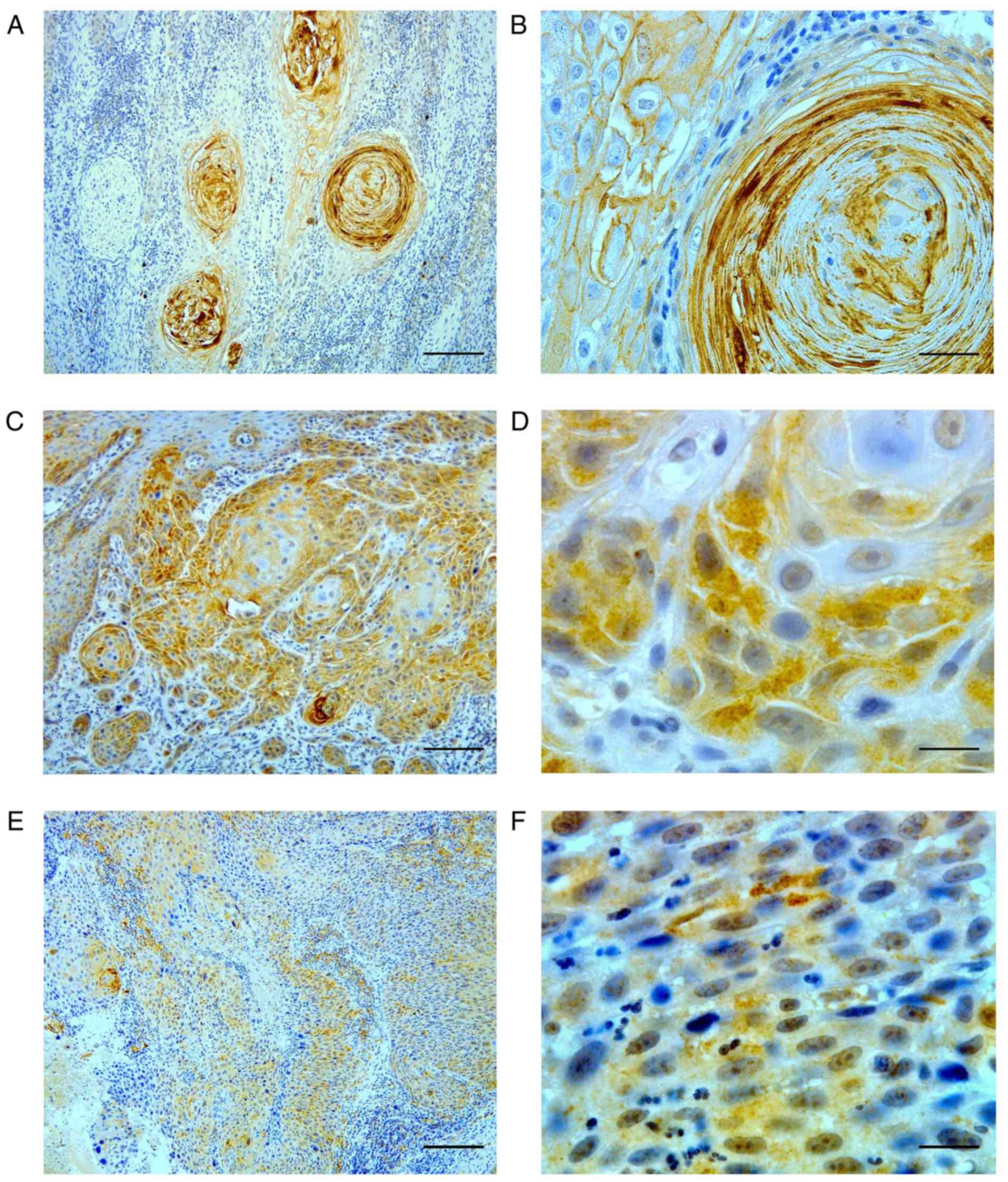
well-differentiated OSCC, intense immunostaining was observed in
the keratinized layers of the epithelial pearl and the
intercellular spaces of the tumor nests (Fig. 2A and B). In moderately
differentiated OSCC, positive staining was often strongly observed
in the nuclei and cytoplasm of cells in the tumor nests that
exhibited some keratinization (Fig. 2C
and D), whereas weak immunoreaction was observed in some tumor
cells that did not exhibit any keratinization. Tumor cells with no
keratinization in poorly differentiated OSCC demonstrated weak
immunoreactions (Fig. 2E and
F).
Expression and concentration of RNase
7 in the cells
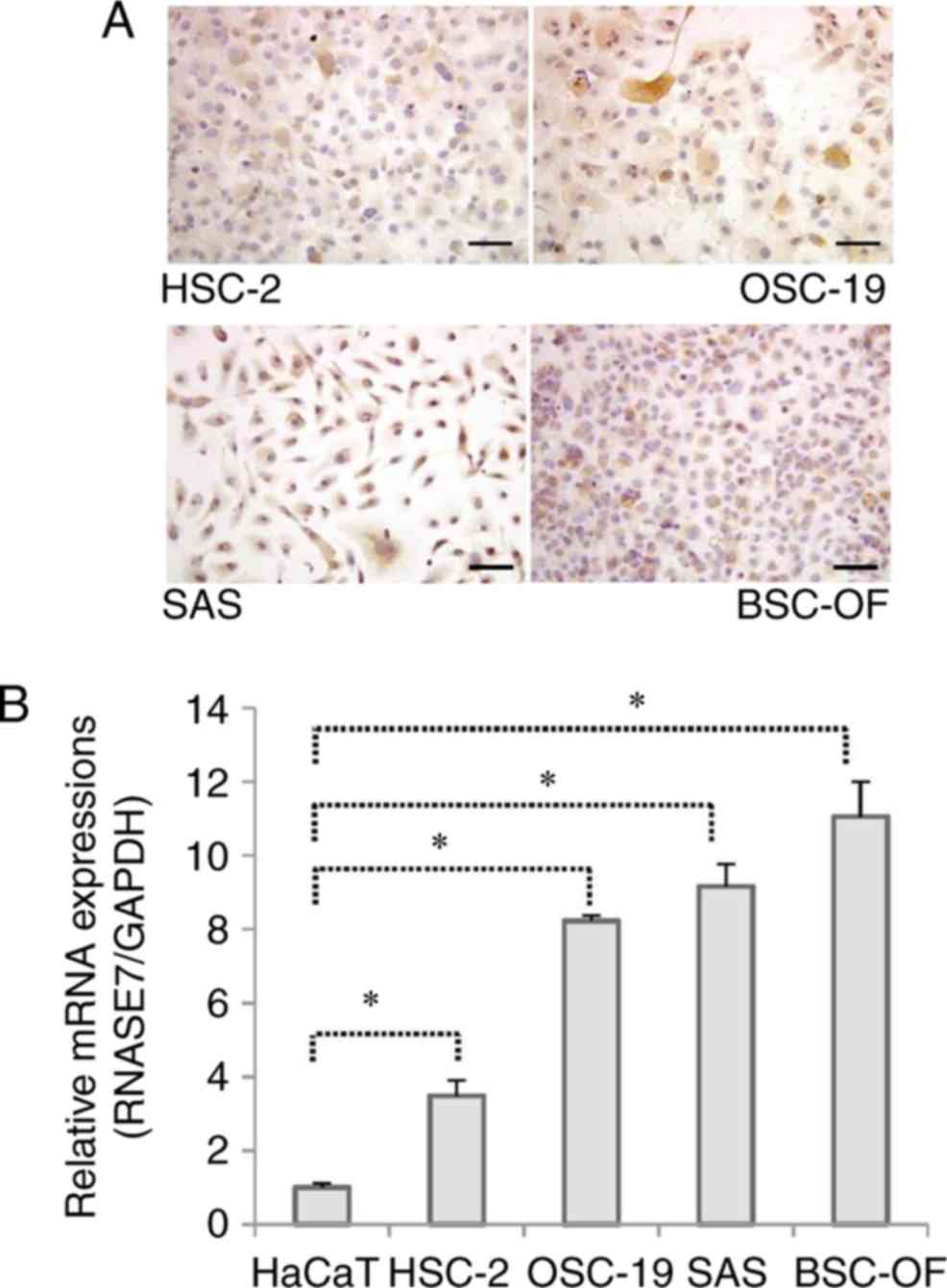
The mRNA expression level of RNase 7 was
significantly higher in the OSCC cell lines (BSC-OF, SAS, OSC-19,
and HSC-2) than in the controls (P<0.05; Fig. 3B). To determine whether the mRNA
expression was translated into protein, the presence of
intracellular RNase 7 was detected by immunocytochemistry (Fig. 3A). The protein concentration was
evaluated in the conditioned medium and the cell extract obtained
from each cell line using ELISA. Positive staining for RNase 7 was
observed in all the cell lines (Fig.
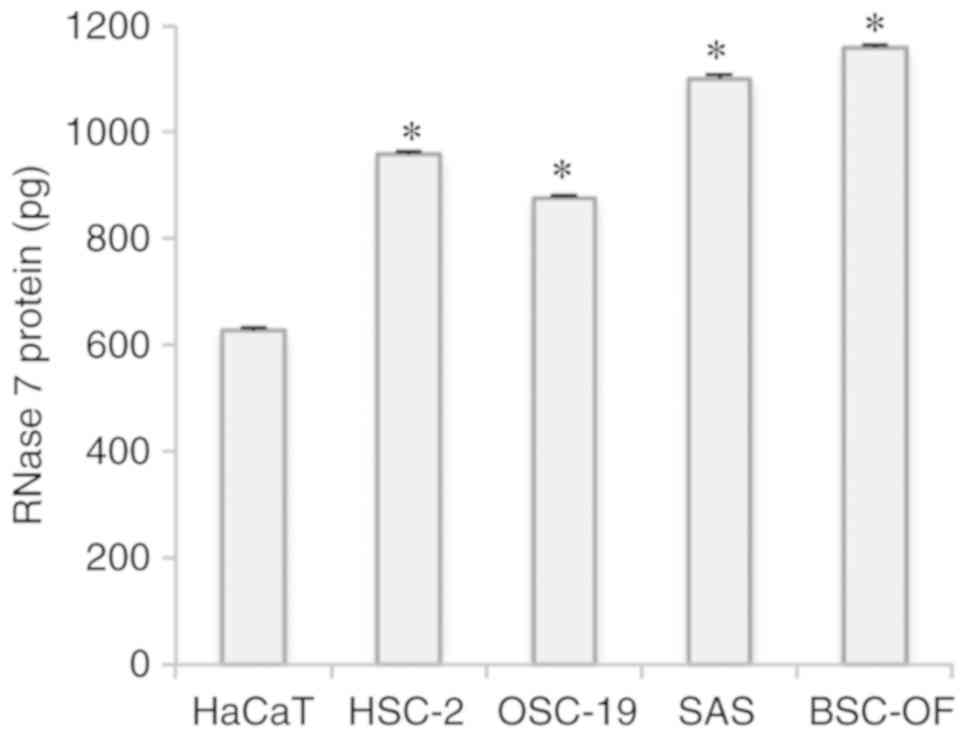
3A). RNase 7 protein was detected in both the conditioned
medium and the cell extracts derived from each cell line. The
concentration of RNase 7 was significantly higher in the
conditioned media compared to the cell extracts (P<0.05;
Fig. S1). The RNase 7 protein
levels were significantly higher in the OSCCs compared to the
control (P<0.05; Fig. 4).
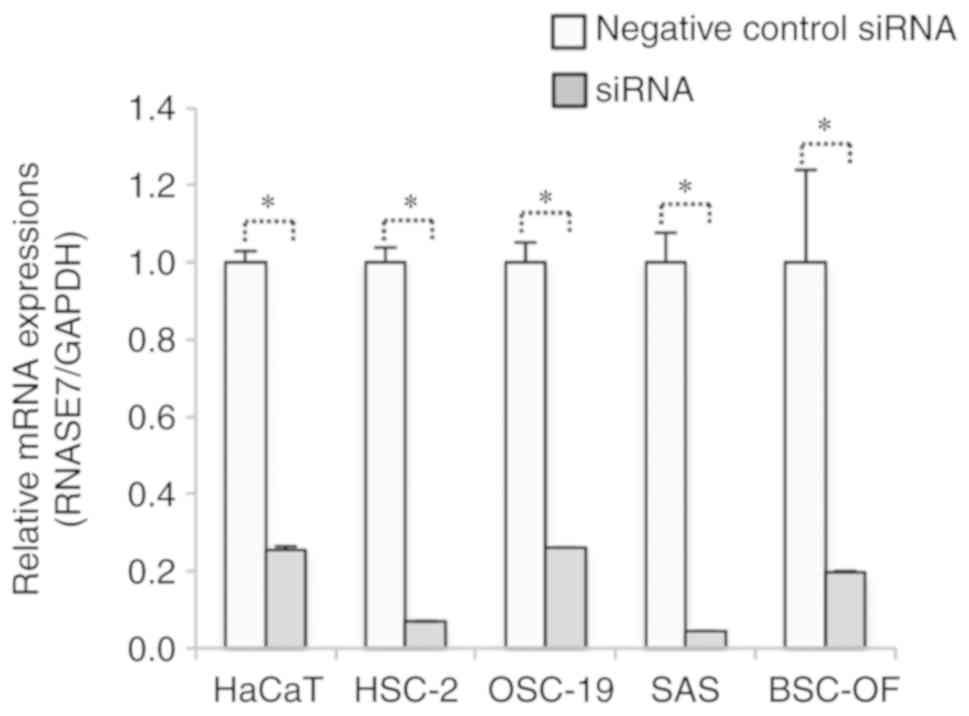
Knockdown of RNase 7 was performed and confirmed by RT-qPCR after
siRNA transfection. The mRNA expression of RNase 7 was
significantly lower in the siRNA-OSCC cells compared to the OSCC
cells (P<0.05; Fig. 5).
Furthermore, cell numbers were significantly higher in the
siRNA-BSC-OF, -SAS, -HSC-2, -OSC-19, and -HaCaT cells at 24 h and
siRNA-BSC-OF, -SAS, and -HSC-2 cells at 48 h when compared with the
control OSCC and HaCaT cells (P<0.05; Fig. 6).
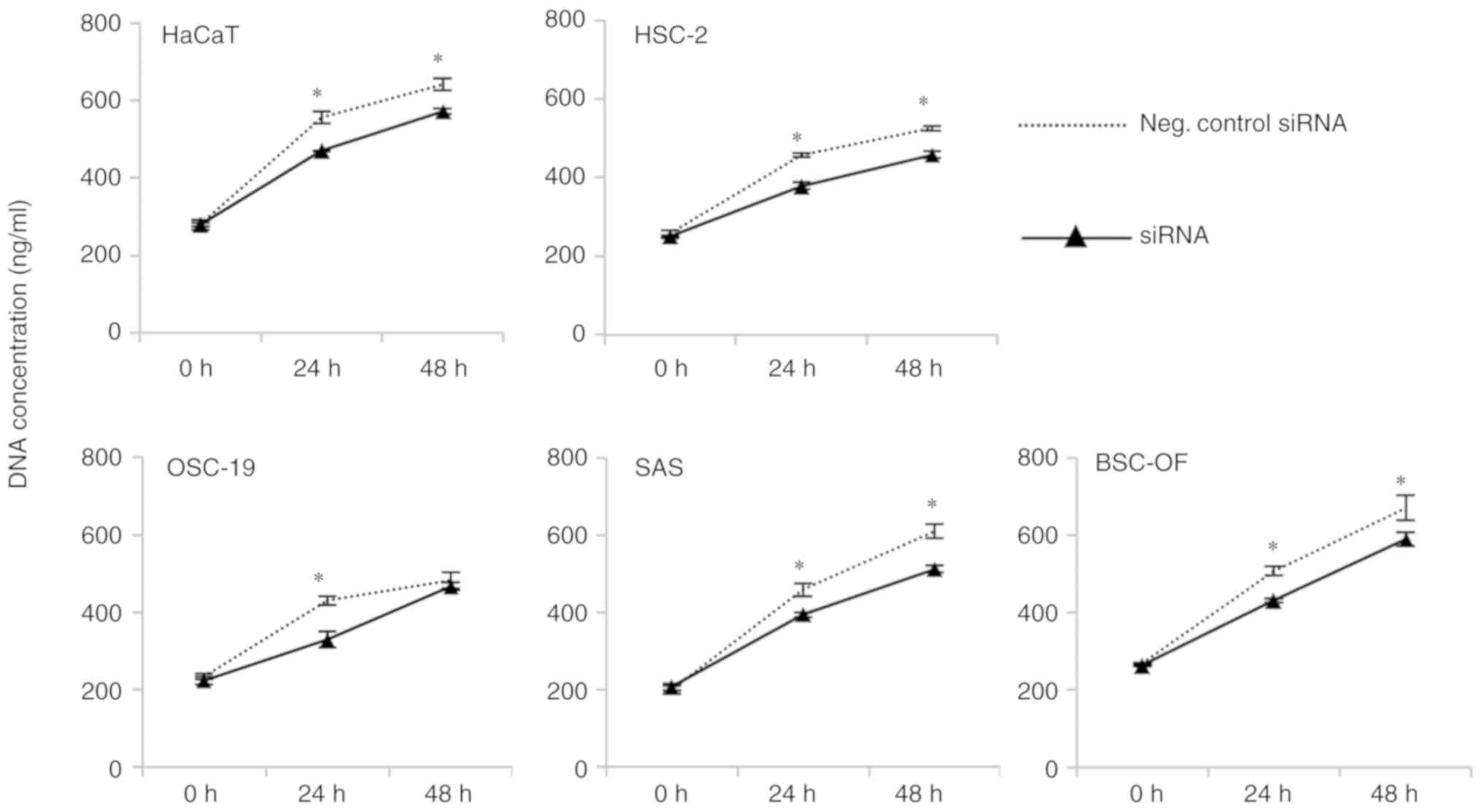 | Figure 6.Proliferation of the knockdown OSCC
and OSCC cells. The cell numbers were significantly higher
(*P<0.05) in the siRNA-BSC-OF, -SAS, -HSC-2, -OSC-19, and -HaCaT
cells compared to the BSC-OF, SAS, HSC-2, OSC-19, and HaCaT cells
at 24 h. At 48 h, the cell numbers were significantly higher
(*P<0.05) in the siRNA-BSC-OF, -SAS, -HSC-2, and -HaCaT cells
compared to the BSC-OF, SAS, HSC-2, and HaCaT cells. Scale bars:
100 µm (A). *P<0.05; Student's t-test. |
Cell invasion and MMP 9 expression
levels
The number of invading cells in the siRNA-BSC-OF,
-SAS, and -HSC-2 samples was significantly higher than those in the
control OSCC and HaCaT samples (P<0.05; Fig. 7B). No significant difference in cell
numbers between siRNA-OSC-19 and the control was observed. The
expression levels of MMP 9 mRNA were significantly higher in the
siRNA-BSC-OF, SAS, and HSC-2 cells than in the control OSCC cells
(P<0.05; Fig. 7C), which was
consistent with the findings of the cell invasion assay. No
significant differences in the expression levels of MMP 9 were
observed between the siRNA-OSC-19 and siRNA-HaCaT cells.
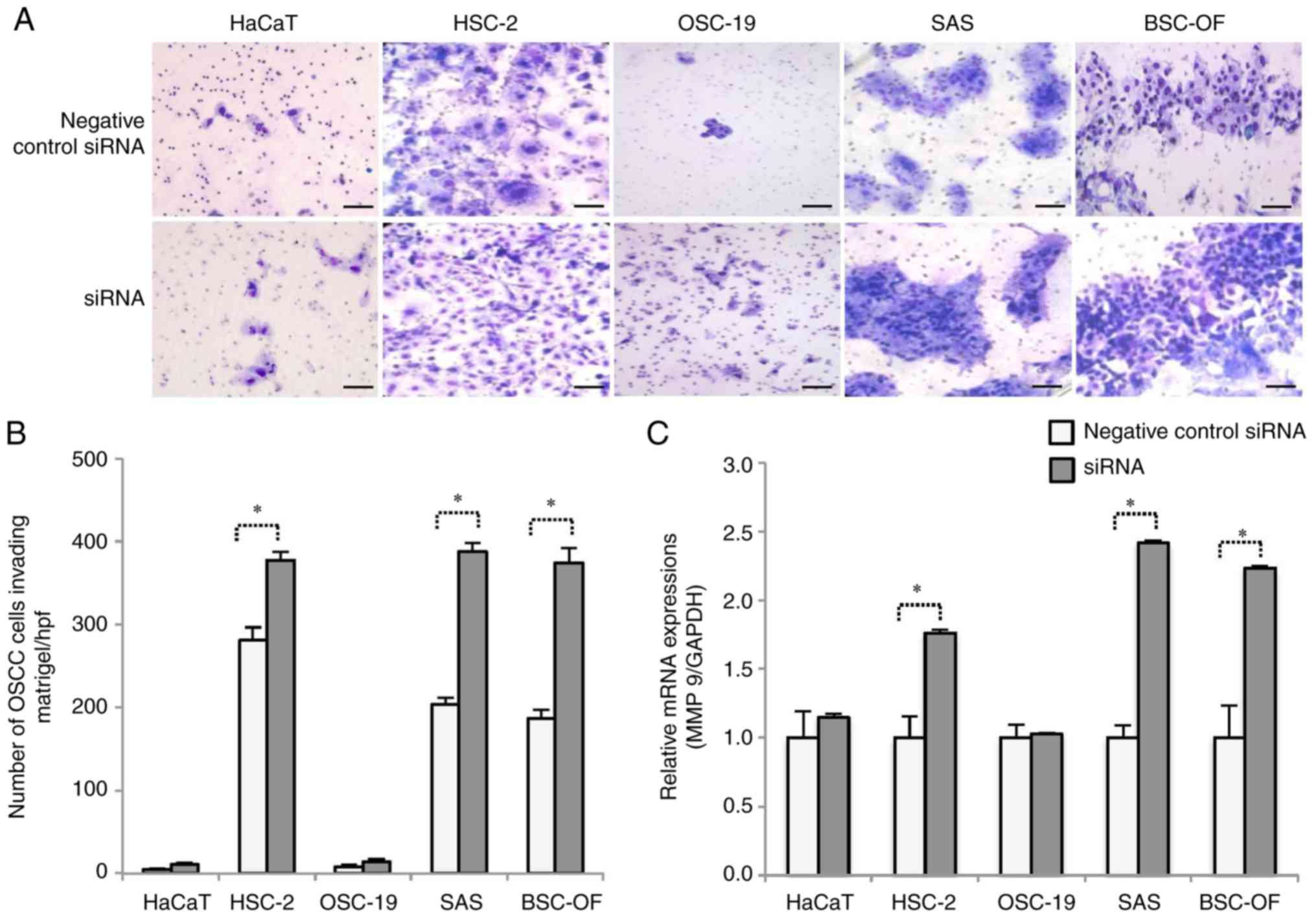 | Figure 7.Cell invasion and MMP 9 expression
levels. Negative control-HaCaT, HSC-2, OSC-19, SAS, BSC-OF, and
siRNA-HaCaT, -HSC-2, -OSC-19, -SAS and -BSC-OF cells invading
through the Matrigel (A). Scale bars: 100 µm. The number of
invading cells among the siRNA-BSC-OF, -SAS, and -HSC-2 cells was
significantly higher (*P<0.05) than that among the BSC-OF, SAS,
and HSC-2 cells. *P<0.05; Student's t-test (B). The expression
level of MMP 9 was significantly higher (*P<0.05) in the
siRNA-BSC-OF, -SAS, and -HSC-2 cells compared to the BSC-OF, SAS,
and HSC-2 cells (C). *P<0.05; Mann Whitney U-test. |
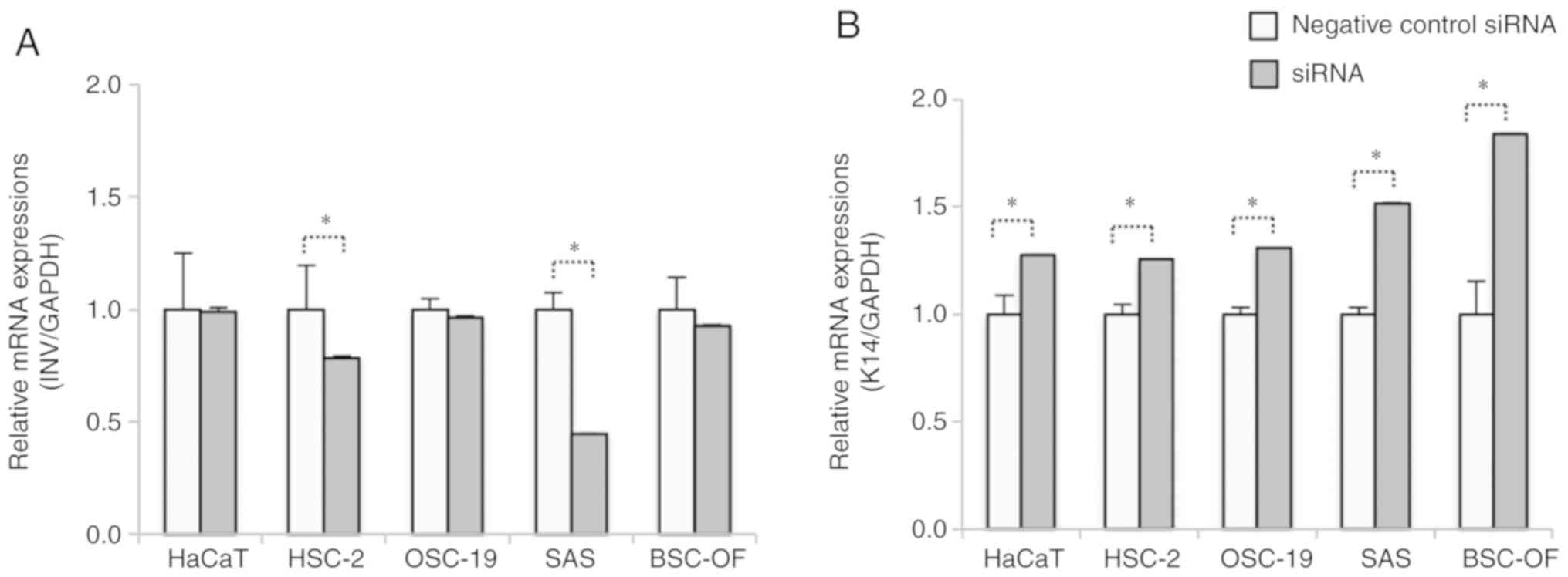
Expression levels of INV and K14 in
the OSCC cells
To examine whether the differentiations were linked
to the invasive abilities, the mRNA expression levels of the
differentiation and dedifferentiation markers INV and K14,
respectively, were evaluated by RT-qPCR. The mRNA expression level
of INV was significantly higher in the HSC-2 and SAS cells when
compared to the siRNA-HSC-2 and -SAS cells (P<0.05; Fig. 8A). No significant differences in INV
expression levels were observed between the siRNA-BSC-OF, -OSC-19,
and -HaCaT cells and the control OSCC and HaCaT cells. All the
siRNA-OSCC, and -HaCaT cell lines showed significantly higher
expression levels of K14 compared to the control OSCC and HaCaT
cells (P<0.05; Fig. 8B).
Discussion
The results of the present study have identified the
expression levels of RNase 7 in OSCC tissues. Although RNase 7 was
localized mainly in the keratinized surface layers of the normal
oral epithelium, it was often observed in the basaloid and
spinous-like cells and the keratinized pearls of the OSCC tissues.
As reported previously in normal and inflamed oral epithelium, the
expression of RNase 7 was linked to keratinocyte differentiation in
the current study (17). The
increased expression of RNase 7 in the keratinized pearl may be due
to the differentiation of the cells. The expression of RNase 7 in
the basaloid and spinous-like OSCC cells may be in response to an
inflammatory stimulus and/or bacterial infection (1,4). OSCC
cells have frequently revealed interstitial inflammatory reactions
around tumor nests, which are easily infected with bacteria
(18,19). The increase in the expression level
of the epidermal growth factor receptor (EGFR) in OSCC may be
another reason for the RNase 7-positive staining in the basaloid
and spinous-like cells. Human carcinomas, including OSCC,
frequently express high levels of EGFR (20). Moreover, the EGFR signaling pathway
is known to induce RNase 7 expression (3); therefore, high expression levels of
EGFR may contribute to an increase in the expression of RNase 7 in
OSCC cells (3,21). Nonetheless, further investigations
are needed to clarify this speculation.
The potential role of hBD2, an epithelial AMP, in
tumor suppression has been previously identified (20). The increased expression of hBD2 in
oral carcinoma cells following gene transfection was found to
inhibit both proliferation and invasion (22). Therefore, we hypothesized that high
expression levels of RNase 7 may play a role in the malignant
potential of OSCC cells. By contrast, RNase 7 knockdown promoted
the proliferation and invasion of OSCC cells in the current study.
Although the invasiveness of the OSC-19 cells was low compared to
that of the siRNA-transfected-OSC-19 cells, no significant
difference was observed. These results indicate that RNase 7 may
function as a tumor suppressor. In addition, other epithelial AMPs
such as hBD1 and hBD2 may contribute to tumor suppression (22,23).
Thus, RNase 7 may suppress OSCC cell growth and invasion in
conjunction with the other epithelial AMPs. Nonetheless, the reason
for the high expression levels of RNase 7 in OSCC cells in the
current study is unclear. The paracrine and autocrine effects
exerted by cancer cells may have induced the expression of this
peptide (24).
The expression levels of RNase 7 may vary among
cancer cells. The expression profiles of hBDs are known to differ
among the various types of cancer cells (25–28);
both high and low expression levels of hBD2 have been reported in
OSCC (26,27). The role of MMP 9 in the promotion of
cell invasion was evaluated because MMP 9 is a key enzyme that
promotes cancer cell invasion (29,30).
The siRNA-OSCCs showed significantly higher levels of MMP 9
compared to the control OSCCs, except for OSC-19. These data are
consistent with those obtained from the invasion assay. The
differentiation of the squamous epithelium may be followed by the
expression of RNase 7 in the tissue sections. Therefore, we
evaluated the level of differentiation of the siRNA-OSCC cells
using the differentiation and undifferentiation markers INV and
K14, respectively. The downregulated expression of RNase 7 in
cancer cells may induce dedifferentiation in OSCC. In general,
undifferentiated cancer cells grow more rapidly than differentiated
cancer cells (31). These results
indicate that RNase 7 may function as a suppressor of tumor growth
and invasion. The associations between MMP 9 expression and
dedifferentiation and the induction of both proliferation and
invasion by the downregulated expression of RNase 7 in OSCC cells
remain unknown. K14 may play a role in maintaining the cell
proliferation potential in the basal layer of the stratified
epithelia by modulating phosphatidylinositol 3-kinase/Akt-mediated
cell proliferation (PI3K/Akt) (32). The expression levels of both RNase 7
and MMP 9 can be regulated by the PI3K/Akt signaling pathway
(33,34). A previous study showed that
siRNA-mediated downregulation of Notch2 in gastric cancer cells
could increase tumor cell invasion and enhance MMP 9 expression via
the PI3K/Akt signaling pathway (35). Therefore, the increase in cell
proliferation, MMP 9 expression, and invasion during the
downregulation of RNase 7 may have been regulated by the PI3K/Akt
signaling pathway in the present study. Further studies were needed
to clarify this hypothesis. The stimulation of keratinocyte
differentiation in vitro has been shown to induce MMP 9
secretion (36). On the contrary,
increased MMP 9 expression followed by keratinocyte
dedifferentiation was observed in the siRNA-OSCC cells in the
present study. The high expression levels of MMP 9 in this study
may have been induced by pathways that are different from those
involved in keratinocyte differentiation. MMP 9 expression is
strongly associated with the EGFR signaling pathway (37). High EGFR expression levels may
contribute to high levels of RNase 7 expression in OSCC. EGFR
stimulation may induce the expression of MMP 9 in cells with
decreased RNase 7 expression levels. Further investigations are
needed to elucidate the mechanisms involved in the promotion of the
proliferation and invasion of siRNA-OSCC cells.
In conclusion, RNase 7, an epithelial antimicrobial
peptide, is widely expressed in OSCC. The upregulated expression of
RNase 7 in OSCC suggests its involvement in tumor development and
growth. However, decreased RNase 7 expression was found to promote
the growth and invasion of the OSCC cells in this study. Thus,
RNase 7 may contribute to the suppression of the malignant
potential of these cells.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant
from funding agencies in the public, commercial, or not-for-profit
sectors.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YA, KY and MS conceived and designed the study. PN
conducted the experiments. YK, TM, and OU analyzed the data and
interpreted the results data and DP, KY and JS performed the
statistical analysis. PN and YA wrote the manuscript. MS, OU, and
KY reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work is appropriately investigated and resolved.
Ethics approval and consent to
participate
Approval for the study was obtained from the ethics
review board of the Institute of Personalized Medical Science,
Health Sciences University of Hokkaido (approval no. 2012-005). All
the patients provided written informed consent for this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harder J and Schroder JM: RNase 7, a novel
innate immune defense antimicrobial protein of healthy human skin.
J Biol Chem. 277:46779–46784. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spencer JD, Schwaderer AL, Dirosario JD,
McHugh KM, McGillivary G, Justice SS, Carpenter AR, Baker PB,
Harder J and Hains DS: Ribonuclease 7 is a potent antimicrobial
peptide within the human urinary tract. Kidney Int. 80:174–180.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amatngalim GD, Broekman W, Daniel NM, van
der Vlugt LE and Hiemstra PS: Cigarette smoke impairs basal cell
mediated epithelial wound repair and induces expression of the
antimicrobial protein RNase 7 via oxidative stress. Eur Respir J.
46 (Suppl 59):PA50472015.
|
|
4
|
Eberhard J, Menzel N, Dommisch H, Winter
J, Jepsen S and Mutters R: The stage of native biofilm formation
determines the gene expression of human beta-defensin-2, psoriasin,
ribonuclease 7 and inflammatory mediators: A novel approach for
stimulation of keratinocytes with in situ formed biofilms. Oral
Microbiol Immunol. 23:21–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Köten B, Simanski M, Gläser R, Podschun R,
Schröder JM and Harder J: RNase 7 contributes to the cutaneous
defense against enterococcus faecium. PLoS One. 4:e64242009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simanski M, Dressel S, Gläser R and Harder
J: RNase 7 protects healthy skin from staphylococcus aureus
colonization. J Invest Dermatol. 130:2836–2848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weinberg A, Jin G, Sieg S and McCormick
TS: The yin and yang of human beta-defensins in health and disease.
Front Immunol. 3:2942012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosh SK, McCormick TS and Weinberg A:
Human beta defensins and cancer: Contradictions and common ground.
Front Oncol. 9:3412019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Bunston C, Hodson N, Resaul J, Sun
PH, Cai S, Chen G, Gu Y, Satherley LK, Bosanquet DC, et al:
Psoriasin promotes invasion, aggregation and survival of pancreatic
cancer cells; association with disease progression. Int J Oncol.
50:1491–1500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Algermissen B, Sitzmann J, LeMotte P and
Czarnetzki B: Differential expression of CRABP II, psoriasin and
cytokeratin 1 mRNA in human skin diseases. Arch Dermatol Res.
288:426–430. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Zou X, Qi G, Tang Y, Guo Y, Si J
and Liang L: Roles and mechanisms of human cathelicidin LL-37 in
cancer. Cell Physiol Biochem. 47:1060–1073. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kahlenberg JM and Kaplan MJ: Little
peptide, big effects: The role of LL-37 in inflammation and
autoimmune disease. J Immunol. 191:4895–4901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ross KF and Herzberg MC: Calprotectin
expression by gingival epithelial cells. Infect Immun.
69:3248–3254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scola N, Gambichler T, Saklaoui H, Bechara
FG, Georgas D, Stücker M, Gläser R and Kreuter A: The expression of
antimicrobial peptides is significantly altered in cutaneous
squamous cell carcinoma and precursor lesions. Br J Dermatol.
167:591–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abiko Y, Okumura K, Ohuchi T, Konishi T,
Kanazawa M and Kaku T: Basaloid-squamous cell carcinoma of the
floor of the mouth: Characterization of a cell line. J Oral Pathol
Med. 26:367–370. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neopane P, Paudel D, Yoshida K, Raj
Adhikari B, Morikawa T, Onishi A, Hiraki D, Uehara O, Sato J,
Nishimura MA, et al: Immunohistochemical localization of RNase 7 in
normal and inflamed oral epithelia and salivary glands. Acta
Histochem Cytochem. 52:35–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abiko Y, Suraweera AK, Nishimura M,
Arakawa T, Takuma T, Mizoguchi I and Kaku T: Differential
expression of human beta-defensin 2 in keratinized and
non-keratinized oral epithelial lesions; immunohistochemistry and
in situ hybridization. Virchows Arch. 438:248–253. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pushalkar S, Ji X, Li Y, Estilo C,
Yegnanarayana R, Singh B, Li X and Saxena D: Comparison of oral
microbiota in tumor and non-tumor tissues of patients with oral
squamous cell carcinoma. BMC Microbiol. 12:1442012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grandis JR and Tweardy DJ: TGF-alpha and
EGFR in head and neck cancer. J Cell Biochem Suppl. 17F:188–191.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheikh Ali MA, Gunduz M, Nagatsuka H,
Gunduz E, Cengiz B, Fukushima K, Beder LB, Demircan K, Fujii M,
Yamanaka N, et al: Expression and mutation analysis of epidermal
growth factor receptor in head and neck squamous cell carcinoma.
Cancer Sci. 99:1589–1594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamino Y, Kurashige Y, Uehara O, Sato J,
Nishimura M, Yoshida K, Arakawa T, Nagayasu H, Saitoh M and Abiko
Y: HBD-2 is downregulated in oral carcinoma cells by DNA
hypermethylation, and increased expression of hBD-2 by DNA
demethylation and gene transfection inhibits cell proliferation and
invasion. Oncol Rep. 32:462–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun CQ, Arnold R, Fernandez-Golarz C,
Parrish AB, Almekinder T, He J, Ho SM, Svoboda P, Pohl J, Marshall
FF and Petros JA: Human beta-defensin-1, a potential chromosome 8p
tumor suppressor: Control of transcription and induction of
apoptosis in renal cell carcinoma. Cancer Res. 66:8542–8549. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsujikawa T, Yaguchi T, Ohmura G, Ohta S,
Kobayashi A, Kawamura N, Fujita T, Nakano H, Shimada T, Takahashi
T, et al: Autocrine and paracrine loops between cancer cells and
macrophages promote lymph node metastasis via CCR4/CCL22 in head
and neck squamous cell carcinoma. Int J Cancer. 132:2755–2766.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kesting MR, Loeffelbein DJ, Hasler RJ,
Wolff KD, Rittig A, Schulte M, Hirsch T, Wagenpfeil S, Jacobsen F
and Steinstraesser L: Expression profile of human beta-defensin 3
in oral squamous cell carcinoma. Cancer Invest. 27:575–581. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshimoto T, Yamaai T, Mizukawa N, Sawaki
K, Nakano M, Yamachika E and Sugahara T: Different expression
patterns of beta-defensins in human squamous cell carcinomas.
Anticancer Res. 23:4629–4633. 2003.PubMed/NCBI
|
|
27
|
Abiko Y, Mitamura J, Nishimura M,
Muramatsu T, Inoue T, Shimono M and Kaku T: Pattern of expression
of beta-defensins in oral squamous cell carcinoma. Cancer Lett.
143:37–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sawaki K, Mizukawa N, Yamaai T, Yoshimoto
T, Nakano M and Sugahara T: High concentration of beta-defensin-2
in oral squamous cell carcinoma. Anticancer Res. 22:2103–2107.
2002.PubMed/NCBI
|
|
29
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells.
Biochim. Biochim Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
30
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bullock S and Hales M: Neoplasia. Babey
AM: Principles of pathophysiology, chapter 4. Pearson Higher
Education AU; pp. 662012
|
|
32
|
Alam H, Sehgal L, Kundu ST, Dalal SN and
Vaidya MM: Novel function of keratins 5 and 14 in proliferation and
differentiation of stratified epithelial cells. Mol Biol Cell.
22:4068–4078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eichler TE, Becknell B, Easterling RS,
Ingraham SE, Cohen DM, Schwaderer AL, Hains DS, Li B, Cohen A,
Metheny J, et al: Insulin and the phosphatidylinositol 3-kinase
signaling pathway regulate ribonuclease 7 expression in the human
urinary tract. Kidney Int. 90:568–579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Wan D, Zhou R, Zhong W, Lu S and
Chai Y: Geraniin inhibits migration and invasion of human
osteosarcoma cancer cells through regulation of PI3K/Akt and ERK1/2
signaling pathways. Anticancer Drugs. 28:959–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo LY, Li YM, Qiao L, Liu T, Du YY, Zhang
JQ, He WT, Zhao YX and He DQ: Notch2 regulates matrix
metallopeptidase 9 via PI3K/AKT signaling in human gastric
carcinoma cell MKN-45. World J Gastroenterol. 18:7262–7270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kobayashi T, Hattori S, Nagai Y and Tajima
S: Differential regulation of the secretions of matrix
metalloproteinase-9 and tissue inhibitor of metalloproteinases-1
from human keratinocytes in culture. IUBMB Life. 50:221–226. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O-charoenrat P, Modjtahedi H, Rhys-Evans
P, Court WJ, Box GM and Eccles SA: Epidermal growth factor-like
ligands differentially up-regulate matrix metalloproteinase 9 in
head and neck squamous carcinoma cells. Cancer Res. 60:1121–1128.
2000.PubMed/NCBI
|