Introduction
Colorectal cancer (CRC) is the third most common
cancer and fourth most common cause of cancer-related deaths
globally (1). Metastatic
dissemination, mainly to the liver and lungs, accounts for most
CRC-related deaths (2). The
pathological process and molecular mechanism of CRC metastasis are
complex and not fully understood. The epithelial-mesenchymal
transition (EMT), which involves the dissolution of cell-cell
adhesions, loss of apical-basal lateral polarity and reorganization
of the cytoskeleton, has been increasingly recognized to play
pivotal and complex roles in promoting carcinoma metastasis
(3,4). Major developmental signalling
pathways, including the TGF-β, Wnt, and growth factor receptor
signalling cascades, have been demonstrated to be involved in some
aspects of the EMT programme (5,6).
Similarly, the MAPK signalling pathway has been confirmed to have
an impact on EMT in a variety of tumours, such as CRC, lung cancer
and breast cancer (7–9).
Paxillin (PXN), a structural protein of 68 kDa,
contains five leucine-aspartic acid (LD) motifs in its N-terminal
end that control most of its signalling activity and four LIM
domains at its C-terminal end that mediate protein-protein
interactions (10). As a focal
adhesion protein, PXN was confirmed to play a prominent role in
regulating cytoskeletal rearrangements, tissue remodelling and cell
motility (11). PXN expression has
been revealed to be negatively correlated with CRC patient
prognosis (12,13). The phosphorylation of PXN, which is
regulated by multiple mRNAs, can promote CRC cell invasion
(14). However, the influence of
PXN on EMT and the underlying mechanism by which PXN promotes
metastasis in CRC remain unknown.
In present study, data was collected from 102
postoperative patients. Immunohistochemistry was performed to
evaluate the relationships between PXN expression and N stage, M
stage and distant site recurrence. Furthermore, differences in PXN
expression between the primary tumour and matched liver metastasis
specimens from 24 patients who underwent concurrent resection were
analysed. SW480 cells were selected to clarify the role of PXN in
cell proliferation, migration and invasion. The relationship
between PXN and EMT was explored, and the results revealed that the
extracellular signal regulated kinase (ERK) signalling pathway may
act as an intermediate between PXN and EMT.
Materials and methods
Tumour samples
The retrospective study was approved by the Peking
University First Hospital Biomedical Research Ethics Committee (No.
2018-15). CRC samples were collected from the Department of Surgery
at Peking University First Hospital between January 2010 and
December 2012. All patients related to this study signed an
informed consent agreement before surgery. The present study
enrolled the patients (including 56 males and 46 females, with a
median age of 68 years) with primary colorectal cancer diagnosed by
pathology, and excluded those with other tumours or that had
received radiotherapy and chemotherapy before surgery. The
clinicopathologic stage was determined according to TNM
classification [American Joint Commission on Cancer (AJCC) 8th
edition]. Follow-up data were collected by telephone and outpatient
interview.
Cell lines and reagents
The SW480, SW620, Caco-2, DLD-1, HCT116, and LoVo
human colon cancer cell lines were obtained from the General
Surgery Laboratory of Peking University First Hospital (Beijing,
China). These cells were routinely cultured in Dulbecco's modified
Eagle's medium (DMEM) with 10% foetal bovine serum (FBS; both from
Gibco; Thermo Fisher Scientific, Inc.) at 37°C and supplemented
with 5% CO2. hEGF (cat. no. E9644), which was used as an
ERK1/2 activator in our research (15), was purchased from Sigma-Aldrich;
Merck KGaA. SCH-772984, a selective inhibitor of Erk1/2
(hereinafter called EI), dissolved in dimethylsulfoxide (DMSO) was
purchased from Selleckchem. Both hEGF and EI were maintained at
−20°C.
Immunohistochemical staining
Paraffin-embedded tumour tissues were cut into
5-µm-thick sections. Anti-PXN antibody (1:100 dilution; product
code ab2264; Abcam) and horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG (cat. no. PV6001; ZSGB-BIO) were used as
primary and secondary antibodies, respectively. DAB staining (5 min
at room temperature; cat. no. ZLI-9018; ZSGB-BIO) was used to
visualize PXN expression, which was evaluated independently by a
pathologist and researcher and scored based on the proportion of
deposited cells with a brown granular appearance as follows:
<10%, score of 0; 11 to 25%, score of 1; 26 to 50%, score of 2;
51 to 75%, score of 3; and 76 to 100%, score of 4. The staining
intensity was assessed and scored as follows: Negative staining,
score of 0; mild positive staining, score of 1; moderate positive
staining, score of 2; and strong positive staining, score of 3. The
total score was equal to the score indicating the proportion of
positive cells multiplied by the score indicating the staining
intensity. Tumour tissues with a total score of 4 or more were
categorized as expressing high levels of PXN, while those with a
score less than 4 were categorized as expressing low levels of
PXN.
Cell transfection
We searched in BLAST (Basic Local Alignment Search
Tool; http://blast.ncbi.nlm.nih.gov/Blast.cgi) and designed
two short hairpin RNAs (shRNA), which specifically knock down the
mRNA of PXN. Plasmids expressing shRNAs specifically targeting PXN
(shPXN) and a negative control shRNA (shNC) were constructed by
Shanghai GenePharma Co., Ltd. The shPXN sequences were
5′-GGGCAGCAACCTTTCTGAACT-3′ (shPXN1) and
5′-GGAGAGTCTCTTGGATGAACT-3′ (shPXN2). Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for
transfection. Cell clones stably expressing shRNA were selected
with G418 (Geneticin; Gibco Thermo Fisher Scientific, Inc.).
Cell proliferation
Cancer cell proliferation was evaluated using the
Cell Counting Kit-8 assay (cat. no. B34304; Bimake). SW480 cells
were seeded in 96-well plates (5×103 cells/well) and
incubated for 1, 2, 3 or 4 days at 37°C. Then, CCK-8 reagent (10
µl) was added to each well. Each plate was incubated for another
1.5 h at 37°C, and the absorbance at 450 nm was measured. Each
experiment was repeated at least three times.
Colony formation assay
SW480 cells were counted and seeded in 6-well plates
at 200 cells/well. The culture medium was replaced every 3 days
over a 2-week incubation. At the time of harvesting, the medium was
removed, and colonies were stained with 0.05% crystal violet for 1
h at room temperature. Samples were allowed to dry at room
temperature, and each plate was photographed with a camera (A7M3;
Sony). Each colony was counted only if it contained more than 50
cells by using ImageJ software (v1.4.3.67; National Institutes of
Health). Each experiment was performed in triplicate.
Wound healing assay
Equivalent numbers of SW480 cells
(n=5×105) were seeded in 6-well plates, and wounds were
generated in the cells covering the plate. Each well was cultured
with serum-free DMEM and the appropriate drugs (hEGF and EI) for 48
h and imaged over time. The scratched area was captured by a light
microscope (magnification, ×40) and measured using ImageJ software
(v1.4.3.67; National Institutes of Health) by finding the edges of
the wound. Each sample was assessed in three fields as three
replicates.
Transwell assays
For the migration assay, a Transwell chamber with
8-µm pores (BD Biosciences) was used to establish a bilayer culture
model. The upper chamber was seeded with 1×105 SW480
cells in serum-free DMEM, while 600 µl of medium containing 20% FBS
was added to the lower chamber. After 24 h of incubation at 37°C,
the cells that had migrated through the filter were fixed with 100%
methanol for 20 min and stained with 0.05% crystal violet for 10
min, at room temperature. Non-migrated cells on the top surface of
the filter were removed, and migrated cells were observed with a
light microscope (magnification, ×100) and counted with ImageJ
software (v1.4.3.67; National Institutes of Health). The Transwell
invasion assay was performed in a similar manner, except that the
top chamber was pre-coated with 50 µl of Matrigel (1:8 dilution
with DMEM; Corning, Inc.). Each experiment was repeated at least
three times.
Western blot analysis
Total cellular protein and phosphorylated protein
samples were prepared from cell lysates (SW480, SW620, Caco-2,
DLD-1, HCT116 and LoVo cells) in lysis buffer (cat. no. KGP2100;
Nanjing KeyGen Biotech, Co., Ltd.). The protein determination of
samples was performed by BCA method. After the protein
concentration of each sample was adjusted, 10% sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis was performed to
separate the proteins (20 µg per lane). Subsequently, the protein
bands were transferred to polyvinylidene difluoride (PVDF)
membranes, and blocked using 5% BSA (Sigma-Aldrich) at room
temperature for 1 h. The membranes were incubated with specific
primary antibodies against PXN (product no. 2542), phosphorylated
(p)-ERK (product no. 4370), ERK (product no. 4695), vimentin
(product no. 5741), Snail (product no. 3879), N-cadherin (product
no. 13116), E-cadherin (product no. 3195) and GAPDH (product no.
5174) at 4°C overnight. All of the aforementioned primary
antibodies were purchased from CST and used at a 1:1,000 dilution.
GAPDH was used as an internal control. Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (cat. no. ZB-2301; ZSGB-BIO;
OriGene Technologies, Inc.) diluted with BSA and diluted 1:10,000
was used as a secondary antibody. The expression levels of the
target proteins were assessed via an electrochemiluminescence (ECL)
detection system (Merck KGaA) on a Syngene Gene Genius gel imaging
system (Syngene Europe).
In vivo assay
The animal experiments were approved by the
Laboratory Animal Ethics Committee of Peking University First
Hospital (No. 2015-55). Male BALB/c nude mice (n=24; weight 18–20
g) at 4–5 weeks of age were purchased from Beijing Vital River Co.,
Ltd. and maintained at SPF (specific pathogen-free) laboratory
barrier facility of the Experimental Animal Center of Peking
University First Hospital, with controlled temperature and humidity
conditions (22±1°C; 40–60%) with food and water ad libitum.
SW480 cells stably expressing shNC and shPXN (1×107
cells per mouse, n=6/group) were injected subcutaneously into the
left flanks of the nude mice to establish a tumour xenograft model.
The tumour volume was measured every 3 days and calculated as
width2 x length/2. After 6 weeks, the mice were
sacrificed by cervical dislocation, and the tumours were excised
for further study. Another group of nude mice was randomly divided
into two groups (n=6/group), and two groups of SW480 cells
transfected with shRNA (1×105 cells per mouse) were
individually injected via the tail vein to establish lung
experimental metastasis models. After 5 weeks, the mice were
sacrificed by cervical dislocation, and their major organs (lung
and liver) were collected and embedded in paraffin. Organ samples
were sliced into five continuous sheets at the maximum
cross-sectional area, embedded in paraffin and stained with
haematoxylin and eosin (H&E, at room temperature for 5 and 1
min, respectively). Metastases in all sections observed by a light
microscope (magnification, ×200) were counted and averaged.
Transmission electron microscopy
The subcutaneous tumours from the nude mice were cut
into 1×1 mm pieces within one minute after separation and fixed in
3% glutaraldehyde at 4°C for 24 h. Subsequent specimen preparation
was completed by the Department of Electron Microscopy of Peking
University First Hospital. Transmission electron microscopy images
were captured using a JEM-1230 microscope (JEOL, Ltd.). Image
acquisition and analysis were completed using DigitalMicrograph
software (v1.8.3; Gatan, Inc.) under the guidance of a pathologist
specializing in electron microscopy.
Immunofluorescence (IF) staining
An equivalent amount of SW480 cells
(n=5×105) was seeded on sterilized glass coverslips.
Briefly, after fixation with methanol for 20 min and blocking with
goat serum (1:10 dilution; cat. no. ZLI-9022; ZSGB-BIO) for 1 h at
room temperature, the coverslips were incubated with primary
antibodies against vimentin, N-cadherin and E-cadherin (1:100
dilution; product nos. 5741, 13116 and 14472, respectively; Cell
Signalling Technology) overnight at 4°C, followed by Alexa
488-conjugated goat anti-rabbit antibody and Alexa 555-conjugated
goat anti-mouse antibody (1:100 dilution; product codes A11008 and
A21422, respectively; Invitrogen; Thermo Fisher Scientific, Inc.)
for 1 h at room temperature. The nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI; ZLI-9557; ZSGB-BIO) for 30 min
at room temperature. The fluorescent expression of the target
markers and the nuclei were evaluated and imaged using a Leica
confocal laser microscope (magnification, ×400).
Statistical analysis
Statistical data analyses were performed using SPSS
24.0 statistical software (IBM Corp.). The values in this study are
reported as the mean ± standard deviation (SD) and are
representative of an average of at least three independent
experiments. Student's t-test and repeated measures analysis of
variance (ANOVA) followed by LSD or Tukey's post hoc tests were
used for statistical analyses. The χ2 test was performed
to evaluate the association between the expression level of PXN and
clinicopathological parameters. P<0.05 was used to indicate a
statistically significant difference.
Results
PXN expression is associated with
clinical pathological parameters in CRC metastasis
There were 102 patients enrolled in the present
study, including 56 males and 46 females, with a median age of 68
years. Of the patients, 16, 26, 36 and 24 patients had pathological
stage I, stage II, stage III and stage IV CRC, respectively. As
revealed in Table I, PXN expression
was significantly associated with N stage (P=0.014) and M stage
(P=0.007), but there was no significant difference depending on age
or sex (P>0.05). The proportion of patients with stage III and
stage IV CRC exhibiting high PXN expression was higher than that of
patients with stage I and stage II CRC (80 vs. 54.8%, respectively,
P=0.006). Three-year follow-up data revealed that in all patients
who underwent R0 resection, the proportion of metastatic patients
with high PXN expression was higher than that of non-metastatic
patients (88.5 vs. 60.6%, respectively, P=0.010).
 | Table I.The expression of PXN is associated
with clinicopathological parameters in CRC metastasis. |
Table I.
The expression of PXN is associated
with clinicopathological parameters in CRC metastasis.
| Clinicopathological
parameters | Total number | Low PXN expression
n (%) | High PXN expression
n (%) | P-value |
|---|
| Age (years, median,
quartiles) | 102 | 70 (66,74) | 67 (53,74) | 0.111 |
| Sex |
|
|
| 0.382 |
|
Male | 56 | 15 (26.8) | 41 (73.2) |
|
|
Female | 46 | 16 (34.8) | 30 (65.2) |
|
| N stage |
|
|
| 0.014 |
| N0 | 44 | 19 (43.2) | 25 (56.8) |
|
| N+ | 58 | 12 (20.7) | 46 (79.3) |
|
| M stage |
|
|
| 0.007 |
| M0 | 78 | 29 (37.2) | 49 (62.8) |
|
| M1 | 24 | 2 (8.3) | 22 (91.7) |
|
| TNM stage |
|
|
| 0.006 |
|
I/II | 42 | 19 (45.2) | 23 (54.8) |
|
|
III/IV | 60 | 12 (20.0) | 48 (80.0) |
|
| Recurrence of
distant sitea |
|
|
| 0.010 |
| No | 66 | 26 (39.4) | 40 (60.6) |
|
|
Yes | 26 | 3 (11.5) | 23 (88.5) |
|
Stage IV CRC patients exhibit higher
PXN expression in liver metastases than in primary tumours
Twenty-four paired specimens from patients with
stage IV CRC who underwent concurrent excision of the primary
lesion and liver metastasis were included. The PXN protein was
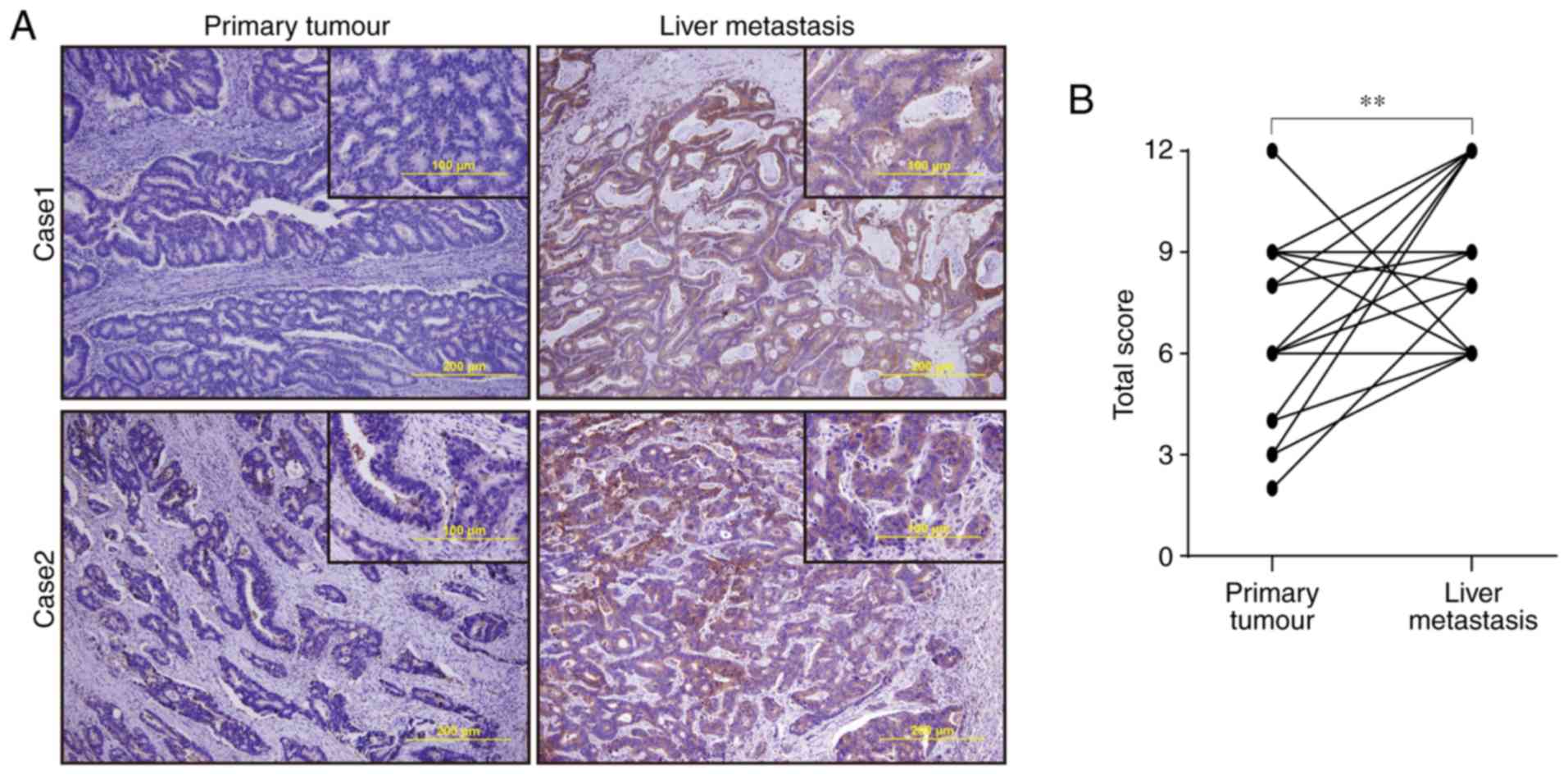
expressed mainly in the cytoplasm (Fig.
1A). The total score reflecting PXN expression was
significantly higher in liver metastases than in paired primary
lesions (9.08±2.38 vs. 6.46±2.57, respectively, P=0.002, Fig. 1B). The number of cancer-associated
fibroblasts (CAFs) in liver metastasis was counted, however, there
was no significant difference between the number of CAFs and PXN
expression (Fig. S1).
Knockdown of PXN suppresses the
proliferation, migration and invasion potential of SW480 cells
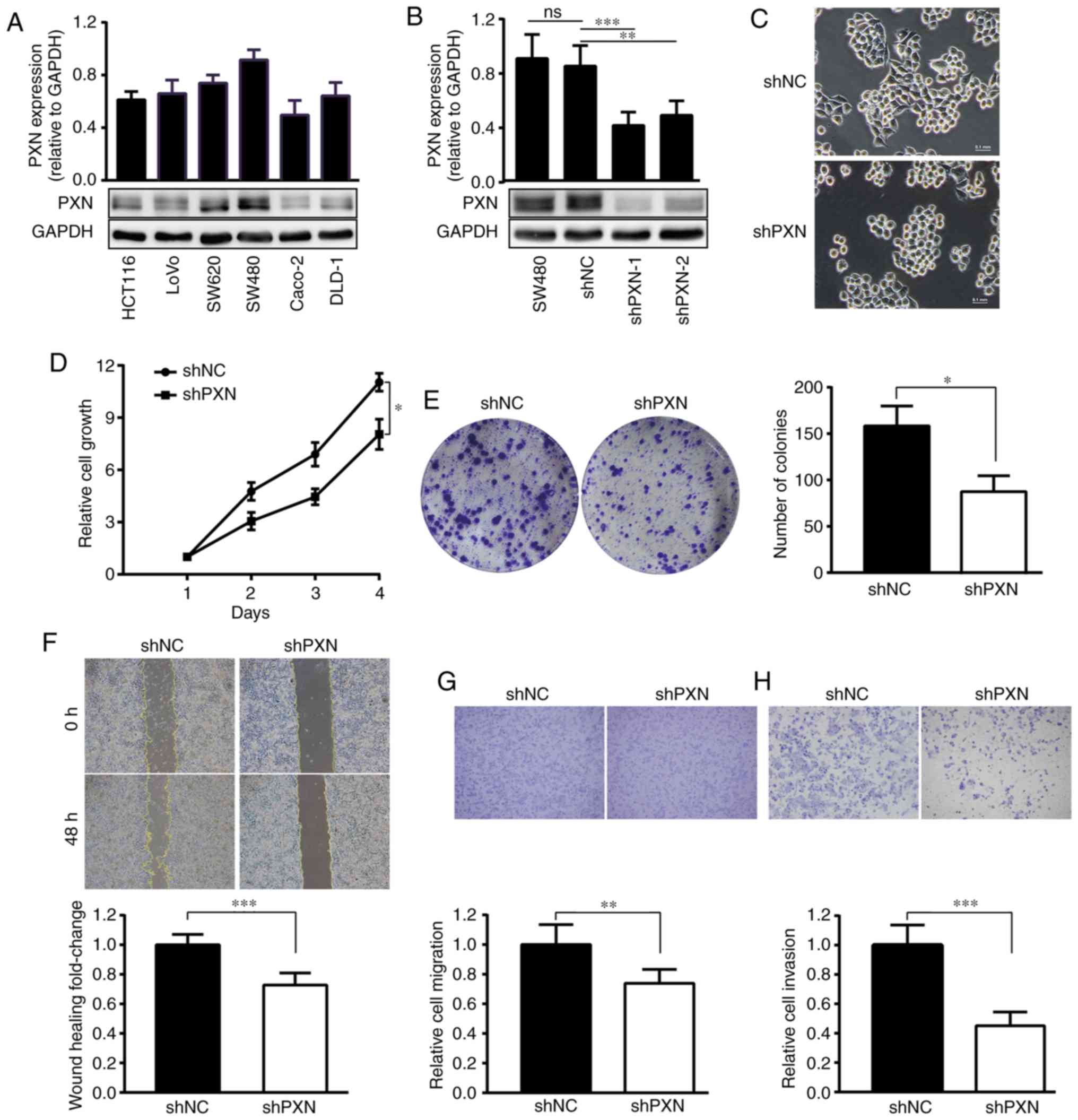
Among Caco-2, LoVo, SW480, HCT116, DLD-1 and SW620
cells, PXN was expressed at the highest level in SW480 cells, which
were used for subsequent experiments (Fig. 2A). Compared with SW480 cells
transfected with shNC, which exhibited no difference in PXN
expression compared with control SW480 cells, SW480 cells
transfected with shPXN exhibited significantly reduced PXN
expression (Fig. 2B). shPXN1 was
selected for subsequent experiments in the present study since it
more effectively suppressed PXN expression.
With PXN knockdown, cells exhibited a more rounded
appearance (Fig. 2C), and the
proportion of migrated cells decreased by approximately 30%
(Fig. 2F and G). The invasion assay
revealed that the number of cells that penetrated the Matrigel was
decreased by more than half (Fig.
2H). Decreased PXN expression also inhibited cell proliferation
(Fig. 2D) and colony formation
(n=84.67±13.53 vs. 151.67±20.59, respectively, P=0.011, Fig. 2E).
Downregulation of PXN inhibits
tumourigenesis in vivo
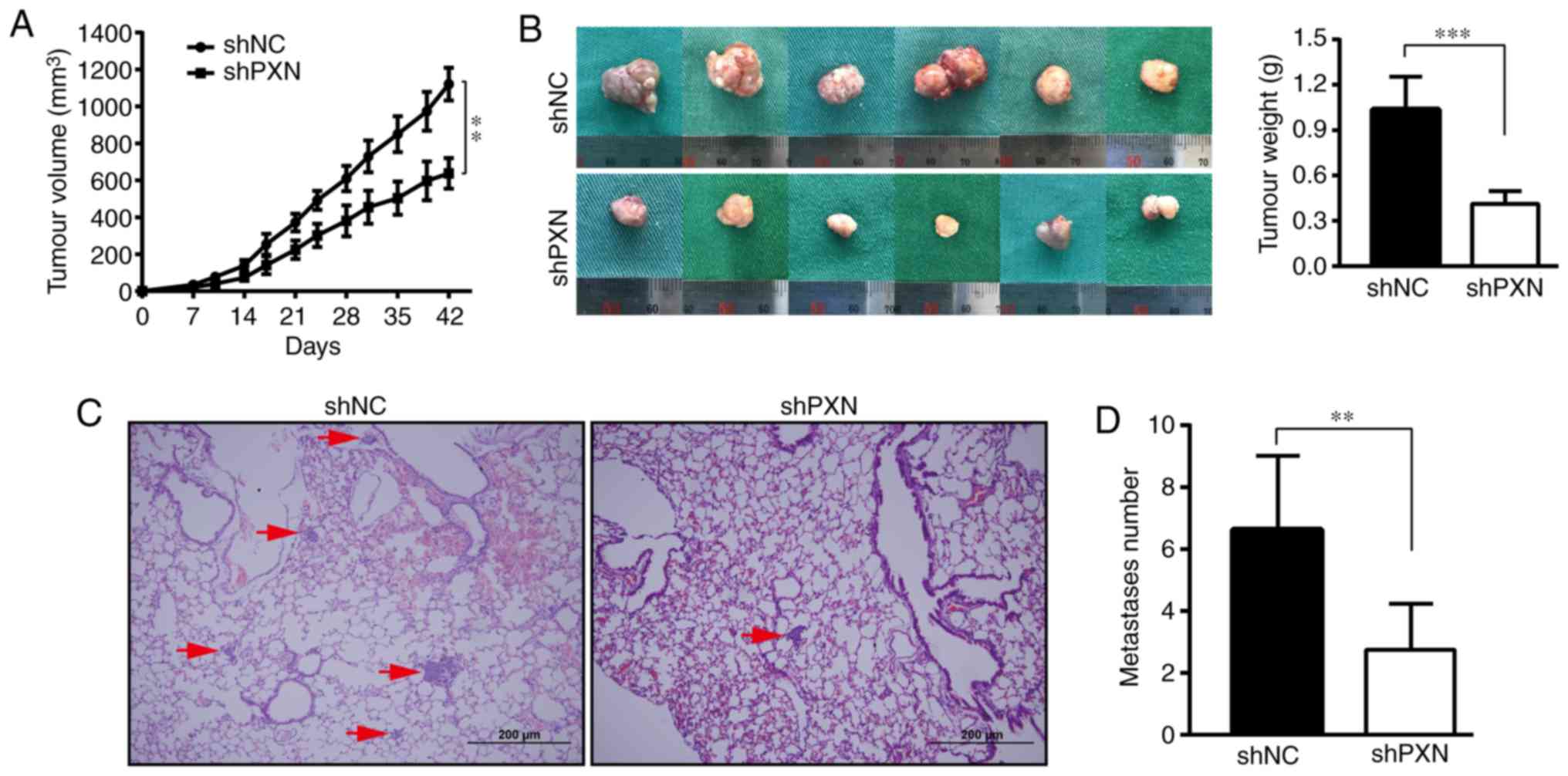
Next, the effects of PXN on tumour proliferation and
metastasis in vivo were explored. The tumour mass was
palpable on approximately the tenth day. As revealed in Fig. 3A, the mice in the shNC group
exhibited faster tumour growth. In contrast, PXN downregulation
resulted in a smaller tumour volume (637.28±82.87 vs. 1120.72±88.39
mm3) and decreased tumour weight (0.41±0.09 vs.
1.04±0.22 mg, Fig. 3B) over the
same growth period, which was in accordance with the results of the
in vitro experiments.
Tumour cells were injected via the tail vein to
observe the effect of PXN on experimental metastasis in
vivo. No metastases were found in the liver. Metastatic nodules
on the lung specimens were not macroscopic but could be clearly
observed and quantified under a microscope. The lung metastases of
the shNC group were larger and denser, and some masses appeared
next to the bronchi, while the tumour sites in the shPXN group were
mainly at the acini (Fig. 3C).
There were significantly fewer experimental metastases in the shPXN
group than in the shNC group (n=2.75±1.49 vs. 6.62±2.38,
respectively, Fig. 3D).
Knockdown of PXN reverses malignant
ultrastructural characteristics
In the shNC group, numerous microvilli, a bulging
arrangement of individual cells, formed on the cell surface, and
glandular lumens were observed between cells in partial microscopic
fields (Fig. 4A-a). These
microvilli were rarely found on the surface of shPXN-transfected
cells, leading to a smooth cytomembrane (Fig. 4A-b). Cell-cell contacts and junction
structures are presented at a higher magnification. The connections
between shNC-transfected cells, which were maintained mainly by
desmosomes and tight connections, were no longer tight (Fig. 4A-c). In contrast, the
shPXN-transfected cells were attached and closer, and their
junction structures were clearer (Fig.
4A-d). As illustrated in Fig.
4B, PXN knockdown induced an increase in the number of
desmosomes (n=3.20±0.39 vs. 1.90±0.23, respectively, P=0.01).
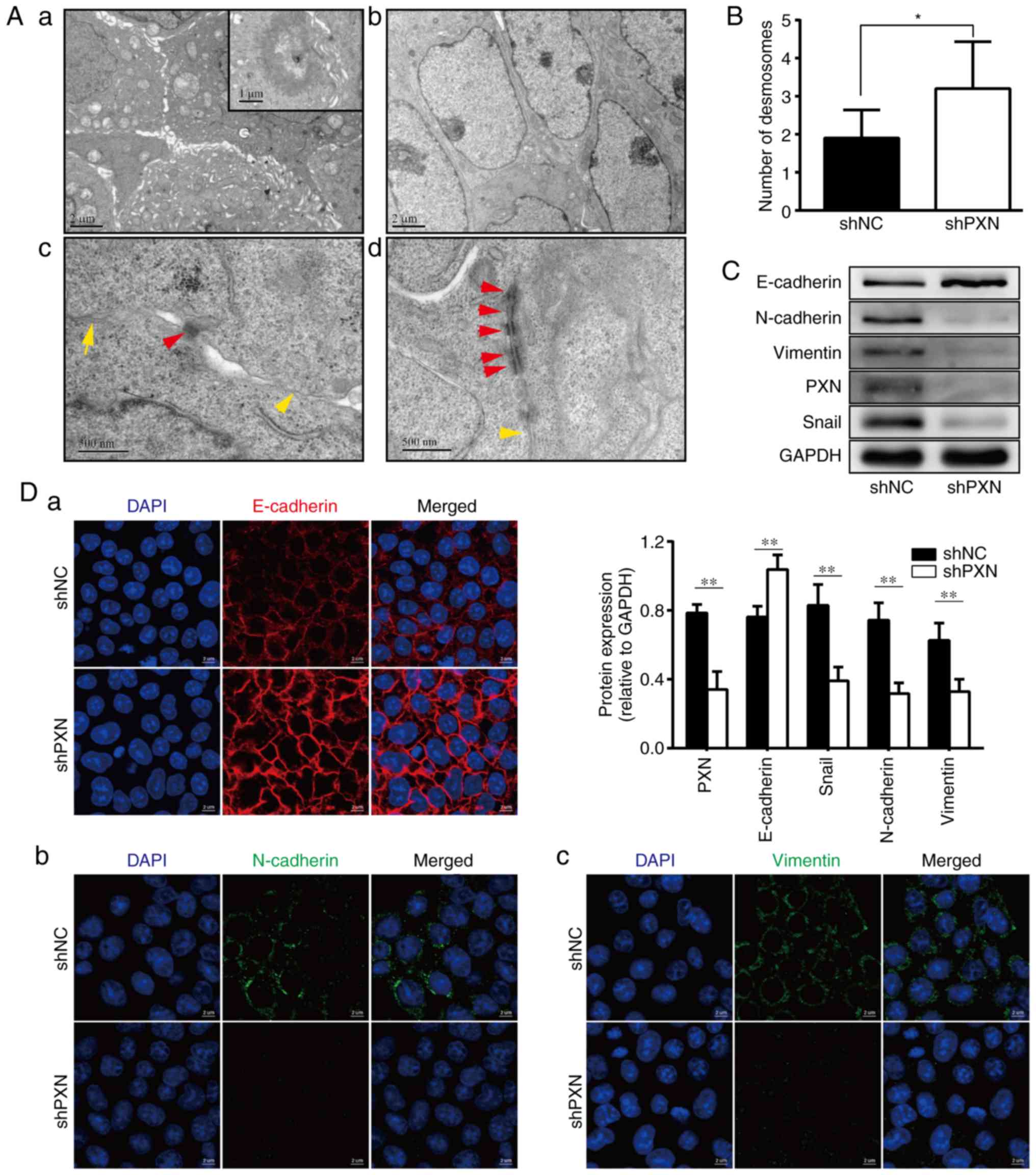 | Figure 4.Knockdown of PXN reverses some
malignant ultrastructural characteristics and regulates the
expression of EMT markers in SW480 cells. (A) The cell
ultrastructure was observed by TEM. The number of microvilli was
sharply decreased in (b) the shPXN group compared with (a) the shNC
group (original magnification, ×10,000). The enlarged figure in (a)
shows a glandular structure (original magnification, ×20,000). (c
and d) The junctions between tumours (red triangle, desmosome;
yellow triangle, tight junction; original magnification, ×60,000).
(B) Desmosomes in ten random images were counted. The data are
presented as the mean ± SD. (C) The knockdown of PXN increased
E-cadherin expression and decreased N-cadherin, vimentin and Snail
expression. The expression of the aforementioned markers was
detected by western blot analysis; the histogram represents the
relative protein expression. (D) The immunofluorescence staining
revealed that knockdown of PXN upregulated the expression of (a,
red) E-cadherin and downregulated the expression of (b, green)
N-cadherin and (c, green) vimentin. Nuclear staining with DAPI
(blue). P-values were obtained by Student's t-test. *P<0.05,
**P<0.01. PXN, paxillin; EMT, epithelial-mesenchymal transition;
DAPI, 4′,6-diamidino-2-phenylindole. |
PXN regulates the expression of EMT
markers
Taking into consideration the change in cell
migration ability, morphology and ultrastructural characteristics
induced by the downregulation of PXN, it was hypothesized that PXN
plays an important role in EMT progression and evaluated the
expression of EMT markers using western blotting (WB) and IF. As
revealed in Fig. 4C, PXN knockdown
decreased the expression of N-cadherin and vimentin, two classic
mesenchymal markers, and increased the expression of E-cadherin. In
addition, the expression of Snail, a transcription factor that
suppresses E-cadherin, was decreased following PXN knockdown. The
IF images of E-cadherin, N-cadherin and vimentin (Fig. 4D) exhibited a similar expression
change as that observed in the WB experiments. Both types of cells
exhibited positive membranous and cytoplasmic immunoreactivity for
E-cadherin. Notably, knockdown of PXN resulted in increased and
more continuous expression of E-cadherin on the cytomembrane,
followed by a tighter cell connection, which was consistent with
the observation in TEM images.
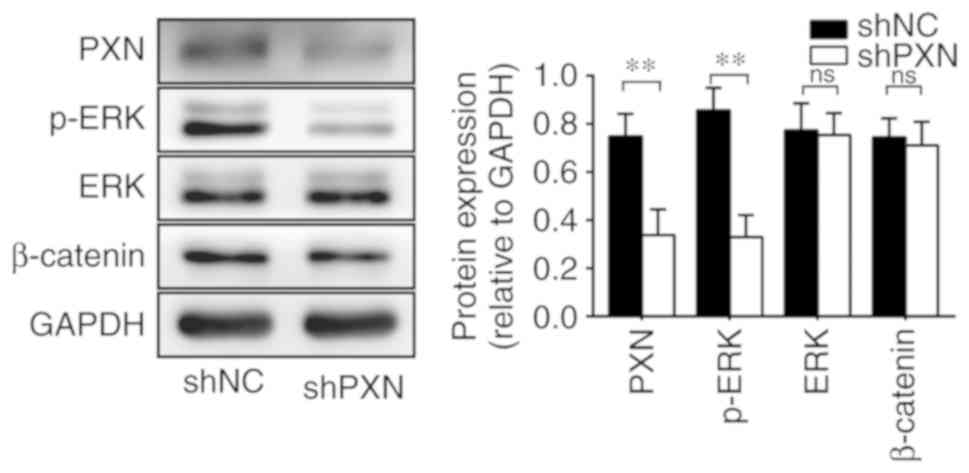
PXN regulates the ERK signalling
pathway
To further explore how PXN regulates EMT, its
effects were verified on two classic pathways, the ERK and
β-catenin signalling pathways. As revealed in Fig. 5, PXN downregulation decreased the
expression of p-ERK, whereas total ERK1/2 levels remained
unchanged, indicating the loss of ERK activation. In contrast, the
knockdown of PXN did not change the expression of β-catenin. These
results demonstrated that PXN silencing could decrease the activity
of the downstream ERK signalling pathway.
Knockdown of PXN inhibits EMT through
the ERK signalling pathway
To verify the hypothesis that PXN regulates EMT
through the ERK signalling pathway, two drugs were applied to
activate (hEGF) or inhibit (EI; SCH-772984) the ERK signalling
pathway and validated their effects in cell experiments.
The viabilities of shNC and shPXN cells under drug
treatments in the serum-free state at 0, 24 and 48 h were assessed.
There was no significant difference in proliferation and viability
of either cells under different treatments (F=0.732, P=0.601,
Fig. S2), which indicated that
within 48 h, tumour proliferation had little effect on the results
of migration and invasion assays. Then, we measured changes in the
migration and invasion abilities of SW480 cells. First, similar to
the aforementioned results (Fig.
2F-H), the downregulation of PXN effectively attenuated tumour
migration and invasion. Second, activation of the ERK signalling
pathway by hEGF increased the migration and invasion abilities of
both groups. In contrast, the migration and invasion abilities of
both groups were decreased following treatment with EI. Finally,
although migration and invasion abilities were reduced by PXN
knockdown, they were restored by ERK activation and further
attenuated by combination treatment with EI. Consistent results
were obtained in wound healing (Fig.
6A-a), migration (Fig. 6A-b)
and invasion (Fig. 6A-c) assays,
despite differences in the proportions of each group of cells in
the three experiments (Fig. 6B
a-c).
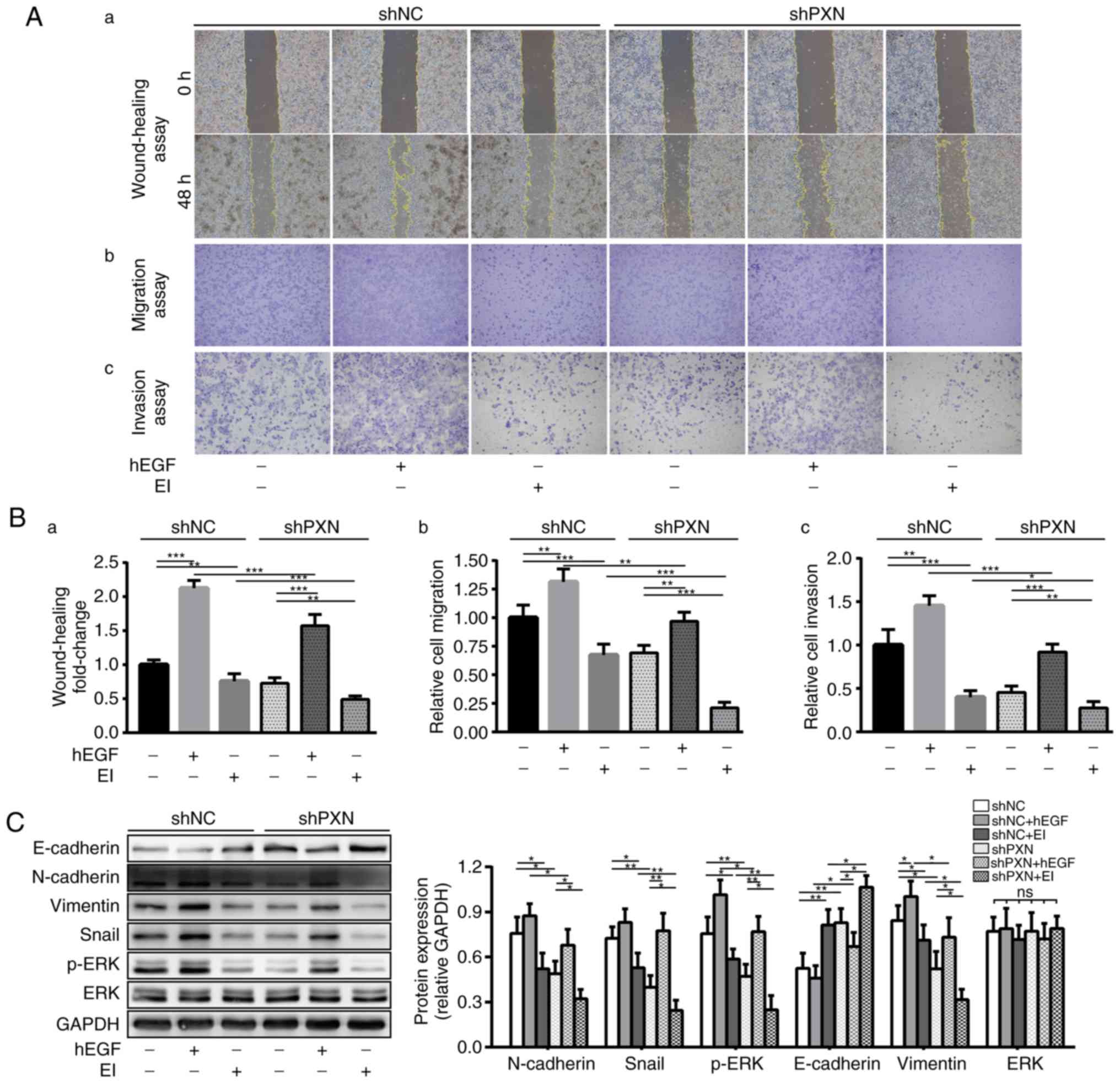 | Figure 6.Knockdown of PXN suppresses the
migration and invasion abilities of SW480 cells by inhibiting EMT
through the ERK signalling pathway. SW480 cells transfected with
shNC and shPXN were treated with an ERK activator (hEGF, 0.6 µg/ml)
or inhibitor (SCH-772984; EI, 0.5 µg/ml). (A) The migration and
invasion abilities of cells were detected using (a) wound healing,
Transwell (b) migration and (c) invasion assays and are
respectively illustrated by (B, a-c) histograms. The histograms
represent the wound width change and the number of migrated and
invaded cells relative to the shNC group. (C) Activation of the ERK
signalling pathways promoted PXN-mediated EMT. In contrast,
inhibition of the ERK signalling pathways further suppressed the
EMT process. The expression levels of p-ERK, ERK and EMT markers
were analysed by western blot assay. The data are presented as the
mean ± SD of at least three independent experiments. The histograms
of the WB experiments represent the relative protein expression.
P-values were obtained using Student's t-test. *P<0.05,
**P<0.01, ***P<0.001. PXN, paxillin; EMT,
epithelial-mesenchymal transition; ERK, extracellular regulated
protein kinase; p-, phosphorylated; EI, ERK inhibitor; ns, no
significance. |
Then, the expression levels of ERK and EMT markers
were investigated. Previous experimental results confirmed that the
downregulation of PXN decreased p-ERK levels and the EMT process.
These experiments were repeated to make the entire study more
rigorous and confirm the previous conclusions. As revealed in
Fig. 6C, in the shNC group, the
expression levels of N-cadherin, vimentin and Snail decreased with
the decrease in p-ERK due to the application of EI; however,
E-cadherin expression exhibited an increasing trend. In the shPXN
group, the expression levels of mesenchymal markers that had
already been reduced due to PXN knockdown were increased by the
effect of the ERK activator, accompanied by a decreased expression
of E-cadherin, and EI further suppressed the expression of these
three mesenchymal markers. Notably, changes in the expression of
these three markers were consistent with changes in only p-ERK
expression, as the total ERK levels remained unchanged.
Collectively, these data indicated that the
knockdown of PXN inhibited EMT through downregulation of the ERK
signalling pathway.
Discussion
Approximately 20% of patients with CRC present with
distant metastasis at the time of diagnosis, and approximately 40%
of patients with localized disease develop metastases within 5
years after surgery (1,16). Metastasis is a multi-step process,
and its first step requires the local invasion of tumour cells
(3). PXN, a multi-domain focal
adhesion adaptor protein, contributes to recruiting structural and
signalling molecules involved in cell movement and invasion
(11).
By analysing clinical data, it was confirmed that
PXN expression was positively associated to the clinicopathological
parameters of metastasis. Moreover, patients who developed distant
site recurrence after radical surgery were more likely to express
high levels of PXN. A previous study reported that PXN expression
in lymph node metastases was more intense than that in primary
adenocarcinoma (17). Similarly,
Ikuta et al (18) confirmed
the significance of CAF expression in metastatic lymph nodes for a
poor prognosis, while the level of genes related to the PXN
signalling pathways was higher in CAFs than in normal fibroblasts
(19), which may be a potential
mechanism by which PXN promotes metastasis. In the present study,
it was further revealed that PXN expression was higher in liver
metastasis compared with the paired primary tumour. However, in a
recent study (20), the expression
of phosphorylated PXN was decreased in liver metastasis compared
with the matched paired primary cancer and correlated with poor
histological grade in CRC patients. This finding may be because the
phosphorylation status of several protein kinases is different in
primary and metastatic matched lesions, due to the activation of
various signalling pathways (21).
All of the aforementioned results indicated that PXN plays an
important and complex role in CRC metastasis.
The in vitro and in vivo experiments
in the present study demonstrated that the knockdown of PXN
inhibited cell migration and invasion. PXN, a downstream binding
partner of FAK/Src, performs critical functions in coordinating the
ligation of integrin and the extracellular matrix (ECM) (22). The phosphorylation of PXN alters the
organization of focal adhesions and increases cell motility
(23). The significant effect of
PXN on metastasis was directly reflected and evidenced in the
ultrastructural changes observed with PXN knockdown. The appearance
of numerous microvilli on the surface of tumour cells is
characteristic of active cells in general (24). More microvilli form in highly
metastatic colon carcinoma cells than in weakly metastatic ones,
indicating that the presence of an increased number of microvilli
closely correlates with the metastatic ability of CRC cells
(25). PXN regulates cell polarity
and the cytoskeleton through binding to the regulators and
effectors of Rho GTPases (26). By
inhibiting Rho signalling, ERK1/2 signalling pathway activation
leads to malignant transformations, such as the transition of
ruffles into active microvilli (27). The present results indicated that
the knockdown of PXN decreased the number of microvilli and
deactivated Erk1/2, accompanied by a decrease in migration and
invasion abilities. In addition, the downregulation of PXN
increased the number of desmosomes and led to clearer junction
structures. Desmosomes, a type of intercellular junction, enhance
adhesion between epithelial cells, and a decreased number of
desmosomes indicates the promotion of cell invasion (28). When the normal epithelium is
transformed to a malignant epithelium, desmosomes become unstable
and easily disrupted, facilitating cell separation (29). We did not further explore how PXN
affects desmosomes; however, the change in the number of desmosomes
is important evidence that PXN promotes cell dissemination.
Previous studies have reported that PXN plays
different roles in EMT in diverse tissues and tumours. PXN δ, which
lacks the key phosphorylation sites of PXN at Y31 and Y118, was
revealed to downregulate TGF-β-induced EMT in normal murine mammary
epithelial cells (30).
Upregulation of the ERK-FAK-PXN axis led to the induction of type 2
EMT-like changes during corneal epithelial wound healing (31). In trabecular meshwork cells, the
knockdown of PXN blocked EMT-like alterations in cellular
characteristics (32). Although the
depletion of PXN in breast cancer cells led to an elongated
morphology, it decreased the ability of cells to undergo
trans-endothelial migration (33).
In the present study, inhibiting the expression of PXN increased
the expression of E-Cadherin and enhanced the adherens junction,
suggesting that the knockdown of PXN suppressed EMT progression. In
addition, as an adaptor protein in the integrin signalling pathway,
PXN has been revealed to provide a necessary link in the
FAK-dependent survival pathway and contribute to anoikis resistance
(34), which promotes EMT and
tumour metastasis (35).
In the present study, the Wnt and ERK signalling
pathways were identified as responsible for the underlying
mechanisms by which PXN regulated EMT. The expression of β-catenin,
a typical marker of the Wnt pathway that regulates Snail (36), was not altered with PXN knockdown;
however, p-ERK expression displayed a decreasing trend. Other
proteins that regulate EMT processes via the ERK signalling pathway
in CRC have been previously reported (8,37). In
addition, several studies have demonstrated that PXN mutation led
to a reduction in cell motility and survival mediated by ERK1/2
(38), and PXN regulated cell
proliferation by modulating ERK signalling and its subsequent
downstream effects (39). ERK has
been revealed to be associated with PXN phosphorylation and mediate
epithelial morphogenesis, indicating the important role of the
ERK-PXN association in cell adhesion and migration. However, the
authors did not verify EMT (40).
Based on these findings, it was hypothesized that the ERK
signalling pathway plays an important role in the mechanism by
which PXN regulates EMT. Following PXN downregulation, ERK was
inactivated to a degree consistent with the decreased expression of
EMT markers. This finding indicated that reactivation of the ERK
pathway restored EMT and that PXN knockdown combined with an ERK
inhibitor further blocked the ERK pathway. Changes to cellular
migration and invasion were also in accordance with the activation
status of ERK. However, changes in the expression of EMT markers
were not completely consistent with changes in cell migration and
invasion, which may be attributed to the fact that PXN is one of
the various regulators of the ERK signalling pathway (23,40).
Furthermore, there were several limitations in present study, such
as the effect of PXN on the specific transcription mechanism of EMT
and the tumour microenvironment during CRC metastasis, which
require further exploration in a following study.
In addition, published studies have indicated that
PXN is not only associated with the microtubule network and the
polarization and motility of lymphoid cells (41,42),
but is also involved in regulating the migration of
antigen-presenting cells, such as macrophages, neutrophils and
dendritic cells (43–45). The knockdown of PXN suppressed the
invasion of colon cancer cell by inhibiting M2 macrophage
polarization (46), which indicates
that PXN could regulate tumour metastasis through immune cells.
In summary, the present findings indicated that the
knockdown of PXN inhibited EMT through the ERK signalling pathway,
thereby reducing the ability of CRC to invade and metastasize. PXN
knockdown may represent a future therapeutic strategy to prevent
the EMT-associated progression and invasion of CRC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Ding-Fang
Bu for technical assistance with the WB assay, Mrs. Xu Zhang for
technical guidance with TEM, and Mrs. Yuanyuan Ma for animal study
assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this article.
Authors' contributions
JZha and XW designed this study. LW and JZhu
completed the experiments. LW and XZ wrote the manuscript. JW, JH
and TY performed sample collection. XZ, YM, TW and SC analysed the
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by Peking University
First Hospital Biomedical Research Ethics Committee (no. 2018-15).
All patients related to this study signed an informed consent
agreement before surgery. The animal experiments were approved by
the Laboratory Animal Ethics Committee of Peking University First
Hospital (no. 2015-55).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester R, Barzi A and Jemal A: Colorectal cancer statistics, 2017.
CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao H, Xu E, Liu H, Wan L and Lai M:
Epithelial-mesenchymal transition in colorectal cancer metastasis:
A system review. Pathol Res Pract. 211:557–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizukoshi K, Okazawa Y, Haeno H, Koyama Y,
Sulidan K, Komiyama H, Saeki H, Ohtsuji N, Ito Y, Kojima Y, et al:
Metastatic seeding of human colon cancer cell clusters expressing
the hybrid epithelial/mesenchymal state. Int J Cancer.
146:2547–2562. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lampropoulos P, Zizi-Sermpetzoglou A,
Rizos S, Kostakis A, Nikiteas N and Papavassiliou AG: TGF-beta
signalling in colon carcinogenesis. Cancer Lett. 314:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li T, Zhu J, Wang X, Chen G, Sun L, Zuo S,
Zhang J, Chen S, Ma J, Yao Z, et al: Long non-coding RNA lncTCF7
activates the Wnt/β-catenin pathway to promote metastasis and
invasion in colorectal cancer. Oncol Lett. 14:7384–7390.
2017.PubMed/NCBI
|
|
7
|
Hong SK, Park JR, Kwon OS, Kim KT, Bae GY
and Cha HJ: Induction of integrin β3 by sustained ERK activity
promotes the invasiveness of TGFβ-induced mesenchymal tumor cells.
Cancer Lett. 376:339–346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanahashi T, Osada S, Yamada A, Kato J,
Yawata K, Mori R, Imai H, Sasaki Y, Saito S, Tanaka Y, et al:
Extracellular signal-regulated kinase and Akt activation play a
critical role in the process of hepatocyte growth factor-induced
epithelial-mesenchymal transition. Int J Oncol. 42:556–564. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith BN, Burton LJ, Henderson V, Randle
DD, Morton DJ, Smith BA, Taliaferro-Smith L, Nagappan P, Yates C,
Zayzafoon M, et al: Snail promotes epithelial mesenchymal
transition in breast cancer cells in part via activation of nuclear
ERK2. PLoS One. 9:e1049872014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deakin NO and Turner CE: Paxillin comes of
age. J Cell Sci. 121:2435–2444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
López-ColoméLee-Rivera I,
Benavides-Hidalgo R and López E: Paxillin: A crossroad in
pathological cell migration. J Hematol Oncol. 10:502017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao CJ, Du SK, Dang XB and Gong M:
Expression of paxillin is correlated with clinical prognosis in
colorectal cancer patients. Med Sci Monit. 21:1989–1995. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du C, Wang X, Zhang J, Liu X, Zhu J and
Liu Y: Paxillin is positively correlated with the
clinicopathological factors of colorectal cancer, and knockdown of
Paxillin improves sensitivity to cetuximab in colorectal cancer
cells. Oncol Rep. 35:409–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen DL, Wang DS, Wu WJ, Zeng ZL, Luo HY,
Qiu MZ, Ren C, Zhang DS, Wang ZQ, Wang FH, et al: Overexpression of
paxillin induced by miR-137 suppression promotes tumor progression
and metastasis in colorectal cancer. Carcinogenesis. 34:803–811.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joo D, Woo JS, Cho KH, Han SH, Min TS,
Yang DC and Yun CH: Biphasic activation of extracellular
signal-regulated kinase (ERK) 1/2 in epidermal growth factor
(EGF)-stimulated SW480 colorectal cancer cells. BMB Rep.
49:220–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi E: The regulation of epithelial
mesenchymal transition in ocular disorders. Nippon Ganka Gakkai
Zasshi. 120:783–790. 2016.(In Japanese). PubMed/NCBI
|
|
17
|
Yang HJ, Chen JZ, Zhang WL and Ding YQ:
Focal adhesion plaque associated cytoskeletons are involved in the
invasion and metastasis of human colorectal carcinoma. Cancer
Invest. 28:127–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ikuta D, Miyake T, Shimizu T, Sonoda H,
Mukaisho KI, Tokuda A, Ueki T, Sugihara H and Tani M: Fibrosis in
metastatic lymph nodes is clinically correlated to poor prognosis
in colorectal cancer. Oncotarget. 9:29574–29586. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi H, Sakakura K, Kawabata-Iwakawa
R, Rokudai S, Toyoda M, Nishiyama M and Chikamatsu K:
Immunosuppressive activity of cancer-associated fibroblasts in head
and neck squamous cell carcinoma. Cancer Immunol Immunother.
64:1407–1417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mantonakis E, Giaginis C, Pikoulis E,
Felekouras E, Patsouris E, Liakakos T, Papalampros E and Theocharis
S: FAK, Src and p-Paxillin expression is decreased in liver
metastasis of colorectal carcinoma patients. J BUON. 22:1097–1106.
2017.PubMed/NCBI
|
|
21
|
Belluco C, Mammano E, Petricoin E,
Prevedello L, Calvert V, Liotta L, Nitti D and Lise M: Kinase
substrate protein microarray analysis of human colon cancer and
hepatic metastasis. Clin Chim Acta. 357:180–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deakin NO, Pignatelli J and Turner CE:
Diverse roles for the paxillin family of proteins in cancer. Genes
Cancer. 3:362–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Devreotes P and Horwitz AR: Signaling
networks that regulate cell migration. Cold Spring Harb Perspect
Biol. 7:a0059592015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng Y, Lu Z, Yu S, Zhang Q, Ma Y and Chen
J: Ezrin promotes invasion and metastasis of pancreatic cancer
cells. J Transl Med. 8:612010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren J, Hamada J, Okada F, Takeichi N,
Morikawa K, Hosokawa M and Kobayashi H: Correlation between the
presence of microvilli and the growth or metastatic potential of
tumor cells. Jpn J Cancer Res. 81:920–926. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hodge RG and Ridley AJ: Regulating Rho
GTPases and their regulators. Nat Rev Mol Cell Biol. 17:496–510.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barros JC and Marshall CJ: Activation of
either ERK1/2 or ERK5 MAP kinase pathways can lead to disruption of
the actin cytoskeleton. J Cell Sci. 118:1663–1671. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alroy J, Pauli BU and Weinstein RS:
Correlation between numbers of desmosomes and the aggressiveness of
transitional cell carcinoma in human urinary bladder. Cancer.
47:104–112. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Collins JE, Taylor I and Garrod DR: A
study of desmosomes in colorectal carcinoma. Br J Cancer.
62:796–805. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tumbarello DA, Brown MC, Hetey SE and
Turner CE: Regulation of paxillin family members during
epithelial-mesenchymal transformation: A putative role for paxillin
delta. J Cell Sci. 118:4849–4863. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kowtharapu BS, Murin R, Jünemann AGM and
Stachs O: Role of corneal stromal cells on epithelial cell function
during wound healing. Int J Mol Sci. 19:4642018. View Article : Google Scholar
|
|
32
|
Takahashi E, Inoue T, Fujimoto T, Kojima S
and Tanihara H: Epithelial mesenchymal transition-like phenomenon
in trabecular meshwork cells. Exp Eye Res. 118:72–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deakin NO and Turner CE: Distinct roles
for paxillin and Hic-5 in regulating breast cancer cell morphology,
invasion, and metastasis. Mol Biol Cell. 22:327–341. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zouq NK, Keeble JA, Lindsay J, Valentijn
AJ, Zhang L, Mills D, Turner CE, Streuli CH and Gilmore AP: FAK
engages multiple pathways to maintain survival of fibroblasts and
epithelia: Differential roles for paxillin and p130Cas. J Cell Sci.
122:357–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simpson CD, Anyiwe K and Schimmer AD:
Anoikis resistance and tumor metastasis. Cancer Lett. 272:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stemmer V, de Craene B, Berx G and Behrens
J: Snail promotes Wnt target gene expression and interacts with
beta-catenin. Oncogene. 27:5075–5080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ha GH, Park JS and Breuer EK: TACC3
promotes epithelial-mesenchymal transition (EMT) through the
activation of PI3K/Akt and ERK signaling pathways. Cancer Lett.
332:63–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Subauste MC, Pertz O, Adamson ED, Turner
CE, Junger S and Hahn KM: Vinculin modulation of paxillin-FAK
interactions regulates ERK to control survival and motility. J Cell
Biol. 165:371–381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sen A, De Castro I, Defranco DB, Deng FM,
Melamed J, Kapur P, Raj GV, Rossi R and Hammes SR: Paxillin
mediates extranuclear and intranuclear signaling in prostate cancer
proliferation. J Clin Invest. 122:2469–2481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishibe S, Joly D, Zhu X and Cantley LG:
Phosphorylation-dependent paxillin-ERK association mediates
hepatocyte growth factor-stimulated epithelial morphogenesis. Mol
Cell. 12:1275–1285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Herreros L, Rodríguez-Fernandez JL, Brown
MC, Alonso-Lebrero JL, Cabañas C, Sánchez-Madrid F, Longo N, Turner
CE and Sanchez-Mateos P: Paxillin localizes to the lymphocyte
microtubule organizing center and associates with the microtubule
cytoskeleton. J Biol Chem. 275:26436–26440. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Romanova LY, Hashimoto S, Chay KO,
Blagosklonny MV, Sabe H and Mushinski JF: Phosphorylation of
paxillin tyrosines 31 and 118 controls polarization and motility of
lymphoid cells and is PMA-sensitive. J Cell Sci. 117:3759–3768.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rose DM, Han J and Ginsberg MH: Alpha4
integrins and the immune response. Immunol Rev. 186:118–124. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rhee I, Zhong MC, Reizis B, Cheong C and
Veillette A: Control of dendritic cell migration, T cell-dependent
immunity, and autoimmunity by protein tyrosine phosphatase PTPN12
expressed in dendritic cells. Mol Cell Biol. 34:888–899. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Parsons SA, Sharma R, Roccamatisi DL,
Zhang H, Petri B, Kubes P, Colarusso P and Patel KD: Endothelial
paxillin and focal adhesion kinase (FAK) play a critical role in
neutrophil transmigration. Eur J Immunol. 42:436–446. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang LL, Zhang LF and Shi YB:
Down-regulated paxillin suppresses cell proliferation and invasion
by inhibiting M2 macrophage polarization in colon cancer. Biol
Chem. 399:1285–1295. 2018. View Article : Google Scholar : PubMed/NCBI
|