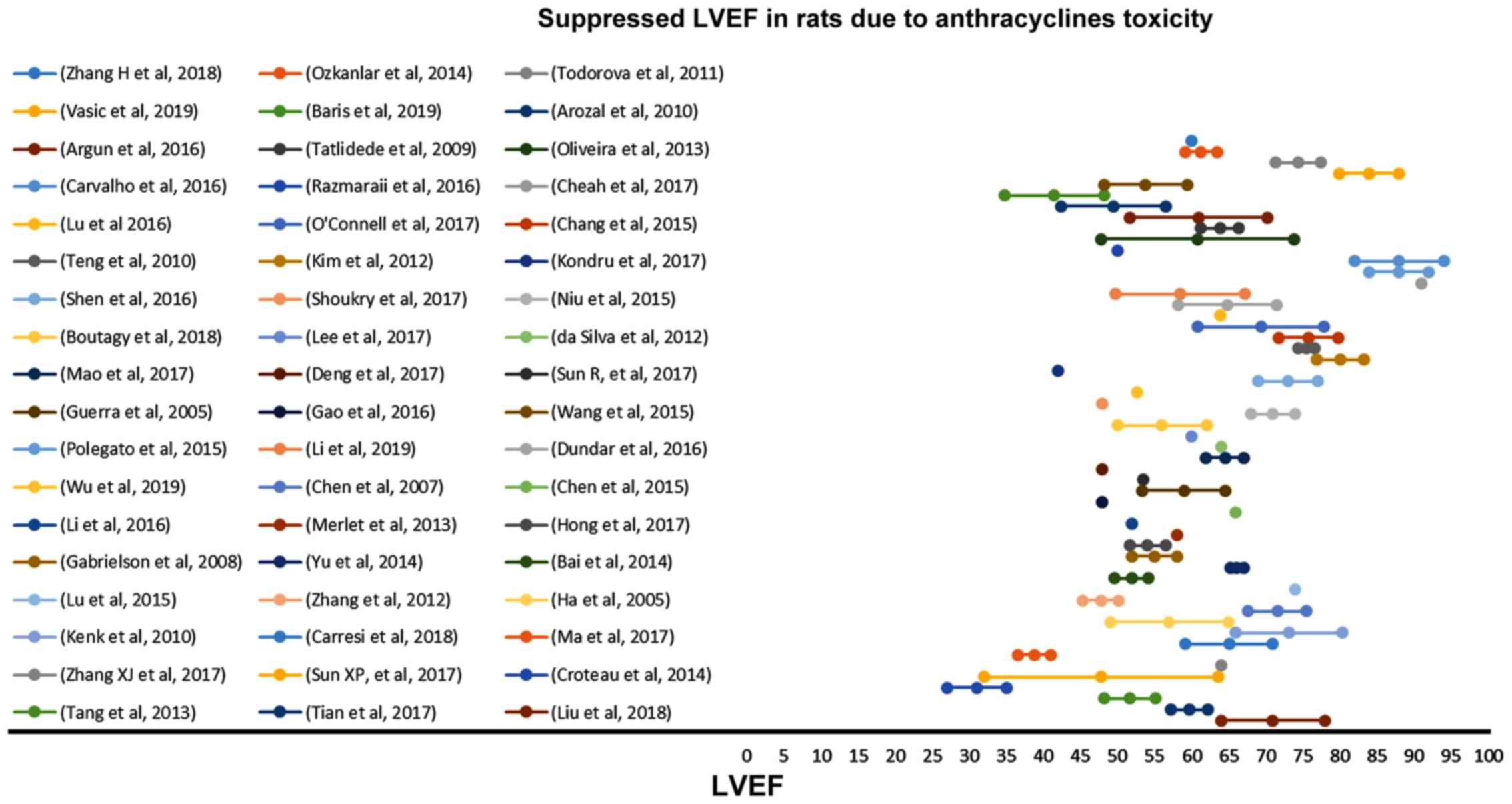
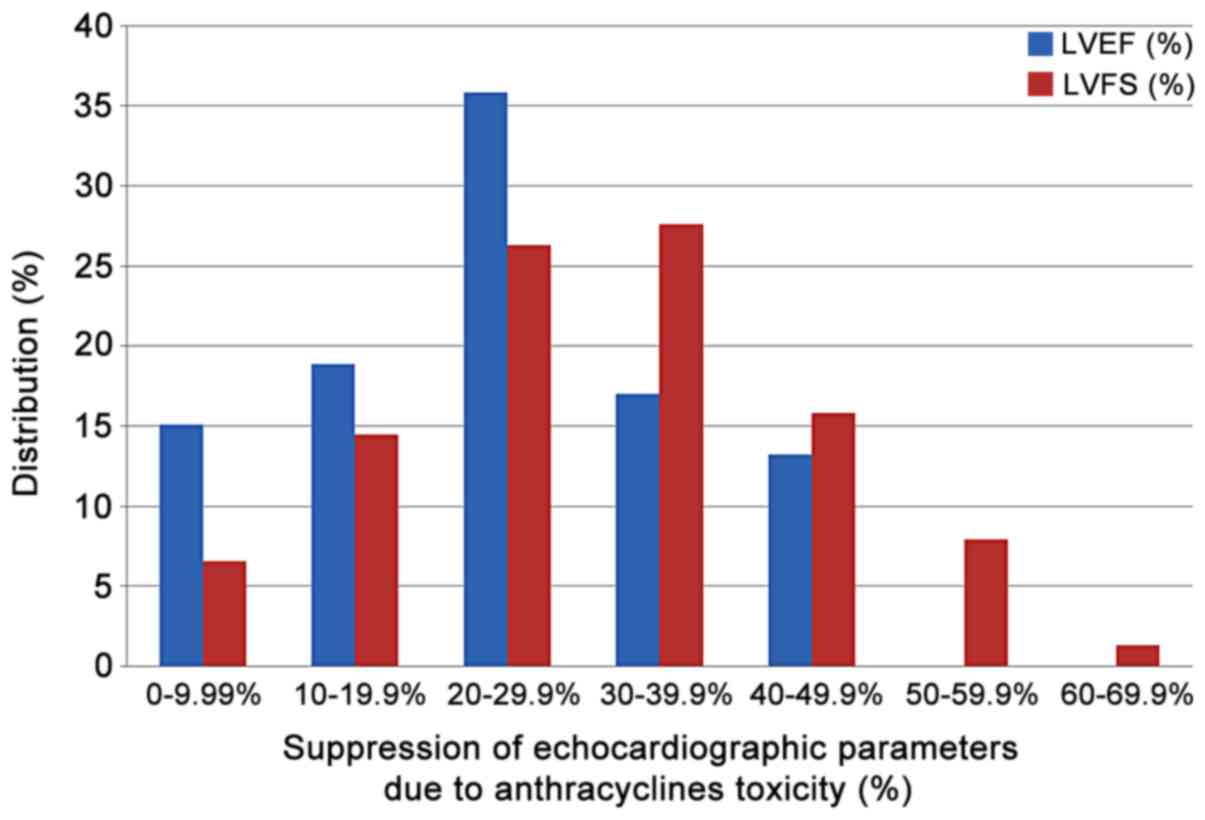
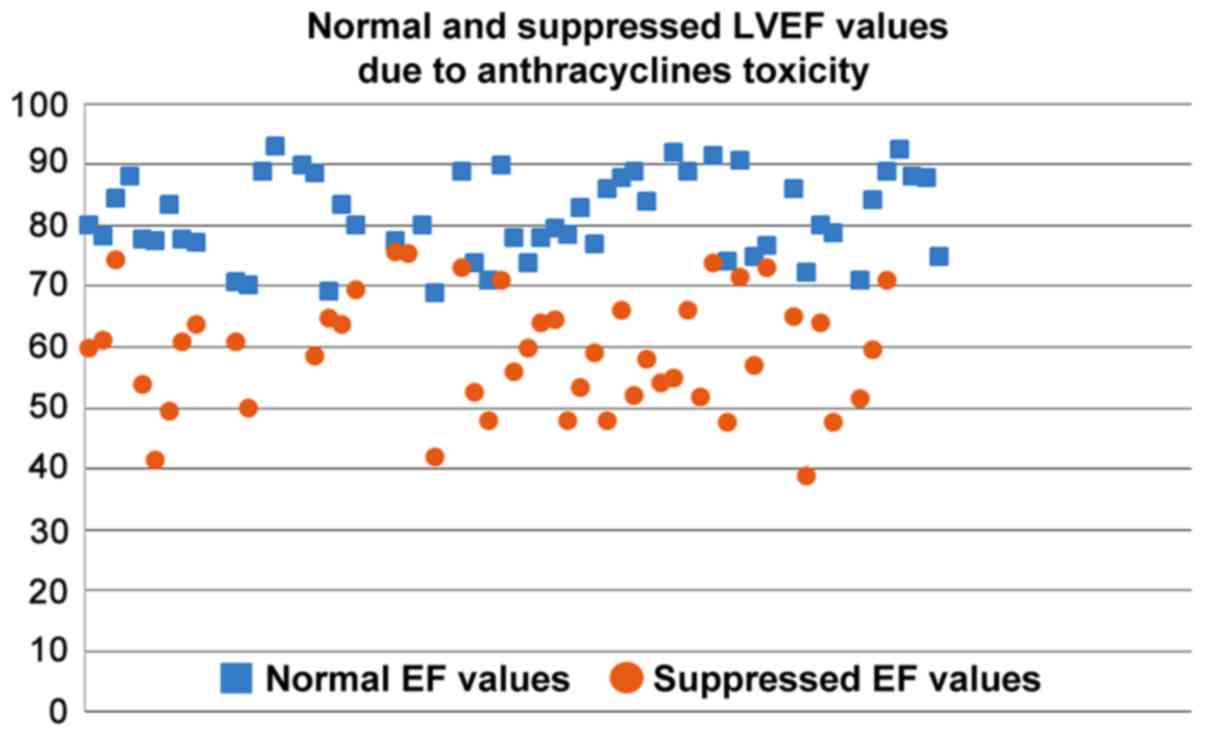
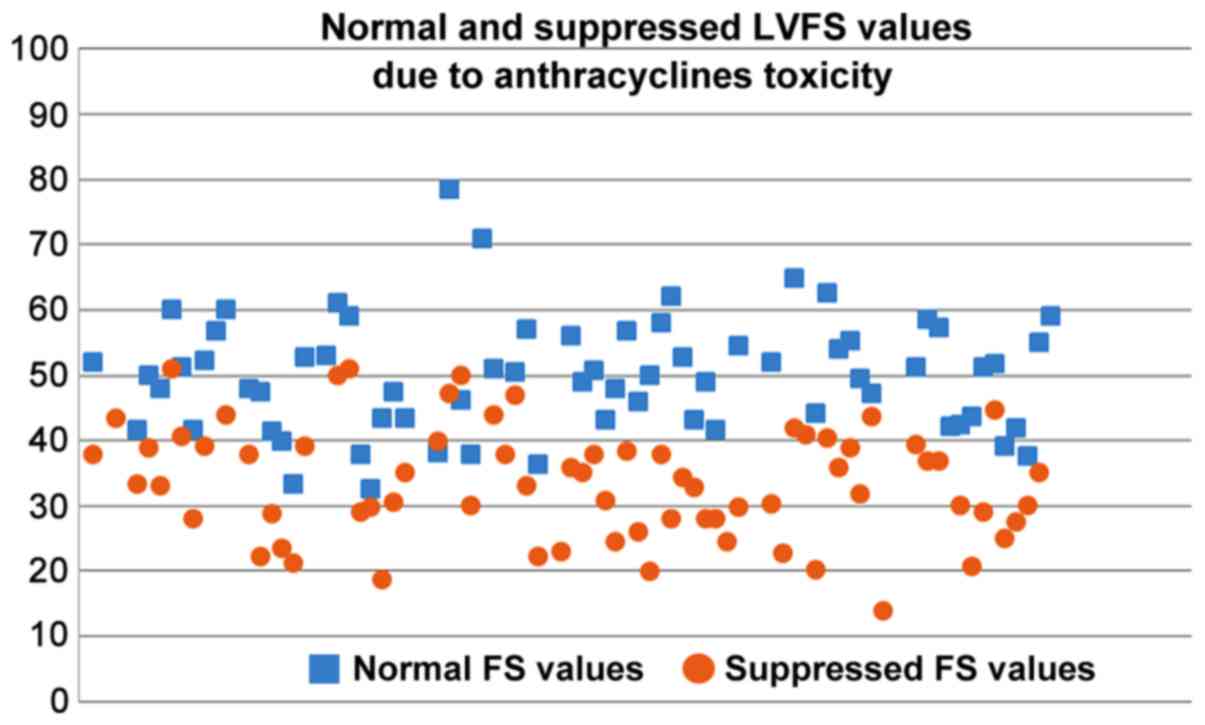
|
1
|
Zamorano JL, Lancellotti P, Rodriguez
Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ,
Lip GYH, Lyon AR, et al ESC Scientific Document Group, : 2016 ESC
Position Paper on cancer treatments and cardiovascular toxicity
developed under the auspices of the ESC Committee for Practice
Guidelines: The Task Force for cancer treatments and cardiovascular
toxicity of the European Society of Cardiology (ESC). Eur Heart J.
37:2768–2801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Plana JC, Galderisi M, Barac A, Ewer MS,
Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP,
et al: Expert consensus for multimodality imaging evaluation of
adult patients during and after cancer therapy: A report from the
American Society of Echocardiography and the European Association
of Cardiovascular Imaging. J Am Soc Echocardiogr. 27:911–939. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pardo Sanz A and Zamorano JL:
‘Cardiotoxicity’: time to define new targets? Eur Heart J.
41:1730–1732. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park CJ, Branch ME, Vasu S and Melendez
GC: The role of cardiac MRI in animal models of cardiotoxicity:
hopes and challenges. J Cardiovasc Transl Res. Apr 4–2020.(Epub
ahead of print). View Article : Google Scholar
|
|
5
|
Sobczuk P, Czerwinska M, Kleibert M and
Cudnoch-Jedrzejewska A: Anthracycline-induced cardiotoxicity and
renin-angiotensin-aldosterone system-from molecular mechanisms to
therapeutic applications. Heart Fail Rev. May 30–2020.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashemzaei M, Karami SP, Delaramifar A,
Sheidary A, Tabrizian K, Rezaee R, Shahsavand S, Arsene AL,
Tsatsakis AM and Mohammad S: Anticancer effects of
co-administration of daunorubicin and resveratrol in MOLT-4, U266
B1 and RAJI cell lines. Farmacia. 64:36–42. 2016.
|
|
7
|
Iranshahi M, Barthomeuf C, Bayet-Robert M,
Chollet P, Davoodi D, Piacente S, Rezaee R and Sahebkar A:
Drimane-type sesquiterpene coumarins from ferula gummosa fruits
enhance doxorubicin uptake in doxorubicin-resistant human breast
cancer cell line. J Tradit Complement Med. 4:118–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwarz ER, Pollick C, Dow J, Patterson M,
Birnbaum Y and Kloner RA: A small animal model of non-ischemic
cardiomyopathy and its evaluation by transthoracic
echocardiography. Cardiovasc Res. 39:216–223. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robert J: Preclinical assessment of
anthracycline cardiotoxicity in laboratory animals: Predictiveness
and pitfalls. Cell Biol Toxicol. 23:27–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Germanakis I, Tsarouhas K, Fragkiadaki P,
Tsitsimpikou C, Goutzourelas N, Champsas MC, Stagos D, Rentoukas E
and Tsatsakis AM: Oxidative stress and myocardial dysfunction in
young rabbits after short term anabolic steroids administration.
Food Chem Toxicol. 61:101–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
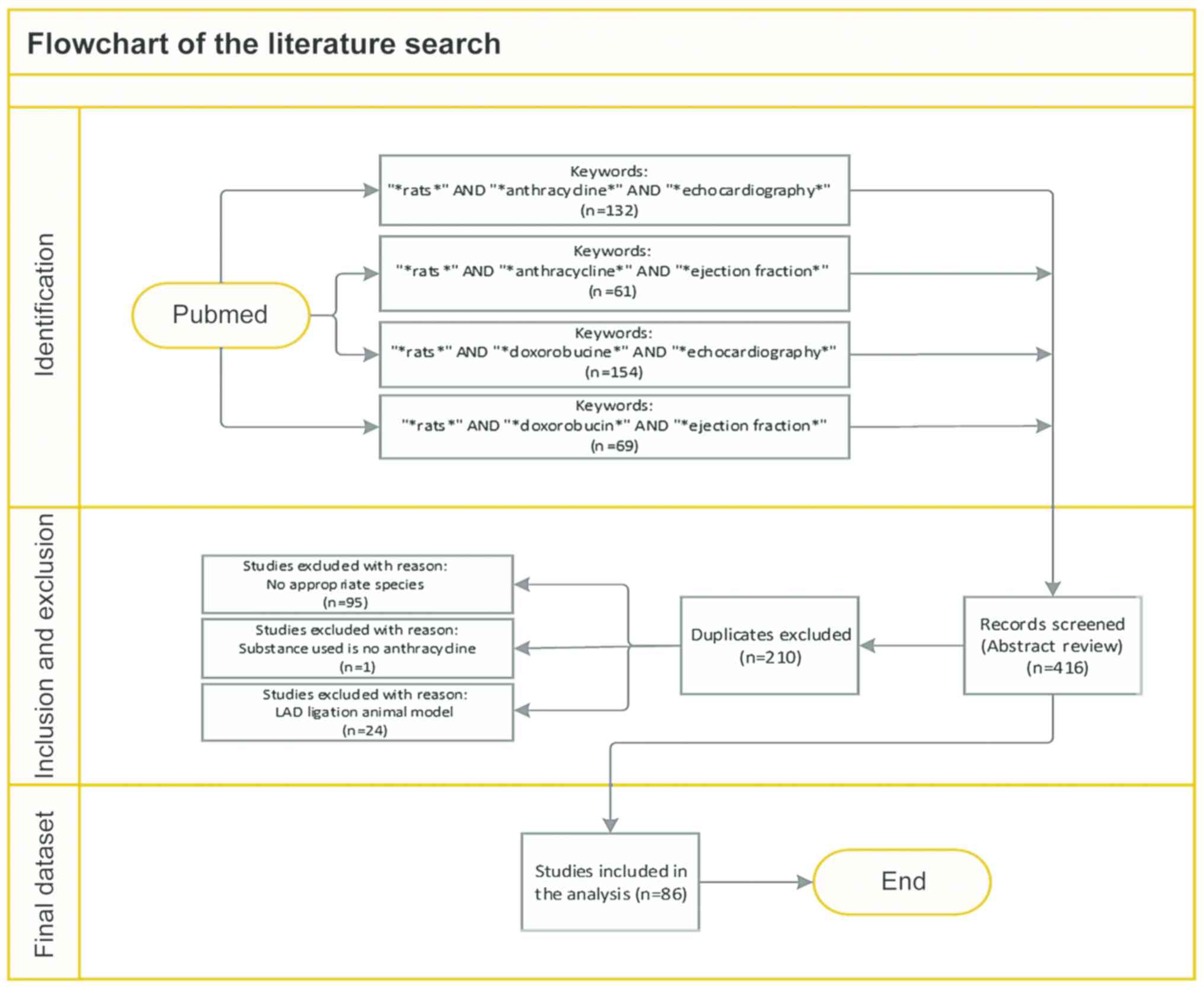
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA; PRISMA-P Group,
: Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4:12015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
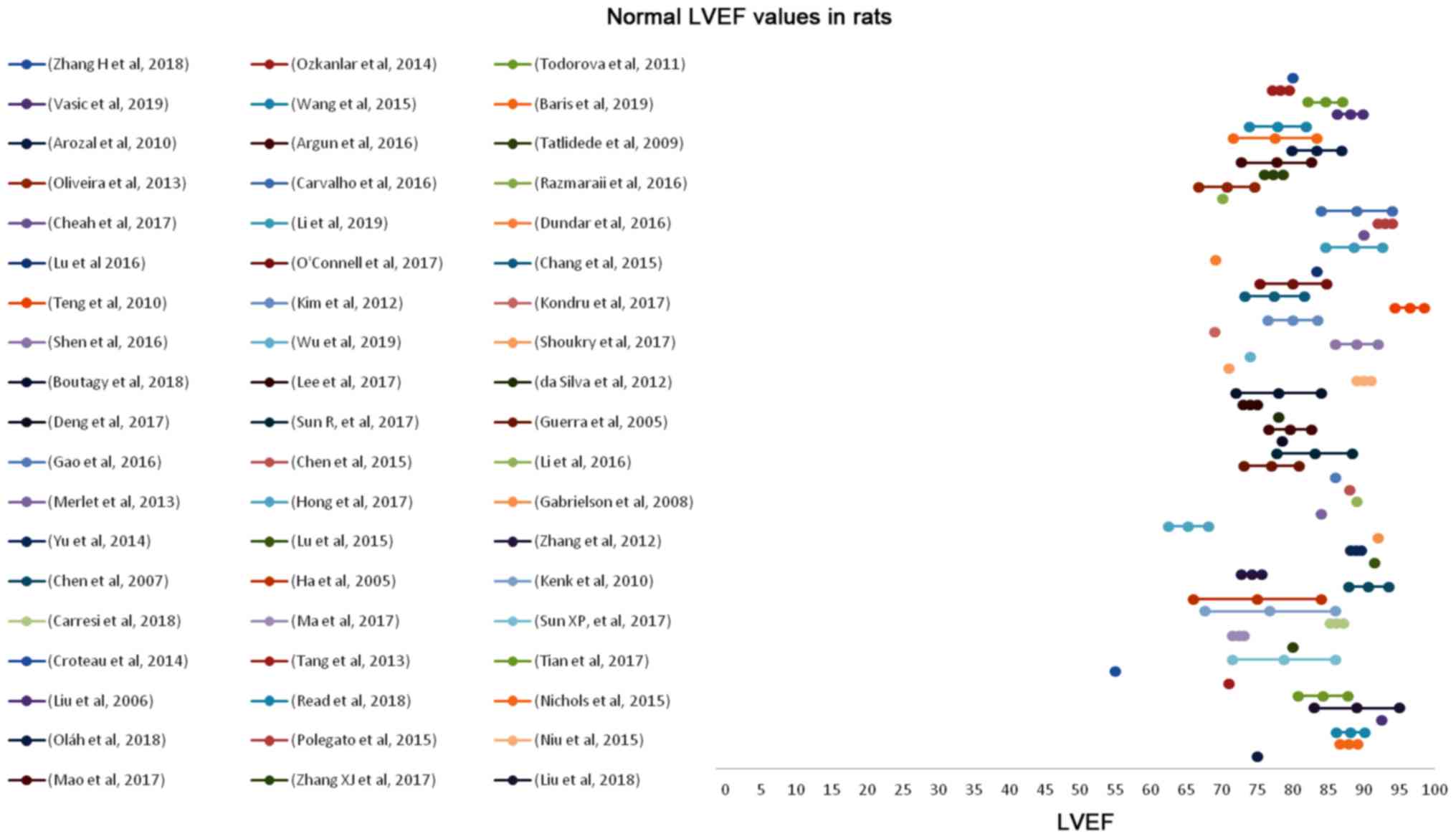
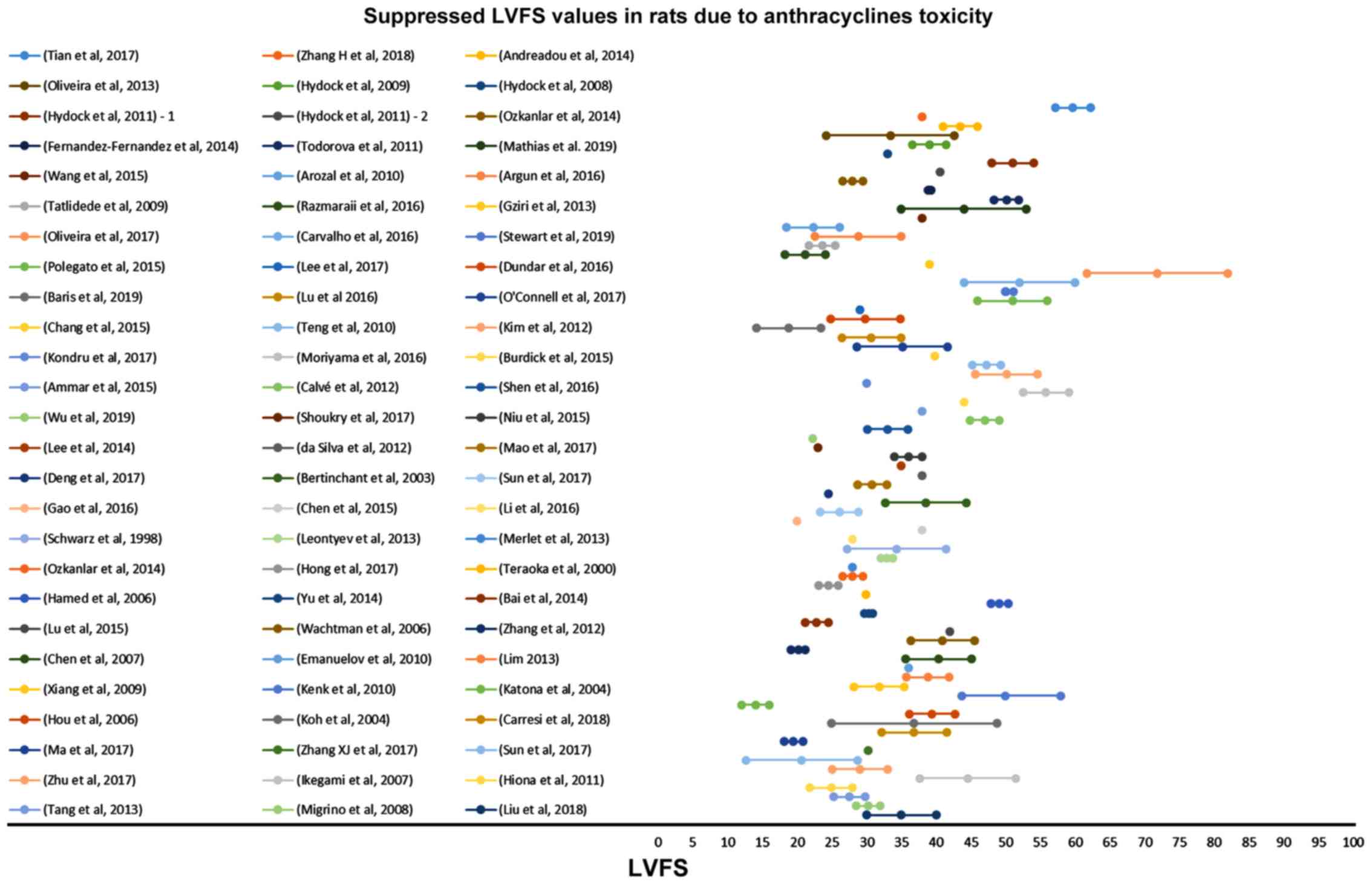
Zacchigna S, Paldino A, Falcao-Pires I,
Daskalopoulos EP, Dal Ferro M, Vodret S, Lesizza P, Cannatà A,
Daniela Miranda-Silva D, et al: Toward standardization of
echocardiography for the evaluation of left ventricular function in
adult rodents: a position paper of the ESC Working Group on
Myocardial Function. Cardiovasc Res. May 4–2020.(Epub ahead of
print). View Article : Google Scholar
|
|
13
|
Liu J and Rigel DF: Echocardiographic
examination in rats and mice. Methods Mol Biol. 573:139–155. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Lu X, Liu Z and Du K:
Rosuvastatin reduces the pro-inflammatory effects of adriamycin on
the expression of HMGB1 and RAGE in rats. Int J Mol Med.
42:3415–3423. 2018.PubMed/NCBI
|
|
15
|
Tian XQ, Ni XW, Xu HL, Zheng L, ZhuGe DL,
Chen B, Lu CT, Yuan JJ and Zhao YZ: Prevention of
doxorubicin-induced cardiomyopathy using targeted MaFGF mediated by
nanoparticles combined with ultrasound-targeted MB destruction. Int
J Nanomedicine. 12:7103–7119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andreadou I, Mikros E, Ioannidis K, Sigala
F, Naka K, Kostidis S, Farmakis D, Tenta R, Kavantzas N, Bibli SI,
et al: Oleuropein prevents doxorubicin-induced cardiomyopathy
interfering with signaling molecules and cardiomyocyte metabolism.
J Mol Cell Cardiol. 69:4–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oliveira MS, Melo MB, Carvalho JL, Melo
IM, Lavor MSI, Gomes DA, de Goes AM and Melo MM: Doxorubicin
cardiotoxicity and cardiac function improvement after stem cell
therapy diagnosed by strain echocardiography. J Cancer Sci Ther.
5:52–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hydock DS, Lien CY and Hayward R:
Anandamide preserves cardiac function and geometry in an acute
doxorubicin cardiotoxicity rat model. J Cardiovasc Pharmacol Ther.
14:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fernandez-Fernandez A, Carvajal DA, Lei T
and McGoron AJ: Chemotherapy-induced changes in cardiac capillary
permeability measured by fluorescent multiple indicator dilution.
Ann Biomed Eng. 42:2405–2415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Todorova VK, Kaufmann Y and Klimberg VS:
Increased efficacy and reduced cardiotoxicity of metronomic
treatment with cyclophosphamide in rat breast cancer. Anticancer
Res. 31:215–220. 2011.PubMed/NCBI
|
|
21
|
Vasić M, Lončar-Turukalo T, Tasić T, Matić
M, Glumac S, Bajić D, Popović B and Japundžić-Žigon N:
Cardiovascular variability and β-ARs gene expression at two stages
of doxorubicin-induced cardiomyopathy. Toxicol Appl Pharmacol.
362:43–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathias LMBS, Alegre PHC, Dos Santos IOF,
Bachiega T, Figueiredo AM, Chiuso-Minicucci F, Fernandes AA, Bazan
SGZ, Minicucci MF, Azevedo PS, et al: Euterpe oleracea Mart.
(Açai) supplementation attenuates acute doxorubicin-induced
cardiotoxicity in rats. Cell Physiol Biochem. 53:388–399. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Chen L, Wang T, Jiang X, Zhang H,
Li P, Lv B and Gao X: Ginsenoside Rg3 antagonizes
adriamycin-induced cardiotoxicity by improving endothelial
dysfunction from oxidative stress via upregulating the Nrf2-ARE
pathway through the activation of akt. Phytomedicine. 22:875–884.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arozal W, Watanabe K, Veeraveedu PT,
Thandavarayan RA, Harima M, Sukumaran V, Suzuki K, Kodama M and
Aizawa Y: Effect of telmisartan in limiting the cardiotoxic effect
of daunorubicin in rats. J Pharm Pharmacol. 62:1776–1783. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Argun M, Üzüm K, Sönmez MF, Özyurt A,
Derya K, Çilenk KT, Unalmış S, Pamukcu Ö, Baykan A, Narin F, et al:
Cardioprotective effect of metformin against doxorubicin
cardiotoxicity in rats. Anatol J Cardiol. 16:234–241.
2016.PubMed/NCBI
|
|
26
|
Tatlidede E, Sehirli O, Velioğlu-Oğünc A,
Cetinel S, Yeğen BC, Yarat A, Süleymanoğlu S and Sener G:
Resveratrol treatment protects against doxorubicin-induced
cardiotoxicity by alleviating oxidative damage. Free Radic Res.
43:195–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Razmaraii N, Babaei H, Mohajjel Nayebi A,
Assadnassab G, Ashrafi Helan J and Azarmi Y: Crocin treatment
prevents doxorubicin-induced cardiotoxicity in rats. Life Sci.
157:145–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gziri MM, Pokreisz P, De Vos R, Verbeken
E, Debiève F, Mertens L, Janssens SP and Amant F: Fetal rat hearts
do not display acute cardiotoxicity in response to maternal
Doxorubicin treatment. J Pharmacol Exp Ther. 346:362–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliveira LF, O'Connell JL, Carvalho EE,
Pulici ECC, Romano MMD, Maciel BC and Simões MV: Comparison between
radionuclide ventriculography and echocardiography for
quantification of left ventricular systolic function in rats
exposed to doxorubicin. Arq Bras Cardiol. 108:12–20.
2017.PubMed/NCBI
|
|
30
|
Carvalho PB, Gonçalves AF, Alegre PH,
Azevedo PS, Roscani MG, Bergamasco CM, Modesto PN, Fernandes AA,
Minicucci MF, Paiva SA, et al: Pamidronate attenuates oxidative
stress and energetic metabolism changes but worsens functional
outcomes in acute doxorubicin-induced cardiotoxicity in rats. Cell
Physiol Biochem. 40:431–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stewart LK, Smoak P, Hydock DS, Hayward R,
O'Brien K, Lisano JK, Boeneke C, Christensen M and Mathias A: Milk
and kefir maintain aspects of health during doxorubicin treatment
in rats. J Dairy Sci. 102:1910–1917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Polegato BF, Minicucci MF, Azevedo PS,
Carvalho RF, Chiuso-Minicucci F, Pereira EJ, Paiva SA, Zornoff LA,
Okoshi MP, Matsubara BB, et al: Acute doxorubicin-induced
cardiotoxicity is associated with matrix metalloproteinase-2
alterations in rats. Cell Physiol Biochem. 35:1924–1933. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee KH, Cho H, Lee S, Woo JS, Cho BH, Kang
JH, Jeong YM, Cheng XW and Kim W: Enhanced-autophagy by exenatide
mitigates doxorubicin-induced cardiotoxicity. Int J Cardiol.
232:40–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheah HY, Šarenac O, Arroyo JJ, Vasić M,
Lozić M, Glumac S, Hoe SZ, Hindmarch CCT, Murphy D, Kiew LV, et al:
Hemodynamic effects of HPMA copolymer based doxorubicin conjugate:
A randomized controlled and comparative spectral study in conscious
rats. Nanotoxicology. 11:210–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Xu G, Wei S, Zhang B, Yao H, Chen Y,
Liu W, Wang B, Zhao J and Gao Y: Lingguizhugan decoction attenuates
doxorubicin-induced heart failure in rats by improving TT-SR
microstructural remodeling. BMC Complement Altern Med. 19:3602019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dundar HA, Kiray M, Kir M, Kolatan E,
Bagriyanik A, Altun Z, Aktas S, Ellidokuz H, Yilmaz O, Mutafoglu K,
et al: Protective effect of acetyl-L-carnitine against
doxorubicin-induced cardiotoxicity in wistar albino rats. Arch Med
Res. 47:506–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barış VO, Gedikli E, Yersal N, Müftüoğlu S
and Erdem A: Protective effect of taurine against
doxorubicin-induced cardiotoxicity in rats: Echocardiographical and
histological findings. Amino Acids. 51:1649–1655. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu PP, Ma J, Liang XP, Guo CX, Yang YK,
Yang KQ, Shen QM, Ma LH and Zhou XL: Xinfuli improves cardiac
function, histopathological changes and attenuate cardiomyocyte
apoptosis in rats with doxorubicin-induced cardiotoxicity. J
Geriatr Cardiol. 13:968–972. 2016.PubMed/NCBI
|
|
39
|
O'Connell JL, Romano MM, Campos Pulici EC,
Carvalho EEV, de Souza FR, Tanaka DM, Maciel BC, Salgado HC,
Fazan-Júnior R, Rossi MA, et al: Short-term and long-term models of
doxorubicin-induced cardiomyopathy in rats: A comparison of
functional and histopathological changes. Exp Toxicol Pathol.
69:213–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang SA, Lim BK, Lee YJ, Hong MK, Choi JO
and Jeon ES: A novel angiotensin type I receptor antagonist,
Fimasartan, prevents Doxorubicin-induced cardiotoxicity in rats. J
Korean Med Sci. 30:559–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Teng LL, Shao L, Zhao YT, Yu X, Zhang DF
and Zhang H: The beneficial effect of n-3 polyunsaturated fatty
acids on doxorubicin-induced chronic heart failure in rats. J Int
Med Res. 38:940–948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim YH, Kim M, Park SM, Kim SH, Lim SY,
Ahn JC, Song WH and Shim WJ: Discordant impairment of
multidirectional myocardial deformation in rats with Doxorubicin
induced cardiomyopathy. Echocardiography. 29:720–728. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kondru SK, Potnuri AG, Allakonda L and
Konduri P: Histamine 2 receptor antagonism elicits protection
against doxorubicin-induced cardiotoxicity in rodent model. Mol
Cell Biochem. 441:77–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moriyama T, Kemi M and Horie T: Elevated
cardiac 3-deoxyglucosone, a highly reactive intermediate in
glycation reaction, in doxorubicin-induced cardiotoxicity in rats.
Pathophysiology. 23:237–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Burdick J, Berridge B and Coatney R:
Strain echocardiography combined with pharmacological stress test
for early detection of anthracycline induced cardiomyopathy. J
Pharmacol Toxicol Methods. 73:15–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ammar HI, Sequiera GL, Nashed MB, Ammar
RI, Gabr HM, Elsayed HE, Sareen N, Rub EA, Zickri MB and Dhingra S:
Comparison of adipose tissue- and bone marrow-derived mesenchymal
stem cells for alleviating doxorubicin-induced cardiac dysfunction
in diabetic rats. Stem Cell Res Ther. 6:1482015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Calvé A, Haddad R, Barama SN, Meilleur M,
Sebag IA and Chalifour LE: Cardiac response to doxorubicin and
dexrazoxane in intact and ovariectomized young female rats at rest
and after swim training. Am J Physiol Heart Circ Physiol.
302:H2048–H2057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shen LJ, Lu S, Zhou YH, Li L, Xing QM and
Xu YL: Developing a rat model of dilated cardiomyopathy with
improved survival. J Zhejiang Univ Sci B. 17:975–983. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu Z, Zhao X, Miyamoto A, Zhao S, Liu C,
Zheng W and Wang H: Effects of steroidal saponins extract from
Ophiopogon japonicus root ameliorates doxorubicin-induced chronic
heart failure by inhibiting oxidative stress and inflammatory
response. Pharm Biol. 57:176–183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shoukry HS, Ammar HI, Rashed LA, Zikri MB,
Shamaa AA, Abou Elfadl SG, Rub EA, Saravanan S and Dhingra S:
Prophylactic supplementation of resveratrol is more effective than
its therapeutic use against doxorubicin induced cardiotoxicity.
PLoS One. 12:e01815352017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Niu QY, Li ZY, Du GH and Qin XM: (1)H NMR
based metabolomic profiling revealed doxorubicin-induced systematic
alterations in a rat model. J Pharm Biomed Anal. 118:338–348. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Boutagy NE, Wu J, Cai Z, Zhang W, Booth
CJ, Kyriakides TC, Pfau D, Mulnix T, Liu Z, Miller EJ, et al: In
vivo reactive oxygen species detection with a novel positron
emission tomography tracer, 18F-DHMT, allows for early detection of
anthracycline-induced cardiotoxicity in rodents. JACC Basic Transl
Sci. 3:378–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee PJ, Rudenko D, Kuliszewski MA, Liao C,
Kabir MG, Connelly KA and Leong-Poi H: Survivin gene therapy
attenuates left ventricular systolic dysfunction in doxorubicin
cardiomyopathy by reducing apoptosis and fibrosis. Cardiovasc Res.
101:423–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
da Silva MG, Mattos E, Camacho-Pereira J,
Domitrovic T, Galina A, Costa MW and Kurtenbach E: Cardiac systolic
dysfunction in doxorubicin-challenged rats is associated with
upregulation of MuRF2 and MuRF3 E3 ligases. Exp Clin Cardiol.
17:101–109. 2012.PubMed/NCBI
|
|
55
|
Mao C, Hou X, Wang B, Chi J, Jiang Y,
Zhang C and Li Z: Intramuscular injection of human umbilical
cord-derived mesenchymal stem cells improves cardiac function in
dilated cardiomyopathy rats. Stem Cell Res Ther. 8:182017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deng B, Wang JX, Hu XX, Duan P, Wang L, Li
Y and Zhu QL: Nkx2.5 enhances the efficacy of mesenchymal stem
cells transplantation in treatment heart failure in rats. Life Sci.
182:65–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bertinchant JP, Polge A, Juan JM,
Oliva-Lauraire MC, Giuliani I, Marty-Double C, Burdy JY,
Fabbro-Peray P, Laprade M, Bali JP, et al: Evaluation of cardiac
troponin I and T levels as markers of myocardial damage in
doxorubicin-induced cardiomyopathy rats, and their relationship
with echocardiographic and histological findings. Clin Chim Acta.
329:39–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun R, Wang J, Zheng Y, Li X, Xie T, Li R,
Liu M, Cao Y, Lu L, Zhang Q, et al: Traditional Chinese medicine
baoxin decoction improves cardiac fibrosis of rats with dilated
cardiomyopathy. Exp Ther Med. 13:1900–1906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guerra J, De Jesus A, Santiago-Borrero P,
Roman-Franco A, Rodríguez E and Crespo MJ: Plasma nitric oxide
levels used as an indicator of doxorubicin-induced cardiotoxicity
in rats. Hematol J. 5:584–588. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gao Y, Yang H, Fan Y, Li L, Fang J and
Yang W: Hydrogen-rich saline attenuates cardiac and hepatic injury
in doxorubicin rat model by inhibiting inflammation and apoptosis.
Mediators Inflamm. 2016:13203652016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen Y, Tang Y, Xiang Y, Xie YQ, Huang XH
and Zhang YC: Shengmai injection improved doxorubicin-induced
cardiomyopathy by alleviating myocardial endoplasmic reticulum
stress and caspase-12 dependent apoptosis. BioMed Res Int.
2015:9526712015.PubMed/NCBI
|
|
62
|
Li H, Mao Y, Zhang Q, Han Q, Man Z, Zhang
J, Wang X, Hu R, Zhang X, Irwin DM, et al: Xinmailong mitigated
epirubicin-induced cardiotoxicity via inhibiting autophagy. J
Ethnopharmacol. 192:459–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Leontyev S, Schlegel F, Spath C, Schmiedel
R, Nichtitz M, Boldt A, Rübsamen R, Salameh A, Kostelka M, Mohr FW,
et al: Transplantation of engineered heart tissue as a biological
cardiac assist device for treatment of dilated cardiomyopathy. Eur
J Heart Fail. 15:23–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Merlet N, Piriou N, Rozec B, Grabherr A,
Lauzier B, Trochu JN and Gauthier C: Increased beta2-adrenoceptors
in doxorubicin-induced cardiomyopathy in rat. PLoS One.
8:e647112013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ozkanlar Y, Aktas MS, Turkeli M, Erturk N,
Oruc E, Ozkanlar S, Kirbas A, Erdemci B and Aksakal E: Effects of
ramipril and darbepoetin on electromechanical activity of the heart
in doxorubicin-induced cardiotoxicity. Int J Cardiol. 173:519–521.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hong YM, Lee H, Cho MS and Kim KC:
Apoptosis and remodeling in adriamycin-induced cardiomyopathy rat
model. Korean J Pediatr. 60:365–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Teraoka K, Hirano M, Yamaguchi K and
Yamashina A: Progressive cardiac dysfunction in adriamycin-induced
cardiomyopathy rats. Eur J Heart Fail. 2:373–378. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hamed S, Barshack I, Luboshits G, Wexler
D, Deutsch V, Keren G and George J: Erythropoietin improves
myocardial performance in doxorubicin-induced cardiomyopathy. Eur
Heart J. 27:1876–1883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gabrielson KL, Mok GS, Nimmagadda S, Bedja
D, Pin S, Tsao A, Wang Y, Sooryakumar D, Yu SJ, Pomper MG, et al:
Detection of dose response in chronic doxorubicin-mediated cell
death with cardiac technetium 99m annexin V single-photon emission
computed tomography. Mol Imaging. 7:132–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yu Q, Li Q, Na R, Li X, Liu B, Meng L,
Liutong H, Fang W, Zhu N and Zheng X: Impact of repeated
intravenous bone marrow mesenchymal stem cells infusion on
myocardial collagen network remodeling in a rat model of
doxorubicin-induced dilated cardiomyopathy. Mol Cell Biochem.
387:279–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bai J, Gu R, Wang B, Zhang N, Kang L and
Xu B: Overexpression of integrin-linked kinase improves cardiac
function in a rat model of doxorubicin-induced chronic heart
failure. Zhonghua Xin Xue Guan Bing Za Zhi. 42:225–229. 2014.(In
Chinese). PubMed/NCBI
|
|
72
|
Lu XL, Tong YF, Liu Y, Xu YL, Yang H,
Zhang GY, Li XH and Zhang HG: Gαq protein carboxyl terminus
imitation polypeptide GCIP-27 improves cardiac function in chronic
heart failure rats. PLoS One. 10:e01210072015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wachtman LM, Browning MD, Bedja D, Pin S
and Gabrielson KL: Validation of the use of long-term indwelling
jugular catheters in a rat model of cardiotoxicity. J Am Assoc Lab
Anim Sci. 45:55–64. 2006.PubMed/NCBI
|
|
74
|
Zhang J, Zhang L, Wu Q, Liu H and Huang L:
Recombinant human brain natriuretic peptide therapy combined with
bone mesenchymal stem cell transplantation for treating heart
failure in rats. Mol Med Rep. 7:628–632. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen X, Chen Y, Bi Y, Fu N, Shan C, Wang
S, Aslam S, Wang PW and Xu J: Preventive cardioprotection of
erythropoietin against doxorubicin-induced cardiomyopathy.
Cardiovasc Drugs Ther. 21:367–374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ha JW, Kang SM, Pyun WB, Lee JY, Ahn MY,
Kang WC, Jeon TJ, Chung N, Lee JD and Cho SH: Serial assessment of
myocardial properties using cyclic variation of integrated
backscatter in an adriamycin-induced cardiomyopathy rat model.
Yonsei Med J. 46:73–77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Emanuelov AK, Shainberg A, Chepurko Y,
Kaplan D, Sagie A, Porat E, Arad M and Hochhauser E: Adenosine A3
receptor-mediated cardioprotection against doxorubicin-induced
mitochondrial damage. Biochem Pharmacol. 79:180–187. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lim SC: Interrelation between expression
of ADAM 10 and MMP 9 and synthesis of peroxynitrite in doxorubicin
induced cardiomyopathy. Biomol Ther (Seoul). 21:371–380. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hydock DS, Lien CY, Schneider CM and
Hayward R: Exercise preconditioning protects against
doxorubicin-induced cardiac dysfunction. Med Sci Sports Exerc.
40:808–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xiang P, Deng HY, Li K, Huang G-Y, Chen Y,
Tu L, Ng PC, Pong NH, Zhao H, Zhang L, et al: Dexrazoxane protects
against doxorubicin-induced cardiomyopathy: Upregulation of Akt and
Erk phosphorylation in a rat model. Cancer Chemother Pharmacol.
63:343–349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kenk M, Thackeray JT, Thorn SL, Dhami K,
Chow BJ, Ascah KJ, DaSilva JN and Beanlands RS: Alterations of pre-
and postsynaptic noradrenergic signaling in a rat model of
adriamycin-induced cardiotoxicity. J Nucl Cardiol. 17:254–263.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Katona M, Boros K, Sántha P, Ferdinandy P,
Dux M and Jancsó G: Selective sensory denervation by capsaicin
aggravates adriamycin-induced cardiomyopathy in rats. Naunyn
Schmiedebergs Arch Pharmacol. 370:436–443. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hydock DS, Parry TL, Jensen BT, Lien CY,
Schneider CM and Hayward R: Effects of endurance training on
combined goserelin acetate and doxorubicin treatment-induced
cardiac dysfunction. Cancer Chemother Pharmacol. 68:685–692. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hou XW, Son J, Wang Y, Ru YX, Lian Q,
Majiti W, Amazouzi A, Zhou YL, Wang PX and Han ZC: Granulocyte
colony-stimulating factor reduces cardiomyocyte apoptosis and
improves cardiac function in adriamycin-induced cardiomyopathy in
rats. Cardiovasc Drugs Ther. 20:85–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hydock DS, Lien CY, Jensen BT, Schneider
CM and Hayward R: Exercise preconditioning provides long-term
protection against early chronic doxorubicin cardiotoxicity. Integr
Cancer Ther. 10:47–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Koh E, Nakamura T and Takahashi H:
Troponin-T and brain natriuretic peptide as predictors for
adriamycin-induced cardiomyopathy in rats. Circ J. 68:163–167.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Carresi C, Musolino V, Gliozzi M, Maiuolo
J, Mollace R, Nucera S, Maretta A, Sergi D, Muscoli S, Gratteri S,
et al: Anti-oxidant effect of bergamot polyphenolic fraction
counteracts doxorubicin-induced cardiomyopathy: Role of autophagy
and c-kitposCD45negCD31neg cardiac stem cell activation. J Mol Cell
Cardiol. 119:10–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ma H, Kong J, Wang YL, Li JL, Hei NH, Cao
XR, Yang JJ, Yan WJ, Liang WJ, Dai HY, et al:
Angiotensin-converting enzyme 2 overexpression protects against
doxorubicin-induced cardiomyopathy by multiple mechanisms in rats.
Oncotarget. 8:24548–24563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang XJ, Cao XQ, Zhang CS and Zhao Z:
17β-estradiol protects against doxorubicin-induced cardiotoxicity
in male Sprague-Dawley rats by regulating NADPH oxidase and
apoptosis genes. Mol Med Rep. 15:2695–2702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sun XP, Wan LL, Yang QJ, Huo Y, Han YL and
Guo C: Scutellarin protects against doxorubicin-induced acute
cardiotoxicity and regulates its accumulation in the heart. Arch
Pharm Res. 40:875–883. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhu HJ, Han ZY, He SF, Jin S-Y, Xu S-J,
Fang X-D and Zhang Y: Specific MicroRNAs comparisons in hypoxia and
morphine preconditioning against hypoxia-reoxgenation injury with
and without heart failure. Life Sci. 170:82–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Croteau E, Tremblay S, Gascon S,
Dumulon-Perreault V, Labbé SM, Rousseau JA, Cunnane SC, Carpentier
AC, Bénard F and Lecomte R: [(11)C]-Acetoacetate PET imaging: A
potential early marker for cardiac heart failure. Nucl Med Biol.
41:863–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ikegami E, Fukazawa R, Kanbe M, Watanabe
M, Abe M, Watanabe M, Kamisago M, Hajikano M, Katsube Y and Ogawa
S: Edaravone, a potent free radical scavenger, prevents
anthracycline-induced myocardial cell death. Circ J. 71:1815–1820.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hiona A, Lee AS, Nagendran J, Xie X,
Connolly AJ, Robbins RC and Wu JC: Pretreatment with
angiotensin-converting enzyme inhibitor improves
doxorubicin-induced cardiomyopathy via preservation of
mitochondrial function. J Thorac Cardiovasc Surg. 142:396–403.e3.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tang DX, Zhao HP, Pan CS, Liu YY, Wei XH,
Yang XY, Chen YY, Fan JY, et al: QiShenYiQi pills, a compound
Chinese medicine, ameliorates doxorubicin-induced myocardial
structure damage and cardiac dysfunction in rats. Evid Based
Complement Alternat Med: eCAM. 2013:4805972013. View Article : Google Scholar
|
|
96
|
Migrino RQ, Aggarwal D, Konorev E,
Brahmbhatt T, Bright M and Kalyanaraman B: Early detection of
doxorubicin cardiomyopathy using two-dimensional strain
echocardiography. Ultrasound Med Biol. 34:208–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu Y, Xu W, Xiong Y, Du G and Qin X:
Evaluations of the effect of HuangQi against heart failure based on
comprehensive echocardiography index and metabonomics.
Phytomedicine. 50:205–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu X, Gu X, Li Z, Li X, Li H, Chang J,
Chen P, Jin J, Xi B, Chen D, et al: Neuregulin-1/erbB-activation
improves cardiac function and survival in models of ischemic,
dilated, and viral cardiomyopathy. J Am Coll Cardiol. 48:1438–1447.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cadeddu Dessalvi C, Pepe A, Penna C,
Gimelli A, Madonna R, Mele D, Monte I, Novo G, Nugara C, Zito C, et
al: Sex differences in anthracycline-induced cardiotoxicity: The
benefits of estrogens. Heart Fail Rev. 24:915–925. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Swain SM, Whaley FS and Ewer MS:
Congestive heart failure in patients treated with doxorubicin: A
retrospective analysis of three trials. Cancer. 97:2869–2879. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Blanter JB and Frishman WH: The preventive
role of angiotensin converting enzyme inhibitors/angiotensin-II
receptor blockers and β-adrenergic blockers in anthracycline- and
trastuzumab-induced cardiotoxicity. Cardiol Rev. 27:256–259. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sharkey LC, Radin MJ, Heller L, Rogers LK,
Tobias A, Matise I, Wang Q, Apple FS and McCune SA: Differential
cardiotoxicity in response to chronic doxorubicin treatment in male
spontaneous hypertension-heart failure (SHHF), spontaneously
hypertensive (SHR), and Wistar Kyoto (WKY) rats. Toxicol Appl
Pharmacol. 273:47–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Georgiadis N, Tsarouhas K, Tsitsimpikou C,
Vardavas A, Rezaee R, Germanakis I, Tsatsakis A, Stagos D and
Kouretas D: Pesticides and cardiotoxicity. Where do we stand?
Toxicol Appl Pharmacol. 353:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Vasilaki F, Tsitsimpikou C, Tsarouhas K,
Germanakis I, Tzardi M, Kavvalakis M, Ozcagli E, Kouretas D and
Tsatsakis AM: Cardiotoxicity in rabbits after long-term nandrolone
decanoate administration. Toxicol Lett. 241:143–151. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Achar S, Rostamian A and Narayan SM:
Cardiac and metabolic effects of anabolic-androgenic steroid abuse
on lipids, blood pressure, left ventricular dimensions, and rhythm.
Am J Cardiol. 106:893–901. 2010. View Article : Google Scholar : PubMed/NCBI
|