Globally, gastric cancer (GC) is the leading cause
of cancer-related mortality and one of the most common types of
cancers (1). The number of newly
discovered GC cases worldwide reached over 1,000,000 in 2018, with
783,000 GC-related deaths, making it the fifth most commonly
diagnosed cancer (2). East Asia is
one of the major regions with a high incidence of GC (3). For example, GC ranks as the second
leading cause of cancer-related death in China (4). Despite a large amount of progress in
diagnosis and therapeutic strategies, the overall survival of GC
patients remains unsatisfactory (5). Furthermore, due to the lack of
sensitive predictive markers at an early stage and the lack of
specific symptoms, the majority of GC patients are diagnosed with
advanced GC or metastasis (6).
Therefore, it is urgent to explore the molecular mechanism and
critical signaling pathways underlying the initiation and
progression of GC.
In recent decades, numerous studies have
demonstrated that non-coding RNAs (ncRNAs), primarily microRNAs
(miRNAs) and long non-coding RNAs (lncRNAs), are closely related to
every stage of GC, including tumorigenesis, growth, development,
apoptosis, invasion, metastasis, and drug resistance (7). The role of ncRNAs as promising
biomarkers in the diagnosis of GC has also been deeply studied
(8). It is worth noting that
activation of the wingless-type MMTV integration site family
(Wnt)/β-catenin signaling pathway has a vital role in a variety of
cancers, such as breast cancer (9),
colon cancer (10) and liver cancer
(11). Indeed, continual activation
of Wnt/β-catenin signaling pathway is closely related to the
carcinogenesis of GC (12–14). Thus, efforts to further understand
the mechanism by which ncRNAs modulate the Wnt/β-catenin signaling
pathway may provide early effective diagnosis and potential novel
therapeutic strategies for GC. Therefore, in this review, we
summarize the role of ncRNAs in regulating the Wnt/β-catenin
signaling pathway in the pathogenesis of GC.
In the current main literature databases, PubMed is
the most widely used premier bibliographic database in the life
sciences and biomedicine fields. In addition, Web of Science is the
largest comprehensive academic information resource with the most
disciplines in the world. Therefore, we identify eligible studies
using the following terms: ‘β-catenin’, ‘Wnt’, ‘gastric cancer’ and
‘non-coding RNA’ in the PubMed and Web of Science databases. The
search began on February 1, 2020, and the last retrieval was on
March 15, 2020. The citation lists associated with the studies were
used to identify additional eligible studies. Reviews and
bibliographies were also manually inspected to identify related
articles. The role of ncRNAs in regulating the Wnt/β-catenin
signaling pathway in the pathogenesis of GC was analyzed.
The Wnt signaling pathway is a highly conserved
extracellular signal transduction pathway that is triggered by the
binding of the ligand protein Wnt to its membrane protein receptor.
In 1982, the Wnt gene was first discovered in mouse breast
cancer. As activation of this gene relies on the insertion of mouse
breast cancer-associated viral genes, it was named ‘Int1’
(15). Subsequent studies
discovered that the Int1 gene plays an essential role in the
normal embryonic development of mice and that its function is
similar to that of the Wingless gene of Drosophila,
which controls axial development of the fruit fly embryo. Due to
the similarity between these two genes, Wingless and
Int1 were combined and named ‘Wnt’ (16–19).
The Wnt pathway is mainly divided into three
pathways: i) The canonical Wnt or β-catenin dependent pathway,
which activates expression of target genes in nucleus planar cells;
ii) the polarity (PCP) pathway, which participates in Jun
N-terminal kinase (JNK) activation and cytoskeletal rearrangement;
and iii) the Wnt/Ca2+ pathway, which participates in
activation of phospholipase C (PLC) and protein kinase C (PKC)
(20–23). The last two pathways together are
called the ‘non-classical’ or ‘β-catenin-independent’ pathways
(24).
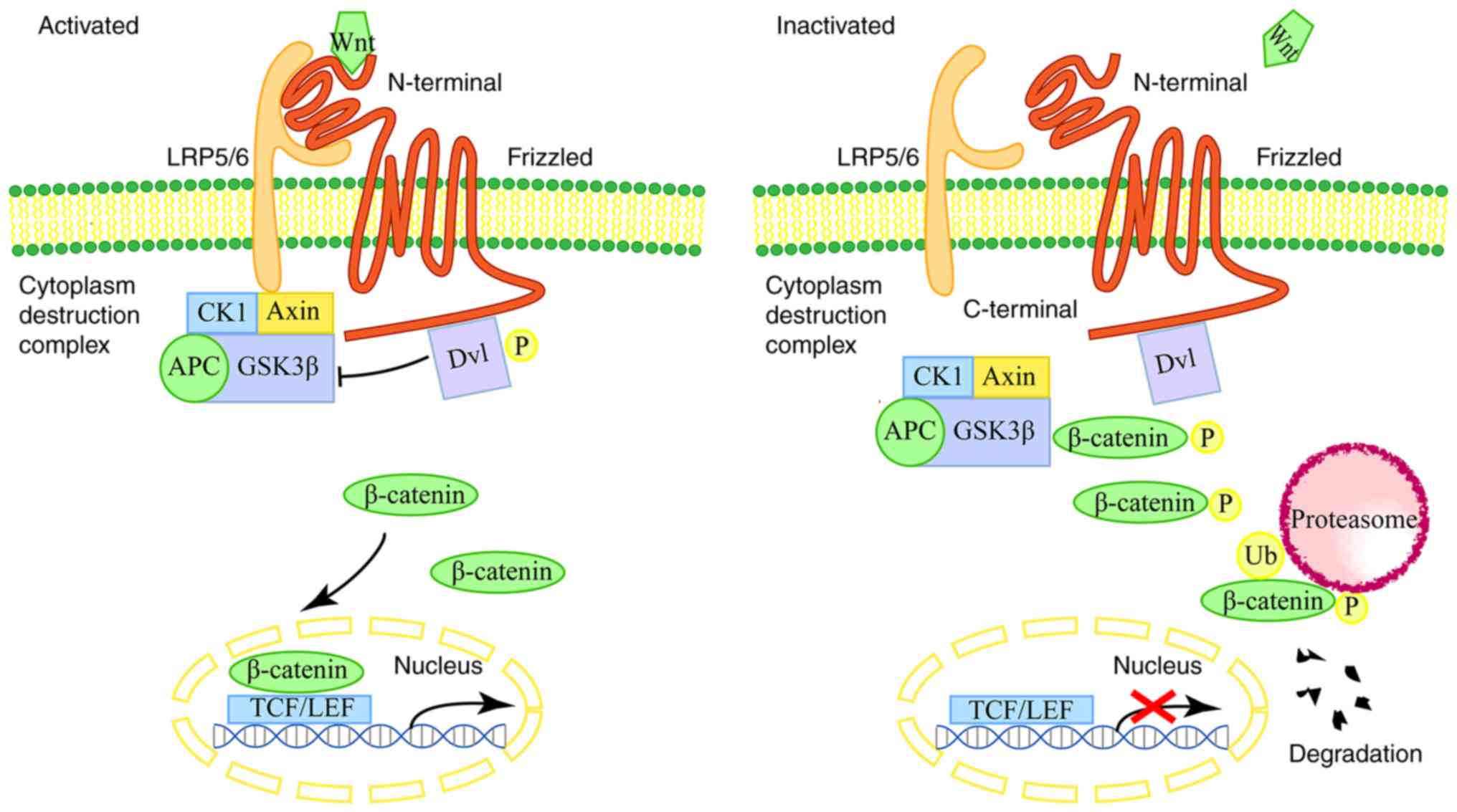
The Wnt/β-catenin signaling pathway is a classical
Wnt pathway by which β-catenin accumulates in the cytoplasm and
eventually translocates to the nucleus and coactivates
transcription with TCF/LEF (T cytokine/lymphoid enhancer factor)
family members (21). The Wnt
signal is induced when the Wnt protein binds to a cysteine-rich
domain of the N-terminal of the Frizzled (Fz) family of receptors
(25). Following gene transduction
and TCF/LEF transcription, the factor induces transcription of the
target gene that ultimately acts on Wnt, inducing subsequent
cellular responses (Fig. 1)
(26,27).
Among these Wnt signaling pathways, the
Wnt/β-catenin signaling pathway is highly evolutionarily conserved.
It is involved in the pathogenesis of gastric carcinoma (28). According to statistics, activation
of Wnt/β-catenin signaling pathway can be found in ~30–50% of GC
tissues (29).
Non-coding RNAs (ncRNAs) are a group of RNA
transcribed from the genome that do not have protein-coding
functions (30). In the past, most
ncRNAs were considered ‘evolutionary junk’, but developments in
molecular biology suggest that mutation or aberrant expression of
ncRNAs has a huge impact on the occurrence and development of
diseases, including cancers (31).
Recently, ncRNAs have attracted widespread attention. It has also
been confirmed that abnormal expression of miRNAs strongly
contributes to the initiation and development of carcinoma
(32). lncRNAs exert potent
tumor-suppressive or -promoting effects on the pathogenic processes
underlying GC tumorigenesis and progression (33). In addition, miRNAs have been
verified to have deep connections to the occurrence, development,
proliferation, metastasis, and invasion of GC (33–37).
Glycogen synthase kinase-3β (GSK-3β) is an
evolutionarily highly conserved serine/threonine kinase that is
ubiquitous in mammalian eukaryotic cells. It acts on many signaling
proteins, structural proteins and transcription factors, regulating
cell differentiation, proliferation, survival and apoptosis
(38,39). One study revealed that lncRNA
LINC01225 activates the Wnt/β-catenin signaling pathway mainly by
inhibiting GSK-3β to promote the proliferation, migration,
invasion and EMT of GC (40). In
another study, GSK-3β was identified as a target gene of
lncRNA small nucleolar RNA host gene 20 (SNHG20) in GC cells.
Promoting expression of lncRNA SNHG20 suppresses that of
GSK-3β to activate the Wnt/β-catenin signaling pathway in GC
(41).
In addition to activating Wnt/β-catenin signaling
pathway, some lncRNAs play a completely opposite role. For
instance, upregulation of lncRNA GATA6 antisense RNA 1 (GATA6-AS1)
suppresses the Wnt/β-catenin signaling pathway by recruiting
enhancer of Zeste homolog 2 (EZH2) to the Frizzled family receptor
4 (FZD4) promoter region, enhancing H3K27me3 and downregulating
expression of FZD4, both of which reduce the occurrence,
development, and invasion of GC (42).
Desmoplakin (DSP), a class of proteins found in
desmosomes, can decrease the level of β-catenin both in the
cytoplasm and nucleus. Upregulation of lncRNA MIR4435-2HG
diminishes the activity of DSP, thus promoting GC growth and
metastasis (43). In addition,
knockdown of lncRNA zinc finger antisense (ZFAS)1 increases
expression of miR-200b, which targets Wnt1 to inhibit the induction
of cell proliferation, cell cycle progression and activation of
Wnt/β-catenin signaling pathway in GC (44,45).
Furthermore, lncRNAs act as tumor suppressors by
altering Wnt/β-catenin signaling pathway via their mediators.
lncRNA LINC01314 is expressed at low levels in GC tissue, and
enhancing its expression was found to inhibit GC tumor growth,
invasion and migration in nude mice by directly suppressing the
Wnt/β-catenin signaling pathway via downregulation of kallikrein 4
(KLK4) (46).
Competing endogenous RNA (ceRNA) refers to a new
mechanism of interaction between RNAs; that is, ceRNA binds to
miRNA via microRNA response elements (MERs) to regulate target gene
expression (47,48). A large number of studies have
demonstrated that ceRNAs may be inextricably linked to multiple
cancers, including GC (49–51).
lncRNA Homeobox A11 (HOXA11) antisense RNA
(HOXA11-AS) serves as a ceRNA by sponging miR-148a, which
deactivates the Wnt/β-catenin signaling pathway by directly
targeting Wnt1. Thus, lncRNA HOXA11-AS promotes the migration and
invasion of GC cells, leading to a poor clinical survival rate
(52). Consistent with this
finding, lncRNA LIN01606 was verified to increase expression of
Wnt3a by acting as a ceRNA of miR-423-5p, thereby promoting cell
migration and invasion of GC though activation of the Wnt/β-catenin
signaling pathway (53).
In addition, some lncRNAs can serve as tumor
suppressors in GC. LINC01133 regulates adenoma colon polyp (APC) by
acting as a ceRNA of miR-106a-3p. LINC01133 also inhibits nuclear
accumulation of the β-catenin protein by inhibiting miR-106a-3p and
promoting APC expression to inhibit activation of Wnt/β-catenin
signaling pathway, thus suppressing EMT and metastasis in GC
(54).
Similarly, lncRNA TOB1 antisense RNA 1 (TOB1-AS1)
functions as a ceRNA of miR-23a to abolish its inhibitory effector
on neuraminidase 1 (NEU1), promoting apoptosis and inhibiting the
metastasis of GC via the Wnt/β-catenin signaling pathway (55).
In addition to participating in Wnt/β-catenin
signaling pathway regulation via lncRNA-mediated mechanisms, some
miRNAs directly exert regulatory effects on the Wnt/β-catenin
signaling pathway in GC. miR-214 was discovered to downregulate
expression of GSK-3β. As a downstream gene of GSK-3β,
β-catenin was significantly upregulated by decreased levels
of GSK-3β, thus facilitating proliferation potency and
suppressing apoptosis in GC cells (56). Additionally, miR-501-5p has been
proven to directly target Dickkopf Wnt signaling pathway inhibitor
1 (DKK1), naked cuticle homolog 1 (NKD1), and GSK-3β and suppress
their expression, which enhances the stem cell-like phenotype in GC
(57).
Wnt ligands, which are involved in a variety of
signaling pathways, have been demonstrated to play an active role
in mediating the occurrence and progression of several types of
human cancers (58). Moreover, the
combination of Wnt1 synergistically activates Wnt/β-catenin
signaling pathway (59). For
instance, miR-140-5p suppresses Wnt/β-catenin signaling pathway
activation by binding to Wnt1 to inhibit GC proliferation and
invasion in vitro (60).
Similar to miR-140-5p, miR-200b and miR-22 act as tumor suppressors
in GC cells by binding to Wnt1. Furthermore, miR-200b and miR-22
synergistically contribute to the efficacy of diallyl disulfide
both in vivo and in vitro (61–63).
Consistent with these findings, miR-491-5p inhibits progression and
induces apoptosis and cell cycle arrest in GC by targeting Wnt3a
through the Wnt/β-catenin signaling pathway (64).
In addition, miRNAs mediate genes downstream of the
Wnt ligand, acting as tumor suppressors in GC. Dishevelled
and axin are both downstream genes of the Wnt ligand. The
Dishevelled-axin domain contains a type of protein named DIXDC1,
which is an activator of the Wnt/β-catenin signaling pathway.
Interestingly, miR-154 can directly inhibit DIXDC1 to suppress the
proliferation and invasion of GC via the Wnt/β-catenin signaling
pathway (65). Another study
revealed that miR-338 decreases c-myc and promotes phosphorylation
of GSK-3β to regulate Wnt/β-catenin signaling pathway, inhibiting
the proliferation, migration, and invasion of GC cells (66). As another inactivator of the
Wnt/β-catenin signaling pathway, miR-503 is beneficial for
inhibiting the progression and invasion of GC through upregulation
of GSK-3β and p-β-catenin (67).
The role of ncRNAs in GC was further clarified by Cong et al
who discovered that expression of miR-200a is downregulated in
gastric carcinoma cells. Furthermore, upregulated miR-200a directly
target β-catenin to suppress activation of the Wnt/β-catenin
signaling pathway, thus inhibiting tumorigenesis in GC (68).
miR-188-5p has been validated as targeting
phosphatase and tensin homolog (PTEN), a tumor suppressor involved
in a variety of cancers, including GC (69–73).
miR-188-5p suppresses PTEN and further promotes phosphorylation of
GSK3β to activate the Wnt/β-catenin signaling pathway, leading to
metastasis and poor prognosis in GC patients (74).
Suppressor of Fused (SUFU), a major negative
regulator of the Hedgehog signaling pathway (75–77),
as well as the Wnt signaling pathway (78–80),
is reported to be abnormally activated in GC (81–83).
Oncogenic miR-194 inhibits SUFU; then, massive amounts of β-catenin
accumulate in the cytoplasm, leading to translocation of β-catenin
to the nucleus, which promotes GC carcinogenesis (84,85).
Consistently, miR-324-3p activates Wnt/β-catenin
signaling pathway by inhibiting expression of the Wnt/β-catenin
signaling pathway antagonist SMAD family member 4 (Smad4) to
promote tumorigenesis and inhibit apoptosis in GC cells. Such
increased expression of miR-324-3p is accompanied by the expansion
and increased proliferation rate of gastric organoids (86).
miRNAs can also act as inhibitors of the
Wnt/β-catenin signaling pathway by targeting its mediators.
Overexpression of miR-200a upregulates expression of E-cadherin by
targeting zinc finger E-box-binding homeobox 1 (ZEB1) and zinc
finger E-box binding homeobox 2 (ZEB2), which transfers β-catenin
from the nucleus to the cytoplasm, suppressing activation of the
pathway (68). Furthermore,
miR-219-5p targets liver receptor homolog-1 (LRH-1) to inhibit
Wnt/β-catenin signaling pathway, further suppressing the
proliferation, migration, and invasion of GC (87).
Moreover, miR-302b inhibits expression of cyclin D1
and c-myc by targeting erythropoietin-producing hepatocellular
(Eph) A2 via the Wnt/β-catenin signaling pathway (88). Consistently, the level of miR-19 in
GC clinical samples is significantly decreased compared with that
noted in normal gastric tissue. Further analysis revealed that the
transcription factor myocyte enhancer factor 2D (MEF2D) functions
as a potential target of miR-19. Indeed, high expression of MEF2D
promotes activation of the Wnt/β-catenin signaling pathway and
contributes to the tumorigenesis and growth of GC. However, such
effects of MEF2D can be reversed by miR-19 (89). Additionally, synergistic
upregulation of miR381 and miR489 exerts phenotypic effects such as
inhibiting cell migration, invasion and EMT through inactivation of
the Wnt/β-catenin signaling pathway by directly targeting cullin 4B
(CUL4B) (90).
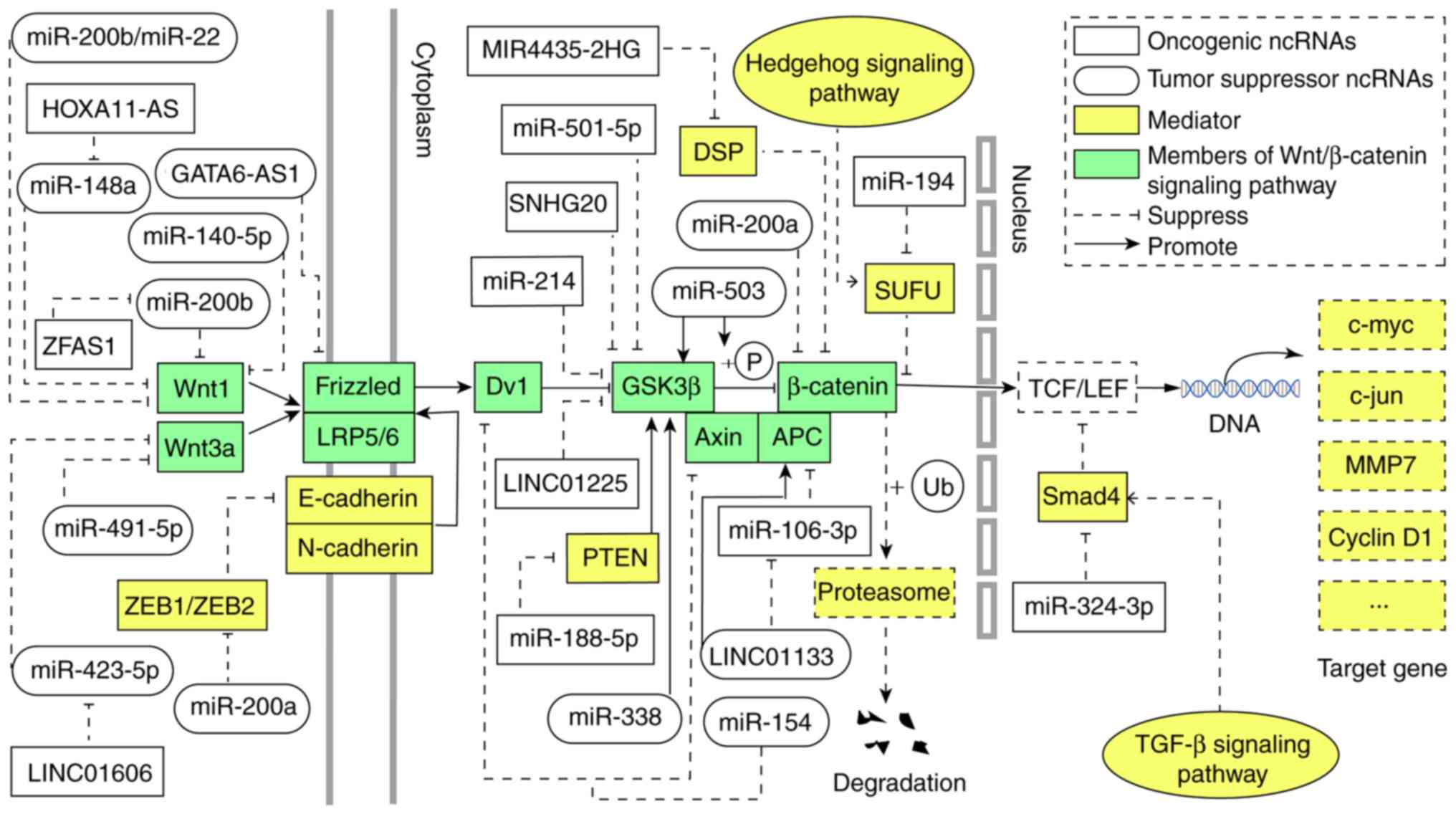
As mentioned above, ncRNAs can serve as underlying
novel biomarkers and therapeutic targets in human GC diagnosis and
treatment. Thus, efforts to thoroughly understand the role of
ncRNAs in modulating the Wnt/β-catenin signaling pathway in GC is
crucial (Fig. 2).
Various ncRNAs contribute to target directly or
indirectly the members of the Wnt/β-catenin signaling pathway in
GC. It remains unknown whether some microRNAs that interacts with
lncRNAs are involved in this regulatory process. Interestingly,
some substances that act as mediators of the ncRNAs are also
regulators of other signaling pathways. It means further study is
needed to explore whether the lncRNA-Wnt/β-catenin signaling
pathway is the main signaling pathway affecting the progression of
GC.
In addition, compared with that in normal gastric
tissue, expression of ncRNAs is abnormal in GC tissue and cells;
hence, detecting changes in ncRNA expression may serve as a
diagnostic factor in the early stage of GC. In addition, ncRNAs
directly or indirectly act on members of the Wnt/β-catenin
signaling pathway to regulate the biological behavior of GC, and
EMT, angiogenesis and other pathogenic processes that may be
involved in GC can serve as effective entry points for GC research.
Thus, determining specific targets is paramount, and the
development of targeted drugs, improvement in the accuracy of
targeted drugs and reduction in drug resistance rates will offer a
glimmer of hope to patients with GC. We firmly believe that a
better understanding of the precise underlying molecular mechanisms
will increase our knowledge of the basic mechanism of GC and
provide new clues to developing valuable diagnostic and therapeutic
strategies for GC.
Not applicable.
The present study was supported by grants from the
Educational Department of Jiangxi Province, China (no.
GJJ20053).
The data used to support the findings of this study
are available from the corresponding author upon request. All
information reported in this Review is based on relevant and
current references.
ZS, DG, LC, WD and QY were all involved in selection
of the review topic, literature data search, writing, figure and
table design, and editing of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Catalano V, Labianca R, Beretta GD, Gatta
G, de Braud F and Van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 71:127–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balakrishnan M, George R, Sharma A and
Graham DY: Changing trends in stomach cancer throughout the world.
Curr Gastroenterol Rep. 19:362017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiel A and Ristimäki A: Gastric cancer:
Basic aspects. Helicobacter. 17 (Suppl 1):S26–S29. 2012. View Article : Google Scholar
|
|
6
|
Tian L, Zhao Z, Xie L and Zhu J:
miR-361-5p inhibits the mobility of gastric cancer cells through
suppressing epithelial-mesenchymal transition via the Wnt/β-catenin
pathway. Gene. 675:102–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie SS, Jin J, Xu X, Zhuo W and Zhou TH:
Emerging roles of non-coding RNAs in gastric cancer: Pathogenesis
and clinical implications. World J Gastroenterol. 22:1213–1223.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang M and Du X: Noncoding RNAs in
gastric cancer: Research progress and prospects. World J
Gastroenterol. 22:6610–6618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang S, Chen L, Cui B, Chuang HY, Yu J,
Wang-Rodriguez J, Tang L, Chen G, Basak GW and Kipps TJ: ROR1 is
expressed in human breast cancer and associated with enhanced
tumor-cell growth. PLoS One. 7:e311272012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagy TA, Wroblewski LE, Wang D, Piazuelo
MB, Delgado A, Romero-Gallo J, Noto J, Israel DA, Ogden SR, Correa
P, et al: β-catenin and p120 mediate PPARδ-dependent proliferation
induced by Helicobacter pylori in human and rodent
epithelia. Gastroenterology. 141:553–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahn HJ and Lee DS: Helicobacter
pylori in gastric carcinogenesis. World J Gastrointest Oncol.
7:455–465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valenzuela MA, Canales J, Corvalán AH and
Quest AF: Helicobacter pylori-induced inflammation and
epigenetic changes during gastric carcinogenesis. World J
Gastroenterol. 21:12742–12756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baker NE: Molecular cloning of sequences
from wingless, a segment polarity gene in Drosophila: The
spatial distribution of a transcript in embryos. EMBO J.
6:1765–1773. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baker NE: Transcription of the
segment-polarity gene wingless in the imaginal discs of
Drosophila, and the phenotype of a pupal-lethal wg mutation.
Development. 102:489–497. 1988.PubMed/NCBI
|
|
17
|
Peifer M and Wieschaus E: The segment
polarity gene armadillo encodes a functionally modular protein that
is the Drosophila homolog of human plakoglobin. Cell.
63:1167–1176. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phillips RG, Roberts IJ, Ingham PW and
Whittle JR: The Drosophila segment polarity gene patched is
involved in a position-signalling mechanism in imaginal discs.
Development. 110:105–114. 1990.PubMed/NCBI
|
|
19
|
Therond P, Busson D, Guillemet E,
Limbourg-Bouchon B, Preat T, Terracol R, Tricoire H and
Lamour-Isnard C: Molecular organisation and expression pattern of
the segment polarity gene fused of Drosophila melanogaster.
Mech Dev. 44:65–80. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Semenov MV, Habas R, Macdonald BT and He
X: SnapShot: Noncanonical Wnt signaling pathways. Cell.
131:13782007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rao TP and Kühl M: An updated overview on
Wnt signaling pathways: A prelude for more. Circ Res.
106:1798–1806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schulte G: International union of basic
and clinical pharmacology. LXXX. The class Frizzled receptors.
Pharmacol Rev. 62:632–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sato N, Meijer L, Skaltsounis L, Greengard
P and Brivanlou AH: Maintenance of pluripotency in human and mouse
embryonic stem cells through activation of Wnt signaling by a
pharmacological GSK-3-specific inhibitor. Nat Med. 10:55–63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ooi CH, Ivanova T, Wu J, Lee M, Tan IB,
Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y, et al: Oncogenic
pathway combinations predict clinical prognosis in gastric cancer.
PLoS Genet. 5:e10006762009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gomes AQ, Nolasco S and Soares H:
Non-coding RNAs: Multi-tasking molecules in the cell. Int J Mol
Sci. 14:16010–16039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li PF, Chen SC, Xia T, Jiang XM, Shao YF,
Xiao BX and Guo JM: Non-coding RNAs and gastric cancer. World J
Gastroenterol. 20:5411–5419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taft RJ, Pang KC, Mercer TR, Dinger M and
Mattick JS: Non-coding RNAs: Regulators of disease. J Pathol.
220:126–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiu MT, Hu JW, Yin R and Xu L: Long
noncoding RNA: An emerging paradigm of cancer research. Tumour
Biol. 34:613–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin F, Li Y, Yan S, Liu S, Qian W, Shen D,
Lin Q and Mao W: MicroRNA-181a inhibits tumor proliferation,
invasiveness, and metastasis and is downregulated in gastric
cancer. Oncol Res. 22:75–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shin VY and Chu KM: miRNA as potential
biomarkers and therapeutic targets for gastric cancer. World J
Gastroenterol. 20:10432–10439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang C, Chen X, Alattar M, Wei J and Liu
H: MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis
of gastric cancer. Cancer Gene Ther. 22:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen S, Zhu J, Yu F, Tian Y, Ma S and Liu
X: Combination of miRNA and RNA functions as potential biomarkers
for gastric cancer. Tumour Biol. 36:9909–9918. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miyashita K, Nakada M, Shakoori A,
Ishigaki Y, Shimasaki T, Motoo Y, Kawakami K and Minamoto T: An
emerging strategy for cancer treatment targeting aberrant glycogen
synthase kinase 3 beta. Anticancer Agents Med Chem. 9:1114–1122.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sahin I, Eturi A, De Souza A, Pamarthy S,
Tavora F, Giles FJ and Carneiro BA: Glycogen synthase kinase-3 beta
inhibitors as novel cancer treatments and modulators of antitumor
immune responses. Cancer Biol Ther. 20:1047–1056. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu Y, Zhang G, Zou C, Qi W, Gong Z, Zhang
G, Ma G, Zhang W and Jiang P: Long non-coding RNA LINC01225
promotes proliferation, invasion and migration of gastric cancer
via Wnt/β-catenin signalling pathway. J Cell Mol Med. 23:7581–7591.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu J, Liu L, Wan JX and Song Y: Long
noncoding RNA SNHG20 promotes gastric cancer progression by
inhibiting p21 expression and regulating the GSK-3β/β-catenin
signaling pathway. Oncotarget. 8:80700–80708. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li ZT, Zhang X, Wang DW, Xu J, Kou KJ,
Wang ZW, Yong G, Liang DS and Sun XY: Overexpressed lncRNA
GATA6-AS1 inhibits LNM and EMT via FZD4 through the Wnt/β-catenin
signaling pathway in GC. Mol Ther Nucleic Acids. 19:827–840. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang H, Wu M, Lu Y, He K, Cai X, Yu X, Lu
J and Teng L: lncRNA MIR4435-2HG targets desmoplakin and promotes
growth and metastasis of gastric cancer by activating Wnt/β-catenin
signaling. Aging (Albany NY). 11:6657–6673. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang F, Li Y, Xu W, He L, Tan Y and Xu H:
Long non-coding RNA ZFAS1 regulates the malignant progression of
gastric cancer via the microRNA-200b-3p/Wnt1 axis. Biosci
Biotechnol Biochem. 83:1289–1299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu X, Zhang P, Zhu H, Li S, Chen X and Shi
L: Long noncoding RNA FEZF1-AS1 indicates a poor prognosis of
gastric cancer and promotes tumorigenesis via activation of Wnt
signaling pathway. Biomed Pharmacother. 96:1103–1108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang L, Wen JB, Wen P, Li X, Gong M and Li
Q: Long non-coding RNA LINC01314 represses cell migration,
invasion, and angiogenesis in gastric cancer via the Wnt/β-catenin
signaling pathway by down-regulating KLK4. Cancer Cell Int.
19:942019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gu W, Ren JH, Zheng X, Hu XY and Hu MJ:
Comprehensive analysis of expression profiles of long non-coding
RNAs with associated ceRNA network involved in gastric cancer
progression. Mol Med Rep. 20:2209–2218. 2019.PubMed/NCBI
|
|
48
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu Y, Zhu J, Ma X, Han S, Xiao D, Jia Y
and Wang Y: ceRNA network construction and comparison of gastric
cancer with or without Helicobacter pylori infection. J Cell
Physiol. 234:7128–7140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Karreth FA and Pandolfi PP: ceRNA
cross-talk in cancer: When ce-bling rivalries go awry. Cancer
Discov. 3:1113–1121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu J, Li Y, Lu J, Pan T, Ding N, Wang Z,
Shao T, Zhang J, Wang L and Li X: The mRNA related ceRNA-ceRNA
landscape and significance across 20 major cancer types. Nucleic
Acids Res. 43:8169–8182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guo T, Yuan X, Liu DF, Peng SH and Xu AM:
lncRNA HOXA11-AS promotes migration and invasion through modulating
miR-148a/WNT1/β-catenin pathway in gastric cancer. Neoplasma.
67:492–500. 2020.PubMed/NCBI
|
|
53
|
Luo Y, Tan W, Jia W, Liu Z, Ye P, Fu Z, Lu
F, Xiang W, Tang L, Yao L, et al: The long non-coding RNA LINC01606
contributes to the metastasis and invasion of human gastric cancer
and is associated with Wnt/β-catenin signaling. Int J Biochem Cell
Biol. 103:125–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J,
Tang Z, Liao QX, Zhang H, Zeng LS and Cui SZ: LINC01133 as ceRNA
inhibits gastric cancer progression by sponging miR-106a-3p to
regulate APC expression and the Wnt/β-catenin pathway. Mol Cancer.
17:1262018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang K, Zhi XH, Ma YY and Zhou LQ: Long
non-coding RNA TOB1-AS1 modulates cell proliferation, apoptosis,
migration and invasion through miR-23a/NEU1 axis via Wnt/β-catenin
pathway in gastric cancer. Eur Rev Med Pharmacol Sci. 23:9890–9899.
2019.PubMed/NCBI
|
|
56
|
Li HL, Liang S, Cui JH and Han GY:
Targeting of GSK-3β by miR-214 to facilitate gastric cancer cell
proliferation and decrease of cell apoptosis. Eur Rev Med Pharmacol
Sci. 22:127–134. 2018.PubMed/NCBI
|
|
57
|
Fan D, Ren B, Yang X, Liu J and Zhang Z:
Upregulation of miR-501-5p activates the wnt/β-catenin signaling
pathway and enhances stem cell-like phenotype in gastric cancer. J
Exp Clin Cancer Res. 35:1772016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Willert K and Nusse R: Wnt proteins. Cold
Spring Harb Perspect Biol. 4:a0078642012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Alok A, Lei Z, Jagannathan NS, Kaur S,
Harmston N, Rozen SG, Tucker-Kellogg L and Virshup DM: Wnt proteins
synergize to activate β-catenin signaling. J Cell Sci.
130:1532–1544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cha Y, He Y, Ouyang K, Xiong H, Li J and
Yuan X: MicroRNA-140-5p suppresses cell proliferation and invasion
in gastric cancer by targeting WNT1 in the WNT/β-catenin signaling
pathway. Oncol Lett. 16:6369–6376. 2018.PubMed/NCBI
|
|
61
|
Tang H, Kong Y, Guo J, Tang Y and Xie X,
Yang L, Su Q and Xie X: Diallyl disulfide suppresses proliferation
and induces apoptosis in human gastric cancer through Wnt-1
signaling pathway by up-regulation of miR-200b and miR-22. Cancer
Lett. 340:72–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Su B, Su J, Zeng Y, Ding E, Liu F, Tan T,
Xia H, Wu YH, Zeng X, Ling H, et al: Diallyl disulfide inhibits
TGF-β1-induced upregulation of Rac1 and β-catenin in
epithelial-mesenchymal transition and tumor growth of gastric
cancer. Oncol Rep. 39:2797–2806. 2018.PubMed/NCBI
|
|
63
|
Xiang SL, Xiao XL, Ling H, Liao QJ, Zhou
XT, Dong L and Su Q: Antitumor effect of diallyl disulfide on human
gastric cancer MGC803 cells xenograft in nude mice. Ai Zheng.
24:940–944. 2005.(In Chinese). PubMed/NCBI
|
|
64
|
Sun R, Liu Z, Tong D, Yang Y, Guo B, Wang
X, Zhao L and Huang C: miR-491-5p, mediated by Foxi1, functions as
a tumor suppressor by targeting Wnt3a/β-catenin signaling in the
development of gastric cancer. Cell Death Dis. 8:e27142017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Song J, Guan Z, Li M, Sha S, Song C, Gao Z
and Zhao Y: MicroRNA-154 inhibits the growth and invasion of
gastric cancer cells by targeting DIXDC1/WNT signaling. Oncol Res.
26:847–856. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song B, Lin HX, Dong LL, Ma JJ and Jiang
ZG: MicroRNA-338 inhibits proliferation, migration, and invasion of
gastric cancer cells by the Wnt/β-catenin signaling pathway. Eur
Rev Med Pharmacol Sci. 22:1290–1296. 2018.PubMed/NCBI
|
|
67
|
Li W, Li J, Mu H, Guo M and Deng H:
miR-503 suppresses cell proliferation and invasion of gastric
cancer by targeting HMGA2 and inactivating WNT signaling pathway.
Cancer Cell Int. 19:1642019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cong N, Du P, Zhang A, Shen F, Su J, Pu P,
Wang T, Zjang J, Kang C and Zhang Q: Downregulated microRNA-200a
promotes EMT and tumor growth through the wnt/β-catenin pathway by
targeting the E-cadherin repressors ZEB1/ZEB2 in gastric
adenocarcinoma. Oncol Rep. 29:1579–1587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Conde-Perez A, Gros G, Longvert C,
Pedersen M, Petit V, Aktary Z, Viros A, Gesbert F, Delmas V, Rambow
F, et al: A caveolin-dependent and PI3K/AKT-independent role of
PTEN in β-catenin transcriptional activity. Nat Commun. 6:80932015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
de Araujo WM, Robbs BK, Bastos LG, de
Souza WF, Vidal FC, Viola JP and Morgado-Diaz JA: PTEN
overexpression cooperates with lithium to reduce the malignancy and
to increase cell death by apoptosis via PI3K/Akt suppression in
colorectal cancer cells. J Cell Biochem. 117:458–469. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jang H, Lee OH, Lee Y, Yoon H, Chang EM,
Park M, Lee JW, Hong K, Kim JO, Kim NK, et al: Melatonin prevents
cisplatin-induced primordial follicle loss via suppression of
PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal
Res. 60:336–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Perumal E, So Youn K, Sun S, Seung-Hyun J,
Suji M, Jieying L and Yeun-Jun C: PTEN inactivation induces
epithelial-mesenchymal transition and metastasis by intranuclear
translocation of β-catenin and snail/slug in non-small cell lung
carcinoma cells. Lung Cancer. 130:25–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ma J, Guo X, Zhang J, Wu D, Hu X, Li J,
Lan Q, Liu Y and Dong W: PTEN gene induces cell invasion and
migration via regulating AKT/GSK-3β/β-catenin signaling pathway in
human gastric cancer. Dig Dis Sci. 62:3415–3425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Y, Yan X, Shi J, He Y, Xu J, Lin L,
Chen W and Lin X and Lin X: Aberrantly expressed miR-188-5p
promotes gastric cancer metastasis by activating Wnt/β-catenin
signaling. BMC Cancer. 19:5052019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Katoh Y and Katoh M: Hedgehog signaling
pathway and gastrointestinal stem cell signaling network (Review).
Int J Mol Med. 18:1019–1023. 2006.PubMed/NCBI
|
|
76
|
Katoh Y and Katoh M: Hedgehog target
genes: Mechanisms of carcinogenesis induced by aberrant hedgehog
signaling activation. Curr Mol Med. 9:873–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Katoh M: Genomic testing, tumor
microenvironment and targeted therapy of hedgehog-related human
cancers. Clin Sci (Lond). 133:953–970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Maniatis T: A ubiquitin ligase complex
essential for the NF-kappaB, Wnt/Wingless, and hedgehog signaling
pathways. Genes Dev. 13:505–510. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Min TH, Kriebel M, Hou S and Pera EM: The
dual regulator Sufu integrates hedgehog and Wnt signals in the
early xenopus embryo. Dev Biol. 358:262–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Taylor MD, Zhang X, Liu L, Hui CC,
Mainprize TG, Scherer SW, Wainwright B, Hogg D and Rutka JT:
Failure of a medulloblastoma-derived mutant of SUFU to suppress WNT
signaling. Oncogene. 23:4577–4583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang L, Huang S, Bian Y, Ma X, Zhang H and
Xie J: Identification of signature genes for detecting hedgehog
signaling activation in gastric cancer. Mol Med Rep. 3:473–478.
2010.PubMed/NCBI
|
|
82
|
Yan R, Peng X, Yuan X, Huang D, Chen J, Lu
Q, Lv N and Luo S: Suppression of growth and migration by blocking
the hedgehog signaling pathway in gastric cancer cells. Cell Oncol
(Dordr). 36:421–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Katoh Y and Katoh M: Hedgehog signaling
pathway and gastric cancer. Cancer Biol Ther. 4:1050–1054. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Peng Y, Zhang X, Ma Q, Yan R, Qin Y, Zhao
Y, Cheng Y, Yang M, Wang Q, Feng X, et al: miRNA-194 activates the
Wnt/β-catenin signaling pathway in gastric cancer by targeting the
negative Wnt regulator, SUFU. Cancer Lett. 385:117–127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Peng Y, Zhang X, Lin H, Deng S, Huang Y,
Qin Y, Feng X, Yan R, Zhao Y, Cheng Y, et al: Inhibition of miR-194
suppresses the Wnt/β-catenin signalling pathway in gastric cancer.
Oncol Rep. 40:3323–3334. 2018.PubMed/NCBI
|
|
86
|
Sun GL, Li Z, Wang WZ, Chen Z, Zhang L, Li
Q, Wei S, Li BW, Xu JH, Chen L, et al: miR-324-3p promotes gastric
cancer development by activating Smad4-mediated Wnt/beta-catenin
signaling pathway. J Gastroenterol. 53:725–739. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li C, Dong J, Han Z and Zhang K:
MicroRNA-219-5p represses the proliferation, migration, and
invasion of gastric cancer cells by targeting the
LRH-1/Wnt/β-catenin signaling pathway. Oncol Res. 25:617–627. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Huang J, He Y, Mcleod HL, Xie Y, Xiao D,
Hu H, Chen P, Shen L, Zeng S, Yin X, et al: miR-302b inhibits
tumorigenesis by targeting EphA2 via Wnt/β-catenin/EMT signaling
cascade in gastric cancer. BMC Cancer. 17:8862017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xu K and Zhao YC: MEF2D/Wnt/β-catenin
pathway regulates the proliferation of gastric cancer cells and is
regulated by microRNA-19. Tumour Biol. 37:9059–9069. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fang Z, Zhong M, Wang Y, Yuan X, Guo H,
Yao Y, Feng M, Chen J, Xiong J and Xiang X: miR-381 and miR-489
suppress cell proliferation and invasion by targeting CUL4B via the
Wnt/β-catenin pathway in gastric cancer. Int J Oncol. 54:733–743.
2019.PubMed/NCBI
|
|
91
|
Arnold M, Park JY, Camargo MC, Lunet N,
Forman D and Soerjomataram I: Is gastric cancer becoming a rare
disease? A global assessment of predicted incidence trends to 2035.
Gut. 69:823–829. 2020. View Article : Google Scholar : PubMed/NCBI
|