Introduction
Oral squamous cell carcinoma (OSCC) is a malignant
epithelial cancer that threatens the health of individuals
worldwide and OSCC frequently occurs in the tongue and buccal
mucosa (1–3). The 5-year survival for human OSCC
patients is approximately 50% and the incidence of OSCC is still
increasing with a reported annual incidence worldwide of
approximately 275,000 (4,5) and 50,000 in China (6). However, the detailed mechanisms of the
tumor formation, progression and metastasis of OSCC require further
in-depth research. Therefore, it has been a difficult quest for
researchers to elucidate the mechanisms of OSCC progression and
metastasis and to identify new methods for the early diagnosis and
treatment for OSCC.
Long non-coding RNAs (lncRNAs) are a diverse class
of RNA transcripts longer than 200 nucleotides that have limited
protein coding capacity (7,8). lncRNAs have been found to participate
in various biological processes in cancers such as cervical cancer,
prostate carcinoma, epithelial ovarian cancer and breast cancer,
including tumorigenesis, metastasis and chemotherapy resistance
(9–12). Recent evidence shows that lncRNAs
play oncogenic or tumor-suppressor roles in OSCC (13–15).
For example, LINC01133 was found to inhibit OSCC metastasis by
regulating GDF15 (14). Zhu et
al demonstrated that the upregulation of lncHAS2-AS1 plays
important roles in the invasiveness of OSCC (13). Jin et al found that lncRNA
MORT inhibited cell proliferation by repressing ROCK1 in OSCC
(15). lncRNA plasmacytoma variant
translocation 1 (PVT1) has been demonstrated to be increased and to
function as an oncogenic gene to promote tumor formation, invasion,
migration and resistance in many types of cancers (16–21),
such as glioma (16), colorectal
cancer (17), gastric cancer
(18), ovarian cancer (19) and gallbladder cancer (20). However, the roles of PVT1 in OSCC
remain unknown.
MicroRNAs (miRNAs/miRs) are approximately 18–22
nucleotides in length and cannot be translated into protein as
well, yet miRNAs are verified to play critical roles in the
biological functions of various diseases via directly targeting
downstream target genes (22–26).
Salmena et al firstly reported that lncRNAs interact with
miRNAs through ‘competing endogenous RNAs’ (ceRNAs), which indicate
that lncRNAs interact with miRNAs (27,28)
and this hypothesis has been proven in various pathological
conditions and diseases (29–33).
miR-150-5p was found to participate in the biological processes of
various cancers (34–36). For example, miR-150-5p is important
in inhibiting the metastasis of human non-small cell lung cancer
(NSCLC) (35). Chen et al
demonstrated that miR-150-5p repressed progression of colorectal
cancer (36). Lu et al
revealed that miR-150-5p participated in the processes of
proliferation, migration and invasion in NSCLC (34). However, whether it plays similar
roles in OSCC remains unknown.
In the present study, we investigated expression
levels of PVT1 in OSCC tissues and adjacent tissues. Then we
observed cell proliferation, invasion and migration in OSCC cells
after PVT1 was overexpressed and downregulated. Bioinformatics
predicted that PVT1 may competitively bind with miR-150-5p, which
could then target by binding with glucose transporter type 1
(GLUT1). GLUT1 was reported to be an oncogenic gene that was
associated with tumor cell proliferation, tumorigenesis, glucose
metabolism and resistance in some cancers, such as urinary bladder,
non-small cell lung and prostate cancer (37–39).
We uncovered that expression of PVT1 was increased in human OSCC
patients and cell lines. As a result, we aimed to ascertain the
functions of PVT1 in OSCC. The results revealed that PVT1
expression is increased in OSCC tissues and it plays some roles in
OSCC, such as regulating cell proliferation, cell apoptosis,
invasion and migration in OSCC.
Materials and methods
Patient tissues
A total of 70 paired cancer and adjacent tissues
were collected from patients with OSCC from October 2012 to
September 2013 at the First Affiliated Hospital of Jinzhou Medical
University (Jinzhou, Liaoning, China). Totally, 34 male and 36
female patients were recruited and their mean age was 48.4±10.3
years. All tissues were cut into small pieces approximately 40 mg
and frozen at −80°C. Two groups were divided according to the mean
expression of PVT1: PVT1 high expression and low expression groups.
The Declaration of Helsinki was consulted during the human study.
We received informed consent from all patients and the study was
approved by the Faculty of Medicine's Ethics Committee of The First
Affiliated Hospital of Jinzhou Medical University (ethical approval
no. JYD160923).
Cell culture
Human normal oral epithelial cell line NOK and OSCC
cell lines, including SCC-090, SCC-25 and CAL-27 were purchased
from the American Type Culture Collection (ATCC). The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml) and
streptomycin (100 µg/ml). The cells were cultured in an incubator
at 37°C with 5% CO2.
Construction of lentivirus and cell
transfection
The full length of human PVT1 cDNA was constructed
into a lentivirus (Shanghai GenePharma Co., Ltd.), resulting in
PVT1 overexpression, and was named LV-PVT1 and the negative control
(LV-NC) was obtained from GenePharma. Furthermore, PVT1-shRNA
(LV-sh PVT1) was synthesized and cloned into a lentivirus, resulted
in PVT1 downregulation and the shRNA negative control (LV-sh NC)
was obtained from GenePharma. The sequences were as follow:
PVT1-shRNA, 5′-CCUGAUGGAUUUACAGUGATT-3′ and NC-shRNA,
5′-GCUACGAUCUGCCUAAGAUTT-3′. LV-PVT1 or LV-NC or LV-sh PVT1 or
LV-sh NC was respectively infected into SCC-25 and CAL-27 cells
according to the manufacturer's instructions. After antibiotic
selection and constructed for 2 weeks, the stable cells with PVT1
overexpression or downregulation were obtained. Cells were plated
in 6-well plates (1×106/well) until reaching 60–70%
confluence. Transfection reagent Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), serum-free DMEM and hsa-miR-150-5p
mimics (Guangzhou RiboBio Co., Ltd, miR10000451-1-5, 5 nmol) were
mixed and incubated at room temperature for 30 min, which were
added into the prepared 6-well plates with complete medium with 10%
FBS.
CCK-8 assay
Cell proliferation abilities were examined using
CCK-8 assay (Dojundo) in accordance with the supplier's protocol.
Cells (1×103/well) were plated in 96-well plates and
transfected with the indicated lentivirus and mimics for 24 h. Each
group was set with three replicate wells. For each well, 10 µl
CCK-8 was added at 0, 1, 2 and 3 days, and then incubation was
carried out in darkness for another 3 h at room temperature.
Finally, the absorbance (OD) value was measured at 450 nm with a
microplate reader.
Cell invasion and migration
assays
Cells (2×105 cells/well) were diluted in
200 µl serum-free DMEM and inoculated onto the upper wells of a
Transwell chamber coated with 20 µl Matrigel (BD Biosciences). DMEM
with 10% FBS was added into the lower chambers, which were
incubated for 48 h. Then the non-invasive cells were removed by
using a cotton swab following overnight incubation. The invasive
cells in the lower chambers were fixed and stained with 100%
pre-cold methanol and 0.1% crystal violet at room temperature for
30 min. Finally, the cells were observed by using an inverted light
microscope (magnification, ×200, Olympus Corp.). For the cell
migration assay, cells were suspended in FBS-free DMEM with 1 µg/ml
Mitomycin C and plated in the upper wells of the Transwell chamber
and DMEM with 10% FBS was mixed into the lower chambers. Then the
following steps were similar to the invasion assay. Five visual
fields of each chamber were randomly chosen and cell number was
counted under a light microscope (magnification ×100; Olympus
Corp.; Japan Nikon Corp.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA of tissues and cells were extracted by
using Trizol (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. For the quantitative detection of
genes, RT was conducted at 37°C for 15 min, which was followed by
85°C for 5 sec with a PrimeScript™ RT Reagent Kit (Takara Bio,
Inc.) according to the manufacturer's protocol. PCR primers were
purchased from ShangHai Gene Pharma and mRNA expression was
detected by SYBR Premix Ex Taq II (Takara Bio, Inc.). Two-step PCR
amplification was performed at 95°C for 180 sec, which were then
followed by 40 cycles at 95°C for 10 sec and 60°C for 30 sec. Final
extension was performed at 72°C for 5 min. Expression levels were
normalized to β-actin and U6. The 2−∆∆Cq method
(40) was used to calculate the
relative gene expression. Gene primers are listed in Table I.
 | Table I.Sequences of primers for qPCR. |
Table I.
Sequences of primers for qPCR.
| Genes | Primer
sequences |
|---|
| PVT1 | F:
5′-TGAGAACTGTCCTTACGTGACC-3′ |
|
| R:
5′-AGAGCACCAAGACTGGCTCT-3′ |
|
miR-150-5p | F:
5′-CGGCGGCAAAGTGCTTACAG-3′ |
|
| R:
5′-GGGCATACATCGGCTAATACA-3′ |
| GLUT-1 | F:
5′-GTCAACACGGCCTTCACTG-3′ |
|
| R:
5′-GGTCATGAGTATGGCACAACC-3′ |
| β-actin | F:
5′-CCAAGGCCAACCGCGAGAAGAT-3′ |
|
| R:
5′-AGGGTACATGGTGGTGCCGCCA-3′ |
| U6 | F:
5′-CGCTTCGGCAGCACATATACT-3′ |
|
| R:
5′-CGCTTCACGAATTTGCGTGTC-3′ |
Protein extraction and western blot
analysis
Total proteins of tissues, mouse tumors and cells
were extracted by using 150 µl PRO-PREP Protein Extraction Solution
(iNtRON), which were measured by BCA protein assay kit (Beyotime
Institute of Biotechnology). Protein (40 µg) was added to 10%
SDS-PAGE and the separated protein was transferred onto
polyvinylidene fluoride membranes (PVDF; EMD Millipore), which were
blocked by 5% non-fat milk for 1 h. Then TBST (Boster) was used to
wash the membranes for three times, which were incubated with
primary antibodies overnight at 4°C, which were purchased from
Abcam. The antibodies used in the western blot analysis are listed
in Table II. After that, they were
incubated with matched secondary antibodies (goat anti-rabbit,
dilution 1:5,000, product code ab150077; goat anti-mouse, dilution
1:5,000; product code ab150113) for 1 h. Finally, the proteins were
visualized and quantified by using Pierce ECL Western blot
substrate (Thermo Fisher Scientific, Inc.) with ECL detection
system (Thermo Fisher Scientific, Inc.) and proteins were
normalized to β-actin. The gray value of the target band was
analyzed by using Image Lab (version 3.1; Bio-Rad Laboratories,
Inc.).
 | Table II.Antibodies used in western blot
analysis. |
Table II.
Antibodies used in western blot
analysis.
| Proteins | Host | kDa | Catalog no. | Antibody
dilution |
|---|
| Ki67 | Rabbit | 359 | ab16667 | 1:5,000 |
| Bcl-2 | Rabbit | 26 | ab182858 | 1:1,000 |
| Bax | Rabbit | 21 | ab32503 | 1:1,000 |
| Cleaved
caspase-3 | Rabbit | 17 | ab49822 | 1:500 |
| MMP-9 | Rabbit | 92 | ab38898 | 1:1,000 |
| MMP-14 | Rabbit | 66 | ab51074 | 1:2,000 |
| GLUT-1 | Rabbit | 55 | ab40084 | 1:5,000 |
| β-actin | Rabbit | 42 | ab179467 | 1:5,000 |
Luciferase assay
StarBase v2.0 database (41) was used to analyze the potential
miRNAs that interact with PVT1. miR-150-5p was identified as a
potential miRNA. Human PVT1-3′-untranslated region (3′-UTR) and
GLUT-1-3′-UTR reporter constructs containing the potential binding
site of miR-150-5p, and their identical sequences with a mutation
in the seed sequence, which were constructed into pmiR-GLO-basic
reporter vector (Promega Corp.). Cells (2×105
cells/well) were plated into 48-well plates for 12 h,
co-transfected with the indicated plasmids and miR-150-5p mimics
for 24 h. The plasmids (200 ng) were mixed with Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), Renilla
luciferase plasmids and DMEM for 30 min and then added into the
prepared cells for 24 h. Finally, the luciferase activities were
measured by the Dual-Luciferase Assay kit (Promega Corp.) according
to the manufacturer's instructions. Every sample was tested for
three times and the whole experiment was repeated for three
times.
In vivo nude mouse xenograft
assay
Twelve female BALB/c nude mice, about 4-weeks of age
and weighing 15–20 g, were purchased from the Animal Center of
Shanghai Jiaotong University (Shanghai, China), and were housed in
humidity- and temperature-controlled rooms (40–80%; 22±2°C) for 7
days prior to the experiments. All animal handling and experimental
procedures were approved by the Animal Ethics Committees of the
First Affiliated Hospital of Jinzhou Medical University and in
accordance with the guidelines of the China Council of Animal Care.
Two groups were randomly divided, as the LV-NC and LV-shPVT1 group.
Mice in each group were injected subcutaneously on the right axilla
with 1×107/0.1 ml of SCC-25 cells transfected with
LV-shPVT1 or LV-NC. After successful transplantation, the tumor
volumes of nude mice in two groups were observed every week. The
tumor volume (V) was calculated as V=π/6 × length × width × height.
After 35 days, mice were sacrificed with isoflurane (4%) for 1 min
and cervical dislocation was performed to verify euthanasia,
finally, the tumor tissues were obtained.
Statistical analysis
All data were analyzed by SPSS 19.0 (IBM Corp.) and
GraphPad Prism 6.0 (GraphPad Software). Student's t-test was used
to analyze the statistical significance between two groups, and
one-way ANOVA and Tukey's post hoc test method were used to analyze
the statistical significance among more than two groups, while
Dunnett's test was used when each group was only compared to the
control group. Count data were processed by Chi-square test.
Correlations between PVT1, miR-150-5p and GLUT-1 were analyzed by
using Pearson's correlation analysis. Survival curves were analyzed
by using Kaplan-Meier survival test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PVT1 is upregulated in OSCC tumor
tissues and cell lines
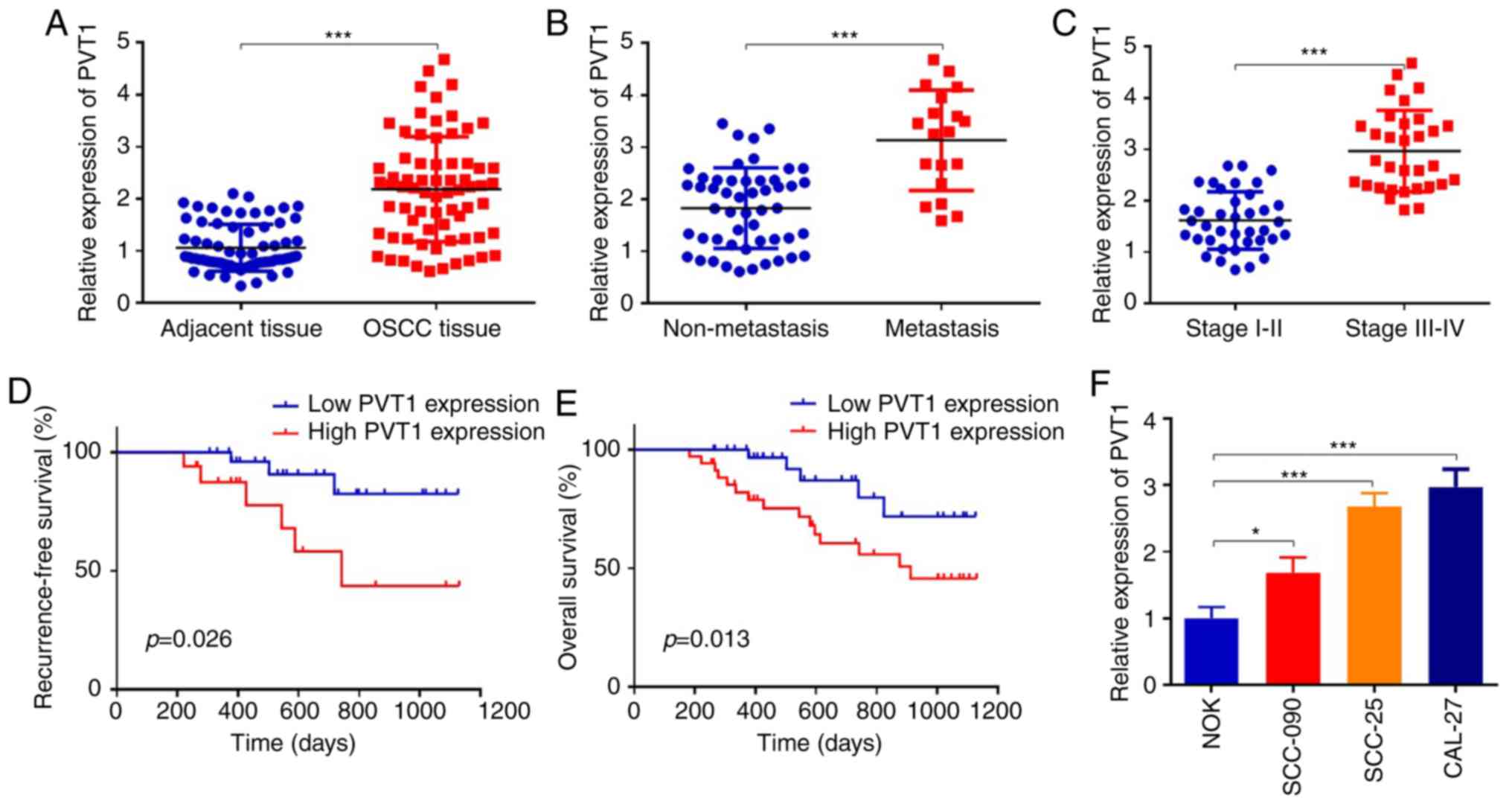
First, we determined the expression of PVT1 in 70
cases of OSCC tissues and adjacent tissues. PVT1 was increased
2.2-fold in OSCC tissues when compared to the adjacent tissues
(Fig. 1A) (P<0.001).
Furthermore, we collected and analyzed the correlations between
PVT1 and clinicopathological features in the OSCC patients. PVT1
upregulation was correlated with advanced TNM stage, metastasis and
recurrence (Table III)
(P<0.05). Furthermore, we found that PVT1 was increased 1.7-fold
in the OSCC tissues with metastasis (n=19), compared to that in
OSCC tissues without metastasis (n=51) (Fig. 1B) (P<0.001). We also divided
patients into two groups according to the TNM stage, and the
results showed that the expression levels of PVT1 in stage III–IV
were significantly higher than those in stage I–II (Fig. 1C) (P<0.001). To explore the
prognostic values of PVT1 in OSCC patients, Kaplan-Meier analysis
was performed. Results revealed that patients with high expression
of PVT1 had a worse recurrence-free and overall survival when
compared with patients in the low PVT1 expression group (Fig. 1D and E). Then we detected PVT1
expression in human normal oral epithelial cell line NOK and OSCC
cell lines, including SCC-090, SCC-25 and CAL-27. The results
revealed that expression of PVT1 was significantly increased in the
human OSCC cells when compared to that in the NOK cells (Fig. 1F). As the expression levels of PVT1
in SCC-25 and CAL-27 were much higher than other cell lines,
therefore, we chose SCC-25 and CAL-27 cell lines for subsequent
experiments. These data indicated that PVT1 was upregulated in
OSCC, and was correlated with a poor prognosis of OSCC patients;
however, the detailed mechanisms remained unknown.
 | Table III.Association between PVT1 expression
and the clinicopathological features of the OSCC patients. |
Table III.
Association between PVT1 expression
and the clinicopathological features of the OSCC patients.
| Parameters | Low expression
(n=35) | High expression
(n=35) | P-value |
|---|
| Sex |
|
| 0.339 |
|
Male | 15 | 19 |
|
|
Female | 20 | 16 |
|
| Age (years) |
|
| 0.147 |
|
<45 | 18 | 12 |
|
|
≥45 | 17 | 23 |
|
| TNM stage |
|
| 0.016 |
| I | 15 | 5 |
|
| II | 10 | 8 |
|
|
III | 7 | 12 |
|
| IV | 3 | 10 |
|
| Metastasis |
|
| 0.015 |
|
Yes | 5 | 14 |
|
| No | 30 | 21 |
|
| Recurrence |
|
| 0.006 |
|
Yes | 7 | 18 |
|
| No | 28 | 17 |
|
Overexpression of PVT1 promotes cell
proliferation, invasion, migration and inhibits apoptosis in OSCC
cells
To explore the functions of PVT1 in OSCC, LV-PVT1
was constructed, which resulted in PVT1 overexpression. After
LV-PVT1 infection, PVT1 expression was significantly increased
compared to the LV-NC group (Fig.
2A) (P<0.001). CCK-8 assay showed that PVT1 upregulation
significantly promoted the cell proliferation of SCC-25 and CAL-27
cells at 3 and 5 days (Fig. 2B and
C). Furthermore, Transwell invasion assays indicated that
increased expression of PVT1 significantly enhanced SCC-25 and
CAL-27 cell invasion (Fig. 2D and
E) (P<0.001), and the same results were found for the
migration assays (Fig. 2F and G)
(P<0.001). Moreover, we detected apoptotic-associated protein
expression, such as Ki67, Bcl-2, Bax and cleaved caspase 3, and
also detected expression levels of migration-associated proteins,
such as MMP-9 and MMP-14 in the OSCC cell lines SCC-25 and CAL-27.
Western blot analysis demonstrated that protein expression of Ki67
and Bcl-2 were significantly increased, the apoptotic genes Bax and
cleaved caspase 3 were significantly decreased and
migration-related MMP-9 and MMP-14 were significantly increased
after LV-PVT1 infection (Fig.
2H-K). Collectively, these data indicated that overexpression
of PVT1 enhanced cell proliferation, invasion, migration and
inhibited apoptosis in OSCC cells.
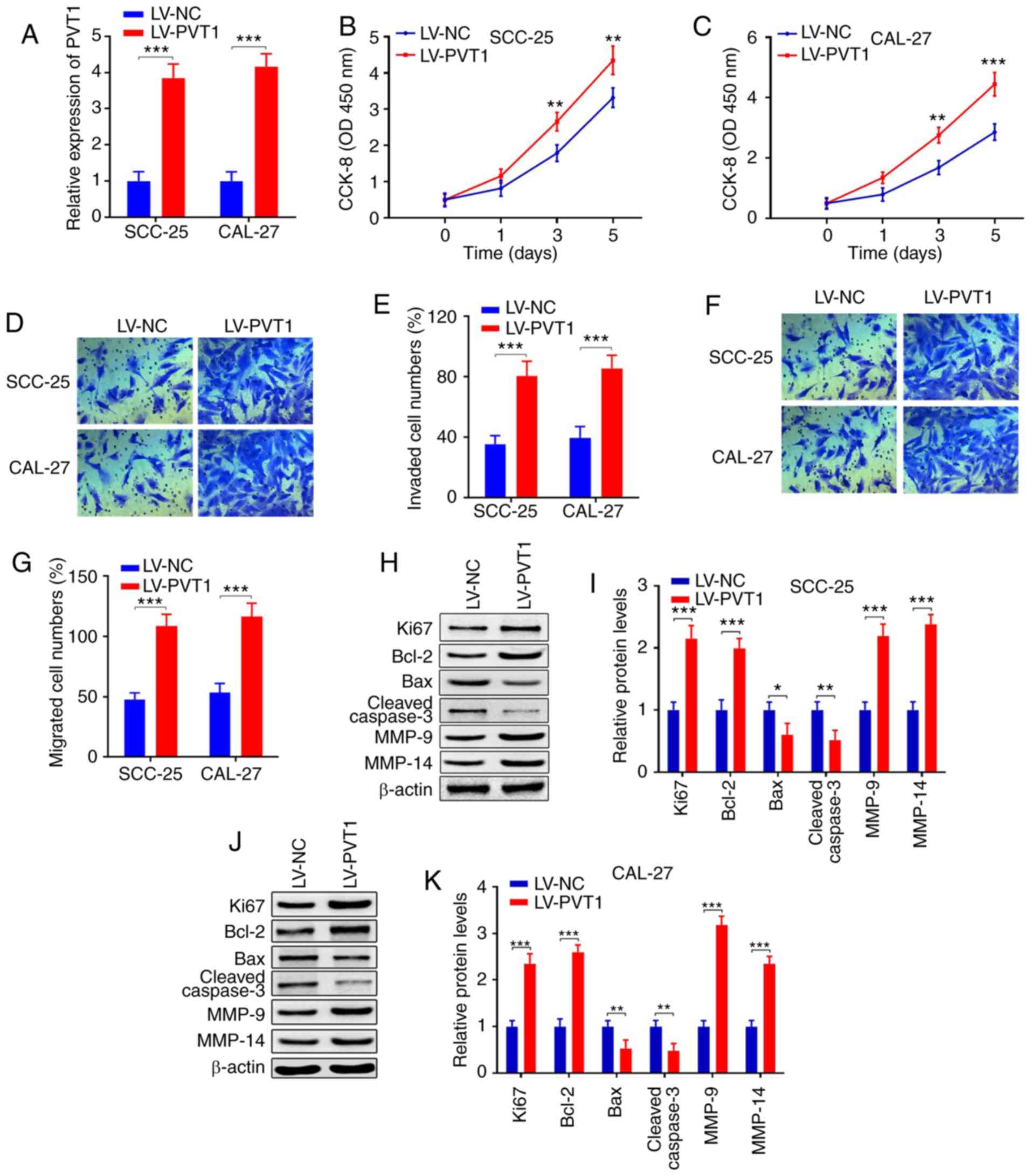 | Figure 2.Overexpression of PVT1 promotes cell
proliferation, invasion, migration and inhibits apoptosis in OSCC.
(A) qPCR showed that PVT1 expression was upregulated in SCC-25 and
CAL-27 cells transfected with LV-PVT1 when compared with the LV-NC
group. (B and C) CCK-8 assay revealed that the proliferation
abilities were promoted in cell lines transfected with LV-PVT1 when
compared with the LV-NC group. (D-G) Transwell assays indicated
that invasion and migration were both enhanced in cell lines
transfected with LV-PVT1 when compared with the LV-NC group. (H-K)
Protein expression levels of Ki67, Bcl-2, Bax, cleaved caspase 3,
MMP-9 and MMP-14 were detected by western blot analysis in SCC-25
and CAL-27 cell lines transfected with LV-PVT1 when compared with
the LV-NC group (*P<0.05; **P<0.01, ***P<0.001). OSCC,
PVT1, lncRNA plasmacytoma variant translocation 1; OSCC, oral
squamous cell carcinoma; Bcl-2, B-cell lymphoma 2; Bax, bcl-2-like
protein 4; MMP, matrix metalloproteinase; qPCR, real-time
quantitative PCR. |
Downregulation of PVT1 suppresses cell
proliferation, invasion, migration and promotes apoptosis in
OSCC
To further verify the functions of PVT1 in OSCC
SCC-25 and CAL-27 cell lines, the LV-shPVT1 was constructed, which
resulted in PVT1 downregulation. After LV-sh PVT1 infection into
SCC-25 and CAL-27, the PVT1 levels were significantly inhibited
compared with the cells transfected with LV-sh NC (Fig. 3A) (P<0.001). CCK-8 assay revealed
that PVT1 downregulation significantly suppressed cell
proliferation compared with the LV-sh NC (Fig. 3B and C). Furthermore, Transwell
assays indicated that downregulation of PVT1 significantly
inhibited cell invasion and migration (Fig. 3D-G) (P<0.001). Moreover, protein
expression levels of Ki67 and Bcl-2 were significantly decreased,
the apoptotic genes Bax and cleaved caspase3 were upregulated and
the migration-associated genes MMP-9 and MMP-14 were significantly
inhibited following with LV-shPVT1 infection when compared with the
LV-sh NC group (Fig. 3H-K).
Collectively, these data indicate that PVT1 is vitally important in
cell apoptosis, proliferation, invasion and migration in OSCC.
However, the detailed mechanisms of PVT1 in tumorigenesis, invasion
and migration in OSCC remain unknown.
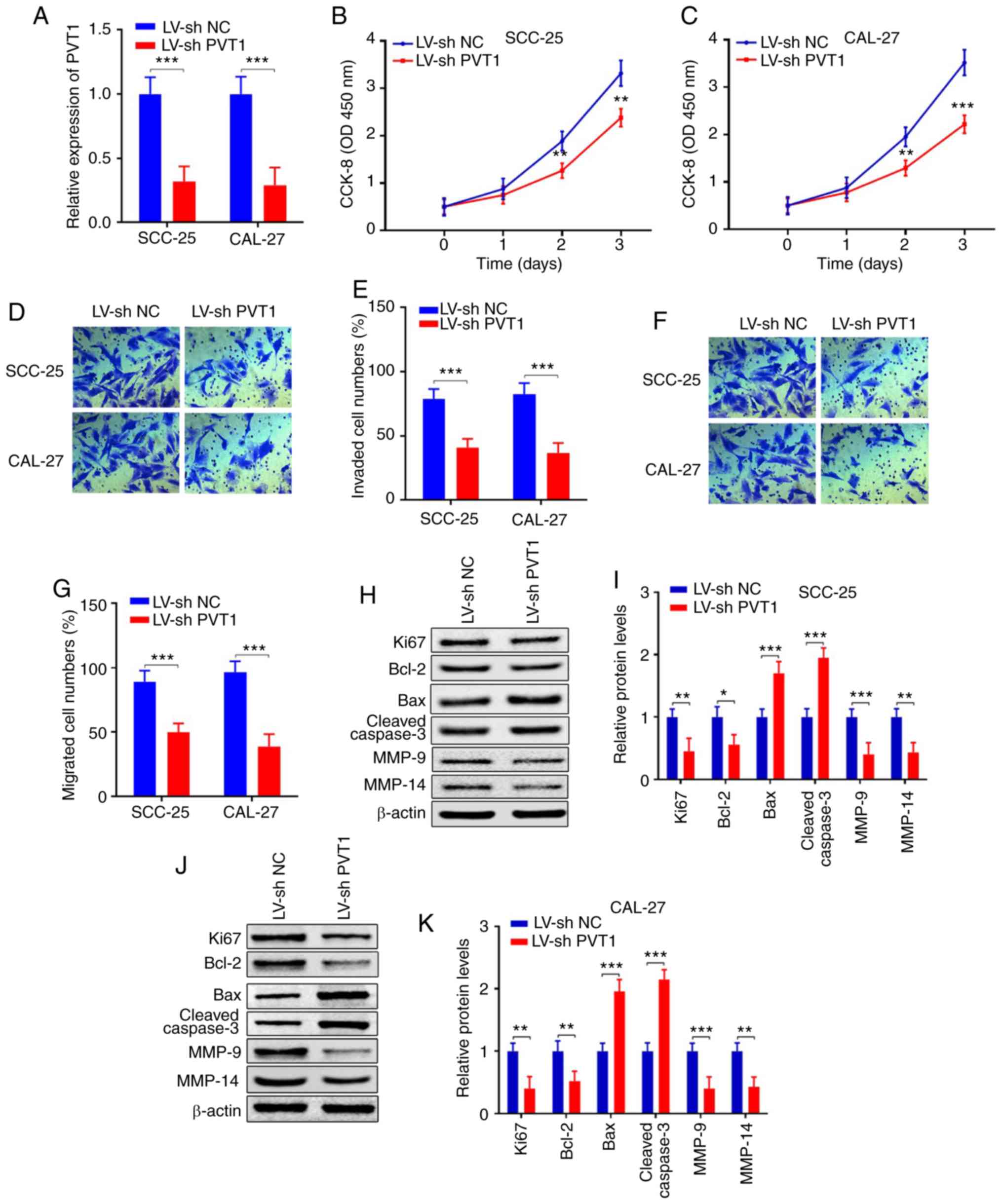 | Figure 3.Downregulation of PVT1 inhibits cell
proliferation, invasion, migration and promotes apoptosis in OSCC.
(A) qPCR showed that PVT1 expression was inhibited in SCC-25 and
CAL-27 cells transfected with LV-shPVT1 compared with the LV-sh NC
group. (B and C) CCK-8 assay revealed that the proliferation
abilities were suppressed in SCC-25 and CAL-27 cells transfected
with LV-shPVT1 compared with the LV-sh NC group. (D-G) Transwell
assays revealed that invasion and migration were suppressed in
SCC-25 and CAL-27 cells transfected with LV-shPVT1 compared with
the LV-sh NC group. (H-K) Protein expression levels of Ki67, Bcl-2,
Bax, cleaved caspase 3, MMP-9 and MMP-14 were detected by western
blot analysis in SCC-25 and CAL-27 cell lines transfected with
LV-PVT1 when compared with the LV-NC group (*P<0.05;
**P<0.01, ***P<0.001). PVT1, lncRNA plasmacytoma variant
translocation 1; OSCC, oral squamous cell carcinoma; Bcl-2, B-cell
lymphoma 2; Bax, bcl-2-like protein 4; MMP, matrix
metalloproteinase; qPCR, real-time quantitative PCR. |
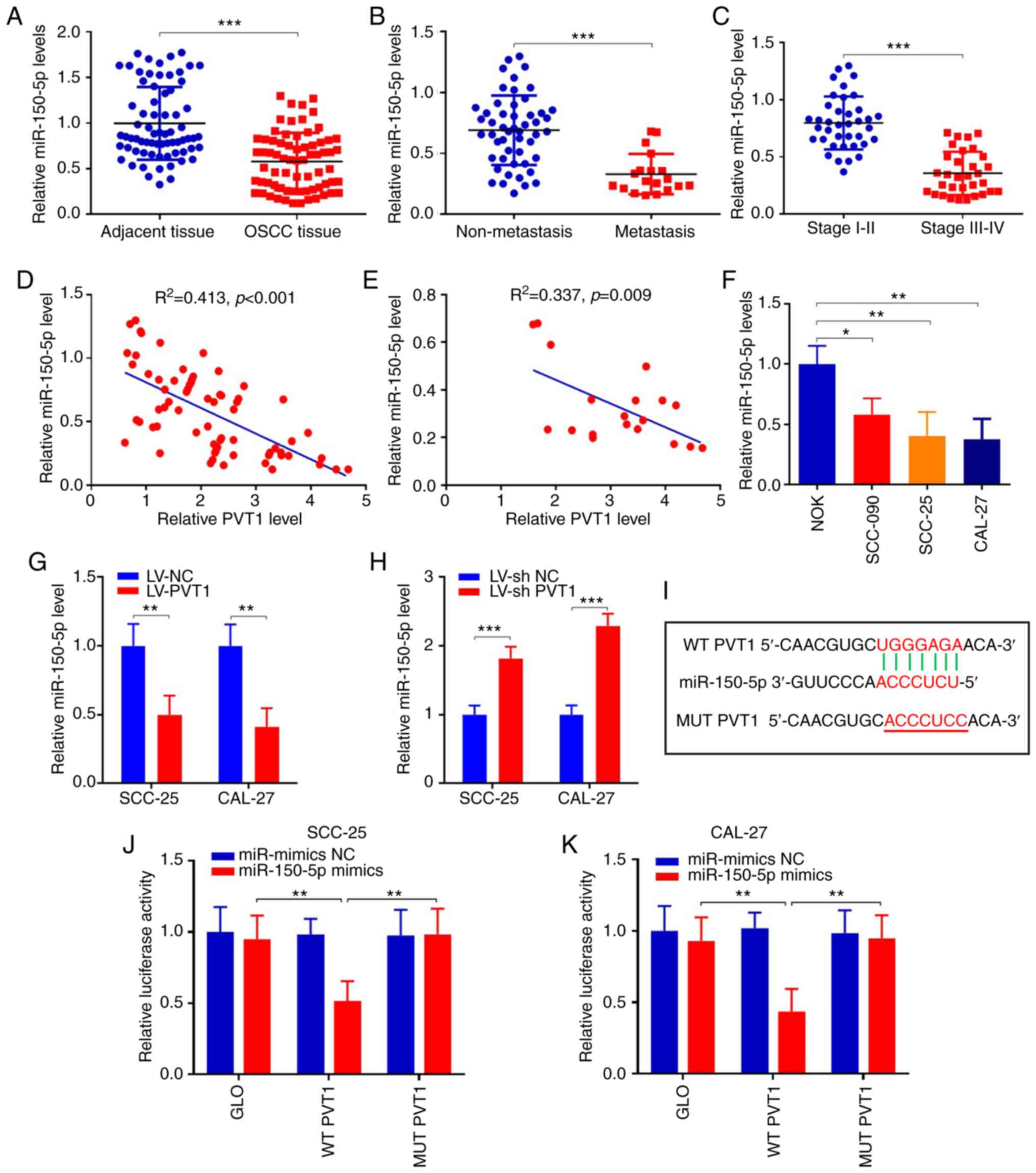
PVT1 directly sponges miR-150-5p in
OSCC
To further explore the detailed mechanism involved
in the participation of PVT1 in the processes of cell
proliferation, invasion and migration in OSCC, starBase v2.0
database (41) was used and
miR-150-5p was found to be a potential downstream miRNA that
contains potential binding sites with PVT1. Then the expression of
miR-150-5p in patients was detected. The results revealed that
miR-150-5p was significantly suppressed in OSCC tumor tissues when
compared with that in the adjacent tissues (Fig. 4A) (P<0.001). Furthermore,
miR-150-5p was decreased 2.0-fold in OSCC patients with metastasis
(n=19), compared to those without metastasis (n=51) (Fig. 4B) (P<0.001). Moreover, expression
of miR-150-5p in stage III–IV tissues was much lower than
expression in stage I–II tissues (Fig.
4C) (P<0.001). In addition, correlation analysis was
performed between PVT1 and miR-150-5p, which showed that miR-150-5p
was negatively correlated with PVT1 in OSCC and metastatic patients
(Fig. 4D and E). We also detected
miR-150-5p expressions in NOK and OSCC cells. The results revealed
that miR-150-5p was significantly suppressed in the OSCC cell lines
when compared with NOK cells (Fig.
4F). In addition, expression of miR-150-5p was significantly
suppressed in SCC-25 and CAL-27 cells following LV-PVT1 infection
(P<0.01), while they were significantly upregulated following
LV-shPVT1 infection (P<0.001), when compared with the relevant
NC group. (Fig. 4G and H). Above
all, these results indicate that PVT1 is negatively interactive
with miR-150-5p, which may be a downstream factor of PVT1 (Fig. 4I). To confirm whether PVT1 could
competitively bind with miR-150-5p, wild-type WT-PVT1 and mutant
MUT-PVT1 sequences were constructed into vectors and the luciferase
gene reporter assay was performed. Results showed that relative
luciferase activity in SCC-25 cells co-transfected with WT-PVT1 and
miR-150-5p mimics was obviously repressed. However, it was reversed
following co-transfected with MUT-PVT1 and miR-150-5p mimics. And
the same result was found in CAL-27 cells (Fig. 4J and K). These data indicate that
PVT1 competitively binds with miR-150-5p in OSCC.
miR-150-5p inhibits cell
proliferation, invasion, migration and promotes apoptosis in
OSCC
We aimed to further ascertain the roles of
miR-150-5p in OSCC. miR-150-5p mimics was respectively transfected
into SCC-25 and CAL-27 cells. The results revealed that miR-150-5p
was significantly increased following transfection with miR-mimics
when compared with the miR-mimics NC group (Fig. 5A) (P<0.001). In addition, the
cell proliferation abilities were significantly suppressed in the
SCC-25 and CAL-27 cell lines following transfection with miR-150-5p
mimics compared with the miR-mimics NC group (Fig. 5B and C) (P<0.01). Transwell
assays revealed that cell invasion and migration abilities were
significantly suppressed following miR-150-5p overexpression
(Fig. 5D-G) (P<0.001). Protein
expression levels of Ki67 and Bcl-2 were significantly inhibited,
apoptotic genes Bax and cleaved caspase3 were upregulated, and
migration and invasion-associated genes MMP-9 and MMP-14 were
inhibited following miR-150-5p overexpression (Fig. 5H-K). The results demonstrated that
miR-150-5p regulated the biological functions in OSCC. As we know
that miRNAs participate in various biological functions by
inhibiting target genes, however, the detailed mechanism of
miR-150-5p in OSCC remained unknown.
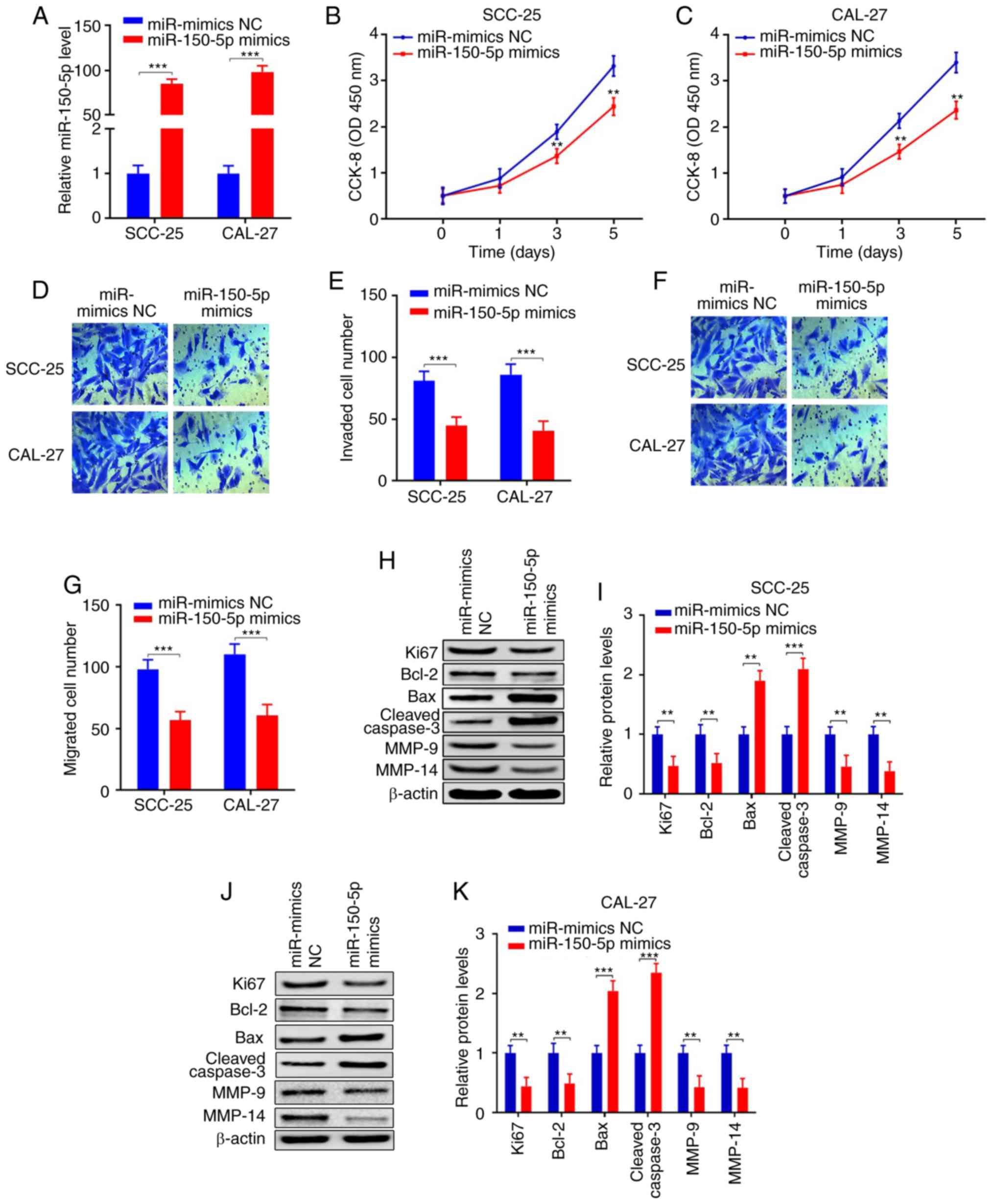 | Figure 5.miR-150-5p inhibits cell
proliferation, invasion, migration and promotes apoptosis in OSCC.
(A) qPCR demonstrate that miR-150-5p was upregulated after
miR-150-5p mimic transfection in the SCC-25 and CAL-27 cell lines
when compared with the miR-mimics NC group. (B and C) CCK-8 assays
revealed that the proliferation abilities were inhibited in the
SCC-25 and CAL-27 cell lines transfected with the miR-150-5p mimics
when compared with the miR-mimics NC group. (D-G) Transwell assays
revealed that invasion and migration were inhibited in the SCC-25
and CAL-27 cell lines transfected with the miR-150-5p mimics when
compared with the miR-mimics NC group. (H-K) Protein expression
levels of Ki67, Bcl-2, Bax, cleaved caspase 3, MMP-9 and MMP-14
were detected by western blot analysis in the SCC-25 and CAL-27
cell lines transfected with the miR-150-5p mimics when compared
with the miR-mimics NC group (**P<0.01, ***P<0.001). OSCC,
oral squamous cell carcinoma; Bcl-2, B-cell lymphoma 2; Bax,
bcl-2-like protein 4; MMP, matrix metalloproteinase; qPCR,
real-time quantitative PCR. |
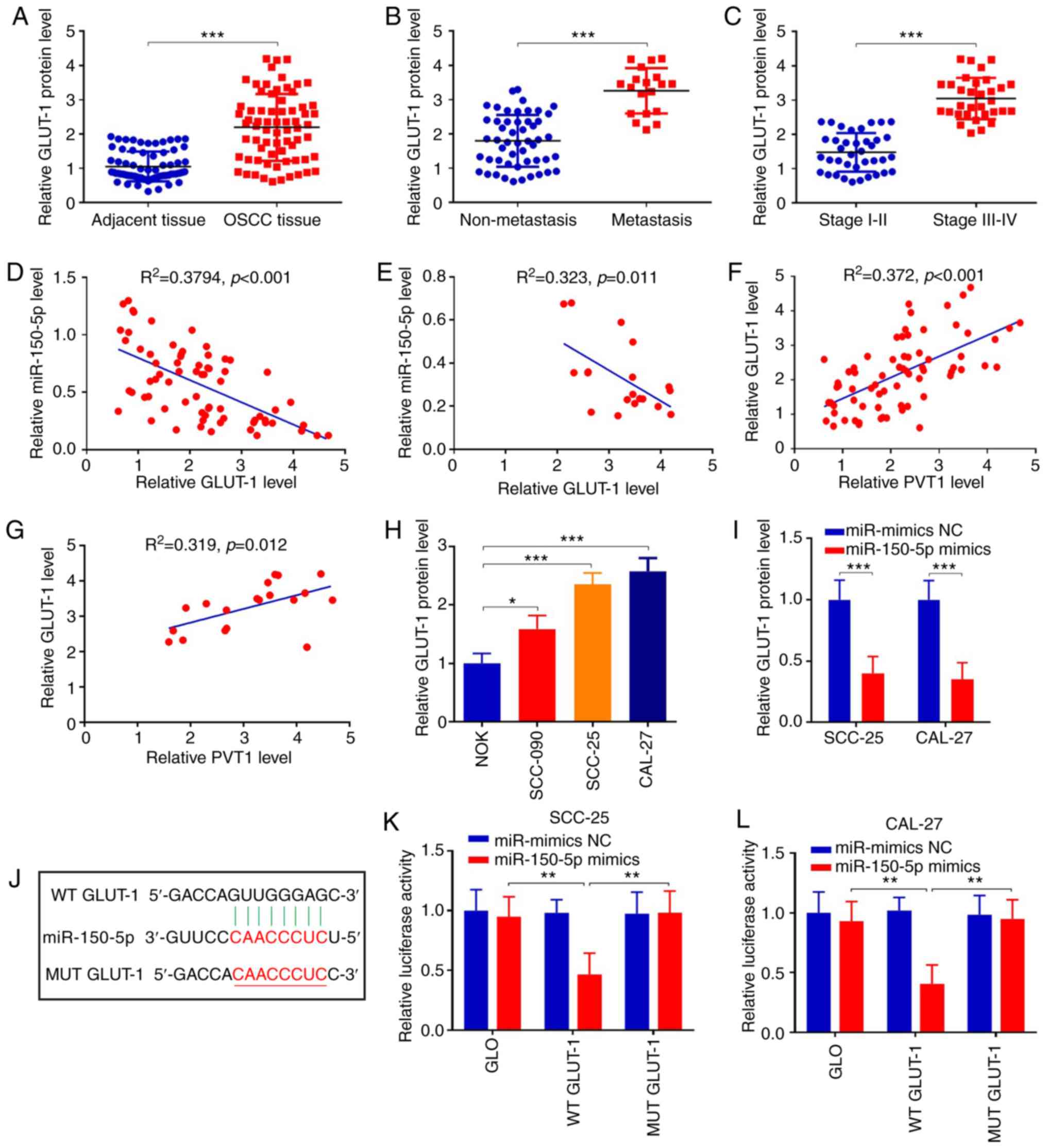
miR-150-5p negatively interacts with
GLUT-1
To further explore the detailed mechanism of
miR-150-5p in OSCC, downstream targets of miR-150-5p were analyzed
using TargetScan database. As a result, GLUT-1 was predicted as a
downstream target of miR-150-5p, which is associated with cell
proliferation and tumorigenesis (37–39).
We first detected the GLUT-1 protein expression in OSCC tumor
tissues and adjacent non-tumor tissues. Western blot analysis
revealed that the protein expression of GLUT-1 was increased
2.1-fold in the OSCC tumor tissues when compared with that in the
adjacent tissues (Fig. 6A)
(P<0.001). Furthermore, GLUT-1 expression was increased 1.8-fold
in the OSCC tissues with metastasis (n=19), compared to those
without metastasis (n=51) (Fig. 6B)
(P<0.001). Moreover, protein expression of GLUT-1 in patients
with stage III–IV were much higher than those at stage I–II
(Fig. 6C) (P<0.001). We also
found that miR-150-5p was negatively correlated with GLUT-1 in OSCC
patients and metastasis patients (Fig.
6D and E). Additionally, PVT1 was positively correlated with
GLUT-1 in OSCC patients and metastasis patients (Fig. 6F and G). In addition, protein
expression of GLUT-1 was increased in OSCC cell lines compared with
that in the NOK cells (Fig. 6H).
Finally, expression of GLUT-1 was suppressed in SCC-25 and CAL-27
cells following miR-150-5p overexpression (Fig. 6I) (P<0.001). Above all,
miR-150-5p was negatively correlated with GLUT-1, which may be a
potential target for miR-150-5p.
To confirm whether miR-150-5p targets GLUT-1 in
OSCC, WT-GLUT-1 and MUT-GLUT-1 sequences were constructed into
vectors and the luciferase gene reporter assay was performed
(Fig. 6J). The results demonstrated
that miR-150-5p overexpression attenuated luciferase activity of
WT-GLUT-1 but not of MUT-GLUT-1 (Fig.
6K and L) (P<0.01). The above data showed that miR-150-5p
could inhibit GLUT-1 and it may be a target of miR-150-5p in OSCC.
Overall, PVT1 may directly bind with miR-150-5p, which targets
GLUT-1 expression, thereby affecting biological functions in
patients with OSCC.
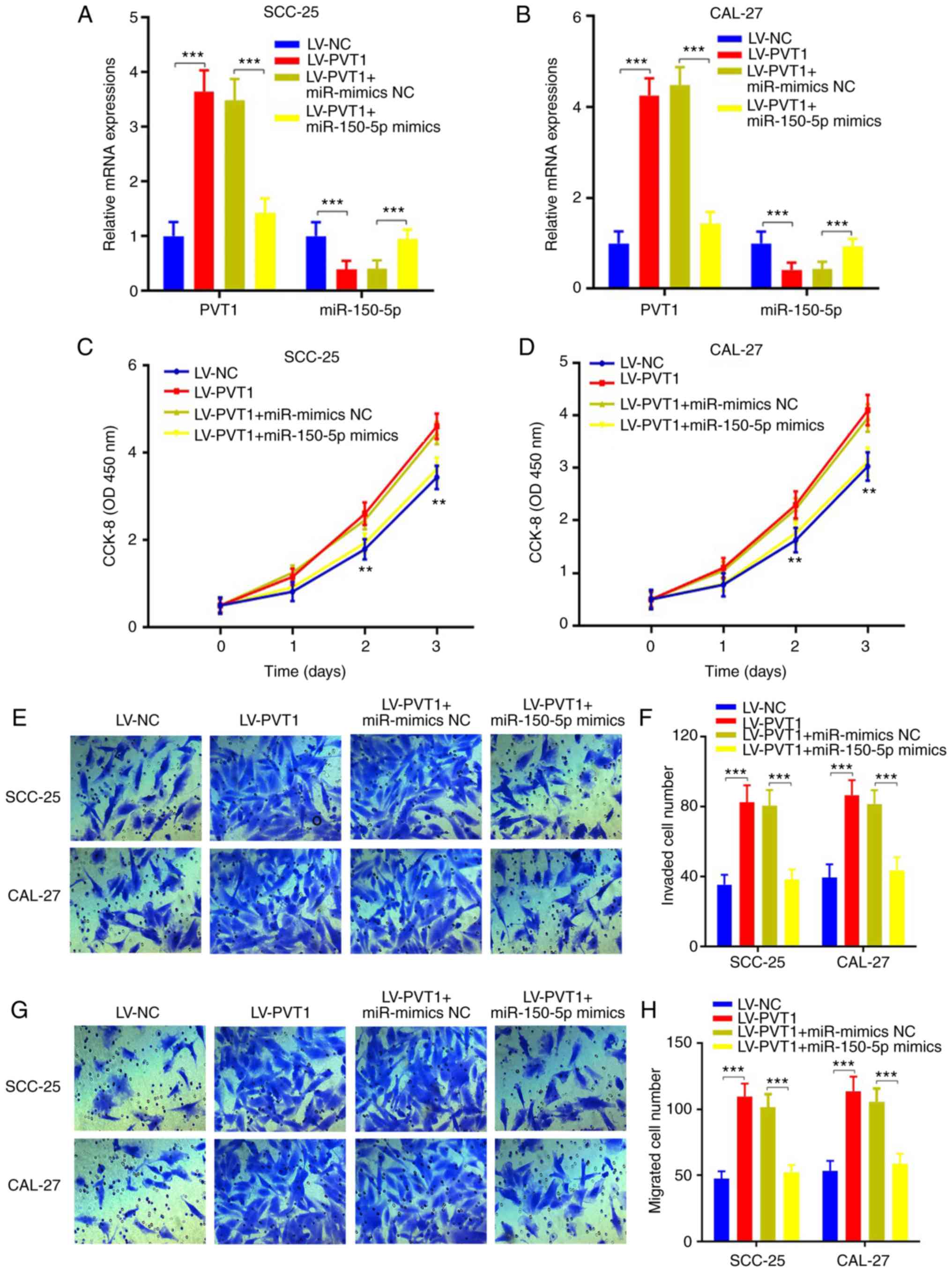
PVT1 promotes cell proliferation,
invasion, migration and inhibits apoptosis via the
miR-150-5p/GLUT-1 axis in patients with OSCC
To confirm our hypothesis, miR-150-5p mimics or
miR-NC was respectively transfected into cells infected with
LV-PVT1, and cell proliferation ability, invasive and migratory
abilities were evaluated. The results showed that PVT1 was
increased and miR-150-5p was repressed in LV-PVT1 transfected
cells, while PVT1 was reduced and miR-150-5p was increased
following miR-150-5p overexpression in the SCC-25 and CAL-27 cells
(Fig. 7A and B) (P<0.001).
Furthermore, CCK-8 assays indicated that the
LV-PVT1-overexpression-enhanced cell proliferation abilities were
suppressed following miR-150-5p overexpression (Fig. 7C and D) (P<0.01). Moreover, the
LV-PVT1-overexpression-enhanced cell invasion and migration were
suppressed following miR-150-5p overexpression (Fig. 7E-H) (P<0.001). In addition, we
also detected the gene expression of GLUT-1, Ki67, Bcl-2, Bax,
cleaved caspase 3, MMP-9 and MMP-14. The results showed that these
protein levels were consistent with our previous results, while
they were reversed following by miR-150-5p overexpression in the
SCC-25 (Fig. 8A and B) and CAL-27
cell line (Fig. 8C and D).
Collectively, PVT1 promotes cell proliferation, invasion, migration
and inhibits apoptosis via the miR-150-5p/GLUT-1 axis in patients
with OSCC.
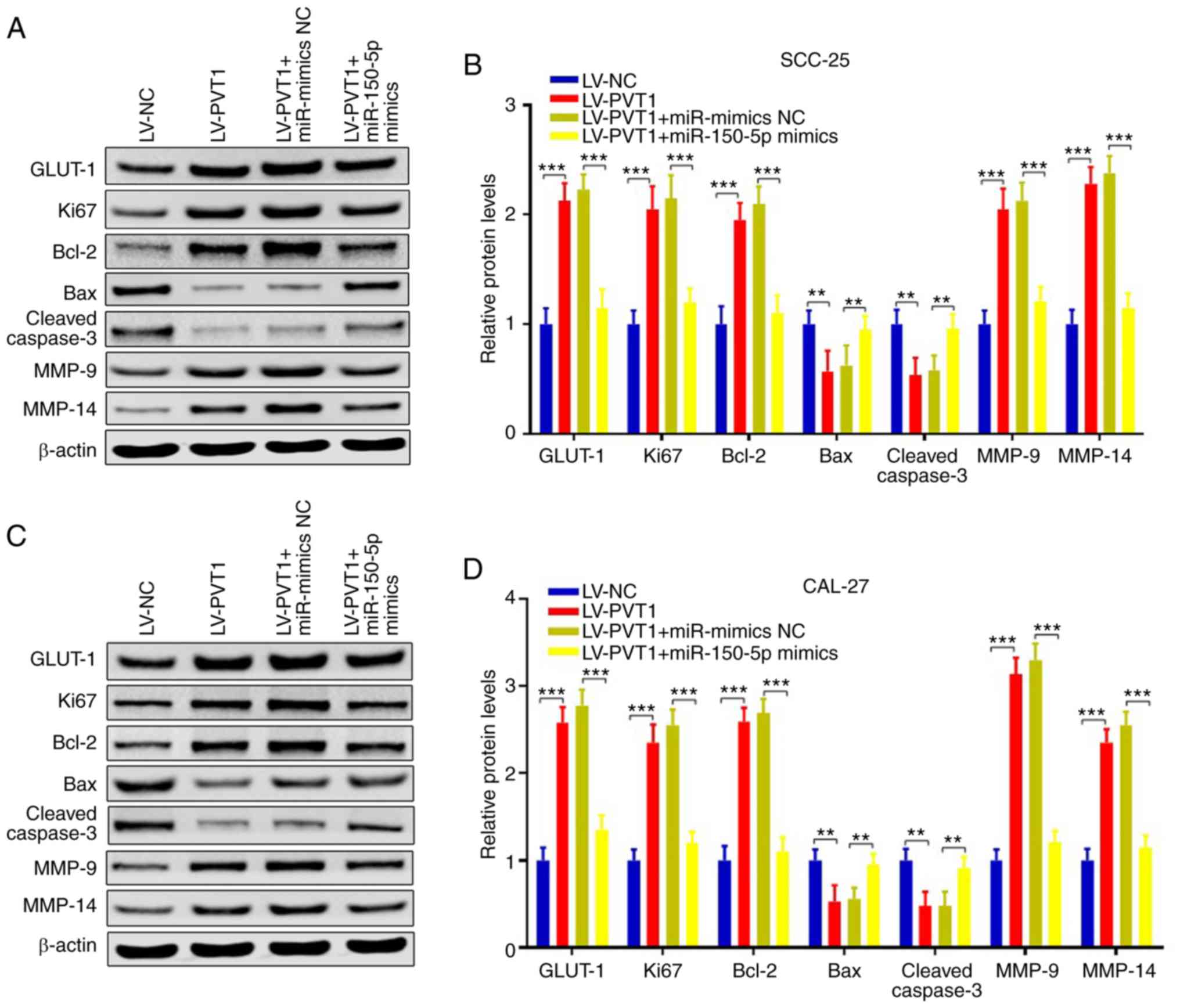 | Figure 8.PVT1 regulates the expression of
proteins associated with proliferation, invasion, apoptosis and
migration via the miR-150-5p/GLUT-1 axis in patients with OSCC.
(A-D) Western blot analyses showed that protein levels of GLUT-1,
Ki67, Bcl-2, Bax, cleaved caspase 3, MMP-9 and MMP-14 were
consistent with our previous results, while they were reversed
following miR-150-5p overexpression. **P<0.01, ***P<0.001).
GLUT1, glucose transporter 1; PVT1, lncRNA plasmacytoma variant
translocation 1; OSCC, oral squamous cell carcinoma; Bcl-2, B-cell
lymphoma 2; Bax, bcl-2-like protein 4; MMP, matrix
metalloproteinase. |
PVT1 inhibition suppresses tumor
growth and expression of invasion and migration-associated genes in
vivo
To verify the functions of PVT1 in vivo, we
injected SCC-25 cells that had been stably infected with LV-shPVT1
or LV-sh NC into nude mice. Tumor volumes were measured every week
until the 5th week. Tumors infected with LV-shPVT1 showed smaller
tumor volume (Fig. 9A). The tumor
volumes in the LV-shPVT1 group were significantly smaller at day 28
and 35 (Fig. 9B) (P<0.001). At
day 35, the mean value of the orthotopic tumor weight of LV-shPVT1
tumors was lighter than that noted in the LV-sh NC group (Fig. 9C) (P<0.001). Furthermore, we
detected expression levels of PVT1, miR-150-5p, GLUT-1, Ki67,
Bcl-2, Bax, cleaved caspase3, MMP-9 and MMP-14 in the tumors
derived from cells transfected with LV-shNC or LV-sh PVT1. The
results showed that PVT1 and GLUT-1 were decreased in the LV-shPVT1
group, while miR-150-5p was increased (Fig. 9D) (P<0.001). The protein
expression levels of Ki67 and Bcl-2 were decreased, while Bax and
cleaved caspase 3 were increased, which suggested that the
proliferation ability was suppressed in the LV-shPVT1 group. The
protein expression levels of EMT markers, including GLUT-1, MMP-9
and MMP-14, were reduced in the LV-shPVT1 group, indicating that
the invasive and migrated abilities were inhibited (Fig. 9E and F). These results confirmed
that PVT1 is critical for tumor growth, invasion and migration via
the miR-150-5p/GLUT-1 pathway in human OSCC.
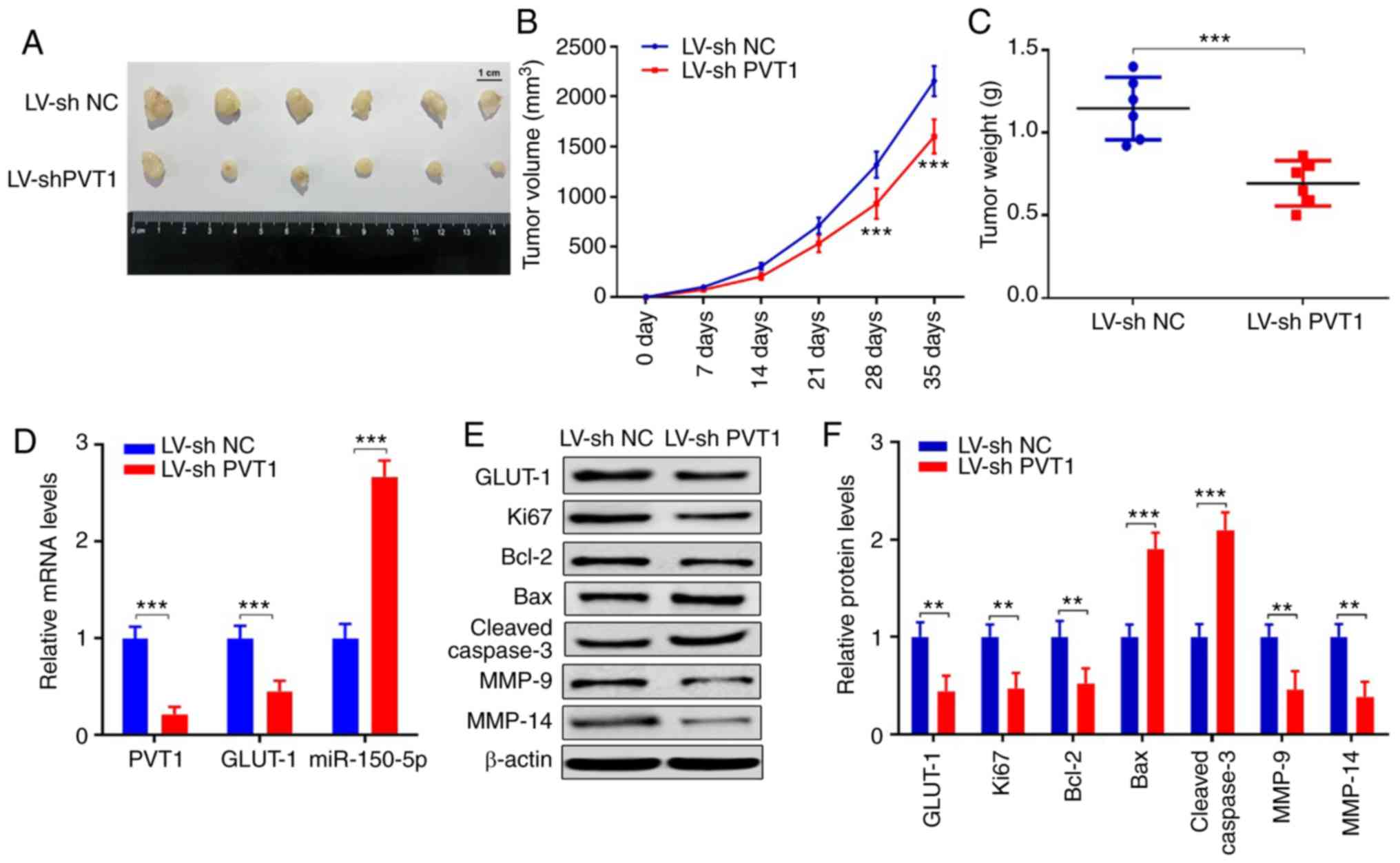 | Figure 9.PVT1 inhibition suppressed tumor
growth and expression of proteins associated with invasion and
migration in vivo. (A) Images of 12 primary tumors at day 35
from the LV-shPVT1 and LV-sh NC groups. (B) Tumor volumes were
measured every week for 35 days. (C) Tumor weights at day 35 after
injection were calculated. (D) mRNA levels of PVT1, miR-150-5p,
GLUT-1 were detected in the tumor tissues of the LV-sh NC and LV-sh
PVT1 groups. (E and F) Protein expression levels of GLUT-1, Ki67,
Bcl-2, Bax, cleaved caspase 3, MMP-9 and MMP-14 were detected by
western blot analysis (**P<0.01, ***P<0.001). GLUT1, glucose
transporter 1; PVT1, lncRNA plasmacytoma variant translocation 1;
OSCC, oral squamous cell carcinoma; Bcl-2, B-cell lymphoma 2; Bax,
bcl-2-like protein 4; MMP, matrix metalloproteinase. |
Discussion
Abnormal expression of long non-coding RNAs
(lncRNAs) and microRNAs (miRNAs/miRs) has been demonstrated to be
related to tumor formation and progression in various types of
cancers (10–12). For example, lncRNA HOTAIR was found
to be suppressed in ovarian cancer cells, which was found to
inhibit the invasion and tumorigenicity by interacting with
miR-200c in mice (11). lncRNA
MIR4435-2HG was found to promote prostate cancer migration and
invasion by upregulating TGF-β1 expression (10). LINC00511 was upregulated in estrogen
receptor (ER)-negative breast cancer, which was associated with
poor prognosis for patients and it could act as an oncogene by
interacting with EZH2 and PRC2 (12). Recently, increasing evidence has
revealed that lncRNAs play oncogenic or tumor-suppressor roles in
oral squamous cell carcinoma (OSCC) (13–15).
lncRNA plasmacytoma variant translocation 1 (PVT1) was confirmed to
participate in the promotion of tumor formation, invasion and
migration in many types of cancers, such as glioma, colorectal,
gastric, ovarian, gallbladder and breast cancer (16–21).
However, the functions of PVT1 in OSCC remains unclear.
In the present study, we found that PVT1 was
upregulated in OSCC tissues and cell lines, and was associated with
metastasis, advanced stages and poor overall patient survival. The
results revealed that PVT1 overexpression promoted cell
proliferation, invasion, migration and apoptosis, while PVT1
downregulation produced the opposite results, which suggests that
PVT1 is an oncogenic gene in OSCC. This finding was consistent with
former reports in various types of cancers, such as glioma,
colorectal, gastric, ovarian, gallbladder and breast cancer
(16–21). However, the underlying mechanisms of
PVT1 in tumorigenesis and migration in OSCC remain unknown.
miRNAs act as oncogenes or tumor suppressors in
cancers, and have been reported to be target genes of lncRNAs
(27,28). We used Starbase v2.0 database which
indicated that miR-150-5p may be a downstream target of PVT1. We
also uncovered that miR-150-5p expression was reduced in OSCC tumor
tissues, and was negatively correlated with PVT1. In order to
determine the interaction between PVT1 and miR-150-5p,
dual-luciferase gene reporter assay was performed and the results
demonstrated that miR-150-5p overexpression attenuated luciferase
activity in the wild-type WT-PVT1 but not mutant MUT-PVT1, which
indicated that PVT1 could function as a competing endogenous RNA
(ceRNA), to competitively bind to miR-150-5p in OSCC. Moreover,
miR-150-5p overexpression inhibited cell proliferation, invasion
and migration in OSCC. Collectively, PVT1 serves as an oncogenic
gene in OSCC via binding with miR-150-5p, which are important for
cancer biology; however, the underlying mechanism remained
unknown.
Glucose transporter type 1 (GLUT1) is an oncogenic
gene that is associated with the biological functions and
progression of various types of cancers, such as bladder, non-small
cell lung and prostate cancer (37–39),
and may be a downstream target for miR-150-5p. GLUT1 is a glucose
membrane transporter that can uptake glucose and regulate the
metabolism in cancers through several mechanisms (42,43).
Firstly, transported glucose will follow the glycolytic pathway to
generate pyruvate, which is converted into lactate under the
condition of anaerobiosis and it will supply energy for cancer
cells (44,45). Secondly, pyruvate can be transformed
into acetyl-coenzyme-A (Acetyl-CoA), which can be supplied to ATP
generation and promote cancer cell proliferation (46–48).
Finally, cancer cells can use substrates from glucose as a carbon
source, such as fatty acids and glutamine (49,50).
In the present study, we found that GLUT1 protein
expression was increased in OSCC tumor tissues, and was negatively
correlated with miR-150-5p. Luciferase assay indicated that
miR-150-5p overexpression attenuated luciferase activity in
WT-GLUT-1 but not MUT-GLUT-1, which suggested that miR-150-5p could
directly bind with GLUT-1 in OSCC. Collectively, we assumed that
PVT1 could directly bind with miR-150-5p, which attenuated GLUT-1
inhibition, thereby promoting cell proliferation, invasion and
migration in OSCC. Collectively, we found that overexpression of
PVT1 promoted cell proliferation, invasion and migration of OSCC
via targeting the miR-150-5p/GLUT-1 pathway.
To verify the functions of PVT1 in vivo, a
nude mouse xenograft model was performed and SCC-25 cells stably
transfected with LV-shPVT1 or LV-NC were injected into the mice.
The results revealed that the tumor volumes and tumor weight were
reduced in the LV-shPVT1 group when compared with the LV-NC group.
Furthermore, GLUT-1 was decreased and miR-150-5p was upregulated in
the LV-shPVT1 group. In addition, the protein expression levels of
Ki67, Bcl-2, GLUT-1, MMP-9 and MMP-14 were decreased, while Bax and
cleaved caspase 3 were increased in the LV-shPVT1 group, suggesting
that the proliferation, invasion and migration capacities were
suppressed in the LV-shPVT1 group in vivo.
In the present study, we performed in situ
hybridization, but the results showed poor specificity and the
quantification was not precise, which was a limitation of this
present study and we will improve the in situ hybridization
experiment in future research. On the other hand, we used RT-qPCR
to detect PVT1 expressions in OSCC tissues and adjacent tissues,
which showed a very high specificity and quantification. According
to the results of the RT-qPCR, we carried out further research to
explore the functions of PVT1, and we found that the elevated PVT1
promoted tumor cell proliferation, invasion, migration and
inhibited apoptosis in the OSCC cells.
In conclusion, the present study revealed that PVT1
is increased in OSCC tissues, and is associated with the poor
prognosis of OSCC patients. We uncovered a previously unappreciated
PVT1/miR-150-5p/GLUT-1 signaling axis in promoting cell
proliferation, invasion and migration in OSCC cells in vitro
and also in vivo, which suggests that the
PVT1/miR-150-5p/GLUT-1 signaling axis may be a target for the
treatment of OSCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XL and HR conceived and designed the study. XL
performed the experiments and wrote the manuscript. HR reviewed and
edited the manuscript. Both authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The protocol for collected the patient samples were
approved by the Faculty of Medicine's Ethics Committee of The First
Affiliated Hospital of Jinzhou Medical University (ethical approval
no. JYD160923). All animal handling and experimental procedures
were approved by the Animal Ethics Committees of the First
Affiliated Hospital of Jinzhou Medical University and in accordance
with the guidelines of the China Council of Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wangmo C, Charoen N, Jantharapattana K,
Dechaphunkul A and Thongsuksai P: Epithelial-mesenchymal transition
predicts survival in oral squamous cell carcinoma. Pathol Oncol
Res. 26:1511–1518. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang F, Xin C, Lei K, Bai H, Li J and
Chen Q: Noncoding RNAs in oral premalignant disorders and oral
squamous cell carcinoma. Cell Oncol (Dordr). June 3–2020.(Epub
ahead of print). View Article : Google Scholar
|
|
3
|
Gharat SA, Momin M and Bhavsar C: Oral
squamous cell carcinoma: Current treatment strategies and
nanotechnology-based approaches for prevention and therapy. Crit
Rev Ther Drug Carrier Syst. 33:363–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Almangush A, Mäkitie AA, Triantafyllou A,
de Bree R, Strojan P, Rinaldo A, Hernandez-Prera JC, Suárez C,
Kowalski LP, Ferlito A and Leivo I: Staging and grading of oral
squamous cell carcinoma: An update. Oral Oncol. 107:1047992020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hauptman N and Glavac D: MicroRNAs and
long non-coding RNAs: Prospects in diagnostics and therapy of
cancer. Radiol Oncol. 47:311–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morris KV and Mattick JS: The rise of
regulatory RNA. Nat Rev Genet. 15:423–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo W, Wang M, Liu J, Cui X and Wang H:
Identification of a six lncRNAs signature as novel diagnostic
biomarkers for cervical cancer. J Cell Physiol. 235:993–1000. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Meng H, Huang X, Tong W, Liang X,
Li J, Zhang C and Chen M: lncRNA MIR4435-2HG promotes cancer cell
migration and invasion in prostate carcinoma by upregulating
TGF-β1. Oncol Lett. 18:4016–4021. 2019.PubMed/NCBI
|
|
11
|
Yang C, Li H, Zhang T, Chu Y, Chen D and
Zuo J: miR-200c overexpression inhibits the invasion and
tumorigenicity of epithelial ovarian cancer cells by suppressing
lncRNA HOTAIR in mice. J Cell Biochem. 121:1514–1523. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Sui S, Wu H, Zhang J, Zhang X, Xu
S and Pang D: The transcriptional landscape of lncRNAs reveals the
oncogenic function of LINC00511 in ER-negative breast cancer. Cell
Death Dis. 10:5992019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu G, Wang S, Chen J, Wang Z, Liang X,
Wang X, Jiang J, Lang J and Li L: Long noncoding RNA HAS2-AS1
mediates hypoxia-induced invasiveness of oral squamous cell
carcinoma. Mol Carcinog. 56:2210–2222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong J, Sun W, Zhu W, Liu C, Zhang H and
Wang H: Long noncoding RNA LINC01133 inhibits oral squamous cell
carcinoma metastasis through a feedback regulation loop with GDF15.
J Surg Oncol. 118:1326–1334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin Z, Jiang S, Jian S and Shang Z: Long
noncoding RNA MORT overexpression inhibits cancer cell
proliferation in oral squamous cell carcinoma by downregulating
ROCK1. J Cell Biochem. Feb 25–2019.(Epub ahead of print).
View Article : Google Scholar
|
|
16
|
Song T, Yan L, Cai K, Zhao T and Xu M:
Downregulation of long noncoding RNA PVT1 attenuates paclitaxel
resistance in glioma cells. Cancer Biomark. 23:447–453. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shang AQ, Wang WW, Yang YB, Gu CZ, Ji P,
Chen C, Zeng BJ, Wu JL, Lu WY, Sun ZJ and Li D: Knockdown of long
noncoding RNA PVT1 suppresses cell proliferation and invasion of
colorectal cancer via upregulation of microRNA-214-3p. Am J Physiol
Gastrointest Liver Physiol. 317:G222–G232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Du P, Cui P, Qin Y, Hu C, Wu J,
Zhou Z, Zhang W, Qin L and Huang G: LncRNA PVT1 promotes
angiogenesis via activating the STAT3/VEGFA axis in gastric cancer.
Oncogene. 37:4094–4109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Q, Yu Y, Sun Z and Pan Y: Long
non-coding RNA PVT1 promotes cell proliferation and invasion
through regulating miR-133a in ovarian cancer. Biomed Pharmacother.
106:61–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Yu Y, Li H, Hu Q, Chen X, He Y,
Xue C, Ren F, Ren Z, Li J, et al: Long non-coding RNA PVT1 promotes
tumor progression by regulating the miR-143/HK2 axis in gallbladder
cancer. Mol Cancer. 18:332019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan C, Chen Y, Kong W, Fu L, Liu Y, Yao Q
and Yuan Y: PVT1-derived miR-1207-5p promotes breast cancer cell
growth by targeting STAT6. Cancer Sci. 108:868–876. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dejene SB, Ohman AW, Du W, Randhawa D,
Bradley A, Yadav N, Elias KM, Dinulescu DM and Setlur SR: Defining
fallopian tube-derived miRNA cancer signatures. Cancer Med.
8:6709–6716. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong G, Lou W, Yao M, Du C, Wei H and Fu
P: Identification of novel mRNA-miRNA-lncRNA competing endogenous
RNA network associated with prognosis of breast cancer.
Epigenomics. 11:1501–1518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
25
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan AQ, Ahmed EI, Elareer NR, Junejo K,
Steinhoff M and Uddin S: Role of miRNA-regulated cancer stem cells
in the pathogenesis of human malignancies. Cells. 8:8402019.
View Article : Google Scholar
|
|
27
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tay Y, Kats L, Salmena L, Weiss D, Tan SM,
Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, et al:
Coding-independent regulation of the tumor suppressor PTEN by
competing endogenous mRNAs. Cell. 147:344–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang S, Liao K, Miao Z, Wang Q, Miao Y,
Guo Z, Qiu Y, Chen B, Ren L, Wei Z, et al: CircFOXO3 promotes
glioblastoma progression by acting as a competing endogenous RNA
for NFAT5. Neuro-oncol. 21:1284–1296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Sha L, Huang L, Yang S, Zhou Q,
Luo X and Shi B: LINC00261 functions as a competing endogenous RNA
to regulate BCL2L11 expression by sponging miR-132-3p in
endometriosis. Am J Transl Res. 11:2269–2279. 2019.PubMed/NCBI
|
|
31
|
Cao C, Xu Y, Du K, Mi C, Yang C, Xiang L,
Xie Y and Liu W: LINC01303 functions as a competing endogenous RNA
to regulate EZH2 expression by sponging miR-101-3p in gastric
cancer. J Cell Mol Med. 23:7342–7348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan J, Jia Y, Chen H, Chen W and Zhou X:
Long non-coding RNA PXN-AS1 suppresses pancreatic cancer
progression by acting as a competing endogenous RNA of miR-3064 to
upregulate PIP4K2B expression. J Exp Clin Cancer Res. 38:3902019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Dong X, Ji T, Chen G and Shan L:
Long non-coding RNA UCA1 promotes cell progression by acting as a
competing endogenous RNA of ATF2 in prostate cancer. Am J Transl
Res. 9:366–375. 2017.PubMed/NCBI
|
|
34
|
Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H,
Kong J, Ding K, Shen HM, Wu H, et al: Long non-coding RNA linc00673
regulated non-small cell lung cancer proliferation, migration,
invasion and epithelial mesenchymal transition by sponging
miR-150-5p. Mol Cancer. 16:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dai FQ, Li CR, Fan XQ, Tan L, Wang RT and
Jin H: miR-150-5p inhibits non-small-cell lung cancer metastasis
and recurrence by targeting HMGA2 and β-catenin signaling. Mol Ther
Nucleic Acids. 16:675–685. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen X, Xu X, Pan B, Zeng K, Xu M, Liu X,
He B, Pan Y, Sun H and Wang S: miR-150-5p suppresses tumor
progression by targeting VEGFA in colorectal cancer. Aging (Albany
NY). 10:3421–3437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Al-Maghrabi JA, Qureshi IA and Khabaz MN:
Immunhistochemical expression of GLUT1 is associated with low grade
and low stage of urinary bladder cancer. Int J Clin Exp Pathol.
12:3049–3057. 2019.PubMed/NCBI
|
|
38
|
Lee SY and Park JY: GLUT1 variants for
predicting prognosis after surgery in non-small cell lung cancer.
Ann Surg Oncol. 25 (Suppl 3):S948–S949. 2018. View Article : Google Scholar
|
|
39
|
Xiao H, Wang J, Yan W, Cui Y, Chen Z, Gao
X, Wen X and Chen J: GLUT1 regulates cell glycolysis and
proliferation in prostate cancer. Prostate. 78:86–94. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42((Database Issue)): D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mueckler M and Thorens B: The SLC2 (GLUT)
family of membrane transporters. Mol Aspects Med. 34:121–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Deng D and Yan N: GLUT, SGLT, and SWEET:
Structural and mechanistic investigations of the glucose
transporters. Protein Sci. 25:546–558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smolková K, Bellance N, Scandurra F, Génot
E, Gnaiger E, Plecitá-Hlavatá L, Jezek P and Rossignol R:
Mitochondrial bioenergetic adaptations of breast cancer cells to
aglycemia and hypoxia. J Bioenerg Biomembr. 42:55–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baffy G: Mitochondrial uncoupling in
cancer cells: Liabilities and opportunities. Biochim Biophys Acta
Bioenerg. 1858:655–664. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Y, Cao Y, Zhang W, Bergmeier S, Qian
Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, et al: A small-molecule
inhibitor of glucose transporter 1 downregulates glycolysis,
induces cell-cycle arrest, and inhibits cancer cell growth in vitro
and in vivo. Mol Cancer Ther. 11:1672–1682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zambrano A, Molt M, Uribe E and Salas M:
Glut 1 in cancer cells and the inhibitory action of resveratrol as
a potential therapeutic strategy. Int J Mol Sci. 20:33742019.
View Article : Google Scholar
|
|
49
|
Samudio I, Harmancey R, Fiegl M,
Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W,
Duvvuri S, Taegtmeyer H and Andreeff M: Pharmacologic inhibition of
fatty acid oxidation sensitizes human leukemia cells to apoptosis
induction. J Clin Invest. 120:142–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vélez J, Hail N Jr, Konopleva M, Zeng Z,
Kojima K, Samudio I and Andreeff M: Mitochondrial uncoupling and
the reprograming of intermediary metabolism in leukemia cells.
Front Oncol. 3:672013. View Article : Google Scholar : PubMed/NCBI
|