Introduction
Colorectal cancer (CRC) is a common digestive system
malignancy and a major cause of cancer-related mortality worldwide
(1). CRC is a fatal disease that is
responsible for more than 600,000 deaths each year (2). Lifestyle and dietary factors
contribute to the increasing incidence of CRC each year (3). In the past decades, combined therapies
for CRC have improved treatment efficacy. These therapies primarily
includes surgical resection, chemotherapy and radiotherapy, but
none have proven to be optimal due to the fact that the majority of
patients with CRC are often diagnosed at an advanced stage
(4,5). Notably, the primary reason for poor
prognosis in patients with advanced CRC is tumor metastasis and
recurrence (6). Thus, elucidating
the molecular mechanisms underlying the progression of CRC may
reveal potential prognostic biomarkers and novel treatment
strategies for CRC.
Long non-coding RNAs (lncRNAs) are a class of
non-protein coding transcripts that are >200 nucleotides in
length. It has been well reported that lncRNAs play significant
roles in cancer biology, including proliferation, invasion,
apoptosis and metastasis (7,8).
Aberrant expression of lncRNAs has been observed in the occurrence
and development of a variety of cancers, suggesting that they may
serve as powerful mediators in carcinogenesis (9–11).
Over the past few decades, researchers have focused on
investigating the function of lncRNAs in the pathogenesis of CRC
(12,13). LINC00958 is a newly discovered
lncRNA, which was identified in recent years (14). The abnormally high expression of
LINC00958 has been demonstrated in patients with gastric cancer,
and was found to be associated with metastasis and unfavorable
prognosis (15). Additionally,
evidence indicates that LINC00958 accelerates the progression of
oral squamous cell carcinoma and pancreatic cancer by acting as a
microRNA (miR/miRNA) sponge (16,17).
However, to the best of our knowledge, the function of LINC00958 in
CRC has not yet been studied, which has peaked our interest.
In the present study, the expression of LINC00958
was detected in several CRC cell lines. Subsequently, the effects
of LINC00958 on proliferation, invasion, migration and apoptosis of
CRC cells, as well as its underlying mechanisms were investigated.
It is hoped that this study provides the theoretical basis for a
novel therapeutic strategy for the treatment of CRC.
Materials and methods
Cell culture
The colon cancer cell lines (GEO, SNU-C1, COLO205,
HCT-116 and SW480), colorectal cancer cell line (HT-29) and normal
colorectal epithelial cell line (NCM460) used in this study were
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences and the cell lines were authenticated
using STR profiles. Cell lines were maintained in Dulbecco's
modified Eagle medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in incubators
containing 5% CO2 at 37°C.
Cell transfection
SW480 cells were seeded in a 24-well plate. When
cell confluence reached 60%, 20 nM of short hairpin RNA (shRNA)
targeted against LINC00958 (shRNA-LINC00958-1 or shRNA-LINC00958-2)
or scramble shRNA (negative control for LINC00958 shRNA, shRNA-NC),
40 nM of miR-3619-5p mimic, miR-3619-5p inhibitor or miRNA negative
control (miR-NC) were transfected into SW480 cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), following the manufacturer's instructions. The
above oligonucleotides were purchased from Shanghai GenePharma Co.,
Ltd. SW480 cells were seeded in a 24-well plate. SW480 cells
without transfection served as the control group. At 48 h
post-transfection, cells were harvested and successful transfection
was detected using reverse transcription-quantitative polymerase
chain reaction (RT-qPCR).
Cell Counting Kit-8 (CCK-8) assay
Cells were grown in 96-well plates and viability was
evaluated using a CCK-8 reagent (Sigma-Aldrich; Merck KGaA),
according to the manufacturer's protocol when 80% confluency was
reached. After transfection for 24, 48 and 72 h, 10 µl CCK-8
solution was added to each well, then the optical density was
measured at 450 nm using a microplate reader.
EdU (5-ethynyl-20-deoxyuridine)
incorporation assay
The proliferation of SW480 cells was detected using
an EdU assay kit (Ribobio, China) in accordance with the
manufacturer's guidelines. Briefly, cells were incubated with 10 µM
EdU and subsequently fixed in 4% paraformaldehyde. After EdU
staining, cell nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI). The images of EDU-positive
cells were observed and captured randomly in five fields under a
confocal fluorescence microscope (magnification, ×100; Nikon,
Tokyo, Japan).
Transwell assay
A Transwell assay was used to determine the invasive
activity of SW480 cells. Briefly, 200 µl serum-free DMEM containing
5×104 cells were plated into the apical chamber, which
consisted of 8-µm pore inserts coated with Matrigel (BD
Biosciences) in culture plates. DMEM supplemented with 10% FBS as a
chemotactic factor was added in the basolateral chambers. Then, 4%
polyformaldehyde was used to fix cells and 0.1% crystal violet was
employed to stain cells. Finally, the number of invasive cells were
counted and images were captured by a light microscope (Olympus
Corporation) at ×200 magnification.
Scratch wound healing assay
SW480 cells were seeded into 6-well plates
(6×105 cells per well) for adherent culture. When cells
reached 80% confluence, a thin wound was created on the cell
monolayer using a 10-µl sterile pipette tip. The detached cells
were washed off twice, and the medium was replaced with DMEM
containing 1% FBS. Subsequently, the migration of cells was
observed and images were acquired at 0 and 24 h after wounding
using a phase contrast microscope (magnification, ×100; IX711;
Olympus Corporation). The relative wound healing closure was
calculated.
Cell apoptosis assay
SW480 cells were seeded into 6-well plates
(6×105 per well). Then, each group of cells was treated
as needed. Following this, cells were double stained with Annexin V
and propidium iodide (PI) using the Annexin V-FITC Apoptosis
Detection kit (Vazyme Biotech Co., Ltd.) in accordance with the
manufacturer's instructions. The activity of Annexin V/PI was then
examined using flow cytometry (BD Biosciences) and quantified by
Flow Jo software (version 7.6.1; FlowJo LLC).
RT-qPCR
Cells were subjected to total RNA extraction using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
total RNA (2 µg) was then reverse transcribed into cDNA using a
Reverse Transcription kit (Beijing Transgen Biotech Co., Ltd.),
according to the manufacturer's protocols. qPCR was performed using
iTaq™ Universal SYBR® Green Supermix (Bio-Rad
Laboratories, Inc.) on an ABI 7500 instrument (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Relative expression of the target
gene was normalized against U6 and GAPDH and calculated based on
the 2−ΔΔCq method (18).
Western blot analysis
Total protein was isolated from SW480 cells using a
protein lysis buffer (RIPA; Beyotime Institute of Biotechnology),
followed by quantification of protein concentration using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Protein samples (40 µg/lane) were loaded on a 10%
gel, resolved via SDS-PAGE, and then subsequently transferred to a
PVDF membrane (EMD Millipore). After blocking with skimmed milk,
these membranes were incubated with primary antibodies overnight at
4°C. Subsequently, these blots were probed with horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.). Finally, the immunoreactive bands were
visualized using the Odyssey Infrared Imaging System (LI-COR
Biosciences). Anti-Bcl-2 (1:1,000 dilution; cat. no. 3498T),
anti-Bax (1:1,000 dilution; cat. no. 5023T), anti-caspase-3
(1:1,000 dilution; cat. no. 14220T) anti-glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; 1:1,000; cat. no. 5174T) antibodies were the
products of Cell Signaling Technology, Inc. GAPDH was used as an
internal control. The intensity of the bands was quantified using
ImageJ software (version 1.52r; National Institutes of Health).
Luciferase reporter assay
The potential miRNAs which may have binding sites
with LINC00958 were predicted using the bioinformatics tool
StarBase version 3.0 (http://starbase.sysu.edu.cn/) (19). Wild-type and mutant reporter
plasmids of LINC00958 (WT-LINC00958 and MUT-LINC00958), which
contained miR-3619-5p mimic or miR-NC binding sites, were
synthesized by Shanghai GenePharma Co., Ltd. (cat. no. H00001).
293T cells were seeded in 24-well plates and cultured at 37°C until
they reached 70% confluence. To perform the luciferase reporter
assay, cells were first co-transfected with LINC00958 3′-UTR-WT or
LINC00958 3′-UTR-MUT and miR-3619-5p mimic or miR-NC using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
following which luciferase activity was detected using a
Dual-Luciferase® Reporter Assay System (Promega Corp.)
48 h after transfection. Renilla luciferase activity was
used as control.
Statistical analysis
Each experiment was repeated in triplicate and data
are presented as the mean ± standard deviation. Data were analyzed
using GraphPad Prism version 6.0 (GraphPad Software, Inc.).
Comparisons between groups were performed using a two-tailed
Student's t-test, whereas multiple comparisons were analyzed with
one-way ANOVA followed by a post hoc Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
LINC00958 is highly expressed in CRC
cell lines
To investigate the role of LINC00958 in CRC, the
expression of LINC00958 was detected using RT-qPCR in several human
CRC cell lines (GEO, SNU-C1, COLO205, HCT-116, HT-29 and SW480) and
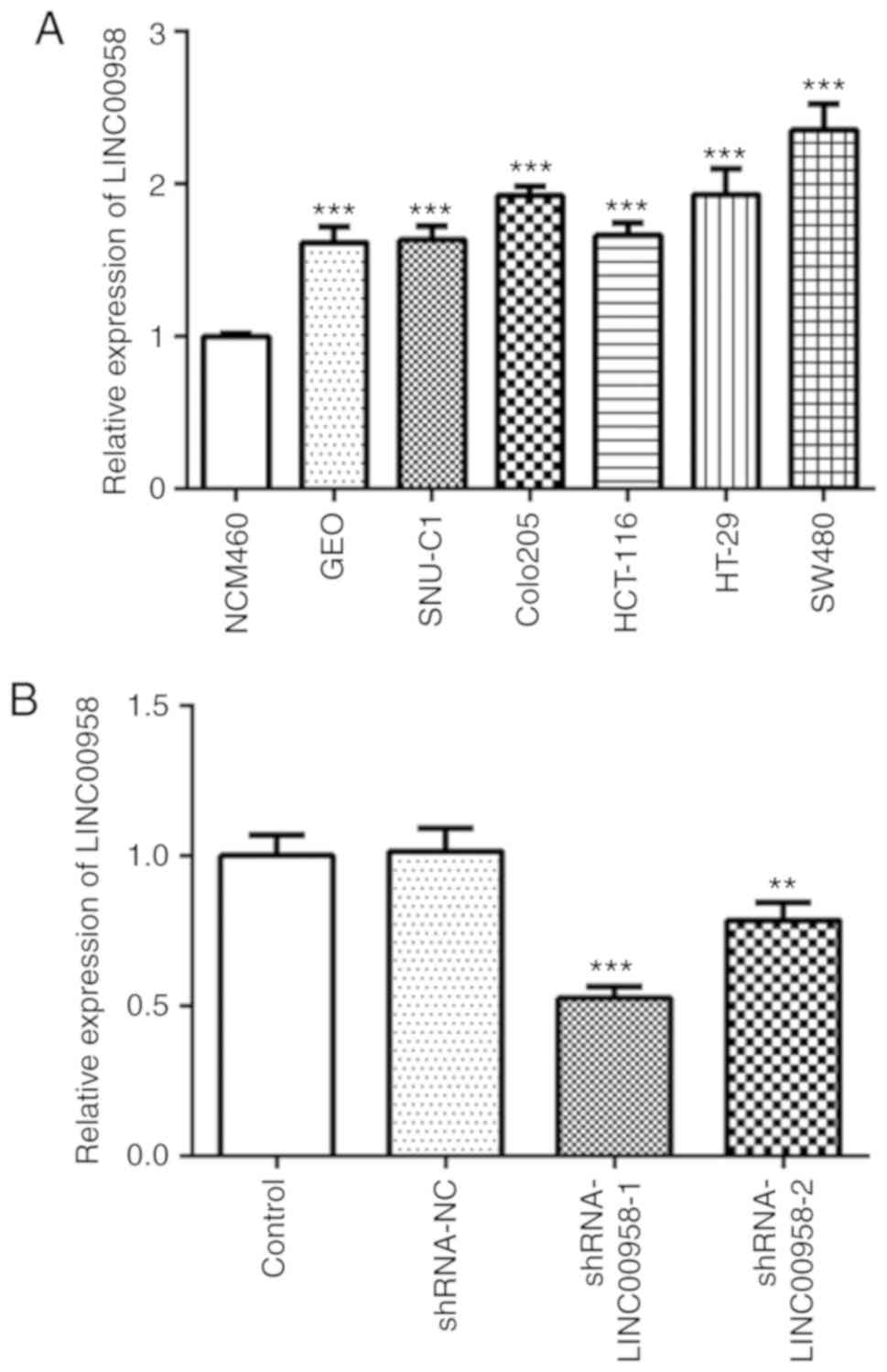
one normal colorectal epithelial cell line (NCM460). As presented
in Fig. 1A, LINC00958 expression
was notably upregulated in CRC cell lines compared with that noted
in the NCM460 cells, and the expression of LINC00958 was the
highest in the SW480 cells. Therefore, SW480 cells were used to
perform the following experiments.
LINC00958 silencing inhibits the
proliferation, invasion and migration of CRC cells
To explore the effects of LINC00958 in regards to
the functions of SW480 cells, shRNA-LINC00958-1 or
shRNA-LINC00958-2 was transfected into the cells. As shown in
Fig. 1B, the expression of
LINC00958 was notably decreased after transfection compared with
the shRNA-NC group. shRNA-LINC00958-1 was used to perform the
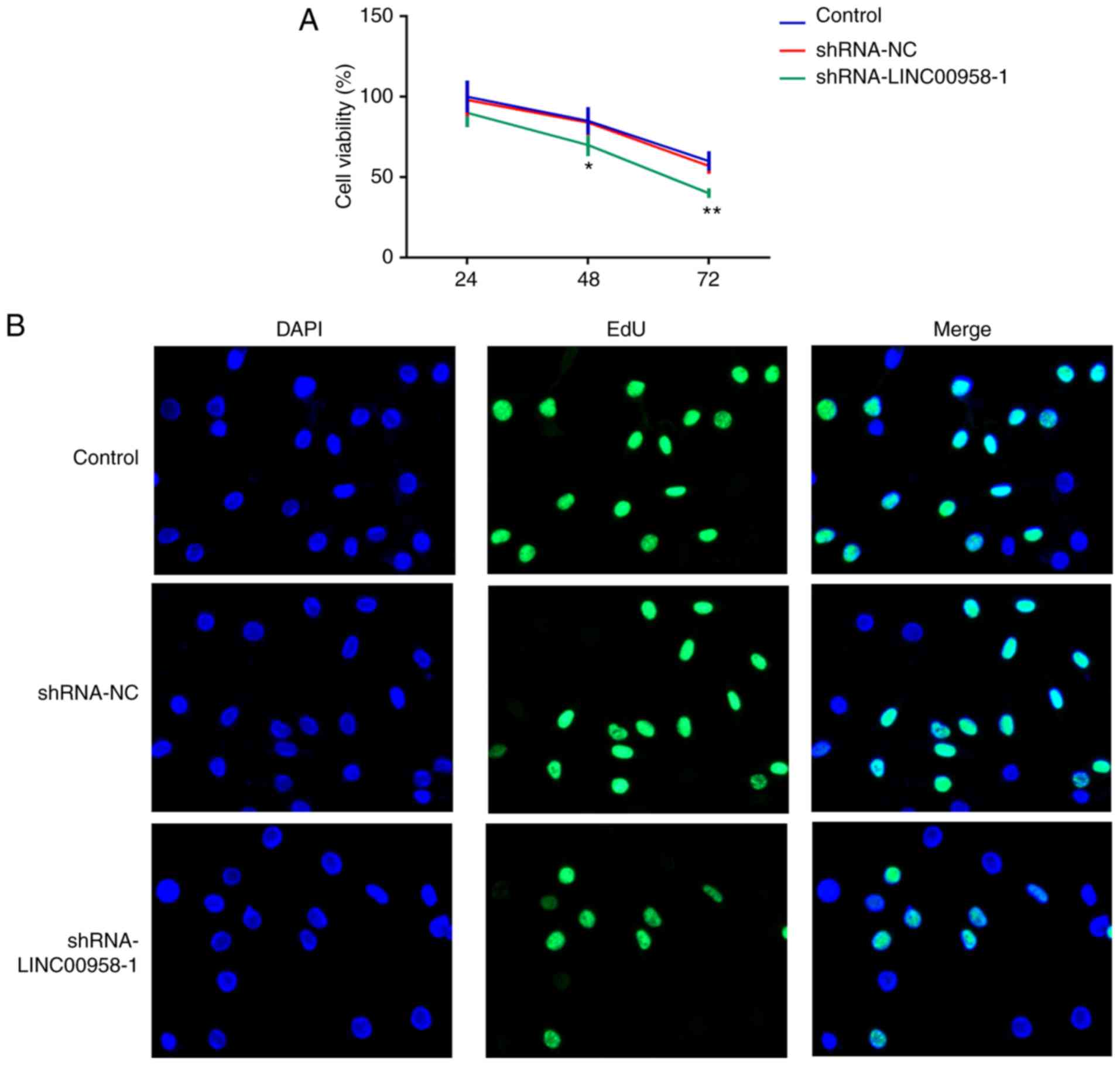
following experiments. A CCK-8 assay was employed to measure the
proliferation of SW-480 cells. As exhibited in Fig. 2A and B, LINC00958 silencing
significantly suppressed the proliferative ability of SW-480 cells
compared with the shRNA-NC group. In addition, Transwell and wound
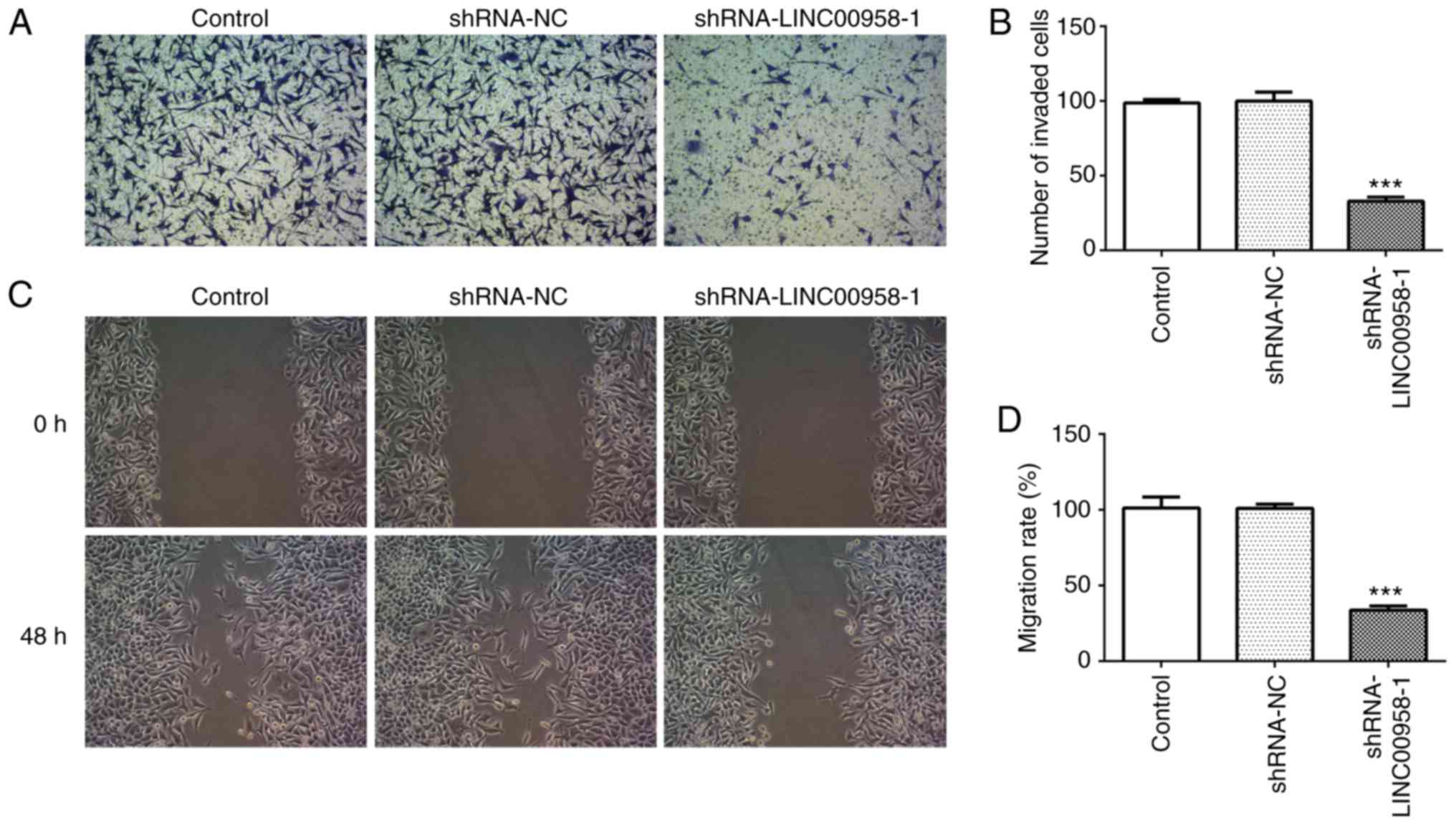
healing assays were performed to evaluate the effects of LINC00958
on the invasion and migration of CRC cells, respectively. As
demonstrated in Fig. 3A and B, the
invasive ability of SW480 cells was significantly decreased
relative to the shRNA-NC group following transfection with
shRNA-LINC00958-1. Concurrently, notable inhibition of SW480 cell
migration was found in the shRNA-LINC00958-1 group compared with
the shRNA-NC group (Fig. 3C and D).
These findings indicated that LINC00958 silencing suppressed the
proliferation, invasion and migration of CRC cells.
LINC00958 silencing promotes the
apoptosis of CRC cells
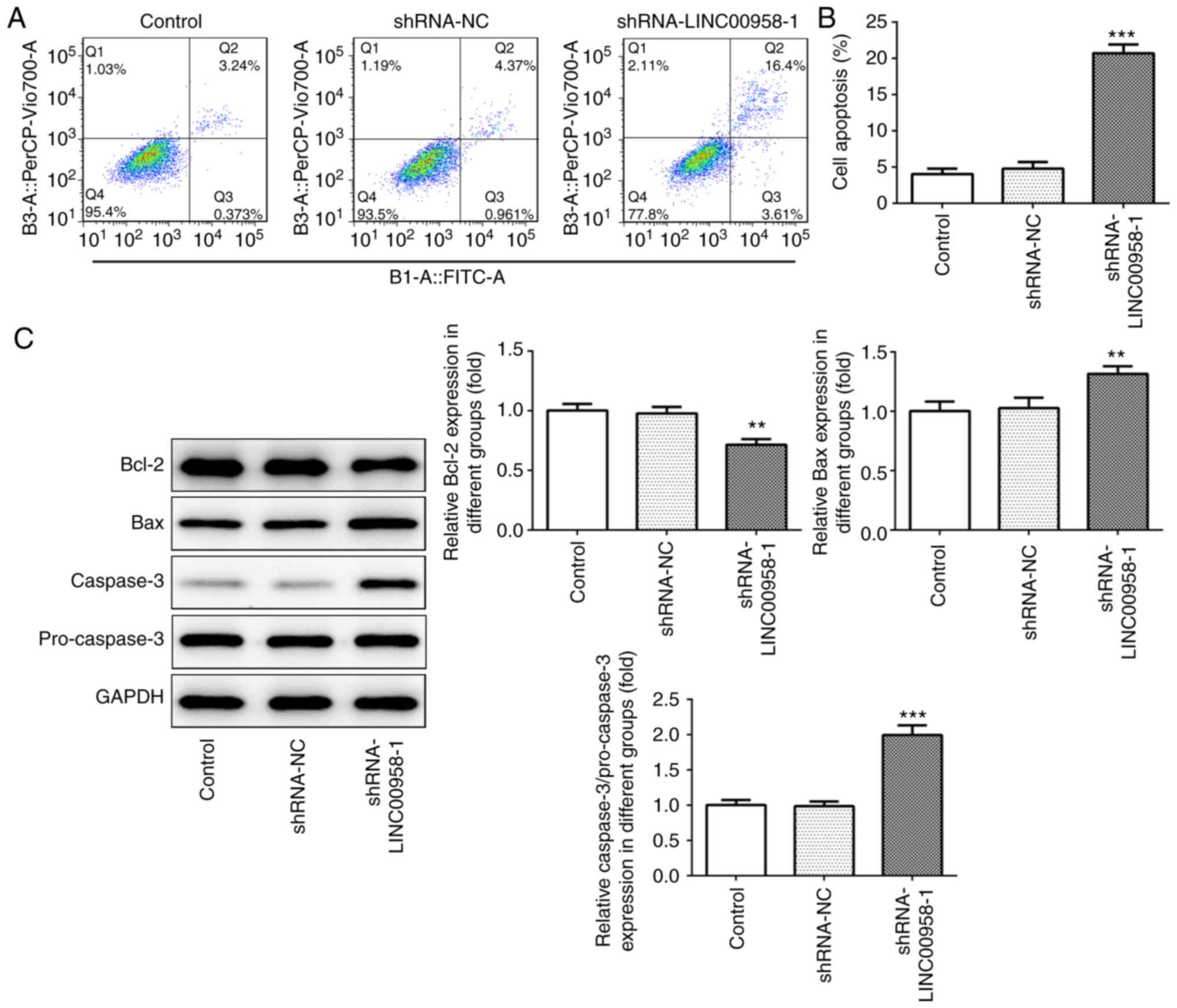
Flow cytometry was performed to examine the effects
of LINC00958-1 transfection on the apoptosis of SW480 cells. As
presented in Fig. 4A and B,
silencing of LINC00958 notably elevated the rate of cell apoptosis
in the SW480 cells compared with the shRNA-NC group. Subsequently,
the expression levels of apoptosis-related proteins were measured
using western blotting. As shown in Fig. 4C, the expression of Bcl-2 was
significantly reduced after transfection with shRNA-LINC00958-1,
which was accompanied by a significant increase in Bax and
caspase-3 expression. These results indicate that downregulation of
LINC00958 promoted the apoptosis of CRC cells.
LINC00958 is identified as a potential
target of miR-3619-5p
To explore the underlying regulatory mechanisms of
LINC00958 in CRC, StarBase bioinformatics database (http://starbase.sysu.edu.cn/) was used to predict the
potential miRNAs which may have binding sites with LINC00958. The
binding site of miR-3619-5p on LINC00958 was predicted by StarBase
(Fig. 5A). The location of binding
site on LINC00958 is chr11:13011075-13011081[-]. Then, a dual
luciferase assay was adopted to verify the target relationship
between LINC00958 and miR-3619-5p. As presented in Fig. 5B, the luciferase intensity was
significantly decreased after co-transfection with a miR-3619-5p
mimic and LINC00958-WT. These observations revealed that LINC00958
is a potential direct target of miR-3619-5p.
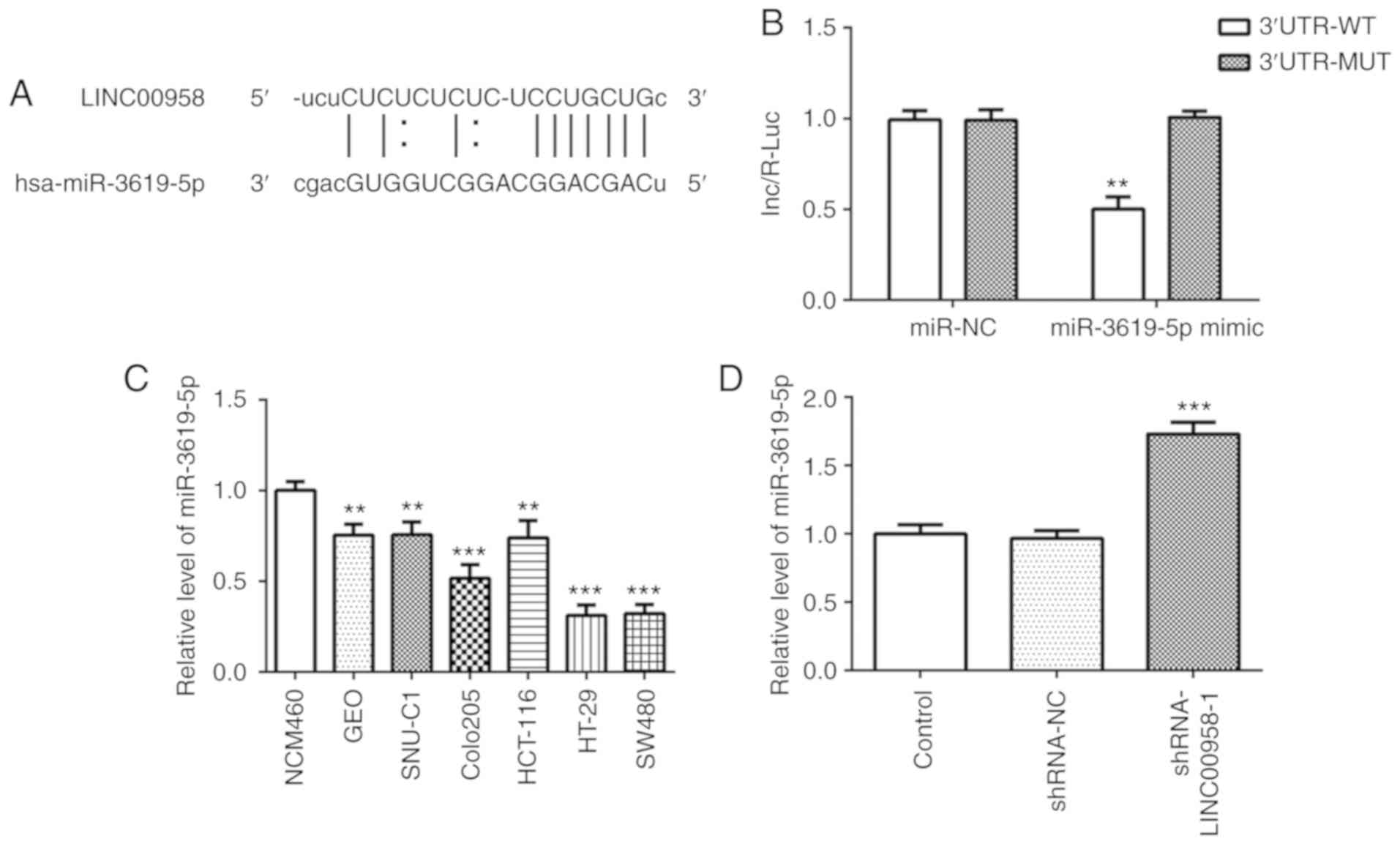 | Figure 5.miR-3619-5p is directly targeted by
LINC00958. (A) Binding region between miR-3619-5p and LINC00958.
(B) A luciferase reporter assay was performed to detect the
relative luciferase activity. Each experiment was repeated three
times independently (N=3). Data are expressed as mean ± standard
deviation. Statistical comparisons were analyzed by a two-tailed
Student's t test. **P<0.01 vs. 3′untranslated region-MUT. (C)
The expression of miR-3619-5p was measured in several CRC cell
lines (GEO, SNU-C1, COLO205, HCT-116, HT-29 and SW480) using
RT-qPCR. The experiments were generated from three independent
repeats (N=3). Data are expressed as mean ± standard deviation.
Statistical comparisons were analyzed by a two-tailed Student's t
test. **P<0.01, ***P<0.001 vs. NCM460. (D) The expression of
miR-3619-5p was detected using RT-qPCR after transfection with
shRNA-LINC00958-1. Each experiment was repeated three times
independently (N=3). Data are expressed as mean ± standard
deviation. Statistical comparisons were analyzed by a two-tailed
Student's t-test. ***P<0.001 vs. shRNA-NC. shRNA, short hairpin
RNA; NC, negative control; miR, microRNA; MUT, mutant; WT,
wild-type; RT-qPCR, reverse transcription-quantitative PCR. |
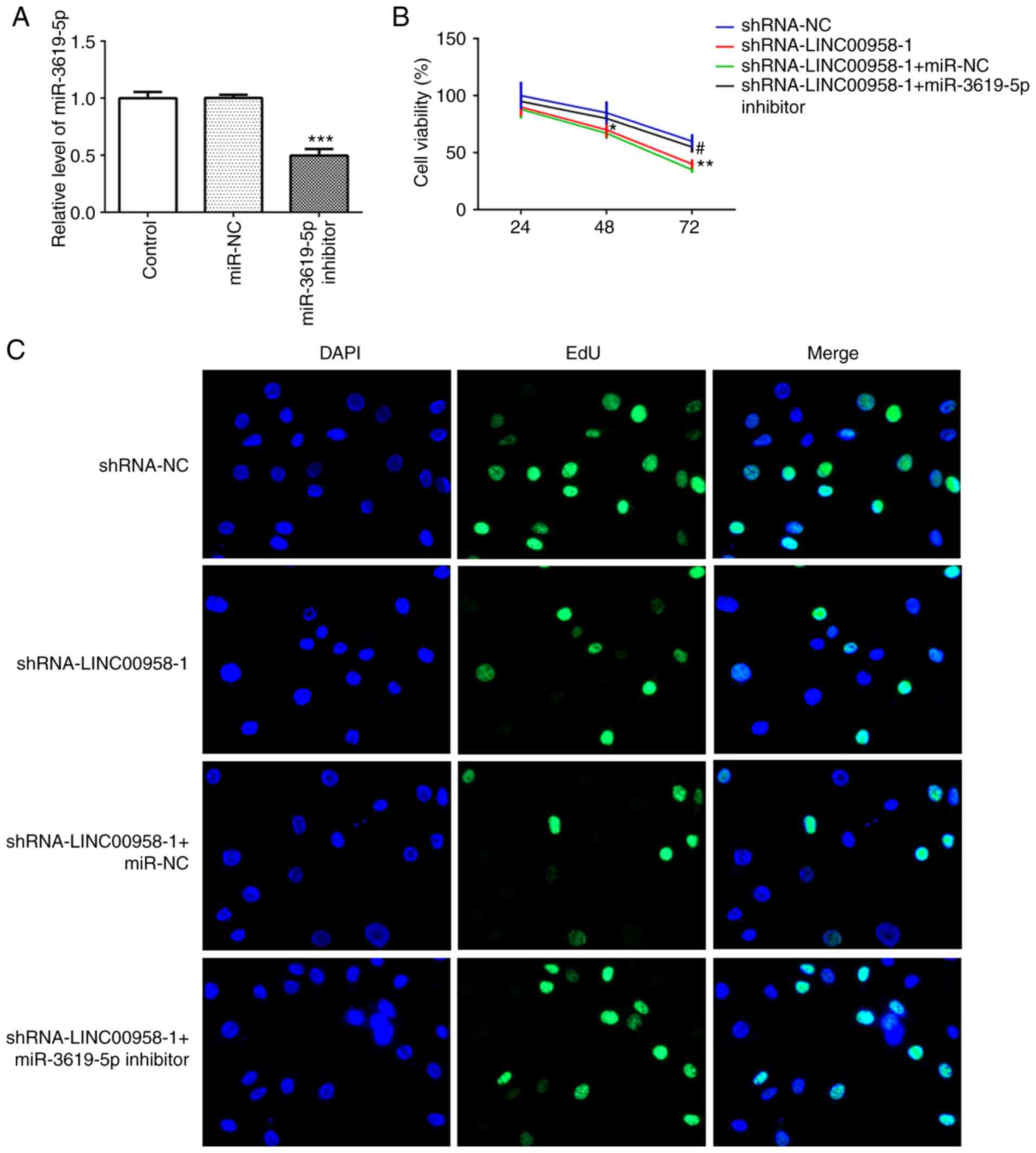
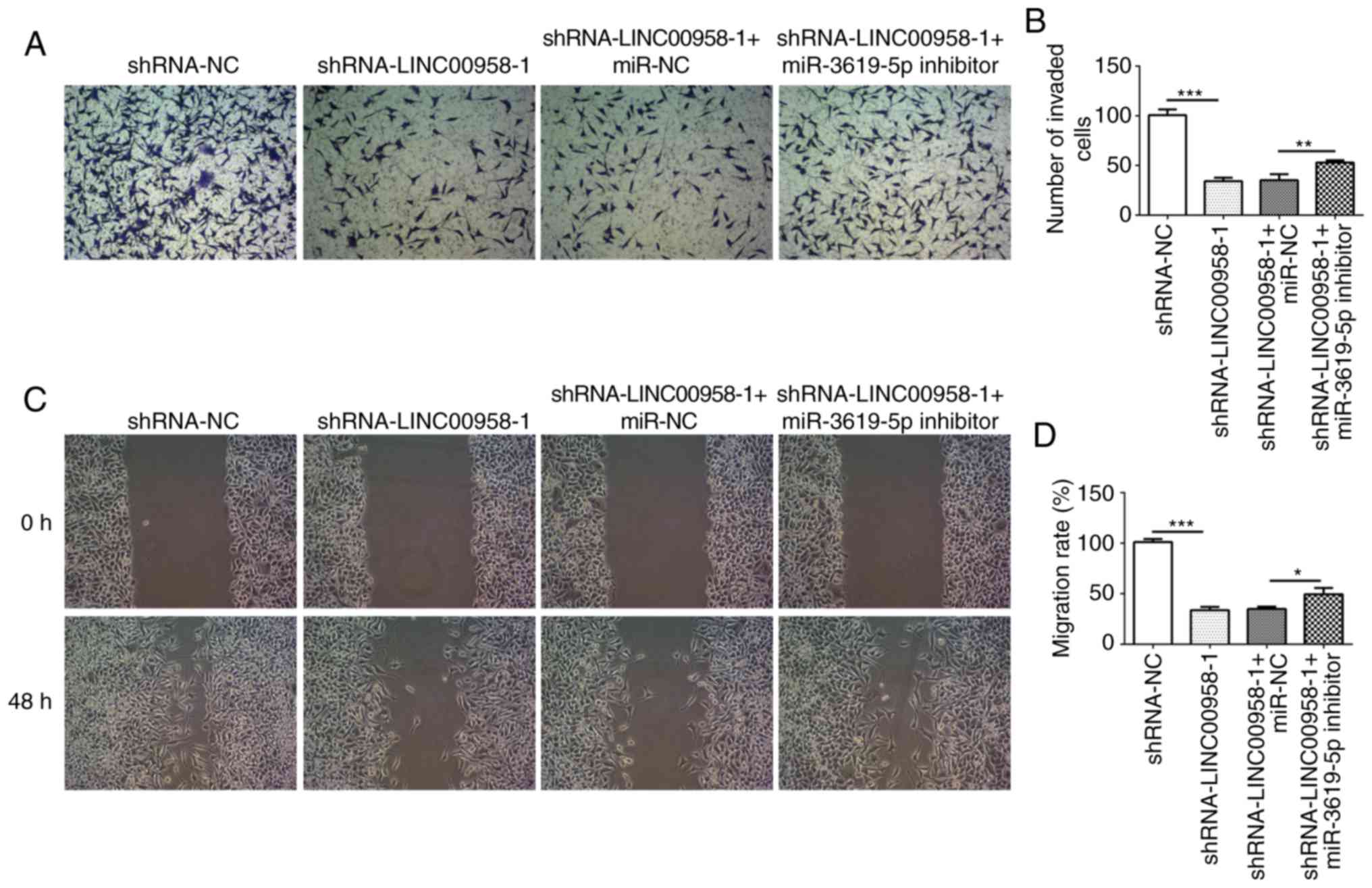
miR-3619-5p inhibitor reverses the
inhibitory effects of LINC00958 silencing on proliferation,
invasion and migration of CRC cells
The expression of miR-3619-5p was assessed in
several cell lines using RT-qPCR. As shown in Fig. 5C, significantly reduced expression
of miR-3619-5p was observed in the CRC cell lines, especially in
SW480 cells. Subsequently, the level of this miRNA was detected in
SW480 cells following transfection with shRNA-LINC00958-1. It was
found that the expression of miR-3619-5p was notably upregulated
following LINC00958 silencing (Fig.
5D). Then, a miR-3619-5p inhibitor was transfected into SW480
cells and successful transfection is presented in Fig. 6A. In addition, the proliferation of
SW480 cells was measured using a CCK-8 assay. As exhibited in
Fig. 6B and C, miR-3619-5p
inhibitor reversed the effects of LINC00958 silencing on cell
viability in SW480 cells. Moreover, the changes in invasive
(Fig. 7A and B) and migratory
activity (Fig. 7C and D) were
consistent with the results of the proliferation assay. These data
indicated that the miR-3619-5p inhibitor attenuated the inhibitory
effects of LINC00958 silencing on proliferation, invasion and
migration of CRC cells.
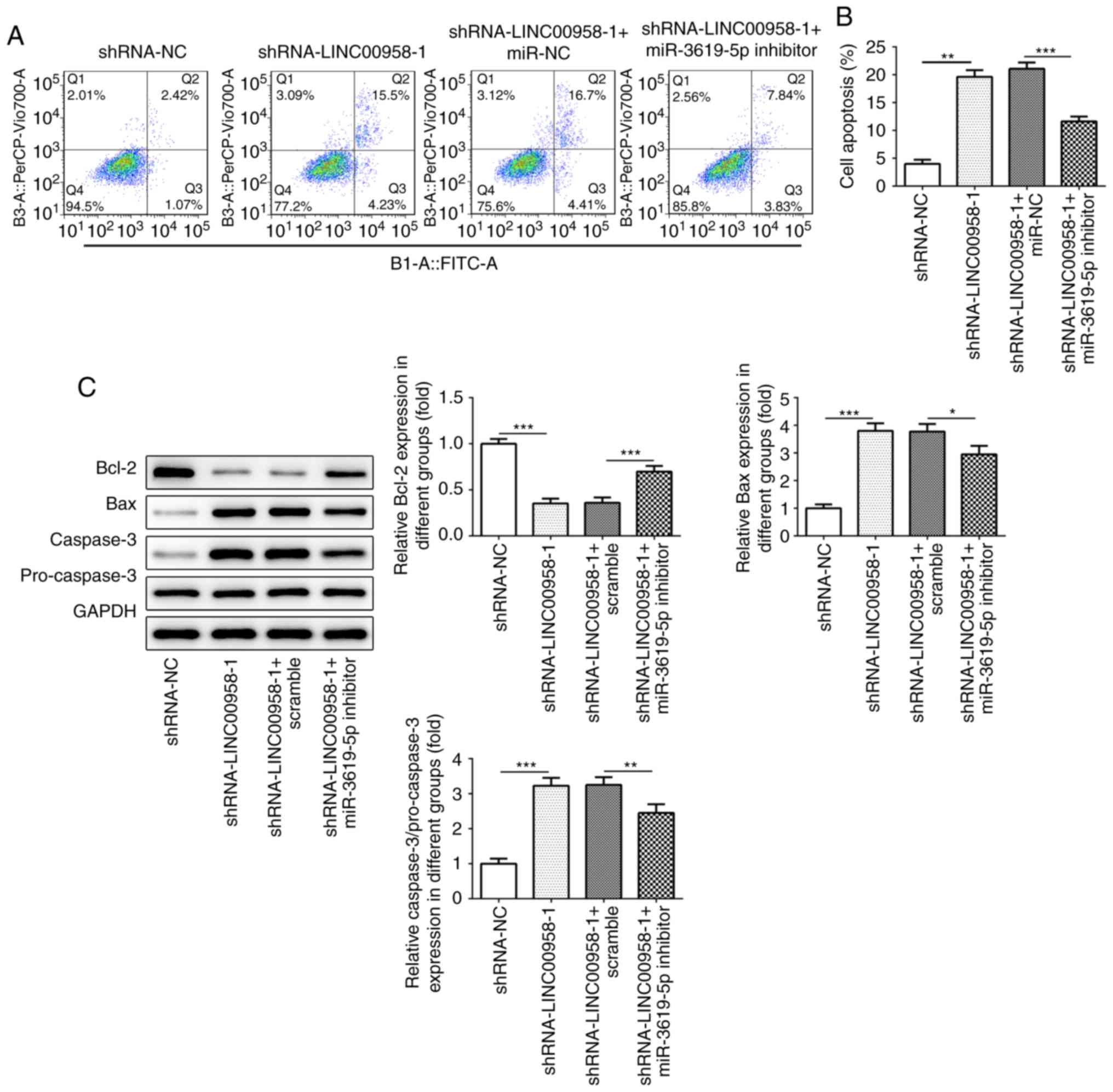
miR-3619-5p inhibitor reverses the
effects of LINC00958 silencing on the apoptosis of CRC cells
To confirm the regulatory effects of miR-3619-5p on
the apoptosis of CRC cells, flow cytometry was performed. As
presented in Fig. 8A and B,
transfection with a miR-3619-5p inhibitor decreased the number of
apoptotic SW480 cells compared with LINC00958 silencing alone.
Meanwhile, the expression of apoptosis-related proteins was
evaluated using western blot analysis. As shown in Fig. 8C, co-transfection with miR-3619-5p
inhibitor and shRNA-LINC00958-1 notably enhanced the expression of
Bcl-2, whereas the expression of Bax and casapase-3 was reduced in
SW480 cells compared with shRNA-LINC00958-1 transfection alone.
Taken together, the results indicated that the miR-3619-5p
inhibitor reversed the effects of LINC00958 silencing on the
apoptosis of CRC cells.
Discussion
Accumulating evidence has demonstrated that lncRNAs
are abnormally expressed in colorectal cancer (CRC) and are
involved in its progression (20,21).
The present study aimed to investigate the roles of LINC00958 and
miR-3619-5p in the proliferation, invasion and migration of CRC
cells. The results demonstrated that LINC00958 was highly expressed
in CRC cells and downregulation of LINC00958 inhibited CRC cell
proliferation, invasion and migration by targeting miR-3619-5p.
An increasing amount of evidence suggests that
dysregulation of lncRNAs are closely implicated in the
pathogenesis, development and metastasis of CRC (22,23).
Emerging evidence confirms that LINC00958 expression is notably
overexpressed in cervical cancer cells, and LINC00958 contributes
to the proliferation and metastasis of cervical cancer cells
(24). In support, a previous study
highlighted the importance of LINC00958 in the occurrence of glioma
(25). Notably, accumulating
evidence has demonstrated that CRC-related lncRNAs regulate the
progression of CRC by inhibiting or promoting proliferation,
invasion, apoptosis and metastasis of CRC cells (26,27).
For example, lncRNA PCAT-1 can modulate cell proliferation,
invasion, migration and apoptosis in CRC by targeting miR-149-5p
(28). Downregulation of
XLOC-010588 reduces CRC cell invasion and migration (29). In the present study, the expression
of LINC00958 was markedly upregulated in CRC cells. Silencing of
LINC00958 inhibited proliferation, invasion and migration, and
promoted apoptosis of CRC cells. These findings demonstrated that
LINC00958 silencing may suppress the progression of CRC.
lncRNAs act as competing endogenous (ce)RNAs to
regulate the activity of miRNAs, which is an important mechanism in
the regulation of tumor development (30,31).
In the present study, the StarBase bioinformatics database
predicted that LINC00958 is a potentially direct target of
miR-3619-5p, which was verified by a luciferase reporter assay.
miR-3619-5p was found to act as a tumor-suppressor gene, which can
prevent proliferation and cisplatin resistance of skin squamous
cell carcinoma cell via regulating the expression of KPNA4
(32). Moreover, a previous study
reported that miR-3619-5p has the ability to inhibit the growth of
prostate cancer cells (33).
Compelling evidence indicates that miR-3619-5p could suppress
β-catenin-mediated cancer growth and invasion in non-small cell
lung cancer cells (34). In support
of these findings, decreased miR-3619-5p expression has been
detected in retinoblastoma cells, and transfection with miR-3619-5p
mimic can improve the progression of retinoblastoma (35). In the present study, miR-3619-5p
inhibitor reversed the effects of LINC00958 silencing on
proliferation, invasion, migration and apoptosis of CRC cells.
Thus, the results of the present study demonstrated that the
downregulation of LINC00958 can suppress the progression of CRC
cells by targeting miR-3619-5p.
In summary, to the best of our knowledge, the
present study demonstrated for the first time that downregulation
of LINC00958 suppresses CRC cell proliferation, invasion and
migration, and promotes apoptosis by targeting miR-3619-5p,
suggesting a potential novel biomarker and target for the diagnosis
and treatment of CRC. However, the lack of studies of LINC00958 in
tumor tissue from CRC patients, the absence of experiments
concerning the miR-3619-5p effect on endogenous LINC00958, the
characteristics of SW480 cells and how this could impact the
extrapolation of our data to in vivo tumors are limitations
of the present research and therefore, a comprehensive analysis is
required in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS designed the experiments. YS, SH and YL performed
the experiments. YL, RW and PH analyzed the data. YC, LC and YS
wrote the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng Y, Nie P and Xu S: Long noncoding
RNA CASC21 exerts an oncogenic role in colorectal cancer through
regulating miR-7-5p/YAP1 axis. Biomed Pharmacother. 121:1096282020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meng S, Jian Z, Yan X, Li J and Zhang R:
lncRNA SNHG6 inhibits cell proliferation and metastasis by
targeting ETS1 via the PI3K/AKT/mTOR pathway in colorectal cancer.
Mol Med Rep. 20:2541–2548. 2019.PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Werf A, Arthey K, Hiesmayr M, Sulz
I, Schindler K, Laviano A, Langius J and de van der Schueren M: The
determinants of reduced dietary intake in hospitalised colorectal
cancer patients. Support Care Cancer. 26:2039–2047. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan Q and Liu B: Identification of the
anticancer effects of a novel proteasome inhibitor, ixazomib, on
colorectal cancer using a combined method of microarray and
bioinformatics analysis. OncoTargets Ther. 10:3591–3606. 2017.
View Article : Google Scholar
|
|
7
|
Niu X, Yang B, Liu F and Fang Q: lncRNA
HOXA11-AS promotes OSCC progression by sponging miR-98-5p to
upregulate YBX2 expression. Biomed Pharmacother. 121:1096232020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bao Y, Tang J, Qian Y, Sun T, Chen H, Chen
Z, Sun D, Zhong M, Chen H, Hong J, et al: Long noncoding RNA BFAL1
mediates enterotoxigenic bacteroides fragilis-related
carcinogenesis in colorectal cancer via the RHEB/mTOR pathway. Cell
Death Dis. 10:6752019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu T, Liu Y, Wei C, Yang Z, Chang W and
Zhang X: lncRNA HULC promotes the progression of gastric cancer by
regulating miR-9-5p/MYH9 axis. Biomed Pharmacother. 121:1096072020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi X, Zhang W, Nian X, Lu X, Li Y, Liu F,
Wang F, He B, Zhao L, Zhu Y, et al: The previously uncharacterized
lncRNA APP promotes prostate cancer progression by acting as a
competing endogenous RNA. Int J Cancer. 146:475–486. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen G, Gu Y, Han P, Li Z, Zhao JL and Gao
MZ: Long noncoding RNA SBF2-AS1 promotes colorectal cancer
proliferation and invasion by inhibiting miR-619-5p activity and
facilitating HDAC3 expression. J Cell Physiol. 234:18688–18696.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun A, Xiao LJ, Zhao XR, Wang JP, Chang XM
and Zhao EH: Effect of lncRNA AK093407 on colorectal cancer cell
line HCT-116. Eur J Immunol. 49:1535–1536. 2019.
|
|
13
|
Bermúdez M, Aguilar-Medina M,
Lizárraga-Verdugo E, Avendaño-Félix M, Silva-Benítez E,
López-Camarillo C and Ramos-Payán R: LncRNAs as regulators of
autophagy and drug resistance in colorectal cancer. Front Oncol.
9:10082019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seitz AK, Christensen LL, Christensen E,
Faarkrog K, Ostenfeld MS, Hedegaard J, Nordentoft I, Nielsen MM,
Palmfeldt J, Thomson M, et al: Profiling of long non-coding RNAs
identifies LINC00958 and LINC01296 as candidate oncogenes in
bladder cancer. Sci Rep. 7:3952017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Song ZJ, Wang Y, Zhong WF, Kang P
and Yang Y: Elevated long non-coding RNA LINC00958 was associated
with metastasis and unfavorable prognosis in gastric cancer. Eur
Rev Med Pharmacol Sci. 23:598–603. 2019.PubMed/NCBI
|
|
16
|
Chen F, Liu M, Yu Y, Sun Y, Li J, Hu W,
Wang X and Tong D: LINC00958 regulated miR-627-5p/YBX2 axis to
facilitate cell proliferation and migration in oral squamous cell
carcinoma. Cancer Biol Ther. 20:1270–1280. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, Chen JZ, Zhang JQ, Chen HX, Qiu
FN, Yan ML, Tian YF, Peng CH, Shen BY, Chen YL and Wang YD:
Silencing of long noncoding RNA LINC00958 prevents tumor initiation
of pancreatic cancer by acting as a sponge of microRNA-330-5p to
down-regulate PAX8. Cancer Lett. 446:49–61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng J, Liu F, Zheng H, Wu Q and Liu S:
Long noncoding RNA ZFAS1 promotes tumorigenesis and metastasis in
nasopharyngeal carcinoma by sponging miR-892b to up-regulate LPAR1
expression. J Cell Mol Med. 24:1437–1450. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Damas ND, Marcatti M, Côme C, Christensen
LL, Nielsen MM, Baumgartner R, Gylling HM, Maglieri G, Rundsten CF,
Seemann SE, et al: SNHG5 promotes colorectal cancer cell survival
by counteracting STAU1-mediated mRNA destabilization. Nat Commun.
7:138752016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C, Liu T, Zhang Y, Li Q and Jin LK:
lncRNA-ZDHHC8P1 promotes the progression and metastasis of
colorectal cancer by targeting miR-34a. Eur Rev Med Pharmacol Sci.
23:1476–1486. 2019.PubMed/NCBI
|
|
22
|
Iguchi T, Uchi R, Nambara S, Saito T,
Komatsu H, Hirata H, Ueda M, Sakimura S, Takano Y, Kurashige J, et
al: A long noncoding RNA, lncRNA-ATB, is involved in the
progression and prognosis of colorectal cancer. Anticancer Res.
35:1385–1388. 2015.PubMed/NCBI
|
|
23
|
Wang X, Mo FM, Bo H, Xiao L, Chen GY, Zeng
PW, Huang YN, Lei Z, Yuan WJ and Chen ZH: Upregulated expression of
long non-coding RNA, LINC00460, suppresses proliferation of
colorectal cancer. J Cancer. 9:2834–2843. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Zhong Y, Yang B, Zhu Y, Zhu X, Xia
Z, Xu J and Xu L: LINC00958 facilitates cervical cancer cell
proliferation and metastasis by sponging miR-625-5p to upregulate
LRRC8E expression. J Cell Biochem. 121:2500–2509. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo E, Liang C, He X, Song G, Liu H, Lv Z,
Guan J, Yang D and Zheng J: Long noncoding RNA LINC00958
accelerates gliomagenesis through regulating miR-203/CDK2. DNA Cell
Biol. 37:465–472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin H, Guo Q, Lu S, Chen J, Li X, Gong M,
Tang L and Wen J: lncRNA SUMO1P3 promotes proliferation and
inhibits apoptosis in colorectal cancer by epigenetically silencing
CPEB3. Biochem Biophys Res Commun. 511:239–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Song Y, Yang C and Wu X:
Overexpression of lncRNA TUSC7 reduces cell migration and invasion
in colorectal cancer. Oncol Rep. 41:3386–3392. 2019.PubMed/NCBI
|
|
28
|
Wang AH, Fan WJ, Fu L and Wang XT: lncRNA
PCAT-1 regulated cell proliferation, invasion, migration and
apoptosis in colorectal cancer through targeting miR-149-5p. Eur
Rev Med Pharmacol Sci. 23:8310–8320. 2019.PubMed/NCBI
|
|
29
|
Wang Y, Kuang H, Xue J, Liao L, Yin F and
Zhou X: lncRNA AB073614 regulates proliferation and metastasis of
colorectal cancer cells via the PI3K/AKT signaling pathway. Biomed
Pharmacother. 93:1230–1237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li S, Chen X, Liu X, Yu Y, Pan H, Haak R,
Schmidt J, Ziebolz D and Schmalz G: Complex integrated analysis of
lncRNAs-miRNAs-mRNAs in oral squamous cell carcinoma. Oral Oncol.
73:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wan X, Ding X, Chen S, Song H, Jiang H,
Fang Y, Li P and Guo J: The functional sites of miRNAs and lncRNAs
in gastric carcinogenesis. Tumour Biol. 36:521–532. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang M, Luo H and Hui L: miR-3619-5p
hampers proliferation and cisplatin resistance in cutaneous
squamous-cell carcinoma via KPNA4. Biochem Biophys Res Commun.
513:419–425. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li S, Wang C, Yu X, Wu H, Hu J, Wang S and
Ye Z: miR-3619-5p inhibits prostate cancer cell growth by
activating CDKN1A expression. Oncol Rep. 37:241–248. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niu X, Liu S, Jia L and Chen J: Role of
miR-3619-5p in β-catenin-mediated non-small cell lung cancer growth
and invasion. Cell Physiol Biochem. 37:1527–1536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan G, Su Y, Ma Z, Yu L and Chen N: Long
noncoding RNA LINC00202 promotes tumor progression by sponging
miR-3619-5p in retinoblastoma. Cell Struct Funct. 44:51–60. 2019.
View Article : Google Scholar : PubMed/NCBI
|