Introduction
Acute kidney injury (AKI) is currently the main
cause of high mortality in patients with acute disease (1). In recent years, the molecular and
pathological mechanisms of AKI has been elucidated; however, most
studies were focused on signaling pathways such as the PI3K/Akt
(2), Nrf2/HO-1 (3), and TLR4/NF-κB (4) pathways. MicroRNAs (miRNAs) have been
reported to play a vital regulatory role in many physiological
pathways, including multiple acute tissue injury, for example the
miR-30b-3p pathway, which is involved in acute lung injury
(5) and the miR-30a-5p pathway,
which can alleviate inflammatory responses and oxidative stress
stimulated by spinal cord injury by targeting the Neurod 1/MAPK/ERK
axis (6). In addition, miR-30a-5p
is involved in the process of inflammation and apoptosis in PC-12
cells mediated by lipopolysaccharide (7). Nevertheless, the regulatory mechanism
of miR-30a-5p in the physiological process of renal I/R injury at
the gene level has not been fully investigated.
The glutamate dehydrogenase 1 (GLUD1) gene
encodes glutamate dehydrogenase (GDH), which is mainly located in
the mitochondria of the liver, myocardium and renal cells (8). It is well known that GDH acts as a
critical enzyme for catalyzing the entry of glutamine into the
tricarboxylic acid cycle (9) and
reducing NAD(P) to NAD(P)H, which can be used in bio-synthetic
pathways (10). As an important
antioxidant and maintainer of intracellular calcium homeostasis
(11), Ginsenoside Rg1 (Rg1) was
demonstrated to alleviate hypoxia/reoxygenation (H/R)-induced cell
damage and mitochondrial dysfunction by regulating GDH imbalance
(12). More importantly, the
bioinformatics prediction website TargetScan (http://www.targetscan.org) demonstrated that the
3′-UTR region of GLUD1 can specifically bind to miR-30a-5p.
Therefore, we hypothesized that miR-30a-5p participated in the H/R
injury by targeting the GLUD1 gene.
In the present study, human renal tubule epithelial
cells (HK-2) were used to construct a H/R cell model in
vitro, to research the molecular mechanism of miR-30a-5p on
oxidative stress and apoptosis from the perspective of the
GLUD1 gene.
Materials and methods
HK-2 cell culture
HK-2 cells were provided by the American Type
Culture Collection (Rockville). RMPI-1640 medium (HyClone) was used
to culture cells in a thermostatic incubator (Thermo Scientific)
with 5% CO2, at 37°C. Fetal bovine serum (10%, Sigma),
penicillin (100 IU/ml), streptomycin (100 µg/ml), and human
recombinant epidermal growth factor (10 ng/ml, Gibco) were added to
the RMPI-1640 medium. At 80% confluency, 0.25% trypsin was used for
digestion and cells were passaged at a ratio of 1:3.
Establishment of the model of
hypoxia/reoxygenation (H/R)
HK-2 cells were incubated in a 96-well plate at a
density of 5×104 cells/well. After 24 h culture, for the
H/R group, HK-2 cells were subjected to hypoxic treatment
(containing 1% O2 and 5% CO2, with 94%
N2, temperature was maintained at 37°C) for 12 h in
serum-free medium without glucose. Subsequently, the cells were
switched to a humidified cell incubator (BB 15; Thermo Electron LED
GmbH) containing 5% CO2 and 95% O2 at 37°C
for 2 h for reoxygenation with normal medium. For the control
group, HK-2 cells were cultured in a humidified cell incubator
containing 5% CO2 at 37°C for 14 h.
HK-2 cell groups and
transfections
HK-2 cells has been assigned to 10 groups and
treated as follows: Experiment 1: i) control group (cells without
any treatment); ii) H/R group (cells exposed to H/R); experiment 2:
iii) H/R + negative control 1 (NC1) group (cells treated with mimic
NC before H/R was induced); iv) H/R + mimic group (cells treated
with miR-30a-5p mimic before H/R was induced; v) H/R + NC2 group
(cells treated with inhibitor NC before H/R was induced); vi) H/R +
inhibitor group (cells treated with miR-30a-5p inhibitor before H/R
was induced); experiment 3: vii) H/R + NC2 + si-control group
(cells treated with inhibitor NC and siRNA NC to GLUD1 before H/R
was induced); viii) H/R + inhibitor + si control group (cells
treated with miR-30a-5p inhibitor and siRNA NC to GLUD1 before H/R
was induced); ix) H/R + NC2 + si-GLUD1 (cells treated with
inhibitor NC and siRNA to GLUD1 before H/R was induced); and x) H/R
+ inhibitor + si-GLUD1 (cells treated with miR-30a-5p inhibitor and
siRNA to GLUD1 before H/R was induced).
Mimic NC, inhibitor NC, miR-30a-5p mimic, miR-30a-5p
inhibitor, siRNA NC and siRNA GLUD1 were provided by GenePharma
Co., Ltd. Cell transfection was conducted following the
instructions in the Lipofectamine 2000 transfection kit (Thermo
Fisher Scientific, Ltd.). Forty-eight hours after transfection,
HK-2 cells were subjected to H/R treatment as mentioned above.
Dual luciferase reporter analysis
Bioinformatics analysis TargetScan (http://www.targetscan.org) was used in the present
study to predict the binding sites of GLUD1 and miR-30a-5p. A
wild-type fragment that contained the binding site for miR-30a-5p
was designed to target the 3′-untranslated region (3′-UTR) of the
GLUD1 gene and synthesized by Genechem Corp. The fragment
was designed to have a restriction endonuclease site HindIII
and SpeI. The target fragment was inserted into the
pMIR-REPORT™ Luciferase vector plasmid (Ambion) using T4 DNA/RNA
ligase and labelled as pMIR-GLUD1-wild-type (WT). The binding site
of miR-30a-5p was mutated in the WT plasmid to produce the
pMIR-GLUD1-mutant-type (MUT) plasmid. HK-2 cells were inoculated in
12-well plates with a density of 2×105 cells/well, and
co-transfected with recombinant plasmids (WT and MUT) and
miR-30a-5p mimic for 48 h according to the groups described above.
Cells were collected as described earlier. A luciferase kit
(Promega) was used to determine firefly luciferase (A) and
Renilla luciferase (B), respectively. The internal reference
was represented by firefly luciferase, meaning that the
fluorescence activity (C) was evaluated by the ratio of B:A.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After total RNA was extracted from the HK-2 cells in
each group using TRIzol® reagent (Promega Corp.) as per
the manufacturer's protocol, it was used to synthesize cDNA using a
reverse transcription kit and following the manufacturer's
instructions (Takara). GLUD1 was detected by RT-qPCR using a
SYBR-Green qPCR Super Mix (Invitrogen). For miR-30a-5p, a Taqman
MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific) was
used to synthesize cDNA and a Taqman Universal Master Mix II (Roche
Applied Science) was used to perform RT-qPCR in accordance with the
manufacturer's protocol. The primer sequences are presented in
Table I. U6 and β-actin were used
as the internal reference for miRNA-30a-5p and GLUD1,
respectively, the 2−ΔΔCq method (13) was utilized to calculate the relative
expression level of the target gene. Experiments were independently
repeated three times.
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Primer
sequences | Forward
(5′→3′) | Reverse
(5′→3′) |
|---|
|
miR-30a-5p | TGTAAACATCCTCGAC
TGGAAG |
TGCGTGTCGTGGAGTC |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| GLUD1 |
TATCCGGTACAGCACTGACG |
GCTCCATGGTGAATCTTCGT |
| β-actin |
GCAGGAGTACGATGAGTCCG |
ACGCAGCTCAGTAACAGTCC |
Western blot analysis
RIPA lysis buffer (Beyotime Biotechnology) was used
to extract total protein from cells. Cells were incubated on ice
for 30 min, centrifuged at 12,000 × g for 10 min at 4°C, then total
protein concentration was measured in the supernatants using a
bicinchoninic acid kit (Pierce). SDS loading buffer was mixed with
the protein samples and boiled at 100°C for 10 min at 50 µg/well.
Proteins were resolved on a 10% SDS-PAGE gel, and then transferred
to polyvinylidene fluoride (PVDF) membranes. After 1 h blocking in
5% bovine serum albumin at 25°C, the membrane was incubated with
primary antibodies (all supplied by Abcam) against GLUD1 (ab166618,
1:1,000), cleaved-caspase-3 (ab32042, 1:500), caspase-3 (ab13847,
1:500), Bax (ab32503, 1:1,000), Bcl-2 (ab32124, 1:1,000) and GAPDH
(ab8245, 1:1,000) overnight at 4°C. Next, the membrane was rinsed
using Tris-buffered saline.
The membrane was washed in Tween-20 (TBST) four
times for 5 min each and treated with secondary antibodies
(1:2,000; Santa Cruz Biotechnology) for 1 h. The signals were
determined using an electrogenerated chemiluminescence reagent (GE
Healthcare Life Sciences) after washing in TBST three times for 5
min. ImageJ software (NIH, Bethesda) was used to detect intensity
of the bands and, with GAPDH as the internal reference, the
expression of these proteins was quantified.
Determination of intracellular ROS
concentration
The fluorescent probe of 2′,7′-dichlorofluolescein
diacetate (DCFH-DA; Sigma) was used to monitor the intracellular
reactive oxygen species (ROS) concentration. After the HK-2 cells
reached a density of 1×104 cells/ml, the culture medium
was removed, and the cells were washed three times with D-Hank's
solution. HK-2 cells were then cultured with 40 µM DCFH-DA for 30
min at 37°C. After incubation, the cells were washed again with
D-Hank's solution three times. To identify the nucleus, the cells
were then stained with Hoechst 33342 (Beyotime Institute of
Biotechnology) at room temperature for 5 min, then washed again
with D-Hank's solution three times and 1 ml of fresh culture medium
was added for resuspension. A fluorescence microplate reader
(Tecan) was used to evaluate the fluorescence intensity, with
excitation wavelengths at 485 nm and emission wavelengths at 535
nm. Experiments were performed in triplicate.
Measurement of SOD, CAT, GPx, GST and
GR activities
To quantify enzyme activity, HK-2 cells were
collected after 48 h culture, cells were lysed on ice with 100 µl
of RIPA buffer for 20 min and the supernatant was collected after
centrifugation at 6,000 × g for 30 min at 4°C. Commercial kits
(JianCheng Bioengineering Institute) were used to quantify the
activity of superoxide dismutase (SOD), catalase (CAT) and
peroxidase (GPx) in accordance with the manufacturer's
instructions. The activity of glutathione S-transferase (GST)
enzyme in the cells was measured according to the method of Habig
et al (14) and the activity
of glutathione-reductase (GR) was detected based on the method
reported by Aviram et al (15). These experiments were repeated in
triplicate.
Flow cytometry
Flow cytometry was employed to measure cell
apoptosis. HK-2 cells were digested with 0.25% trypsin after 48 h
in culture and centrifuged at 1,000 × g for 5 min at room
temperature. The cell pellet was resuspended in phosphate-buffered
saline (PBS) solution and the cell density was adjusted to
12×105 cells/ml. Cell suspension (100 µl) was removed
and stained with AnnexinV-FITC/PI staining kit (Carlsbad) according
to the manufacturer's instructions. BD FACSCalibur flow cytometer
(BD Biosciences) was used to analyze the stained cells and
calculate the number of apoptotic cells in each group. All
experiments were repeated three times.
Statistical analysis
Data were analyzed using SPSS 21.0 statistical
software (SPSS Inc.), and conducted three times to calculate the
mean value and standard deviation. Results are presented as mean ±
standard deviation (SD) and the Student's t-test was used to
calculate the difference between two groups, while one-way ANOVA
and Turkey's post-hoc test were utilized to analyze the differences
between multiple groups. In cases of non-normal distribution,
Mann-Whitney and Kruskal Wallis tests were performed. P<0.05 was
considered statistically significant.
Results
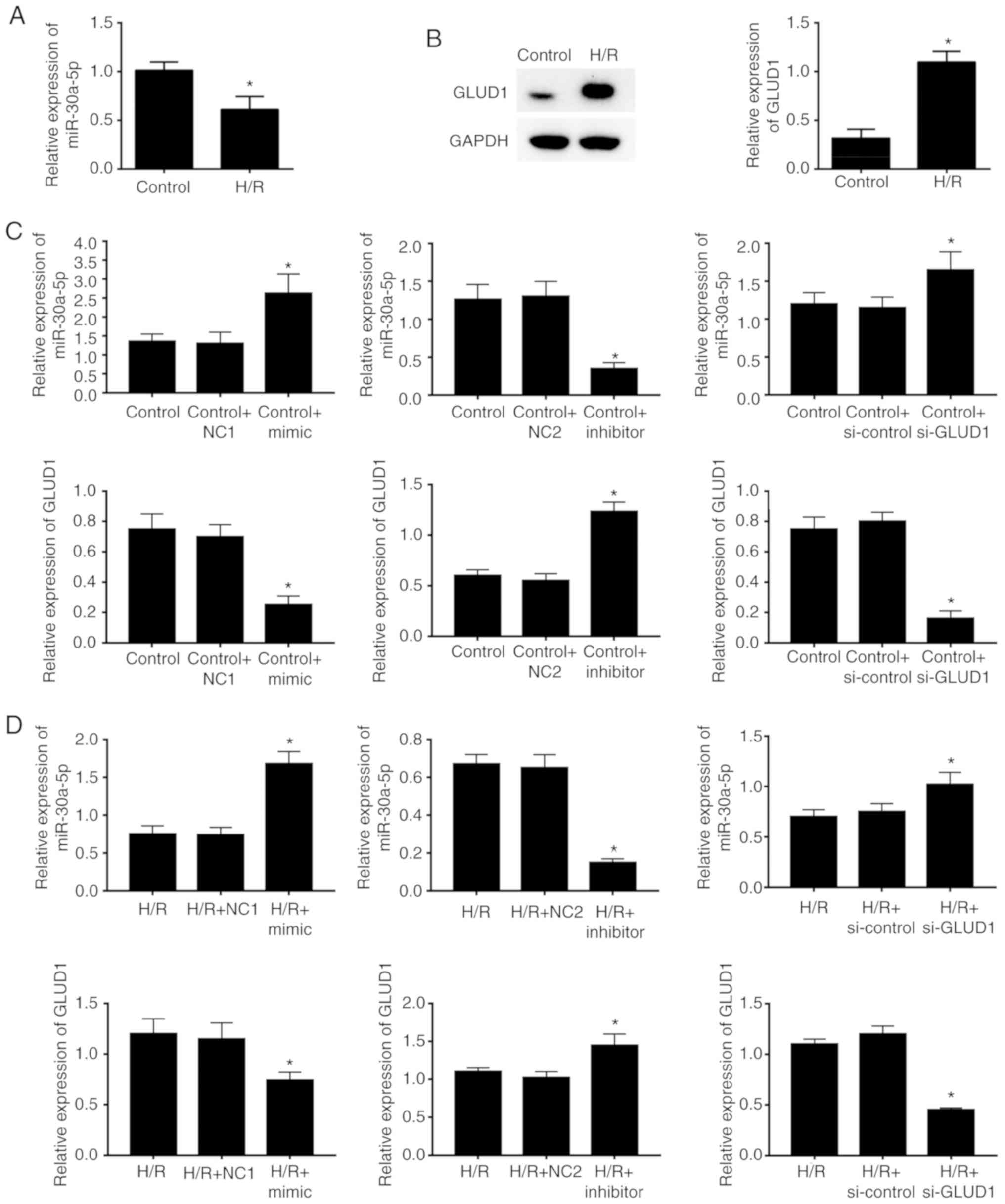
Decreased expression of miR-30a-5p
inversely correlated with GLUD1 protein expression in HK-2 cells
with or without H/R stimulation
Expression of miR-30a-5p and GLUD1 was
examined in HK-2 cells from each group. H/R-stimulated HK-2 cells
were decreased in miR-30a-5p expression when compared with cells
with no treatment (P<0.05; Fig.
1A). Furthermore, the H/R group had a significant increase in
GLUD1 expression compared with the control group (P<0.05;
Fig. 1B). This result suggested
that miR-30-5p and the GLUD1 gene have vital effects on
renal injury. To investigate whether miR-30a-5p and GLUD1
were involved in the process of H/R injury, overexpression and
inhibition of miR-30a-5p as well as silencing of GLUD1 gene
were performed in HK-2 cells. RT-qPCR determination showed that in
the cells with or without H/R stimulation, mimics transfected with
miR-30a-5p markedly promote the expression of miR-30a-5p and
inhibited the expression of GLUD1, while inhibitors transfected
with miR-30a-5p notably reduced the expression of miR-30a-5p and
increased the expression of GLUD1 (all P<0.05). In addition,
silencing GLUD1 by introducing GLUD1 siRNA distinctly
suppressed the expression of GLUD1 in HK-2 cells (P<0.05;
Fig. 1C-D).
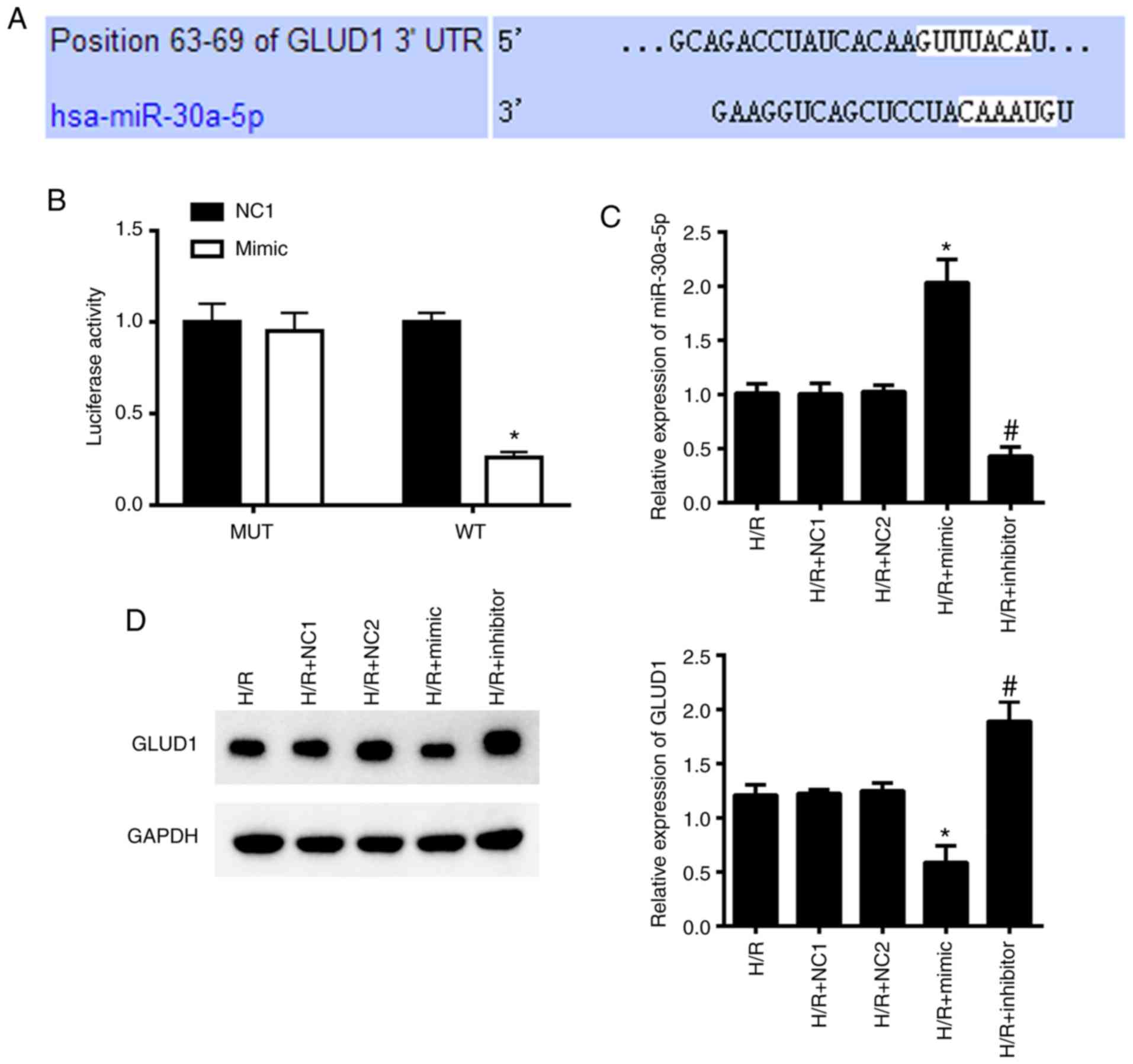
GLUD1 is a target gene of
miR-30a-5p
Bioinformatics prediction analysis (http://www.targetscan.org) demonstrated that the
3′-UTR region of GLUD1 can specifically bind to miR-30a-5p
(Fig. 2A). Dual luciferase reporter
analysis was conducted to confirm this hypothesis, and revealed
that compared with HK-2 cells in the NC1 + WT group, the mimic + WT
group showed a sharp decline in luciferase activity (P<0.05),
whereas cells in the mimic + MUT and NC1 + MUT had no changes in
luciferase activity (Fig. 2B; all
P>0.05).
Furthermore, we determined the protein levels of
GLUD1 in HK-2 cells following either up- or downregulation of
miR-30a-5p. RT-qPCR results showed that the mimic group increased
the level of miR-30a-5p, and the inhibitor group decreased the
level of miR-30a-5p compared with the NC1 and NC2 groups,
respectively (Fig. 2C). In
addition, cells transfected with mimic were associated with a
decrease in GLUD1 protein expression and increased GLUD1 protein
expression in the inhibitor group, as determined by western blot
analysis (Fig. 2D). These results
suggested that GLUD1 could function as the target gene for
miR-30a-5p.
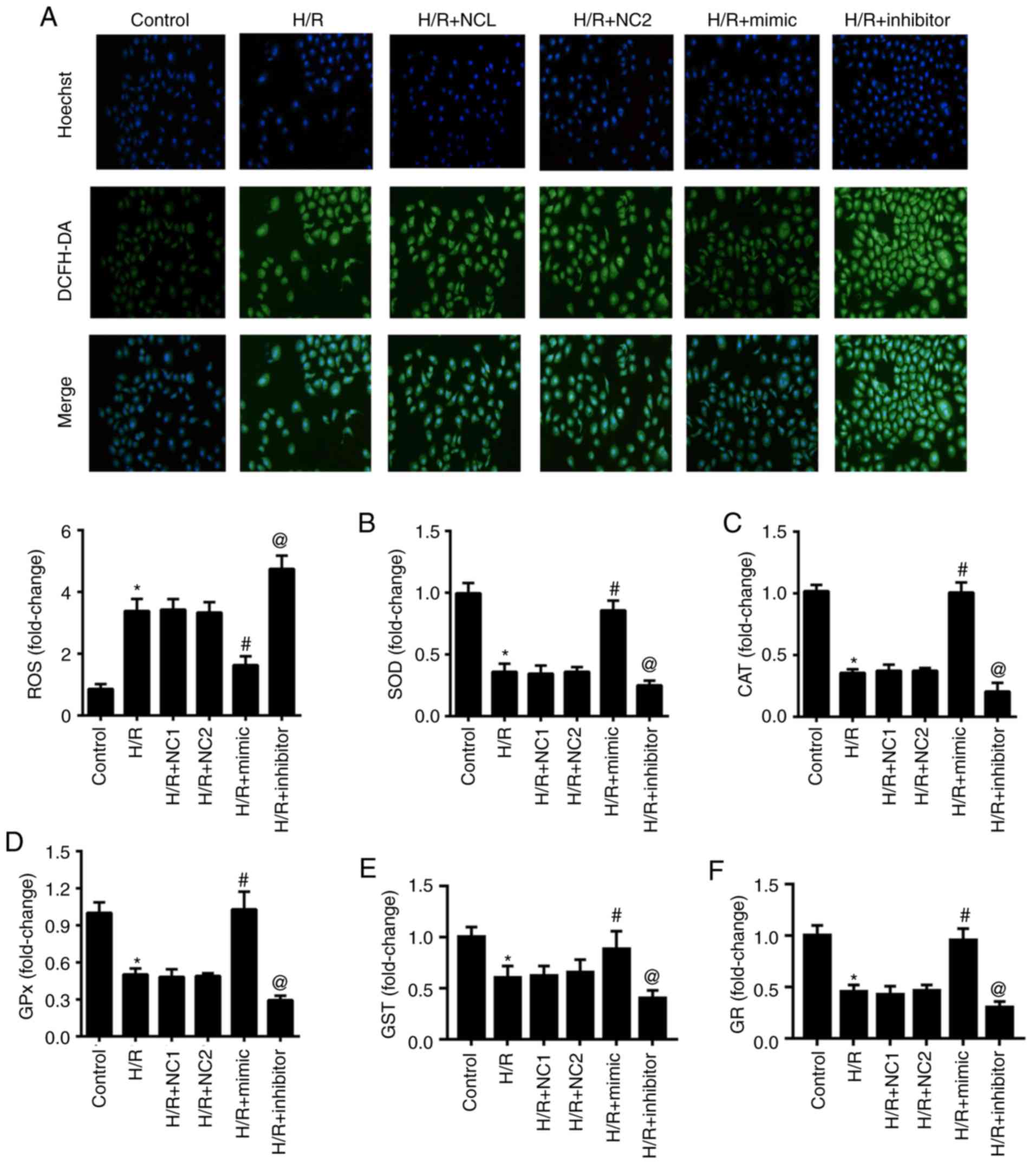
miR-30a-5p inhibits oxidative stress
in H/R-stimulated HK-2 cells
In order to investigate the influence of miR-30a-5p
in oxidative damage in HK-2 cells that undergo H/R, we investigated
intracellular ROS generation and the activities of SOD, CAT, GPx,
GST and GR in each group. As shown in Fig. 3, Hoechst 33342 was used to identify
the nucleus, which appears blue and intracellular ROS generation
was appears green. Intracellular ROS generation significantly
increased in the H/R group with decreased activities of SOD, CAT,
GPx, GST and GR when compared with the normal control group (all
P<0.05). Moreover, the levels of the oxidative stress indicators
in the H/R group have no significant difference compared with the
NC groups (all P>0.05). Compared with the NC group, the H/R +
mimic group showed a decrease in intracellular ROS generation and
significantly elevated levels for SOD, CAT, GPx, GST and GR. By
contrast, the H/R + inhibitor group had the completely opposite
result (all P<0.05). This result demonstrated the negative
action of miR-30a-5p in oxidative stress in H/R-stimulated HK-2
cells.
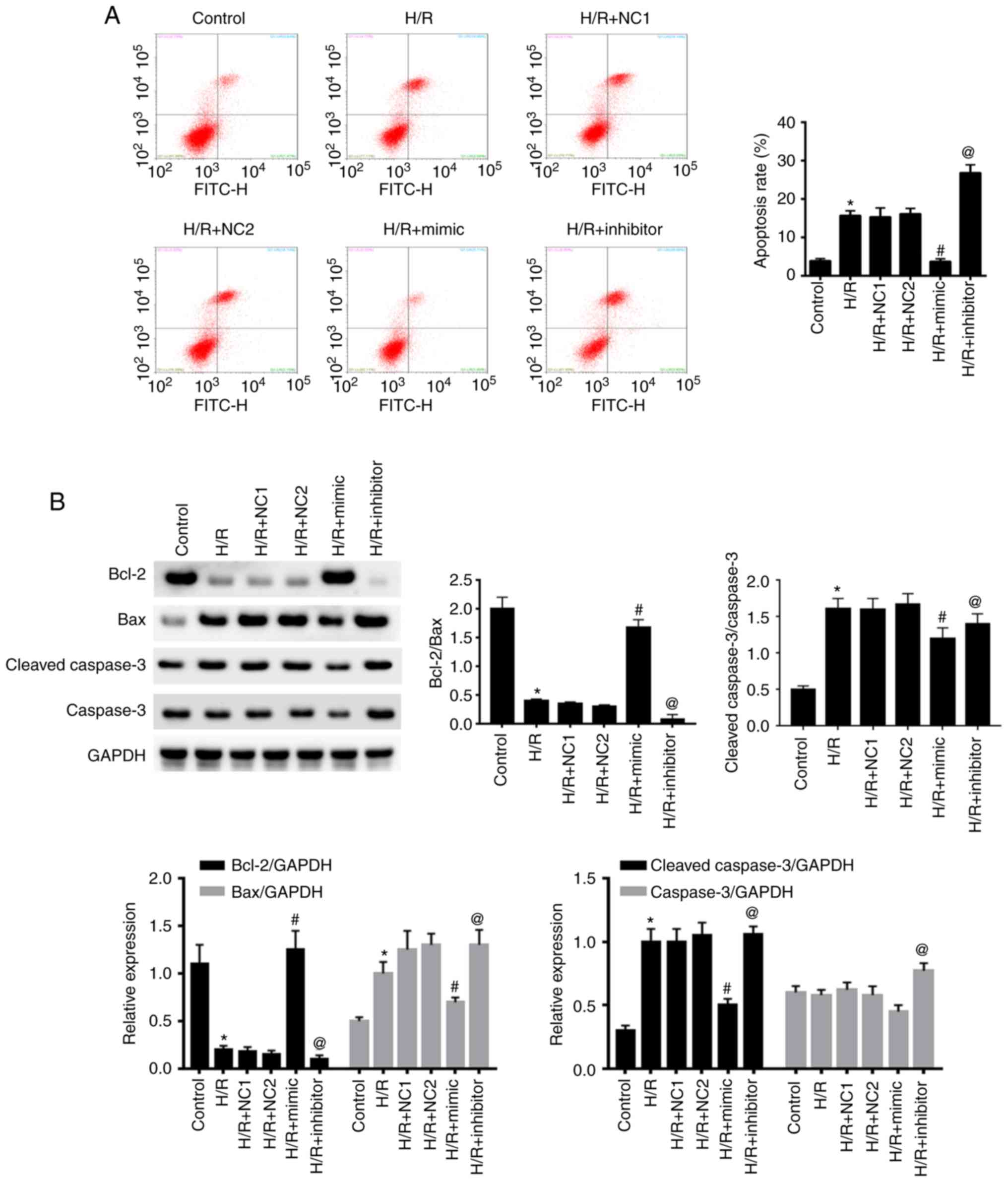
miR-30a-5p repressed H/R-induced
apoptosis in HK-2 cells
Flow cytometry was utilized to evaluate apoptosis in
HK-2 cells in each group (Fig. 4A).
There was a significant increase in cell apoptosis in the H/R group
compared with the control group (P<0.05), but there was no
difference between the NC groups and H/R (all P>0.05). In
comparison to the NC groups, H/R + mimic showed a downregulation in
apoptosis whereas the H/R + inhibitor group had upregulation in
apoptosis (all P<0.05).
Western blot analysis revealed a significant
increase in Bax/Bcl-2 and cleaved caspase-3/caspase-3 expression in
the H/R and NC groups (all P<0.05). Furthermore, Bax/Bcl-2 and
cleaved caspase-3/caspase-3 expression were significantly decreased
in the H/R + mimic group compared to the NC group (all P<0.05).
Nevertheless, the trend of Bax/Bcl-2 and cleaved caspase-3/caspase
were evidently contrary in the H/R + inhibitor group (all
P<0.05; Fig. 4B). Notably,
miR-30a-5p suppressed cell apoptosis in H/R-stimulated HK-2
cells.
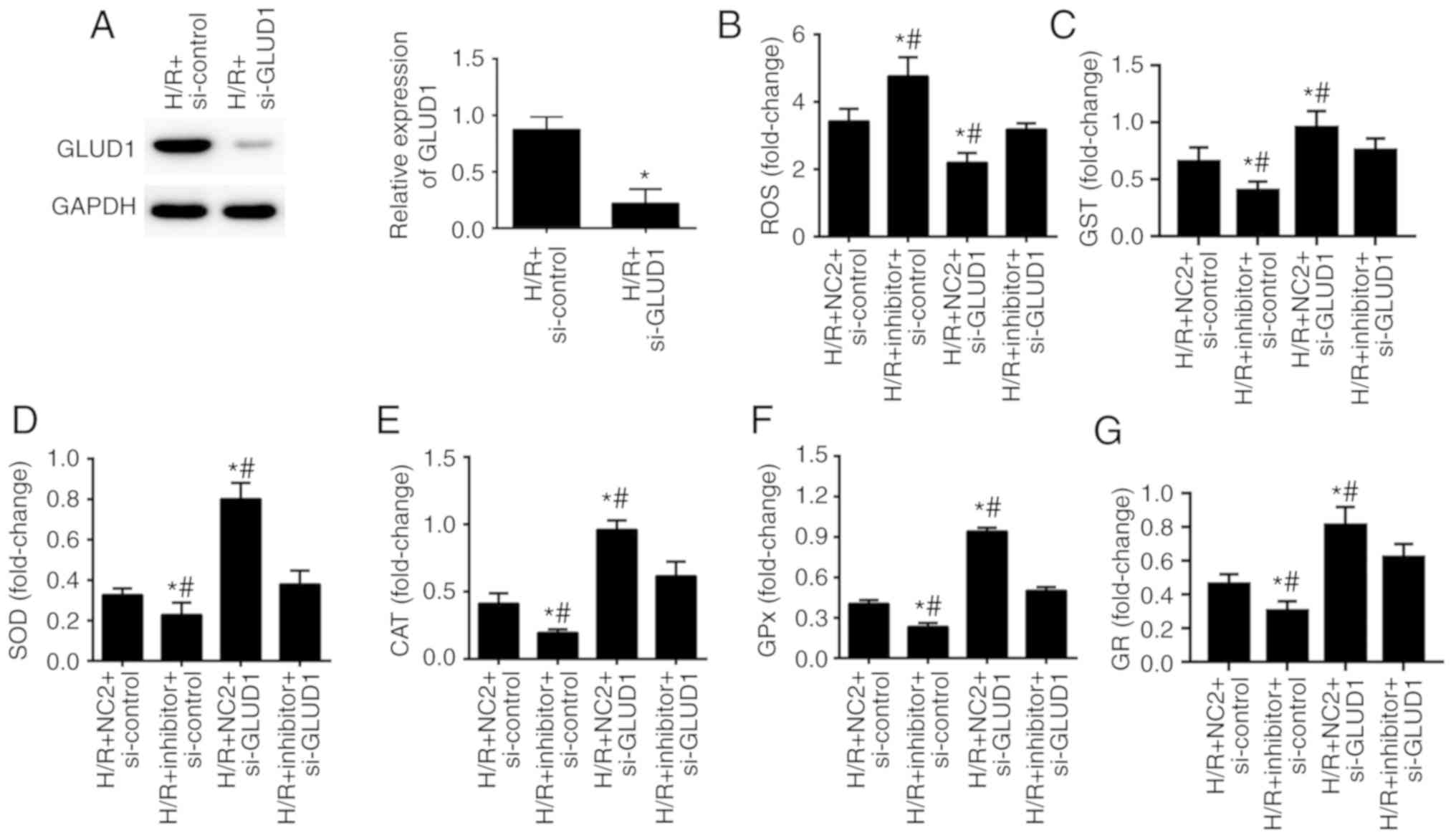
Inhibition of GLUD1 rescues the
influence of miR-30a-5p inhibitor in oxidative damage stimulated by
H/R
To assess whether miR-30a-5p contributed to
oxidative stress in HK-2 cells exposed to H/R by regulating GLUD1,
we suppressed the expression of GLUD1 in HK-2 cells along with
inhibition of miR-30a-5p. We found that expression of GLUD1 protein
was notably reduced when GLUD1 gene was downregulated with
the siRNA (P<0.05; Fig. 5A).
Furthermore, when compared with the H/R + NC2 + si-control group,
intracellular ROS generation was significantly increased in the H/R
+ inhibitor + si-control group with decreased activities of GST,
SOD, CAT, GPx and GR. By contrast, reduced generation of
intracellular ROS and GST as well as increased activities of SOD,
CAT, GPx and GR were observed in the H/R + NC2 + si-GLUD1 group
(P<0.05). Simultaneously, interference of GLUD1 expression
abolished miR-30a-5p inhibitor effects on H/R-induced oxidative
stress in the H/R + inhibitor + si-GLUD1 group (P<0.05; Fig. 5B-G). Thus, inhibition of GLUD1
rescues the impact of miR-30a-5p inhibitor on oxidative stress in
HK-2 cells.
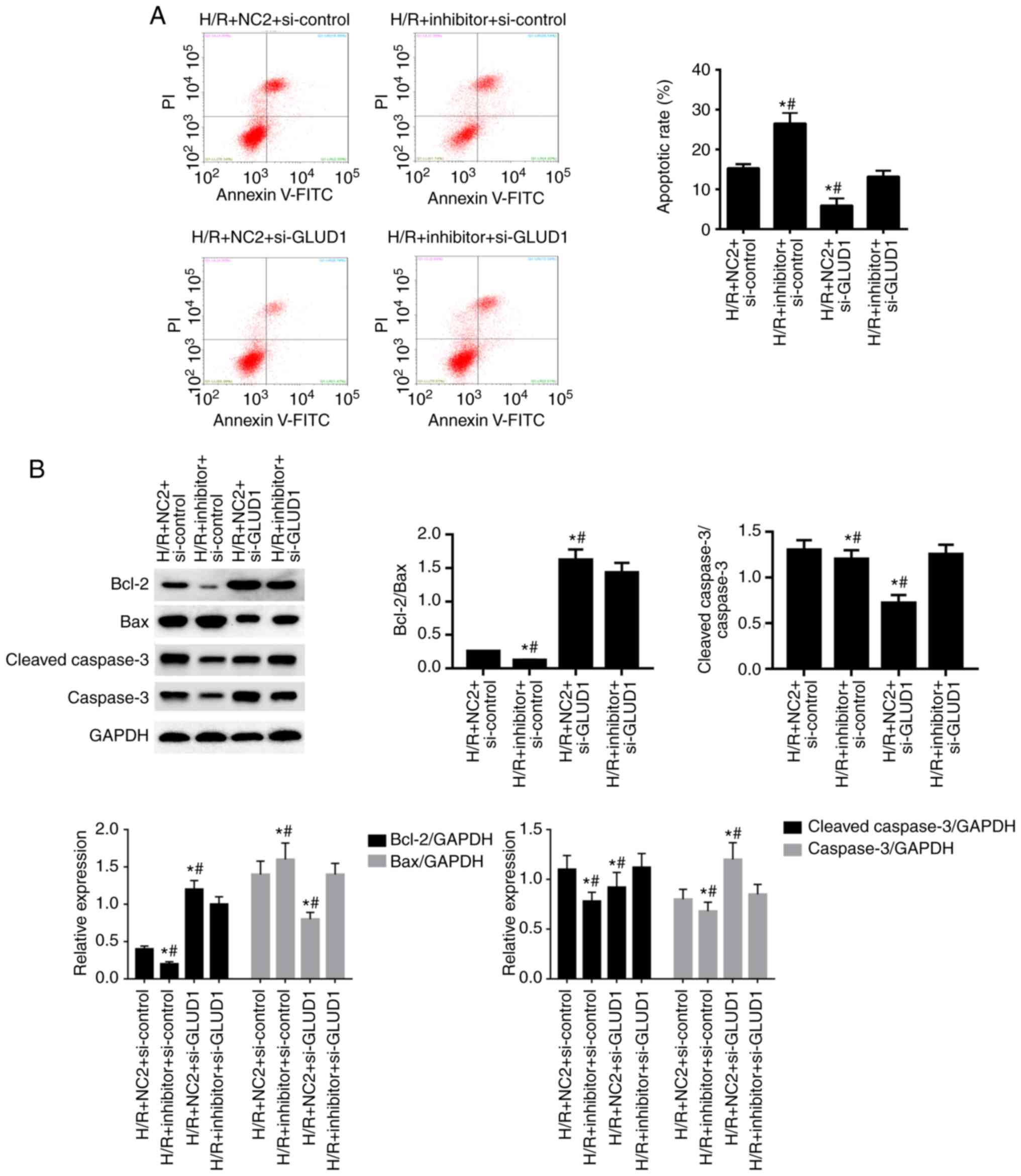
Inhibition of GLUD1 rescues the role
of miR-30a-5p inhibitor in apoptosis in HK-2 cells
As shown in Fig. 6A,
when compared with the H/R + NC2 + si-control group, the rate of
apoptosis in HK-2 cells was increased with inhibition of miR-30a-5p
in the H/R + inhibitor + si-control group and reduced by the
suppression of GLUD1 in the H/R + NC2 + si-GLUD1 group (all
P<0.05). Moreover, the downregulated expression of GLUD1
mitigated the function of miR-30a-5p inhibitor on apoptosis in HK-2
cells in the H/R + inhibitor + si-GLUD1 group (all P<0.05).
Consistent with annexin V/PI assay, the ratio of
Bax/Bcl-2 and the ratio of cleaved-caspase-3/caspase were markedly
increased in the H/R + inhibitor + si-control group when compared
with the H/R + NC2 + si-control group. However, the ratio of
Bax/Bcl-2 and the ratio of cleaved-caspase-3/caspase-3 were
reversed in the H/R + NC2 + si-GLUD1 group (Fig. 6B).
A reduced ratio of Bax/Bcl-2 and
cleave-caspase-3/caspase-3 was observed in the H/R + inhibitor +
si-GLUD1 group compared with the H/R + inhibitor + si-control group
(all P<0.05; Fig. 6C-E). These
results indicated that silencing the GLUD1 gene
significantly attenuated the influence of miR-30a-5p inhibitor on
apoptosis in HK-2 cells.
Discussion
An important effect associated with oxidative damage
is that the renal tubular epithelial cells are the first involved
in acute renal injury (16,17). Emerging evidence indicates that
miR-30a-5p acts as a suppressor for cancer, regulating the growth,
migration, and invasion of various tumors, including maintaining
renal function during acute renal injury (18–20).
This study aimed to explore the miR-30a-5p effect on HK-2 renal
tubular epithelial cells in renal injury.
miR-30a-5p binding to the 3′-UTR region of the
GLUD1 gene was confirmed by dual-luciferase reporter assay
in the present study. GDH, encoded by GLUD1, is an important
mitochondrial enzyme that is associated with amino acid metabolism
and the tricarboxylic acid cycle (21,22).
It has been reported that GDH is mainly distributed in the
mitochondria of liver cells and only increases significantly when
liver cells are damaged (23,24).
Thus, overexpression of the GLUD1 gene was suspected to be
involved in the course of renal injury. Our results demonstrated
that HK-2 cells under H/R exposure reduced the level of miR-30a-5p,
which upregulated the expression of its target gene, GLUD1.
This is similar to previous studies (23,24).
Results of the present study laid the foundation for studying the
mechanism of miR-30a-5p affecting renal H/R damage on a molecular
basis.
Oxidative stress is directly related to the
occurrence and development of I/R injury in myocardial cells and
can be regarded as an important treatment target for I/R injury
(25). In addition to the marked
increase in ROS production and GST, which affects cellular
mitochondrial damage and renal dysfunction (26,27),
decreased activity of the intracellular antioxidants SOD, CAT, GPx
and GR, were observed leading to oxidative stress (28,29).
To further investigate the effect of the downregulation of GLUD1 by
miR-30a-5p on H/R injury, the status of oxidative stress in HK-2
cells was analyzed. In the model of H/R injury, HK-2 cells
transfected with miR-30a-5p mimic and GLUD1 siRNA enhanced the
production of ROS along with reduced activities of SOD, CAT, GPx,
GST and GR, whereas cells in the miR-30a-5p inhibitor group
aggravated the oxidative stress induced by H/R. Consistent with the
present study, Fu et al (30) reported that miR-30a-5p relieved
oxidative stress in spinal cord by regulating Neurod 1 and the
mitogen-activated protein kinases/extracellular signal-regulated
kinase (MAPK/ERK) signaling pathway. Zhou et al (5) reported that miR-30b-5p exerts a
critical role in acute lung injury in children. More importantly,
we also found that inhibition of GLUD1 attenuated the effect of
miR-30a-5p inhibitor on oxidative stress in HK-2 cells. This
suggests that miR-30-5p influences oxidative stress in the
processes of renal injury by negative regulation of GLUD1.
As a consequence of oxidative stress, apoptosis is
significantly involved in the pathogenesis of renal injury and by
reducing cell apoptosis can effectively ameliorate renal injury
(31,32). Mitochondria are not only the site of
oxidative metabolism and energy transformation, but also have the
function of regulating programmed cell death. Oxidative stress
directly leads to mitochondrial damage, which causes the initiation
of the mitochondrial apoptosis program. Bax/Bcl-2 as well as
cleaved-caspase-3/caspase-3 are critical markers of apoptosis. The
former promotes apoptosis, while the latter inhibits apoptosis and
they regulate apoptosis by controlling the permeability of
mitochondrial membranes (33). In
addition, the Bcl-2 gene can regulate the activity of
cleaved-caspase-3, which eventually leads to apoptotic cascade
(34,35). Our research showed that, when the
level of miR-30a-5p was upregulated or the level of GLUD1 was
suppressed, the cell apoptosis rate stimulated by H/R was reduced.
The opposite results occurred after miR-30a-5p expression was
inhibited. Western blot results revealed that cells containing
miR-30a-5p mimics and GLUD1 siRNA have a significant reduction in
the ratio of Bax/Bcl-2 and the ratio of
cleaved-caspase-3/caspase-3. The results were the opposite in HK-2
cells when treated with miR-30a-5p inhibitor. Similar to our
result, Chien et al (35)
found that after renal ischemia reperfusion in rats, the rate of
apoptosis in renal cells increased with time as well as the
increasing ratio of Bax/Bcl-2 and the ratio of
cleaved-caspase-3/caspase 3. Furthermore, reducing the expression
of GLUD1 weakened the impact of miR-30a-5p inhibitor on apoptosis
in HK-2 cells treated with H/R. Thus, miR-30a-5p affects apoptosis
induced by renal injury by targeting the GLUD1 gene.
However, there remain several limitations to our study. For
example, lack of antioxidant enzyme activity detection in H/R cells
as well as the detection of convincing indicators of cell
apoptosis, which may complement and refine the results in the
current manuscript. More importantly, whether the downstream
signaling pathway of miR-30a-5p plays a role in H/R injury was not
thoroughly investigated, and may be explored in a further
study.
In conclusion, the findings of our study indicate
that, during H/R miR-30-5p helps to avoid oxidative stress and
prevent apoptosis by negatively regulating GLUD1. Therefore,
miR-30-5p mimic is potentially therapeutic for renal injury and
worth further study.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH conceived and designed this study. XL, DC and TZ
performed the experiments and collected data. ZY and RX analyzed
and interpreted the experimental data. YH and XL wrote the
manuscript. YH reviewed and revised the paper. All authors approved
the version to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Schiffl H: Discontinuation of renal
replacement therapy in critically ill patients with severe acute
kidney injury: Predictive factors of renal function recovery. Int
Urol Nephrol. 50:1845–1851. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiang HL, Xue WJ, Li Y, Zheng J, Ding C,
Dou M and Wu X: C1q/TNF-related protein 6 (CTRP6) attenuates renal
ischemia-reperfusion injury through the activation of PI3K/Akt
signaling pathway. Clin Exp Pharmacol Physiol. 47:1030–1040. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng J, Kong R, Xie L, Lu W, Zhang Y, Dong
H and Jiang H: Clemaichinenoside protects renal tubular epithelial
cells from hypoxia/reoxygenation injury in vitro through activating
the Nrf2/HO-1 signalling pathway. Clin Exp Pharmacol Physiol.
47:495–502. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai J, Zhao J, Cui D, Wang F, Song Y,
Cheng L, Gao K, Wang J, Li L, Li S, et al: Protective effect of
hydroxysafflor yellow a against acute kidney injury via the
TLR4/NF-κB signaling pathway. Sci Rep. 8:91732018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou T and Chen YL: The functional
mechanisms of miR-30b-5p in acute lung injury in children. Med Sci
Monit. 25:40–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu XD, Shen Y, Wang WL and Li X:
miR-30a-5p ameliorates spinal cord injury-induced inflammatory
responses and oxidative stress by targeting neurod 1 through
MAPK/ERK signalling. Clin Exp Pharmacol Physiol. 45:68–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu S, Zhou Z, Li Z, Shao J, Jiao G, Huang
YE and Lin Y: Suppression of LINC00707 alleviates
lipopolysaccharide-induced inflammation and apoptosis in PC-12
cells by regulated miR-30a-5p/neurod 1. Biosci Biotechnol Biochem.
83:2049–2056. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Brien PJ, Slaughter MR, Polley SR and
Kramer K: Advantages of glutamate dehydrogenase as a blood
biomarker of acute hepatic injury in rats. Lab Anim. 36:313–321.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mastorodemos V, Kotzamani D, Zaganas I,
Arianoglou G, Latsoudis H and Plaitakis A: Human GLUD1 and GLUD2
glutamate dehydrogenase localize to mitochondria and endoplasmic
reticulum. Biochem Cell Biol. 87:505–516. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spanaki C, Kotzamani D, Petraki Z, Drakos
E and Plaitakis A: Expression of human GLUD1 and GLUD2 glutamate
dehydrogenases in steroid producing tissues. Mol Cell Endocrinol.
415:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu D, Wu L, Li CR, Wang XW, Ma YJ, Zhong
ZY, Zhao HB, Cui J, Xun SF, Huang XL, et al: Ginsenoside Rg1
protects rat cardiomyocyte from hypoxia/reoxygenation oxidative
injury via antioxidant and intracellular calcium homeostasis. J
Cell Biochem. 108:117–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong G, Chen T, Ren X, Zhang Z, Huang W,
Liu L, Luo P and Zhou H: Rg1 prevents myocardial
hypoxia/reoxygenation injury by regulating mitochondrial dynamics
imbalance via modulation of glutamate dehydrogenase and mitofusin
2. Mitochondrion. 26:7–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real- time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Habig WH, Pabst MJ and Jakoby WB:
Glutathione S-transferases. The first enzymatic step in mercapturic
acid formation. J Biol Chem. 249:7130–7139. 1974.PubMed/NCBI
|
|
15
|
Aviram M, Kent UM and Hollenberg PF:
Microsomal cytochromes P450 catalyze the oxidation of low density
lipoprotein. Atherosclerosis. 143:253–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang S, Li R, Dong W, Yang H, Zhang L,
Chen Y, Wang W, Li C, Wu Y, Ye Z, et al: RIPK3 mediates renal
tubular epithelial cell apoptosis in endotoxin-induced acute kidney
injury. Mol Med Rep. 20:1613–1620. 2019.PubMed/NCBI
|
|
17
|
Yang L, Chang B, Guo Y, Wu X and Liu L:
The role of oxidative stress-mediated apoptosis in the pathogenesis
of uric acid nephropathy. Ren Fail. 41:616–622. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei W, Yang Y, Cai J, Cui K, Li RX, Wang
H, Shang X and Wei D: miR-30a-5p suppresses tumor metastasis of
human colorectal cancer by targeting ITGB3. Cell Physiol Biochem.
39:1165–1176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiong J, Wei B, Ye Q and Liu W:
miR-30a-5p/UBE3C axis regulates breast cancer cell proliferation
and migration. Biochem Biophys Res Commun. 516:1013–1018. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Wang K, Han L, Zhang A, Shi Z,
Zhang K, Zhang H, Yang S, Pu P, Shen C, et al: PRDM1 is directly
targeted by miR-30a-5p and modulates the Wnt/β-catenin pathway in a
Dkk1-dependent manner during glioma growth. Cancer Lett.
331:211–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hensley CT, Wasti AT and DeBerardinis RJ:
Glutamine and cancer: Cell biology, physiology, and clinical
opportunities. J Clin Invest. 123:3678–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YQ, Wang HL, Xu J, Tan J, Fu LN, Wang
JL, Zou TH, Sun DF, Gao QY, Chen YX and Fang JY: Sirtuin5
contributes to colorectal carcinogenesis by enhancing
glutaminolysis in a deglutarylation-dependent manner. Nat Commun.
9:5452018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Palladino AA and Stanley CA: The
hyperinsulinism/hyperammonemia syndrome. Rev Endocr Metab Disord.
11:171–178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frederiks WM and Marx F: Changes in
cytoplasmic and mitochondrial enzymes in rat liver after ischemia
followed by reperfusion. Exp Mol Pathol. 47:291–299. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perrelli MG, Pagliaro P and Penna C:
Ischemia/reperfusion injury and cardioprotective mechanisms: Role
of mitochondria and reactive oxygen species. World J Cardiol.
3:186–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu MM, Chiou HY, Wang TW, Hsueh YM, Wang
IH, Chen CJ and Lee TC: Association of blood arsenic levels with
increased reactive oxidants and decreased antioxidant capacity in a
human population of northeastern Taiwan. Environ Health Perspect.
109:1011–1017. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Wang X, Zhang XS and Liang CZ:
Cryptotanshinone attenuates oxidative stress and inflammation
through the regulation of Nrf-2 and NF-KB in mice with unilateral
ureteral obstruction. Basic Clin Pharmacol Toxicol. 123:714–720.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu X, Shen Y, Wang W and Li X: miR-30a-5p
ameliorates spinal cord injury-induced inflammatory responses and
oxidative stress by targeting neurod 1 through MAPK/ERK signalling.
Clin Exp Pharmacol Physiol. 45:68–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Xun L, Jin G and Shi L: Salidroside
protects renal tubular epithelial cells from hypoxia/reoxygenation
injury in vitro. J Pharmacol Sci. 137:170–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye S, Zhu Y, Ming Y, She X, Liu H and Ye
Q: Glycyrrhizin protects mice against renal ischemia-reperfusion
injury through inhibition of apoptosis and inflammation by
downregulating p38 mitogen-activated protein kinase signaling. Exp
Ther Med. 7:1247–1252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Ai L, Hai B, Cao Y, Li R, Li H and
Li Y: Tempol alleviates chronic intermittent hypoxia-induced
pancreatic injury through repressing inflammation and apoptosis.
Physiol Res. 68:445–455. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shrivastava A, Tiwari M, Sinha RA, Kumar
A, Balapure AK, Bajpai VK, Sharma R, Mitra K, Tandon A and Godbole
MM: Molecular iodine induces caspase-independent apoptosis in human
breast carcinoma cells involving the mitochondria-mediated pathway.
J Biol Chem. 281:19762–19771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chien CT, Hsu SM, Chen CF, Lee PH and Lai
MK: Prolonged ischemia potentiates apoptosis formation during
reperfusion by increase of caspase 3 activity and free radical
generation. Transplant Proc. 32:2065–2066. 2000. View Article : Google Scholar : PubMed/NCBI
|