Introduction
Propofol is a commonly used anesthetic in the clinic
(1). Previous studies have
investigated the inhibitory effect of propofol on cancer cells in a
variety of tumors, such as breast (2), lung (3) pancreatic (4), ovarian (5), hepatic (6) and gastric cancer (GC) (7,8). In
GC, Yang et al (7) reported
that propofol suppressed the proliferation of SGC-7901 and MGC-803
cells by promoting inhibitor of growth 3 (7). Peng and Zhang (8) indicated that propofol inhibited
proliferation, but induced apoptosis of SGC-7901 cells by
regulating matrix metalloproteinase (MMP)2 (8). These previous studies have mostly been
limited to the pharmacodynamic effects of propofol on phenotypes,
and the underlying mechanisms of the antitumor effects still need
to be explored.
Circular RNAs (circRNAs) are a series of endogenous
non-coding RNAs with a covalently closed loop structure (9). A previous study confirmed that
circRNAs were more stable compared with linear mRNAs due to the
lack of a 5′cap and 3′polyadenylate tail (10). There is evidence that circRNAs are
specifically expressed in hematological malignancies or certain
developmental stages, indicating that circRNAs have considerable
regulatory potential by serving as post-transcriptional regulators
(11,12). CircRNA-PVT1 (circ-PVT1) has been
reported to function as an oncogenic circRNA in tumors, such as
oral squamous cell carcinoma, head and neck squamous cell
carcinoma, and osteosarcoma (13–15).
Circ-PVT1 is highly expressed in GC tissues and cells, and is also
associated with poor prognosis; therefore, Chen et al
(16) predicted that circ-PVT1 was
a novel proliferative factor and prognostic indicator in GC.
However, to the best of our knowledge, there are few reports on the
mechanism of circ-PVT1 in regulating the proliferation, apoptosis
and invasion of GC cells (17). In
addition, the association between propofol and circ-PVT1 has not
been reported.
Accumulating evidence has indicated that microRNAs
(miRNAs or miRs) act as regulators by regulating oncogene
expression in malignant cancers (18–20).
Studies have reported that miR-195-5p is downregulated in GC
tissues and cells, and as a tumor suppressor, miR-195-5p can
inhibit cell proliferation, invasion and resistance in GC cells
(21–23). A previous study revealed that the
expression of miR-195-5p in the tissues and serum of patients with
GC was associated with tumor diameter, tumor-node-metastasis stage
and differentiation (24). In
addition, miR-195-5p overexpression can suppress the proliferation
and invasion, and promote the apoptosis of GC cells (24). In another study by Wang et al
(23), it was identified that the
expression level of miR-195-5p was negatively correlated with basic
fibroblast growth factor (bFGF) in human GC tissues. In addition,
miR-195-5p inhibited the migration and invasion of SNU-1 and KATO-3
cells by downregulating bFGF (23).
Nevertheless, the regulatory role of miR-195-5p in GC has not been
fully clarified.
E-26 factor has been shown to play an essential role
in numerous biological processes, such as proliferation,
differentiation, transformation and apoptosis (25–29).
E26 oncogene homolog 1 (ETS1) is a gene associated with invasion
and metastasis in tumorigenesis via transcriptional factors
(30). ETS1 has been shown to
mediate MMPs and integrins in numerous types of cancer cells and
tissues (31–36). For example, the upregulation of ETS1
can promote invasion and progression of breast cancer by regulating
MMP-9 (37).
Considering the aforementioned studies, the present
study was designed to improve understanding of the molecular
mechanism of propofol in inhibiting GC progression, as well as the
associations among circ-PVT1, miR-195-5p and ETS1.
Materials and methods
Sample collection
The present study received approval from the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China). A total of 30 GC tissue and paired adjacent
tissue samples (located >5 cm away from the tumor) were
collected from patients with GC who underwent total gastrectomy at
The First Affiliated Hospital of Zhengzhou University between June
2015 and March 2018. A total of 30 patients were included in the
study, including 10 females and 20 males, with an age range of
25–75 years, and the median age at the time of diagnosis was 53
years. The main inclusion criteria were: i) Patients who were
diagnosed with GC through pathological examination; ii) patients
who were diagnosed and treated for GC for the first time; and iii)
patients who were willing to join the study. The exclusion criteria
were: i) History of other malignant disease; and ii) recurrent or
treated GC. Written informed consent was obtained from each patient
before chemotherapy. All samples were collected and stored at
−80°C.
Cell culture and propofol
administration
The human gastric mucosa cell line (GES-1) and GC
cell lines (HGC-27, AGS, SNU5 and MKN-45) were obtained from
Shanghai Berry Innovation Biotechnology Co., Ltd. (Shanghai,
China). All cells were cultured in 90% Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2. The HGC-27 and AGS cells were divided into
the following groups: i) Control group, without propofol; ii) 2.5
µg/ml propofol (Sigma-Aldrich; Merck KGaA) group; iii) 5 µg/ml
propofol group; and iv) 10 µg/ml propofol group, which were treated
for 24, 48 and 72 h at 37°C. For subsequent experiments, HGC-27 and
AGS cells were exposed to 5 µg/ml propofol for 48 h at 37°C.
MTT assay
The viability of GC cells was evaluated by MTT
assay. Briefly, HGC-27 and AGS cells (3×103/well) with
or without transfection were seeded into 96-well plates (Corning,
Inc.) and maintained in an incubator with 5% CO2 at 37°C
for 24 h. Then, MTT (20 µl; Sigma-Aldrich; Merck KGaA) was added to
each well for 4 h. Subsequently, the supernatant of each well was
discarded and DMSO (150 µl; Sigma-Aldrich; Merck KGaA) was added to
dissolve the formazan crystals. Finally, a microplate absorbance
reader (Thermo Fisher Scientific, Inc.) was used to measure the
optical density value at 490 nm.
Flow cytometry assay
Annexin V-FITC/PI Apoptosis Detection kit (Vazyme
Biotech Co., Ltd.) was used for the flow cytometry assay. In brief,
cells (1×105/well) were seeded into 6-well plates,
treated with 0.25% trypsin and washed with pre-cooled PBS.
Subsequently, 2×105 cells were resuspended in 100 µl 1X
binding buffer and then incubated with 5 µl Annexin V-FITC and PI
staining solution for 10 min at room temperature without light.
Finally, the apoptotic cells were assessed using a flow cytometer
(BD Biosciences).
Transwell assay
The rate of cell invasion was investigated using a
Transwell chamber (Corning Inc.) coated with Matrigel at 4°C
overnight. The lower chamber was filled with DMEM with 10% FBS,
while the transfected HGC-27 and AGS cells (1×105/well)
were added to the upper chamber with 100 µl serum-free medium.
After incubation for 24 h at 37°C, paraformaldehyde (Sigma-Aldrich;
Merck KGaA) was used to attach cells located on lower surface of
the upper chamber for 20 min at the room temperature. Cells were
analyzed under a fluorescence microscope (magnification, ×100)
after staining with crystal violet for 30 min at the room
temperature.
Western blotting
Western blotting was performed as described
previously (38). Briefly, protein
samples were extracted with RIPA lysis buffer (Thermo Fisher
Scientific, Inc.) and then quantified by BCA Protein assay kit
(Thermo Fisher Scientific, Inc.). Subsequently, each protein sample
(50 µg/lane) was separated by 10% SDS-PAGE and then transferred
onto polyvinylidene fluoride membranes (EMD Millipore). Then, the
membranes were incubated with primary antibodies overnight at 4°C
after blocking with 5% non-fat milk for 1 h at room temperature.
The next day, the corresponding secondary antibody was used for 1 h
at room temperature, and the signals were visualized using an
enhanced chemiluminescence kit (EMD Millipore). Primary antibodies
against the following were used: Cyclin-dependent kinase inhibitor
P21 (P21; catalog no. ab109520; 1:1,000; Abcam), B-cell lymphoma-2
(Bcl-2; catalog no. ab32124; 1:1,000; Abcam), MMP9 (catalog no.
ab76003; 1:2,500; Abcam), ETS1 (catalog no. ab26096; 1:2,500;
Abcam) and GAPDH (catalog no. ab9485; 1:2,500; Abcam). In addition,
the horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (catalog no. ab205718; 1:5,000; Abcam) was detected with
the enhanced chemiluminescence kit and analyzed with Quantity One
v4.6.2 software (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from HGC-27 and AGS cells was extracted
using TRIzol reagent (Thermo Fisher Scientific, Inc.).
Primer-Script RT-PCR kit (Takara Bio, Inc.) or miRNA Reverse
Transcription kit (GeneCopoeia, Inc.) was used, according to the
manufacturers' protocols, to synthesize the first-strand
complementary DNA of miR-195-5p and circ-PVT1 or ETS1,
respectively. The levels of miR-195-5p and circ-PVT1 or ETS1 were
assessed via SYBR Premix Dimer Eraser kit (Takara Bio, Inc.). The
thermocycling conditions were as follows: Denaturation at 95°C for
10 min, followed by 40 cycles of denaturation at 95°C for 30 sec,
annealing at 60°C for 30 sec and extension at 72°C for 1 min. The
primer sequences used were as follows: circ-PVT1 forward,
5′-GGTTCCACCAGCGTTATTC-3′ and reverse, 5′-CAACTTCCTTTGGGTCTCC-3′;
miR-195-5p forward, 5′-CGTAGCAGCACAGAAAT-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; ETS1 forward, 5′-AGCCGACTCTCACCATCATC-3′
and reverse, 5′-CAAGGCTTGGGACATCATTT-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; and GAPDH forward,
5′-CAATGACCCCTTCATTGACC-3′ and reverse, 5′-TGGAAGATGGTGATGGGATT-3′.
The levels of miR-195-5p, circ-PVT1 and ETS1 were calculated by the
2−ΔΔCq method (39), and
GAPDH or U6 were used as an internal control for circ-PVT1 and
miR-195-5p or ETS1, respectively.
Cell transfection
For circRNA downregulation, small interfering RNA
against circ-PVT1 (si-circ-PVT1; 3′-CUGUCAGCUGCAUGGAGCUUCGU-5′) and
its negative control (si-NC; 3′-AAUUCUCCGAACGUGUCACGU-5′) were
constructed by Shanghai Genepharma Co., Ltd.. For circ-PVT1
upregulation, circ-PVT1 sequences were amplified by PCR and
inserted into a pcDNA vector (Invitrogen; Thermo Fisher Scientific,
Inc.) to generate fusion plasmids, and a pcDNA empty vector was
used as the control. For miR-195-5p enrichment or inhibition,
miR-195-5p mimic (5′-UAGCAGCACAGAAAUAUUGGC-3′), miR-195-5p
inhibitor (anti-miR-195-5p: 5′-GCCAAUAUUUCUGUGCUGCUA-3′) and the
negative controls (miR-NC, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′; and
anti-miR-NC, 5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased from
Guangzhou RiboBio Co., Ltd.. All plasmids (2 µg) and
oligonucleotides (40 nM) were transfected into HGC-27 and AGS cells
using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.). At 48 h post-transfection, HGC-27 and AGS cells were
collected and used for further analyses.
Bioinformatics analysis and
dual-luciferase reporter assay
StarBase v2.0 online software (http://starbase.sysu.edu.cn/starbase2/)
was used to predict the potential binding relationship between
miR-195-5p and circ-PVT1 or ETS1. The associations between
miR-195-5p and circ-PVT1 or ETS1 were verified by dual-luciferase
reporter assay. In brief, the sequences of circ-PVT1 wild type (WT)
containing the binding sites with miR-195-5p and corresponding
circ-PVT1 mutant (MUT) sequences were amplified and cloned into the
pRL-CMV vector (Promega Corporation), and termed WT-circ-PVT1 and
MUT-circ-PVT1, respectively. Subsequently, WT-circ-PVT1 and
MUT-circ-PVT1 were introduced into HGC-27 and AGS cells together
with miR-195-5p or miR-NC using Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Following 48 h, the luciferase
activity was detected using a Dual-Luciferase Reporter assay kit
(Promega Corporation). Similarly, ETS1-3′UTR-WT containing the
binding site for miR-195-5p and ETS1-3′UTR-MUT were also
constructed and used for luciferase activity analysis, according to
the previous method. Renilla luciferase activities were used
as the internal control for the normalization of firefly luciferase
activity.
Xenograft tumor model
Firstly, a sh-circ-PVT1 lentivirus plasmid was
generated by transfecting a shRNA sequence of circ-PVT1
(5′-GAATGCCTCATGGATTCTTAC-3′) into a pLKO.1 lentivirus vector
(Addgene, Inc.). A pLKO.1 empty vector was used as a negative
control (sh-control). Subsequently, sh-circ-PVT1 or sh-control
vector (40 nM) was transfected into AGS cells along with the
lentivirus packaging plasmids (psPAX2 and pMD2 G; Addgene, Inc.)
using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.), followed by incubation for 72 h. The transfection efficiency
of sh-circ-PVT1 was determined by RT-qPCR.
A total of 32 BALB/c male nude mice (age, 5 weeks,
weight 16–22 g) were purchased from Charles River Laboratories
Inc., followed by maintenance under specific pathogen-free
conditions with a 12-h light/dark cycle, and a constant and
suitable temperature (25°C) and humidity (60%), with easy access to
food and water. The mice were randomly divided into the following
four groups (n=8 per group); i) Sh-control + PBS group; ii)
sh-control + propofol group; iii) sh-circ-PVT1 + PBS group; and iv)
sh-circ-PVT1 + propofol group. AGS cells (2×106) were
stably transfected with 40 nM sh-circ-PVT1 (forward,
5′-GATCCGAATGCCTCATGGATTCTTACCTCGAGCATTCTTAGGTACTCCGTAAGTTTTTG-3′
and reverse,
5′-AATTCAAAAAGAATGCCTCATGGATTCTTACCTCGAGCATTCTTAGGTACTCCGTAAGG-3′)
using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.), and were injected subcutaneously into the left hind back of
nude mice. The control groups received an equal amount of
sh-control-transfected cells. After 7 days, 30 mg/kg propofol or
PBS was intraperitoneally injected every 3 days. The tumor volume
was measured using a caliper every 4 days. At day 27
post-inoculation, the mice were sacrificed by cervical dislocation
following deep anesthesia with 2% isoflurane (Baxter Healthcare),
and tumor tissues were collected to measure the tumor weight and
for RT-qPCR. The experiments were approved by the Experimental
Animal Ethics Committee of The First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China).
Statistical analysis
Data were collected from at least three independent
experiments and analyzed by GraphPad Prism 7.0 (GraphPad Software
Inc.). Data are presented as the mean ± standard deviation.
Pearson's correlation analysis was used to analyze the correlation
between expression levels. The analyses between two groups were
performed using Student's t-test. One-way analysis of variance
followed by Tukey's post-hoc test was used to compare the
differences among three or more groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Propofol suppresses cell viability and
invasion, but induces apoptosis of GC cells
The present study detected the expression level of
circ-PVT1 in the human gastric mucosa cell line GES-1 and the GC
cell lines SNU5, MKN-45, HGC-27 and AGS. As presented in Fig. 1A, circ-PVT1 level was significantly
higher in GC cell lines (HGC-27, AGS, SNU5 and MKN-45) compared
with GES-1 cells, especially for HGC-27 and AGS cells. Therefore,
HGC-27 and AGS cells were selected for further experiments. To
validate the function of propofol on the progression of GC cells,
an MTT assay was employed to detect the viability of HGC-27 and AGS
cells treated with various concentrations of propofol for different
durations. Compared with the control group, the viability of HGC-27
and AGS cells treated with propofol significantly decreased in a
concentration-dependent manner (Fig. 1B
and C). To analyze the rate of cell apoptosis, flow cytometry
was performed with cells administrated propofol and the results
demonstrated that propofol significantly promoted apoptosis in a
dose-dependent manner (Fig. 1D). A
Transwell assay was performed to assess the invasive abilities of
HGC-27 or AGS cells treated with various concentrations of propofol
for 48 h. It was identified that treatment with significantly
reduced cell invasion in a concentration-dependent manner compared
with the control group (Fig. 1E).
Subsequently, western blotting was performed to analyze the protein
expression of P21, Bcl-2 and MMP9, which revealed that Bcl-2 and
MMP9 expression levels were significantly decreased following
treatment with propofol in a dose-dependent manner, whereas the
level of P21 was significantly increased as the dose of propofol
increased (Fig. 1F and G).
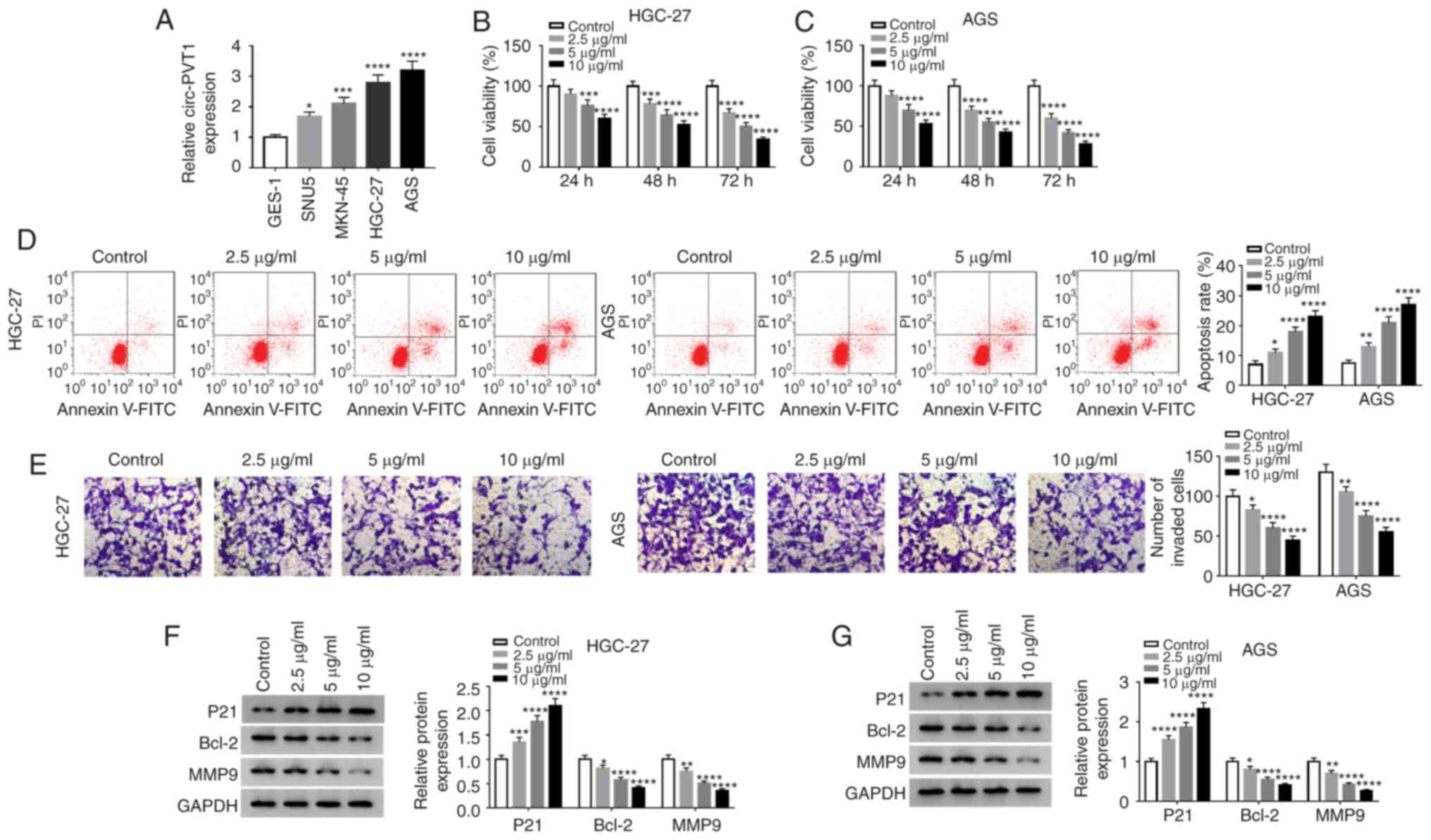 | Figure 1.Propofol suppresses the viability and
invasion, but induces apoptosis of GC cells. (A) Circ-PVT1 level
was detected in a human gastric mucosa cell line (GES-1) and GC
cell lines (SNU5, MKN-45, HGC-27 and AGS) by reverse
transcription-quantitative PCR. MTT assay was performed to assess
the cell viability of (B) HGC-27 and (C) AGS cells at 24, 48 and 72
h after treatment with 0, 2.5, 5 or 10 µg/ml; propofol (D) Flow
cytometry was performed to measure the rate of apoptosis when cells
were treated with propofol. (E) Transwell assay was performed to
evaluate the invasion of HGC-27 and AGS cells following treatment
with propofol (Magnification, ×100). Western blotting was used to
detect the expression of P21, Bcl-2 and MMP9 in (F) HGC-27 and (G)
AGS cells. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
vs. control. Circ-PVT1, circular RNA-PVT1; GC, gastric cancer; p21,
cyclin-dependent kinase inhibitor p21; Bcl-2, B-cell lymphoma-2;
MMP9, matrix metalloproteinase 9. |
Circ-PVT1 is upregulated in GC tissues
and cells, and circ-PVT1 reverses the propofol-mediated effects on
GC cells
Next, the potential role of circ-PVT1 in GC was
investigated, and RT-qPCR revealed that circ-PVT1 expression was
significantly higher in GC tissues (Fig. 2A) and cells (Fig. 2B) compared with the normal controls.
In addition, propofol significantly reduced the expression level of
circ-PVT1 in a dose-dependent manner in GC cells (Fig. 2C). To further confirm the
association between circ-PVT1 and propofol, rescue assays with
HGC-27 and AGS cells were performed. As presented in Fig. 2D, circ-PVT1 overexpression reversed
the propofol-induced repression of circ-PVT1 in HGC-27 and AGS
cells. Circ-PVT1 overexpression also significantly reversed the
suppressive effect of propofol on the proliferation (Fig. 2E and F) and invasion (Fig. 2H) of HGC-27 and AGS cells. In
addition, circ-PVT1 overexpression significantly reversed the
increased apoptotic rate induced by propofol in GC cells (Fig. 2G). Additionally, the aberrant
expression levels of P21, Bcl-2 and MMP9 were significantly
reversed by circ-PVT1 overexpression in HGC-27 and AGS cells
(Fig. 2I and J).
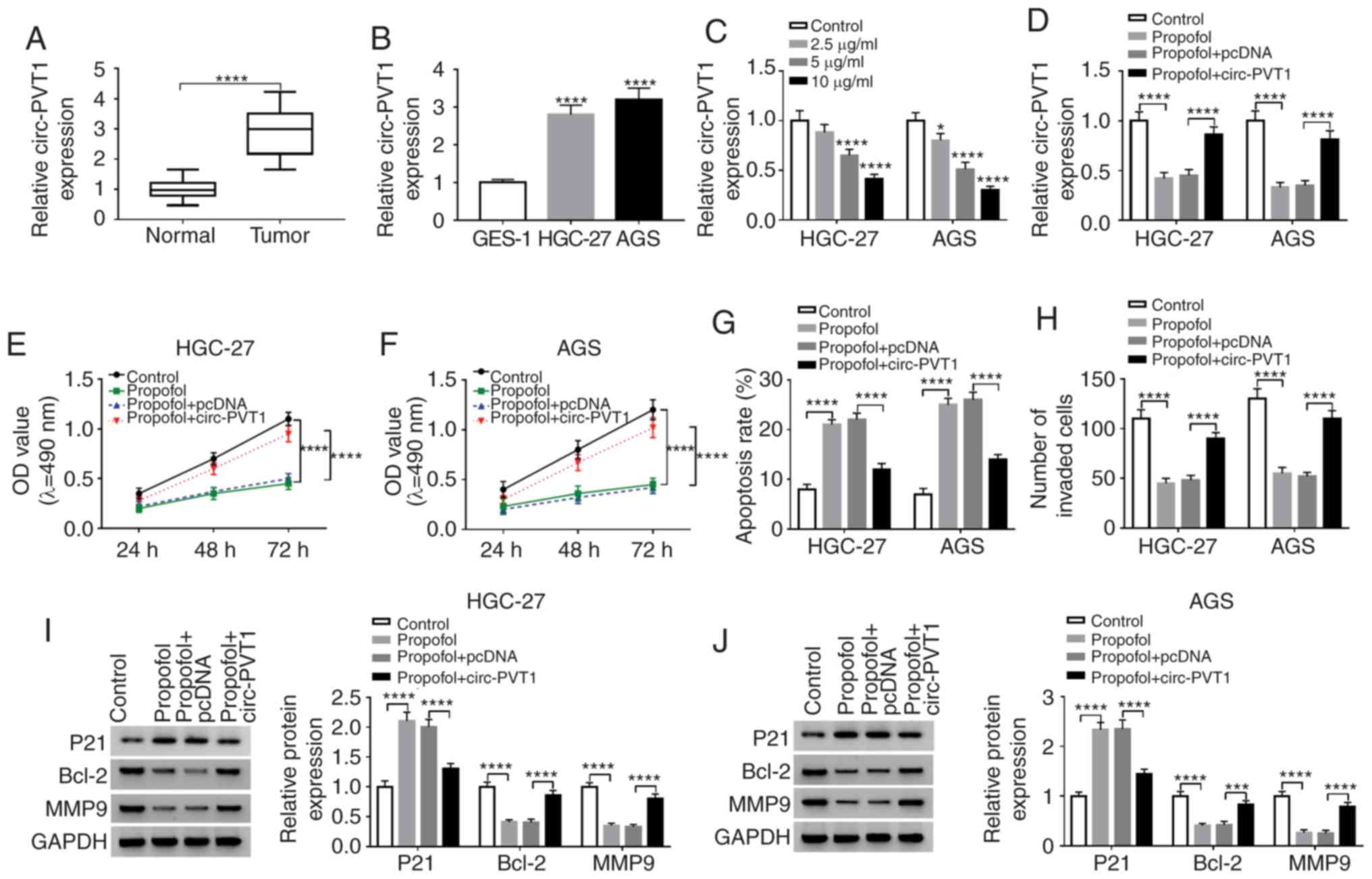 | Figure 2.Circ-PVT1 is upregulated in GC
tissues and cells, and circ-PVT1 restores the propofol-mediated
effects on GC cells. Circ-PVT1 is expressed at a higher level in GC
(A) tissues or (B) cells (HGC-27 and AGS) compared with the
corresponding controls. (C) Circ-PVT1 level was decreased in a
concentration-dependent manner when cells were treated with
propofol. (D) Rescued circ-PVT1 expression in HGC-27 and AGS cells
following propofol treatment was demonstrated by reverse
transcription-quantitative PCR. Rescued cell viability of (E)
HGC-27 and (F) AGS cells was investigated by MTT assay. (G) The
effect of circ-PVT1 on the apoptosis of HGC-27 and AGS cells was
confirmed by flow cytometry assay. (H) The invasion of cells was
rescued by circ-PVT1 overexpression as determined by Transwell
assay. Western blotting revealed that the expression levels of P21,
Bcl-2and MMP9 were restored by circ-PVT1 overexpression in (I)
HGC-27 and (J) AGS cells treated with propofol. *P<0.05,
***P<0.001, ****P<0.0001 vs. control. Circ-PVT1, circular
RNA-PVT1; GC, gastric cancer; P21, cyclin-dependent kinase
inhibitor P21; Bcl-2, B-cell lymphoma-2; MMP9, matrix
metalloproteinase 9; OD, optical density. |
Circ-PVT1 directly binds to
miR-195-5p
In order to identify a specific miRNA that is
regulated by circ-PVT1, bioinformatics analysis and a
dual-luciferase reporter assay were performed. First, the results
of bioinformatics analysis with starBase v2.0 suggested that
miR-195-5p, which contains the putative binding sites, was a target
of circ-PVT1 (Fig. 3A). To further
demonstrate that miR-195-5p is a target of circ-PVT1, the
dual-luciferase reporter assay was applied. The fragment of
circ-PVT1 containing miR-195-5p binding sites (WT-circ-PVT1) or
mutant fragment (MUT-circ-PVT1) was inserted into plasmids. Then,
cells were co-transfected with plasmid and miR-195-5p or miR-NC. As
presented in Fig. 3B and C, the
luciferase activity in WT-circ-PVT1-transfected cells was
significantly decreased by miR-195-5p. However, there was no change
in cells transfected with MUT-circ-PVT1. Subsequently, the
expression level of miR-195-5p was measured in tissues and cells,
which revealed that miR-195-5p was significantly lower in GC
tissues or cells compared with normal controls (Fig. 3D and E). In addition, a significant
negative correlation was identified between miR-195-5p and
circ-PVT1 in 30 GC tumor tissues (Fig.
3F). Subsequently, the level of circ-PVT1 was decreased or
increased in HGC-27 and AGS cells by transfection with si-circ-PVT1
or circ-PVT1 vector for loss- and gain-of function, respectively
(Fig. 3G). Following transfection,
the level of miR-195-5p in GC cells transfected with si-circ-PVT1
or circ-PVT1 vector was assessed, and it was identified that the
level of miR-195-5p was significantly increased in
circ-PVT1-knockdown HGC-27 and AGS cells, and significantly
decreased in circ-PVT1-upregulated HGC-27 and AGS cells (Fig. 3H). In the rescue assays, knockdown
of miR-195-5p significantly inhibited the miR-195-5p expression
(Fig. 3I), and significantly
increased the proliferation (Fig. 3J
and K) and invasion (Fig. 3M)
of si-circ-PVT1-transfected HGC-27 and AGS cells. Furthermore,
miR-195-5p-knockdown significantly reversed the increase in
apoptosis rate induced by si-circ-PVT1 transfection (Fig. 3L). Additionally, decreased
expression of Bcl-2 and MMP9, and increased expression of P21
induced by circ-PVT1-knockdown could be eliminated by
miR-195-5p-knockdown (Fig. 3N and
O).
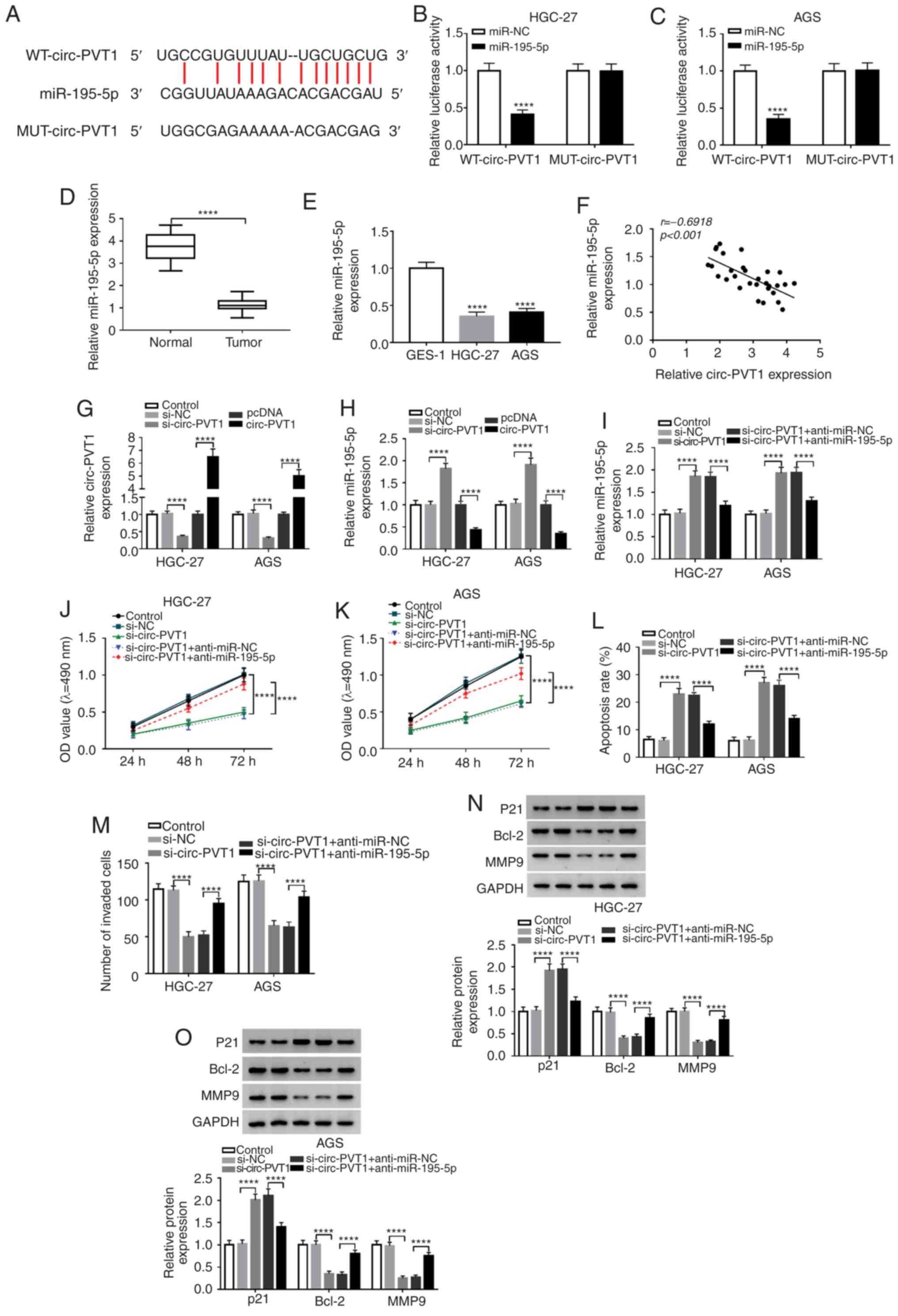 | Figure 3.miR-195-5p is a target of circ-PVT1.
(A) The putative binding sites between miR-195-5p and circ-PVT1
were predicted by starBase v2.0. The predicted sites were confirmed
by dual-luciferase reporter assay with (B) HGC-27 and (C) AGS
cells. Expression of miR-195-5p in GC (D) tissues and (E) cells.
(F) The correlation between circ-PVT1 and miR-195-5p expression in
GC tissues was analyzed by Pearson's correlation analysis. (G) The
expression of circ-PVT1 in HGC-27 and AGS cells was detected by
RT-qPCR following circ-PVT1-knockdown or overexpression. (H) The
expression of miR-195-5p in HGC-27 and AGS cells was detected by
RT-qPCR following circ-PVT1-knockdown or overexpression.
Anti-miR-195-5p was used for the rescue experiments of
si-circ-PVT1. (I) Recovered expression of miR-195-5p was observed
following anti-miR-195-5p transfection. The effect of si-circ-PVT1
on viability was reversed by transfection of (J) HGC-27 and (K) AGS
cells with anti-miR-195-5p. (L and M) The effect of anti-miR-195-5p
on apoptosis was detected by flow cytometry assay. (N and O) The
si-circ-PVT1-induced effects on the expression levels of P21, Bcl-2
and MMP9 were reversed by anti-miR-195-5p, as revealed by western
blotting. ****P<0.0001 vs. control. miR-195-5p, microRNA-195-5p;
Circ-PVT1, circular RNA-PVT1; GC, gastric cancer; P21,
cyclin-dependent kinase inhibitor P21; Bcl-2, B-cell lymphoma-2;
MMP9, matrix metalloproteinase 9; OD, optical density; RT-qPCR,
reverse transcription-quantitative PCR; anti-miR-195-5p, miR-195-5p
inhibitor; WT, wild type; MUT, mutant; NC, negative control. |
miR-195-5p inhibitor reverses
propofol-mediated effects on the proliferation, apoptosis and
invasion of GC cells
To investigate the association between miR-195-5p
and propofol, RT-qPCR assays demonstrated that miR-195-5p
expression was significantly increased as the concentration of
propofol increased (Fig. 4A), and
that miR-195-5p inhibitor could significantly reverse the increased
expression of miR-195-5p induced by propofol in GC cells (Fig. 4B). This indicated that the
tumor-suppressive effect of propofol occurred in a
miR-195-5p-dependent manner. Subsequently, MTT, flow cytometry and
Transwell assays revealed that miR-195-5p inhibitor reversed the
effects of propofol on the proliferation (Fig. 4C and D), invasion (Fig. 4F) and apoptosis (Fig. 4E) of HGC-27 and AGS cells.
Similarly, the decrease of Bcl-2 and MMP9, and increase of P21
induced by propofol could be significantly reversed by miR-195-5p
silencing (Fig. 4G and H).
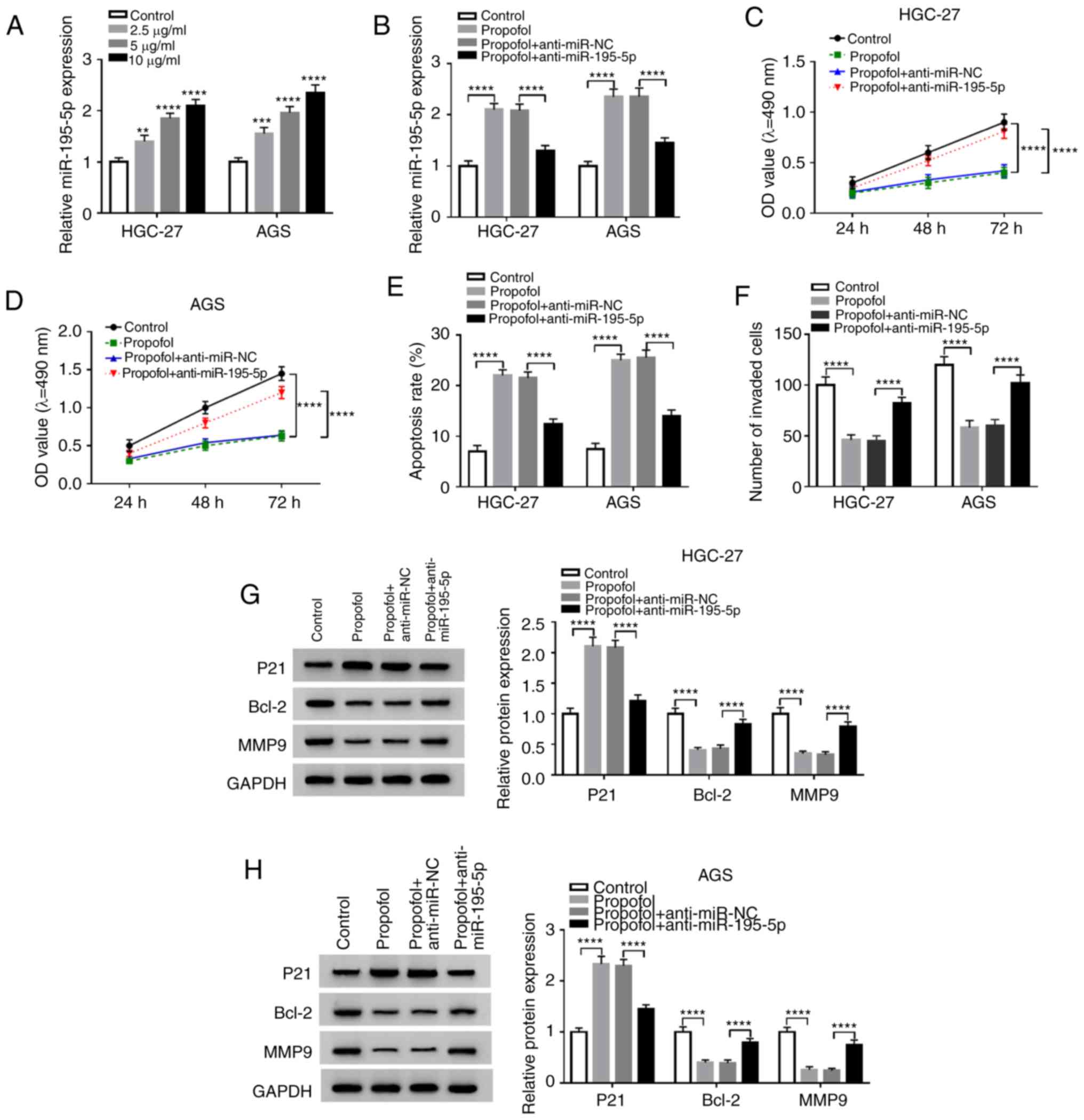 | Figure 4.miR-195-5p inhibitor reverses the
propofol-mediated effects on the proliferation, apoptosis and
invasion of gastric cancer cells. (A) RT-qPCR was used to detect
the miR-195-5p expression in cells treated with increasing
concentrations of propofol. (B) RT-qPCR was performed to detect the
effects of anti-miR-195-5p on the propofol-induced expression of
miR-195-5p in HGC-27 and AGS cells. Anti-miR-195-5p revered the
propofol-induced effects on the viability of (C) HGC-27 and (D) AGS
cells, and the (E) apoptosis and (F) invasion, which was measured
by MTT, flow cytometry and Transwell assays, respectively. Western
blot assay was performed to assess the effects of anti-miR-195-5p
on the propofol-induced changes in the protein expression of P21,
Bcl-2 and MMP9 in (G) HGC-27 and (H) AGS cells. **P<0.05,
***P<0.001, ****P<0.0001 vs. control. Circ-PVT1, circular
RNA-PVT1; P21, cyclin-dependent kinase inhibitor P21; Bcl-2, B-cell
lymphoma-2; MMP9, matrix metalloproteinase 9; OD, optical density;
miR-195-5p, microRNA-195-5p; RT-qPCR, reverse
transcription-quantitative PCR; anti-miR-195-5p, miR-195-5p
inhibitor; NC, negative control. |
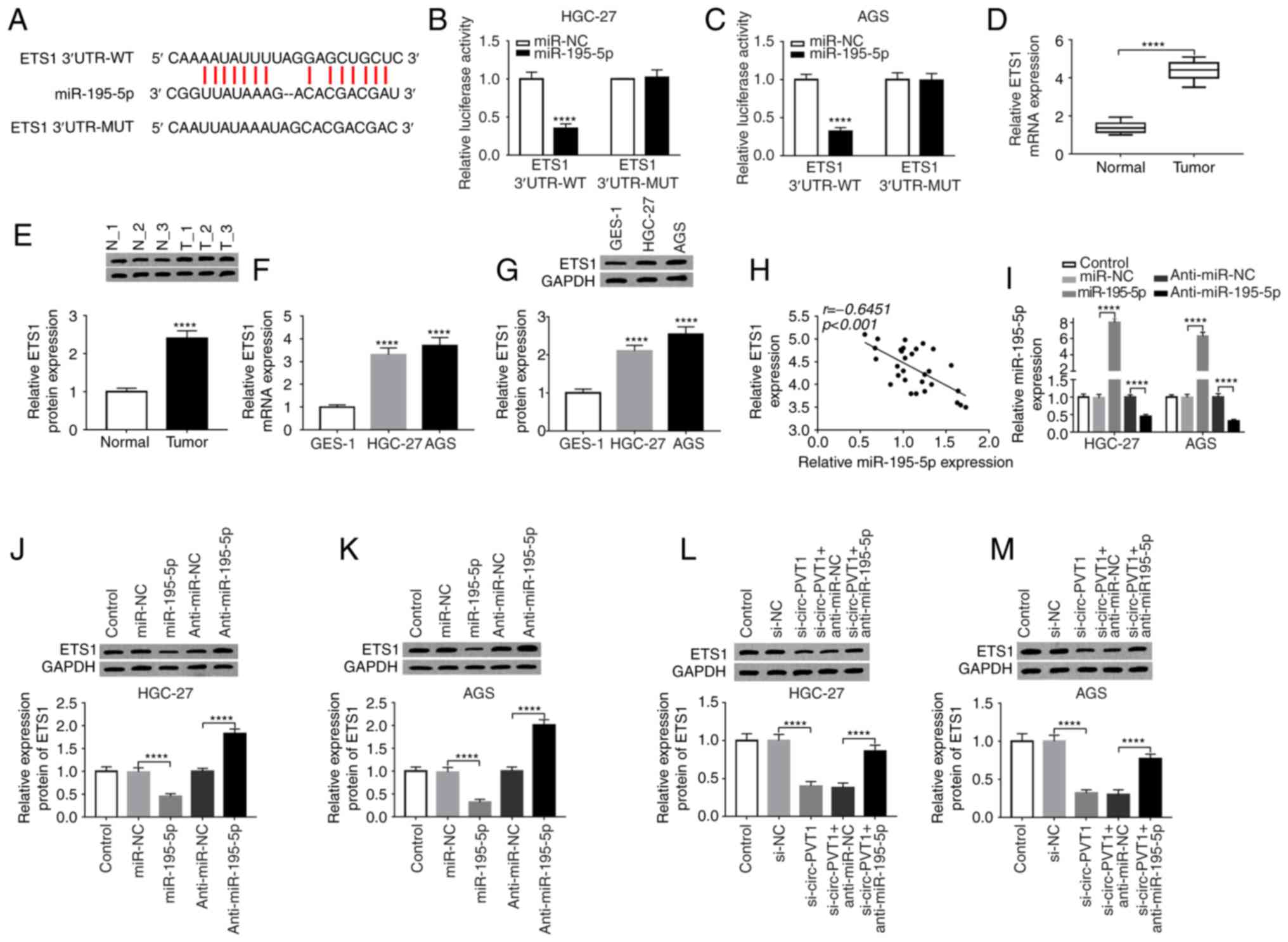
ETS1 is a direct target of
miR-195-5p
StarBase v2.0 was used to identify the potential
targets of miR-195-5p on the 3′-UTR of ETS1 mRNA (Fig. 5A). Subsequently, a dual-luciferase
activity assay was performed to further confirm the association
between miR-195-5p and ETS1. The results demonstrated that the
luciferase activity of ETS1-3′UTR-WT was inhibited by miR-195-5p,
as no distinct change of ETS1-3′UTR-MUT luciferase activity was
detected (Fig. 5B and C). In
addition, ETS1 expression was significantly higher in GC tissues
and cells compared with normal controls (Fig. 5D-G). Notably, the relative ETS1
expression was significantly negatively correlated with that of
miR-195-5p in 30 GC tumor tissues (Fig.
5H). The efficiency of transfection with miR-195-5p and
anti-miR-195-5p vectors was assessed by RT-qPCR (Fig. 5I). The protein level of ETS1 in
HGC-27 and AGS cells was measured by a western blot assay, and the
results revealed that the ETS1 level was significantly
downregulated by miR-195-5p and significantly upregulated by
anti-miR-195-5p (Fig. 5J and K). It
was also demonstrated that si-circ-PVT1 and anti-miR-195-5p
co-transfection significantly reversed the reduced ETS1 expression
induced by si-circ-PVT1 transfection (Fig. 5L and M).
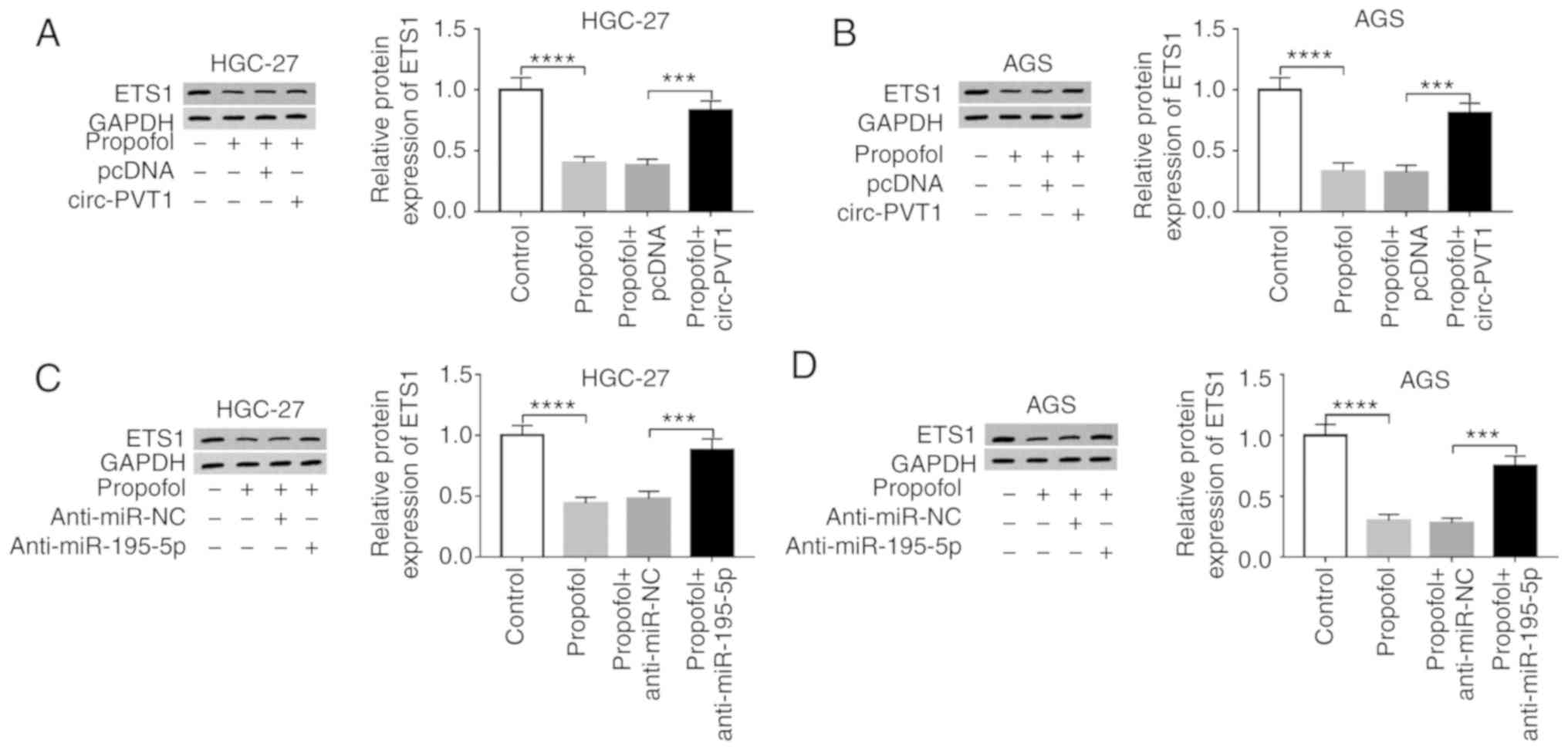
Circ-PVT1 overexpression and
miR-195-5p downregulation reverses the propofol-induced effects on
ETS1 expression in GC cells
To determine whether propofol exerts its function
via the circ-PVT1/miR-195-5p/ETS1 axis, the experimental groups
(Control, Propofol, Propofol + pcDNA, and Propofol + circ-PVT1)
were designed. As presented in Fig. 6A
and B, downregulation of ETS1 induced by propofol in HGC-27 and
AGS cells could be restored by circ-PVT1 overexpression.
Furthermore, western blotting results suggested that silencing
miR-195-5p could abolish the inhibitory action of propofol on ETS1
protein level in HGC-27 (Fig. 6C)
and AGS (Fig. 6D) cells.
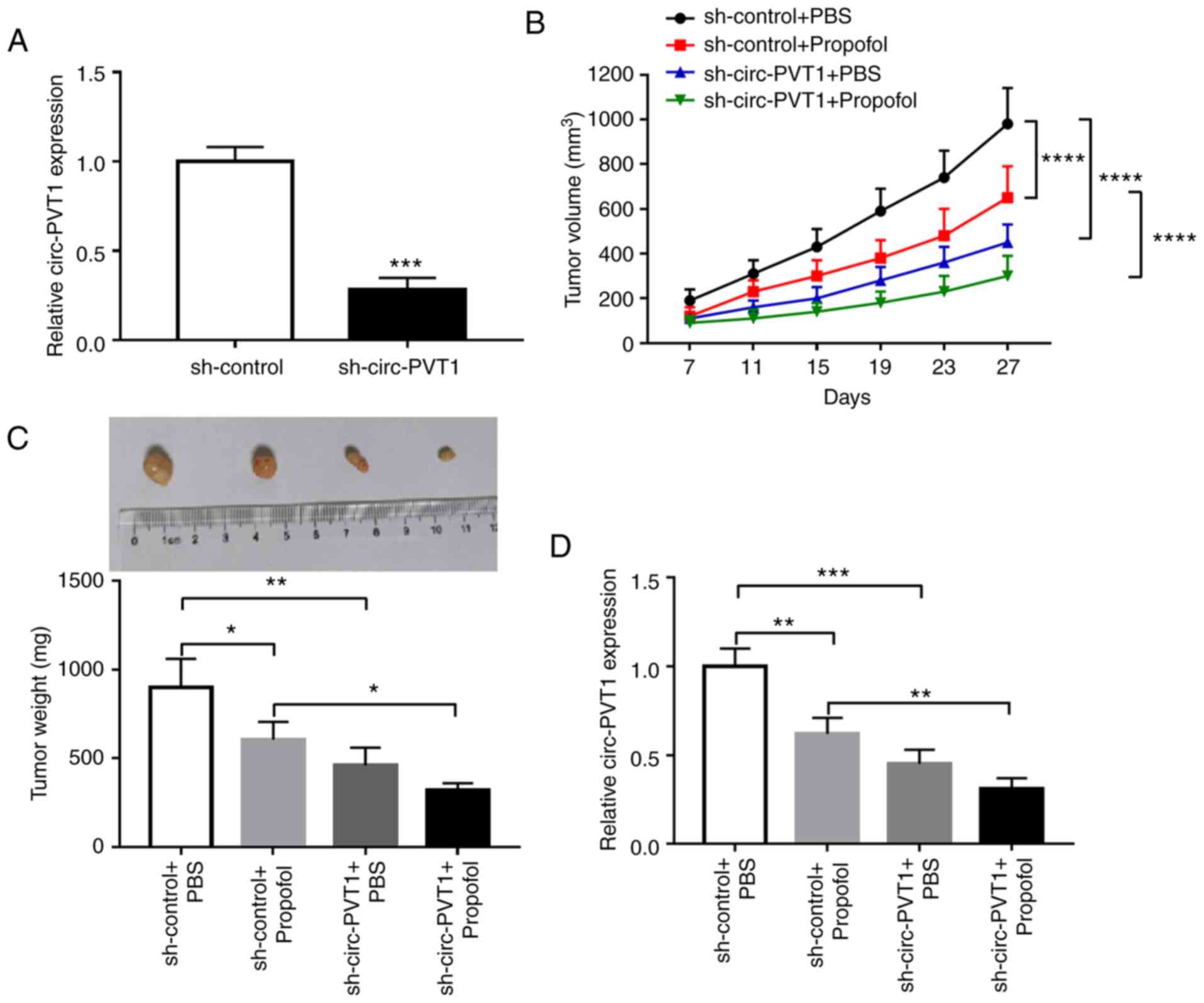
Circ-PVT1-knockdown improves the
anti-GC effect of propofol in vivo
To examine the effectiveness of
circ-PVT1-overexpression on tumor growth in vivo,
sh-circ-PVT1 stably transfected AGS cells were constructed. The
transfection efficiency of sh-circ-PVT1 was examined and presented
in Fig. 7A. Subsequently, the
transfected AGS cells were inoculated into nude mice, and tumor
volume was monitored. The sh-circ-PVT1+ propofol group generated
smaller tumors compared with the sh-control + propofol group
(Fig. 7B). In addition, the
sh-circ-PVT1+ propofol group exhibited the smallest tumor weight
among all groups (Fig. 7C). The
RT-qPCR results demonstrated that the level of circ-PVT1 in tumors
from the sh-circ-PVT1+ propofol group was significantly smaller
compared with the sh-control + propofol group (Fig. 7D). These results indicated that
circ-PVT1 silencing improves the anti-GC effect of propofol in
vivo.
Discussion
Propofol inhibits cell viability and proliferation,
but induces apoptosis of MKN45 GC (40). The present study investigated the
GC-suppressive effects of propofol on viability, apoptosis and
invasion. It was demonstrated that propofol inhibited the
proliferation and invasion, but promoted the apoptosis of HGC-27
and AGS cells. It has been reported that P21, Bcl-2 and MMP9
participate in physiological processes. P21 is associated with
linking DNA damage to arrest the cell cycle, and Bcl-2 is a member
of the Bcl-2 family of regulator proteins that regulate cell death,
by either inhibiting or inducing apoptosis (41,42).
MMP9 is regarded as a key invasion-associated protein (43). In the present study, propofol could
enhance the expression of P21 and reduce the levels of Bcl-2 and
MMP9.
Circ-PVT1 is a cancer susceptibility locus, located
on chromosome 8q24 (16). A
previous study has demonstrated that circ-PVT1 exhibits a high
expression in oral squamous cell carcinoma (OSCC) tissues and
cells, which could indicate a potential prognostic importance of
circ-PVT1 for patients with OSCC (13). Consistent with the previous study,
the current study observed increased circ-PVT1 expression in GC
tissues and cells, and the circ-PVT1 level could be decreased by
propofol administration in HGC-27 and AGS cells. In addition, in
HGC-27 and AGS cells, propofol could restrain the proliferative and
invasive abilities, but promoted the apoptotic rate. Furthermore,
propofol led to a significant increase in P21 protein level, and a
significant decrease in the protein levels of Bcl-2 and MMP9. In a
rescue experiment, overexpression of circ-PVT1 reversed the
aforementioned effects of propofol.
A study by Yang et al (44) indicated that the expression of
miR-195-5p was decreased in breast cancer cells and tissues. The
present study predicted and demonstrated that miR-195-5p was a
targeted miRNA for circ-PVT1, and miR-195-5p exhibited a low
expression in GC tissues and cells. Furthermore, a downregulated
level of miR-195-5p restored the tumor suppressor phenotype
following si-circ-PVT1 transfection. It was also identified that
propofol led to upregulation of miR-195-5p expression. In the
rescue experiment, miR-195-5p was knocked down, which revealed that
downregulated miR-195-5p reversed the inhibitory effects of
propofol on GC.
EST1 is considered to be a pro-oncogene that
regulates the pathophysiological progress of GC (45). It has been reported that EST1 is
involved in the regulation of proliferation, apoptosis and
metastasis, with a higher expression in GC tissues and cells
(46,47). In the current study, EST1 was shown
to be a downstream target of miR-195-5p. Furthermore, downregulated
miR-195-5p restored EST1 expression induced by the downregulation
of circ-PVT1. Additionally, downregulation of EST1 by propofol was
restored by circ-PVT1 overexpression or miR-195-5p-knockdown.
In the final xenograft experiment, the volume and
weight of the tumor decreased significantly following treatment
with propofol in the circ-PVT1-knockdown group, and demonstrated a
low level of circ-PVT1 expression, which also indicated that the
knockdown of circ-PVT1 increased the sensitivity of propofol to
tumor suppression.
In conclusion, the present study reported that
propofol could inhibit GC progression in vitro and in
vivo. Mechanism analysis revealed that propofol suppressed the
expression of ETS1 in GC cells by regulating the
circ-PVT1/miR-195-5p axis, suggesting that propofol inhibited the
GC process through modulating the circ-PVT1/miR-195-5p/ETS1 axis. A
limitation of the present study is a small tissue sample size. In
addition, ETS1 has been reported to be involved in the
PI3K/AKT/mTOR pathway in colorectal cancer cells (48). Therefore, in future studies, we will
focus on whether the regulatory role of circ-PVT1/miR-195-5p/ETS1
on the differentiation and metastasis is mediated by the
PI3K/AKT/mTOR pathway in propofol-treated GC cells in
vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed data during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
CZ and ZL designed the study and experiments. ZL and
JY generated and analyzed the data. HS and JY performed the
experiments and validated the results. HS, CZ and ZL wrote,
reviewed and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study received approval from the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China). Written informed consent was obtained from each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chidambaran V, Costandi A and D'Mello A:
Correction to: Propofol: A review of its role in pediatric
anesthesia and sedation. CNS Drugs. 32:8732018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siddiqui RA, Zerouga M, Wu M, Castillo A,
Harvey K, Zaloga GP and Stillwell W: Anticancer properties of
propofol-docosahexaenoate and propofol-eicosapentaenoate on breast
cancer cells. Breast Cancer Res. 7:R645–R654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui WY, Liu Y, Zhu YQ, Song T and Wang QS:
Propofol induces endoplasmic reticulum (ER) stress and apoptosis in
lung cancer cell H460. Tumour Biol. 35:5213–5217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang ZT, Gong HY, Zheng F, Liu DJ and Dong
TL: Propofol suppresses proliferation and invasion of pancreatic
cancer cells by upregulating microRNA-133a expression. Genet Mol
Res. 14:7529–7537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang P, Chen J, Mu LH, Du QH, Niu XH and
Zhang MY: Propofol inhibits invasion and enhances
paclitaxel-induced apoptosis in ovarian cancer cells through the
suppression of the transcription factor slug. Eur Rev Med Pharmacol
Sci. 17:1722–1729. 2013.PubMed/NCBI
|
|
6
|
Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX,
Jin HY and Zhu SM: Propofol exerts anti-hepatocellular carcinoma by
microvesicle-mediated transfer of miR-142-3p from macrophage to
cancer cells. J Transl Med. 12:2792014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang C, Gao J, Yan N, Wu B, Ren Y, Li H
and Liang J: Propofol inhibits the growth and survival of gastric
cancer cells in vitro through the upregulation of ING3.
Oncol Rep. 37:587–593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng Z and Zhang Y: Propofol inhibits
proliferation and accelerates apoptosis of human gastric cancer
cells by regulation of microRNA-451 and MMP-2 expression. Genet Mol
Res. 15:2016. View Article : Google Scholar
|
|
9
|
Ebbesen KK, Hansen TB and Kjems J:
Insights into circular RNA biology. RNA Biol. 14:1035–1045. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Yang L and Chen LL: Life without
A tail: New formats of long noncoding RNAs. Int J Biochem Cell
Biol. 54:338–349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonizzato A, Gaffo E, Te Kronnie G and
Bortoluzzi S: CircRNAs in hematopoiesis and hematological
malignancies. Blood Cancer J. 6:e4832016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dykes IM and Emanueli C: Transcriptional
and post-transcriptional gene regulation by long non-coding RNA.
Genomics Proteomics Bioinformatics. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He T, Li X, Xie D and Tian L:
Overexpressed circPVT1 in oral squamous cell carcinoma promotes
proliferation by serving as a miRNA sponge. Mol Med Rep.
20:3509–3518. 2019.PubMed/NCBI
|
|
14
|
Verduci L, Ferraiuolo M, Sacconi A, Ganci
F, Vitale J, Colombo T, Paci P, Strano S, Macino G, Rajewsky N and
Blandino G: The oncogenic role of circPVT1 in head and neck
squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD
transcription-competent complex. Genome Biol. 18:2372017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kun-Peng Z, Xiao-Long M and Chun-Lin Z:
Overexpressed circPVT1, a potential new circular RNA biomarker,
contributes to doxorubicin and cisplatin resistance of osteosarcoma
cells by regulating ABCB1. Int J Biol Sci. 14:321–330. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Li Y, Zheng Q, Bao C, He J, Chen
B, Lyu D, Zheng B, Xu Y, Long Z, et al: Circular RNA profile
identifies circPVT1 as a proliferative factor and prognostic marker
in gastric cancer. Cancer Lett. 388:208–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YY, Zhang LY and Du WZ: Circular RNA
circ-PVT1 contributes to paclitaxel resistance of gastric cancer
cells through the regulation of ZEB1 expression by sponging
miR-124-3p. Biosci Rep. 39:BSR201930452019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pang JC, Kwok WK, Chen Z and Ng HK:
Oncogenic role of microRNAs in brain tumors. Acta Neuropathol.
117:599–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mott JL: MicroRNAs involved in tumor
suppressor and oncogene pathways: Implications for hepatobiliary
neoplasia. Hepatology. 50:630–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tutar Y, Özgür A, Tutar E, Tutar L,
Pulliero A and Izzotti A: Regulation of oncogenic genes by
MicroRNAs and pseudogenes in human lung cancer. Biomed
Pharmacother. 83:1182–1190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nie H, Mu J, Wang J and Li Y: miR1955p
regulates multidrug resistance of gastric cancer cells via
targeting ZNF139. Oncol Rep. 40:1370–1378. 2018.PubMed/NCBI
|
|
22
|
Wang F, Ruan L, Yang J, Zhao Q and Wei W:
TRIM14 promotes the migration and invasion of gastric cancer by
regulating epithelial-to-mesenchymal transition via activation of
AKT signaling regulated by miR1955p. Oncol Rep. 40:3273–3284.
2018.PubMed/NCBI
|
|
23
|
Wang J, Li L, Jiang M and Li Y:
MicroRNA-195 inhibits human gastric cancer by directly targeting
basic fibroblast growth factor. Clin Transl Oncol. 19:1320–1328.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao DL and Wu QL: Effect of inhibition to
Yes-related proteins-mediated Wnt/β-catenin signaling pathway
through miR-195-5p on apoptosis of gastric cancer cells. Eur Rev
Med Pharmacol Sci. 23:6486–6496. 2019.PubMed/NCBI
|
|
25
|
Yordy JS and Muise-Helmericks RC: Signal
transduction and the Ets family of transcription factors. Oncogene.
19:6503–6513. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu D, Wilson TJ, Chan D, De Luca E, Zhou
J, Hertzog PJ and Kola I: Ets1 is required for p53 transcriptional
activity in UV-induced apoptosis in embryonic stem cells. EMBO J.
21:4081–4093. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wolvetang EJ, Wilson TJ, Sanij E,
Busciglio J, Hatzistavrou T, Seth A, Hertzog PJ and Kola I: ETS2
overexpression in transgenic models and in Down syndrome
predisposes to apoptosis via the p53 pathway. Hum Mol Genet.
12:247–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Lu JY, Zhao LQ, Wang XQ, Liu GL, Liu
Z, Zhou CN, Wu M and Liu ZH: Overexpression of ETS2 in human
esophageal squamous cell carcinoma. World J Gastroenterol.
9:205–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou J, Ng AY, Tymms MJ, Jermiin LS, Seth
AK, Thomas RS and Kola I: A novel transcription factor, ELF5,
belongs to the ELF subfamily of ETS genes and maps to human
chromosome 11p13-15, a region subject to LOH and rearrangement in
human carcinoma cell lines. Oncogene. 17:2719–2732. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Chen Y, Mei H, Jiao W, Song H, Ye L,
Fang E, Wang X, Yang F, Huang K, et al: Ets-1 promoter-associated
noncoding RNA regulates the NONO/ERG/Ets-1 axis to drive gastric
cancer progression. Oncogene. 37:4871–4886. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baillat D, Bègue A, Stéhelin D and
Aumercier M: ETS-1 transcription factor binds cooperatively to the
palindromic head to head ETS-binding sites of the stromelysin-1
promoter by counteracting autoinhibition. J Biol Chem.
277:29386–29398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rutter JL, Mitchell TI, Butticè G, Meyers
J, Gusella JF, Ozelius LJ and Brinckerhoff CE: A single nucleotide
polymorphism in the matrix metalloproteinase-1 promoter creates an
Ets binding site and augments transcription. Cancer Res.
58:5321–5325. 1998.PubMed/NCBI
|
|
33
|
Nakada M, Yamashita J, Okada Y and Sato H:
Ets-1 positively regulates expression of urokinase-type plasminogen
activator (uPA) and invasiveness of astrocytic tumors. J
Neuropathol Exp Neurol. 58:329–334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Trojanowska M: Ets factors and regulation
of the extracellular matrix. Oncogene. 19:6464–6471. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Z and Klominek J: Regulation of matrix
metalloprotease activity in malignant mesothelioma cell lines by
growth factors. Thorax. 58:198–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fink K and Boratyński J: The role of
metalloproteinases in modification of extracellular matrix in
invasive tumor growth, metastasis and angiogenesis. Postepy Hig Med
Dosw (Online). 66:609–628. 2012.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nazir SU, Kumar R, Singh A, Khan A, Tanwar
P, Tripathi R, Mehrotra R and Hussain S: Breast cancer invasion and
progression by MMP-9 through Ets-1 transcription factor. Gene.
711:1439522019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Q, Liu H, Cheng H, Li Y, Li X and Zhu
C: Downregulation of long noncoding RNA TUG1 inhibits proliferation
and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder
cancer cells. Onco Targets Ther. 10:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W, Wang Y, Zhu Z, Zheng Y and Song
B: Propofol inhibits proliferation, migration and invasion of
gastric cancer cells by up-regulating microRNA-195. Int J Biol
Macromol. 120:975–984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pisonero-Vaquero S, Soldati C, Cesana M,
Ballabio A and Medina DL: TFEB modulates p21/WAF1/CIP1 during the
DNA damage response. Cell. 9:11862020. View Article : Google Scholar
|
|
42
|
Skommer J, Brittain T and Raychaudhuri S:
Bcl-2 inhibits apoptosis by increasing the time-to-death and
intrinsic cell-to-cell variations in the mitochondrial pathway of
cell death. Apoptosis. 15:1223–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, Wang C, Li Q, Guo C, Sun W, Zhao
D, Jiang S, Hao L, Tian Y, Liu S and Sun MZ: miR-429-CRKL axis
regulates clear cell renal cell carcinoma malignant progression
through SOS1/MEK/ERK/MMP2/MMP9 pathway. Biomed Pharmacother.
127:1102152020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang R, Xing L, Zheng X, Sun Y, Wang X and
Chen J: The circRNA circAGFG1 acts as a sponge of miR-195-5p to
promote triple-negative breast cancer progression through
regulating CCNE1 expression. Mol Cancer. 18:42019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chou NH, Lo YH, Wang KC, Kang CH, Tsai CY
and Tsai KW: MiR-193a-5p and −3p play a distinct role in gastric
cancer: miR-193a-3p suppresses gastric cancer cell growth by
targeting ETS1 and CCND1. Anticancer Res. 38:3309–3318. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu Y, Zhang YC, Zhang WZ, Shen LS, Hertzog
P, Wilson TJ and Xu DK: Ets1 as a marker of malignant potential in
gastric carcinoma. World J Gastroenterol. 9:2154–2159. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng L, Qi T, Yang D, Qi M, Li D, Xiang
X, Huang K and Tong Q: microRNA-9 suppresses the proliferation,
invasion and metastasis of gastric cancer cells through targeting
cyclin D1 and Ets1. PLoS One. 8:e557192013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meng S, Jian Z, Yan X, Li J and Zhang R:
LncRNA SNHG6 inhibits cell proliferation and metastasis by
targeting ETS1 via the PI3K/AKT/mTOR pathway in colorectal cancer.
Mol Med Rep. 20:2541–2548. 2019.PubMed/NCBI
|