Introduction
Colorectal cancer (CRC) is the most common malignant
tumor of the digestive system and has the third-highest incidence
rate among all cancers (1). CRC has
various characteristics, including a fast reproductive rate,
exuberant energy metabolism and propensity for invasion (2). Approximately half of all patients are
diagnosed during the final stages, and the patients commonly
present with metastases to multiple organs, such as the lymph nodes
and liver (3). At present, surgical
resection and chemotherapy are considered to be the most effective
treatment strategies for CRC. However, postoperative invasion and
metastasis of CRC may lead to recurrence and even death (4).
Over the past 30 years, 5-fluorouracil (5-FU) has
been the primary chemotherapeutic drug used to treat CRC (5), and it is widely used as first-line
chemotherapy for advanced CRC (6).
However, 95% of patients with CRC exhibit resistance to 5-FU, which
markedly reduces the cure rate (7).
Resistance and associated side effects of chemotherapy remain
unsolved problems in the clinical treatment of CRC. Therefore,
novel effective low-toxicity anti-CRC drugs from natural antitumor
compounds have become an attractive research target.
Curcumin is a type of polyphenol extracted from the
roots of turmeric plants, and it is the main component of turmeric
(8). In 1985, Kuttan et al
(9) were the first to propose the
use of curcumin in the treatment of tumors. Subsequently, a large
number of studies (8,10) demonstrated that curcumin may possess
anti-infection, anti-inflammatory, antioxidant and tumor growth
inhibitory properties. Curcumin has been referred to as a
third-generation anticancer drug due to its broad anticancer
spectrum, high efficiency and low toxicity (10).
Cell apoptosis, also known as programmed cell death,
is an essential process for cells to maintain life activities.
Cysteinyl aspartate-specific proteinases (caspases) are a group of
proteins that play a key role in promoting apoptosis (11). There are three classical signaling
pathways that can induce cancer cell apoptosis: The death receptor,
mitochondrial and endoplasmic reticulum signaling pathways
(12). Fas receptor-mediated
apoptosis is one of the most important death receptor signaling
pathways. The cancer stem cell theory of tumor growth suggests that
Fas signaling may be involved in cell apoptosis, cell senescence
and tumor maintenance (13).
The malignant degree of CRC is determined by
hematogenous and lymphatic metastasis, as well as local invasion.
The pathogenesis of CRC is currently a clinical research focus. It
has been reported that epithelial-to-mesenchymal transition (EMT)
is crucial for the development and progression of malignant tumors,
mainly manifesting as the disruption of the tight connections
between marginal tumor cells (14).
Additionally, claudin and matrix metalloproteinase (MMP) protein
regulation is associated with tumor metastasis (15,16).
The activation of the nuclear factor (NF)-κB signaling pathway
promotes the transcription of inflammatory factors, chemokines,
adhesion molecules and growth factor-related genes, thus leading to
tumor development (17), and it may
represent an effective antitumor strategy for inducing tumor cell
apoptosis and inhibiting tumor cell activity and invasion.
The aim of the present study was to investigate the
antitumor effects of curcumin on CRC cell proliferation, migration
and apoptosis, explore the possible underlying molecular
mechanisms, and compare the antitumor efficacy of curcumin with
that of 5-FU, in order to determine whether curcumin may be
considered as a potential drug for the treatment of patients with
CRC.
Materials and methods
Cells and animals
The HCT-116 cell line was purchased from the China
Center for Type Culture Collection. The cells were maintained in
RPMI-1640 medium (HyClone; Cytiva) supplemented with 10% FBS
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.) and
antibiotics (100 U/ml streptomycin and 100 U/ml penicillin) in an
incubator at 37°C with 5% Co2. A total of 24 Kunming
mice, aged 4 weeks and weighing 20±2 g, were purchased from the
Hubei Provincial Center for Disease Control and Prevention. The
mice were housed at room temperature with 45–55% humidity and with
a 10/14-h light/dark cycle under pathogen-free conditions. The
health status of the mice was monitored daily. The humane endpoints
were deterioration of their general condition, and the mice were
sacrificed in the event of a body weight loss of >20%. All
animal protocols were approved by the Hubei University of
Traditional Chinese Medicine Ethics Committee.
Cell experimental groups
The following five treatment groups were used in the
experiments: Control (no curcumin or 5-FU); CUR(L) (10 µM
curcumin); CUR(M) (20 µM curcumin); CUR(H) (30 µM curcumin); and
5-FU (500 µM 5-FU) groups. The control and 5-FU groups were used as
blank and positive controls, respectively. The 5-FU injection was
purchased from Tianjin Jinyao Amino Acid Co., Ltd. Curcumin
(Sigma-Aldrich; Merck KGaA) was dissolved in DMSO.
Cell counting Kit-8 (CCK-8) assay
The proliferation ability of HCT-116 cells that were
treated with different doses of curcumin and 5-FU for 24, 36 and 48
h was analyzed using a CCK-8 assay. After the HCT-116 cells were
seeded in a 96-well culture plate (Wuxi NEST Biotechnology Co.,
Ltd.) at a density of 5×103 cells/well for 24 h, the
cells were treated with three doses of curcumin and 5-FU for 24, 36
and 48 h. Subsequently, 10 µl CCK-8 dye (Dalian Meilun
Biotechnology Co., Ltd.) was added to each well, and the cells were
placed in an incubator for 3 h at 37°C. Finally, the absorbance was
measured at 450 nm on a microplate reader (Bio-Rad Laboratories,
Inc.). The inhibition rate of HCT-116 cell viability with different
treatments was calculated according to the following equation:
Inhibition rate (%)=[optical density (OD)control
group-ODtreatment group]/ODcontrol
group ×100%.
Colony formation assay
Cell viability was detected using a colony formation
assay. HCT-116 cells were seeded into a 6-well plate at a density
of 3×102 cells/well for 24 h, followed by treatment with
three different doses of curcumin and 5-FU for 12 h. HCT-116 cells
were cultured with new medium for 2 weeks after the drug-containing
medium was discarded. The cells were fixed with methanol-glacial
acetic acid stationary solution (3:1) at room temperature for 10
min and stained with Giemsa at room temperature for 15 min. The
following formula was used to calculate the colony formation
inhibition rate: Colony formation inhibition rate=(control group
colony number-experimental group colony number)/control group
colony number ×100%.
Transwell assay
The migration ability of HCT-116 cells was detected
using a Transwell assay. Following treatment with curcumin and 5-FU
for 12 h, HCT-116 cells were collected and seeded into the upper
chamber of a Transwell plate at a density of 1×105 cells
in 200 µl serum-free medium and incubated for 24 h at 37°C. Medium
containing 10% FBS (700 µl) was added to the lower chamber.
Subsequently, HCT-116 cells that had migrated to the lower surface
of the membrane were fixed in methanol-glacial acetic acid
stationary solution (3:1) at room temperature for 10 min and
stained with Giemsa at room temperature for 15 min. The number of
migrated cells was counted in four random fields under an inverted
microscope (Olympus CK-40; Olympus Corporation) at a magnification
of ×100.
Apoptosis assay based on Annexin
V-FITC/propidium iodide (PI) flow cytometry
Flow cytometry can distinguish early apoptotic
cells, late apoptotic cells and normal cells. The cells were seeded
in 6-well plates at a density of 1×106 cells/well,
followed by a 24-h incubation at 37°C. The cells were then treated
with different concentrations of curcumin and 5-FU for 24 and 48 h.
The assay was performed using the Annexin V-FITC apoptosis
detection kit (BestBio; Nanjing Fengfeng Biological Medicine
Technology Co., Ltd.) according to the manufacturer's protocol.
Subsequently, the cells were analyzed by flow cytometry (BD Accuri
C6; BD Biosciences), and the data were analyzed using FlowJo™
software (version 10; FlowJo LLC).
Acridine orange/ethidium bromide
(AO/EB) dual staining assay
Normal, early apoptotic, late apoptotic and necrotic
cells may be detected by fluorescence microscopy with green
fluorescence. The HCT-116 cells were seeded at a density of
5×105 cells/well in 6-well plates in which a round cover
slide was placed, followed by a 24-h incubation at 37°C.
Subsequently, the cells were treated with different concentrations
of curcumin and 5-FU for 24 h. The cover slides to which the cells
attached were stained with 20 µl AO/EB (Sigma-Aldrich; Merck KGaA)
and observed under a green fluorescence microscope (Nikon 80i;
Nikon Corporation) at a magnification of ×200. A total of 1,000
cells in each group were counted, and the apoptosis rate was
calculated according to the following equation: Apoptosis
rate=(early apoptotic cells + late apoptotic cells)/1,000
×100%.
Cell cycle assay based on flow
cytometry
The HCT-116 cell culture, drug treatment and cell
collection methods were as described for the apoptosis assay based
on Annexin V-FITC/PI flow cytometry. The cells were fixed and
incubated with 400 µl PI dye solution and 100 µl RNase (100 µg/ml)
at 4°C for 30 min. The cells were filtered using a 300 mesh (70 µm)
cell strainer and analyzed by flow cytometry. The experimental
results were analyzed using Modfit LT software (version 3.1; Verity
Software House).
Zymography assay
MMP-9 expression in HCT-116 cells was analyzed using
a zymography assay. Following drug treatment for 12 h, the
medicated medium was discarded, and the cells were washed twice
with PBS. Subsequently, the cells were cultured with serum-free
medium for another 24 h. The medium was harvested, and a 20-µl
medium sample was analyzed in a zymography assay. The zymography
assay was performed as previously described (18). The results were obtained using a gel
imaging system (ChemiDoc XRS+; Bio-Rad Laboratories,
Inc.).
Colon cancer cell metastasis in mouse
lung samples
Following curcumin and 5-FU treatment for 12 h,
HCT-116 cells were collected and washed with PBS. The cells were
suspended in PBS to a density of 4×106/ml. The cancer
cells were then labeled with 5 µM carboxyfluorescein succinimidyl
amino ester (CFSE) in a 37°C incubator for 10 min. After washing
with serum-free medium and PBS, the cancer cells were again
suspended in PBS to a density of 2.5×106/ml. The
4-week-old Kunming mice were divided into three groups (n=8 per
group), and 5×105 CFSE-labeled HCT-116 cells were
injected via the tail vein. At 6 and 24 h after injection, the 24
mice were anesthetized by intraperitoneal injection of 10% chloral
hydrate (300 mg/kg body weight) and immediately sacrificed by
cervical dislocation. The lungs were then harvested, dissected and
fixed with 4% paraformaldehyde for 6 h at 4°C. Subsequently, the
mouse lung samples were dehydrated in 20% sucrose. Frozen mouse
lung sections were observed under a fluorescence microscope (Nikon
80i; Nikon Corporation; magnification, ×100) at 488 nm.
Additionally, 20 fields of view of lung tissue sections were
randomly selected, and the number of fluorescent nodules observed
in each section was counted (19).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from HCT-116 cells treated
with curcumin (20 µM) and 5-FU (500 µM) for 24 h using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). cDNA
was synthesized from 1 µg RNA using the Bestar™ qPCR RT kit
(DBI® Bioscience). mRNA expression levels were assessed
with a qPCR assay using SYBR Green Realtime PCR Master mix on a
Bio-Rad CFX96 Real-Time PCR system (Bio-Rad Laboratories, Inc.).
The assay was performed according to the manufacturer's protocol.
The housekeeping gene used for normalization was β-actin. The
primers (Tsinke Biological Technology Co., Ltd.) used were as
follows: Fas forward, 5′-TCTGGTTCTTACGTCTGTTGC-3′ and reverse,
5′-CTGTGCAGTCCCTAGCTTTCC-3′; Fas-associated via death domain (FADD)
forward, 5′-GGGAGTCACTGAGAATCTGGAA-3′ and reverse,
5′-GGCCTGCTGAACCTCTTGTAC-3′; caspase-8 forward,
5′-TTTCTGCCTACAGGGTCATGC-3′ and reverse,
5′-GCTGCTTCTCTCTTTGCTGAA-3′; caspase-3 forward,
5′-CATGGAAGCGAATCAATGGACT-3′ and reverse,
5′-CTGTACCAGACCGAGATGTCA-3′; and β-actin forward,
5′-TGCTGTCCCTGTATGCCTCT-3′ and reverse, 5′-TTTGATGTCACGCACGATTT-3′.
The following thermocycling conditions were used: 95°C for 60 sec,
followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec.
Relative mRNA expression levels were determined using the
2−∆∆Cq method (2). The
experiments were performed with four repeats for each sample.
Western blotting
The effects of curcumin and 5-FU on the expression
levels of Fas, FADD, caspase-8, caspase-3, NF-κB, E-cadherin and
claudin-3 were analyzed using western blotting. Protein samples
were obtained from HCT-116 cells that were treated with different
concentrations of curcumin and 5-FU for 24 h using cell lysis
buffer (Wuhan Servicebio Biotechnology Co., Ltd.). The protein
concentration was detected using the BCA method (Beyotime
Biotechnology Co., Ltd.). Total protein (20 µg) was separated using
SDS-PAGE (12% gel) and transferred onto a nitrocellulose membrane
(Millipore; Merck KGaA). Following blocking with Tris-buffered
saline containing 1% Tween-20 and 5% fat-free milk powder, the
membrane was incubated with the corresponding antibodies. The assay
protocol has been previously described in detail (2). The following antibodies were used:
CD95/Fas (cat. no. bs-0215R; rabbit polyclonal; 1:1,000 dilution;
Beijing Biosynthesis Biotechnology Co., Ltd.), FADD (cat. no.
SC-271520; mouse polyclonal; 1:1,000 dilution; Santa Cruz
Biotechnology, Inc.), caspase-8 (cat. no. bsm33190M; rabbit
polyclonal; 1:1,000 dilution; Beijing Biosynthesis Biotechnology
Co., Ltd.), cleaved caspase-8 (cat. no. Asp384; mouse polyclonal;
1:1,000 dilution; Cell Signaling Technology, Inc.), caspase-3 (cat.
no. BS1067; rabbit polyclonal; 1:1,000 dilution; Santa Cruz
Biotechnology, Inc.), cleaved caspase-3 (cat. no. Asp175; rabbit
polyclonal; 1:1,000 dilution; Cell Signaling Technology, Inc.),
NF-κB (cat. no. bs-19789R; rabbit polyclonal; 1:1,000 dilution;
Beijing Biosynthesis Biotechnology Co., Ltd.), E-cadherin (cat. no.
3195S; rabbit polyclonal; 1:1,000 dilution; Cell Signaling
Technology, Inc.), claudin-3 (cat. no. 3195S; rabbit polyclonal;
1:1,000 dilution; Bioworld Technology, Inc.), β-actin (cat. no.
SC-47778; mouse polyclonal; 1:1,000 dilution; Santa Cruz
Biotechnology, Inc.) and horseradish peroxidase immunoglobulin G
antibody (cat. no. SE134, goat anti-rabbit, 1:1,000 dilution; and
cat. no. SESE131, goat anti-mouse, 1:1,000 dilution; Beijing
Solarbio Science & Technology Co., Ltd.). Human β-actin was
used for normalization. Following incubation with secondary
antibody and chemiluminescence reagent (Wuhan Servicebio
Biotechnology Co., Ltd.), the blots of the proteins of interest
were scanned using a gel imaging system (ChemiDoc XRS+;
Bio-Rad Laboratories, Inc.) and the analysis was performed using
Image Lab™ software (version 5.0; MCM DESIGN). The relative protein
expression levels were calculated as follows: Relative expression
of protein=(gray value of the band in the experimental group)/(gray
value of the band in the control group).
Statistical analysis
All experiments were repeated three times. Data were
analyzed using GraphPad Prism software (version 6.0; GraphPad
Software, Inc.). Student's t-test and ANOVA followed by Tukey's
post hoc test were used to compare different groups. P<0.05 was
considered to indicate a statistically significant difference. Data
are presented as the mean ± standard deviation (n=3).
Results
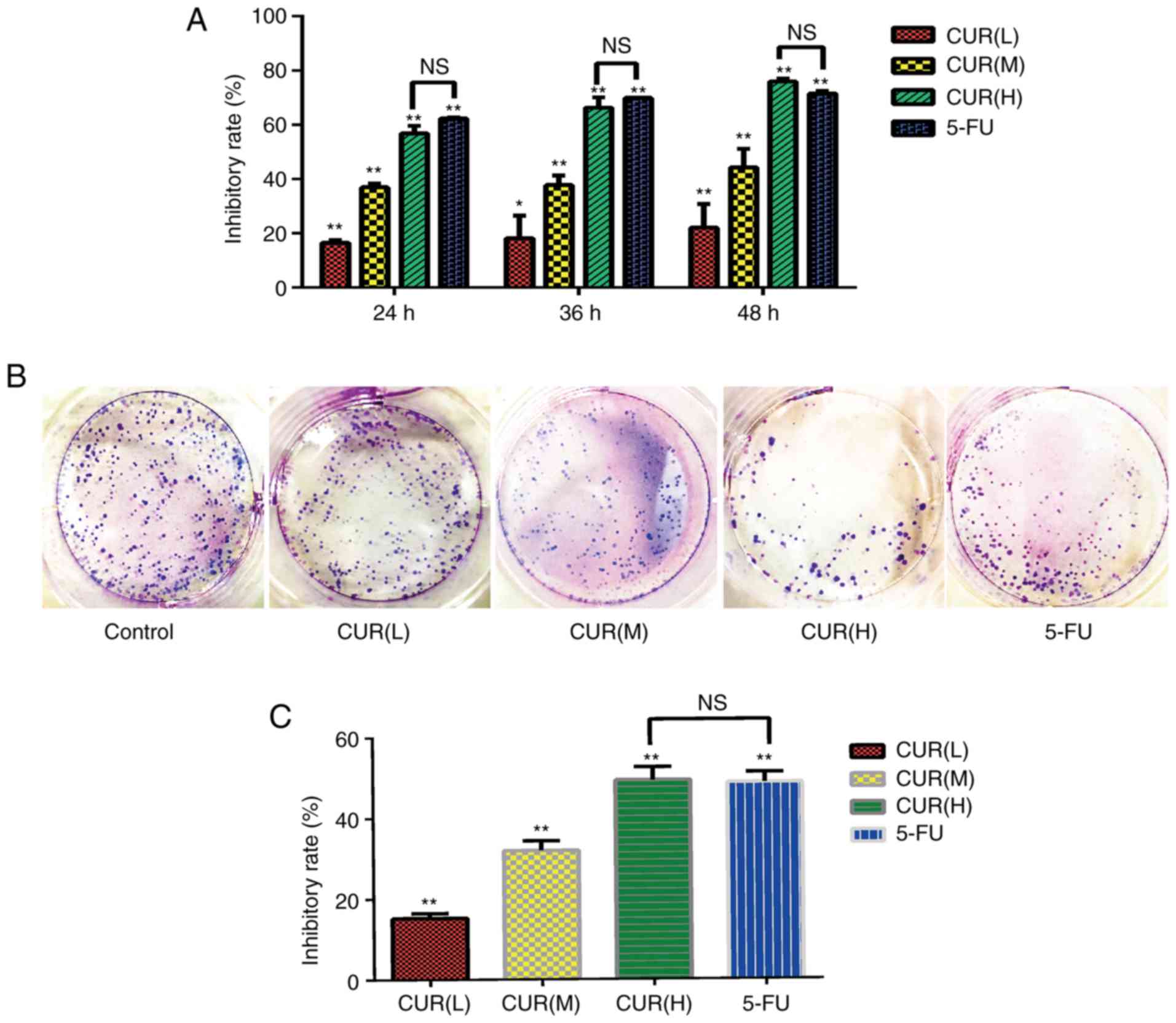
Curcumin inhibits the proliferation
and viability of HCT-116 cells
The results of the CCK-8 assay revealed that the
proliferation of cells in the curcumin and 5-FU groups differed
compared with that in the blank control group (Fig. 1A). The inhibitory effect increased
gradually with increasing curcumin concentration in a dose- and
time-dependent manner (P<0.05). The cell proliferation
inhibition rates of the CUR(H) group at 24, 36 and 48 h were not
significantly different from those of the 5-FU group. Additionally,
according to the colony formation assay results (Fig. 1B and C), the cell viability in each
group was significantly inhibited compared with the blank control
group (P<0.05), and the inhibition rate of CUR(H) (49.4±3.15%)
was higher compared with that of 5-FU (48.8±2.50%). These data
indicated that curcumin inhibited the viability and proliferation
of HCT-116 cells, and its effect was comparable to that of
5-FU.
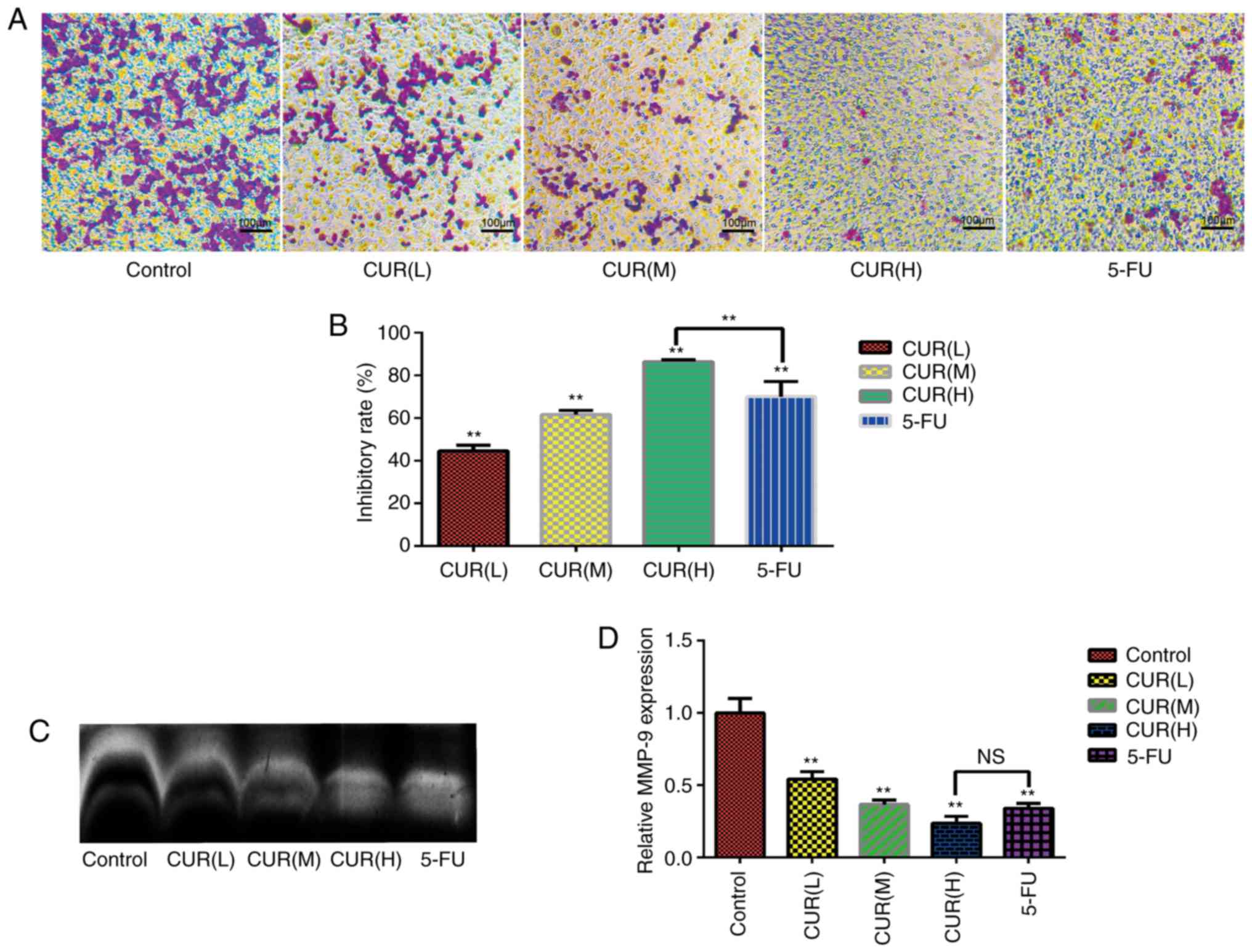
Curcumin inhibits migration and MMP-9
expression in HCT-116 cells
The results of the Transwell assay (Fig. 2A) revealed that numerous cells in
the control group migrated into the membrane of the upper chamber,
whereas curcumin treatment markedly reduced the cell migration rate
(Fig. 2B). Notably, the inhibitory
effect on migration increased gradually with increasing curcumin
concentration in a dose-dependent manner (P<0.05). The
inhibition rate in the Transwell assays was markedly increased from
that of the control group to 44.64±2.60% in the CUR(L), 61.59±2.13%
in the CUR(M), 86.53±0.72% in the CUR(H) and 70.15±7.04% in the
5-FU treatment groups. Furthermore, the zymography assay
demonstrated that curcumin treatment decreased MMP-9 expression in
HCT-116 cells (Fig. 2C and D).
Overall, the data demonstrated that curcumin markedly inhibited the
migration of HCT-116 cells. Notably, the inhibition of migration
and MMP-9 expression in HCT-116 cells following curcumin treatment
reached the levels observed with 5-FU treatment.
Curcumin induces apoptosis of HCT-116
cells
As shown in Fig. 3A and
B, early and late apoptotic cells may be selected by flow
cytometry. The apoptosis rate of each group was significantly
different from that of the blank control group (P<0.05; Fig. 3C). The proportion of apoptotic cells
increased from 3.6±0.60% in the control group to 23.7±1.01% in the
CUR(L), 27.2±2.00% in the CUR(M), 34.6±3.00% in the CUR(H) and
34.9±3.01% in the 5-FU experimental groups following treatment for
24 h. The proportion of apoptotic cells increased from 22.07±2.00%
in the control group to 37.08±2.51% in the CUR(L), 58.80±2.01% in
the CUR(M), 89.20±2.03% in the CUR(H) and 90.02±4.51% in the 5-FU
experimental groups following treatment for 48 h. At 24 and 48 h,
the apoptosis rate of the CUR(H) group was not significantly
different from that of the 5-FU group. Therefore, curcumin induced
apoptosis of the HCT-116 cells in a dose- and time-dependent manner
(P<0.05), and the ability of curcumin to induce the apoptosis of
HCT-116 cells was comparable to that of 5-FU.
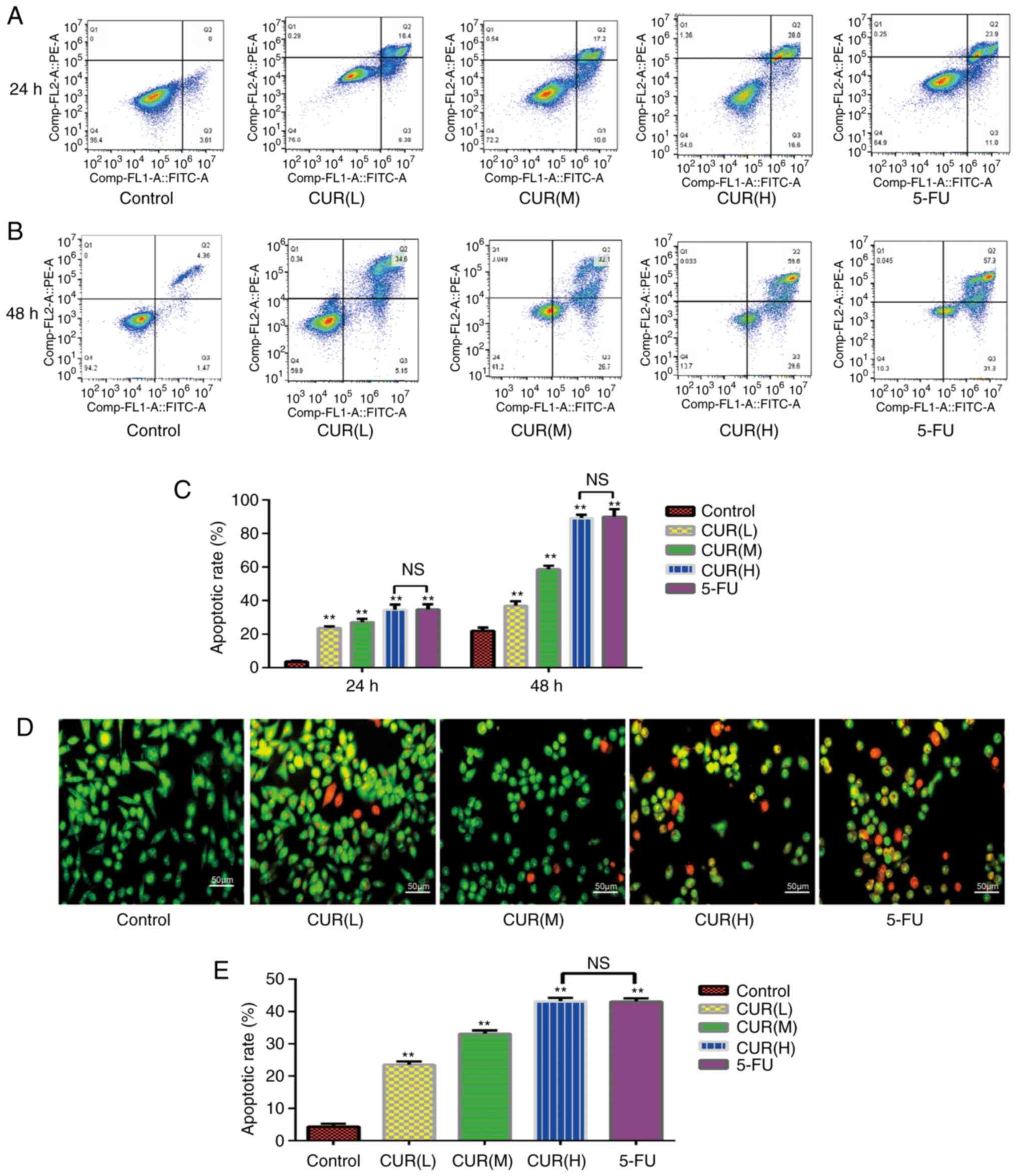 | Figure 3.Curcumin induces apoptosis of HCT-116
cells. (A and B) Following treatment with three different doses of
curcumin (10, 20 and 30 µM) and 5-FU (500 µM) for 24 and 48 h, the
apoptosis rate of HCT-116 cells was determined using Annexin
V-FITC/PI dual-staining flow cytometry. (C) Quantification of the
apoptosis rate in HCT-116 cells detected by Annexin V-FITC/PI
dual-staining flow cytometry. (D) Morphological alterations in
HCT-116 cells treated with three different concentrations of
curcumin for 24 h, as observed using fluorescence microscopy with
AO/EB staining. (E) Quantification of the results of the AO/EB
dual-staining assay. Results are presented as the mean ± standard
error of the mean (n≥3). **P<0.01 vs. control group. 5-FU,
5-fluorouracil; AO/EB, acridine orange/ethidium bromide; CUR(L), 10
µM curcumin; CUR(M), 20 µM curcumin; CUR(H), 30 µM curcumin; NS,
not significant; PI, propidium iodide. |
Curcumin induces morphological changes
in HCT-116 cells
Following staining with AO/EB, normal cells emitted
green or yellow-green fluorescence, early apoptotic cells emitted
orange fluorescence, and late apoptotic cells emitted red
fluorescence, with nuclear debris and apoptotic bodies. As shown in
Fig. 3D, the morphology of cells in
each group was markedly altered. The HCT-116 cells became smaller
and more rounded following curcumin and 5-FU treatment compared
with cells in the control group. Not only did the cell density
decrease with the increase in drug concentration, but the
proportion of early and late apoptotic cells also increased
gradually. The apoptosis rates were as follows: Control,
4.30±0.86%; CUR(L), 23.576±1.01%; CUR(M), 33.16±1.02%; CUR(H),
43.2±1.08%; and 5-FU, 43.1±0.95%. The apoptosis rate of each group
was significantly different from that of the blank control group
(P<0.05), and the apoptosis rate of the CUR(H) group was not
significantly different from that of the 5-FU group (Fig. 3E). Curcumin induced apoptosis in the
HCT-116 cells in a dose-dependent manner (P<0.05). The
percentages of early and late apoptotic cells increased gradually
with an increase in drug concentration. These results were
consistent with the flow cytometry results, in that curcumin
induced apoptosis of HCT-116 cells, and its effect was comparable
to that of 5-FU treatment.
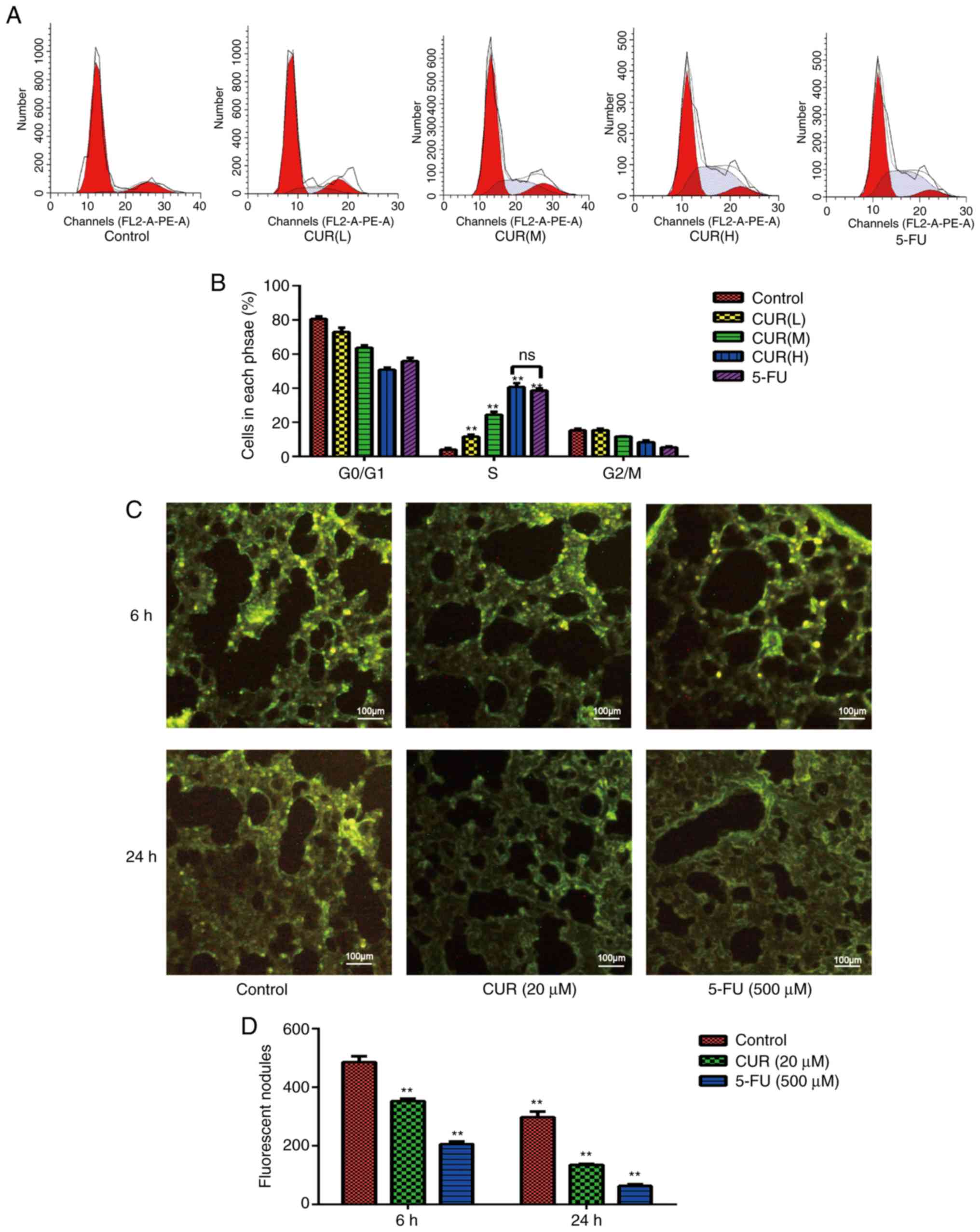
Curcumin induces S-phase arrest in
HCT-116 cells
As shown in Fig. 4A,
the proportion of cells that were arrested in the S-phase in each
experimental group was significantly different from that in the
blank control group (P<0.05), and this proportion increased in a
dose-dependent manner (P<0.05; Fig.
4B). The proportions of cells that were arrested in the
G0/G1 and G2/M phase were lower
compared with those in the blank control group. The proportion of
cells in S-phase increased from 17±0.60% in the control group to
23±0.81% in the CUR(L), 26.4±1.00% in the CUR(M), 45.83±0.40% in
the CUR(H) and 42.8±3.00% in the 5-FU treatment groups. These data
indicated that curcumin induced S-phase arrest in HCT-116 cells,
and this effect reached the level of 5-FU treatment.
Curcumin inhibits HCT-116 cell
metastasis to mouse lungs
Tumor cells migrate through the blood to the lungs,
where they accumulate by penetrating through the wall of the
vessels. HCT-116 cells were fluorescence-labeled with CFSE and were
injected into the mice via the tail vein. When the lungs were
surgically removed from the mice, the ability of cells to aggregate
and invade into the lungs were evaluated by counting the number of
fluorescent nodules in the lungs. At 6 h after HCT-116 cells were
injected (Fig. 4C), the numbers of
cancer cells that had invaded the lungs of the treatment groups (20
µM curcumin and 500 µM 5-FU) were markedly decreased compared with
those in the control group. As shown in Fig. 4D, the number of fluorescent nodules
decreased from 255±2% in the control group to 150±2% (CUR 20 µM)
and 160±2% (5-FU) in the experimental groups. At 24 h after
injection, the body's immune system quickly cleared a large number
of HCT-116 cells (Fig. 4C and D),
and the numbers of HCT-116 cells in lung tissue samples from each
group were reduced compared with those at 6 h. Only a small
percentage of HCT-116 cells invaded the lung tissue, and their
behavior determined the number of metastases. These data indicated
that curcumin inhibited CRC cell metastasis in vivo.
Curcumin affects the expression levels
of the Fas death receptor pathway-associated genes
The present study aimed to explore the possible
molecular mechanisms of apoptosis through western blot analysis.
Compared with the blank control group, the expression levels of
Fas, FADD, caspase-8 and caspase-3 were found to be significantly
upregulated (P<0.05) following curcumin (20 µM) and 5-FU (500
µM) treatment for 24 h (Fig.
5A).
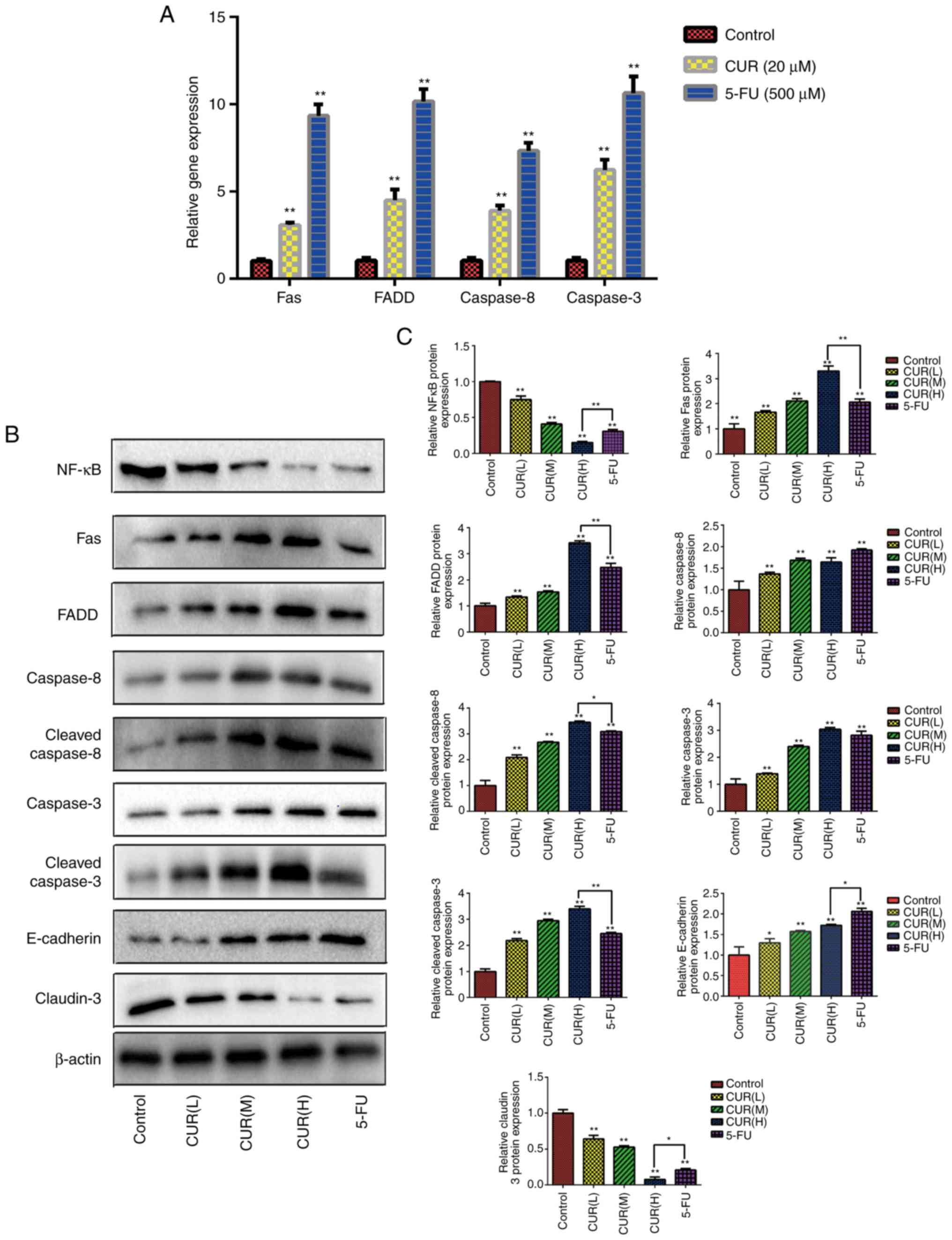 | Figure 5.Curcumin affects the expression
levels of Fas death receptor pathway-associated proteins/genes and
tumor metastasis-associated proteins. (A) Following treatment with
curcumin (20 µM) and 5-FU (500 µM), the expression levels of Fas
death receptor signaling pathway-associated genes were assessed in
HCT-116 cells using quantitative PCR analysis. (B) Following
treatment with three different doses of curcumin (10, 20 and 30 µM)
and 5-FU (500 µM) for 24 h, western blotting was used to determine
the expression levels of various proteins (NF-κB, Fas, FADD,
caspase-8, cleaved-caspase-8, caspase-3, cleaved-caspase-3,
E-cadherin and claudin-3) in HCT-116 cells. (C) Semi-quantification
of the relative protein expression levels. Results are presented as
the mean ± standard error of the mean (n≥3). *P<0.05 and
**P<0.01 vs. control group. 5-FU, 5-fluorouracil; CUR(L), 10 µM
curcumin; CUR(M), 20 µM curcumin; CUR(H), 30 µM curcumin; FADD,
Fas-associated protein with death domain; NF-κB, nuclear
factor-κB. |
Curcumin affects the expression levels
of Fas death receptor pathway-associated proteins and tumor
metastasis-associated proteins
Compared with the blank control group, the
expression levels of Fas, FADD, cleaved caspase-8 and cleaved
caspase-3 in each drug treatment group were significantly
upregulated (P<0.05). As shown in Fig. 5B and C, the expression levels of
Fas, FADD, cleaved caspase-8 and cleaved caspase-3 increased
gradually with increasing curcumin concentrations (P<0.05).
These data indicated that curcumin promoted the activation of the
Fas death receptor signaling pathway to induce apoptosis in HCT-116
cells. Additionally, the relative protein expression levels of
NF-κB and claudin-3 decreased gradually with increasing curcumin
concentrations (Fig. 5B and C).
Conversely, curcumin treatment upregulated the relative expression
levels of E-cadherin. These data indicated that curcumin treatment
not only regulated the apoptosis, but also the metastasis of CRC
cells. Notably, the relative expression levels of these proteins in
the CUR(H) group were significantly different from those in the
5-FU group (P<0.05). The data indicated that the regulatory
effect of curcumin on the Fas death receptor signaling pathway and
metastasis of HCT-116 cells could match or even exceed that of 5-FU
treatment.
Discussion
The treatment options for CRC include surgery,
radiotherapy, and a combination of chemotherapy and targeted
therapy, as well as expensive cytotoxic drugs with various
non-therapeutic effects. Although there have been advances in the
treatment of CRC, the recurrence and mortality rates of CRC remain
high (20). 5-FU has a wide
antitumor spectrum and may be used in the treatment of digestive
tract malignant tumors, as well as breast, ovarian, lung, cervical,
bladder and skin cancer. In recent years, numerous studies have
investigated the molecular mechanism of action of 5-FU in tumors,
and a number of potential molecular mechanisms have been implicated
in its effects. However, 5-FU inhibits bone marrow hematopoiesis
and reduces the secretion of cytokines, such as interleukin-2,
interferon-γ and tumor necrosis factor-α (21). Furthermore, it has been demonstrated
that the effective concentration range of 5-FU is narrow, and a
high blood concentration is likely to cause treatment-related side
effects, whereas a low blood concentration will not achieve the
desired chemotherapeutic efficacy in the treatment of CRC (22).
Curcumin, a naturally occurring phytotherapeutic
agent, has demonstrated therapeutic efficacy in the treatment of
cancer, and it has attracted attention as an antitumor agent
against gastric cancer, liver cancer and CRC (23). Curcumin may be combined with or
replace other chemotherapeutic drugs, such as 5-FU, in oncotherapy
(24,25). Studying these molecular targets also
offers the possibility of developing novel drugs to replace 5-FU in
treatment. The safety of curcumin has been studied in animals,
healthy individuals and patients (26,27),
and curcumin is generally recognized as a safe substance. In cell
culture studies, normal cell proliferation and viability may be
affected by curcumin, and they would be reduced at certain
concentrations. However, curcumin was more cytotoxic against cancer
cells rather than normal cells, which indicated the potential of
curcumin as an antitumor agent (28). Therefore, experiments were performed
on the basis that curcumin is non-toxic or low-toxic.
The main antitumor mechanisms of curcumin that have
been described thus far are as follows: Inhibition of proliferation
by blocking the cell cycle (29),
regulation of signaling pathways inhibiting proliferation (30), and inhibition of tumor metastasis
(31). Pro-apoptotic proteins, such
as Bcl-2 and Bax, induce cell apoptosis (32). In addition, activation of the tumor
suppressor gene p53 (33), and
inhibition of EMT, MMP, NF-κB and angiogenesis (34–36),
can regulate tumor cell invasion and metastasis. However, to the
best of our knowledge, the anticancer effect of curcumin in CRC
cells based on the death receptor signaling pathway has rarely been
reported to date. The purpose of the present study was to determine
the effects of curcumin on the proliferation, migration and
apoptosis of HCT-116 cells. Additionally, the possible molecular
mechanisms through which curcumin inhibits HCT-116 cell apoptosis
and migration were investigated. Furthermore, the inhibitory effect
of curcumin on HCT-116 cells was compared to that of 5-FU.
The results of the CCK-8 and colony formation assays
revealed that curcumin exerted an inhibitory effect on cell
viability and proliferation in a dose- and time-dependent manner.
The results were consistent with the hypothesis of Aaron et
al (37) and Shakibaei et
al (32), who hypothesized that
curcumin could inhibit the differentiation of cells and decrease
the numbers of stem-like cancer cells by affecting the whole cell
population. Furthermore, the inhibitory effect of curcumin on the
growth of HCT-116 cells was comparable to that of 5-FU.
The basic process of cell life is supported by a
complete cell cycle and generally develops in the order of
G1-S-G2-M phase. When certain factors impair
the integrity of the cell DNA, cells cannot pass through the
G1/S and G2/M detection points, which
eventually inhibits cell proliferation and migration (38). Shehzad et al reported that
curcumin may cause cell cycle arrest by decreasing the expression
levels of certain genes to inhibit tumor cell proliferation
(39). Flow cytometry demonstrated
that curcumin triggered S-phase arrest in HCT-116 cells and
inhibited cell proliferation in a dose-dependent manner. The
results were consistent with the results of a previous study that
demonstrated that 5-FU incorporates DNA double chains when it
replicates during the S phase of the cell cycle and leads to cell
death (32,40). Additionally, curcumin was able to
achieve the effect of 5-FU treatment on S-phase arrest of HCT-116
cells.
Apoptosis is considered to be the key to an
effective anticancer treatment regimen. In the present study,
curcumin treatment induced apoptosis of HCT-116 cells. The
morphological observation and flow cytometry data suggested that
curcumin induced apoptosis of HCT-116 cells in a dose- and
time-dependent manner, as did 5-FU treatment, and the regulatory
ability of curcumin treatment in HCT-116 cells was comparable to
that of 5-FU treatment. Therefore, curcumin inhibited the growth of
HCT-116 cells by inducing apoptosis.
To the best of our knowledge, caspases modulate cell
growth and apoptosis (41), and
apoptosis can be promoted by caspase regulation. The caspase family
is the initiator and the executor of programmed cell death in
mammals. The caspase initiator is first activated by apoptosis
signals, followed by activation of caspase effector molecules of
the downstream cascade. Finally, a series of substrates in cells
are specifically hydrolyzed, which leads to cell disintegration.
Caspase-3 is the most important apoptotic effector molecule, and it
is at the hub of each signaling pathway. Following the activation
of caspase-3, cell death is inevitable (42). RT-qPCR and western blot analyses
indicated that curcumin treatment markedly upregulated the
expression levels of Fas in a dose-dependent manner, which
activated the Fas death receptor signaling pathway and regulated
FADD expression to activate caspase-8 and caspase-3. Overexpression
of caspase-3 induced apoptosis in HCT-116 cells, which was
consistent with other apoptosis-related experiments, such as the
analysis of morphological changes and Annexin V-FITC/PI dual
staining flow cytometry. Notably, curcumin treatment matched or
even exceeded the effect of 5-FU treatment on the caspase
expression levels in HCT-116 cells.
It is well known that invasion and metastasis are
key biological characteristics of malignant tumors. Adhesion
molecules play a crucial role in the occurrence, invasion and
metastasis of malignant tumors. Tumor cells can accumulate in the
target organ by penetrating through the blood vessel wall, thus
activating tumor invasion (43).
Transwell assays demonstrated that curcumin suppressed the
migration and invasion of HCT-116 cells in a dose-dependent manner.
Furthermore, in the present study, an artificial lung metastasis
model was constructed using tail vein injection. The artificial
metastasis model has the advantages of detecting tumor cell
adhesion and the ability to pass through the blood vessel walls of
target organs (44). Tail vein
injection is mainly used to establish the tumor lung metastasis
model (45). According to the
characteristics of the artificial metastasis model, metastasis of
HCT-116 cells to the lungs of mice was observed, but not to other
tissues or organs. The results of the CFSE fluorescence labeling
assay indicated that curcumin markedly inhibited HCT-116 cell
aggregation and invasion of mouse lung tissues. The inhibitory
effect of curcumin treatment was similar to that of 5-FU
treatment.
NF-κB is one of the most important cell
transcription factors. It is involved in the transcriptional
regulation of numerous genes and is closely associated with the
development of tumors (46).
Overexpression of NF-κB activates the transcription of cyclin D1,
which regulates the cell cycle and enhances cell proliferation
(32,47). Qazi et al (17) revealed that inhibiting the
activation of the NF-κB signaling pathway can regulate related
signaling pathways, inhibit inflammatory factors, activate
apoptotic signals and promote cell apoptosis, thereby inhibiting
tumor progression. A subsequent study (48) demonstrated that activation of the
NF-κB signaling pathway can block chemokines and induce EMT and
invasion of tumor cells. Therefore, inhibition of NF-κB can
effectively inhibit tumor metastasis.
The tight junction is a vital membrane junction
complex between adjacent cells, and claudin is the main structural
protein (49). Abnormal protein
expression of claudin has been reported in a variety of malignant
tumors (15), and related studies
on claudin-3 expression in CRC have been performed (50). MMP-9 can mediate the degradation of
the extracellular matrix and provides suitable conditions for the
infiltration and diffusion of tumor cells (16). Additionally, the expression of the
intercellular adhesion factor E-cadherin is considered to be a
marker of EMT (51). Overall, when
investigating the possible molecular mechanism of cell metastasis,
the zymography assay and western blotting demonstrated that
curcumin treatment downregulated the expression levels of NF-κB,
claudin-3 and MMP-9 in HCT-116 cells, and also upregulated the
expression levels of E-cadherin in a dose-dependent manner.
Based on the observation of the present study that
curcumin altered the expression levels of tumor
metastasis-associated molecular targets, it was hypothesized that
the possible molecular mechanism of action of curcumin involved the
NF-κB signaling pathway, which could regulate MMP expression to
induce EMT. Incidentally, this hypothesis regarding the possible
molecular regulatory mechanism has been presented in a previous
study (52). However, the
conclusions regarding the mechanism of NF-κB and EMT regulation
have rarely been presented in previous studies on curcumin
treatment in HCT-116 cells. Furthermore, the observation that the
NF-κB signaling pathway can induce anti-apoptotic signaling
pathways (32,53) was confirmed in the present study.
Shakibaei et al (32)
reported that curcumin regulated Bax and Bcl-2 to induce apoptosis
in HCT-116 cells. However, in the present study, regarding the
possible molecular mechanism of action of curcumin, it was
hypothesized that NF-κB inhibited the Fas signaling pathway to
induce apoptosis. NF-κB, Fas and E-cadherin regulate the
proliferation, migration and invasion of CRC cells. Therefore,
agents that downregulate the expression levels of NF-κB and
upregulate the expression levels of Fas and E-cadherin may be a
possible strategy for inhibiting the progression of CRC.
In conclusion, the antitumor effects, including the
effects on cell migration and proliferation, of curcumin on HCT-116
cells were investigated. Curcumin did not only inhibit the
proliferation and migration of HCT-116 cells, but also induced
apoptosis. In terms of the possible molecular mechanism underlying
these antitumor effects, curcumin likely suppressed the NF-κB
signaling pathway to induce the activation of the Fas death
receptor signaling pathway and inhibit EMT, through paracrine
regulation of MMP-9 and cell tight junctions. Notably, the present
study demonstrated that the inhibitory effect of curcumin on the
HCT-116 cells was similar to that of 5-FU treatment in
vitro. Most of the indicators were detected in vitro,
and a single CRC cell line (HCT-116) was used in the present study.
Another limitation was we did not use inhibitors against either
Fas, caspase-8, or NF-κB, and did not investigate the expression of
additional EMT markers, such as N-cadherin and vimentin. Therefore,
the present study only preliminarily indicated the effect of
curcumin on EMT, and the NF-κB signaling pathway is a possible
molecular mechanism through which curcumin regulated the
proliferation and migration of HCT-116 cells. In subsequent
studies, the effects and the mechanism of curcumin treatment on CRC
must be verified through the use of inhibitors, and the
investigation of additional EMT markers in other CRC cell lines and
in vivo.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Project of Hubei Provincial Department of Education
(grant no. D20162001).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LX performed the experiments, analyzed the data and
drafted the manuscript. BH conducted western blotting experiments
and flow cytometry. QL, DDH, WJL and RCL conducted the cell culture
assay. GZ and QW designed the study, conceived the experiments,
laid out the experimental scheme, edited and revised the paper. All
the authors have read and approved the final version of the
manuscript to be published.
Ethics approval and consent to
participate
All animal protocols were approved by the Hubei
University of Traditional Chinese Medicine Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
of interest.
References
|
1
|
Duan Y, Fang Z, Shi Z and Zhang L:
Knockdown of lncRNA CCEPR suppresses colorectal cancer progression.
Exp Ther Med. 18:3534–3542. 2019.PubMed/NCBI
|
|
2
|
Yu F, Zhou C, Zeng H, Liu Y and Li S: BMI1
activates WNT signaling in colon cancer by negatively regulating
the WNT antagonist IDAX. Biochem Biophys Res Commun. 496:468–474.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krijger ID, Mekenkamp LJ, Punt CJ and
Nagtegaal LD: MicroRNAs in colorectal cancer metastasis. J Pathol.
224:438–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bengmark S and Hafström L: The natural
history of primary and secondary malignant tumors of the liver. I.
The prognosis for patients with hepatic metastases from colonic and
rectal carcinoma by laparotomy. Cancer. 23:198–202. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nordman IC, Iyer S, Joshua AM and Clarke
SJ: Advances in the adjuvant treatment of colorectal cancer. ANZ J
Surg. 76:373–380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Apostolou P, Toloudi M, Kalliara I,
Kipourou V, Tourna I and Papasotiriou I: Gene expression changes in
colorectal cancer during metronomic chemotherapy and
high-concentration drug administration. J Cancer Ther. 6:679–689.
2015. View Article : Google Scholar
|
|
7
|
Alfarouk KO: Tumor metabolism, cancer cell
transporters, and microenvironmental resistance. J Enzyme Inhib Med
Chem. 31:859–866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta SC, Patchva S and Aggarwal BB:
Therapeutic roles of curcumin: lessons learned from clinical
trials. AAPS J. 15:195–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuttan R, Bhanumathy P, Nirmala K and
George MC: Potential anticancer activity of turmeric (Curcuma
longa). Cancer Lett. 29:197–202. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai XZ, Yin HT, Sun LF, Hu X, Zhou C, Zhou
Y, Zhang W, Huang XE and Li XC: Potential therapeutic efficacy of
curcumin in liver cancer. Asian Pac J Cancer Prev. 14:3855–3859.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Merlin JLP: Biochemical characterization
of caspase-12 in apoptosis and a way for its purification using
fusion proteins. J Chosun Nat Sci. 7:103–112. 2014. View Article : Google Scholar
|
|
12
|
Dasmahaparea G, Almenara JA and Grant S:
Flavopiridol and histone deacetylase inhibitors promote
mitochondrial injury and cell death in human leukemia cells that
overexpress Bcl-2. Mol Pharmacol. 69:2882006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szarynska M, Olejniczak A, Wierzbicki P,
Kobiela J, Laski D, Sledzinski Z, Adrych K, Guzek M and Kmiec Z:
FasR and FasL in colorectal cancer. Int J Oncol. 51:975–986. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sánchez-Tilló E, de Barrios O, Siles L,
Cuatrecasas M, Castells A and Postigo A: β-catenin/TCF4 complex
induces the epithelial-to-mesenchymal transition (EMT)-activator
ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA.
108:19204–19209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karabulut M, Alis H, Bas K, Karabulut S,
Afsar CU, Oguz H, Gunaldi M, Akarsu C, Kones O and Aykan NF:
Clinical significance of serum claudin-1 and claudin-7 levels in
patients with colorectal cancer. Mol Clin Oncol. 3:1255–1267. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benedicto A, Romayor I and Arteta B: Role
of liver ICAM-1 in metastasis. Oncol Lett. 14:3883–3892. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qazi AK, Hussain A, Aga MA, Ali S, Taneja
SC, Sharma PR, Saxena AK, Mondhe DM and Hamid A: Cell specific
apoptosis by RLX is mediated by NFκB in human colon carcinoma
HCT-116 cells. BMC Cell Biol. 15:362014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gogly B, Groult N, Hornebeck W, Godeaua G
and Pellata B: Collagen zymography as a sensitive and specific
technique for the determination of subpicogram levels of
interstitial collagenase. Anal Biochem. 255:211–216. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoefel D, Grooby WL, Monis PT, Andrews S
and Saint CP: A comparative study of carboxyfluorescein diacetate
and carboxyfluorescein diacetate succinimidyl ester as indicators
of bacterial activity. J Microbiol Methods. 52:379–388. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chidambaram M, Manavalan R and Kathiresan
K: Nanotherapeutics to overcome conventional cancer chemotherapy
limitations. J Pharm Pharm Sci. 14:67–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren M, Ye L, Hao X, Ren Z, Ren S, Xu K and
Li J: Polysaccharides from Tricholoma matsutake and Lentinus edodes
enhance 5-fluorouracil-mediated H22 cell growth inhibition. J
Tradit Chin Med. 34:309–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suttle AB, Ball HA, Molimard M, Hutson TE,
Carpenter C, Rajagopalan D, Lin Y, Swann S, Amado R and Pandite L:
Relationships between pazopanib exposure and clinical safety and
efficacy in patients with advanced renal cell carcinoma. Br J
Cancer. 111:1909–1916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quispe-Soto1 ET and Calaf GM: Effect of
curcumin and paclitaxel on breast carcinogenesis. Int J Oncol.
49:2569–2577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He B, Wei W, Liu J, Xu YD and Zhao G:
Synergistic anticancer effect of curcumin and chemotherapy regimen
FP in human gastric cancer MGC-803 cells. Oncol Lett. 14:3387–3394.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vinod BS, Antony J, Nair HH,
Puliyappadamba VT, Saikia M, Narayanan SS, Bevin A and Anto RJ:
Mechanistic evaluation of the signaling events regulating
curcumin-mediated chemosensitization of breast cancer cells to
5-fluorouracil. Cell Death Dis. 4:e5052013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aggarwal ML, Chacko KM and Kuruvilla BT:
Systematic and comprehensive investigation of the toxicity of
curcuminoid-essential oil complex: A bioavailable turmeric
formulation. Mol Med Rep. 13:592–604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Panahi Y, Saadat A, Beiraghdar F, Hosseini
Nouzari SM, Jalalian HR and Sahebkar A: Antioxidant effects of
bioavailability-enhanced curcuminoids in patients with solid
tumors: A randomized double-blind placebo-controlled trial. J Funct
Foods. 6:615–622. 2014. View Article : Google Scholar
|
|
28
|
Kunwar A, Barik A, Mishra B, Rathinasamy
K, Pandey R and Priyadarsini KI: Quantitative cellular uptake,
localization and cytotoxicity of curcumin in normal and tumor
cells. Biochim Biophys Acta. 1780:673–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sivanantham B, Sethuraman S and Krishnan
UM: Combinatorial effects of curcumin with an anti-neoplastic agent
on head and neck squamous cell carcinoma through the regulation of
EGFR-ERK1/2 and apoptotic signaling pathways. ACS Comb. 18:22–35.
2016. View Article : Google Scholar
|
|
30
|
Arumuggam N, Bhowmick NA and Rupasinghe
HP: A review: Phytochemicals targeting JAK/STAT signaling and IDO
expression in cancer. Phytother Res. 29:805–817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar G, Mittal S, Sak K and Tuli HS:
Molecular mechanisms underlying chemopreventive potential of
curcumin: Current challenges and future perspectives. Life Sci.
148:313–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shakibaei M, Mobasheri A, Lueders C, Busch
F, Shayan P and Goel A: Curcumin enhances the effect of
chemotherapy against colorectal cancer cells by inhibition of NF-κB
and Src protein kinase signaling pathways. PLoS One. 8:e572182013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li YB, Gao JL, Zhong ZF, Hoi PM, Lee SM
and Wang YT: Bisdemethoxycurcumin suppresses MCF-7 cells
proliferation by inducing ROS accumulation and modulating
senescence-related pathways. Pharmacol Rep. 65:700–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun W, Liu DB, Li WW, Zhang LL, Long GX,
Wang JF, Mei Q and Hu GQ: Interleukin-6 promotes the migration and
invasion of nasopharyngeal carcinoma cell lines and upregulates the
expression of MMP-2 and MMP-9. Int J Oncol. 44:1551–1560. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang J, Kim E, Kim W, Seong KM, Youn H,
Kim JW, Kim J and Youn B: Rhamnetin and cirsiliol induce
radiosensitization and inhibition of epithelial-mesenchymal
transition (EMT) by miR-34a-mediated suppression of Notch-1
expression in non-small cell lung cancer cell lines. J Biol Chem.
288:27343–27357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fu Z, Chen X, Guan S, Yan Y, Lin H and Hua
ZC: Curcumin inhibits angiogenesis and improves defective
hematopoiesis induced by tumor-derived VEGF in tumor model through
modulating VEGF-VEGFR2 signaling pathway. Oncotarget.
6:19469–19482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aaron JS, John O and Dino P: Multiple
Actions of curcumin including anticancer, anti-inflammatory,
antimicrobial and enhancement via cyclodextrin. J Cancer Ther.
6:257–272. 2015. View Article : Google Scholar
|
|
38
|
Siegel R, Desantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shehzad A, Khan S, Shehzad O and Lee YS:
Curcumin therapeutic promises and bioavailability in colorectal
cancer. Drugs Today (Barc). 46:523–532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Harrison DE and Lerner CP: Most primitive
hematopoietic stem cells are stimulated to cycle rapidly after
treatment with 5-fluorouracil. Blood. 78:1237–1240. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang Q, Li F, Liu X, Li W, Shi W, Liu FF,
O'Sullivan B, He Z, Peng Y, Tan AC, et al: Caspase 3-mediated
stimulation of tumor cell repopulation during cancer radiotherapy.
Nat Med. 17:860–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hyoudou K, Nishikawa M, Umeyama Y,
Kobayashi Y, Yamashita F and Hashida M: Inhibition of metastatic
tumor growth in mouse lung by repeated administration of
polyethylene glycol-conjugated catalase: Quantitative analysis with
firefly luciferase-expressing melanoma cells. Clin Cancer Res.
10:7685–7691. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Teicher BA: Tumor models in cancer
research. Springer Science & Business Media; 2010
|
|
45
|
Lorger M and Felding-Habermann B:
Capturing changes in the brain microenvironment during initial
steps of breast cancer brain metastasis. Am J Pathol.
176:2958–2971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hayden MS and Sankar G: NF-κB, the first
quarter-century: Remarkable progress and outstanding questions.
Genes Dev. 26:203–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Meng Q, Zhi T, Chao Y, Nie E, Xu X, Shi Q,
Hua L, Wang L, Zhan W, Wang Y, et al: Bex2 controls proliferation
of human glioblastoma cells through NF-κB signaling pathway. J Mol
Neurosci. 53:262–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zheng Y, Miu Y, Yang X, Yang X and Zhu M:
CCR7 mediates TGF-β1-induced human malignant glioma invasion,
migration, and epithelial-mesenchymal transition by activating
MMP2/9 through the nuclear factor kappaB signaling pathway. DNA
Cell Biol. 36:853–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang H and Yang X: The expression patterns
of tight junction protein claudin-1, −3, and −4 in human gastric
neoplasms and adjacent non-neoplastic tissues. Int J Clin Exp
Patho. 8:881–887. 2015.
|
|
50
|
Oliveira SS and Morgado-Díaz JA: Claudins:
Multifunctional players in epithelial tight junctions and their
role in cancer. Cell Mol Life Sci. 64:17–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Downey C, Craig DH and Basson MD:
Isoform-specific modulation of pressure-stimulated cancer cell
proliferation and adhesion by α-actinin. Am J Surg. 202:520–523.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shant J, Cheng K, Marasa BS, Wang JY and
Raufman JP: Akt-dependent NF-kappaB activation is required for bile
acids to rescue colon cancer cells from stress-induced apoptosis.
Exp Cell Res. 315:432–450. 2009. View Article : Google Scholar : PubMed/NCBI
|