Introduction to the sorting nexin
family
The sorting nexin (SNX) family comprises cytoplasmic
and membrane-associated proteins that are involved in endocytosis
and protein trafficking through these membranous compartments
(1). The hallmark of the SNX family
is the presence of a phospholipid-binding motif (PX domain) that
contains a conserved sequence of 100–130 amino acids. The PX motif
binds to various phosphatidylinositol phosphates and then mediates
the transportation of these proteins to specific cellular
membranes. At present, 25 human SNXs involved in membrane
trafficking regulation have been identified (2). Based on their common domain
structures, the SNX family is divided into three subgroups: The
first group, including SNX1, 2, 4, 5, 6, 7, 8, 9, 13, 14, 15, 16
and 18, contains 1–3 coiled-coil domains that may be involved in
protein-protein interactions as well as homo- and/or
hetero-oligomerization with other SNXs. The second group, which
includes SNX3, 10, 11, 12, 22, 23 and 24, only contains a PX domain
and acts as a cargo protein adaptor in retromer-dependent
recycling. The remaining sorting nexins, including SNX17, 19, 21,
25 and 27, contain various membrane targeting sequences, G-protein
regulatory sequences or protein-protein interaction sequences, and
may be involved in endosomal sorting and signal transduction
(1).
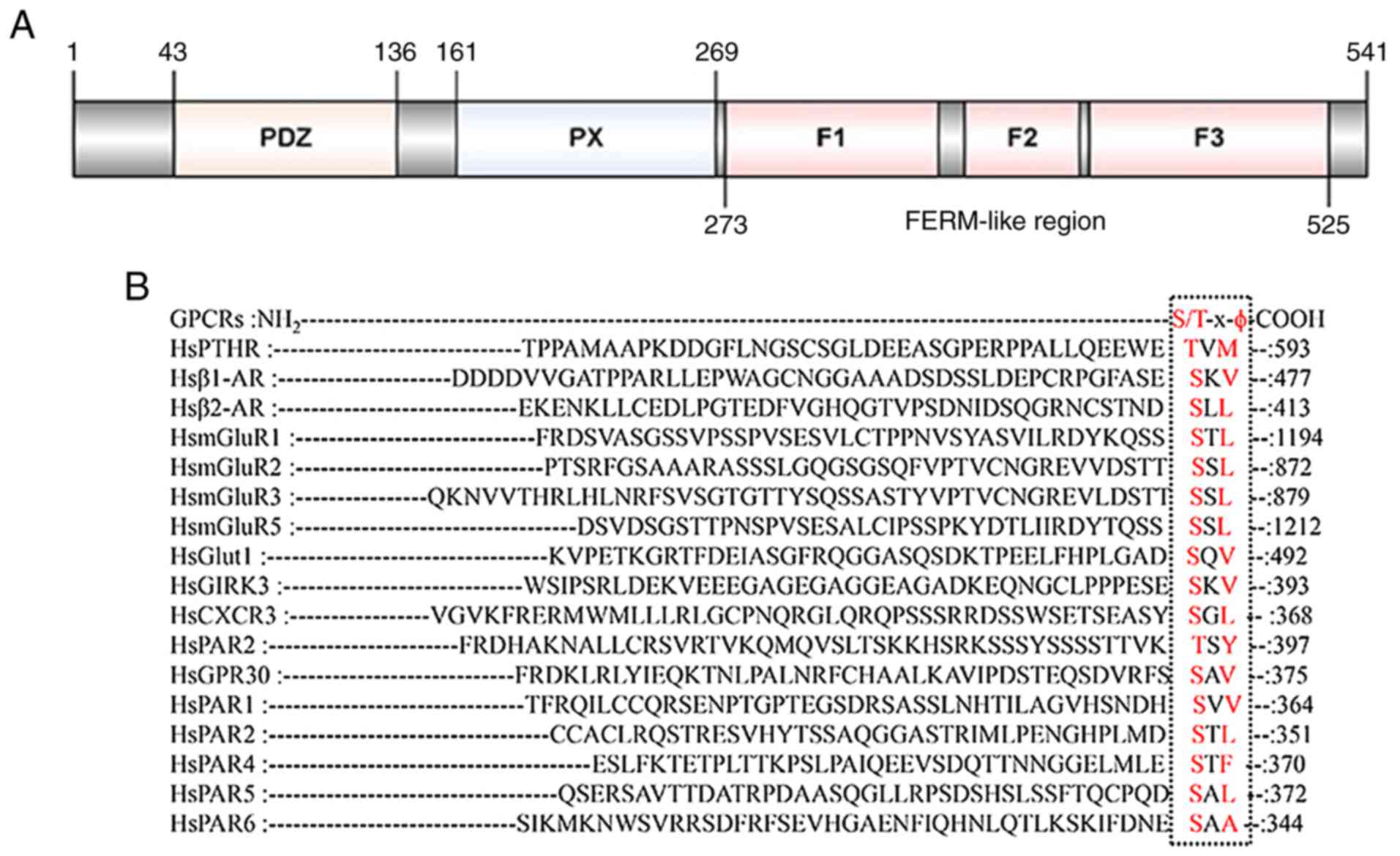
The hallmark of SNX27 is the presence of both a PX
domain, a postsynaptic density protein 95/discs large 1/zona
occludens 1 (PDZ) domain and
four-point-one-protein-erzin-moesin-radixin (FERM) domain, which is
linked to the endosomal trafficking of a range of transmembrane
cargoes (Fig. 1A) (1,2). The
PDZ domain, which distinguishes SNX27 from other SNX proteins,
allows SNX27 to recognize and bind with internalized proteins with
unique PDZ binding motif ‘S/T-x-ϕ’ (x, any amino acids; ϕ, any
hydrophobic amino acids) located in their C-terminal tails
(Fig. 1B). Furthermore, SNX27 acts
as a ‘cargo selector’ to form the actin-sorting nexin 27-retromer
tubule (ASRT) complex and participates in G protein-coupled
receptor (GPCR) recycling from endosomes to the plasma membrane
after internalization (3).
Components internalized from the plasma membrane can be transported
to the plasma membrane for rapid recycling, to lysosomes for
degradation, or to endosomes for short-term storage via endosomal
signal transduction before being trafficked to the cell surface
(4). Interestingly, several latest
research reports have indicated that SNX27 may contribute to cancer
development via recycling cancer-associated proteins (5,6).
GPCRs and their activation modes
GPCRs, which are also known as seven transmembrane
spanning receptors, constitute the largest family of membrane
proteins in mammalian cells. On the basis of their sequence
homology and functional similarity (7), the GPCR superfamily is divided into
six principal classes (A to F) via an alternative classification
system called glutamate, rhodopsin, adhesion, frizzled/taste2,
secretin. A diverse array of components involved in extracellular
stimulation, including light-sensitive compounds, odors, peptides,
hormones, neurotransmitters and large proteins are converted into
intracellular signals via GPCR activation and regulate various
physiological or pathological processes, including proliferation,
differentiation, chemotaxis and communication (8). Recently, certain modes of GPCR
activation have been used to explain the complexity of their
behaviors. The canonical GPCR activation signaling pathway, which
is termed the ‘GPCR-G protein’ activation mode, is dependent on the
interaction of GPCRs with intracellular G proteins, which is
mediated by GPCR ligands. Compared to the canonical ‘GPCR-G
protein’ activation, GPCRs may be biased towards either G
protein-dependent pathways or β-arrestin-dependent pathways, which
is referred to as the biased activation mode (8).
In canonical GPCR signaling, each Gα subfamily binds
GTP and dissociates from Gβ and Gγ to initiate downstream signaling
cascades via second messengers. Gβ and Gγ contribute to the
termination of canonical signaling by binding to β-arrestin, which
is recruited near the G protein and mediates the internalization of
the agonist-GPCR complex. Subsequently, desensitization is mediated
by phosphorylation of the GPCR by a specific kinase (4).
In addition to the canonical signaling pathway,
accumulating evidence has provided support suggesting that
activation of the G protein initiated by a GPCR occurs both in the
plasma membrane, but also in endosomes or the Golgi membrane, and
this is called intracellular activation. In this mode, β-arrestin
serves a central role in mediating GPCR trafficking. In 1999,
β2-adrenergic receptor (β2-AR) was shown to initiate ERK1/2
activation following internalization and was found to be mediated
by β-arrestin on clathrin-coated pits (9). Similar phenomena have also been found
in other GPCRs, such as proteinase-activated receptor 2 (10) and angiotensin II type 1A receptor
(11). Prolonging the lifetime of
arrestin-bound clathrin-coated pits by inhibiting subsequent
endocytosis enhances the intensity of the signal (12). Based on the strength of their
interaction with β-arrestin, GPCRs are divided into two classes:
Class A (β2-AR and biogenic amine receptors), which exhibit
unstable interactions and display transient endocytosis, and class
B (including peptide receptors), which form stable complexes with
β-arrestin and undergo sustained internalization (13). After internalization, complexes are
sorted to endosomes or the Golgi apparatus through the
trans-Golgi-network (TGN) by the SNXs family. The receptors then
recruit G proteins to active adenylyl cyclase, which in turn
produces cAMP and induces transient or prolonged activation
(14).
Other novel modes of GPCR activation, including
biased activation, dimerization activation, transactivation and
biphasic activation, have been addressed in a recent review
(15). These activation modes may
serve as potent drug targets to treat types of cancer with this
mode of activation (16), as
previous studies have shown that GPCRs regulate a broad range of
signal transduction pathways that contribute to several
characteristics of cancer development, including initiation,
growth, invasion, migration and angiogenesis (17,18).
SNX27-dependent GPCR endosome-to-plasma
membrane recycling is involved in cancer progression and
metastasis
Aberrant expression and activation of GPCRs has been
linked to oncogenicity since 1986, when the Mas oncogene was
identified, which was predicted to encode an internal protein
exhibiting the characteristics of a GPCR (19). At present, multiple lines of
evidence have shown that several GPCRs may be involved in tumor
progression and metastasis via activating excessive signaling
cascade (Table I). These
cancer-associated GPCRs are recycled from the cytoplasm to the
plasma membrane in several types of tumor cells in a
SNX27-dependent manner owing to the PDZ binding motifs. Some
proteins regulated by SNX27 belong to the classical GPCR
superfamily, such as parathyroid hormone receptor (PTHR) (20), β-adrenergic receptor 1 (β1-AR)
(21,22) and β2-AR (2). Second, metabotropic glutamate
receptors (mGluRs), including mGluR1-8, are class B, synaptic GPCRs
that contain large extracellular ligand binding domains and form
constitutive dimers. The trafficking and expression of mGluRs in
the dorsal horns is primarily dependent on SNX27 modifications
(23). Third, the recycling of ion
channels, such as protein-coupled inwardly rectifying potassium
channels (GIRKs), is regulated by SNX27 through a combination of
GIRK subunits (24,25). Additionally, Frizzled receptors
(FZDs), which are a family of GPCRs, interact with SNX27 and
consequently mediate the canonical Wnt signaling pathway (26).
 | Table I.Recycling GPCRs in a SNX27-dependent
manner are involved in cancer. |
Table I.
Recycling GPCRs in a SNX27-dependent
manner are involved in cancer.
| Subfamily | GPCRs | Cancers | Expression | Activating
signals | Effects | Refs. |
|---|
| Classic GPCRs | PTHR | Breast cancer | High | ERK1/2 | Proliferation | (27–30) |
|
|
| Prostate
cancer |
| MAPK | Metastasis |
|
|
|
| Colorectal
carcinomas |
| PI3K/AKT | Invasion |
|
|
| β1-AR | Breast cancer
Melanoma | High | TGF-β/Smad2(3) | Proliferation | (31–33) |
|
|
|
|
| cAMP/PKA/CREB | Migration |
|
|
|
|
|
| Ras/ERK |
|
|
|
| β2-AR | Squamous cell
carcinoma | High |
| Proliferation | (34,35) |
|
|
| Pancreatic
cancer |
|
| Migration |
|
|
|
| Gastric cancer |
|
| Angiogenesis |
|
|
|
| Breast cancer |
|
|
|
|
| Transporter | mGluRSs | Glioma
Hepatocellular carcinoma | High | MAPK | Proliferation | (36–41) |
|
|
| Prostate
cancer |
| PI3K/AKT | Metastasis |
|
|
|
| Melanoma |
| PKC |
|
|
| Ion channels | GIRKs | Breast cancer | High | β-adrenergic
signaling | Proliferation | (42–46) |
|
|
| Lung cancer |
|
| Migration |
|
|
|
| Hepatocellular
carcinoma |
|
|
|
|
| FZDs |
| Prostate
cancer | High | Wnt/β-catenin | Proliferation | (26,47–49) |
|
|
| Glioma |
|
| Metastasis |
|
|
|
| Lung cancer |
|
|
|
|
|
|
| Hepatocellular
carcinoma |
|
|
|
|
|
|
| Gastric
carcinoma |
|
|
|
|
| Others | Chemokine | Breast cancer | High | ERK1/2/MMP-7 | Proliferation | (50–52) |
|
| receptors | Colorectal
cancer |
|
| Metastasis |
|
|
| PARs | Prostate
cancer | High | p38 MAPK | Metastasis | (53,54) |
|
|
| Breast cancer |
| TGF-β |
|
|
|
|
| Pancreatic
cancer |
|
|
|
|
|
|
| Glioma |
|
|
|
|
|
| LPARs | Breast cancer | High | NOTCH0 | Proliferation | (55–58) |
|
|
| Liver cancer |
| PI3K/PAK1/ERK | Migration |
|
|
|
| Gastric cancer |
| LPAR signaling |
|
|
|
|
| Ovarian cancer |
|
|
|
|
|
| GPR30 | Fibroblasts | High | ERK1/2 | Proliferation | (59) |
|
|
| Thyroid cancer |
| NOTCH | Anti-apoptosis |
|
|
|
| Ovarian cancer |
|
|
|
|
|
|
| Endometrial
cancer |
|
|
|
|
|
|
| Renal cell
cancer |
|
|
|
|
Classic GPCRs and cancer
PTHR, a receptor of parathyroid hormone or
parathyroid hormone-related protein (PTHrP) is occasionally
secreted by cancer cells and is involved in cancer progression.
Immunohistochemistry analysis has demonstrated that PTHR1 is highly
expressed in the plasma membrane of certain cancer cells (27). PTHR typically activates a range of
mitogenic pathways, including the ERK1/2, MAPK and PI3K/AKT
signaling pathways, and modulates cell cycle progression by
inducing the expression of cyclin D1 (28). Additionally, hypercalcemia, which is
caused by an increase in PTHrP and PTHR, contributes to
epithelial-mesenchymal-transition (EMT) progression and skeletal
metastasis of breast and prostate cancer cells (29,30).
As with other classical GPCRs, β-ARs are associated
with the transduction of multiple intracellular signals, such as
adrenalin/HuR/TGF-β/Smad2 (3),
cAMP/PKA/CREB (or VEGF) and cAMP/Ras/ERK, and are involved in the
progression of several types of cancer (31–33).
β-ARs have also been demonstrated to stimulate tumor progression
via several cellular and molecular processes, such as recruitment
of macrophages into tumor tissues or increasing the expression of
inflammatory cytokines (34,35).
Transporters and cancer
Glutamate receptors on neuronal cell membranes are
responsible for regulating glutamate-mediated postsynaptic
excitation. Interestingly, in the last few decades, it has been
reported that glutamate receptors are involved in tumor development
in both neural and non-neural cancer tissues (36). Glutamate receptors are composed of
two groups, mGluRs and ionotropic glutamate receptors iGluRs.
Excluding the activation iGluRs in a G protein-independent manner,
mGluRs (mGluR1-8), which belong to the GPCR superfamily, are
ubiquitously expressed in both neuronal tissues and various
non-neuronal human tissues, such as the skin, liver, heart and
adrenal gland (37). Abundant
expression of mGluRs has been confirmed to regulate
glutamate-mediated signals and enhance malignant tumor phenotypes.
For example, both mGluR1 and mGluR5 can be coupled to Gαq, which
stimulates phospholipase C β and activated PKC, resulting in the
phosphorylation of downstream targets (38). Additionally, mGluR2-4 and 6–8
couples with Gαi/o leading to the inhibition of adenylyl cyclase,
which attenuates several different pathways, including the MAPK and
PI3K/Akt signaling pathways (39,40).
Inhibition of mGluR5 by the selective antagonist MPEP promotes
glioma cell death by facilitating the generation of a hypoxic
microenvironment (41). Notably,
through a PDZ-dependent mechanism, SNX27 promotes the recycling and
membrane insertion of the glutamatergic receptor. For example,
mGluR5 recycling in a vacuolar protein sorting-associated protein
26 (VPS26)-SNX27-dependent manner serves a role in the development
of neuropathic pain (23).
Ion channels and cancer
G protein-coupled inwardly rectifying K+
channels (GIRKs), as classical G protein effectors, are special
potassium ion channels, the activation of which results in
hyperpolarization of the cell membrane, thereby regulating cellular
activity. GIRK channels are known to be activated by GPCRs coupled
to the Gi/o subclass, which also inhibit voltage-dependent
Ca2+ channels and adenylate cyclase (42). Over the last decade, numerous
studies have suggested that two of the gene loci that encode GIRK
subunits in humans are related to tumorigenesis and tumor growth.
For example, 69% of patients with non-small cell lung cancers
exhibit high levels of GIRK1 gene expression and an increased
likelihood of cancer progression compared with patients with low
GIRK1 levels (43). Overexpression
of KCNJ3 (a GIRK1 subunit) contributes to invasion, metastasis and
angiogenesis in breast cancer cell lines (44). Stimulation of GIRK1 or GIRK2
channels may activate the β-adrenergic signaling pathway in both
small cell lung cancer and breast cancer (44,45).
Interestingly, SNX27 also appears to promote the movement of GIRKs
through early endosomes to the cell surface and leads to
alterations in their expression (46).
FZDs and cancer
Frizzled receptors are structurally similar to
GPCRs, with seven transmembrane-spanning domains, and they serve a
vital role in development and tissue homeostasis (47). The canonical Wnt/β-catenin signal
cascade, which is a pathway involved in vital aspects of cell
proliferation and differentiation, occurs via a combination of a
single Wnt ligand and multiple FZDs (26,48).
There is some evidence indicating that FZDs are frequently
overexpressed in tumor tissues, and this upregulation is associated
with a poor prognosis (26). Amino
acid sequence analysis has shown that most FZDs contain a
PDZ-binding motif at the C-terminal tail (49). Therefore, FZDs may bind to SNX27
through their PDZ domain and are then internalized and undergo
vesicular trafficking to regulate canonical Wnt signaling in cancer
cells.
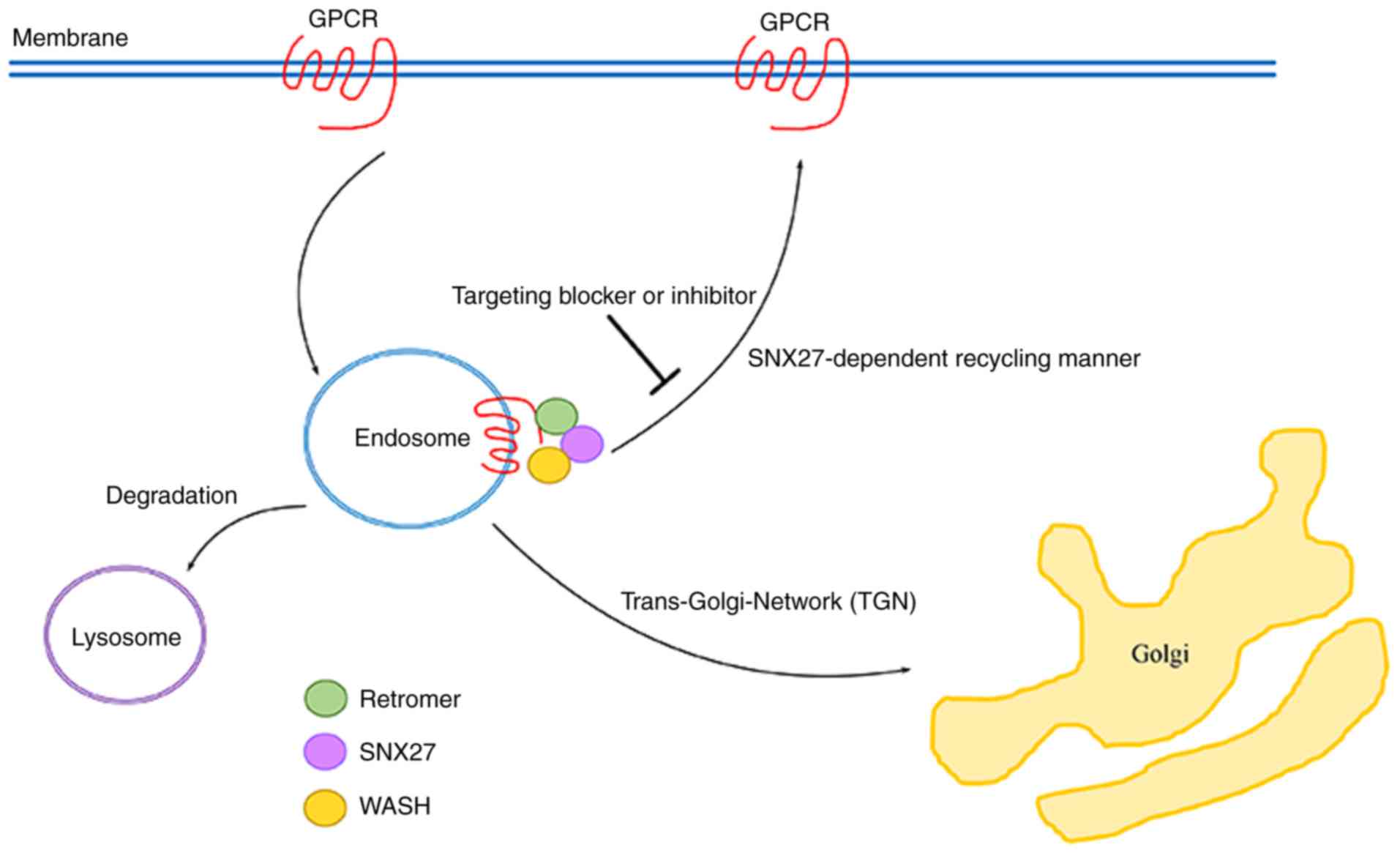
Other cancer-associated GPCRs
dependent on SNX27-mediated recycling
As described above, aberrant expression of several
SNX27-related GPCRs is closely associated with cancer development
and progression. Additionally, there are the other
cancer-associated GPCRs which are dependent on SNX27-mediated
recycling and trafficking to the membrane as naïve receptors. For
example, chemokine receptors are well-documented receptors that
facilitate cell growth, survival, migratory capability and cancer
metastasis (50–52). Protease-activated receptors (PARs)
are a unique class of GPCRs involved in cancer that can transmit
signals to extracellular proteases. Thrombin acts on PAR1, 2 and 4,
and has been shown to affect cancer progression via activation of
the PAR pathway (53,54). Most lysophosphatidic acid receptors
(LPARs) are GPCRs and several studies have shown that the
activation of the LPAR signaling axis is involved in cell
proliferation and invasion in several types of cancer (55–58).
Recent studies have shown that activation of GPCR30 (GPR30) results
in cancer cell growth, including in breast cancer-associated
fibroblasts, thyroid cancer cells, ovarian cancer cells,
endometrial cancer cells and renal cell cancer cells (59). It is currently unknown whether these
cancer-associated GPCRs are dependent on endosome-to-plasma
membrane recycling via the SXN27-dependent pathway, but it is
hypothesized that these cancer-associated GPCRs may undergo
SNX27-mediated recycling and trafficking to the membrane as naïve
receptors, which is hypothesized to enhance cancer signaling
pathways owing to the presence of similar PDZ binding motifs
(Fig. 2).
SNX27 and cancer
SNX27, as a scaffold protein which mediates
protein-protein interaction in membrane remodeling, signaling,
intracellular trafficking, tight junctions, organelle motility and
cell movement, potentially exhibits its roles sequentially during
tumorigenesis, cancer progression and metastasis. Sharma et
al (6) investigated the
expression pattern of SNX27 in datasets obtained from The Cancer
Genome Atlas and found significantly higher levels of SNX27
expression in invasive breast tumor tissue compared with normal
breast tissue. Furthermore, the higher expression of SNX27 was
inversely correlated with patient survival. SNX27 knockdown
dramatically decreased cell motility owing to increased expression
of E-cadherin and β-catenin, which contributes to adhesion
formation and mesenchymal-epithelial transition. Studies have
further shown that SNX27 regulates matrix invasion by cancer cells
by recycling matrix metalloprotease depending on its direct
interaction (6,60). Additionally, SNX27 is involved in
regulating energy substance uptake in cancer cells via recycling
energy transport receptors. For example, due to the roles for SNX27
in glutamine uptake and amino acid-stimulated mTORC1 activation via
modulation of alanine-, serine-, cysteine-preferring transporter 2
intracellular trafficking, knockdown of SNX27 in breast cancer
cells significantly decreased cell proliferation in vitro,
inhibited tumor growth and prolonged animal survival in xenograft
nude mouse models (5,61). Additionally, via the PDZ domain,
SNX27 is able to bind to and regulate the localization and
expression of GLUT1, which facilitates the transport of glucose
across the plasma membrane to support cell growth (62). Taken together, the abundance of
SNX27 may serve important roles in tumorigenesis, cancer
progression and metastasis.
Mechanism of SNX27-dependent GPCR
recycling
At present, the mechanisms responsible for the
trafficking of internalized GPCRs are not well understood. PTHR, a
retromer, is a component of the endosomal sorting complex actin/SNX
27/retromer tubule (ASRT), regulates the sustained generation of
cAMP triggered by the internalization of PTHR and results in the
movement of internalized receptors from endosomes to the Golgi
apparatus (63). In general,
internalized receptors do not exert any functions following
termination of endosomal signaling. To maintain quantitative
receptor homeostasis, inactive receptors undergo two definite modes
of postendocytic sorting: First, transfer into the lysosome for
degradation and downregulation of receptors, and second, recycling
from the endosome to the membrane in an ASRT-dependent manner or
via the TGN to the Golgi and then back to the cell surface in an
ASRT-independent manner (4,64,65).
In the second mode, recycled GPCRs on the cell surface, which act
as naïve receptors, may be directly or indirectly ready to receive
another stimulus (66).
Accordingly, it is critical to comprehensively
understand the molecular basis of GPCR recycling as it may
contribute to several receptor-associated diseases. As the
SNX27/retromer recycling pathway occurs in multiple tissues,
particularly in neurons, the loss of SNX27 contributes to several
neurological diseases, such as Alzheimer's disease, Parkinson's
disease, Down syndrome, epilepsy and cancer (67–70).
Low expression of SNX27 also reduces the membrane levels of β2-AR,
NMDARs and AMPARs, resulting in relevant disorders (71,72).
Although transfection of SNX27 small interfering (si)RNA does not
inhibit the recycling of FLAG-tagged β1-AR in HEK293 cells
(22,73), selective depletion of SNX27 reduces
recycling of the most relevant GPCRs and results in the subsequent
downregulation of membrane receptors. Emerging evidence suggests
that SNX27 interacts with a multitude of proteins and forms the
ASRT complex to perform GPCR recycling from the endosome to the
cell membrane (4). Receptors such
as β-2 AR contain a PDZ ligand at the C-terminus called the
PDZ-binding-motif (PBM), which can interact with SNX27 and
subsequently control the recycling process (74,75).
The PDZ domain of SNX27 binds to PBM as a cargo selector, while two
Bin-Amphiphysin-Rvs domains interact with retromer, which consists
of the vacuolar protein sorting (Vps) proteins Vps26-Vps29-Vps35,
constituting the ASRT complex (2).
Interaction analysis between GFP-tagged Vps26 and SNX27 indicates
that SNX27 directly interacts with retromer via Vps26. The PX and
FERM domains of SNX27 are hypothesized to be involved in recruiting
the ASRT complex to the Wiskott-Aldrich syndrome protein and SCAR
homologue complex, which activates Arp2/3-mediated actin
polymerization on endosomes (62,76).
These findings strongly support the relevant mechanism that SNX27,
as a cargo selector, serves important roles in recycling GPCRs from
endosomes to the plasma membrane.
Concluding remarks and future
perspectives
Several GPCRs have been demonstrated to undergo
recycling in a SNX27-dependent manner and are suggested to
critically regulate cancer progression and development. GPCRs have
been used as potential targets for cancer treatment. Several
humanized monoclonal antibody drugs, such as mogamulizumab, which
targets CCR4 have been approved by FDA to treat T-cell lymphoma.
Several small molecules, such as plerixafor, which targets CXCR4,
brigatinib which targets EGFR have been used to treat myeloma and
lung cancer patients. In addition, several known GPCR-targeted
drugs such as β-blockers, are reported to contribute to improvement
in the prognosis of numerous cancers, which is currently in phase
II clinical trials (17).
Additionally, since the PDZ domain is involved in protein-protein
interactions and abnormal intracellular signaling, small molecule
drugs, including intrabodies, peptides and siRNA, have been used to
block the interaction between proteins and PDZ domains for cancer
treatment (77). The specific
small-molecule inhibitor compound 3289–8625 strongly binds the
disheveled PDZ domain and effectively blocks Wnt/β-catenin
signaling, which impacts the growth rate of prostate cancer cells
(78). The cell-permeable
lipopeptide CR1166 blocks the PDZ domain of GIPC, and prevents
pancreatic and breast cancer development (79). The peptide PSD95, which binds to
syntenin tandem PDZ domains (PDZ1 and PDZ2) with high affinity,
significantly inhibits cancer cell proliferation, migration and
invasion (80).
Recent research has indicated that a specific
small-molecule inhibitor that targets the PDZ1 domain of
MDA-9/Syntenin (SDCBP) reduces prostate cancer cell invasion,
migration and metastasis, thus exhibiting significant therapeutic
potential (81). Additionally, the
association of SNX27 with retromer (VPS26) can be mechanistically
blocked by PTEN via PTEN-PDZ binding motif, which controls the
Glut1 recycling pathway and contributes to the tumor-suppresser
function of PTEN (82). Hence, the
interaction between cancer-associated proteins and SNX27 via
recognition of the PDZ binding motif can be a therapeutic drug
target for cancer treatment. Selective inhibition of the PDZ domain
can prevent cancer progression by blocking SNX27-dependent
recycling manner and reducing aberrant expression of
cancer-associated proteins at the membrane.
Thus, further research is required to identify the
universality of SNX27-dependent recycling, and whether it applies
across the GPCR superfamily and also other membrane proteins,
particularly those associated with tumorigenesis. Additionally,
understanding whether different types of cancer express elevated
levels of SNX27 and its relationship with prognosis of cancer
patients will provide sufficient evidence that SNX27 and the PDZ
binding motif are potential anticancer drug targets.
In summary, several highly expressed GPCRs on the
plasma membrane of cancer cells are primarily dependent on
SNX27-mediated endosome-to-membrane recycling, and are involved in
cancer progression. As GPCR recycling participates in cancer
progression and GPCRs are currently the most extensively
investigated drug targets in pharmaceutical studies, the targeting
of SNX27 may be of great pharmaceutical interest, as
endosome-to-plasma membrane recycling occurs in a SNX27-dependent
manner. Therefore, the discovery of compounds, antibodies or small
molecules that bind to functional SNX27 may provide novel avenues
for targeted therapy of cancer.
Acknowledgements
Not applicable.
Funding
The authors acknowledge the financial support by the
National Natural Science Foundation of China (81772909) and the
Tezhi Plan from the Organization Department of Anhui Provincial
Party Committee (2019-14).
Availability of data and materials
All information included in this Review is
documented by recent and valid references.
Authors' contributions
ZB and HZ conceptualized and co-wrote the
manuscript. ZB and SZ searched the literature, organized and wrote
various sections of the manuscript. HZ is the PI and grant holder.
All authors read and approved the final manuscript
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
On behalf of all authors, the corresponding author
states that there are no competing interests.
References
|
1
|
Gallon M and Cullen PJ: Retromer and
sorting nexins in endosomal sorting. Biochem Soc Trans. 43:33–47.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Temkin P, Lauffer B, Jäger S, Cimermancic
P, Krogan NJ and von Zastrow M: SNX27 mediates retromer tubule
entry and endosome-to-plasma membrane trafficking of signalling
receptors. Nat Cell Biol. 13:715–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clairfeuille T, Mas C, Chan AS, Yang Z,
Tello-Lafoz M, Chandra M, Widagdo J, Kerr MC, Paul B, Mérida I, et
al: A molecular code for endosomal recycling of phosphorylated
cargos by the SNX27-retromer complex. Nat Struct Mol Biol.
23:921–932. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavlos NJ and Friedman PA: GPCR signaling
and trafficking: The long and short of it. Trends Endocrinol Metab.
28:213–226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Li K, Zhang Y, Lu R, Wu S, Tang
J, Xia Y and Sun J: Deletion of sorting nexin 27 suppresses
proliferation in highly aggressive breast cancer MDA-MB-231 cells
in vitro and in vivo. BMC Cancer. 19:5552019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P, Parveen S, Shah LV, Mukherjee M,
Kalaidzidis Y, Kozielski AJ, Rosato R, Chang JC and Datta S:
SNX27-retromer assembly recycles MT1-MMP to invadopodia and
promotes breast cancer metastasis. J Cell Biol. 219:e2018120982020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bjarnadóttir TK, Gloriam DE, Hellstrand
SH, Kristiansson H, Fredriksson R and Schiöth HB: Comprehensive
repertoire and phylogenetic analysis of the G protein-coupled
receptors in human and mouse. Genomics. 88:263–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lefkowitz RJ: A brief history of G-protein
coupled receptors (Nobel Lecture). Angew Chem Int Ed Engl.
52:6366–6378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luttrell LM, Ferguson SS, Daaka Y, Miller
WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K,
Luttrell DK, et al: Beta-arrestin-dependent formation of beta2
adrenergic receptor-Src protein kinase complexes. Science.
283:655–661. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeFea KA, Zalevsky J, Thoma MS, Déry O,
Mullins RD and Bunnett NW: Beta-arrestin-dependent endocytosis of
proteinase-activated receptor 2 is required for intracellular
targeting of activated ERK1/2. J Cell Biol. 148:1267–1281. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McDonald PH, Chow CW, Miller WE, Laporte
SA, Field ME, Lin FT, Davis RJ and Lefkowitz RJ: Beta-arrestin 2: A
receptor-regulated MAPK scaffold for the activation of JNK3.
Science. 290:1574–1577. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eichel K, Jullié D and von Zastrow M:
β-Arrestin drives MAP kinase signalling from clathrin-coated
structures after GPCR dissociation. Nat Cell Biol. 18:303–310.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oakley RH, Laporte SA, Holt JA, Caron MG
and Barak LS: Differential affinities of visual arrestin, beta
arrestin1, and beta arrestin2 for G protein-coupled receptors
delineate two major classes of receptors. J Biol Chem.
275:17201–17210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomsen ARB, Plouffe B, Cahill TJ III,
Shukla AK, Tarrasch JT, Dosey AM, Kahsai AW, Strachan RT, Pani B,
Mahoney JP, et al: GPCR-G protein-β-arrestin super-complex mediates
sustained G protein signaling. Cell. 166:907–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Qiao Y and Li Z: New insights into
modes of GPCR activation. Trends Pharmacol Sci. 39:367–386. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomsen ARB, Jensen DD, Hicks GA and
Bunnett NW: Therapeutic targeting of endosomal G-protein-coupled
receptors. Trends Pharmacol Sci. 39:879–891. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nieto Gutierrez A and McDonald PH: GPCRs:
Emerging anti-cancer drug targets. Cell Signal. 41:65–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bar-Shavit R, Maoz M, Kancharla A, Nag JK,
Agranovich D, Grisaru-Granovsky S and Uziely B: G protein-coupled
receptors in cancer. Int J Mol Sci. 17:13202016. View Article : Google Scholar
|
|
19
|
Young D, Waitches G, Birchmeier C, Fasano
O and Wigler M: Isolation and characterization of a new cellular
oncogene encoding a protein with multiple potential transmembrane
domains. Cell. 45:711–719. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan AS, Clairfeuille T, Landao-Bassonga
E, Kinna G, Ng PY, Loo LS, Cheng TS, Zheng M, Hong W, Teasdale RD,
et al: Sorting nexin 27 couples PTHR trafficking to retromer for
signal regulation in osteoblasts during bone growth. Mol Biol Cell.
27:1367–1382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakagawa T and Asahi M: β1-adrenergic
receptor recycles via a membranous organelle, recycling endosome,
by binding with sorting nexin27. J Membr Biol. 246:571–579. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nooh MM, Mancarella S and Bahouth SW:
Identification of novel transplantable GPCR recycling motif for
drug discovery. Biochem Pharmacol. 120:22–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin TB, Lai CY, Hsieh MC, Wang HH, Cheng
JK, Chau YP, Chen GD and Peng HY: VPS26A-SNX27
interaction-dependent mGluR5 recycling in dorsal horn neurons
mediates neuropathic pain in rats. J Neurosci. 35:14943–14955.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balana B, Maslennikov I, Kwiatkowski W,
Stern KM, Bahima L, Choe S and Slesinger PA: Mechanism underlying
selective regulation of G protein-gated inwardly rectifying
potassium channels by the psychostimulant-sensitive sorting nexin
27. Proc Natl Acad Sci USA. 108:5831–5836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nassirpour R and Slesinger PA:
Subunit-specific regulation of Kir3 channels by sorting nexin 27.
Channels (Austin). 1:331–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng CM, Chen Z and Fu L: Frizzled
receptors as potential therapeutic targets in human cancers. Int J
Mol Sci. 19:15432018. View Article : Google Scholar
|
|
27
|
Lupp A, Klenk C, Röcken C, Evert M, Mawrin
C and Schulz S: Immunohistochemical identification of the PTHR1
parathyroid hormone receptor in normal and neoplastic human
tissues. Eur J Endocrinol. 162:979–986. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calvo N, Martin MJ, de Boland AR and
Gentili C: Involvement of ERK1/2, p38 MAPK, and PI3K/Akt signaling
pathways in the regulation of cell cycle progression by PTHrP in
colon adenocarcinoma cells. Biochem Cell Biol. 92:305–315. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boras-Granic K and Wysolmerski JJ: PTHrP
and breast cancer: More than hypercalcemia and bone metastases.
Breast Cancer Res. 14:3072012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ongkeko WM, Burton D, Kiang A, Abhold E,
Kuo SZ, Rahimy E, Yang M, Hoffman RM, Wang-Rodriguez J and Deftos
LJ: Parathyroid hormone related-protein promotes
epithelial-to-mesenchymal transition in prostate cancer. PLoS One.
9:e858032014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Coelho M, Soares-Silva C, Brandão D,
Marino F, Cosentino M and Ribeiro L: β-adrenergic modulation of
cancer cell proliferation: Available evidence and clinical
perspectives. J Cancer Res Clin Oncol. 143:275–291. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu HC, Wang C, Xie N, Zhuang Z, Liu X,
Hou J and Huang H: Activation of adrenergic receptor β2 promotes
tumor progression and epithelial mesenchymal transition in tongue
squamous cell carcinoma. Int J Mol Med. 41:147–154. 2018.PubMed/NCBI
|
|
33
|
Pu J, Zhang X, Luo H, Xu L, Lu X and Lu J:
Adrenaline promotes epithelial-to-mesenchymal transition via
HuR-TGFβ regulatory axis in pancreatic cancer cells and the
implication in cancer prognosis. Biochem Biophys Res Commun.
493:1273–1279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cole SW and Sood AK: Molecular pathways:
Beta-adrenergic signaling in cancer. Clin Cancer Res. 18:1201–1206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang D, Ma QY, Hu HT and Zhang M:
β2-adrenergic antagonists suppress pancreatic cancer cell invasion
by inhibiting CREB, NFκB and AP-1. Cancer Biol Ther. 10:19–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du J, Li XH and Li YJ: Glutamate in
peripheral organs: Biology and pharmacology. Eur J Pharmacol.
784:42–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Skerry TM and Genever PG: Glutamate
signalling in non-neuronal tissues. Trends Pharmacol Sci.
22:174–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robert SM and Sontheimer H: Glutamate
transporters in the biology of malignant gliomas. Cell Mol Life
Sci. 71:1839–1854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prickett TD and Samuels Y: Molecular
pathways: Dysregulated glutamatergic signaling pathways in cancer.
Clin Cancer Res. 18:4240–4246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Iacovelli L, Bruno V, Salvatore L,
Melchiorri D, Gradini R, Caricasole A, Barletta E, De Blasi A and
Nicoletti F: Native group-III metabotropic glutamate receptors are
coupled to the mitogen-activated protein
kinase/phosphatidylinositol-3-kinase pathways. J Neurochem.
82:216–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu B, Zhao S, Qi C, Zhao X, Liu B, Hao F
and Zhao Z: Inhibition of metabotropic glutamate receptor 5
facilitates hypoxia-induced glioma cell death. Brain Res.
1704:241–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Touhara KK and MacKinnon R: Molecular
basis of signaling specificity between GIRK channels and GPCRs.
Elife. 7:e429082018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takanami I, Inoue Y and Gika M: G-protein
inwardly rectifying potassium channel 1 (GIRK 1) gene expression
correlates with tumor progression in non-small cell lung cancer.
BMC Cancer. 4:792004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rezania S, Kammerer S, Li C,
Steinecker-Frohnwieser B, Gorischek A, DeVaney TT, Verheyen S,
Passegger CA, Tabrizi-Wizsy NG, Hackl H, et al: Overexpression of
KCNJ3 gene splice variants affects vital parameters of the
malignant breast cancer cell line MCF-7 in an opposing manner. BMC
Cancer. 16:6282016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Plummer HK III, Dhar MS, Cekanova M and
Schuller HM: Expression of G-protein inwardly rectifying potassium
channels (GIRKs) in lung cancer cell lines. BMC Cancer. 5:1042005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Munoz MB and Slesinger PA: Sorting nexin
27 regulation of G protein-gated inwardly rectifying K(+) channels
attenuates in vivo cocaine response. Neuron. 82:659–669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Katanaev VL: The Wnt/Frizzled GPCR
signaling pathway. Biochemistry (Mosc). 75:1428–1434. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chakravarthi BVSK, Chandrashekar DS,
Hodigere Balasubramanya SA, Robinson AD, Carskadon S, Rao U,
Gordetsky J, Manne U, Netto GJ, Sudarshan S, et al: Wnt receptor
Frizzled 8 is a target of ERG in prostate cancer. Prostate.
78:1311–1320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun L, Hu X, Chen W, He W, Zhang Z and
Wang T: Sorting nexin 27 interacts with Fzd7 and mediates Wnt
signalling. Biosci Rep. 36:e002962016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu J, Li L, Liu J, Wang Y, Wang Z, Wang Y,
Liu W, Zhou Z, Chen C, Liu R and Yang R: CC chemokine receptor 7
promotes triple-negative breast cancer growth and metastasis. Acta
Biochim Biophys Sin (Shanghai). 50:835–842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin HY, Sun SM, Lu XF, Chen PY, Chen CF,
Liang WQ and Peng CY: CCR10 activation stimulates the invasion and
migration of breast cancer cells through the ERK1/2/MMP-7 signaling
pathway. Int Immunopharmacol. 51:124–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bai M, Chen X and Ba YI: CXCL10/CXCR3
overexpression as a biomarker of poor prognosis in patients with
stage II colorectal cancer. Mol Clin Oncol. 4:23–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wojtukiewicz MZ, Hempel D, Sierko E,
Tucker SC and Honn KV: Protease-activated receptors (PARs)-biology
and role in cancer invasion and metastasis. Cancer Metastasis Rev.
34:775–796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Arakaki AKS, Pan WA and Trejo J: GPCRs in
cancer: Protease-activated receptors, endocytic adaptors and
signaling. Int J Mol Sci. 19:18862018. View Article : Google Scholar
|
|
55
|
Wang J, Sun Y, Qu JK, Yan Y, Yang Y and
Cai H: Roles of LPA receptor signaling in breast cancer. Expert Rev
Mol Diagn. 16:1103–1111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lopane C, Agosti P, Gigante I, Sabbà C and
Mazzocca A: Implications of the lysophosphatidic acid signaling
axis in liver cancer. Biochim Biophys Acta Rev Cancer.
1868:277–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ren Z, Zhang C, Ma L, Zhang X, Shi S, Tang
D, Xu J, Hu Y, Wang B, Zhang F, et al: Lysophosphatidic acid
induces the migration and invasion of SGC-7901 gastric cancer cells
through the LPA2 and Notch signaling pathways. Int J Mol Med.
44:67–78. 2019.PubMed/NCBI
|
|
58
|
Yu X, Zhang Y and Chen H: LPA receptor 1
mediates LPA-induced ovarian cancer metastasis: An in vitro and in
vivo study. BMC Cancer. 16:8462016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Feldman RD and Limbird LE: GPER (GPR30): A
nongenomic receptor (GPCR) for steroid hormones with implications
for cardiovascular disease and cancer. Annu Rev Pharmacol Toxicol.
57:567–584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Noll B, Benz D, Frey Y, Meyer F, Lauinger
M, Eisler SA, Schmid S, Hordijk PL and Olayioye MA: DLC3 suppresses
MT1-MMP-dependent matrix degradation by controlling RhoB and actin
remodeling at endosomal membranes. J Cell Sci. 132:jcs2231722019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang Z, Follett J, Kerr MC, Clairfeuille
T, Chandra M, Collins BM and Teasdale RD: Sorting nexin 27 (SNX27)
regulates the trafficking and activity of the glutamine transporter
ASCT2. J Biol Chem. 293:6802–6811. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Steinberg F, Gallon M, Winfield M, Thomas
EC, Bell AJ, Heesom KJ, Tavaré JM and Cullen PJ: A global analysis
of SNX27-retromer assembly and cargo specificity reveals a function
in glucose and metal ion transport. Nat Cell Biol. 15:461–471.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Feinstein TN, Wehbi VL, Ardura JA, Wheeler
DS, Ferrandon S, Gardella TJ and Vilardaga JP: Retromer terminates
the generation of cAMP by internalized PTH receptors. Nat Chem
Biol. 7:278–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Irannejad R and von Zastrow M: GPCR
signaling along the endocytic pathway. Curr Opin Cell Biol.
27:109–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Eichel K and von Zastrow M: Subcellular
organization of GPCR signaling. Trends Pharmacol Sci. 39:200–208.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gupta MK, Mohan ML and Naga Prasad SV: G
protein-coupled receptor resensitization paradigms. Int Rev Cell
Mol Biol. 339:63–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vardarajan BN, Breusegem SY, Harbour ME,
Inzelberg R, Friedland R, St George-Hyslop P, Seaman MN and Farrer
LA: Identification of Alzheimer disease-associated variants in
genes that regulate retromer function. Neurobiol Aging.
34:2231.e15–2231.e30. 2012. View Article : Google Scholar
|
|
68
|
Tsika E, Glauser L, Moser R, Fiser A,
Daniel G, Sheerin UM, Lees A, Troncoso JC, Lewis PA, Bandopadhyay
R, et al: Parkinson's disease-linked mutations in VPS35 induce
dopaminergic neurodegeneration. Hum Mol Genet. 23:4621–4638. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang X, Zhao Y, Zhang X, Badie H, Zhou Y,
Mu Y, Loo LS, Cai L, Thompson RC, Yang B, et al: Loss of sorting
nexin 27 contributes to excitatory synaptic dysfunction by
modulating glutamate receptor recycling in Down's syndrome. Nat
Med. 19:473–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Damseh N, Danson CM, Al-Ashhab M,
Abu-Libdeh B, Gallon M, Sharma K, Yaacov B, Coulthard E, Caldwell
MA, Edvardson S, et al: A defect in the retromer accessory protein,
SNX27, manifests by infantile myoclonic epilepsy and
neurodegeneration. Neurogenetics. 16:215–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hussain NK, Diering GH, Sole J, Anggono V
and Huganir RL: Sorting Nexin 27 regulates basal and
activity-dependent trafficking of AMPARs. Proc Natl Acad Sci USA.
111:11840–11845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Choy RW, Park M, Temkin P, Herring BE,
Marley A, Nicoll RA and von Zastrow M: Retromer mediates a discrete
route of local membrane delivery to dendrites. Neuron. 82:55–62.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
McGarvey JC, Xiao K, Bowman SL, Mamonova
T, Zhang Q, Bisello A, Sneddon WB, Ardura JA, Jean-Alphonse F,
Vilardaga JP, et al: Actin-sorting nexin 27 (SNX27)-retromer
complex mediates rapid parathyroid hormone receptor recycling. J
Biol Chem. 291:10986–11002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lauffer BE, Melero C, Temkin P, Lei C,
Hong W, Kortemme T and von Zastrow M: SNX27 mediates PDZ-directed
sorting from endosomes to the plasma membrane. J Cell Biol.
190:565–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rincón E, Santos T, Avila-Flores A, Albar
JP, Lalioti V, Lei C, Hong W and Mérida I: Proteomics
identification of sorting nexin 27 as a diacylglycerol kinase
zeta-associated protein: New diacylglycerol kinase roles in
endocytic recycling. Mol Cell Proteomics. 6:1073–1087. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Seaman MN, Gautreau A and Billadeau DD:
Retromer-mediated endosomal protein sorting: All WASHed up! Trends
Cell Biol. 23:522–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dev KK: Making protein interactions
druggable: Targeting PDZ domains. Nat Rev Drug Discov. 3:1047–1056.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Grandy D, Shan J, Zhang X, Rao S, Akunuru
S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, et al: Discovery
and characterization of a small molecule inhibitor of the PDZ
domain of dishevelled. J Biol Chem. 284:16256–16263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Patra CR, Rupasinghe CN, Dutta SK,
Bhattacharya S, Wang E, Spaller MR and Mukhopadhyay D: Chemically
modified peptides targeting the PDZ domain of GIPC as a therapeutic
approach for cancer. ACS Chem Biol. 7:770–779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu J, Qu J, Zhou W, Huang Y, Jia L, Huang
X, Qian Z, Xia J and Yu Y: Syntenin-targeted peptide blocker
inhibits progression of cancer cells. Eur J Med Chem. 154:354–366.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Das SK, Kegelman TP, Pradhan AK, Shen XN,
Bhoopathi P, Talukdar S, Maji S, Sarkar D, Emdad L and Fisher PB:
Suppression of prostate cancer pathogenesis using an MDA-9/Syntenin
(SDCBP) PDZ1 small-molecule inhibitor. Mol Cancer Ther.
18:1997–2007. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shinde SR and Maddika S: PTEN regulates
glucose transporter recycling by impairing SNX27 retromer assembly.
Cell Rep. 21:1655–1666. 2017. View Article : Google Scholar : PubMed/NCBI
|