Introduction
Lymphoma is one of the most common hematological
malignancies (1). Despite it being
a potentially curable disease, the survival rate of lymphoma
depends on multiple factors (1).
Burkitt lymphoma (BL), a B-cell tumor subtype, is characterized by
aggressive proliferation, and patients with BL who have a poor
survival prognosis require intensive chemotherapy (2). Thus far, no effective targeted drug
has been approved for the treatment of BL (3). Therefore, most studies have focused on
identifying effective targets for the treatment of BL.
Sperm-associated antigen 6 (SPAG6) was first
detected in human testicular tissue (4). Its main function is to participate in
the maturation of germ cells and maintain sperm viability and
fertility (5,6). Recent studies have identified SPAG6 as
a new cancer-testis antigen, and it is considered as a tumor marker
and a potential drug candidate for the treatment of solid tumors,
such as lung and breast cancer (7,8). In
contrast to normal marrow, 7 genes, including Wilms' tumor 1
(WT1) and SPAG6, were found to be significantly
upregulated in patients with acute myeloid leukemia (AML) through
comprehensive whole-genome sequencing and gene expression analysis
(9). In addition, a recent study
demonstrated that SPAG6 was overexpressed in patients with
myelodysplastic syndromes (MDSs) (10). Yang et al reported that the
growth of malignant bone marrow cells SKM-1 and K562 was
significantly inhibited after silencing SPAG6 expression (11). However, the roles and underlying
molecular mechanisms of action in SPAG6 in other hematological
malignancies, particularly lymphomas, have been less extensively
investigated.
The present study examined whether there is a
correlation between the expression of SPAG6 and the prognosis of
patients with lymphoma via analysis of The Cancer Genome Atlas
(TCGA) database, in the hope of elucidating the role of SPAG6 in
the occurrence and development of BL and exploring its molecular
mechanisms of action, thereby identifying new targets for the
clinical treatment of BL.
Materials and methods
Survival of BL patients in the TCGA
database
The preprocessed level 3 RNA-seq data and
corresponding clinical information of lymphoma patients were
collected from TCGA database (http://cancergenome.nih.gov/). Data from TCGA were
downloaded to integrate the expression of SPAG6 and survival data.
Survival analysis was performed with the survival R package and
using Kaplan-Meier analysis (log-rank test) (12).
Cell culture
All the cell lines were purchased from Nanjing Kaiji
Bio-tech Co., Ltd. The human normal peripheral-blood B-lymphocyte
(IM-9) and four BL cell lines (CA46, NAMALWA, Daudi and Raji) were
cultured in RPMI-1640 medium (HyClone; Cytiva) supplemented with
10% FBS (HyClone; Cytiva) and 1% penicillin and streptomycin
(Beyotime Institute of Biotechnology). All cells were maintained at
37°C in 5% CO2 during all experiments.
Transfection and stable cell
lines
SPAG6 shRNA and scrambled control-shRNA were
purchased from Shanghai GenePharma Company. For shRNA transfection,
293T cells were grown to 30–50% confluence in 6-well culture plates
and transfected with scrambled shRNA or SPAG6 shRNA using
Lipofectamine™ LTX reagent with PLUS™ reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) for 48 h. Then, the supernatant from 293T
cells was harvested and added to Daudi and Raji cells for 72 h,
followed by selection with 1 µg/ml puromycin for 1 month. Plasmids
(pcDNA3.1-SPAG6 and pcDNA3.1) were purchased from Obio Technology
Co., Ltd. For plasmid transfection, CA46 and NAMALWA cells (70%
confluence) were plated in 6-well culture plates. Plasmids were
purified and transfected with pcDNA3.1-SPAG6 or pcDNA3.1 for 72 h
using Lipofectamine™ LTX reagent with PLUS™ reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), followed by selection with 1 µg/ml
puromycin for 1 month.
Cell proliferation
Cell proliferation was tested with the Cell Counting
Kit-8 (CCK-8) Assay (Dojindo Molecular Technologies, Inc.).
Briefly, cells (2,000–5,000) were seeded in 96-well plates. After
incubation for 24, 48, 72 and 96 h, 10 µl CCK-8 reagent was added
into each well of the 96-well plates. After incubation with the
CCK-8 solution for 1 h, the final optical density (OD) values at
450 nm were calculated.
Flow cytometry assay
Daudi and Raji cells (2×105 cells/well)
were cultured in 6-well culture plates. After incubation, the cells
were washed with PBS and centrifuged at 500 × g for 5 min at 4°C.
Then, the cell suspension was re-suspended with binding buffer,
followed by the addition of 5 µl Annexin V and 5 µl propidium
iodide (PI) (Invitrogen; Thermo Fisher Scientific, Inc.) for 15 min
in the dark. Cells subsequently were counted by a flow cytometer
(BD Biosciences Inc.).
Reverse transcription-quantitative PCR
(RT-PCR) analysis
Cells were harvested and total RNA was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA (1 µg) was reverse-transcribed at 37°C for
15 min and 85°C for 5 sec using the PrimeScript Reverse
Transcriptase system (Takara Biotechnology Co., Ltd.). qPCR
amplification was performed using SYBR Green Master Mix (Takara
Biotechnology Co., Ltd.) under the following conditions: 94°C for 1
min, 30 cycles at 94°C for 30 sec, 50°C for 30 sec, 72°C for 45
sec, 72°C for 5 min. The relative expression of SPAG6 was
calculated using the 2−ΔΔCq method. Primers for PCR
included: SPAG6, 5′-GACGTGGTCCCAACAATTCAA-3′ (forward) and
5′-ACAGCTTCTGCTAGGTCATCAT-3′ (reverse); GAPDH,
5′-TGACTTCAACAGCGACACCCA-3′ (forward) and 5′-ACCCTGTTGCTGTAGCCAAA-3
(reverse).
Western blot assay
Cells were harvested and lysed using RIPA lysis
buffer (Beyotime Institute of Biotechnology) with protease
inhibitors (Thermo Fisher Scientific, Inc.). The protein
concentration was determined by a BCA kit (Thermo Fisher
Scientific, Inc.). For western blotting, samples were
electrophoresed on 8–12% acrylamide gradient Tris-Tricine Ready
Gels and transferred to PVDF membranes (EMD Millipore). Then, the
PVDF membranes were blocked with 5% milk for 1 h at room
temperature and incubated with the primary antibodies anti-SPAG6
(Abcam, ab155653, 1:1,000 dilution), anti-β-actin (Sigma-Aldrich;
Merck KGaA, A5441, 1:5,000 dilution), anti-Bcl-2 [Cell Signaling
Technology, Inc. (CST), cat. no. 3498, 1:1,000 dilution], anti-Bax
(CST, cat. no. 5023, 1:1,000 dilution), anti-caspase-8 (CST, cat.
no. 4790, 1:1,000 dilution), anti-cleaved-caspase-8 (CST, cat. no.
9748, 1:1,000 dilution), anti-poly(ADP-ribose) polymerase (PARP;
CST, cat. no. 9542, 1:1,000 dilution), anti-cleaved-PARP (CST, cat.
no. 52873, 1:1,000 dilution), anti-caspase-3 (CST, cat. no. 14220,
1:1,000 dilution), anti-cleaved-caspase-3 (CST, cat. no. 9664,
1:1,000 dilution), anti- phosphatase and tensin homolog (PTEN; CST,
cat. no. 9188, 1:1,000 dilution), anti-AKT (CST, cat. no. 4685,
1:1,000 dilution) and anti-p-AKT (CST, cat. no. 4060, 1:1,000
dilution) overnight at 4°C, followed by incubation with secondary
antibodies (CST, cat. no. 7074, 1:5,000 dilution) at room
temperature for 2 h.
Animal experiments
Four-week-old male severe combined immunodeficiency
(SCID) mice were obtained from Beijing Vital River Laboratory
Animal Technology Co., Ltd. SPAG6 stable knockdown or control Raji
cell lines (5×106 cells in 50 µl DMEM) were
subcutaneously injected into the left axilla of 15 SCID mice. When
the tumors reached 100 mm3, the mice were randomized
into three groups: i) nc/DMSO group, intraperitoneal injection of
DMSO (50 µl daily) (n=5); ii) shSPAG6/DMSO group, intraperitoneal
injection of DMSO (50 µl daily) (n=5); and iii) shSPAG6/SF1670
group, intraperitoneal injection of SF1670 (10 µmol/kg diluted in
50 µl DMSO; n=5) (13). The length
(a) and width (b) of the tumors were calculated every 3 days and
the volume (V) was calculated using the formula
V=1/2ab2. After 3 weeks of treatment, the mice were
euthanized by CO2 asphyxiation at a flow rate of 25%
volume/min of gas displacement in a chamber for 5 min in December
of 2019. Death of all mice were confirmed before removing them from
the chamber. Then the tumors were harvested and weighed. During the
entire animal experiment, we performed this experiment following
the Ethical Guidelines of Animal Experiment version 1.0 of the
Affiliated Huaian No. 1 People's Hospital of Nanjing Medical
University released on October 28th, 2019.
Immunohistochemistry
The expression of proliferation- associated antigens
Ki67 and proliferating cell nuclear antigen (PCNA) in tissues was
measured by immunohistochemistry assay. After conventional paraffin
embedding, sectioning, dewaxing and hydration, the tumor tissues
were treated with antigen repair buffer (pH 6.0). Following
treatment with 0.3% H2O2 for 20 min, the
tumor tissue sections were blocked with 5% BSA (Sigma-Aldrich;
Merck KGaA) and incubated with primary antibodies (anti-Ki-67,
Abcam, ab15580, 1:200 dilution; anti-PCNA, Dako, M0879, 1:50
dilution) overnight at 4°C. On the following day, the tissue
sections were washed with PBS three times and incubated with
secondary antibodies at 37°C for 1 h, followed by treatment with
DAB chromogen.
TUNEL assay
TUNEL staining was performed to assess the tumor
cell apoptosis. For the TUNEL assay, tissues were fixed with 4%
formalin for 24 h at 4°C and then embedded in paraffin; TUNEL
staining was carried out using a TUNEL kit (Roche Diagnostics).
Specifically, the tumor tissue sections were deparaffinized in
xylene and hydrated through graded alcohols. The tissue slides were
blocked with PBST solution (PBS with 0.1% Triton X-100) for 20 min.
After washing with PBS three times, the slides were incubated with
TUNEL reaction mixture (5 µl of enzyme solution and 45 µl of label
solution) for 2 h in the dark at 37°C. Then, the tissue sections
were washed and incubated with DAPI (1:1,000 dilution, Beyotime
Institute of Biotechnology) for 20 min in the dark.
Statistical analysis
GraphPad Prism 7.0 (GraphPad Software, Inc.) was
used to create all graphs and perform statistical analyses. The
two-tailed Student's t-test and one-way ANOVA were used to
performed statistical analyses. P<0.05 was considered to
indicate statistically significant differences.
Results
SPAG6 is significantly upregulated in
BL cells and increased SPAG6 expression is associated with poor
prognosis
In order to examine the effects of SPAG6 and its
clinical relevance in BL patients, the association between the
levels of SPAG6 and the survival of BL patients was investigated in
the TCGA database (12). The result
indicated that higher expression of SPAG6 is associated with worse
prognosis of lymphoma patients (Fig.
1A), indicating that SPAG6 expression is closely linked to the
development and progression of lymphoma.
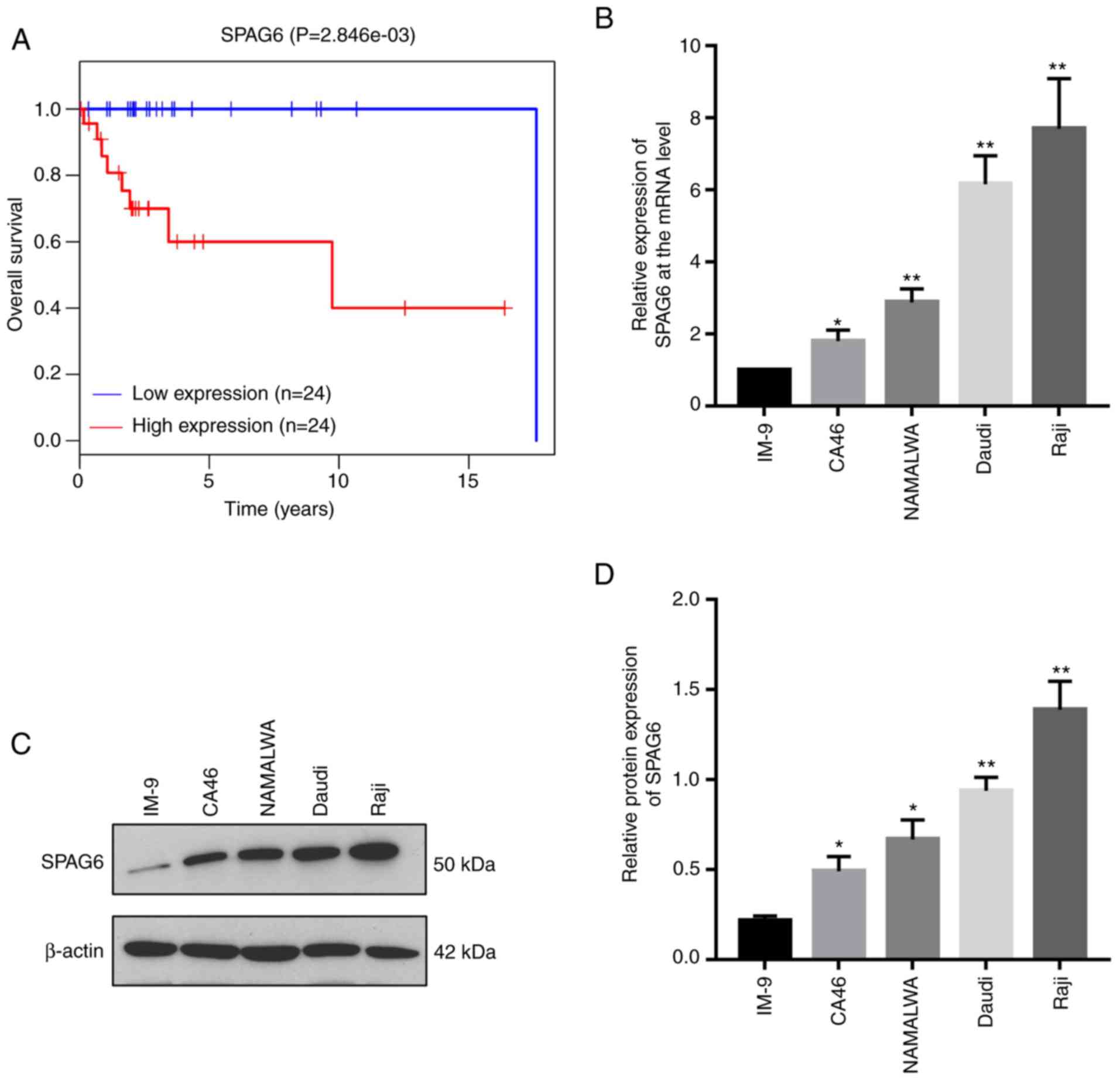 | Figure 1.Increased SPAG6 expression is
associated with the poor prognosis of patients with lymphoma and
SPAG6 is significantly upregulated in BL cells. (A) The association
between the levels of SPAG6 and the overall survival of patients
with lymphoma was examined using TCGA database. Data from TCGA were
downloaded to integrate the expression and survival data. Survival
analysis was performed with the survival R package and using
Kaplan-Meier analysis (log-rank test). (B) The mRNA levels of SPAG6
in human normal peripheral-blood B-lymphocytes (IM-9) and BL cell
lines (CA46, NAMALWA, Daudi and Raji) were determined by
quantitative PCR assay. (C and D) The protein levels of SPAG6 in
IM-9, CA46, NAMALWA, Daudi and Raji cells were determined by
western blot assay. *P<0.05, **P<0.01. The levels of SPAG6 of
CA46, NAMALWA, Daudi and Raji cells were compared with IM-9 cells.
SPAG6, sperm-associated antigen 6; BL, Burkitt lymphoma; TCGA, The
Cancer Genome Atlas. |
BL, a germinal center B-cell-derived tumor, is the
most frequently occurring non-Hodgkin lymphoma (NHL), accounting
for 40% of childhood NHLs (14,15).
Despite the fact that a high-dose combination of chemotherapy is an
effective treatment strategy for BL, a poor prognosis is
unavoidable. Furthermore, the exact mechanism underlying the
development and progression of BL remains unclear. Hence, BL was
selected as the study focus to assess the role of SPAG6. The
expression of SPAG6 was analyzed in human normal peripheral blood
B-lymphocytes (IM-9) and BL cell lines (CA46, NAMALWA, Daudi and
Raji). The RT-PCR result revealed that the mRNA levels of SPAG6 in
CA46, NAMALWA, Daudi and Raji cells were 1.80±0.26; 2.88±0.31;
6.17±0.65 and 7.70±1.14 times higher, respectively, compared with
that noted in the IM-9 cells (P<0.05; Fig. 1B). Moreover, the protein levels of
SPAG6 were significantly increased in the BL cell lines compared
with this level in the IM-9 cells (P<0.05) as detected by
western blot analysis (Fig. 1C and
D).
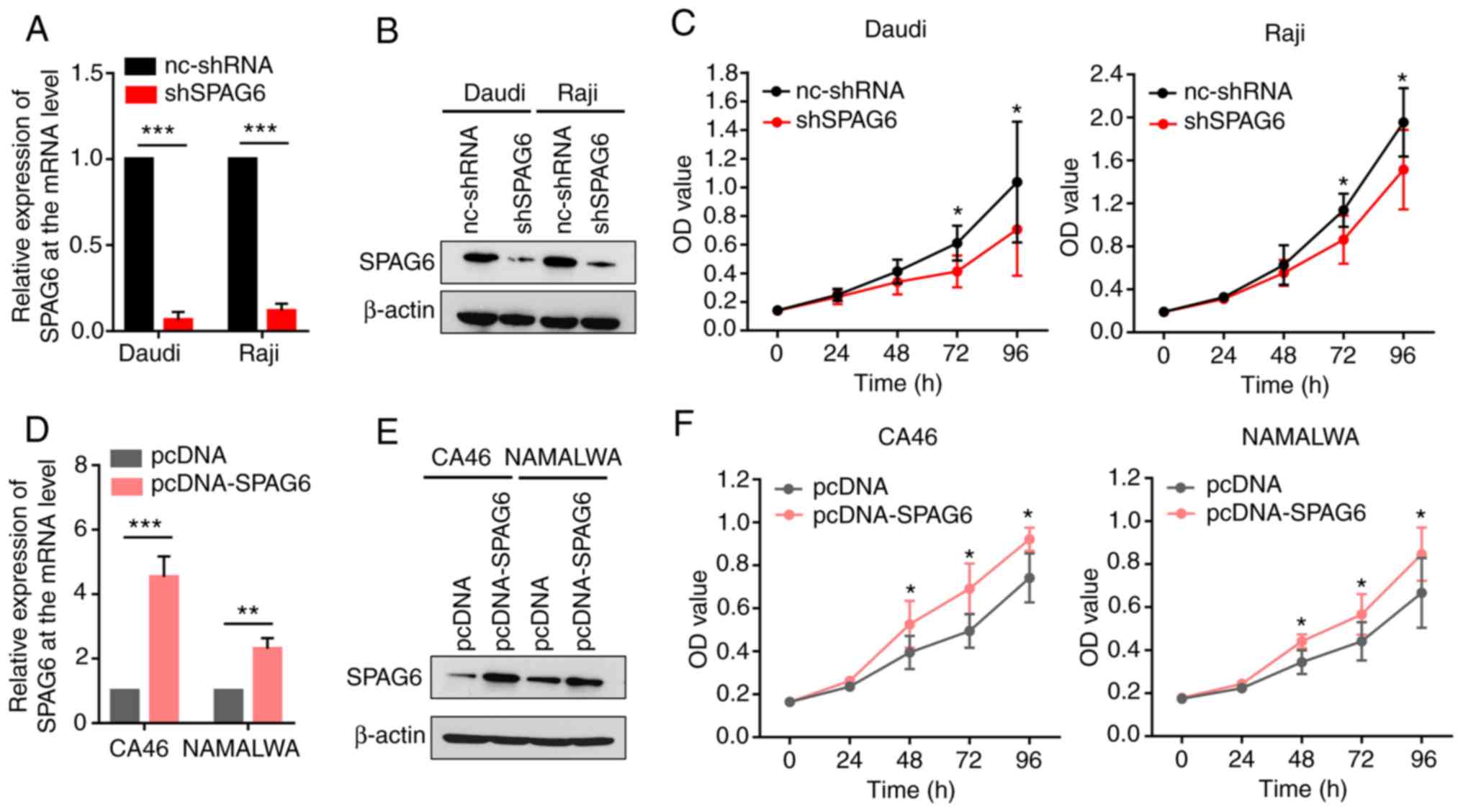
SPAG6 expression promotes the
proliferation of BL cells
As shown in Fig.
1B-D, the expression of SPAG6 was the highest in the Daudi and
Raji cells. To ascertain whether SPAG6 expression is associated
with the proliferative activity of BL cells, SPAG6 expression was
first knocked down in Daudi and Raji cells using shRNAs. The
results revealed that the expression of SPAG6 was significantly
reduced in the knockdown groups compared with the control groups in
both Daudi and Raji cells, as shown by RT-PCR and western blot
assays (Fig. 2A and B).
Furthermore, a proliferation assay was performed to determine
whether SPAG6 depletion could affect the proliferation ability of
Daudi and Raji cells. As shown in Fig.
2C, cell viability was markedly decreased at 72 h in the
shSPAG6 groups compared with the control groups, as shown by the
CCK-8 assay.
Based on the expression of SPAG6 in BL cell lines,
CA46 and NAMALWA cells were selected to construct cell lines stably
overexpressing SPAG6 (Fig. 1B-D).
Using plasmid transfection, CA46 and NAMALWA cell lines that stably
overexpressed SPAG6 were generated and the transfection efficiency
testing indicated that the levels of SPAG6 were significantly
higher in the SPAG6 overexpression groups compared with those in
the control groups, as shown by RT-PCR and western blot assays
(Fig. 2D and E). By contrast, the
CCK-8 assay revealed that overexpression of SPAG6 significantly
enhanced cell proliferation from 48 h (Fig. 2F). These results suggest that the
expression of SPAG6 is correlated with the proliferation of BL
cells.
SPAG6 expression inhibits the
apoptosis of BL cells
Abnormal apoptosis, including resistance to
apoptosis, is considered to be an important mechanism underlying
tumorigenesis (16). Previous
studies have found that, in most types of lymphoma, apoptosis is
significantly reduced and abnormal or reduced apoptosis is the main
cause behind the occurrence and development of lymphoma (17). Therefore, promoting the apoptosis of
lymphoma cells is one of the strategies employed for treating
lymphoma. We next sought to investigate whether the expression of
SPAG6 is also associated with reduced apoptosis of BL cells. The
cells were stained with Annexin V-PI solution to evaluate the
apoptosis rate by flow cytometry. Specifically, the percentage of
apoptotic cells in the blank control and SPAG6-knockdown groups of
Daudi cells was 4.62±0.28 and 12.32±2.34%, respectively (P<0.01)
(Fig. 3A). An increased apoptosis
rate was also observed in the SPAG6-silenced group compared with
the non-transfected group of Raji cells (P<0.01; Fig. 3B).
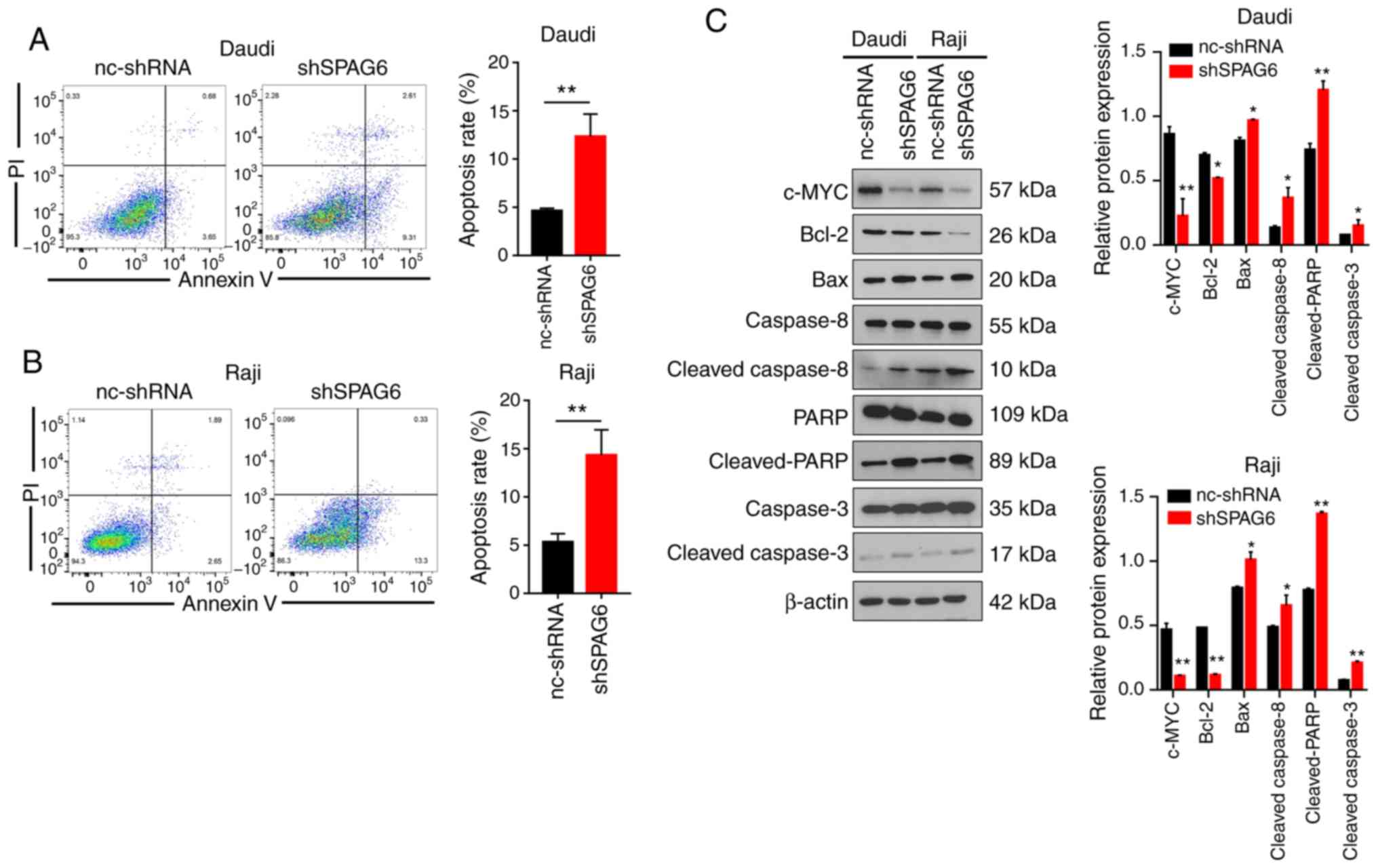 | Figure 3.SPAG6 expression inhibits the
apoptosis of BL cells. (A and B) Annexin V-PI staining was used to
detect the apoptosis rate of control groups (nc-shRNA) and SPAG6
knockdown (shSPAG6) groups by flow cytometry assay in Daudi and
Raji cells. (C) The levels of apoptosis-related proteins (c-MYC,
Bcl-2, Bax, caspase-8, cleaved-caspase-8, caspase-3,
cleaved-caspase-3, PARP and cleaved-PARP) were examined by western
blot analysis after knockdown of SPAG6 in Daudi and Raji cells.
*P<0.05, **P<0.01. The relative expression of
apoptosis-related proteins of shSPAG6 group was compared with the
nc-shRNA group. SPAG6, sperm-associated antigen 6; BL, Burkitt
lymphoma; PARP, poly(ADP-ribose) polymerase. |
c-MYC is an established indicator of BL (18), and it also plays an important role
in numerous biological processes, including cell growth and
proliferation, cell cycle progression and apoptosis (19). The expression of c-MYC was reduced
after the knockdown of SPAG6 in Daudi and Raji cells, as shown by
western blot analysis (Fig. 3C). In
addition, the western blot results demonstrated that the expression
of Bcl-2 (a well-known anti-apoptotic protein) in the
SPAG6-knockdown group of Daudi and Raji cells was significantly
downregulated, while the expression of Bax (a well-known
pro-apoptotic protein) was markedly enhanced (Fig. 3C). Moreover, the protein levels of
cleaved-caspase-8, cleaved-caspase-3 and cleaved-PARP were
obviously increased in the SPAG6-knockdown groups compared with the
control groups in Daudi and Raji cells (Fig. 3C). In summary, the aforementioned
data indicate that SPAG6 expression is correlated with the
apoptosis of BL cells.
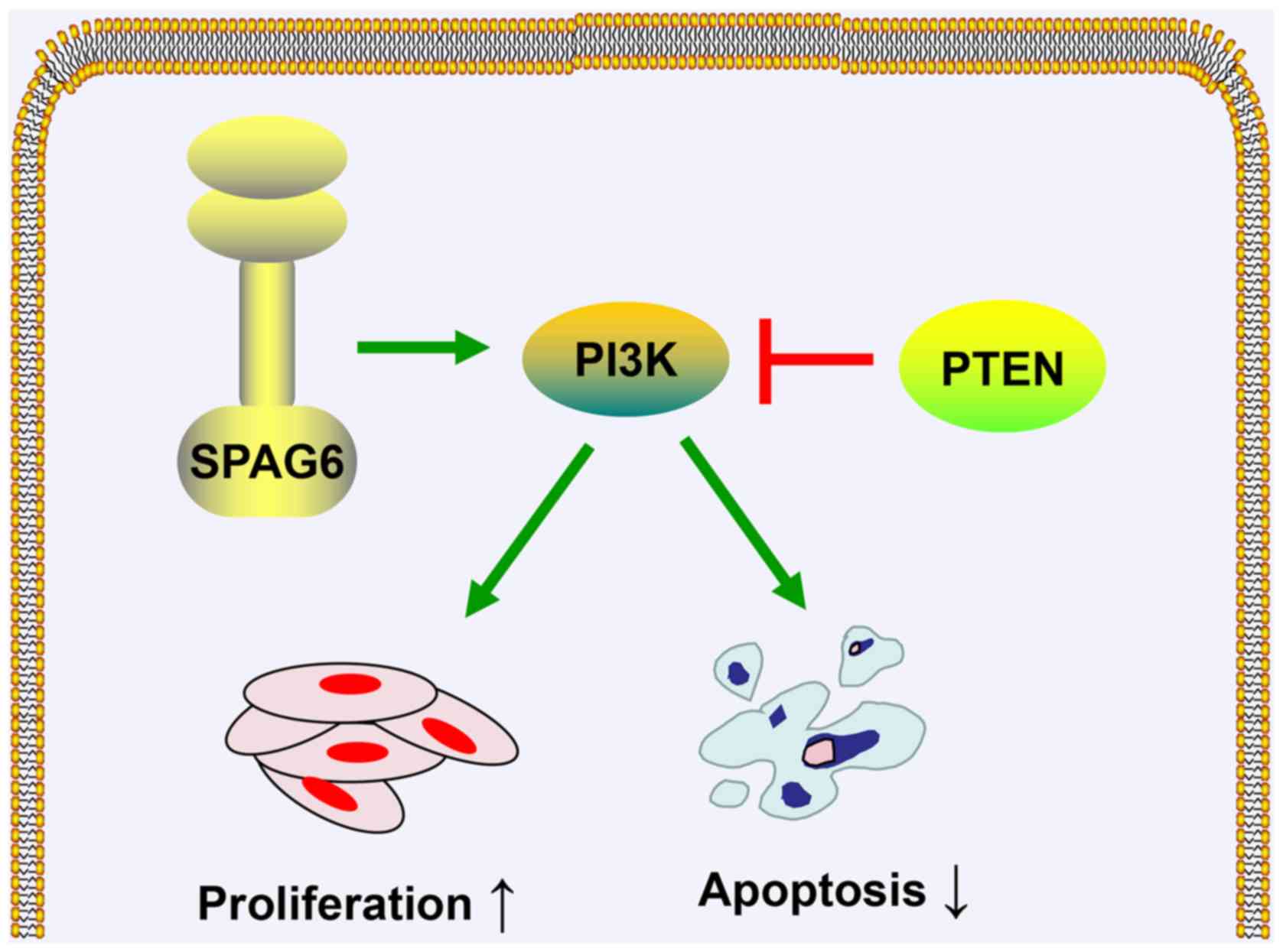
SPAG6 promotes proliferation of BL
cells through the PTEN/phosphoinositide 3-kinase (PI3K) protein
kinase B (AKT) pathway
The PTEN/PI3K/AKT signaling pathway plays a key role
in the development of various cancers and the regulation of
essential tumor cell functions (20). In particular, PTEN, a tumor
suppressor, can negatively regulate the PI3K/AKT signaling pathway
(21). To confirm whether the
effects of SPAG6 on the viability of BL cells were mediated via the
modulation of the PI3K/AKT pathway, AKT, p-AKT and PTEN were tested
in SPAG6-depleted or SPAG6-overexpressed cells by western blotting.
The experimental results uncovered that the level of PTEN protein
was significantly increased, while p-AKT protein was markedly
reduced in the SPAG6-knockdown group (shSPAG6) compared with the
blank control group (nc-shSPAG6) in Daudi and Raji cells, as shown
by western blot analysis (Fig. 4A).
Conversely, the expression of PTEN was obviously decreased and the
protein levels of p-AKT was significantly increased in the
SPAG6-overexpression group (pcDNA-SPAG6) compared with the control
group (pcDNA) in CA46 and NAMALWA cells (Fig. 4E). We further explored whether the
PI3K/PTEN/AKT pathway is implicated in the SPAG6-mediated
enhancement of cell proliferation. For that purpose, PTEN was
knocked down by siRNAs in Daudi and Raji cells (Fig. 4B). Interestingly, silencing of SPAG6
suppressed the proliferation of Daudi and Raji cells, whereas PTEN
knockdown (siPTEN) rescued the tumor-inhibiting effect of SPAG6
depletion (Fig. 4C). Similarly,
SF1670, a specific PTEN inhibitor, also reversed the
anti-proliferative effect exerted by SPAG6 knockdown (Fig. 4D).
 | Figure 4.SPAG6 promotes proliferation of BL
cells through the PTEN/PI3K/AKT pathway. (A) The levels of PI3K/AKT
pathway-related proteins (AKT, p-AKT and PTEN) were examined in
SPAG6-depleted (shSPAG6) or control (nc-shRNA) Daudi and Raji cells
by western blot analysis. (B) Stable SPAG6-knockdown Daudi and Raji
cells were transfected with PTEN siRNA (siPTEN) and the knockdown
efficiency was confirmed by western blot assay. (C) Cell viability
in the nc-shRNA, shSPAG6 and shSPAG6/siPTEN groups was examined
using the CCK-8 assay. (D) Cells were treated with DMSO or SF1670
(10 µM) for 24 h and cell viability in the nc-shRNA/DMSO,
shSPAG6/DMSO and shSPAG6/SF1670 groups was examined using the CCK-8
assay. (E) The levels of PI3K/AKT pathway-related proteins (AKT,
p-AKT and PTEN) were examined in SPAG6-overexpressing (pcDNA-SPAG6)
or control (pcDNA) CA46 and NAMALWA cells by western blot analysis.
(F) Stable SPAG6-overexpression CA46 and NAMALWA cells were
transfected with PTEN overexpression plasmid (PTEN) and the
overexpression efficiency was confirmed by western blot anallysis.
(G) Cell viability in the pcDNA, pcDNA-SPAG6 and pcDNA-SPAG6/PTEN
groups was examined using the CCK-8 assay. (H) Cells were treated
with DMSO or LY294002 (20 µM) for 24 h and cell viability in the
pcDNA/DMSO, pcDNA-SPAG6/DMSO and pcDNA-SPAG6/LY294002 groups was
examined using the CCK-8 assay. *P<0.05, **P<0.01;
***P<0.001; ns, not significant. SPAG6, sperm-associated antigen
6; BL, Burkitt lymphoma; PTEN, phosphatase and tensin homolog;
PI3K, phosphoinositide 3-kinase; CCK-8, Cell Counting Kit-8. |
Next, PTEN overexpression was achieved using
plasmids in CA46 and NAMALWA cells (Fig. 4F). SPAG6 overexpression promoted the
proliferation of CA46 and NAMALWA cells, whereas PTEN
overexpression rescued the SPAG6 overexpression-mediated
tumor-promoting effect (Fig. 4G).
Furthermore, stably overexpressing SPAG6 cell lines were treated
using the PI3K-specific inhibitor LY294002, and reversal of the
proliferative effect of SPAG6 overexpression on CA46 and NAMALWA
cells was observed by inhibiting the PI3K pathway (Fig. 4H). Overall, these data indicate that
SPAG6 may promote the proliferation of BL cells via the
PTEN/PI3K/AKT pathway.
SPAG6 inhibits apoptosis of BL cells
via the PTEN/PI3K/AKT pathway
It was verified that SPAG6 induced growth promotion
in human BL cells by activating the PTEN/PI3K/Akt signaling
pathway. We further examined whether SPAG6 promotes the AKT
pathways associated with apoptosis. Raji cells had highest
expression of SPAG6 in the BL cell lines (Fig. 1C and D), so this cell line was
chosen for the subsequent apoptosis experiments. Annexin V-PI
staining was performed to detect the apoptosis rate in four groups
of Raji cells (nc-shRNA, shSPAG6, shSPAG6/siPTEN and
shSPAG6/SF1670) by flow cytometry assay. It was demonstrated that
the apoptotic rates in the four groups were 4.91±0.98, 11.34±2.31,
6.22±1.81 and 5.63±0.73, respectively (Fig. 5A). SPAG6 knockdown enhanced the
apoptosis of Raji cells, while PTEN knockdown by siRNA or inhibitor
reversed the apoptosis activation caused by SPAG6 depletion. The
apoptosis-related protein levels detected by western blotting
confirmed these results. As shown in Fig. 5B, the protein levels of
cleaved-caspase-3 and cleaved-PARP were obviously increase in the
SPAG6-knockdown group compared with the control group, while the
increased protein levels induced by SPAG6 knockdown were partly
reversed after silencing of PTEN. These results indicated that
SPAG6 may inhibit the apoptosis of BL cells though the
PTEN/PI3K/AKT pathway.
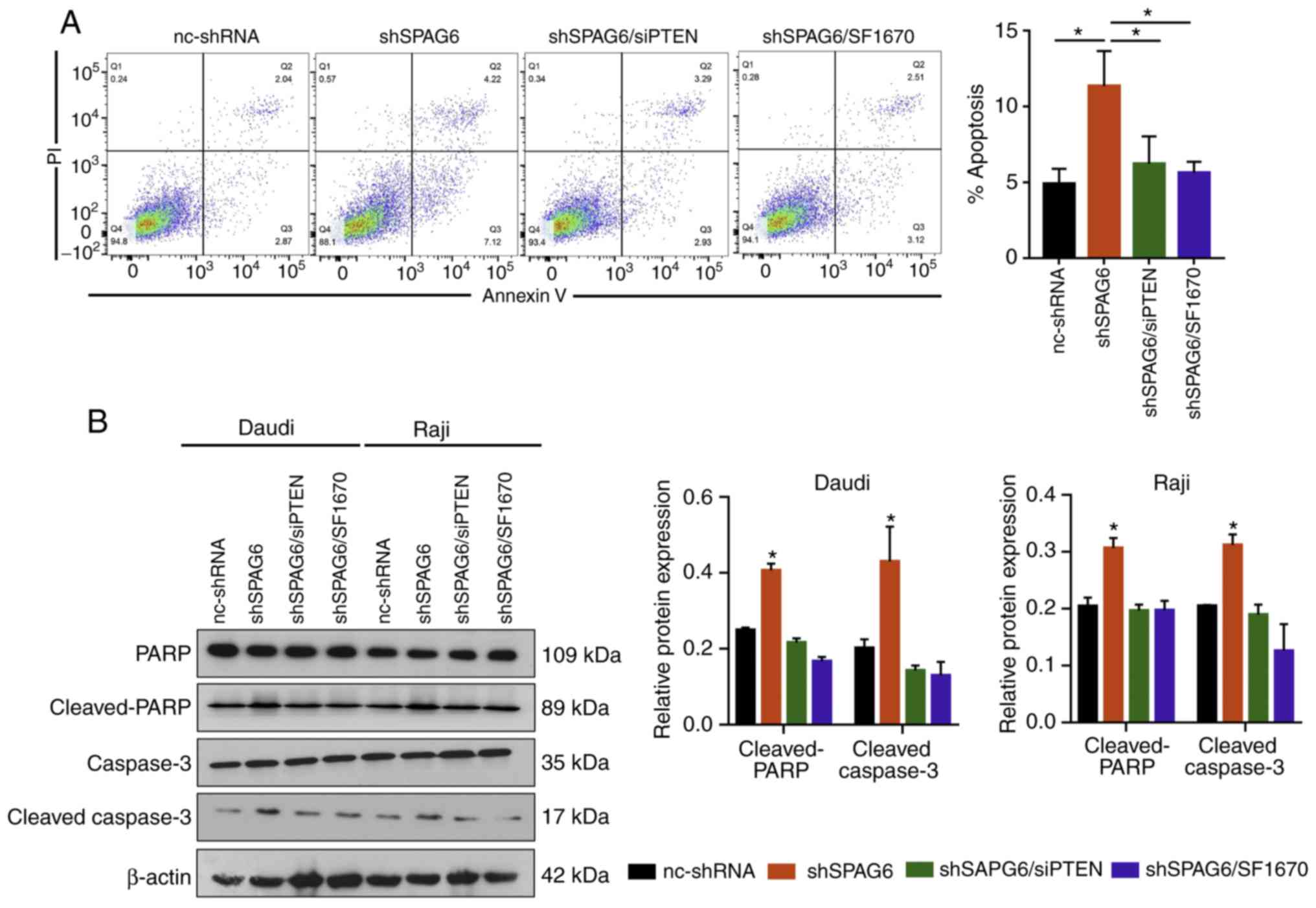 | Figure 5.SPAG6 inhibits apoptosis of BL cells
via the PTEN/PI3K/AKT pathway. (A) The apoptosis rate in the
nc-shRNA, shSPAG6, shSPAG6/siPTEN and shSPAG6/SF1670 groups was
examined using flow cytometry assay. (B) The levels of
apoptosis-related proteins (caspase-3, cleaved-caspase-3, PARP and
cleaved-PARP) were examined by western blot assay in the nc-shRNA,
shSPAG6, shSPAG6/siPTEN and shSPAG6/SF1670 groups. *P<0.05.
SPAG6, sperm- associated antigen 6; BL, Burkitt lymphoma; PARP,
poly(ADP-ribose) polymerase; PTEN, phosphatase and tensin homolog;
PI3K, phosphoinositide 3-kinase. |
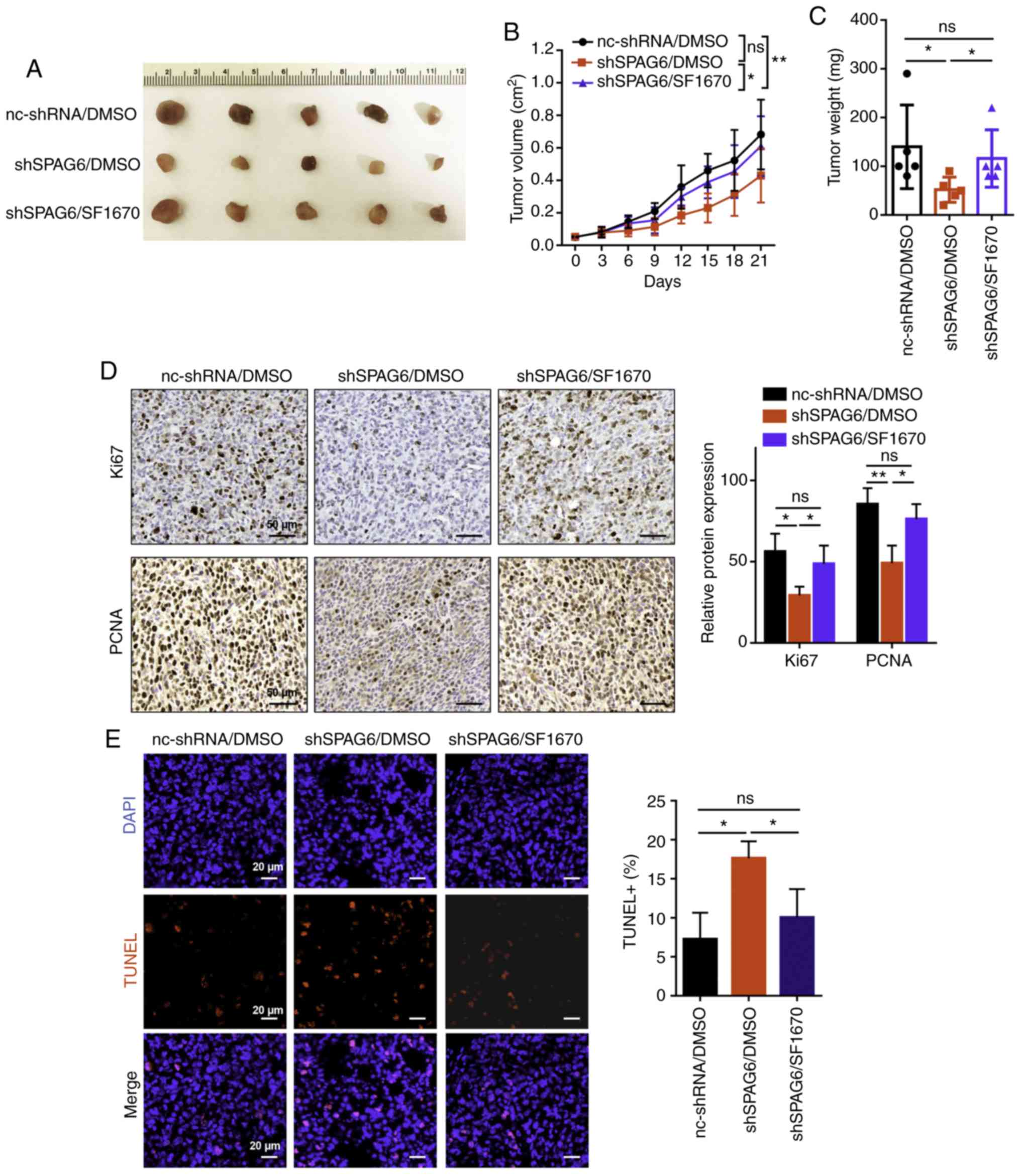
SPAG6 promotes tumor growth via the
PTEN/PI3K/AKT pathway in vivo
As mentioned above, SPAG6 was shown to promote cell
proliferation and inhibit cell apoptosis via the PTEN/PI3K/AKT
pathway in BL cells in vitro (Fig. 6). To observe whether SPAG6 exerts
functional effects on BL progression in vivo, a Raji BL cell
xenograft mouse model was established. The mice were divided into
three groups: nc-shRNA/DMSO group (injection with control cells and
treatment with DMSO); shSPAG6/DMSO group (injection with SPAG6
stable knockdown cells and treatment with DMSO); and shSPAG6/SF1670
group (injection with SPAG6 stable knockdown cells and treatment
with SF1670). It was confirmed that the tumor size and weight in
the tumor tissues from the SPAG6-knockdown (shSPAG6/DMSO) group
were smaller compared with those in the control tissues
(nc-shRNA/DMSO group) (Fig. 7A-C).
Consistently, the expression of the proliferation-related proteins
Ki67 and PCNA in BL tumor tissues was reduced following SAPG6
knockdown compared with the blank group, as demonstrated by
immunohistochemistry (Fig. 7D).
Furthermore, TUNEL assay revealed that the apoptotic cell numbers
were obviously increased in the shSPAG6/DMSO group (17.64±1.76)
compared with the nc-shRNA/DMSO group (7.27±2.77; P<0.05;
Fig. 7E). Interestingly, silencing
of PTEN using the inhibitor SF1670 (shSPAG6/SF1670 group) reversed
the tumor growth induced by SPAG6 depletion compared with the
shSPAG6/DMSO group (Fig. 7A-C).
Moreover, there was no difference in the expression of Ki67 or PCNA
between the shSPAG6/SF1670 and the nc-shRNA/DMSO groups, whereas
the expression of Ki67 and PCNA was higher in the shSPAG6/SF1670
group compared with the shSPAG6/DMSO group (Fig. 7D). In addition, PTEN knockdown in
the shSPAG6/SF1670 group (10.06±2.96) partly rescued the apoptotic
ratio mediated by SPAG6 depletion compared with the shSPAG6/DMSO
group (17.64±1.76), as shown by the TUNEL assay (Fig. 7E). Collectively, these results
indicate that SPAG6 promotes tumor growth via the PTEN/PI3K/AKT
pathway in vivo.
Discussion
Sperm-associated antigen 6 (SPAG6) is an
ortholog of chlamydia PF16, located at the chromosomal region
10p12.2 (5). SPAG6 was originally
identified in human testicular tissues and plays a role in
regulating germ cell maturation and flagellar movement (4,6).
Subsequently, it was found to be expressed in cilia-containing
tissues, such as the lung and brain, and highly expressed in the
embryonic spinal cord (22). SPAG6
also plays an important role in neuronal migration in mice
(23), as well as in the process of
neuronal proliferation and differentiation (24). Over the past decade, the amount of
research focusing on the association between SPAG6 and cancer has
markedly increased. Recent studies have demonstrated that abnormal
expression of SPAG6 is associated with the clinical outcome of
breast and lung cancer (7,8). Mulaw et al reported that SPAG6
is highly expressed at the chromosomal regions t(10;11)(p12;q14) of
CALM/AF10-positive acute myelogenous leukemia (AML) (25). Furthermore, lower expression of
SPAG6 was found to be associated with longer relapse-free survival
in patients with AML (26). These
studies suggested that SPAG6 may act as an oncogene and may be a
useful prognostic marker and a therapeutic predictor.
To the best of our knowledge, the present study was
the first to examine the prognostic value of SPAG6 in lymphoma
patients according to the TCGA database. A poor prognosis of
lymphoma was found to be significantly correlated with
overexpression of SPAG6. These data suggest that the SPAG6 may, at
least in part, contribute to the progression of lymphoma. Moreover,
high levels of SPAG6 were observed in Burkitt lymphoma (BL) cell
lines compared with those in normal human peripheral-blood
B-lymphocytes. It was next demonstrated that SPAG6 enhanced cell
proliferation and repressed cell apoptosis, in vitro as well
as in vivo.
There are several pathways through which SPAG6 can
affect cell proliferation and apoptosis. First, TRAIL is a member
of the tumor necrosis factor family and it can mediate apoptosis
(27). Li et al found that
the levels of FADD and TRAIL were increased in SPAG6-knockdown
SKM-1 cells in myelodysplastic syndromes (MDSs), suggesting that
SPAG6 may modulate TRAIL activity to regulate cell apoptosis
(28). Furthermore, modulation of
DNA methylation has been implicated in cell proliferation.
Altenberger et al reported that SPAG6 expression is
regulated by DNA hypomethylation, which may lead to the
proliferation of non-small cell lung cancer (NSCLC) cells (8). In addition, AKT is an important
mediator of the cell cycle in malignant tumors, and cell cycle
arrest is a key event in repression of cell proliferation (29). FoxO is a major downstream molecule
of the PI3K/Akt signaling pathway and was found to be primarily
regulated by AKT (30). A previous
study reported that SPAG knockdown could downregulate the
phosphorylation of AKT and FoxO, resulting in inhibition of cell
proliferation and cell cycle arrest in MDS, indicating that SPAG6
may participate in cell cycle regulation through the AKT/FoxO
pathway (31).
Phosphatase and tensin homolog (PTEN), as a negative
regulator of the PI3K/AKT pathway, has been shown to be missing or
inactivated in various tumors (20,21).
It has been demonstrated that PTEN also plays a key role in
regulating cell viability and apoptosis, not only in solid tumors,
but also in MDS and chronic myeloid leukemia cells (32–34).
It was previously demonstrated that PTEN is a key factor that may
regulate PI3K activity in BL (35).
AKT acts as a downstream factor of PI3K, and the observed
inhibition of AKT phosphorylation suggests activation of the
PI3K/AKT signaling pathway (36).
To investigate whether the effects of SPAG6 on the proliferation
and apoptosis of BL cells were associated with the activation of
the PI3K/AKT pathway, the present study demonstrated that the PTEN
protein was significantly upregulated and p-AKT protein was
significantly downregulated after the knockdown of SPAG6 expression
in Daudi and Raji cells. The results of the present study also
uncovered that silencing of SPAG6 suppressed viability and promoted
apoptosis of BL cells, whereas PTEN knockdown rescued the
tumor-inhibiting effects of SPAG6 depletion, in vitro as
well as in vivo. These results suggested that SPAG6 may
exert tumor-promoting effects on BL cells though the PTEN/PI3K/AKT
pathway.
In conclusion, the present study demonstrated that
the expression of SPAG6 was correlated with the prognosis of
patients with BL. It was also observed that SPAG6 promotes the
proliferation and inhibits the apoptosis of BL cells via the
PTEN/PI3K/AKT pathway in vitro and in vivo. These
results indicate that SPAG6 plays a key role in the development of
BL and may be of value as a predictive and prognostic biomarker in
patients with BL.
Acknowledgements
Not applicable.
Funding
This study was financially supported by Huaian Key
Laboratory of Pediatric Respiratory Diagnosis and Treatment
(HAP201607).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RZ and ZT designed the study. RZ, HZ and YY
performed the experiments. RZ and YW analyzed the data. RZ and ZT
discussed the project. RZ and ZT drafted, and HZ proofread and
revised the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal experiments conducted in the present
study were approved by the Animal Care Committee of the Affiliated
Huaian No. 1 People's Hospital of Nanjing Medical University
(approval no. DWP201900101).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jaffe ES: The 2008 WHO classification of
lymphomas: Implications for clinical practice and translational
research. Hematology Am Soc Hematol Educ Program. 523–531. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molyneux EM, Rochford R, Griffin B, Newton
R, Jackson G, Menon G, Harrison CJ, Israels T and Bailey S:
Burkitt's lymphoma. Lancet. 379:1234–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferry JA: Burkitt's lymphoma:
Clinicopathologic features and differential diagnosis. Oncologist.
11:375–383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neilson LI, Schneider PA, Van Deerlin PG,
Kiriakidou M, Driscoll DA, Pellegrini MC, Millinder S, Yamamoto KK,
French CK and Strauss JF III: cDNA cloning and characterization of
a human sperm antigen (SPAG6) with homology to the product of the
Chlamydomonas PF16 locus. Genomics. 60:272–280. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sapiro R, Kostetskii I, Olds-Clarke P,
Gerton GL, Radice GL and Strauss IJ: Male infertility, impaired
sperm motility, and hydrocephalus in mice deficient in
sperm-associated antigen 6. Mol Cell Biol. 22:6298–6305. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z, Jones BH, Tang W, Moss SB, Wei Z,
Ho C, Pollack M, Horowitz E, Bennett J, Baker ME and Strauss JF
III: Dissecting the axoneme interactome: The mammalian orthologue
of Chlamydomonas PF6 interacts with sperm-associated antigen 6, the
mammalian orthologue of Chlamydomonas PF16. Mol Cell Proteomics.
4:914–923. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lonergan KM, Chari R, Deleeuw RJ, Shadeo
A, Chi B, Tsao MS, Jones S, Marra M, Ling V, Ng R, et al:
Identification of novel lung genes in bronchial epithelium by
serial analysis of gene expression. Am J Respir Cell Mol Biol.
35:651–661. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Altenberger C, Heller G, Ziegler B,
Tomasich E, Marhold M, Topakian T, Müllauer L, Heffeter P, Lang G,
End-Pfützenreuter A, et al: SPAG6 and L1TD1 are transcriptionally
regulated by DNA methylation in non-small cell lung cancers. Mol
Cancer. 16:12017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steinbach D, Schramm A, Eggert A, Onda M,
Dawczynski K, Rump A, Pastan I, Wittig S, Pfaffendorf N, Voigt A,
et al: Identification of a set of seven genes for the monitoring of
minimal residual disease in pediatric acute myeloid leukemia. Clin
Cancer Res. 12:2434–2441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Fidler C, Nadig N, Giagounidis A,
Della Porta MG, Malcovati L, Killick S, Gattermann N, Aul C,
Boultwood J and Wainscoat JS: Genome-wide analysis of copy number
changes and loss of heterozygosity in myelodysplastic syndrome with
del(5q) using high-density single nucleotide polymorphism arrays.
Haematologica. 93:994–1000. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang B, Wang L, Luo X, Chen L, Yang Z and
Liu L: SPAG6 silencing inhibits the growth of the malignant myeloid
cell lines SKM-1 and K562 via activating p53 and caspase
activation-dependent apoptosis. Int J Oncol. 46:649–656. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Jensen MA and Zenklusen JC: A
practical guide to the cancer genome atlas (TCGA). Methods Mol
Biol. 1418:111–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Prasad A, Jia Y, Roy SG, Loison F,
Mondal S, Kocjan P, Silberstein LE, Ding S and Luo HR: Pretreatment
with phosphatase and tensin homolog deleted on chromosome 10 (PTEN)
inhibitor SF1670 augments the efficacy of granulocyte transfusion
in a clinically relevant mouse model. Blood. 117:6702–6713. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Li Y, Que X, Gao X, Gao Q, Yu M, Ma
K, Xi Y and Wang T: Prognostic significances of overexpression MYC
and/or BCL2 in R-CHOP-treated diffuse large B-cell lymphoma: A
systematic review and meta-analysis. Sci Rep. 8:62672018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rochford R and Moormann AM: Burkitt's
lymphoma. Curr Top Microbiol Immunol. 390:267–285. 2015.PubMed/NCBI
|
|
16
|
Martin GS: Cell signaling and cancer.
Cancer Cell. 4:167–174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adem J, Eray M, Eeva J, Nuutinen U and
Pelkonen J: The combination of TRAIL and MG-132 induces apoptosis
in both TRAIL-sensitive and TRAIL-resistant human follicular
lymphoma cells. Leuk Res. 66:57–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaffe ES and Pittaluga S: Aggressive
B-cell lymphomas: A review of new and old entities in the WHO
classification. Hematology Am Soc Hematol Educ Program.
2011:506–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vervoorts J, Luscher-Firzlaff J and
Luscher B: The ins and outs of MYC regulation by posttranslational
mechanisms. J Biol Chem. 281:34725–34729. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Waniczek D, Snietura M, Lorenc Z,
Nowakowska-Zajdel E and Muc-Wierzgon M: Assessment of PI3K/AKT/PTEN
signaling pathway activity in colorectal cancer using quantum dot-
conjugated antibodies. Oncol Lett. 15:1236–1240. 2018.PubMed/NCBI
|
|
21
|
Capodanno A, Camerini A, Orlandini C,
Baldini E, Resta ML, Bevilacqua G and Collecchi P: Dysregulated
PI3K/Akt/PTEN pathway is a marker of a short disease-free survival
in node- negative breast carcinoma. Hum Pathol. 40:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamada T, Teraoka M, Imaki J, Ui-Tei K,
Ladher RK and Asahara T: Gene expression of Spag6 in chick central
nervous system. Anat Histol Embryol. 39:227–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan R, Hu X, Zhang Q, Song L, Zhang M,
Zhang Y and Zhao S: Spag6 negatively regulates neuronal migration
during mouse brain development. J Mol Neurosci. 57:463–469. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu X, Yan R, Cheng X, Song L, Zhang W, Li
K and Zhao S: The function of sperm-associated antigen 6 in
neuronal proliferation and differentiation. J Mol Histol.
47:531–540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mulaw MA, Krause A, Deshpande AJ, Krause
LF, Rouhi A, La Starza R, Borkhardt A, Buske C, Mecucci C, Ludwig
WD, et al: CALM/AF10-positive leukemias show upregulation of genes
involved in chromatin assembly and DNA repair processes and of
genes adjacent to the breakpoint at 10p12. Leukemia. 26:1012–1019.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Steinbach D, Bader P, Willasch A,
Bartholomae S, Debatin KM, Zimmermann M, Creutzig U, Reinhardt D
and Gruhn B: Prospective validation of a new method of monitoring
minimal residual disease in childhood acute myelogenous leukemia.
Clin Cancer Res. 21:1353–1359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalvez F and Ashkenazi A: New insights
into apoptosis signaling by Apo2L/TRAIL. Oncogene. 29:4752–4765.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Yang B, Wang L, Chen L, Luo X and
Liu L: SPAG6 regulates cell apoptosis through the TRAIL signal
pathway in myelodysplastic syndromes. Oncol Rep. 37:2839–2846.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY
and Chiang CW: Regulation of phosphorylation of Thr-308 of Akt,
cell proliferation, and survival by the B55alpha regulatory subunit
targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol
Chem. 283:1882–1892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramezani A, Nikravesh H and Faghihloo E:
The roles of FOX proteins in virus-associated cancers. J Cell
Physiol. 234:3347–3361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang M, Chen Y, Deng L, Luo X, Wang L and
Liu L: Upregulation of SPAG6 in myelodysplastic syndrome: Knockdown
inhibits cell proliferation via AKT/FOXO Signaling Pathway. DNA
Cell Biol. 38:476–484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao L, Shan Y, Liu B, Li Y and Jia L:
Retraction note: Functional screen analysis reveals miR-3142 as
central regulator in chemoresistance and proliferation through
activation of the PTEN-AKT pathway in CML. Cell Death Dis.
11:1212020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin J, Li X, Zhang Z, Luo X, Wang L and
Liu L: SPAG6 silencing induces apoptosis in the myelodysplastic
syndrome cell line SKM1 via the PTEN/PI3K/AKT signaling pathway
in vitro and in vivo. Int J Oncol. 53:297–306.
2018.PubMed/NCBI
|
|
35
|
Gehringer F, Weissinger SE, Moller P,
Wirth T and Ushmorov A: Physiological levels of the PTEN-PI3K-AKT
axis activity are required for maintenance of Burkitt lymphoma.
Leukemia. 34:857–871. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim AH, Khursigara G, Sun X, Franke TF and
Chao MV: Akt phosphorylates and negatively regulates apoptosis
signal- regulating kinase 1. Mol Cell Biol. 21:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|