Introduction
Breast cancer is the most commonly diagnosed form of
cancer and is a main cause of cancer-associated mortality worldwide
(1). Compared with estrogen
receptor (ER)-positive breast cancers, which can be treated with
estrogen and aromatase inhibitors, ER-negative breast tumors are
associated with poorer clinical outcomes, exhibiting low survival
rates and a high probability of metastasis into multiple distal
organs (2). Treatment options for
patients with ER-negative breast cancer with metastases include
surgical resection, radiotherapy and chemotherapy, and these
treatment modalities have improved over the past decade (3). Despite these advances, ER-negative
breast cancer cases continue to account for a large proportion of
breast cancer-associated deaths (4). Therefore, safe and effective molecules
of natural origin for the treatment of ER-negative breast cancer
are urgently required.
The aryl hydrocarbon receptor (AhR) is a ligand
activated transcription factor that is ubiquitously expressed in
mammalian cells and tissues (5).
AhR was first reported as a high affinity receptor of
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; also termed dioxin),
which is involved in a number of toxicological outcomes (6). Since then, studies have focused on the
endogenous role of AhR in human cancer, including its
tumor-specific pro-oncogenic and tumor-suppressor functions that
can be targeted by AhR antagonists and agonists, respectively
(7–9). Notably, several AhR ligands have been
identified as potential antitumor agents by enhancing apoptosis, as
monitored by analyses of DNA fragmentation and caspase activation
(10,11). Cytochrome P450 family 1 subfamily A
member 1 (CYP1A1) has been reported as a prototypical marker of the
AhR-mediated cellular response to TCDD and other AhR agonists
(12).
In addition to binding exogenous molecules, AhR can
also bind endogenous biochemical or pharmaceutical molecules
(13). However, only few
AhR-targeting drugs have been clinically used, such as laquinimod
and aminoflavone (NSC686288), which have been used in the treatment
of multiple sclerosis and breast cancer, respectively (14). Therefore, discovering novel natural
AhR ligands would be a great clinical asset.
The present study examined the effects of luteolin
(3′,4′,5,7-tetrahydroxyflavone), a non-toxic naturally occurring
plant flavonoid with diverse biological activities (15,16),
on MDA-MB-231 breast cancer cells in vitro and in
vivo. The results indicated that luteolin can suppress the
viability of MDA-MB-231 breast cancer cells and reduce their
metastatic capability in vitro and in vivo. Notably,
luteolin exhibited a marked anti-metastatic effect in a xenograft
mouse model.
Materials and methods
Cell culture and MTT assay
Human cell lines with known invasive ability,
including HCT116 human colon carcinoma cells, MDA-MB-231 human
breast carcinoma cells, A549 human lung carcinoma cells, PC-3 human
prostatic carcinoma cells and ES-2 human ovarian clear cell
adenocarcinoma cells, and B16-F10 murine melanoma cells were
purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. B16-F10 cells were cultured in
DMEM-high glucose (Gibco; Thermo Fisher Scientific, Inc.) and all
other cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.). All cell cultures were supplemented with 10%
(v/v) fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.)
and 2 mM L-glutamine, and cells were incubated in a humidified
incubator with 5% CO2 at 37°C.
Cell viability was assessed using a MTT assay.
Briefly, 5,000 HCT116, MDA-MB-231, A549, PC-3 or ES-2 cells/well
were plated in a 96-well plate and incubated in a humidified
incubator with 5% CO2 at 37°C, allowed to adhere for 4
h, then treated with different concentrations of luteolin (1.562,
3.125, 6.25,12.5, 25, 50, 100, 250 an 500 mM) (Shanghai Aladdin
Bio-Chem Technology Co., Ltd.). Suberoylanilide hydroxamic acid
(SAHA) (catalog no. S1047; Selleck Chemicals) was used as a
positive control. After 48 h, 0.5% MTT solution was added to each
well and incubated for 4 h. DMSO was then added to dissolve the
formazan, and the optical density was measured at 570 nm using an
EnSpire plate reader (PerkinElmer, Inc.).
Cell cycle analysis
MDA-MB-231 cells were seeded in 6-well plates at a
density of 4×105/well. Following incubation overnight,
cells were treated with 3, 10 or 30 µM Luteolin dissolved in DMSO
solution in a humidified incubator with 5% CO2 at 37°C.
Control wells were treated with equal volumes of DMSO. Following 48
h of treatment, cells were harvested and fixed with 70% (v/v)
ethanol/phosphate buffer at 4°C overnight. The cells were washed
with PBS twice and incubated with DNase-free RNase A (Beijing
Solarbio Science & Technology, Co., Ltd.) at a concentration of
1 mg/ml for 30 min, then stained with propidium iodide (PI; 50
µg/ml; Beijing Solarbio Science & Technology, Co., Ltd.) for 30
min at room temperature in the dark. The DNA content was measured
using a flow cytometer (FACS Aria III; BD Biosciences) and analyzed
using FlowJo V10 software (FlowJo LLC).
Apoptosis analysis
Apoptosis analysis was performed by Annexin V-FITC
staining, according to the manufacturer's protocol of FITC Annexin
V Apoptosis Detection Kit I (BD Biosciences). Cells were treated as
described for the cell cycle analysis. Following treatment for 48
h, cells were harvested and washed twice with PBS, then resuspended
with binding buffer (0.01 M HEPES, pH 7.4; 0.14 M NaCl; 2.5 mM
CaCl2), and incubated with Annexin V-FITC and PI in the
dark at room temperature for 30 min. Samples were analyzed using a
flow cytometer and FlowJo V10 software (FlowJo LLC).
Cell invasion assay
The in vitro invasion assay was performed as
described previously (17).
Briefly, 50 µl Matrigel (BD Biosciences) diluted in serum-free
RPMI-1640 medium (1:19) was used to coat each Transwell invasion
chamber (8-µm pore size; Corning Inc.) for 2 h at 37°C. A total of
1×105 MDA-MB-231 cells supplemented with different
concentrations of luteolin (0, 3, 10 or 30 µM), with or without 1
µM StemRegenin 1 (SR1; AhR inhibitor; Selleck Chemicals), were
added to the upper chamber. The lower chamber was filled with
RPMI-1640 medium containing 10% fetal calf serum (Gibco; Thermo
Fisher Scientific, Inc.). The Matrigel on the upper side of the
filter was removed after 20 h of incubation, and the cells on the
bottom of the membrane were fixed with methanol and stained with 1
mg/ml crystal violet dye for 10 min at room temperature. Images of
invaded cells were obtained using a fluorescence microscope (Nikon;
magnification, ×200). The membrane was washed with 33% acetic acid,
and the eluent absorbance was measured at 590 nm using a plate
reader (EnSpire; Perkin Elmer Corporation). The data were then
analyzed with GraphPad Prism 7 (GraphPad Software, Inc.).
In vivo tumor growth assay
A total of 32 C57BL/6 mice (male; 6–8-weeks-old;
20±1 g) were purchased from Beijing HFK Bio-Technology Co., Ltd.
The animals were housed at an ambient temperature of 23±1°C,
relative humidity of 45±5%, under a 12 h light/dark cycle and
provided access to water and food ad libitum. The animals
were housed under pathogen-free conditions and were acclimated for
1 week prior to tumor implantation. The research procedures were in
accordance with the institutional guidelines of the Animal Care and
Use Committee at Shandong Analysis and Test Center, Shandong
Academy of Sciences, (Jinan, China). Previously, cultured B16-F10
melanoma cells were harvested with trypsin and resuspended in PBS
to reach the desired concentration. A total of 5×104
cells were injected via the tail vein into C57BL/6 mice. The mice
were randomized into four groups, with 8 mice per group: i) DMSO
group, melanoma cell-injected mice treated with DMSO; ii) SAHA
group, melanoma cell-injected mice treated with 40 mg/kg SAHA; iii)
luteolin-20 mg/kg group, melanoma cell-injected mice treated with
20 mg/kg luteolin; and iv) luteolin-40 mg/kg group, melanoma
cell-injected mice treated with 40 mg/kg luteolin. Mice were
treated with the desired dose of DMSO, SAHA or luteolin by
intraperitoneal injection 1 day after B16-F10 cell injection.
Animals were weighed every day throughout the study.
The mice were sacrificed at day 14, and the lungs
were extracted and washed with PBS as previously described
(18). Endpoints of the animal
experiments were discussed in the approved protocol, including
maximum tumor burden, body weight loss, major organs failure and
other severe pathological and cachexia conditions. None of the
experimental mice were identified to reach these endpoints. In
addition, a large volume of solid tumor was not established, and
the maximum tumor volume observed in the study was ~12
mm3. Briefly, mice were sacrificed by CO2;
the flow of CO2 from the gas cylinder was at a rate that
displaced 10–30% of the chamber volume/min, which was maintained
for ≥3 min. Subsequently, death was verified by checking for no
heartbeat, and cervical dislocation was performed to ensure death.
Following extraction of lungs, the number of surface tumor nodules
was counted under a dissecting microscope (magnification, ×6.7).
Sections of the lungs were also used for western blot analysis and
reverse transcription (RT)-semi-quantitative PCR (qPCR). The animal
experiments were approved by the Research Ethics Committee of
Shandong Analysis and Test Center (approval no.
ECAESDATC-2016-011).
RT-semi-qPCR
MDA-MB-231 cells were seeded in a 6-well plate
(4×105 cells/well) and incubated overnight prior to
treatment with different concentrations of luteolin (0, 3, 10 or 30
µM) for 48 h at 37°C. Cells were collected by trypsinization and
washed with PBS. In addition, mouse lung tissue samples were
homogenized using a tissue homogenizer. Total RNA was isolated from
cells and tissue samples using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and 5 mg extracted RNA was used for cDNA
synthesis using M-MLV enzyme (Invitrogen; Fisher Scientific, Inc.).
qPCR was performed with SYBR Green Master (Roche Diagnostics) in a
Roche LightCycler480 II using the following thermocycling
conditions: 95°C for 10 min; 45 cycles of 95°C for 10 sec and 60°C
for 15 sec (with single detection mode); and a final extension at
72°C for 10 min. GAPDH and β-actin were used as reference genes.
The primer sequences are presented in Table I. The qPCR products were separated
on 1% agarose gel and detected using SYBRGreen staining (Thermo
Fisher Scientific, Inc.). Densitometric analysis of bands was
performed with BandScan 5.0 software (ProZyme, Inc.).
 | Table I.Primers used for reverse
transcription-semi-quantitative PCR. |
Table I.
Primers used for reverse
transcription-semi-quantitative PCR.
| Species | Gene | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| Human | CYP1A1 |
CCATGTCGGCCACGGAGTT |
ACAGTGCCAGGTGCGGGTT |
|
| MMP-2 |
GTTCATTTGGCGGACTGT |
AGGGTGCTGGCTGAGTAG |
|
| MMP-9 |
AATCTCACCGACAGGCAGCT |
CCAAACTGGATGACGATGTC |
|
| β-actin |
TCATGAAGTGTGACGTGGACATC |
CAGGAGGAGCAATGATCTTGATC |
|
| CXCR4 |
TTCTACCCCAATGACTTGTG |
ATGTAGTAAGGCAGCCAACA |
|
| GAPDH |
GAAGGTGAAGGTCGGAGT |
CATGGGTGGAATCATATTGGAA |
|
| AhR |
ACTCCACTTCAGCCACCATC |
ATGGGACTCGGCACAATAAA |
| Mouse | MMP-2 |
GATAACCTGGATGCCGTCGTG |
CTTCACGCTCTTGAGACTTTGGTT |
|
| MMP-9 |
GCCCTGGAACTCACACGACA |
TTGGAAACTCACACGCCAGAAG |
|
| CXCR4 |
ACCTCTACAGCAGCGTTCTCA |
GGTGGCGTGGACAATAG |
|
| MITF |
CCCGTCTCTGGAAACTTGATCG |
CTGTACTCTGAGCAGCAGGTG |
|
| TYR |
CCAGAAGCCAATGCACCTAT |
ATAACAGCTCCCACCAGTGC |
|
| β-actin |
AGAGGGAAATCGTGCGTGAC |
CAATAGTGATGACCTGGCCGT |
Western blot analysis
MDA-MB-231 cells were treated with luteolin (3, 10
or 30 mM) or luteolin and SR1 (1 mM) for 24 h at 37°C, and mouse
lung tissues from different treatment groups (DMSO, SAHA, Lut-20
and Lut-40) were collected and lysed with lysis buffer (Beijing
Solarbio Science & Technology, Co., Ltd.) for 30 min. The
lysates were centrifuged at 12,000 × g for 15 min at 4°C, and the
supernatant containing protein extracts was transferred to a new
tube. The protein concentration was determined using a
bicinchoninic acid assay (Beyotime Institute of Biotechnology). A
total of 30 µg total protein was loaded per lane and resolved by
12% SDS-PAGE (a 10% gel was used for AhR) and transferred onto PVDF
membranes (catalog no. IPVH00010; EMD Millipore). The membranes
were blocked with 5% milk in PBST buffer (PBS with 0.1% Tween-20)
for 1 h at room temperature, then incubated overnight at 4°C with
1:1,000 dilutions of primary antibodies. Primary antibodies against
the following were used: MMP-2 (catalog no. sc-10736; Santa Cruz
Biotechnology, Inc.), MMP-9 (catalog no. sc-10737; Santa Cruz
Biotechnology, Inc.), C-X-C chemokine receptor type 4 (CXCR4;
sc-9046, Santa Cruz Biotechnology, Inc.), β-actin (catalog no.
ab8227; Abcam), GAPDH (catalog no; KM9002T, Tianjin Sungene Biotech
Co., Lt.) and AhR (catalog no. 13790s' Cell Signaling Technology,
Inc.). The membrane was then washed three times with PBST, then
incubated with goat-HRP-conjugated anti-mouse (catalog no. GB23301;
Wuhan Servicebio Technology Co., Ltd.) or anti-rabbit secondary
antibodies (catalog no. GB23303; Wuhan Servicebio Technology Co.,
Ltd.) (both 1:2,000) for 2 h at room temperature. Following three
washes with PBST, the membrane was developed with enhanced
chemiluminescence reagent (catalog no. WBKLS0050; EMD Millipore)
and the results were analyzed using BandScan 5.0 software (ProZyme,
Inc.).
Statistical analysis
Three biological replicates were performed for each
condition, and the results are presented as the mean ± standard
deviation. The data was analyzed with GraphPad Prism 7 (GraphPad
Software, Inc.). One-way analysis of variance followed by Tukey's
post hoc test was used for comparisons among multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Luteolin inhibits the viability of
MDA-MB-231 cells
Previous studies have demonstrated that luteolin
exhibits anti-proliferation effects with an IC50 range
of 15–50 µM in several cell lines, including 3T3-L1 and HL-60 cells
(19,20). In the present study, the effect of
luteolin on cell viability was evaluated using a MTT assay in other
cancer cell lines, including HCT116 (colorectal cancer), MDA-MB-231
(breast cancer), A549 (lung cancer), PC-3 (prostatic carcinoma),
ES-2 (ovarian carcinoma) cells. SAHA was used as positive control.
As presented in Table II, luteolin
exerted a moderate anti-proliferation effect on different cell
lines, among which MDA-MB-231 cells showed the highest sensitivity
(IC50=27.54 µM). Therefore, MDA-MB-231 cells were
selected for further experiments to further characterize the
effects of luteolin on ER-negative breast cancer cells.
 | Table II.IC50 of luteolin and SAHA
in various human cultured cell lines determined by MTT assay. |
Table II.
IC50 of luteolin and SAHA
in various human cultured cell lines determined by MTT assay.
|
| IC50,
µM |
|---|
|
|
|
|---|
| Drug | Structure | HCT116 | MDA-MB-231 | A549 | PC-3 | ES-2 |
|---|
| Luteolin |  | 43.92±3.54 | 27.54±2.05 | 122.1±10.0 | 230.0±16.7 | >500 |
| SAHA |  | 27.68±1.25 | 1.38±0.09 | 1.69±0.03 | 8.95±0.19 | 5.58±0.41 |
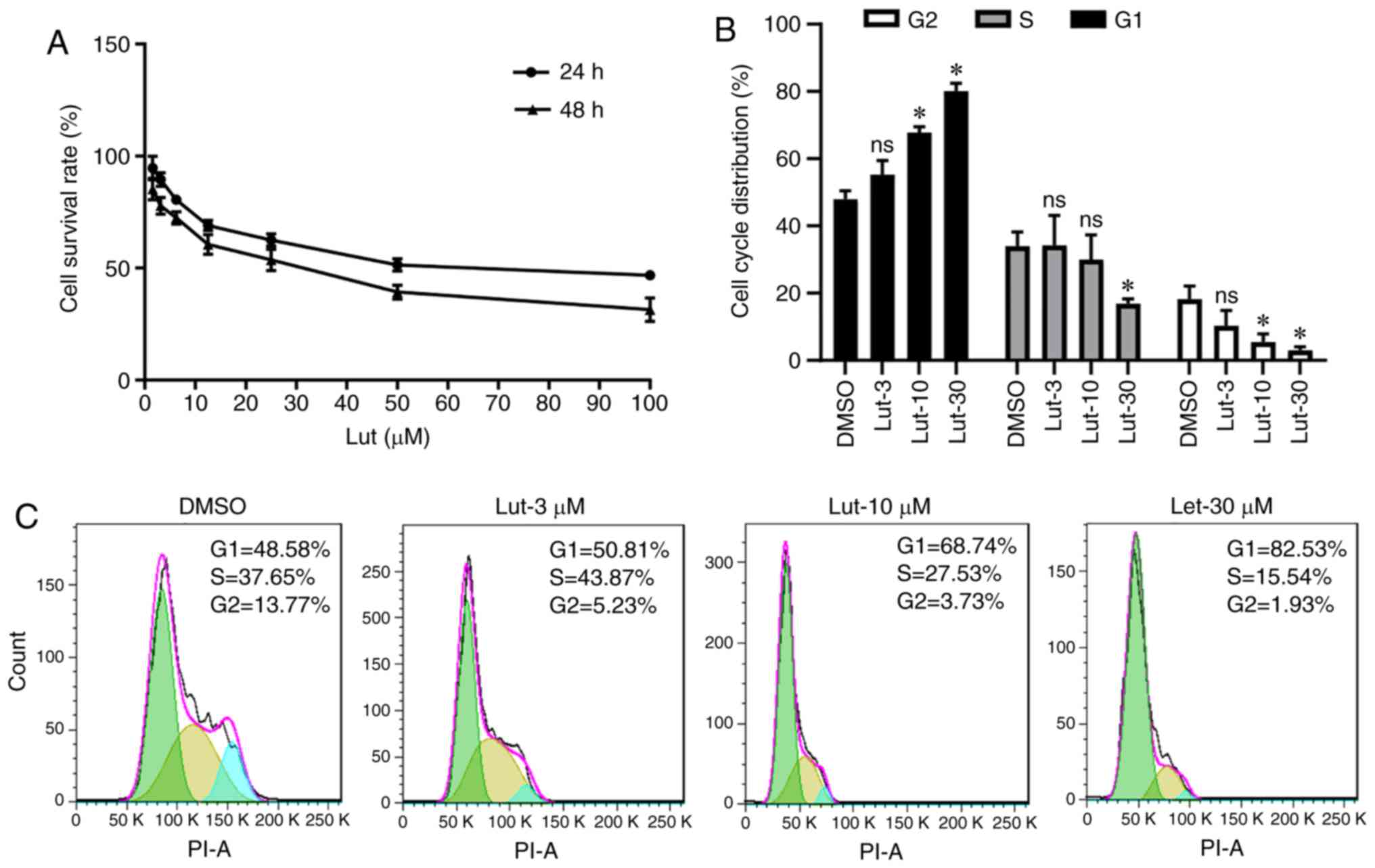
As demonstrated in Fig.
1A, increasing concentrations of luteolin inhibited the
viability of MDA-MB-231 cells after 24 and 48 h. In addition,
luteolin increased MDA-MB-231 cell cycle arrest at the G1 phase and
reduced the cell population in the S phase following treatment with
different of concentrations of luteolin (Fig. 1B and C). These results demonstrated
that luteolin can inhibit the growth of breast cancer cells through
the induction of cell cycle arrest in vitro.
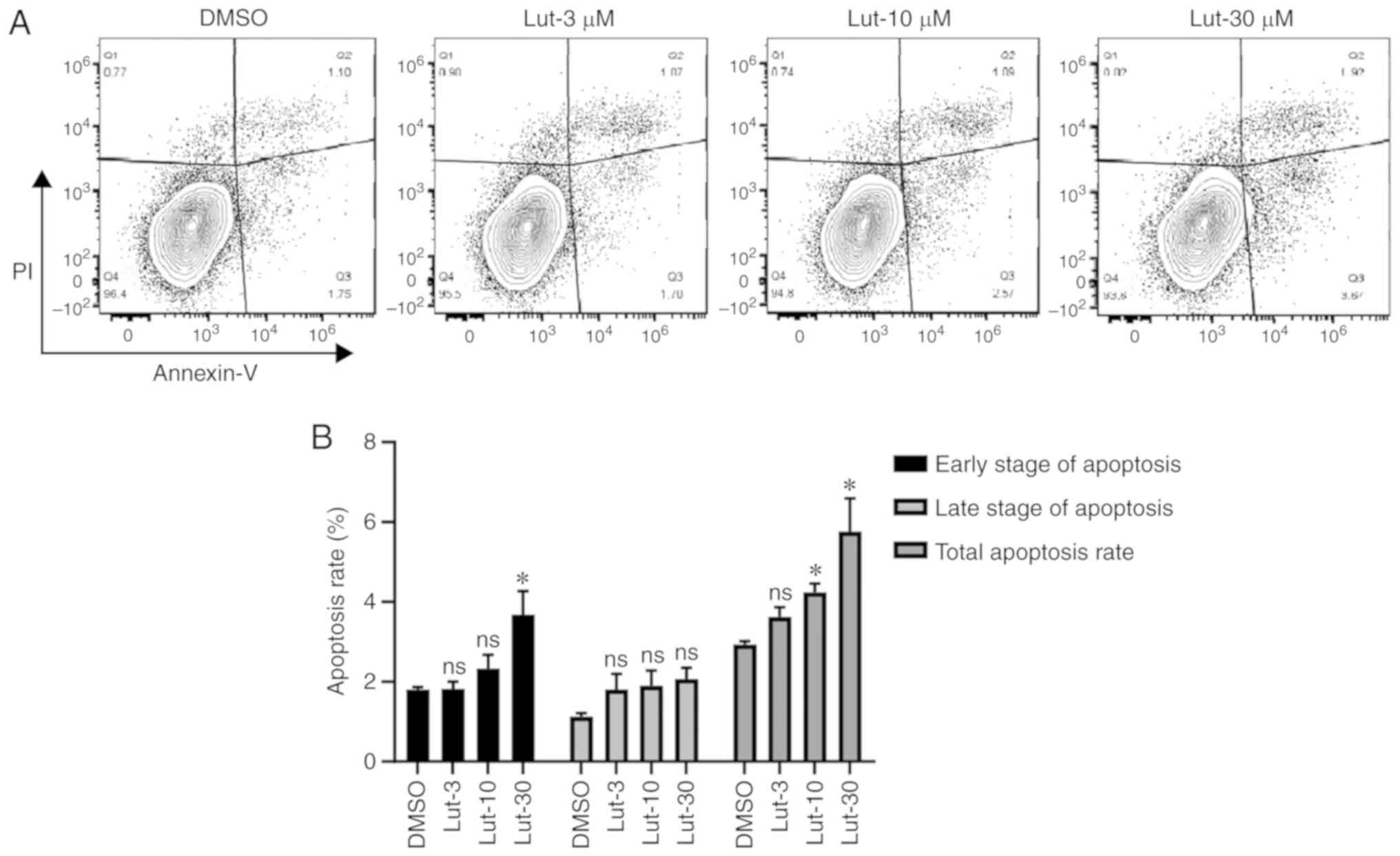
Subsequently, the pro-apoptotic effects of luteolin
on breast cancer cells were examined using an Annexin V/PI staining
assay. As presented in Fig. 2A,
compared with the DMSO control group, MDA-MB-231 cells treated with
luteolin for 24 h exhibited a dose-dependent increase in apoptosis.
A significant increase in total apoptosis was demonstrated at ≥10
µM (P<0.05; Fig. 2B). These
results indicated that luteolin can promote apoptosis of breast
cancer cells.
Luteolin-induced tumor inhibition is
mediated via AhR in MDA-MB-231 cells
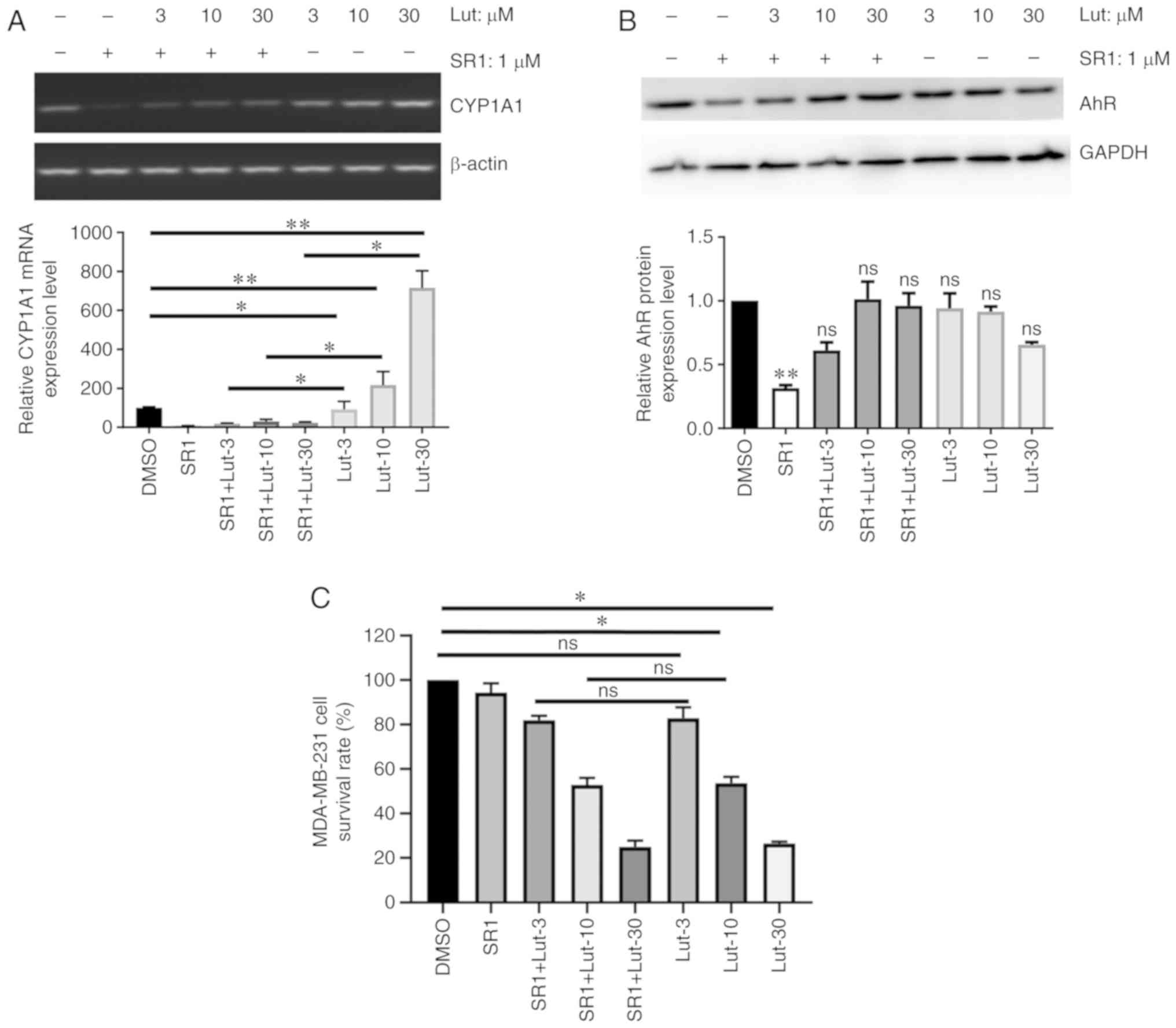
CYP1A1 is a prototypical marker of AhR-mediated
cellular response to TCDD and other AhR agonists (12). Thus, the expression of CYP1A1 and
AhR in luteolin-treated MDA-MB-231 cells was examined to
investigate the implication of AhR signaling in the effects of
luteolin. Compared with control cells, treatment of MDA-MB-231
cells with luteolin significantly increased the mRNA expression of
CYP1A1 in a dose-dependent manner after 24 h (P<0.05). This
effect was significantly reversed by treatment with the AhR
antagonist SR1 (Fig. 3A). Notably,
it was observed that the AhR protein level was not significantly
affected by luteolin in MDA-MB cells, in the presence or absence of
SR1 (Fig. 3B). Furthermore, SR1
treatment did not reverse luteolin-mediated growth inhibition of
MDA-MB-231 cells, according to MTT assay (Fig. 3C), which indicates that the effect
of luteolin on the proliferation of these cells is
AhR-independent.
Luteolin inhibits MDA-MB-231 cell
migration and invasion in an AhR-dependent manner
AhR overexpression has previously been shown to
increase the migration and invasion of immortalized mammary
epithelial cells (12). Therefore,
a Transwell assay was performed to investigate the effect of
luteolin on invasion of breast cancer cells. Indeed, cell invasion
deceased significantly after 24 h of luteolin treatment compared
with control cells (Fig. 4A and B;
P<0.05). In addition, the antagonist SR1 significantly reversed
the effects of 10 and 30 µM luteolin on the invasion of MDA-MB-231
cells, which indicates that the luteolin-induced inhibition of
invasion is AhR-dependent in breast cancer cells.
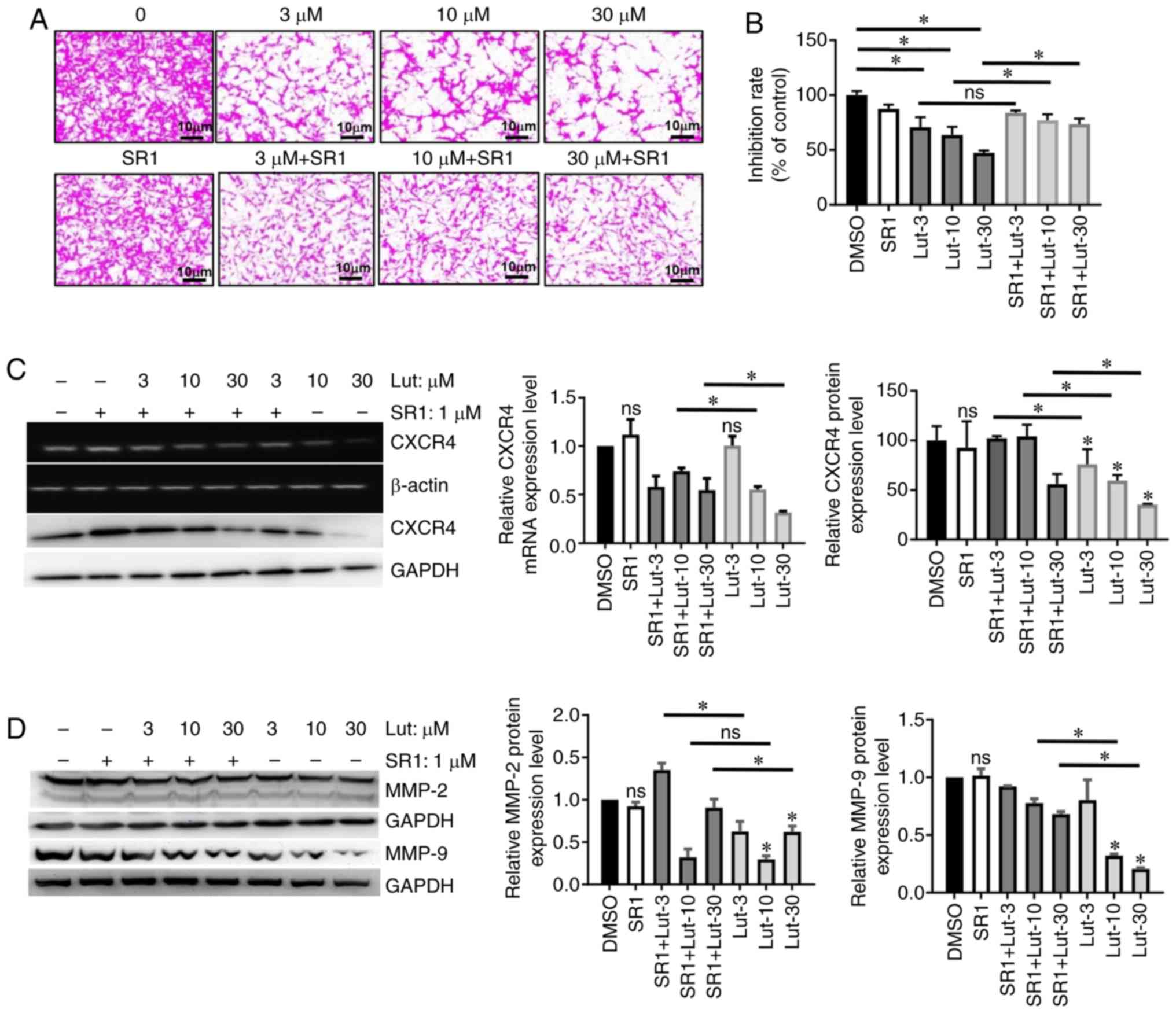 | Figure 4.Lut inhibits MDA-MB-231 cell
metastasis in an AhR-dependent manner. (A) Representative images of
cell invasion assay, in which Transwell chambers coated with
Matrigel were used. Cells were treated for 24 h during the assay
with 0, 3, 10 or 30 µM of lut, with or without SR1. (B)
Quantitative results of the invasion assay. Cancer cell invasion is
presented relative to the untreated control cells. Expression
levels of (C) CXCR4, (D) MMP-2 and MMP-9 were measured by reverse
transcription-semi-quantitative PCR and western blotting.
MDA-MB-231 cells were treated with or without with SR1, then
treated with lut for 24 h. Data are presented as mean ± standard
deviation of at least three independent experiments. *P<0.05.
ns, not significant compared with DMSO-treated cells; SR1,
StemRegenin 1; AhR, aryl hydrocarbon receptor; lut, luteolin;
CXCR4, C-X-C chemokine receptor type 4; MMP, matrix
metalloproteinase. |
Subsequently, the effect of luteolin on the
expression of the chemokine receptor CXCR4 was examined, which has
been reported as a molecular marker of metastasis in several cancer
types, including breast cancer (21). Significant decreases in the mRNA and
protein expression levels of CXCR4 were observed in MDA-MB-231
cells following treatment with 10 and 30 µM luteolin (Fig. 4C). While co-treatment with the
antagonist SR1 significantly reversed these effects of luteolin on
CXCR4 expression at the protein level; and the mRNA level of CXCR4
was moderately reversed by SR1 treatment (Fig. 4C).
It has been reported that MMP-2 and MMP-9 promote
metastasis in several cancer types (22). As presented in Fig. 4D, significant decreases in MMP-2 and
MMP-9 protein expression levels were observed in MDA-MB-231 cells
following treatment with 10 and 30 µM luteolin, which was reversed
by the antagonist SR1. However, the decrease in MMP-9 expression
following treatment with 3 µM luteolin was not reversed by the
antagonist SR1.
Luteolin inhibits metastasis of
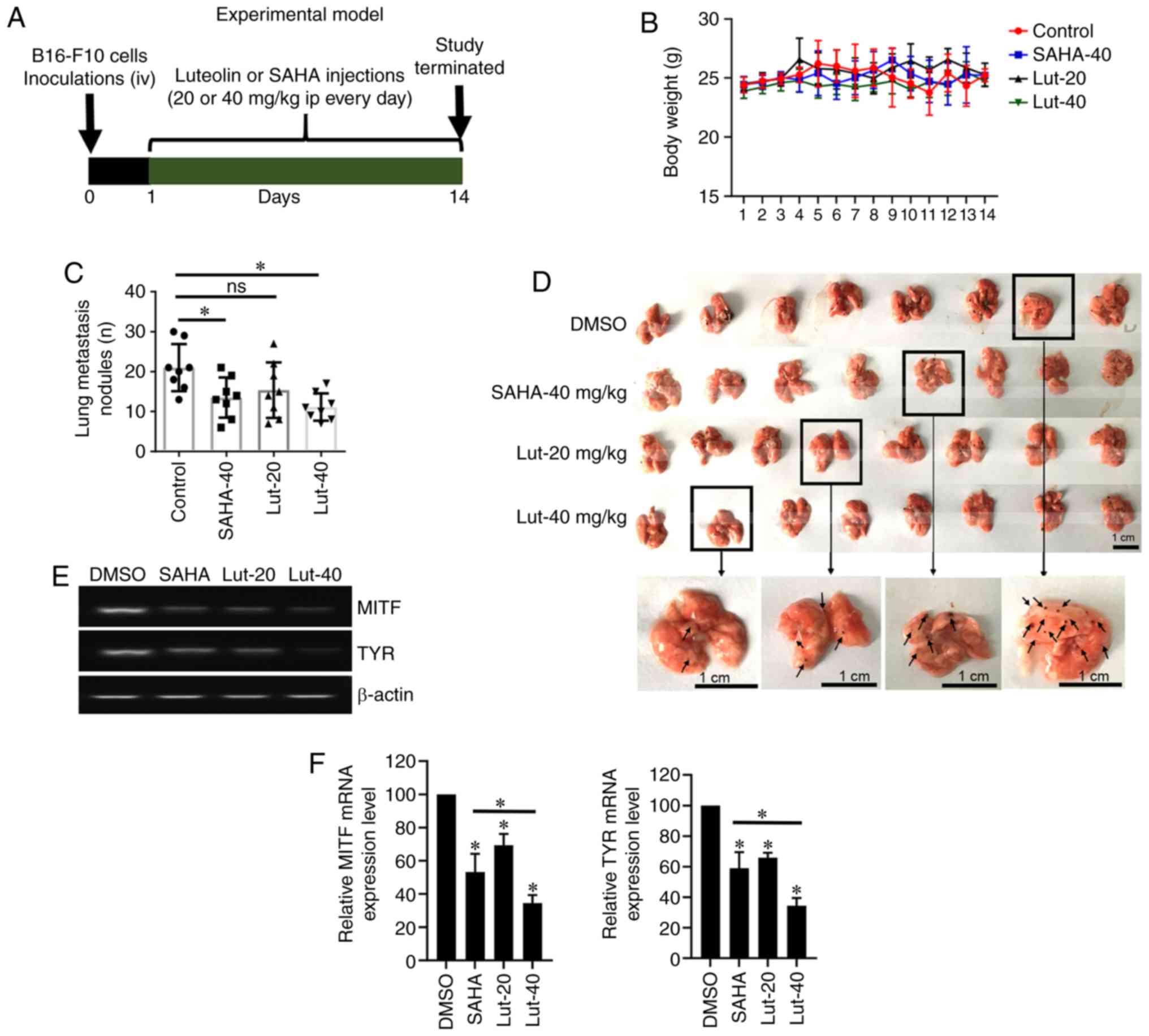
B16-F10 cells in vivo
As reported previously, luteolin exerts its effect
via AhR engagement (23,24). To investigate the effects of
targeting AhR on metastasis in vivo, luteolin was
administered to nude mice that were subcutaneously injected with
5×104 B16-F10 melanoma cells. The metastatic load in the
lung was determined 2 weeks after treatment. SAHA, a widely used
anticancer drug, was used as a positive control for comparison
(Fig. 5A). Mice injected
intraperitoneally with 40 mg/kg luteolin exhibited a significant
reduction in the number of lung metastatic nodules, while no
significant differences in body weight were observed between groups
(Fig. 5B and C). Notably, the
reduction was more significant in the luteolin-treated mice
compared with the SAHA-treated mice (Fig. 5C and D). The representative images
of lung metastatic nodules (Fig.
5D) demonstrated that the number of lung metastatic nodules
from luteolin-treated mice was fewer than that in the DMSO group.
The microphthalmia-associated transcription factor (MITF) is
essential for melanoblast survival and for the expression of
melanogenic enzymes (25). Thus, as
a target of MITF, tyrosinase (TYR) (26) was measured in response to luteolin
treatment in lung tissues. Compared with SAHA, luteolin treatment
exhibited a stronger inhibitory effect on MITF and TYP mRNA
expression, which suggests the mechanism of how luteolin inhibits
B16-F10 metastasis (Fig. 5E and
F).
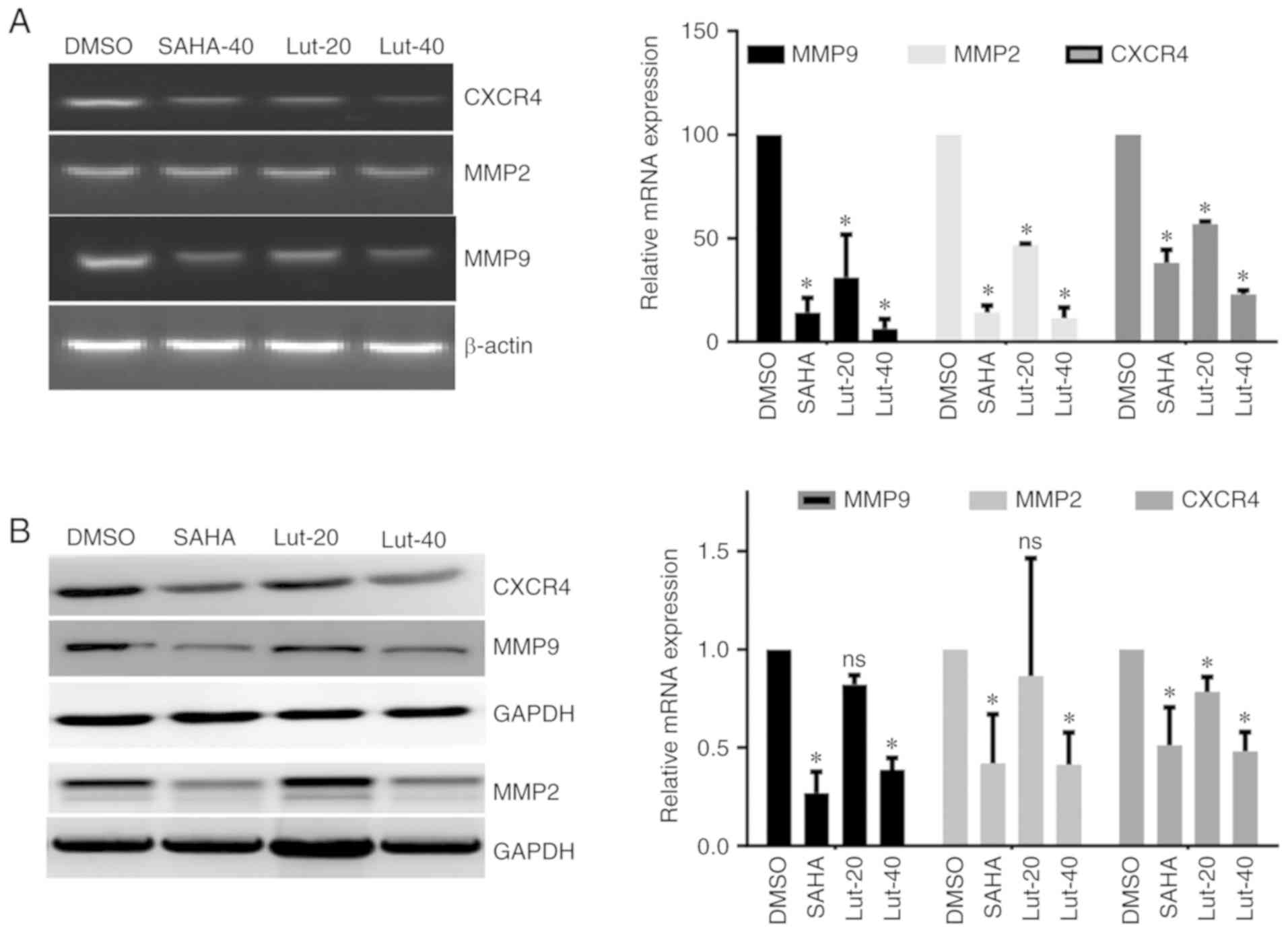
To investigate the effects of luteolin on
metastasis-related genes, the lung tissues were isolated from
tumor-bearing nude mice following luteolin treatment, and the mRNA
and protein levels of MMP-2, MMP-9 and CXCR4 were detected. It was
identified that 40 µM luteolin treatment significantly decreased
the expression levels of MMP-9, MMP-2 and CXCR4 in the lung
tissues, and the inhibition of these genes was comparable to the
group treated with the positive control SAHA. While 20 µM luteolin
inhibited mRNA levels of MMP-9, MMP-2 and CXCR4, which exhibited a
weaker effect compared with SAHA, also the protein level of CXCR4
was decreased, while the protein levels of MMP-2 and MMP-9 were not
affected by 20 µM luteolin treatment (Fig. 6A and B). These results suggest that
luteolin efficiently inhibits the expression of metastasis-related
genes in vivo.
Discussion
The present study demonstrated that
luteolin-mediated AhR activation exerted anti-metastatic effects
via downregulation of CXCR4 and MMP-9 in vitro in the
ER-negative breast cancer cell line MDA-MB-231. Notably, these
results were highly consistent with those obtained by the xenograft
experiments in nude mice.
A number of studies have demonstrated that the AhR
is a promising target for the treatment of ER-negative breast
cancer (27,28). It has reported that an AhR agonist,
SAhRM MCDF, can inhibit the ER-induced growth and/or metastasis of
ER-negative breast cancer in animal models (29). In addition, structurally diverse AhR
ligands demonstrated AhR-mediated inhibition of cell invasion in
ER-negative breast cancer cells, along with a downregulation of the
pro-metastatic genes CXCR4 and MMP-9 (30–32).
Nevertheless, AhR-targeting drugs have been rarely used in clinical
applications. In this regard, several studies have demonstrated
that certain natural products, especially flavonoids, exhibit the
capability of activation and/or inactivation of AhR and
AhR-dependent signaling pathways. While most of these natural
products exhibit an antagonistic and completive activity, some of
them have been reported to activate AhR (33–35).
Given the urgent need for a safer and more effective treatment of
ER-negative breast cancers, the present study investigated luteolin
as a potential molecule that can block ER-negative breast tumor
growth and metastasis. Luteolin is a flavone that exists in
numerous types of plants, including fruits, vegetables and
medicinal herbs (36). Due to their
low toxicity, such compounds confer significant promising
advantages over currently used drugs.
It has been reported that luteolin exerts multiple
beneficial effects, including cardiovascular protection,
anti-inflammatory activity and anticancer activity (37–39).
Luteolin has been reported to block lung metastasis in vivo
and cell migration in vitro (18). In addition, previous studies have
demonstrated that luteolin can inhibit AhR transformation and
CYP1A1 expression, while inducing heme oxygenase-1 expression in
hepatic cells (23). However, to
the best of our knowledge, the relationship between luteolin and
AhR in human cancer cell lines has not been fully clarified. The
present study used a well-established xenograft model of lung
metastasis and examined the effects of luteolin on the
lung-metastatic MDA-MB-231 cell line.
Primarily, the antiproliferative effects of luteolin
were examined in five human tumor cell lines (HCT116, MDA-MB-231,
A549, PC-3 and ES-2). Notably, it was observed that the
IC50 of luteolin was relatively low, ~30 µM, in the
breast cancer MDA-MB-231 cell line, which exhibits high basal rates
of migration and invasion. Although luteolin was observed to
promote apoptosis in a dose-dependent manner, SR1 treatment did not
reverse luteolin-mediated growth inhibition in MDA-MB-231 cells,
which indicates the complex apoptosis pathways regulated by AhR.
Notably, while AhR expression was not affected by luteolin, the
luteolin-mediated inhibition of MDA-MB-231 cell invasion were
reversed by co-treatment with the AhR antagonist SR1. Based on the
association between invasion and AhR, this could be related to the
function of luteolin as a potential natural ligand of AhR. This is
further supported by the results demonstrating that the
luteolin-mediated downregulation of the pro-metastatic genes CXCR4
and MMP-9 was attenuated by co-treatment with the AhR antagonist
SR1.
These in vitro assays were further supported
in vivo by the inhibition of lung metastasis of B16-F10
melanoma cells in mice treated with luteolin. Previous studies have
reported that luteolin inhibits metastasis via downregulation of
β-catenin or suppression of Notch4 signaling in human TNBC cells,
colorectal cancer and glioblastoma in mouse models in vivo
(18,40–42).
The present study demonstrated that luteolin potentially inhibits
the metastasis of B16-F10 melanoma cells in a well-established
xenograft model. Indeed, luteolin reduced the number of lung
colonies at a dose of 40 mg/kg, compared with the DMSO-treated
control mice. In addition, MITF and TYR mRNA levels in the lung
tissues of luteolin-treated mice were significantly lower compared
with the control group, and the inhibition effect was more marked
compared with that in SAHA-treated mice at the same dose. Similar
results were also observed regarding MMP-9 and CXCR-4 expression.
However, the effect of luteolin on other genes and nuclear
cofactors required for AhR-mediated signaling were not investigated
in cancer cells. Nevertheless, the present results suggest that the
anticancer activities of luteolin are at least partly mediated in
an AhR-independent manner.
In summary, the present results indicate that
luteolin-mediated AhR activation can efficiently inhibit the growth
and metastasis of cancer in vitro and in vivo by
inducing apoptotic cell death and cell cycle arrest. In addition,
the inhibition of AhR reduced the sensitivity of breast cancer to
luteolin, which further supports the role of AhR in mediating the
effects of luteolin on cancer cells. Notably, luteolin
administration resulted in a potent anti-metastatic effect in a
xenograft mouse model, which indicates that luteolin could be a
potential compound for cancer chemotherapy.
Acknowledgements
Not applicable.
Funding
This work was supported by the Chinese National
Natural Science Foundation (grant nos. 81803539 and 81800187) and
the Science and Technology Program of Shandong Province (grant nos.
2017GSF19111 and 2018YYSP022).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW designed the study. JF, TZ and TH performed the
experiments. ZH, CL, BH, AX and XS analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Research
Ethics Committee of Shandong Analysis and Test Center (Jinan,
China; approval no. ECAESDATC-2016-011).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AhR
|
aryl hydrocarbon receptor
|
|
SR1
|
StemRegenin 1
|
|
MMP-2
|
matrix metalloproteinase 2
|
|
MMP-9
|
matrix metalloproteinase 9
|
|
CXCR4
|
C-X-C chemokine receptor type 4
|
|
SAHA
|
suberoylanilide hydroxamic acid
|
|
MITF
|
microphthalmia-associated
transcription factor
|
References
|
1
|
Fernández-Nogueira P, Mancino M, Fuster G,
Bragado P, Puig MP, Gascón P, Casado FJ and Carbó N: Breast
mammographic density: Stromal implications on breast cancer
detection and therapy. J Clin Med. 9:7762020. View Article : Google Scholar
|
|
2
|
Arvold ND, Taghian AG, Niemierko A, Abi
Raad RF, Sreedhara M, Nguyen PL, Bellon JR, Wong JS, Smith BL and
Harris JR: Age, breast cancer subtype approximation, and local
recurrence after breast-conserving therapy. J Clin Oncol.
29:3885–3891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Mahmood S, Sapiezynski J, Garbuzenko OB
and Minko T: Metastatic and triple-negative breast cancer:
Challenges and treatment options. Drug Deliv Transl Res.
8:1483–1507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denkert C, Liedtke C, Tutt A and von
Minckwitz G: Molecular alterations in triple-negative breast
cancer-the road to new treatment strategies. Lancet. 389:2430–2442.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Denison MS, Pandini A, Nagy SR, Baldwin EP
and Bonati L: Ligand binding and activation of the Ah receptor.
Chem Biol Interact. 141:3–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poland A, Glover E and Kende AS:
Stereospecific, high affinity binding of
2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence
that the binding species is receptor for induction of aryl
hydrocarbon hydroxylase. J Biol Chem. 251:4936–4946.
1976.PubMed/NCBI
|
|
7
|
Murray IA, Patterson AD and Perdew GH:
Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat
Rev Cancer. 14:801–814. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolluri SK, Jin UH and Safe S: Role of the
aryl hydrocarbon receptor in carcinogenesis and potential as an
anti-cancer drug target. Arch Toxicol. 91:2497–2513. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vacher S, Castagnet P, Chemlali W,
Lallemand F, Meseure D, Pocard M, Bieche I and Perrot-Applanat M:
High AHR expression in breast tumors correlates with expression of
genes from several signaling pathways namely inflammation and
endogenous tryptophan metabolism. PLoS One. 13:e01906192018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ronnekleiv-Kelly SM, Nukaya M, Díaz-Díaz
CJ, Megna BW, Carney PR, Geiger PG and Kennedy GD: Aryl hydrocarbon
receptor-dependent apoptotic cell death induced by the flavonoid
chrysin in human colorectal cancer cells. Cancer Lett. 370:91–99.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Donnell EF, Koch DC, Bisson WH, Jang HS
and Kolluri SK: The aryl hydrocarbon receptor mediates
raloxifene-induced apoptosis in estrogen receptor-negative hepatoma
and breast cancer cells. Cell Death Dis. 5:e10382014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Amato NC, Rogers TJ, Gordon MA, Greene
LI, Cochrane DR, Spoelstra NS, Nemkov TG, D'Alessandro A, Hansen KC
and Richer JK: A TDO2-AhR signaling axis facilitates anoikis
resistance and metastasis in triple-negative breast cancer. Cancer
Res. 75:4651–4664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brinkmann V, Ale-Agha N, Haendeler J and
Ventura N: The Aryl hydrocarbon receptor (AhR) in the aging
process: Another puzzling role for this highly conserved
transcription factor. Front Physiol. 10:15612019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Denison MS and Nagy SR: Activation of the
aryl hydrocarbon receptor by structurally diverse exogenous and
endogenous chemicals. Annu Rev Pharmacol Toxicol. 43:309–334. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cook MT, Mafuvadze B, Besch-Williford C,
Ellersieck MR, Goyette S and Hyder SM: Luteolin suppresses
development of medroxyprogesterone acetate-accelerated
7,12-dimethylbenz(a)anthracene-induced mammary tumors in
Sprague-Dawley rats. Oncol Rep. 35:825–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cook MT, Liang Y, Besch-Williford C,
Goyette S, Mafuvadze B and Hyder SM: Luteolin inhibits
progestin-dependent angiogenesis, stem cell-like characteristics,
and growth of human breast cancer xenografts. SpringerPlus.
4:4442015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tester AM, Waltham M, Oh SJ, Bae SN, Bills
MM, Walker EC, Kern FG, Stetler-Stevenson WG, Lippman ME and
Thompson EW: Pro-matrix metalloproteinase-2 transfection increases
orthotopic primary growth and experimental metastasis of MDA-MB-231
human breast cancer cells in nude mice. Cancer Res. 64:652–658.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cook MT, Liang Y, Besch-Williford C and
Hyder SM: Luteolin inhibits lung metastasis, cell migration, and
viability of triple-negative breast cancer cells. Breast Cancer
(Dove Med Press). 9:9–19. 2017.PubMed/NCBI
|
|
19
|
Ko WG, Kang TH, Lee SJ, Kim YC and Lee BH:
Effects of luteolin on the inhibition of proliferation and
induction of apoptosis in human myeloid leukaemia cells. Phytother
Res. 16:295–298. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sabzichi M, Hamishehkar H, Ramezani F,
Sharifi S, Tabasinezhad M, Pirouzpanah M, Ghanbari P and Samadi N:
Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB
231 cells to doxorubicin by suppressing Nrf2 mediated signalling.
Asian Pac J Cancer Prev. 15:5311–5316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Bo Q, Wang W, Wang R, Li Y, Chen
S, Xia Y, Wang W, Wang Y, Zhu K, et al: CCL17-CCR4 axis promotes
metastasis via ERK/MMP13 pathway in bladder cancer. J Cell Biochem.
Sep 19–2018.(Epub ahead of print).
|
|
22
|
Tang Y, Lv P, Sun Z, Han L and Zhou W:
14-3-3β promotes migration and invasion of human hepatocellular
carcinoma cells by modulating expression of MMP2 and MMP9 through
PI3K/Akt/NF-κB pathway. PLoS One. 11:e01460702016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang T, Kimura Y, Jiang S, Harada K,
Yamashita Y and Ashida H: Luteolin modulates expression of
drug-metabolizing enzymes through the AhR and Nrf2 pathways in
hepatic cells. Arch Biochem Biophys. 557:36–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koper JEB, Loonen LMP, Wells JM, Troise
AD, Capuano E and Fogliano V: Polyphenols and tryptophan
metabolites activate the Aryl hydrocarbon receptor in an in vitro
model of colonic fermentation. Mol Nutr Food Res. 63:e18007222019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi MH, Jo HG, Yang JH, Ki SH and Shin
HJ: Antioxidative and anti-melanogenic activities of bamboo stems
(Phyllostachys nigra variety henosis) via PKA/CREB-mediated MITF
downregulation in B16F10 melanoma cells. Int J Mol Sci. 19:4092018.
View Article : Google Scholar
|
|
26
|
Hartman ML, Talar B, Noman MZ,
Gajos-Michniewicz A, Chouaib S and Czyz M: Gene expression
profiling identifies microphthalmia-associated transcription factor
(MITF) and Dickkopf-1 (DKK1) as regulators of
microenvironment-driven alterations in melanoma phenotype. PLoS
One. 9:e951572014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Piwarski SA, Thompson C, Chaudhry AR,
Denvir J, Primerano DA, Fan J and Salisbury TB: The putative
endogenous AHR ligand ITE reduces JAG1 and associated NOTCH1
signaling in triple negative breast cancer cells. Biochem
Pharmacol. 174:1138452020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baker JR, Sakoff JA and McCluskey A: The
aryl hydrocarbon receptor (AhR) as a breast cancer drug target. Med
Res Rev. 40:972–1001. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang S, Kim K, Jin UH, Pfent C, Cao H,
Amendt B, Liu X, Wilson-Robles H and Safe S: Aryl hydrocarbon
receptor agonists induce microRNA-335 expression and inhibit lung
metastasis of estrogen receptor negative breast cancer cells. Mol
Cancer Ther. 11:108–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Subramaniam V, Ace O, Prud'homme GJ and
Jothy S: Tranilast treatment decreases cell growth, migration and
inhibits colony formation of human breast cancer cells. Exp Mol
Pathol. 90:116–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Subramaniam V, Chakrabarti R, Prud'homme
GJ and Jothy S: Tranilast inhibits cell proliferation and migration
and promotes apoptosis in murine breast cancer. Anticancer Drugs.
21:351–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poormasjedi-Meibod MS, Salimi Elizei S,
Leung V, Baradar Jalili R, Ko F and Ghahary A: Kynurenine modulates
MMP-1 and type-I collagen expression via Aryl hydrocarbon receptor
activation in dermal fibroblasts. J Cell Physiol. 231:2749–2760.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang T, Feng YL, Chen L, Vaziri ND and
Zhao YY: Dietary natural flavonoids treating cancer by targeting
aryl hydrocarbon receptor. Crit Rev Toxicol. 49:445–460. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lv Q, Shi C, Qiao S, Cao N, Guan C, Dai Y
and Wei Z: Alpinetin exerts anti-colitis efficacy by activating
AhR, regulating miR-302/DNMT-1/CREB signals, and therefore
promoting Treg differentiation. Cell Death Dis. 9:8902018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao H, Chen L, Yang T, Feng YL, Vaziri
ND, Liu BL, Liu QQ, Guo Y and Zhao YY: Aryl hydrocarbon receptor
activation mediates kidney disease and renal cell carcinoma. J
Transl Med. 17:3022019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu Q, Zhang M, Ying Q, Xie X, Yue S, Tong
B, Wei Q, Bai Z and Ma L: Decrease of AIM2 mediated by luteolin
contributes to non-small cell lung cancer treatment. Cell Death
Dis. 10:2182019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cordaro M, Cuzzocrea S and Crupi R: An
update of palmitoylethanolamide and luteolin effects in preclinical
and clinical studies of neuroinflammatory events. Antioxidants
(Basel). 9:2162020. View Article : Google Scholar
|
|
38
|
Manzoor MF, Ahmad N, Ahmed Z, Siddique R,
Zeng XA, Rahaman A, Muhammad Aadil R and Wahab A: Novel extraction
techniques and pharmaceutical activities of luteolin and its
derivatives. J Food Biochem. 43:e129742019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahmed S, Khan H, Fratantonio D, Hasan MM,
Sharifi S, Fathi N, Ullah H and Rastrelli L: Apoptosis induced by
luteolin in breast cancer: Mechanistic and therapeutic
perspectives. Phytomedicine. 59:1528832019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin D, Kuang G, Wan J, Zhang X and Li H,
Gong X and Li H: Luteolin suppresses the metastasis of
triple-negative breast cancer by reversing
epithelial-to-mesenchymal transition via downregulation of
beta-catenin expression. Oncol Rep. 37:895–902. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu Y, Chen X, Li Z, Zheng S and Cheng Y:
Thermosensitive in situ gel containing luteolin micelles is a
promising efficient agent for colorectal cancer peritoneal
metastasis treatment. J Biomed Nanotechnol. 16:54–64. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yao Y, Rao C, Zheng G and Wang S: Luteolin
suppresses colorectal cancer cell metastasis via regulation of the
miR384/pleiotrophin axis. Oncol Rep. 42:131–141. 2019.PubMed/NCBI
|