Introduction
Bladder cancer (BC) is a cancer arising from the
urinary bladder. BC is one of the most common malignant cancers
worldwide with high morbidity and mortality, representing a huge
economic burden. As of 2018, BC affected approximately 1.6 million
people globally with 550,000 new cases and 200,000 deaths (1). More than 50% of patients relapse
within 6–12 years after initial diagnosis (2–4). The
increased incidence of BC and poor outcomes underscore attempts to
understand the underlying pathological mechanisms of BC
progression. Emerging evidence has revealed that
epithelial-mesenchymal transition (EMT) is an important process in
the development of BC, and EMT-related molecules may become new
targets for treatment of chemoresistance (5–7). EMT
is a process in which cells lose their epithelial features and
acquire mesenchymal characteristics, and where cells with
mesenchymal characteristics can migrate more efficiently and invade
other tissues. In cancer, EMT is associated with tumor occurrence,
metastasis, tumor stemness and resistance to treatment (8,9).
EMT can be induced by several cell signaling
transduction pathways. The transforming growth factor (TGF)-β
pathway has been revealed to induce EMT in several cancer types
(6,8,10–13).
The TGF-β ligand binds to TGF-β receptors, resulting in
phosphorylation of SMAD2 and SMAD3 (14). Activated SMAD2-SMAD3 forms complexes
with SMAD4, and these complexes translocate to the nucleus to
regulate the expression of TGF-β target genes, including a large
number of genes involved in EMT, invasion, motility and
proliferation (9,11,12,14).
Potential cancer therapies targeting TGF-β signaling pathways have
been investigated, and several promising therapies are being tested
in clinical trials (6,14).
Long noncoding RNAs (lncRNAs) are noncoding RNAs
longer than 200 nucleotides (nts) (15–17).
lncRNAs can act as scaffolds or competing endogenous RNAs (ceRNAs)
by interacting with microRNAs (miRNAs or miRs), circRNAs and
proteins (18–21). In addition, lncRNAs recruit
chromatin remodeling and modification complexes to guide epigenetic
regulations (21,22). Several lncRNAs have been reported to
serve as oncogenes in BC, including H19, MALAT1, TUG1, UCA1, and
HOTAIR (23–27), highlighting the potential for
lncRNAs to serve as biomarkers and therapeutic targets in BC.
Cancer susceptibility candidate 9 (CASC9) is located
on human chromosome 8q21.11 (28).
It was originally identified in esophageal squamous cell carcinoma
(ESCC) and is predicted to be a novel putative oncogene (28). Subsequently, CASC9 expression has
been reported to be aberrantly upregulated in numerous human
malignancies, including esophageal cancer (28–30),
pancreatic ductal adenocarcinoma (31), gastric cancer (32), nasopharyngeal carcinogenesis
(33), and non-small cell lung
cancer (34). The upregulation of
CASC9 in human cancer indicates its potential tumorigenic
properties. In ESCC, CASC9 has been revealed to facilitate cell
growth by negatively regulating PDCD4 and promote metastasis
through upregulating LAMC2 expression (29,30).
CASC9 has been demonstrated to interact with HIF1α and enhance the
stabilization of HIF1α in nasopharyngeal carcinoma (33). However, the role of CASC9 in BC has
not been characterized, especially during EMT.
In this study, we aimed to elucidate the expression
of CASC9 in BC tissues and cell lines, its association with the
depth of bladder tumor invasion and prognosis, and to determine the
role of CASC9 in the development and progression of BC.
Materials and methods
Sample collection
In total, 49 pairs of BC tissues and corresponding
adjacent normal bladder tissues were collected from Peking
University Shenzhen Hospital (Shenzhen, China) from January 2010 to
November 2011. Tissue specimens were collected from 49 patients
(aged 30 to 80 years old) with BC who underwent cystectomy; 40 male
patients and 9 female patients. All patients were diagnosed as
transitional cell carcinoma clinically and pathologically. The
exclusion criteria included patients with other tumors, patients
with a history of other cancer treatments, or patients with bladder
cancer who had received chemotherapy or radiation therapy before
surgery. All human tissue samples were obtained with informed
consent. The Ethics Committee of Peking University Shenzhen
Hospital in China approved this study (approval no. 20090017).
Cell lines and cell cultures
All cell lines were obtained from the American Type
Culture Collection and maintained using standard media and
conditions. Human BC cells (T24, TCCSUP, UM-UC-3, J82 and 5637),
human normal bladder epithelial cells (SV-HUC-1) and 293T cells
were maintained in Roswell Park Memorial Institute (RPMI)-1640
medium, Dulbecco's modified Eagle's medium (DMEM) or F-12K
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (PS) (all from Gibco; Thermo Fisher
Scientific Inc.). All cells were cultured at 37°C in a 5%
CO2 incubator.
Cell transfection
Cells were transfected with 100 nM small interfering
(si)RNA or mimics or inhibitors using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 24–48 h at 37°C.
Then, the transfected cells were analyzed. All siRNAs, mimics and
inhibitors were synthesized by Suzhou GenePharma Co., Ltd. The
sequences of siRNAs were as follows: Negative control (NC),
5′-UUCUCCGAACGUGUCACGUTT-3′; siCASC9-1,
5′-CAACUGGAUUCCAACUUUAUU-3′; siCASC9-2,
5′-CAAGAAGUUUAGUAAACCAUU-3′; siCASC9-3,
5′-GAGAUCAUUAAGCCCAGAAUU-3′; mimics NC,
5′-UUGUACUACACAAAAGUACUG-3′; inhibitor NC,
5′-CAGUACUUUUGUGUAGUACAA-3′; mimics miR-758-3p,
5′-UUUGUGACCUGGUCCACUAACC-3′; inhibitor miR-758-3p,
5′-GGUUAGUGGACCAGGUCACAAA-3′.
RNA extraction, cDNA synthesis and
reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted from cells or tissue
specimens using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The cDNA was synthesized with random primers
using PrimeScript RT reagent Kit (Takara Biotechnology, Co., Ltd.)
or miScript II RT kit (Qiagen GmbH) according to the manufacturer's
instructions. Quantitative RT-PCR was performed on a Roche
Lightcycler 480 (Roche Diagnostics) using SYBR Premix Ex Taq kit
(Takara Biotechnology, Co., Ltd.) according to the manufacturer's
instructions. Quantitative RT-PCR amplification was performed
according to the following thermocycling conditions: 30 sec at 95°C
for initial denaturation; 40 cycles of 5 sec at 95°C for
denaturation and 31 sec at 60°C for extension; and 10 min at 60°C
for final extension. Human EMT RT2 Profiler PCR Array
(Qiagen GmbH) was used to analyze the expression of genes involved
in EMT. The relative expression levels of candidate genes were
analyzed using the 2−ΔΔCq method (35). The primers for AHNAK, CTNNB1, EGFR,
FN1, ITGAV, PDGFRB and SNAI3 were purchased from Qiagen, Inc. The
primer sequences were as follows: AHNAK forward,
5′-CAGGCATTGGTGTTCAAGGC-3′ and reverse, 5′-TCTGCCCAGTTGGGAGTTTC-3′;
CTNNB1 forward, 5′-TTGTGCGGCGCCATTTTAAG-3′ and reverse,
5′-TCCTCAGACCTTCCTCCGTC-3′; EGFR forward, 5′-AAGGCACGAGTAACAAGC-3′
and reverse, 5′-AGGGCAATGAGGACATAA-3′; FN1 forward,
5′-TGTGCCAAAGCTTTACTACTGT-3′ and reverse,
5′-TATTTCCCCCGAAGGTGTCT-3′; ITGAV forward,
5′-TCACTAAGCGGGATCTTGCC-3′ and reverse, 5′-AAGCACTGAGCAACTCCACA-3′;
PDGFRB forward, 5′-GCTGTTACCCACTCTGGGAC-3′ and reverse,
5′-TGGTGTCCTTGCTGCTGATG-3′; SNAI3 forward,
5′-GCACAACTACCTCTCAGCCA-3′ and reverse, 5′-ATAGACGTGTGACATGGGGC-3′;
CASC9 forward, 5′-CCAGACAGCAGCAAAGCAAT-3′ and reverse,
5′-GGAAGCAGCAAATGTGTCCAT-3′; TGF-β2 forward,
5′-CGACGAAGAGTACTACGCCA-3′ and reverse, 5′-GATGGCATTTTCGGAGGGGA-3′;
GAPDH forward, 5′-CGCTCTCTGCTCCTCCTGTTC-3′ and reverse,
5′-ATCCGTTGACTCCGACCTTCAC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-ACGCTTCACGAATTTGCGT-3′; miR-758-3p forward,
5′-ACACTCCAGCTGGGTTTGTGACCTGGTCCA-3′ and reverse,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGTTAGTG-3′.
Cell Counting Kit 8 (CCK-8) and
5-ethynyl-2′-deoxyuridine (EdU) assays
Cell Counting Kit-8 (CCK-8; US Everbright, Inc.) and
EdU assay kit (Guangzhou RiboBio Co., Ltd.) were used to assess
cell proliferation. Experiments were performed as previously
described (36).
Wound healing, Transwell and flow
cytometric assays
Cell migration was determined using wound healing
assays. Transwell assays without or with Matrigel were used to
assess BC cell migration and invasion abilities, respectively.
Wound healing, Transwell and flow cytometric assays were performed
as previously described (36).
Serum-free medium was used in the wound healing experiment.
Dual luciferase report assay
The CASC9 (or TGF-β2) fragment containing the
predicted miR-758-3p binding site or a fragment with a mutated
binding site were cloned into the psiCHECK-2 luciferase reporter
vector (Wuhan GeneCreate Biological Engineering Co., Ltd.). Then, 1
µg/ml luciferase reporter vector psiCHECK-2-CASC9-WT (wild type) or
psiCHECK-2-CASC9-MT (mutant type) or psiCHECK-2-TGF-β2-WT or
psiCHECK-2-TGF-β2-MT and 100 nM miRNA mimic or mimic NC were
co-transfected into 293T cells using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h at 37°C.
Renilla and firefly luciferase activities were detected
using the Dual luciferase reporter assay system (Promega
Corporation). miRNA mimic and mimic NC sequences were as follows:
mimics NC, 5′-UUGUACUACACAAAAGUACUG-3′; and mimics miR-758-3p,
5′-UUUGUGACCUGGUCCACUAACC-3′.
Antibodies and western blotting
Anti-E-cadherin (product code ab15148),
anti-N-cadherin (product code ab18203) and anti-GAPDH (product code
ab9485) were purchased from Abcam. Goat anti-mouse IgG-HRP (cat.
no. sc-2005) and goat anti-rabbit IgG-HRP (cat. no. sc-2004) were
purchased from Santa Cruz Biotechnologies. Western blotting was
performed as previously described (36).
RNA fluorescence in situ hybridization
(FISH)
FISH assay was performed using the Ribo™ FISH Kit
(Guangzhou RiboBio Co., Ltd.) according to the manufacturer's
instructions. The lncRNA CASC9 probe was designed and synthesized
by Guangzhou RiboBio Co., Ltd. and was labeled with Cy3 fluorescent
dye. BC cells were seeded onto sterile coverslips until cells
reached 30–60% confluence. The cells were washed with PBS, fixed
with 4% paraformaldehyde for 10 min at 25°C, and then permeabilized
with 0.5% Triton X-100 (PBS) for 10 min at 4°C. Next, the cells
were blocked with prehybridization buffer for 30 min at 37°C and
then incubated in 0.5 µM lncRNA CASC9 probe in hybridization buffer
at 37°C overnight. The cells were then washed with saline sodium
citrate (SSC) buffer solution and stained with DAPI for 10 min at
25°C. Finally, the cell slides were removed from the plate and
fixed on a glass slide for detection by fluorescence microscopy
(magnification, ×400).
Statistical analysis
The data were presented as the mean ± standard error
of mean (SEM). Log-rank test, chi-square test, one-way ANOVA with
Bonferroni post hoc test, paired and unpaired Student's t-test were
employed for statistical analysis. Kaplan-Meier survival analysis
from http://gepia.cancer-pku.cn (37) was used to reveal that the
relationship between CASC9 expression and the prognosis of bladder
cancer patients. Survival analysis was performed using log-rank
test. Chi-square test was used to assess the association between
CASC9 expression and clinicopathological characteristics of bladder
cancer patients. When comparing the population means of only two
groups, the Student's t-test was used, and when means of more than
two groups were compared, ANOVA was selected. Paired Student's
t-test was used to assess CASC9 expression in 49 pairs of BC
tissues (Tumor) and matched adjacent normal tissues (Normal). The
association of CASC9 expression and BC tumor invasion depth was
calculated by one-way ANOVA with Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were carried out with Graphpad
Prism 6 (GraphPad Software, Inc.).
Data sets
Bioinformatics tools (LncBase v2 and miRDB) were
used to predict potential target miRNAs of CASC9 (38,39).
Computational algorithms (TargetScan 7.1 and miRDB) were used to
search for potential miR-758-3p target genes (39,40).
Results
Upregulation of CASC9 in BC tissues is
significantly associated with BC tumor invasion depth and poor
prognosis
To investigate the role of CASC9 in BC, the
expression levels of CASC9 in 49 BC tissues and adjacent normal
bladder tissues from patients were first analyzed by RT-qPCR.
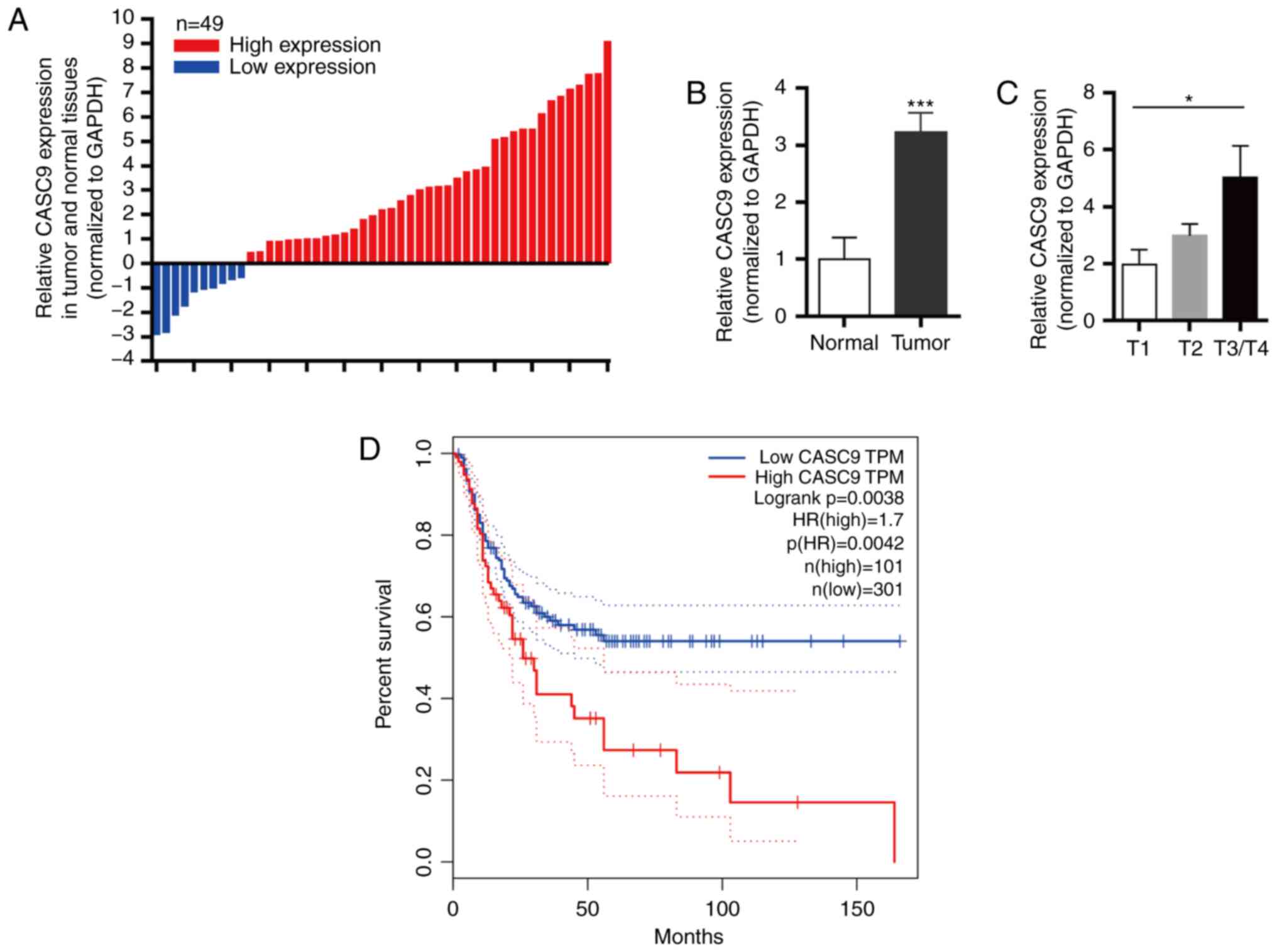
Compared with paired adjacent normal tissues, 79.6% (39/49) of
human BC tissues had upregulated CASC9 expression (Fig. 1A and B; P<0.001). In addition,
the expression level of CASC9 in T3/T4 patients was higher than
that in T1 patients, which may indicate that CASC9 is related to
cell invasion ability (Fig. 1C;
P<0.05).
The correlation between CASC9 expression levels and
the tumor invasion depth in BC patients was further analyzed. As
revealed in Table I, CASC9
upregulation was significantly associated with BC tumor invasion
depth (n=49; P<0.05) and age (n=49, P<0.05), however sex and
tumor size were not associated with CASC9 expression levels. These
results indicated that CASC9 may play a carcinogenic role in
BC.
 | Table I.Associations between CASC9 expression
and clinicopathological characteristics of bladder cancer
patients. |
Table I.
Associations between CASC9 expression
and clinicopathological characteristics of bladder cancer
patients.
|
|
| Expression of
CASC9 |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Total | High (n=39) | Low (n=10) | P-value |
|---|
| Sex |
|
|
| 0.069 |
|
Male | 40 | 34 | 6 |
|
|
Female | 9 | 5 | 4 |
|
| Tumor size
(cm) |
|
|
| 0.719 |
|
<4 | 19 | 16 | 3 |
|
| ≥4 | 30 | 23 | 7 |
|
| Age (years) |
|
|
| 0.029 |
|
≤60 | 22 | 14 | 8 |
|
|
>60 | 27 | 25 | 2 |
|
| Tumor invasion
depth (T) |
|
|
| 0.025 |
| Tis,
Ta, T1 | 18 | 11 | 7 |
|
| T2, T3
or above | 31 | 28 | 3 |
|
| TNM stage |
|
|
| 0.247 |
|
0/I | 15 | 10 | 5 |
|
|
II/III/IV | 34 | 29 | 5 |
|
In addition, Kaplan-Meier survival analysis revealed
that the disease-free survival (DFS) of patients with high CASC9
expression was significantly decreased compared with patients with
low CASC9 expression (Fig. 1D;
analysis from GEPIA). Collectively, the present results revealed
that CASC9 was upregulated in BC, and the expression level of CASC9
could serve as a predictor of prognosis in BC patients.
CASC9 promotes BC cell
proliferation
As revealed in Fig.
2A, CASC9 expression was significantly upregulated in BC cell
lines (T24, TCCSUP, UM-UC-3, J82 and 5637) compared with normal
human bladder epithelial cells (SV-HUC-1) (Fig. 2A). Notably, compared with SV-HUC-1
cells, the expression of CASC9 in J82 and 5637 cells was
significantly increased by >40-fold (Fig. 2A). Therefore, 5637 and J82 cells
were selected for further investigation.
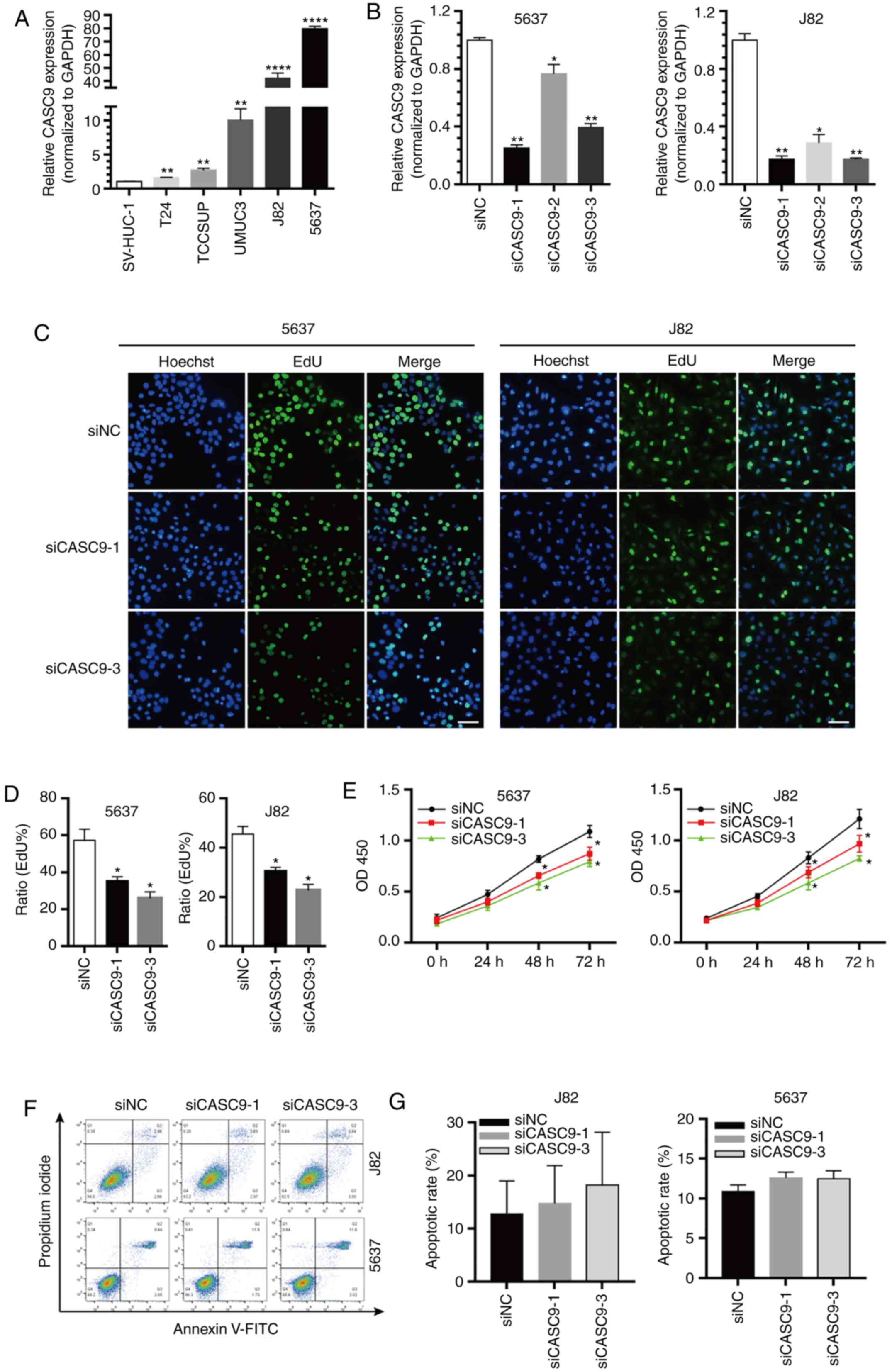 | Figure 2.CASC9 promotes BC cell proliferation.
(A) The relative expression levels of CASC9 in BC cell lines (T24,
TCCSUP, UM-UC-3, J82 and 5637) compared with normal human bladder
epithelial cells (SV-HUC-1). (B) The efficacy of CASC9 siRNAs. (C
and D) The proliferation rate of 5637 and J82 cells transfected
with 50 nM siCASC9-1, siCASC9-3 or siNC measured by EdU assay.
Scale bars, 100 µm. (E) The proliferation rate of 5637 and J82
cells transfected with 50 nM siCASC9-1, siCASC9-3 or siNC measured
by CKK-8 assay reported as the means ± SEM from 3 independently
repeated experiments. (F and G) The apoptotic rate of siCASC9- and
siNC-transfected cells (P>0.05). Unpaired Student's t-test was
used and data are presented as the mean ± SEM. *P<0.05,
**P<0.01 and ****P<0.0001. CASC9, cancer susceptibility
candidate 9; BC, bladder cancer; siRNAs, small interfering RNAs;
NC, negative control; EdU, 5-ethynyl-2′-deoxyuridine; CCK-8, Cell
Counting Kit-8; SEM, standard error of mean. |
The marked upregulated expression of CASC9 in BC
tissues and various BC cell lines prompted us to further explore
the role of CASC9 in tumorigenesis. Three siRNAs that specifically
targeted CASC9 were first designed, and the knockdown efficiency of
the siRNAs was quantified by RT-qPCR. siCASC9-1 and siCASC9-3 were
efficient in depleting CASC9 compared with the negative control (NC
treatment) or siCASC9-2 (Fig. 2B).
Therefore, siCASC9-1 and siCASC9-3 were selected for further
experiments. The effect of CASC9 on BC cell proliferation was
further assessed using EdU and CCK-8 assays. EdU assay results
revealed that CASC9 knockdown (siCASC9-1 or siCASC9-3 transfection)
in 5637 and J82 cells significantly attenuated cell proliferation
(Fig. 2C and D). Similar results
were observed using CCK-8 assays (Fig.
2E). Collectively, the results demonstrated that CASC9
knockdown inhibited BC cell proliferation.
Flow cytometric assays were performed to assess
whether CASC9 knockdown promotes BC cell apoptosis. However, no
statistically significant differences in apoptotic rates were
observed between 5637 cells transfected with siCASC9 and siNC
(P>0.05, Fig. 2F and G). Similar
results were observed in J82 cells (P>0.05; Fig. 3F and G).
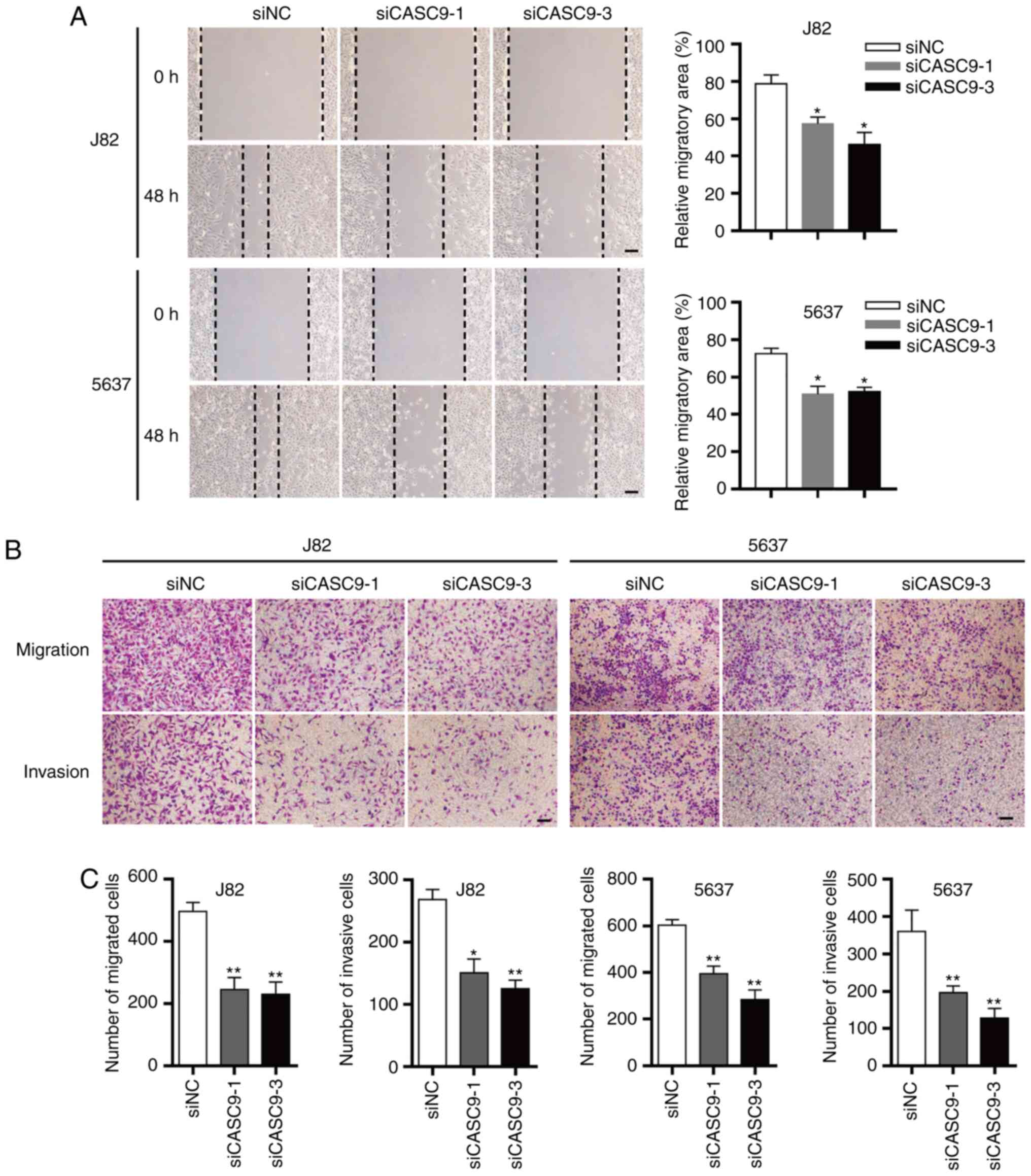 | Figure 3.CASC9 promotes BC cell migration and
invasion. (A) Representative images of wound healing assays of 5637
and J82 cells transfected with 50 nM siCASC9-1, siCASC9-3 or siNC.
Magnification, ×200; scale bars, 100 µm. *P<0.05. (B)
Representative images of Transwell assays of 5637 and J82 cells
transfected with 50 nM siCASC9-1, siCASC9-3 or siNC. Magnification,
×200; scale bars, 100 µm. (C) Quantification of relative migration
and invasion of 5637 and J82 cells transfected with 50 nM
siCASC9-1, siCASC9-3 or siNC. Unpaired Student's t-test was used
and data are presented as the mean ± SEM. *P<0.05, **P<0.01
and. CASC9, cancer susceptibility candidate 9; BC, bladder cancer;
si, small interfering; NC, negative control; SEM, standard error of
mean. |
CASC9 promotes BC cell migration and
invasion
Wound healing and Transwell assays (without Matrigel
coating) were utilized to assess the effect of CASC9 on BC cell
migration. In wound healing assays, the open wound area of siCASC9
(siCASC9-1 or siCASC9-3)-transfected cells was significantly
increased compared with siNC-transfected cells 48 h after
scratching (Fig. 3A). Transwell
migration assays (without Matrigel) revealed that the number of
migrated cells in siCASC9-treated groups was significantly
decreased compared with siNC-treated groups (Fig. 3B and C). These results indicated
that CASC9 promoted BC cell migration.
The effects of CASC9 on BC cell invasion were
assessed using Transwell assays (with Matrigel). The number of
invasive cells in the siCASC9 groups was reduced by ~50% in 5637
and J82 cells (Fig. 3B and C).
These results indicated that CASC9 promoted BC cell invasion.
CASC9 functions as a sponge for
miR-758-3p
Increasing evidence indicates that lncRNAs may act
as sponges for miRNAs, thereby regulating their downstream targets
(19,21). Bioinformatics tools (LncBase v2 and
miRDB) were used to predict potential target miRNAs of CASC9
(38,39). The predicted miRNAs were screened
and the candidate to miR-758-3p was narrowed down. The results
revealed that miR-758-3p expression levels were significantly
decreased in BC cell lines (T24, TCCSUP, UM-UC-3, J82 and 5637)
compared with normal human bladder epithelial cells (SV-HUC-1)
(Fig. 4A).
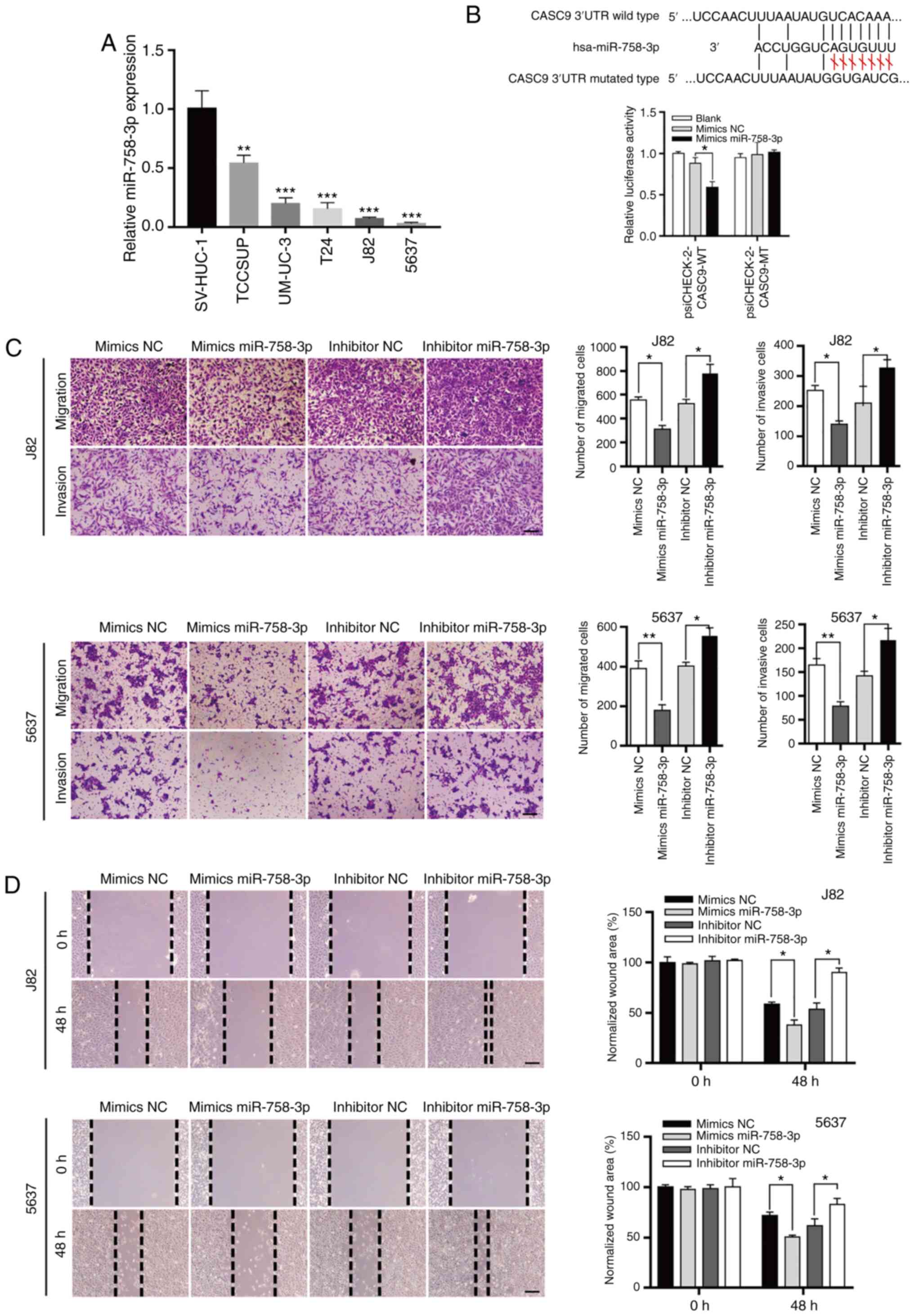 | Figure 4.CASC9 functions as a sponge for
miR-758-3p. (A) The relative expression levels of miR-758-3p in BC
cell lines (TCCSUP, UM-UC-3, T24, J82 and 5637) compared with
normal human bladder epithelial cells (SV-HUC-1). (B) CASC9
functions as a sponge for miR-758-3p. Upper panel, schematic
diagrams of the mutual interactions between miR-758-3p and CASC9.
Bottom panel, 293T cells were transfected with miRNA mimics in
combination with luciferase reporters harboring wild-type or
mutated miRNA binding sites on CASC9. The effects of miR-758-3p on
luciferase activity were determined by luciferase reporter assays.
The activities of firefly luciferase were normalized to
Renilla luciferase. *P<0.05. (C) Transwell assay of 5637
and J82 cells transfected with mimics NC, mimics miR-758-3p,
inhibitor NC or inhibitor miR-758-3p as indicated reported as the
means ± SD from 3 independently repeated experiments. (D) Wound
healing assays of 5637 and J82 cells transfected with NC mimics,
miR-758-3p mimics, NC inhibitor or miR-758-3p inhibitor as
indicated. Unpaired Student's t-test was used and data are
presented as the mean ± SEM. Scale bars, 100 µm. *P<0.05,
**P<0.01 and ***P<0.001. CASC9, cancer susceptibility
candidate 9; miR-758-3p, microRNA-758-3p; BC, bladder cancer; NC,
negative control; SEM, standard error of mean. |
Subsequent luciferase reporter assays revealed that
miR-758-3p overexpression reduced the luciferase activity of a
luciferase reporter harboring wild-type (WT) CASC9 but not the
reporter carrying mutant (MT) CASC9 (Fig. 4B). Collectively, these results
indicated that CASC9 acted as a sponge for miR-758-3p.
miR-758-3p suppresses BC cell
proliferation
To understand the roles of miR-758-3p in BC, wound
healing and Transwell assays were used to assess the effect of
miR-758-3p on BC cell migration and invasion. Consistent with the
theory that CASC9 functions as an oncogene, it was revealed that
miR-758-3p mimics suppressed BC cell migration and invasion, while
a miR-758-3p inhibitor promoted BC cell migration and invasion
(Fig. 4C and D).
The effect of miR-758-3p on cell proliferation was
further evaluated using EdU assays. As revealed in Fig. 5A-C, the proliferation of BC cells
transfected with miR-758-3p mimics was significantly inhibited
compared to the NC group (P<0.05), while BC cell proliferation
in the miR-758-3p inhibitor group was increased compared with the
NC group (P<0.05). The rate of BC cell apoptosis was also
quantified using flow cytometric assays. However, no statistical
significance was observed between any groups of BC cells
(P>0.05; Fig. 5E).
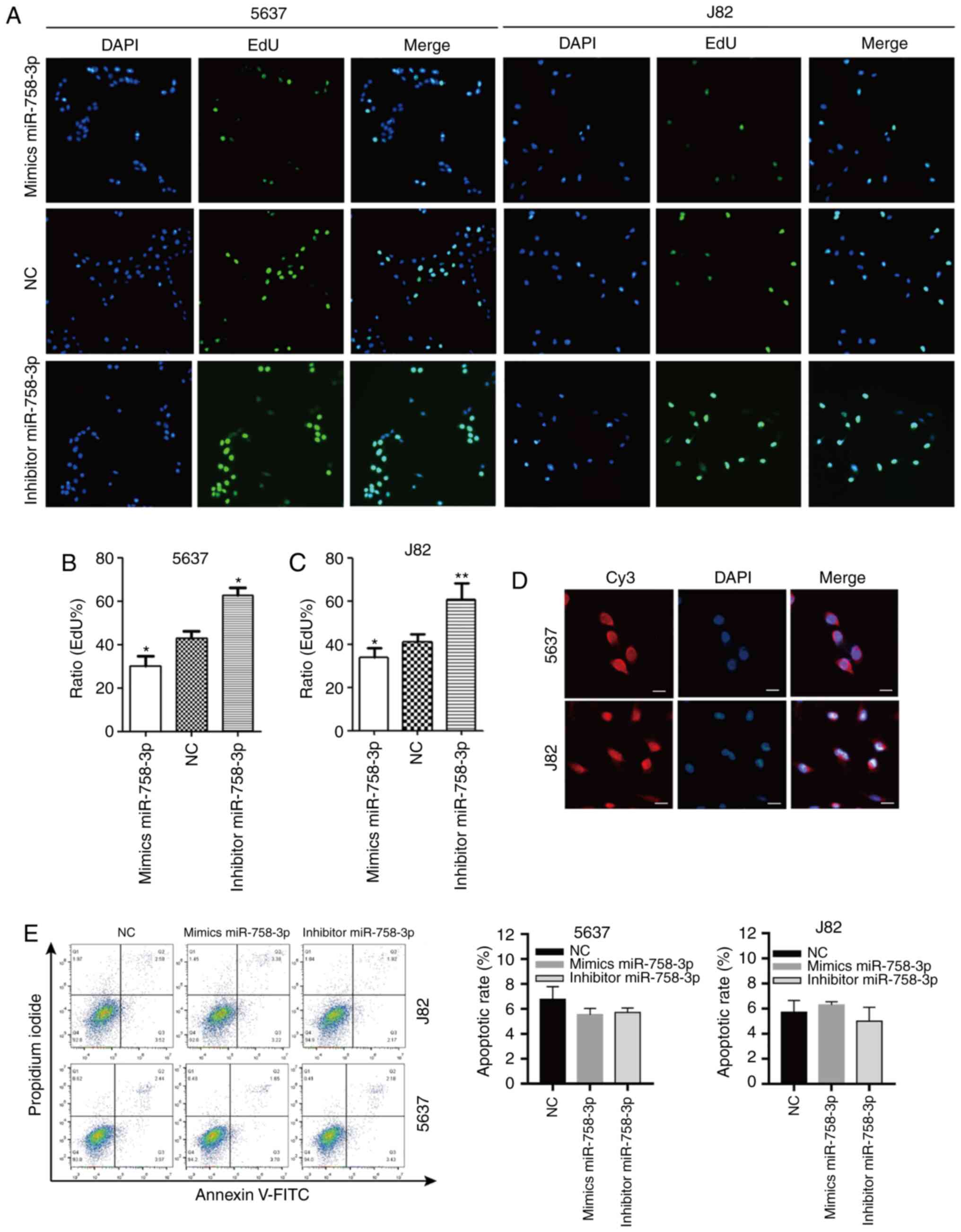 | Figure 5.miR-758-3p inhibits BC cell
proliferation. (A-C) The proliferation rate of 5637 and J82 cells
transfected with 50 nM mimics miR-758-3p, inhibitor miR-758-3p or
NC measured by EdU assay. Scale bars, 100 µm. (D) Subcellular
localization of CASC9 in 5637 and J82 detected by RNA FISH. Scale
bars, 20 µm. (E) The apoptotic rate of miR-758-3p mimic-,
miR-758-3p inhibitor- and NC-transfected cells (P>0.05).
Unpaired Student's t-test was used and data are presented as the
mean ± SEM. *P<0.05 and **P<0.01. miR-758-3p,
microRNA-758-3p; BC, bladder cancer; NC, negative control; EdU,
5-ethynyl-2′-deoxyuridine; FISH, fluorescence in situ
hybridization; SEM, standard error of mean. |
lncRNA CASC9 sponges miR-758-3p to
promote EMT of BC by upregulating TGF-β2
An RNA FISH experiment was also performed to
determine the cellular localization of CASC9. CASC9 was distributed
in both the cytoplasm and nucleus (Fig.
5D), indicating that CASC9 may function in the nucleus and/or
cytoplasm.
In order to explore the function of miR-758-3p,
computational algorithms (TargetScan and miRDB) were used to search
for potential miR-758-3p target genes (Fig. 6B). After knocking down the
expression of CASC9, the expression changes of EMT-related genes
were detected by the Human EMT RT2 Profiler PCR Array
(Fig. 6A). A miR-758-3p-binding
site was revealed in the 3′-UTR of TGF-β2 (Fig. 6C). The TGF-β pathway plays a central
role in inducing EMT in BC (41).
To verify whether TGF-β2 is the direct target of miR-758-3p, the
TGF-β2 3′-UTR sequence was subcloned into the luciferase reporter
vector pSicheck-2. It was revealed that miR-758-3p overexpression
suppressed the luciferase activity of the luciferase reporter
harboring wild-type (WT) TGF-β2 3′-UTR but not the reporter
carrying mutant (MT) TGF-β2 (Fig.
6C).
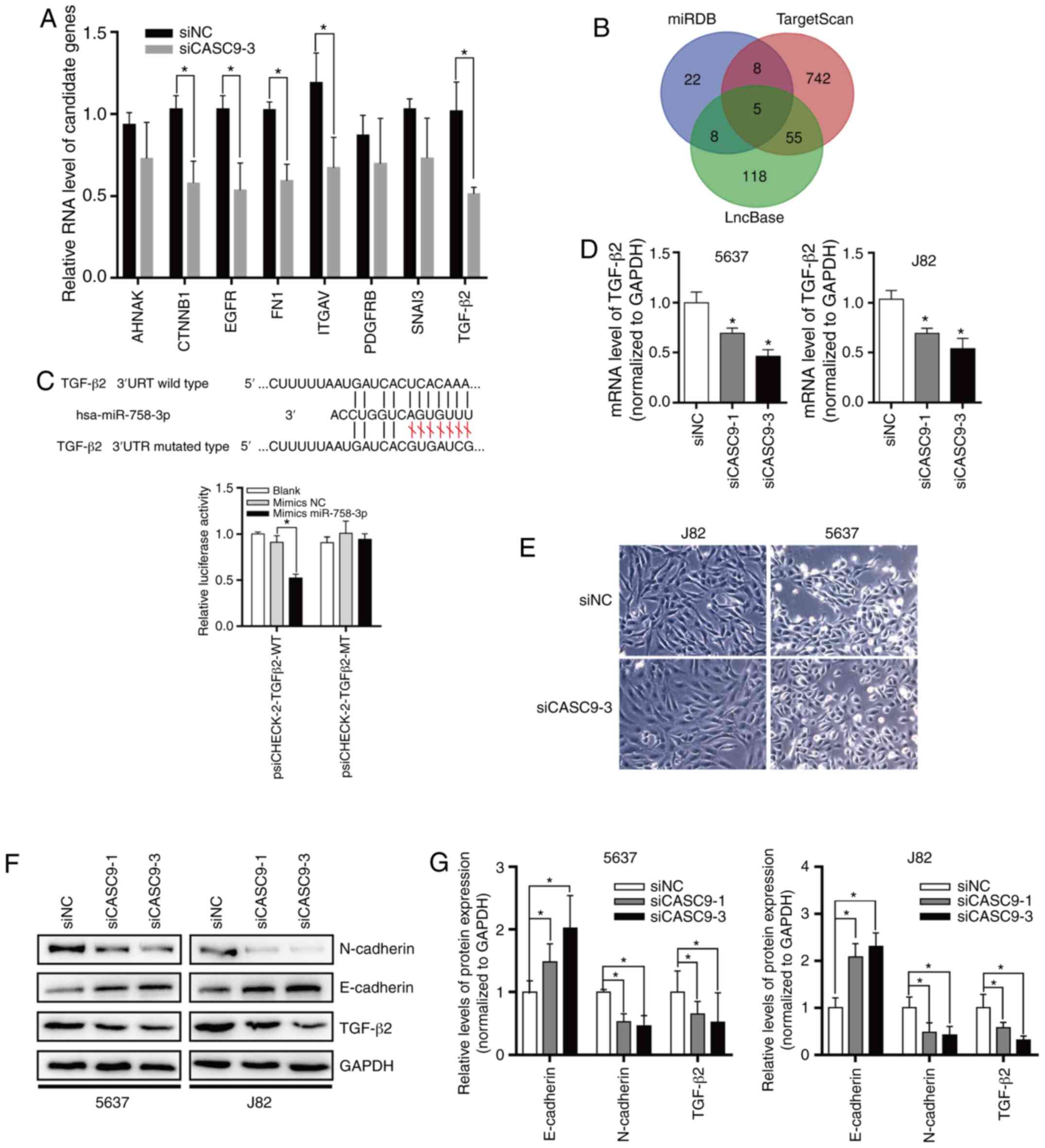 | Figure 6.CASC9 sponges miR-758-3p to promote
EMT in BC by upregulating TGF-β2. (A) After knocking down the
expression of CASC9, the expression changes of EMT-related genes
were detected by the Human EMT RT2 Profiler PCR Array.
(B) Target genes of CASC9 predicted by miRDB, TargetScan and
LncBase. (C) TGF-β2 is a direct target of miR-758-3p. Upper panel,
schematic diagrams of the mutual interactions between miR-758-3p
and TGF-β2 3′UTR. Lower panel, luciferase reporter assay was
performed to examine the effect of CASC9 on antagonizing
miR-758-3p-mediated suppression of TGF-β2 expression. (D) CASC9 was
transfected into BC cells (J82 and 5637), and TGF-β2 mRNA levels
were evaluated by RT-qPCR. (E) Morphological changes of 5637 and
J82 after knocking down CASC9 levels. (F and G) After transfection
with CASC9, the protein levels of TGF-β2 and EMT markers were
evaluated by western blotting. Unpaired Student's t-test was used
and data are presented as the mean ± SEM. *P<0.05. CASC9, cancer
susceptibility candidate 9; miR-758-3p, microRNA-758-3p; EMT,
epithelial-mesenchymal transition; BC, bladder cancer; TGF,
transforming growth factor; RT-qPCR, reverse
transcription-quantitative PCR; SEM, standard error of mean; si,
small interfering; NC, negative control. |
To assess the effect of CASC9 on TGF-β2 expression,
CASC9 was knocked down in 5637 and J82 cells, and it was determined
that CASC9 knockdown reduced TGF-β2 mRNA and protein levels
(Fig. 6D-F). Subsequently, the
protein levels of EMT markers were also determined by western
blotting. The results revealed that after knockdown of CASC9,
E-cadherin protein levels were significantly increased. After
knocking down the expression of CASC9, 5637 and J82 cells exhibited
stromal cell morphological characteristics (Fig. 6E), and N-cadherin protein expression
was significantly decreased in 5637 and J82 cells (Fig. 6F and G).
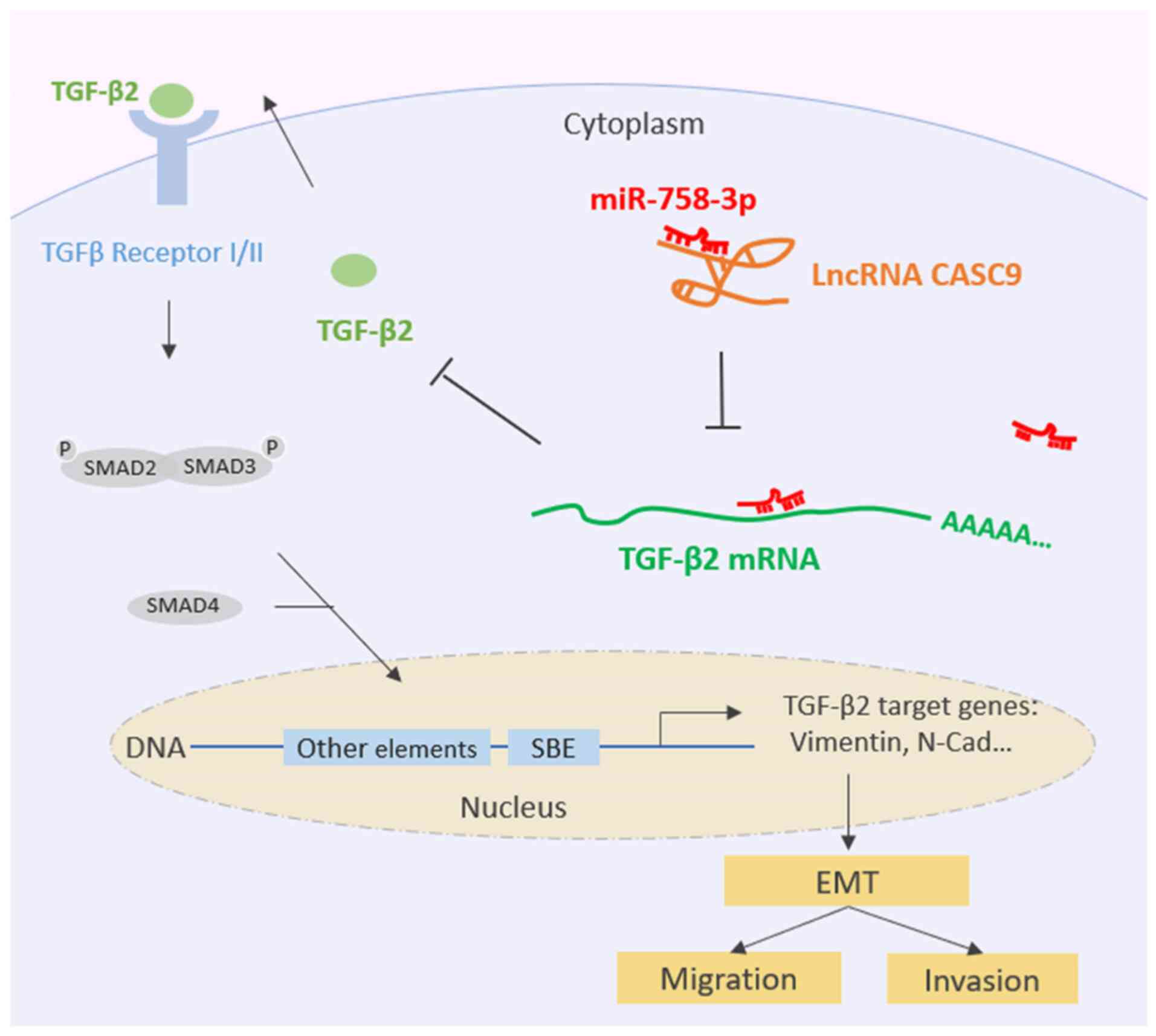
Collectively, all of the aforementioned data
indicated that CASC9 sponges miR-758-3p to promote EMT in BC by
upregulating TGF-β2 (Fig. 7).
Discussion
Previous research has highlighted the potential of
lncRNAs as biomarkers and therapeutic targets in BC (42). The lncRNA CASC9 is located in a gene
desert region that is devoid of nearby protein-coding genes
(30). CASC9 is distributed in both
the cytoplasm and nucleus (30),
suggesting that CASC9 may play a role in the cytoplasm and nucleus
and regulate gene expression in different ways. However, the
relationship between CASC9 and BC remains unknown.
This study is the first, to the best of our
knowledge, to explore the cellular functions of CASC9 in BC. It was
revealed that CASC9 expression was increased in BC tissues, and
CASC9 upregulation was significantly associated with the depth of
bladder tumor invasion. CASC9 knockdown inhibited BC cell
proliferation, migration and invasion. However, knockdown of CASC9
had no effect on BC cell apoptosis. Recent studies have
demonstrated that upregulated CASC9 expression is a poor prognostic
factor for esophageal cancer (28–30),
pancreatic ductal adenocarcinoma (31), gastric cancer (32), nasopharyngeal carcinogenesis
(33), and non-small cell lung
cancer (34). These data support
the present findings that CASC9 functions as an oncogene and plays
a key role in the progression of BC.
Recently, it has been reported that lncRNAs can
function as ceRNAs. Such ceRNAs regulate the distribution of
miRNAs, thereby exerting an additional level of
post-transcriptional regulation (43–46).
In the present study, it was confirmed that CASC9 was upregulated
in BC cells and CASC9 functioned as an effective miRNA (miR-758-3p)
sponge. Consistent with the role of CASC9 as an oncogene, it was
revealed that miR-758-3p was downregulated in BC. The
downregulation of miR-758-3p has also been observed in
hepatocellular carcinoma (HCC), papillary thyroid cancer (PTC)
(47), gastric cancer (GC)
(48) and non-small cell lung
cancer (NSCLC) (49). However, the
molecular mechanisms that underlie the tumor suppressive role of
miR-758-3p remains unknown. In the present study, it was
demonstrated that miR-758-3p downregulation activated TGF-β2 and
promoted cell growth and metastasis in BC.
Increased expression of TGF-β isoforms, receptors,
and signaling components is reported in high-grade invasive BC
cells expressing vimentin and lacking E-cadherin (50). TGF-β activation promotes BC
metastasis (12). Upregulation of
phosphorylated SMAD2 has been reported in advanced invasive BC and
associated with more frequent recurrence and poor survival
(50). In human glioma, TGF-β2
initiated autophagy via SMAD and non-SMAD pathways, thereby
promoting the invasion of glioma cells (51).
In the nucleus, CASC9 was revealed to recruit CBP
and modulate H3K27ac levels of the LAMC2 promoter, thereby
promoting LAMC2 transcription and stimulating ESCC cell growth
(30). In the cytoplasm, CASC9 was
revealed to sponge miR-758-3p to regulate the expression of TGF-β2,
which activated the TGF-β signaling pathway and promoted
proliferation and EMT in BC. In addition to interactions with
miRNAs, lncRNAs also act as protein scaffolds to mediate protein
interactions (18–21). More functions of CASC9 remain to be
revealed. Notably, CASC9 appears to utilize various mechanisms to
achieve its functions (28–30). Interactions with other proteins or
even other nucleic acids (e.g., miRNAs, mRNAs, ncRNAs, or DNA)
increase the spectrum of CASC9 functions. The function of CASC9
depends on the molecular context of the corresponding cancer cell.
CASC9 plays different roles by interacting with other proteins or
nucleic acids, such as miRNA, mRNA, ncRNA or DNA (28–30).
In summary, the present study revealed that CASC9
sponged miR-758-3p to regulate the expression of TGF-β2, a cytokine
that activates the TGF-β signaling pathway and promoted
proliferation and EMT in BC. These data allowed us to conclude that
CASC9 plays an important role in the complex regulatory interaction
network that controls the progression of BC. This regulatory
mechanism facilitates our understanding of EMT of BC as well as
other relevant human diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guangdong
Basic and Applied Basic Research Fund (Guangdong Natural Science
Fund, grant no. 2019A1515110766), and Longhua Science and
Technology Innovation Program (grant no. 2017029).
Availability of data and materials
The data that support the findings of this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JY and YL conceived the study and analyzed the
findings. ZZ, FC and YL performed all of the experiments. ZZ and FC
wrote the manuscript. FC, HZ, LC, TX and QD assisted in performing
the experiments. QD provided partial funding support and manuscript
modification. All the authors reviewed and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The collection and use of all tissues were approved
by the Ethic Committee of Peking University Shenzhen Hospital. All
human tissue samples were obtained with informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richters A, Aben KKH and Kiemeney L: The
global burden of urinary bladder cancer: An update. World J Urol.
38:1895–1904. 2020. View Article : Google Scholar
|
|
2
|
Grayson M: Bladder cancer. Nature. 551
(Suppl 1):S332017. View
Article : Google Scholar
|
|
3
|
Smith ZL and Guzzo TJ: Urinary markers for
bladder cancer. F1000Prime Rep. 5:212013. View Article : Google Scholar
|
|
4
|
Kamat AM, Hegarty PK, Gee JR, Clark PE,
Svatek RS, Hegarty N, Shariat SF, Xylinas E, Schmitz-Dräger BJ,
Lotan Y, et al: ICUD-EAU international consultation on bladder
cancer 2012: Screening, diagnosis, and molecular markers. Eur Urol.
63:4–15. 2013. View Article : Google Scholar
|
|
5
|
Santamaria PG, Moreno-Bueno G, Portillo F
and Cano A: EMT: Present and future in clinical oncology. Mol
Oncol. 11:718–738. 2017. View Article : Google Scholar
|
|
6
|
Singh M, Yelle N, Venugopal C and Singh
SK: EMT: Mechanisms and therapeutic implications. Pharmacol Ther.
182:80–94. 2018. View Article : Google Scholar
|
|
7
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21:9652016. View Article : Google Scholar
|
|
8
|
Pastushenko I, Brisebarre A, Sifrim A,
Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D,
Moers V, Lemaire S, et al: Identification of the tumour transition
states occurring during EMT. Nature. 556:463–468. 2018. View Article : Google Scholar
|
|
9
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar
|
|
10
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar
|
|
11
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar
|
|
12
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: TGF-β-induced upregulation of malat1 promotes bladder
cancer metastasis by associating with suz12. Clin Cancer Res.
20:1531–1541. 2014. View Article : Google Scholar
|
|
13
|
Calon A, Espinet E, Palomo-Ponce S,
Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung
P, Zhang XH, et al: Dependency of colorectal cancer on a
TGF-β-driven program in stromal cells for metastasis initiation.
Cancer Cell. 22:571–584. 2012. View Article : Google Scholar
|
|
14
|
Papageorgis P: TGFβ signaling in tumor
initiation, epithelial-to-mesenchymal transition, and metastasis. J
Oncol. 2015:5871932015. View Article : Google Scholar
|
|
15
|
Kapranov P, Cheng J, Dike S, Nix DA,
Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J,
Hofacker IL, et al: RNA maps reveal new RNA classes and a possible
function for pervasive transcription. Science. 316:1484–1488. 2007.
View Article : Google Scholar
|
|
16
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long noncoding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016. View Article : Google Scholar
|
|
17
|
Xiong T, Li J, Chen F and Zhang F: PCAT-1:
A novel oncogenic Long Non-coding RNA in human cancers. Int J Biol
Sci. 15:847–856. 2019. View Article : Google Scholar
|
|
18
|
Flynn RA and Chang HY: Long noncoding RNAs
in cell-fate programming and reprogramming. Cell Stem Cell.
14:752–761. 2014. View Article : Google Scholar
|
|
19
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar
|
|
20
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar
|
|
21
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar
|
|
22
|
Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao
SD, Zhang Y, Yang L, Shan W, He Q, et al: Comprehensive genomic
characterization of long non-coding RNAs across human cancers.
Cancer Cell. 28:529–540. 2015. View Article : Google Scholar
|
|
23
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar
|
|
24
|
Han Y, Liu Y, Nie L, Gui Y and Cai Z:
Inducing cell proliferation inhibition, apoptosis, and motility
reduction by silencing long noncoding ribonucleic acid
metastasis-associated lung adenocarcinoma transcript 1 in
urothelial carcinoma of the bladder. Urology. 81:209.e1–e7. 2013.
View Article : Google Scholar
|
|
25
|
Han Y, Liu Y, Gui Y and Cai Z: Long
intergenic non-coding RNA TUG1 is overexpressed in urothelial
carcinoma of the bladder. J Surg Oncol. 107:555–559. 2013.
View Article : Google Scholar
|
|
26
|
Yang C, Li X, Wang Y, Zhao L and Chen W:
Long non-coding RNA UCA1 regulated cell cycle distribution via CREB
through PI3-K dependent pathway in bladder carcinoma cells. Gene.
496:8–16. 2012. View Article : Google Scholar
|
|
27
|
Yan TH, Lu SW, Huang YQ, Que GB, Chen JH,
Chen YP, Zhang HB, Liang XL and Jiang JH: Upregulation of the long
noncoding RNA HOTAIR predicts recurrence in stage Ta/T1 bladder
cancer. Tumour Biol. 35:10249–10257. 2014. View Article : Google Scholar
|
|
28
|
Pan Z, Mao W, Bao Y, Zhang M, Su X and Xu
X: The long noncoding RNA CASC9 regulates migration and invasion in
esophageal cancer. Cancer Med. 5:2442–2447. 2016. View Article : Google Scholar
|
|
29
|
Wu Y, Hu L, Liang Y, Li J, Wang K, Chen X,
Meng H, Guan X, Yang K and Bai Y: Up-regulation of lncRNA CASC9
promotes esophageal squamous cell carcinoma growth by negatively
regulating PDCD4 expression through EZH2. Mol Cancer. 16:1502017.
View Article : Google Scholar
|
|
30
|
Liang Y, Chen X, Wu Y, Li J, Zhang S, Wang
K, Guan X, Yang K and Bai Y: LncRNA CASC9 promotes esophageal
squamous cell carcinoma metastasis through upregulating LAMC2
expression by interacting with the CREB-binding protein. Cell Death
Differ. 25:1980–1995. 2018. View Article : Google Scholar
|
|
31
|
Yu X, Lin Y, Sui W, Zou Y and Lv Z:
Analysis of distinct long noncoding RNA transcriptional
fingerprints in pancreatic ductal adenocarcinoma. Cancer Med.
6:673–680. 2017. View Article : Google Scholar
|
|
32
|
Shang C, Sun L, Zhang J, Zhao B, Chen X,
Xu H and Huang B: Silence of cancer susceptibility candidate 9
inhibits gastric cancer and reverses chemoresistance. Oncotarget.
8:15393–15398. 2017. View Article : Google Scholar
|
|
33
|
Su X, Li G and Liu W: The long noncoding
RNA cancer susceptibility candidate 9 promotes nasopharyngeal
carcinogenesis via stabilizing HIF1α. DNA Cell Biol. 36:394–400.
2017. View Article : Google Scholar
|
|
34
|
Ma P, Zhang M, Nie F, Huang Z, He J, Li W
and Han L: Transcriptome analysis of EGFR tyrosine kinase
inhibitors resistance associated long noncoding RNA in non-small
cell lung cancer. Biomed Pharmacother. 87:20–26. 2017. View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative pcr and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Li Y, Quan J, Chen F, Pan X, Zhuang C,
Xiong T, Zhuang C, Li J, Huang X, Ye J, et al: MiR-31-5p acts as a
tumor suppressor in renal cell carcinoma by targeting
cyclin-dependent kinase 1 (CDK1). Biomed Pharmacother. 111:517–526.
2019. View Article : Google Scholar
|
|
37
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: Gepia: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar
|
|
38
|
Paraskevopoulou MD, Vlachos IS, Karagkouni
D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P,
Floros E, Dalamagas T and Hatzigeorgiou AG: Diana-lncbase v2:
Indexing microrna targets on non-coding transcripts. Nucleic Acids
Res. 44:D231–D238. 2016. View Article : Google Scholar
|
|
39
|
Chen Y and Wang X: MiRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48:D127–D131. 2020. View Article : Google Scholar
|
|
40
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar
|
|
41
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar
|
|
42
|
Chandra Gupta S and Nandan Tripathi Y:
Potential of long non-coding RNAs in cancer patients: From
biomarkers to therapeutic targets. Int J Cancer. 140:1955–1967.
2017. View Article : Google Scholar
|
|
43
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar
|
|
44
|
Kumar MS, Armenteros-Monterroso E, East P,
Chakravorty P, Matthews N, Winslow MM and Downward J: HMGA2
functions as a competing endogenous RNA to promote lung cancer
progression. Nature. 505:212–217. 2014. View Article : Google Scholar
|
|
45
|
Jeyapalan Z, Deng Z, Shatseva T, Fang L,
He C and Yang BB: Expression of CD44 3′-untranslated region
regulates endogenous microRNA functions in tumorigenesis and
angiogenesis. Nucleic Acids Res. 39:3026–3041. 2011. View Article : Google Scholar
|
|
46
|
Cesana M, Cacchiarelli D, Legnini I,
Santini T, Sthandier O, Chinappi M, Tramontano A and Bozzoni I: A
long noncoding RNA controls muscle differentiation by functioning
as a competing endogenous RNA. Cell. 147:358–369. 2011. View Article : Google Scholar
|
|
47
|
Chen J, Xu Z, Yu C, Wu Z, Yin Z, Fang F
and Chen B: MiR-758-3p regulates papillary thyroid cancer cell
proliferation and migration by targeting TAB1. Pharmazie.
74:235–238. 2019.
|
|
48
|
Guo J, Zhang Z, Pan L and Zhou Y:
Identification of miR-758-3p as potential modulator of CBX5
expression in gastric cancer. Technol Cancer Res Treat.
17:15330338188160612018. View Article : Google Scholar
|
|
49
|
Wang S and Jiang M: The long non-coding
RNA-DANCR exerts oncogenic functions in non-small cell lung cancer
via miR-758-3p. Biomed Pharmacother. 103:94–100. 2018. View Article : Google Scholar
|
|
50
|
Gupta S, Hau AM, Al-Ahmadie HA, Harwalkar
J, Shoskes AC, Elson P, Beach JR, Hussey GS, Schiemann WP, Egelhoff
TT, et al: Transforming growth factor-beta is an upstream regulator
of mammalian target of rapamycin complex 2-dependent bladder cancer
cell migration and invasion. Am J Pathol. 186:1351–1360. 2016.
View Article : Google Scholar
|
|
51
|
Zhang C, Zhang X, Xu R, Huang B, Chen AJ,
Li C, Wang J and Li XG: TGF-β2 initiates autophagy via Smad and
non-Smad pathway to promote glioma cells' invasion. J Exp Clin
Cancer Res. 36:1622017. View Article : Google Scholar
|