Introduction
The unprecedented development of chemotherapeutics
has been effective in the treatment of malignant leukemia; however,
in 2013 the overall survival rate of patients with hematological
malignancies was unsatisfactory, at only ~40% (1–3), as
multidrug-resistance (MDR) disease eventually develops in the
majority of patients. Intrinsic or treatment-induced acquired MDR,
which is a phenomenon characterized by resistance to structurally
and mechanistically unrelated drugs, is considered to be the
primary reason for chemotherapeutic failure and leads to the death
of patients with advanced leukemia (4). ATP-binding cassette (ABC) transporters
have been widely considered to play pivotal roles in clinical drug
resistance (5,6). The overexpression of ABC transporter
proteins, which function as active drug efflux pumps, can reduce
the efficacy of anticancer drugs.
Among the ABC-protein family, the proteins encoded
by the ABCB1 (P-gp), ABCC1 (MRP1) and ABCG2 (BCRP) genes have been
associated with MDR. Previous studies have demonstrated that ABCG2
was associated with unsatisfactory chemotherapeutic effects,
leading to a lower sensitivity to chemotherapy, an increased risk
of relapse and a shorter disease-free survival rate in patients
with acute lymphoblastic leukemia (7,8). ABCG2
could be a promising therapeutic target for the eradication of
leukemia stem cells (LSCs) (9),
based on the evidence that ABCG2 was responsible for the
identification and isolation of the ‘side population’ (SP)
phenotype using flow cytometry, due to the efflux of Hoechst 33342
from tumor cells, with cancer stem cell characteristics (10). SP cells are highly rich in LSCs,
which are responsible for asymmetric cell division, self-renewal
capacity and the maintenance of leukemia (11,12).
Furthermore, ABCG2 facilitated the effusion of antineoplastic
agents out of LSCs and plays a crucial role in the differentiation,
proliferation and self-renewal of LSCs (13,14).
These results suggested that ABCG2 may be a promising therapeutic
target for the eradication of LSCs.
On account of their favorable therapeutic responses,
tyrosine kinase inhibitors (TKIs) have been regarded as promising
agents for the majority of patients with leukemia (15). TKIs have been found to not only
directly inhibit the growth and metastasis of cancer cells
(16), but also to enhance the
anti-cancer effects of traditional chemotherapeutic agents by
suppressing the drug transport activity of ABCG2, or by directly
reducing the expression of ABCG2 (17). Therefore, combined treatment with
TKIs and conventional cytotoxic agents may improve survival
times.
Tucatinib (also known as ONT-380, ARRY380 or
irbinitinib) is a small molecule taken orally (480.532 g/mol), and
a selective HER2 inhibitor for the treatment of HER2-positive solid
tumors, including breast cancer (18) and colorectal cancer (19). Tucatinib was approved in the USA in
April 2020 and in Switzerland in May 2020 for the treatment of
HER2-positive breast cancer, which is the most aggressive type of
breast cancer (20). Tucatinib has
been proven to inhibit HER2 kinase activity, with nanomolar potency
and to provide notable selectivity for HER2 compared with that for
the related receptor tyrosine kinase, EGFR (19). Tucatinib is active as a single agent
in multiple HER2+ tumor models and exhibits increased
antitumor activity, when used in combination with conventional
chemotherapeutic drugs, thereby increasing the rate of partial and
complete tumor regression (21).
Furthermore, tucatinib may provide an efficient therapeutic effect
for patients with breast cancer and brain metastases, as it can
penetrate the blood-brain barrier (22). Therefore, from these pre-clinical
data, the present study aimed to determine whether tucatinib could
also exert an inhibitory effect on hematological malignancies, with
a high incidence of brain metastasis. The aim of current study was
to investigate the effect of tucatinib on conventional
chemotherapeutic agent retention in ABCG2-overexpressing leukemia
cells and leukemia stem cells and characterize the interactions of
tucatinib with ABCG2 transporters in primary leukemic blasts. The
findings of the present study may provide new prospects for
overcoming MDR of LSCs.
Materials and methods
Chemicals and reagents
Tucatinib (purity 99.38%) was purchased from Selleck
Chemicals. Topotecan, Hoechst 33342, mitoxantrone, cisplatin and
fumitremorgin C (FTC) were purchased from Sigma-Aldrich (Merck
KGaA). RPMI-1640, DMEM, BSA, FBS, penicillin/streptomycin and 0.25%
trypsin were purchased from Hyclone (GE Healthcare Life Sciences).
[3H]-mitoxantrone (4 Ci/mmol) was purchased from Moravek
Biochemicals, Inc. The monoclonal antibody against ABCG2 was
purchased from Santa Cruz Biotechnology, Inc., while the
AlexaFluor488-conjugated goat anti-mouse IgG secondary antibody was
purchased from Signet Laboratories Inc. HRP-conjugated rabbit
anti-sheep IgG secondary antibody was purchased from Sigma-Aldrich
(Merck KGaA), while the monoclonal antibody against GAPDH was
purchased from Kangcheng BioTech Co., Ltd. Dimethylsulfoxide
(DMSO), MTT and paraformaldehyde were purchased from Sigma-Aldrich
(Merck KGaA). Mitoxantrone, topotecan and FTC were used as positive
controls to confirm the mechanism of drug resistance in LSC models.
Cisplatin (a non-substrate of ABCG2) was used as a negative
control.
Cell lines and cell culture
The human HL60 and K562 leukemia cell lines were
purchased from the Institute of Hematology and Blood Diseases
Hospital, Chinese Academy of Medical Sciences and Peking Union
Medical College (Tianjin, China). The HL60/ABCG2 and K562/ABCG2
cell lines (which overexpress ABCG2) were established, in the
present study, by the transduction of the HL60 and K562 cell lines,
respectively, with a HaMyBCRP retrovirus that contains Myc-tagged
human BCRP (ABCG2) cDNA in Ha retrovirus vector (23–25).
Subsequently, the transfected cells were selected using 4.0 µM
mitoxantrone for 7 days. The cell lines were cultured in RPMI-1640
containing 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin,
at 37°C in a humidified atmosphere with 5% CO2, as
previously described (23).
Cytotoxicity evaluation using a MTT
assay
MTT (purity 99.59%) reagent was used to determine
the cell sensitivity to different chemotherapeutic agents
(mitoxantrone, topotecan, cisplatin and tucatinib) with minor
modifications (26). The
IC50, which is defined as the drug concentration
resulting in 50% cell death, was calculated from the cell survival
curves using the Bliss method (27–29).
The cells were collected and seeded in 96-well plates at
5×103 cells/well in 160 µl medium. After culturing for
24 h, the cells were pre-incubated with 0.1, 0.2 and 0.4 µM
tucatinib for a further 72 h at 37°C. The cells were then treated
with various concentrations of the chemotherapeutic agents by
2-fold dilution (mitoxantrone, 0.5–50 µM; topotecan, 0.5–50 µM; and
cisplatin, 0.5–50 µM) for a further 68 h. Subsequently, 20 µl MTT
solution (5 mg/ml) was added to the cells, followed by further
incubation for 4 h at 37°C then, the medium was discarded, and 200
µl DMSO was added to dissolve the formazan product formed from the
metabolism of MTT. The absorbance was determined at 540 nm, with a
background subtraction at 670 nm, using a Model550 microplate
reader (Bio-Rad Laboratories, Inc.) (30). The resistance fold change was
calculated by dividing the IC50 values of the
substrates, in the presence or absence of tucatinib, by the
IC50 of the parental cells without the inhibitor
treatment (23).
Patient samples
The present study was approved by the Ethics Review
Committee at Sun Yat-Sen University. A total of 15 patients with
leukemia were recruited into the study between June 2017 and April
2018; however, the bone marrow samples were obtained from 6
patients, at diagnosis, and randomly selected from a study pool of
blast samples and examined. The patient population consisted of 2
males and 4 females, with a median age of 27 years. According to
the French-American-British classification (31), three of the patients were diagnosed
with acute lymphocytic leukemia, two of the patients were diagnosed
with acute myeloid leukemia, and one patient was diagnosed with T
lymphoblastic lymphoma. After the patients provided written
informed consent, leukemia blasts were isolated using
Ficoll-Hypaque density gradient centrifugation (at 300 × g for 25
min at room temperature) and cultured in RPMI-1640, containing 20%
FBS, with penicillin (100 U/ml) and streptomycin (100 U/ml) at
37°C. Western blot analysis was performed to detect the protein
expression level of ABCG2 in the patient samples. The patient
characteristics are summarized in Table S1.
SP analysis and sorting
The sorting and analysis of the SP cells was
performed according to the methods described by Vieyra et al
(32). In brief, the cells
(1×106 cells/ml) were cultured in prepared DMEM,
containing 2% FBS and 10 mmol/I HEPES. Subsequently, 5 µg/ml
Hoechst 33342 dye was added to the cells in the presence or absence
of 10 µmol/l FTC. Following incubation for 90 min, with
intermittent shaking at 37°C, the cells were washed twice with
ice-cold PBS. The Hoechst dye was excited at 355 nm, and the
fluorescence profile was measured during the analysis (blue,
402–446 nm; red, 650–670 nm; MoFlo™ XDP; Beckman Coulter). The
Summit v5.2 Software (Beckman Coulter) was used for the
analysis.
Intracellular Hoechst 33342
accumulation assay
The HL60/ABCG2 cell lines (1×105) were
cultured in 6-well plates, containing various concentrations of
tucatinib (0.1, 0.2 and 0.4 µM) for 24 h. Subsequently, 0.5 µg/ml
Hoechst 33342 dye was added, followed by a further incubation for
30 min at 37°C. Finally, the cells were washed with ice-cold PBS,
three times and re-suspended in PBS for flow cytometric analysis
(MoFlo™ XDP; Beckman Coulter). The Summit v5.2 Software (Beckman
Coulter) was used for the analysis.
[3H]-mitoxantrone
accumulation assay
The [3H]-mitoxantrone intracellular
accumulation assay was used for the assessment of the reversal
effects of tucatinib. In brief, the HL60, K562, HL60/ABCG2 and
K562/ABCG2 cells (5×105) were suspended in RPMI 1640
medium, then incubated with or without 0.1 and 0.4 µM tucatinib or
2.5 µM FTC for 1 h at 37°C. Subsequently, the cells were cultured
in medium containing 0.1 µM [3H]-mitoxantrone for a
further 2 h at 37°C. After washing twice with 10 ml ice-cold PBS
and lysed with 1% SDS (Ph 7.4), the radioactivity of the cells was
measured. Each sample was placed in scintillation fluid (5 ml),
then the radioactivity was analyzed using a Packard TRI-CARB 1900CA
liquid scintillation analyzer (PerkinElmer, Inc.), as described
previously (33).
[3H]-mitoxantrone efflux
assay
Following the accumulation assay, the HL60, K562,
HL60/ABCG2 and K562/ABCG2 cells (5×105) were suspended
in RPMI 1640 medium, pre-incubated with or without 0.1 and 0.4 µM
tucatinib or 2.5 µM FTC for 2 h at 37°C. Subsequently, 0.1 µM
[3H]-mitoxantrone was added to each sample for 2 h at
37°C, and following washing with ice-cold PBS, the cells were lysed
at various time points (0, 30, 60 and 120 min). Each sample was
placed in scintillation fluid and the radioactivity was analyzed
(Packard TRI-CARB 1900CA liquid scintillation analyzer;
PerkinElmer, Inc.), as previously described (33). The Spectra Works2™ v2.0 STD and
Quanta-Smart v5.2 Software (PerkinElmer, Inc.) were used for the
analysis.
ATPase assay
The ABCG2-associated ATPase activity assay was
determined using a SB-BRCP-M-PREDEASY-ATPase kit (TEBU-BIO nv,),
with modified protocols (34). Cell
membranes that overexpressed ABCG2 were incubated with an assay
buffer containing 5 mM sodium azide, 1 mM ouabain, 2 mM
dithiothreitol, 10 mM MgCl2, 50 mM potassium chloride, 2
mM ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetra acetic
acid, 50 mM pH 6.8 2-(N-morpholino) ethanesulfonic acid MES and 0.3
mM sodium orthovanadate (Na3VO4) at 37°C for
5 min. Na3VO4 was used as an ATPase
inhibitor. Various concentrations of tucatinib (0–40 µM) were
incubated with the membranes for 5 min at 37°C. The ATPase reaction
was initiated by the addition of 5 mM Mg2+ ATP.
Luminescent signals of free phosphate group (Pi) were initiated and
measured at 880 nm using a spectrophotometer (Bio Rad Laboratories,
Inc.) following incubation at 37°C for 40 min with brief mixing.
The changes in relative light units were determined by comparing
Na3VO4-treated samples with the
tucatinib-treated groups, using the colorimetric method (35).
Western blot analysis
Western blot analysis was performed to determine the
protein expression levels of ABCG2, following treatment with 0.1,
0.2, 0.4 and 1 µM tucatinib for 48 h or with 0.4 µM tucatinib for
0, 24, 36, 48 and 72 h. The HL60/ABCG2 cells were incubated with 0,
0.1, 0.2, 0.4 and 1 µM tucatinib for 12 h, then harvested and
washed twice with ice-cold PBS. The cell extracts were collected
using a cell lysis buffer and protein concentration was determined
using a BCA Protein assay kit (both from Thermo Fisher Scientific,
Inc.) (36). Equal amounts of total
cell lysates (30 µg protein) were resolved using a 10% SDS-PAGE and
electrophoretically transferred onto PVDF membranes (EMD
Millipore). The membranes were blocked with 5% skimmed milk
dissolved in TBS-Tween-20 (TBST) buffer (10 mmol/l Tris-HCl, 150
mmol/l NaCl and 0.1% Tween-20, pH 8.0) for 2 h at room temperature.
The membranes were then incubated with the primary monoclonal
antibodies against GAPDH (cat. no. KC-5G4; 1:1,000) or ABCG2 (cat.
no. MAB4145; clone BXP-34; 1:200) at 4°C overnight, then with
HRP-conjugated secondary antibody (cat. no. AP147P; 1:1,000) at
room temperature for 2 h. Subsequently, the protein-antibody
complexes were washed three times with TBST and protein bands were
visualized using an enhanced chemiluminescence detection system
(Phototope TM-HRP Detection kit; Cell Signaling Technology, Inc.)
and the protein bands were analyzed using Scion Image v4.0.3
software (Scion Corporation). GAPDH was used as a loading
control.
Immunofluorescence staining
The HL60/ABCG2 cells were cultured overnight in
24-well plates, then 0.4 µM tucatinib was added to each well for
further incubation for 72 h. After washing with PBS, the treated
cells were fixed with 4% paraformaldehyde for 15 min and
permeabilized using 0.1% Triton X-100 for 10 min, both at room
temperature. Subsequently, the cells were incubated with a
monoclonal antibody against ABCG2 (cat. no. MAB4145; clone BXP-34;
(1:500), overnight at 4°C. Following incubation, the cells were
washed with ice-cold PBS and incubated with an Alexa Fluor
488-conjugated goat anti-mouse IgG secondary antibody (cat. no.
A-10684; 1:1,000) for 1 h, at 4°C. Immunofluorescence images were
obtained using an inverted confocal microscope, and 6–8 random
microscopic fields (magnification, ×400; model IX70; Olympus
Corporation) with IX-FLA fluorescence and a Charge Coupled Devices
camera.
Statistical analysis
Statistical analysis was performed using SPSS v16.0
software (SPSS, Inc.) and data are presented as the mean ± SD, from
3–5 independent experiments. Statistical differences between 2
groups were determined using an unpaired Student's t-test. One-way
ANOVA was used to determine differences between the means of
multiple samples, with a Dunnett's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Tucatinib markedly potentiates the
cytotoxicity of ABCG2 substrate anticancer drugs in leukemia
cells
Prior to investigating the cytotoxicity of
tucatinib, the protein expression levels of ABCG2 in the
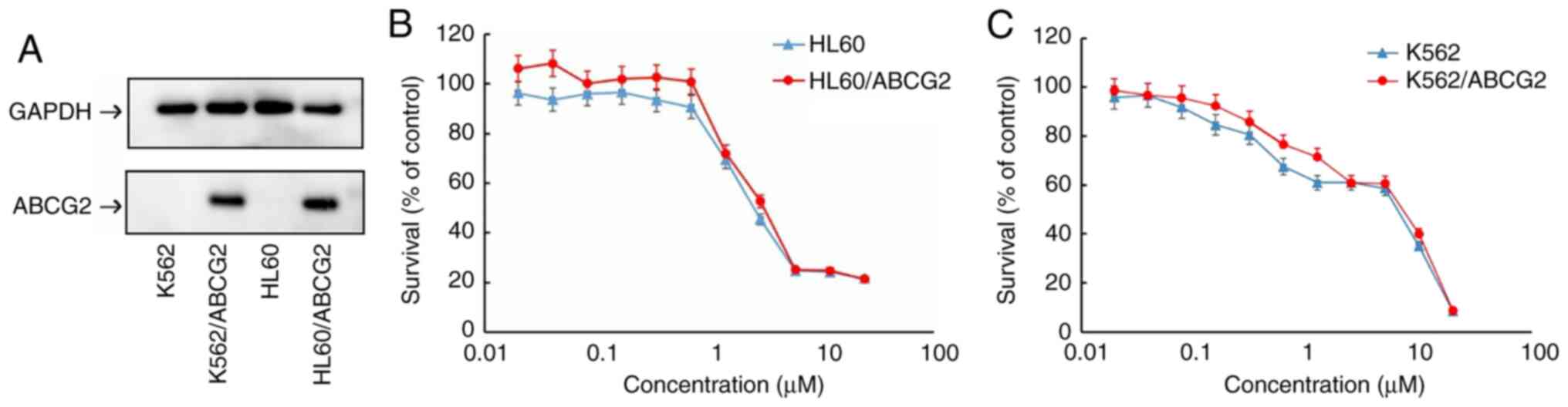
transfected cell lines were confirmed using western blot analysis.
The protein expression levels of ABCG2 were overexpressed in the
HL60/ABCG2 and K562/ABCG2 cell lines compared with that in the HL60
and K562 parental cell lines, respectively (Fig. 1A).
To investigate the effects of tucatinib on leukemia
cells, MTT assays were subsequently performed to determine the
cytotoxicity of tucatinib on 2 leukemia cell lines. As shown in
Fig. 1B and C, >80% of the
HL60/ABCG2 and K562/ABCG2 ABCG2-overexpressing cell lines, and
their parental cell lines, HL60 and K562, survived 0.4 µM tucatinib
treatment. Therefore, 0.4 µM tucatinib was selected as the maximum
working concentration for further experiments. Subsequently,
whether tucatinib, at various concentrations, could increase the
sensitivity of ABCG2-overexpressing leukemia drug resistant cells
to mitoxantrone and topotecan was investigated. As shown in
Tables I and II, the ABCG2-overexpressing HL60/ABCG2
and K562/ABCG2 cell lines showed higher IC50 values to
the ABCG2 substrates, mitoxantrone and topotecan compared with that
in their parental cell lines, respectively. In the presence of 0.1
and 0.2 µM tucatinib, there was a significant increase in
sensitivity of the cell lines to the two drugs. Tucatinib (0.4 µM)
further increased the sensitivity of leukemia cells to the two
drugs in both the ABCG2-overexpressing HL60/ABCG2 and K562/ABCG2
cell lines, and its efficacy was comparable to that of the known
ABCG2 inhibitor, FTC (2.5 µM). Conversely, tucatinib did not
significantly alter the IC50 value of cisplatin in all
the leukemia cell lines, which is a non-ABCG2 substrate. Taken
together, these results suggested that tucatinib may significantly
sensitize ABCG2-overexpressing leukemia cells to become
anti-neoplastic.
 | Table I.Effect of tucatinib on reversing
ABCG2-mediated MDR in the HL60/ABCG2 cells lines. |
Table I.
Effect of tucatinib on reversing
ABCG2-mediated MDR in the HL60/ABCG2 cells lines.
|
| IC50 ±
SDa, µM | Resistance
fold |
|---|
|
|
|
|
|---|
| Compound | HL60 | HL60/ABCG2 | HL60 | HL60/ABCG2 |
|---|
| Mitoxantrone | 0.727±0.058 | 32.612±1.020 | 1.00b | 44.858b |
|
+Tucatinib 0.1 µM | 0.793±0.081 | 6.522±0.098 | 1.091 | 8.971d |
|
+Tucatinib 0.2 µM | 0.730±0.092 | 1.855±0.064 | 1.004 | 2.552d |
|
+Tucatinib 0.4 µM | 0.677±0.089 | 0.603±0.043 | 0.931 | 0.829d |
| +FTC
2.5 µM | 0.433±0.035 | 0.509±0.038 | 0.596c | 0.700d |
| Topotecan | 0.684±0.044 | 28.960±1.004 | 1.00b | 42.339b |
|
+Tucatinib 0.1 µM | 0.496±0.053 | 7.283±0.083 | 0.725 | 10.645d |
|
+Tucatinib 0.2 µM | 0.533±0.045 | 4.021±0.079 | 0.779 | 5.877d |
|
+Tucatinib 0.4 µM | 0.401±0.039 | 1.829±0.063 | 0.586c | 2.674d |
| +FTC
2.5 µM | 0.376±0.053 | 0.915±0.051 | 0.550c | 1.337d |
| Cisplatin | 15.561±0.971 | 18.611±0.899 | 1.00b | 1.00b |
|
+Tucatinib 0.4 µM | 14.873±0.915 | 19.799±0.926 | 0.956 | 0.94 |
 | Table II.Effect of tucatinib on reversing
ABCG2-mediated multidrug resistance in K562/ABCG2 cells lines. |
Table II.
Effect of tucatinib on reversing
ABCG2-mediated multidrug resistance in K562/ABCG2 cells lines.
|
| IC50 ±
SDa, µM |
Resistance-foldb |
|---|
|
|
|
|
|---|
| Compound | K562 | K562/ABCG2 | K562 | K562/ABCG2 |
|---|
| Mitoxantrone | 1.003±0.013 | 19.433±0.459 | 1.00b | 19.375b |
|
+Tucatinib 0.1 µM | 0.841±0.017 | 5.608±0.087 | 0.838 | 5.591d |
|
+Tucatinib 0.2 µM | 0.649±0.018 | 3.052±0.055 | 0.647 | 3.043d |
|
+Tucatinib 0.4 µM | 0.295±0.020 | 0.979±0.075 | 0.294c | 0.976d |
| +FTC
2.5 µM | 0.219±0.004 | 1.302±0.019 | 0.218c | 1.298d |
| Topotecan | 0.919±0.047 | 18.772±0.761 | 1.00b | 20.427b |
|
+Tucatinib 0.1 µM | 0.799±0.023 | 4.771±0.079 | 0.869 | 5.191d |
|
+Tucatinib 0.2 µM | 0.589±0.022 | 2.600±0.018 | 0.641 | 2.829d |
|
+Tucatinib 0.4 µM | 0.278±0.031 | 0.898±0.054 | 0.303c | 0.977d |
| +FTC
2.5 µM | 0.317±0.027 | 0.811±0.014 | 0.345c | 0.882d |
| Cisplatin | 15.810±1.733 | 17.870±1.903 | 1.00b | 1.00b |
|
+Tucatinib 0.4 µM | 14.664±1.653 | 15.829±1.833 | 0.927 | 0.886 |
Tucatinib significantly increases the
cytotoxicity of conventional chemotherapeutic agents in leukemia
blast cells, derived from patients with leukemia
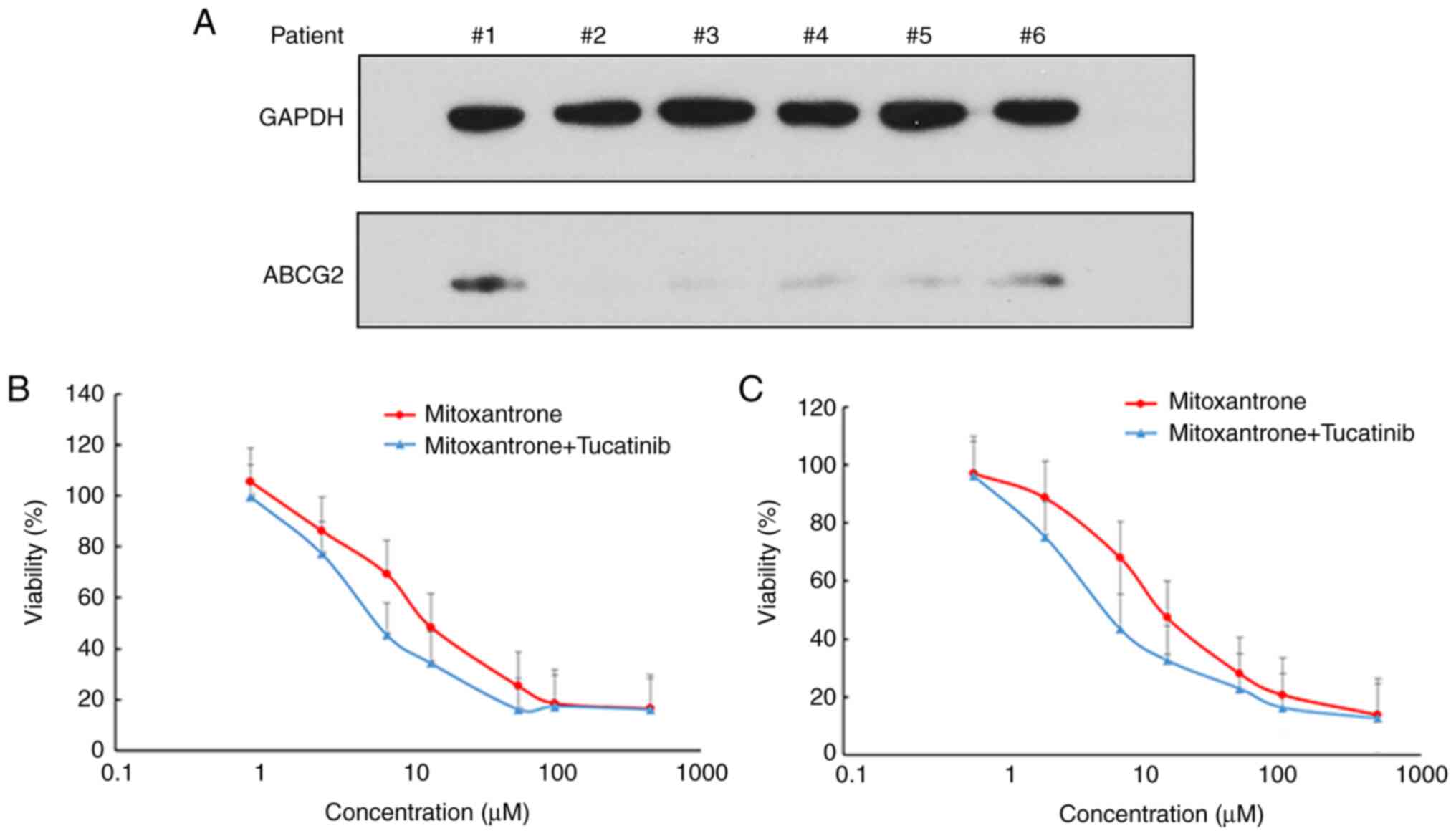
ABCG2 is abundantly expressed in patients with acute
leukemia and in LSCs (9). Thus, the
present study examined the protein expression levels of ABCG2 and
analyzed the cytotoxicity of mitoxantrone, with or without
tucatinib treatment in leukemia blast cells derived from patients
with leukemia (Fig. 2). The results
demonstrated that 2/6 patient samples exhibited detectable
expression levels of ABCG2 (Fig.
2A). Notably, treatment of the leukemia blast cells, with
tucatinib, effectively increased their sensitivity to the
cytotoxicity of mitoxantrone in two patient samples (Fig. 2B and C). These results suggested
that the combined use of tucatinib and mitoxantrone may achieve
positive clinical effects.
Tucatinib significantly decreases the
proportion of SP cells
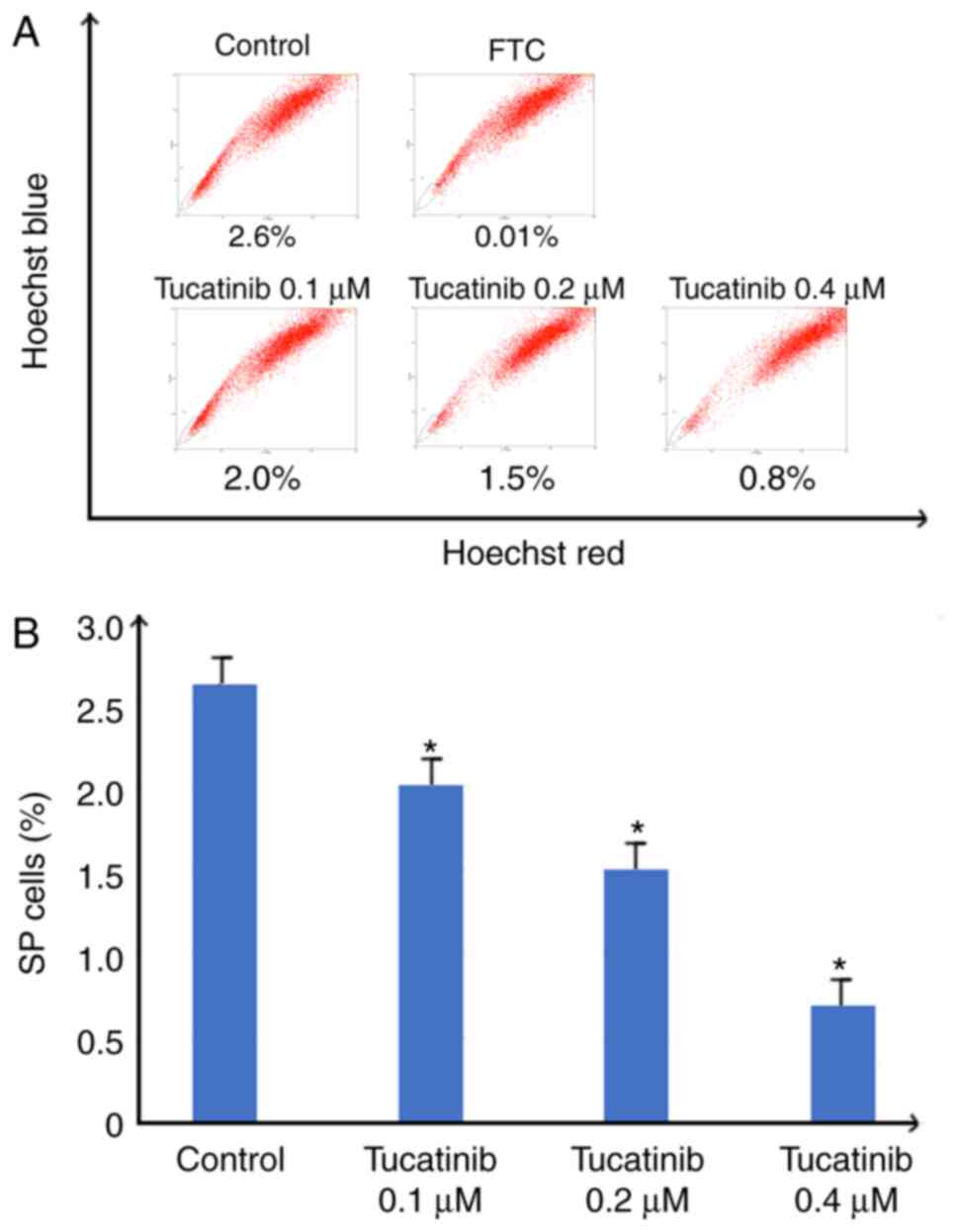
In the present study, to investigate whether
tucatinib has the potential to inhibit the proportion of SP cells,
the population of SP cells in the HL60 cell line, treated with
various concentrations of tucatinib were analyzed using flow
cytometry. As shown in Fig. 3A and
B, following treatment with 0.4 µM tucatinib, the proportion of
SP cells in the HL60 cell line was significantly decreased from 2.6
to 0.8% (P=0.041) compared with that in the control cells,
respectively. These results indicated that tucatinib significantly
decreased the SP cell fraction in the HL60 cell line, in a
dose-dependent manner.
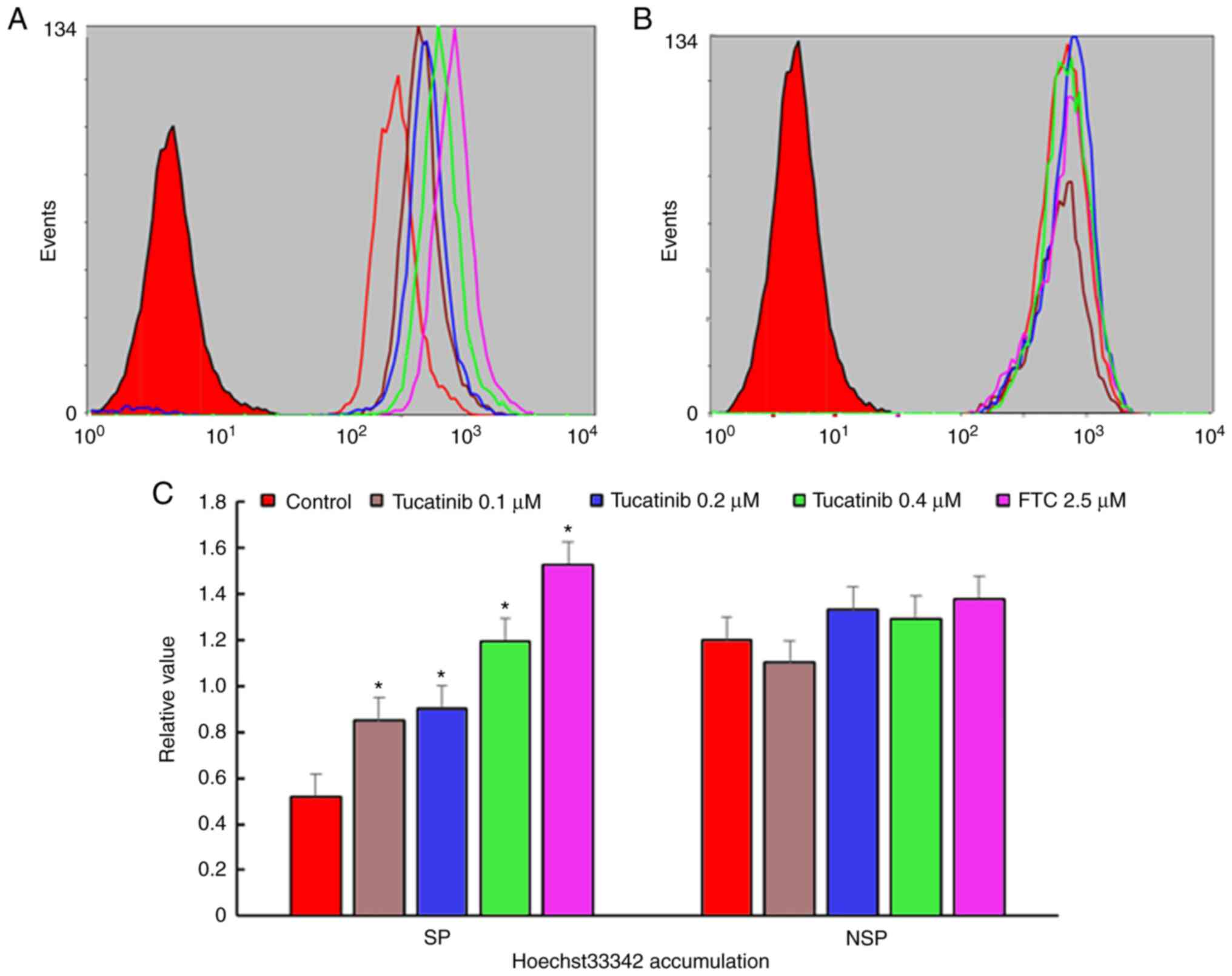
Tucatinib significantly increases the
intracellular levels of Hoechst 33342 in SP cells
The potentiation of antitumor activity by
transporter inhibitor is typically mediated by inhibiting
transporter-mediated drugs, resulting in increased intracellular
drug accumulation (37). To
determine whether tucatinib may potentiate the chemotherapeutic
efficacy in SP cells, the intracellular accumulation levels of
Hoechst 33342 were determined (known as fluorescent substrates of
ABCG2) using flow cytometry. The results revealed that in the
presence of 0.1, 0.2 and 0.4 µM tucatinib, the relative values of
Hoechst 33342 in SP cells were significantly increased by 0.85
(P=0.035)-, 0.92 (P=0.028)- and 1.195 (P=0.024)-fold, respectively
(Fig. 4A and C). These results
indicated that tucatinib may significantly elevated the
intracellular accumulation of Hoechst 33342 in a
concentration-dependent manner in SP cells. Conversely, there was
no significant difference in the intracellular levels of Hoechst
33342 in non-SP (NSP) cells treated with tucatinib and FTC.
Tucatinib effectively enhances the
intracellular levels of anti-neoplastic drugs and antagonizes the
drug efflux in ABCG2-overexpressing leukemia cell lines
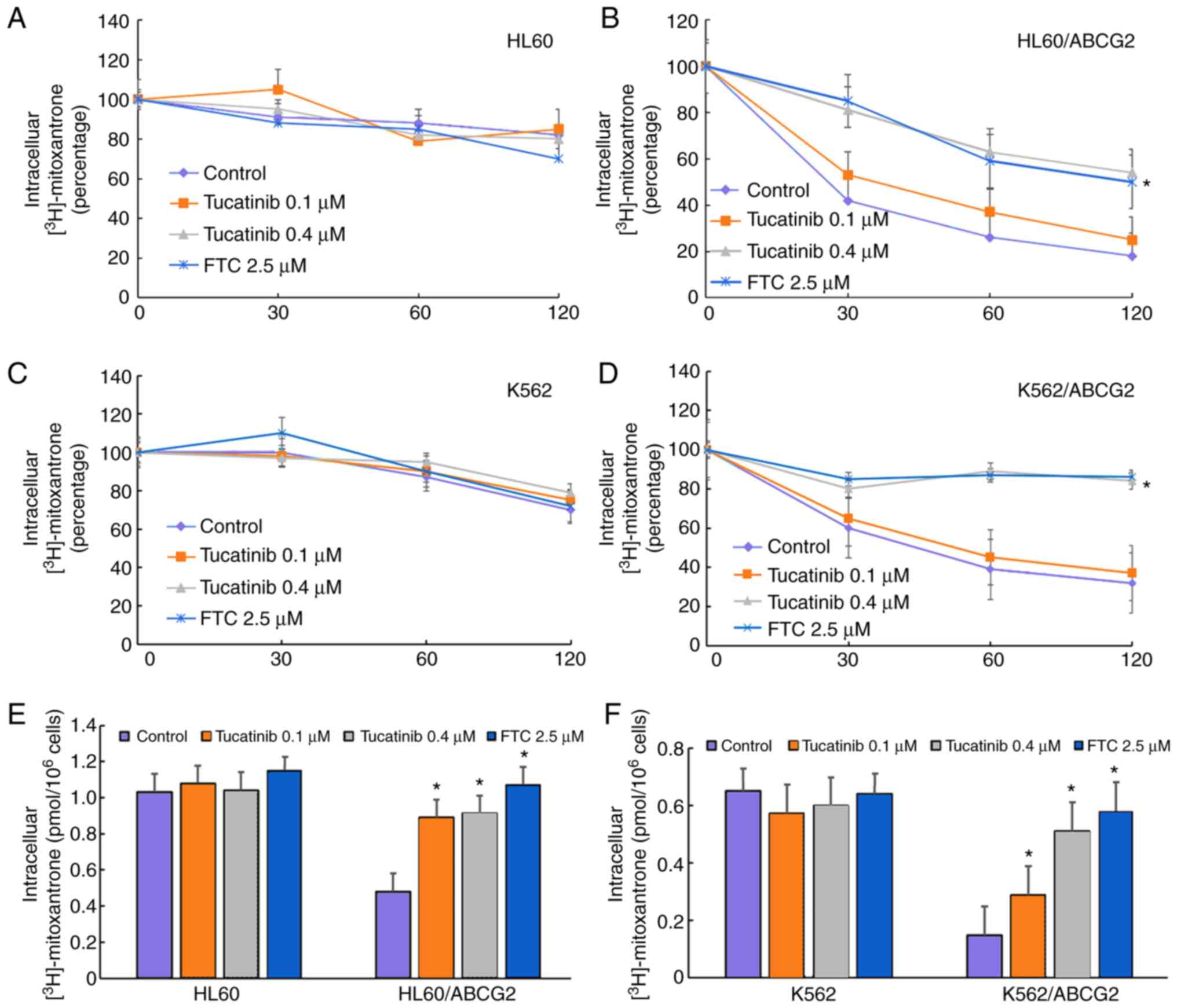
To investigate the potential traverse mechanism by
which tucatinib sensitized ABCG2-overexpressing leukemia cell
lines, the intracellular levels of [3H]-mitoxantrone
were analyzed in the presence or absence of tucatinib. In the
presence of 0.1 and 0.4 µM, tucatinib significantly increased the
intracellular levels of [3H]-mitoxantrone in the
HL60/ABCG2 cell lines, but the accumulative effect of
[3H]-mitoxantrone following 0.4 µM tucatinib was lower
compared with that of FTC, at 2.5 µM, respectively (Fig. 5E). In addition, tucatinib, at 0.4
µM, significantly reduced the efflux of
[3H]-mitoxantronein close to the effect of FTC at 2.5 µM
in the HL60/ABCG2 cell lines (Fig.
5B). Similarly, pretreatment of tucatinib also effectively
improved the intracellular levels of [3H]-mitoxantrone
and decreased its efflux in K562/ABCG2 cells (Fig. 5D and F). Neither tucatinib nor FTC
significantly affected the intracellular levels of
[3H]-mitoxantrone in the parental HL60 and K562 cells
(Fig. 5A and C). These results
indicated that tucatinib increased the intracellular levels of the
antitumor drugs in a dose-dependent manner.
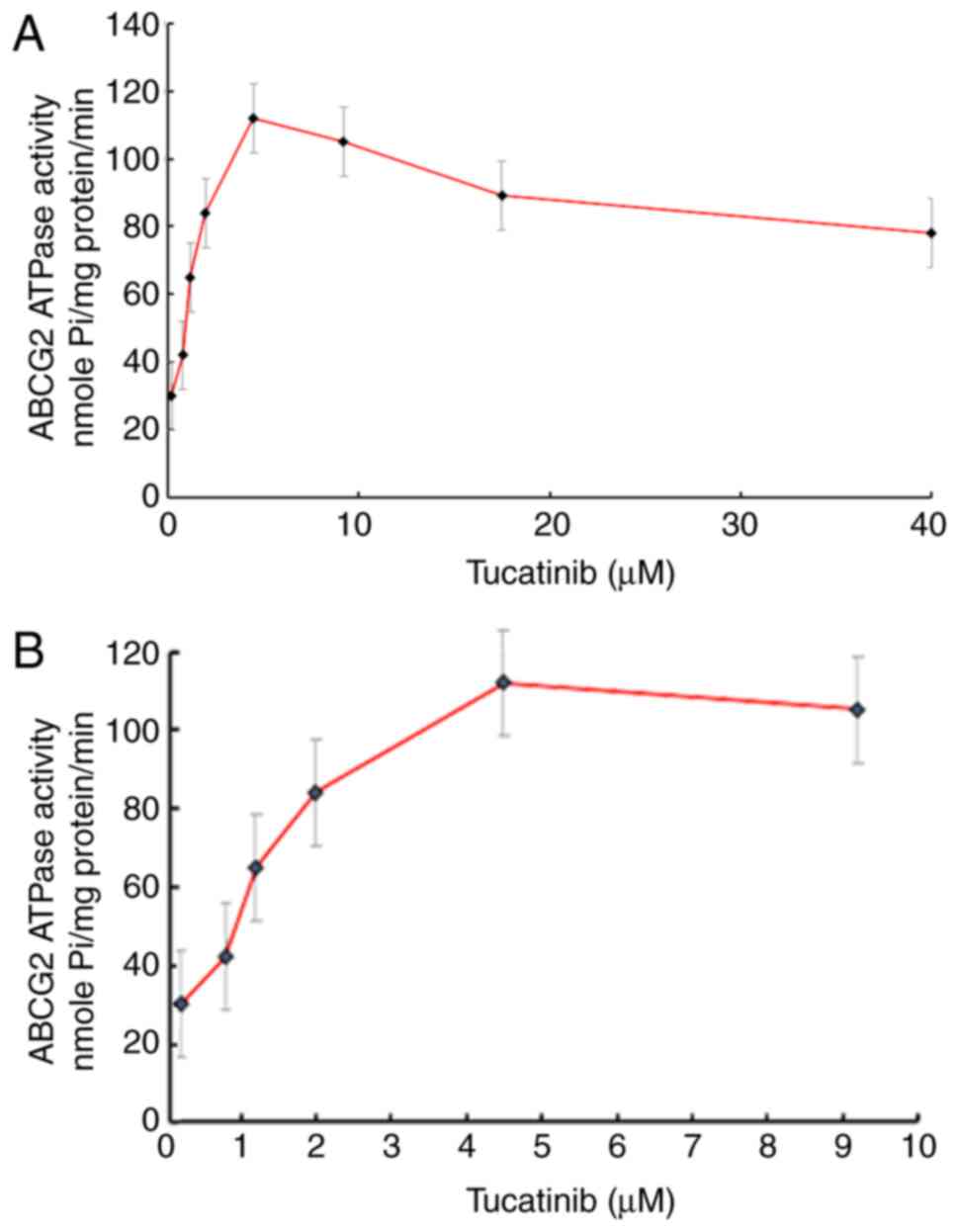
Tucatinib stimulates the ATPase
activity of ABCG2
The drug efflux function of ABCG2 has been
associated with ATP hydrolysis, which is mediated by the presence
of its substrates or inhibitors (34). To further investigate the mechanisms
involved for the potential of tucatinib to overcome MDR, the
efficacy of tucatinib on the ATPase activity of ABCG2 transporters
was determined, by measuring BCRP-mediated ATP hydrolysis in the
presence or absence of 0–40 µM tucatinib (Fig. 6A). As shown in Fig. 6B, using the colorimetric method, the
maximum ATPase activities of ABCG2 increased to 114.28±8.91 nmoles
Pi/mg protein/min in the presence of tucatinib (from
0–10 µM), the maximum stimulation was 4.28-fold greater compared
with that at the basal level. The concentration of tucatinib
required to obtain 50% stimulation was 2.7 µM. These results
suggested that tucatinib stimulated the ATPase activity of ABCG2 by
interacting with the drug substrate-binding site, thereby
restricting the efflux function of ABCG2.
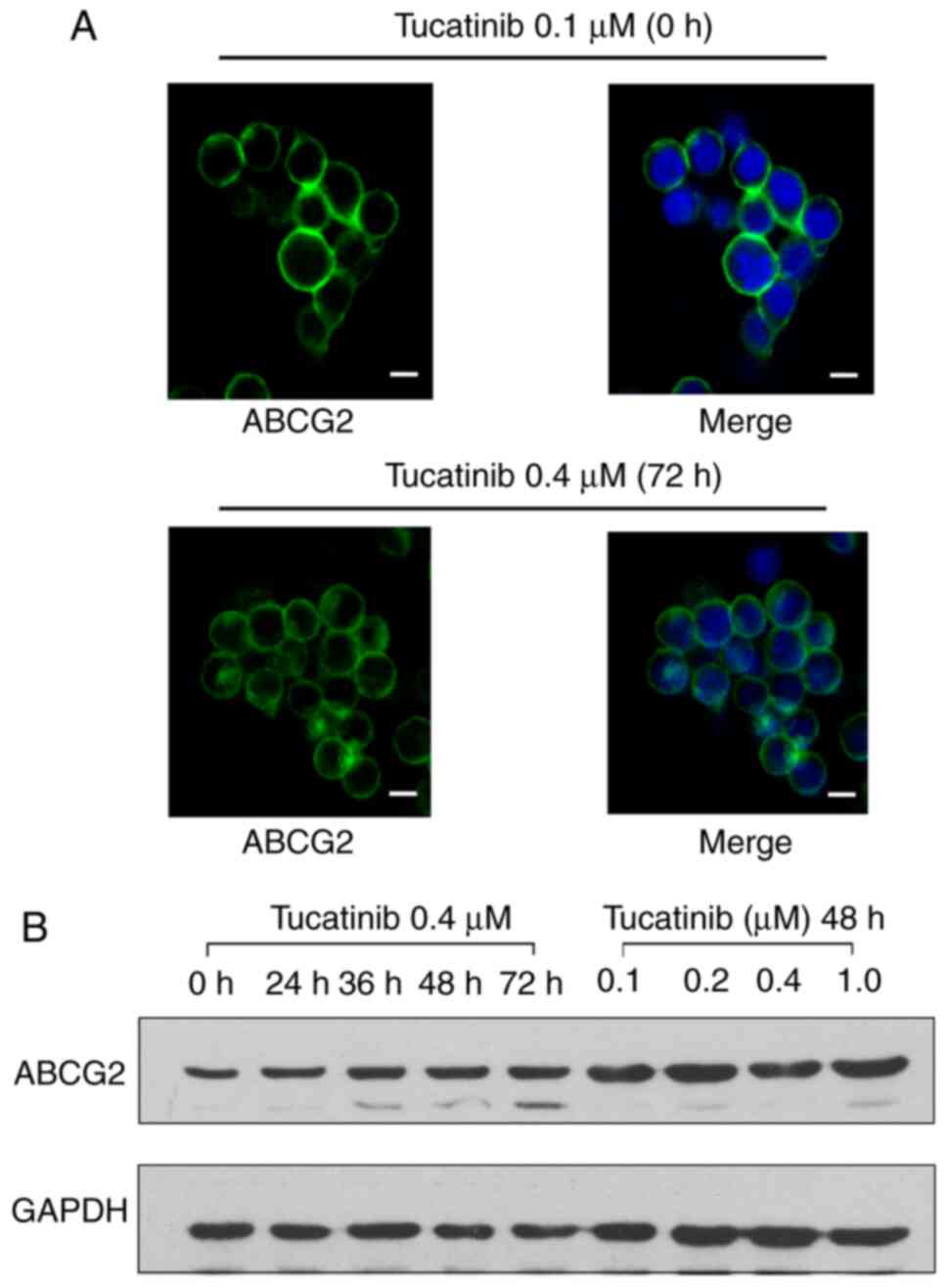
Tucatinib does not alter the protein
intracellular localization nor the expression levels of ABCG2 in
the HL60/ABCG2 cell line
To determine whether the antagonistic effects of
tucatinib on MDR were achieved by altering ABCG2 protein expression
levels or changing its subcellular localization, western blot and
immunofluorescence analyses were performed. There were no notable
changes in the cellular localization of ABCG2 transporters,
following incubation with 0.4 µM tucatinib for 72 h in the
HL60/ABCG2 cell line (Fig. 7A). In
addition, the ABCG2 protein expression level was not altered
following 72 h of treatment with 0.4 µM tucatinib or following
treatment with 0.1, 0.2, 0.4 or 1 µM tucatinib for 48 h in the
HL60/ABCG2 cell line. (Fig. 7B).
These results indicated that the reversal effects of tucatinib were
neither accomplished by altering the expression levels nor by
affecting the intracellular localization of ABCG2 in the HL60/ABCG2
cell line.
Discussion
Over the past few decades, significant developments
in chemotherapeutics have markedly improved clinical treatment
efficacy for patients with acute leukemia (38). Standard induction therapy typically
achieves short-term complete hematological remission; however, it
has failed to improve overall survival times (5,39).
This may be due to the combined use of various chemotherapeutics,
which leads to intrinsic or treatment-induced acquired MDR. MDR is
a formidable impediment for achieving long-term remission in
leukemia, and patients with MDR have to confront treatment failure,
relapse and a poor prognosis (40).
The main mechanism of MDR is the aberrant activation of the agent
efflux pumps of the ABC transporters, which leads to a decrease in
the intracellular concentration of antineoplastic drugs and weakens
the cytotoxicity of the drugs (41). In humans, two members of the ABC
family of transporters, the breast cancer resistance protein (BCRP
or ABCG2) and multidrug resistance protein (ABCB1) have been
established to play pivotal roles in the anti-neoplastic resistance
of malignant leukemia (42,43). ABCG2 has attracted increasing
attention due to its multiple pharmacological binding sites for
nilotinib, imatinib and dasatinib. For example, a previous
meta-analysis revealed that ABCG2 was a potential predictor of the
efficacy of chemotherapy in chronic myeloid leukemia (CML), as
overexpressed ABCG2 conferred poor effects to conventional
anticancer drugs (44). In
addition, accumulating data has suggested that ABCG2 was abundantly
expressed in LSCs, and could be responsible for the proliferation
and self-renewal ability of LSCs (14,45).
ABCG2 protects the LSCs from cytotoxicity by reducing the
intracellular concentrations of antitumor drugs (42). Therefore, a subpopulation of LSCs
may survive standard chemotherapy, maintaining malignant potential,
leading to an eventual cancer recurrence. Furthermore, the
fluorescent dye Hoechst 33342, as one of the ABCG2 substrates, has
been widely used to identify and isolate the cancer stem cell
population in pharmacological assays (46). Thus, ABCG2 may have potential for
use in the development of novel chemical sensitizers targeting
drug-resistant leukemia cells and for eradicating LSCs.
TKIs are a new type of highly specific and promising
antitumor drugs, which have been demonstrated to be safe and
effective agents for the treatment of various malignancies
(47,48). Furthermore, it has been demonstrated
that dasatinib or nilotinib (second generation ABL-TKIs) may
efficiently reduce the number of CML stem cells (49). Tucatinib is a new type of
ATP-competitive and reversible HER2-targeted small-molecular TKI,
which was approved in the USA in April 2020 and in Switzerland in
May 2020 for the treatment of HER2-positive breast cancer, is
pending regulatory review in the EU, Australia, Canada and
Singapore, and approved in combination with other drugs, in
patients with HER2-positive breast cancer and brain metastases
(20). Tucatinib is active as a
single agent in multiple HER2+ tumor models and it
exhibits potent antitumor activity in combined treatment with
conventional chemotherapeutic drugs, thereby increasing the rate of
partial and complete tumor regression (18). Therefore, the aim of the present
study was to investigate the interaction of tucatinib with the
ABCG2 transporter and its ability to eradicate LSCs.
The results from the cytotoxicity assays
demonstrated that tucatinib, at a non-toxic concentration,
significantly potentiated the cytotoxicity of conventional
chemotherapeutic agents in ABCG2-overexpressing leukemia cells in a
dose-dependent manner. However, tucatinib treatment did not affect
the cytotoxicity of cisplatin (a non-substrate of ABCG2). These
results suggested that the preferable anti-neoplastic effects of
tucatinib on leukemia cells may be attributed to its specific
effect on ABCG2 transporters. The present study also revealed that
tucatinib treatment significantly enhanced the cytotoxicity of
mitoxantrone in ABCG2-overexpressing primary leukemia blast cells
derived from patients with leukemia. In addition, flow cytometric
assays revealed that tucatinib significantly decreased the
proportion of LSCs-like SP cells in the HL60 cell line, which are
typically used in the identification and isolation of cancer
stem-like cells. In the SP cells, isolated from leukemia cells, the
intracellular levels of Hoechst 33342, which is an ABCG2 substrate,
were significantly elevated.
The significant antitumor activity of transporter
inhibitors is typically regulated by the inhibition of the
transporter-mediated efflux, leading to an increase in the
intracellular drug levels (28).
Therefore, the intracellular levels and efflux of
[3H]-mitoxantrone were analyzed in leukemia cells in the
presence or absence of tucatinib. The results demonstrated that
tucatinib inhibited the efflux of [3H]-mitoxantrone and,
hence, there were higher levels of [3H]-mitoxantrone in
the HL60/ABCG2 and K562/ABCG2 cell lines.
It has been previously proposed that ABCG2
inhibitors can be divided into two subtypes: One that only inhibits
ABCG2 activity and the other that suppresses ABCG2 activity in
addition to reducing the expression levels of ABCG2 (17). Thus, the protein expression level
and the intracellular location of ABCG2, at various concentrations
of tucatinib was analyzed using western blot and immunofluorescence
analyses. The results revealed that there was no notably decrease
in the protein expression levels of ABCG2 following 72 h incubation
with 0.4 µM tucatinib or following tucatinib treatment at 0.1, 0.2,
0.4 and 1 µM for 48 h in the HL60/ABCG2 cell line. Furthermore,
treatment with 0.4 µM tucatinib did not affect the intracellular
locations of the ABCG2 transporter.
Several studies have indicated that the
HER2-specific TKIs, which bind to the extracellular domains of
HER2, can compete with the ATP-TKIs domain, block tyrosine
phosphorylation and signal events downstream of ligand binding
(50). Tucatinib, which belongs to
the ATP-competitive and highly selective small-molecule TKIs of
anti-HER2 compounds, may exert similar effects. Thus, the present
study investigated the effects of tucatinib on the ATPase
activities of ABCG2 transporters in the HL60/ABCG2 cell line. The
results demonstrated that tucatinib could stimulate the ATPase
activity of ABCG2 transporters. These results were consistent with
those of previous studies on the TKI pathway (51). Tucatinib may act as a substrate of
ABCG2, which can compete with chemotherapeutic agents to occupy the
binding site of ABCG2, increase ATPase activity and replace the
antitumor drugs from the ABCG2 transporter. As a result, it
suppresses the efflux function and increases the intracellular
accumulation of substrate agents, leading to the reversal of
MDR.
In recent years, a number of reversal reagents have
been developed to suppress the ABC transporter-mediated MDR;
however, due to the unpredictable results of their combined use
with chemotherapeutic drugs, the majority of these reagents have
failed in clinical practice (37,40).
Nevertheless, tucatinib, as an oral selective HER2 TKI, may have
the ability to cure leukemia, when used in combination with other
anti-tumor drugs, to reverse MDR induced by ABCG2 overexpression.
Recent clinical trials indicated that tucatinib combined with
trastuzumab and capecitabine, resulted in better progression-free
survival and overall survival outcomes than without adding
tucatinib in HER2-positive metastatic breast cancer (52), and with and without brain metastases
(53). Thereby, the results from
the present study suggests that the combined use of tucatinib with
ABCG2 substrate-drugs may be a potential prospective treatment
strategy to overcome resistance in patients with leukemia.
In conclusion, tucatinib may significantly enhance
the chemosensitivity of ABCG2-overexpressing leukemia cells and
LSCs to classic anti-tumor agents by inhibiting the drug efflux
functions of ABCG2. Notably, the combined use of tucatinib with
ABCG2 substrate anticancer drugs may be beneficial for patients
with leukemia to elude the MDR of leukemia cells. In addition, the
combined use of tucatinib with the ABCG2 substrate drugs may
enhance the anti-tumor effects of anticancer drugs in vitro
and in patient-derived leukemic blast cells. Thus, the results from
the present study suggested that the use of tucatinib in
combination with conventional chemotherapeutic drugs may prove to
be a potential therapeutic strategy to improve the cytotoxicity of
anti-tumor drugs and decrease the recurrence rate of
ABCG2-overexpressing leukemia.
In summary, combination of tucatinib with the ABCG2
substrate drugs increased the antitumor effect of anticancer drugs
in vitro and patient-derived leukemic blast cells.
Therefore, this may be a potential therapeutic strategy to improve
the cytotoxicity of antitumor drugs and decrease the recurrence
rate in ABCG2 overexpressing leukemia cells.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during this study
are included within the article.
Authors' contributions
ZW and SP designed the research and revised the
manuscript. WJ, MZ and RC designed the methods and experiments,
performed the laboratory experiments, analyzed the data,
interpreted the results and wrote the paper. XY, XS, JL and WL
co-designed the experiments and discussed the analyses,
interpretation and presentation of the data. All authors approved
the final version of the manuscript.
Ethics approval and consent to
participate
The experimental protocol for the use of patient
samples was reviewed and approved by the Ethics Review Committee at
Sun Yat-Sen University. Informed consent was provided by each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bouvy C, Wannez A, Laloy J, Chatelain C
and Dogné JM: Transfer of multidrug resistance among acute myeloid
leukemia cells via extracellular vesicles and their microRNA cargo.
Leuk Res. 62:70–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hochhaus A, Ernst T, Eigendorff E and La
Rosée P: Causes of resistance and treatment choices of second- and
third-line treatment in chronic myelogenous leukemia patients. Ann
Hematol. 94 (Suppl 2):S133–S140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeshita A: Efficacy and resistance of
gemtuzumab ozogamicin for acute myeloid leukemia. Int J Hematol.
97:703–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tesfatsion DA: Dendritic cell vaccine
against leukemia: Advances and perspectives. Immunotherapy.
6:485–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valera ET, Scrideli CA, de Paula Queiroz
RG, Mori BM and Tone LG: Multiple drug resistance protein (MDR-1),
multidrug resistance-related protein (MRP) and lung resistance
protein (LRP) gene expression in childhood acute lymphoblastic
leukemia. Sao Paulo Med J. 122:166–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steinbach D and Legrand O: ABC
transporters and drug resistance in leukemia: Was P-gp nothing but
the first head of the hydra? Leukemia. 21:1172–1176. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plasschaert SL, Van Der Kolk DM, De Bont
ES, Vellenga E, Kamps WA and De Vries EG: Breast cancer resistance
protein (BCRP) in acute leukemia. Leuk Lymphoma. 45:649–654. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bram EE, Stark M, Raz S and Assaraf YG:
Chemotherapeutic drug-induced ABCG2 promoter demethylation as a
novel mechanism of acquired multidrug resistance. Neoplasia.
11:1359–1370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Damiani D, Tiribelli M, Geromin A,
Michelutti A, Cavallin M, Sperotto A and Fanin R: ABCG2
overexpression in patients with acute myeloid leukemia: Impact on
stem cell transplantation outcome. Am J Hematol. 90:784–789. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith PJ, Furon E, Wiltshire M, Campbell
L, Feeney GP, Snyder RD and Errington RJ: ABCG2-associated
resistance to Hoechst 33342 and topotecan in a murine cell model
with constitutive expression of side population characteristics.
Cytometry A. 75:924–933. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moshaver B, Wouters RF, Kelder A,
Ossenkoppele GJ, Westra G, Kwidama Z, Rutten AR, Kaspers G,
Zweegman S, Cloos J and Schuurhuis GJ: Relationship between
CD34/CD38 and side population (SP) defined leukemia stem cell
compartments in acute myeloid leukemia. Leuk Res. 81:27–34. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanekamp D, Cloos J and Schuurhuis GJ:
Leukemic stem cells: Identification and clinical application. Int J
Hematol. 105:549–557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abbott BL: ABCG2 (BCRP): A cytoprotectant
in normal and malignant stem cells. Clin Adv Hematol Oncol.
4:63–72. 2006.PubMed/NCBI
|
|
14
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2-cancer cells are similarly
tumorigenic. Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yazdi MH, Faramarzi MA, Nikfar S and
Abdollahi M: Comparative safety and efficacy of tyrosine kinase
inhibitors (TKIs) in the treatment setting of different types of
leukemia, and different types of adenocarcinoma. Biomed
Pharmacother. 95:1556–1564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hegedus C, Ozvegy-Laczka C, Apáti A,
Magócsi M, Német K, Orfi L, Kéri G, Katona M, Takáts Z, Váradi A,
et al: Interaction of nilotinib, dasatinib and bosutinib with ABCB1
and ABCG2: Implications for altered anti-cancer effects and
pharmacological properties. Br J Pharmacol. 158:1153–1164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XK, He Jh, Xu Jh, Ye S, Wang F, Zhang
H, Huang Zc, To KK and Fu Lw: Afatinib enhances the efficacy of
conventional chemotherapeutic agents by eradicating cancer
stem-like cells. Cancer Res. 74:4431–4445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murthy R, Borges VF, Conlin A, Chaves J,
Chamberlain M, Gray T, Vo A and Hamilton E: Tucatinib with
capecitabine and trastuzumab in advanced HER2-positive metastatic
breast cancer with and without brain metastases: A non-randomised,
open-label, phase 1b study. Lancet Oncol. 19:880–888. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kulukian A, Lee P, Taylor J, Rosler R, de
Vries P, Watson D, Forero-Torres A and Peterson S: Preclinical
activity of HER2-selective tyrosine kinase inhibitor tucatinib as a
single agent or in combination with trastuzumab or docetaxel in
solid tumor models. Mol Cancer Ther. 19:976–987. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee A: Tucatinib: First approval. Drugs.
80:1033–1038. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borges VF, Ferrario C, Aucoin N, Falkson
C, Khan Q, Krop I, Welch S, Conlin A, Chaves J, Bedard PL, et al:
Tucatinib combined with ado-trastuzumab emtansine in advanced
ERBB2/HER2-positive metastatic breast cancer: A phase 1b clinical
trial. Jama Oncol. 4:1214–1220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Filho OM, Leone JP, Li T, Tan-Wasielewski
Z, Trippa L, Barry WT, Younger J, Lawler E, Walker L, Freedman RA,
et al: Phase I dose-escalation trial of tucatinib in combination
with trastuzumab in patients with HER2-positive breast cancer brain
metastases. Ann Oncol. 31:1231–1239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Kumar P, Anreddy N, Zhang YK, Wang
YJ, Chen Y, Talele TT, Gupta K, Trombetta LD and Chen ZS:
Quizartinib (AC220) reverses ABCG2-mediated multidrug resistance:
In vitro and in vivo studies. Oncotarget. 8:93785–93799. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugimoto Y, Tsukahara S, Imai Y, Sugimoto
Y, Ueda K and Tsuruo T: Reversal of breast cancer resistance
protein-mediated drug resistance by estrogen antagonists and
agonists. Mol Cancer Ther. 2:105–112. 2003.PubMed/NCBI
|
|
25
|
Wang DS, Patel A, Shukla S, Zhang YK, Wang
YJ, Kathawala RJ, Robey RW, Zhang L, Yang DH, Talele TT, et al:
Icotinib antagonizes ABCG2-mediated multidrug resistance, but not
the pemetrexed resistance mediated by thymidylate synthase and
ABCG2. Oncotarget. 5:4529–4542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi Z, Tiwari AK, Shukla S, Robey RW,
Singh S, Kim IW, Bates SE, Peng X, Abraham I, Ambudkar SV, et al:
Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug
resistance. Cancer Res. 71:3029–3041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XH, Wang XK, Liang YJ, Shi Z, Zhang
JY, Chen LM and Fu LW: A cell-based screen for anticancer activity
of 13 pyrazolone derivatives. Chin J Cancer. 29:980–987. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi Z, Liang YJ, Chen ZS, Wang XH, Ding Y,
Chen LM and Fu LW: Overexpression of survivin and XIAP in MDR
cancer cells unrelated to P-glycoprotein. Oncol Rep. 17:969–976.
2007.PubMed/NCBI
|
|
29
|
Shi Z, Parmar S, Peng XX, Shen T, Robey
RW, Bates SE, Fu LW, Shao Y, Chen YM, Zang F and Chen ZS: The
epidermal growth factor tyrosine kinase inhibitor AG1478 and
erlotinib reverse ABCG2-mediated drug resistance. Oncol Rep.
21:483–489. 2009.PubMed/NCBI
|
|
30
|
Ji N, Yang Y, Cai CY, Lei ZN, Wang JQ,
Gupta P, Teng QX, Chen ZS, Kong D and Yang DH: VS-4718 antagonizes
multidrug resistance in ABCB1- and ABCG2-overexpressing cancer
cells by inhibiting the efflux function of ABC transporters. Front
Pharmacol. 9:12362018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Percival ME, Lai C, Estey E and Hourigan
CS: Bone marrow evaluation for diagnosis and monitoring of acute
myeloid leukemia. Blood Rev. 31:185–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vieyra DS, Rosen A and Goodell MA:
Identification and characterization of side population cells in
embryonic stem cell cultures. Stem Cells Dev. 18:1155–1166. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji N, Yang Y, Lei ZN, Cai CY, Wang JQ,
Gupta P, Xian X, Yang DH, Kong D and Chen ZS: Ulixertinib (BVD-523)
antagonizes ABCB1- and ABCG2-mediated chemotherapeutic drug
resistance. Biochem Pharmacol. 158:274–285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dai Cl, Tiwari AK, Wu CP, Su XD, Wang SR,
Liu Dg, Ashby CJ JR, Huang Y, Robey RW, Liang YJ, et al: Lapatinib
(Tykerb, GW572016) reverses multidrug resistance in cancer cells by
inhibiting the activity of ATP-binding cassette subfamily B member
1 and G member 2. Cancer Res. 68:7905–7914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai CY, Zhai H, Lei ZN, Tan CP, Chen BL,
Du ZY, Wang JQ, Zhang YK, Wang YJ, Gupta P, et al: Benzoyl indoles
with metabolic stability as reversal agents for ABCG2-mediated
multidrug resistance. Eur J Med Chem. 179:849–862. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jing W, Zhang X, Chen R, Ye X, Zhou M, Li
W, Yan W, Xuyun X and Peng J: KD025, an anti-adipocyte
differentiation drug, enhances the efficacy of conventional
chemotherapeutic drugs in ABCG2-overexpressing leukemia cells.
Oncol Lett. 20:3092020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Litman T, Druley TE, Stein WD and Bates
SE: From MDR to MXR: New understanding of multidrug resistance
systems, their properties and clinical significance. Cell Mol Life
Sci. 58:931–959. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Spagnuolo P: Interactions between
nutraceutical supplements and standard acute myeloid leukemia
chemotherapeutics. J Pharm Pharm Sci. 18:339–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
List AF: The role of multidrug resistance
and its pharmacological modulation in acute myeloid leukemia.
Leukemia. 10 (Suppl 1):S36–S38. 1996.PubMed/NCBI
|
|
40
|
Polgar O and Bates SE: ABC transporters in
the balance: Is there a role in multidrug resistance? Biochem Soc
Trans. 33:241–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Robey RW, Pluchino KM, Hall MD, Fojo AT,
Bates SE and Gottesman MM: Revisiting the role of ABC transporters
in multidrug-resistant cancer. Nat Rev Cancer. 18:452–464. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Robey RW, To KK, Polgar O, Dohse M, Fetsch
P, Dean M and Bates SE: ABCG2: A perspective. Adv Drug Deliv Rev.
61:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carrillo IO, Peñafiel CR, Peralta EM,
Fuller ER, Ipiña JJ, Cruz FC, Guerrero EG, Jaloma JC, Vargas KN and
Tovar AM: Clinical significance of the ABCB1 and ABCG2 gene
expression levels in acute lymphoblastic leukemia. Hematology.
22:286–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang ZP, Zhao XL, Takahashi N, Angelini
S, Dubashi B, Sun L and Xu P: Trough concentration and ABCG2
polymorphism are better to predict imatinib response in chronic
myeloid leukemia: A meta-analysis. Pharmacogenomics. 18:35–56.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang F, Wang XK, Shi CJ, Zhang H, Hu YP,
Chen YF and Fu LW: Nilotinib enhances the efficacy of conventional
chemotherapeutic drugs in CD34+CD38− stem
cells and ABC transporter overexpressing leukemia cells. Molecules.
19:3356–3375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li D, Su D, Xue L, Liu Y and Pang W:
Establishment of pancreatic cancer stem cells by flow cytometry and
their biological characteristics. Int J Clin Exp Pathol.
8:11218–11223. 2015.PubMed/NCBI
|
|
47
|
Russo A, Franchina T, Ricciardi GR,
Smiroldo V, Picciotto M, Zanghì M, Rolfo C and Adamo V: Third
generation EGFR TKIs in EGFR-mutated NSCLC: Where are we now and
where are we going. Crit Rev Oncol Hematol. 117:38–47. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kouhpeikar H, Butler AE, Bamian F, Barreto
GE, Majeed M and Sahebkar A: Curcumin as a therapeutic agent in
leukemia. J Cell Physiol. 234:12404–12414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Arrigoni E, Del RM, Galimberti S, Restante
G, Rofi E, Crucitta S, Baratè C, Petrini M, Danesi R and Di Paolo
A: Concise review: Chronic myeloid leukemia: Stem cell niche and
response to pharmacologic treatment. Stem Cells Transl Med.
7:305–314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dohse M, Scharenberg C, Shukla S, Robey
RW, Volkmann T, Deeken JF, Brendel C, Ambudkar SV, Neubauer A and
Bates SE: Comparison of ATP-binding cassette transporter
interactions with the tyrosine kinase inhibitors imatinib,
nilotinib, and dasatinib. Drug Metab Dispos. 38:1371–1380. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang X, Goldstein D, Crowe PJ and Yang JL:
Next-Generation EGFR/HER tyrosine kinase inhibitors for the
treatment of patients with non-small-cell lung cancer harboring
EGFR mutations: A review of the evidence. Onco Targets Ther.
9:5461–5473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Murthy RK, Loi S, Okines A, Paplomata E,
Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, et
al: Tucatinib, trastuzumab, and capecitabine for HER2-positive
metastatic breast cancer. N Engl J Med. 382:597–609. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin NU, Borges V, Anders C, Murthy RK,
Paplomata E, Hamilton E, Hurvitz S, Loi S, Okines A, Abramson V, et
al: Intracranial efficacy and survival with tucatinib plus
trastuzumab and capecitabine for previously treated HER2-positive
breast cancer with brain metastases in the HER2CLIMB trial. J Clin
Oncol. 38:2610–2619. 2020. View Article : Google Scholar : PubMed/NCBI
|