Introduction
Thyroid cancer (TC), is the most common endocrine
malignancy (1), with papillary TC
accounting for 80–90% of all TC cases (2). While the 5-year survival rate of the
patients with TC is >95%, the local invasion or distant
metastases of these tumors can result in poor outcomes, as these
tumors generally respond poorly to standard treatments (3,4). It is
thus essential that the mechanistic basis for TC onset and
progression be better understood in an effort to develop reliable
approaches for the treatment of patients suffering from these
tumors.
HOX antisense intergenic RNA myeloid 1 (HOTAIRM1) is
a type of lncRNA, which has reported to be associated with tumor
metastasis. HOTAIRM1 may function as regulator of gene expression,
which is expressed from HOXA genomic cluster between HOXA1 and
HOXA2. For example, HOTAIRM1 has been reported to exert a crucial
effect on multiply types of cancer, such as breast (5), colorectal cancer (6) and glioma (7). However, it remains unclear whether
HOTAIRM1 contributes to the malignant progression of TC. To explore
the effect and biology function of HOTAIRM1 in TC, the present
study assessed its expression, biological functions and the
underlying molecular pathways in TC cells.
MicroRNAs (miRNAs or miRs, 18–22 nucleotides in
length) are endogenous short noncoding single-stranded RNAs, which
have been been reported to induce messenger RNA (mRNA) degradation
or block translation by interacting with the 3′-untranslated region
(UTR) of target mRNAs (8,9). miRNAs have been reported regulate
cellular physiological processes via complex mechanisms. Multiple
studies have demonstrated that miR-148a is involved in cervical
(10), pancreatic (11), colorectal (12) and gastric cancer (13). However, to date, there is no
evidence of an association between HOTAIRM1 and miR-148a in TC, at
least to the best of our knowledge.
Herein, it was found that the long non-coding RNA
(lncRNA) HOTAIRM1 was significantly upregulated in TC tumor tissues
and cells. The knockdown of this lncRNA in TC cell lines was
sufficient to impair their proliferative and invasive activity,
while simultaneously promoting the upregulation of miR-148a and the
downregulation of Wnt10b. The inhibition of miR-148a was sufficient
to reverse this reduction in Wnt10b expression, and miR-148
overexpression exerted the opposite effect, owing to the ability of
this miRNA to directly bind to the Wnt10b 3′-UTR. The
overexpression of Wnt10b reversed the effect of miR-148a mimics on
both TPC-1 and BCPAP cells. Taken together, the data thus suggest
that HOTAIRM1 knockdown can suppress the proliferation and
metastasis of TC cells via modulating the miR-148a/Wnt10b axis.
Materials and methods
Tissue specimens
A total of 52 pairs clinical tissues were obtained
from patients with TC at the First Hospital of Jilin University
from March 2019 to November 2019. Written informed consent was
obtained from all study subjects. The present study conformed to
the principles presented in the Declaration of Helsinki and was
approved (no. 20190318) by the Ethics Committee of the First
Hospital of Jilin University (Jilin, China).
Cells and cell culture
Human TPC-1 [cat. no. CC-Y1522; Meiyan (Shanghai)
Biological Technology Co., Ltd.], BCPAP [cat. no. CC-Y1064; Meiyan
(Shanghai) Biological Technology Co., Ltd.] and Nthy-ori 3-1 [cat.
no. CC-Y1708; Meiyan (Shanghai) Biological Technology Co., Ltd.]
cells were grown in DMEM supplemented 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2
incubator.
Construction of lentiviruses
The primer sequences targeting lncRNA HOTAIRM1
(Table I) were chemically
synthesized, and each was inserted into the
AgeI-EcoRI site of the pLKO.1-Puro vector purchased
from Beijing Solarbio Science & Technology Co., Ltd. The coding
sequence (CDS) of Wnt10b was synthesized from GenePharma Co., Ltd.,
and inserted into the EcoRI-BamHI site of the
pLVX-Puro vector (Shanghai Yubo Biotechnology Co., Ltd.).
Subsequently, HOTAIRM1-specific shRNAs (GenePharma Co., Ltd.) or
pLKO.1-Puro vector of the lentiviral particles were co-transfected
(1:1.5) into 293T cells (ATCC) with the mixed set of packaging
plasmids (SPAX2 and MD2G) using Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Following 48 h of incubation at
37°C, viral particles were achieved by ultracentrifugation (12,000
× g for 1 min at room temperature).
 | Table I.shRNA sequences used for HOTAIRM1. |
Table I.
shRNA sequences used for HOTAIRM1.
| shRNA name | Sequence (5′-3′) |
|---|
| shHOTAIRM1-1 |
CCGGGCCTCTATTACCAATTTAAATTCTCGAGTTAAATTGGTAATAGAGGCAGTTTTTTG |
| shHOTAIRM1-2 |
AATTCAAAAAACTGCCTCTATTACCAATTTAACTCGAGATTTAAATTGGTAATAGAGGC |
Cell treatment
TPC-1 and BCPAP cells were transduced with
lentiviruses encoding HOTARIRM1-specific shRNAs (shHOTAIRM1-1 and
shHOTAIRM1-2), corresponding negative controls (shNC), or a Wnt10b
overexpression (Wnt10b) vector (all from RiboBio Co. Ltd.) at
1:1.2, after which reverse transcription-quantitative PCR (RT-qPCR)
was used to determine the efficacy of HOTAIRM1 knockdown or Wnt10b
overexpression.
Following lentiviral transfection with shHOTAIRM1-1
(5′-AGAAACUCCGUGUUACUCAUU-3′), shHOTAIRM1-2
(5′-GCCAGAAACCAGCCAUAGU-3′) or shNC, MTT and Transwell assays were
respectively used to determine the proliferation and invasion of
the transduced cells. Furthermore, the expression of Wnt10b and
specific miRNAs in these cells was analyzed. miR-148a mRNA, Wnt10b
expression and luciferase activity were assessed following
transfection with miR-148a mimic or inhibitor at 1:1.5 at room
temperature.
For individual experiments, cells were transfected
with lentiviral constructs and miR-148a inhibitors/mimic and
respective negative controls (Guangzhou RiboBio Co. Ltd.) using
DharmaFECT 1 (Qcbio Science & Technologies Co., Ltd.)
transfection reagent at 1:1.5 with the following combinations:
shNC, shNC + miR-148a inhibitor, shHOTAIRM1, shHOTAIRM1 + miR-148a
inhibitor. Moreover, cells were stimulated with miR-148a mimics,
miR-148a mimics + Wnt10b, NC + Wnt10b, or NC alone. Following the
indicated treatments, cellular proliferation, invasion and gene
expression were analyzed.
RT-qPCR
Total RNA was extracted from the TC cells and
tissues using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). A total of 1 µg RNA was reverse transcribed into cDNA using
the PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.).
RT-qPCR was performed using the CFX96 system using SYBR®
Premix Ex Taq™ II kit (Takara Biotechnology Co., Ltd.). The
thermocycling conditions were as follows: 95°C for 30 sec, 95°C for
10 sec, 60°C for 30 sec, 35 cycles. GAPDH was used as the
endogenous control. The Cq values was calculated through the
2−ΔΔCq method (14).
Likewise, miRNAs were reverse transcribed and
miRNA-specific primers (10 µM) (Table
II). miRNA expression was detected using the TaqMan microRNA
assay kit (Applied Biosystems; Thermo Fisher Scientific, Inc.),
with an ABI7900 real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: 95°C for 5 min, 95°C for 30 sec, 58°C for 20 sec, 40
cycles. U6 was used as the endogenous control.
 | Table II.Primers for the target gene and
primers for Wnt10b 3′UTR used for RT-qPCR and in the luciferase
report assay. |
Table II.
Primers for the target gene and
primers for Wnt10b 3′UTR used for RT-qPCR and in the luciferase
report assay.
| Gene | Sequence (5′-3′) |
|---|
| miR-148a | F:
GGCAGCAAAGTTCTGAGACAC |
|
| R:
GTGCAGGGTCCGAGGTATTC |
| miR-141-3p | F:
ACACTCCAGCTGGGCATCTTCCAG |
|
| R:
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCCAAC |
| miR-1 | F:
CAGTGCGTGTCGTGGAGT |
|
| R:
GGCCTGGAATGTAAAGAAGT |
| miR-21 | F:
GCACCGTCAAGGCTGAGAAC |
|
| R:
CAGCCCATCGACTGGTG |
| Wnt 10b | F:
TGGAAGAATGCGGCTCTGAC |
|
| R:
AGAGTGACCTTGGAAGGAAATC |
| GAPDH | F: TCACCA
GGGCTGCTTTTAAC |
|
| R: TGACGGTGCCA
TGGAATTTG |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| Wt 3′UTR of
Wnt10b | F:
CTAGCTAGCGGCCGCTAGTTGGACTAAGATG AAATGCACTGTG |
|
| R:
TCGACACAGTGCATTTCATCTTAGTCCAACTA GCGGCCGCTAG |
| Mut 3′UTR of
Wnt10b | F:
CTAGCTAGCGGCCGCTAGTTGGACTAAGATG AAAAGGAGTCTG |
|
| R:
TCGACAGACTCCTTTTCATCTTAGTCCAACTA GCGGCCGCTAG |
Western blot analysis
Total protein was extracted from the treated and
untreated TC cells using RIPA buffer (Beijing Solarbio Science
& Technology Co., Ltd.). Following quantification using a BCA
quantification kit (Beijing Solarbio Science & Technology Co.,
Ltd.), 50 µg protein were separated in a 10% SDS-PAGE gels and
transferred onto a polyvinylidene fluoride (PVDF) membranes (EMD
Millipore). The membranes were blocked for 1 h at room temperature
with 5% no-fat milk (BD Biosciences), followed by incubation with
the primary antibodies overnight at 4°C. The primary antibodies
used were as follows: Against Wnt10b (1:1,000; ab70816; Abcam) and
GAPDH (1:2,000; ab9485; Abcam). The following day, after 3 washes
with 0.1% TBST at room temperature, the membranes were incubated
with HRP-conjugated goat anti-rabbit IgG (1:2,000; ab6721; Abcam)
for 2 h at room temperature. Following 3 washes with 0.1% TBST, the
blots were incubated for 3 min at room temperature with ECL reagent
(EMD Millipore) and exposed on an imaging system (Tanon Science
& Technology Co., Ltd.). Protein density values were assessed
and calculated using ImageJ software (version 1.47; National
Institutes of Health).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
assay
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to assess TC cell proliferation following the
indicated treatments. Briefly, at 0, 24, 48 or 72 h post-treatment,
the cells were treated with MTT (5 mg/ml) for 4 h. Dimethyl
sulfoxide was then used to dissolve the resultant formazan
crystals, and the absorbance (OD) values at 570 nm were measured
using a plate reader (ELX800; Biotek Instruments, Inc.).
Transwell invasion assay
The upper chamber of a Transwell plate insert was
coated with Matrigel (BD Biosciences). Cells (5×104)
appropriately treated in serum-free medium were added to the upper
chamber at 24 h post-treatment, while medium containing 10% FBS was
added to the lower chamber. Following 48 h of incubation at 37°C,
cells that remained in the upper chamber were carefully removed
using cotton swab, while 4% paraformaldehyde was used to stain the
remaining cells for 10 min at room temperature, and invasive cells
in 6 random fields of view per samples were counted using a light
microscope (Olympus Corporation).
Luciferase reporter assay
The cells (5×105 per well) were added to
6-well plates for 24 h at 37°C, after which they were transfected
with 1.5 µg of a luciferase plasmid (pGL3-promoter Wnt10b) and 5 µl
of NC miRNA (5′-CAGUACUUUUGUGUAGUACAA-3′), 1.5 µg of luciferase
plasmid and 5 µl of miR-148a inhibitor, or 1.5 µg luciferase
plasmid and 5 µl miR-148a mimics for 6 h at 37°C using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.). At
48 h post-transfection, a Dual-Luciferase Reporter Assay System
(Promega Corporation) was used to assess the luciferase activity in
these cells, with Renilla luciferase activity being used for
normalization.
Targeting of 148a
TargetScan (http://www.targetscan.org) software program was used
to search for miR-148a target genes.
Statistical analysis
SPSS 22.0 software (IBM, Inc.) was used for all
statistical analyses. All data are the means ± the standard
deviation (SD), and were compared using Student's t-tests or
one-way analysis of variance (ANOVA) with the Tukey's post hoc
test. The Kaplan-Meier analysis and the log rank test were used for
the survival analysis. HOTAIRM1 expression was analyzed using
Fisher's exact probability test in Excel. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
PTC tissues and cells exhibit HOTAIRM1
upregulation
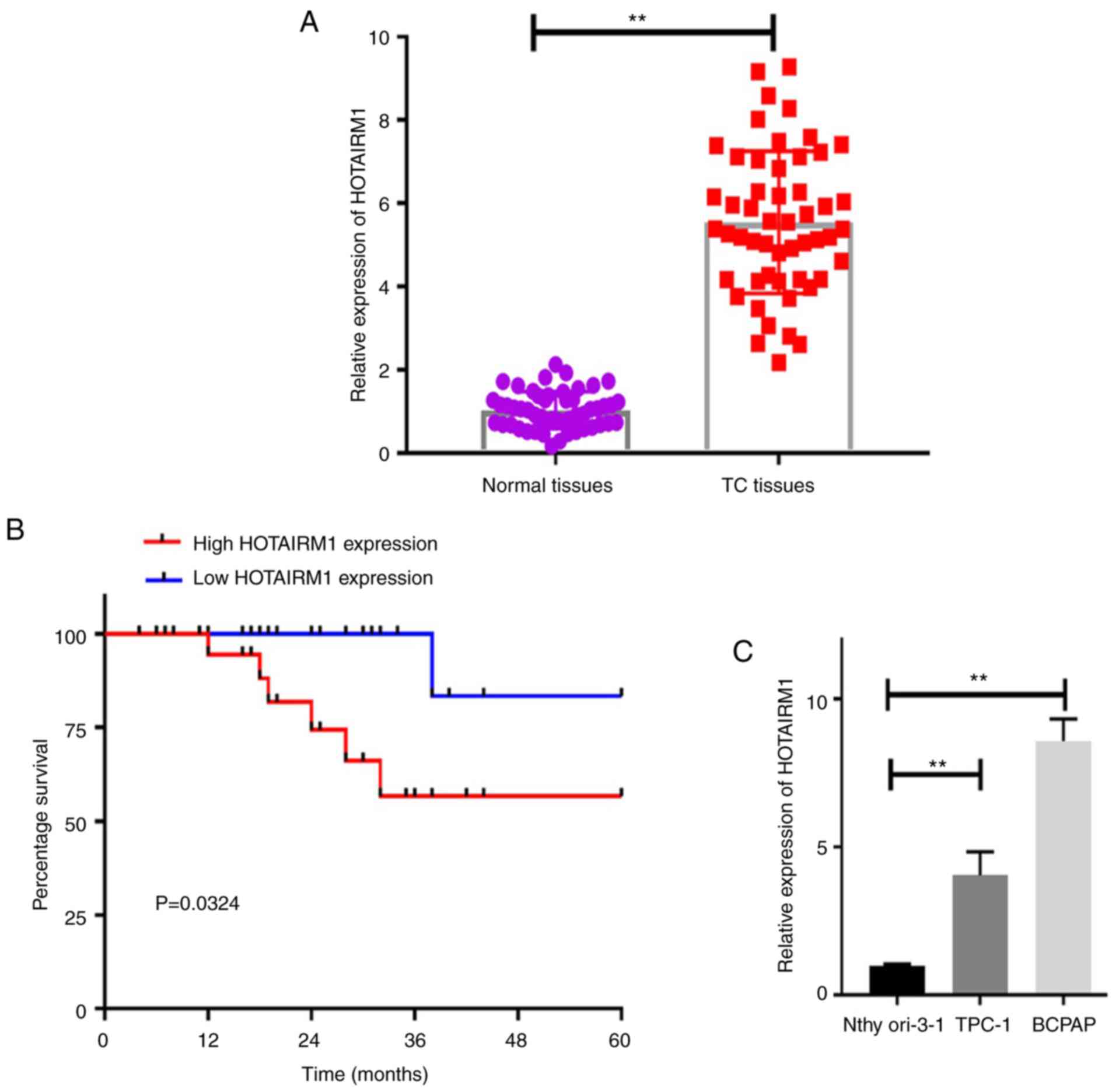
The present study began by comparing the expression
of HOTAIRM1 in human TC tumor and paracancerous tissue samples,
revealing that this lncRNA was markedly upregulated in cancerous
tissues (Fig. 1A; P<0.01). It
was also found that HOTAIRM1 upregulation was closely associated
with patient TNM stage and lymph node metastasis, whereas it was
not associated with patient age, sex, or tumor size (Table III; P<0.05). The long-term
survival of patients expressing higher HOTAIRM1 levels was also
significantly decreased relative to that of patients expressing
lower levels of this lncRNA (Fig.
1B; P<0.05).
 | Table III.Association between
clinicopathological features and HOTAIRM1 expression in 52 patients
with thyroid carcinoma. |
Table III.
Association between
clinicopathological features and HOTAIRM1 expression in 52 patients
with thyroid carcinoma.
|
|
| HOTAIRM1
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | No. of
patients | High (%) | Low (%) | P-value |
|---|
| Age (years) |
|
|
| 0.976 |
|
<60 | 23 | 12 (52.17) | 11 (47.83) |
|
|
≥60 | 29 | 13 (44.83) | 16 (55.17) |
|
| Sex |
|
|
| 0.373 |
|
Male | 17 | 5 (29.41) | 12 (70.59) |
|
|
Female | 35 | 20 (57.14) | 15 (42.86) |
|
| TNM stage |
|
|
| 0.018 |
|
I–II | 18 | 4 (22.22) | 14 (77.78) |
|
|
III–IV | 34 | 21 (61.67) | 13 (38.23) |
|
| Tumor size |
|
|
| 0.427 |
| <1
cm | 21 | 9 (42.86) | 12 (57.14) |
|
| ≥1
cm | 31 | 16 (51.61) | 15 (48.39) |
|
| Lymph node
metastasis |
|
|
| 0.014 |
|
Yes | 33 | 22 (66.67) | 11 (33.33) |
|
| No | 19 | 3 (15.79) | 16 (84.21) |
|
The expression of HOTAIRM1 was then compared in TC
and control cell lines, revealing that this lncRNA was expressed at
significantly higher levels in the TPC-1 and BCPAP PTC cell lines
relative to the control Nthy-ori 3-1 cell line (Fig. 1C; P<0.01). Thus, the TPC-1 and
BCPAP cells were selected for use in further analyses in order to
better understand the functional relevance of HOTAIRM1 in TC.
Knockdown of HOTAIRM1 impairs TC cell
proliferation and invasion
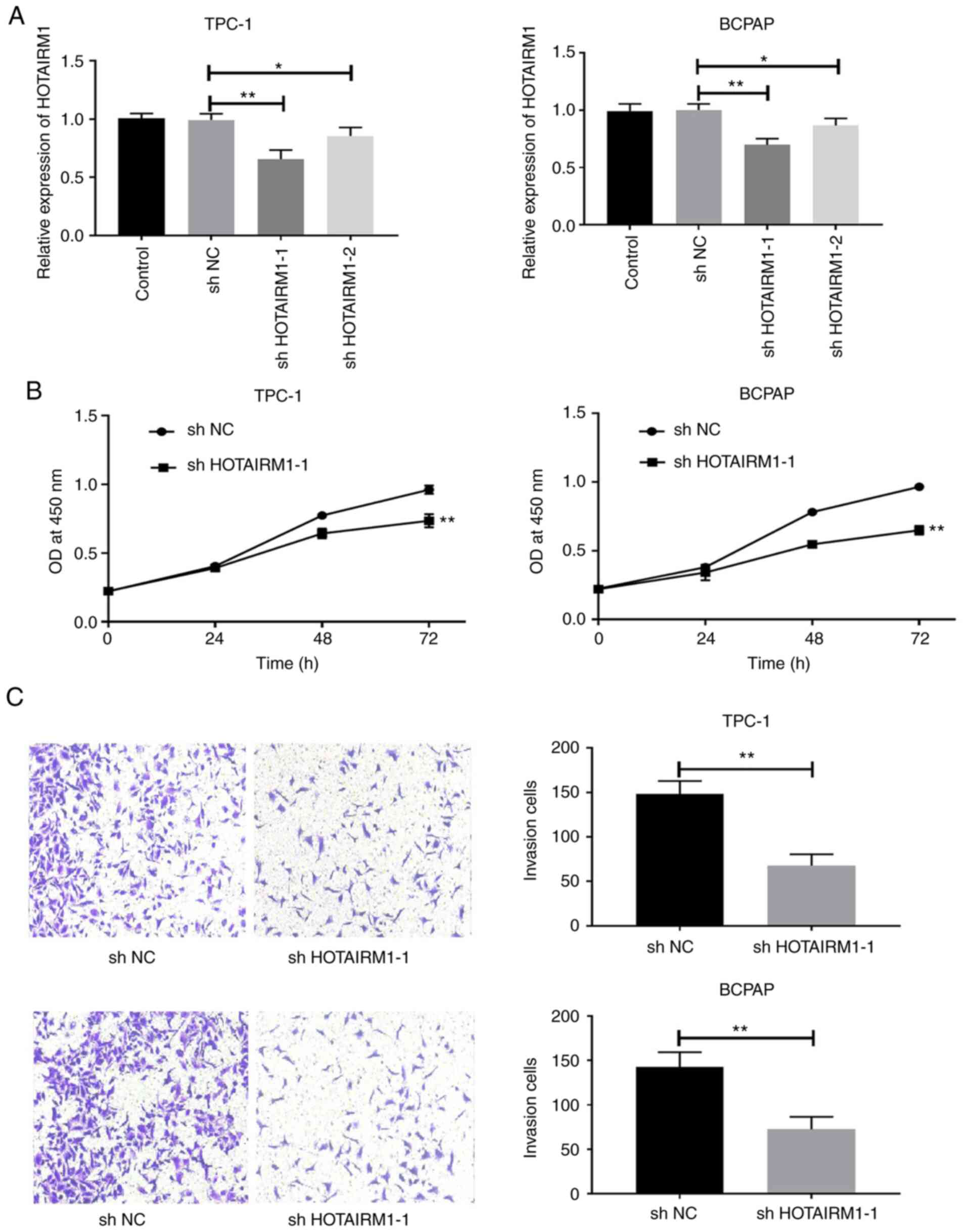
Subsequently, HOTAIRM1 expression was successfully
knocked down in TC cell lines using lentiviruses encoding
shHOTAIRM1 (Fig. 2A; P<0.05).
The knockdown of this lncRNA significantly impaired TPC-1 and BCPAP
cell proliferation (Fig. 2B,
P<0.05) and invasion (Fig. 2C,
P<0.05). Thus, HOTAIRM1 may drive TC cell proliferation and
metastasis.
miR-148a represents a putative
HOTAIRM1 downstream target in TC
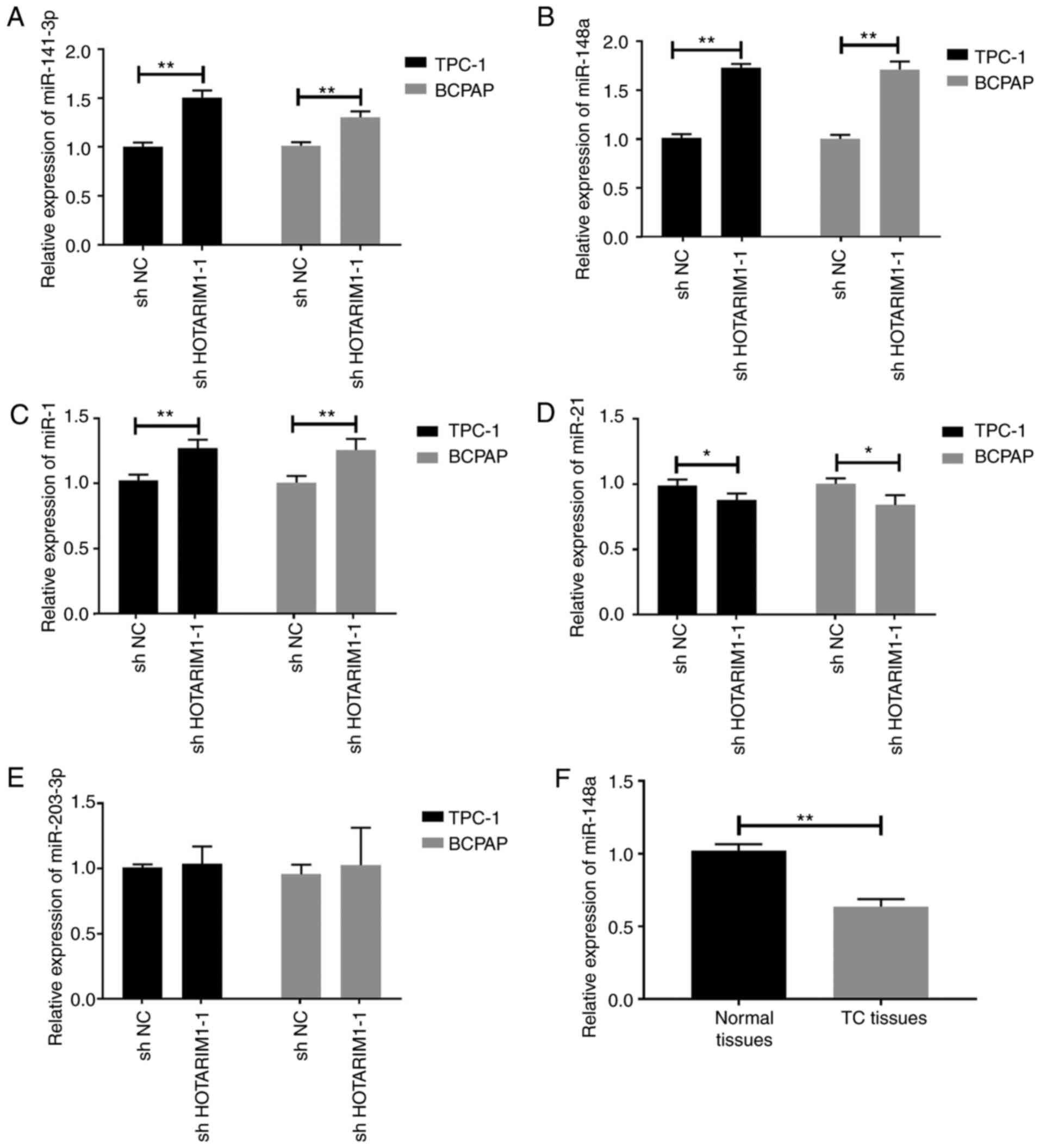
The miRNA expression patterns in TC cells following
the knockdown of HOTAIRM1 were then evaluated. HOTAIRM1 knockdown
promoted the upregulation of miR-141-3p (Fig. 3A, P<0.05), miR-148a (Fig. 3B, P<0.05) and miR-1 (Fig. 3C, P<0.05) in TC cells, whereas it
suppressed the expression of miR-21 (Fig. 3D, P<0.05). No marked changes in
the expression of miR-202-3p were observed as a function of
HOTAIRM1 knockdown (Fig. 3E,
P>0.05). Of the miRNAs examined, miR-148a exhibited the most
marked changes relative to baseline expression, and it was also
found that miR-148a was significantly downregulated in tissues from
patients with TC relative to the paracancerous tissue samples
(Fig. 3F, P<0.01).
Inhibition of miR-148a reverses the
effects of HOTAIRM1 knockdown on TC cells
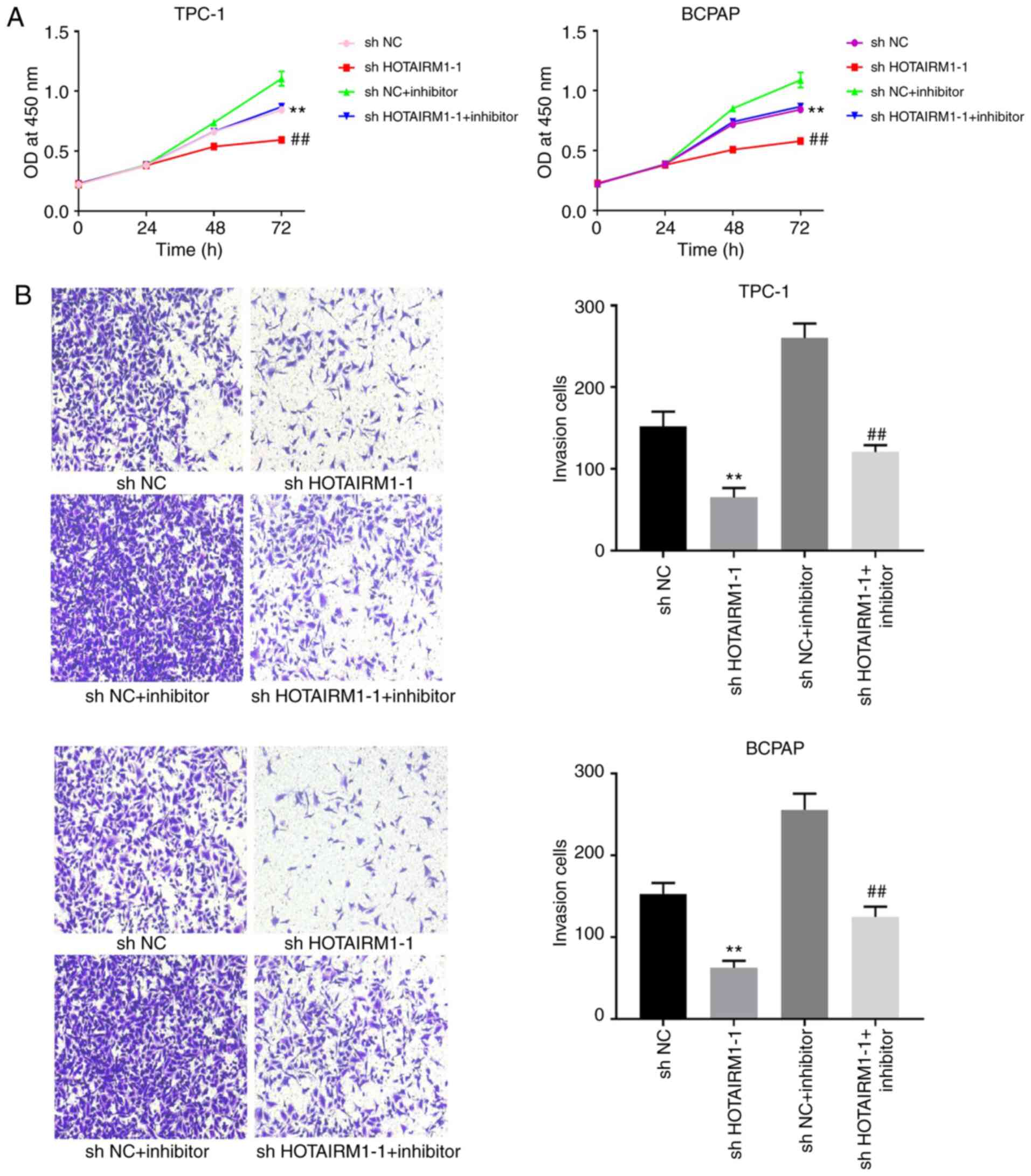
The potential association between HOTAIRM1 and
miR-148a was then evaluated in TC cells. It was found that
transfection with miR-148a inhibitor following HOTAIRM1 knockdown
was sufficient to restore TC cell proliferation (Fig. 4A, P<0.01) and invasion (Fig. 4B, P<0.01), indicating that this
lncRNA controls TC cell proliferation and invasion, at least in
part by regulating miR-148a.
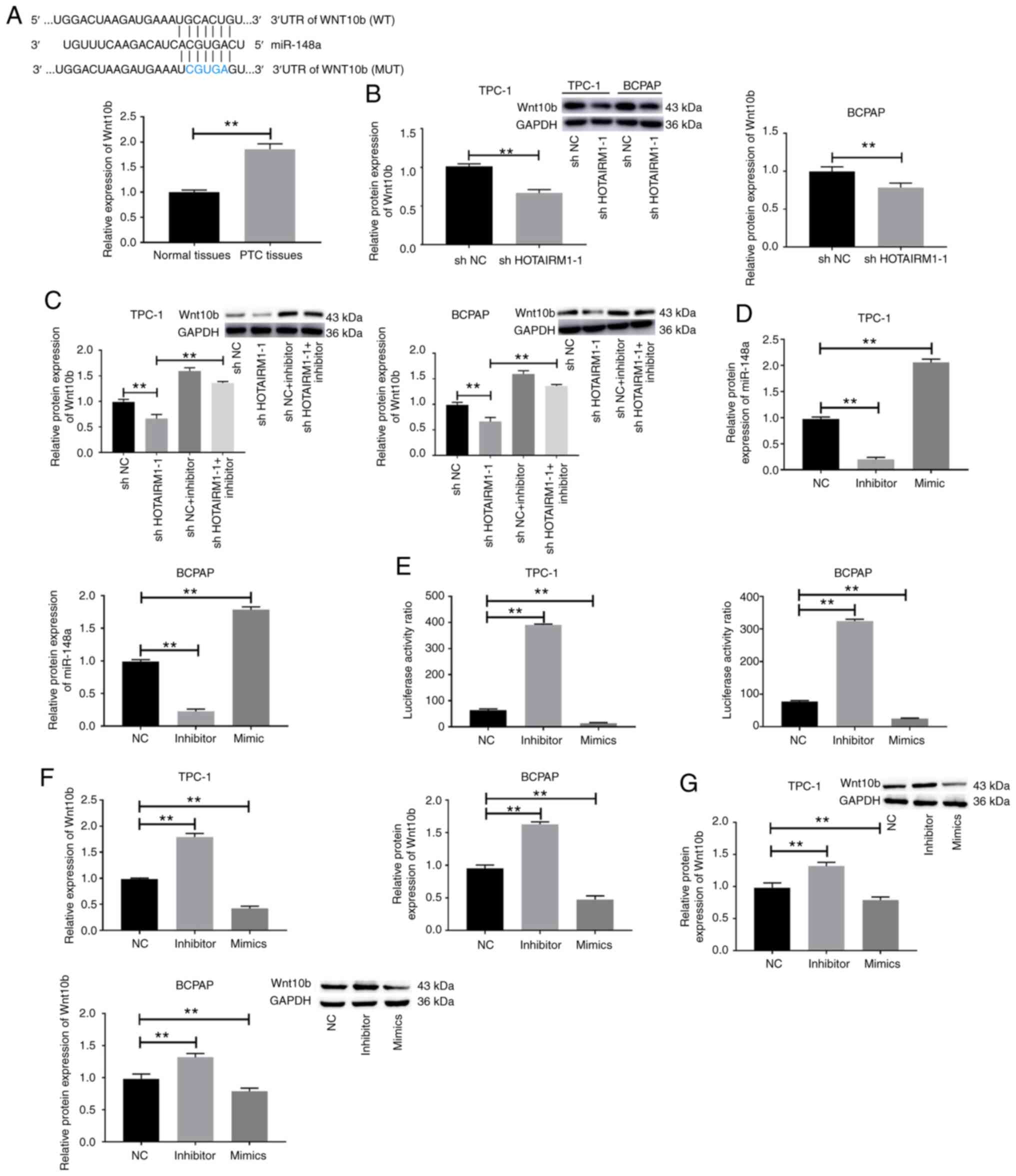
miR-148a directly suppresses Wnt10b
expression in TC cells
Subsequently, the mechanisms whereby miR-148a
affects TC cells were evaluated. TargetScan software revealed a
putative miR-148a binding site within the Wnt10b 3′-UTR. In line
with this prediction, it was found that Wnt10b was significantly
upregulated in TC tumor tissues relative to paracancerous control
tissues (Fig. 5A, P<0.01).
Importantly, the knockdown of HOTAIRM1 decreased the protein
expression level of Wnt10b in TPC-1 and BCPAP cells (Fig. 5B, P<0.01), whereas transfection
with miR-148a inhibitor reversed this effect (Fig. 5C, P<0.01). Following miR-148a
overexpression or inhibition in TC cell lines (Fig. 5D, P<0.01), a luciferase reporter
assay was then conducted, which revealed that the overexpression of
miR-148a suppressed the luciferase activity and the expression of
Wnt10b expression, while miR-148a inhibition resulted in the
opposite effect (Fig. 5E-G,
P<0.01). Taken together, these findings suggested that miR-148a
suppressed Wnt10b expression via binding to the Wnt10b 3′-UTR in TC
cells.
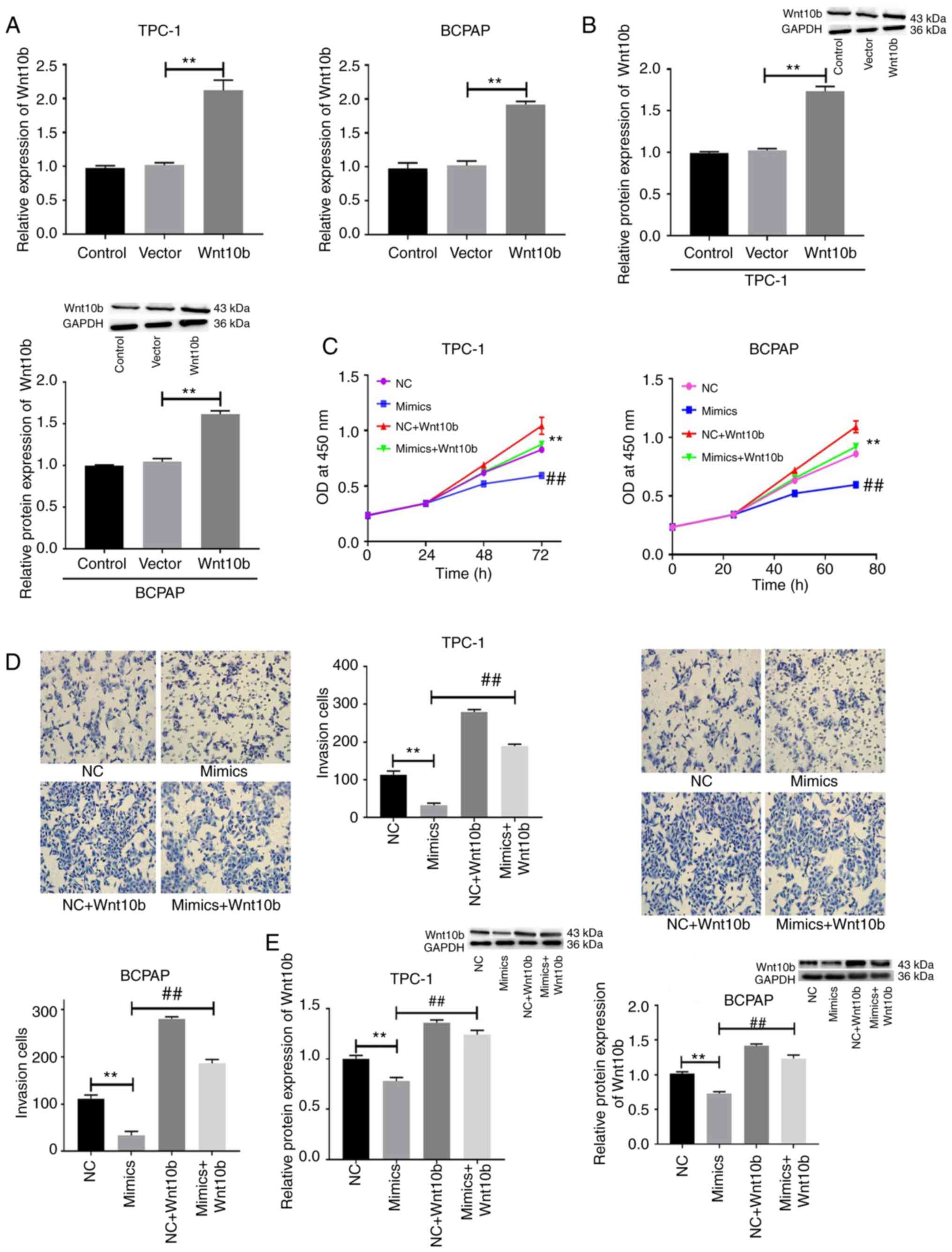
miR-148a targets Wnt10b to control TC
cell proliferation and invasion
Finally, the functional impact of the overexpression
of Wnt10b in TC cells was evaluated using a lentiviral construct
(Fig. 6A-C). Following Wnt10b
overexpression and simultaneous transfection with miR-148a mimic,
it was found that the overexpression of miR-148a was sufficient to
impair cell proliferation (Fig. 6C,
P<0.01) and invasion (Fig. 6D,
P<0.01), while also suppressing Wnt10b expression (Fig. 6E, P<0.01) in TPC-1 and BCPAP
cells. Wnt10b overexpression, however, was sufficient to reverse
these effects induced by miR-148a mimic, thus suggesting that the
miR-148a-mediated suppression of Wnt10b expression is an important
regulator of TC cell proliferation and invasion.
Discussion
Herein, it was found that both HOTAIRM1 and Wnt10b
were significantly upregulated in TC tissues and cells, whereas
miR-148a was downregulated in these samples. The knockdown of
HOTAIRM1 and the overexpression miR-148a were both sufficient to
suppress the proliferation and invasion of TC cells and to reduce
Wnt10b expression in these cells. As such, these data suggested
that HOTAIRM1, miR-148a and Wnt10b may serve as key regulators of
TC progression.
While the mechanistic basis for TC development
remains complex and incompletely understood, several lncRNAs have
been shown to regulate key oncogenic processes in this context,
including cellular proliferation, migration and
epithelial-mesenchymal transition (15–18).
As such, the present study sought to evaluate the functional
relevance of lncRNAs in TC.
Previous studies have demonstrated that miRNAs can
also regulate TC pathogenesis (19–21).
For example, miR-141-3p suppresses the growth and metastasis of TC
cells (22), while miR-1 serves as
a tumor suppressor that constrains the migration and proliferation
of TC cells (23). Similarly,
miR-148a impairs TC cell proliferation, migration and invasion
(24,25), while miR-21 and miR-202-3p also
serve as regulators of this cancer type (26,27).
Based on the above-mentioned reports, the present
study observed that several miRNAs exert marked effects on TC,
particularly miR-148a. Furthermore, miR-148a expression in TC cells
was negatively regulated by HOTAIRM1, which is consistent with the
findings of previous studies (28,29).
From these results, it was hypothesized that miR-148a functions
downstream of HOTAIRM1. Furthermore, the inhibitory effects of
HOTAIRM1 knockdown on the proliferation and invasion of TC cells
were potently counteracted by the inhibition of miR-148a. These
findings indicate that the HOTAIRM1-regulated cell proliferative
and invasive ability in TC is likely modulated by miR-148a.
It was found that miR-148a upregulation in TC cells
directly suppressed Wnt10b expression by binding to the Wnt10b
3′-UTR. As such, Wnt10b is a miR-148a target gene that may be
linked to TC pathogenesis. Previous studies have demonstrated that
a range of miRNAs control cellular proliferation and metastasis by
targeting Wnt10b (12,30). This result was consistent with the
current finding that transfection with miR-148a mimic suppressed TC
cell proliferation and invasion, whereas Wnt10b overexpression
reversed this effect. Taken together, the data suggested that this
HOTAIRM1/miR-148a/Wnt10b axis controls TC progression. By knocking
down HOTAIRM1, it may be possible to inhibit TC cell proliferation
and invasion through the miR-148a-mediated suppression of Wnt10b
expression.
In conclusion, the present study investigated
explored the potential role of lncRNA HOTAIRM1 in inhibiting TC
cell proliferation and suppressing the invasive ability by
modulating the invasion of TC cells by controlling miR-148a and
Wnt10b. This lncRNA mediated the regulation of Wnt10b expression.
Taken together, these results suggest that targeting this
HOTAIRM1/miR-148a/Wnt10b axis may be represent a potential
therapeutic strategy for the treatment of TC.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of Jilin Province (grant no. 20180101138JC).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
CL and GC conceived and designed the study. CL, GC,
XC and TL performed the experiments. CL and GC wrote the
manuscript. CL, GC, XC and TL reviewed and edited the manuscript.
All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study conformed to the principles
presented in the Declaration of Helsinki and was approved (no.
20190318) by the Ethics Committee of the First Hospital of Jilin
University (Jilin, China). Written informed consent was obtained
from all study subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu H, Lv Z, An C, Shi M, Pan W, Zhou L,
Yang W and Yang M: Onco-lncRNA HOTAIR and its functional genetic
variants in papillary thyroid carcinoma. Sci Rep. 6:319692016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jendrzejewski J, Thomas A, Liyanarachchi
S, Eiterman A, Tomsic J, He H, Radomska HS, Li W, Nagy R, Sworczak
K and de la Chapelle A: PTCSC3 is involved in papillary thyroid
carcinoma development by modulating S100A4 gene expression. J Clin
Endocrinol Metab. 100:1370–1377. 2015. View Article : Google Scholar
|
|
4
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sørensen KP, Thomassen M, Tan Q, Bak M,
Cold S, Burton M, Larsen MJ and Kruse TA: Long non-coding RNA
HOTAIR is an independent prognostic marker of metastasis in
estrogen receptor-positive primary breast cancer. Breast Cancer Res
Treat. 142:529–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Svoboda M, Slyskova J, Schneiderova M,
Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z,
Protivankova M, et al: HOTAIR long non-coding RNA is a negative
prognostic factor not only in primary tumors, but also in the blood
of colorectal cancer patients. Carcinogenesis. 35:1510–1515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang JX, Han L, Bao ZS, Wang YY, Chen LY,
Yan W, Yu SZ, Pu PY, Liu N, et al: HOTAIR, a cell cycle-associated
long noncoding RNA and a strong predictor of survival, is
preferentially expressed in classical and mesenchymal glioma. Neuro
Oncol. 15:1595–1603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu ZM, Wu ZY, Li WH, Wang LQ, Wan JN and
Zhong Y: MiR-96-5p promotes the proliferation, invasion and
metastasis of papillary thyroid carcinoma through down-regulating
CCDC67. Eur Rev Med Pharmacol Sci. 23:3421–3430. 2019.PubMed/NCBI
|
|
9
|
Zhou SL, Tang QL, Zhou SX and Ren RZ:
MiR-296-5p suppresses papillary thyroid carcinoma cell growth via
targeting PLK1. Eur Rev Med Pharmacol Sci. 23:2084–2091.
2019.PubMed/NCBI
|
|
10
|
Zhang Y, Sun B, Zhao L, Liu Z, Xu Z, Tian
Y and Hao C: Up-Regulation of miRNA-148a inhibits proliferation,
invasion, and migration while promoting apoptosis of cervical
cancer cells by down-regulating RRS1. Biosci Rep.
39:BSR201818152019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y, Zhu Q, Zhou M, Yang W, Shi H, Shan
Y, Zhang Q and Yu F: Restoration of miRNA-148a in pancreatic cancer
reduces invasion and metastasis by inhibiting the Wnt/β-catenin
signaling pathway via downregulating maternally expressed gene-3.
Exp Ther Med. 17:639–648. 2019.PubMed/NCBI
|
|
12
|
Shi L, Xi J, Xu X, Peng B and Zhang B:
MiR-148a suppressed cell invasion and migration via targeting
WNT10b and modulating β-catenin signaling in cisplatin-resistant
colorectal cancer cells. Biomed Pharmacother. 109:902–909. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi H, Chen X, Jiang H, Wang X, Yu H, Sun
P and Sui X: MiR-148a suppresses cell invasion and migration in
gastric cancer by targeting DNA methyltransferase 1. Oncol Lett.
15:4944–4950. 2018.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choy M, Guo Y, Li H, Wei G, Ye R, Liang W,
Xiao H, Li Y and Guan H: Long noncoding RNA LOC100129940-N is
upregulated in papillary thyroid cancer and promotes the invasion
and progression. Int J Endocrinol. 2019:70435092019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo K, Chen L, Wang Y, Qian K, Zheng X,
Sun W, Sun T, Wu Y and Wang Z: Long noncoding RNA RP11-547D24.1
regulates proliferation and migration in papillary thyroid
carcinoma: Identification and validation of a novel long noncoding
RNA through integrated analysis of TCGA database. Cancer Med.
1:3105–3119. 2019. View Article : Google Scholar
|
|
17
|
Liang M, Jia J, Chen L, Wei B, Guan Q,
Ding Z, Yu J, Pang R and He G: LncRNA MCM3AP-AS1 promotes
proliferation and invasion through regulating miR-211-5p/SPARC axis
in papillary thyroid cancer. Endocrine. 27:318–326. 2019.
View Article : Google Scholar
|
|
18
|
Song B, Li R, Zuo Z, Tan J, Liu L, Ding D,
Lu Y and Hou D: LncRNA ENST00000539653 acts as an oncogenic factor
via MAPK signalling in papillary thyroid cancer. BMC Cancer.
19:2972019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang XZ, Hang YK, Liu JB, Hou YQ, Wang N
and Wang MJ: Over-Expression of microRNA-375 inhibits papillary
thyroid carcinoma cell proliferation and induces cell apoptosis by
targeting ERBB2. J Pharmacol Sci. 130:78–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Wang J, Li X, Ma J, Shi C, Zhu H,
Xi Q, Zhang J, Zhao X and Gu M: MiR-204-5p suppresses cell
proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma.
Biochem Biophys Res Commun. 457:621–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Zhao L, Zhang Z, Zhang H, Ding C and
Su Z: Roles of microRNA let-7b in papillary thyroid carcinoma by
regulating HMGA2. Tumour Biol. 39:1010428317719274. 2017.
View Article : Google Scholar
|
|
22
|
Fang M, Huang W, Wu X, Gao Y, Ou J, Zhang
X and Li Y: MiR-141-3p suppresses tumor growth and metastasis in
papillary thyroid cancer via targeting yin yang 1. Anat Rec
(Hoboken). 302:258–268. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leone V, D'Angelo D, Rubio I, de Freitas
PM, Federico A, Colamaio M, Pallante P, Medeiros-Neto G and Fusco
A: MiR-1 is a tumor suppressor in thyroid carcinogenesis targeting
CCND2, CXCR4, and SDF-1alpha. J Clin Endocrinol Metab.
96:E1388–E1398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han C, Zheng W, Ge M, Wang K, Xiang Y and
Wang P: Downregulation of cyclin-dependent kinase 8 by
microRNA-148a suppresses proliferation and invasiveness of
papillary thyroid carcinomas. Am J Cancer Res. 7:2081–2090.
2017.PubMed/NCBI
|
|
25
|
Xu Y, Han YF, Zhu SJ, Dong JD and Ye B:
MiRNA-148a inhibits cell growth of papillary thyroid cancer through
STAT3 and PI3K/AKT signaling pathways. Oncol Rep. 38:3085–3093.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han J, Zhang M, Nie C, Jia J, Wang F, Yu
J, Bi W, Liu B, Sheng R, He G, et al: MiR-215 suppresses papillary
thyroid cancer proliferation, migration, and invasion through the
AKT/GSK-3β/Snail signaling by targeting ARFGEF1. Cell Death Dis.
10:1952019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Yin J, Liu J, Zhu RX, Zheng Y and
Wang XL: MiR-202-3p functions as a tumor suppressor and reduces
cell migration and invasion in papillary thyroid carcinoma. Eur Rev
Med Pharmacol Sci. 23:1145–1150. 2019.PubMed/NCBI
|
|
28
|
Xiao Y, Yan X, Yang Y and Ma X:
Downregulation of long noncoding RNA HOTAIRM1 variant 1 contributes
to osteoarthritis via regulating miR-125b/BMPR2 axis and activating
JNK/MAPK/ERK pathway. Biomed Pharmacother. 109:1569–1577. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Dong C, Cui J, Wang Y and Hong X:
Over-Expressed lncRNA HOTAIRM1 promotes tumor growth and invasion
through up-regulating HOXA1 and sequestering G9a/EZH2/Dnmts away
from the HOXA1 gene in glioblastoma multiforme. J Exp Clin Cancer
Res. 37:2652018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng L, Liu Z, Xiao J, Tu Y, Wan Z, Xiong
H, Li Y and Xiao W: MicroRNA-148a suppresses epithelial-mesenchymal
transition and invasion of pancreatic cancer cells by targeting
wnt10b and inhibiting the Wnt/β-catenin signaling pathway. Oncol
Rep. 38:301–308. 2017. View Article : Google Scholar : PubMed/NCBI
|