Introduction
Multiple myeloma (MM) accounts for ~10% of all
hematological malignancies (1,2). As a
complex plasma cell neoplasm, patients with MM exhibit highly
heterogenous molecular characteristics (3,4).
Chemotherapy, targeted therapy, immunotherapy and hematopoietic
stem cell transplantation are common therapeutic strategies for MM.
Recently, proteasome inhibitors and immunomodulatory drugs have
also been used for MM therapy (5).
However, the median survival rate for patients with MM is ~50%
(6). Furthermore, MM treatment
leads to various adverse effects, including neutropenia,
myelosuppression and thrombocytopenia (7,8). Thus,
understanding the factors involved in the progression of MM is of
utmost importance for the development of novel therapies and for
improving patient prognosis.
Epigenetic dysregulation plays an important role in
the progression of MM (9). Abnormal
methylation and the overproduction of misfolded proteins have been
widely observed in MM tissues and cells (10,11).
For example, histone deacetylase (HDAC) inhibitors have become a
focus of research in the treatment of MM (12). Panobinostat, an inhibitor of HDACs,
exhibits cytotoxic activity against MM cells in combination with
bortezomib in vitro and in vivo (13). HDAC3 can regulate the expression of
DNA methyltransferase 1 (DNMT1) to trigger the proliferation of MM
cells (14). Various micro (mi)RNAs
and long non-coding (lnc)RNAs can also regulate the in vitro
and in vivo progression of MM by targeting downstream
signaling molecules (15).
Jumonji domain-containing proteins (JMJDs), which
can recognize methylated histone as substrates, have attracted
immense interest since previous findings showed their significant
roles in the progression of a wide range of cancers (16,17).
JMJD2C, also known as KDM4C, can demethylate histone 3 lysine 9
trimethylation to relieve chromatin compaction by recruiting
epigenetic writers and their readers, such as heterochromatin
protein 1 α and KRAB-associated protein 1 (18). JMJD2C can promote cell migration and
invasion by modulating cullin-4A expression in lung cancer
(19). It is required for the
expression of interleukin-3 receptor subunit α and the survival of
acute myeloid leukemia cells (20).
However, the potential roles of JMJD, particularly those of JMJD2C,
in the progression of MM have not yet been well defined.
The present study examined the expression of JMJD2
A/B/C in MM tissues and healthy controls. Furthermore, the
potential effects of JMJD2C on the growth of MM cells were
investigated. The results indicated that JMJD2C promoted the
malignancy of MM via the activation of the β-catenin pathway.
Materials and methods
Patient sample collection
All patient and healthy control samples were
collected at the Zaozhuang Municipal Hospital (Zaozhuang, China)
between February 2017 and September 2018 following the approval of
the Human Ethics Committee of Zaozhuang Municipal Hospital
(approval no. ZZ-2018002). The patient group comprised 20 patients
with newly-diagnosed MM (NDMM) who had not received any treatment
and 20 healthy controls who had no history of basic or chronic
diseases. The clinical characteristics of the participating
patients and healthy controls are presented in Table I. All patients and healthy controls
signed written informed consent forms in accordance with the
Declaration of Helsinki. Mononuclear cells were separated from bone
marrow by gradient density centrifugation, and plasma cells were
then enriched from the bone marrow samples using CD138-coated
magnetic beads (Miltenyi Biotech, Inc.) according to the
manufacturer's instructions to ensure >90% plasma cell purity.
Subsequently, the mRNA expression of JMJD2 was examined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
assay.
 | Table I.Clinical characteristics of the study
cohorts. |
Table I.
Clinical characteristics of the study
cohorts.
| Parameter | HC | MM | Total |
|---|
| n | 20 | 20 | 40 |
| Age, years |
| Mean ±
SD | 47.9±9.4 | 52.1±11.2 | 50.1±10.5 |
|
Range | 33–71 | 31–75 | 31–75 |
| Sex |
| Male, n
(%) | 13 (65%) | 12 (60%) | 25 (63%) |
| Female,
n (%) | 7
(35%) | 8
(40%) | 15 (37%) |
Oncomine database and Kaplan-Meier
plotter analysis
The online microarray database Oncomine™ was used to
explore the mRNA expression levels of JMJD2C in MM and adjacent
normal tissues (https://www.oncomine.org/resource/login.html). The
conditions for filter setting were as follows: Gene, ‘JMJD2C’;
Cancer Type, ‘Myeloma’. The studies incorporating mRNA expression
data of JMJD2C were obtained. Kaplan-Meier plotter (KM
plotter, www.kmplot.com), which assesses the
effect of 54,675 genes on survival using >10,000 cancer samples,
was used to analyze survival data based on an online database
(21). The time from beginning of
surgery to death was defined as overall survival (OS). All patients
were split into two groups according to the median level of the
genes (high vs. low expression). The statistical analysis was
carried out using a log-rank test. Kaplan-Meier survival plots were
automatically generated.
RNA extraction and RT-qPCR
Total RNA was separated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance
with the manufacturer's instructions. The concentration and purity
of the RNA were ascertained by UV spectrophotometry. Complementary
DNA was synthesized using 1 µg total RNA and the PrimeScript RT
Reagent Kit with gDNA Eraser (Takara Bio, Inc.), according to the
manufacturer's instructions. The mRNA expression of target genes
was quantified using the SYBR-Green PCR kit (Takara Biotechnology
Co., Ltd.) using an ABI 7500 Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the primers listed
in the primerbank (http://pga.mgh.harvard.edu/primerbank/). The following
primers were used: JMJD2A forward, 5′-ATCCCAGTGCTAGGATAATGACC-3′
and reverse, 5′-ACTCTTTTGGAGGAACAACCTTG-3′; JMJD2B forward,
5′-ACTTCAACAAATACGTGGCCTAC-3′ and reverse,
5′-CGATGTCATCATACGTCTGCC-3′; JMJD2C forward,
5′-CGAGGTGGAAAGTCCTCTGAA-3′ and reverse,
5′-GGGCTCCTTTAGACTCCATGTAT-3′; CTNNB1 (encodes β-catenin) forward,
5′-AAAGCGGCTGTTAGTCACTGG-3′ and reverse,
5′-CGAGTCATTGCATACTGTCCAT-3′; casein kinase 1α (CK1α) forward,
5′-AGTGGCAGTGAAGCTAGAATCT-3′ and reverse,
5′-CGCCCAATACCCATTAGGAAGTT-3′; GSK3β forward,
5′-GGCAGCATGAAAGTTAGCAGA-3′ and reverse,
5′-GGCGACCAGTTCTCCTGAATC-3′; GPADH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′; and pre-JMJD2C forward,
5′-AATTTGGCATCCAACCTGAG-3′ and reverse, 5′-CATCTAACCCAGCCCACACT-3′.
The PCR cycling conditions were 15 min at 95°C, followed by 40
cycles for 10 sec at 95°C, 30 sec at 60°C, and 1 sec at 72°C, and 1
cycle of cooling for 30 sec at 50°C. The relative gene expression
was calculated using the 2−ΔΔCq method (22). GAPDH was used as the internal
reference for normalization.
Cell culture and transfection
Human U266 cells (established from the peripheral
blood of a patient with an IgE myeloma) (23), RPMI8226 cells (derived from the
peripheral blood of a 61-year-old male with MM) (24) and H929 cells (a human plasma cell
myeloma culture having a rearranged cellular myc proto-oncogene)
(25), which are all B cell
maturation antigen-positive MM cell lines, were purchased from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences and maintained in the authors' laboratory. All cell lines
were authorized by short tandem repeat genotyping. Cells were
cultured in RPMI-1640 (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; Nichirei
Biosciences, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a 5%
CO2 humidified atmosphere. The vector control
pcDNA3.1-NC was obtained from Chang Sha Axybio Bio-tech Co., Ltd.
The cDNA of JMJD2C and GSK3β were amplified by general PCR and
cloned into the expression vector pcDNA3.1 between the BamHI
and EcoRI restriction sites using a Cold Fusion kit (System
Biosciences, LLC). Small interfering (si)RNA targeting β-catenin
(5′-CACCUCCCAAGUCCUUUAU-3′ and 5′-UUCUGCAGCUUCCUUGUCCUG-3′) and a
negative control (5′-UAGCGACUAAACACAUCAA-3′) were purchased from
Shanghai GenePharma Co., Ltd. For transfection, cells were seeded
at the density of 5×105 cells per well in 6-well plates
and cultured until the confluence reached 70–80%. Next, cells were
transfected with 20 µM of each construct or siRNAs using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions and
maintained at 37°C for 6 h. Then, medium was replaced with fresh
DMEM containing 10% FBS. After 24 h of transfection, cells were
used for further experiments.
Inhibitor treatment
U266 cells were pre-transfected with vector control
or JMJD2C construct for 12 h as described above, and further
treated with inhibitors of NF-κB [10 µM BAY 11-7082 (BAY)],
GSK3β/β-catenin (10 µM LiCl), PI3K/Akt [10 µM LY294002 (LY)],
ERK1/2 [10 µM PD98059 (PD)] and EGFR [10 µM AG1478 (AG)] for 48 h,
and subsequently cell viability was determined
Cell viability assay
Cell viability was assessed with an MTT assay as
described in previous studies (26,27).
Briefly, cells were seeded in 96-well culture plates
(1×104 cells/well) following pre-transfection with
vector control or pcDNA/JMJD2C for 48 h. Following incubation with
MTT for 4 h, the optical density of viable cells was measured at
450 nm using a SpectraMAX M5 spectrophotometer (Molecular Devices
LLC).
Western blot analysis
Cells were lysed using radio-immunoprecipitation
assay lysis buffer containing protease inhibitors (Beyotime
Institute of Biotechnology). The proteins concentration was
measured using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Proteins (25 µg) were separated by
SDS-PAGE on 12% gel, and then electrotransferred to polyvinylidene
difluoride membranes (EMD Millipore). The membranes were blocked
with 5% (w/v) non-fat milk at room temperature for 2 h, and
incubated overnight at 4°C with the following polyclonal primary
antibodies (all from Abcam at a dilution of 1:1,000): Anti-JMJD2C
(cat. no. ab226480), anti-proliferating cell nuclear antigen (PCNA;
cat. no. ab18197), anti-β-catenin (cat. no. ab6302),
anti-phosphorylated (p)-β-catenin (cat. no. ab81305), anti-H2A.X
(cat. no. ab229914), anti-CK1α (cat. no. ab206652) and anti-GSK3β
(cat. no. ab32391). Subsequently, membranes were incubated with a
HRP-conjugated secondary antibody (1:10,000; cat. no. ab7090;
Abcam) for 2 h at room temperature. The enhanced chemiluminescence
system (EMD Millipore) was used to identify the protein bands and
visualized using a Gel imager camera (Bio-Rad Laboratories, Inc.).
The subcellular localization of β-catenin was examined using the
Nuclear and Cytoplasmic Protein Extraction kit (Beyotime Institute
of Biotechnology) and western blot analysis. Semi-quantification
was performed using ImageJ software (version 1.46; National
Institutes of Health), and the signal of control was set to 100%
for normalization. Results were obtained in uncalibrated units.
mRNA and protein stability
In order to evaluate the effects of JMJD2C on the
stability of β-catenin, cells were treated with 10 µg/ml ActD
(Sigma-Aldrich; Merck KGaA), CHX (Sigma-Aldrich; Merck KGaA), or an
equal volume of solvent (DMSO) as the control. The mRNA and protein
expression of β-catenin was then examined by RT-qPCR and western
blotting, respectively, according to the same methods outlined
above. Relative expression levels were calculated to the levels of
GAPDH.
Chromatin immunoprecipitation (ChIP)
assay
The ChIP-PCR assay for the enrichment of β-catenin
promoter in JMJD2C was performed as previously described (28) using a ChIP assay kit (cat. no.
17-10086; Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol. Briefly, cells were cultured to 90–100%
confluence. After washing three times with PBS, cells were
cross-linked with 1% formaldehyde (Sigma-Aldrich; Merck KGaA),
lysed with 2 ml lysis buffer (50 mM Tris-HCl, pH 8.1/1% SDS/10 mM
EDTA protease inhibitors) at 4°C, and sonicated 4×15 times at 4°C.
The chromatin fragments were incubated with 3 µg affinity-purified
antibodies against JMJD2C (cat. no. ab27532; Abcam) or IgG (cat.
no. ab2410; Abcam) at 4°C overnight and precipitated using protein
A/G beads (cat. no. sc-2002; Santa Cruz Biotechnology, Inc.)
coupled to magna beads. The DNA fragments were extracted using
phenol/chloroform and used as templates for PCR. As to PCR, 1 µl
from a 50 µl DNA extraction and 38 cycles of amplification were
used. The primer sequences for the CTNNB1 promoter was as follows:
Forward, 5′-GTAGAGACGGGGTTTCACCA-3′ and reverse,
5′-CCTGGGCAATAAGAGCAAAA-3′. cycle quantification was determined for
both immunoprecipitated DNA and known amount of DNA from input
sample using the 2−ΔΔCq method (22). The products were confirmed by 3%
agarose gel electrophoresis. Each experiment was performed in
triplicate.
Promoter activity assay
The promoter activities of β-catenin were analyzed
by dual-luciferase reporter assay according to the protocol of a
previous study (29). Cells were
co-transfected with pGL-CTNNB1, pRL-TK, vector control or JMJD2C
construct for 24 h, and then the promoter activity was measured
using a dual-luciferase reporter assay. Briefly, the −1,000-bp
PCR-generated promoter fragment of β-catenin was inserted into the
pGL3-Basic vector (Promega Corporation). Cells (5×103
cells/well) seeded in 96-well plate were transfected with a plasmid
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Luciferase activity was examined using the Dual
Luciferase Reporter Assay System (Promega Corporation) according to
the manufacturer's protocols. The firefly luciferase was normalized
to a control reporter Renilla luciferase in the same
sample.
Statistical analysis
Each experiment was performed in triplicate. Data
are presented as the means ± standard deviation, and were analyzed
using GraphPad Prism 6.0 software (GraphPad Software, Inc.).
Differences between groups were evaluated using a Student's t-test
for two groups or one-way ANOVA followed by Bonferroni's post hoc
test when making pairwise comparisons among ≥3 groups of data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
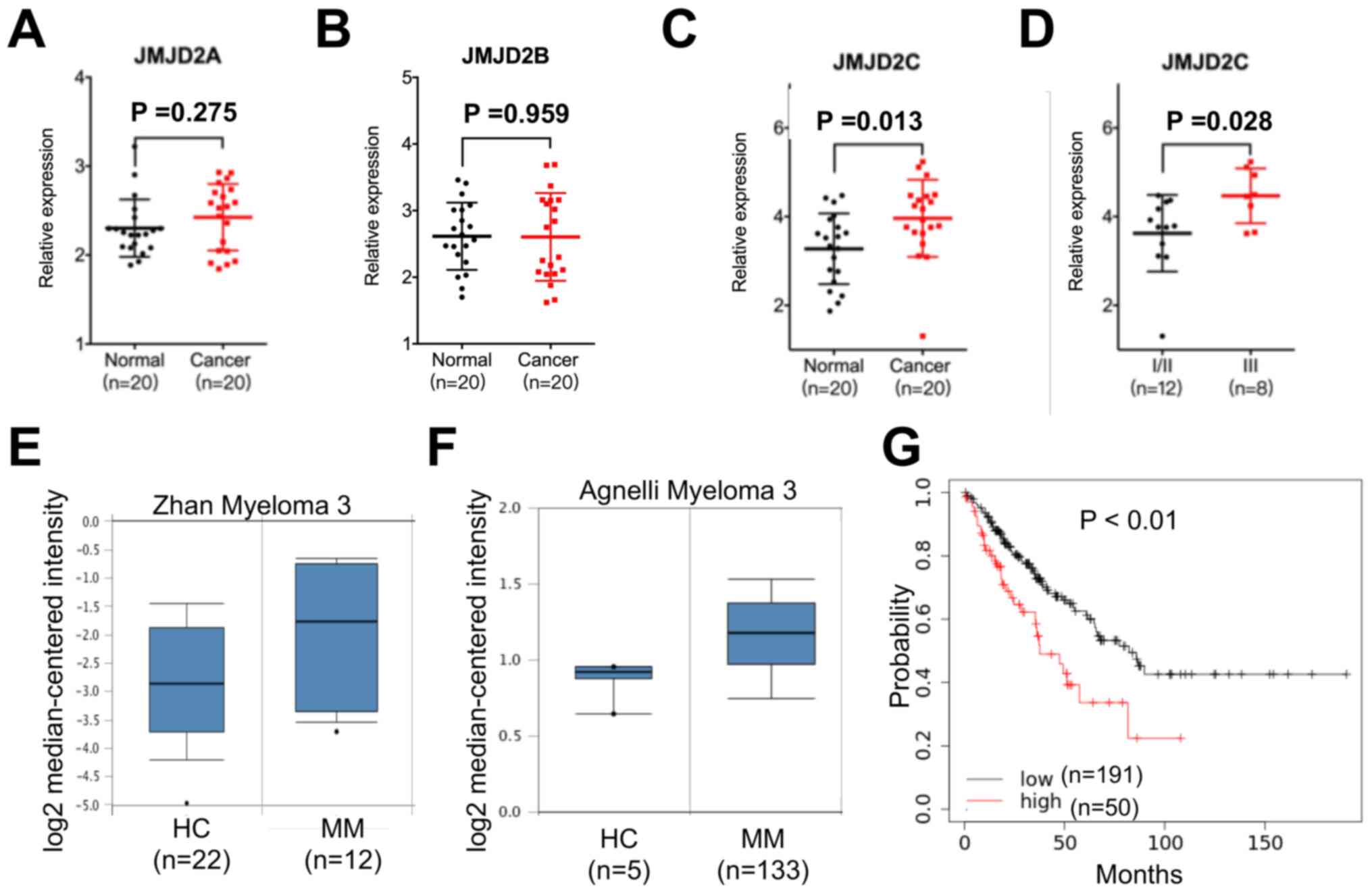
JMJD2C is upregulated in MM
tissues
In order to evaluate the role of JMJD2 in the
progression of MM, the mRNA expression levels of JMJD2A, JMJD2B and
JMJD2C in the bone marrow samples of 20 healthy controls and 20
patients with MM were examined. The results revealed that compared
with the healthy controls, JMJD2C expression was significantly
upregulated in the samples from patients with MM, while the
expression of JMJD2A or JMJD2B in the samples from patients with MM
or healthy controls exhibited no significant difference (Fig. 1A-C). In addition, an association
analysis between JMJD2C expression and the clinicopathological
features of patients with MM was performed, and the results
demonstrated that JMJD2C expression was not related to sex, age or
renal insufficiency in the patients with MM (data not shown).
However, the patients with MM with higher international staging
system (ISS) stages (30) had
significantly higher levels of JMJD2C than those with lower ISS
stages (Fig. 1D). The data from the
Oncomine database revealed that the increased expression of JMJD2C
in MM tissues compared with adjacent normal tissues on the basis of
the data from Zhan (Fig. 1E) and
Agnelli (Fig. 1F) on myeloma. Using
the online bioinformatics tool Kaplan-Meier plotter (31), it was found that patients with MM
with increased expression of JMJD2C showed significantly reduced OS
(Fig. 1G). All these data indicated
that JMJD2C was upregulated in MM tissues.
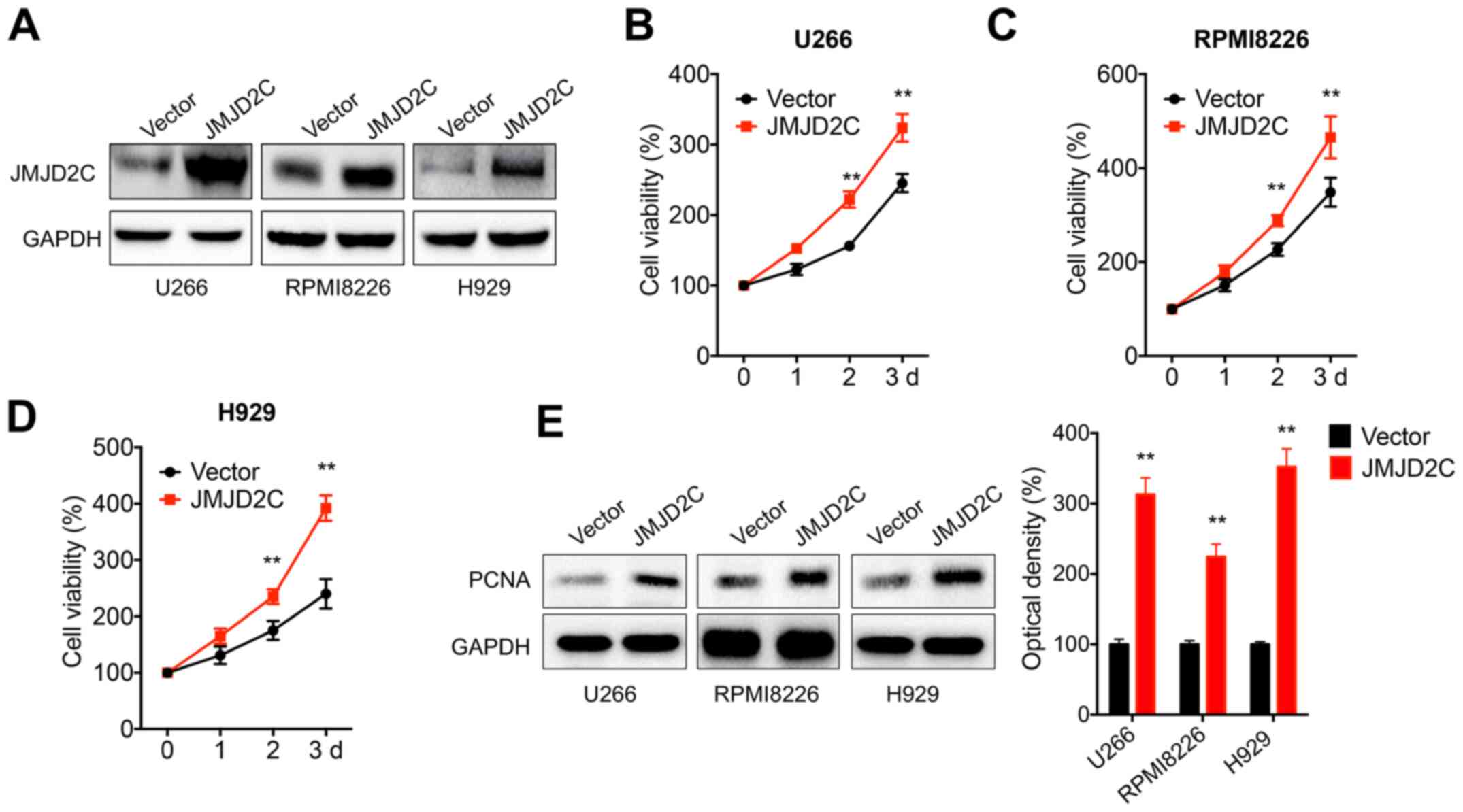
JMJD2C increases cell viability of MM
cells
In order to evaluate the potential roles of JMJD2C
in the progression of MM, human MM U266, RPMI8226 and H929 cells
were transfected with JMJD2C constructs (Fig. 2A). The data demonstrated that the
overexpression of JMJD2C significantly increased the viability of
U266 (Fig. 2B), RPMI8226 (Fig. 2C) and H929 (Fig. 2D) cells. In addition, the expression
of proliferating cell nuclear antigen (PCNA), a proliferation
marker, was significantly increased in MM cells transfected with
JMJD2C (Fig. 2E). These results
indicated that JMJD2C increased the malignancy of MM cells.
β-catenin signals are involved in the
JMJD2C-induced growth of MM cells
It has been reported that various signaling
pathways, such as PI3K/Akt, ERK1/2, NF-κB, GSK3β/β-catenin and EGFR
can trigger the malignancy of MM (7). The present study found that LiCl, an
inhibitor of GSK3β, abolished the JMJD2C-induced cell viability of
U266 cells (Fig. 3A). The
inhibitors of other pathways, such as PI3K/Akt, ERK1/2, NF-κB and
EGFR, had no effect on JMJD2C-induced cell viability of U266 cells
(Fig. 3A). Therefore, further
assays focused on investigating the potential roles of
GSK3β/β-catenin in JMJD2C-regulated malignancy of MM cells.
Furthermore, LiCl attenuated the JMJD2C-induced cell viability of
RPMI8226 (Fig. 3B) and H929
(Fig. 3C) cells. In addition, the
expression of β-catenin was knocked down in U266 cells using
specific siRNA (Fig. 3D). The data
demonstrated that the knockdown of β-catenin suppressed the
viability of U266 cells (Fig. 3E)
and attenuated the JMJD2C-induced cell viability of U266 cells
(Fig. 3F). Consistently, the
knockdown of β-catenin in RPMI8226 cells (Fig. 3G) also suppressed cell viability
(Fig. 3H) and attenuated
JMJD2C-induced cell viability (Fig.
3I).
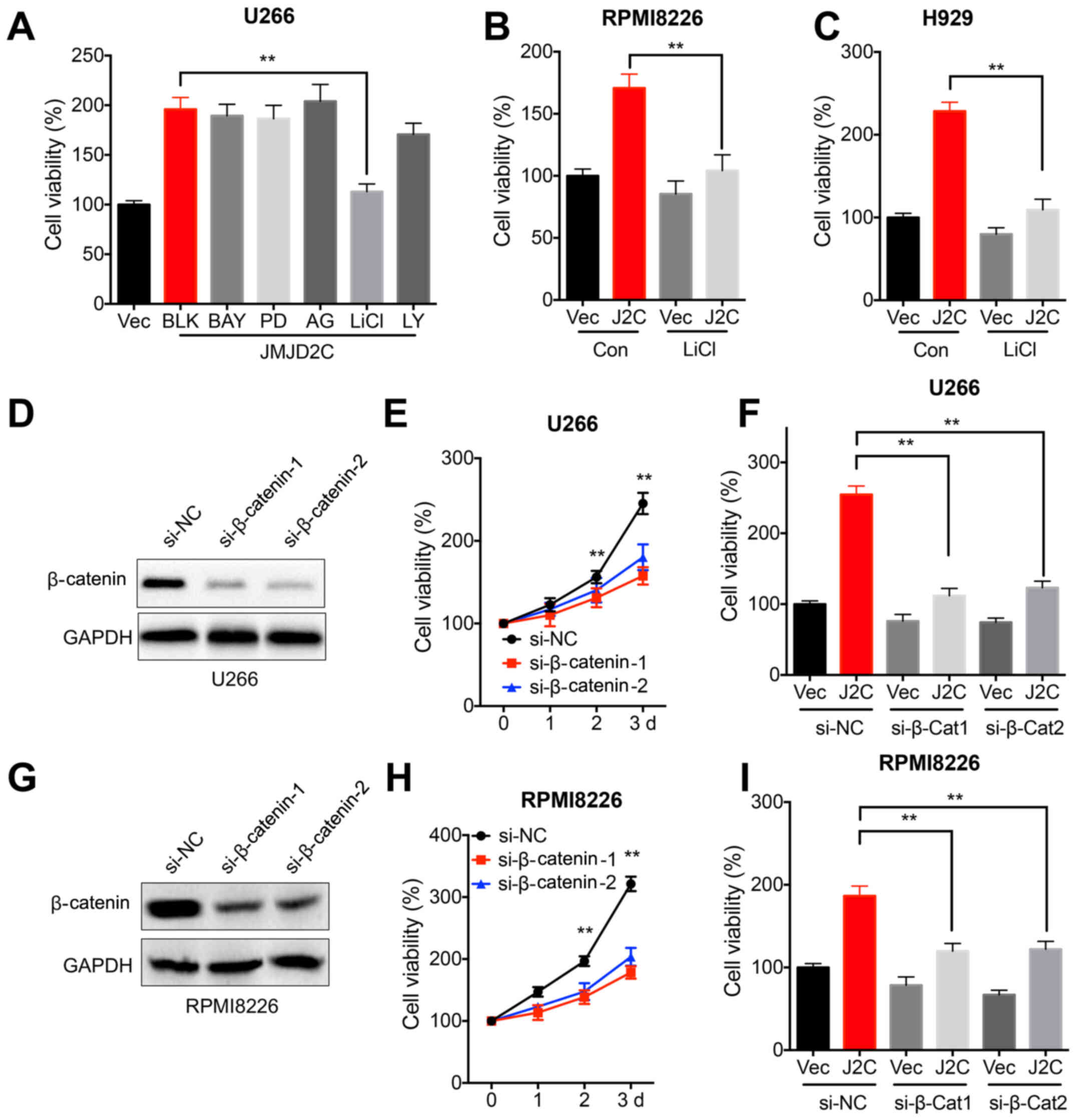 | Figure 3.β-catenin signals are involved in
JMJD2C-induced cell viability of multiple myeloma cells. (A) U266
cells were pre-transfected with vector control or JMJD2C construct
for 12 h and further treated with inhibitors of NF-κB (10 µM BAY),
GSK3β/β-catenin (10 µM LiCl), PI3K/Akt (10 µM LY), ERK1/2 (10 µM
PD) and EGFR (10 µM AG) for 48 h, and subsequently cell viability
was determined. (B) RPMI8226 or (C) H929 cells were pre-transfected
with vector control or JMJD2C construct for 12 h and further
treated with or without 10 µM LiCl for 48 h, and subsequently cell
viability was determined. (D) U266 cells were transfected with
si-NC or si-β-catenin-1/-2 for 24 h. (E) U266 cells were
transfected with si-NC or si-β-catenin-1/-2 for the indicated time
periods, and cell viability was measured. (F) Cells were
co-transfected with vector control, JMJD2C construct, si-NC or
si-β-catenin-1/-2 for 48 h, following which, cell viability was
detected. (G) RPMI8226 cells were transfected with si-NC or
si-β-catenin-1/-2 for 24 h. (H) RPMI8226 cells were transfected
with si-NC or si-β-catenin-1/-2 for the indicated time periods, and
then cell viability was determined. (I) RPMI8226 cells were
co-transfected with vector control, JMJD2C construct, si-NC or
si-β-catenin-1/-2 for 48 h, and subsequently cell viability was
measured. Data are presented as means ± SD of three independent
experiments. **P<0.01 vs. si-NC or as indicated. JMJD2C, Jumonji
C domain-containing 2; BAY, BAY 11-7082; LY, LY294002; PD, PD98059;
AG, AG1478; si-, small interfering RNA; NC, negative control. |
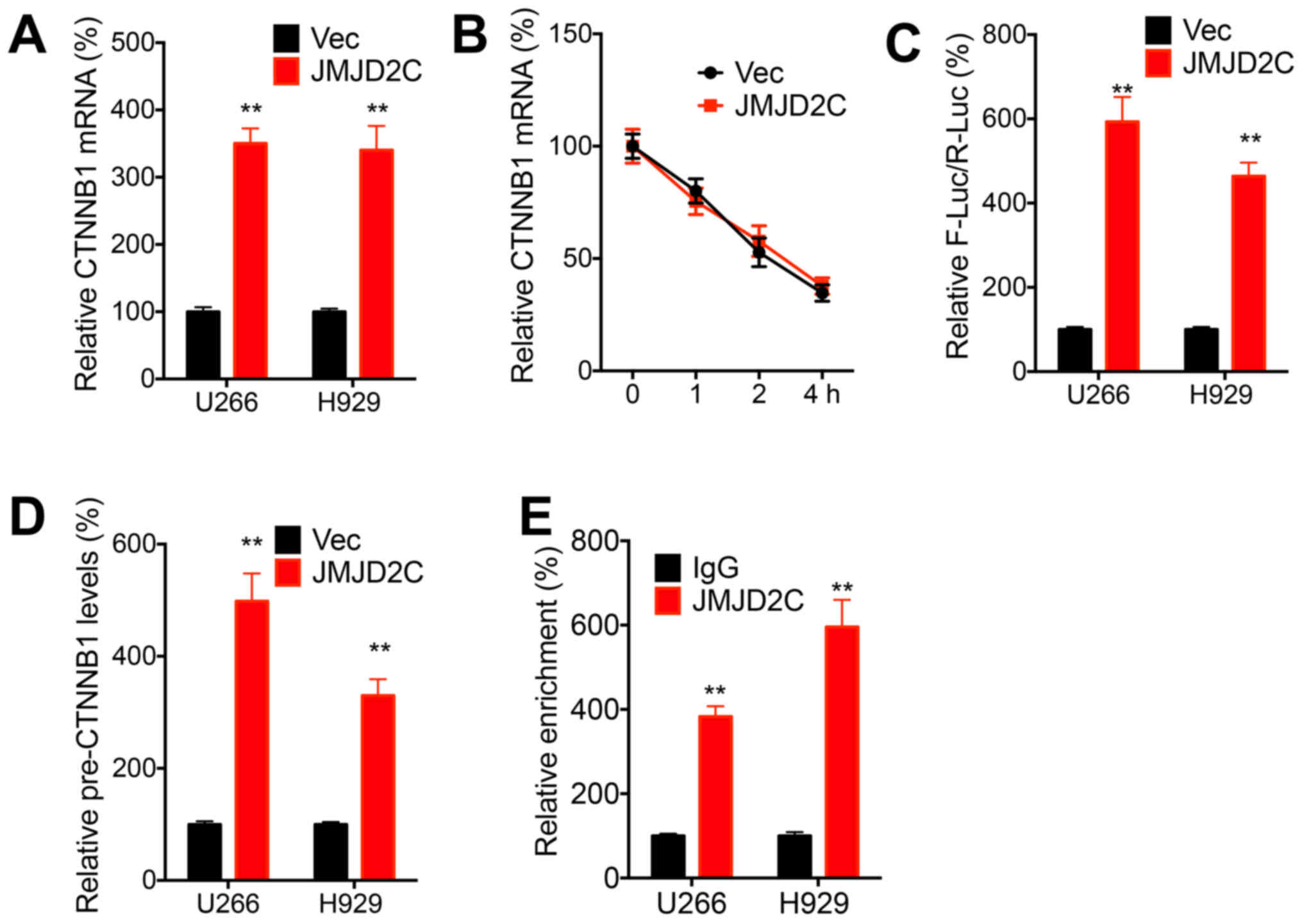
JMJD2C increases the transcription of
β-catenin in MM cells
The potential roles of JMJD2C in the expression and
activation of β-catenin were further evaluated. The data
demonstrated that the overexpression of JMJD2C increased the mRNA
expression of CTNNB1 in both the U266 and H929 cells (Fig. 4A). However, the overexpression of
JMJD2C had no significant effect on the mRNA stability of CTNNB1 in
U266 cells (Fig. 4B), which
indicated that JMJD2C may regulate the transcription of CTNNB1 in
MM cells. The luciferase reporter assay revealed that JMJD2C
increased the promoter activities of CTNNB1 in both U266 and H929
cells (Fig. 4C). Consistently, the
overexpression of JMJD2C increased the precursor mRNA expression of
CTNNB1 in both U266 and H929 cells (Fig. 4D). ChIP-PCR revealed that the
promoter of CTNNB1 was directly enriched with JMJD2C antibody
(Fig. 4E). These results indicated
that JMJD2C increased the transcription of β-catenin in MM
cells.
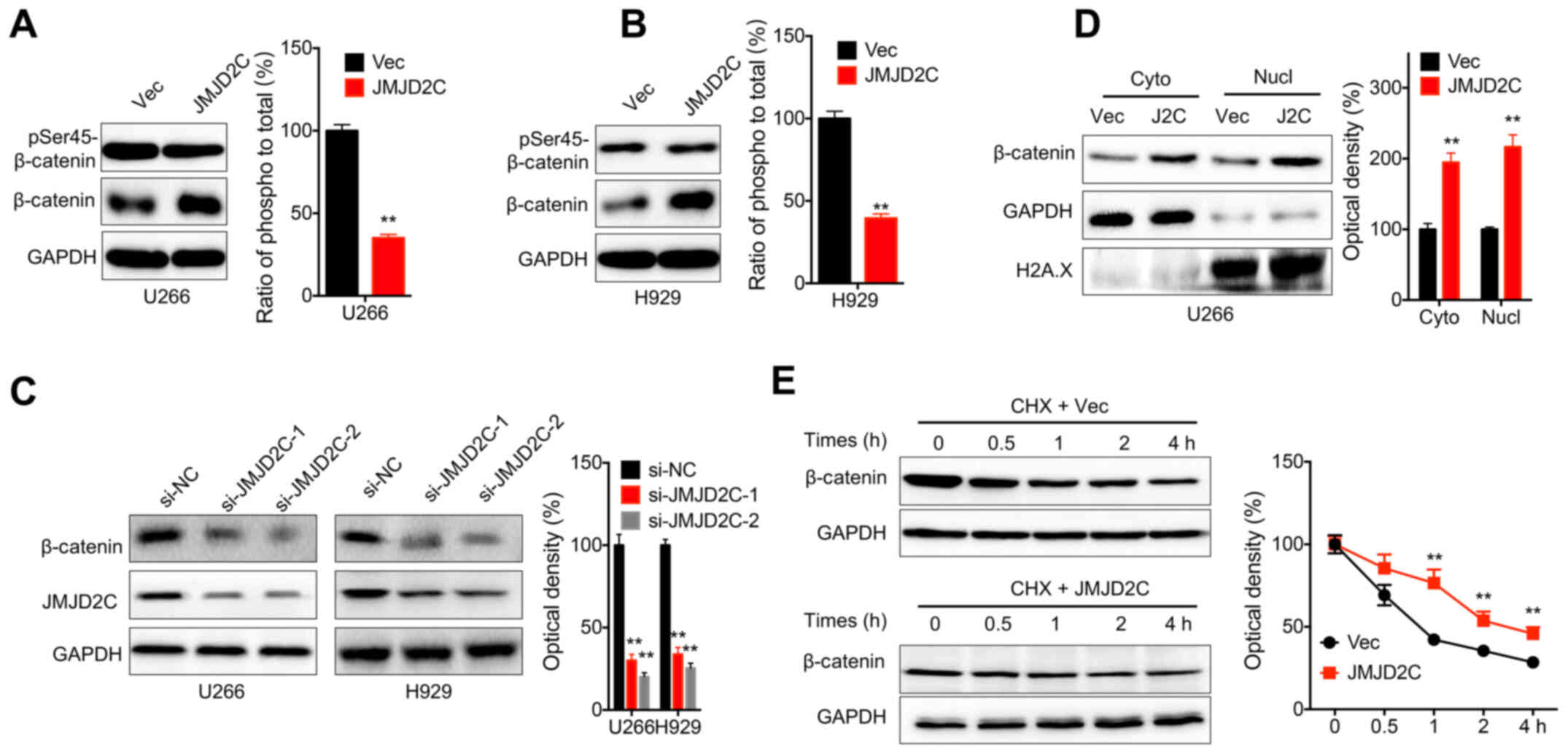
JMJD2C activates β-catenin in MM
cells
Phosphorylation is critical for the activation and
subcellular localization of β-catenin in human cells (32). In the present study, western blot
analysis revealed that the overexpression of JMJD2C notably
increased the expression of β-catenin in both U266 (Fig. 5A) and H929 (Fig. 5B) cells. The relative
phosphorylation of β-catenin was significantly decreased in cells
transfected with JMJD2C constructs (Fig. 5A and B). Consistently, the knockdown
of JMJD2C significantly decreased the expression of β-catenin in
both U266 and H929 cells (Fig. 5C).
Furthermore, the overexpression of JMJD2C increased the nuclear
localization of β-catenin in U266 cells (Fig. 5D). In addition, the overexpression
of JMJD2C increased the protein stability of β-catenin in U266
cells (Fig. 5E). All these data
indicated that JMJD2C increased the expression and activation of
β-catenin in MM cells.
GSK3β is involved in the
JMJD2C-induced activation of β-catenin and malignancy of MM
cells
CK1α and GSK3β are crucial for the phosphorylation
of β-catenin in cancer cells (33).
The data of the present study demonstrated that JMJD2C
overexpression significantly decreased the mRNA (Fig. 6A) and protein (Fig. 6B) expression of GSK3β, but not that
of CK1α, in both U266 and H929 cells. Furthermore, the
overexpression of GSK3β attenuated the JMJD2C-induced decrease in
the phosphorylation of β-catenin in U266 cells (Fig. 6C). However, the overexpression of
GSK3β had no effect on the JMJD2C-induced mRNA expression of CTNNB1
(Fig. 6D). The cell viability assay
revealed that the overexpression of GSK3β attenuated the
JMJD2C-induced increase in the cell viability of U266 (Fig. 6E) and H929 (Fig. 6F) cells. All these results confirmed
that GSK3β was involved in the JMJD2C-induced activation of
β-catenin and the malignancy of MM cells.
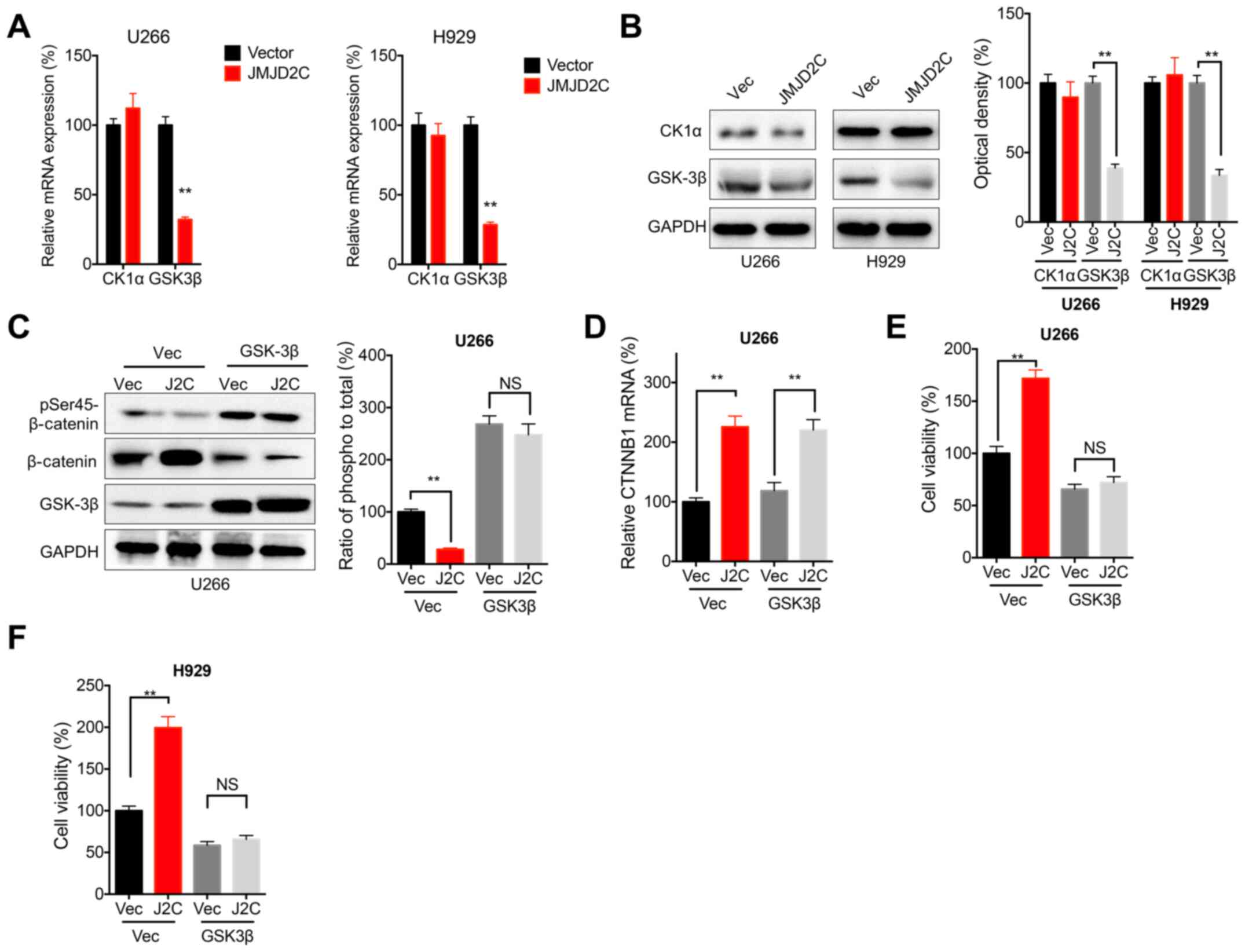 | Figure 6.GSK3β is involved in JMJD2C-induced
activation of β-catenin and malignancy of multiple myeloma cells.
U266 or H929 cells were transfected with vector control or JMJD2C
construct for 24 h, and then the (A) mRNA and (B) protein
expression of CK1α and GSK3β were determined. U266 cells were
co-transfected with vector control, pcDNA/JMJD2C and pcDNA/GSK3β
alone or together for 24 h, following which, the (C)
phosphorylation and (D) mRNA of β-catenin were detected. (E) U266
or (F) H929 cells were co-transfected with vector control,
pcDNA/JMJD2C and pcDNA/GSK3β alone or together for 48 h, and
subsequently cell viability was measured. Data are presented as
means ± SD of three independent experiments. **P<0.01 vs. vector
control or as indicated. NS, not significant; JMJD2C, Jumonji C
domain-containing 2; CK1α, casein kinase 1α; p-, phosphorylated;
si-, small interfering RNA; NC, negative control. |
Discussion
The data of the present study demonstrated that
JMJD2C was upregulated in MM tissues and promoted the malignancy of
MM cells. As one of the most important histone demethylases, JMJD2C
can play crucial roles in the progression of various types of
cancer, such as breast (34),
prostate (35) and lung (19) cancer. For example, the knockdown of
JMJD2C has been reported to inhibit the proliferation of breast
cancer cells in vitro and in vivo (36). In colorectal cancer cells, JMJD2C
has been found to stimulate the proliferation of colon cancer cells
by upregulating the levels of cyclin D1 and Fos-related antigen 1
(36). Consistently, the present
study confirmed that the expression of JMJD2C was upregulated in MM
tissues. The overexpression of JMJD2C increased the cell viability
of MM cells. The present study confirmed the oncogenic roles of
JMJD2C in the progression of MM.
The present study found that β-catenin was involved
in the JMJD2C-induced malignancy of MM cells, which was evidenced
by the finding that the targeted inhibition of β-catenin signals
abolished the JMJD2C-induced cell viability of MM cells. As a
highly conserved signal transduction pathway, β-catenin can
regulate a wide range of cellular processes, including
proliferation and the cell cycle (37). The hyperactivation of β-catenin
signaling is considered one of the hallmarks of MM (38). Several previous studies revealed
that the inhibition of β-catenin signals can suppress the
progression of MM and may be potentially beneficial for patients
with MM (39–41). The present study also demonstrated
that the inhibition of β-catenin significantly suppressed the cell
viability of MM cells.
The data of the present study indicated that JMJD2C
increased the transcription and activation of β-catenin in MM
cells, which was evidenced by the results that JMJD2C positively
regulated its promoter activity and protein stability. Notably,
β-catenin has been demonstrated to bind to the JMJD2C promoter to
increase its transcription in colon cancer cells (42). It is suggested that there may be a
positive feedback loop between JMJD2C and β-catenin in human cancer
cells. Furthermore, lysine demethylase 5A interacts with
CCAAT/enhancer-binding protein β and their interaction
cooperatively inhibits Wnt6 transcription and the activation of the
Wnt/β-catenin pathway (43). The
interaction between JMJDs and the β-catenin pathway warrants
further investigations.
In conclusion, the present study demonstrated that
JMJD2C promoted the malignancy of MM cells via the activation of
β-catenin. Although the interaction between JMJDs and β-catenin
requires further investigation, the data of the present study
indicated that the targeted inhibition of JMJD2C may be a potential
therapeutic approach for MM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and QL conceived and designed the present study,
and confirm the authenticity of all the raw data. ML was involved
in acquisition of data and QL analyzed and interpreted the data. ML
and QL wrote, reviewed and revised the manuscript. Both authors
read and reviewed the final manuscript.
Ethics approval and consent to
participate
Approval for the present study was received from the
Human Ethics Committee of Zaozhuang Municipal Hospital (Zaozhuang,
China). All patients and healthy controls signed written informed
consent forms in accordance with the Declaration of Helsinki.
Patient consent for publication
Written informed consent for publication was
obtained from all participants
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moreau P, San Miguel J, Sonneveld P,
Mateos MV, Zamagni E, Avet-Loiseau H, Hajek R, Dimopoulos MA,
Ludwig H, Einsele H, et al ESMO Guidelines Committee, : Multiple
myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 28 (Suppl_4):iv52–iv61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merz AMA, Merz M, Hillengass J, Holstein
SA and McCarthy P: The evolving role of maintenance therapy
following autologous stem cell transplantation in multiple myeloma.
Expert Rev Anticancer Ther. 19:889–898. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gandolfi S, Vekstein C, Laubach JP,
O'Brien A, Masone K, Munshi NC, Anderson KC and Richardson PG: The
evolving role of transplantation in multiple myeloma: The need for
a heterogeneous approach to a heterogeneous disease. Clin Adv
Hematol Oncol. 16:564–574. 2018.PubMed/NCBI
|
|
4
|
Robiou du Pont S, Cleynen A, Fontan C,
Attal M, Munshi N, Corre J and Avet-Loiseau H: Genomics of Multiple
Myeloma. J Clin Oncol. 35:963–967. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chim CS, Kumar SK, Orlowski RZ, Cook G,
Richardson PG, Gertz MA, Giralt S, Mateos MV, Leleu X and Anderson
KC: Correction: Management of relapsed and refractory multiple
myeloma: novel agents, antibodies, immunotherapies and beyond.
Leukemia. 33:1058–1059. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brioli A, Klaus M, Sayer H, Scholl S,
Ernst T, Hilgendorf I, Scherag A, Yomade O, Schilling K, Hochhaus
A, et al: The risk of infections in multiple myeloma before and
after the advent of novel agents: A 12-year survey. Ann Hematol.
98:713–722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marino S, Petrusca DN and Roodman GD:
Therapeutic targets in myeloma bone disease. Br J Pharmacol. Oct
24–2019.(Epub ahead of print). doi: 10.1111/bph.14889. PubMed/NCBI
|
|
8
|
Webb SL and Edwards CM: Novel therapeutic
targets in myeloma bone disease. Br J Pharmacol. 171:3765–3776.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barwick BG, Gupta VA, Vertino PM and Boise
LH: Cell of Origin and Genetic Alterations in the Pathogenesis of
Multiple Myeloma. Front Immunol. 10:11212019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moreau P, Attal M and Facon T: Frontline
therapy of multiple myeloma. Blood. 125:3076–3084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yaqub S, Ballester G and Ballester O:
Frontline therapy for multiple myeloma: A concise review of the
evidence based on randomized clinical trials. Cancer Invest.
31:529–537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greig SL: Panobinostat: A Review in
Relapsed or Refractory Multiple Myeloma. Target Oncol. 11:107–114.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hideshima T, Richardson PG and Anderson
KC: Mechanism of action of proteasome inhibitors and deacetylase
inhibitors and the biological basis of synergy in multiple myeloma.
Mol Cancer Ther. 10:2034–2042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harada T, Ohguchi H, Grondin Y, Kikuchi S,
Sagawa M, Tai YT, Mazitschek R, Hideshima T and Anderson KC: HDAC3
regulates DNMT1 expression in multiple myeloma: Therapeutic
implications. Leukemia. 31:2670–2677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pourhanifeh MH, Mahjoubin-Tehran M,
Shafiee A, Hajighadimi S, Moradizarmehri S, Mirzaei H and Asemi Z:
MicroRNAs and exosomes: Small molecules with big actions in
multiple myeloma pathogenesis. IUBMB Life. 72:314–333. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van Rechem C and Whetstine JR: Examining
the impact of gene variants on histone lysine methylation. Biochim
Biophys Acta. 1839:1463–1476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Liu L, Yuan X, Wei Y and Wei X:
JMJD3 in the regulation of human diseases. Protein Cell.
10:864–882. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ayrapetov MK, Gursoy-Yuzugullu O, Xu C, Xu
Y and Price BD: DNA double-strand breaks promote methylation of
histone H3 on lysine 9 and transient formation of repressive
chromatin. Proc Natl Acad Sci USA. 111:9169–9174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li N and Jiang D: Jumonji domain
containing 2C promotes cell migration and invasion through
modulating CUL4A expression in lung cancer. Biomed Pharmacother.
89:305–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agger K, Miyagi S, Pedersen MT, Kooistra
SM, Johansen JV and Helin K: Jmjd2/Kdm4 demethylases are required
for expression of Il3ra and survival of acute myeloid leukemia
cells. Genes Dev. 30:1278–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Györffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nilsson K, Bennich H, Johansson SG and
Pontén J: Established immunoglobulin producing myeloma (IgE) and
lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin
Exp Immunol. 7:477–489. 1970.PubMed/NCBI
|
|
24
|
Pellat-Deceunynk C, Amiot M, Bataille R,
van Riet I, van Camp B, Omede P and Boccadoro M: Human myeloma cell
lines as a tool for studying the biology of multiple myeloma: A
reappraisal 18 years after. Blood 86: 4001, 1995. Published Erratum
Blood. 131:1542018.PubMed/NCBI
|
|
25
|
Gazdar AF, Oie HK, Kirsch IR and Hollis
GF: Establishment and characterization of a human plasma cell
myeloma culture having a rearranged cellular myc proto-oncogene.
Blood. 67:1542–1549. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanasugi J, Hanamura I, Ota A, Karnan S,
Lam VQ, Mizuno S, Wahiduzzaman M, Rahman ML, Hyodo T, Konishi H, et
al: Biallelic loss of FAM46C triggers tumor growth with concomitant
activation of Akt signaling in multiple myeloma cells. Cancer Sci.
111:1663–1675. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wahiduzzaman M, Ota A, Karnan S, Hanamura
I, Mizuno S, Kanasugi J, Rahman ML, Hyodo T, Konishi H, Tsuzuki S,
et al: Novel combined Ato-C treatment synergistically suppresses
proliferation of Bcr-Abl-positive leukemic cells in vitro and in
vivo. Cancer Lett. 433:117–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soriano AA, de Cristofaro T, Di Palma T,
Dotolo S, Gokulnath P, Izzo A, Calì G, Facchiano A and Zannini M:
PAX8 expression in high-grade serous ovarian cancer positively
regulates attachment to ECM via Integrin β3. Cancer Cell Int.
19:3032019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hui RC, Francis RE, Guest SK, Costa JR,
Gomes AR, Myatt SS, Brosens JJ and Lam EW: Doxorubicin activates
FOXO3a to induce the expression of multidrug resistance gene ABCB1
(MDR1) in K562 leukemic cells. Mol Cancer Ther. 7:670–678. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised International Staging System
for Multiple Myeloma: A Report From International Myeloma Working
Group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abdel-Magid AF: Wnt/β-Catenin Signaling
Pathway Inhibitors: A Promising Cancer Therapy. ACS Med Chem Lett.
5:956–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daugherty RL and Gottardi CJ:
Phospho-regulation of Beta-catenin adhesion and signaling
functions. Physiology (Bethesda). 22:303–309. 2007.PubMed/NCBI
|
|
34
|
Liu G, Bollig-Fischer A, Kreike B, van de
Vijver MJ, Abrams J, Ethier SP and Yang ZQ: Genomic amplification
and oncogenic properties of the GASC1 histone demethylase gene in
breast cancer. Oncogene. 28:4491–4500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kahl P, Gullotti L, Heukamp LC, Wolf S,
Friedrichs N, Vorreuther R, Solleder G, Bastian PJ, Ellinger J,
Metzger E, et al: Androgen receptor coactivators lysine-specific
histone demethylase 1 and four and a half LIM domain protein 2
predict risk of prostate cancer recurrence. Cancer Res.
66:11341–11347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ye Q, Holowatyj A, Wu J, Liu H, Zhang L,
Suzuki T and Yang ZQ: Genetic alterations of KDM4 subfamily and
therapeutic effect of novel demethylase inhibitor in breast cancer.
Am J Cancer Res. 5:1519–1530. 2015.PubMed/NCBI
|
|
37
|
Nusse R and Clevers H: Wnt/β-Catenin
Signaling, Disease, and Emerging Therapeutic Modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van Andel H, Kocemba KA, Spaargaren M and
Pals ST: Aberrant Wnt signaling in multiple myeloma: Molecular
mechanisms and targeting options. Leukemia. 33:1063–1075. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Gong Y, Liang L, Xiao L, Yi H, Ye
M, Roy M, Xia J, Zhou W, Yang C, et al: Lycorine targets multiple
myeloma stem cell-like cells by inhibition of Wnt/β-catenin
pathway. Br J Haematol. 189:1151–1164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Su N, Wang P and Li Y: Role of
Wnt/β-catenin pathway in inducing autophagy and apoptosis in
multiple myeloma cells. Oncol Lett. 12:4623–4629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schmeel LC, Schmeel FC, Kim Y, Endo T, Lu
D and Schmidt-Wolf IG: Targeting the Wnt/beta-catenin pathway in
multiple myeloma. Anticancer Res. 33:4719–4726. 2013.PubMed/NCBI
|
|
42
|
Yamamoto S, Tateishi K, Kudo Y, Yamamoto
K, Isagawa T, Nagae G, Nakatsuka T, Asaoka Y, Ijichi H, Hirata Y,
et al: Histone demethylase KDM4C regulates sphere formation by
mediating the cross talk between Wnt and Notch pathways in colonic
cancer cells. Carcinogenesis. 34:2380–2388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo L, Guo YY, Li BY, Peng WQ and Tang QQ:
Histone demethylase KDM5A is transactivated by the transcription
factor C/EBPβ and promotes preadipocyte differentiation by
inhibiting Wnt/β-catenin signaling. J Biol Chem. 294:9642–9654.
2019. View Article : Google Scholar : PubMed/NCBI
|