Introduction
Acute myeloid leukemia (AML) is a hematological
cancer type that is characterized by the clonal expansion and
differentiation arrest of myeloid progenitor cells (1). The standard treatment for AML is
induction chemotherapy, which is based on a backbone of cytarabine
plus anthracycline treatment (2).
However, the survival time for most patients who receive
conventional therapy is short. Especially, the median survival of
patients aged ≥65 years is only 6 months (3). The 5-year survival rate of patients with
AML has not significantly increased despite significant advances in
targeted therapy and immunotherapy over recent years (4). Therefore, there is an urgent requirement
for the further identification of novel agents and therapeutics for
AML.
Phytochemicals, which are natural compounds from
plants, have been recognized as vital resources for novel drugs
(5). For example, curcumin (6), epigallocatechin gallate (EGCG) (7), genistein (8) and resveratrol (9) have been reported to possess anti-AML
properties. Curcumin is the main polyphenol component extracted
from rhizomes of the plant Curcuma longa, and its
therapeutic benefit has been demonstrated in various cancer types,
including AML (10). However, the
underlying mechanism is complex and remains poorly understood, as
curcumin has multiple targets and is involved in various signaling
pathways (11). Previous studies have
reported that curcumin can exert its antitumor effects by acting as
an inhibitor of kinases, such as protein kinase B (AKT/PKB) in head
and neck cancer cells (12), JAK1 in
retinoblastoma cells (13) and
p38MAPK in endothelial cells (14).
In the present study, protein phosphorylation profiling using an
antibody array demonstrated that curcumin treatment increased the
phosphorylation levels of 14 proteins but decreased those of four
proteins. Among the 18 proteins, AKT/PKB was found to be the main
target of curcumin. Moreover, it was identified that curcumin
promoted cell cycle arrest and apoptosis of AML cells by
inactivating AKT.
Materials and methods
Chemicals and antibodies
Curcumin, genistein, epigallocatechin gallate
(EGCG), resveratrol and decitabine were purchased from Target
Molecule Corp. Afuresertib (GSK2110183) and SC79 were purchased
from Selleck Chemicals. Antibodies against phosphorylated (p)-P70S6
kinase (P70S6K; T421/S424; cat. no. AP0540), p-AKT1(S473; cat. no.
AP0140), total AKT1 (cat. no. A11016), poly(ADP-ribose) polymerase
(PARP; cat. no. A11010), ACTB (cat. no. AF0198) and caspase 3 (cat.
no. A2156) were obtained from ABclonal Biotech Co., Ltd. Antibodies
against p-RAF-1 (S301; cat. no. AF0047), p-proline-rich Akt
substrate, 40 kDa (PRAS40; T246; cat. no. AF2387), p-p27/Kip1
(T198; cat. no. AF3325), p-eukaryotic translation initiation factor
4E-binding protein 1 (4E-BP1; T36; cat. no. AF3431), β-tubulin
(cat. no. AF7011) and Ki67 (cat. no. AF0198) were obtained from
Affinity Biosciences. PE-conjugated (clone HI30; cat. no. 560975;
BD Bioscience) and unconjugated mouse anti-human CD45 antibody
(clone HI30; cat. no. 555480; BD Bioscience) were used for flow
cytometry and immunohistochemistry (IHC), respectively. A FITC
TUNEL cell apoptosis detection kit was purchased from Wuhan
Servicebio Technology Co., Ltd.
Cell lines and culture
AML cell lines (HL-60, ML-2, MOLM-13, OCI-AML3,
OCI-AML5 and U937) were obtained from the American Type Culture
collection, and were cultured according to the manufacturer's
instructions. All cell lines were mycoplasma-free and were
authenticated by Yubo Biological Technology Co., Ltd. using short
tandem repeat analysis.
Cytotoxicity assay
Cells were cultured in a 96-well plate until the
cell confluence reached ~70%, and then cells were treated with
different concentrations (0, 5, 10, 20, 40 and 80 µM) of curcumin,
genistein, EGCG, resveratrol or decitabine. After 48 h, cell
viability was determined using a MTT assay as described previously
(15). Based on the results of the
MTT assay, the half maximal inhibitory concentration
(IC50) of each chemical was calculated.
Cell cycle and apoptosis analyses
As reviewed by Kouhpeikar et al (16), in vitro examination of the
efficacy of curcumin against AML cells was conducted using 10–50 µM
curcumin to treat cells for 24–48 h. In the present study, cells
were treated with 25 µM curcumin for 24 h. After treatment, cell
cycle and apoptosis were analyzed using a PI staining kit [Hangzhou
Multi Sciences (Lianke) Biotech Co., Ltd.] and an Annexin V-FITC/PI
staining kit (Invitrogen; Thermo Fisher Scientific, Inc.),
respectively, according to the manufacturer's instructions. After
staining, cells were analyzed using a flow cytometer (CytoFlex;
Beckman Coulter, Inc.).
Phosphorylation profiling
A human phosphorylation pathway profiling array
(cat. no. AAH-PPP-1-4) was purchased from RayBiotech, Inc., which
can detect 55 phosphorylated proteins in five signaling pathways:
MAPK, AKT, JAK/STAT, NF-κB and TGF-β. ML-2 cells were cultured in a
10-cm dish until cells reached 90% confluence, and then cells were
treated with or without curcumin (25 µM) for 6 h. After treatment,
the cells were harvested and lysed using the cell lysis buffer with
a protease inhibitor cocktail and a phosphatase inhibitor cocktail.
Phosphorylation array analysis was performed according to the
manufacturer's protocol. The array was sequentially incubated with
the sample and horseradish peroxidase-conjugated antibodies
(provided within the kit), and then scanned with ImageQuant LAS4000
Scanner (Cytiva). In total, two biological replicates were
performed, and the average expression levels were compared between
the treatment and control samples.
Western blotting
Cells were cultured in a 12-well plate until the
cell confluence reached ~90%, and then cells were treated with the
indicated chemicals. Cell lysates were prepared using the cell
lysis buffer with a protease inhibitor cocktail and a phosphatase
inhibitor cocktail (Cell Signaling Technology). After
quantification of the protein concentration, cell lysates
containing equal amounts of total protein were denatured and
separated on 10–12% SDS-PAGE. Following separation, the proteins
were blotted onto PVDF membranes and blocked. After sequentially
incubated with primary antibodies and appropriate secondary
antibodies, the membranes were exposed to Pierce ECL Western
Blotting Substrate (Thermo Fisher Scientific, Inc.) and were imaged
using a gel imaging system (Tanon 4600SF; Tanon Science and
Technology Co., Ltd.).
Xenograft mouse models of AML
A total of 20 male NOD/SCID mice (age, 5–6 weeks;
average weight, 23 mg) were purchased from Hunan Slaccas Jingda
Laboratory Animal Co. Ltd., and housed in groups of 5 per cage with
water and food ad libitum, in a specific-pathogen-free room
with filtered air and controlled light/dark cycle (12/12 h),
temperature (24±2°C) and relative humidity (45–65%). All mice were
pretreated with an intraperitoneal injection of 20 mg/kg busulfan
(APExBIO Technology LLC) 24 h before inoculation, and were then
injected intravenously with 1×106 ML2 cells. At 15 days
after inoculation, the mice were randomly divided into four groups
(5 mice per group), and then treated with vehicle, curcumin (2
mg/mouse), afuresertib (1 mg/mouse) or curcumin (2 mg/mouse) +
afuresertib (1 mg/mouse) via oral gavage every other day for 16
days. Curcumin and afuresertib were dissolved with 5% DMSO + 10%
PEG300 + 5% Tween-80. The humane endpoints were defined by body
weight loss of 20%. All mice were euthanized by asphyxiation
(CO2 displacement rate was ~20% vol/min) 4 days after
the last treatment, and the death was verified by respiratory
arrest and cardiac arrest for >10 min. The experiments were
performed from to July 10 to August 12. The spleens were fixed in
10% formalin and processed for hematoxylin and eosin (H&E)
staining, immunohistochemistry (IHC) analysis and TUNEL assay, as
described previously (17,18). Bone marrow (obtained from tibias and
femurs) was crushed in PBS and created into single cell suspensions
for flow cytometry analysis.
Statistical analysis
RStudio (https://rstudio.com) was used for statistical
analysis. ANOVA and Tukey's post hoc test were performed to
evaluate the significance of difference between samples, adjust
P<0.05 was considered as the level of significance.
Results
Screening for anti-AML
phytochemicals
The four phytochemicals (curcumin, EGCG, genistein
and resveratrol) have been reported to function as epigenetic
modulating agents in cancer (19),
while the DNA methyltransferase inhibitor decitabine is an
FDA-approved chemical for the treatment for AML. Thus, the present
study compared the cytotoxicity of these four phytochemicals with
decitabine in six AML cell lines (HL-60, ML-2, MOLM-13, OCI-AML3,
OCI-AML5 and U937). Cell viabilities at 48 h after exposure to
various concentrations of drugs were determined using MTT assays,
and IC50 values were calculated. Compared with
decitabine, curcumin had a similar or lower IC50 in the
AML cell lines (Table I). Moreover,
curcumin had the strongest cytotoxic activity against AML cells
(except for OCI-AML3) among the four phytochemicals, and ML-2 cells
were the most sensitive to curcumin. Therefore, curcumin was
selected for further study of its function and mechanism in
AML.
 | Table I.IC50 values (mean ± SD) of
four phytochemicals and decitabine/curcumin against AML cell
lines. |
Table I.
IC50 values (mean ± SD) of
four phytochemicals and decitabine/curcumin against AML cell
lines.
| Cell line | Decitabine | Curcumin | EGCG | Genistein | Resveratrol |
|---|
| HL-60 | 69.13±13.65 | 46.98±0.79 |
111.94±34.72d | 90.24±15.65 | 60.55±3.67 |
| ML-2 |
33.67±1.57d |
21.51±0.46b |
34.65±1.81d |
40.10±2.13b,d |
28.70±1.29a,d |
| MOLM-13 | 54.02±11.89 | 53.18±5.87 |
102.11±2.77b,d | 59.65±10.02 | 64.93±5.01 |
| OCI-AML3 | 55.39±8.37 | 71.43±10.12 | 78.65±18.07 |
100.01±20.15a | 48.01±8.11 |
| OCI-AML5 |
126.76±8.54d |
38.45±0.38b |
120.17±21.37d |
64.59±2.13b,d |
70.26±0.32b,c |
| U937 | 56.10±2.17 | 59.80±1.34 |
74.44±1.40b,d |
95.32±5.40b,d |
74.32±4.20b,d |
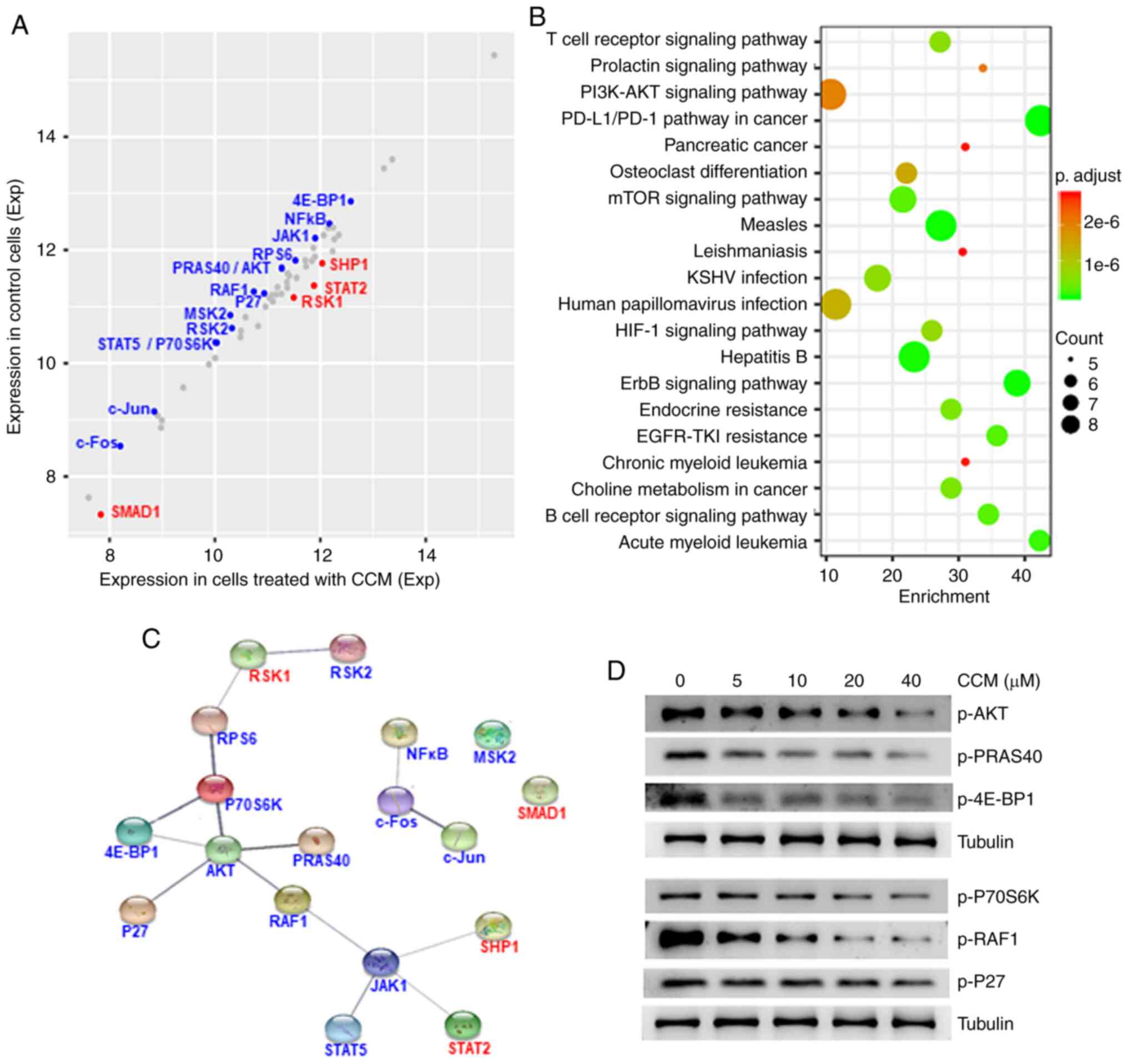
Phosphorylation array analysis
indicates AKT is the key target of curcumin
It has been reported that curcumin can affect
protein phosphorylation (12–14). Thus, the influence of curcumin
treatment on protein phosphorylation was examined using a human
phosphorylation pathway profiling array, which can detect 55
phosphorylated proteins in five signaling pathways: MAPK, AKT,
JAK/STAT, NF-κB and TGF-β. The results demonstrated that the
phosphorylation levels of 14 proteins were downregulated (fold
change ≤0.83), while those of four proteins were upregulated (fold
change ≥1.2) after treatment with curcumin for 6 h (Fig. 1A; Table
SI). Functional annotation analysis based on the Kyoto
Encyclopedia of Genes and Genomes (KEGG) database identified that
the five most enriched pathway were the ‘PD-L1/L1 pathway’, ‘AML’,
‘ErbB signaling pathway’, ‘EGFR tyrosine kinase inhibitor (TKI)
resistance’ and ‘B cell receptor signaling pathway’ (Fig. 1B; Table
SII).
To understand the association between these 18
proteins, a protein-protein interaction (PPI) network was
constructed using STRING software (https://string-db.org). In a PPI network, the nodes
(proteins) with multiple edges (interactions) are defined as hubs,
and hubs are more essential for the global network structure
compared with non-hubs (20). In the
present network (Fig. 1C), the AKT
node had the highest number of edges, indicating that AKT was the
key target of curcumin. Moreover, AKT was involved in the top 20
pathways affected by curcumin (Table
SII).
To confirm the result of the phosphorylation array
analysis, western blotting was conducted to detect the influence of
curcumin (CCM) on the phosphorylation status of AKT and its
interacting proteins in ML-2 cells. Curcumin suppressed the
phosphorylation of AKT1, PRAS40, 4E-BP1, P70S6K, RAF-1 and p27 in a
dose-dependent manner (Fig. 1D).
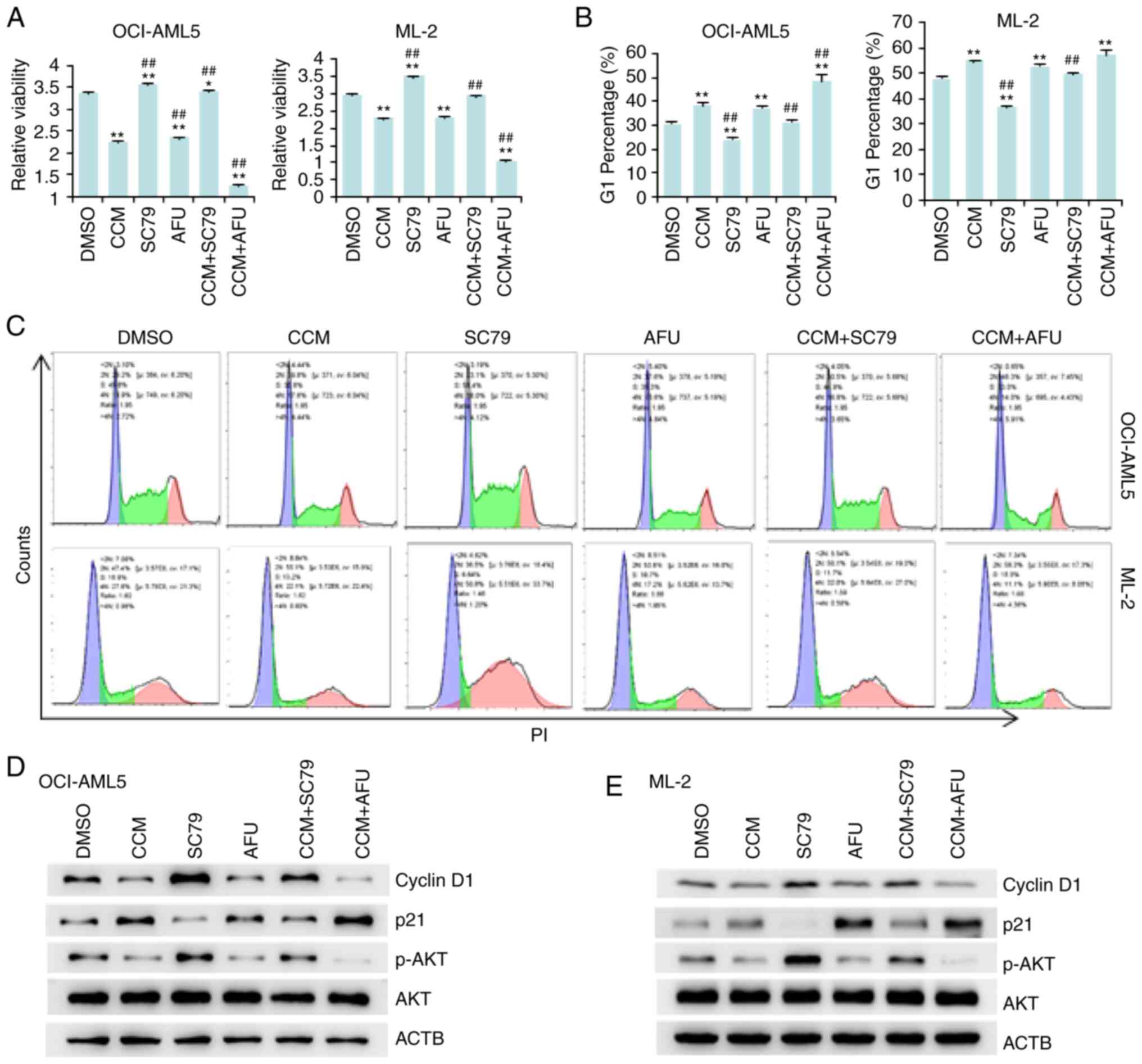
Curcumin promotes AML cell arrest in
the G1 phase by inactivating AKT
To determine whether the cytotoxicity of curcumin is
dependent on AKT activity, AML cells were treated with curcumin
(CCM), an AKT activator (SC-79) or an AKT inhibitor (afuresertib)
alone (AFU) or in combination (CCM+SC79/CCM+AFU). Based on the
results of the MTT assay (Fig. 2A),
SC-79 reversed the antitumor effects of curcumin, while afuresertib
augmented its cytotoxicity on both OCI-AML5 and ML-2 cells. Flow
cytometry results demonstrated that treatment with curcumin or the
AKT inhibitor afuresertib led to cell cycle arrest in the
G1 phase, while treatment with the AKT activator SC-79
promoted cell division (Fig. 2B and
C). Moreover, AKT activation by SC-79 rescued the
curcumin-induced cell cycle arrest, while AKT inhibition by
afuresertib enhanced this effect (Fig. 2B
and C). These results suggested that curcumin suppressed AML
cell arrest in the G1 phase by inactivating AKT.
The influence of curcumin, SC-79 and afuresertib on
cyclin D1 and p21, which are positive and negative regulators of
the cell cycle progression from the G1 to S phase
(21,22), respectively, was then examined.
Treatment of the AML cell lines with curcumin or afuresertib
suppressed the expression of cyclin D1, but increased the
expression of p21 (Fig. 2D and E).
However, SC-79 produced the opposite results. Thus, the effects of
curcumin were enhanced by afuresertib but attenuated by SC-79.
Curcumin promotes AML cell apoptosis
by inactivating AKT
Annexin V and PI labeling followed by flow cytometry
were used to detect apoptotic cells. The results demonstrated that
both curcumin and afuresertib promoted apoptosis, while SC-79
suppressed apoptosis. Moreover, curcumin-induced apoptosis was
stimulated by afuresertib, but diminished by SC-79 (Fig. 3A and B).
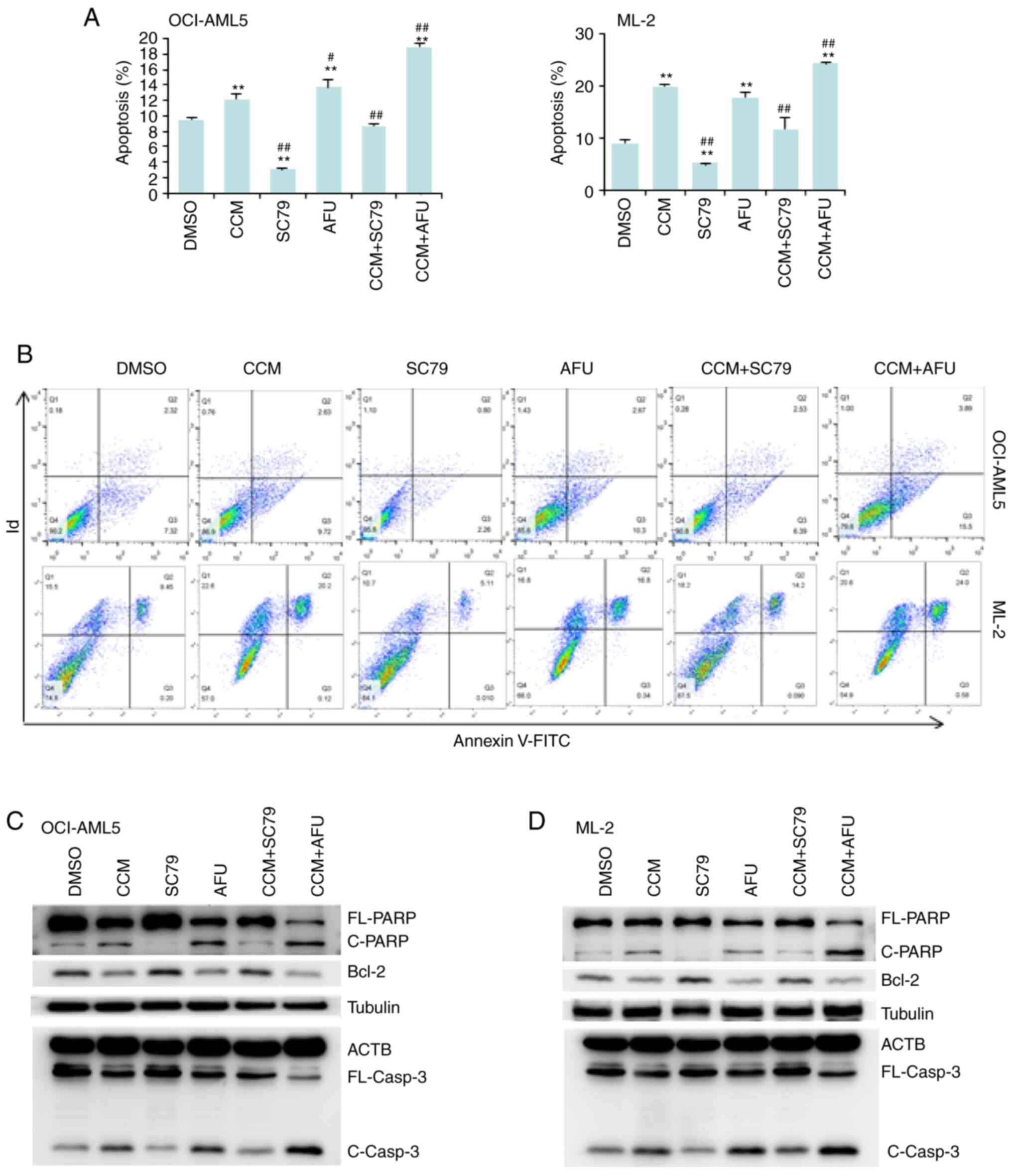 | Figure 3.Curcumin (CCM) promotes AML cell
apoptosis by inactivating AKT. ML-2 and OCI-AML5 cells were treated
with CCM (25 µM), SC-79 (10 µM) or AFU (10 µM) alone or in
combination for 24 h. After treatment, flow cytometry and western
blotting were performed. (A) Percentage of apoptosis is presented
as the mean ± SD of triplicate experiments. (B) Representative
images of flow cytometry. (C and D) Western blot analysis of
FL-PARP, C-PARP, FL-casp3 and C-Casp3. **P≤0.01 vs. DMSO;
#P≤0.05 and ##P≤0.01 vs. CCM. FL-PARP, full
length PARP; AFU, afuresertib; CCM, curcumin; AML, acute myeloid
leukemia; C-PARP, cleaved-PARP; FL-casp3, full length caspase-3;
C-Casp3, cleaved caspase-3; PARP, poly(ADP-ribose) polymerase. |
To identify the proteins involved in
curcumin-induced apoptosis, the expression of three
apoptosis-related proteins, including Bcl-2, caspase-3 and PARP,
were examined. The results indicated that curcumin treatment
decreased the antiapoptotic Bcl-2 protein expression but increased
the cleavage of caspase-3 (C-Casp3) and PARP (C-PARP) (Fig. 3C and D). Furthermore, the influence of
curcumin on these three proteins could be enhanced by afuresertib,
but was abrogated by SC-79.
The above results suggested that curcumin promoted
AML cell arrest and apoptosis by inactivating AKT. However,
IC50 values of curcumin were very weakly correlated with
the levels of phosphorylated AKT in AML cell lines (Fig. S1). Thus suggested that curcumin also
exerted antitumor roles via other pathways, besides the AKT
pathway.
Curcumin and afuresertib
synergistically reduce the leukemia burden in an AML xenograft
mouse model
Next, the in vivo efficacy of curcumin and
afuresertib for the treatment of AML was evaluated. NOD/SCID mice
were intravenously injected with 1×106 ML-2 cells. Drug
treatment began 15 days after injection and continued every other
day for 16 days. After treatment, peripheral blood mononuclear
cells (PBMCs) and bone marrow mononuclear cells (BMMCs) were
isolated and evaluated for human hematopoietic (hCD45) chimerism
via flow cytometry (Fig. 4). Compared
with the control group (VEH), the mice treated with curcumin (CCM)
or afuresertib (AFU) either alone or in combination (CCM+AFU) had
fewer human CD45+ cells in the bone marrow and
peripheral blood. Moreover, combination drug therapy was more
effective than single drug therapy in reducing the chimerism of
hCD45 (Fig. 4). These results
indicated that curcumin and afuresertib synergistically suppressed
the engraftment of AML cells.
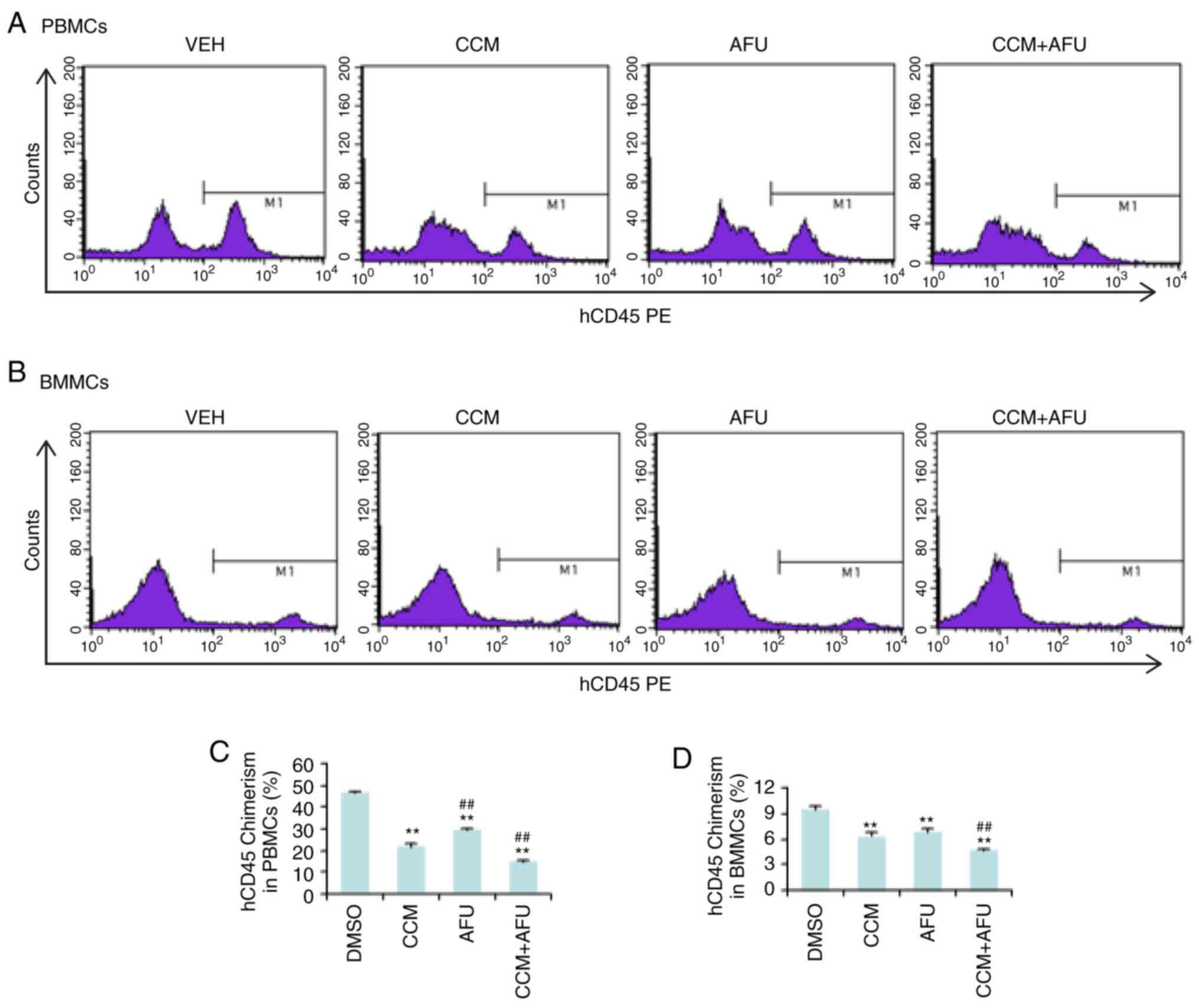 | Figure 4.Curcumin (CCM) and afuresertib (AFU)
synergistically inhibit engraftment of AML cells in PB and BM of
mice. NOD/SCID mice were pretreated with intraperitoneal injection
of 20 mg/kg busulfan 24 h before inoculation, and were then
injected intravenously with 1×106 ML-2 cells. At 15 days
after inoculation, the mice were randomly divided into four groups
(5 mice per group), and were treated with VEH, CCM, AFU or CCM+AFU
via oral gavage every other day for 16 days. PBMCs and BMMCs were
isolated and evaluated for human hematopoietic (hCD45) chimerism
via flow cytometry. (A and B) Representative images from flow
cytometry. (C and D) Data are presented as the mean ± SD of three
mice. **P≤0.01 vs. VEH; ##P≤0.01 vs. CCM. PB, peripheral
blood; BM, bone marrow; VEH, vehicle; PBMCs, peripheral blood
mononuclear cells; BMMCs, bone marrow mononuclear cells; AFU,
afuresertib; CCM, curcumin; AML, acute myeloid leukemia. |
The mice treated with curcumin and afuresertib
either alone or in combination had a smaller and lighter spleen
compared with the control mice (Fig. 5A
and B). Thus, it was suggested that treatment with curcumin or
afuresertib could decrease splenomegaly in AML mice. IHC using an
anti-hCD45 antibody demonstrated that, compared with control mice,
the mice treated with curcumin or afuresertib had decreased
dissemination of AML cells in the spleen, and the combinational use
of curcumin and afuresertib was more effective compared with the
use of a single drug (Fig. 5C).
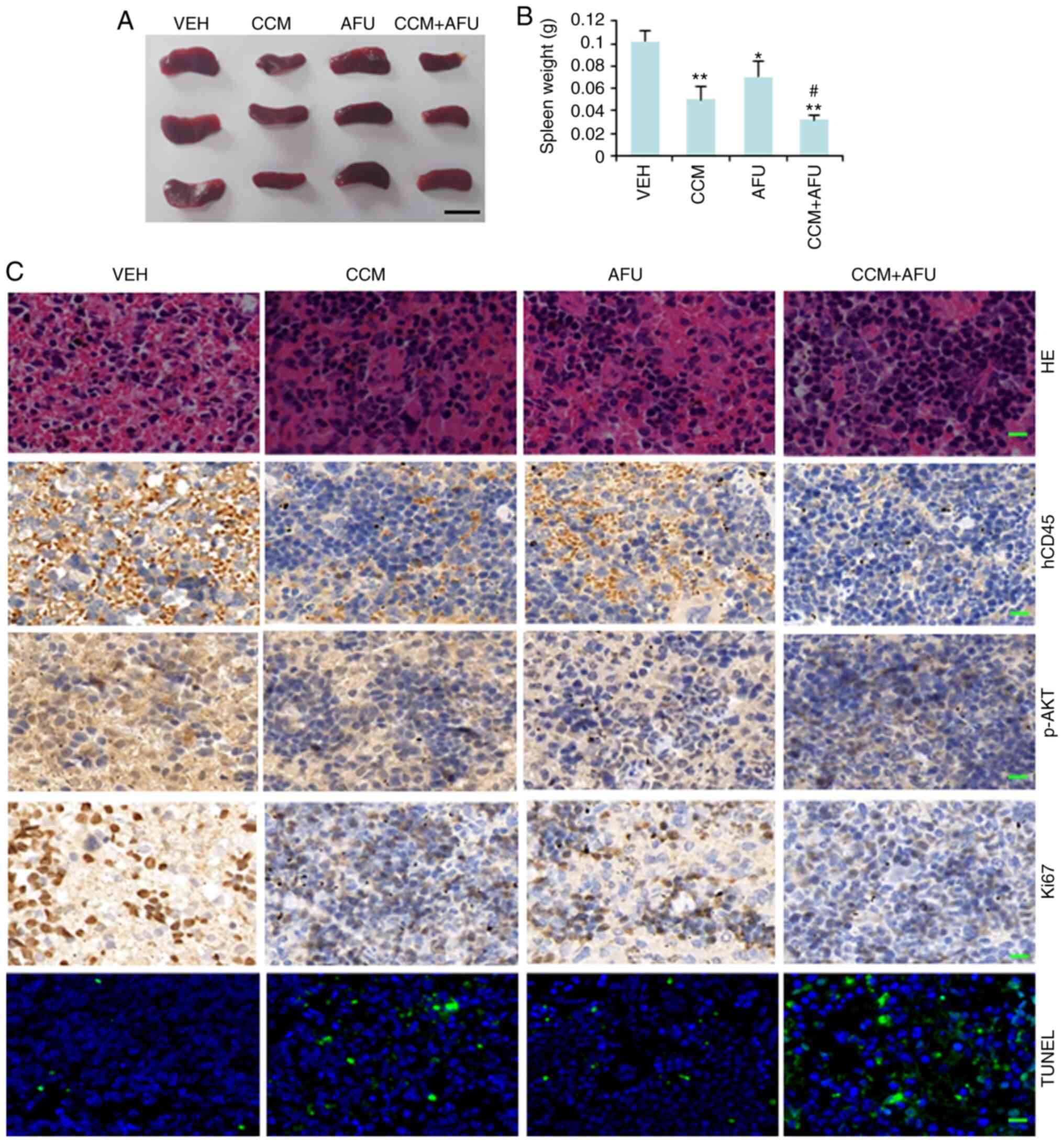 | Figure 5.Curcumin (CCM) and afuresertib (AFU)
synergistically inhibit engraftment, proliferation and survival of
AML cells in the spleens of mice. NOD/SCID mice were pretreated
with intraperitoneal injection of 20 mg/kg busulfan 24 h before
inoculation and were then injected intravenously with
1×106 ML-2 cells. At 15 days after inoculation, the mice
were randomly divided into four groups (5 mice per group), and were
treated with VEH, CCM, AFU or CCM+AFU via oral gavage every other
day for 16 days. (A) Gross appearance of spleen. Scale bar, 1 cm.
(B) Average weight of spleen. (C) H&E, IHC and TUNEL assays of
the spleen. Scale bar, 50 µm. *P≤0.05 and **P≤0.01 vs. VEH;
#P≤0.05 vs. CCM. VEH, vehicle; AFU, afuresertib; CCM,
curcumin; AML, acute myeloid leukemia; H&E, hematoxylin and
eosin; IHC, immunohistochemistry. |
Subsequently, Ki-67 staining and TUNEL assay were
conducted to evaluate cell proliferation and apoptosis,
respectively. The results (Fig. 5C)
demonstrated that treatment with curcumin or afuresertib
significantly increased apoptosis but decreased AKT phosphorylation
and the cell proliferation rate in spleen, while treatment with
both drugs had stronger effects compared with treatment with a
single drug. These findings suggested that treatment with curcumin
or afuresertib suppressed the engraftment, proliferation and
survival of AML cells, and that combination therapy had increased
efficacy compared with monotherapy.
Discussion
The present study compared the cytotoxicity of four
phytochemicals (curcumin, EGCG, genistein and resveratrol) and
identified that curcumin had the strongest anti-acute myeloid
leukemia (AML) efficacy. It has been reported that curcumin has
multiple targets and exerts its role via different molecular
mechanism in various cancer types (11). Recently, several studies have revealed
that curcumin can inhibit the phosphorylation of certain kinases,
such as AKT (12), JAK1 (13) and p38MAPK (14). To identifying the targets of curcumin
in AML, the present study performed a phosphorylation antibody
array to detect the influence of curcumin on 55 phosphorylated
proteins in five signaling pathways (MAPK, AKT, JAK/STAT, NF-κB and
TGF-β). The present results suggested that curcumin decreased the
phosphorylation levels of 14 proteins but increased the
phosphorylation levels of four proteins. Then, a protein-protein
interaction (PPI) network of these 18 proteins was conducted, in
which AKT was a hub, indicating that AKT was a main target of
curcumin.
Protein kinase B (AKT/PKB) is frequently
overactivated in AML, and its phosphorylation is an independent
poor prognostic factor of overall survival in adult de novo
AML (23). AKT is a serine threonine
kinase that contains three isoforms: AKT1, AKT2 and AKT3. It has
been reported to serve roles in various cellular pathways,
including proliferation, apoptosis and angiogenesis. Cyclin D1,
which regulates the G1/S check point of the cell cycle,
has been reported to be upregulated by the AKT/glycogen synthase
kinase 3β axis (24). However, p21, a
negative regulator of the cell cycle G1/S transition, is
negatively regulated by AKT (25).
AKT also promotes leukemia T cells by enhancing the transcription
of Bcl-2 (26). The present results
suggested that curcumin treatment increased AKT phosphorylation and
p21 expression but decreased the expression levels of cyclin D1 and
Bcl-2 in AML cells. Moreover, the effects of curcumin on the
expression levels of p21, cyclin D1 and Bcl-2 were enhanced by the
AKT inhibitor but were suppressed by the AKT activator. Therefore,
it was indicated that curcumin may function via AKT. However, the
sensitivities to curcumin of AML cell lines were not significantly
correlated with their levels of AKT phosphorylation, suggesting
that curcumin still functioned via other pathways, besides tbe AKT
pathway.
The present study demonstrated the anti-AML effect
of curcumin both in vitro and in vivo, and this
effect was increased by the combination with afuresertib.
Afuresertib has been reported to exert antitumor effects in ovarian
cancer (27), malignant pleural
mesothelioma (28) and chronic
lymphocytic leukemia (29). However,
to the best of our knowledge, its role in AML has not been
previously reported. The present study was the first report that
afuresertib could potentially be used for the treatment of AML.
In conclusion, the present study demonstrated that
curcumin decreased the survival and proliferation of AML cells
in vitro, as well as AML cell proliferation in hematopoietic
tissue and dissemination into non-hematopoietic tissues.
Mechanistically, curcumin treatment suppressed AKT activation,
leading to cell cycle arrest and apoptosis.
Supplementary Material
Supporting Data
Acknowledgements
We would like to thank RayBiotech Inc. (Guangzhou,
China) for the assistance in the phosphorylation array
analysis.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81872256 and 82070155) and
Hunan Key Laboratory of Pharmacodynamics and Safety Evaluation of
New Drugs.
Availability of data and materials
All data generated and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
CZ and XD conceived and designed the study. HZ, YN
and GZ performed the experiments. HZ, YN, CZ and XD analyzed and
interpreted the data. HZ, CZ and XD wrote the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Hunan Normal University and performed according
to institutional animal care guidelines (no. 2018-037).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tallman MS, Wang ES, Altman JK, Appelbaum
FR, Bhatt VR, Bixby D, Coutre SE, De Lima M, Fathi AT, Fiorella M,
et al: Acute myeloid leukemia, version 3.2019, NCCN clinical
practice guidelines in oncology. J Natl Compr Cancer Netw.
17:721–749. 2019. View Article : Google Scholar
|
|
3
|
Oran B and Weisdorf DJ: Survival for older
patients with acute myeloid leukemia: A population-based study.
Haematologica. 97:1916–1924. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sami SA, Darwish NHE, Barile ANM and Mousa
SA: Current and future molecular targets for acute myeloid leukemia
therapy. Curr Treat Options Oncol. 21:32020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang XJ, Chen JY, Fu LQ and Yan MJ: Recent
advances in natural therapeutic approaches for the treatment of
cancer. J Chemother. 32:53–65. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kian MM, Salemi M, Bahadoran M, Haghi A,
Dashti N, Mohammadi S, Rostami S, Chahardouli B, Babakhani D and
Nikbakht M: Curcumin combined with thalidomide reduces expression
of STAT3 and Bcl-xL, leading to apoptosis in acute myeloid leukemia
cell lines. Drug Des Devel Ther. 14:185–194. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang K, Bae KH, Nambu A, Dutta B, Chung
JE, Osato M and Kurisawa M: A two-pronged anti-leukemic agent based
on a hyaluronic acid-green tea catechin conjugate for inducing
targeted cell death and terminal differentiation. Biomater Sci.
8:497–505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Blas E, Estañ MC, Del Carmen Gomez de
Frutos M, Ramos J, Del Carmen Boyano-Adánez M and Aller P: Selected
polyphenols potentiate the apoptotic efficacy of glycolytic
inhibitors in human acute myeloid leukemia cell lines. Regulation
by protein kinase activities. Cancer Cell Int. 16:702016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Guo Y, Feng Z, Bergan R, Li B, Qin
Y, Zhao L, Zhang Z and Shi M: Involvement of the PI3K/Akt/Nrf2
signaling pathway in resveratrol-mediated reversal of drug
resistance in HL-60/ADR cells. Nutr Cancer. 71:1007–1018. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giordano A and Tommonaro G: Curcumin and
cancer. Nutrients. 11:23762019. View Article : Google Scholar
|
|
11
|
Liczbinski P, Michałowicz J and Bukowska
B: Molecular mechanism of curcumin action in signaling pathways:
Review of the latest research. Phytother Res. 34:1992–2005. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borges GA, Elias ST, Amorim B, de Lima CL,
Coletta RD, Castilho RM, Squarize CH and Guerra EN: Curcumin
downregulates the PI3K-AKT-mTOR pathway and inhibits growth and
progression in head and neck cancer cells. Phytother Res.
34:3311–3324. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Sun W, Han N, Zou Y and Yin D:
Curcumin inhibits proliferation, migration, invasion and promotes
apoptosis of retinoblastoma cell lines through modulation of
miR-99a and JAK/STAT pathway. BMC Cancer. 18:12302018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hosseini A, Rasmi Y, Rahbarghazi R,
Aramwit P, Daeihassani B and Saboory E: Curcumin modulates the
angiogenic potential of human endothelial cells via FAK/P-38 MAPK
signaling pathway. Gene. 688:7–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Duan H, Xie Y, Ning Y, Zhang X,
Hui N, Wang C, Zhang J and Zhou J: MiR-193a-5p targets the coding
region of AP-2alpha mRNA and induces cisplatin resistance in
bladder cancers. J Cancer. 7:1740–1746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kouhpeikar H, Butler AE, Bamian F, Barreto
GE, Majeed M and Sahebkar A: Curcumin as a therapeutic agent in
leukemia. J Cell Physiol. 234:12404–12414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou C, Zhao XM, Li XF, Wang C, Zhang XT,
Liu XZ, Ding XF, Xiang SL and Zhang J: Curcumin inhibits
AP-2γ-induced apoptosis in the human malignant testicular germ
cells in vitro. Acta Pharmacol Sin. 34:1192–1200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang L, Qiu J, Xiao Y, Hu X, Liu Q, Chen
L, Huang W, Li X, Li L, Zhang J, et al: AP-2βinhibits
hepatocellular carcinoma invasion and metastasis through Slug and
Snail to suppress epithelial-mesenchymal transition. Theranostics.
8:3707–3721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carlos-Reyes A, Lopez-Gonzalez JS,
Meneses-Flores M, Gallardo-Rincón D, Ruíz-García E, Marchat LA,
Astudillo-de la Vega H, de la Cruz ON and López-Camarillo C:
Dietary compounds as epigenetic modulating agents in cancer. Front
Genet. 10:792019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He X and Zhang J: Why do hubs tend to be
essential in protein networks? PLoS Genet. 2:e882006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masamha CP and Benbrook DM: Cyclin D1
degradation is sufficient to induce G1 cell cycle arrest despite
constitutive expression of cyclin E2 in ovarian cancer cells.
Cancer Res. 69:6565–6572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Georgakilas AG, Martin OA and Bonner WM:
p21: A two-faced genome guardian. Trends Mol Med. 23:310–319. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prijic S, Ugrina I, Labar B, Nemet D,
Batinić J, Zadro R, Ries S, Gjadrov-Kuvedžić K, Davidović S and
Batinić D: Prognostic significance of constitutive
phosphatidylinositol 3-kinase/akt and mitogen-activated protein
kinase phosphorylation in acute myeloid leukemia. Leuk Lymphoma.
56:2281–2288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin Z, Li Y, Li Y and Liu G: Tumor
necrosis factor alpha stimulates proliferation of dental pulp stem
cells via akt/glycogen synthase kinase-3β/cyclin D1 signaling
pathway. J Endod. 41:1066–1072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu Z, Long T, Ma Y, Zhu J, Gao L, Zhong Y,
Wang X, Wang X and Li Z: Downregulation of GLYR1 contributes to
microsatellite instability colorectal cancer by targeting p21 via
the p38MAPK and PI3K/AKT pathways. J Exp Clin Cancer Res.
39:762020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan YJ, Yang Y, Leng QL, Lan B, Jia HY,
Liu YH, Zhang CZ and Cao Y: Vav1 increases bcl-2 expression by
selective activation of rac2-akt in leukemia T cells. Cell Signal.
26:2202–2209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blagden SP, Hamilton AL, Mileshkin L, Wong
S, Michael A, Hall M, Goh JC, Lisyanskaya AS, DeSilvio M, Frangou
E, et al: Phase IB dose escalation and expansion study of AKT
inhibitor afuresertib with carboplatin and paclitaxel in recurrent
platinum-resistant ovarian cancer. Clin Cancer Res. 25:1472–1478.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamaji M, Ota A, Wahiduzzaman M, Karnan S,
Hyodo T, Konishi H, Tsuzuki S, Hosokawa Y and Haniuda M: Novel
ATP-competitive Akt inhibitor afuresertib suppresses the
proliferation of malignant pleural mesothelioma cells. Cancer Med.
6:2646–2659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen CI, Paul H, Le LW, Wei EN, Snitzler
S, Wang T, Levina O, Kakar S, Lau A, Queau M, et al: A phase 2
study of ofatumumab (Arzerra®) in combination with a
pan-AKT inhibitor (afuresertib) in previously treated patients with
chronic lymphocytic leukemia (CLL). Leuk Lymphoma. 60:92–100. 2019.
View Article : Google Scholar : PubMed/NCBI
|