Introduction
Glioblastoma (GBM) is the most deadly yet common
form of primary central nervous system tumours, and accounts for
approximately 53.7% of all gliomas (1). Only 4.75% of patients diagnosed with GBM
survive for 5 years (2,3). The inevitable therapeutic failure and
poor prognosis partially result from the highly invasive feature of
GBM by infiltrating surrounding structures through preferential
anatomical pathways, which leads to incomplete surgical resection
and consequent tumour recurrence (4–6). Although
a significant amount of work has been conducted to find the
intracellular factors facilitating the infiltrative nature of GBM
cells, the underlying molecular mechanisms remain unclear.
Calcium (Ca2+) is reported to be involved
in the tumorigenesis, survival, invasion and apoptosis of cancer
cells (7,8). Calpains are a group of
Ca2+-activated cysteine proteinases that consist of a
regulatory and a catalytic subunit (9). Various studies have documented that
tumour invasion can be enhanced by calpains (10–13).
Calpains are also abundantly expressed in GBM cells and are
implicated in cell invasion and migration of GBM (14–17). The
in vivo evidence has revealed that inhibition of calpains
can suppress the angiogenesis of pulmonary microvascular
endothelial cells via vascular endothelial growth factor (18). Jang et al demonstrated by in
vitro Transwell assays that reduced calpain 2 expression
decreased the invasive capacity of GBM cells (14). Calpains have also been revealed to be
involved in regulating cancer cell apoptosis and executing necrosis
(19).
Apoptosis is a well-known type of programmed cell
death that plays a crucial role in cancer maintenance and
development as well as inhibition during chemotherapy (20). In response to chemotherapeutic drugs,
overexpression of proapoptotic proteins could provide GBM cells a
survival advantage (21). Wick et
al and Stegh et al have also demonstrated that apoptosis
resistance through overexpression of Bcl-2 (an antiapoptotic
protein) in GBM cells increased tumour migration and invasion
(22,23). The intrinsic pathways leading to
apoptosis include mitochondrial dysfunction, oxidative stress,
endoplasmic reticulum (ER) stress and production of reactive oxygen
species (ROS) (24–29). It has recently been documented that
calpains trigger an apoptotic cascade via several pathological
factors, such as oxidative stress, induction of mitochondrial
injury and activation of ER stress (29–34). Guan
et al have demonstrated that expression and activation of
calpains facilitate mitochondrial fission, inducing cardiomyocyte
apoptosis. Inhibition of calpains by calpastatin (an endogenous
calpain inhibitor) enhanced mitophagy and mitochondrial fusion, and
inhibited apoptosis and excessive mitochondrial fission (35). Cho et al have reported that
inhibition of calpain activity alleviated DNA cleavage induced by
oxidative stress, and apoptosis in pancreatic acinar cells
(36).
In our previous study, it was reported that
treatment with dopamine receptor D1 (DRD1) agonist, SKF83959,
yielded a therapeutic effect against GBM both in vitro and
in vivo (37). In the present
study, the complex interplay between DRD1-agonist-induced GBM cell
apoptosis and possible signalling pathways related to downstream
molecules of Ca2+ accumulation were explored. The aim of
the present study, was to investigate an alternative avenue for the
design of future GBM therapies.
Materials and methods
Collection of human GBM samples and
control brain tissues
A total of 4 female and 6 male patients (mean age,
56.3±7.7 years) diagnosed as GBM with both typical clinical
symptoms (including headache, nausea, vomiting, motor or sensory
disturbance, speech or swallowing difficulties or other
manifestations of focal neurological deficits) and MRI scans
(diagnosed by at least two radiologists) were hospitalized at the
Department of Neurosurgery, the Second Hospital of Dalian Medical
University (DMU; Dalian, China) between January 2016 and January
2018. The surgical resection and collection of GBM samples were
approved by the Ethics Committee of the Second Hospital of Dalian
Medical University (approval no. 2018052), following the ethical
guidelines of the Declaration of Helsinki. Written consent was
obtained from all patients. The resected GBM samples were diagnosed
as the World Health Organization (WHO) grade IV by at least two
pathologists. The control brain tissues (n=10) were obtained from
negative margins of intracranial hematoma patients (4 females and 6
males; mean age: 59.4±6.8 years), who had no neuropathological
evidence of brain tumours or history of brain trauma, encephalitis,
meningitis or epilepsy.
Cell culture and measurement of cell
viability
The human U87 GBM (GBM of unknown origin; cat. no.
TCHu138) and mouse N2a neuroblastoma (cat. no. TCM29) cell lines
were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China), which has authenticated the cell lines
by STR profiling. Cells were cultured in Dulbecco's modified
Eagle's medium (C11995500BT; Gibco; Thermo Fisher Scientific, Inc.)
containing 10% foetal bovine serum, with 5% CO2
supplementation at 37°C. Measurement of cell viability was
conducted with CCK-8 assay (product code CK04; Dojindo Molecular
Technologies, Inc.). The compounds applied were: SKF83959
hydrobromide (cat. no. 2074; Tocris Bioscience; 0–50 µM) was used
at 37°C for 0–72 h; BAPTA-AM (cat. no. S7534; Selleck Chemicals;
500 nM) was applied at 37°C for 48 h; U73122 (cat. no. S8011;
Selleck Chemicals; 1–2 µM) was used at 37°C for 48 h, and
calpastatin (cat. no. 2950; Tocris Bioscience; 25 nM-1 µM) was used
at 37°C for 48 h. After the addition of CCK-8 reagent (10 µl) to
all wells where cells were plated, the reaction plates were
incubated for 2 h at 37°C. A microplate reader (Tecan Infinite
F200/M200) was used to detect the absorbance at 450 nm.
Western blotting
Cells were collected and lysed in RIPA buffer
(product no. P0013C; Beyotime Institute of Biotechnology). A BCA
protein assay kit (cat. no. T9300A-3; Takara Biotechnology Co.,
Ltd.) was used to evaluate the protein concentration. Western
blotting was conducted according to standard protocols. Cellular
proteins (50 µg) were separated by gradient 4–15% SDS-PAGE gels
(cat. no. 4561086; Bio-Rad Laboratories, Inc.). The transferred
PVDF membranes (Immobilon; EMD Millipore) were incubated with
primary antibodies at 4°C overnight and secondary antibodies at
room temperature (RT) for 1 h. The primary antibodies were
purchased and used as followings: Anti-cleaved caspase-3 (product
no. 9661; 1:1,000), anti-caspase-3 (product no. 9662; 1:1,000),
anti-cleaved caspase-8 (product no. 9496; 1:1,000), anti-caspase-8
(product no. 4790; 1:1,000), anti-CHOP (product no. 2895T;
1:1,000), anti-BiP (product no. 3177T; 1:1,000), anti-GAPDH
(product no. 2118; 1:1,000), and anti-cytochrome c (Cyt
c) (product no. 4272s; 1:1,000) all from Cell Signaling
Technology, Inc., anti-COX IV (product code ab14744; 1:1,000;
Abcam), anti-Bcl-2 (product code ab692; 1:1,000; Abcam),
anti-calpains (cat. no. sc-58326, 1:500; Santa Cruz Biotechnology,
Inc.), and anti-β-actin (product no. A5441; 1:5,000; Sigma-Aldrich;
Merck KGaA). The secondary antibodies were anti-rabbit/mouse IgG,
HRP-linked antibody (product nos. 7076 and 7074; 1:2,000; Cell
Signaling Technology, Inc.). ECL was used as the visualisation
reagent (product no. P0018FM; Beyotime Institute of Biotechnology).
A FluorChem Q system was used to quantify the target protein bands,
which were then evaluated by Alpha View SA software (both from
ProteinSimple).
Mitochondria and cytoplasm
extraction
The mitochondrial and cytosolic proteins were
extracted with Tissue Mitochondria Isolation Kit (product no.
C3601; Beyotime Institute of Biotechnology). Cells
(2×106) were washed with PBS and collected by
centrifugation (600 × g for 5 min, 4°C), and then resuspended with
1.5 ml mitochondrial extraction buffer and stored on ice for 10
min. The cellular suspension was homogenized with a Teflon-glass
homogenizer with 10–15 up-and-down passes of the pestle. The
homogenate was then centrifuged at 750 × g for 10 min (4°C). The
supernatants were centrifuged at 11,000 × g for 10 min (4°C). The
sediments were suspended to acquire mitochondria, while the
resulting supernatants were centrifuged at 12,000 × g for 10 min
(4°C) to acquire the cytosolic proteins.
Immunohistochemistry
Formaldehyde-fixed (10%, at 4°C, overnight),
paraffin-embedded GBM tissue sections were boiled in sodium citrate
buffer (pH 6.0) for 30 min using a microwave histoprocessor for
antigen retrieval. Tissue sections were dehydrated and subjected to
peroxidase blocking (3% hydrogen peroxide, at RT for 10 min). All
brain tissues were cut into 4-µm sections. Then they were incubated
with primary antibody at RT for 1 h and then were incubated with
100 µl enhanced HRP-conjugated secondary antibody at RT for 20 min
(product no. PV-9002; ZSGB-BIO; OriGene Technologies, Inc.). The
primary antibody used was anti-calpain (cat. no. sc-58326; 1:500;
Santa Cruz Biotechnology, Inc.). After washing, sections were
covered with mounting medium and imaged with a fluorescence
microscope.
Detection of intracellular
Ca2+ levels
The Fluo-4 AM detection kit (product no. S1060;
Beyotime Institute of Biotechnology) was used to evaluate the
intracellular Ca2+ level in U87 cells. Cells
(5×104) were firstly plated in 96-well culture plates
for 24 h. After washing with PBS, cells were loaded with Fluo-4 AM
reagent for 30 min at 37°C in Hank's Balanced Salt Solution (HBSS;
Invitrogen; Life Technologies; Thermo Fisher Scientific, Inc.) and
incubated for another 30 min at room temperature. The cells were
washed with HBSS followed by fluorescence recording at 37°C with a
Flouroskan Ascent (emission at 538 nm and excitation at 485 nm;
Thermo Electronic Corporation; Thermo Fisher Scientific, Inc.)
every 6 sec. Fluorescence microscopy was also used to visualize the
fluorescent intensity of Fluo-4 AM probe in SKF83959-treated U87
cells.
ROS detection
Cells (5×104) were incubated in PBS with
10 µM DCFH-DA (product no. S0033; ROS Assay Kit; Beyotime Institute
of Biotechnology) and 5.5 mM glucose supplement for 20 min at 37°C.
After incubation in common culture medium for another 10 min, the
ROS levels in cells were detected using a microplate reader (Tecan
Group, Ltd.).
Mitochondrial transmembrane potential
Assay
The Mito-Tracker Red CMXRos kit (product no. C1035;
Beyotime Institute of Biotechnology) was used to evaluate the
mitochondrial transmembrane potential in U87 cells. Cells
(5×105) were firstly treated with SKF83959 (35 µM)
and/or BAPTA (500 nM) for 24 h. After washing with PBS, cells were
stained with 50 nM Mito-Tracker Red CMXRos reagent for 30 min at
37°C and nuclei were stained with Hoechst at RT for 5 min. Red
fluorescence images were recorded under fluorescence microscopic
observation and the fluorescence intensity was measured using
ImageJ software 2.1 (National Institutes of Health).
Flow cytometric analysis
After treatment with SKF83959 (35 µM) at 37°C for 72
h, U87 and N2a cells (2×106) were collected and the
Annexin V and PI staining-based fluorescein isothiocyanate Annexin
V Apoptosis Detection kit (BD Biosciences) was used to assess cell
apoptosis, according to the manufacturer's instructions. The flow
cytometry (BD FACSCanto II; BD Biosciences) and BD FACSDiva™ 7.0
software (BD Biosciences) were used for analysis for early + late
apoptotic cells.
Cell cycle analysis
Pretreated cells (2×106) were collected
and maintained in 70% ethanol at 4°C for at least 24 h. After RNA
degradation by RNase A (100 µg/ml; product no. GE101-01; TransGen
Biotech Co., Ltd.) at 37°C for 30 min, cells were then stained with
PI (50 µg/ml; BD Pharmingen; BD Biosciences) on ice for 30 min and
assessed by flow cytometry (BD FACSCanto II; BD Biosciences). The
data was collected by BD FACSDiva™ 7.0 software (BD Biosciences)
and analyzed by ModFit LT 4.0 software (BD Biosciences) according
to the manufacturer's instructions.
Xenograft experiments
A total of 10 female BALB/c mice (weight, 18–20 g)
were purchased from the Institute of Genome-Engineered Animal
Models of Dalian Medical University and kept under specific
pathogen-free conditions. The housing conditions were as follows:
Temperature 22±2°C, 12-h light/12-h dark cycle, relative humidity
60±15% and autonomous intake of water and food. U87 cells
(1×107) mixed in PBS were subcutaneously injected into
the flanks of 5-week-old immunocompromised athymic nude mice. The
mice were intraperitoneally treated with SKF83959 (1 mg/kg/day) or
the same amount of saline (n=5 in each group). The tumour volumes
were measured every other day. The mice were sacrificed humanely by
CO2 asphyxiation with displacement of CO2 (in
30%/min) at the same end point. The protocol was approved by the
Animal Ethics Committee of Dalian Medical University (approval no.
2018112).
Statistical analysis
Statistical analysis was conducted with GraphPad
Prism 6 (GraphPad Software, Inc.). Unpaired Student's t-tests were
used to determine statistical differences between treatment and
control groups. Comparisons among multiple groups were performed
using one-way analysis of variance (ANOVA) followed by Tukey's (for
multiple comparison between 4 or more groups) and Dunnett's (for
comparison with one control group) post hoc tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
DRD1 agonist leads to apoptotic GBM
cell death
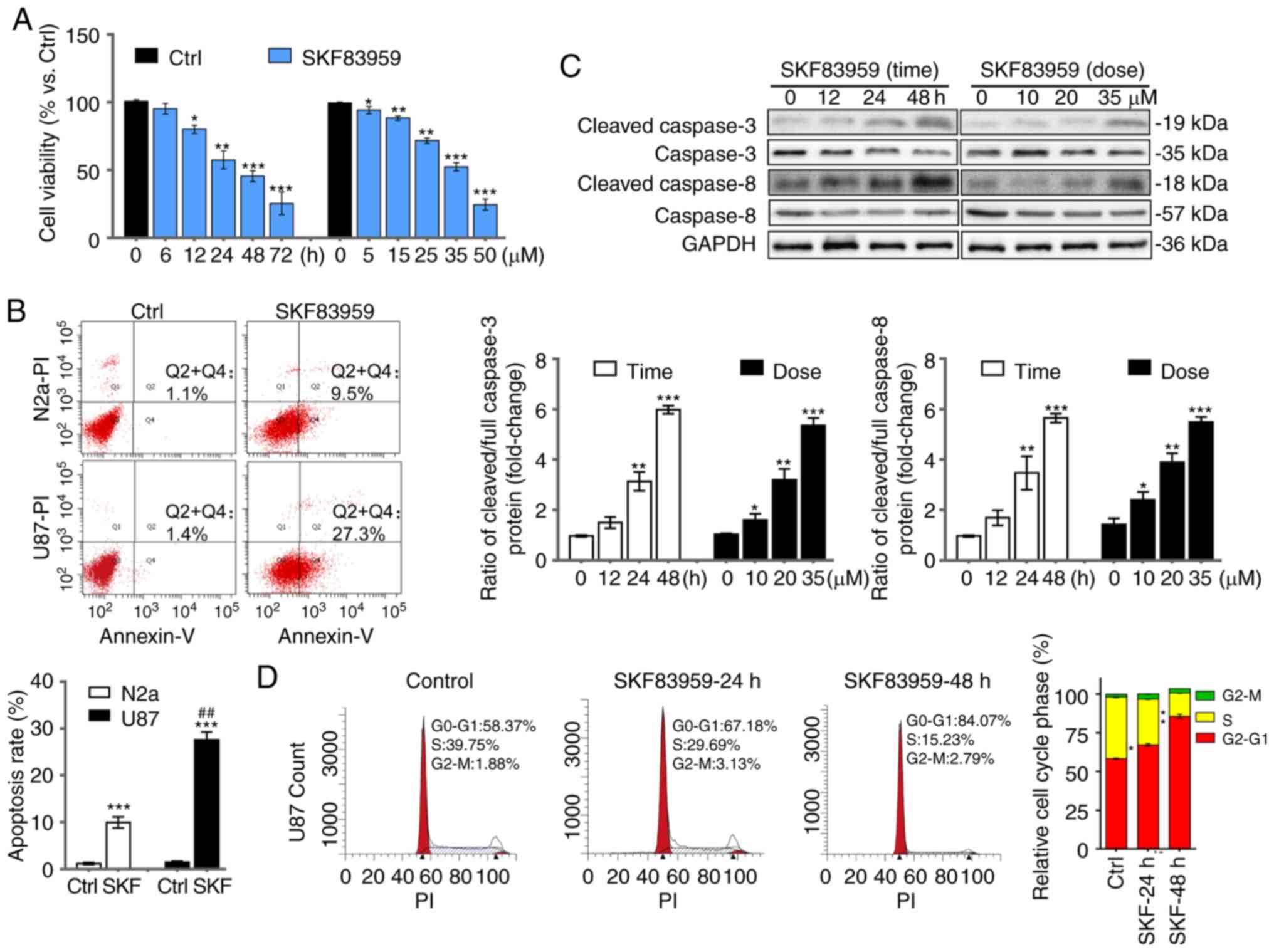
A CCK-8 assay was used to evaluate the cytotoxicity
of DRD1 agonist SKF83959 in human GBM cell line U87; one of the
most common GBM cell models to date. SKF83959 revealed a
significant inhibitory effect in a time-dependent (0–72 h) and
concentration-dependent (0–50 µM) manner (Fig. 1A). The proportion of viable U87 cells
was markedly decreased to 51.8% by SKF83959 at 35 µM for 48 h.
Therefore, 35 µM was selected as the intervention concentration for
the following experiments. To investigate whether apoptotic death
was involved in the inhibitory effect, flow cytometric analysis was
used to compare the apoptotic rate between U87 cells treated with
DRD1 agonist and the non-GBM cell line N2a as a control.
Quantification of apoptosis is the early + late apoptosis rate
(n=3). SKF83959 treatment at 35 µM for 72 h induced 27.3% apoptotic
cell death, while the apoptotic rate of N2a cells was only 9.5%,
which validated the selectivity of SKF83959-induced apoptosis
towards GBM cells (Fig. 1B). Western
blotting revealed the protein levels of full and cleaved caspase-8
and caspase-3, typical apoptosis markers (36). To determine the apoptotic status
regarding caspase-3 and caspase-8 activation, the ratio between
cleaved and full caspase-3/8 (and rationalized to endogenous
control) was analysed. The results revealed that the ratios were
increased in U87 cells treated with SKF83959 in a time-dependent
(0–48 h) and concentration-dependent (0–35 µM) manner (Fig. 1C). DNA content was further analysed in
U87 cells treated with SKF83959 (35 µM) and a worsening
G0/G1 arrest over time was detected (Fig. 1D), suggesting that cell cycle arrest
was triggered by this agonist, which may have been followed by
apoptosis.
DRD1 agonist increases PLC-dependent
intracytosolic Ca2+ levels and induces oxidative
stress
D1-like receptors are known to upregulate PLC, which
is a major contributor to intracellular Ca2+ release
(38–41). An increased level of intracytosolic
Ca2+ after SKF83959 treatment (0, 10, 20, 35 µM) was
observed as demonstrated by increased fluorescent intensity in
treated Fluo-4 AM-loaded U87 cells (Fig.
2A). Then, the PLC inhibitor, U73122, was used to evaluate
whether PLC signalling was involved. By measuring the
intracytosolic Ca2+ after SKF83959 treatment (35 µM), a
significant reversal of intracytosolic Ca2+ level in the
presence of 2 µM U73122 was observed (Fig. 2B). GBM cell inhibition by SKF83959 was
also ameliorated when co-treated with U73122, which suggested the
involvement of PLC in the apoptotic signalling pathway (Fig. 2C).
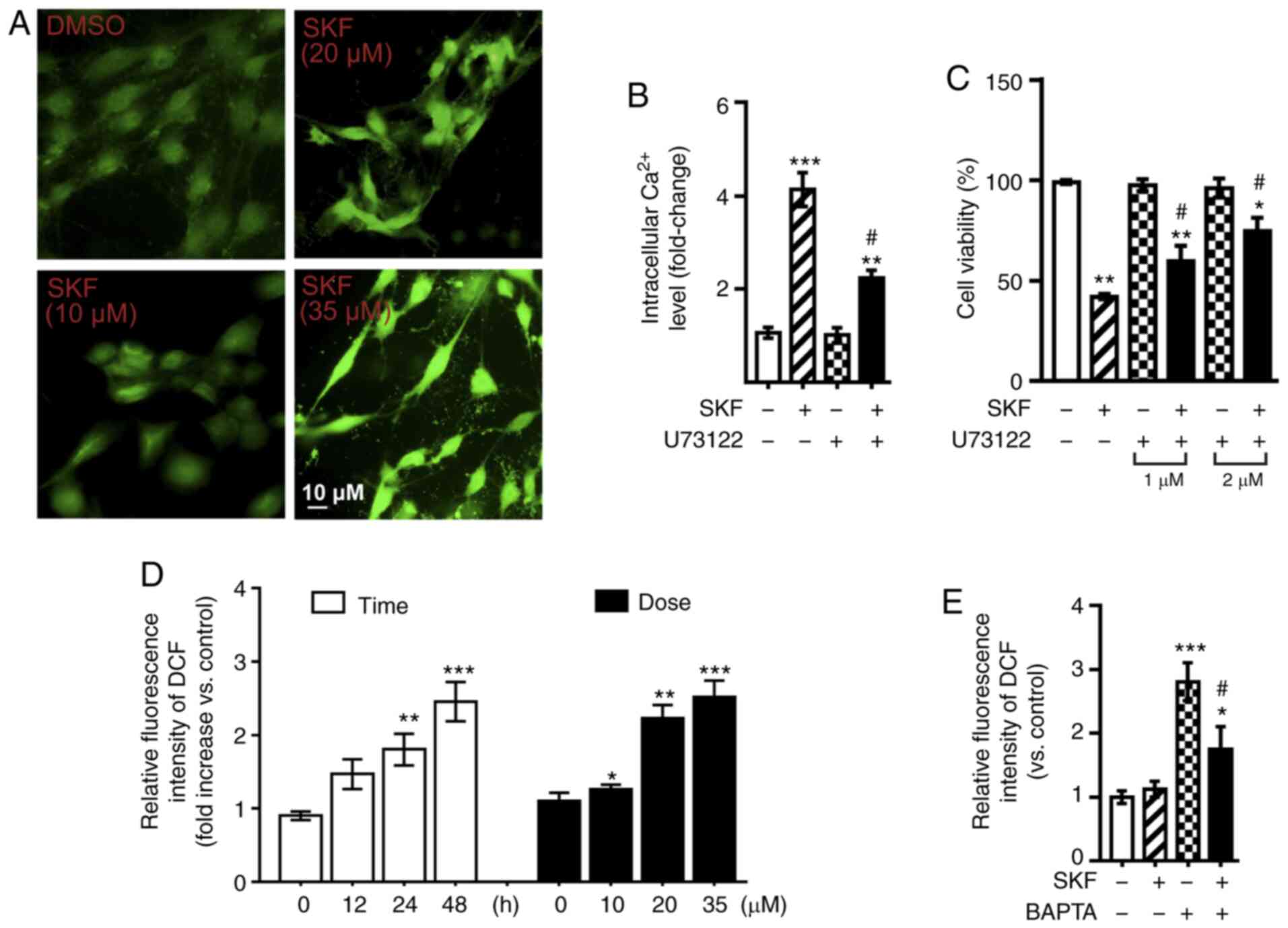 | Figure 2.DRD1 agonist increases PLC-dependent
intracytosolic Ca2+ levels and induces oxidative stress.
(A) Fluorescence microscopy was used to measure intracellular
Ca2+ in Fluo-4 AM-loaded U87 cells treated with various
doses of SKF83959 (0, 10, 20, and 35 µM) for 30 min. Scale bar, 10
µm. (B) Intracellular Ca2+ measurements in
Fluo-4-AM-loaded U87 cells treated with SKF83959 (35 µM) and/or
U73122 (2 µM,) for 60 min. (C) Viability of U87 cells treated with
SKF83959 and/or U73122 (1–2 µM) for 48 h. (D) Relative fluorescence
intensity of DCF (representing ROS levels) was analysed in U87
cells treated with SKF83959 at 0–35 µM or for 0–48 h. (E) Relative
fluorescence intensity of DCF (representing ROS level) was analysed
in U87 cells treated with SKF83959 and/or BAPTA (500 nM) for 48 h.
*P<0.05, **P<0.01, and ***P<0.001 (vs. Ctrl);
#P<0.05 (vs. U87 cells treated with SKF83959). DRD1,
dopamine receptor D1; PLC, phospholipase C; ROS, reactive oxygen
species; Ctrl, control; SKF, SKF83959. |
Oxidative stress was then focused on, such as ROS
generation, an important intrinsic pathway leading to apoptotic
cell death, which can be induced by an increased intracytosolic
Ca2+ level (24–29). ROS assays were used to detect ROS, and
increased levels were revealed in U87 cells treated with SKF83959
in a time- (0–48 h) and concentration- (0–35 µM) dependent manner
(Fig. 2D). An intracellular calcium
chelator, BAPTA-AM (500 nM), was used to pre-treat U87 cells, and
the ROS production induced by SKF83959 was decreased, indicating
that inhibition of intracellular Ca2+ level prevented
the aberrantly over-increased oxidative stress (Fig. 2E).
DRD1-agonist-induced-GBM cell
apoptosis is related to ER stress and mitochondrial
dysfunction
To investigate the mechanism involved in
DRD1-agonist-induced apoptosis of U87 cells, the downstream
molecules of increased intracellular Ca2+ level and ROS
generation were investigated. Excessive oxidative stress has been
implicated in activation of ER stress and destruction of
mitochondrial outer membrane, triggering apoptosis (28,34).
Therefore, to reveal the effect of SKF83959 treatment on
mitochondria, the mitochondrial membrane potential-dependent
staining assay (Mito-Tracker Red CMXRos kit) was firstly employed
to evaluate the mitochondrial transmembrane potential in U87 cells.
The red fluorescence intensity was significantly decreased by
SKF83959 treatment (35 µM), suggesting probable mitochondrial
injury. However, co-treatment with BAPTA-AM (500 nM) partially
reversed the fluorescence intensity in U87 cells compared with
SKF83959 treatment alone, suggesting the aforementioned signalling
changes were dependent on the increase in intracellular
Ca2+ level (Fig. 3A).
Then, COX IV, which was attached to the surface of the
mitochondrial membrane and Bcl-2, which suppressed activation of
caspases by inhibiting the release of Cyt c into the
cytoplasm, were assessed (28). The
protein level of COX IV was markedly increased while the protein
level of Bcl-2 was significantly decreased in U87 cells under
SKF83959 treatment (Fig. 3B).
Furthermore, the protein levels of Cyt c in U87 cells under
different doses of SKF83959 treatment (0, 10, 20, 35 µM) were
measured after the mitochondrial and cytosolic fraction. The
mitochondrial Cyt c was decreased while the cytosolic Cyt
c was significantly increased, which suggested damage in the
mitochondrial membrane in response to activation of mitochondrial
apoptosis (Fig. 3C). Western blotting
was also used to assess the ER stress markers BiP (GRP78) and CHOP,
and the results revealed significant increases in their protein
levels in SKF83959-treated U87 cells in a time-dependent (0–48 h)
and concentration-dependent (0–35 µM) manner (Fig. 3D) (42,43).
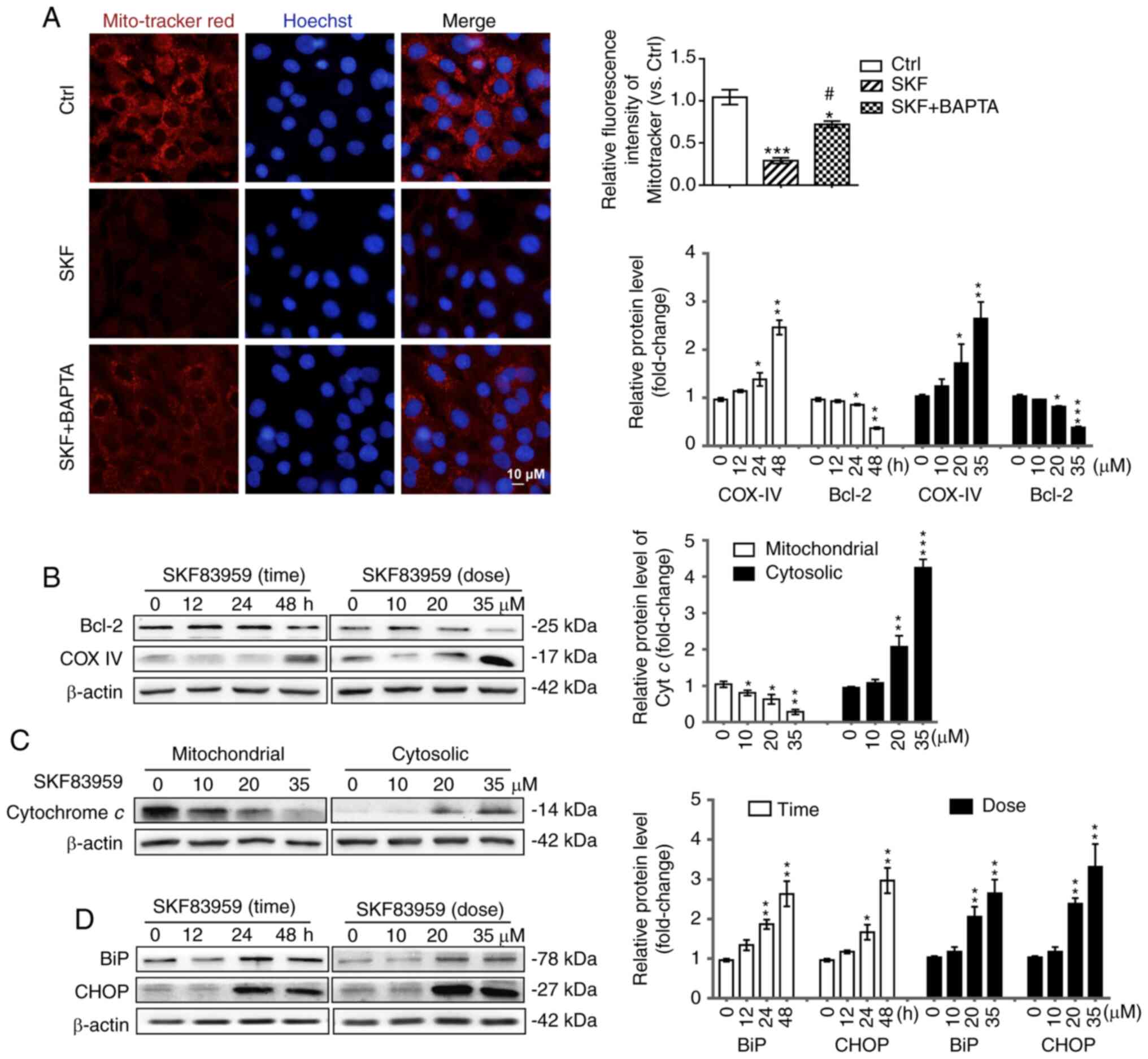 | Figure 3.DRD1-agonist-induced-GBM cell
apoptosis is related to ER stress and mitochondrial dysfunction.
(A) Mitochondrial membrane potential was detected using
Mito-tracker Red. Fluorescence microscopy was used to measure
fluorescence intensity in U87 cells treated with SKF83959 (35 µM)
and/or BAPTA (500 nM) for 24 h. Scale bars, 10 µm. (B) The protein
levels of COX IV and Bcl-2 in U87 cells treated with SKF83959 at
0–35 µM or for 0–48 h were determined by western blotting.
Quantification of relative protein levels is presented on the right
(n=3). (C) Mitochondrial and cytosolic protein levels of Cyt
c in U87 cells treated with various doses of SKF83959 (0,
10, 20, 35 µM) for 48 h were measured by western blotting. (D)
Expression of BiP, CHOP, and β-actin in U87 cells treated with
SKF83959 at 0–35 µM or for 0–48 h was determined by western
blotting. Quantification of relative protein levels is presented on
the right (n=3). *P<0.05, **P<0.01, and ***P<0.001 (vs.
Ctrl); #P<0.05 (vs. U87 cells treated with SKF83959).
DRD1, dopamine receptor D1; GBM, glioblastoma; ER, endoplasmic
reticulum; Cyt c, cytochrome c; Ctrl, control; SKF,
SKF83959. |
DRD1 agonist-activated calpain
signalling, induces ER stress and mitochondrial dysfunction and
eventual apoptosis
Activation of PLC leads to Ca2+ release
from intracellular Ca2+ stores including ER, and
increased intracytosolic Ca2+ concentration activates
calpains (9,39). Calpain 1 (µ-calpain) and calpain 2
(m-calpain) both contain a small regulatory 30-kDa subunit, which
is activated by increased intracellular Ca2+ (9,44).
Increased calpain levels were determined by western blotting in U87
cells treated with SKF83959 in a time-dependent (0–48 h) and
concentration-dependent (0–35 µM) manner (Fig. 4A). Calpain inhibitor calpastatin was
applied to reduce calpain activation. Western blotting verified the
inhibitory effect of calpastatin on calpains by decreasing protein
levels of the small regulatory subunit of calpains in the absence
and presence of SKF83959 treatment (Fig.
4B). CCK-8 assays revealed that co-treatment with calpastatin
decreased the cytotoxic effect of DRD1 agonist on U87 cells in a
concentration-dependent manner (25 nM-1 µM) (Fig. 4C). The microscopic in vitro
images displayed the reversed inhibitory effect of SKF83959, (35
µM, incubation for 48 h) when cotreated with calpastatin (500 nM,
incubation for 48 h), on U87 cells (Fig.
4D). The aforementioned observations suggest the important role
of calpains in the SKF83959 treatment-induced GBM cell apoptosis.
To determine whether calpains were also involved in the ER stress
and mitochondrial dysfunction leading to apoptotic cascade, the
respective markers including cleaved caspase-3 and BiP were
assessed by western blotting. Compared with SKF83959 treatment
alone, co-treatment with the calpain inhibitor, calpastatin,
significantly decreased the expression levels of cleaved caspase-3
and BiP, which suggested that the over-activated calpain signalling
contributed to mitochondrial dysfunction and ER stress in U87
cells, followed by apoptosis during SKF83959 treatment (Fig. 4E).
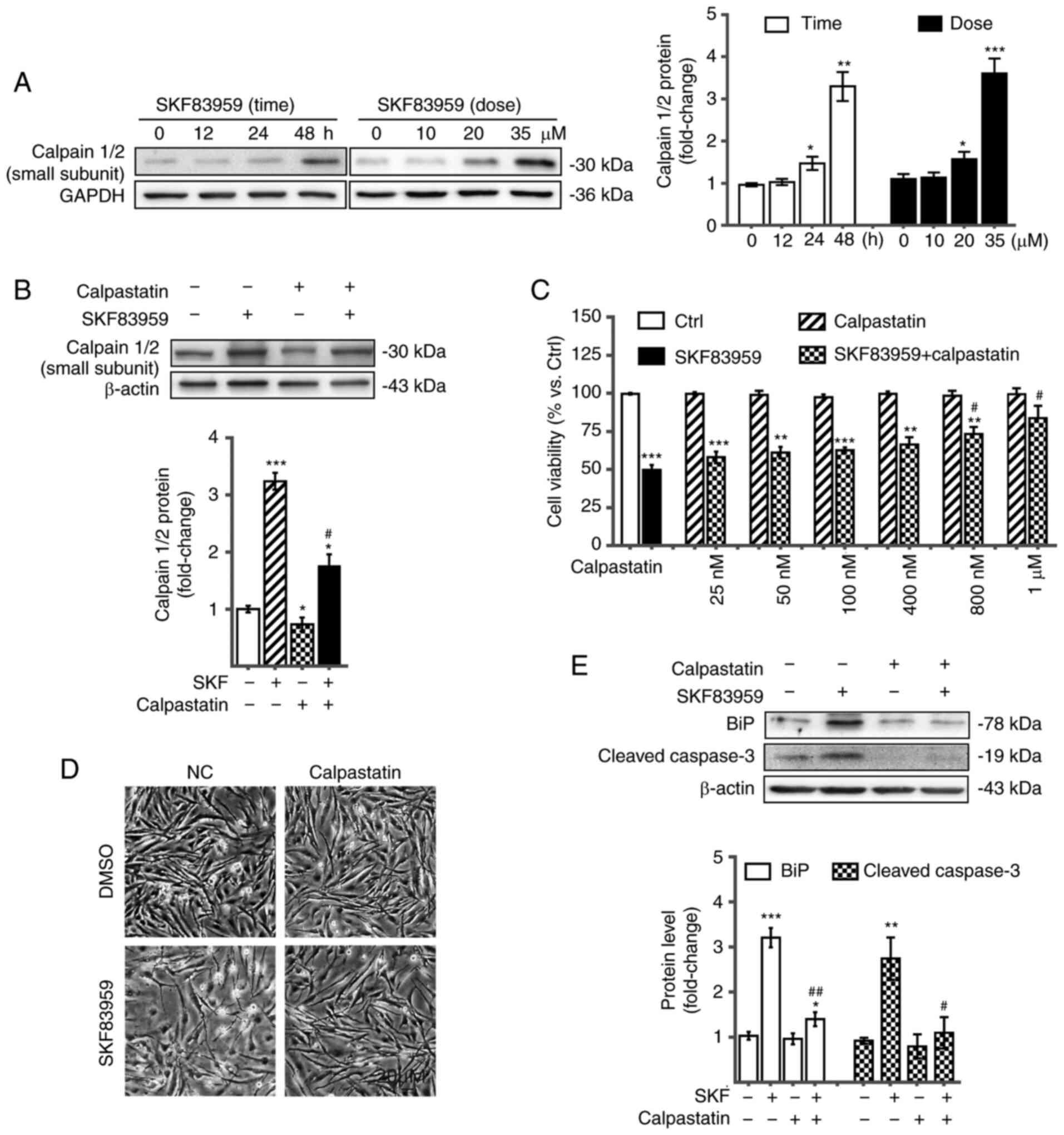 | Figure 4.DRD1 agonist-activated calpain
signalling, induces ER stress and mitochondrial dysfunction, which
leads to apoptosis. (A) Expression of the small subunit of calpains
in U87 cells treated with SKF83959 at 0–35 µM or for 0–48 h was
determined by western blotting. Quantification of the relative
protein levels is presented on the right (n=3). (B) Expression of
the small subunit of calpains in U87 cells treated with SKF83959 in
the presence or absence of calpastatin, a calpain inhibitor, was
determined by western blotting. (C) Viability of U87 cells with
SKF83959 co-treated with the calpain inhibitor calpastatin at 25
nM-1 µM for 48 h. (D) Microscopic images of U87 cells treated with
calpastatin (500 nM) and/or SKF83959 (35 µM) for 48 h. Scale bars,
20 µm. (E) Expression of BIP and cleaved caspase-3 in U87 cells
treated with SKF83959 in the presence or absence of calpastatin.
*P<0.05, **P<0.01, and ***P<0.001 (vs. Ctrl);
#P<0.05 and ##P<0.01 (vs. U87 cells
treated with SKF83959). DRD1, dopamine receptor D1; ER, endoplasmic
reticulum; Ctrl, control; SKF, SKF83959. |
DRD1-agonist-induced-calpain
activation, followed by GBM growth inhibition, is observed in
vivo
The in vivo inhibitory efficacy of SKF83959
was verified by observing a 36.3% reduction of tumour size in GBM
xenograft models (U87 cells injected into the flanks of
immunocompromised BALB/c nude mice) receiving SKF83959 treatment (1
mg/kg/day) compared with the control group (mice treated with the
same amount of saline, n=5 in each group) (Fig. 5A). In order to investigate the in
vivo molecular pathway involving calpain activation, ER stress
and mitochondrial dysfunction, western blot analysis of calpains,
BiP and Cyt c was performed in tumours treated with SKF83959
or control saline. It was revealed that increases in protein levels
of calpains, BiP and Cyt c in SKF83959-treated GBM tumours
were consistent with the in vitro observations in U87 cells
(Fig. 5B). In addition,
immunohistochemical (IHC) staining of calpains on xenograft GBM
sections was performed, and it was revealed that calpains exhibited
higher expression in tumours treated with SKF83959 compared with
controls (percentage of calpain-positive cells of the SKF83959
group was 39.1 vs. 12.6% in the control group), which provided
in vivo evidence for the upregulation of calpains under
SKF83959 treatment (Fig. 5C).
Considering that DRD1 agonist induced GBM cell apoptosis by
dysregulated calpain activation, IHC staining of calpains was
performed on human GBM tissues, and evidence of increased calpain
expression compared with control brain tissues, was determined
(Fig. 5D). These data indicated that
calpains may be involved in GBM progression, and that therapeutic
approaches regarding calpain expression and activity regulation may
be a potential avenue for GBM treatment.
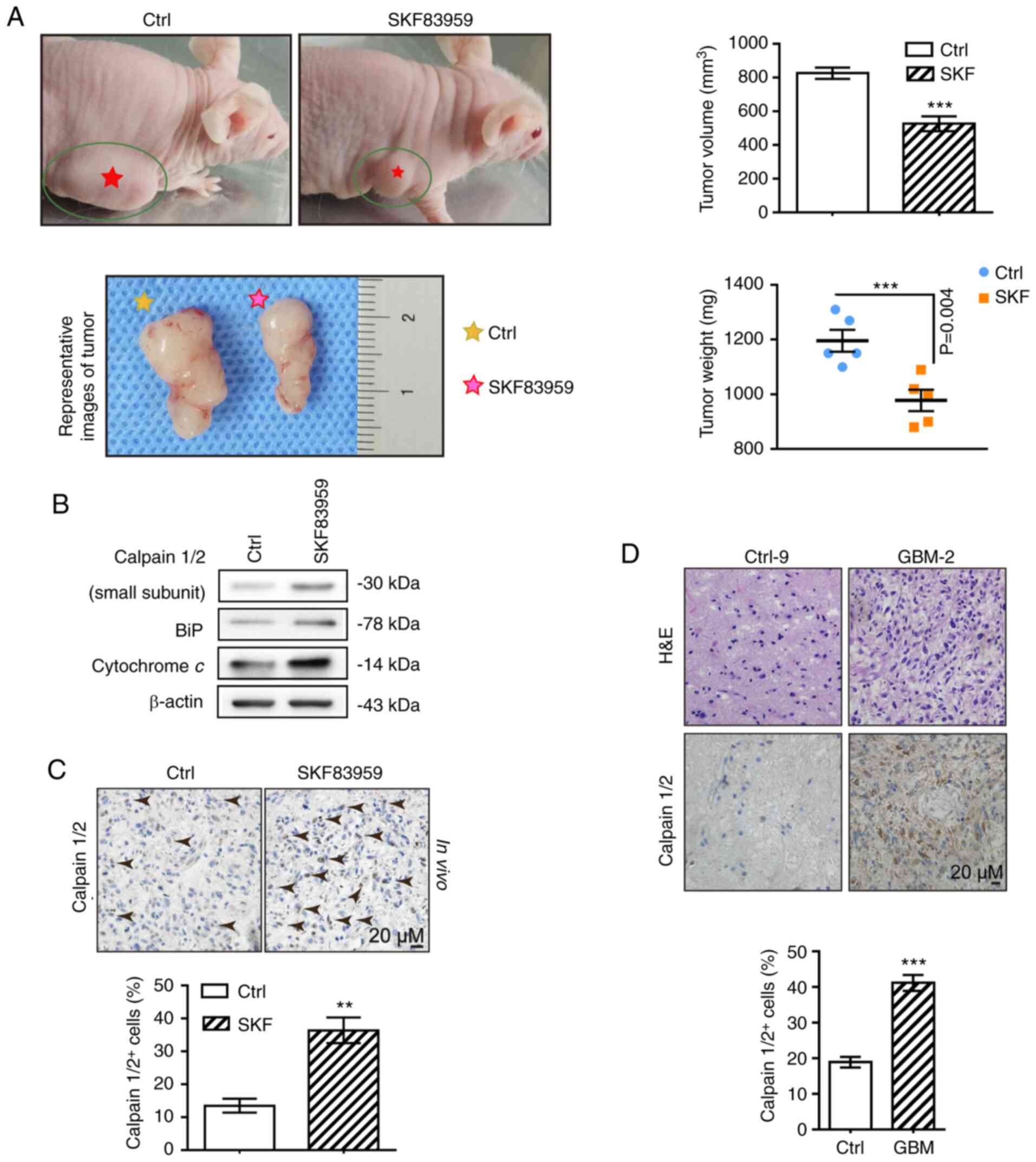 | Figure 5.DRD1-agonist-induced-calpain
activation, ER stress and mitochondrial dysfunction are observed
in vivo. (A) Representative GBM xenograft mice bearing
subcutaneously implanted tumours as well as images of tumours that
had received SKF83959 treatment or control saline are presented at
the endpoint (images on the left). Statistical analysis of tumour
volume and weight under different treatment conditions was
performed (images on the right). (B) Expression of calpain1/2, BiP
and Cyt c in tumours measured across two groups by western
blotting. (C) IHC staining for calpains in xenograft tumours
treated with SKF83959 or saline. Scale bars, 20 µm. (D) IHC
staining for calpain1/2 in GBM and control brain tissues.
H&E-stained GBM sections revealing necrosis, dense cellularity
and perinecrotic pseudo-palisading cells. Scale bars, 20 µM.
**P<0.01, and ***P<0.001 (vs. Ctrl). DRD1, dopamine receptor
D1; ER, endoplasmic reticulum; GBM, glioblastoma; Ctrl, control;
SKF, SKF83959. |
Discussion
Management of glioblastoma remains challenging;
thus, it is mandatory to investigate novel treatments for improved
patient outcome. Growing evidence implicates the role of
dopaminergic signalling in pathogenesis of GBM, and in
dopamine-receptor-regulator-mediated chemotherapy involving
apoptosis of GBM (45,46). Calpains are also implicated in tumour
migration and invasion of GBM (9,47). It is
documented that through activation of ER stress, oxidative stress,
and mitochondrial injury, calpains trigger apoptosis (29,33,34).
However, the role of calpains in
dopamine-receptor-regulator-mediated apoptosis in GBM was not fully
elucidated. In the present study, the DRD1 agonist, SKF83959, was
applied to GBM cells and ER stress and mitochondrial
injury-dependent GBM cell apoptosis were observed. The mechanistic
study revealed that the well expressed intracytosolic
Ca2+-activated calpains in GBM cells were the key
signalling factors during the apoptotic cascade. Application of
calpain inhibitor calpastatin significantly reversed the increase
in mitochondrial injury and ER stress markers and eventually
ameliorated the GBM cell apoptosis during SKF83959 treatment.
Previous studies have described that DRs can bind to
Gq protein, stimulate PLC and result in hydrolysis of
phosphoinositide (48,49). SKF83959, a selective DRD1 agonist,
activates the PLC/IP3 pathway, which is followed by an increase in
cytosolic Ca2+ (50–52). The
present data validated the signalling pathway and it was observed
that SKF83959-stimulated increases in Ca2+ levels, and
even apoptosis, were abolished by the PLC inhibitor, U73122, in U87
cells, which was a novel finding regarding intracellular
Ca2+ release via PLC activation in human GBM cell lines.
The increased intracellular Ca2+ levels upregulated
calpains, which are intracellular cysteine proteases (9,39). The
present data suggested that increased Ca2+ levels
following PLC activation could enhance calpain activity after
SKF83959 treatment. The calcium chelator, BAPTA-AM, reversed the
increased calpain levels, calpain-mediated mitochondrial injury,
and ER stress, suggesting a pivotal role of intracellular
Ca2+ between DRD1 agonist and calpain-induced GBM
apoptosis.
Calpains are multi-subunit,
Ca2+-activated proteases and are necessary for various
cellular functions such as genetic regulation, cytoskeletal
remodelling and cell mobility. However, there are still many
counterviews regarding the role of calpains in GBM cell migration
and survival (12,16,17,53). For
instance, calpain inhibitors were revealed to have a protective
effect on neurons under ischemic or cytotoxic conditions (54,55). In
the GBM cell line, C6, the cytotoxic effect of Crocus
sativus L. was significantly reversed by MDL-28170 (a calpain
inhibitor), implicating calpains as possible cell death mediators
(54). However, Jang et al
reported a positive effect of calpain 2 on GBM invasion by
identifying the complex signalling transduction of tumour cells in
the brain environment (14). Calpains
have also been revealed to activate matrix metalloproteinase
secretion and enhance invasive potential through transformation of
growth factor-β-inducible gene-h3 and integrin α5β1 in U87 cells
(17). Another in vivo study
revealed, by conducting a xenograft GBM model in zebrafish brain,
that calpains are also necessary for the dispersal and migration of
GBM cells along the blood vessels (16).
The pivotal factor defining the effects of calpains
is the impact of Ca2+ elevation on their activation, and
the uncontrolled elevation of Ca2+ level may lead to
prolonged and dysregulated calpain activation, followed by cellular
damage (9,56). Previous studies have reported that
under pathological conditions, the dysregulation of calpain
activity could cause uncontrolled protein cleavage, resulting in
irreversible cellular injury. For instance, under environmental
stress stimuli, the hydrolysis of Beclin-1 mediated by calpains was
revealed in nerve tissues (56–59). The
present data support the hypothesis that activation of calpains by
high levels of intracellular Ca2+ and oxidative stress
negatively affect cell fate and survival of GBM, suggesting a
potential therapeutic target for human GBM treatment regarding
calpain expression and activity regulation.
Mechanistic studies concerning the signalling
transduction between calpains and apoptosis through oxidative
stress, induction of mitochondrial injury, and activation of ER
stress have recently been reported (60–62).
Fluoride-induced bone damage has been revealed to be related to
apoptosis induced by calpain-dependent ER stress and mitochondrial
dysfunction (60). Lv et al
demonstrated that taurine attenuated hypoxia-induced cardiomyocyte
damage by supressing calpain-mediated, mitochondria-related
apoptosis (61). Activation of ER
stress and calpains was detected during hyperuricemia-triggered
cellular apoptosis in vivo, which could be alleviated by
calpain inhibitors or knockdown of calpain 1 expression (62). In human retinal epithelial cells, the
decreased expression of calpain 2 has also been reported to inhibit
starvation-triggered apoptosis (63).
The present data were consistent with the aforementioned evidence
that calpain activation induced by a DRD1 agonist could trigger an
apoptotic cascade through damage to mitochondrial function and
exacerbation of ER stress.
In summary, GBM cell apoptosis in human GBM U87
cells under treatment with DRD1 agonist SKF83959 was demonstrated.
SKF83959 administration increased the intracellular Ca2+
level through PLC signalling, and the downstream calpains were
activated and dysregulated accordingly, which led to mitochondrial
injury and ER stress, followed by apoptosis. These findings
suggested the potential therapeutic effect of DRD1 agonist against
GBM and may require further investigations for human GBM treatment
regarding calpain expression and activity regulation.
Acknowledgements
Not applicable.
Funding
The present research was funded by the National
Natural Science Foundation of China (NSFC) (grant nos. 81430021 and
81771521).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
KY and WL designed the study. KY and RX collected
human GBM samples and provided the nude mice. KY performed
experiments (cell culture analysis, immunohistochemistry, flow
cytometric analysis, cell cycle, and xenograft experiments) and
analysed the data. KY wrote and edited the manuscript. RX and WL
edited and reviewed the manuscript for important intellectual
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Hospital of Dalian Medical University (DMU) (Dalian,
China) (approval no. 2018052) and followed the ethical guidelines
of the Declaration of Helsinki. Written informed consent was
obtained from all patients whose tissues were used in this study.
Mice were purchased from the Institute of Genome-Engineered Animal
Models of DMU and were kept under specific pathogen-free
conditions. The study protocol was approved by the Animal Ethics
Committee of DMU (approval no. 2018112).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostrom QT, Gittleman H, Xu J, Kromer C,
Wolinsky Y, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the united states in 2009–2013. Neuro Oncol. 18 (Suppl
5):v1–v75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tabatabai G and Wakimoto H: Glioblastoma:
State of the art and future perspectives. Cancers (Basel).
11:10912019. View Article : Google Scholar
|
|
4
|
Claes A, Idema AJ and Wesseling P: Diffuse
glioma growth: A guerilla war. Acta Neuropathol. 114:443–458. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Groot JF, Fuller G, Kumar AJ, Piao Y,
Eterovic K, Ji Y and Conrad CA: Tumor invasion after treatment of
glioblastoma with bevacizumab: Radiographic and pathologic
correlation in humans and mice. Neuro Oncol. 12:233–242. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee DH, Ryu HW, Won HR and Kwon SH:
Advances in epigenetic glioblastoma therapy. Oncotarget.
8:18577–18589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clapham DE: Calcium signaling. Cell.
131:1047–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim KW, Choi CH, Kim TH, Kwon CH, Woo JS
and Kim YK: Silibinin inhibits glioma cell proliferation via
Ca2+/ROS/MAPK-dependent mechanism in vitro and glioma tumor growth
in vivo. Neurochem Res. 34:1479–1490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goll DE, Thompson VF, Li H, Wei W and Cong
J: The calpain system. Physiol Rev. 83:731–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carragher NO and Frame MC: Calpain: A role
in cell transformation and migration. Int J Biochem Cell Biol.
34:1539–1543. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roumes H, Leloup L, Dargelos E, Brustis
JJ, Daury L and Cottin P: Calpains: Markers of tumor
aggressiveness? Exp Cell Res. 316:1587–1599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cortesio CL, Chan KT, Perrin BJ, Burton
NO, Zhang S, Zhang ZY and Huttenlocher A: Calpain 2 and PTP1B
function in a novel pathway with Src to regulate invadopodia
dynamics and breast cancer cell invasion. J Cell Biol. 180:957–971.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mamoune A, Luo JH, Lauffenburger DA and
Wells A: Calpain-2 as a target for limiting prostate cancer
invasion. Cancer Res. 63:4632–4640. 2003.PubMed/NCBI
|
|
14
|
Jang HS, Lal S and Greenwood JA: Calpain 2
is required for glioblastoma cell invasion: Regulation of matrix
metalloproteinase 2. Neurochem Res. 35:1796–1804. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vo TM, Burchett R, Brun M, Monckton EA,
Poon HY and Godbout R: Effects of nuclear factor I phosphorylation
on calpastatin (CAST) gene variant expression and subcellular
distribution in malignant glioma cells. J Biol Chem. 294:1173–1188.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lal S, La Du J, Tanguay RL and Greenwood
JA: Calpain 2 is required for the invasion of glioblastoma cells in
the zebrafish brain microenvironment. J Neurosci Res. 90:769–781.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma J, Cui W, He SM, Duan YH, Heng LJ, Wang
L and Gao GD: Human U87 astrocytoma cell invasion induced by
interaction of βig-h3 with integrin α5β1 involves calpain-2. PLoS
One. 7:e372972012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su Y, Cui Z, Li Z and Block ER: Calpain-2
regulation of VEGF-mediated angiogenesis. FASEB J. 20:1443–1451.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kritis A, Pourzitaki C, Klagas I,
Chourdakis M and Albani M: Proteases inhibition assessment on PC12
and NGF treated cells after oxygen and glucose deprivation reveals
a distinct role for aspartyl proteases. PLoS One. 6:e259502011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Samarghandian S, Tavakkol Afshari J and
Davoodi S: Suppression of pulmonary tumor promotion and induction
of apoptosis by Crocus sativus L. extraction. Appl Biochem
Biotechnol. 164:238–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scaffidi C, Kischkel FC, Krammer PH and
Peter ME: Analysis of the CD95 (APO-1/Fas) death-inducing signaling
complex by high-resolution two-dimensional gel electrophoresis.
Methods Enzymol. 322:363–373. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wick W, Wild-Bode C, Frank B and Weller M:
BCL-2-induced glioma cell invasiveness depends on furin-like
proteases. J Neurochem. 91:1275–1283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stegh AH, Kim H, Bachoo RM, Forloney KL,
Zhang J, Schulze H, Park K, Hannon GJ, Yuan J, Louis DN, et al:
Bcl2L12 inhibits post-mitochondrial apoptosis signaling in
glioblastoma. Genes Dev. 21:98–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sastry PS and Rao KS: Apoptosis and the
nervous system. J Neurochem. 74:1–20. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zimmermann KC, Bonzon C and Green DR: The
machinery of programmed cell death. Pharmacol Ther. 92:57–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ashe PC and Berry MD: Apoptotic signaling
cascades. Prog Neuropsychopharmacol Biol Psychiatry. 27:199–214.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao RV, Ellerby HM and Bredesen DE:
Coupling endoplasmic reticulum stress to the cell death program.
Cell Death Differ. 11:372–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Charlier E, Relic B, Deroyer C, Malaise O,
Neuville S, Collée J, Malaise MG and De Seny D: Insights on
molecular mechanisms of chondrocytes death in osteoarthritis. Int J
Mol Sci. 17:21462016. View Article : Google Scholar
|
|
29
|
Lepetsos P and Papavassiliou AG:
ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys
Acta. 1862:576–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shiraishi H, Okamoto H, Yoshimura A and
Yoshida H: ER stress-induced apoptosis and caspase-12 activation
occurs downstream of mitochondrial apoptosis involving Apaf-1. J
Cell Sci. 119:3958–3966. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li D, Xie G and Wang W: Reactive oxygen
species: The 2-edged sword of osteoarthritis. Am J Med Sci.
344:486–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bolisetty S and Jaimes EA: Mitochondria
and reactive oxygen species: Physiology and pathophysiology. Int J
Mol Sci. 14:6306–6344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu L, Liu H, Li L, Liu H, Cheng Q, Li H
and Huang H: Mitochondrial pathology in osteoarthritic
chondrocytes. Curr Drug Targets. 15:710–719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye W, Zhu S, Liao C, Xiao J, Wu Q, Lin Z
and Chen J: Advanced oxidation protein products induce apoptosis of
human chondrocyte through reactive oxygen species-mediated
mitochondrial dysfunction and endoplasmic reticulum stress
pathways. Fundam Clin Pharmacol. 31:64–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guan L, Che Z, Meng X, Yu Y, Li M, Yu Z,
Shi H, Yang D and Yu M: MCU Up-regulation contributes to myocardial
ischemia-reperfusion Injury through calpain/OPA-1-mediated
mitochondrial fusion/mitophagy Inhibition. J Cell Mol Med.
23:7830–7843. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cho SO, Lim JW and Kim H: Oxidative stress
induces apoptosis via calpain- and caspase-3-mediated cleavage of
ATM in pancreatic acinar cells. Free Radic Res. Aug 30–2019.(Epub
ahead of print). doi: 10.1080/10715762.2019.1655145. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang K, Wei M, Yang Z, Fu Z, Xu R, Cheng
C, Chen X, Chen S, Dammer E and Le W: Activation of dopamine
receptor D1 inhibits glioblastoma tumorigenicity by regulating
autophagic activity. Cell Oncol (Dordr). 43:1175–1190. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin LQ, Goswami S, Cai G, Zhen X and
Friedman E: SKF83959 selectively regulates
phosphatidylinositol-linked D1 dopamine receptors in rat brain. J
Neurochem. 85:378–386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu J, Wang F, Huang C, Long LH, Wu WN,
Cai F, Wang JH, Ma LQ and Chen JG: Activation of
phosphatidylinositol-linked novel D1 dopamine receptor contributes
to the calcium mobilization in cultured rat prefrontal cortical
astrocytes. Cell Mol Neurobiol. 29:317–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Panchalingam S and Undie AS: SKF83959
exhibits biochemical agonism by stimulating [(35)S]GTP gamma S
binding and phosphoinositide hydrolysis in rat and monkey brain.
Neuropharmacology. 40:826–837. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tran TD, Gimble JM and Cheng H:
Vasopressin-induced Ca(2+) signals in human adipose-derived stem
cells. Cell Calcium. 59:135–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: A vicious cycle or a
double-edged sword? Antioxid Redox Signal. 9:2277–2293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Uehara Y, Hirose J, Yamabe S, Okamoto N,
Okada T, Oyadomari S and Mizuta H: Endoplasmic reticulum
stress-induced apoptosis contributes to articular cartilage
degeneration via C/EBP homologous protein. Osteoarthritis
Cartilage. 22:1007–1017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Williams A, Sarkar S, Cuddon P, Ttofi EK,
Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, et
al: Novel targets for Huntington's disease in an mTOR-independent
autophagy pathway. Nat Chem Biol. 4:295–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moreno-Smith M, Lu C, Shahzad MM, Pena GN,
Allen JK, Stone RL, Mangala LS, Han HD, Kim HS, Farley D, et al:
Dopamine blocks stress-mediated ovarian carcinoma growth. Clin
Cancer Res. 17:3649–3659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lan YL, Wang X, Xing JS, Yu ZL, Lou JC, Ma
XC and Zhang B: Anti-cancer effects of dopamine in human glioma:
Involvement of mitochondrial apoptotic and anti-inflammatory
pathways. Oncotarget. 8:88488–88500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Franco SJ and Huttenlocher A: Regulating
cell migration: Calpains make the cut. J Cell Sci. 118:3829–3838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Friedman E, Jin LQ, Cai GP, Hollon TR,
Drago J, Sibley DR and Wang HY: D1-like dopaminergic activation of
phosphoinositide hydrolysis is independent of D1A dopamine
receptors: Evidence from D1A knockout mice. Mol Pharmacol. 51:6–11.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Panchalingam S and Undie AS:
Physicochemical modulation of agonist-induced [35s]GTPgammaS
binding: Implications for coexistence of multiple functional
conformations of dopamine D1-like receptors. J Recept Signal
Transduct Res. 25:125–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhen X, Goswami S and Friedman E: The role
of the phosphatidyinositol-linked D1 dopamine receptor in the
pharmacology of SKF83959. Pharmacol Biochem Behav. 80:597–601.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ming Y, Zhang H, Long L, Wang F, Chen J
and Zhen X: Modulation of Ca2+ signals by
phosphatidylinositol-linked novel D1 dopamine receptor in
hippocampal neurons. J Neurochem. 98:1316–1323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rashid AJ, So CH, Kong MM, Furtak T,
El-Ghundi M, Cheng R, O'Dowd BF and George SR: D1-D2 dopamine
receptor heterooligomers with unique pharmacology are coupled to
rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA.
104:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yousefi S, Perozzo R, Schmid I, Ziemiecki
A, Schaffner T, Scapozza L, Brunner T and Simon HU:
Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis.
Nat Cell Biol. 8:1124–1132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Giakoumettis D, Pourzitaki C, Vavilis T,
Tsingotjidou A, Kyriakoudi A, Tsimidou M, Boziki M, Sioga A,
Foroglou N and Kritis A: Crocus sativus L. causes a non
apoptotic calpain dependent death in C6 rat glioma cells,
exhibiting a synergistic effect with temozolomide. Nutr Cancer.
71:491–507. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schumacher PA, Siman RG and Fehlings MG:
Pretreatment with calpain inhibitor CEP-4143 inhibits calpain I
activation and cytoskeletal degradation, improves neurological
function, and enhances axonal survival after traumatic spinal cord
injury. J Neurochem. 74:1646–1655. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim JS, Wang JH, Biel TG, Kim DS,
Flores-Toro JA, Vijayvargiya R, Zendejas I and Behrns KE:
Carbamazepine suppresses calpain-mediated autophagy impairment
after ischemia/reperfusion in mouse livers. Toxicol Appl Pharmacol.
273:600–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim JS, Nitta T, Mohuczy D, O'Malley KA,
Moldawer LL, Dunn WA Jr and Behrns KE: Impaired autophagy: A
mechanism of mitochondrial dysfunction in anoxic rat hepatocytes.
Hepatology. 47:1725–1736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Russo R, Berliocchi L, Adornetto A, Varano
GP, Cavaliere F, Nucci C, Rotiroti D, Morrone LA, Bagetta G and
Corasaniti MT: Calpain-mediated cleavage of Beclin-1 and autophagy
deregulation following retinal ischemic injury in vivo. Cell Death
Dis. 2:e1442011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Song F, Han X, Zeng T, Zhang C, Zou C and
Xie K: Changes in beclin-1 and micro-calpain expression in
tri-ortho-cresyl phosphate-induced delayed neuropathy. Toxicol
Lett. 210:276–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang J, Yang J, Cheng X, Xiao R, Zhao Y,
Xu H, Zhu Y, Yan Z, Ommati MM, Manthari RK and Wang J: Calcium
alleviates fluoride-induced bone damage by inhibiting endoplasmic
reticulum stress and mitochondrial dysfunction. J Agric Food Chem.
67:10832–10843. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lv Q, Yang J, Wang Y, Liu M, Feng Y, Wu G,
Lin S, Yang Q and Hu J: Taurine prevented hypoxia induced chicken
cardiomyocyte apoptosis through the inhibition of mitochondrial
pathway activated by Calpain-1. Adv Exp Med Biol. 1155:451–462.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yan M, Chen K, He L, Li S, Huang D and Li
J: Uric acid induces cardiomyocyte apoptosis via activation of
Calpain-1 and endoplasmic reticulum stress. Cell Physiol Biochem.
45:2122–2135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Y, Ren S, Liu Y, Gao K, Liu Z and
Zhang Z: Inhibition of starvation-triggered endoplasmic reticulum
stress, autophagy, and apoptosis in ARPE-19 cells by taurine
through modulating the expression of Calpain-1 and Calpain-2. Int J
Mol Sci. 18:21462017. View Article : Google Scholar
|