Introduction
Breast cancer is the most prevalent malignant
disease among women worldwide, and the second most common cause of
cancer-associated mortality (1,2). Breast
cancer is a heterogeneous disease characterized by distinct
molecular and morphological traits, and is classified into five
subtypes based on the expression profiles of estrogen receptor α
(ER-α), progesterone receptor (PR) and human epidermal growth
factor receptor 2 (HER2) (3,4). Triple-negative breast cancers (TNBCs),
which do not express ER-α, PR or HER2, are some of the most
aggressive breast cancers, accounting for 10–20% of all observed
breast cancer cases (5,6). Due to the lack of these major target
receptors, patients with TNBCs tend to have the worst overall
prognosis among the luminal A, B, HER2 and TNBC breast cancer
subtypes (4,6). Therefore, a number of clinical trials
have assessed the efficacy of various therapeutic options for
TNBCs.
Celastrol is a quinine methide triterpene isolated
from root extracts of the Asian perennial vine Tripterygium
wilfordii (7). Celastrol exhibits a
wide range of bioactivities, including the inhibition of cellular
proliferation, as well as anti-angiogenesis, antitumor and
anti-inflammatory properties (7–9). Celastrol
also acts as a potent inhibitor of constitutive and inducible
STAT3, and contributes to the inhibition of various genes involved
in cellular proliferation and survival by suppressing STAT3
activity in human hepatocellular carcinoma (8). In addition, celastrol inhibits a wide
variety of inflammatory mediators, including IL10 and IL13, via
suppression of the PI3K/AKT/mTOR signaling pathway (10,11).
However, the regulatory mechanism by which celastrol influences
TNBC cell invasiveness and migration is not known.
Elevated levels of pro-inflammatory cytokines affect
tumor growth, survival, angiogenesis and metastatic potential
during cancer progression (12,13).
Interleukins (ILs) are representative inflammatory cytokines
associated with, for example, inflammation and invasion in estrogen
receptor negative (ER-) breast cancer (14,15).
Abnormal IL1B induction is associated with poor prognosis in the
majority of the cancer types, including breast, colon and lung
cancer (16). previously, Tulotta
et al (17) reported that the
endogenous production of IL1B provided a bone metastatic niche,
thereby promoting tumor cell proliferation in breast cancer. In
addition, IL1 stimulates the production of IL8, which increases
cellular invasiveness and the metastatic potential of both ER- and
ER+ breast cancers (18,19). Our previous study also reported that
elevated IL1B expression enhances the motility of TNBC cells
(15).
The aim of the present study was to investigate the
pharmacological effect of celastrol on TNBC cells. Herein, the
present study aimed to demonstrate the possibility of celastrol as
an anti-inflammatory drug to inhibit IL1B in TNBC cells. In
addition, the regulatory role of celastrol on IL1B expression was
investigated.
Materials and methods
Reagents
Cell culture media and antibiotics were purchased
from Thermo Fisher Scientific, Inc., and fetal bovine serum (FBS)
was purchased from Hyclone; Cytiva. Celastrol was acquired from
Sigma-Aldrich (Merck KGaA), and MEK162, from Selleck Chemicals.
LY294002, SP600125, GM6001 and Bay11-7082 were purchased from
Tocris Bioscience. The anti-matrix metalloproteinases (MMP) 1
antibody was purchased from Abcam (cat. no. ab137332; dilution,
1:1,000); anti-β-actin antibodies were purchased from Santa Cruz
Biotechnology, Inc. (cat. no. sc-47778; dilution, 1:1,000); and
anti-phospho (p-)ERK and total (t-)ERK antibodies (cat. no. 9102
(t); and 4370 (p); dilution, 1:1,000) were purchased from Cell
Signaling Technology, Inc. The human IL8 (cat. no. K0331216) IL1B
(cat. no. K1331800) ELISA kits were purchased from Koma Biotech,
Inc.
Cell culture
All breast cancer cells were obtained from the
American Type Culture. T47D, ZR75-1, BT-474, SK-BR-3, HCC1143 and
HCC1806 human breast cancer cells were cultured in RPMI1640 medium
supplemented with 10% FBS, 2 mM glutamine, 100 IU/ml penicillin and
100 µg/ml streptomycin, and maintained at 37°C in a humidified
atmosphere (95% air and 5% CO2). MCF7, MDA-MB-157,
MDA-MB-231, and Hs578T human breast cancer cells were maintained in
DMEM under the same culture conditions.
MTT assay
As previously described (15), TNBC cells were trypsinized and counted
using a Countess™ automated cell counter (Invitrogen; Thermo Fisher
Scientific, Inc.). Each cell line was seeded into 96-well plates at
a density of 1×104 cells/well. After 24 h at 37°C in a
cell culture incubator, fresh culture media with the indicated
concentrations of celastrol were added, followed by further
incubation for 24 h. Viable cells were photometrically quantified
at 570 nm using a SpectraMax 190 microplate reader (Molecular
Devices, LLC).
Colony formation assay
Hs578T TNBC cells were plated into 6-well culture
plates (1×103 cells/well). After 24 h at 37°C, the cells
were treated with celastrol at the indicated concentrations,
followed by an additional 7 days of incubation. The colonies were
subsequently fixed with 10% ethanol for 5 min at RT and stained
with 0.01% crystal violet for 30 min at RT (20).
Proteome profiler human cytokine
array
As previously described (20), Hs578T TNBC cells (1×106
cells/plate) were seeded into two separate 100-mm dishes, and
treated with or without 0.5 µM celastrol in fresh serum-free media
for 24 h. The conditioned culture media were collected 24 h later,
and 300 µl was immediately used with The Proteome Profiler™ Human
Cytokine Array Kit (R&D Systems, which was conducted according
to manufacturer's instructions.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from cells using
TRIzol® Reagent (Molecular Research Center, Inc.)
according to the manufacturer's protocol. Isolated RNA samples (1
µg total RNA) were reverse-transcribed into cDNA in 20-µl reaction
volumes using a first-strand cDNA synthesis kit (Fermentas; Thermo
Fisher Scientific, Inc.) per the manufacturer's protocol. IL1B and
IL8 gene expression were quantified by qPCR using the SensiMix SYBR
Kit (Bioline) with 100 ng cDNA per reaction. The primer sets used
for analysis were as follows: Human IL1B forward,
5′-GCCCTAAACAGATGAAGTGCTC-3′ and reverse,
5′-GAACCAGCATCTTCCTCAG-3′; human IL8 forward,
5′-AGGGTTGCCAGATGCAATAC-3′ and reverse, 5′-AAACCAAGGCACAGTGGAAC-3′;
human MMP-1 forward, 5′-ATTCTACTGATATCGGGGCTTTGA-3′ and reverse,
5′-ATGTCCTTGGGGTATCCGTGTAG-3′; human MMP-2 forward,
5′-GGCCTCGTATACCGCATCAATC-3′ and reverse,
5′-GGCCTCTCCTGACATTGACCTT-3′; human MMP-9 forward,
5′-CCCGGACCAAGGATACAG-3′ and reverse, 5′-GGCTTTCTCTCGGTACTG-3′; and
GAPDH forward, 5′-ATTGTTGCCATCAATGACCC-3′ and reverse,
5′-AGTAGAGGCAGGGATGATGT-3′. An annealing temperature of 60°C was
used for all primers, and PCR were performed using a standard
384-well-plate format with an ABI 7900HT real-time PCR detection
system. The following thermocycling conditions were used: 95°C for
10 min, 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for
15 sec. For data analysis, the raw threshold cycle
(CT) value was normalized to the housekeeping
gene for each sample to obtain ΔCT. Normalized
ΔCT was calibrated to control cell samples to
calculate ΔΔCT (13,15).
IL8 and IL1B ELISA
IL8 and IL1B protein levels were determined using
ELISA kits for human IL8 and IL1B (Koma Biotech, Inc.) according to
the manufacturer's instructions. A microtiter plate reader was used
to measure the absorbance at 450 nm (15).
Western blotting
Conditioned culture media (serum-free) and cell
lysates were used for the western blot analysis of MMP-1, p-ERK,
t-ERK and β-actin. The cells were lyzed using PRO-PREP™ Protein
Extraction Solution (Intron Biotechnology, Inc.) and centrifuged
(16,100 × g for 15 min). Total protein concentration was quantified
by Bradford assay (Bio-Rad Laboratories, Inc.). The proteins were
boiled for 5 min in Laemmli sample buffer and then electrophoresed
onto 10% SDS-PAGE gels. The separated proteins (30 mg) were
transferred to PVDF membranes, which were then blocked with 10%
skim milk in 0.01% TBS-Tween (TBS/T) for 15 min at RT. The blots
were incubated with anti-MMP-1, -p-ERK, -t-ERK and -β-actin
antibodies in 1% TBS/T, at 4°C overnight. The blots were washed 3–4
times in TBS/T buffer, and then incubated with an anti-rabbit or
anti-mouse HRP-conjugated antibody (1:2,000 dilution) in TBS/T.
After a 1-h incubation at room temperature (RT), the blots were
washed three times, and ECL prime reagents (Amersham; Cytiva) were
used for development. The levels of protein expression (p/t-ERK
ratio) were quantified using ImageJ version 2 (National Institutes
of Health).
Zymography
Conditioned culture media (serum-free) were mixed
with loading buffer and run on a 10% SDS-PAGE gel containing 0.5
mg/ml gelatin without prior denaturation. After electrophoresis,
the gels were washed to remove residual SDS, and incubated in
renaturing buffer (50 mM Tris, 5 mM CaCl2, 0.02%
NaN3 and 1% Triton X-100) for 30 min at RT. Next, the
gels were incubated for 48 h at 37°C in developing buffer (50 mM
Tris, 5 mM CaCl2, 0.15 M NaCl and 1% Triton X-100), and
subsequently stained with Coomassie brilliant blue G-250, destained
in 30% methanol, and flooded with 10% acetic acid until bands can
clearly visible (19).
Boyden chamber assay
Matrigel-precoated filter inserts (pore size, 8-µm);
Becton, Dickinson and Company) were inserted into 24-well invasion
chambers. Breast cancer cells were resuspended in culture medium
(2×105 cells/well) and added to the upper compartment of
the invasion chamber in the presence or absence of 20 ng/ml IL-1β,
and/or 0.5 µM celastrol or 10 µM GM6001. Fresh culture medium (700
µl) was added to the lower compartment of the chamber. The chambers
were incubated at 37°C for 24 h. After incubation, the cells on the
upper side of the filter were removed using cotton swabs, and the
lower filters were fixed in 4% paraformaldehyde for 10 min and
stained in 0.5% Toluidine blue for 1 h at RT (Sigma-Aldrick; Merck
KGaA). The cells on the underside of the filter were photographed
in four separate fields per condition using a CK40 inverted
microscope (Olympus Corporation). The values obtained were
calculated by averaging the total number of cells from four filters
(15,21).
Statistical analysis
Data analysis was performed using Excel 2016
(Microsoft Corporation) and GraphPad Prism version 8 (GraphPad
Software, Inc.). Statistical significances between two groups of
data were calculated using unpaired two-tailed Student's t-test and
Tukey's multiple comparison test was used for comparisons among
multiple groups. Results are presented as the mean ± S.E.M. All
quoted P-values are two-tailed, and P<0.05 was considered to
indicate a statistically significant difference (20).
Results
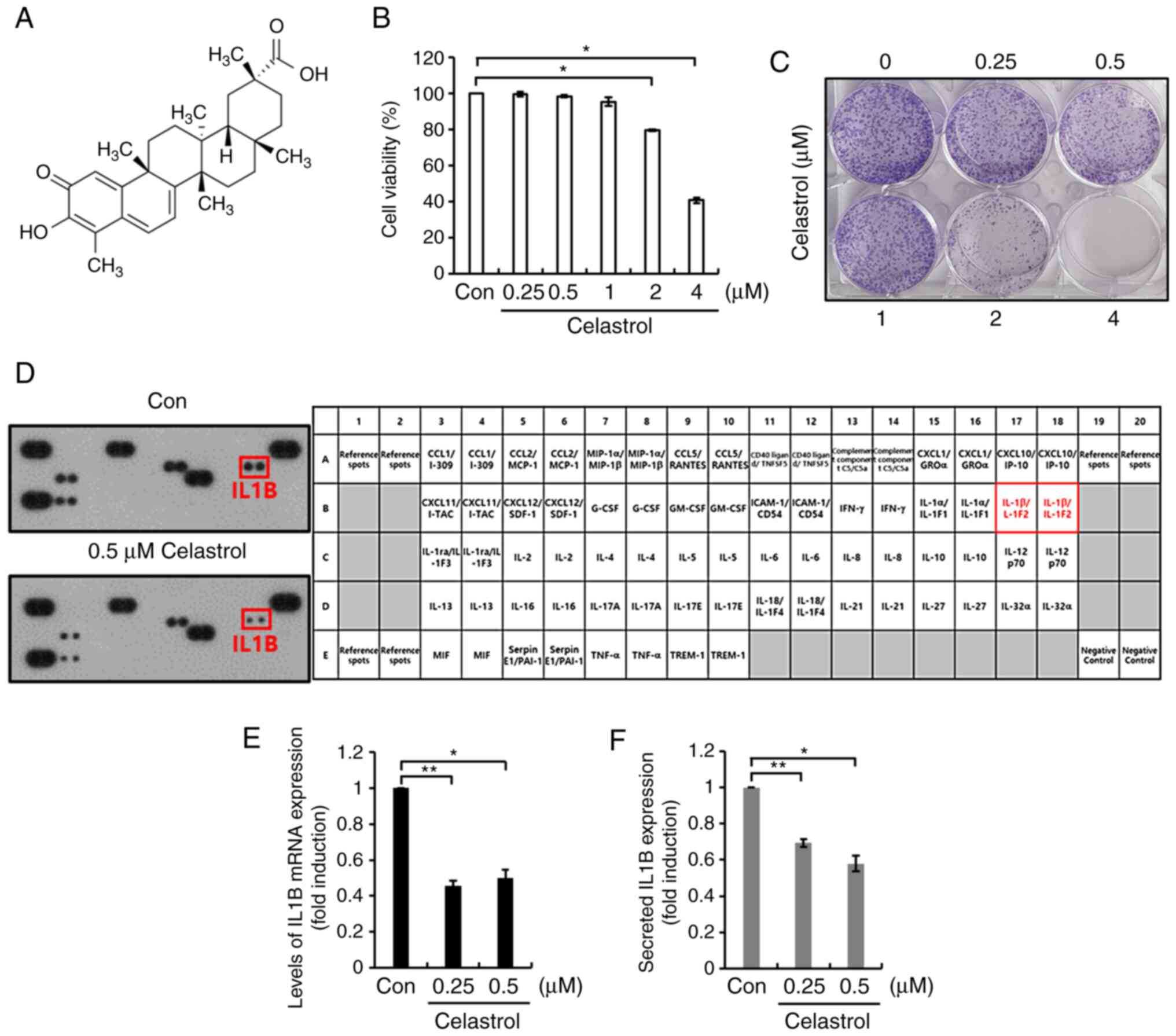
Celastrol decreases basal levels of
IL1B expression in Hs578T TNBC cells
To investigate the effect of celastrol on TNBC
cells, Hs578T cells that had been subjected to serum starvation for
24 h were treated with celastrol at the indicated concentrations
for a further 24 h, after which cell viability was evaluated. The
chemical structure of celastrol is shown in Fig. 1A. As shown in Fig. 1B, cell viability was significantly
decreased following treatment with 2 and 4 µM celastrol. Moreover,
the colony formation capacity of Hs578T TNBC cells treated with 2
and 4 µM celastrol was markedly reduced, compared with that of the
untreated cells (Fig. 1C). Therefore,
0.25 and 0.5 µM celastrol were used for subsequent experimentation.
Using the Proteome Profiler Human Cytokine Array Kit, the effect of
celastrol on cytokine expression was evaluated using Hs578T TNBC
cells. As shown in Fig. 1D, the
amount of secreted IL1B protein was considerably decreased by
celastrol treatment. Under similar conditions, the levels of IL1B
mRNA and protein expression were examined in Hs578T TNBC cells. As
predicted, treatment with celastrol significantly decreased the
basal levels of IL1B mRNA and protein expression (Fig. 1E and F). Treatment with 0.5 µM
celastrol decreased the levels of IL1B mRNA and protein expression
0.49±0.03- (Fig. 1E) and
0.58±0.04-fold (Fig. 1F),
respectively, relative to the control. Celastrol also reduced the
levels of IL1B mRNA expression (Fig.
S1) in HCC1143, MDA231 and HCC1806 TNBC cells. Consequently,
these results demonstrate that celastrol attenuated the
inflammatory response in TNBC cells by suppressing IL1B
expression.
Elevated IL1B upregulates the levels
of IL8 and MMPs in TNBC cells
In the present study, IL1B and IL8 mRNA expression
were found to be significantly higher in TNBC cells (MDA-157,
Hs578T, MDA-231, HCC1143 and HCC1806) compared with non-TNBC cells
(MCF7, T47D, ZR75-1, BT474 and SK-BR-3) (Fig. 2A). The effect of IL1B on IL8
expression in Hs578T and HCC1143 TNBC cells was also determined.
After serum starvation for 24 h, the two cell lines were treated
with or without 20 ng/ml IL1B for 24 h in fresh serum-free media.
An increase in IL8 expression was observed subsequent to IL1B
treatment in Hs578T and HCC1143 TNBC cells (Fig. 2B and C). By contrast, IL8 treatment
had no effect on IL1B expression (Fig.
S2A and B). Compared with the control, treatment with 20 ng/ml
IL1B led to a 14.9±2.8-fold and 585.6±50.6-fold increase in IL8
mRNA expression in Hs578T and in HCC1143 cells, respectively
(Fig. 2B). In addition, the
expression of secreted IL8 protein in the conditioned culture
medium was assessed. IL1B treatment increased the level of secreted
IL8 protein to 71.4±0.4 pg/ml in Hs578T cells and 72.9±1.2 pg/ml in
HCC1143 cells, compared with the control (Fig. 2C). Under similar conditions, IL1B
significantly increased the levels of MMPs, including MMP-1 and
MMP-9, which play a pivotal role in the metastatic and invasion
abilities of various cancer cell types (Fig. 2D and E). Moreover, a significant
increase in TNBC cell invasiveness was observed in response to IL8
treatment (Fig. S2C). These results
indicate that IL1B functions as a key mediator of inflammation and
TNBC cell invasiveness through the induction of IL8, MMP-1 and
MMP-9.
Celastrol regulates IL1B expression
through the MEK/ERK-dependent pathway in TNBC cells
The mechanism underlying celastrol-regulated IL1B
expression in TNBC cells was investigated. The cells that had been
previously subjected to serum starvation for 24 h, both in the
absence and presence of 5 µM specific inhibitors, were selected for
analysis. As shown in Fig. 3A,
MEK162, a MEK1/2-specific inhibitor, significantly decreased IL1B
mRNA expression in Hs578T cells, though no effect was observed
following treatment with Bay11-7082, LY294002 or SP600125. MEK162
decreased IL1B mRNA expression 0.53±0.02-fold (Hs578T cells) and
0.5±0.01-fold (HCC1143 cells), respectively (Fig. 3A). Under similar conditions, the
amount of secreted IL1B protein in response to MEK162 treatment was
analyzed in Hs578T and HCC1143 cells. As shown in Fig. 3B, basal levels of IL1B protein
expression were decreased following treatment with 5 µM MEK162.
These findings demonstrate that basal levels of IL1B expression are
regulated through a MEK/ERK-dependent pathway in TNBC After serum
starvation for 24 h, the cells were treated with celastrol at the
indicated concentrations for a further 24 h. As shown in Fig. 3C, ERK phosphorylation was suppressed
by celastrol treatment in both Hs578T and HCC1143 TNBC cells. In
addition, the effect of celastrol was investigated at shorter time
points, indicating that the levels of ERK phosphorylation began to
decrease from 2 h in Hs578T cells (Fig.
3D). These results suggest that celastrol downregulated IL1B
expression through the suppression of the MEK/ERK signaling pathway
in TNBC cells.
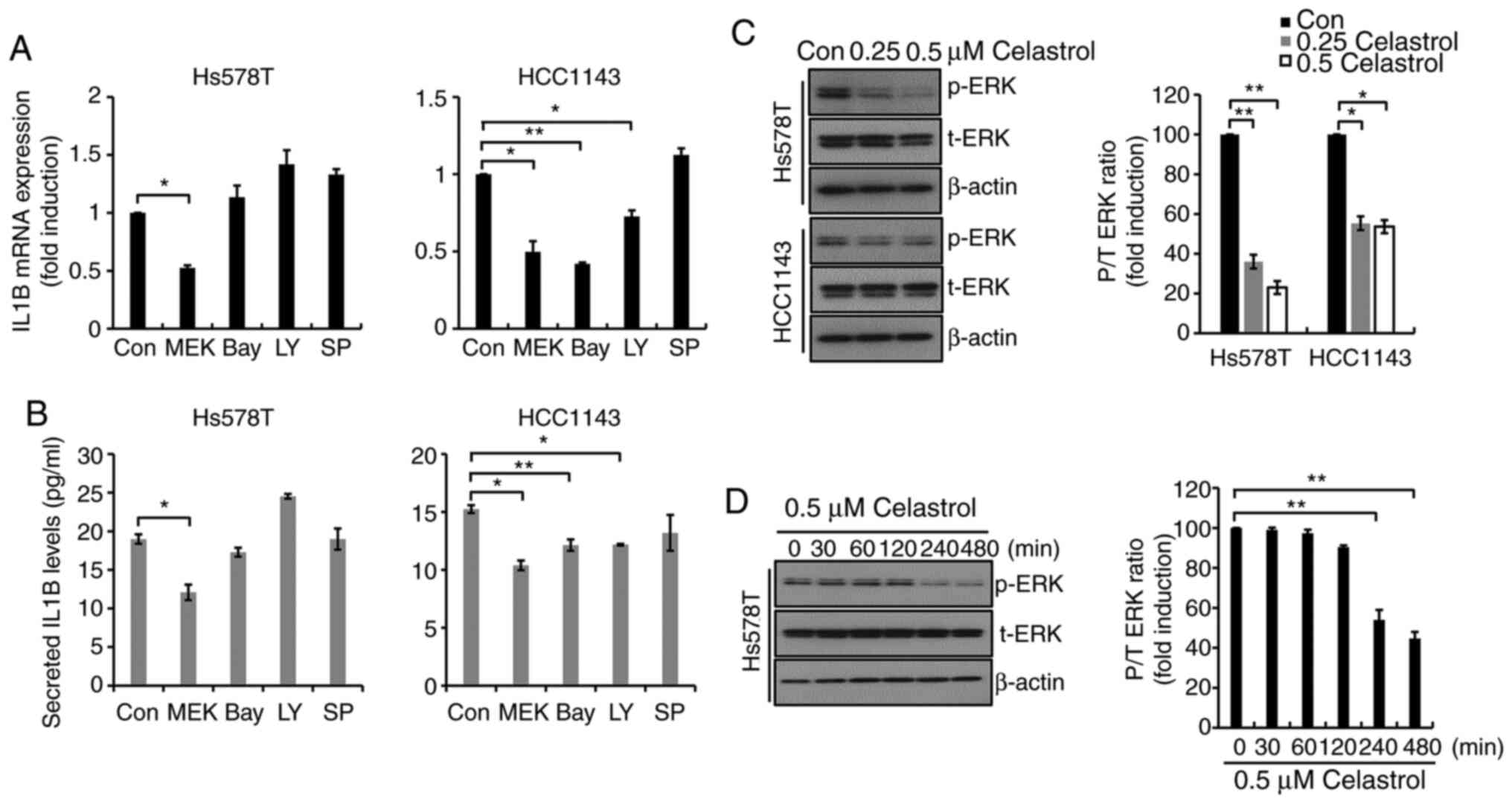 | Figure 3.Celastrol regulates IL1B expression
through a MEK/ERK-dependent mechanism in TNBC cells. After serum
starvation for 24 h, cells were treated with 5 µM specific
inhibitors for 24 h. (A) IL1B mRNA expression was determined by
reverse transcription-quantitative PCR. (B) Conditioned culture
media from the TNBC cells were harvested to detect secreted IL1B,
which was analyzed by ELISA. Values shown are the mean ± S.E.M. (C)
After serum starvation for 24 h, cells were treated with celastrol
for a further 24 h. (D) After serum starvation for 24 h, cells were
treated with 0.5 µM celastrol for the indicated time. Using the
whole cell lysates, p-ERK and t-ERK expression levels were
determined by western blotting. Results represent the mean of three
independent experiments. *P<0.05 and **P<0.01. Con, control.
TNBC, triple-negative breast cancer; p-, phosphorylated; t-, total;
MEK, MEK162; Bay, Bay11-7082; LY, LY294002; SP, SP600125. |
Celastrol decreases IL1B-induced IL8,
MMP-1 and MMP-9 expression, and the invasive ability of TNBC
cells
Finally, the pharmacological effects of celastrol on
IL1B-induced IL8 expression and cellular invasiveness were
investigated. Cells that had been subjected to serum starvation for
24 h were pretreated with 0.5 µM celastrol for 3 h, followed by the
addition of 20 ng/ml IL1B with incubation for a further 24 h. The
effect of celastrol on MMP-1 and −9 expression was determined, as
both proteins play an important role in cellular invasive and
metastatic potential. The induction of IL1B-associated MMP-1 and −9
mRNA expression was decreased in response to celastrol treatment in
Hs578T and HCC1143 TNBC cells (Fig.
4A). As anticipated, IL1B-induced MMP-1 and −9 protein
expression was also decreased in the presence of celastrol
(Fig. 4B). Furthermore, the effect of
celastrol on IL1B-induced IL8 expression was investigated. As shown
in Fig. 4C, celastrol decreased
IL1B-induced IL8 mRNA expression. Under the control conditions (no
celastrol), IL1B increased IL8 mRNA expression 133.5±6.9-fold in
Hs578T TNBC cells and 765.4±127.6-fold in HCC1143 TNBC cells.
Pretreatment with 0.5 µM celastrol decreased IL1B-induced IL8
expression by 64.8±4.3-fold in Hs578T TNBC cells and 7.6±1.9-fold
in HCC1143 TNBC cells (Fig. 4C). In
addition, celastrol suppressed IL1B-induced cell invasiveness
(Fig. 4D). Furthermore, the effect of
GM6001 (a reversible broad-spectrum MMP inhibitor) on IL1B-induced
cell invasiveness was investigated, which was found to be
suppressed (Fig. 4E). Therefore, it
was demonstrated that celastrol suppressed cellular invasiveness by
inhibiting IL1B-induced IL8, MMP-1 and MMP-9 expression in TNBC
cells.
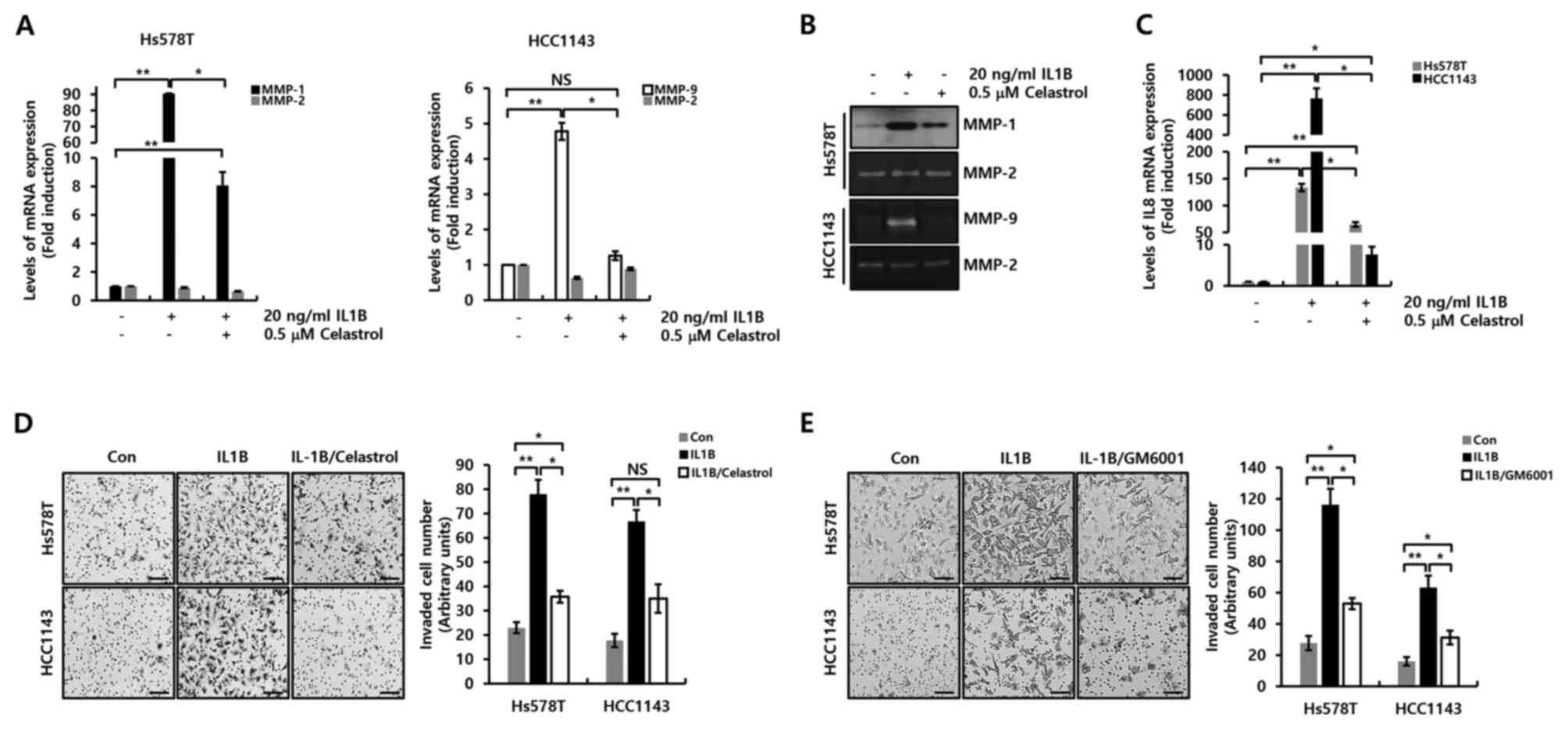 | Figure 4.Celastrol decreases IL1B-induced IL8,
MMP-1 and MMP-9 expression in TNBC cells. After serum starvation
for 24 h, cells were pretreated with the indicated celastrol
concentrations for 1 h, and then treated with 20 ng/ml IL1B for 24
h. Levels of IL8, MMP-1, MMP-2 and MMP-9 mRNA expression in (A)
Hs578T and (C) HCC1143 cells were determined by reverse
transcription-quantitative PCR. (B) Conditioned culture media from
the TNBC cells were harvested to detect MMP-1, MMP-2 and MMP-9
expression, and then analyzed by western blotting and Zymography.
After being seeded into Transwell chambers, cells were treated with
20 ng/ml IL1B and/or (D) 0.5 µM celastrol or (E) 10 µM GM6001 for
24 h. Invasive cells on the underside of the filter were
photographed using a CK40 inverted microscope. Scale bars, 20 µm.
Results represent the mean ± S.E.M of three independent
experiments. *P<0.05 and **P<0.01. Con, control; NS, not
significant; TNBC, triple-negative breast cancer; MMP, matrix
metalloproteinase. |
Discussion
In the past few decades, various researchers have
revealed that inflammation plays a pivotal role in tumor
development and progression (22).
ILs are involved in mediating both acute and chronic inflammatory
responses, as well as intercellular communication (including in
cellular invasion, proliferation and adhesion) in various cancer
cell types (23,24). IL1A, IL1B and IL1 receptor antagonist
(IL-1RA) are frequently expressed in breast cancer cells, tissues
and in the tumor microenvironment (24,25). In
particular, IL1B plays a major role in human malignancy and is
often associated with tumor invasiveness (26). The levels of IL1B expression have been
reported to be significantly higher in invasive carcinomas compared
with benign and ductal carcinoma in situ lesions (27). In the present study, IL1B mRNA
expression levels were significantly higher in TNBC compared with
non-TNBC cells, although IL1B protein expression could not be
quantified due to the radical proliferation rates of the cancer
cell lines. However, no notable difference in IL1A mRNA expression
was observed in breast cancer cells (data not shown). Therefore, it
has been demonstrated that aberrant IL1B induction may be
associated with the aggressiveness of TNBC cells in association
with an increase in inflammatory responses.
Secreted IL1B binds to IL1R, which triggers multiple
signaling pathways that involve p38, MAPK, JNK and NF-κB activation
(28,29). Elevated levels of IL1Bβ promote the
expression of multiple inflammatory genes, such as cyclooxygenase-2
(COX2), phospholipase A2, prostaglandin E2 and IL-8 (13,30). Hou
et al (31) reported that
IL1B-induced COX2 expression was decreased by IL1RA in a
dose-dependent manner. Although the effectiveness of cellular
invasion through the knockdown/overexpression of IL1B was not
verified due to technical limitations, IL1RA decreased the
invasiveness of Hs578T and MDA-MB231 TNBC cells (13). Therefore, we hypothesized that IL1B
production was essential for cellular migration and invasiveness of
these TNBC cell types.
Previous studies have reported IL8 as a
multifunctional pro-inflammatory chemokine, and demonstrated a
correlation between IL8 expression and breast cancer metastasis and
poor prognosis (15,19,32). In
addition, it has been demonstrated that aberrant IL8 expression
significantly increases cellular invasion and migration ability in
tamoxifen-resistant and TNBC cells (15,19).
Consistent with a previous report, in the present study, it was
observed that IL1B treatment markedly increased IL8 expression in
TNBC cells, and elevated IL8-associated invasiveness. However, IL8
had no effect on IL1B expression (Fig.
S2). Therefore, the results of the present study revealed that
IL1B was directly associated with cellular invasiveness and
migration through the induction of IL8 expression in TNBC
cells.
The expression of MMPs is regulated by various
stimuli, including growth factors, cytokines, chemical agents and
oncogenic transformation (33). MMPs
play a role in tumor growth and metastasis by degrading matrix
barriers and enhancing angiogenesis (33). Excessive induction of MMP-1 protein
has been reported in several tumors, with the development of more
aggressive characteristics such as cellular invasion and migration
(34). MMP-9 acts as a tumor promoter
in the process of carcinogenesis and has been demonstrated to
decrease the incidence of carcinogenesis in MMP-9-knockdown mouse
models (35). Consistent with these
reports, it was observed that IL1B triggered the induction of MMP-1
and MMP-9, but not MMP-2 in the present study. These results
suggest that IL1B may enhance the metastatic potential of TNBC
cells by promoting MMP-1 and −9 production.
Celastrol is a triterpene with numerous
pharmacological properties, including demonstrable anticancer and
anti-inflammatory activities in various cancer cell types, such as
breast, lung, myeloma and gastric cancer cells (8,36,37). In addition, celastrol has been
reported to completely block epithelial-to-mesenchymal transition
in A549 non-small cell lung cancer cells by inhibiting the TGF-β1
signaling pathway (38), and to
promote inflammatory responses in orbital fibroblasts and retinal
pigment epithelial cells (39,40).
Consistent with these reports, the results of the current study
showed that celastrol suppressed TNBC cell proliferation in a
dose-dependent manner. In particular, celastrol dramatically
reduced basal levels of IL1B and IL8 expression (important
mediators of the inflammatory response) in TNBC cells. Furthermore,
IL1B-induced MMP-1 and −9 expression was also decreased by
celastrol. Consequently, celastrol is proposed as an effective drug
for reducing inflammatory responses in TNBC cells.
In addition, previous studies have demonstrated the
possible potential of celastrol in modulating different signaling
cascades, including the STAT3, 5′AMP-activated protein kinase,
NF-κB and AKT/mTOR pathways (37,41). Park
et al (42) reported that
ERK1/2 activation was closely associated with cancer cell
migration, invasiveness and metastatic potential. In a previous
study, Hashimoto et al (43)
reported that the IL1B promoter contains various transcription
factor binding sites, such as those for AP-1 and NF-kB. The results
of the present study showed that IL1B expression was reduced by
Bay11-7082 in HCC1143 cells, while there was no significant change
in Hs578T cells. However, the effect of MEK inhibition commonly
suppresses IL1B expression in both cell types. Based on previous
reports, it is also believed that the activity of AP-1 is regulated
by the MAPK (MEK/ERK) pathway, and increases IL1B promoter activity
in TNBC cells. Herein, it was observed that celastrol decreased the
level of ERK phosphorylation in TNBC cells, and that basal levels
of IL1B expression were decreased through inhibition of the
MEK/ERK-dependent pathway. Thus, it is hypothesized that celastrol
suppresses IL1B expression by inhibiting ERK1/2 activity.
Furthermore, it was found that IL1B-induced IL8,
MMP-1 and −9 expression was completely suppressed by celastrol. The
MEK inhibitor, MEK162, completely suppressed IL1B-induced IL8,
MMP-1 and MMP-9 expression. Furthermore, it was revealed that
IL1B-dependent activation of the MEK/ERK pathway plays an important
role in IL8, MMP-1 and MMP-9 expression, which subsequently
inhibits TNBC cell motility.
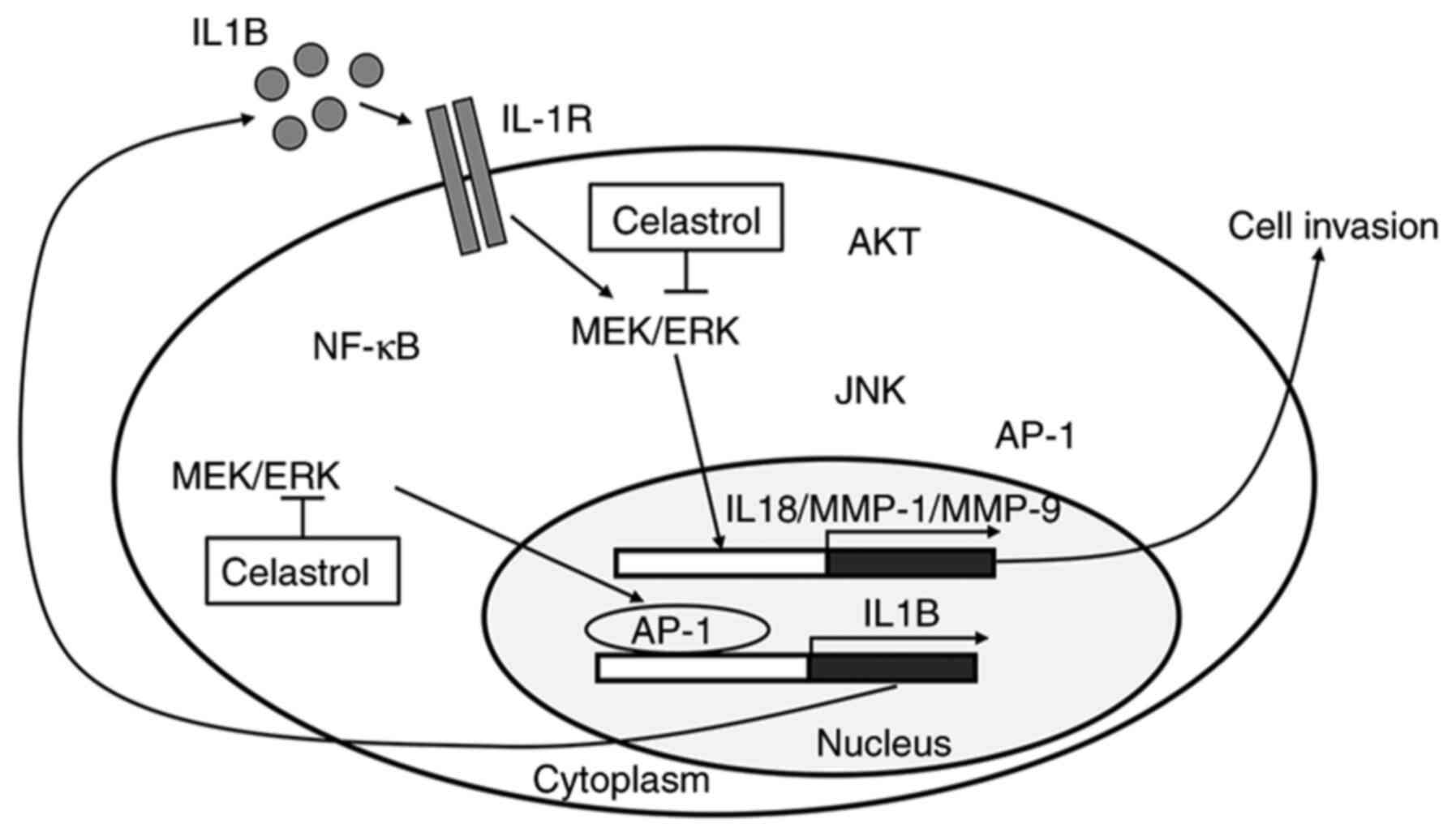
In conclusion, the pharmacological effects of
celastrol in TNBC cells were investigated. IL1B mRNA expression
levels were observed to be higher in TNBC cells compared with
non-TNBC cells. As shown in Fig. 5,
elevated IL1B enhances TNBC cell invasiveness through the secretion
of IL8 and MMPs. Currently, extensive clinical research is being
conducted with a variety of agents that reduce IL-1 activity. In
the current study, celastrol was found to suppress basal expression
of IL1B in TNBC cells, and to inhibit IL1B-induced IL8, MMP-1 and
MMP-9 expression. Taken together, the results of the present study
demonstrated that celastrol is an effective drug for the treatment
of TNBC by reducing IL-1 activity or associated signaling
pathways.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ministry of
Science and ICT (Korea) under the ICT Creative Consilience program
(grant no. IITP-2021-2020-0-01821) supervised by the IITP
(Institute for Information & communications Technology Planning
& Evaluation), and by the Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Education (grant no. 2016R1D1A1B01010508).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SK, SWK, SJN and JEL contributed to the experimental
design and analyzed the results. SK and JEL wrote the manuscript.
SK, DY, YJ, SYY and SAK performed the experiments and analyzed the
results. All authors are responsible for the authenticity of the
raw data. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ciriello G, Gatza ML, Beck AH, Wilkerson
MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, et
al: Comprehensive molecular portraits of invasive lobular breast
cancer. Cell. 163:506–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kohler BA, Sherman RL, Howlader N, Jemal
A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, et
al: Annual report to the nation on the status of cancer, 1975–2011,
featuring incidence of breast cancer subtypes by race/ethnicity,
poverty, and state. J Natl Cancer Inst. 107:djv0482015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perou CM, Sorlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boyle P: Triple-negative breast cancer:
Epidemiological considerations and recommendations. Ann Oncol. 23
(Suppl 6):vi7–vi12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HW, Jang KS, Choi HJ, Jo A, Cheong JH
and Chun KH: Celastrol inhibits gastric cancer growth by induction
of apoptosis and autophagy. BMB Rep. 47:697–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rajendran P, Li F, Shanmugam MK, Kannaiyan
R, Goh JN, Wong KF, Wang W, Khin E, Tergaonkar V, Kumar AP, et al:
Celastrol suppresses growth and induces apoptosis of human
hepatocellular carcinoma through the modulation of STAT3/JAK2
signaling cascade in vitro and in vivo. Cancer Prev Res (Phila).
5:631–643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin L, Sun Y, Wang D, Zheng S, Zhang J and
Zheng C: Celastrol ameliorates ulcerative colitis-related
colorectal cancer in mice via suppressing inflammatory responses
and epithelial-mesenchymal transition. Front Pharmacol.
6:3202015.PubMed/NCBI
|
|
10
|
Zhao J, Sun Y, Shi P, Dong JN, Zuo LG,
Wang HG, Gong JF, Li Y, Gu LL, Li N, et al: Celastrol ameliorates
experimental colitis in IL-10 deficient mice via the up-regulation
of autophagy. Int Immunopharmacol. 26:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Broide DH: Molecular and cellular
mechanisms of allergic disease. J Allergy Clin Immunol. 108((2
Suppl)): S65–S71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dranoff G: Cytokines in cancer
pathogenesis and cancer therapy. Nat Rev Cancer. 4:11–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeon M, Han J, Nam SJ, Lee JE and Kim S:
Elevated IL-1β expression induces invasiveness of triple negative
breast cancer cells and is suppressed by zerumbone. Chem Biol
Interact. 258:126–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao N, Lu Z, Zhang Y, Wang M, Li W, Hu Z,
Wang S and Lin Y: Interleukin-8 upregulates integrin β3 expression
and promotes estrogen receptor-negative breast cancer cell invasion
by activating the PI3K/Akt/NF-κB pathway. Cancer Lett. 364:165–172.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han J, Bae SY, Oh SJ, Lee J, Lee JH, Lee
HC, Lee SK, Kil WH, Kim SW, Nam SJ, et al: Zerumbone suppresses
IL-1β-induced cell migration and invasion by inhibiting IL-8 and
MMP-3 expression in human triple-negative breast cancer cells.
Phytother Res. 28:1654–1660. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lewis AM, Varghese S, Xu H and Alexander
HR: Interleukin-1 and cancer progression: The emerging role of
interleukin-1 receptor antagonist as a novel therapeutic agent in
cancer treatment. J Transl Med. 4:482006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tulotta C, Lefley DV, Freeman K, Gregory
WM, Hanby AM, Heath PR, Nutter F, Wilkinson JM, Spicer-Hadlington
AR, Liu X, et al: Endogenous production of IL-1Β by breast cancer
cells drives metastasis and colonization of the bone
microenvironment. Clin Cancer Res. 25:2769–2782. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Todorović-Rakovic N and Milovanović J:
Interleukin-8 in breast cancer progression. J Interferon Cytokine
Res. 33:563–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim S, Lee J, Jeon M, Lee JE and Nam SJ:
MEK-dependent IL-8 induction regulates the invasiveness of
triple-negative breast cancer cells. Tumour Biol. 37:4991–4999.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim S, You D, Jeong Y, Yoon SY, Kim SA,
Kim SW, Nam SJ and Lee JE: WNT5A augments cell invasiveness by
inducing CXCL8 in HER2-positive breast cancer cells. Cytokine.
135:1552132020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim S, You D, Jeong Y, Yu J, Kim SW, Nam
SJ and Lee JE: Berberine down-regulates IL-8 expression through
inhibition of the EGFR/MEK/ERK pathway in triple-negative breast
cancer cells. Phytomedicine. 50:43–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dmitrieva OS, Shilovskiy IP, Khaitov MR
and Grivennikov SI: Interleukins 1 and 6 as main mediators of
inflammation and cancer. Biochemistry (Mosc). 81:80–90. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pantschenko AG, Pushkar I, Anderson KH,
Wang Y, Miller LJ, Kurtzman SH, Barrows G and Kreutzer DL: The
interleukin-1 family of cytokines and receptors in human breast
cancer: Implications for tumor progression. Int J Oncol.
23:269–284. 2003.PubMed/NCBI
|
|
25
|
Miller LJ, Kurtzman SH, Anderson K, Wang
Y, Stankus M, Renna M, Lindquist R, Barrows G and Kreutzer DL:
Interleukin-1 family expression in human breast cancer:
Interleukin-1 receptor antagonist. Cancer Invest. 18:293–302. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elaraj DM, Weinreich DM, Varghese S,
Puhlmann M, Hewitt SM, Carroll NM, Feldman ED, Turner EM and
Alexander HR: The role of interleukin 1 in growth and metastasis of
human cancer xenografts. Clin Cancer Res. 12:1088–1096. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A,
Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, et al:
Expression of interleukin-1beta in human breast carcinoma. Cancer.
80:421–434. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma L, Lan F, Zheng Z, Xie F, Wang L, Liu
W, Han J, Zheng F, Xie Y and Huang Q: Epidermal growth factor (EGF)
and interleukin (IL)-1β synergistically promote ERK1/2-mediated
invasive breast ductal cancer cell migration and invasion. Mol
Cancer. 11:792012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshimura H, Nakahama K, Safronova O,
Tanaka N, Muneta T and Morita I: Transforming growth factor-beta
stimulates IL-1beta-induced monocyte chemoattractant protein-1
expression in human synovial cells via the ERK/AP-1 pathway.
Inflamm Res. 55:543–549. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hou Z, Falcone DJ, Subbaramaiah K and
Dannenberg AJ: Macrophages induce COX-2 expression in breast cancer
cells: Role of IL-1β autoamplification. Carcinogenesis. 32:695–702.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Collins TS, Lee LF and Ting JP: Paclitaxel
up-regulates interleukin-8 synthesis in human lung carcinoma
through an NF-kappaB- and AP-1-dependent mechanism. Cancer Immunol
Immunother. 49:78–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Westermarck J and Kahari VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jackson BC, Nebert DW and Vasiliou V:
Update of human and mouse matrix metalloproteinase families. Hum
Genomics. 4:194–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kannaiyan R, Hay HS, Rajendran P, Li F,
Shanmugam MK, Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, et
al: Celastrol inhibits proliferation and induces chemosensitization
through down-regulation of NF-κB and STAT3 regulated gene products
in multiple myeloma cells. Br J Pharmacol. 164:1506–1521. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JH, Won YS, Park KH, Lee MK, Tachibana
H, Yamada K and Seo KI: Celastrol inhibits growth and induces
apoptotic cell death in melanoma cells via the activation
ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT
signaling. Apoptosis. 17:1275–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mou H, Zheng Y, Zhao P, Bao H, Fang W and
Xu N: Celastrol induces apoptosis in non-small-cell lung cancer
A549 cells through activation of mitochondria- and
Fas/FasL-mediated pathways. Toxicol In Vitro. 25:1027–1032. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li H, Yuan Y, Zhang Y, He Q, Xu R, Ge F
and Wu C: Celastrol inhibits IL-1β-induced inflammation in orbital
fibroblasts through the suppression of NF-κB activity. Mol Med Rep.
14:2799–2806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang J, Zhou K, Zhang X, Zhou Y, Li Z and
Shang F: Celastrol ameliorates inflammation in human retinal
pigment epithelial cells by suppressing NF-κB signaling. J Ocul
Pharmacol Ther. 35:116–123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim Y, Kang H, Jang SW and Ko J: Celastrol
inhibits breast cancer cell invasion via suppression of
NF-kB-mediated matrix metalloproteinase-9 expression. Cell Physiol
Biochem. 28:175–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park S, Jung HH, Park YH, Ahn JS and Im
YH: ERK/MAPK pathways play critical roles in EGFR ligands-induced
MMP1 expression. Biochem Biophys Res Commun. 407:680–686. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hashimoto K, Otero M, Imagawa K, de Andrés
MC, Coico JM, Roach HI, Oreffo RO, Marcu KB and Goldring MB:
Regulated transcription of human matrix metalloproteinase 13
(MMP13) and interleukin-1β (IL-1Β) genes in chondrocytes depends on
methylation of specific proximal promoter CpG sites. J Biol Chem.
288:10061–10072. 2013. View Article : Google Scholar : PubMed/NCBI
|