Introduction
Pancreatic cancer (PC) is one of the most aggressive
types of cancer and its prognosis is particularly poor, among
gastrointestinal cancers (1). The
main reasons for this are late diagnosis and early recurrence, even
after curative resection (2,3). Our previous research demonstrated that,
among several types of recurrence, prognosis after peritoneal
recurrence and liver metastasis was markedly poor compared with
other types of recurrence (3).
Previous studies have also revealed that these malignant
recurrences may be predicted by measuring the glucose activity of
cancer cells via the maximum standardized uptake values (SUVmax)
obtained from 18-fluorodeoxyglucose positron emission
tomography/computed tomography (18F-FDG PET/CT) (4,5). The data
suggest that high glucose uptake promotes metastasis of PC cells,
causing poor prognosis. However, the detailed mechanism of
metastasis remains unclear.
Stomatin-like protein 2 (SLP-2) is mainly located in
the mitochondrial inner membrane (6,7). It has
been reported to play important roles in regulating mitochondrial
membrane stability (7), the formation
of mitochondrial respiratory chain super-complexes (8), and in modulating mitochondrial
sodium-calcium exchange (9). SLP-2 is
also required for stress-induced mitochondrial hyperfusion (SIMH)
and its expression is upregulated under conditions of mitochondrial
stress (10). This stress condition
stimulates mitochondrial biogenesis and function (11), providing the energetic requirements of
activation.
Several previous studies have demonstrated that
increased SLP-2 expression induces poor prognosis (12–22). An
increasing number of studies have revealed that SLP-2 is implicated
in tumor progression and development. The depletion of SLP-2 has
been revealed to inhibit the capability of cells to proliferate in
colorectal cancer and esophageal squamous cell carcinoma (15,23).
Migration and invasion activities have also been revealed to be
inhibited after SLP-2 suppression in glioma and liver cancer
(18,24). Furthermore, SLP-2 inhibited
chemotherapy-induced apoptosis in cervical cancer and in head and
neck squamous cell carcinoma (22,25). These
findings indicated that the role and the mechanism of SLP-2 in
inducing poor prognosis differs according to the origin of the
cancer.
It was previously reported by our research group
that SLP-2 is a novel prognostic biomarker of PC, based on the
results of proteomic analysis (26).
However, the function and molecular mechanism of SLP-2 in PC had
not been thoroughly explored to date, and no other correlation
between SLP-2 and PC had been reported.
The aim of the present study was to explore the
function of SLP-2 in PC, using in vitro and in vivo
assays. The level of SLP-2 expression at metastatic sites compared
to that at primary sites was analyzed, to evaluate its metastatic
potential.
Materials and methods
Antibodies, reagents, and cell
lines
Antibodies against SLP-2 were purchased from
ProteinTech Group, Inc. (cat. no. 10348-1-AP); GAPDH from Cell
Signaling Technology, Inc. (product no. 2118S); GFPT2 from Abcam
(product code ab190966); and anti-rabbit IgG as a secondary
antibody (cat. no. A0545) was obtained from Sigma-Aldrich; Merck
KGaA. The cell lines AsPC-1, BxPC-3, SUIT-2, and SW1990 were
purchased from the American Type Culture Collection. The cell line
PANC-1 was obtained from RIKEN and MIA-PaCa2 was provided from Cell
Resource Center for Biomedical Research, Institute of Development,
Aging and Center, Tohoku University (Sendai, Japan). Cells were
expanded within 3 passages after being purchased, and multiple lots
were stocked at −80°C. Mycoplasma contamination check tests were
performed using e-Myco plus Mycoplasma PCR Detection Kit (iNtRON
Biotechnology, Inc.). Cells were used at least <20 passages, but
were not independently authenticated.
Transfection with short hairpin
(sh)RNA
The sequences of SLP-2 shRNAs are presented in
Table SI. The shRNAs were inserted
into pBAsi-hU6 NEO plasmids (cat. no. 3227; Takara Bio, Inc.),
which carry a neomycin resistance gene. Lipofectamine 2000 reagent
(cat. no. 11668019; Thermo Fisher Scientific, Inc.) was used for
plasmid transfections into PANC-1 and AsPC-1, according to the
manufacturer's protocol. In short, 20 µg plasmids with 2 ml
Opti-MEM (cat. no. 31985070; Thermo Fisher Scientific, Inc.) and 30
µl Lipofectamine 2000 with Opti-MEM were produced. Then, these
mediums were combined at room temperature for 20 min. After
mixture, this medium was placed in a 10-mm dish and cells were
cultured at 37°C for 12 h. After exposure, the medium was changed
to RPMI-1640 medium supplemented with 1,000 mg/ml
Geneticin® (cat. no. 10131-027; Thermo Fisher
Scientific, Inc.). The blank pBAsi-hU6 NEO plasmid was also
transfected as a negative control. Transfected clones, on RPMI-1640
medium supplemented with 1,000 mg/ml Geneticin®, were
selected over a period of 3 weeks, after which a single colony was
selected and cultured.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cancer cells using the
Nucleospin RNA kit (cat. no. 740955; Takara Bio, Inc.). Reverse
transcription reactions were set up using PrimeScript RT Master Mix
(cat. no. RR036A; Takara Bio, Inc.), according to the
manufacturer's protocol under the following thermocycling
conditions: 37°C for 15 min, followed by 85°C for 5 sec, and the
products were used as templates for RT-qPCR. The gene products were
amplified using TB Green Premix Ex Taq II, ROX Plus (cat. no.
RR82LR; Takara Bio, Inc.) under the following thermocycling
conditions: 95°C for 30 sec, followed by 40 cycles at 95°C for 5
sec for denaturation and 60°C for 30 sec for annealing/extension.
The expression levels of each target gene were calculated using the
2−ΔΔCq method (27).
Relative quantities were calculated after normalizing for GAPDH
expression. The primers used in the present study are presented in
Table SI.
RNA preparation and microarray
analyses
RNA isolates were obtained from AsPC-1cont,
AsPC-1sh1, and AsPC-1sh2 cells using the Nucleospin RNA kit and
assessed for quality using a NanoDrop spectrophotometer (Thermo
Fisher Scientific, Inc.). The GeneChip™ WT PLUS Reagent Kit (cat.
no. 902281; Thermo Fisher Scientific, Inc.) was used to prepare the
RNA samples; for whole-transcriptome expression analyses, the
Clariom™ S assay (cat. no. 902926; Thermo Fisher Scientific, Inc.)
was used. The GeneChip was analyzed using a GeneChip™ Scanner 3000
7G system, while the gene expression was analyzed using a
GeneTitan™ instrument (both from Thermo Fisher Scientific, Inc.).
The transcriptomic array data set was analyzed using the
Transcriptome Analysis Console software Ver 4.0 (Thermo Fisher
Scientific, Inc.). Genes with fold-changes of <-20 or >20 and
with a P-value <0.05 in comparisons between AsPC-1cont and
AsPC-1sh1 or AsPC-1sh2 were selected as candidate genes associated
with SLP-2 expression. The data of microarray analysis are
available in Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE162981.
Western blotting
Cells were lysed with RIPA buffer (Thermo Fisher
Scientific, Inc.), and the concentrations of protein samples were
evaluated using a BCA kit (Thermo Fisher Scientific, Inc.). A total
of 20 µg of proteins were separated on SDS-PAGE gels (4-15%) and
subsequently transferred onto PVDF membranes (Bio-Rad Laboratories,
Inc.). The membranes were then blocked at room temperature for 1 h
using Western Blocking Reagent (Thermo Fisher Scientific, Inc.) and
incubated with primary antibodies (SLP-2 and GFPT-2) at room
temperature for 1 h at dilutions of 1:2,000. PVDF membranes were
then incubated with secondary antibodies at room temperature for 30
min (HRP-conjugated) at dilutions of 1:5,000, and signals were
detected using the Clarity ECL Western Substrate (cat. no. 1705062;
Bio-Rad Laboratories, Inc.). Protein bands were visualized using an
ImageQuant LAS 4000 mini system (GE Healthcare; Cytiva).
Cell proliferation assay
A total concentration of 5×103 cells was
seeded in 96-well plates and cultured with 100 µl of RPMI-1640
medium with 10% FBS, 1% penicillin, and 1,000 mg/ml geneticin. The
MTS assays were performed using CellTiter 96-well assay reagent
(cat. no. G358B; Promega Corporation), according to the
manufacturer's recommendations. The absorbance was measured at a
wavelength of 490 nM. The experiment was performed in
triplicate.
Wound-healing assay
Cells grown on petri dishes were starved in
serum-free medium for 24 h and then scratched with the tip of a
sterile 10-µl pipette. Following this, the cultured cells were
rinsed and incubated with RPMI-1640 medium containing 10% FBS, 1%
penicillin, and 1,000 mg/ml geneticin. After 12 h, the
wound-closure distances were measured at three independent wound
sites per group using a light microscope, and the average was
calculated. The data are expressed as a relative index considering
the differences in wound length at the initial time-point. The
assays were performed in triplicate.
Transwell cell migration and invasion
assay
Transwell assays were performed using the QCM
24-well Fluorometric Cell Migration Assay kit (cat. no. ECM509; EMD
Millipore) for the migration assay and the QCM 24-well Cell
Invasion Assay kit using Matrigel®-coated Transwell
chambers (cat. no. ECM554; EMD Millipore) for the invasion assay.
The cells were pretreated with serum-free medium for 24 h, and then
harvested in a serum-free medium. Then, 300-µl cell samples were
transferred to the upper chambers of the kit (1×106/ml),
and 500 µl of medium containing 10% FBS for the migration assay and
20% FBS for the invasion assay was added to the lower chamber.
After incubation for 24 h, the cells were dislodged using 225 µl of
cell detachment solution from the underside of the upper chamber
for 30 min at 37°C. Thereafter, the samples were stained with
sufficient lysis buffer/dye solution for 15 min at 20–25°C. The
results were quantified using a fluorescence plate reader fitted
with a 480/520 nm filter. The data are expressed using a relative
index, by setting the migrated or invasive control cells to 100%.
This assay was performed in triplicate.
Cytotoxicity assay
Cells were plated at 5×103 cells/well in
96-well plates. After 24 h, the medium was replaced by another
containing from 1×10−4 to 1×103 µM
gemcitabine hydrochloride (FUJIFILM WAKO Pure Chemical
Corporation). Cells were cultured for 120 h and then cell viability
was measured by MTS assays using the CellTiter 96-well assay
reagent (Promega Corporation), as recommended by the manufacturer.
The absorbance was measured at a wavelength of 490 nM. Each test
was carried out in triplicate.
Glucose uptake assay
The Glucose Uptake-Glo Assay (cat. no. J1342;
Promega Corporation) was applied to cells grown in 96-well plates.
Glucose-free media were used throughout the steps of this assay. A
total of 50 µl of 1 mM 2-deoxyglucose was added to each well to
initiate the assay. The uptake reaction was stopped according to
the manufacturer's protocol, after 2 h of incubation. The
luminescence was recorded using 1-sec integration on a luminometer.
These experiments were performed in triplicate.
In vivo experiments
The Institutional Animal Experiment Committee of
Tohoku University (Sendai, Japan) approved the present study
protocol on September 20, 2017. AsPC-1 stable cells (blank or
shRNA-transfected) were harvested from 80% confluent culture
dishes, resuspended in PBS, and maintained on ice. Six-week-old
SCID mice (C.B-17/Icr-scid/scid Jcl; male; weight, 23 g)
were used for this experiment. A triple-mixed anesthetic was
prepared, consisting of medetomidine (1 mg/ml), midazolam (5
mg/ml), and butorphanol (5 mg/ml); this solution was injected
intraperitoneally into the mice (0.1 ml per 10 g body weight per
mouse). An incision was made to exteriorize the spleens, to inject
5×105 cells per 100 µl slowly into them. A cotton swab
was held over the injection site for at least 5 min to avoid
leakage and bleeding. After confirming that the bleeding had
stopped, the spleens were returned into the peritoneal cavities,
and the abdominal wounds were closed. Six weeks after the
injection, the mice were sacrificed using 60 µl of pentobarbital
(50 mg/ml), and their livers were removed. The number of metastatic
sites on the liver surface was counted. Three biological replicates
were used for each experiment.
Immunohistochemistry
All specimens for immunohistochemistry were fixed
for 24 h in 10% formalin and embedded in paraffin wax. Two
specialists from the Department of Pathology of Tohoku University
performed the SLP-2 immunostaining procedures and scored the
immunoreactivity. The staining intensity combined with the positive
cell percentages were used to obtain a semiquantitative analysis of
immunoreactivity. The staining intensity was scored as 0
(negative), 1 (weak), 2 (moderate), or 3 (strong) (Fig. S1); the positive cell percentage was
scored as 0 (0%), 1 (<10%), 2 (10-50%), 3 (51-80%), or 4
(>80%). The staining intensity and positive cell percentages
were multiplied to evaluate the immunoreactive scores (IRS), which
ranged from 0 to 12.
Clinical data concerning 279 patients (166 male and
113 female patients; aged 27–88 years old; median age, 67 years)
with pancreatic ductal adenocarcinoma, who underwent surgical
resection between January 1, 2006 and December 31, 2014, were
obtained from records at our institute on January 1, 2020. The
study included resectable to unresectable PDAC, NAC and non-NAC as
well as R0 to R2 patients. Radiographic resection status was
defined by the National Comprehensive Cancer Network guidelines for
pancreatic cancer, Version 1 (2020) (28) and pathological status was diagnosed by
the Union for International Cancer Control TNM classification (7th
edition) (29). The Institutional
Review Board of Tohoku University (Sendai, Japan) approved the
present study design on May 25, 2016 (2016-1-151). As this was a
retrospective study, the requirement for informed consent was
waived and an opt-out method was used instead. The present study
was conducted in accordance with the STROBE guidelines (www.strobe-statement.org) (30).
Statistical analysis
Statistical analysis was conducted on the JMP pro
software v14 (SAS Institute). Data are expressed as the mean ±
standard deviation (SD). Student's t-test was used to compare the
means of two groups, while ANOVA followed by Dunnett's post hoc
test were used to compare the means of multiple experimental
groups. The binomial variables of clinicopathological factors were
compared using Pearson's chi-square test. P-values <0.05 were
considered to indicate a statistically significant difference.
Results
Cloning a stable SLP-2 silencing cell
line
SLP-2 expression was evaluated thrice in six types
of PC cell lines (PANC-1, MIA-PaCa2, AsPC-1, BXPC-3, SUIT-2, and
SW1990) (Fig. 1A). SLP-2 expression
in AsPC-1 and PANC-1 cells was relatively high compared with that
in other cell lines; hence, these cell lines were selected for
further analysis.
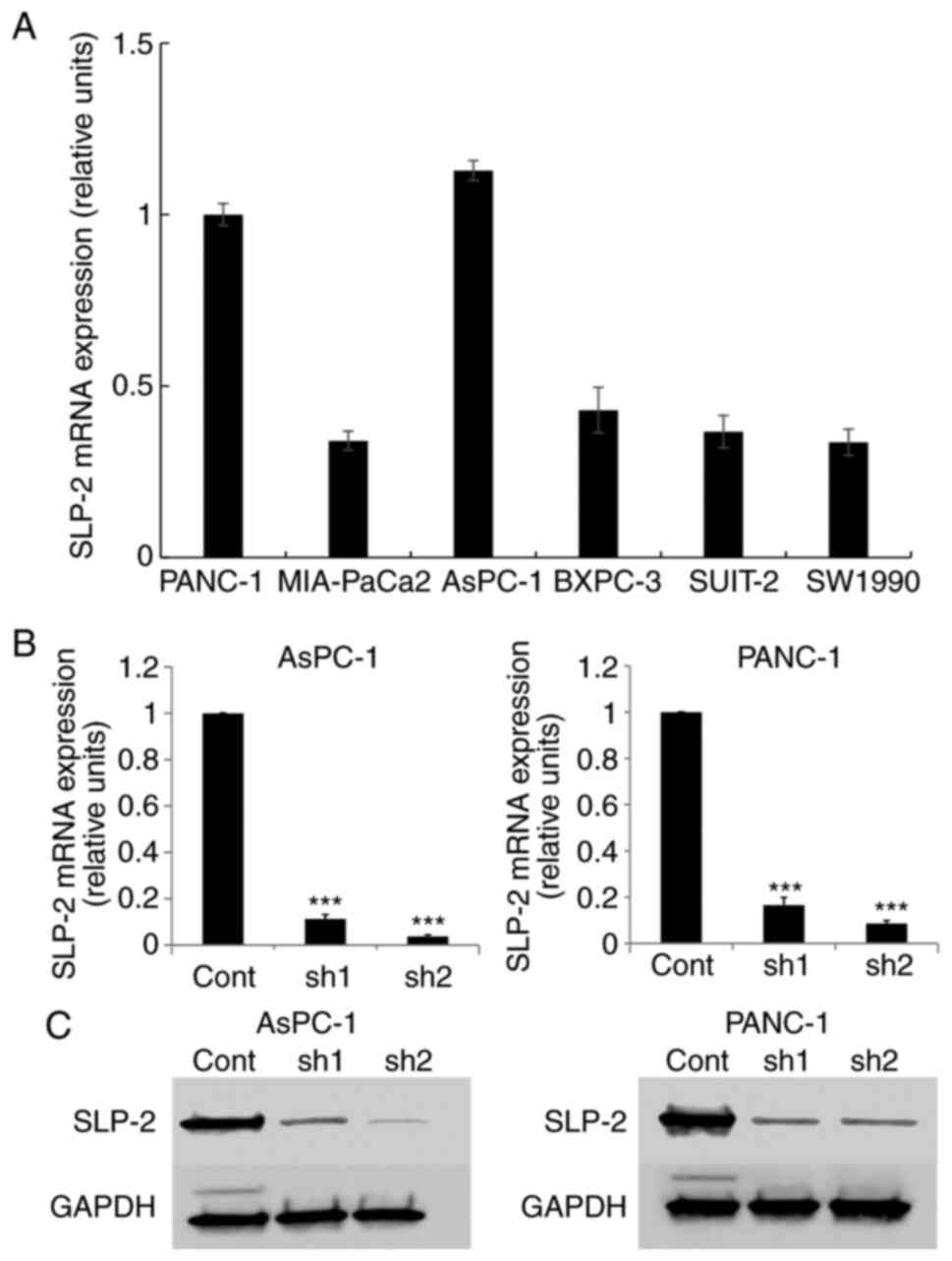 | Figure 1.SLP-2 expression in PC cells. (A)
SLP-2 expression in PANC-1, MIA-PaCa2, AsPC-1, BXPC-3, SUIT-2, and
SW1990 cells were evaluated by RT-qPCR. Relative expression level
was calculated after normalizing to GAPDH expression. (B and C)
SLP-2 expression suppressed by shRNA transfection in both AsPC-1
and PANC-1 cells. (B) RT-qPCR and (C) western blotting. GAPDH was
an internal control. RT-qPCR and western blotting were performed
thrice. ***P<0.001. SLP-2, stomatin-like protein 2; PC,
pancreatic cancer; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; cont, control; sh, short hairpin. |
The designed shRNA was inserted into the pBAsi-hU6
NEO plasmid and transfected into AsPC-1 and PANC-1 cells. By
RT-qPCR (Fig. 1B) and western
blotting (Figs. 1C and S2A), it was revealed that SLP-2 expression
was significantly decreased in shRNA-transfected cells.
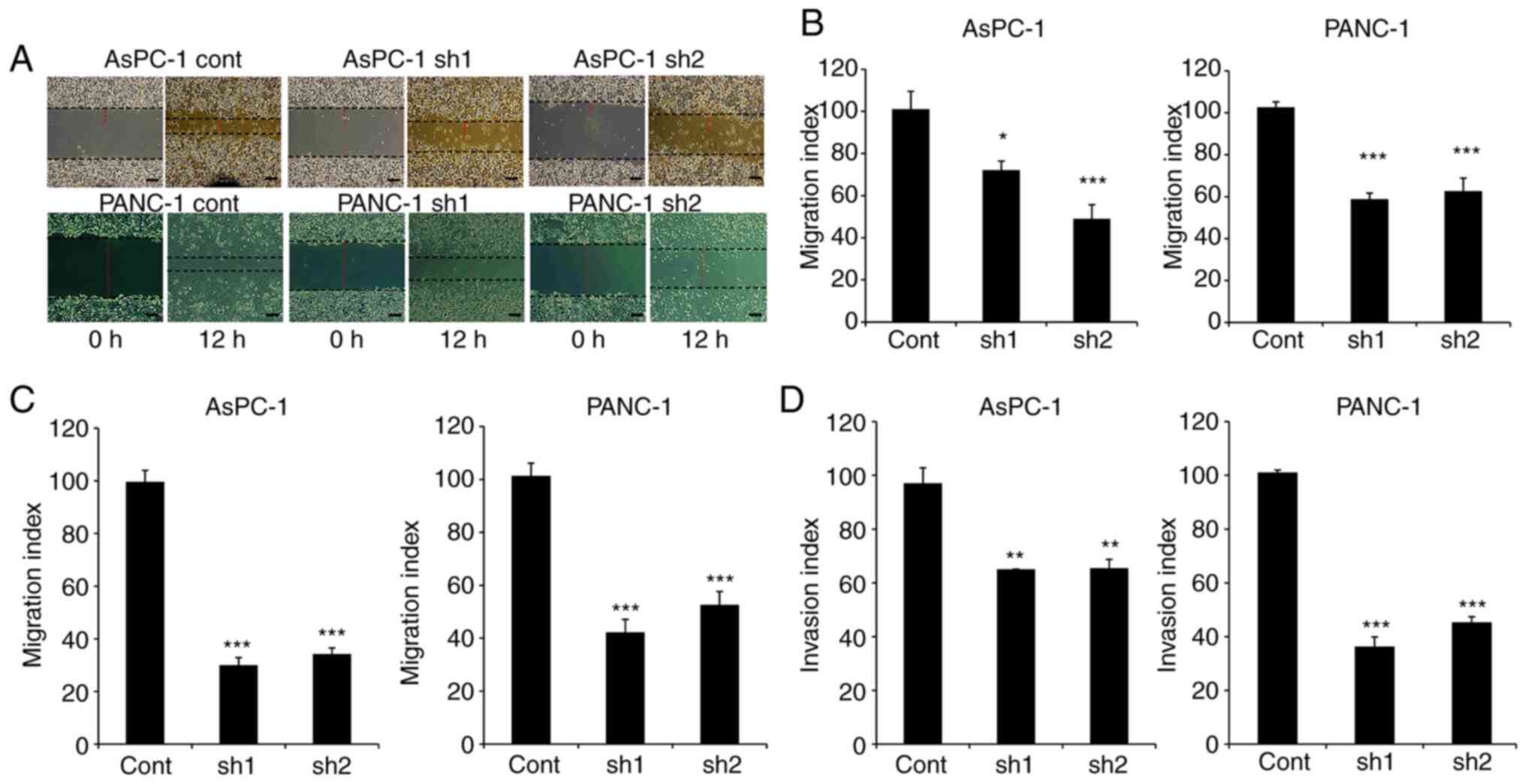
SLP-2 silencing reduces cell migration
and invasion abilities
To evaluate the effect of SLP-2 expression on cell
motility, in vitro wound-healing assays were performed
(Fig. 2A and B). The results revealed
that wound widths were decreased in AsPC-1sh1 (P=0.017) and
AsPC-1sh2 (P<0.001) compared with the widths observed in
AsPC-1cont cells. Similar results were also revealed in PANC-1sh1
(P<0.001) and PANC-1sh2 (P<0.001) cells. In addition,
Transwell cell migration assays also demonstrated that the
migration abilities of AsPC-1sh1 and AsPC-1sh2 cells were decreased
to 30.0% (P<0.001) and 34.2% (P<0.001), respectively, in
comparison with the corresponding migration ability of AsPC-1cont
cells (Fig. 2C). The same results
were also demonstrated in PANC-1 cells, with migration abilities
decreased to 41.6% (P<0.001) in PANC-1sh1 and 51.8% (P<0.001)
in PANC-1sh2 cells. Furthermore, the results of invasion assay
using Matrigel®-coated Transwell chambers revealed that
the invasive activity of AsPC-1sh1 and AsPC-1sh2 cells was
decreased to 67.2% (P=0.002) and 67.9% (P=0.002), respectively,
compared to the corresponding activity level in control cells
(Fig. 2D). The results in PANC-1
cells also confirmed a decrease in activity to 35.8% (P<0.001)
in PANC-1sh1 cells and 44.7% (P<0.001) in PANC-1sh2 cells.
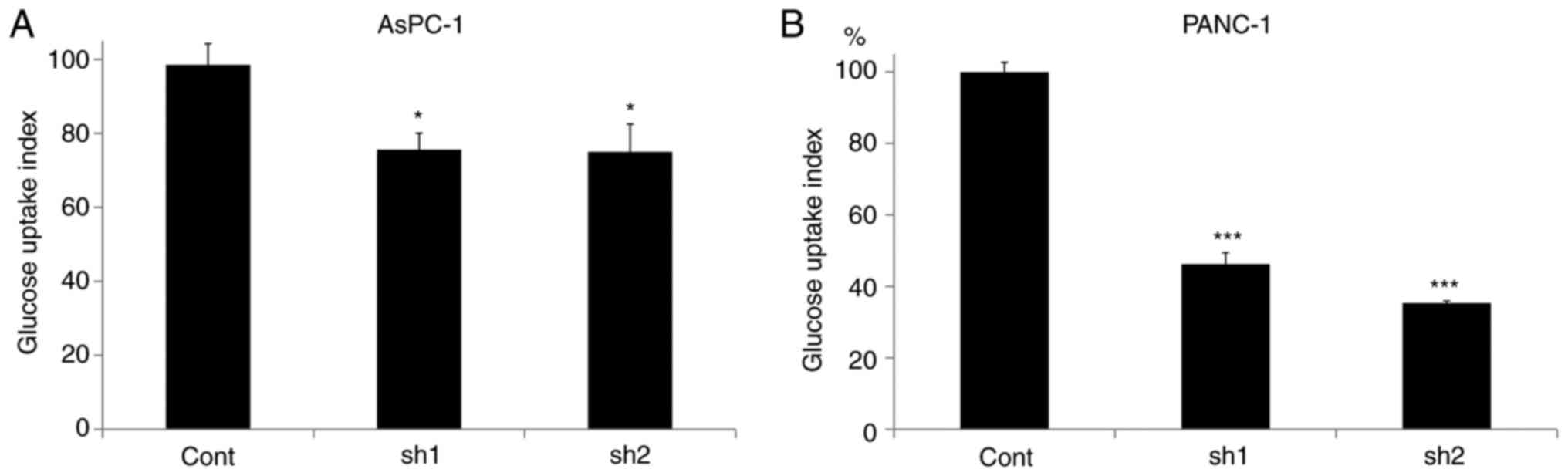
Inhibition of SLP-2 expression reduces
the glucose uptake in PC cells
Glucose uptake was evaluated in different
SLP-2-expressing PC cells. As revealed in Fig. 3A, compared to AsPC-1cont cells,
glucose uptake was significantly decreased to 75.6% in AsPC-1sh1
(P=0.042) and 75.0% in AsPC-1sh2 cells (P=0.038). The same was
observed in PANC-1 cells; it was decreased to 46.3% in PANC-1sh1
(P<0.001) and to 35.3% in PANC-1sh2 cells (P<0.001) (Fig. 3B).
SLP-2 does not affect cell
proliferation and chemosensitivity to gemcitabine
An MTS assay demonstrated that the cell growth
curves were almost identical regardless of the expression of SLP-2
in both AsPC-1 and PANC-1 cells. Additionally, no significant
differences were found in the four-day growth rate of cells among
AsPC-1cont, AsPC-1sh1 and AsPC-1sh2 cells (P=0.944) (Fig. S3A). Similar results were revealed
among PANC-1cont cells, PANC-1sh1and PANC-1sh2 cells (P=0.532)
(Fig. S3B).
To investigate whether SLP-2 affects the
chemosensitivity of PC, cells were treated with different doses of
gemcitabine. The present findings revealed no significant
differences in the IC50 values of AsPC-1cont (0.069 µM),
AsPC-1sh1 (0.066 µM), and AsPC-1sh2 cells (0.090 µM) (P=0.713)
(Fig. S4A). No significant
differences were also observed in the IC50 values of
PANC-1cont cells (0.053 µM), PANC-1sh1 (0.053 µM), and PANC-1sh2
cells (0.053 µM) (P=0.989) (Fig.
S4B).
SLP-2 expression was significantly
positively correlated with liver metastasis in PC
To investigate the role of SLP-2 during liver
metastasis, different SLP-2-expressing PC cells were injected into
the spleen of SCID mice. A previous study revealed that only AsPC-1
could cause liver metastasis in SCID mice, among several PC cell
lines such as PANC-1, MIA PaCa-2, AsPC-1, and BxPC-3 (31). In agreement with the previous study,
PANC-1 cells did not cause liver metastasis, even in control cells
(data not shown). Therefore, AsPC-1 cells were used to evaluate the
potential of SLP-2 to cause liver metastasis. Six weeks after the
injections, the mean number of liver metastases was significantly
decreased from 22 in the AsPC-1cont cells, to 2.3 (P<0.001) in
AsPC-1sh1, and 1.6 in AsPC-1sh2 (P<0.001) (Fig. 4A and B).
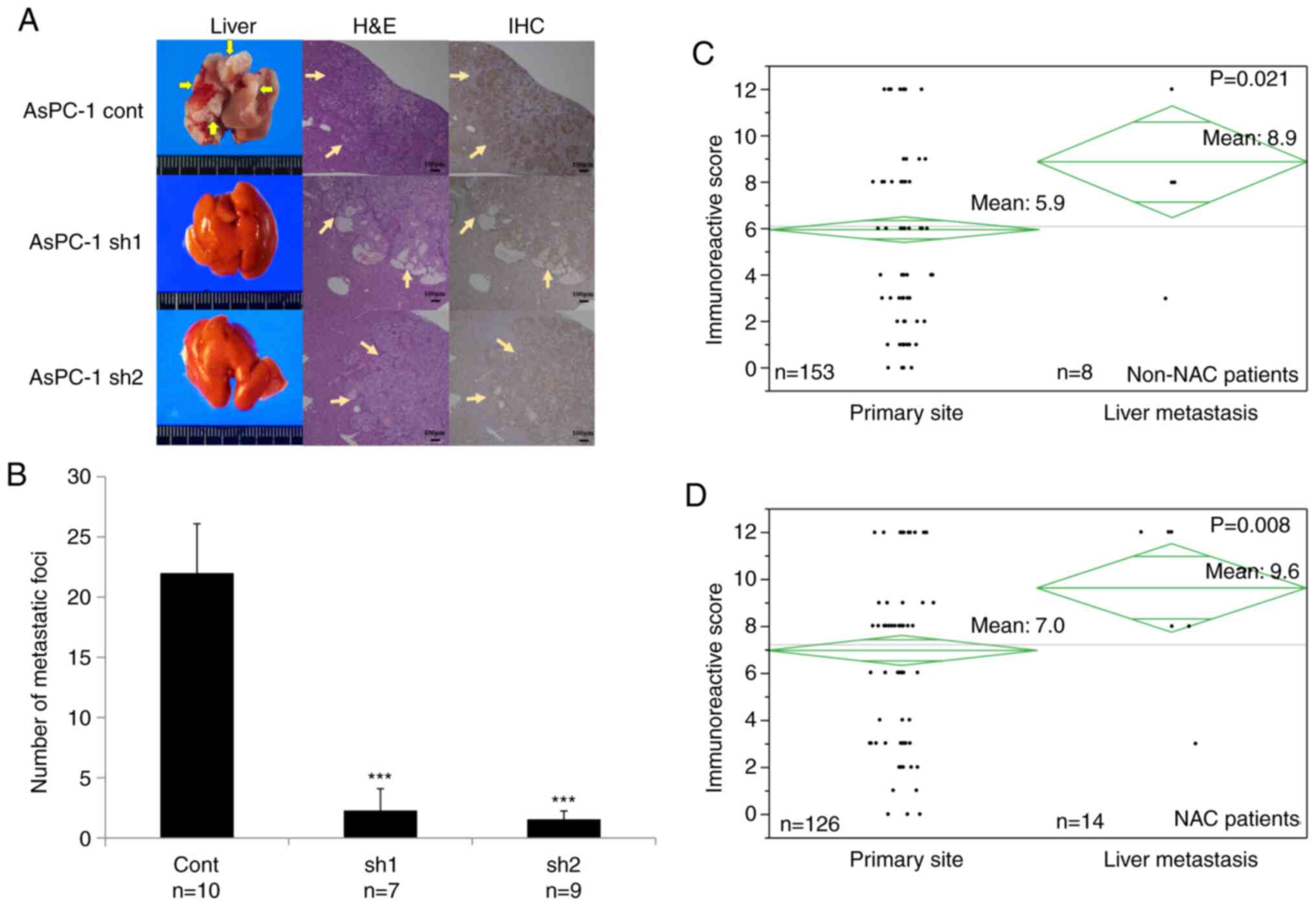 | Figure 4.SLP-2 expression promotes liver
metastasis in PC. (A and B) Stable cells of AsPC-1 were injected
into the spleen of mice, and, after 6 weeks, the number of
metastatic foci on the liver surface were counted. (A) Images
revealing liver metastasis, hematoxylin and eosin staining, and
immunohistochemical analysis of SLP2. Arrows indicate the
metastatic tumor. (B) Values are represented by the mean ± SD.
***P<0.001. The biological replicate number was 10 for
AsPC-1cont cells, 7 for AsPC-1sh1 cells, and 9 for AsPC-1sh2 cells.
(C and D) SLP-2 expression level was compared between the primary
site and the liver metastatic site using the Immunoreactive Score.
(C) SLP-2 expression was compared in the patients without
neoadjuvant chemotherapy. The mean score of the liver metastatic
site (n=8) was significantly higher than that of the primary site
(n=153) (8.9 vs. 5.9; P=0.021). (D) SLP-2 expression was
compared in the patients undergoing neoadjuvant chemotherapy. The
mean score of the liver metastatic site (n=14) was significantly
higher than that of the primary site (n=126) (9.6 vs. 7.0;
P=0.008). SLP-2, stomatin-like protein 2; PC, pancreatic
cancer; cont, control; sh, short hairpin; H&E, hematoxylin and
eosin staining; IHC, immunohistochemical analysis; NAC, neoadjuvant
chemotherapy. |
To validate the results of the in vivo
analysis, SLP-2 expression level was measured from the primary
sites and liver metastatic sites in PC tissue samples from
patients, using immunohistochemistry. First, the SLP-2 expression
of the two tissues from the group without neoadjuvant chemotherapy
(non-NAC) was compared. The IRS was significantly increased in the
metastatic site compared with that at the primary site (8.9 vs.
5.9; P=0.021) (Fig. 4C). Next, the
IRS of the two sites was compared in patients after neoadjuvant
chemotherapy (the NAC group). Similarly, the IRS was significantly
increased at the metastatic site compared with that at the primary
site (9.6 vs. 7.0; P=0.008) (Fig.
4D).
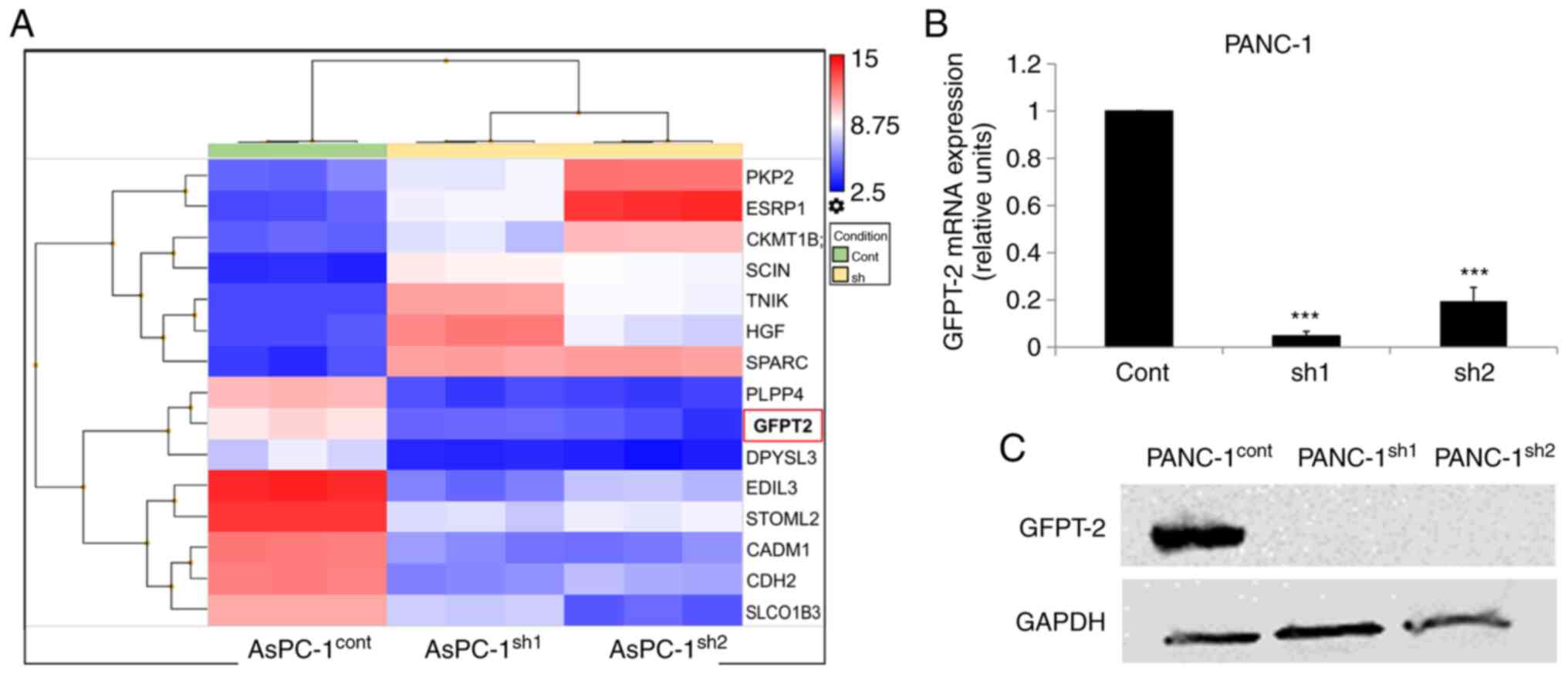
SLP-2 expression is associated with
the expression of GFPT2
To define the SLP-2-associating factor in PC cells,
a microarray analysis was performed using AsPC-1cont cells and
AsPC-1sh1 or AsPC-1sh2 cells. The data of microarray analysis are
available in Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE162981.
It was determined that 8 candidate genes were overexpressed by
>20-fold and 7 genes were downregulated by >20-fold in
AsPC-1cont cells compared with the corresponding expression levels
of the genes in SLP-2-suppressed cells (Fig. 5A). In the present study, the
expression of glutamine-fructose-6-phosphate transaminase 2 (GFPT2)
as a new candidate gene associated with SLP-2 expression was
evaluated; this is because, from the literature (32–34), it
appears that only GFPT2 could regulate cell motility and glucose
uptake activity. Expression of the GFPT2 gene was confirmed in
PANC-1 cells using RT-qPCR and western blotting and was revealed to
be downregulated in SLP-2-suppressed cells (Figs. 5B and C, and S2B).
SLP-2 expression is increased after
NAC
Table I reveals the
clinicopathological characteristics of both the non-NAC and NAC
groups. The distribution between the two groups revealed a
significant difference in resectability status. This is because
borderline resectable or unresectable cases usually underwent
preoperative treatment before surgical resection. To evaluate
whether SLP-2 expression differed according to tumor progression,
SLP-2 expression was analyzed for each resectability status. For
patients in the non-NAC group, the mean IRS did not exhibit
significant differences among the three groups (R vs. BR vs. UR,
6.1 vs. 5.8 vs. 6.0; P=0.839) (Fig.
S5A). The same results were also revealed in the NAC group (R
vs. BR vs. UR, 6.3 vs. 7.1 vs. 7.3; P=0.534) (Fig. S5B). These results demonstrated that
SLP-2 expression did not differ according to the resectability
status. However, when the SLP-2 expression profile of the non-NAC
group was compared with that of the NAC group, the IRS was
significantly higher in the NAC group (5.9 vs. 7.0; P=0.019)
(Fig. S5C). All these results
demonstrated that NAC itself may increase SLP-2 expression.
 | Table I.Distribution between non-NAC and NAC
patients. |
Table I.
Distribution between non-NAC and NAC
patients.
|
|
|
| Distribution
between non-NAC and NAC patients |
|---|
|
|
|
|
|
|---|
|
Clinical/pathological variables |
| Total (n=279) | non-NAC
(n=153) | NAC (n=126) | P-value |
|---|
| Resectability | Resectable | 117 | 88 | 29 | <0.001 |
|
| Borderline | 138 | 64 | 74 |
|
|
| Resectable |
|
|
|
|
|
| Unresectable | 24 | 1 | 23 |
|
| Pretreatment CA19-9
(U/ml) | >100 | 119 | 71 | 48 | 0.163 |
|
| ≤100 | 160 | 82 | 78 |
|
| Tumor position | ph | 195 | 102 | 93 | 0.196 |
|
| pbt | 84 | 51 | 33 |
|
| Tumor size
(mm) | >30 | 138 | 69 | 69 | 0.108 |
|
| ≤30 | 141 | 84 | 57 |
|
| UICC-T | 1,2 | 14 | 9 | 5 | 0.141 |
|
| 3 | 265 | 144 | 121 |
|
| UICC-N | 0 | 77 | 37 | 40 | 0.371 |
|
| 1 | 202 | 116 | 86 |
|
| UICC-M | 0 | 224 | 125 | 99 | 0.176 |
|
| 1 | 55 | 28 | 27 |
|
| Residual
cancer | R0 | 244 | 134 | 110 | 0.944 |
|
| R1,2 | 35 | 19 | 16 |
|
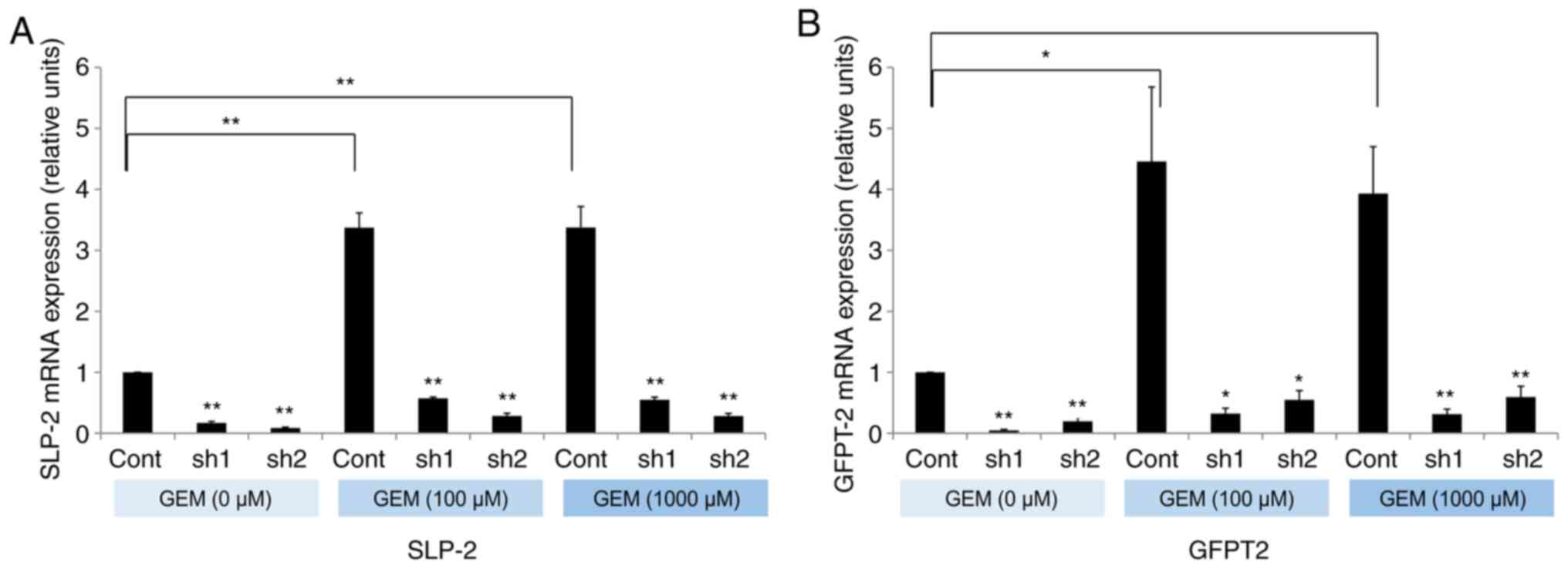
Gemcitabine exposure increases SLP-2
expression in PC cells
To evaluate the hypothesis that NAC itself promotes
the expression of SLP-2, its expression was evaluated after
exposure to gemcitabine. SLP-2 expression was significantly
increased in PANC-1 cells after exposure to 100 or 1,000 µM
gemcitabine (Fig. 6A). Next, the
effect of SLP-2 on GFPT2 expression was analyzed. GFPT2 expression
was also significantly enhanced after gemcitabine exposure, and
inhibition of SLP-2 suppressed the expression of GFPT2 (Fig. 6B). These results demonstrated that
gemcitabine promoted SLP-2 expression in PC cells, and subsequently
increased the expression of GFPT2. Suppression of SLP-2 could also
inhibit gemcitabine-induced GFPT2 expression.
Discussion
In the present study, with regard to PC, suppression
of SLP-2 did not alter the cell proliferation or chemosensitivity
to gemcitabine. Conversely, it reduced cell migration and invasion
activities. Suppression of SLP-2 significantly decreased the number
of liver metastases in the mouse xenograft model. Furthermore,
immunohistochemical results revealed that SLP-2 expression was
increased at the site of liver metastasis. This confirmed that
SLP-2 expression plays an important role in liver metastasis and
that increased SLP-2 expression is linked to liver metastasis in
PC, which may explain the poor prognosis.
SLP-2 has been revealed to regulate cell migration
and invasion activities in several types of cancer. Dowling et
al revealed that SLP-2 is one of the 16 most upregulated
proteins in extremely invasive cancer cells, implying that SLP-2
may be active during cancer metastasis (35). However, the signaling pathway
affecting cell migration and invasion activities differs according
to the type of cancer. In liver cancer, SLP-2 expression was
revealed to promote EMT progression (18). SLP-2 facilitated migration activity
via regulating the Wnt/β-catenin pathway in colorectal cancer
(15). In glioma and liver cancer,
SLP-2 was revealed to regulate cell motility via the NF-κB pathway,
targeting MMP2 or MMP9 (17,24). In the present study, to elucidate the
mechanism in PC cells, microarray analyses were performed using
SLP-2-suppressed PC cells. Among 15 candidate genes selected by the
expression level of SLP-2, only GFPT2 was associated with cell
death and glucose uptake activity. Therefore, GFPT2 was selected as
an optimal new candidate genes regulated by SLP-2 expression to
promote cell migration and invasion activities in PC cells.
GFPT2 is the first, and rate-limiting, enzyme of the
hexosamine biosynthesis pathway (HBP). HBP is a branch of glucose
metabolism that usually consumes approximately 2–5% of total
glucose (36). It has also been
revealed that GFPT2 is correlated with glucose uptake and is
associated with glucose-driven metabolic pathways (32). Thus, increased GFPT2 expression could
activate HBP by supplying the glucose itself and also by modulating
the glucose substrate to this pathway. The HBP contributes to the
provision of a substrate for glycosylation modification and has a
wide range of effects on cellular function (32). Uridine diphosphate N-acetylglucosamine
(UDP-GlcNAc), an end-product of the HBP, is catalyzed by
O-GlcNAc transferase to form O-GlcNAc, which binds to
serine/threonine residues of the target proteins in glycosylation
modification (33). Increased
O-GlcNAcylation can activate various cancer-related factors
such as β-catenin (33), p53
(37), or c-Myc (38). In particular, O-GlcNAcylation
of β-catenin enhances intranuclear β-catenin expression and
facilitates signal activation, including EMT promotion and cancer
invasion (33). Several previous
studies have also demonstrated that GFPT2 can regulate cell
migration and invasion activities by activating the EMT mechanism
(32,34). Therefore, high SLP-2 expression may
promote cell motility by regulating HBP through GFPT2 expression.
However, the present study presented no evidence of the HBP
modulation in vitro or in vivo; hence, further
metabolic studies are warranted to evaluate the role of SLP-2 in
affecting the GFPT2 downstream pathway in HBP.
SLP-2 has been reported to be localized in
mitochondria and upregulated under several conditions of
mitochondrial stress (7). This
increased expression plays an important role in SIMH, which
promotes mitochondrial ATP production and leads to stress
resistance in cells (39). Exposure
to high concentrations of cisplatin was revealed to increase SLP-2
expression and induce an anti-apoptotic effect on cells (25). This response is one possible mechanism
for stress-resistant regulation of SLP-2 expression. The present
study revealed that SLP-2 expression was increased after NAC. When
PC cells were exposed to gemcitabine, expression was increased for
SLP-2 and GFPT2. Furthermore, GFPT2 expression was decreased in
SLP-2-suppressed cells, even in a stressed condition (such as
exposure to anticancer drug gemcitabine). All these data indicated
that SLP-2 expression may be increased by chemotherapy itself;
additionally, this increase may induce activation of the HBP
through upregulation of GFPT2. Recently, several studies have
raised the possibility of chemotherapy-induced metastasis (40) and have suggested that cancer cells
tend to become metastatically aggressive following exposure to
chemotherapy (41). Although SLP-2
suppression did not alter chemosensitivity to gemcitabine,
increased expression of SLP-2 after NAC may contribute to promoting
liver metastasis. Further study should be conducted to investigate
this hypothesis.
The present study has several limitations. First,
only the function of SLP-2 was evaluated by inhibiting its
expression. SLP-2 suppression did not significantly change the
proliferation and chemosensitivity of PC cells to gemcitabine
unlike previous findings (24,42).
Overexpression of SLP-2 increased the ability of cells to produce
ATP, which is necessary for proliferation and resistance to stress
(11,23). Exposure of PC cells to gemcitabine
also increased the expression of SLP-2, which may be a response to
stress. Considering these data, overexpression may lead to diverse
effects on cell functions. Second, the present study used G418
(geneticin) to select shRNA-transfected PC cells, and this agent
may influence the growth and metabolism of cell lines (43). However, several examinations were
performed, using two types of cell lines and two types of
shRNA-transfected cell lines, to mitigate the effects of G418 and
minimize this bias.
In conclusion, to the best of our knowledge, the
present study is the first to analyze the effects of SLP-2 in PC.
The results obtained herein demonstrated the involvement of SLP-2
in liver metastasis via regulation of the migration and invasion
activities of PC cells. Furthermore, SLP-2 expression promoted
glucose uptake activity and may regulate HBP, a branch of glucose
metabolism, which can contribute to the activation of cancer cell
motility. Furthermore, SLP-2 expression was increased under
cytotoxic stress, implying that the aforementioned functions may be
enhanced under the cellular stress caused by chemotherapy
treatments. All these results indicated that the poor prognosis of
high SLP-2 expression was caused by activated metastatic potential.
Further investigations on HBP activation and its promotion of
metastasis are warranted to determine whether these signals could
provide novel therapeutic targets for PC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by KAKENHI
Grants-in-Aid for young scientists (B) (grant no.
K.A.16K19911).
Availability of data and materials
The data of microarray analysis are available in
Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE162981
and can be accessed with GSE162981. The other datasets used and/or
analyzed during this study are available from the corresponding
author on reasonable request.
Authors' contributions
DC, KA, HO, KMi, XJY performed in vivo and
in vitro analysis. KA, MM, TT, MI, KMa, SM, TMi, MM, KN,
TMo, TK, MU interpreted the patient data regarding the pancreatic
ductal adenocarcinoma. SS and FF performed the pathological
examination of the pancreatic ductal adenocarcinoma samples. KA,
TT, MI, KMa, SM, TMi, MM, KN, TMo, TK, MU were major contributors
in writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The Institutional Animal Experiment Committee of
Tohoku University (Sendai, Japan) approved the present study
protocol on September 20, 2017. The Institutional Review Board of
Tohoku University (Sendai, Japan) approved the present study design
on May 25, 2016 (2016-1-151). This was a retrospective study,
therefore, the requirement for informed consent was waived and an
opt-out method was used instead.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
GFPT2
|
glutamine-fructose-6-phosphate
transaminase 2
|
|
HBP
|
hexosamine biosynthesis pathway
|
|
IRS
|
immunoreactive score
|
|
NAC
|
neoadjuvant chemotherapy
|
|
PC
|
pancreatic cancer
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SIMH
|
stress-induced mitochondrial
hyperfusion
|
|
SLP-2
|
stomatin-like protein 2
|
References
|
1
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neoptolemos JP, Stocken DD, Friess H,
Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C,
Lacaine F, et al: A randomized trial of chemoradiotherapy and
chemotherapy after resection of pancreatic cancer. N Engl J Med.
350:1200–1210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ariake K, Motoi F, Ohtsuka H, Fukase K,
Masuda K, Mizuma M, Hayashi H, Nakagawa K, Morikawa T, Maeda S, et
al: Predictive risk factors for peritoneal recurrence after
pancreatic cancer resection and strategies for its prevention. Surg
Today. 47:1434–1442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ariake K, Motoi F, Shimomura H, Mizuma M,
Maeda S, Terao C, Tatewaki Y, Ohtsuka H, Fukase K, Masuda K, et al:
18-fluorodeoxyglucose positron emission tomography predicts
recurrence in resected pancreatic ductal adenocarcinoma. J
Gastrointest Surg. 22:279–287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto T, Sugiura T, Mizuno T, Okamura
Y, Aramaki T, Endo M and Uesaka K: Preoperative FDG-PET predicts
early recurrence and a poor prognosis after resection of pancreatic
adenocarcinoma. Ann Surg Oncol. 22:677–684. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hájek P, Chomyn A and Attardi G:
Identification of a novel mitochondrial complex containing
mitofusin 2 and stomatin-like protein 2. J Biol Chem.
282:5670–5681. 2007. View Article : Google Scholar
|
|
7
|
Da Cruz S, Parone PA, Gonzalo P, Bienvenut
WV, Tondera D, Jourdain A, Quadroni M and Martinou JC: SLP-2
interacts with prohibitins in the mitochondrial inner membrane and
contributes to their stability. Biochim Biophys Acta. 1783:904–911.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitsopoulos P, Chang YH, Wai T, König T,
Dunn SD, Langer T and Madrenas J: Stomatin-like protein 2 is
required for in vivo mitochondrial respiratory chain supercomplex
formation and optimal cell function. Mol Cell Biol. 35:1838–1847.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Da Cruz S, De Marchi U, Frieden M, Parone
PA, Martinou JC and Demaurex N: SLP-2 negatively modulates
mitochondrial sodium-calcium exchange. Cell Calcium. 47:11–18.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tondera D, Grandemange S, Jourdain A,
Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke
I, Merkwirth C, et al: SLP-2 is required for stress-induced
mitochondrial hyperfusion. EMBO J. 28:1589–1600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christie DA, Lemke CD, Elias IM, Chau LA,
Kirchhof MG, Li B, Ball EH, Dunn SD, Hatch GM and Madrenas J:
Stomatin-like protein 2 binds cardiolipin and regulates
mitochondrial biogenesis and function. Mol Cell Biol. 31:3845–3856.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang D, Ma K, Gong M, Cui Y, Liu ZH, Zhou
XG, Zhou CN and Wang TY: SLP-2 overexpression is associated with
tumour distant metastasis and poor prognosis in pulmonary squamous
cell carcinoma. Biomarkers. 15:104–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu D, Zhang L, Shen Z, Tan F, Hu Y, Yu J
and Li G: Increased levels of SLP-2 correlate with poor prognosis
in gastric cancer. Gastric Cancer. 16:498–504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li XH, He F, Yan SM, Li Y, Cao Y, Huang CY
and Zhou ZW: Increased expression of stomatin-like protein 2
(STOML2) predicts decreased survival in gastric adenocarcinoma: A
retrospective study. Med Oncol. 31:7632014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou C, Li Y, Wang G, Niu W, Zhang J, Wang
G, Zhao Q and Fan L: Enhanced SLP-2 promotes invasion and
metastasis by regulating Wnt/β-catenin signal pathway in colorectal
cancer and predicts poor prognosis. Pathol Res Pract. 215:57–67.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang WX, Lin QF, Shen D, Liu SP, Mao WD,
Ma G and Qi WD: Clinicopathological significance of SLP-2
overexpression in human gallbladder cancer. Tumour Biol.
35:419–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu W, Li W, Geng Q, Wang X, Sun W, Jiang
H and Pu X: Silence of stomatin-like protein 2 represses migration
and invasion ability of human liver cancer cells via inhibiting the
nuclear factor kappa B (NF-κB) pathway. Med Sci Monit.
24:7625–7632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Y, Chen Y, Lin X, Lin Q, Han M and
Guo G: Clinical significance of SLP-2 in hepatocellular carcinoma
tissues and its regulation in cancer cell proliferation, migration,
and EMT. OncoTargets Ther. 10:4665–4673. 2017. View Article : Google Scholar
|
|
19
|
Cao W, Zhang B, Li J, Liu Y, Liu Z and Sun
B: SLP-2 overexpression could serve as a prognostic factor in node
positive and HER2 negative breast cancer. Pathology. 43:713–718.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Song X, Li C and Tian Y:
Expression and clinical significance of SLP-2 in ovarian tumors.
Oncol Lett. 17:4626–4632. 2019.PubMed/NCBI
|
|
21
|
Deng H, Deng Y, Liu F, Chen J, Li Z, Zhao
K, Guan X and Liang W: Stomatin-like protein 2 is overexpressed in
cervical cancer and involved in tumor cell apoptosis. Oncol Lett.
14:6355–6364. 2017.PubMed/NCBI
|
|
22
|
Qu H, Jiang W, Wang Y and Chen P: STOML2
as a novel prognostic biomarker modulates cell proliferation,
motility and chemo-sensitivity via IL6-Stat3 pathway in head and
neck squamous cell carcinoma. Am J Transl Res. 11:683–695.
2019.PubMed/NCBI
|
|
23
|
Wang Y, Cao W, Yu Z and Liu Z:
Downregulation of a mitochondria associated protein SLP-2 inhibits
tumor cell motility, proliferation and enhances cell sensitivity to
chemotherapeutic reagents. Cancer Biol Ther. 8:1651–1658. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song L, Liu L, Wu Z, Lin C, Dai T, Yu C,
Wang X, Wu J, Li M and Li J: Knockdown of stomatin-like protein 2
(STOML2) reduces the invasive ability of glioma cells through
inhibition of the NF-κB/MMP-9 pathway. J Pathol. 226:534–543. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu G, Zhang J, Xu F, Deng H, Zhang W, Kang
S and Liang W: Stomatin-like protein 2 inhibits cisplatin-induced
apoptosis through MEK/ERK signaling and the mitochondrial apoptosis
pathway in cervical cancer cells. Cancer Sci. 109:1357–1368. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takadate T, Onogawa T, Fukuda T, Motoi F,
Suzuki T, Fujii K, Kihara M, Mikami S, Bando Y, Maeda S, et al:
Novel prognostic protein markers of resectable pancreatic cancer
identified by coupled shotgun and targeted proteomics using
formalin-fixed paraffin-embedded tissues. Int J Cancer.
132:1368–1382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen DT: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
National Comprehensive Cancer Network, .
NCCN practice guidelines for pancreatic cancer. Version 1.
(2020).https://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdfSeptember
1–2020
|
|
29
|
UICC, . TNM Classification of Malignant
Tumours. 7th edition. Sobin LH, Gospodarowicz MK and Wittekind C:
Wiley Blackwell; Hoboken: 2009
|
|
30
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP; STROBE Initiative, : The
strengthening the reporting of observational studies in
epidemiology (STROBE) statement: Guidelines for reporting
observational studies. J Clin Epidemiol. 61:344–349. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suemizu H, Monnai M, Ohnishi Y, Ito M,
Tamaoki N and Nakamura M: Identification of a key molecular
regulator of liver metastasis in human pancreatic carcinoma using a
novel quantitative model of metastasis in NOD/SCID/gammacnull (NOG)
mice. Int J Oncol. 31:741–751. 2007.PubMed/NCBI
|
|
32
|
Zhang W, Bouchard G, Yu A, Shafiq M,
Jamali M, Shrager JB, Ayers K, Bakr S, Gentles AJ, Diehn M, et al:
GFPT2-expressing cancer-associated fibroblasts mediate metabolic
reprogramming in human lung adenocarcinoma. Cancer Res.
78:3445–3457. 2018.PubMed/NCBI
|
|
33
|
Zhou L, Luo M, Cheng LJ, Li RN, Liu B and
Linghu H: Glutamine-fructose-6-phosphate transaminase 2 (GFPT2)
promotes the EMT of serous ovarian cancer by activating the
hexosamine biosynthetic pathway to increase the nuclear location of
β-catenin. Pathol Res Pract. 215:1526812019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szymura SJ, Zaemes JP, Allison DF, Clift
SH, D'Innocenzi JM, Gray LG, McKenna BD, Morris BB, Bekiranov S,
LeGallo RD, et al: NF-κB upregulates glutamine-fructose-6-phosphate
transaminase 2 to promote migration in non-small cell lung cancer.
Cell Commun Signal. 17:242019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dowling P, Walsh N and Clynes M: Membrane
and membrane-associated proteins involved in the aggressive
phenotype displayed by highly invasive cancer cells. Proteomics.
8:4054–4065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marshall S, Bacote V and Traxinger RR:
Discovery of a metabolic pathway mediating glucose-induced
desensitization of the glucose transport system. Role of hexosamine
biosynthesis in the induction of insulin resistance. J Biol Chem.
266:4706–4712. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang WH, Kim JE, Nam HW, Ju JW, Kim HS,
Kim YS and Cho JW: Modification of p53 with O-linked
N-acetylglucosamine regulates p53 activity and stability. Nat Cell
Biol. 8:1074–1083. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Itkonen HM, Minner S, Guldvik IJ, Sandmann
MJ, Tsourlakis MC, Berge V, Svindland A, Schlomm T and Mills IG:
O-GlcNAc transferase integrates metabolic pathways to regulate the
stability of c-MYC in human prostate cancer cells. Cancer Res.
73:5277–5287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghose P, Park EC, Tabakin A,
Salazar-Vasquez N and Rongo C: Anoxia-reoxygenation regulates
mitochondrial dynamics through the hypoxia response pathway,
SKN-1/Nrf, and stomatin-like protein STL-1/SLP-2. PLOS Genet.
9:e10040632013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Karagiannis GS, Condeelis JS and Oktay MH:
Chemotherapy-induced metastasis: Mechanisms and translational
opportunities. Clin Exp Metastasis. 35:269–284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang CT, Li JM, Li LF, Ko YS and Chen JT:
Stomatin-like protein 2 regulates survivin expression in non-small
cell lung cancer cells through β-catenin signaling pathway. Cell
Death Dis. 9:4252018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yallop CA and Svendsen I: The effects of
G418 on the growth and metabolism of recombinant mammalian cell
lines. Cytotechnology. 35:101–114. 2001. View Article : Google Scholar : PubMed/NCBI
|