Introduction
Cholangiocarcinoma (CCA) originates from the
epithelium lining of the biliary tree and is classified into
intrahepatic cholangiocarcinoma (iCCA) and extrahepatic
cholangiocarcinoma (eCCA), which is further stratified into
perihilar (pCCA) and distal (dCCA) cholangiocarcinoma (1,2).
Cholangiocarcinoma is the second most common primary hepatobiliary
malignancy after hepatocellular carcinoma (HCC) (3,4). Most
patients with CCA are diagnosed in the advanced and metastatic
stages of the disease due to lack of signs and symptoms in the
early stage (3). Unfortunately, CCA
is an invasive malignancy with a median survival of less than 2
years from diagnosis (5). This fact,
as well as the adverse outcomes of the current use of local and
systemic therapy, is the cause of poor prognosis in CCA patients
and strongly supports the need for new therapeutic drugs and
strategies (6). The molecular
mechanisms of CCA have been partially identified in recent years,
including isocitrate dehydrogenase (IDH1 and IDH2)
mutations and fibroblast growth factor receptor 2 (FGFR2) fusions,
as well as gene mutations involved in chromatin remodeling, such as
AT-rich interaction domain 1A (ARID1A), protein poly-bromo 1
(PBRM1), and BRCA1-associated protein 1 (BAP1)
(7,8).
Elucidation of key molecules involved in CCA development,
inhibition of certain mutated genes or inhibition of related
signaling pathways through specific inhibitors opens new horizons
for novel therapeutic approaches (9,10). Thus, a
deeper understanding of CCA molecular mechanisms is needed to lay
the foundation for targeted therapy.
Germ cell-specific gene 2 protein (GSG2), also
termed histone H3 phosphorylated by GSG2 at threonine-3, is mainly
expressed in haploid germ cells (11,12). GSG2
has been shown to be weakly expressed in proliferating normal
somatic cells but plays a crucial role in mitosis, where it
specifically phosphorylates Thr-3 in histone H3 (H3T3) (12–14). On
the other hand, GSG2 does not belong to the family of eukaryotic
protein kinase, which is a structurally unique kinase and may
result in fewer off-target effects (15). GSG2 RNAi in tumor cells prevents
chromosome alignment and normal mitosis, suggesting that GSG2
inhibitors may be a novel anti-mitotic agent that prevents cancer
cell proliferation (16,17). For instance, GSG2 knockdown was found
to inhibit progression and development of pancreatic cancer in
vitro and in vivo (18).
Recently, Yu et al found that GSG2 knockdown inhibited cell
proliferation, colony formation and induced apoptosis, and may
serve as a potential therapeutic target for prostate cancer therapy
(19). Ample evidence suggests that
identifying specific GSG2 inhibitors may be feasible and useful for
basic biological studies and as candidates for cancer therapy
(20–23). Therefore, we were committed to
exploring the molecular mechanisms of GSG2 in CCA to determine
whether GSG2 inhibitors have the potential to be molecular
anticancer drugs against CCA.
In the present study, the role and mechanisms of
GSG2 in the regulation of CCA progression and development were
explored. First, we found that GSG2 was abundantly expressed in CCA
and its expression was positively correlated with pathological
grade. Additionally, it was revealed that GSG2 knockdown inhibited
cell proliferation, migration, promoted cell apoptosis and arrested
the cell cycle in the G2 phase. These findings highlight the
significance of GSG2 in CCA and confirm its therapeutic
potential.
Materials and methods
Tissue microarray chip
A total of 80 cases/80 points of microarray chips of
CCA were purchased from Xi'an Alina Biotechnology Co., Ltd. (Xi'an,
China). These included 75 cases of tumor tissues (48 cases of eCCA,
27 cases of iCCA) and 5 cases of para-carcinoma tissues
(intrahepatic bile duct tissue). These paraffin-embedded human
tissue chips were 1.5 mm in diameter and 5 µm in thickness and
stored immediately at −4°C for later use. The study was approved by
the Ethics Committee of The IRB of The Third Xiangya Hospital,
Central South University (no. 2019-S435).
Cell culture
The human CCA cell lines HCCC-9810, QBC939 and
HuCCT1 were obtained from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). Human intrahepatic bile duct epithelial
cells (HIBECs) were purchased from Beina Biotechnology Research
Institute (http://www.bnbio.com/pro/p1/1/p_3391.html, Beijing,
China). These cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) together with 10% fetal bovine
serum (FBS), 100 mg/ml streptomycin plus, and 100 IU/ml of
penicillin (Gibco; Thermo Fisher Scientific, Inc.) in an atmosphere
of 5% CO2 at 37°C.
Immunohistochemical (IHC)
staining
The tissue microarray chips were stained with DAB
solution and then with hematoxylin. In brief, the tissue microarray
chip was immersed in xylene and ethanol in turn dewaxed and
rehydrated. The chip was boiled in 10 mM sodium citrate buffer (pH
6. 0) and maintained for 10 min. After that, the chip was cooled
and soaked in distilled water for cleaning. To permeabilize the
tissue, the chip was washed twice with 1% animal serum in PBS with
0.4% Triton X-100 (PBS-T). The primary antibody GSG2 (dilution
1:200, Bioss, cat. # bs-15413R) was diluted in 1% animal serum in
PBS-T and incubated at room temperature for 2 h. The incubation was
continued overnight at 4°C in a humidified chamber. Subsequently,
the secondary antibody goat anti-rabbit (dilution 1:200, Beyotime
Institute of Biotechnology, cat. # A0208) was immersed for 2 h at
room temperature. Subsequently, the chips were stained with DAB
solution as well as hematoxylin, and photographed with a microscope
(magnification, ×200 and ×400) (MicroPublisher 3.3RTV; Olympus,
Tokyo, Japan), and viewed with ImageScope (ScanScope XT) and
CaseViewer. IHC total scores were determined by staining percentage
scores [classified as: 1 (1-24%), 2 (25-49%), 3 (50-74%), 4
(75-100%)] and staining intensity scores (scored as 0, slight
color; 1, brown; 2, light yellow; 3, dark brown). Finally, high or
low expression of GSG2 was determined by the median of the IHC
experimental scores of all tissues.
Cell transfection, lentivirus
production and infection
For knockdown of GSG2, small interfering RNAs
specifically targeting GSG2 (shGSG2-1, shGSG2-2, shGSG2-3)
(Table SI) were designed by Shanghai
YiBeiRui Biomedical Science and Technology Co., Ltd. and negative
controls were scramble siRNAs (shCtrl) (sequences are detailed in
Table SI). The shGSG2 sequences were
inserted into BR-V108 vectors (Shanghai YiBeiRui, China) containing
green fluorescent protein (GFP) which acted as a detectable
marker.
HCCC-9810, QBC939 and HuCCT1 cells were seeded into
6-well plates (Corning Inc.) at an approximate density of
2×105 cells per well. Subsequent to a 24-h cultivation,
the cells were infected with 100 µl lentiviral vectors
(1×107 TU/ml) added to ENI.S and polybrene (10 µg/ml,
Sigma-Aldrich; Merck KGaA). Next, the reconstructed vectors were
introduced into 293T cells for the generation of lentiviruses,
together with pHelper 1.0 and pHelper 2.0 (Shanghai YiBeiRui
Biomedical Science and Technology) as packing vectors. Following
infection for 72 h, the supernatants containing the lentivirus
expressing shGSG2 or shCtrl were harvested. Subsequently, qPCR
analysis and western blot analysis were used to evaluate the GSG2
knockdown efficiency. Finally, the successfully infected cells were
subjected to the following function assays.
qPCR
HCCC-9810 and QBC939 cell RNA was isolated with
Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) with
DNase I (Vazyme) according to the manufacturer using a standard
procedure. RNA was converted into cDNA using the M-MLV RT kit
(Promega Corp.). cDNA was amplified with SYBR Green Master Mix kit
(Vazyme) and Bio-Rad CFX96 sequence detection system (Bio-Rad
Laboratories, Inc.). Sequences are detailed in Table SII and GAPDH was used as an internal
reference. Results of qPCR were evaluated using the
2−ΔΔCq method (24) and
converted into fold change.
Western blotting (WB)
HCCC-9810 and QBC939 cells were fully lysed in
ice-cold RIPA buffer (Millipore) to obtain protein. The protein
concentration detection was performed using the HyClone-Pierce™ BCA
Protein Assay kit (Thermo Fisher Scientific, Inc., cat. # 23225).
Protein (20 µg) per group was separated by 10% SDS-PAGE,
transferred onto PVDF membranes, and analyzed with required primary
antibodies and the corresponding secondary antibodies in turn
(antibodies are detailed in Table
SIII) at room temperature for 2 h. The blots were visualized by
Amersham ECL plus TM Western Blot system (GE Healthcare Life
Sciences) and the density of the protein band was analyzed by
ImageJ (National Institutes of Health, v1.8.0).
MTT assay
Following trypsinization of the HCCC-9810 and QBC939
cells in each experimental group while in the logarithmic growth
phase, cells (2,000 cells/well) were seeded onto a 96-well plate
overnight. A total of 20 µl of a 5 mg/ml MTT solution (Genview,
cat. # JT343) was added to each well 4 h prior to termination of
the culture. After incubation for 4 h, 100 µl dimethyl sulfoxide
(DMSO) was added to each well. Following that, formazan was
quantified at 24, 48, 72, 96 and 120 h by measuring the absorbance
at 490 nm with a microplate reader. The absorbance was associated
with the percentage of viable cells, and the cell viability ratio
was calculated according to the following formula: Cell viability
(%) = optical density (OD) treated/OD control ×100%.
Cell cycle analysis by flow
cytometry
HCCC-9810 and QBC939 cells were inoculated in 6-well
plates (Corning Inc.) until cell density reached 85%. Afterwards,
these cells were harvested, centrifuged (1,200 × g), and
resuspended. The cells were fixed with pre-cooled 70% ethanol (4°C)
for at least 1 h, the ethanol was removed, and the cells were
washed once with PBS. Subsequently, the cells were stained with 1
ml cell staining solution [40X PI (BD Biosciences), 2 mg/ml: 100X
RNase, 10 mg/ml: 1X PBS = 25:10:1,000) for 30 min. Fluorescence
activated cell analysis (FACS)/FACScan and FlowJo 7.6.1 (FlowJo,
LLC)/CellQuest Pro software (BD Biosciences) were used for
analysis. The percentage of the cells in the G0-G1, S, and G2-M
phases were counted and compared.
Cell apoptosis analysis by flow
cytometry
After HCCC-9810 and QBC939 cells were inoculated in
6-well plates at a seeding density of 1×103 cells/ml for
10 day, washed with PBS and harvested by centrifugation at 3,000 ×
g for 10 min, the supernatant was discarded. The cells were washed
once again with PBS, centrifuged, and the supernatant was
discarded, and the cells were resuspended by adding 500 µl of
diluted 1X Annexin V Binding Buffer working solution. Annexin V-APC
(10 µl) was added for staining for 10–15 min at room temperature
without light. The percentage of cells in the different phases was
measured using FACSCanto II Flow Cytometry (BD Bioscience) to
assess the apoptotic rate, and the results were analyzed.
Human apoptosis antibody array
For signal pathway gene detection, the Human
Apoptosis Antibody Array (Abcam, cat. # ab134001) was applied
following the manufacturer's instructions. Briefly, QBC939 cells
were lysed in cold RIPA buffer (Millipore), and the protein
concentration was detected by BCA Protein Assay kit
(HyClone-Pierce; Thermo Fisher Scientific, Inc.). Proteins were
incubated with a blocked array antibody membrane overnight at 4°C.
After washing, Detection Antibody Cocktail (1:100) was added and
incubation was carried out for 1 h, followed by incubated with HRP
linked streptavidin conjugate for 1 h. All spots were visualized by
enhanced ECL and the signal densities were analyzed with Image J
software (National Institute of Health, v1.8.0).
Wound-healing assay
HCCC-9810 and QBC939 cells were cultivated into
6-well plates and were grown until reaching 90% confluence. On the
following day, a 10-µl pipette was used to scratch a wound at the
middle of each well. Then, the medium was substituted with 1%
FBS-containing fresh medium. Images of the wounds were captured at
pre-set time points (4, 8, 24 and 48 h). The cell migration rate of
each group was calculated based on the images and analyzed using
NIH ImageJ software.
Animal xenograft model
Animal experiments were approved by the Ethics
Committee of The IRB of The Third Xiangya Hospital, Central South
University and conducted in accordance with guidelines and
protocols for animal care and protection. The four-week-old male
BALB/c nude mice (15±0.73 g) (Shanghai Lingchang Biotechnology Co.,
Ltd) were housed under pathogen-free condition at room temperature
for the xenograft model. Twenty mice were injected with
4×106 HuCCT1 cells and randomly divided into two groups,
shCtrl and shGSG2. Mice weight and tumor volume were detected twice
a week after 10 days of subcutaneous injection. Tumor volume = π/6
× L × W × W, where L is the long diameter and W is the short
diameter. On the 32th day after cell injection, 0.7% pentobarbital
sodium at a dose of 40 mg/kg was injected into the abdominal cavity
to anesthetize the mice (25–27), and the bioluminescence imaging
intensity (IVIS spectral imaging system, emission wavelength of 510
nm) was observed. After 32 days of subcutaneous injection, the
experimental animals were sacrificed by cervical dislocation
ensuring that the mice died instantly and without suffering. The
tumors were removed and weighed.
Ki67 staining
Mouse tumor tissues were fixed in 10% formalin and
then were paraffin-embedded. Sections (5-µm) were cut and immersed
in xylene and ethanol. Tissue slides were blocked with 3%
PBS-H2O2 and incubated with anti-Ki67
(dilution 1:200, Abcam, cat. # ab16667) and HRP goat anti-rabbit
IgG (dilution 1:400, Abcam, cat. # ab6721). Subsequently, the
slides were stained with DAB solution as well as hematoxylin, and
examined at ×100 and ×200 with an objective lens microscope.
Statistical analysis
All experiments were conducted in triplicate, and
data are shown as mean ± SD. Statistical analyses and graphical
representations were carried out by GraphPad Prism 7.0 (Graphpad
Software, Inc.) and a P-value <0.05 was indicative of a
statistically significant difference. The significance difference
between groups was determined using the two-tailed Student's t-test
or one-way ANOVA followed by Bonferroni's post hoc test analysis.
GSG2 expression in tumor tissues and normal tissues revealed in the
IHC assay were analyzed with Sign test. Relationship between GSG2
expression and tumor characteristics in patients with CCA was
analyzed using the Chi-square test or Fisher's exact test.
Results
High expression of GSG2 in CCA
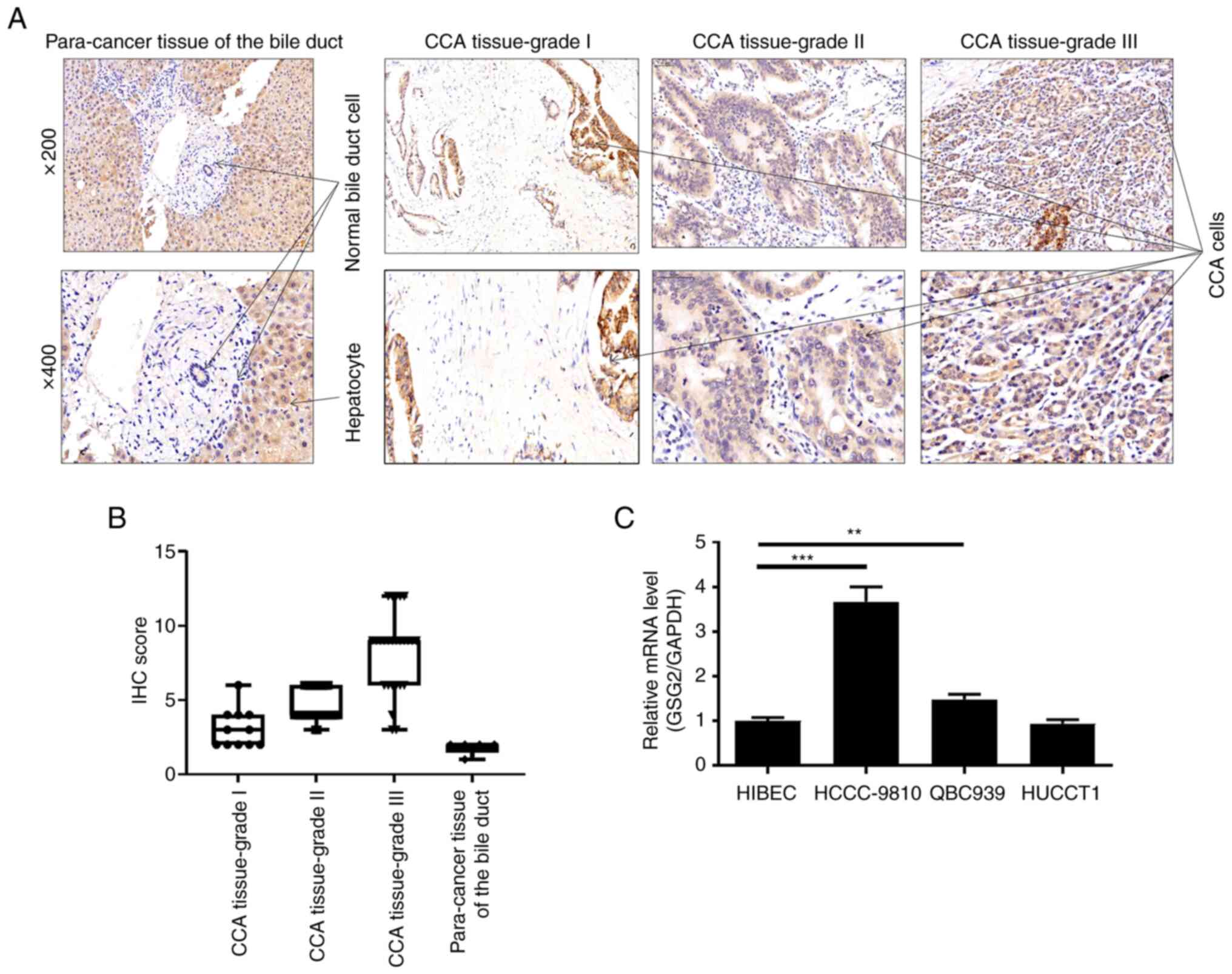
According to the results of the IHC staining,
expression of GSG2 in CCA tissues was significantly higher than
that in normal tissues (P<0.001) (Table I, Fig.
1A). Subsequently, the results of the Chi-square test or
Fisher's exact test revealed a significant correlation between GSG2
expression and pathological tumor grade (Table II, Fig.
1B). Notably, the pathological grade of cholangiocarcinoma was
classified according to the protocol provided in the literature
(28). Consistently, Spearman grade
correlation analysis further confirmed that GSG2 expression was
positively correlated with pathological grade (Table III). More specifically, the increase
in GSG2 expression was accompanied by CCA deterioration. Besides,
we also found that mRNA levels of GSG2 were significantly highly
expressed in CCA cell lines HCCC-9810 and QBC939 when compared to
the HIBEC cell line (Fig. 1C). Taken
together, high expression of GSG2 in CCA has significant clinical
significance in predicting disease deterioration.
 | Table I.Expression patterns in
cholangiocarcinoma cancer tissues and para-carcinoma tissues
revealed by immunohistochemistry analysis. |
Table I.
Expression patterns in
cholangiocarcinoma cancer tissues and para-carcinoma tissues
revealed by immunohistochemistry analysis.
|
| Tumor tissue | Para-carcinoma
tissue |
|
|---|
|
|
|
|
|
|---|
| GSG2
expression | Cases | Percentage | Cases | Percentage | P-value |
|---|
| Low | 37 | 49.3% | 5 | 100% | <0.001 |
| High | 38 | 50.7% | 0 | – |
|
 | Table II.Relationship between GSG2 expression
and tumor characteristics in patients with cholangiocarcinoma
cancer. |
Table II.
Relationship between GSG2 expression
and tumor characteristics in patients with cholangiocarcinoma
cancer.
|
|
| GSG2
expression |
|
|---|
|
|
|
|
|
|---|
| Features | No. of
patients | Low | High | P-value |
|---|
| All patients | 75 | 37 | 38 |
|
| Age (years) |
|
|
| 0.7301456 |
|
<59 | 37 | 19 | 18 |
|
|
≥59 | 38 | 18 | 20 |
|
| Sex |
|
|
| 0.7253323 |
|
Male | 39 | 20 | 19 |
|
|
Female | 36 | 17 | 19 |
|
| Tumor grade |
|
|
| 1.1793e-06 |
| 1 | 10 | 9 | 1 |
|
| 2 | 38 | 23 | 15 |
|
| 3 | 23 | 2 | 21 |
|
| Lymphatic
metastasis (N) |
|
|
| 0.06172829 |
| N0 | 58 | 32 | 26 |
|
| N1 | 17 | 5 | 12 |
|
| T infiltrate |
|
|
| 0.1400056 |
| T1 | 6 | 5 | 1 |
|
| T2 | 34 | 13 | 21 |
|
| T3 | 32 | 18 | 14 |
|
| T4 | 3 | 1 | 2 |
|
 | Table III.Correlation between GSG2 expression
and tumor characteristics in patients with cholangiocarcinoma
cancer. |
Table III.
Correlation between GSG2 expression
and tumor characteristics in patients with cholangiocarcinoma
cancer.
|
|
| GSG2 |
|---|
| Grade | Pearson
related | 0.575 |
|
| Significance
(double tail) | <0.001 |
|
| N | 71 |
Construction of the GSG2-knockdown CCA
cell model
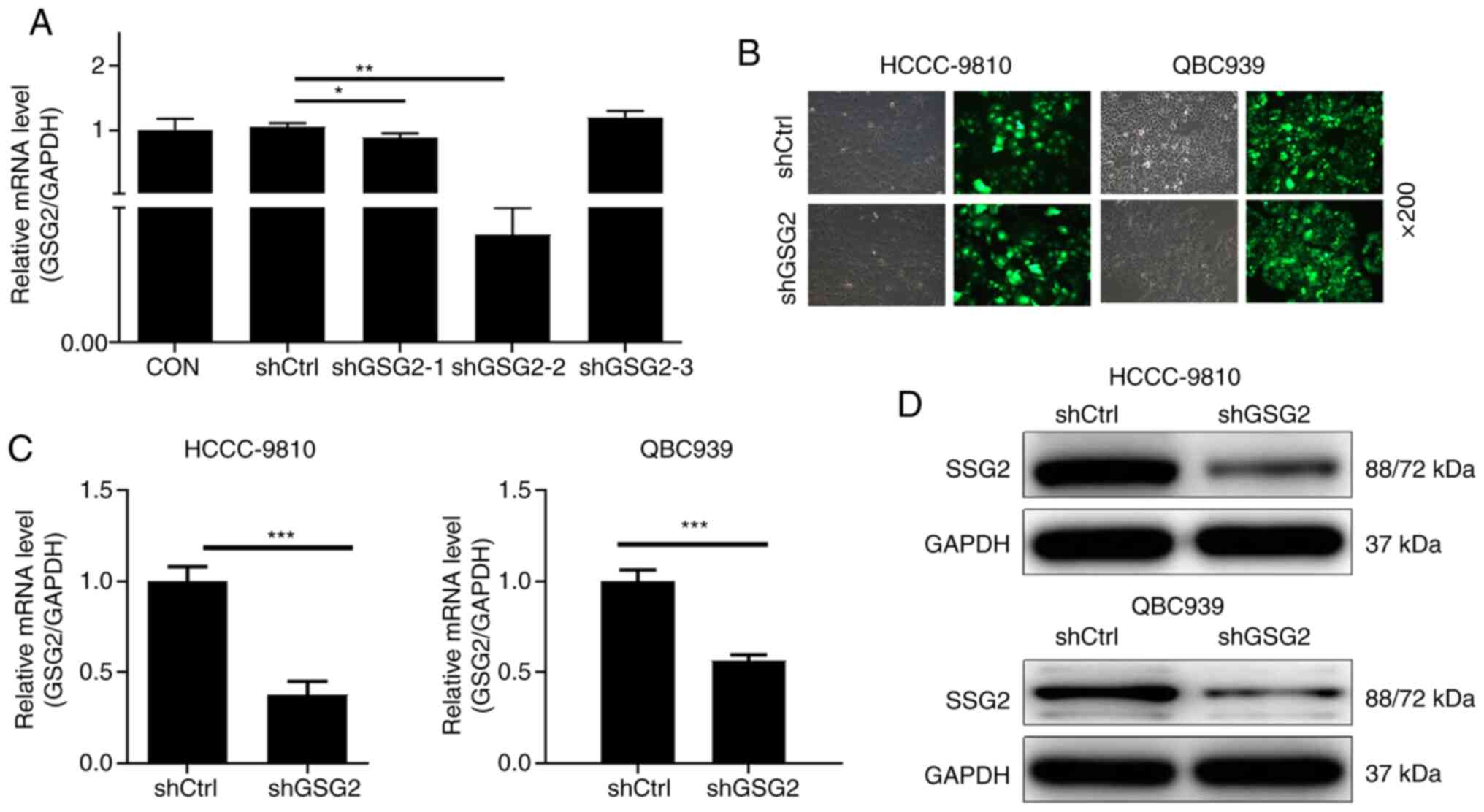
Firstly, qPCR analysis determined that the
transfection efficiency of GSG2 in the shGSG2-2 group was 99.6% and
it was used in the following experiments (P<0.01) (Fig. 2A). Furthermore, the percentage of
GFP-positive cells infected with shCtrl or shGSG2 for 72 h observed
under fluorescence microscope was more than 80% (Fig. 2B). The results of qPCR showed that in
the HCCC-9810 and QBC939 cells, compared with the relevant shCtrl
group, the knockdown efficiency of GSG2 in the shGSG2 group was
62.4% (P<0.001) and 43.6% (P<0.001), respectively (Fig. 2C). Not surprisingly, the results of
the WB analysis showed a consistent downregulation of protein
expression in HCCC-9810 and QBC939 cells compared with the controls
(Fig. 2D). The above results clearly
revealed that the CCA cell model of GSG2 knockdown was successfully
constructed.
Knockdown of GSG2 inhibits CCA cell
proliferation in vitro
The results of the MTT assays are presented as
(P<0.001) Fig. 3A. Cell
proliferation of the HCCC-9810 and QBC939 cells in the shGSG2 group
was obviously slower compared with the shCtrl group. These results
indicated that viable cells were both reduced as time goes on after
knockdown of GSG2. All in all, GSG2 knockdown has a certain
inhibitory effect on CCA cell proliferation.
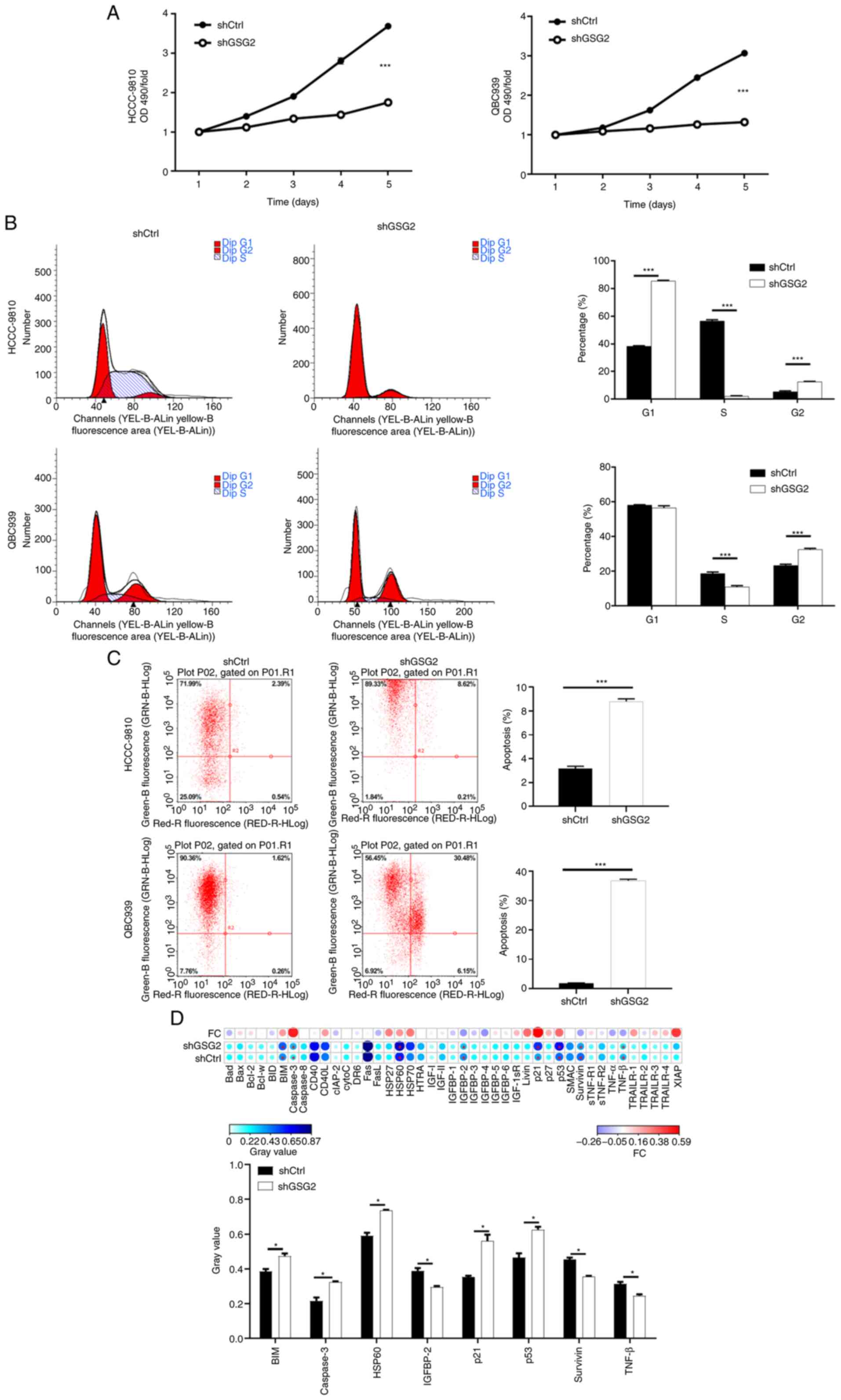 | Figure 3.Knockdown of GSG2 inhibits cell
proliferation, arrests cell cycle at G2 and promotes apoptosis in
CCA cells. (A) Cell proliferation of CCA HCCC-9810 and QBC939 cells
with or without knockdown of GSG2 was evaluated by MTT assay.
***P<0.001, shGSG2 vs. shCtrl group. (B and C) Flow cytometric
analysis based on Annexin V-APC staining was utilized to detect
cell cycle distribution (B) and cell apoptotic ratio (C) in the
HCCC-9810 and QBC939 cells. (D) Human apoptosis antibody array
analysis was performed using QBC939 cells with or without GSG2
knockdown. Data are presented as mean ± SD (n=3), *P<0.05,
***P<0.001. GSG2, germ cell-specific gene 2 protein; CCA,
cholangiocarcinoma; BIM, Bcl-2-like protein 11; HSP60, heat shock
protein 60; IGFBP-2, insulin-like growth factor-binding protein 2;
TNF-β, tumor necrosis factor-β. |
Knockdown of GSG2 arrests cell cycle
and promotes CCA cell apoptosis in vitro
Cell cycle and cell apoptosis were assessed using
flow cytometry. The results of the cell cycle distribution
detection showed that the percentages of cells in the S phase were
significantly decreased whereas the percentages of cells in the G2
phase were significantly increased in the shGSG2 group, compared
with the shCtrl groups (P<0.001) (Fig.
3B). Moreover, the ratio of apoptotic cells in the shGSG2
groups of HCCC-9810 and QBC939 cells was significantly higher than
that in the shCtrl groups (P<0.001) (Fig. 3C). Thus, the comprehensive results
suggest that GSG2 knockdown arrests the cell cycle in the G2 phase
and promotes the apoptosis of CCA cells. The expression of related
proteins in the human apoptosis signaling pathway was detected
after the knockdown of GSG2 in QBC939 cells, showing that the
protein expression levels of Bcl-2-like protein 11, commonly called
BIM, caspase3, heat shock protein 60 (HSP60), p21, p53 were
significantly upregulated, while the protein expression of
insulin-like growth factor-binding protein 2 (IGFBP-2), survivin
and tumor necrosis factor (TNF)-β was obviously downregulated
(P<0.05) (Fig. 3D).
Knockdown of GSG2 inhibits CCA cell
migration in vitro
The migration capacity of CCA cells with or without
GSG2 knockdown was identified by wound-healing assay. The results
displayed that the migration rate of HCCC-9810 cells in the shGSG2
group at 24 h was decreased by 57% compared with the shCtrl group
(P<0.001). Meanwhile, the migration rate of QBC939 cells at 48 h
was decreased by 83% (P<0.001) (Fig.
4A). Additionally, the expression of EMT biomarkers was
detected by WB, indicating that the protein level of E-cadherin was
upregulated in the shGSG2 group compared with the shCtrl group in
the HCCC-9810 and QBC939 cells; contrarily, protein expression of
N-cadherin and vimentin were downregulated (Fig. 4B). Obviously, knockdown of GSG2
inhibited CCA cell migration by suppressing N-cadherin and
vimentin.
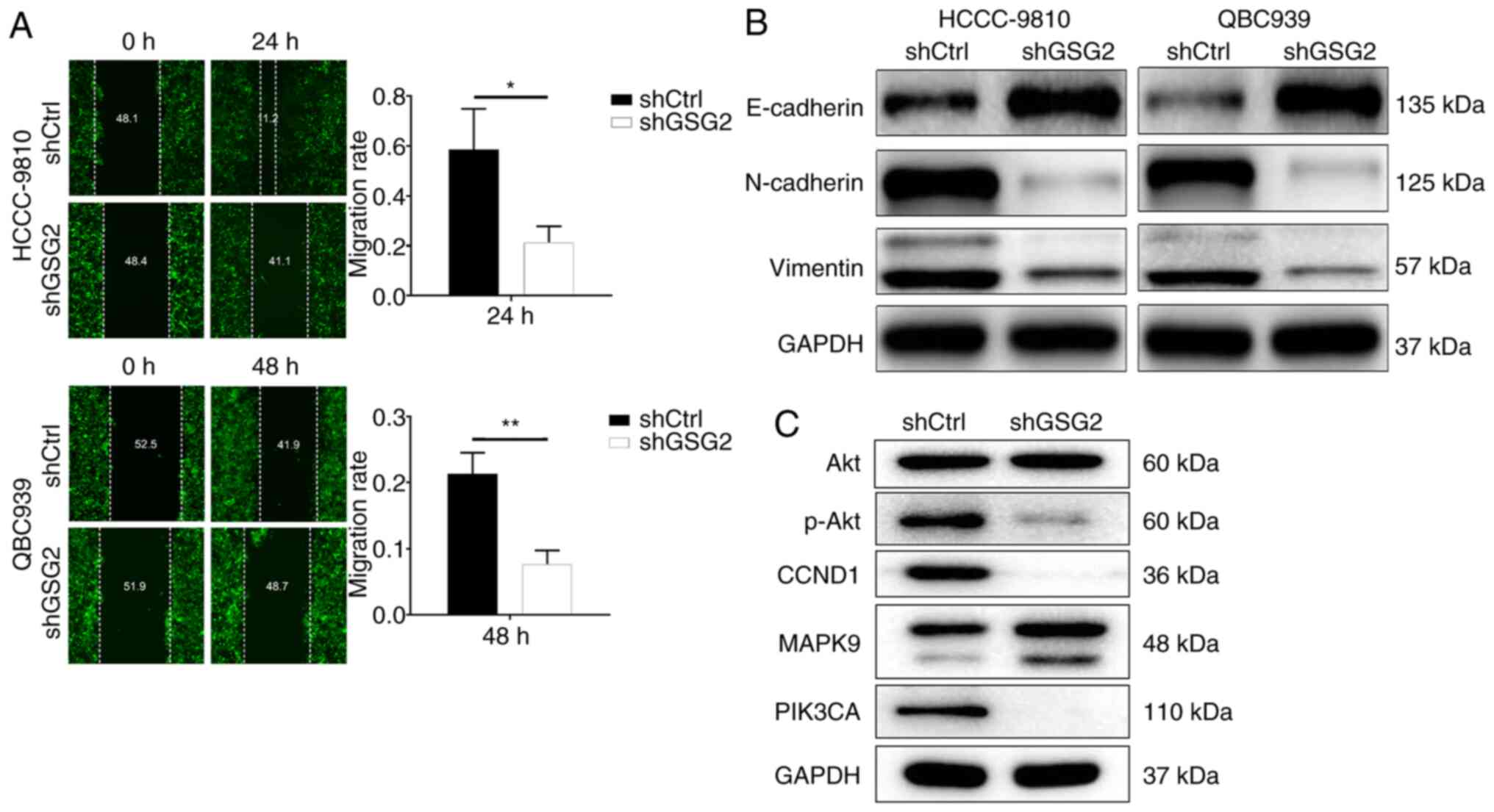 | Figure 4.Effects of GSG2 knockdown on CCA cell
migration and downstream molecular mechanisms. (A) Cell migration
of CAA HCCC-9810 and QBC939 cells with or without knockdown of GSG2
was evaluated by wound healing assay. (B) EMT marker proteins of
HCCC-9810 and QBC939 cells with or without knockdown of GSG2 were
detected by WB. (C) The expression of the downstream protein
pathway was observed by WB in QBC939 cells with or without GSG2
knockdown. Data are presented as mean ± SD (n=3), *P<0.05,
**P<0.01. GSG2, germ cell-specific gene 2 protein; CCA,
cholangiocarcinoma; EMT, epithelial-to-mesenchymal transition; WB,
western blotting; CCND1, cyclin D1; PIK3CA,
phosphatidylinositol-4,5-bisphosphate 3-kinase; MAPK9,
mitogen-activated protein kinase 9. |
Exploration of downstream molecular
mechanism of GSG2 in CCA
The downstream molecular mechanism of GSG2 in CCA
cell was elicited through WB (Fig.
4C). The results showed that the protein expression of
phosphorylated (p-)Akt, cyclin D1 (CCND1) and
phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK3CA) was
downregulated in the experimental group compared with the control
group; while mitogen-activated protein kinase 9 (MAPK9) protein
expression was upregulated, and there was no significant alteration
in Akt. In brief, GSG2 is involved in the progression of CCA by
regulating apoptosis-related factors and downstream signaling.
Knockdown of GSG2 in CCA cells impairs
tumor growth in vivo
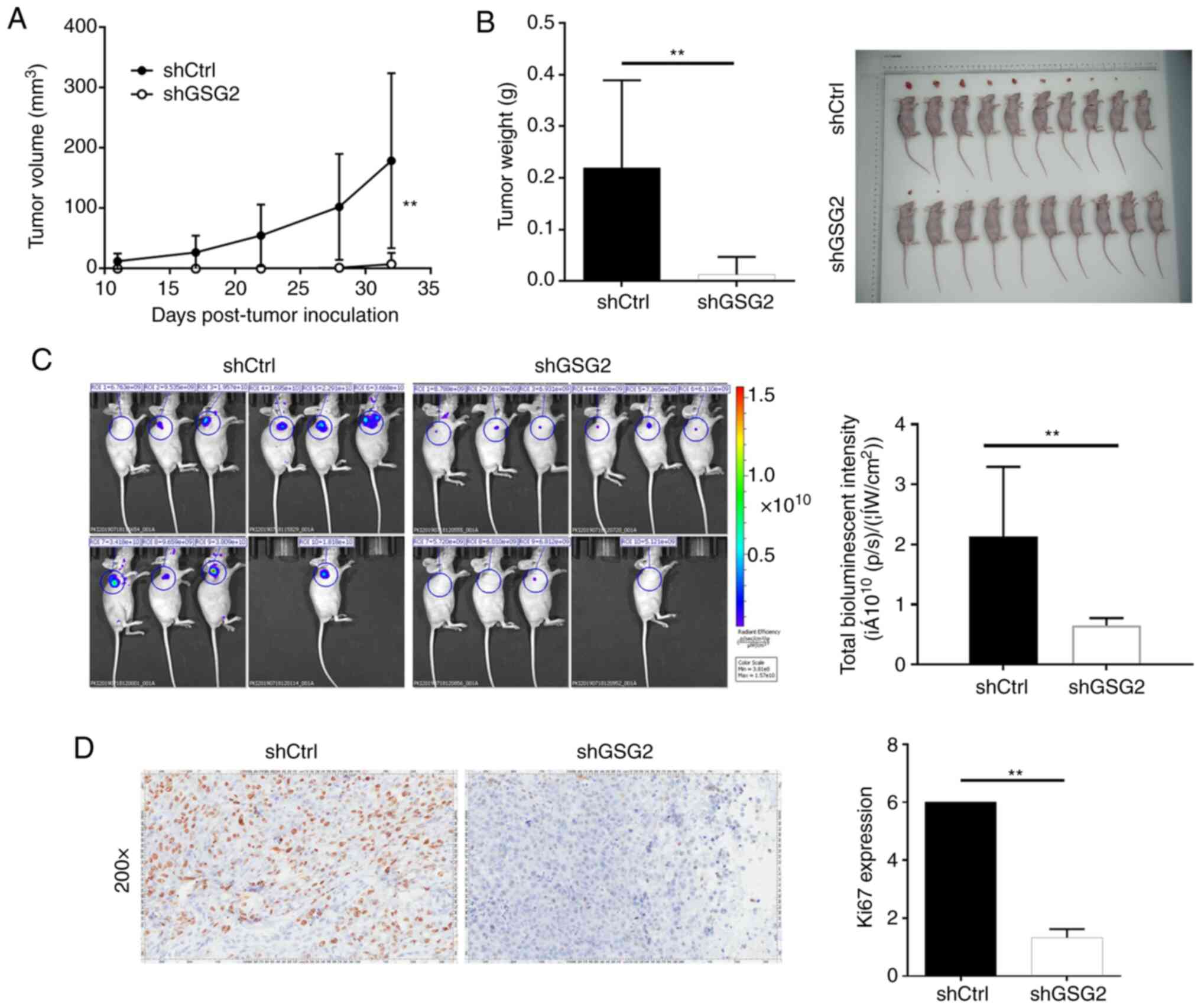
HuCCT1 cells infected with shGSG2 or shCtrl were
subcutaneously injected into nude mice to establish the xenograft
model, and the GSG2 expression of shGSG2 and shCtrl in mouse tumor
tissue was detected by WB (Fig. S1).
The results showed that the expression of GSG2 in the shGSG2 tumor
group was significantly lower than that in the shCtrl group, which
confirmed the inhibition efficiency of GSG2 in the targeted
xenografts derived from the injected HuCCT1 cells.
Importantly, the average tumor volume in the shGSG2
group was significantly reduced by 33.85±10.92 mm3
compared with the shCtrl group (P<0.01) (Fig. 5A). In particular, the average tumor
weight of mice inoculated with shGSG2 cells was significantly lower
than that of the shCtrl group (P<0.01) (Fig. 5B). Additionally, in vivo
imaging indicated that bioluminescence expression was apparently
weaker in the shGSG2 group than that in the shCtrl group
(P<0.01), also indicating the lower tumor burden in the shGSG2
group (Fig. 5C). Moreover, Ki67
staining displayed that the proliferative activity of tumors in the
shGSG2 group was significantly lower than that in the shCtrl group
(P<0.01) (Fig. 5D). In a word,
knockdown of GSG2 impaired tumorigenicity in vivo, which was
in accordance with the aforementioned in vitro results.
Discussion
The physiological function of germ cell-specific
gene 2 protein (GSG2) has not been well illustrated, and the
underlying mechanism associated with tumor progression is far from
clear. In the present study, it was demonstrated that GSG2 promoted
the development of cholangiocarcinoma (CCA). Through
loss-of-function experiments, it was demonstrated that GSG2
knockdown significantly suppressed cell proliferation, migration
and tumor growth. Conversely, CCA cell apoptosis was obviously
promoted upon GSG2 knockdown, which may have resulted from the
regulation of apoptosis-related proteins such as BIM, caspase3,
HSP60, p21, p53, IGFBP-2, survivin and TNF-β.
Unlimited growth, aggressiveness, reduced apoptosis
and cell cycle disorders are markers of cancer and play an
important role in the development of cancer (29). Moreover, apoptosis is a key biological
process by which to prevent uncontrolled cell proliferation and
eliminate harmful cells, and anti-apoptotic stimulation is a
hallmark of various types of cancer (30,31).
Mechanisms of apoptosis and their effector proteins include
pro-apoptotic protein, anti-apoptotic Bcl-2 family members, and
inhibitor of apoptosis proteins (IAP) (31). BIM, caspase3, HSP60, p21 and p53 are
all pro-apoptotic proteins, which may contribute to apoptosis
induction (32–35). Caspase3 functions as an executor of
apoptosis by activating DNA fragmentation (36). Alternatively, IGFBP-2 plays an
important role in cell proliferation, invasion, angiogenesis and
apoptosis (37). Simultaneously,
survivin, as an important member of the IAP family, is considered
to be a regulator of apoptosis-related proteins and prevents
apoptosis, and it was strongly expressed in CCA (38–40). TNF-β
also was identified as a key mediator between apoptosis and cancer
cell progression (41). Thus, it was
possible that GSG2 knockdown initiated the process of apoptosis
through balancing the expression of pro-apoptotic and
anti-apoptotic factors.
We further revealed that GSG2 may regulate cell
migration by influencing EMT-related proteins. Research has
confirmed that epithelial-to-mesenchymal transition (EMT) promotes
invasion and metastasis in various types of tumors (42). This process involves the
downregulation of epithelial-specific marker E-cadherin and
upregulation of mesenchymal markers including vimentin, and
N-cadherin (43). In our study,
knockdown of GSG2 inhibited CCA cell migration by inducing EMT,
which included E-cadherin upregulation and N-cadherin and vimentin
downregulation.
Moreover, we estimated that GSG2 was involved in CCA
progression via Akt signaling. Previous studies have revealed that
PI3K/Akt, CCND1/CDK6 and MAPK pathways play a key role in the
development of CCA (44–47). For example, Wang et al
clarified that TSPAN1 is involved in CCA progression via the
PI3K/Akt pathway (47). Zhang et
al suggested that S100A11 promotes cell proliferation by the
p38/MAPK signaling pathway in iCCA (48). This study discovered that GSG2
knockdown contributed to downregulation of P-Akt, CCND1, PIK3CA,
and upregulation of MAPK9. Therefore, we suggest that GSG2 exerts
effects on CCA cells by modulating protein pathways, such as
PI3K/Akt, CCND1/CDK6 and MAPK9.
The present study found that expression of GSG2 was
positively associated with pathological grade. Importantly, we
revealed that GSG2 knockdown inhibited CCA cell progression by
regulating cell proliferation, apoptosis, cell cycle distribution,
and cell migration. In summary, the role and preliminary regulatory
mechanisms of GSG2 in CCA were demonstrated, suggesting that GSG2
may be a potential therapeutic target for CCA patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
According to reasonable request, the datasets used
in this study are available from the corresponding author.
Authors' contributions
RH designed the research study. JZ, JY and CW
conducted the cell experiments. WN performed the animal
experiments. ZZ and LM carried out the data collection and
analysis. JZ produced the manuscript which was checked and revised
by RH. All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Animal experiments were approved by the Ethics
Committee of The IRB of The Third Xiangya Hospital, The Central
South University and conducted in accordance with guidelines and
protocols for animal care and protection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lowery MA, Ptashkin R, Jordan E, Berger
MF, Zehir A, Capanu M, Kemeny NE, O'Reilly EM, El-Dika I, Jarnagin
WR, et al: Comprehensive molecular profiling of intrahepatic and
extrahepatic cholangiocarcinomas: Potential targets for
intervention. Clin Cancer Res. 24:4154–4161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Esnaola NF, Meyer JE, Karachristos A,
Maranki JL, Camp ER and Denlinger CS: Evaluation and management of
intrahepatic and extrahepatic cholangiocarcinoma. Cancer.
122:1349–1369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel N and Benipal B: Incidence of
cholangiocarcinoma in the USA from 2001 to 2015: A US Cancer
Statistics Analysis of 50 States. Cureus. 11:e39622019.PubMed/NCBI
|
|
4
|
Goldaracena N, Gorgen A and Sapisochin G:
Current status of liver transplantation for cholangiocarcinoma.
Liver Transpl. 24:294–303. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ustundag Y and Bayraktar Y:
Cholangiocarcinoma: A compact review of the literature. World J
Gastroenterol. 14:6458–6466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blechacz B: Cholangiocarcinoma: Current
knowledge and new developments. Gut Liver. 11:13–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fouassier L, Marzioni M, Afonso MB, Dooley
S, Gaston K, Giannelli G, Rodrigues CMP, Lozano E, Mancarella S,
Segatto O, et al: Signalling networks in cholangiocarcinoma:
Molecular pathogenesis, targeted therapies and drug resistance.
Liver Int. 39 (Suppl 1):S43–S62. 2019. View Article : Google Scholar
|
|
8
|
Simile MM, Bagella P, Vidili G, Spanu A,
Manetti R, Seddaiu MA, Babudieri S, Madeddu G, Serra PA, Altana M
and Paliogiannis P: Targeted therapies in cholangiocarcinoma:
Emerging evidence from clinical trials. Medicina (Kaunas).
55:422019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Labib PL, Goodchild G and Pereira SP:
Molecular pathogenesis of cholangiocarcinoma. BMC Cancer.
19:1852019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato S, Maeda C, Hattori N, Yagi S, Tanaka
S and Shiota K: DNA methylation-dependent modulator of Gsg2/Haspin
gene expression. J Reprod Dev. 57:526–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka H, Yoshimura Y, Nozaki M, Yomogida
K, Tsuchida J, Tosaka Y, Habu T, Nakanishi T, Okada M, Nojima H and
Nishimune Y: Identification and characterization of a haploid germ
cell-specific nuclear protein kinase (Haspin) in spermatid nuclei
and its effects on somatic cells. J Biol Chem. 274:17049–17057.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higgins JM: The Haspin gene: Location in
an intron of the integrin alphaE gene, associated transcription of
an integrin alphaE-derived RNA and expression in diploid as well as
haploid cells. Gene. 267:55–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eswaran J, Patnaik D, Filippakopoulos P,
Wang F, Stein RL, Murray JW, Higgins JM and Knapp S: Structure and
functional characterization of the atypical human kinase haspin.
Proc Natl Acad Sci USA. 106:20198–20203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higgins JM: Haspin-like proteins: A new
family of evolutionarily conserved putative eukaryotic protein
kinases. Protein Sci. 10:1677–1684. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai J, Sultan S, Taylor SS and Higgins JM:
The kinase haspin is required for mitotic histone H3 Thr 3
phosphorylation and normal metaphase chromosome alignment. Genes
Dev. 19:472–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patnaik D, Jun X, Glicksman MA, Cuny GD,
Stein RL and Higgins JM: Identification of small molecule
inhibitors of the mitotic kinase haspin by high-throughput
screening using a homogeneous time-resolved fluorescence resonance
energy transfer assay. J Biomol Screen. 13:1025–1034. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han X, Kuang T, Ren Y, Lu Z, Liao Q and
Chen W: Haspin knockdown can inhibit progression and development of
pancreatic cancer in vitro and vivo. Exp Cell Res. 385:1116052019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu F, Lin Y, Xu X, Liu W, Tang D, Zhou X,
Wang G, Zheng Y and Xie A: Knockdown of GSG2 inhibits prostate
cancer progression in vitro and in vivo. Int J Oncol.
57:139–150. 2020.PubMed/NCBI
|
|
20
|
Yi Q, Chen Q, Yan H, Zhang M, Liang C,
Xiang X, Pan X and Wang F: Aurora B kinase activity-dependent and
-independent functions of the chromosomal passenger complex in
regulating sister chromatid cohesion. J Biol Chem. 294:2021–2035.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Otto T and Sicinski P: Cell cycle proteins
as promising targets in cancer therapy. Nat Rev Cancer. 17:93–115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asghar U, Witkiewicz AK, Turner NC and
Knudsen ES: The history and future of targeting cyclin-dependent
kinases in cancer therapy. Nat Rev Drug Discov. 14:130–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thu KL, Soria-Bretones I, Mak TW and
Cescon DW: Targeting the cell cycle in breast cancer: Towards the
next phase. Cell Cycle. 17:1871–1885. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taylor SC, Nadeau K, Abbasi M, Lachance C,
Nguyen M and Fenrich J: The ultimate qPCR experiment: Producing
publication quality, reproducible data the first time. Trends
Biotechnol. 37:761–774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu X, Nie J, Lu L, Du C, Meng F and Song
D: LINC00337 promotes tumor angiogenesis in colorectal cancer by
recruiting DNMT1, which suppresses the expression of CNN1. Cancer
Gene Ther. Dec 16–2020.(Online ahead of print). View Article : Google Scholar
|
|
26
|
Yatziv SL, Yudco O, Dickmann S and Devor
M: Patterns of neural activity in the mouse brain: Wakefulness vs.
General anesthesia. Neurosci Lett. 735:1352122020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Washington MK, Berlin J, Branton PA,
Burgart LJ, Carter DK, Compton CC, Frankel WL, Jessup JM, Kakar S,
Minsky B, et al: Protocol for the examination of specimens from
patients with carcinoma of the intrahepatic bile ducts. Arch Pathol
Lab Med. 134:e14–e18. 2010. View Article : Google Scholar
|
|
28
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drago JZ, Chandarlapaty S and Jhaveri K:
Targeting apoptosis: A new paradigm for the treatment of estrogen
receptor-positive breast cancer. Cancer Discov. 9:323–325. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mott JL, Bronk SF, Mesa RA, Kaufmann SH
and Gores GJ: BH3-only protein mimetic obatoclax sensitizes
cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis. Mol
Cancer Ther. 7:2339–2347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang S, Meng J, Yang Y, Liu H, Wang C, Liu
J, Zhang Y, Wang C and Xu H: A HSP60-targeting peptide for cell
apoptosis imaging. Oncogenesis. 5:e2012016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bene A and Chambers TC: p21 functions in a
post-mitotic block checkpoint in the apoptotic response to
vinblastine. Biochem Biophys Res Commun. 380:211–217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen J: The cell-cycle arrest and
apoptotic functions of p53 in tumor initiation and progression.
Cold Spring Harb Perspect Med. 6:a0261042016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Snigdha S, Smith ED, Prieto GA and Cotman
CW: Caspase-3 activation as a bifurcation point between plasticity
and cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chakrabarty S and Kondratick L:
Insulin-like growth factor binding protein-2 stimulates
proliferation and activates multiple cascades of the
mitogen-activated protein kinase pathways in NIH-OVCAR3 human
epithelial ovarian cancer cells. Cancer Biol Ther. 5:189–197. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhong F, Yang J, Tong ZT, Chen LL, Fan LL,
Wang F, Zha XL and Li J: Guggulsterone inhibits human
cholangiocarcinoma Sk-ChA-1 and Mz-ChA-1 cell growth by inducing
caspase-dependent apoptosis and downregulation of survivin and
Bcl-2 expression. Oncol Lett. 10:1416–1422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang Q, Liu ZR, Wang DY, Kumar M, Chen YB
and Qin RY: Survivin expression induced by doxorubicin in
cholangiocarcinoma. World J Gastroenterol. 10:415–418. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Endo T, Abe S, Seidlar HB, Nagaoka S,
Takemura T, Utsuyama M, Kitagawa M and Hirokawa K: Expression of
IAP family proteins in colon cancers from patients with different
age groups. Cancer Immunol Immunother. 53:770–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y and Weinberg RA:
Epithelial-to-mesenchymal transition in cancer: Complexity and
opportunities. Front Med. 12:361–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh M, Yelle N, Venugopal C and Singh
SK: EMT: Mechanisms and therapeutic implications. Pharmacol Ther.
182:80–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song X, Liu X, Wang H, Wang J, Qiao Y,
Cigliano A, Utpatel K, Ribback S, Pilo MG, Serra M, et al: Combined
CDK4/6 and Pan-mTOR inhibition is synergistic against intrahepatic
cholangiocarcinoma. Clin Cancer Res. 25:403–413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Samukawa E, Fujihara S, Oura K, Iwama H,
Yamana Y, Tadokoro T, Chiyo T, Kobayashi K, Morishita A, Nakahara
M, et al: Angiotensin receptor blocker telmisartan inhibits cell
proliferation and tumor growth of cholangiocarcinoma through cell
cycle arrest. Int J Oncol. 51:1674–1684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Y, Ji G, Han S, Shao Z, Lu Z, Huo L,
Zhang J, Yang R, Feng Q, Shen H, et al: Tip60 suppresses
cholangiocarcinoma proliferation and metastasis via PI3k-AKT. Cell
Physiol Biochem. 50:612–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peng R, Zhang PF, Zhang C, Huang XY, Ding
YB, Deng B, Bai DS and Xu YP: Elevated TRIM44 promotes intrahepatic
cholangiocarcinoma progression by inducing cell EMT via MAPK
signaling. Cancer Med. 7:796–808. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Liang Y, Yang G, Lan Y, Han J,
Wang J, Yin D, Song R, Zheng T, Zhang S, et al: Tetraspanin 1
promotes epithelial-to-mesenchymal transition and metastasis of
cholangiocarcinoma via PI3K/AKT signaling. J Exp Clin Cancer Res.
37:3002018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang MX, Gan W, Jing CY, Zheng SS, Yi Y,
Zhang J, Xu X, Lin JJ, Zhang BH and Qiu SJ: S100A11 promotes cell
proliferation via P38/MAPK signaling pathway in intrahepatic
cholangiocarcinoma. Mol Carcinog. 58:19–30. 2019. View Article : Google Scholar : PubMed/NCBI
|