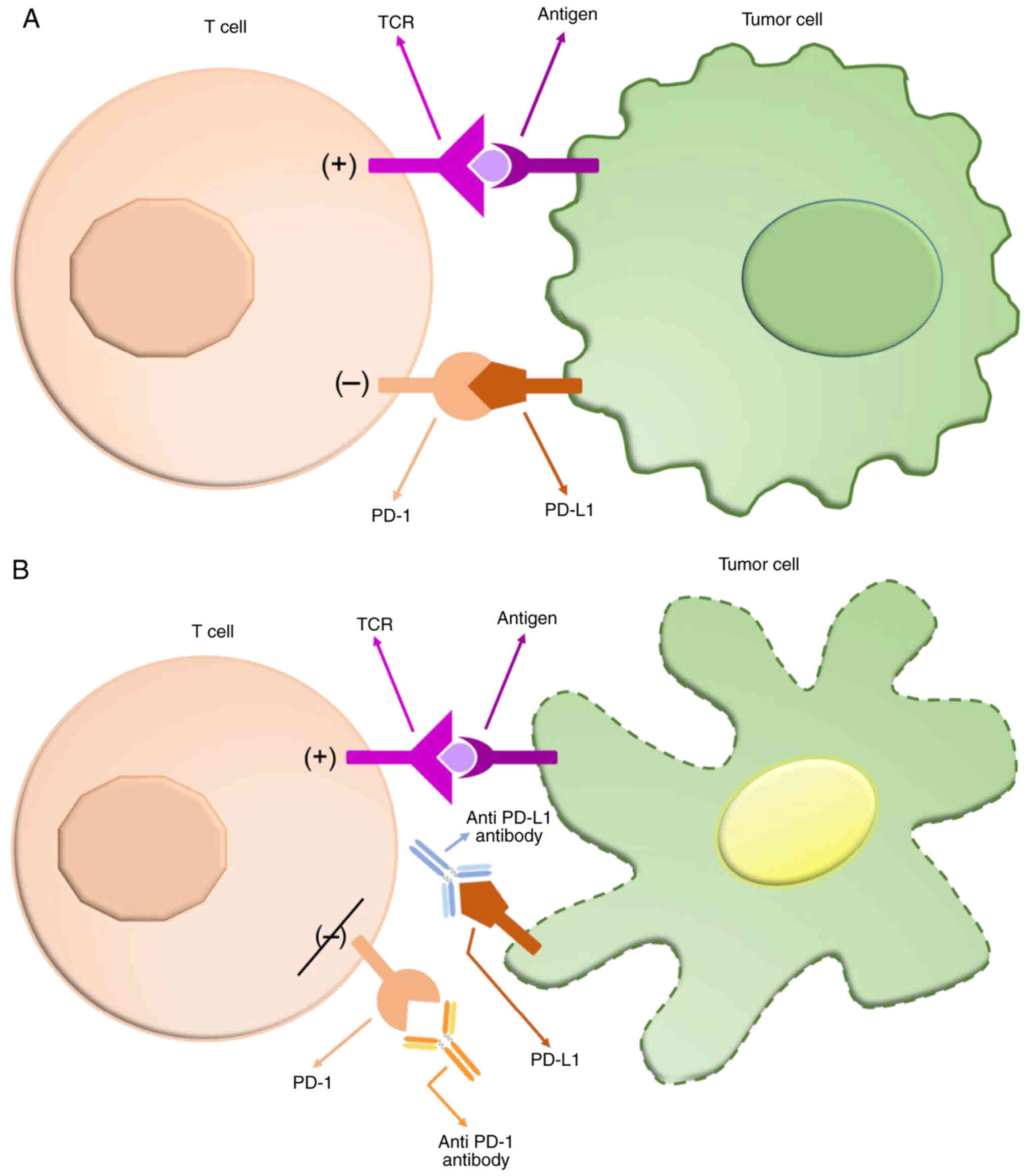
Colorectal cancer (CRC) is one of the most
frequently diagnosed types of cancer globally and one of the most
prevalent causes of cancer-related death (1). CRC has a high percentage of morbidity
and mortality (2), and has been shown
to be associated with age, bacterial and viral infections, smoking,
alcohol consumption, ulcerative colitis, genetic mutations,
sedentary lifestyles and immunosuppression (3).
Surgery, chemotherapy, biological agents and/or
radiotherapy comprise the existing modes of treatment for CRC. For
second-line therapy, immunotherapy is garnering increasing
attention and use, with anti-programmed cell death protein 1
(PD-1)/programmed death ligand-1 (PD-L1) immunotherapy showing
promising results in melanoma and other types of solid tumors
(4).
Since 2011, six antibodies against PD-1 or PD-L1
have been used as anti-cancer treatments following approval by the
FDA (5): Atezolizumab (PD-L1
inhibitor), nivolumab (PD-1 inhibitor), durvalumab (PD-L1
inhibitor), avelumab (PD-L1 inhibitor), pembrolizumab (PD-1
inhibitor) and cemiplimab (PD-1 inhibitor). Pembrolizumab has been
in use since 2017 for the treatment of microsatellite instability
(MSI)-high metastatic CRC if the disease progresses following
treatment with 5-FU, oxaliplatin or irinotecan based regimens, with
promising results (6). For refractory
MSI-high metastatic CRC, the CheckMate 142 phase II clinical trial
showed that nivolumab may adequately control the disease (7).
It is possible that these novel molecular targets
may revolutionize the management of solid tumors by adding an
immunomodulatory component to the existing cytotoxic arsenal. In
this context, the present review aims to summarize the current body
of knowledge regarding targeting of PD-1/PD-L1 in the treatment of
colorectal neoplasms, and to explore their significance in changing
the landscape of medical oncology.
PD-1 is a checkpoint protein and a member of the
CD28 family that was discovered and named by Ishida et al
(8) in 1992. It is expressed on
activated T cells and interacts with its two ligands, PD-L1
(B7-H1/CD274) and programmed death ligand 2 (PD-L2/B7-DC),
inhibiting the activation of T-cells and the production of
cytokines (9) (Fig. 1).
PD-1 and its respective ligand molecule are immune
checkpoint molecules that deliver co-inhibitory signals that
suppress exaggerated immune responses (10). In solid tumors, overactivation of the
intracellular signaling cascades involving MAPK and PI3K-Akt
pathways, as well as increased activity of the STAT3 transcription
factors all lead to increased expression of PD-L1 on the cellular
membranes of cancer cells (11).
Moreover, the presence of inflammatory mediators, such as
interferon-γ and interleukin-6 further enhances PD-L1 transcription
(12). Consequently, the presence of
PD-L1 on the propagating tumor front binds to PD-1 molecules
present on the surrounding populations of CD8+ T-cells
suppressing their clonal expansion and thus evading the suppressive
innate immune response (13).
In CRC, PD-L1 is primarily expressed on
tumor-infiltrating cells- such as B and T lymphocytes, macrophages,
dendritic cells, other bone marrow-derived innate immune cells and
vascular endothelial cells, providing valuable targets for the
development of immune-oriented therapies (14–17). In a
recent study, PD-L1 expression was more prevalent in liver and lung
metastatic foci compared to the primary tumor (18).
In CRC, high densities of tumor-infiltrating
lymphocytes (TILs) have been detected (19), and an association between the density
of T cell subpopulations (CD8+, CD45RO+ and
FOXP3+) and a favorable clinical outcome has been
previously shown (20). Within the
microenvironment of the tumor, accumulation of CD8+TILs
(termed a ‘hot’ tumor microenvironment) has been shown to be
correlated with MSI-high tumors, absence of perineural invasion,
lymph node metastases, and high grade and proximal colon tumors.
Tumors that are PD-L1+/TIL+ may benefit more
from PD-1/PD-L1 blockade immunotherapy combined with conventional
chemotherapy (20). Subsequently,
TILs may possibly be used as a prognostic biomarker and a means of
tumor classification (21). Galon
et al (22) showed that CRC
patients that had low densities of CD3+ cells and
CD45RO+ memory T cells in both the center of the tumor
and the invasive margin had a similarly poor prognosis to patients
that had synchronous distant metastases.
It is well known that MSI-high tumors are commonly
high grade proximal tumors that produce mucin, and exhibit
increased inflammatory reactions and an exophytic pattern of growth
(23–25). These tumors are also associated with a
decreased risk of metastasis and improved prognosis (26,27).
Patients with MSI-high or MMR deficient CRC exhibit improved
responses to PD-1/PD-L1 immunotherapy and improved survival rates,
suggestive of upregulation of tumor CD274 expression due to a high
mutation burden in the tumor (28,29). Wyss
et al (30) showed that
increased PD-L1 expression was primarily associated with
right-sided CRC tumors, females and elder patients. Interestingly,
PD-L1 expression was significantly higher in MSI-high tumors,
particularly in those harboring a BRAF mutation.
The expression status of PTEN in comparison with
PD-L1 expression and clinical variables in CRC was studied by Song
et al (31). Increased PD-L1
expression was correlated with distant metastases (P<0.01) and
less favorable pathological features (P<0.01). Furthermore,
PD-L1 expression was correlated with PTEN expression, whereas PTEN
expression was not correlated with staging variables or overall and
metastasis-free survival. A possible interplay between PTEN and
PD-L1 expression is hinted by the results of their study, and
possibly a trend towards increased rates of tumor dissemination in
patients with PD-L1 positive CRC can be inferred, although the lack
of prospective clinically relevant data is a major caveat.
Several types of cancer have been analyzed with
regard to their PD-1 or PD-L1 expression profiles, and the effects
of the subsequent therapy with anti-PD-1/anti-PD-L1 antibodies have
been demonstrated in patients with melanoma, non-small cell lung
and renal cancer (32–38).
Nonetheless, evidence now suggests improved patient
outcomes may be achieved using PD-1/PD-L1 blockade therapy in
several gastrointestinal malignancies, such as esophageal, gastric,
liver and CRC (39). Results from the
KEYNOTE-158 Phase II Clinical Study showed pembrolizumab
administration improved outcomes in patients with non-resectable
MSI-high non-CRC following failure of standard therapy (40). Similarly, in gastric cancer, it is
evident that PD-L1 positive staining (>5% on tumor cells) is
indicative of a poorer prognosis. When PD-L1 expression is combined
with the MSI status or the extent of CD8+TIL invasion,
the PD-L1(+)/non-MSI and PD-L1(+)/CD8-low subgroups were correlated
with poor prognosis, with higher HR compared with the
PD-L1(+)-alone subgroup, emphasizing the potential of these
biomarkers in distinguishing aggressive disease subtypes (41).
Other neoplasms that can also be treated with anti-
PD-1/PD-L1 immunotherapy are: Hodgkin's lymphoma, head and neck
squamous cell carcinoma, hepatocellular carcinoma, mediastinal
lymphoma (large B-cell), cancer of the urinary bladder, Merkel cell
carcinoma and MSI-high or MMR-deficient solid tumors (17).
Taking into account the biomechanics of the
interaction between PD-1 and PD-L1, analysis of two different
expression profiles is mandated to evaluate a relationship between
expression of these biomarkers and patient survival. Specifically,
PD-L1 overexpression gains significance when it is observed in the
tumor infiltrating front, whereas expression of its molecular
receptor is primarily relevant in the surrounding inflammatory
cells of the innate immune system (TILs) (43). Based on this paradigm, increased
expression of PD-L1 leads to immune evasion and suppressed
expression of PD-1 results in enhanced penetration of the tumor by
cytotoxic immune cells.
A group of 572 patients diagnosed with stage II CRC
were studied with the aim of identifying patients that may benefit
from adjuvant therapy (49). The 5%
cut-off of PD-L1 positive staining on tumor cells was used, as in
previous studies (50–52). Overall, 6% of the colon tumors were
classed as high PD-L1, and high PD-L1 expression was significantly
associated with females, higher grade cancer, right sided tumors
and MSI (high PD-L1 in 18% of MSI vs. 1% in MSS tumors), but not
with survival.
When reviewed in tandem, the previously discussed
studies exhibited a greater degree of variability with regard to
the effect of PD-1/PD-L1 expression on stage-stratified CRC patient
survival. Although a correlation was identified, the retrospective
nature of these studies does not allow for the establishment of a
direct causal relationship between PD-1/PD-L1 expression and poor
survival, and hence, no concrete conclusions can be drawn from the
current body of literature. PD-1 and PD-L1 overexpression may
likely be another facet of the aggressive neoplastic phenotype that
encompasses perineural and microscopic lymphovascular invasion, and
consequently, may not be directly implicated in the biological
process of disease propagation and metastatic dissemination.
PD-L1 expression can also be utilized as an
independent factor for assessing the suitability of patients for
immunotherapy, as PD-L1 expression is commonly expressed in
colorectal metastases in comparison with the primary tumors (81.8%
in metastatic nodes vs. 40.9% in primary CRC) (18). This difference can be attributed to
genomic alterations that accrue during the metastatic spread of the
tumor (53,54) and post-translational modifications
(phosphorylation, glycosylation and/or ubiquitination) that
upregulate the expression of PD-L1 (55).
Of note, cancer patients had a worse prognosis when
treated with anti-PD-1/PD-L1 if they suffered from disorders of the
intestinal flora (63), revealing a
modulating role of the gut microbiota in the responses to immune
checkpoint blockade (64). Further
studies are required to determine the role of the intestinal flora
in relation to immunotherapy.
Serrated adenomas and hyperplastic polyps have been
considered as precursors of colorectal carcinomas that appear in
the right colon and are MSI-high (65,66).
However, a study by Tuppurainen et al (67) showed there was no significant
difference in MSI status between serrated and non-serrated
carcinomas. The prognostic importance of PD-L1 expression in
serrated adenocarcinomas was studied by Zhu et al (68); the 3-year overall survival was 58.4%
in serrated adenocarcinomas with high PD-L1 expression compared
with 72.5% in serrated adenocarcinomas with low PD-L1 expression,
although the difference was not significant.
Rectal cancer, is a separate subtype of CRC, and the
rate of local recurrence is reduced by neoadjuvant chemotherapy,
radiotherapy and total mesorectal excision (69–71).
Studies have attempted to survey the difference in the expression
of PD-L1 in patients with rectal cancer before and after
neoadjuvant chemo/radiotherapy, and to assess the potential use of
PD-L1 as predictive marker or therapeutic target (72–74).
The relationship between PD-L1 positive staining on
tumor cells and clinical as well as pathological variables in
rectal cancer following neoadjuvant therapy was retrospectively
studied by Saigusa et al (74). High PD-L1 expression was associated
with tumor recurrence and vascular invasion. Additionally, high
PD-L1 expression was associated with poor recurrence free and
overall survival rates. Thus, PD-L1 inhibition may reduce tumor
recurrence and improve outcomes in patients with rectal cancer who
undergo surgical resection following neoadjuvant therapy.
These results are in agreement with studies on other
types of cancer. Which showed that chemo/radiotherapy results in
upregulation of PD-L1 expression (75–78).
Further studies are required for patients with rectal cancer to
confirm this hypothesis. Existing studies suggest that different
patient survival profiles are expected dependent on whether PD-L1
expression is upregulated in the tumor itself or in the surrounding
reactive immune front. Based on existing studies, the former is
associated with a worse survival outcomes (74), whereas the latter is associated with
increased survival and improved outcomes following surgery
(73). Ultimately, PD-L1 profiling
may serve as a valuable therapeutic tool in patients with rectal
cancer and low PD-L1 expression that may potentially be eligible
for organ sparing surgery.
There are six anti-PD-1 and anti-PD-L1 therapeutic
antibodies currently in therapeutic use. Several studies have
assessed the efficacy of these agents as a monotherapy or in
combination with chemotherapy (81),
radiotherapy (82), targeted
therapies (83), antiviral drugs,
neoantigen tumor vaccines (84) or
with other immune-modulatory drugs (85).
Nivolumab, is an antibody that blocks PD-1 and has
been used for treating patients with advanced or metastatic
melanoma and metastatic squamous-cell lung cancer (86,87), with
potential effectiveness for treatment of Hodgkin's lymphoma
(88) and hepatocellular carcinoma
(89). Several studies that included
patients with CRC showed that nivolumab is well tolerated and that
PD-L1 expression on tumor cells may serve as a predictive biomarker
of response to therapy (32,90,91).
CRC in a phase II study. In this study, 74 patients
were enrolled. A median follow-up of 12 months was assessed and 51
of the patients had favorable disease control for ≥12 weeks
(12-month overall survival, 73%). A study assessing
co-administration of two immunotherapy drugs, nivolumab and
ipilimumab (anti-CTLA-4 antibody), was also performed by Overman
et al (92). Progression-free
survival was 76% after 9 months and 71% after 12 months. Overall
survival was estimated to be 87 and 85% respectively, highlighting
a promising combination treatment for MSI-H/MMR-deficient
patients.
Pembrolizumab, is an anti-PD-1 antibody that is
currently used for treating patients diagnosed with advanced stage
melanoma (93), urothelial cancer
(advanced or metastatic) (94),
advanced non-small cell lung cancer (95), Hodgkin's lymphoma (96) and squamous cell carcinoma of the head
and neck (97). In the multicohort
KEYNOTE-028 trial, patients with advanced CRC were treated with
pembrolizumab, regardless of their MSI status (98). Median progression free survival was
1.8 months, with 6 and 12-month progression free survival rates of
17.4 and 4.3%, respectively. Median overall survival was 5.3
months, with 6 and 12-month overall survival rates of 43.5 and
29.8%, respectively. No serious side effects were reported,
highlighting the safety profile of the drug.
There are three anti-PD-L1 antibodies currently used
for treatment of other types of cancer other than CRC: Atezolizumab
(100–103), avelumab (104,105)
and duravulumab (106,107). Atezolizumab may show promising
results when administered to patients with MMR-deficient colorectal
tumors, particularly when co-administered with chemotherapy and/or
targeted therapy (108).
Several clinical trials are in progress assessing
the efficacy of anti-PD-L1 antibodies, particularly in patients
with MSI-high colorectal tumors, and they are showing encouraging
results (109), even though the
MSI-high/MMR-deficient patient subgroup constitutes only ~5% of
those diagnosed with metastatic cancer.
PD-1/PD-L1 interaction is a remarkable regulatory
immune system mechanism, with PD-L1 being primarily expressed on
tumor cells in several types of tumor, including CRC (57). Subsequently, inhibition of the
interaction between PD-1 and PD-L1 can induce an anti-tumor effect.
Additionally, integration of these novel immune therapies may
radically change the landscape of preoperative therapy,
particularly in cases of rectal cancer, in which organ preserving
surgery is recommended.
At present, there are several studies in progress
assessing the safety and efficacy of drugs targeting immune
checkpoints in association with PD-L1 and PD-1 expression in CRC.
The primary issue that needs to be answered before these drugs can
be used clinically are: What group of patients will benefit most
from these therapies with regard to their MSI/MMR profile, the
extent of CD8+TIL infiltration and the status of
PD-1/PD-L1 expression. Combinatorial approaches should be taken
into consideration, taking into account the safety of the discussed
therapeutic regimens. We are now in a new era of cancer therapies
with the hope of improved survival and quality of life.
Not applicable.
No funding was received.
The datasets generated and analysed during the
current study are all available in the PUBMED repository.
VN, KS and EP conceived the study. VN, DP, PF and IP
designed the study. DP, AP, VN and IA acquired and analyzed the
data. DP, PF, KS and VN interpreted the data. VN, DP, PF and EP
drafted the manuscript. IA designed and executed the schematic
presentation of Figure 1. All authors
revised, read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Araghi M, Soerjomataram I, Jenkins M,
Brierley J, Morris E, Bray F and Arnold M: Global trends in
colorectal cancer mortality: Projections to the year 2035. Int J
Cancer. 144:2992–3000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yaghoubi N, Soltani A, Ghazvini K,
Hassanian SM and Hashemy SI: PD-1/PD-L1 blockade as a novel
treatment for colorectal cancer. Biomed Pharmacother. 110:312–318.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gandini S, Massi D and Mandalà M: PD-L1
expression in cancer patients receiving anti PD-1/PD-L1 antibodies:
A systematic review and meta-analysis. Crit Rev Oncol Hematol.
100:88–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee HT, Lee SH and Heo YS: Molecular
interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in
immuno-oncology. Molecules. 24:11902019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimura H, Okazaki T, Tanaka Y, Nakatani
K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N
and Honjo T: Autoimmune dilated cardiomyopathy in PD-1
receptor-deficient mice. Science. 291:319–322. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akbay EA, Koyama S, Carretero J, Altabef
A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp
EM, Pugh TJ, et al: Activation of the PD-1 pathway contributes to
immune escape in EGFR-driven lung tumors. Cancer Discov.
3:1355–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flies DB and Chen L: Modulation of immune
response by B7 family molecules in tumor microenvironments. Immunol
Invest. 35:395–418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Black M, Barsoum IB, Truesdell P,
Cotechini T, Macdonald-Goodfellow SK, Petroff M, Siemens DR, Koti
M, Craig AW and Graham CH: Activation of the PD-1/PD-L1 immune
checkpoint confers tumor cell chemoresistance associated with
increased metastasis. Oncotarget. 7:10557–10567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valentini AM, Pinto FDi, Cariola F, Guerra
V, Giannelli G, Caruso ML and Pirrelli M: PD-L1 expression in
colorectal cancer defines three subsets of tumor immune
microenvironments. Oncotarget. 9:8584–8596. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Re. 20:5064–5074. 2014. View Article : Google Scholar
|
|
16
|
Patel SP and Kurzrock R: PD-L1 Expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L
and Liu X: Application of PD-1 blockade in cancer immunotherapy.
Comput Struct Biotechnol J. 17:661–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang HB, Yao H, Li CS, Liang LX, Zhang Y,
Chen YX, Fang JY and Xu J: Rise of PD-L1 expression during
metastasis of colorectal cancer: Implications for immunotherapy. J
Dig Dis. 18:574–581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koelzer VH, Lugli A, Dawson H, Hädrich M,
Berger MD, Borner M, Mallaev M, Galván JA, Amsler J, Schnüriger B,
et al: CD8/CD45RO T-cell infiltration in endoscopic biopsies of
colorectal cancer predicts nodal metastasis and survival. J Transl
Med. 12:812014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang CY, Chiang SF, Ke TW, Chen TW, You
YS, Chen WT and Chao KSC: Clinical significance of programmed death
1 ligand-1 (CD274/PD-L1) and intra-tumoral CD8+ T-cell
infiltration in stage II–III colorectal cancer. Sci Rep.
8:156582018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bupathi M and Wu C: Biomarkers for immune
therapy in colorectal cancer: Mismatchrepair deficiency and others.
J Gastrointest Oncol. 7:713–720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alexander J, Watanabe T, Wu TT, Rashid A,
Li S and Hamilton SR: Histopathological identification of colon
cancer with microsatellite instability. Am J Pathol. 158:527–535.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ward R, Meagher A, Tomlinson I, O'Connor
T, Norrie M, Wu R and Hawkins N: Microsatellite instability and the
clinicopathological features of sporadic colorectal cancer. Gut.
48:821–829. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang JT, Huang KC, Cheng AL, Jeng YM, Wu
MS and Wang SM: Clinicopathological and molecular biological
features of colorectal cancer in patients less than 40 years of
age. Br J Surg. 90:205–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin CC, Lin JK, Lin TC, Chen WS, Yang SH,
Wang HS, Lan YT, Jiang JK, Yang MH and Chang SC: The prognostic
role of microsatellite instability, codon-specific KRAS, and BRAF
mutations in colon cancer. J Surg Oncol. 110:451–457. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gryfe R, Kim H, Hsieh ET, Aronson MD,
Holowaty EJ, Bull SB, Redston M and Gallinger S: Tumor
microsatellite instability and clinical outcome in young patients
with colorectal cancer. N Engl J Med. 342:69–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosenbaum MW, Bledsoe JR, Morales-Oyarvide
V, Huynh TG and Mino-Kenudson M: PD-L1 expression in colorectal
cancer is associated with microsatellite instability, BRAF
mutation, medullary morphology and cytotoxic tumor-infiltrating
lymphocytes. Mod Pathol. 29:1104–1112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wyss J, Dislich B, Koelzer VH, Galván JA,
Dawson H, Hädrich M, Inderbitzin D, Lugli A, Zlobec I and Berger
MD: Stromal PD-1/PD-L1 expression predicts outcome in colon cancer
patients. Clin Colorectal Cancer. 18:e20–e38. 2019. View Article : Google Scholar
|
|
31
|
Song M, Chen D, Lu B, Wang C, Zhang J,
Huang L, Wang X, Timmons CL, Hu J, Liu B, et al: PTEN loss
increases PD-L1 protein expression and affects the correlation
between PD-L1 expression and clinical parameters in colorectal
cancer. PLoS One. 8:e658212013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tykodi SS: PD-1 as an emerging therapeutic
target in renal cell carcinoma: Current evidence. Onco Targets
Ther. 7:1349–1359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Massari F, Santoni M, Ciccarese C, Santini
D, Alfieri S, Martignoni G, Brunelli M, Piva F, Berardi R,
Montironi R, et al: PD-1 blockade therapy in renal cell carcinoma:
Current studies and future promises. Cancer Treat Rev. 41:114–121.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ivashko IN and Kolesar JM: Pembrolizumab
and nivolumab: PD-1 inhibitors for advanced melanoma. Am J Health
Syst Pharm. 73:193–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martin-Liberal J, Kordbacheh T and Larkin
J: Safety of pembrolizumab for the treatment of melanoma. Expert
Opin Drug Saf. 14:957–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sui H, Ma N, Wang Y, Li H, Liu X, Su Y and
Yang J: Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer:
Toward personalized medicine and combination strategies. J Immunol
Res. 2018:69849482018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia L, Liu Y and Wang Y: PD-1/PD-L1
blockade therapy in advanced non-small-cell lung cancer: Current
status and future directions. Oncologist. 24 (Suppl 1):S31–S41.
2019. View Article : Google Scholar
|
|
39
|
Myint ZW and Goel G: Role of modern
immunotherapy in gastrointestinal malignancies: A review of current
clinical progress Ahmed Tarhini; Timothy Burns; Rahul Parikh;
Guarvel Goel; Annie im. J Hematol Oncol. 10:1–12. 2017. View Article : Google Scholar
|
|
40
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, de Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morihiro T, Kuroda S, Kanaya N, Kakiuchi
Y, Kubota T, Aoyama K, Tanaka T, Kikuchi S, Nagasaka T, Nishizaki
M, et al: PD-L1 expression combined with microsatellite
instability/CD8+ tumor infiltrating lymphocytes as a
useful prognostic biomarker in gastric cancer. Sci Rep. 9:46332019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang B, Chen L, Bao C, Sun C, Li J, Wang
L and Zhang X: The expression status and prognostic significance of
programmed cell death 1 ligand 1 in gastrointestinal tract cancer:
A systematic review and meta-analysis. Onco Targets Ther.
8:2617–2625. 2015.PubMed/NCBI
|
|
43
|
Ko YS and Pyo JS: Clinicopathological
significance and prognostic role of tumor-infiltrating lymphocytes
in colorectal cancer. Int J Biol Markers. 34:132–138. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Droeser RA, Hirt C, Viehl CT, Frey DM,
Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A,
Rosso R, et al: Clinical impact of programmed cell death ligand 1
expression in colorectal cancer. Eur J Cancer. 49:2233–2242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Y, Liang L, Dai W, Cai G, Xu Y, Li X,
Li Q and Cai S: Prognostic impact of programed cell death-1 (PD-1)
and PD-ligand 1 (PD-L1) expression in cancer cells and tumor
infiltrating lymphocytes in colorectal cancer. Mol Cancer.
15:552016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Enkhbat T, Nishi M, Takasu C, Yoshikawa K,
Jun H, Tokunaga T, Kashihara H, Ishikawa D and Shimada M:
Programmed cell death ligand 1 expression is an independent
prognostic factor in colorectal cancer. Anticancer Res.
38:3367–3373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Udager AM, Liu TY, Skala SL, Magers MJ,
McDaniel AS, Spratt DE, Feng FY, Siddiqui J, Cao X, Fields KL, et
al: Frequent PD-L1 expression in primary and metastatic penile
squamous cell carcinoma: Potential opportunities for
immunotherapeutic approaches. Ann Oncol. 27:1706–1712. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Slater NA and Googe PB: PD-L1 expression
in cutaneous squamous cell carcinoma correlates with risk of
metastasis. J Cutan Pathol. 43:663–670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eriksen AC, Sørensen FB, Lindebjerg J,
Hager H, dePont Christensen R, Kjær-Frifeldt S and Hansen TF:
Programmed death ligand-1 expression in stage II colon
cancer-experiences from a nationwide populationbased cohort. BMC
Cancer. 19:1422019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim JH, Park HE, Cho NY, Lee HS and Kang
GH: Characterisation of PD-L1-positive subsets of
microsatellite-unstable colorectal cancers. Br J Cancer.
115:490–496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Koganemaru S, Inoshita N, Miura Y, Miyama
Y, Fukui Y, Ozaki Y, Tomizawa K, Hanaoka Y, Toda S, Suyama K, et
al: Prognostic value of programmed death-ligand 1 expression in
patients with stage III colorectal cancer. Cancer Sci. 108:853–858.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee KS, Kwak Y, Ahn S, Shin E, Oh HK, Kim
DW, Kang SB, Choe G, Kim WH and Lee HS: Prognostic implication of
CD274 (PD-L1) protein expression in tumor-infiltrating immune cells
for microsatellite unstable and stable colorectal cancer. Cancer
Immunol Immunother. 66:927–939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mehta KR, Nakao K, Zuraek MB, Ruan DT,
Bergsland EK, Venook AP, Moore DH, Tokuyasu TA, Jain AN, Warren RS,
et al: Fractional genomic alteration detected by array-based
comparative genomic hybridization independently predicts survival
after hepatic resection for metastatic colorectal cancer. Clin
Cancer Res. 11:1791–1797. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang H, Liang L, Fang JY and Xu J: Somatic
gene copy number alterations in colorectal cancer: New quest for
cancer drivers and biomarkers. Oncogene. 35:2011–2019. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo
CW, Khoo KH, Chang SS, Cha JH, Kim T, et al: Glycosylation and
stabilization of programmed death ligand-1 suppresses T-cell
activity. Nat Commun. 7:126322016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu P, Wu D, Li L, Chai Y and Huang J:
PD-L1 and survival in solid tumors: A meta-analysis. PLoS One.
10:e01314032015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M,
Meng YL, Yang AG and Wen WH: B7-H1 Expression is associated with
poor prognosis in colorectal carcinoma and regulates the
proliferation and invasion of HCT116 colorectal cancer cells. PLoS
One. 8:e760122013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhu J, Chen L, Zou L, Yang P, Wu R, Mao Y,
Zhou H, Li R, Wang K, Wang W, et al: miR-20b, −21, and −130b
inhibit PTEN expression resulting in B7-H1 over-expression in
advanced colorectal cancer. Hum Immunol. 75:348–353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shen Z, Gu L, Mao D, Chen M and Jin R:
Clinicopathological and prognostic significance of PD-L1 expression
in colorectal cancer: A systematic review and meta-analysis. World
J Surg Oncol. 17:42019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang Y, Ahn YH, Chen Y, Tan X, Guo L,
Gibbons DL, Ungewiss C, Peng DH, Liu X, Lin SH, et al: ZEB1
sensitizes lung adenocarcinoma to metastasis suppression by PI3K
antagonism. J Clin Invest. 124:2696–2708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cao H, Wang Q, Gao Z, Yu Z, Wu Y and Lu Q:
Programmed death-ligand 1 and survival in colorectal cancers: A
meta-analysis. Int J Biol Markers. 34:356–363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li Y, He M, Zhou Y, Yang C, Wei S, Bian X,
Christopher O and Xie L: The Prognostic and clinicopathological
roles of PD-L1 expression in colorectal cancer: A systematic review
and meta-analysis. Front Pharmacol. 10:1392019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Helmink BA, Khan MAW, Hermann A,
Gopalakrishnan V and Wargo JA: The microbiome, cancer, and cancer
therapy. Nat Med. 25:377–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Goldstein NS, Bhanot P, Odish E and Hunter
S: Hyperplastic-like colon polyps that preceded
microsatellite-unstable adenocarcinomas. Am J Clin Pathol.
119:778–796. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Iino H, Jass JR, Simms LA, Young J,
Leggett B, Ajioka Y and Watanabe H: DNA microsatellite instability
in hyperplastic polyps, serrated adenomas, and mixed polyps: A mild
mutator pathway for colorectal cancer? J Clin Pathol. 52:5–9. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tuppurainen K, Mäkinen JM, Junttila O,
Liakka A, Kyllönen AP, Tuominen H, Karttunen TJ and Mäkinen MJ:
Morphology and microsatellite instability in sporadic serrated and
non-serrated colorectal cancer. J Pathol. 207:285–294. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhu H, Qin H, Huang Z, Li S, Zhu X, He J,
He J, Yang J, Yu X and Yi X: Clinical significance of programmed
death ligand-1 (PD-L1) in colorectal serrated adenocarcinoma. Int J
Clin Exp Pathol. 8:9351–9359. 2015.PubMed/NCBI
|
|
69
|
Guillem JG, Chessin DB, Cohen AM, Shia J,
Mazumdar M, Enker W, Paty PB, Weiser MR, Klimstra D, Saltz L, et
al: Long-term oncologic outcome following preoperative combined
modality therapy and total mesorectal excision of locally advanced
rectal cancer. Ann Surg. 241:828–829. 2005. View Article : Google Scholar
|
|
70
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC; EORTC Radiotherapy Group Trial 22921, : Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sauer R, Becker H, Hohenberger W, Rodel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative versus postoperative chemoradiotherapy for
rectal cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jomrich G, Silberhumer GR, Marian B, Beer
A and Mullauer L: Programmed death-ligand 1 expression in rectal
cancer. Eur Surg. 48:352–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hecht M, Büttner-Herold M,
Erlenbach-Wünsch K, Haderlein M, Croner R, Grützmann R, Hartmann A,
Fietkau R and Distel LV: PD-L1 is upregulated by radiochemotherapy
in rectal adenocarcinoma patients and associated with a favourable
prognosis. Eur J Cancer. 65:52–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Saigusa S, Toiyama Y, Tanaka K, Inoue Y,
Mori K, Ide S, Imaoka H, Kawamura M, Mohri Y and Kusunoki M:
Implication of programmed cell death ligand 1 expression in tumor
recurrence and prognosis in rectal cancer with neoadjuvant
chemoradiotherapy. Int J Clin Oncol. 21:946–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lim SH, Hong M, Ahn S, Choi YL, Kim KM, Oh
D, Ahn YC, Jung SH, Ahn MJ, Park K, et al: Changes in tumour
expression of programmed death-ligand 1 after neoadjuvant
concurrent chemoradiotherapy in patients with squamous oesophageal
cancer. Eur J Cancer. 52:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wu CT, Chen WC, Chang YH, Lin WY and Chen
MF: The role of PD-L1 in the radiation response and clinical
outcome for bladder cancer. Sci Rep. 6:197402016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sheng J, Fang W, Yu J, Chen N, Zhan J, Ma
Y, Yang Y, Huang Y, Zhao H and Zhang L: Expression of programmed
death ligand-1 on tumor cells varies pre and post chemotherapy in
non-small cell lung cancer. Sci Rep. 6:200902016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wimberly H, Brown JR, Schalper K, Haack H,
Silver MR, Nixon C, Bossuyt V, Pusztai L, Lannin DR and Rimm DL:
PD-L1 expression correlates with tumor-infiltrating lymphocytes and
response to neoadjuvant chemotherapy in breast cancer. Cancer
Immunol Res. 3:326–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hamada T, Cao Y, Qian ZR, Masugi Y, Nowak
JA, Yang J, Song M, Mima K, Kosumi K, Liu L, et al: Aspirin use and
colorectal cancer survival according to tumor CD274 (programmed
cell death 1 ligand 1) expression status. J Clin Oncol.
35:1836–1844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li P, Wu H, Zhang H, Shi Y, Xu J, Ye Y,
Xia D, Yang J, Cai J and Wu Y: Aspirin use after diagnosis but not
prediagnosis improves established colorectal cancer survival: A
meta-analysis. Gut. 64:1419–1425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lake RA and Robinson BW: Immunotherapy and
chemotherapy-a practical partnership. Nat Rev Cancer. 5:397–405.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Simeone E, Grimaldi AM, Festino L,
Giannarelli D, Vanella V, Palla M, Curvietto M, Esposito A,
Palmieri G, Mozzillo N and Ascierto PA: Correlation between
previous treatment with BRAF inhibitors and clinical response to
pembrolizumab in patients with advanced melanoma. Oncoimmunology.
6:e12834622017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sahin U and Tureci O: Personalized
vaccines for cancer immunotherapy. Science. 359:1355–1360. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Callahan MK, Postow MA and Wolchok JD:
CTLA-4 and PD-1 pathway blockade: Combinations in the Clinic. Front
Oncol. 4:3852014.
|
|
86
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Horn L, Spigel DR, Vokes EE, Holgado E,
Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L,
et al: Nivolumab versus docetaxel in previously treated patients
with advanced non-small-cell lung cancer: Two-year outcomes from
two randomized, open-label, Phase III trials (CheckMate 017 and
CheckMate 057). J Clin Oncol. 35:3924–3933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Armand P, Engert A, Younes A, Fanale M,
Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R,
Cohen JB, et al: Nivolumab for Relapsed/refractory classic hodgkin
lymphoma after failure of autologous Hematopoietic cell
transplantation: Extended follow-Up of the multicohort single-arm
Phase II checkmate 205 Trial. J Clin Oncol. 36:1428–1439. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Brahmer JR, Drake CG, Wollner I, Powderly
JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller
TL, et al: Phase I study of single-agent anti-programmed death-1
(MDX-1106) in refractory solid tumors: Safety, clinical activity,
pharmacodynamics, and immunologic correlates. J Clin Oncol.
28:3167–3175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yamamoto N, Nokihara H, Yamada Y, Shibata
T, Tamura Y, Seki Y, Honda K, Tanabe Y, Wakui H and Tamura T: Phase
I study of Nivolumab, an anti-PD-1 antibody, in patients with
malignant solid tumors. Invest New Drugs. 35:207–216. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-Deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ribas A, Hamid O, Daud A, Hodi FS, Wolchok
JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al:
Association of pembrolizumab with tumor response and survival among
patients with advanced melanoma. JAMA. 315:1600–1609. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gallacher D, Armoiry X, Auguste P, Court
R, Mantopoulos T, Patterson J, De Santis M, Cresswell J and Mistry
H: Pembrolizumab for previously treated advanced or metastatic
urothelial cancer: An evidence review group perspective of a NICE
single technology appraisal. Pharmacoeconomics. 37:19–27. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder J, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Armand P, Shipp MA, Ribrag V, Michot JM,
Zinzani PL, Kuruvilla J, Snyder ES, Ricart AD, Balakumaran A, Rose
S and Moskowitz CH: Programmed Death-1 Blockade with pembrolizumab
in patients with classical hodgkin lymphoma after brentuximab
vedotin failure. J Clin Oncol. 34:3733–3739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tahara M, Muro K, Hasegawa Y, Chung HC,
Lin CC, Keam B, Takahashi K, Cheng JD and Bang YJ: Pembrolizumab in
Asia-Pacific patients with advanced head and neck squamous cell
carcinoma: Analyses from KEYNOTE-012. Cancer Sci. 109:771–776.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
O'Neil BH, Wallmark JM, Lorente D, Elez E,
Raimbourg J, Gomez-Roca C, Ejadi S, Piha-Paul SA, Stein MN, Abdul
Razak AR, et al: Safety and antitumor activity of the anti-PD-1
antibody pembrolizumab in patients with advanced colorectal
carcinoma. PLoS One. 12:e01898482017. View Article : Google Scholar
|
|
99
|
Le DT, Kim TW, Van Cutsem E, Geva R, Jäger
D, Hara H, Burge M, O'Neil B, Kavan P, Yoshino T, et al: Phase II
Open-label study of pembrolizumab in treatment-refractory,
microsatellite instability-high/mismatch repair-deficient
metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 38:11–19.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
McDermott DF, Sosman JA, Sznol M, Massard
C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV,
et al: Atezolizumab, an anti-programmed death-ligand 1 antibody, in
metastatic renal cell carcinoma: Long-term safety, clinical
activity, and immune correlates from a phase ia study. J Clin
Oncol. 34:833–842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bernard-Tessier A, Bonnet C, Lavaud P,
Gizzi M, Loriot Y and Massard C: Atezolizumab
(Tecentriq®): Activity, indication and modality of use
in advanced or metastatic urinary bladder carcinoma. Bull Cancer.
105:140–145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
No authors listed, . Atezolizumab Extends
Survival for Breast Cancer. Cancer Discov. Jun. 7:OF102017.
|
|
104
|
D'Angelo SP, Russell J, Lebé C,
Chmielowski B, Gambichler T, Grob JJ, Kiecker F, Rabinowits G,
Terheyden P, Zwiener I, et al: Efficacy and safety of first-line
avelumab treatment in patients with stage iv metastatic merkel cell
carcinoma: A preplanned interim analysis of a clinical trial. JAMA
Oncol. 4:e1800772018. View Article : Google Scholar
|
|
105
|
Gulley JL, Rajan A, Spigel DR, Iannotti N,
Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, et
al: Avelumab for patients with previously treated metastatic or
recurrent non-small-cell lung cancer (JAVELIN Solid Tumor):
Dose-expansion cohort of a multicentre, open-label, phase 1b trial.
Lancet Oncol. 18:599–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Siu LL, Even C, Mesia R, Remenar E, Daste
A, Delord JP, Krauss J, Saba NF, Nabell L, Ready NE, et al: Safety
and efficacy of durvalumab with or without tremelimumab in patients
with PD-L1-Low/Negative recurrent or metastatic HNSCC: The phase 2
CONDOR randomized clinical trial. JAMA Oncol. 5:195–203. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et
al: Overall survival with durvalumab after chemoradiotherapy in
stage III NSCLC. N Engl J Med. 379:2342–2350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tapia Rico G and Price TJ: Atezolizumab
for the treatment of colorectal cancer: The latest evidence and
clinical potential. Expert Opin Biol Ther. 18:449–457. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Oliveira AF, Bretes L and Furtado I:
Review of PD-1/PD-L1 inhibitors in metastatic DMMR/MSI-H colorectal
cancer. Front Oncol. 9:3962019. View Article : Google Scholar : PubMed/NCBI
|