Introduction
Breast cancer is the second leading cause of
cancer-related mortality in women (1). The disease may be characterized as
estrogen receptor (ER) and progesterone receptor (PR)-positive,
human epidermal growth factor receptor 2 (HER2)-positive, or
triple-negative (ER-, PR- and HER2-negative). HER2-positive breast
cancer is diagnosed in 15–20% of breast cancer patients and is more
aggressive than hormone receptor (HR)-positive breast cancer
(2). Although the prognosis of
HER2-positive breast cancer patients has dramatically improved by
the development of HER2-targeted therapy (trastuzumab), there are
still major issues that remain to be resolved such as adverse side
effects, which include cardiotoxicity and drug resistance (3,4). Drug
resistance remains a key obstacle to the treatment of relapsed
breast cancer (5,6). Furthermore, many HER2-positive breast
cancer patients fail to respond to trastuzumab (7), and thus, a novel therapeutic strategy is
needed to address adverse side effects and the development of drug
resistance in HER2-positive breast cancer.
Metastasis is another important determining factor
of prognosis and recurrence in cancer. Although the 5-year survival
rate in breast cancer patients has been increased by therapeutic
advancements, the 5-year survival rate of patients diagnosed with
distant metastasis is appreciably lower than that of non-metastatic
breast cancer patients (8).
Combinatorial treatments with trastuzumab and other
drugs, such as doxorubicin and paclitaxel, have been used to reduce
recurrence risk and improve prognosis in HER2-positive breast
cancer (9,10). However, adverse side effects and
metastasis rates remain unacceptably high. Furthermore, several
investigators have reported that tumor relapse and metastasis
should be promoted by chemotherapy (11). Therefore, efforts are needed to
identify novel drug candidates with anticancer and antimetastatic
activities.
Morin (2′,3,4′,5,7-pentahydroxyflavone) is a
flavonoid isolated from almonds, bilberries, members of the
Moraceae family, and the leaves of Cudranaia tricuspidata
Buread. Several investigators have reported that it has
hepatoprotective, antihypertensive, anti-inflammatory, and
antiangiogenic activities (12–14).
Furthermore, we showed previously that morin exhibits
antimetastatic activity in 12-O-tetradecanoylphorbol-13-acetate
(TPA)-treated MCF-7 breast cancer cells (15), and Jin et al reported that
morin inhibited the invasion and growth of metastatic human breast
cancer MDA-MB-231 cells (16).
However, the therapeutic effect of morin in HER2-positive breast
cancer treatment has not been evaluated. Therefore, we assessed the
effects of morin on endothelial growth factor (EGF)-induced
metastatic potential and cell death in HER2-overexpressing human
breast cancer SK-BR-3 cells to confirm whether it should be used in
the treatment of all types of breast cancer.
Materials and methods
Materials
Morin and sulforhodamine B (SRB) were obtained from
Sigma-Aldrich; Merck KGaA, and trichloroacetic acid (TCA) was
obtained from Samchun Pure Chemical Co., Ltd. Dulbecco's modified
Eagle's medium (DMEM), antibiotic/antimycotic solution and trypsin
were purchased from Welgene. Fetal bovine serum (FBS) was obtained
from Cytiva (HyClone), and 30% polyacrylamide solution, protease
inhibitor cocktail, and phosphatase inhibitor cocktail were from
GenDEPOT. Epidermal growth factor (EGF) was purchased from R&D
Systems. Primary antibodies for Akt (cat. no. 4691), phospho
(p)-Akt (cat. no. 4060), EGFR (cat. no. 4267), p-EGFR (cat. no.
3777), extracellular signal-regulated kinase (ERK1/2; cat. no.
4695), p-ERK1/2 (cat. no. 4370), HER2 (cat. no. 2248), p-HER2 (cat.
no. 2247), c-Jun N-terminal kinase (JNK; cat. no. 9258), p-JNK
(cat. no. 4668), p38 mitogen-activated protein kinase (MAPK; cat.
no. 8690), p-p38 MAPK (cat. no. 4511), microtubule-associated
protein 1A/1B-light chain 3 (LC-3; cat. no. 4108), mammalian target
of rapamycin (mTOR; cat. no. 2972), p-mTOR (cat. no. 2971), NF-κB
(cat. no. 8242), p-NF-κB (cat. no. 3033), c-Jun (cat. no. 9165),
p-H2A.X (cat. no. 9718), 5′adenosine monophosphate-activated
protein kinase (AMPK; cat. no. 2532), p-AMPK (cat. no. 2535), ATG7
(cat. no. 8558), beclin-1 (cat. no. 3495), ribosomal protein S6
(cat. no. 2217), p-S6 (cat. no. 4858), survivin (cat. no. 2808),
signal transducer and activator of transcription 3 (STAT3; cat. no.
4904), p-STAT3 (cat. no. 9145), p62 (cat. no. 7695), B-cell
lymphoma-extra large (Bcl-xL; cat. no. 2764), Bcl-2 associated X
protein (Bax; cat. no. 2772), caspase-3 (cat. no. 9662), caspase-7
(cat. no. 9492), poly(ADP-ribose) polymerase (PARP; cat. no. 9542),
cytochrome c (cat. no. 11940), cytochrome c oxidase
(COX) IV (cat. no. 4850), and GAPDH (cat. no. 5174) were purchased
from Cell Signaling Technology. DNA repair protein RAD51 homolog 1
(RAD51; cat. no. sc-8349) and goat anti-mouse immunoglobulin
G-horseradish peroxidase-conjugated antibody (cat. no. sc-2005)
were from Santa Cruz Biotechnology, Inc. and goat anti-rabbit
immunoglobulin G-horseradish peroxidase-conjugated antibody (cat.
no. 31460) was from Thermo Fisher Scientific, Inc.
Cell culture
Human breast cancer SK-BR-3 cells were purchased
from the Korean Cell Line Bank (Seoul, Korea). Cells were routinely
grown in DMEM supplemented with 10% FBS and 1%
antimycotic/antibiotic solution at 37°C in a 5% CO2
atmosphere.
Analysis of the cytotoxicity of
morin
The cytotoxicity of morin was assessed using an SRB
assay. Briefly, 5×103 HER2-overexpressing SK-BR-3 cells
suspended in culture medium (DMEM supplemented with 10% FBS) were
seeded into the wells of a 96-well plate and cultured for 24 h to
facilitate attachment. Then, the culture medium was replaced with
5% FBS supplemented-DMEM containing morin (50 to 200 µM) and the
cells were further cultured for 24, 48, or 72 h. Cells were then
fixed with 20% TCA for 1 h, washed with tap water, and dried for 2
h. Then, cells were stained by 0.4% SRB solution at room
temperature for 30 min. The stained cells were washed by 1% acetic
acid and dried completely in room temperature. SRB was dissolved by
100 µl of 10 mM Tris-HCl (pH 5.0) buffer, and absorption at 510 nm
was measured by SpectraMax M2e (Molecular Devices).
Cell migration assay
The cells (1×106/well) were plated into
collagen-coated 6-well plates and grown for 24 h in 10%
FBS-supplemented DMEM. Cell monolayers were scratched using a 1-ml
micropipette tip, washed twice with phosphate-buffered saline, and
further incubated in serum-free DMEM for 24 h. The serum-free DMEM
was then removed and cell monolayers were incubated in 1%
FBS-supplemented DMEM containing different concentrations (50–200
µM) of morin for 1 h and then treated with 20 ng/ml of EGF. Plates
were then further kept for 48 h. Scratches were photographed at 0
and 48 h after EGF treatment using a Nikon light microscope
(magnification, ×40; Nikon Corp.).
Gelatin zymography
Matrix metalloproteinase-9 (MMP-9) activity was
evaluated by gelatin zymography. The cells (5×105/well)
were seeded into 6-well plates and allowed to attach for 24 h.
Cells were serum-starved for 24 h and then treated with various
concentrations (50–200 µM) of morin dissolved in serum-free DMEM
for 24 h. The serum-free DMEM was then transferred to new 15-ml
conical tubes, centrifuged to remove floating debris, and subjected
to 8% non-reducing SDS-polyacrylamide gel (containing 0.1% (v/v)
gelatin) electrophoresis (PAGE). The SDS in polyacrylamide gel was
then removed by immersing in 0.25% Triton X-100 solution for 40 min
and then kept in reaction buffer [5 mM CaCl2, 0.04%
NaN3 and 50 mM Tris-HCl] overnight at 37°C. The gel was
then stained with Coomassie brilliant blue R solution for 1 h to
visualize the bands, which were photographed using an LAS-4000
image analyzer (Fujifilm Corp.). Band densities were determined
using Scion Image software (Alpha 4.0.3.2; Scion Corp.).
Western blotting
HER-2-overexpressing SK-BR-3 cells were seeded into
6-well plates at 5×105 cells/well, allowed to attach for
24 h, and then serum-starved for 6 h. The cells were treated with
various concentrations (50–200 µM) of morin dissolved in serum-free
DMEM for 24 h, and then treated with 20 ng/ml of EGF for 30 min to
investigate the effect of morin on the metastasis-regulating
signaling pathway triggered by EGF. To study the effect of morin on
cell survival, HER-2-overexpressing SK-BR-3 cells were treated with
50 to 200 µM of morin in DMEM supplemented with 2% FBS for 24, 48,
or 72 h. Cells were then lysed with RIPA lysis buffer [50 mM
Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1% sodium
deoxycholate, 0.1% SDS, and 2 mM ethylenediaminetetraacetic acid]
(Biosesang) containing protease inhibitor cocktail and phosphatase
inhibitor cocktail, and lysates were centrifuged at 13,000 rpm for
10 min at 4°C. Supernatants (whole cell lysates) were removed and
stored at −80°C until required. Total protein concentrations in
whole cell lysates were calculated using the bicinchoninic acid
(BCA) method using Pierce™ BCA Protein Assay Kits (Thermo Fischer
Scientific, Inc.). Same amounts of total protein were subjected to
6, 8, 10 or 15% SDS-PAGE and transferred to polyvinylidene fluoride
membranes. Membranes were incubated in Tris-buffered saline-Tween
(50 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20; TBS-T) containing 1%
BSA. Target proteins were probed overnight by the antibodies
diluted at 1:3,000 in 5% non-fat dry milk or 1% BSA in TBS-T at
4°C. Then, secondary antibody solutions diluted at 1:5,000 in TBS
were covered and incubated onto the membrane for 1 h after washing
three times for 5 min by TBST. Handmade chemiluminescent substrate
[100 mM Tris (pH 8.5; BioShop, Canada, Inc.), 1.25 mM luminol, 198
µM coumaric acid and 0.01% hydrogen peroxide (all from
Sigma-Aldrich; Merck KGaA)] was used for detecting the target
protein bands and each band was photographed using Luminescent
Image Analyzer LAS-4000 (Fujifilm Corp.). Band densities were
determined using Scion Image software (Alpha 4.0.3.2).
Mitochondrial fractionation
HER-2-overexpressing SK-BR-3 cells
(1×106) were plated into 100-mm dishes and allowed to
attach for 24 h. Culture medium was then removed; cells were
treated with morin at 50–200 µM for 48 h at 37°C. After detaching
cells with a scraper, they were collected by centrifugation at 800
× g for 5 min. Mitochondrial fractionation was performed using a
Mitochondria/Cytosol Fractionation Kit (BioVision Inc.).
Fluorescent immunocytochemistry
The cellular expression levels of LC-3I/II and
SQSTM1/p62 in SK-BR-3 cells were assessed by fluorescent
immunocytochemistry to confirm whether morin had triggered
autophagy. Initially, coverslips were sterilized with 70% ethanol
in PBS and UVB radiated for 10 min, coated with collagen, and
placed in 6-well plates. Cells were seeded onto coverslips, allowed
to attach for 24 h, treated with morin at 0 or 200 µM for 24 or 48
h, serially fixed with ice-cold methanol and acetone for 4 min and
2 min, respectively, blocked in PBS containing 10% FBS for 2 h, and
washed three times with PBS for 5 min. Cells were then treated with
1:200 diluted primary antibodies for LC-3I/II and SQSTM1/p62 in
PBS, incubated overnight at 4°C, and probed for 2 h in a dark with
1:200 diluted Alexa 488 (Green)-conjugated goat anti-rabbit
antibody in PBS. DAPI containing antifade mounting solution was
dropped onto the coverslips, and expression levels of LC-3I/II and
SQSTM1/p62 in cells were photographed under a fluorescence
microscope (magnification, ×200; Carl Zeiss).
Statistical analysis
The data were statistically analyzed with one-way
ANOVA test, followed by Turkey post-hoc test, using SPSS V20.0
software (IBM Corp.). The values are presented as the means ± SD.
P-value of less than 0.05 was considered to indicate a significant
difference.
Results
Effects of morin on cell viability and
metastatic potential
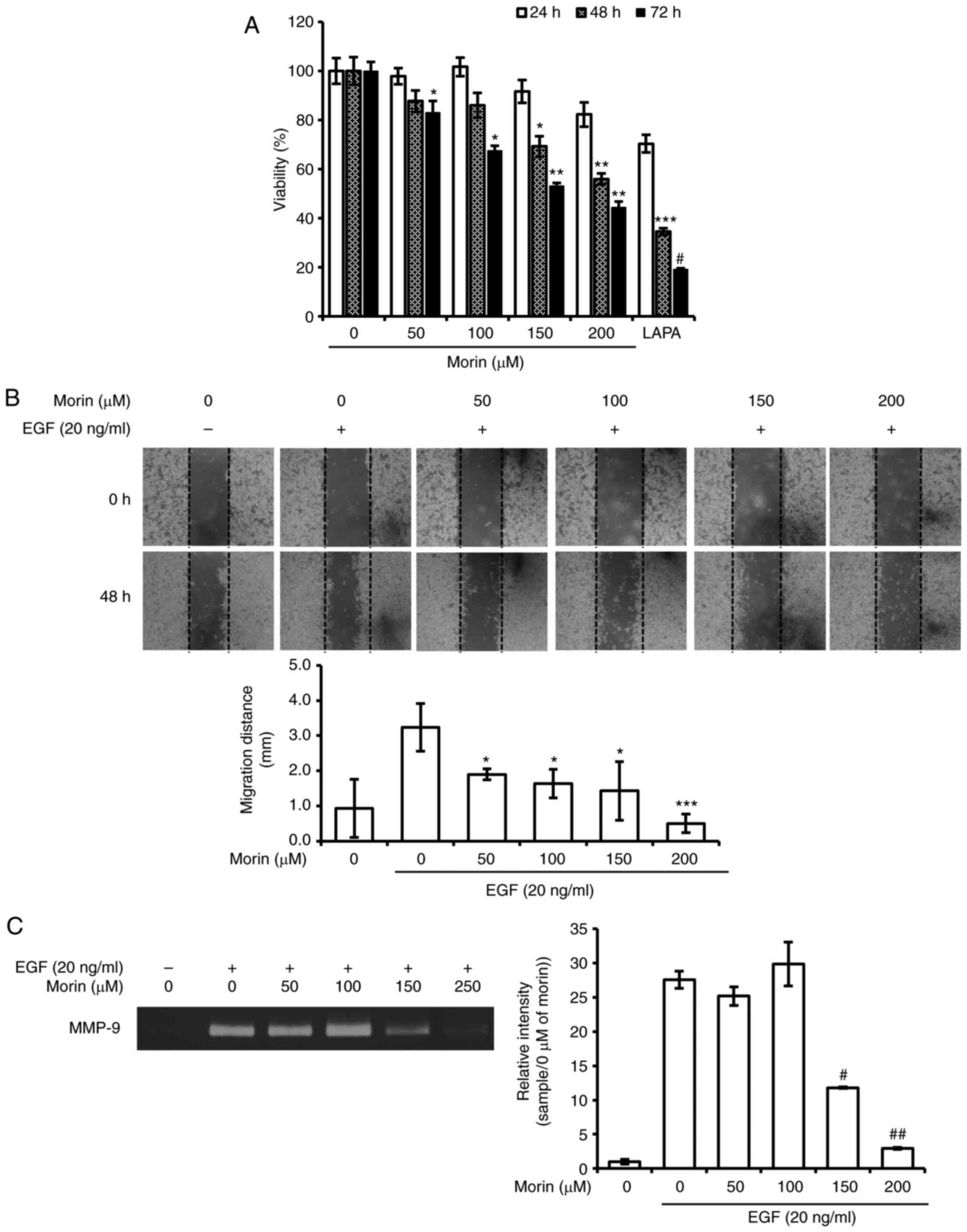
Cytotoxicity of morin in HER-2-overexpressing
SK-BR-3 cells was evaluated using the SRB assay. Results showed
that SK-BR-3 cell viability was significantly and
concentration-dependently reduced by morin after treatment for 48
and 72 h (Fig. 1A), whereas only a
slight decrease in cell viability was observed at 24 h (Fig. 1A). In the wound healing assay,
EGF-induced cell migration was effectively and
concentration-dependently suppressed by morin (Fig. 1B). Furthermore, MMP-9 activity, which
is an important factor in breast cancer metastasis, was
significantly reduced by 150 and 200 µM of morin. We also evaluated
the effect of morin on MMP-2 activity but the activity was not
detectable (data not shown). These results suggest that morin
suppressed EGF-induced metastatic potential in SK-BR-3 cells by
inhibiting cell migration and MMP-9 activity.
Morin inhibits EGF-induced metastatic
potential mediated by the EGFR signaling pathway
EGF-triggered metastatic potential is primarily
governed by the EGFR signaling pathway. Therefore, the effect of
morin on the EGF-triggered EGFR signaling pathway was investigated.
As shown in Fig. 2, the EGF-induced
phosphorylation of EGFR (p-EGFR) was significantly suppressed by
morin at concentrations >150 µM. Furthermore, EGF-induced levels
of p-STAT3 and p-JNK1/2 were concentration-dependently suppressed
by morin, which also reduced c-Jun protein levels (Fig. 2). However, the significant
phosphorylation of Akt was not observed. These results suggest that
the antimetastatic effects of morin in EGF-treated SK-BR-3 cells
were mediated by inhibition of the EGFR signaling pathway.
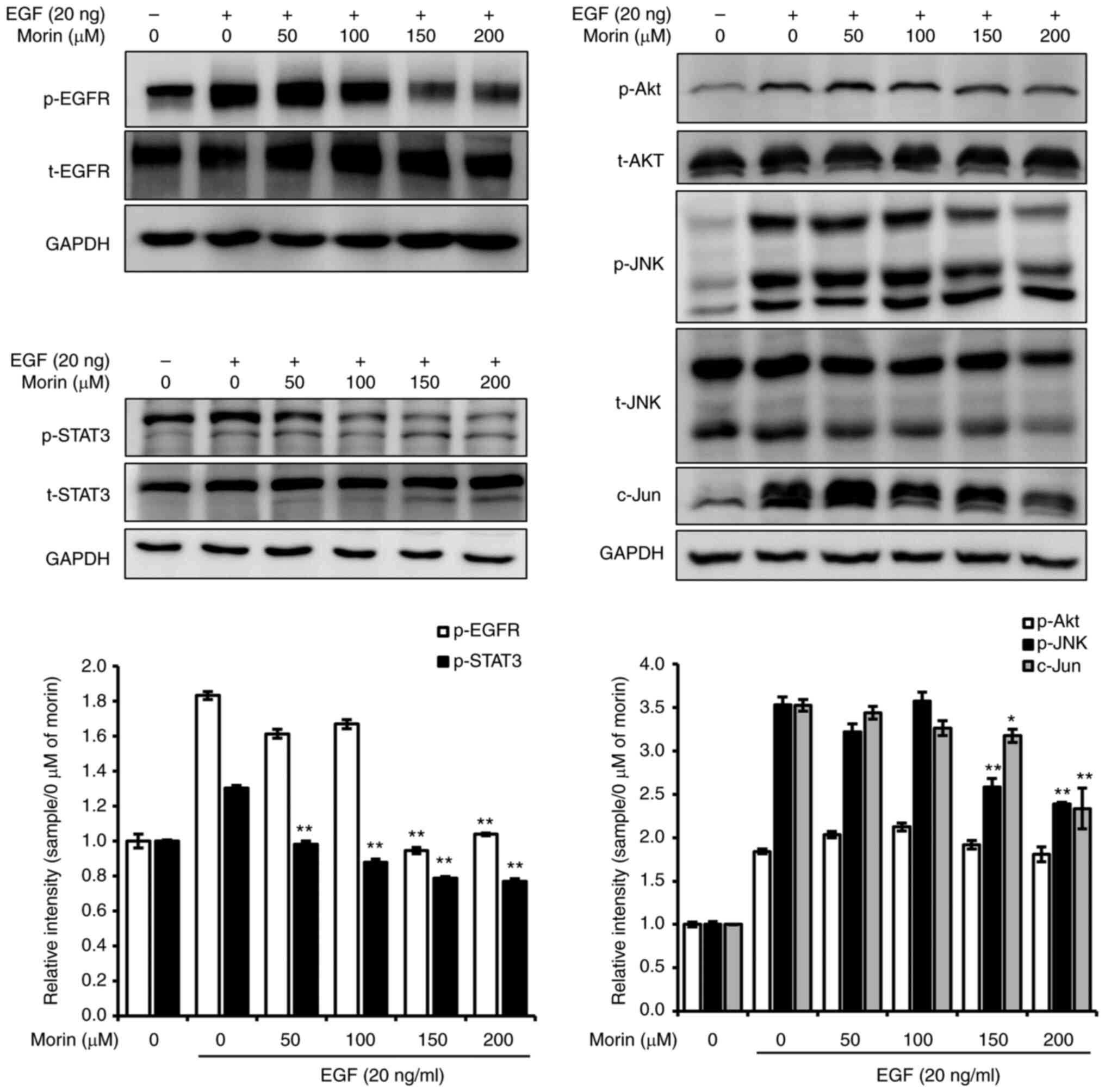 | Figure 2.Effects of morin on the EGF-induced
EGFR signaling pathway. Cells were seeded into 6-well plates and
allowed to attach for 24 h, serum-starved for 24 h, treated with
various concentrations of morin (0–200 µM) in serum-free media for
24 h, and then with EGF (20 ng) for 30 min. Cells were harvested
using RIPA lysis buffer, centrifuged at 14,000 × g for 10 min, and
supernatants (whole cell lysates) were collected and stored at
−80°C until required. Whole-cell lysates were subjected to 6, 8, or
10% SDS-PAGE. Bands were observed and captured using an LAS-4000
image analyzer. GAPDH was used as the internal control. The
experiments were performed independently in triplicate. Band
densities were normalized vs. GAPDH and data are presented as means
± SD; *P<0.05 and **P<0.01 vs. 0 µM of morin with EGF,
respectively. EGFR, epidermal growth factor receptor; STAT3, signal
transducer and activator of transcription 3; JNK, c-Jun N-terminal
kinase; p-, phosphorylated; t-total. |
Morin reduces the protein expression
levels of HER2 and EGFR
HER2 protein is targeted to treat
HER2-overexpressing breast cancer and plays important roles in cell
growth and proliferation (7), and
EGFR is also used as a therapeutic target in several other cancer
types (17). Therefore, we evaluated
the effect of morin on the expression and phosphorylation of HER2
and EGFR. The result showed that morin (150–200 µM) decreased the
protein expression and the phosphorylation of HER2 after treatment
for 24 and 48 h and reduced the phosphorylation of EGFR at 24 and
48 h (Fig. 3). In addition, decreased
EGFR expression by morin (100–200 µM) was also observed after
treatment for 48 h. Furthermore, mTOR phosphorylation at 24 and 48
h and protein expression at 48 h were significantly reduced by
morin (Fig. 3). This result shows
that the observed morin-induced reduction in SK-BR-3 cell viability
was associated with inhibition of the HER2/EGFR signaling
pathway.
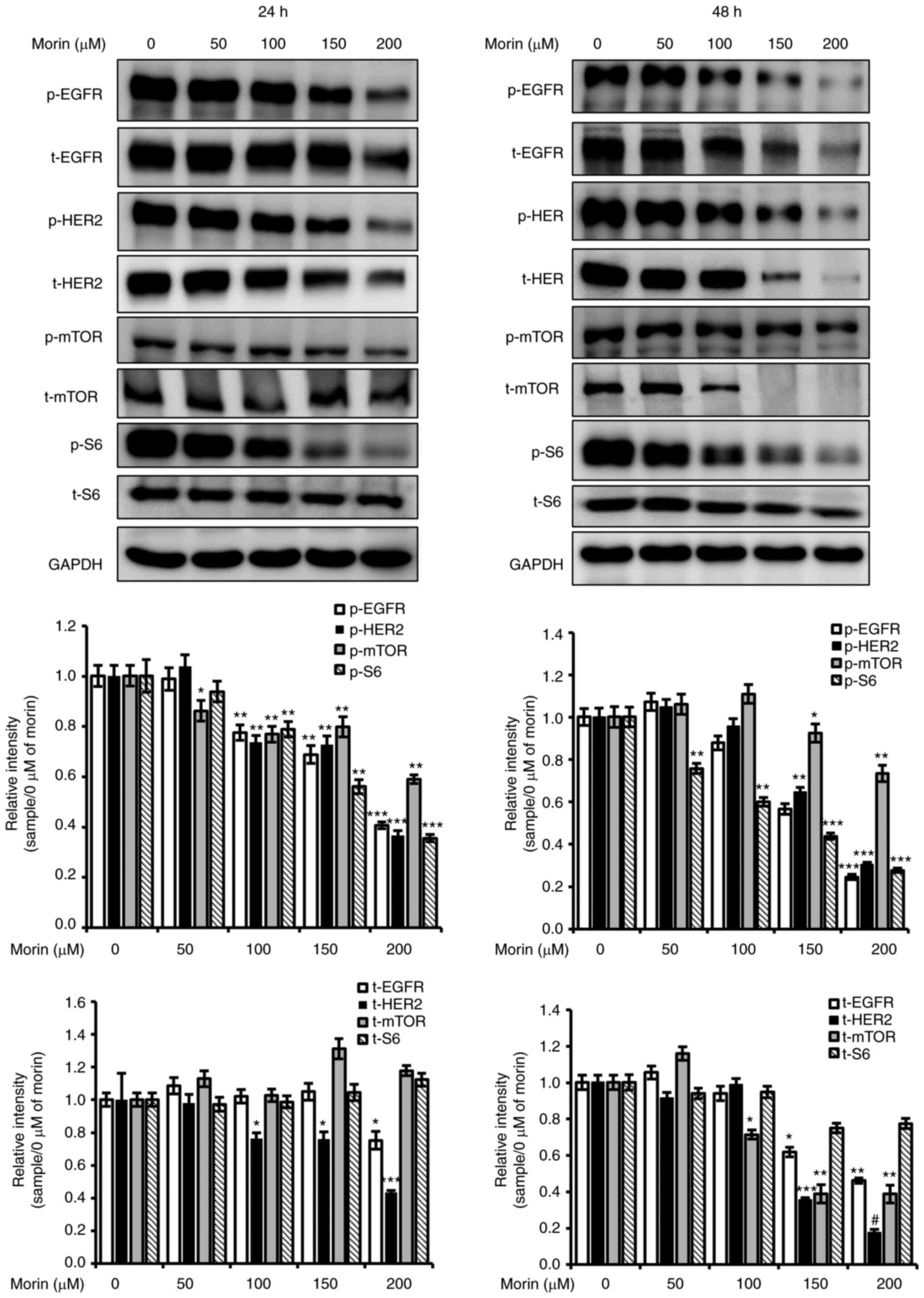 | Figure 3.Effects of morin on the
phosphorylation and expression of HER2 and EGFR. SK-BR-3 cells were
seeded into 6-well plates and allowed to attach for 24 h. Cells
were then treated with various concentrations of morin in media
supplemented with 5% FBS for 24 or 48 h, harvested with RIPA lysis
buffer, and centrifuged at 14,000 × g for 10 min. The supernatants
(whole cell lysates) were collected and stored at −80°C until
required. Whole-cell lysates were subjected to 6 or 10% SDS-PAGE.
Bands were observed and captured using an LAS-4000 image analyzer.
GAPDH was used as the internal control. The experiments were
performed independently in triplicate. Band densities were
normalized vs. GAPDH and data are presented as means ± SD;
*P<0.05, **P<0.01, ***P<0.005 and #P<0.001
vs. 0 µM of morin, respectively. EGFR, EGF, epidermal growth
factor; mTOR, mammalian target of rapamycin; p-, phosphorylated;
t-total. |
Morin-induced cell death is associated
with the induction of DNA damage
Previous investigations have shown that the death of
HER2-overexpressing breast cancer cells is induced by the
downregulation of HER2, the induction of DNA damage, and the
downregulation of DNA repair enzyme (18,19).
Therefore, we investigated whether morin induces DNA damage in
SK-BR-3 cells. As shown Fig. 4A,
H2A.X phosphorylation, a marker for DNA damage, was
concentration-dependently increased by morin (100–200 µM) following
24 or 48 h of treatment. Moreover, TUNEL-positive cells were
observed in morin-treated SK-BR-3 cells (Fig. S1). In addition, levels of RAD51 and
survivin proteins, which participate in DNA repair, were diminished
by morin treatment (Fig. 4A). These
results regarding the suppression of the protein expression levels
of RAD51 and survivin imply that morin induced DNA damage
accumulation.
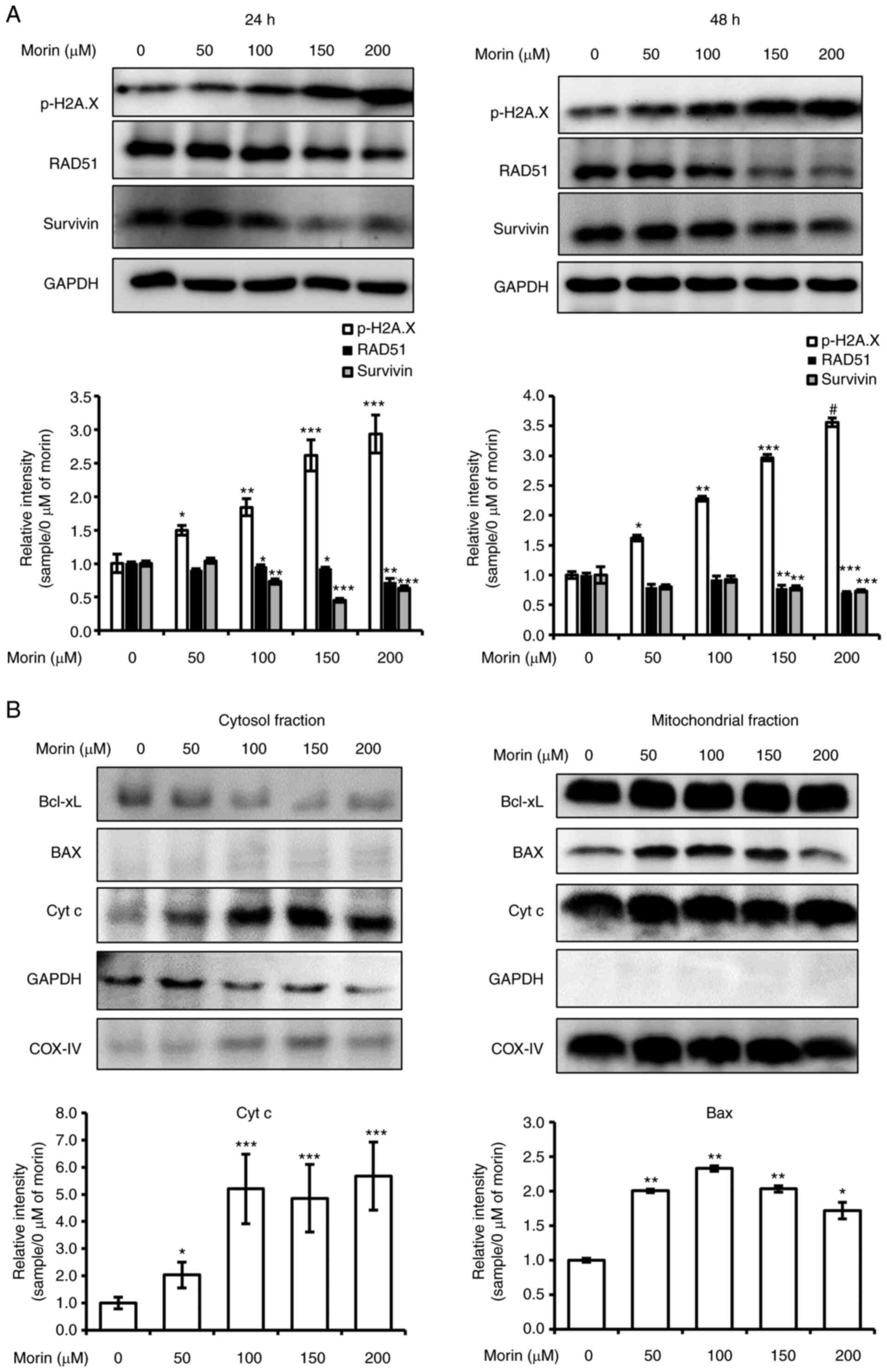 | Figure 4.Effects of morin on DNA damage and
mitochondrial Bcl-xL, BAX, and cytochrome c levels. (A and
B) SK-BR-3 cells were seeded into 6-well plates, allowed to attach
for 24 h, and treated with various concentrations of morin (0–200
µM) in media supplemented with 5% FBS for 24 or 48 h. (A) Cells
were harvested with RIPA lysis buffer, centrifuged at 14,000 × g
for 10 min, and supernatants (whole cell lysates) were collected
and stored at −80°C until required. Whole-cell lysates were
subjected by 10 or 15% SDS-PAGE. Bands were observed and captured
using an LAS-4000 image analyzer, and GAPDH was used as the
internal control. The experiments were performed independently in
triplicate. Band densities were normalized vs. GAPDH and data are
presented as means ± SD; *P<0.05, **P<0.01, ***P<0.005 and
#P<0.001 vs. 0 µM of morin, respectively. (B) Cells
were harvested with a scraper and collected by centrifugation at
800 × g for 5 min and separated into mitochondrial and cytosolic
fractions. All fractions were subjected to 10 or 15% SDS-PAGE.
Bands were observed and captured using an LAS-4000 image analyzer.
COX–IV and GAPDH were used as internal controls for mitochondrial
and cytosolic fractions, respectively. The experiments were
performed independently in triplicate. Band densities were
normalized vs. COX–IV or GAPDH, and results are presented as means
± SD; *P<0.05, **P<0.01 and ***P<0.005 vs. 0 µM of morin,
respectively. Bcl-xL, B-cell lymphoma-extra large; Bax, Bcl-2
associated X protein; Cyt c, cytochrome c; RAD51, DNA repair
protein RAD51 homolog 1; p-, phosphorylated. |
Accumulated DNA damage should lead to apoptotic cell
death mediated by mitochondrial dysfunction (20,21).
Therefore, we tested the effect of morin on the expression levels
of Bcl-2 family members (Bcl-xL, and Bax) and on mitochondrial
cytochrome c release by mitochondrial fractionation assay.
The mitochondrial fractionation assay showed cytosolic Bcl-xL
levels were diminished by morin (100–200 µM) and that cytochrome
c had been released to the cytosol after 48 h of treatment
at 50–200 µM (Fig. 4B). However,
mitochondrial Bcl-xL and cytochrome c levels were unaffected
by morin treatment. These results demonstrated that morin-induced
cell death was linked with DNA damage and mitochondrial dysfunction
in the HER-2-overexpressing SK-BR-3 cells.
Morin-induced cell death is associated
with caspase- mediated PARP cleavage
Mitochondrial dysfunction is closely associated with
caspase-dependent apoptosis (22),
and thus, we assessed the effect of morin on the cleavages of
caspase and PARP. As shown in Fig. 5,
although significant cleavages of caspase-3, caspase-7 and PARP
were not observed at 24 h, morin treatment for 48 h provoked the
cleavages of caspase-3 and −7 and PARP, which suggested that
morin-induced cell death was caused by activation of the
caspase-dependent apoptotic signaling pathway.
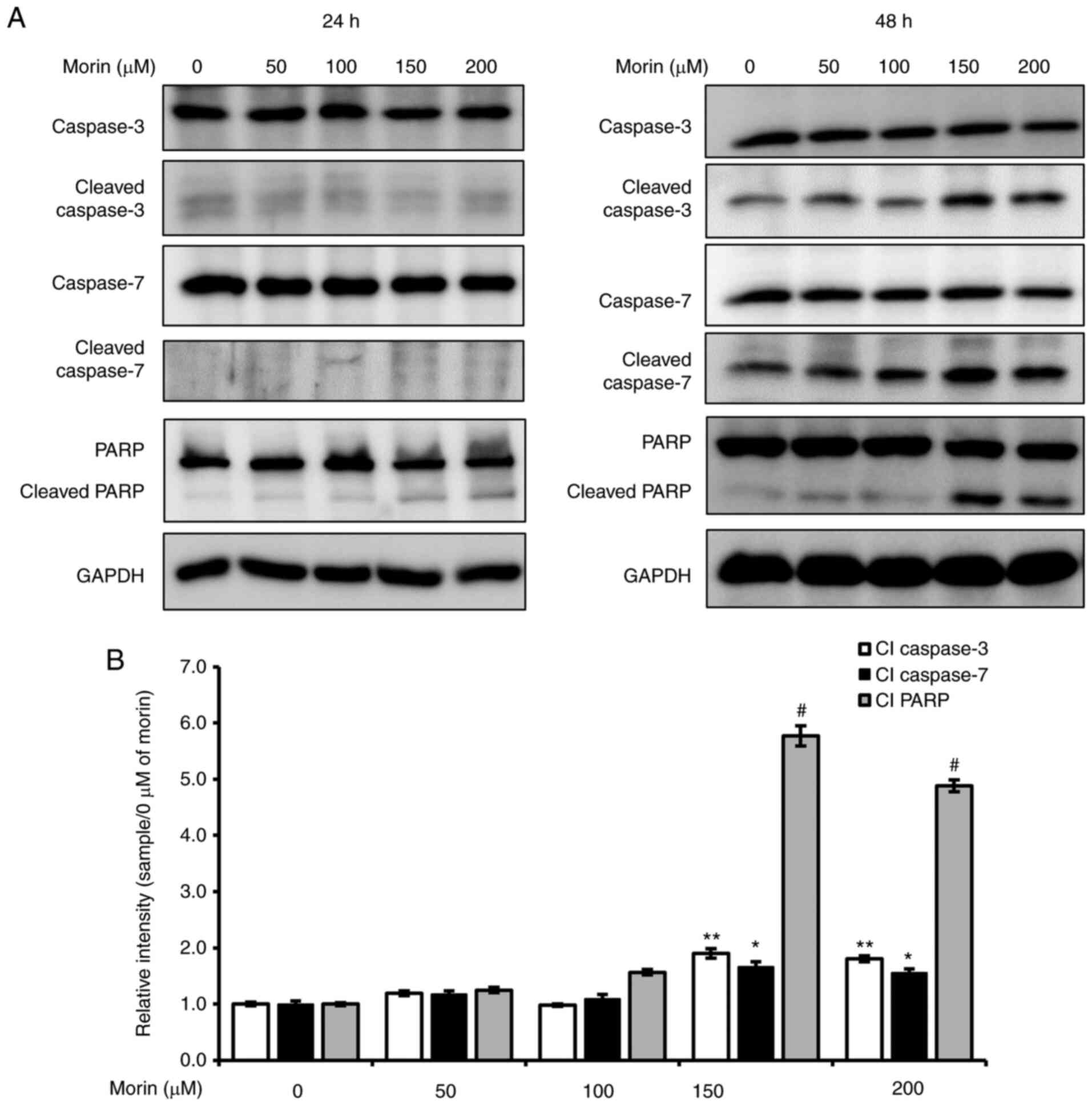 | Figure 5.Effects of morin on caspases and PARP
cleavage. SK-BR-3 cells were seeded into 6-well plates, allowed to
attach for 24 h, and treated with various concentrations of morin
(0–200 µM) in medium supplemented with 5% FBS for 24 or 48 h. Cells
were harvested with RIPA lysis buffer and centrifuged at 14,000 × g
for 10 min. Supernatants (whole cell lysates) were stored at −80°C
until required. Whole-cell lysates were subjected by 8 or 15%
SDS-PAGE, and bands were observed and captured using an LAS-4000
image analyzer. GAPDH was used as the internal control. (B)
Relative intensities of cleaved form level of caspase-3, caspase-7
and PARP vs./0 µM of morin at 48 h. The experiments were performed
independently in triplicate. Band densities were normalized vs.
GAPDH and data are presented as means ± SD; *, **and indicate
*P<0.05, **P<0.01 and #P<0.001 vs. 0 µM of
morin, respectively. PARP, poly(ADP-ribose) polymerase; Cl, cleaved
form. |
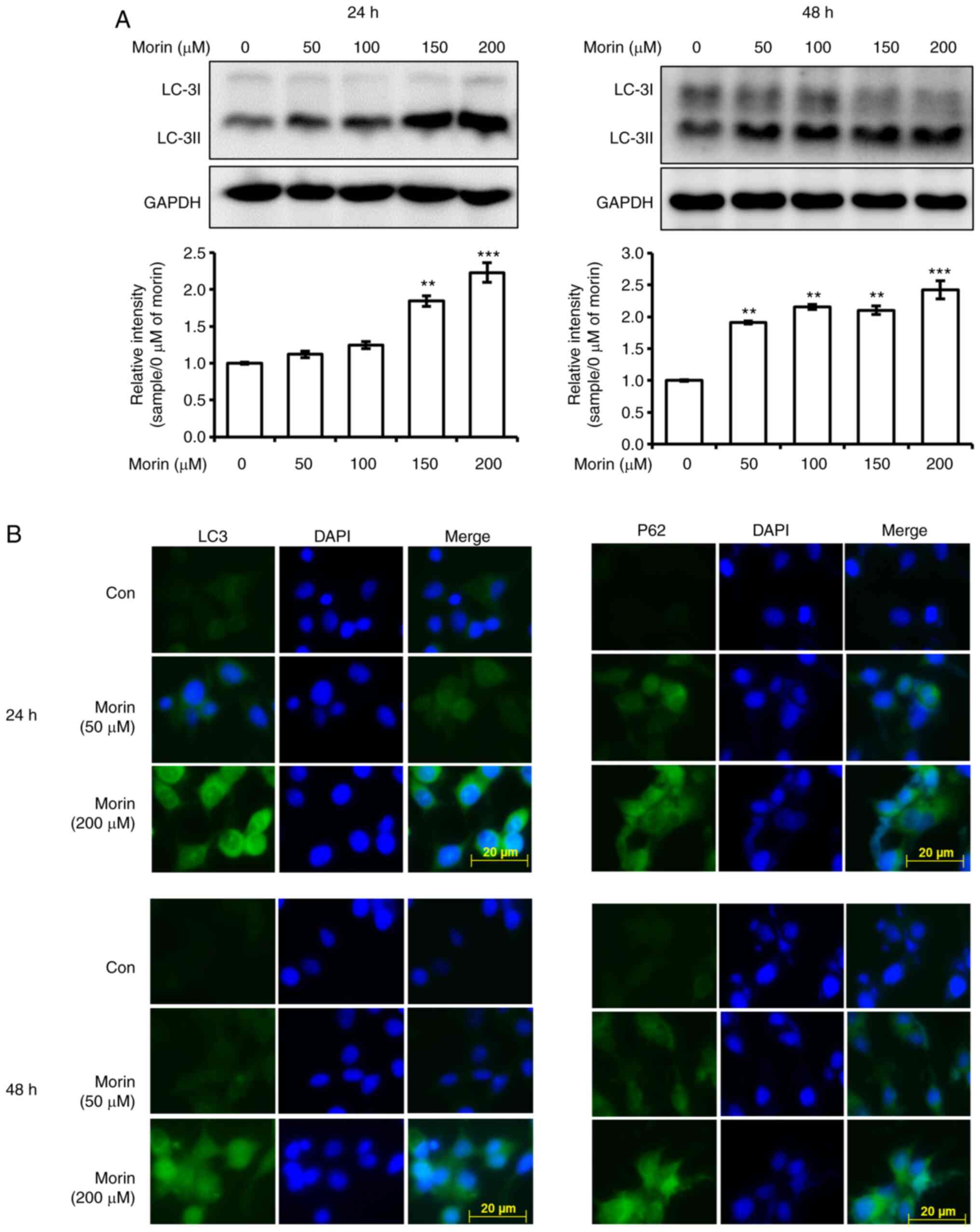
Autophagy is induced after 24 h of
morin treatment
We also investigated whether the observed
morin-induced reduction in cell viability is associated with
autophagy. Morin treatment for 24 h was found to enhance the
expression levels of LC3-II in a concentration-dependent manner at
24 h, and the increased expression level was maintained at 48 h in
western blot analysis (Fig. 6A) and
increased cellular LC3 and p62 levels at 24 and 48 h were observed
by fluorescent immune cytochemistry (Fig.
6B). Furthermore, the decrease in cell viability by 200 µM of
morin treatment for 48 h was recovered by autophagy inhibitor,
chloroquine (Fig. S2). In addition,
morin treatment for 24 increased AMPK phosphorylation (Fig. 7A) but not the expression of beclin-1
and ATG7 (Fig. 7A). These results
imply that the observed morin-induced reduction in cell viability
was associated with the activation of autophagy.
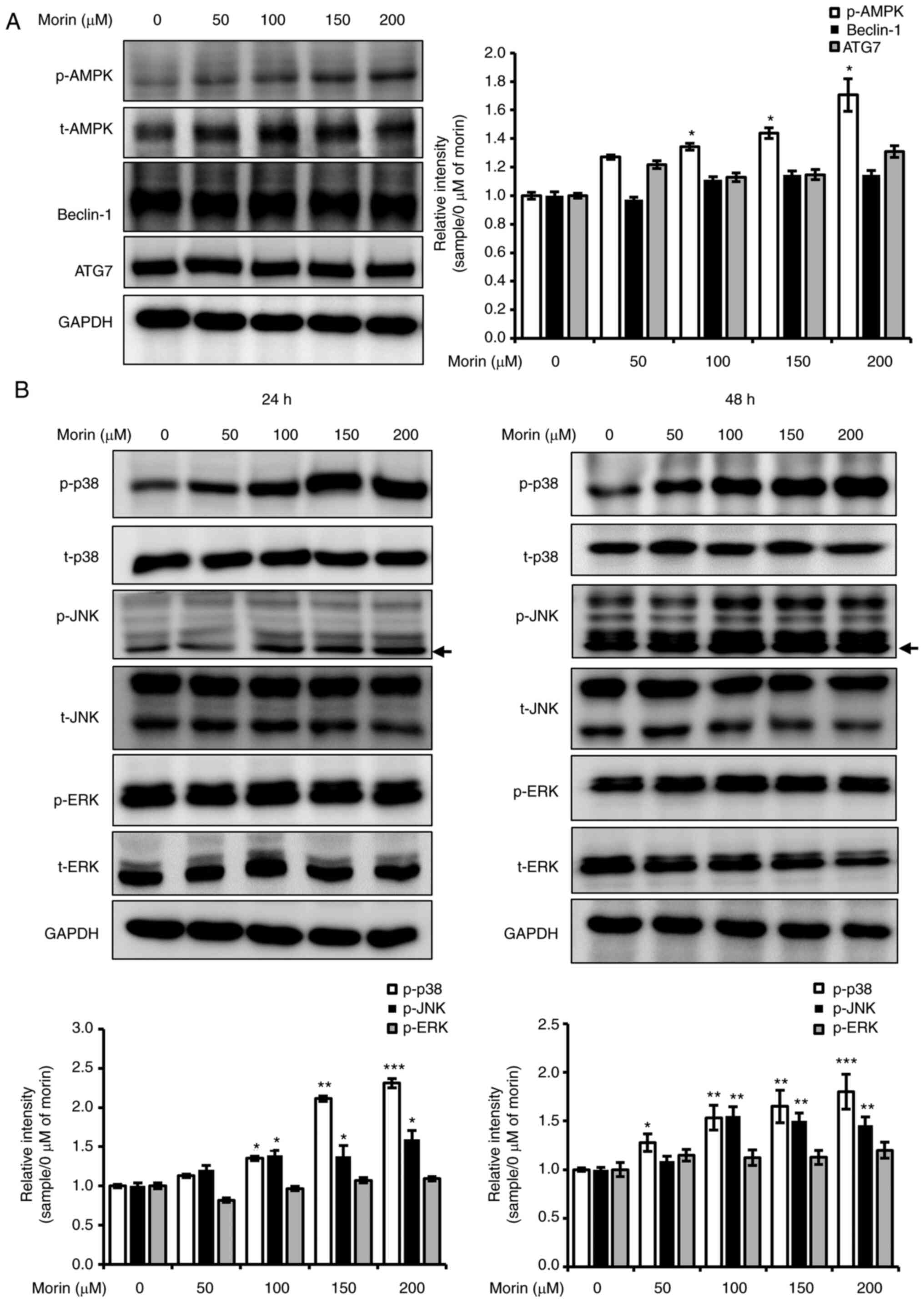 | Figure 7.Effects of morin on the MAPK
signaling pathway contributed to morin-induced cell death. (A)
SK-BR-3 cells were seeded into 6-well plates, allowed to attach for
24 h, and treated with various concentrations of morin (0–200 µM)
in media supplemented with 5% FBS for 24 h. (B) SK-BR-3 HER-2 cells
were seeded into 6-well plates, allowed to attach for 24 h, and
treated with various concentrations of morin (0–200 µM) in media
supplemented with 5% FBS for 24 or 48 h. Arrows indicate
non-specific band. (A and B) Cells were harvested with RIPA lysis
buffer and centrifuged at 13,000 rpm for 10 min. Supernatants
(whole cell lysates) were collected and stored at −80°C until
required. Whole-cell lysates were subjected by 8 or 10% SDS-PAGE,
and bands were observed and captured using an LAS-4000 image
analyzer. GAPDH was used as the internal control. The experiments
were performed independently in triplicate. Band densities were
normalized vs. GAPDH and data are presented as means ± SD;
*P<0.05, **P<0.01 and ***P<0.005 vs. 0 µM of morin,
respectively. ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; p-, phosphorylated; t-, total. |
Morin-induced cell death is associated
with increases in the phosphorylation of JNK and p38
MAPKs are key regulators of cell survival,
proliferation, growth, and death (23,24).
Therefore, we studied the effect of morin on the phosphorylation of
MAPKs (ERK, JNK, and p38). The phosphorylation levels of JNK and
p38 were concentration-dependently increased after 24 or 48 h of
morin treatment but that of ERK were unchanged (Fig. 7B). This result suggests that increased
phosphorylation levels of JNK and p38 are important in the cell
death signaling pathway induced by morin.
Discussion
As mentioned above, metastasis is important in
determining therapeutic efficacy and prognosis in cancer (8), and many investigators have tried to
identify potent antimetastatic agents among natural products
(25–27). In a previous study, we found that
morin reduced 12-O-tetradecanoylphorbol-13-acetate-induced
metastatic potential in HR-positive human breast cancer MCF-7 cells
(15). The present investigation
demonstrated that EGF-induced metastatic potential in epidermal
growth factor receptor 2 (HER2)-overexpressing breast cancer
SK-BR-3 cells was also diminished by morin treatment due to
suppressions of cell migration and MMP-9 activity). These results
demonstrated that natural substances provide a source of agents
that can suppress metastasis, and showed that morin should be
considered a potent antimetastatic agent in hormone receptor
(HR)-positive and HER2-overexpressing breast cancer. The metastatic
potential in HER2-overexpressing breast cancer was found to be
regulated by the EGFR/STAT3 and MAPK signaling pathways (28–30). In
the present study, morin was found to inhibit the EGF-induced
phosphorylation of EGFR and STAT3 and downregulated EGF-induced JNK
phosphorylation and c-Jun expression. These results indicate that
the induction of metastatic potential in SK-BR-3 cells by EGF is
suppressed by morin via suppression of the EGFR/STAT3 and JNK/c-Jun
signaling pathways. However, further investigations in cellular and
animal models are necessary to clarify the antimetastatic potential
of morin in breast cancer as metastasis is a complex process that
cannot be explained simply by the migration assay and the analysis
of MMP-9 activity and is regulated by various signaling
pathways.
HER2 and EFGR are well-known treatment targets in
cancers of the breast and lung and colorectal cancer (31–34).
Trastuzumab provides effective treatment for HER2-overexpressing
breast cancer patients, but experience in regards to the use of
trastuzumab plus cytotoxic agents to treat metastatic
HER2-overexpressing breast cancer have demonstrated that
trastuzumab cannot effectively control metastasis (35). For this reason, lapatinib, a small
molecule HER2 and EGFR inhibitor, is also used to treat
HER2-overexpressing metastatic breast cancer in combination with
trastuzumab (36). In this
investigation, we found that morin inhibited cell viability and
EGF-induced metastatic potential and suppressed EGFR
phosphorylation in SK-BR-3 cells. Furthermore, the expression
levels and phosphorylation of HER2 and EGFR were significantly
reduced after 48 h of morin treatment with concomitant reductions
in the phosphorylation and expression of mTOR and S6. In addition,
previous studies demonstrated that morin did exhibit cytotoxicity
in cancer cells but not in normal epithelial cells (16,37).
Therefore, these results show that the morin-induced reductions in
metastatic potential and cell viability were mediated by inhibition
of the HER2/EGFR signaling pathway without cytotoxicity in normal
cells.
DNA damage is a major cause of cancer cell death
whether caused by chemicals, reactive oxygen species, alkylating
agents, or radiation exposure, and when DNA is damaged, the
expression levels of DNA repair enzymes are increased
appropriately. RAD51 is an important DNA repair protein and
survivin participates in the activation of DNA repair (37–40). In
fact, some authors have suggested that RAD51 and survivin should be
considered selective targets for cancer therapy in glioma cells
(38,39,41).
Accordingly, the accumulation of DNA damage by downregulating
repair enzymes appears to present a therapeutic strategy. In the
present study, morin enhanced the phosphorylation of H2A.X (a DNA
damage marker) and inhibited the expression of RAD51 and survivin
in the SK-BR-3 cells. Furthermore, increased TUNEL-positive cells
were observed in the morin-treated cells. Consequently, our results
suggest morin-induced cell death is caused by an accumulation of
DNA damage and inhibition of DNA repair.
DNA damage-linked cell death usually involves
apoptosis, and DNA damage-mediated apoptosis is associated with
mitochondrial disruption caused by cytochrome c release to
the cytosol (42). Many investigators
have shown that mitochondrial damage increases caspase activity in
cancer cells (43,44), and the release of cytochrome c
by mitochondria is known to be induced by the activation of
caspases, which is a key player in apoptotic cell death (45,46). When
SK-BR-3 HER-2 cells were treated with morin for 48 h, mitochondrial
Bax levels were increased and cytochrome c was released to the
cytosol. Furthermore, treatment with morin for 48 h induced PARP
cleavage and activation of caspase-3 and −7. Our results indicate
that morin-induced SK-BR-3 HER-2 cell death was mediated by
caspase-dependent apoptosis.
Although the cell death mechanism initiated by morin
at 48 h in the HER-2-overexpressing SK-BR-3 cells was elucidated,
the effect of morin at 24 h remained unclear. The viabilities of
SK-BR-3 cells were slightly reduced after treatment with morin at
150 or 200 µM, and this decrease was not associated with caspase
activation, which suggested that apoptosis did not contribute to
reduced cell viability at 24 h. Interestingly, LC-3II and p62
expression was enhanced by morin treatment for 24 h, and this
enhancement was maintained at 48 h. These two proteins are key
autophagy markers, and thus, our observations indicate that morin
induced autophagy at 24 h that this was sustained at 48 h.
Autophagy is a protective process associated with the formation of
autophagolysosomes, which remove damaged cellular components, and
autophagy has been reported to suppress apoptosis (47,48). On
the other hand, some investigators have suggested that some natural
substances might induce autophagy-mediated apoptosis in cancer
cells (49–51). In the present investigation, morin
enhanced autophagy at 24 h and this was maintained at 48 h when
caspase-mediated apoptosis was active. Moreover, autophagy
inhibitor, chloroquine, suppressed morin-induced cell death. Hence,
our results suggest that morin-induced cell death in SK-BR-3 cells
is governed by autophagy-mediated caspase-dependent apoptosis.
Beclin 1 and ATG7 play important roles during
autophagosome formation and in the inductions of LC-3II and p62.
Although morin promoted the expression of LC-3II and p62, it did
not affect the expression levels of beclin 1 or ATG7. Some
investigators have reported that apoptosis can be triggered by
beclin 1-independent autophagy (52,53). In a
previous study, we found that DNA damage contributes to the
activation of autophagy and increased LC3-II expression but not to
beclin 1 or ATG7 expression in UV-exposed HaCaT human keratinocytes
(54). In the present study, morin
induced DNA damage and reduced RAD51 and survivin expressions.
Consequently, our results imply that morin-induced DNA damage might
cause beclin 1- and AtG7-independent autophagy by inducing the
expression of LC-3 II and p62.
The AMPK and mTOR signaling pathways participate in
the regulation of autophagy. Puissant et al demonstrated
that resveratrol-mediated autophagic cell death was promoted by
JNK-mediated p62 expression by the AMPK signaling pathway (55). We also previously found that
autophagic cell death was associated with increased JNK
phosphorylation in HaCaT human keratinocytes (54), and in the present study, morin
increased the phosphorylated AMPK and JNK levels, decreased mTOR
phosphorylation, and increased p38 MAPK phosphorylation. Although
the role of p38 MAPK in autophagy has not been fully elucidated,
some investigators have shown that activation of JNK and p38 MAPK
contributes to autophagy and apoptosis in H9C2 and HepG2 cells
(56,57). Therefore, the present investigation
demonstrated that the induction of autophagy and apoptosis by morin
is mediated by the activation of AMPK, JNK, and p38 MAPK signaling
pathways and the suppression of the mTOR signaling pathway without
the involvement of beclin-1 or ATG7.
In summary, our results revealed that morin inhibits
EGF-induced metastatic potential by suppressing the EGFR signaling
pathway and induces cell death via autophagy-mediated apoptosis and
HER2/EGFR1 signaling pathway inhibition. Taken together, this
investigation suggests that morin offers a potential means of
enhancing the efficacy of HER2-overexpressing breast cancer
treatments by suppressing metastatic potential and inducing cancer
cell death by targeting HER2 and EGFR. However, further
investigation using animal models is needed to assess the
pharmaceutical effects of morin on HER2-overexpressing breast
cancer in a biological system.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education
(2018R1D1A1B07047758).
Availability of data and materials
All the original data generated for this manuscript
are available upon request.
Authors' contributions
KSL ad KSN designed the experiments. KSL and MGL
performed experiments. KSL, MGL and KSN analyzed the data. KSL
wrote the manuscript. KSL and KSN reviewed the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pernas S and Tolaney SM: HER2-positive
breast cancer: New therapeutic frontiers and overcoming resistance.
Ther Adv Med Oncol. 11:17588359198335192019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Telli ML, Hunt SA, Carlson RW and Guardino
AE: Trastuzumab-related cardiotoxicity: Calling into question the
concept of reversibility. J Clin Oncol. 25:3525–3533. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eckstein N: Platinum resistance in breast
and ovarian cancer cell lines. J Exp Clin Cancer Res. 30:912011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murray S, Briasoulis E, Linardou H,
Bafaloukos D and Papadimitriou C: Taxane resistance in breast
cancer: Mechanisms, predictive biomarkers and circumvention
strategies. Cancer Treat Rev. 38:890–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren W, Liu Y, Wan S, Fei C, Wang W, Chen
Y, Zhang Z, Wang T, Wang J, Zhou L, et al: BMP9 inhibits
proliferation and metastasis of HER2-positive SK-BR-3 breast cancer
cells through ERK1/2 and PI3K/AKT pathways. PLoS One. 9:e968162014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee KS, Shin JS and Nam KS: Starfish
polysaccharides downregulate metastatic activity through the MAPK
signaling pathway in MCF-7 human breast cancer cells. Mol Biol Rep.
40:5959–5966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Savornin Lohman E, Linn SC and Kok M:
Low-dose doxorubicin combined with trastuzumab in HER2-positive
metastatic breast cancer: A single institution experience. J Clin
Oncol. 32:e115982014. View Article : Google Scholar
|
|
10
|
Tolaney SM, Barry WT, Dang CT, Yardley DA,
Moy B, Marcom PK, Albain KS, Rugo HS, Ellis M, Shapira I, et al:
Adjuvant paclitaxel and trastuzumab for node-negative,
HER2-positive breast cancer. N Engl J Med. 372:134–141. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Alterio C, Scala S, Sozzi G, Roz L and
Bertolini G: Paradoxical effects of chemotherapy on tumor relapse
and metastasis promotion. Semin Cancer Biol. 60:351–361. 2020.
View Article : Google Scholar
|
|
12
|
Kang DG, Moon MK, Sohn EJ, Lee DH and Lee
HS: Effects of morin on blood pressure and metabolic changes in
fructose-induced hypertensive rats. Biol Pharm Bull. 27:1779–1783.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung HJ, Kim SJ, Song YS, Park EH and Lim
CJ: Evaluation of the antiangiogenic, anti-inflammatory, and
antinociceptive activities of morin. Planta Med. 76:273–275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HS, Jung KH, Park IS, Kwon SW, Lee DH
and Hong SS: Protective effect of morin on
dimethylnitrosamine-induced hepatic fibrosis in rats. Dig Dis Sci.
54:782–788. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KS, Nam GS, Baek J, Kim S and Nam KS:
Inhibition of TPA-induced metastatic potential by morin hydrate in
MCF-7 human breast cancer cells via the Akt/GSK-3β/c-Fos signaling
pathway. Int J Oncol. 56:630–640. 2020.PubMed/NCBI
|
|
16
|
Jin H, Lee WS, Eun SY, Jung JH, Park HS,
Kim G, Choi YH, Ryu CH, Jung JM, Hong SC, et al: Morin, a flavonoid
from Moraceae, suppresses growth and invasion of the highly
metastatic breast cancer cell line MDA-MB-231 partly through
suppression of the Akt pathway. Int J Oncol. 45:1629–1637. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vecchione L, Jacobs B, Normanno N,
Ciardiello F and Tejpar S: EGFR-targeted therapy. Exp Cell Res.
317:2765–2771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scola G, Fernandes Correia Laurino CC,
Menin E and Salvador M: Suppression of oncoprotein Her-2 and DNA
damage after treatment with Flavan-3-ol Vitis labrusca extract.
Anticancer Agents Med Chem. 13:1088–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seoane S, Montero JC, Ocaña A and
Pandiella A: Effect of multikinase inhibitors on
caspase-independent cell death and DNA damage in
HER2-overexpressing breast cancer cells. J Natl Cancer Inst.
102:1432–1446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mercer JR, Cheng KK, Figg N, Gorenne I,
Mahmoudi M, Griffin J, Vidal-Puig A, Logan A, Murphy MP and Bennett
M: DNA damage links mitochondrial dysfunction to atherosclerosis
and the metabolic syndrome. Circ Res. 107:1021–1031. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuzefovych LV, Solodushko VA, Wilson GL
and Rachek LI: Protection from palmitate-induced mitochondrial DNA
damage prevents from mitochondrial oxidative stress, mitochondrial
dysfunction, apoptosis, and impaired insulin signaling in rat L6
skeletal muscle cells. Endocrinology. 153:92–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Q, Gong B and Almasan A: Distinct
stages of cytochrome c release from mitochondria: Evidence for a
feedback amplification loop linking caspase activation to
mitochondrial dysfunction in genotoxic stress induced apoptosis.
Cell Death Differ. 7:227–233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dhanasekaran DN and Johnson G: MAPKs:
Function, regulation, role in cancer and therapeutic targeting.
Oncogene. 26:3097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho HH, Chang CS, Ho WC, Liao SY, Wu CH and
Wang CJ: Anti-metastasis effects of gallic acid on gastric cancer
cells involves inhibition of NF-κB activity and downregulation of
PI3K/AKT/small GTPase signals. Food Chem Toxicol. 48:2508–2516.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Ye T, Yu X, Lei Q, Yang F, Xia Y,
Song X, Liu L, Deng H, Gao T, et al: Nifuroxazide exerts potent
anti-tumor and anti-metastasis activity in melanoma. Sci Rep.
6:202532016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garg S, Afzal S, Elwakeel A, Sharma D,
Radhakrishnan N, Dhanjal JK, Sundar D, Kaul SC and Wadhwa R: Marine
carotenoid fucoxanthin possesses anti-metastasis activity:
Molecular evidence. Mar Drugs. 17:3382019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Liu FB, Huang M, Xie K, Xie QS, Liu
CH, Shen MJ and Huang Q: Circular RNA ciRS-7 promotes the
proliferation and metastasis of pancreatic cancer by regulating
miR-7-mediated EGFR/STAT3 signaling pathway. Hepatobiliary Pancreat
Dis Int. 18:580–586. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gariboldi MB, Ravizza R, Molteni R, Osella
D, Gabano E and Monti E: Inhibition of Stat3 increases doxorubicin
sensitivity in a human metastatic breast cancer cell line. Cancer
Lett. 258:181–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim A, Choi DK, Sung ES, Yun JS, Kwon MH
and Kim YS: Interfering transbody-mediated Her2 gene silencing
induces apoptosis by G0/G1 cell cycle arrest in Her2-overexpressing
SK-BR-3 breast cancer cells. Biotechnol Bioprocess Eng. 17:413–419.
2012. View Article : Google Scholar
|
|
31
|
Li D, Ambrogio L, Shimamura T, Kubo S,
Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A,
Himmelsbach F, et al: BIBW2992, an irreversible EGFR/HER2 inhibitor
highly effective in preclinical lung cancer models. Oncogene.
27:4702–4711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Robichaux JP, Elamin YY, Tan Z, Carter BW,
Zhang S, Liu S, Li S, Chen T, Poteete A, Estrada-Bernal A, et al:
Mechanisms and clinical activity of an EGFR and HER2 exon
20-selective kinase inhibitor in non-small cell lung cancer. Nat
Med. 24:638–646. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Anido J, Matar P, Albanell J, Guzmán M,
Rojo F, Arribas J, Averbuch S and Baselga J: ZD1839, a specific
epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor,
induces the formation of inactive EGFR/HER2 and EGFR/HER3
heterodimers and prevents heregulin signaling in
HER2-overexpressing breast cancer cells. Clin Cancer Res.
9:1274–1283. 2003.PubMed/NCBI
|
|
34
|
LaBonte MJ, Wilson PM, Fazzone W, Russell
J, Louie SG, El-Khoueiry A, Lenz HJ and Ladner RD: The dual
EGFR/HER2 inhibitor lapatinib synergistically enhances the
antitumor activity of the histone deacetylase inhibitor
panobinostat in colorectal cancer models. Cancer Res. 71:3635–3648.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hortobagyi GN: Trastuzumab in the
treatment of breast cancer. N Engl J Med. 353:1734–1735. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu ZQ, Zhang Y, Li N, Liu PJ, Gao L, Gao X
and Tie XJ: Efficacy and safety of lapatinib and trastuzumab for
HER2-positive breast cancer: A systematic review and meta-analysis
of randomised controlled trials. BMJ Open. 7:e0130532017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YJ, Kim WI, Kim SY, Cho SW, Nam HS,
Lee SH and Cho MK: Flavonoid morin inhibits proliferation and
induces apoptosis of melanoma cells by regulating reactive oxygen
species, Sp1 and Mcl-1. Arch Pharm Res. 42:531–542. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee MG, Lee KS and Nam KS: The association
of changes in RAD51 and survivin expression levels with the proton
beam sensitivity of Capan-1 and Panc-1 human pancreatic cancer
cells. Int J Oncol. 54:744–752. 2019.PubMed/NCBI
|
|
39
|
Short SC, Giampieri S, Worku M,
Alcaide-German M, Sioftanos G, Bourne S, Lio KI, Shaked-Rabi M and
Martindale C: Rad51 inhibition is an effective means of targeting
DNA repair in glioma models and CD133+ tumor-derived
cells. Neuro Oncol. 13:487–499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang G, Ren B, Xu L, Song S, Zhu C and Ye
F: Survivin may enhance DNA double-strand break repair capability
by up-regulating Ku70 in human KB cells. Anticancer Res.
29:223–228. 2009.PubMed/NCBI
|
|
41
|
Jane EP, Premkumar DR, Sutera PA, Cavaleri
JM and Pollack IF: Survivin inhibitor YM155 induces mitochondrial
dysfunction, autophagy, DNA damage and apoptosis in Bcl-xL silenced
glioma cell lines. Mol Carcinog. 56:1251–1265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim G, Kim W, Kim K and Lee J: DNA damage
and mitochondria dysfunction in cell apoptosis induced by
nonthermal air plasma. Appl Phys Lett. 96:0215022010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma Y, Karunakaran T, Veeraraghavan VP,
Mohan SK and Li S: Sesame inhibits cell proliferation and induces
apoptosis through inhibition of STAT-3 translocation in thyroid
cancer cell lines (FTC-133). Biotechnol Bioprocess Eng. 24:646–652.
2019. View Article : Google Scholar
|
|
44
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Ann Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bossy-Wetzel E, Newmeyer DD and Green DR:
Mitochondrial cytochrome c release in apoptosis occurs upstream of
DEVD-specific caspase activation and independently of mitochondrial
transmembrane depolarization. EMBO J. 17:37–49. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schuler M, Bossy-Wetzel E, Goldstein JC,
Fitzgerald P and Green DR: p53 induces apoptosis by caspase
activation through mitochondrial cytochrome c release. J Biol Chem.
275:7337–7342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie W, Zhang L, Jiao H, Guan L, Zha J, Li
X, Wu M, Wang Z, Han J and You H: Chaperone-mediated autophagy
prevents apoptosis by degrading BBC3/PUMA. Autophagy. 11:1623–1635.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Castino R, Bellio N, Follo C, Murphy D and
Isidoro C: Inhibition of PI3k class III-dependent autophagy
prevents apoptosis and necrosis by oxidative stress in dopaminergic
neuroblastoma cells. Toxicol Sci. 117:152–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bhoopathi P, Chetty C, Gujrati M, Dinh DH,
Rao JS and Lakka S: Cathepsin B facilitates autophagy-mediated
apoptosis in SPARC overexpressed primitive neuroectodermal tumor
cells. Cell Death Diff. 17:1529–1539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ranjan A and Srivastava SK: Penfluridol
suppresses pancreatic tumor growth by autophagy-mediated apoptosis.
Sci Rep. 6:261652016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Scarlatti F, Maffei R, Beau I, Codogno P
and Ghidoni R: Role of non-canonical Beclin 1-independent autophagy
in cell death induced by resveratrol in human breast cancer cells.
Cell Death Diff. 15:1318–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Al Dhaheri Y, Attoub S, Ramadan G, Arafat
K, Bajbouj K, Karuvantevida N, AbuQamar S, Eid A and Iratni R:
Carnosol induces ROS-mediated beclin1-independent autophagy and
apoptosis in triple negative breast cancer. PLoS One.
9:e1096302014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee KS, Lee MG, Woo YJ and Nam KS: The
preventive effect of deep sea water on the development of cancerous
skin cells through the induction of autophagic cell death in
UVB-damaged HaCaT keratinocyte. Biomed Pharmacother. 111:282–291.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Puissant A, Robert G, Fenouille N, Luciano
F, Cassuto JP, Raynaud S and Auberger P: Resveratrol promotes
autophagic cell death in chronic myelogenous leukemia cells via
JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res.
70:1042–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu J, Chang F, Li F, Fu H, Wang J, Zhang
S, Zhao J and Yin D: Palmitate promotes autophagy and apoptosis
through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun.
463:262–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chun SY, Nam KS and Lee KS: Proton beam
induces P53-mediated cell cycle arrest in HepG2 hepatocellular
carcinoma cells. Biotechnol Bioprocess Eng. 25:141–148. 2020.
View Article : Google Scholar
|