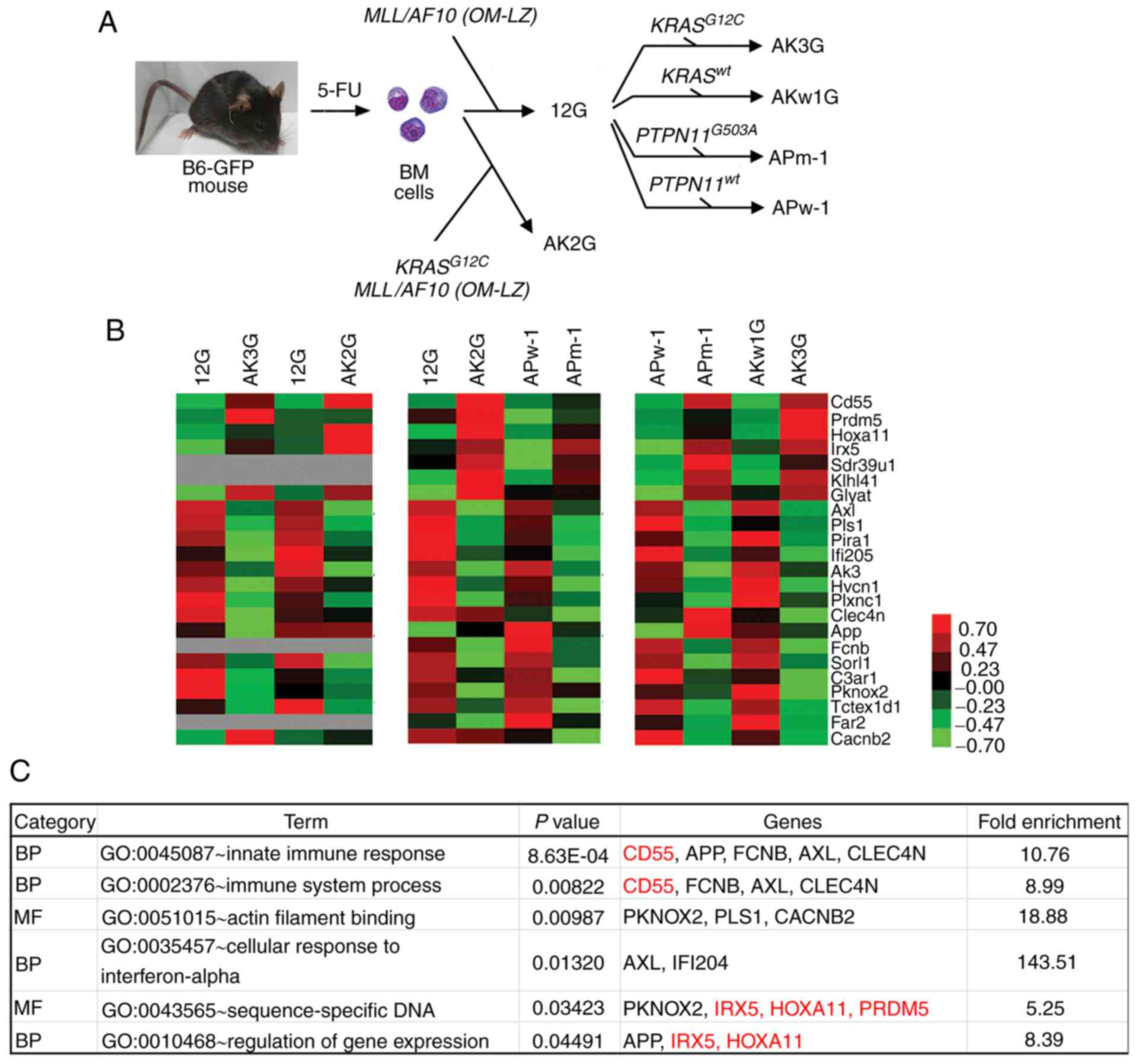
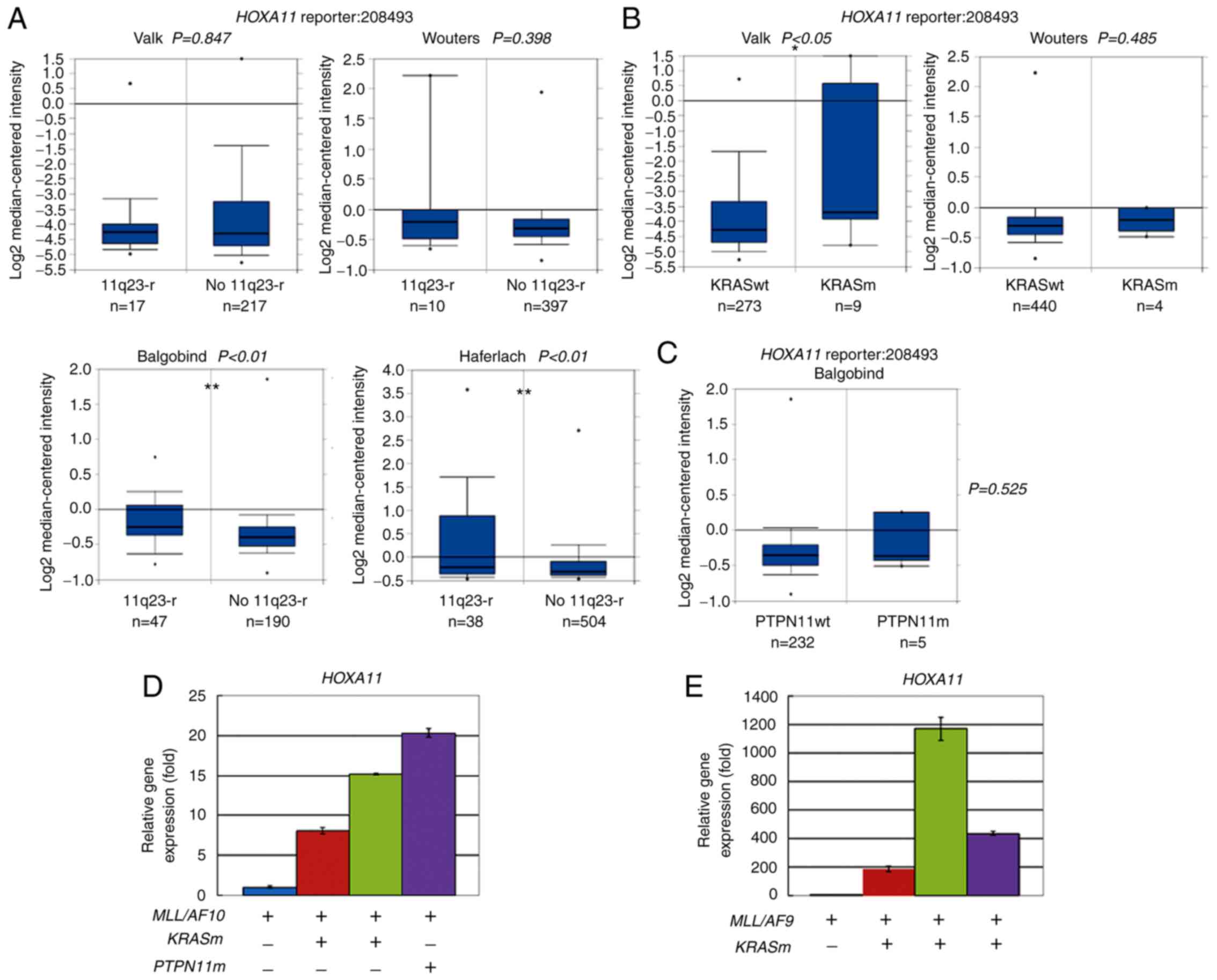
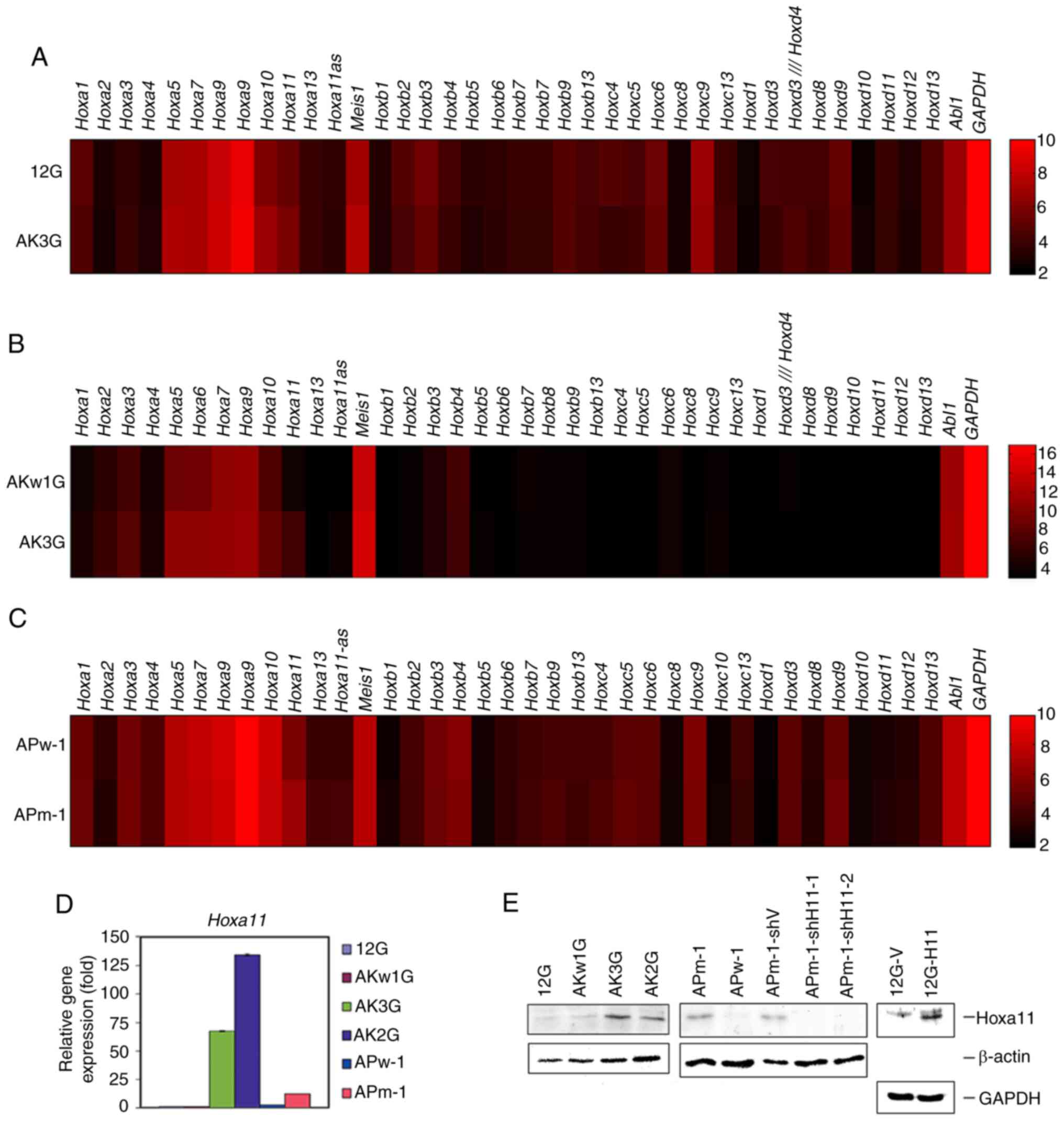
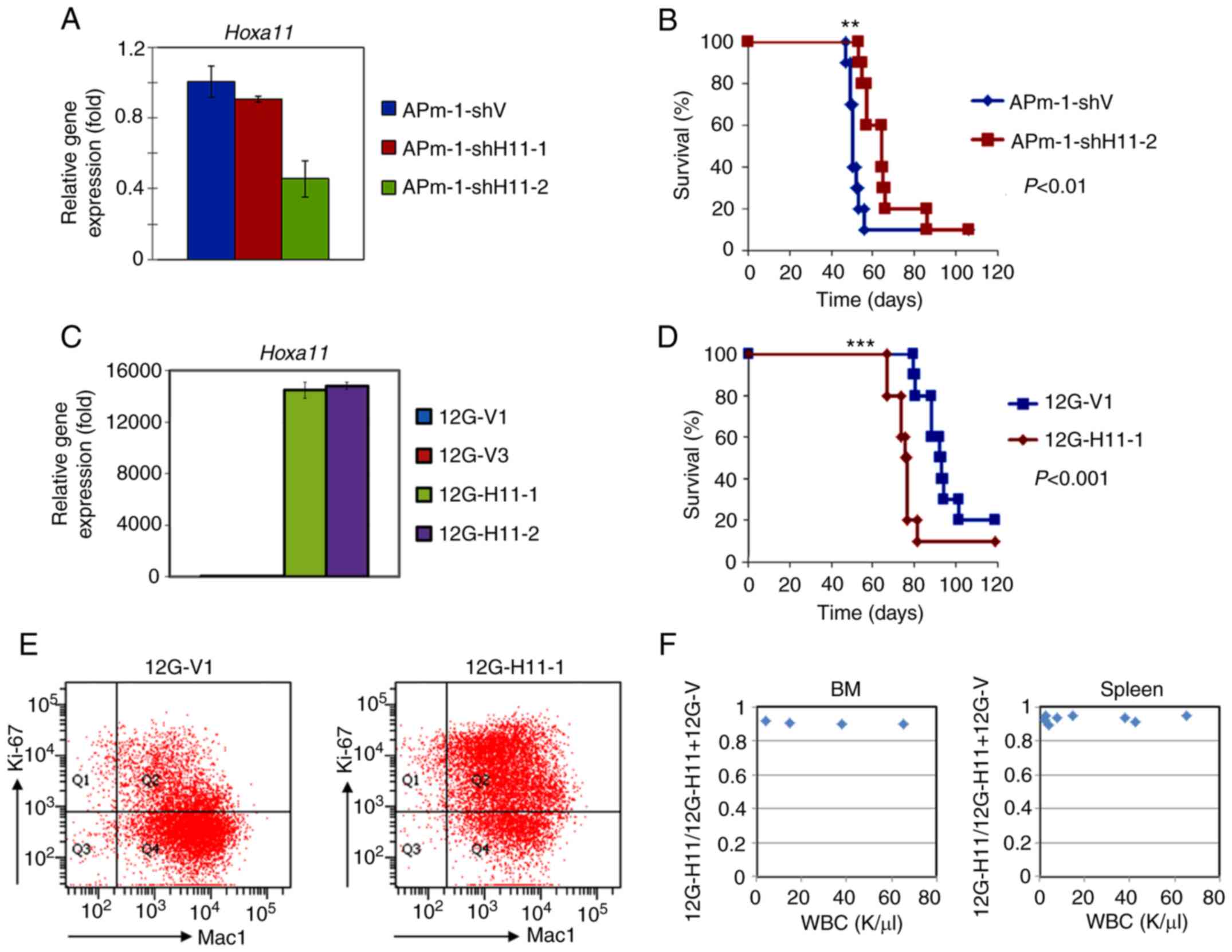
|
1
|
Meyer C, Hofmann J, Burmeister T, Gröger
D, Park TS, Emerenciano M, Pombo de Oliveira M, Renneville A,
Villarese P, Macintyre E, et al: The MLL recombinome of acute
leukemias in 2013. Leukemia. 27:2165–2176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyer C, Burmeister T, Gröger D, Tsaur G,
Fechina L, Renneville A, Sutton R, Venn NC, Emerenciano M,
Pombo-de-Oliveira MS, et al: The MLL recombinome of acute leukemias
in 2017. Leukemia. 32:273–284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balgobind BV, Raimondi SC, Harbott J,
Zimmermann M, Alonzo TA, Auvrignon A, Beverloo HB, Chang M,
Creutzig U, Dworzak MN, et al: Novel prognostic subgroups in
childhood 11q23/MLL-rearranged acute myeloid leukemia: Results of
an international retrospective study. Blood. 114:2489–2496. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grimwade D, Hills RK, Moorman AV, Walker
H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ and Burnett
AK; National Cancer Research Institute Adult Leukaemia Working
Group, : Refinement of cytogenetic classification in acute myeloid
leukemia: Determination of prognostic significance of rare
recurring chromosomal abnormalities among 5876 younger adult
patients treated in the United Kingdom Medical Research Council
trials. Blood. 116:354–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu BD, Hess JL, Horning SE, Brown GA and
Korsmeyer SJ: Altered Hox expression and segmental identity in
Mll-mutant mice. Nature. 378:505–508. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohi MG, Williams IR, Dearolf CR, Chan G,
Kutok JL, Cohen S, Morgan K, Boulton C, Shigematsu H, Keilhack H,
et al: Prognostic, therapeutic, and mechanistic implications of a
mouse model of leukemia evoked by Shp2 (PTPN11) mutations.
Cancer Cell. 7:179–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horton SJ, Grier DG, McGonigle GJ,
Thompson A, Morrow M, De Silva I, Moulding DA, Kioussis D, Lappin
TR, Brady HJ and Williams O: Continuous MLL-ENL expression is
necessary to establish a ‘Hox Code’ and maintain immortalization of
hematopoietic progenitor cells. Cancer Res. 65:9245–9252. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki A, Ito Y, Sashida G, Honda S,
Katagiri T, Fujino T, Nakamura T and Ohyashiki K: t(7;11)(p15;p15)
Chronic myeloid leukaemia developed into blastic transformation
showing a novel NUP98/HOXA11 fusion. British J Haematol.
116:170–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizoguchi Y, Fujita N, Taki T, Hayashi Y
and Hamamoto K: Juvenile myelomonocytic leukemia with
t(7;11)(p15;p15) and NUP98-HOXA11 fusion. Am J Hematol. 84:295–297.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martino V, Bianchera A, Reia L, Bussolati
O, Fazzina R, Marino F, Montemurro L, Tonelli R, Pession A, Gazzola
GC and Sala R: Down-regulation of HOXA4, HOXA7, HOXA10, HOXA11 and
MEIS1 during monocyte-macrophage differentiation in THP-1 cells.
Mol Med Rep. 2:241–244. 2009.PubMed/NCBI
|
|
11
|
Zhao Y and Potter SS: Functional
comparison of the Hoxa 4, Hoxa 10, and Hoxa 11 homeoboxes. Dev
Biol. 244:21–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang DC, Shih LY, Fu JF, Li HY, Wang HI,
Hung IJ, Yang CP, Jaing TH, Chen SH and Liu HC: K-Ras mutations and
N-Ras mutations in childhood acute leukemias with or without
mixed-lineage leukemia gene rearrangements. Cancer. 106:950–956.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grossmann V, Schnittger S, Poetzinger F,
Kohlmann A, Stiel A, Eder C, Fasan A, Kern W, Haferlach T and
Haferlach C: High incidence of RAS signalling pathway mutations in
MLL-rearranged acute myeloid leukemia. Leukemia. 27:1933–1936.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Driessen EM, van Roon EH,
Spijkers-Hagelstein JA, Schneider P, de Lorenzo P, Valsecchi MG,
Pieters R and Stam RW: Frequencies and prognostic impact of RAS
mutations in MLL-rearranged acute lymphoblastic leukemia in
infants. Haematologica. 98:937–944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andersson AK, Ma J, Wang J, Chen X, Gedman
AL, Dang J, Nakitandwe J, Holmfeldt L, Parker M, Easton J, et al:
The landscape of somatic mutations in infant MLL-rearranged acute
lymphoblastic leukemias. Nat Genet. 47:330–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balgobind BV, Zwaan CM, Pieters R and Van
den Heuvel-Eibrink MM: The heterogeneity of pediatric
MLL-rearranged acute myeloid leukemia. Leukemia.
25:1239–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barbosa TC, Andrade FG, Lopes BA, de
Andrade CF, Mansur MB, Emerenciano M and Pombo-de-Oliveira MS:
Impact of mutations in FLT3, PTPN11 and RAS genes on the overall
survival of pediatric B cell precursor acute lymphoblastic leukemia
in Brazil. Leuk Lymphoma. 55:1501–1509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tamai H, Miyake K, Takatori M, Miyake N,
Yamaguchi H, Dan K, Shimada T and Inokuchi K: Activated K-Ras
protein accelerates human MLL/AF4-induced leukemo-lymphomogenicity
in a transgenic mouse model. Leukemia. 25:888–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim WI, Matise I, Diers MD and Largaespada
DA: RAS oncogene suppression induces apoptosis followed by more
differentiated and less myelosuppressive disease upon relapse of
acute myeloid leukemia. Blood. 113:1086–1096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ono R, Kumagai H, Nakajima H, Hishiya A,
Taki T, Horikawa K, Takatsu K, Satoh T, Hayashi Y, Kitamura T and
Nosaka T: Mixed-lineage-leukemia (MLL) fusion protein collaborates
with Ras to induce acute leukemia through aberrant Hox expression
and Raf activation. Leukemia. 23:2197–2209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu JF, Yen TH, Chen Y, Huang YJ, Hsu CL,
Liang DC and Shih LY: Involvement of Gpr125 in the myeloid sarcoma
formation induced by cooperating MLL/AF10(OM-LZ) and oncogenic KRAS
in a mouse bone marrow transplantation model. Int J Cancer.
133:1792–1802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu JF, Liang ST, Huang YJ, Liang KH, Yen
TH, Liang DC and Shih LY: Cooperation of MLL/AF10(OM-LZ) with
PTPN11 activating mutation induced monocytic leukemia with a
shorter latency in a mouse bone marrow transplantation model. Int J
Cancer. 140:1159–1172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rojas JM and Santos E: Ras genes and human
cancer: Different implications and different roles. Curr Genomics.
3:2002. View Article : Google Scholar
|
|
24
|
Schubbert S, Lieuw K, Rowe SL, Lee CM, Li
X, Loh ML, Clapp DW and Shannon KM: Functional analysis of
leukemia-associated PTPN11 mutations in primary
hematopoietic cells. Blood. 106:311–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Z, Li Y, Yin F and Chan RJ:
Activating PTPN11 mutants promote hematopoietic progenitor
cell-cycle progression and survival. Exp Hematol. 36:1285–1296.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu JF, Hsu CL and Shih LY:
MLL/AF10(OM-LZ)-immortalized cells expressed cytokines and induced
host cell proliferation in a mouse bone marrow transplantation
model. Int J Cancer. 126:1621–1629. 2010.PubMed/NCBI
|
|
27
|
Valk PJ, Verhaak RG, Beijen MA, Erpelinck
CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM,
Beverloo HB, Moorhouse MJ, van der Spek PJ, Löwenberg B and Delwel
R: Prognostically useful gene-expression profiles in acute myeloid
leukemia. N Engl J Medicine. 350:1617–1628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wouters BJ, Löwenberg B,
Erpelinck-Verschueren CA, van Putten WL, Valk PJ and Delwel R:
Double CEBPA mutations, but not single CEBPA mutations, define a
subgroup of acute myeloid leukemia with a distinctive gene
expression profile that is uniquely associated with a favorable
outcome. Blood. 113:3088–3091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haferlach T, Kohlmann A, Wieczorek L,
Basso G, Kronnie GT, Béné MC, De Vos J, Hernández JM, Hofmann WK,
Mills KI, et al: Clinical utility of microarray-based gene
expression profiling in the diagnosis and subclassification of
leukemia: Report from the international microarray innovations in
leukemia study group. J Clin Oncol. 28:2529–2537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balgobind BV, Hollink IH, Arentsen-Peters
ST, Zimmermann M, Harbott J, Beverloo HB, von Bergh AR, Cloos J,
Kaspers GJ, de Haas V, et al: Integrative analysis of type-I and
type-II aberrations underscores the genetic heterogeneity of
pediatric acute myeloid leukemia. Haematologica. 96:1478–1487.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leonetti A, Baroli G, Fratini E,
Pietropaoli S, Marcoli M, Mariottini P and Cervelli M: Epileptic
seizures and oxidative stress in a mouse model over-expressing
spermine oxidase. Amino Acids. 52:129–139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu JF, Yen TH, Huang YJ and Shih LY: Ets1
plays a critical role in MLL/EB1-mediated leukemic transformation
in a mouse bone marrow transplantation model. Neoplasia.
21:469–481. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heuser M, Wingen LU, Steinemann D, Cario
G, von Neuhoff N, Tauscher M, Bullinger L, Krauter J, Heil G,
Döhner H, et al: Gene-expression profiles and their association
with drug resistance in adult acute myeloid leukemia.
Haematologica. 90:1484–1492. 2005.PubMed/NCBI
|
|
35
|
Sun Y, Zeng C, Gan S, Li H, Cheng Y, Chen
D, Li R and Zhu W: LncRNA HOTTIP-Mediated HOXA11 expression
promotes cell growth, migration and inhibits cell apoptosis in
breast cancer. Int J Mol Sci. 19:4722018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo X, Yan B and Qiu Y: HOXA11 regulates
chemoresistance by modulating p53 gene expression in acute myeloid
leukemia. Blood. 134:5182. 2019. View Article : Google Scholar
|
|
37
|
Fiegl H, Windbichler G, Mueller-Holzner E,
Goebel G, Lechner M, Jacobs IJ and Widschwendter M: HOXA11 DNA
methylation--a novel prognostic biomarker in ovarian cancer. Int J
Cancer. 123:725–729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hwang JA, Lee BB, Kim Y, Park SE, Heo K,
Hong SH, Kim YH, Han J, Shim YM, Lee YS and Kim DH: HOXA11
hypermethylation is associated with progression of non-small cell
lung cancer. Oncotarget. 4:2317–2325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cui Y, Gao D, Linghu E, Zhan Q, Chen R,
Brock MV, Herman JG and Guo M: Epigenetic changes and functional
study of HOXA11 in human gastric cancer. Epigenomics. 7:201–213.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Q, Chen C, Ren X and Sun W: DNA
methylation profiling identifies the HOXA11 gene as an early
diagnostic and prognostic molecular marker in human lung
adenocarcinoma. Oncotarget. 8:33100–33109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Se YB, Kim SH, Kim JY, Kim JE, Dho YS, Kim
JW, Kim YH, Woo HG, Kim SH, Kang SH, et al: Underexpression of
HOXA11 is associated with treatment resistance and poor prognosis
in glioblastoma. Cancer Res Treat. 49:387–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Cui Y, Sheng J, Yang Y, Kuang G,
Fan Y, Jin J and Zhang Q: Epigenetic inactivation of HOXA11, a
novel functional tumor suppressor for renal cell carcinoma, is
associated with RCC TNM classification. Oncotarget. 8:21861–21870.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xia B, Shan M, Wang J, Zhong Z, Geng J, He
X, Vu T, Zhang D and Pang D: Homeobox A11 hypermethylation
indicates unfavorable prognosis in breast cancer. Oncotarget.
8:9794–9805. 2017. View Article : Google Scholar : PubMed/NCBI
|