Introduction
Lung cancer is the most common form of cancer and
represents a leading cause of cancer-related deaths worldwide
(1). Lung adenocarcinoma (LUAD)
accounts for approximately 40% of all cases involving lung cancer
and is the most heterogeneous and aggressive form of non-small cell
lung cancer (2,3). Despite new developments in the past few
years in the diagnosis and treatment of LUAD, the prognosis of
patients with malignant LUAD is poor; their 5-year survival rate,
is <18% (4). Exploring the
molecular mechanisms underlying the pathogenesis of LUAD and
identifying new diagnostic biomarkers are essential to improve the
prognosis of these patients.
The investigation of immune checkpoint inhibitors
(ICIs) has led to significant changes in the therapeutic methods
used in oncology, and in particular, the blockade of interactions
involving programmed cell death-ligand 1 (PD-L1)/programmed cell
death protein-1 (PD-1) (5). Tumor
cells, by expressing PD-L1 and binding to PD-1 molecules on the
surface of T cells, can activate negative co-stimulatory signals in
T lymphocytes, thus inhibiting the activation and proliferation of
T cells and the induction of apoptosis, thus allowing tumors to
evade the surveillance of the immune system (6). The blockade of PD-L1/PD-1 has
demonstrated favorable overall efficacies for the treatment of
various types of tumor, and has been used clinically to treat
non-small cell lung cancer (NSCLC) (7), melanoma (8), head and neck squamous cell carcinoma
(HNSCC) (9,10), renal cell carcinoma (11), urothelial carcinoma (12), gastric cancer (13), microsatellite instability-high (MSI-H)
cancers (14) and mismatch-repair
deficiency (15). Because PD-L1 is
expressed by tumor cells to inhibit T cells and survive their
cytotoxic activities, the expression of PD-L1 in tumor tissue has
been widely used as a favorable predictive biomarker for the
diagnosis and prognosis of cancer (16,17).
Hypoxia is the main feature of solid tumors. Studies have revealed
that hypoxia can induce the expression of PD-L1 in tumor cells and
facilitate the evasion of tumors from immune attack (18–20).
Ubiquitin-specific peptidase 22 (USP22) is a subunit
of the human SPT-ADA-Gcn5 acetyltransferase (SAGA) complex and is a
deubiquitinating enzyme (21). USP22
has been revealed to exhibit the transcriptional characteristics of
different genes and can cause aggressive growth, metastasis, and
treatment resistance, in numerous forms of human cancers, including
lung cancer (22). A recent study
revealed that USP22 could induce angiogenesis, growth and the
metastasis of LUAD (23), thereby
resulting in the development of LUAD. Study has also revealed that
USP22 can stabilize PD-L1 protein expression in Human non-small
cell lung cancer (24). However,
whether USP22 can regulate the expression of PD-L1 in LUAD has yet
to be elucidated.
Research has revealed that PD-1/PD-L1 plays a key
role in the evasion of tumors from immune attack. Therefore,
researchers have made numerous attempts to target this particular
immune checkpoint (25). MicroRNAs
(miRNAs or miRs) are short, non-coding, and evolutionarily
conserved RNAs. miRNAs have been revealed to regulate gene
expression at the post-transcription level by binding to the
3′-untranslated region (3′-UTR) of mRNAs (26). Multiple studies have reported that
miRs can regulate PD-L1 expression via multiple pathways (27,28). Of
these, miR-30-5p was reported to inhibit cell chemoresistance and
stemness in colorectal cancer by targeting the USP22/Wnt/β-catenin
signaling axis (29). Furthermore,
miR-30-5p has also been revealed to function as a tumor suppressor
in numerous different cancers (30).
However, the relationship among miR-30-5p and USP22 and PD-L1 in
LUAD cells has yet to be investigated.
Therefore, the aim of the present study was to
investigate whether USP22 is the direct target of miR-30a-5p,
miR-30b-5p, miR-30c-5p, miR-30d-5p and miR-30e-5p, and the
relationship of inhibition of USP22 with the promotion of PD-L1
induced by hypoxia. It is theorized that the miR-30-5p family may
represent a new treatment target for LUAD.
Materials and methods
Cell culture and transfection of
oligomers
A549 cells, an important strain of LUAD cells were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). These cells were cultured in Roswell Park
Memorial Institute (RPMI)-1640 medium (Sigma-Aldrich; Merck KGaA)
with 10% fetal bovine serum (FBS) (Hyclone; Cytiva), 100 U/ml
penicillin and 100 U/ml streptomycin at 37°C. Incubation was
carried out in a 5% CO2 incubator with either 20%
O2 or 1% O2. The 293T cell line was purchased
from the American Type Culture Collection and cultured at 37°C with
5% CO2 in Dulbecco's modified Eagle's medium (DMEM)
(Hyclone; Cytiva) supplemented with 10% FBS, 1%
penicillin-streptomycin and 2 mM l-glutamine.
To perform miRNA mimics transfection, one day before
transfection with miRNA mimics, 1×106 cells were seeded
into 6-well plates and cultured at 37°C with 5% CO2. The
transfection was performed when the cells had reached approximately
70% confluence using Lipofectamine RNAiMAX (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions, and cells were cultured at 37°C with 5%
CO2 for 48 h. Then, the level of miRNA was confirmed by
RT-qPCR at 48 h post transfection. The miRNA oligonucleotides and
control used in the study are listed in Table I. The final working concentration of
the miRNA mimics was 100 nM. In addition, A549 cells were
co-transfected with miR-30-5p mimics and a hypoxia response element
(HRE) reporter gene plasmid (HRE-LUC). In addition, miR-30-5p
mimics and Flag-USP22 or USP22 catalytic domain inactivated mutant
plasmid (USP22-HH/AA), purchased from Shanghai GenePharma Co., Ltd,
were co-transfected into A549 cells and cultured under hypoxic
conditions. Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for plasmid transfections following the
manufacturer's instructions. For MG132 treatment, 5 mM stock
solution was prepared by dissolving MG132 (Calbiochem; Merck KGaA)
in DMSO. Then, the cells were treated with MG132 at a final
concentration 25 µM for 24 h and collected for western blot
assay.
 | Table I.The sequences of transfected
oligomers. |
Table I.
The sequences of transfected
oligomers.
| Name of miRNA | Sequences of
transfected oligomers (5′-3′) |
|---|
| miR-30a-5p |
UGUAAACAUCCUCGACUGGAAGAAG |
| miR-30b-5p |
UGUAAACAUCCUACACUCAGCU |
| miR-30c-5p |
UGUAAACAUCCUACACUCUCAGC |
| miR-30d-5p |
UGUAAACAUCCCCGACUGGAAG |
| miR-30e-5p |
UGUAAACAUCCUUGACUGGAAG |
| miR-30e-5p
control |
UUCUCCGAACGUGUCACGUTT |
Luciferase assay
The wild-type (WT) USP22 3UTR or mutant (MUT) USP22
3UTR containing the putative miR-30-5p binding site was synthesized
(Sangon Biotech Co., Ltd.), and the fragments were cloned into the
pMIR-Report Luciferase vector (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The 293T cell line was used for luciferase
reporter assays. Cells were plated and the cell density was allowed
to reach approximately 50%. Then, 100 ng pMIR/USP22 WT or
pMIR/USP22 MUT, 2 ng Renilla luciferase plasmid (cat. no.
E6921; Promega Corporation) containing Renilla luciferase
and 100 nM miRNA mimics were co-transfected using Lipofectamine™
2000 reagent. A total of 24 h after transfection, the firefly and
Renilla luciferase activities were assessed using the
Dual-Glo Luciferase Assay System (cat. no. E2920) and a
GloMax® 20/20 Luminometer (E5311; both from Promega
Corporation).
Reverse transcription-quantitative
(RT-q) PCR
Total RNAs from A549 cells were extracted using
RNAiso Plus (Takara Bio, Inc.). mRNAs were then reversely
transcribed into complementary DNA with a reverse transcription kit
(cat. no. RR036A; Takara Bio, Inc.) according to the manufacturer's
instructions. The expression levels of mRNA and miRNA were then
detected via TB Green II (cat. no. RR820Q; Takara Bio, Inc.). The
thermocycling conditions were as follows: 94°C for 4 min, followed
by 35 cycles of 20 sec at 94°C, 30 sec at 60°C and 30 sec at 72°C.
β-actin and U6 were applied as internal controls. The relative
expression levels were calculated with the 2−ΔΔCq method
(31). The primers are presented in
Table II.
 | Table II.Primers for reverse
transcription-quantitative PCR. |
Table II.
Primers for reverse
transcription-quantitative PCR.
| Gene name | Primer
sequences |
|---|
| USP22 | F:
5′-GGACAACTGGAAGCAGAACC-3′ |
|
| R:
5′-TGAAACAGCCGAAGAAGACA-3′ |
| PD-L1 | F:
5′-TAAGACCACCACCACCAA-3′ |
|
| R:
5′-TGACTATGATAGGCAGACATC-3′ |
| β-actin | F:
5′-CACTGTGCCCATCTACGAGG-3′ |
|
| R:
5′-TAATGTCACGCACGATTTCC-3′ |
| miR-30a-5p | F:
5′-ACACTCCAGCTGGGTGTAAACATCCTCGAC-3′ |
|
| R:
5′-CAGTGCGTGTCGTGGAGT-3′ |
| miR-30b-5p | F:
5′-ACGGGCAAAAATACTCCAGCTCTCAAT-3′ |
|
| R:
5′-CTCTGGAAAACTGGTGTCGACTGGTGTC-3′ |
| miR-30c-5p | F:
5′-GCCGCTGTAAACATCCTACACT-3′ |
|
| R:
5′-GTGCAGGGTCCGAGGT-3′ |
| miR-30d-5p | F:
5′-GGTGTAAACATCCCCGAC-3′ |
|
| R:
5′-CAGTGCGTGTCGTGGAG-3′ |
| miR-30e-5p | F:
5′-TGTAAACATCCTTGACTGGAAGG-3′ |
|
| R:
5′-CCAGTGCGAATACCTCGGAC-3′ |
| U6 | F:
5′-GCTTCGGCAGCACATATACT-3′ |
|
| R:
5′-GTGCAGGGTCCGAGGTATTC-3′ |
Western blot analysis
Cellular protein was extracted by RIPA Lysis Buffer
(cat. no. P0013B; Beyotime Institute of Biotechnology) and protein
concentrations were assessed with a BCA Protein Assay kit (cat. no.
P0010; Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. A total of 30 µg protein was loaded
per lane, and then the proteins were separated by 12% sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene fluoride (PVDF) membranes. Then
membranes were blocked with 5% skim milk-TBST for 1 h at room
temperature. Primary antibodies that were specific to PD-L1
(product no. 13684; 1:1,000; Cell Signaling Technology, Inc.),
USP22 (cat. no. 55110-1-AP; 1:1,000; ProteinTech Group, Inc.),
hypoxia-inducible factor (HIF)-1α (product no. 36169; 1:1,000; Cell
Signaling Technology, Inc.), and β-actin (product no. 3700;
1:1,000; Cell Signaling Technology, Inc.), were incubated with the
PVDF membranes for 12 h at 4°C. PVDF membranes were incubated with
HRP-conjugated goat anti-rabbit (cat. no. SA00001-2; 1:2,000) and
goat anti-mouse (cat. no. SA00001-1; 1:2,000; ProteinTech Group,
Inc.) IgG (H+L) secondary antibodies for 1 h at room temperature.
Then, after being washed three times in PBS containing 0.05%
Tween-20 (PBST), the membranes were visualized to demonstrate the
positive binding antibody using BeyoECL Plus (cat. no. P0018S;
Beyotime Institute of Biotechnology) and a gel imaging system
(Bio-Rad Laboratories, Inc.). ImageJ v1.48 (National Institutes of
Health) was then used to calculate the gray values of the
images.
T-cell-mediated tumor cell killing
assay
According to an experiment previously reported
(32), T cells were activated using
anti-CD3 antibody (cat. no. 14-0037-82; 100 ng/ml; eBioscience;
Thermo Fisher Scientific, Inc.) and interleukin-2 (cat. no.
PHC0023; 10 ng/ml; Thermo Fisher Scientific, Inc.). After
transfection with miRNA mimics, the cells were pre-treated at 37°C
under hypoxic conditions for 24 h, and the tumor cells and T cells
were then co-cultured at 37°C for 3 days. Next, the wells were
washed twice with PBS to remove the T cells, and the surviving
tumor cells were fixed with 4% paraformaldehyde for 20 min and
stained with 1% crystal violet solution for 15 min at room
temperature.
Cycloheximide (CHX) chase assay
Cells were treated with CHX (50 µg/ml;
MedChemExpress) at 37°C and harvested at 0, 4 and 8 h. After CHX
treatment, cells were lysed in ice-cold RIPA cell lysates (CST) and
the lysates were analyzed by western blotting with anti-HIF-1α,
anti-USP22, anti-PD-L1 or anti-β-actin antibodies.
Co-immunoprecipitation (Co-IP)
Cells were collected and immunoprecipitation was
carried out with a Pierce™ Classic Magnetic IP/Co-IP kit (cat. no.
88804; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's instructions. Accordingly, cells were lysed in lysis
buffer (0.025 M Tris, 0.15 M NaCl, 0.001 M EDTA, 1% NP-40, and 5%
glycerin) for 5 min on ice, and then proteins were centrifugated at
13,000 × g for 10 min. Next, the samples were incubated with rabbit
USP22 antibody (cat. no. 55110-1-AP; 4 µg; ProteinTech Group, Inc.)
or rabbit isotype control IgG provided in the kit with agitated
rotation at 4°C overnight. Next, magnetic beads (0.25 mg) were
added to the proteins and rotated at room temperature for 1 h. The
magnetic beads were subsequently washed with buffer solution and
eluted. Western blotting was then carried out as
aforementioned.
Chromatin immunoprecipitation (ChIP)
assay
Next, a Pierce Agarose ChIP kit (cat. no. 26156;
Thermo Fisher Scientific, Inc.), and an HIF-1α or isotype control
mAb (product no. 3900; Cell Signaling Technology, Inc.), were used
for immunoprecipitation according to the manufacturer's
instructions. Accordingly, the cultured cells were incubated with
1% formaldehyde for 10 min at 37°C followed by incubation with 1X
Glycine solution for 5 min at room temperature. Then, cells were
collected, lysed, and digested by MNase. Next, protein-chromatin
complexes were immunoprecipitated with 5 µg antibodies overnight at
4°C with agitated rotation. Complexes were separated by incubation
with ChIP grade proteinA/G agarose at 4°C with agitation rotation
for 1 h. Chromatin DNA fragments were then eluted and purified for
quantitative real-time PCR. The products were then amplified using
the following primers: HRE1 forward, 5′-TACCATGCAGTAAGATGGGCAATA-3′
and reverse, 5′-GAACCCCAAAATGGAGTCCAAA-3′; HRE2 forward,
5′-GTAATAGGAAGTATCAAAGTGCCC-3′ and reverse,
5′-TCCCTCTTAGTGCCTCTCCAA-3′; HRE3 forward,
5′-TGCATACAGTGGTTTTGGGA-3′ and reverse, 5′-AGGAGTTCTACTTCCCTGAGT3′.
anti-MYCN antibody (product no. 51705; Cell Signaling Technology,
Inc.).
Statistical analysis
Results are expressed as the mean ± standard
deviation (SD) of three independent experiments unless otherwise
specified. Data were analyzed using Graph Prism version 8.2
software (GraphPad Software, Inc.). The unpaired Student's t-test
and one-way analysis of variance (ANOVA) with Tukey's post hoc test
were used to analyze differences between and among groups,
respectively. P<0.05 was considered to indicate a statistically
significant difference.
Results
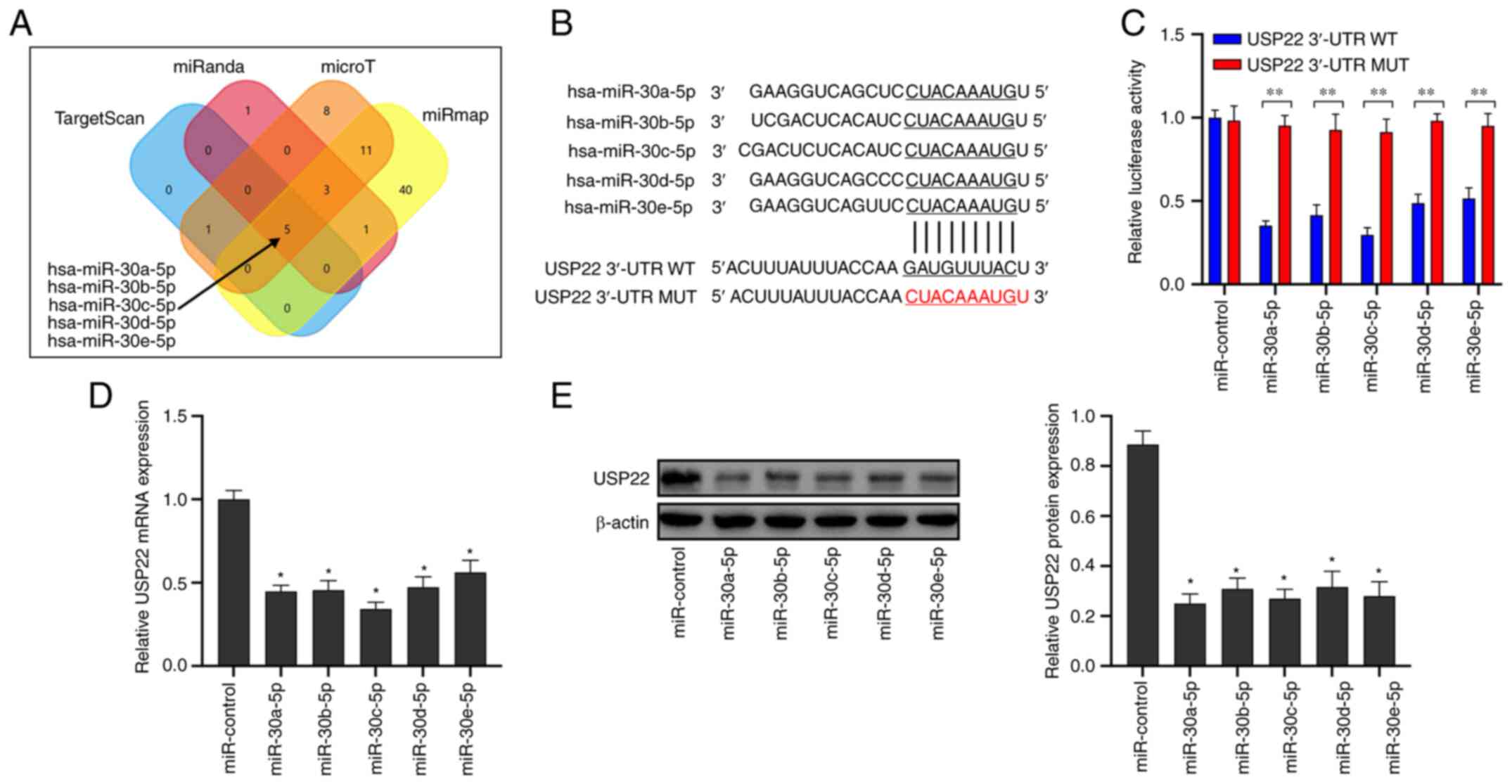
miR-30-5p directly targets USP22
The miRNA targeted by USP22 was firstly predicted
and a Venn diagram showing the overlapping parts of the four
databases was generated (Fig. 1A).
Analysis revealed that the miR-30-5p family targeted the 3′UTR
bound to USP22 (Fig. 1B). The dual
luciferase reporter results revealed that miR-30-5p significantly
reduced the luciferase activity of wild-type USP22 3′-UTR, but did
not affect the mutant USP22 3′-UTR (Fig.
1C; P<0.05). Transfection of miR-30-5p mimics significantly
inhibited USP22 mRNA (Fig. 1D;
P<0.05) and protein expression (Fig.
1E; P<0.05) in A549 cells. These results indicated that
miR-30-5p directly targeted USP22 in A549 cells.
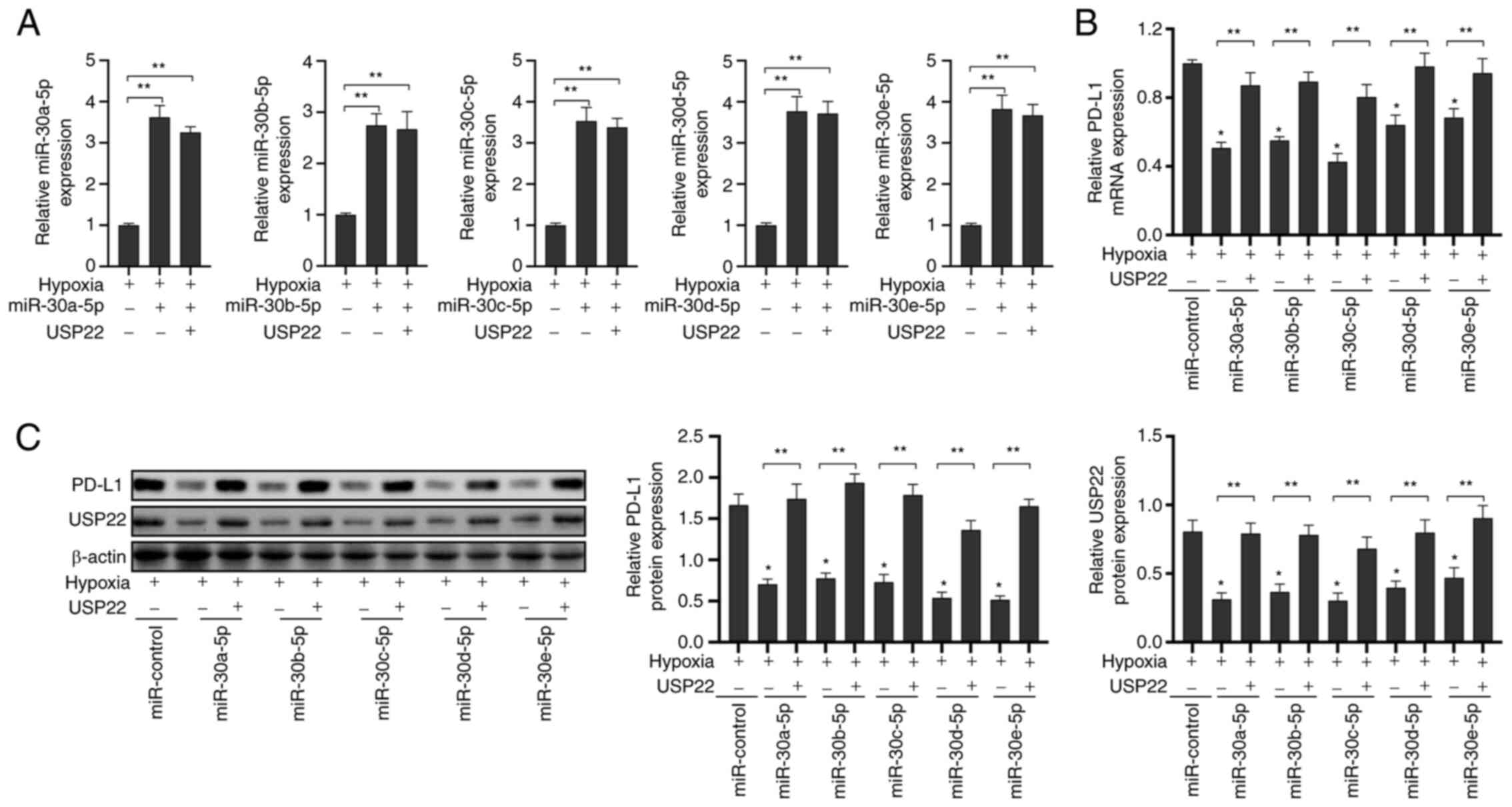
Expression of PD-L1 is inhibited in
A549 cells under hypoxic conditions via the expression of
miR-30-5p
Previous studies have reported that hypoxia induced
the expression of PD-L1 and thus allows tumor cells to evade attack
by the immune system (18–20). Firstly, miR-30a-5p mimics, miR-30b-5p
mimics, miR-30c-5p mimics, miR-30d-5p mimics, miR-30e-5p mimics and
the corresponding control were transfected in A549 cells. The
transfection efficiency is revealed in Fig. S1. Next, it was investigated whether
miR-30-5p could affect PD-L1 expression under hypoxic conditions
(Fig. 2A; P<0.05). It was revealed
that the transfection of miR-30-5p mimics inhibited the expression
of PD-L1 mRNA (Fig. 2B; P<0.05)
and protein (Fig. 2C; P<0.05) in
A549 cells under hypoxic conditions; however, the overexpression of
USP22 reversed this effect. These results indicated that miR-30-5p
targeted USP22 to inhibit the expression of PD-L1 in A549 cells
under hypoxic conditions.
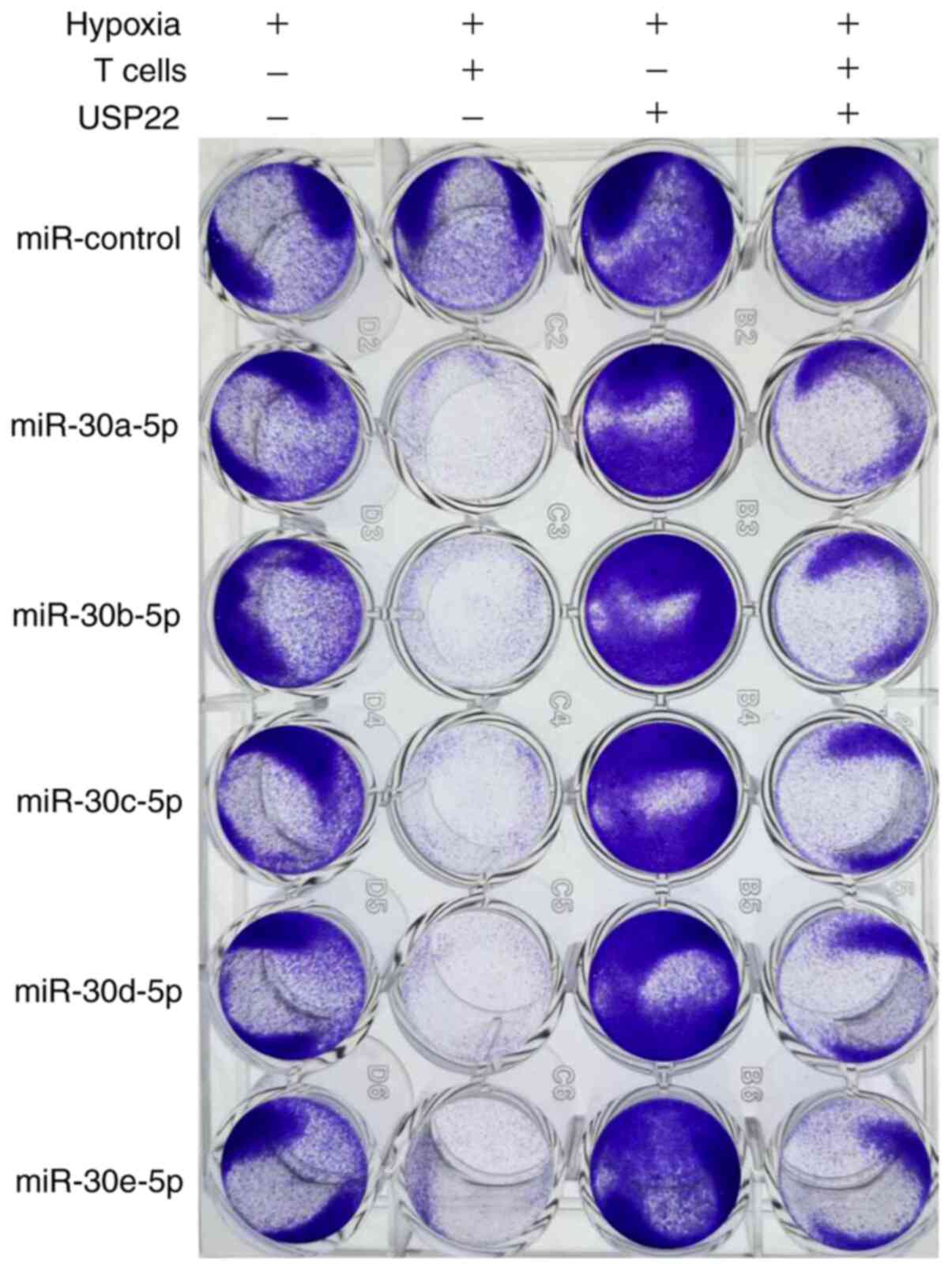
Inducing the destruction of T
cell-mediated A549 cells by targeting miR-30-5p via USP22 under
hypoxia
Given that the binding of PD-L1 to PD-1 can inhibit
T cell activation, and that miR-30-5p downregulated the expression
of PD-L1 in A549 cells under hypoxia, it was next investigated
whether miR-30-5p may have an effect on the mechanism by which A549
cells escape T cell-mediated cancer cell destruction. Our
experiments demonstrated that incubating A549 cells with activated
T cells could induce the death of T cells in A549 cells by the
transfection of miR-30-5p mimics under hypoxic conditions; the
overexpression of USP22 reversed this effect (Fig. 3). Therefore, this experiment indicated
that targeting miR-30-5p via USP22 may induce LUAD cell destruction
by T cell-mediation.
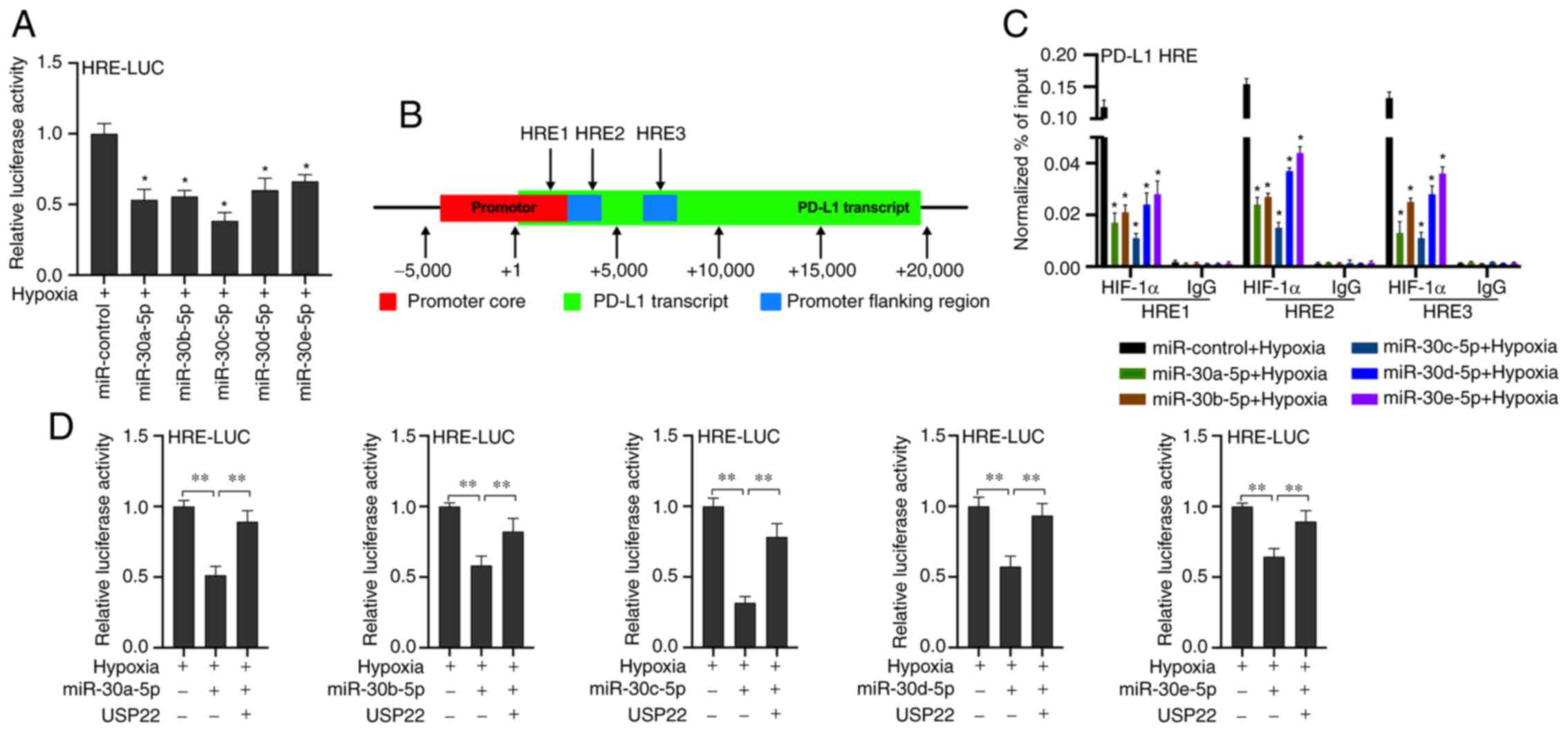
miR-30-5p inhibits HIF-1α binding to
the HRE of the PD-L1 promoter region by down-regulating USP22 under
hypoxia
It was revealed that miR-30-5p inhibits
hypoxia-induced PD-L1 mRNA expression. A previous study reported
that PD-L1 is the target gene of HIF-1α (19). It was hypothesized that the inhibition
of hypoxia-induced PD-L1 mRNA expression by miR-30-5p may be
related to HIF-1α. After co-transfected with miR-30-5p mimics and
response element (HRE) reporter gene plasmid (HRE-LUC), the results
showed that miR-30-5p mimics inhibited the luciferase activity of
HRE-LUC (Fig. 4A; P<0.05). A
previous study reported a potential HRE site on the PD-L1 gene
(33) (Fig.
4B). Our chromatin immunoprecipitation results revealed that
miR-30-5p inhibited the binding of HIF-1α to the PD-L1 gene HRE
(Fig. 4C, P<0.05). These results
indicated that miR-30-5p inhibited HIF-1α binding to the HRE of the
PD-L1 promoter region under hypoxic conditions. To further verify
whether miR-30-5p acts by targeting USP22, our experiments
demonstrated that the co-transfection of USP22 and miR-30-5p mimics
reversed the inhibitory effect of miR-30-5p mimics on the
luciferase activity of HRE-LUC (Fig.
4D; P<0.05), thus indicating that miR-30-5p inhibited HIF-1α
binding to the HRE of the PD-L1 promoter region by targeting USP22
under hypoxia.
miR-30-5p inhibits the stabilizing
effect on HIF-1α and PD-L1 protein by down-regulating USP22 under
hypoxia
Next, it was analyzed whether miR-30-5p affected the
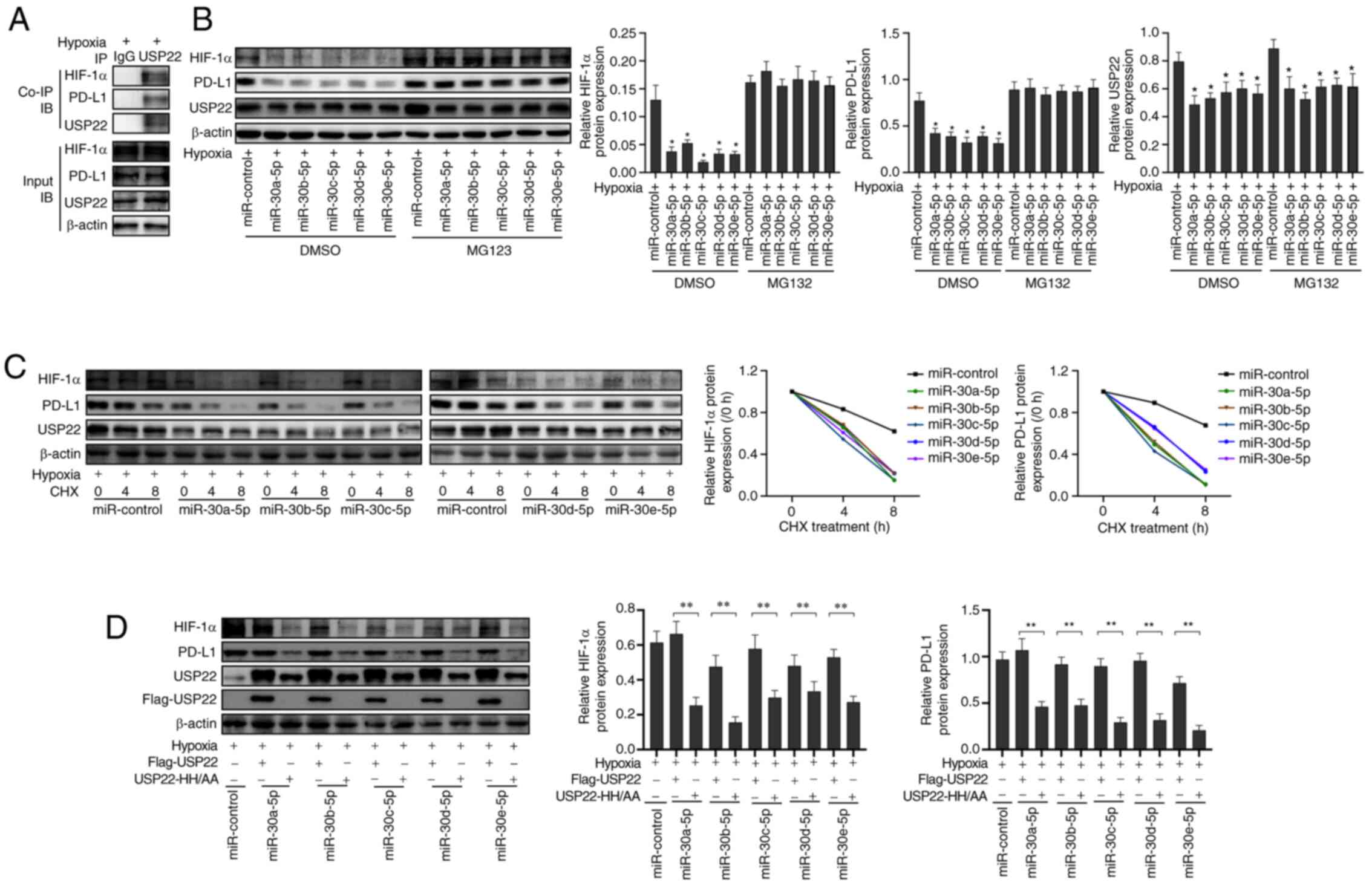
stability of the PD-L1 and HIF-1α protein in A549 cells. Co-IP
revealed that USP22 interacted with the PD-L1 and HIF-1α proteins
in A549 cells under hypoxic conditions (Fig. 5A). Treatment with MG132, a proteasome
inhibitor, reversed the inhibitory effect of miR-30-5p on PD-L1 and
HIF-1α (Fig. 5B; P<0.05).
Furthermore, miR-30-5p treatment shortened the half-life of both
PD-L1 and HIF-1α (Fig. 5C). Our
aforementioned results revealed that the co-transfection of
miR-30-5p mimics and USP22 increased the expression of PD-L1 in
A549 cells. Therefore, miR-30-5p mimics and Flag-USP22 or USP22
catalytic domain inactivated mutant plasmid (USP22-HH/AA) were
co-transfected into A549 cells and cultured under hypoxic
conditions. Compared with the co-transfection of miR-30-5p mimics
and Flag-USP22 plasmids, the co-transfection of miR-30-5p mimics
and USP22-HH/AA plasmid reduced the expression of PD-L1 and HIF-1α
in A549 cells (Fig. 5D; P<0.05).
These results indicated that miR-30-5p downregulated the expression
of USP22 under hypoxic conditions, thus inhibiting its stabilizing
effect on PD-L1 and HIF-1α protein, thereby inhibiting the
expression of PD-L1 protein in A549 cells.
Discussion
The high mortality rate associated with LUAD is due
to the lack of specific diagnostic biomarkers and effective
therapeutic strategies. Understanding the novel mechanisms
underlying the development of LUAD and identifying new targets to
prevent the progression of LUAD represent key challenges in the
improvement of LUAD treatment. MiRNAs are endogenous, non-coding,
single-stranded, and small RNAs of 20–24 nucleotides in eukaryotes.
MiRNAs play an important role in signal transduction, cell
differentiation, proliferation, apoptosis, blood vessel formation
and development, inflammation, and tumorigenesis, in vivo
(34–36). The miR-30 family is an important
member of the miRNA family. Mature miR-30 family members have a
common seed sequence near the 5′-end, but have different
compensation sequences near the 3′-end, allowing miR-30 family
members to target different genes and pathways to perform
corresponding biological functions (37). miR-30-5p has been revealed to function
as a tumor suppressor in numerous different cancers (30). In particular, a previous study has
demonstrated that the miR-30-5p family plays an important role in
adenocarcinoma (38). For example,
urinary miR-30a-5p was reported to be expressed at higher levels in
stage I–II ovarian serous adenocarcinoma samples than that in stage
III–IV samples, and also in well or moderately differentiated
ovarian serous adenocarcinoma samples than in poorly differentiated
samples (39). miR-30b-5p has been
revealed to act as a tumor suppressor miRNA and to regulate cell
proliferation and the cell cycle in esophageal squamous cell
carcinoma (40). miR-30c-5p has been
revealed to inhibit the aggressiveness of pancreatic ductal
adenocarcinoma (PDAC) cells (38).
Moreover, miR-30e-5p has been reported to reduce angiogenesis and
metastasis targeting AEG-1 in head and neck squamous cell carcinoma
(41). In colon cancer, miR-30-5p
targeted the USP 22/Wnt/β-catenin signaling axis and thus increased
chemo-sensitivity (29). Moreover,
miR-30e-5p has been also reported to suppress non-small cell lung
cancer tumorigenesis by regulating USP22-mediated Sirt1/JAK/STAT3
signaling (42). USP22 is a
cytoplasmic and nuclear deubiquitinating enzyme that has been
revealed to serve as an oncogene in a number of different types of
cancer, including non-small cell lung cancer (42,43),
papillary thyroid carcinoma (44) and
glioma (45). Moreover, USP22
expression was revealed to be positively associated with PD-L1
expression in human non-small cell lung cancer samples (46). Furthermore, USP22 was revealed to
upregulate MDMX, an murine double minute 2-related protein and
inhibit p53 subsequently to promote NSCLC tumorigenesis (47). USP22 could also promote the
proliferation of NSCLC cells via the stable expression of
cyclooxygenase-2 (COX-2) (43). USP22
could also be targeted by miRNA-101 to inhibit the tumorigenesis of
papillary thyroid carcinoma (44).
However, the precise relationship between miR-30-5p and USP22 in
LUAD has yet to be fully investigated. In the present study, it was
revealed that the miR-30 family directly targeted USP22 in LUAD,
thus indicating that miR-30-5p could regulate the progression of
LUAD by targeting USP22.
Study has revealed that the expression of PD-L1 is
closely related to tumor grade in several types of malignant
tumors, and has become a new biomarker for tumor diagnosis and
prognosis (48). PD-L1 is highly
expressed in tumor cells and binds to TCR PD-1 leading to the
negative regulation of T cell responses. This leads to tumor
antigen-specific T cell-induced apoptosis, thus allowing cancer
cells to evade immune surveillance and cell death (16,49–51).
Hypoxia is the main feature of solid tumors. Studies have reported
that hypoxia can induce tumor cells to express PD-L1 (18–20). In
the present study, it was demonstrated that USP22 could stabilize
hypoxia-induced PD-L1 expression in LUAD cells, thus indicating
that USP22 plays a key role in the immune evasion of LUAD.
In the present study, the role of USP22 in the
immune evasion of LUAD was examined. It was revealed that the
miR-30-5p family inhibited the expression of PD-L1 in A549 cells by
targeting USP22 under hypoxic conditions, thereby enhancing the
destruction of A549 cells by activated T cells. Furthermore,
miR-30-5p family regulated PD-L1 expression through USP22 at the
level of transcription and by post-translational modification.
Targeting USP22 with the miR-30-5p family directly inhibited its
stabilizing effect on PD-L1 protein and regulated the expression of
PD-L1 at the post-translational modification level. The miR-30-5p
family also inhibited its stabilizing effect on HIF-1α protein,
thereby inhibiting the transcription of its target gene PD-L1 by
regulating USP22. In addition, the miR-30-5p family regulated the
expression of PD-L1 at the transcription level. These findings
indicated that the miR-30-5p family could inhibit the
hypoxia-induced expression of PD-L1 in LUAD cells, which may serve
as a target for inhibiting the hypoxia-induced immune evasion of
LUAD cells.
Collectively, our experiments demonstrated that the
miR-30 family plays an important role in the hypoxic-induced immune
evasion of LUAD by targeting USP22. Consequently, USP22 may serve
as an inhibitor of LUAD immune escape. Thus, our present findings
provided new options for the treatment of lung adenocarcinoma.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the Qingdao
Municipal Commission of Health and Family Planning (grant no.
DTR2017Y18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH designed the study and wrote the manuscript. XH,
HC and CW performed the experiments. XS and AW were in charge of
confirming the authenticity of the raw data and data analysis. ZZ
was the project leader, responsible for the design of the project
and the revision of the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Senosain MF and Massion PP: Intratumor
heterogeneity in early lung adenocarcinoma. Front Oncol.
10:3492020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JM and Chen DS: Immune escape to
PD-L1/PD-1 blockade: Seven steps to success (or failure). Ann
Oncol. 27:1492–1504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldberg SB, Gettinger SN, Mahajan A,
Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A,
Jilaveanu L, et al: Pembrolizumab for patients with melanoma or
non-small-cell lung cancer and untreated brain metastases: Early
analysis of a non-randomised, open-label, phase 2 trial. Lancet
Oncol. 17:976–983. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cohen EEW, Soulières D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab
for recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bauml J, Seiwert TY, Pfister DG, Worden F,
Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, et al:
Pembrolizumab for platinum-and cetuximab-refractory head and neck
cancer: Results from a single-arm, phase II study. J Clin Oncol.
35:1542–1549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Powles T, Durán I, van der Heijden MS,
Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano
D, Bamias A, et al: Atezolizumab versus chemotherapy in patients
with platinum-treated locally advanced or metastatic urothelial
carcinoma (IMvigor211): A multicentre, open-label, phase 3
randomised controlled trial. Lancet. 391:748–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Overman MJ, Lonardi S, Wong KY, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Velcheti V, Schalper KA, Carvajal DE,
Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L
and Rimm DL: Programmed death ligand-1 expression in non-small cell
lung cancer. Lab Invest. 94:107–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+
T lymphocytes are prognostic factors of human ovarian cancer. Proc
Natl Acad Sci USA. 104:3360–3365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vito A, El-Sayes N and Mossman K:
Hypoxia-driven immune escape in the tumor microenvironment. Cells.
9:9922020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18:102019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
You L, Wu W, Wang X, Fang L, Adam V,
Nepovimova E, Wu Q and Kuca K: The role of hypoxia-inducible factor
1 in tumor immune evasion. Med Res Rev. 41:1622–1643. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfoh R, Lacdao IK and Saridakis V:
Deubiquitinases and the new therapeutic opportunities offered to
cancer. Endocr Relat Cancer. 22:T35–T54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melo-Cardenas J, Zhang Y, Zhang DD and
Fang D: Ubiquitin-specific peptidase 22 functions and its
involvement in disease. Oncotarget. 7:44848–44856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang K, Yang L, Wang J, Sun T, Guo Y,
Nelson R, Tong TR, Pangeni R, Salgia R and Raz DJ:
Ubiquitin-specific protease 22 is critical to in vivo angiogenesis,
growth and metastasis of non-small cell lung cancer. Cell Commun
Signal. 17:1672019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang X, Zhang Q, Lou Y, Wang J, Zhao X,
Wang L, Zhang X, Li S, Zhao Y, Chen Q, et al: USP22 Deubiquitinates
CD274 to suppress anticancer immunity. Cancer Immunol Res.
7:1580–1590. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sunshine J and Taube JM: PD-1/PD-L1
inhibitors. Curr Opin Pharmacol. 23:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santos RM, Moreno C and Zhang WC:
Non-Coding RNAs in lung tumor initiation and progression. Int J Mol
Sci. 21:27742020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Jiang CC, Jin L and Zhang XD:
Regulation of PD-L1: A novel role of pro-survival signalling in
cancer. Ann Oncol. 27:409–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong P, Xiong Y, Yu J, Chen L, Tao T, Yi
S, Hanley SJ, Yue J, Watari H and Sakuragi N: Control of PD-L1
expression by miR-140/142/340/383 and oncogenic activation of the
OCT4-miR-18a pathway in cervical cancer. Oncogene. 37:5257–5268.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang S, Miao D, Wang M, Lv J, Wang Y and
Tong J: miR-30-5p suppresses cell chemoresistance and stemness in
colorectal cancer through USP 22/Wnt/β-catenin signaling axis. J
Cell Mol Med. 23:630–640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao JJ, Lin J, Zhu D, Wang X, Brooks D,
Chen M, Chu ZB, Takada K, Ciccarelli B, Admin S, et al: miR-30-5p
functions as a tumor suppressor and novel therapeutic tool by
targeting the oncogenic Wnt/β-catenin/BCL9 pathway. Cancer Res.
74:1801–1813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun X, Li CW, Wang WJ, Chen MK, Li H, Lai
YJ, Hsu JL, Koller PB, Chan LC, Lee PC, et al: Inhibition of c-MET
upregulates PD-L1 expression in lung adenocarcinoma. Am J Cancer
Res. 10:564–571. 2020.PubMed/NCBI
|
|
33
|
Avendaño-Ortiz J, Maroun-Eid C,
Martín-Quirós A, Toledano V, Cubillos-Zapata C, Gómez-Campelo P,
Varela-Serrano A, Casas-Martin J, Llanos-González E, Alvarez E, et
al: PD-L1 overexpression during endotoxin tolerance impairs the
adaptive immune response in septic patients via HIF1α. J Infect
Dis. 217:393–404. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin YH: MicroRNA networks modulate
oxidative stress in cancer. Int J Mol Sci. 20:44972019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chandan K, Gupta M and Sarwat M: Role of
host and pathogen-derived microRNAs in immune regulation during
infectious and inflammatory diseases. Front Immunol. 10:30812020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao Z, Sun W, Guo Z, Zhang J, Yu H and
Liu B: Mechanisms of lncRNA/microRNA interactions in angiogenesis.
Life Sci. 254:1169002020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mao L, Liu S, Hu L, Jia L, Wang H, Guo M,
Chen C, Liu Y and Xu L: miR-30 family: A promising regulator in
development and disease. Biomed Res Int. 2018:96234122018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanaka T, Okada R, Hozaka Y, Wada M,
Moriya S, Satake S, Idichi T, Kurahara H, Ohtsuka T and Seki N:
Molecular pathogenesis of pancreatic ductal adenocarcinoma: Impact
of miR-30c-5p and miR-30c-2-3p regulation on oncogenic genes.
Cancers (Basel). 12:27312020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou J, Gong G, Tan H, Dai F, Zhu X, Chen
Y, Wang J, Liu Y, Chen P, Wu X and Wen J: Urinary microRNA-30a-5p
is a potential biomarker for ovarian serous adenocarcinoma. Oncol
Rep. 33:2915–2923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu J, Lv H, Zhang B, Xu F, Zhu H, Chen B,
Zhu C and Shen J: miR-30b-5p acts as a tumor suppressor microRNA in
esophageal squamous cell carcinoma. J Thorac Dis. 11:3015–3029.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang S, Li G, Liu C, Lu S, Jing Q, Chen
X, Zheng H, Ma H, Zhang D, Ren S, et al: miR-30e-5p represses
angiogenesis and metastasis by directly targeting AEG-1 in squamous
cell carcinoma of the head and neck. Cancer Sci. 111:356–368. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu G, Cai J, Wang L, Jiang L, Huang J, Hu
R and Ding F: MicroRNA-30e-5p suppresses non-small cell lung cancer
tumorigenesis by regulating USP22-mediated Sirt1/JAK/STAT3
signaling. Exp Cell Res. 362:268–278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiao H, Tian Y, Yang Y, Hu F, Xie X, Mei J
and Ding F: USP22 acts as an oncogene by regulating the stability
of cyclooxygenase-2 in non-small cell lung cancer. Biochem Biophys
Res Commun. 460:703–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao H, Tang H, Huang Q, Qiu B, Liu X, Fan
D, Gong L, Guo H, Chen C, Lei S, et al: miR-101 targets USP22 to
inhibit the tumorigenesis of papillary thyroid carcinoma. Am J
Cancer Res. 6:2575–2586. 2016.PubMed/NCBI
|
|
45
|
Li ZH, Yu Y, DU C, Fu H, Wang J and Tian
Y: RNA interference-mediated USP22 gene silencing promotes human
brain glioma apoptosis and induces cell cycle arrest. Oncol Lett.
5:1290–1294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Sun Q, Mu N, Sun X, Wang Y, Fan S,
Su L and Liu X: The deubiquitinase USP22 regulates PD-L1
degradation in human cancer cells. Cell Commun Signal. 18:1122020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ding F, Bao C, Tian Y, Xiao H, Wang M, Xie
X, Hu F and Mei J: USP22 promotes NSCLC tumorigenesis via MDMX
up-regulation and subsequent p53 inhibition. Int J Mol Sci.
16:307–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mischinger J, Comperat E, Schwentner C,
Stenzl A and Gakis G: Inflammation and cancer: What can we
therapeutically expect from checkpoint inhibitors? Curr Urol Rep.
16:592015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thompson RH, Gillett MD, Cheville JC,
Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen
L, et al: Costimulatory B7-H1 in renal cell carcinoma patients:
Indicator of tumor aggressiveness and potential therapeutic target.
Proc Natl Acad Sci USA. 101:17174–17179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Boland JM, Kwon ED, Harrington SM,
Wampfler JA, Tang H, Yang P and Aubry MC: Tumor B7-H1 and B7-H3
expression in squamous cell carcinoma of the lung. Clin Lung
Cancer. 14:157–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cooper WA, Tran T, Vilain RE, Madore J,
Selinger CI, Kohonen-Corish M, Yip P, Yu B, O'Toole SA, McCaughan
BC, et al: PD-L1 expression is a favorable prognostic factor in
early stage non-small cell carcinoma. Lung Cancer. 89:181–188.
2015. View Article : Google Scholar : PubMed/NCBI
|