Introduction
In 2018, 432,000 deaths resulting from pancreatic
cancer were recorded worldwide (1).
It is difficult to detect pancreatic cancer in its early stages; as
a result, patients are often already in the advanced stage of the
disease at the time of diagnosis, which accounts for the 5-year
overall survival rate of <5% (2).
Currently, although surgical resection combined with chemotherapy
is the predominant method for the treatment of pancreatic cancer,
surgical stress can affect the immune and neuroendocrine systems
and induce inadvertent seeding of tumor cells during surgery, which
are the main causes of tumor recurrence (3). Anesthesia management is an essential
part of the perioperative period, and the use of anesthetics can
affect different physiological and pathophysiological functions,
such as cell proliferation, angiogenesis and apoptosis (4). Recently, a meta-analysis has shown that
propofol-based total intravenous anesthesia improved the
recurrence-free survival rate (pooled HR, 0.78; 95% CI, 0.65–0.94;
P<0.01) and the overall survival rate (pooled HR, 0.76; 95% CI,
0.63–0.92; P<0.01) for various cancer types (5), suggesting that propofol may be involved
in tumor suppression. Therefore, the aim of the present study was
to examine the mechanisms associated with this phenomenon.
A disintegrin and metalloproteinase 8 (ADAM8) is a
type-I transmembrane glycoprotein the expression levels of which in
normal tissue are typically low and limited to a few distinct cell
types in the lymphatic organs, which are components of the immune
system (6), and in the central
nervous system (7). However, under
pathological stimuli, ADAM8 protein expression levels are
upregulated in several diseases, including osteosarcoma, colorectal
cancer, gastric cancer and pancreatic cancer, which suggests that
ADAM8 may be pathophysiologically relevant. Once upregulated, ADAM8
is proteolytically active and results in enhanced shedding of cell
adhesion molecules, cytokine receptors and extracellular matrix
components (8). In our previous
study, propofol downregulated ADAM8 expression under hypoxic
conditions (9), which partially
inhibited its activity; this was not observed with the control
drug, batimastat, BB-94) (9, 10). For these reasons, it is possible
that other mechanisms participate in the effect of propofol on
pancreatic cancer through ADAM8.
Specificity protein 1 (SP1) is a widely studied
transcription factor, which regulates target gene expression by
binding to GC boxes with the consensus sequence
5′-G/T-GGGCGG-G/A-G/A-C/T-3′ or 5′-G/T-G/A-GGCG-G/T-G/A-G/A-C/T-3′
within their promoter regions (11).
SP1 affects both tumor-suppressor genes and oncogenes, suggesting
that it may play an important role in tumor development and
metastasis. Recent studies have also demonstrated that SP1 has an
impact on tumor invasion and metastasis. Indeed, in oral squamous
cell carcinoma, SP1 was found to promote cell invasion and
migration by upregulating Annexin A2 transcription (12). Additionally, it has been demonstrated
that inhibition of SP1/Syncytin1 axis inhibits the proliferation
and metastasis through the AKT and ERK1/2 signaling pathways in
non-small cell lung cancer (13).
The aim of the present study was to determine whether SP1 mediates
the effects of ADAM8 on pancreatic cancer cells following treatment
with propofol.
Materials and methods
Cell culture and lentiviral
transduction
The human pancreatic cancer cell lines Panc-1 and
Bxpc3 were purchased from the American Type Culture Collection.
Panc-1 and Bxpc3 cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) in a humidified atmosphere containing 5%
CO2 at 37°C. Panc-1 is a human pancreatic cancer cell
line isolated from a pancreatic carcinoma of ductal cell origin;
Bxpc3 is a human pancreatic cancer cell line used in the study of
pancreatic adenocarcinomas. We had repeated the experiment using
the Bxpc3 cell line. SP1 knockdown cell lines were established by
transfecting the cells with short hairpin RNA (shRNA) lentiviral
plasmids. The lentiviral shRNA plasmid targeting SP1 (shSP1) was
purchased from Vigene Biosciences, and the control sequence
(shCtrl) from Sigma-Aldrich (Merck KGaA).
For transduction, a total of 5×104
cells/well were seeded in 6-well plates. The shSP1 or shCtrl
lentivirus was added to the cells in the presence of 5 µl polybrene
(Sigma-Aldrich; Merck KGaA). After 96 h, the transduced cells were
selected with 1 µg/ml puromycin. Subsequently, the selected cells
were treated with different concentrations of propofol according to
our previous study (10). In order
to avoid the possible adverse effects of lipid emulsion, pure
propofol was obtained from Sigma-Aldrich (Merck KGaA).
Cell viability assay
Cell viability was assessed using MTT assays. Cells
were seeded in 96-well plates (6×103 cells/well) and
treated with propofol for 48 h. MTT solution (Sigma-Aldrich; Merck
KGaA) was added for 4 h at 37°C. The precipitate was then dissolved
in 200 µl DMSO. The absorbance was measured at 490 nm using a
Multiskan Spectrum plate reader (Thermo Fisher Scientific,
Inc.).
Wound healing assay
In a 6-well plate, Panc-1 and Bxpc3 cells
(1×106 cells/well) were incubated in DMEM overnight in
order to create a cell monolayer. A scratch was made in the middle
of the well using a pipette tip (7 mm) and the debris was washed
away prior to the addition of new medium to the wells. Using an
optical microscope (magnification, ×400), the cells were imaged,
and the initial area of the scratch in the field of view was
determined as the length multiplied by the width. A total of three
fields of view were examined. The plate was incubated at 37°C for
24 h, after which the same field of view was imaged and the scratch
area was measured again using the same methodology. The final area
of the scratch wound was divided by the initial area to determine
the percentage wound remaining of the initial area covered by
migrating cells over the 24-h culture period.
Cell cycle analysis
Cells were incubated with the indicated doses of
propofol for 24 h, and then washed with cold PBS. Subsequently, the
cells were fixed using cold 75% ethanol overnight at 4°C, washed
with cold PBS and stained with propidium iodide (PI) for 30 min at
37°C. After staining, the cells were analyzed using flow cytometry
(BD FACSDiva™; BD Biosciences).
Total RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from cell lines using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. A NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
measure RNA concentration. RT reactions were performed using the
PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd.) and
qPCR was performed using FastStart Universal SYBR-Green Master
(Roche Diagnostics GmbH) and the Step One Plus Real-time PCR system
(Applied Biosystems). According to the manufacturer's protocol, the
thermocycling conditions were as follows: i) Initial denaturation,
95°C for 30 sec; ii) amplification, 95°C for 5 sec, 60°C for 20
sec, 40 cycles; and iii) dissociation curve, 95°C for 60 sec, 55°C
for 30 sec, 95°C for 30 sec. The 2−ΔΔCq method was used
to determine the relative mRNA expression levels, which were
normalized to those of β-actin (14). All experiments were performed
independently three times and set up in triplicate. The following
primer sequences were used: i) ADAM8 forward,
5′-ACAATGCAGAGTTCCAGATGC-3′ and reverse,
5′-GGACCACACGGAAGTTGAGTT-3′; ii) SP1 forward,
5′-CGGAATTCATGAGCGACCAAGATCACTCCATG-3′ and reverse,
5′-CGGAATTCTTGGACCCATGCTACCTTGCATCC-3′; and iii) GAPDH forward,
5′-GTCAGTGGTGGACCTGACCT-3′ and reverse,
5′-TGGTGCTCAGTTTAGCCCAGG-3′.
Western blot analysis
Cells were lysed using RIPA buffer (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) to extract total protein. The
extracted protein (50 µg) was separated using SDS-PAGE on 10% gels,
and then transferred to PVDF membranes. The membranes were
incubated with antibodies against ADAM8 (cat. no. ab255608;
dilution 1:1,000; Abcam), SP1 (cat. no. ab231778; dilution 1:1,000;
Abcam) and β-actin (cat. no. ab8226; dilution 1:1,000; Abcam)
overnight at 4°C, then with goat anti-rabbit HRP-conjugated
antibody (cat. no. ab181662; dilution 1:2,000; Abcam). The protein
bands were visualized using the Odyssey system (LI-COR
Biosciences).
Lentiviral infection
Lentiviral vectors for SP1 knockdown (shSP1) were
purchased from Vigene Biosciences (cat. no. P100029). A total of
50×104 cells/well were seeded in 6-well plates. The
shSP1 or shCtrl virus (cat. no. TL506569V) was added to the cells
in the presence of 5 µl polybrene (Sigma-Aldrich; Merck KGaA) to
increase the efficiency of infection. After 96 h, the transduced
cells were selected using 1 µg/ml puromycin for 2 months. The
selected cells were then treated with different concentrations of
propofol, the transfection efficiencies in all cell lines are over
80%. The following primer sequences were used: i) SP1-shRNA1,
5′-GCAAGTTCTGACAGGACTACCTTCAAGAGAGGTAGTCCTGTCAGAACTTGCTTTTTT-3′;
ii) SP1-shRNA2,
5′-GCAACATCATTGCTGCTATGCTTCAAGAGAGCATAGCAGCAATGATGTTGCTTTTTT-3′;
iii) SP1-shRNA3,
5′-GCAGACCTTTACAACTCAAGCTTCAAGAGAGCTTGAGTTGTAAAGGTCTGCTTTTTT-3′.
Dual-luciferase reporter assays
Dual luciferase reporter assays were performed as
previously described (15).
Wild-type or mutant ADAM8 (containing mutations in the putative
binding site for SP1 located in 3′-untranslated region), together
with a synthesized promoter mimic or vector, were co-transfected
for 48 h. The transfected cells were then harvested to determine
luciferase activity using a dual luciferase reporter assay system
(Promega Corp.).
Co-immunoprecipitation (Co-IP)
assay
Panc-1 cells were lysed using RIPA buffer (50 mM
Tris-HCl, pH 7.8; 150 mM NaCl; 5 mM EDTA; 0.1% Triton X-100; 0.05%
NP-40). Subsequently, the lysates were incubated overnight at 4°C
in an orbital shaker with 2 µg anti-ADAM8 or anti-SP1 antibody
alongside a negative control containing 2 µg rabbit IgG antibody.
Cell lysate without any antibody (input) was used as a positive
control. After incubation, the mixture was incubated with agarose
beads at 4°C for 3 h. The beads were collected and sequentially
washed five times with 1 ml RIPA lysis buffer, then analyzed by
western blotting using anti-ADAM8 or anti-SP1 antibodies. The
intensity of the specific bands was evaluated using ImageJ
software, version 1.46 (National Institutes of Health). The assays
were repeated at least three times.
Statistical analysis
The data are presented as the mean ± SD of three
independent experiments. SSPS 20.0 software (IBM Corp.) was used
for statistical analysis. One-way ANOVA followed by Duncan's post
hoc test and unpaired Student's t-tests were used to compare the
experimental groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Propofol inhibits the viability of
pancreatic cancer cells
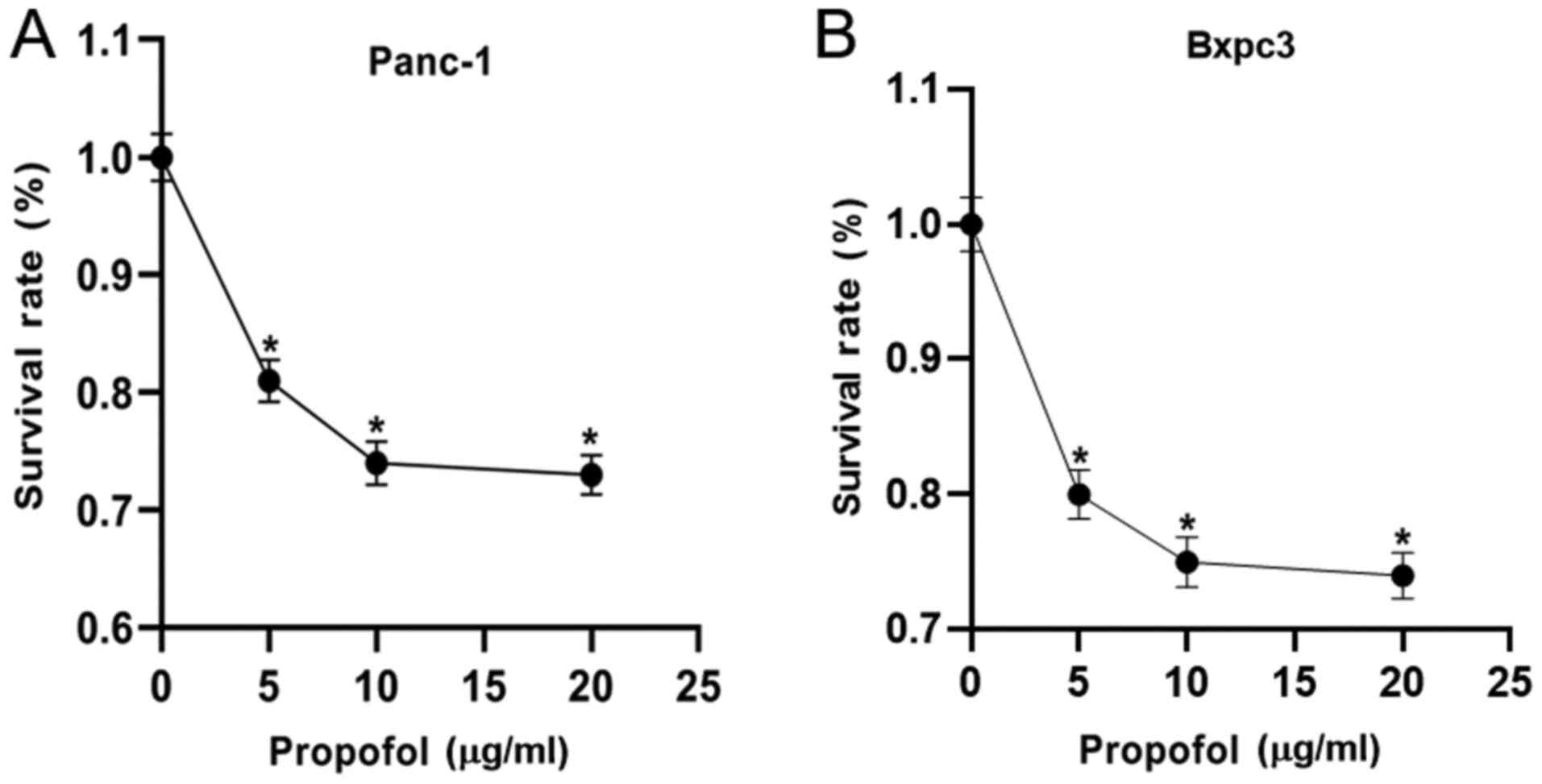
Firstly, the effect of 0 (negative control), 5, 10
and 20 µg/ml propofol on Panc-1 and Bxpc3 cell viability was
evaluated using MTT assays. As shown in Fig. 1, propofol significantly suppressed
the viability of Panc-1 and Bxpc3 cells when compared with the
negative control. It was also observed that 10 µg/ml propofol
resulted in the lowest viability. These findings indicated that
propofol treatment could inhibit the viability of pancreatic cancer
cells.
Propofol reduces the number of
pancreatic cancer cells in the S-phase of the cell cycle
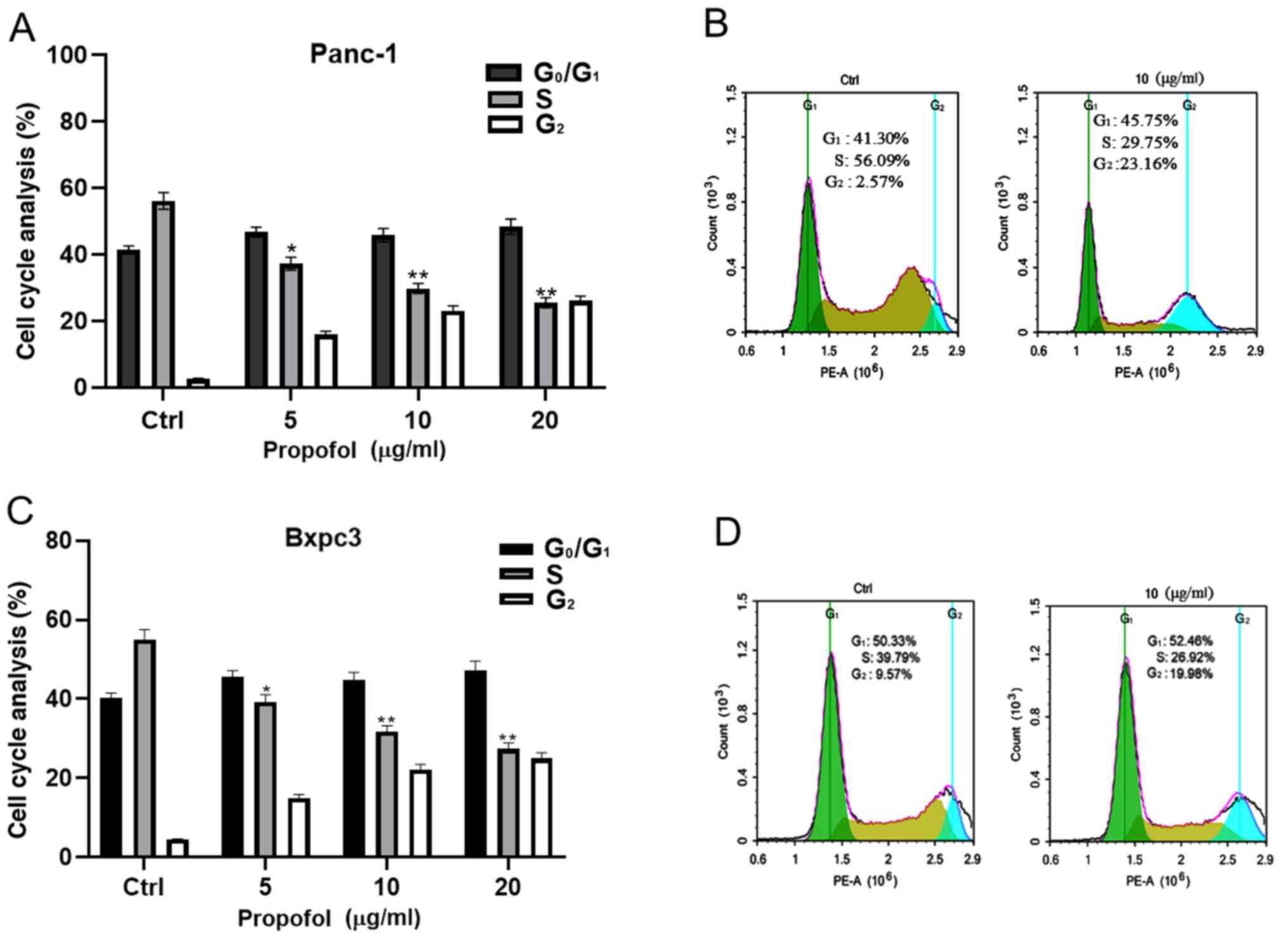
The role of propofol on the cell cycle progression
of Panc-1 and Bxpc3 cells was examined. Panc-1 and Bxpc3 cells were
treated with 0, 5, 10 and 20 µg/ml propofol, and the distribution
of Panc-1 and Bxpc3 cells in different phases of the cell cycle was
examined using flow cytometry. The results indicated that propofol
affected the cell cycle progression of Panc-1 and Bxpc3 cells, as
evidenced by a significant gradual reduction in the number of
pancreatic cancer cells in the S-phase with increasing propofol
concentration (Fig. 2). While the
number of cells in the G1-phase appeared to be increased, this was
not statistically significant. This demonstrated that cells were
blocked at the S-phase, which may indicate a relative decrease in
DNA synthesis and replication.
Propofol inhibits the migration of
pancreatic cancer cells
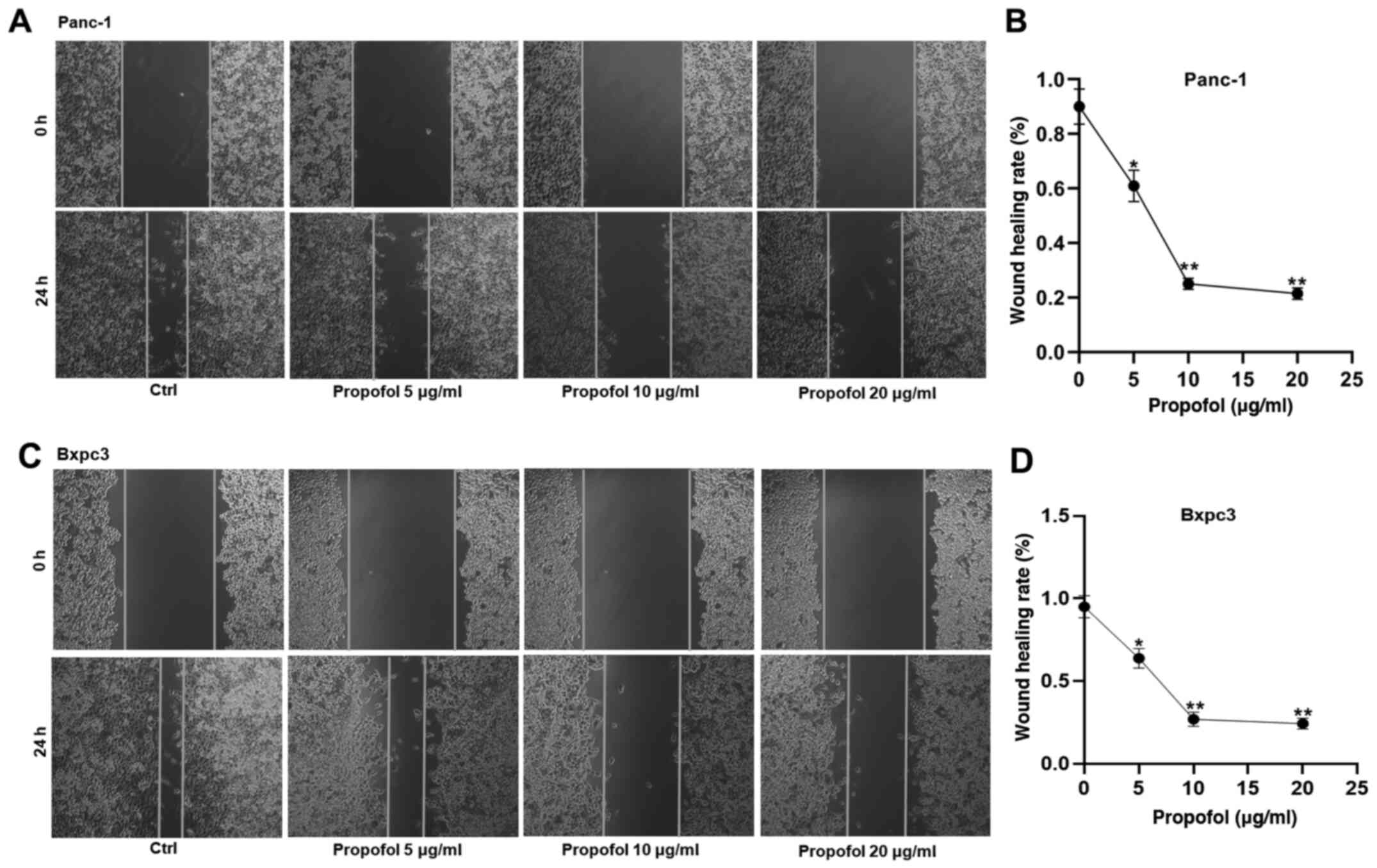
In addition to its inhibitory effects on cell
viability, the potential role of propofol on the malignant behavior
of Panc-1 and Bxpc3 cells was also examined. For this purpose,
wound healing assays were performed. The results demonstrated that
propofol treatment significantly suppressed the migration of Panc-1
and Bxpc3 cells (Fig. 3). Indeed,
the wounds healed at a slower rate following treatment with higher
concentrations of propofol.
Propofol inhibits the expression of
ADAM8
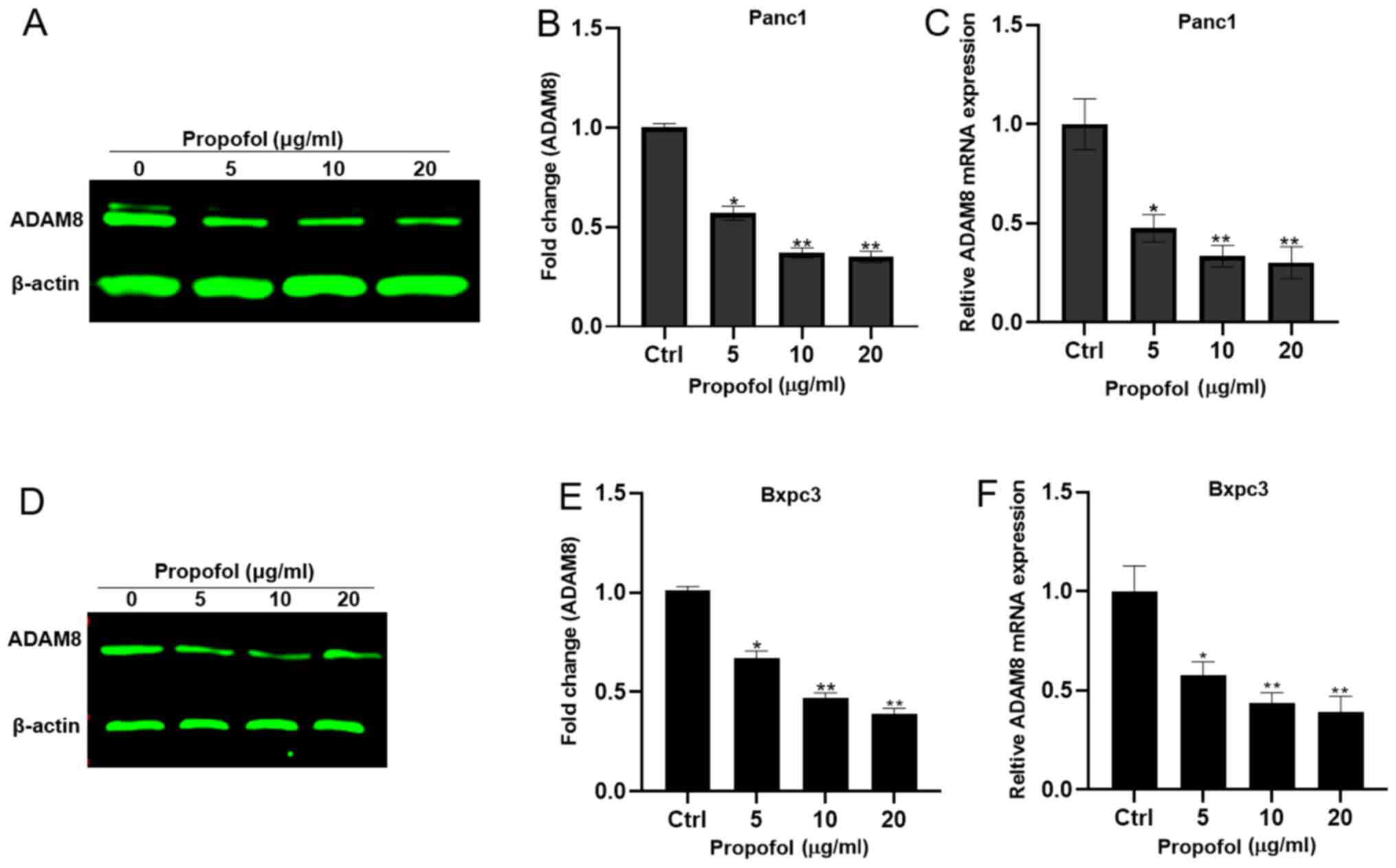
To investigate the effects of propofol on ADAM8,
mRNA and protein were extracted from Panc-1 and Bxpc3 cells treated
with 0, 5, 10 and 20 µg/ml propofol. It was observed that propofol
treatment significantly reduced the ADAM8 mRNA and protein
expression levels in a dose-dependent manner (Fig. 4).
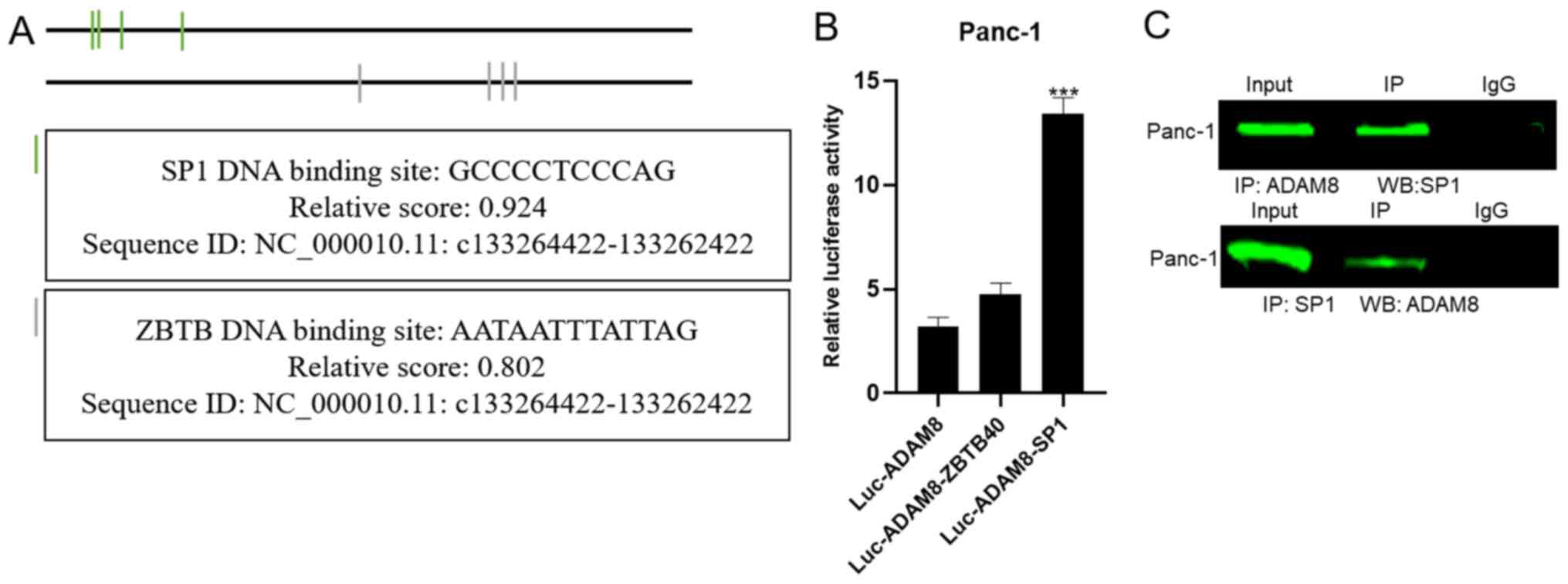
Verification of the direct interaction between SP1
and ADAM8. In the University of California Santa Cruz database, the
promoter region of ADAM8 was predicted to be located at
chr10:133,262,422-133,264,422 (GRCh38). The luciferase reporter
vectors containing the indicated genomic fragments of the ADAM8
gene were constructed. To investigate the potential regulators
involved in ADAM8 expression, potential transcription factor
binding sites in the ADAM8 promoter were identified using three
online software packages: PubMed (https://pmlegacy.ncbi.nlm.nih.gov/gene/101), JASPAR
(http://jaspar.genereg.net/) and
GeneCards (http://genecards.org) (16). Binding sites for the transcription
factor SP1 and zinc finger and BTB domain containing 40 were found
in the promoter region of ADAM8 (Fig.
5A). Of the two candidate transcription factors, only SP1
mimics markedly enhanced luciferase activity (Fig. 5B). Co-IP experiments were performed
in order to confirm whether SP1 binds to the promoter region of
ADAM8. The results indicated that SP1 and ADAM8 were detectable in
the corresponding precipitated protein complexes, indicating that
SP1 interacted directly with ADAM8 (Fig.
5C). This suggested that the SP1 transcription factor may be
targeted by propofol.
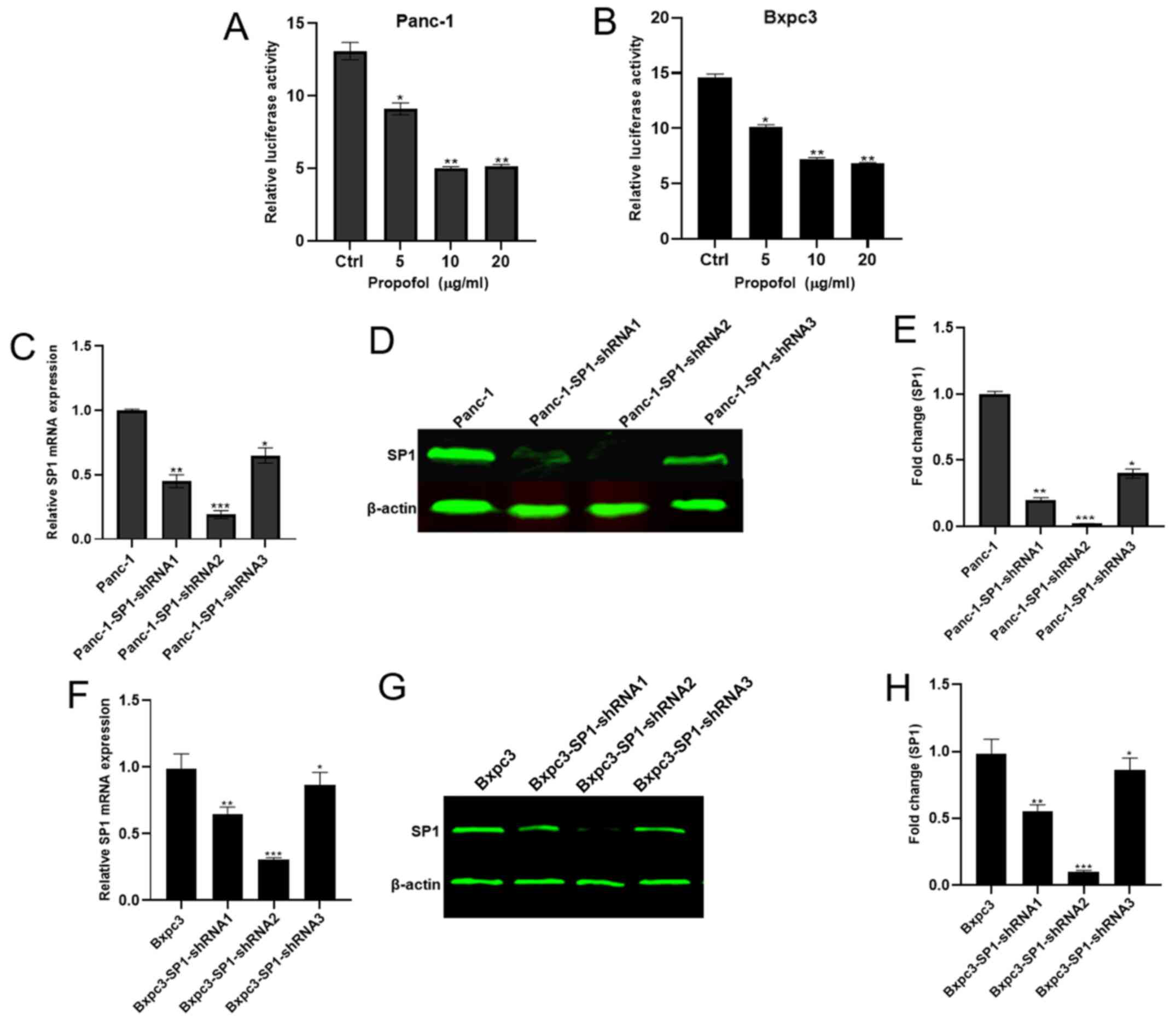
Propofol potentially targets SP1 to regulate ADAM8.
To investigate whether propofol acts through ADAM8 via SP1, Panc-1
and Bxpc3 cells were treated with 0, 5, 10 and 20 µg/ml propofol
and used in a dual luciferase reporter assay. The luciferase
activity was significantly inhibited, with a concentration of 10
µg/ml propofol resulting in the lowest luciferase activity
(Fig. 6A and B). Additionally,
Panc-1 shCtrl and three Panc-1 shSP1 cell lines (Panc-1-SP1-shRNA1,
2 and 3) were established, and the shSP1 cell line expressing the
lowest mRNA and protein levels of SP1 was used in the subsequent
experiments (Fig. 6C-H). Protein and
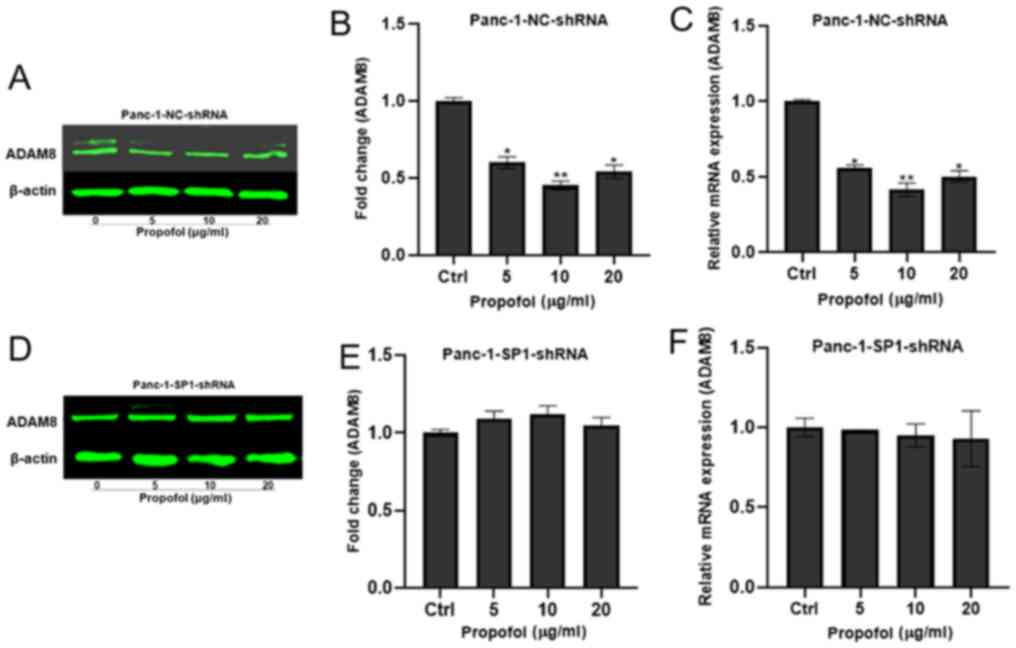
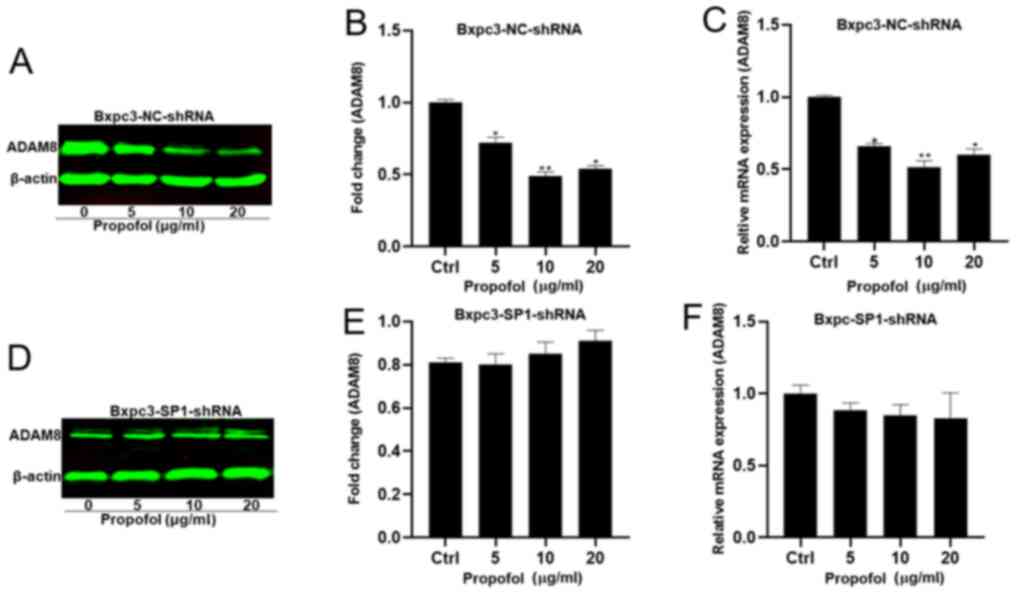
mRNA were extracted from the Panc-1/Bxpc3-shSP1-shRNA and the
Panc-1/Bxpc3-NC-shRNA lines to determine whether the expression of
ADAM8 was regulated by propofol in the absence of SP1. The
expression of ADAM8 was decreased at different concentrations of
propofol in the control groups (Figs.
7A-C and 8A-C), but not in the
experimental groups (Figs. 7D-F and
8D-F).
Discussion
Previous studies have shown that propofol not only
affects epigenetic pathways, such as those involving histone
acetylation, microRNAs and long non-coding RNAs (17,18), but
also modulates signaling pathways, including the SLUG, MAPK,
nuclear factor erythroid 2 like 2 and NF-κB pathways (19). The present study demonstrated that
propofol could inhibit the viability, block the cell cycle at the
S-phase and suppress the migration of pancreatic cancer cells. To
obtain deeper insight into the associated molecular mechanism,
several transcription factors for A disintegrin and
metalloproteinase 8 (ADAM8) were investigated. Interestingly,
specificity protein 1 (SP1) was found to regulate ADAM8 expression,
which was affected by propofol treatment in Panc-1 and Bxpc3 cells.
Thus, the effect of propofol on pancreatic cancer cells was
mediated by ADAM8 via SP1.
Propofol is a commonly used intravenous
sedative-hypnotic agent. In addition to its multiple anesthetic
advantages, propofol exerts a number of non-anesthetic effects.
Indeed, accumulating evidence suggests that it may affect cancer
development in direct as well as indirect manners. A number of
studies have indicated that propofol suppresses the malignancy of a
variety of human cancer types, such as hepatocellular carcinoma
(20), breast cancer (21) and lung cancer (22). Moreover, a previous study suggested a
possible correlation between propofol and chemotherapy, although
the mechanism remains unclear (23).
The previous study conducted by our research group demonstrated
that propofol inhibits pancreatic tumor growth via ADAM8 (9) and determined that propofol specifically
inhibited ADAM8 expression and activation in response to hypoxia in
pancreatic cancer (10). The results
of the present study are consistent with these previous
reports.
ADAM8 is a proteolytically active member of the ADAM
protease family. Increased expression of ADAM8 has been observed in
breast cancer (24), lung
adenocarcinoma (25) and pancreatic
cancer (26). ADAM8 has been shown
to cleave important extracellular matrix components of the tumor
stroma, such as growth factors or cell surface proteins (27).
Epidermal growth factor has been demonstrated to
reduce cell attachment, cell-cell interaction and cell spreading,
as well as to inhibit the expression levels of cyclin A, D1 and
cdk2 (28). Cyclins play an
important role in cell proliferation, pluripotency and
determination of cell fate. Since DNA synthesis and replication are
an important part of the S-phase, the reduced percentage of cells
in the S-phase identified in this study could indicate that
propofol suppresses pancreatic cancer cell growth through the
repression of ADAM8 via SP1, which is a hypothesis that requires
further study.
SP1 is involved in basal transcriptional regulation
of various genes. SP1 contains three highly homologous C2H2
regions, which directly bind to DNA, thus promoting gene
transcription (29). In the present
study, SP1 interacted directly with ADAM8, and propofol did not
inhibit Panc-1 cell migration and ADAM8 expression in Panc-1 cells
following SP1 knockdown by shRNA. Additionally, luciferase activity
was reduced with increasing concentrations of propofol in cells
transfected with luciferase reporter vectors and SP1 mimics. These
results suggest that SP1 directly mediates the expression and the
function of ADAM8 following propofol treatment (Figs. S1 and S2).
There were limitations in this study. First, we did
not perform in vivo experiment, however, our previous study
had demonstrated propofol remarkably retarded xenograft tumor
progression and inhibited the expression of angiogenesis mediators
by ADAM8 in vivo (9,10); second, our previous study confirmed
that propofol inhibited invasion of pancreatic cancer cell by
Transwell assays (10), thus we did
not present the results in this manuscript.
In conclusion, the present study findings suggest
that propofol plays a critical role in inhibiting the viability and
migration of pancreatic cancer cells and also blocks their cell
cycle progression at the S-phase by targeting SP1 to regulate
ADAM8. These findings may expand the current knowledge in the field
of perioperative anesthetics and their effects on tumor cells.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science
Foundation of China (grant no. 82060239; GPPH-NSFC-2020-9); Guizhou
Provincial High-level creative talents cultivation plan: Thousand
plan (grant no. GZSYQCC [2016]001).
Availability of data and materials
All data and materials are available without
restriction. Researchers can obtain data by contacting the
corresponding authors.
Authors' contribution
XY and YBD contributed to the conception and design
of the study. YG, CW and YZ contributed to perform the experiments,
data acquisition and interpretation. KMS was involved in the
bioinformation analysis. YG drafted the manuscript. XY, YBD and KMS
reviewed the manuscript critically. All authors contributed to the
interpretation of the findings, and reviewed, edited and approved
the final manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dudley B and Brand RE: Pancreatic cancer
surveillance: Who, when, and how. Curr Treat Options Gastroenterol.
17:681–691. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horowitz M, Neeman E, Sharon E and
Ben-Eliyahu S: Exploiting the critical perioperative period to
improve long-term cancer outcomes. Nat Rev Clin Oncol. 12:213–226.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aguirre JA, Lucchinetti E, Clanachan AS,
Plane F and Zaugg M: Unraveling interactions between anesthetics
and the endothelium: Update and novel insights. Anesth Analg.
122:330–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yap A, Lopez-Olivo MA, Dubowitz J, Hiller
J and Riedel B; Global Onco-Anesthesia Research Collaboration
Group, : In reply: Comment on ‘Anesthetic technique and cancer
outcomes: A meta-analysis of total intravenous versus volatile
anesthesia’. Can J Anaesth. 67:152–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelly K, Hutchinson G, Nebenius-Oosthuizen
D, Smith AJ, Bartsch JW, Horiuchi K, Rittger A, Manova K, Docherty
AJ and Blobel CP: Metalloprotease-disintegrin ADAM8: Expression
analysis and targeted deletion in mice. Dev Dyn. 232:221–231. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schlomann U, Rathke-Hartlieb S, Yamamoto
S, Jockusch H and Bartsch JW: Tumor necrosis factor alpha induces a
metalloprotease-disintegrin, ADAM8 (CD 156): Implications for
neuron-glia interactions during neurodegeneration. J Neurosci.
20:7964–7971. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schlomann U, Wildeboer D, Webster A,
Antropova O, Zeuschner D, Knight CG, Docherty AJ, Lambert M,
Skelton L, Jockusch H, et al: The metalloprotease disintegrin
ADAM8. Processing by autocatalysis is required for proteolytic
activity and cell adhesion. J Biol Chem. 277:48210–48219. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Y, Yu X, Zhang F and Dai J: Propofol
inhibits pancreatic cancer progress under hypoxia via ADAM8. J
Hepatobiliary Pancreat Sci. 26:219–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu X, Shi J, Wang X and Zhang F: Propofol
affects the growth and metastasis of pancreatic cancer via ADAM8.
Pharmacol Rep. 72:418–426. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Safe S, Imanirad P, Sreevalsan S, Nair V
and Jutooru I: Transcription factor Sp1, also known as specificity
protein 1 as a therapeutic target. Expert Opin Ther Targets.
18:759–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang Y, Nagaraja AS, Armaiz-Pena GN,
Dorniak PL, Hu W, Rupaimoole R, Liu T, Gharpure KM, Previs RA,
Hansen JM, et al: Adrenergic stimulation of DUSP1 impairs
chemotherapy response in ovarian cancer. Clin Cancer Res.
22:1713–1724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Fu Y, Xia X, Zhang X, Xiao K, Zhuang
X and Zhang Y: Knockdown of SP1/Syncytin1 axis inhibits the
proliferation and metastasis through the AKT and ERK1/2 signaling
pathways in non-small cell lung cancer. Cancer Med. 8:5750–5759.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang S, Zhen L, Liu Z, Ai Q, Ji Y, Du G,
Wang Y and Bu Y: Identification and analysis of the promoter region
of the human HAS3 gene. Biochem Biophys Res Commun. 460:1008–1014.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vlieghe D, Sandelin A, De Bleser PJ,
Vleminckx K, Wasserman WW, van Roy F and Lenhard B: A new
generation of JASPAR, the open-access repository for transcription
factor binding site profiles. Nucleic Acids Res. 34:D95–D97. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu X, Gao Y and Zhang F: Propofol inhibits
pancreatic cancer proliferation and metastasis by up-regulating
miR-328 and down-regulating ADAM8. Basic Clin Pharmacol Toxicol.
125:271–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao Y, Yurievich UA and Yosypovych SV:
Long noncoding RNA XIST is a prognostic factor in colorectal cancer
and inhibits 5-fluorouracil-induced cell cytotoxicity through
promoting thymidylate synthase expression. Oncotarget.
8:83171–83182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng C, Song L, Wang J, Li D, Liu Y and
Cui X: Propofol induces proliferation partially via downregulation
of p53 protein and promotes migration via activation of the Nrf2
pathway in human breast cancer cell line MDA-MB-231. Oncol Rep.
37:841–848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ou W, Lv J, Zou X, Yao Y, Wu J, Yang J,
Wang Z and Ma Y: Propofol inhibits hepatocellular carcinoma growth
and invasion through the HMGA2-mediated Wnt/β-catenin pathway. Exp
Ther Med. 13:2501–2506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ecimovic P, Murray D, Doran P and Buggy
DJ: Propofol and bupivacaine in breast cancer cell function in
vitro - role of the NET1 gene. Anticancer Res. 34:1321–1331.
2014.PubMed/NCBI
|
|
22
|
Cui WY, Liu Y, Zhu YQ, Song T and Wang QS:
Propofol induces endoplasmic reticulum (ER) stress and apoptosis in
lung cancer cell H460. Tumour Biol. 35:5213–5217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Lu Y, Pang Y, Li M, Cheng X and Chen
J: Propofol enhances the cisplatin-induced apoptosis on cervical
cancer cells via EGFR/JAK2/STAT3 pathway. Biomed Pharmacother.
86:324–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romagnoli M, Mineva ND, Polmear M, Conrad
C, Srinivasan S, Loussouarn D, Barillé-Nion S, Georgakoudi I, Dagg
Á, McDermott EW, et al: ADAM8 expression in invasive breast cancer
promotes tumor dissemination and metastasis. EMBO Mol Med.
6:278–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishikawa N, Daigo Y, Yasui W, Inai K,
Nishimura H, Tsuchiya E, Kohno N and Nakamura Y: ADAM8 as a novel
serological and histochemical marker for lung cancer. Clin Cancer
Res. 10:8363–8370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schlomann U, Koller G, Conrad C, Ferdous
T, Golfi P, Garcia AM, Höfling S, Parsons M, Costa P, Soper R, et
al: ADAM8 as a drug target in pancreatic cancer. Nat Commun.
6:61752015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Conrad C, Benzel J, Dorzweiler K, Cook L,
Schlomann U, Zarbock A, Slater EP, Nimsky C and Bartsch JW: ADAM8
in invasive cancers: Links to tumor progression, metastasis, and
chemoresistance. Clin Sci (Lond). 133:83–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao L, Yao Y, Lee V, Kiani C, Spaner D,
Lin Z, Zhang Y, Adams ME and Yang BB: Epidermal growth factor
induces cell cycle arrest and apoptosis of squamous carcinoma cells
through reduction of cell adhesion. J Cell Biochem. 77:569–583.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagaoka M, Shiraishi Y and Sugiura Y:
Selected base sequence outside the target binding site of zinc
finger protein Sp1. Nucleic Acids Res. 29:4920–4929. 2001.
View Article : Google Scholar : PubMed/NCBI
|