Introduction
Prostate cancer (PCa) has become a leading cause of
cancer-related mortality among men worldwide (1–3). In
addition, as the population ages, prostate cancer rates are likely
to increase (4). The progression
of PCa to hormone-dependent disease is associated with advanced
metastasis (5). As such, the
5-year survival rate of most patients with a PCa diagnosis is
>90%; however, the survival rate of patients with metastatic
disease is only 29% (6). A number
of patients are diagnosed with PCa at an advanced stage where
treatment is usually less effective (7). In addition, due to the high incidence
rate of overdiagnosis and overtreatment using prostate-specific
antigen (8,9), there is an urgent requirement to
improve the understanding of the molecular mechanisms underlying
PCa to assist in the development of new diagnostic and prognostic
markers, and treatment strategies.
Mind bomb 1 (MIB1) is a well-known E3 ubiquitin
ligase (10). Previous studies
have primarily focused on its ubiquitination in various pathways.
For example, in 2017, Mizoguchi et al (10) demonstrated that MIB1 ubiquitinated
CTNND1, with MIB1 contributing to persistent directional cell
migration by regulating the CTNND1/Rac1 pathway. In addition,
Berndt et al (11) reported
that MIB1 served a role in the regulation of the Wnt/β-catenin
signaling pathway via the ubiquitination and degradation of
receptor-like tyrosine kinase. MIB1 has been reported to regulate
apoptosis by interacting with death-associated protein kinase
(12) and cellular FLICE-like
inhibitory protein (13).
Furthermore, it was revealed that MIB1 was necessary for the
efficient activation of Notch signaling via the ubiquitination of
Notch receptors (14). However,
little is known on the interaction between MIB1 and microRNAs
(miRNAs/miRs) in cancer, with only Ray et al (15) demonstrating that MIB1 knockdown
recapitulated the effects of miR-198 on the proliferation and
colony formation of DU145 and LNCaP cells lines.
miRNAs are small non-coding RNAs, 18–22 nucleotides
in length, that serve important functions in cell physiology by
regulating the expression of mRNAs (16). An increasing amount of evidence has
suggested that miR-195-5p functions as a tumor suppressor in
numerous types of cancer, including non-small cell lung (NSCLC),
cervical and prostate cancers (17–24).
For example, in NSCLC, miR-195-5p overexpression inhibited cell
proliferation and induced apoptosis by directly targeting CIAPIN1
(17) and CEP55 (18), and inhibited cell proliferation,
migration, and invasion by directly targeting CPNE1 (19). Liu et al (20) demonstrated that miR-195-5p
inhibited the tumor development of cervical cancer by targeting
YAP1. The current study investigated the expression pattern, role
and potential functional mechanism of MIB1 and miR-195-5p in PCa.
The association between MIB1 and miR-195-5p was then further
determined in the context of PCa cell proliferation, migration and
invasion.
Materials and methods
Clinical sample selection and ethics
statement
Surgically resected PCa tissues and adjacent normal
tissues (distance, >3 cm) were collected from 40 patients with
PCa between March 2018 and November 2019 at the Affiliated Hospital
of Zunyi Medical University (Guizhou, China). All 40 patients were
pathologically confirmed. Pathological grading was based on Gleason
scoring in accordance with the World Health Organization
histopathological classification standard for prostate cancer
(25,26). Exclusion criteria were as follows:
i) A history of preoperative radiotherapy or chemotherapy; ii)
benign prostatic hyperplasia or urolithiasis and iii) a history of
other types of cancer or systemic immune disease. The clinical data
of these patients were collected from their medical records and are
presented in Table SI. None of
the patients received radiotherapy or chemotherapy prior to
resection. The current research was approved by the Ethics
Committee of Zunyi Medical University (Guizhou, China) and all
patients provided written informed consent. All PCa tissues and
adjacent normal tissues were immediately snap-frozen in liquid
nitrogen and stored at −80°C.
Cell culture and transfection
The PCa cell lines, including PC-3 [American Type
Culture Collection (ATCC) no. CRL-1435)], VCaP (ATCC no. CRL-2876),
22Rv1 (ATCC no. CRL-2505), DU145 (ATCC no. HTB-81) and LNCaP (ATCC
no. CRL-1740), along with the normal human prostate epithelial cell
line RWPE1 (ATCC no. CRL-11609), were purchased from the ATCC. The
RWPE1 cell line was cultured in Keratinocyte Serum-Free medium
(Gibco; Thermo Fisher Scientific, Inc.), while the PC-3, VCaP,
22Rv1, DU145 and LNCaP cell lines were maintained in minimum
essential medium (MEM) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified incubator with 5%
CO2.
The primer sequences for cell transfection were
synthesized by Shanghai GenePharma Co., Ltd. The cells were divided
into groups according to their treatments as follows: 50 nM
miR-195-5p mimics (transfection with miR-195-5p mimics;
5′-UAGCAGCACAGAAAUAUUGGC-3′ and 5′-CAAUAUUUCUGUGCUGCUAUU-3′), 50 nM
miR-negative control (NC; non-targeting; transfection with
miR-195-5p mimics NC; 5′-UCACAACCUCCUAGAAAGAGUAGA-3′ and
5′-CAGUACUUUUGUGUAGUACAA-3′); 50 nM anti-miR-195-5p (transfection
with miR-195-5p inhibitor; 5′-GCCAAUAUUUCUGUGCUGCUA-3′), 50 nM
anti-miR-NC (transfection with NC miR-195-5p inhibitor;
5′-CAGUACUUUUGUGUAGUACAA-3′); 2 µg short hairpin (sh) RNA targeting
MIB1 (transfection with sh-MIB1 plasmid;
5′-GGAUAAAGAUGGUGAUAGATT-3′ and 5′-UCUAUCACCAUCUUUAUCCTT-3′), 2 µg
sh-NC (non-targeting; transfection with NC sh-MIB1 plasmid;
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′); 2 µg
overexpression (OE)-MIB1 (transfection with OE-MIB1 plasmid), 2 µg
OE-NC (transfection with OE-MIB1 NC plasmid); miR-195-5p mimic +
OE-NC (transfection with miR-195-5p mimic and OE-NC plasmid),
miR-195-5p mimic + OE-MIB1 (transfection with miR-195-5p mimic and
OE-MIB1 plasmid); anti-miR-195-5p + sh-MIB1 (transfection with
miR-195-5p inhibitor and sh-MIB1) and anti-miR-195-5p + sh-NC
(transfection with miR-195-5p inhibitor and sh-NC). Full length
MIB1 sequences were amplified via PCR and subcloned into pcDNA3.1
vector (Invitrogen; Thermo Fisher Scientific, Inc.) to generate OE
MIB1 plasmids. The recombinant plasmid vectors expressing the
double-stranded shRNA targeting MIB1 (sh-MIB1) or the shRNA control
(sh-NC) were purchased from Shanghai GenePharma Co., Ltd. The PCa
cells were seeded into a 6-well plate (1×106 cells/well)
and the aforementioned oligonucleotides were transfected into each
PCa cell line using Lipofectamine® 3000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48
h, according to the manufacturer's protocol. At 48 h post
transfection, the transfection efficiency was detected using
reverse transcription-quantitative PCR (RT-qPCR).
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from tumor tissues and cell
lines using TRIzol® (Invitrogen, Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
The concentration and purity of the extracted RNA was then
determined at an absorbance ratio of 260/280 nm using a NanoDrop
2000 spectrophotometer (Invitrogen; Thermo Fisher Scientific,
Inc.). Subsequently, 1 µg RNA was reverse transcribed into cDNA
using the PrimeScript™ RT reagent kit (Takara Bio, Inc.) according
to the manufacturer's protocol. qPCR reactions were performed using
a SYBR® Premix Ex Taq™ II kit (Takara Bio, Inc.) with an
ABI 7500 real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were
used: Initial denaturation at 95°C for 5 min, followed by 40 cycles
at 95°C for 1 min, 60°C for 30 sec and 72°C for 30 sec. A final
cycle was implemented at 72°C for 5 min. GAPDH and U6 small nuclear
RNA served as internal controls for MIB1 and miR-195-5p expression,
respectively. The primers used for qPCR are listed in Table I. Relative expression levels were
calculated using the 2−ΔΔCq method (27).
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Name | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| miR-195-5p |
ACACTCCAGCTGGGTAGCAGCACAGAAAT |
TGGTGTCGTGGAGTCG |
| MIB1 |
TAACCGGGTGATGGTGGAAG |
GTGCCGTTGTCCCACACTA |
| GAPDH |
TCAAGGCTGAGAACGGGAAG |
TCGCCCCACTTGATTTTGGA |
| U6 |
ATTGGAACGATACAGAGAAGATT |
GGAACGCTTCACGAATTTG |
Cell proliferation, migration and
invasion analyses
The proliferation of the PCa cell lines was
evaluated using a Cell Counting Kit-8 (CCK-8) assay (Beyotime
Institute of Biotechnology). Following transfection, the VCaP and
DU145 cell lines were cultured in 96-well plates, at a density of
1×106 cells/well, and incubated for 0, 24, 48 or 72 h
before adding 10 µl CCK-8 solution to each well. The cells were
subsequently incubated at 37°C for 2 h. Cell proliferation was
measured using a microplate reader at an absorbance of 450 nm
(Thermo Fisher Scientific, Inc.).
The migration and invasion of the PCa cell lines was
assessed using Transwell and Matrigel assays. Following
transfection, the VCaP and DU145 cell lines were seeded into the
upper chamber at a density of 1×105 cells/well and then
incubated for 24 h at 37°C. The Transwell chambers were pre-coated
with 100 µl Matrigel (BD Biosciences) for the evaluation of cell
invasion at 37°C for 1 h. The lower chamber was filled with 500 µl
MEM supplemented with 10% FBS. The PCa cells that migrated or
invaded through the membranes into the lower chamber were fixed
with 100% methanol for 10 min at room temperature and stained with
0.5% crystal violet solution for 10 min at room temperature.
Finally, the number of migrated and invaded cells was determined
using a light microscope (Olympus Corporation) in three randomly
selected fields of view. The statistical analysis of cell counts
was performed using ImageJ 1.47v software (National Institutes of
Health).
Dual-luciferase reporter assay
Potential binding sites between MIB1 and miR-195-5p
were identified using TargetScan (http://www.targetscan.org/). MIB1 sequences containing
the binding sites [MIB1 wild-type (WT)] and non-binding sites [MIB1
mutant (MUT)] of miR-195-5p were amplified and subcloned into a
psiCHECK2 luciferase vector (Promega Corporation). The MIB1-WT or
MIB1-MUT reporter vector was subsequently co-transfected into the
VCaP and DU145 cell lines using with the aforementioned miR-195-5p
mimics and its corresponding NC Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). Following culture for
48 h, luciferase activity was examined using the Dual-Luciferase
Reporter Assay kit (Promega Corporation). Subsequently, the
luciferase activity was normalized to the firefly luciferase
internal control.
Western blot analysis
The tumor tissues or PCa cell lines were harvested
and lysed using RIPA buffer (Beijing Solarbio Science and
Technology Co., Ltd.). The protein concentration was quantified
using a BCA protein assay kit (Sangon Biotech Co., Ltd.). Then,
protein samples (30 µg per lane) were separated using 12% SDS-PAGE
and transferred onto PVDF membranes following electrophoresis for 2
h. The membranes were subsequently blocked using 5% skimmed milk in
TBS and 0.1% Tween-20 (TBST) at 37°C for 2 h, following which they
were incubated with GAPDH (1:1,000; cat. no. 3781) or MIB1
(1:1,000; cat. no. 1202) (both from Generay Biotech Co., Ltd.)
primary antibodies overnight at 4°C. After washing with TBST three
times, the membranes were incubated with corresponding horseradish
peroxidase-conjugated secondary antibodies (1:10,000; cat. no.
ZB-2306; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
for 1 h at room temperature. The signals were visualized using the
Pierce™ ECL Western Blotting Substrate (Thermo Fisher Scientific,
Inc.) and quantified using ImageJ v1.8.0 software (National
Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS v22.0
(IBM Corp.) and GraphPad Prism v8.0 (GraphPad Software, Inc.). All
experiments were performed in triplicate and the data are presented
as the mean ± SD. Differences between tumor tissues and their
adjacent normal tissues were compared using a paired Student's
t-test. Unpaired Student's t-test was used for comparisons between
unpaired groups and one-way ANOVA followed by Tukey's post hoc test
was used for comparisons among multiple groups. The association
between miR-195-5p and MIB1 expression was analyzed using the
Pearson's correlation coefficient. P<0.05 was considered to
indicate a statistically significant difference.
Results
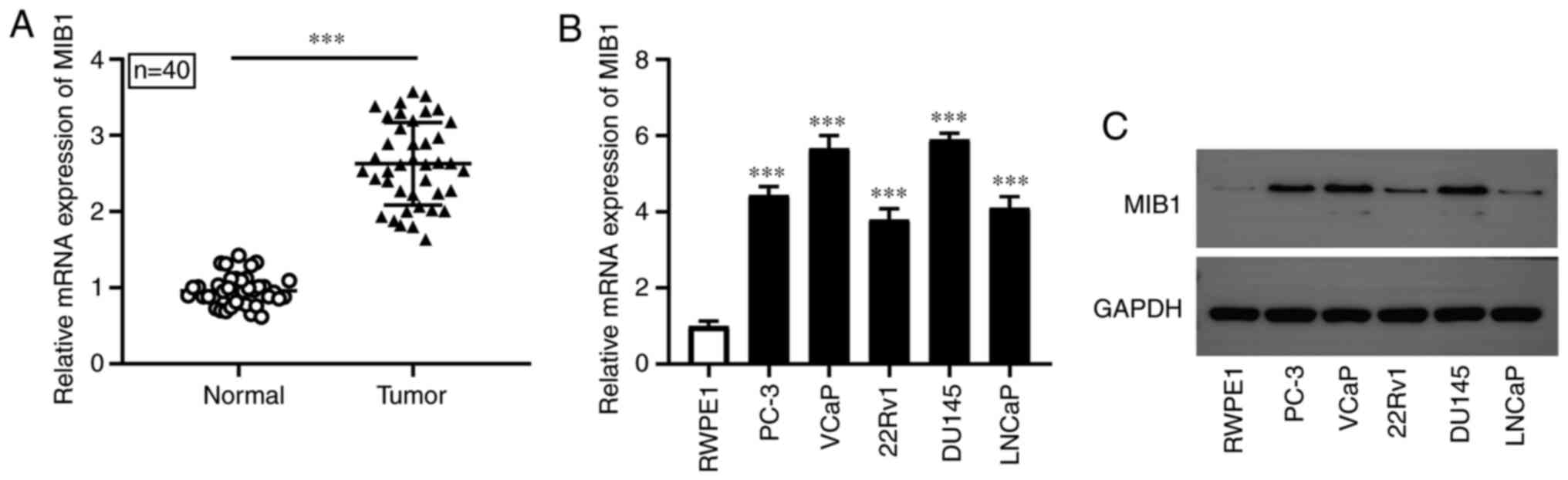
MIB1 expression is upregulated in PCa
tissues and cell lines
RT-qPCR was conducted to detect the mRNA expression
level of MIB1 in PCa tissues and cell lines. Compared with that in
the adjacent normal tissues, the mRNA expression level of MIB1 was
significantly increased in PCa tissues (n=40; Fig. 1A). Furthermore, the results of
RT-qPCR and western blot analysis revealed that MIB1 mRNA and
protein expression level was markedly increased in the PCa cell
lines (PC-3, VCaP, 22Rv1, DU145 and LNCaP) compared with that in
the normal human prostate epithelial cell line, RWPE1, respectively
(Fig. 1B and C). The results
suggested that MIB1 may serve a role in the occurrence and
development of PCa.
MIB1 overexpression markedly promotes
PCa cell proliferation, migration and invasion
Due to the higher expression level of MIB1 in the
VCaP and DU145 cell lines when compared with that in the other
three PCa cell lines, they were selected for further functional
analysis. To determine the effect of MIB1 on the proliferation,
migration and invasion of PCa cells, the VCaP and DU145 cell lines
were transfected with OE-MIB1, sh-MIB1, OE-NC or sh-NC. As
presented in Fig. 2A, the mRNA and
protein expression level of MIB1 was notably increased in the VCaP
and DU145 cell lines following OE-MIB1 transfection. However, the
expression level was markedly decreased following transfection with
sh-MIB1. The CCK-8 assay results indicated that, compared with that
in the sh-NC group, the stable knockdown of MIB1 significantly
inhibited the proliferation of the VCaP and DU145 cell lines, with
MIB1 overexpression significantly promoting the proliferation of
the VCaP and DU145 cell lines compared with that in the OE-NC group
(Fig. 2B). The results of the
Transwell and Matrigel assays confirmed that the inhibition of MIB1
markedly repressed the mobility and invasiveness of the VCaP and
DU145 cell lines, and that MIB1 overexpression significantly
promoted cell migration and invasion (Fig. 2C and D). These data suggested that
MIB1 may promote the proliferation, migration and invasion of PCa
cells.
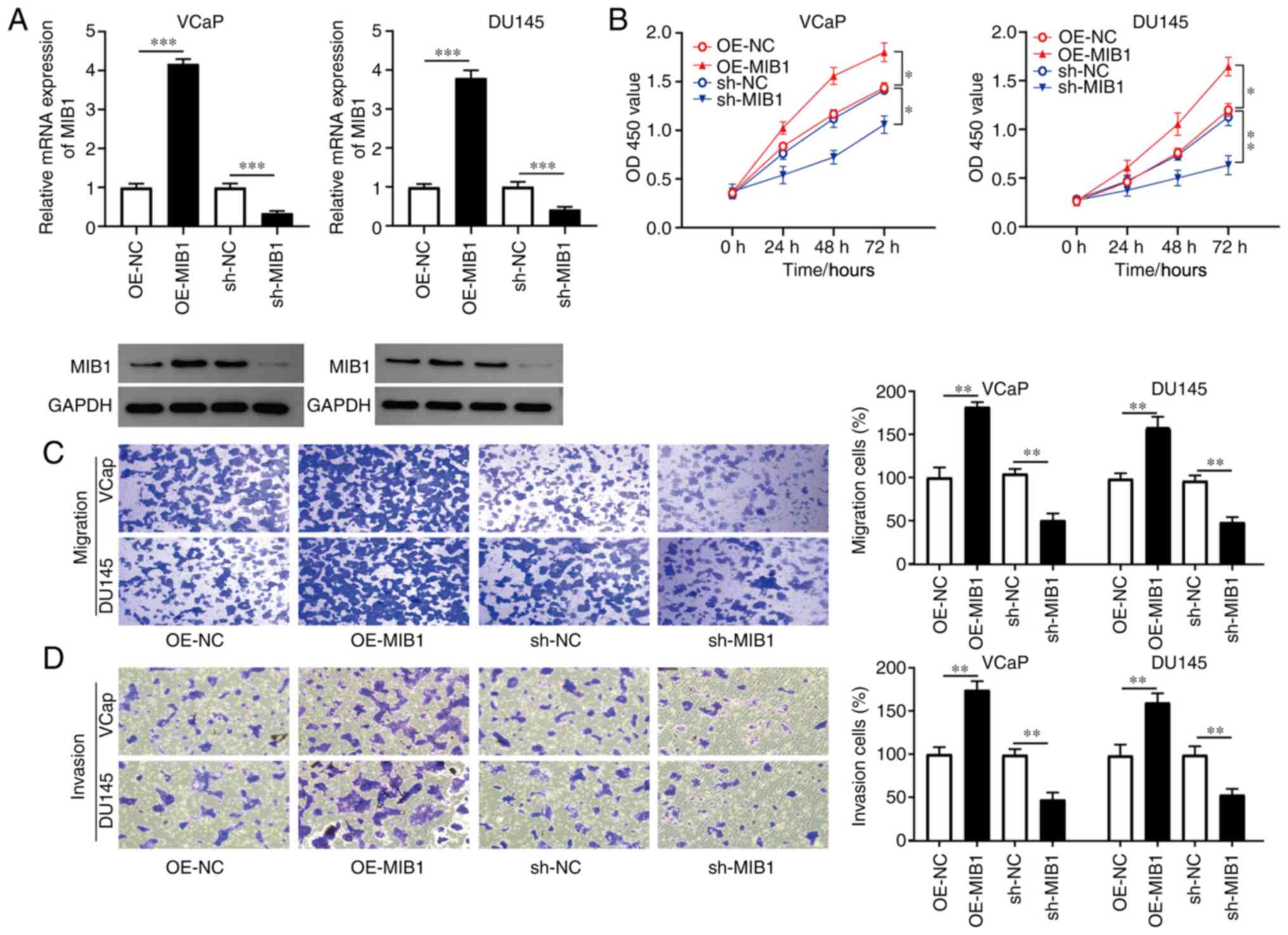 | Figure 2.MIB1 promotes PCa cell proliferation,
migration and invasion. To elucidate the function of MIB1 in PCa,
the VCaP and DU145 cells were transfected with OE-MIB1, sh-MIB1,
OE-NC or sh-NC. (A) mRNA and protein expression level of MIB1 in
the transfected VCaP and DU145 cells was measured using reverse
transcription-quantitative PCR and western blot analysis,
respectively. (B) Cell Counting Kit-8 assay was used to assess the
proliferation of the transfected VCaP and DU145 cell lines.
Transwell assays were used to evaluate the (C) migration and (D)
invasion of the transfected VCaP and DU145 cell lines. *P<0.05,
**P<0.01 and ***P<0.001. MIB1, mind bomb 1; PCa, prostate
cancer; OE, overexpression; sh, short hairpin RNA; NC, negative
control; OD, optical density. |
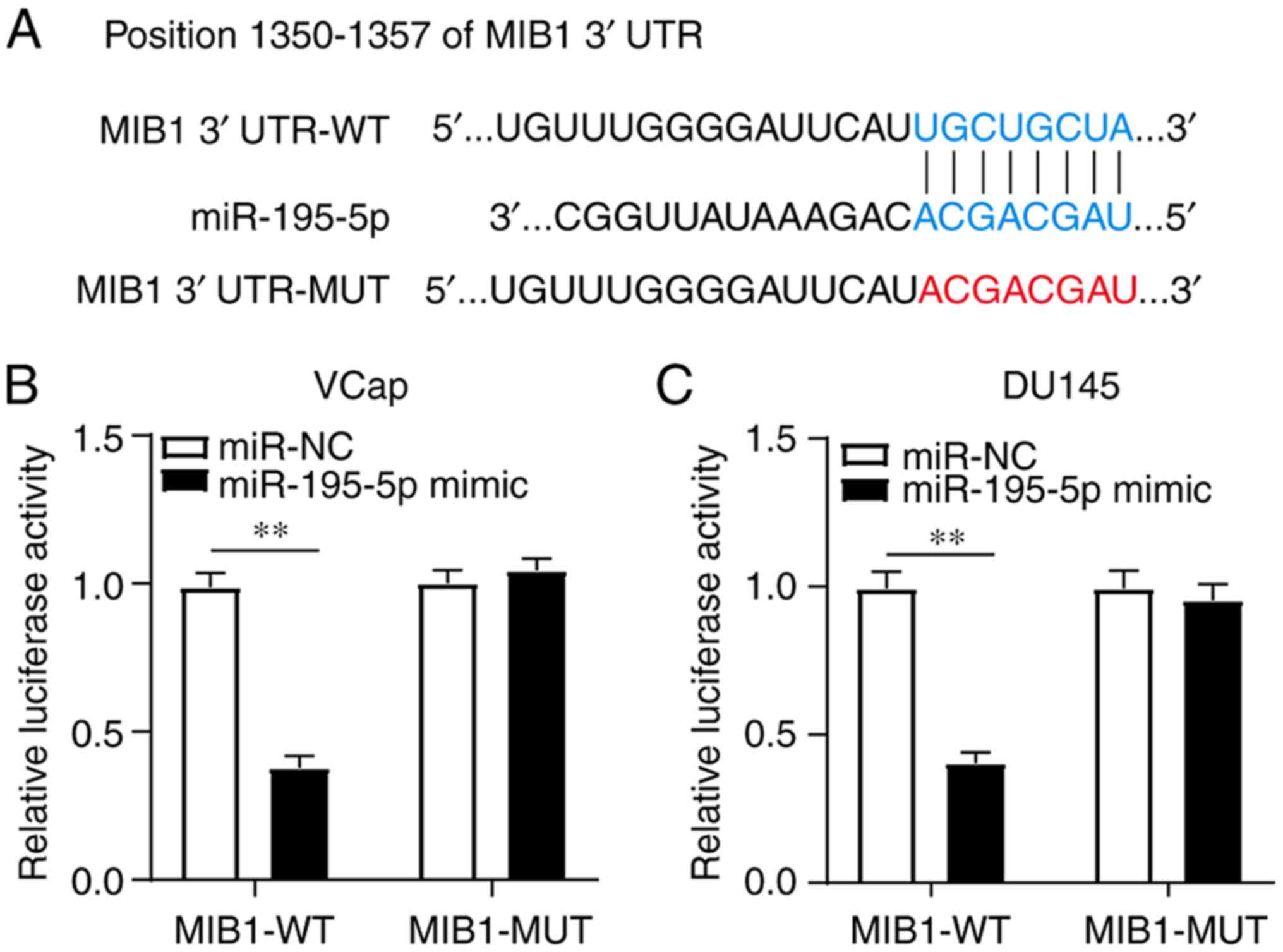
MIB1 is targeted by miR-195-5p
To further investigate the regulatory network of
MIB1 in PCa, TargetScan was used to identify the miRNAs that could
potentially target MIB1. The putative binding sites between MIB1
and miR-195-5p are presented in Fig.
3A. To confirm whether there was a direct interaction,
luciferase reporter vectors for MIB1-WT and MIB1-MUT were
constructed and subsequently co-transfected into the VCaP and DU145
cell lines alongside miR-195-5p mimics or miR-NC. The results
indicated that transfection with miR-195-5p mimics significantly
decreased the luciferase activity of the MIB1-WT reporter, but had
no effect on the luciferase activity of the MIB1-MUT reporter,
suggesting that there was a targeted regulatory relationship
between MIB1 and miR-195-5p in both cells lines (Fig. 3B and C). Taken together, these
results demonstrated that miR-195-5p could directly bind with
MIB1.
miR-195-5p inhibits the expression
level of MIB1 in the PCa cell lines
The mRNA expression level of miR-195-5p in the PCa
tissues and cell lines (PC-3, VCaP, 22Rv1, DU145 and LNCaP) was
detected using RT-qPCR. The results demonstrated that miR-195-5p
mRNA expression level was significantly decreased in PCa tissues
(n=40) and cell lines (PC-3, VCaP, 22Rv1, DU145 and LNCaP) compared
with that in adjacent normal tissues and the normal human prostate
epithelial cell line, RWPE1, respectively (Fig. 4A and B). The correlation between
miR-195-5p and MIB1 expression was subsequently assessed using
Pearson's correlation analysis. The results indicated that there
was a moderate negative correlation between miR-195-5p and MIB1
mRNA expression levels (r, −0.5978; P<0.001; Fig. 4C). The VCaP and DU145 cell lines
were then transfected with miR-195-5p mimics and its inhibitor
(miR-195-5p and anti-miR-195-5p) or their corresponding negative
controls (miR-NC and anti-miR-NC). RT-qPCR and western blot
analyses revealed that MIB1 mRNA and protein expression level,
respectively, was markedly reduced in the VCaP and DU145 cell lines
transfected with miR-195-5p mimics, and was notably increased
following knockdown of miR-195-5p expression (Fig. 4D). These data suggested that MIB1
may serve a potential role in PCa, and that miR-195-5p suppressed
the expression level of MIB1 in a targeted manner.
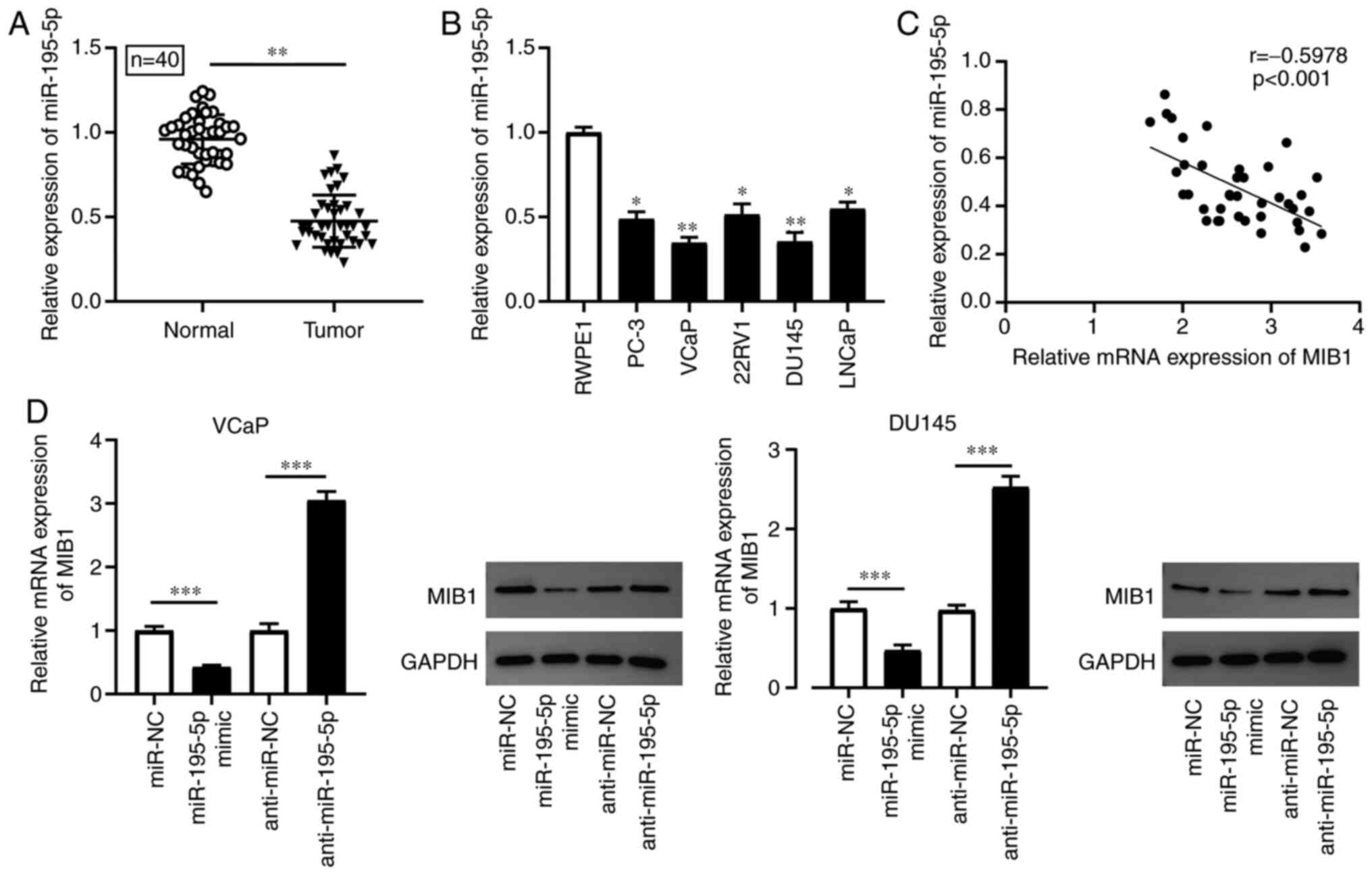 | Figure 4.miR-195-5p expression level is
elevated in PCa tissues and cell lines, and MIB1 is directly
regulated by miR-195-5p. The mRNA expression level of miR-195-5p
was examined using RT-qPCR in (A) PCa tissues and adjacent normal
tissues (n=40), as well as in (B) PCa cell lines (PC-3, VCaP,
22Rv1, DU145 and LNCaP) and a normal human prostate epithelial cell
line (RWPE1). (C) Pearson's analysis was used to assess the
correlation between MIB1 and miR-195-5p expression in PCa. (D) MIB1
expression was measured using RT-qPCR and western blot anlaysis in
VCaP and DU145 cells transfected with miR-195-5p mimics,
anti-miR-195-5p, miR-NC or anti-miR-NC. *P<0.05, **P<0.01 and
***P<0.001. miR, microRNA; PCa, prostate cancer; MIB1, mind bomb
1; RT-qPCR, reverse transcription-quantitative PCR; NC, negative
control. |
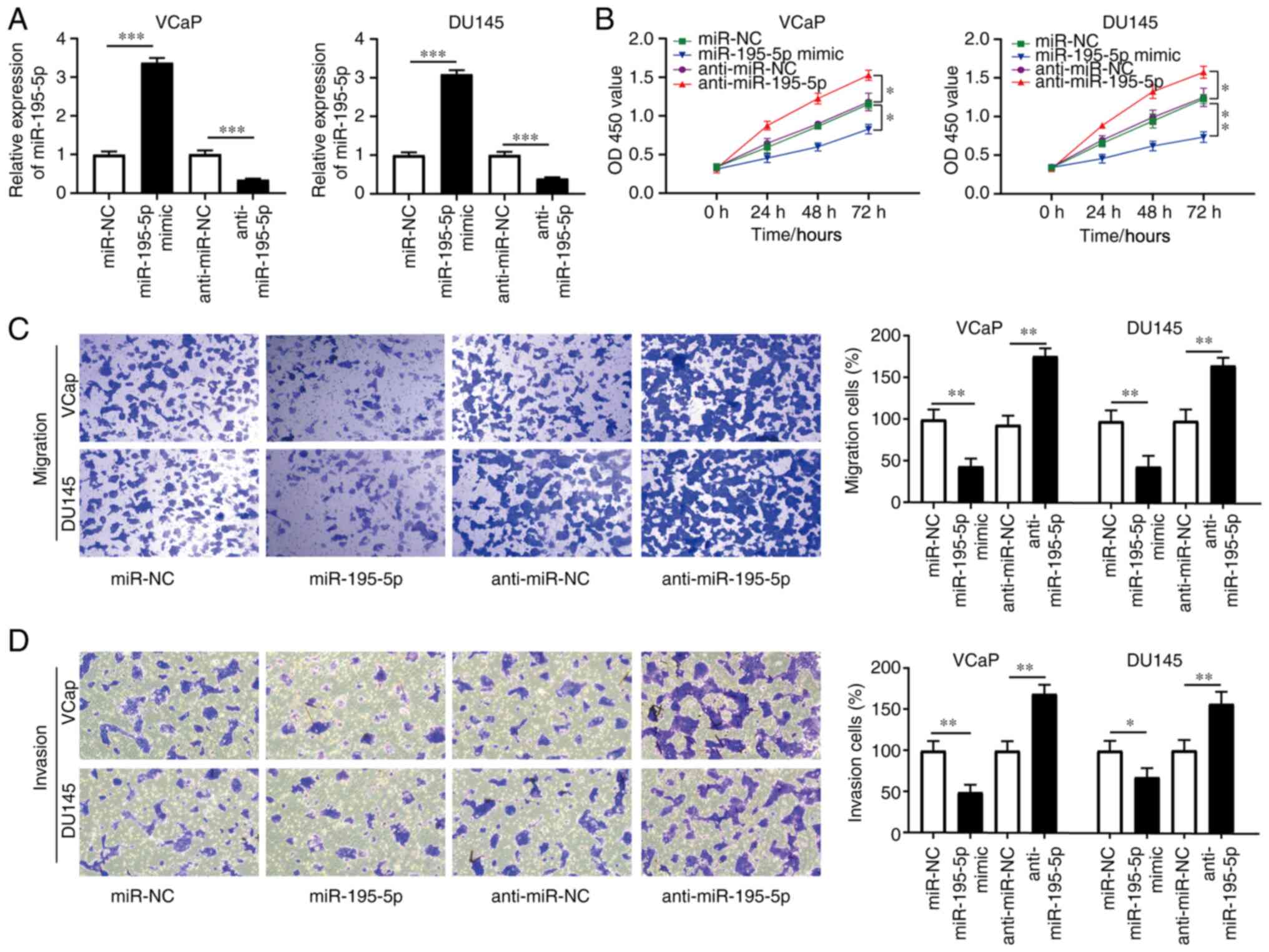
miR-195-5p overexpression inhibits PCa
cell proliferation, migration and invasion
To investigate the biological function of miR-195-5p
in PCa cells, the VCaP and DU145 cell lines were transfected with
miR-195-5p mimics, anti-miR-195-5p, miR-NC or anti-miR-NC. The
results of RT-qPCR demonstrated that the mRNA expression level of
miR-195-5p was markedly increased in the VCaP and DU145 cells
following miR-195-5p mimics transfection, and that the mRNA
expression level of miR-195-5p was significantly decreased
following transfection with anti-miR-195-5p (Fig. 5A). The results of the CCK-8 assay
indicated that miR-195-5p mimics significantly inhibited cell
proliferation, and that miR-195-5p knockdown markedly promoted the
viability of the VCaP and DU145 cell lines (Fig. 5B). In addition, the results of the
Transwell and Matrigel assays revealed that VCaP and DU145 cell
migration and invasion following miR-195-5p mimics transfection was
markedly reduced compared with that in the cells transfected with
NC. Furthermore, miR-195-5p knockdown promoted cell migration and
invasion compared with that in cells transfected with the NC
(Fig. 5C and D). These data
indicated that miR-195-5p regulated the proliferation, migration
and invasion of PCa cells in vitro.
miR-195-5p regulates cell
proliferation and invasion by promoting MIB1 expression
To confirm that miR-195-5p serves a role in the
regulation of MIB1, miR-195-5p and MIB1 were overexpressed and
knocked down at the same time. RT-qPCR and western blot analyses
revealed that MIB1 overexpression reversed the inhibition of
endogenous MIB1 expression in the VCaP and DU145 cell lines
transfected with miR-195-5p mimics (Fig. 6A). In addition, MIB1 knockdown
(sh-MIB1) reversed the promotion of MIB1 expression in the VCaP and
DU145 cells transfected with anti-miR-195-5p (Fig. 6B). According to the results of the
CCK-8 assay, miR-195-5p mimic transfection suppressed the
proliferation of the VCaP and DU145 cell lines, and co-transfection
of miR-195-5p mimics and OE-MIB1 reversed the effect of miR-195-5p
mimics on cell proliferation (Fig.
6C). Anti-miR-195-5p transfection promoted the proliferation of
the VCaP and DU145 cell lines, and the co-transfection of
anti-miR-195-5p and sh-MIB1 abolished the effect of miR-195-5p
knockdown on cell proliferation (Fig.
6D). The overexpression of MIB1 rescued the miR-195-5p
overexpression-induced anti-invasive effects of the VCaP and DU145
cell lines (Fig. 6E) and
co-transfection of anti-miR-195-5p and sh-MIB1 reversed the effect
of miR-195-5p knockdown on cell invasion (Fig. 6F). The results demonstrated that
miR-195-5p regulated cell proliferation and invasion by promoting
MIB1 expression in PCa.
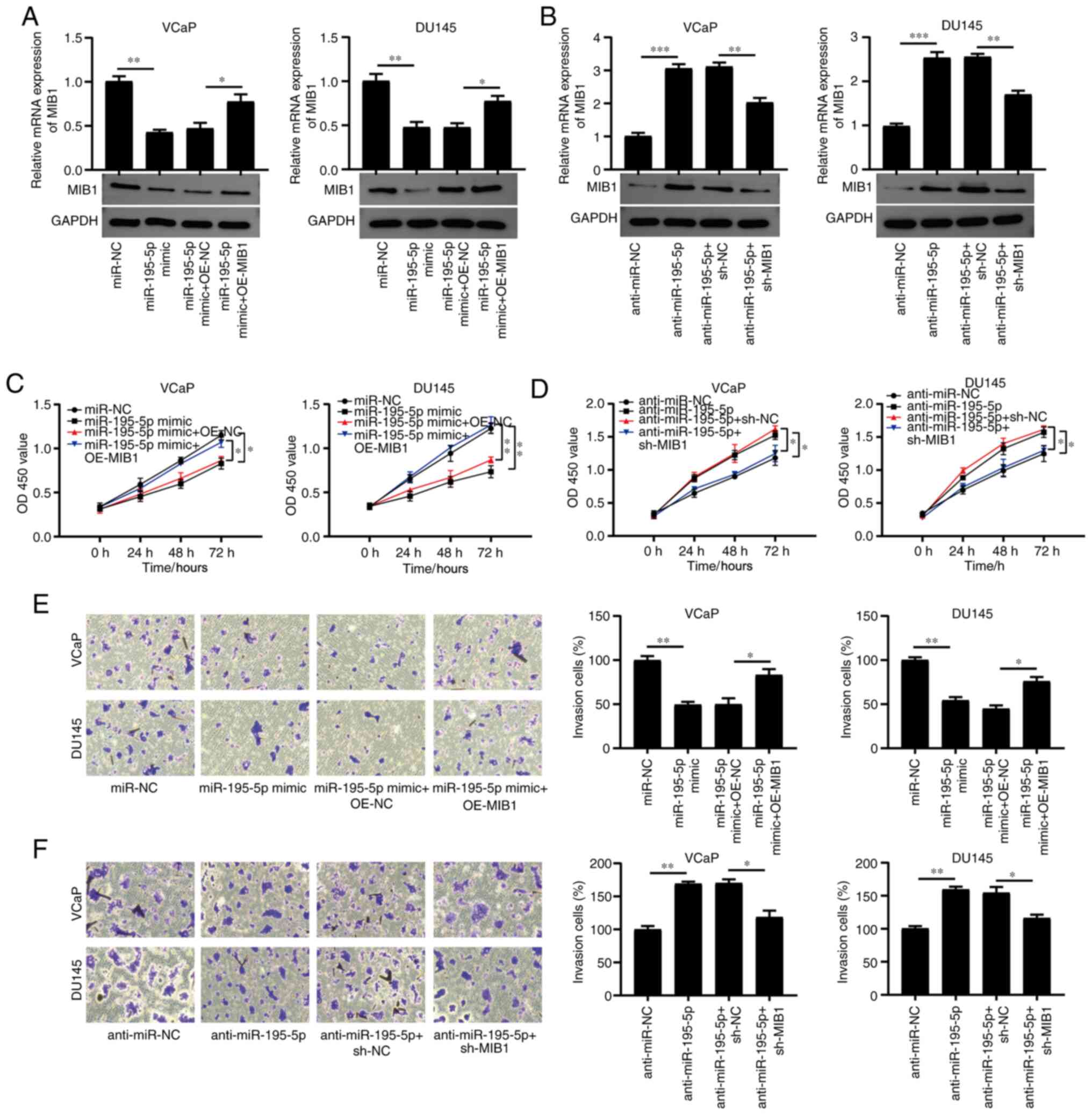 | Figure 6.miR-195-5p promotes the proliferation
and invasion of PCa cells via MIB1. To elucidate the function of
the miR-195-5p/MIB1 axis in PCa, the VCaP and DU145 cell lines were
transfected with miR-195-5p mimics, miR-NC, anti-miR-195-5p,
anti-miR-NC, miR-195-5p mimics + OE-NC, miR-195-5p mimics +
OE-MIB1, anti-miR-195-5p + sh-MIB1 or anti-miR-195-5p + sh-NC. The
mRNA and protein expression levels of MIB1 was determined in the
VCaP and DU145 cell lines using reverse transcription-quantitative
PCR and western blot analysis, respectively following transfection
with (A) miR-195-5p mimics and OE-MIB1, and (B) anti-miR-195-5p and
sh-MIB1. Cell Counting Kit-8 assay was used to assess the
proliferation of VCaP and DU145 cells transfected with (C)
miR-195-5p mimics, miR-NC, miR-195-5p mimics + OE-NC, miR-195-5p
mimic + OE-MIB1, and (D) anti-miR-195-5p, anti-miR-NC,
anti-miR-195-5p + sh-MIB1 or anti-miR-195-5p + sh-NC. Transwell
assay was used to detect the invasion of VCaP and DU145 cells
transfected with (E) miR-195-5p mimics, miR-NC, miR-195-5p mimics +
OE-NC, miR-195-5p mimics + OE-MIB1 and (F) anti-miR-195-5p,
anti-miR-NC, anti-miR-195-5p + sh-MIB1 or anti-miR-195-5p + sh-NC.
*P<0.05, **P<0.01 and ***P<0.001. miR, microRNA; PCa,
prostate cancer; MIB1, mind bomb 1; NC, negative control; OE,
overexpression; sh, short hairpin RNA; OD, optical density. |
Discussion
PCa is considered to be a common malignant tumor,
that poses a major threat to the health of men worldwide (1,2).
Therefore, designing novel treatment strategies is important for
the effective therapy of patients with PCa. The current study aimed
to identify novel biomarkers that could improve current treatment
strategies. The results revealed the essential role of MIB1 in PCa
cell proliferation, migration and invasion, and identified that
miR-195-5p was a regulator of MIB1 expression. RT-qPCR indicated
that miR-195-5p mRNA expression level was decreased in PCa tissues
and cell lines, while MIB1 expression was elevated. By targeting
MIB1, it was demonstrated that miR-195-5p inhibited the
proliferation and invasion of PCa cells. Collectively, the findings
of the current study indicated that MIB1 may be crucial for PCa
progression and that miR-195-5p may inhibit the proliferation of
PCa.
MIB1 is an E3 ubiquitin ligase. Previous studies
have indicated that a number of molecules, such as catenin δ-1
(CTNND1) (10), receptor-like
tyrosine kinase (11),
death-associated protein kinase (12), δ (14), Werner syndrome protein (28), Jagged (29) and polo-like kinase 4 (30), were ubiquitinated by MIB1. For
example, MIB1 may directly regulate cell migration by
ubiquitinating CTNND1 to modulate GTPase activity (10). The present study verified that the
MIB1 mRNA and protein expression level was significantly increased
in PCa tissues and cell lines (PC-3, VCaP, 22Rv1, DU145 and LNCaP).
Furthermore, MIB1 overexpression markedly promoted VCaP and DU145
cell proliferation, migration and invasion. Bioinformatics analysis
and the dual-luciferase reporter assay demonstrated that MIB1 was a
direct target gene of miR-195-5p. However, further studies on the
role of MIB1 and miR-195-5p in PCa are required.
Over the past few years, supporting lines of
evidence have indicated that miRNAs serve crucial roles in prostate
tumorigenesis using a range of mechanisms (31–36),
and this has attracted the interest of numerous researchers who aim
to determine their underlying mechanisms of action. miR-195-5p
belongs to the miR-15 family, and has been demonstrated to exhibit
a low expression level in various types of cancer, serving a key
negative regulatory role (17–21).
In PCa, miR-195-5p suppressed the migration and invasion of the
DU145 and PC3 cell lines by targeting Fos-related antigen 1
(22). Furthermore, miR-195
inhibited the proliferation of the PC-3, LNCaP and DU145 cell lines
by targeting ribosomal protein S6 kinase B1 (23). Cai et al (24) demonstrated that miR-195 inhibited
DU145 and LNCaP cell proliferation and angiogenesis by
downregulating the expression of proline rich 11. In addition,
hsa_circular RNA_0062019 promoted the proliferation, migration and
invasion of PCa cells via the miR-195-5p/high-mobility group
AT-hook 2 axis (37). Long
non-coding (lnc)RNA AFAP1 antisense RNA 1 has also been
demonstrated to modulate the sensitivity of paclitaxel-resistant
PCa cells (PC3 and DU145) to paclitaxel via the miR-195-5p/FKBP
prolyl isomerase 1A axis (38).
lncRNA LINC00473 additionally contributed to cell proliferation by
regulating the miR-195-5p/SEPT2 axis (39). Consistent with these studies, the
present study confirmed that miR-195-5p was significantly decreased
in PCa tissues and cell lines (PC-3, VCaP, 22Rv1, DU145 and LNCaP),
and revealed that miR-195-5p overexpression inhibited the
proliferation, migration and invasion of VCaP and DU145 cells. The
results indicated that miR-195-5p regulated cell proliferation and
invasion by promoting MIB1 expression.
However, the present study still has some
limitations, which require further investigation. First, whether
the expression level of MIB1 and miR-195-5p is associated with
reduced overall survival, recurrence-free survival or
metastasis-free survival in patients with PCa should be analyzed
using Kaplan-Meier survival analysis. Unfortunately, this was not
achieved in the present study due to insufficient collection of
relevant data. Second, future investigations are required to
determine the more specific functions of MIB1 and miR-195-5p in PCa
cells, such as their role in cell apoptosis with in vivo
experimental verification.
In conclusion, the current study demonstrated that
miR-195-5p overexpression markedly suppressed VCaP and DU145 cell
proliferation, migration and invasion. The results are supported by
previous studies (22–24) and indicate that miR-195-5p may
serve an essential role in cancer development and progression. More
importantly, the current study revealed that MIB1 overexpression
markedly promoted the proliferation, migration and invasion of VCaP
and DU145 cells. The results additionally suggested that miR-195-5p
overexpression inhibited the proliferation and invasion of PCa
cells by targeting MIB1, providing a novel promising molecular
target for the treatment of PCa.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was funded by the National Natural
Science Foundation of China (grant no. 31860242) and the
Construction Projects of Medical Biomaterial Research and
Development Talent Base in Guizhou Province and Zunyi City [grant
nos. (2018)3, and (2019)69].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
BC and GB confirm the authenticity of all the raw
data. BC designed the study, analyzed the experiments and wrote the
paper. GB, XM and LT collected the data and formed data analysis.
HX designed the study, and wrote and revised the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Zunyi Medical
University Ethics Committee. All participants provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cai JR, Chen Z, Chen XL, Huang H, Lin X
and Miao B: Coexpression network analysis identifies a novel
nine-RNA signature to improve prognostic prediction for prostate
cancer patients. Biomed Res Int. 2020:42642912020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wild CP, Espina C, Bauld L, Bonanni B,
Brenner H, Brown K, Dillner J, Forman D, Kampman E, Nilbert M, et
al: Cancer prevention Europe. Mol Oncol. 13:528–534. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Chen R, Sun T, Zhao L, Liu F, Ren
S, Wang H, Lu X, Gao X, Xu C and Sun Y: Efficacy and safety of
combined androgen blockade with antiandrogen for advanced prostate
cancer. Curr Oncol. 26:e39–e47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corona G, Baldi E and Maggi M: Androgen
regulation of prostate cancer: Where are we now? J Endocrinol
Invest. 34:232–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Damber JE and Aus G: Prostate cancer.
Lancet. 371:1710–1721. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steele CB, Li J, Huang B and Weir HK:
Prostate cancer survival in the United States by race and stage
(2001–2009): Findings from the CONCORD-2 study. Cancer. 123 (Suppl
24):S5160–S5177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moyer VA; U.S. Preventive services task
force, : Screening for prostate cancer: U.S. Preventive services
task force recommendation statement. Ann Intern Med. 157:120–134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loeb S, Bjurlin MA, Nicholson J, Tammela
TL, Penson DF, Carter HB, Carroll P and Etzioni R: Overdiagnosis
and overtreatment of prostate cancer. Eur Urol. 65:1046–1055. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Welch HG and Albertsen PC: Reconsidering
prostate cancer mortality-the future of PSA screening. N Engl J
Med. 382:1557–1563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizoguchi T, Ikeda S, Watanabe S, Sugawara
M and Itoh M: Mib1 contributes to persistent directional cell
migration by regulating the Ctnnd1-Rac1 pathway. Proc Natl Acad Sci
USA. 114:E9280–E9289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berndt JD, Aoyagi A, Yang P, Anastas JN,
Tang L and Moon RT: Mindbomb 1, an E3 ubiquitin ligase, forms a
complex with RYK to activate Wnt/β-catenin signaling. J Cell Biol.
194:737–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin Y, Blue EK, Dixon S, Shao Z and
Gallagher PJ: A death-associated protein kinase (DAPK)-interacting
protein, DIP-1, is an E3 ubiquitin ligase that promotes tumor
necrosis factor-induced apoptosis and regulates the cellular levels
of DAPK. J Biol Chem. 277:46980–46986. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L and Gallagher PJ: Mind bomb 1
regulation of cFLIP interactions. Am J Physiol Cell Physiol.
297:C1275–C1283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itoh M, Kim CH, Palardy G, Oda T, Jiang
YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et
al: Mind bomb is a ubiquitin ligase that is essential for efficient
activation of Notch signaling by delta. Dev Cell. 4:67–82. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ray J, Hoey C, Huang X, Jeon J, Taeb S,
Downes MR, Boutros PC and Liu SK: MicroRNA-198 suppresses prostate
tumorigenesis by targeting MIB1. Oncol Rep. 42:1047–1056.
2019.PubMed/NCBI
|
|
16
|
Hu YM, Lou XL, Liu BZ, Sun L, Wan S, Wu L,
Zhao X, Zhou Q, Sun MM, Tao K, et al: TGF-β1-regulated miR-3691-3p
targets E2F3 and PRDM1 to inhibit prostate cancer progression.
Asian J Androl. 23:188–196. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng J, Xu TT, Chen F and Zhang Y:
miRNA-195-5p functions as a tumor suppressor and a predictive of
poor prognosis in non-small cell lung cancer by directly targeting
CIAPIN1. Pathol Oncol Res. 25:1181–1190. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo JH, Pan JS, Jin Y, Li MY and Chen M:
miR-195-5p inhibits proliferation and induces apoptosis of
non-small cell lung cancer cells by targeting CEP55. Onco Targets
Ther. 12:11465–11474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du W, Liu T, Zhang Y, Zeng Y, Zhu J, Tang
H, Liu Z and Huang JA: miR-195-5p is a potential factor responsible
for CPNE1 differential expression between subtypes of non-small
cell lung cancer. J Cancer. 11:2610–2620. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu XM, Zhou Y, Ning YE, Gu H, Tong YX and
Wang N: miR-195-5p inhibits malignant progression of cervical
cancer by targeting YAP1. Onco Targets Ther. 13:931–944. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Ren CX, Zhang JM, Xin XY, Hua T and
Wang HB and Wang HB: The effects of miR-195-5p/MMP14 on
proliferation and invasion of cervical carcinoma cells through TNF
signaling pathway based on bioinformatics analysis of microarray
profiling. Cell Physiol Biochem. 50:1398–1413. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu J, Ji A, Wang X, Zhu Y, Yu Y, Lin Y,
Liu Y, Li S, Liang Z, Xu X, et al: MicroRNA-195-5p, a new regulator
of Fra-1, suppresses the migration and invasion of prostate cancer
cells. J Transl Med. 13:2892015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai C, Chen QB, Han ZD, Zhang YQ, He HC,
Chen JH, Chen YR, Yang SB, Wu YD, Zeng YR, et al: miR-195 inhibits
tumor progression by targeting RPS6KB1 in human prostate cancer.
Clin Cancer Res. 21:4922–4934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai C, He H, Duan X, Wu W, Mai Z, Zhang T,
Fan J, Deng T, Zhong W, Liu Y, et al: miR-195 inhibits cell
proliferation and angiogenesis in human prostate cancer by
downregulating PRR11 expression. Oncol Rep. 39:1658–1670.
2018.PubMed/NCBI
|
|
25
|
Alexandratou E, Yova D, Gorpas D, Maragos
P, Agrogiannis G and Kavantzas N: Texture analysis of tissues in
Gleason grading of prostate cancer. Int Soc Opt Photon.
6859:6859042008.
|
|
26
|
Ozkan TA, Eruyar AT, Cebeci OO, Memik O,
Ozcan L and Kuskonmaz I: Interobserver variability in Gleason
histological grading of prostate cancer. Scand J Urol. 50:420–424.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Liu B, Yi J, Yang Y, Wang J, Zhu WG
and Luo J: MIB1-mediated degradation of WRN promotes cellular
senescence in response to camptothecin treatment. FASEB J.
34:11488–11497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ,
Moon JS, Kim YW, Kwon MC, Yoo KW, Kong MP, et al: Mind bomb 1 is
essential for generating functional Notch ligands to activate
Notch. Development. 132:3459–3470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cajánek L, Glatter T and Nigg EA: The E3
ubiquitin ligase Mib1 regulates Plk4 and centriole biogenesis. J
Cell Sci. 128:1674–1682. 2015.PubMed/NCBI
|
|
31
|
Zhang X, Zhou J, Xue D, Li Z, Liu Y and
Dong L: miR-515-5p acts as a tumor suppressor via targeting TRIP13
in prostate cancer. Int J Biol Macromol. 129:227–232. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhan B, Huang L, Chen Y, Ye W, Li J, Chen
J, Yang S and Jiang W: miR-196a-mediated downregulation of p27
protein promotes prostate cancer proliferation and relates to
biochemical recurrence after radical prostatectomy. Prostate.
80:1024–1037. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Y, Jia B, Zhao X, Wang Y and Ye W:
miR-93-5p may be an important oncogene in prostate cancer by
bioinformatics analysis. J Cell Biochem. 120:10463–10483. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nam RK, Benatar T, Wallis CJD, Kobylecky
E, Amemiya Y, Sherman C and Seth A: MicroRNA-139 is a predictor of
prostate cancer recurrence and inhibits growth and migration of
prostate cancer cells through cell cycle arrest and targeting IGF1R
and AXL. Prostate. 79:1422–1438. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du Y, Liu XH, Zhu HC, Wang L, Ning JZ and
Xiao CC: miR-543 promotes proliferation and epithelial-mesenchymal
transition in prostate cancer via targeting RKIP. Cell Physiol
Biochem. 41:1135–1146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu F, Li Q, Wang ZY and Cao XM: Sinomenine
inhibits proliferation, migration, invasion and promotes apoptosis
of prostate cancer cells by regulation of miR-23a. Biomed
Pharmacother. 112:1085922019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang P, Zhang L, Yin S, Xu Y, Tai S, Zhang
LI and Liang C: hsa_circ_0062019 promotes the proliferation,
migration, and invasion of prostate cancer cells via the
miR-195-5p/HMGA2 axis. Acta Biochim Biophys Sin (Shanghai).
12:815–822. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leng W, Liu Q, Zhang S, Sun D and Guo Y:
lncRNA AFAP1-AS1 modulates the sensitivity of paclitaxel-resistant
prostate cancer cells to paclitaxel via miR-195-5p/FKBP1A axis.
Cancer Biol Ther. 21:1072–1080. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xing ZS, Li SL, Liu ZX, Zhang C, Meng MJ
and Bai ZM: The long non-coding RNA LINC00473 contributes to cell
proliferation via JAK-STAT3 signaling pathway by regulating
miR-195-5p/SEPT2 axis in prostate cancer. Biosci Rep.
40:BSR201918502020. View Article : Google Scholar : PubMed/NCBI
|